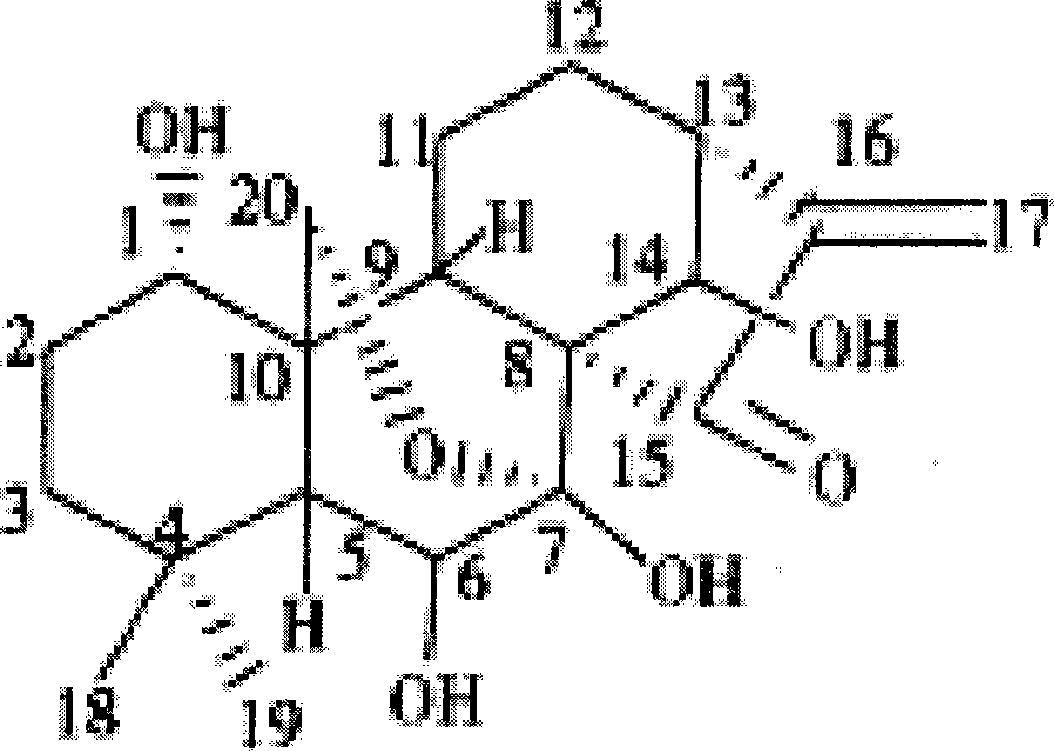
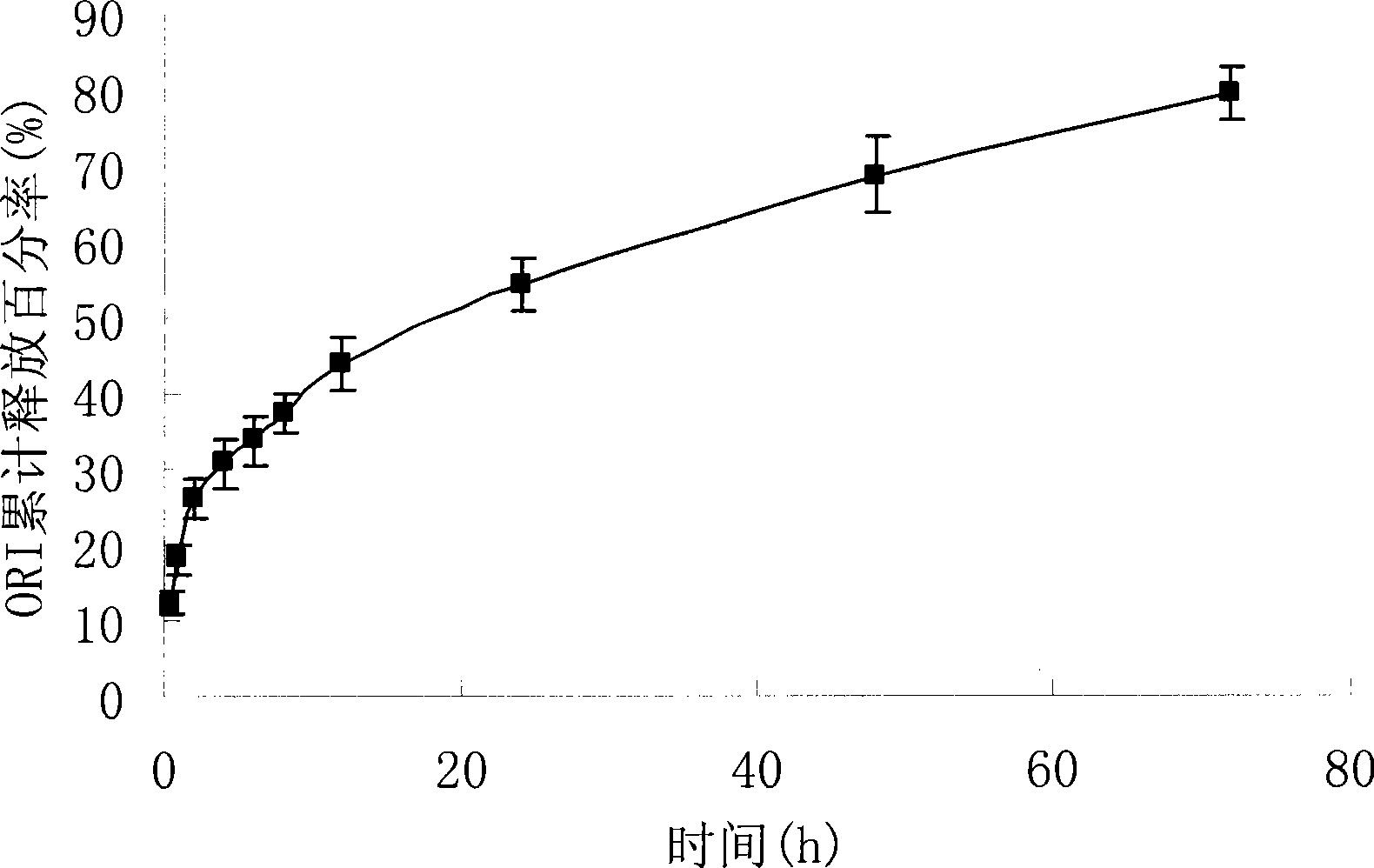
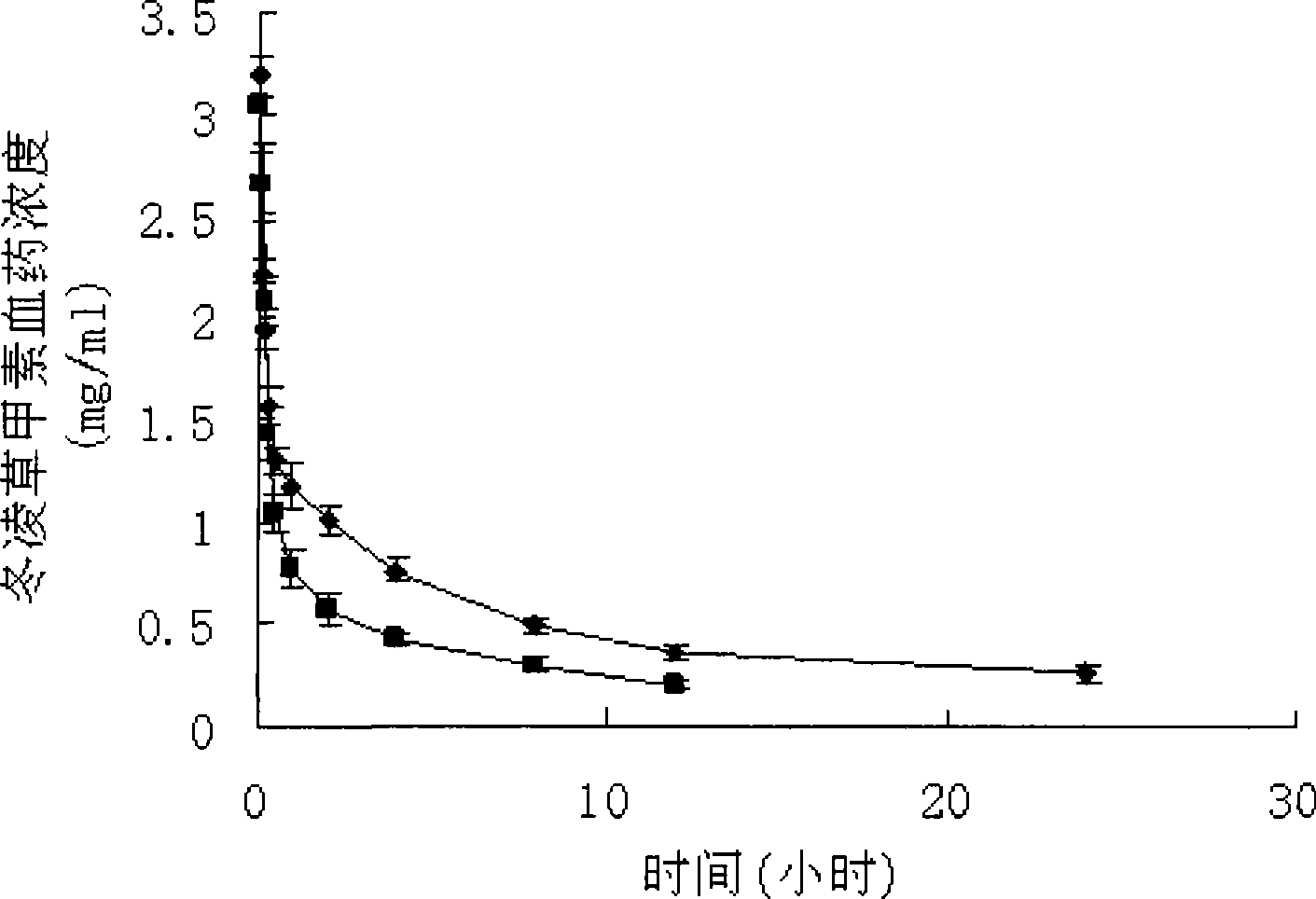
Oridonin polymer micelle administration preparation and preparation method thereof
A technology of oridonin and polymer glue, which is applied in the field of drug delivery preparation and preparation of oridonin polymer micelles, and can solve the problem of unsatisfactory solubilization effect and high toxicity of low-molecular surfactants. problems, to achieve the effects of easy enrichment, reduced toxicity, and extended circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of amphiphilic block copolymer mPEG-PDLLA
[0036] (1) Add 2.5g D, L-lactide and 4g polyethylene glycol monomethyl ether (molecular weight 5000) into a three-necked bottle equipped with a magnetic stirrer, and heat the oil bath to 140°C for melting (decompression vacuum ), add a mass percentage of 0.05% stannous octoate catalyst, react for 8h, stop the reaction, cool to room temperature, and dissolve the product with 10ml of dichloromethane or chloroform, precipitate with 200ml of cold ether, filter, and vacuum dry at 30°C for 24-48 hour, the product mPEG-PDLLA was obtained, and the molecular weight of the PDLLA block was calculated to be 3000 by NMR. The mPEG-PDLLA with a molecular weight of 8000 was obtained 8000
[0037] (2) Add 4.5g D, L-lactide and 4g polyethylene glycol monomethyl ether (molecular weight 5000) into a three-necked bottle equipped with a magnetic stirrer, and heat the oil bath to 140°C for melting (decompression vacuum )...
Embodiment 2
[0039] Embodiment 2: Preparation of amphiphilic block copolymer mPEG-PLLA
[0040] Add 4.5g of L-lactide and 4g of polyethylene glycol monomethyl ether (molecular weight: 5000) into a three-necked bottle equipped with a magnetic stirrer, heat the oil bath to 140°C for melting (decompression and vacuum), then add mass The percentage is 0.05% stannous octoate catalyst, react for 10h, stop the reaction, cool to room temperature, dissolve the product with 10ml of dichloromethane or chloroform, precipitate with 200ml of cold ether, filter, and vacuum dry at 30°C for 24-48 hours to obtain the product mPEG - PLLA, the molecular weight of the PLLA block is calculated to be 5000 by nuclear magnetic spectrum. The mPEG-PLLA with a molecular weight of 10000 was obtained 10000
Embodiment 3
[0041] Embodiment 3: Preparation of amphiphilic block copolymer mPEG-PLGA
[0042] (1) Add 4.5g of D, L-lactide and glycolide mixture (LA / GA=50 / 50) and 4g of polyethylene glycol monomethyl ether (molecular weight 5000) to a three-necked magnetic stirrer In the bottle, after the oil bath is heated to 140°C for melting (decompression and vacuum), add the stannous octoate catalyst that is 0.05% by mass percentage, react for 10h, stop the reaction, cool to room temperature, and the product is dissolved with 10ml of dichloromethane or chloroform. Precipitate with 200ml of cold ether, filter, and vacuum dry at 30°C for 24-48 hours to obtain the product mPEG-PLGA. The molecular weight of the PLGA block is calculated to be 5000 by NMR. The mPEG-PLGA with a molecular weight of 10000 was obtained 10000(50 / 50)
[0043] (2) Add 4.5g of D, L-lactide and glycolide mixture (LA / GA=75 / 25) and 4g of polyethylene glycol monomethyl ether (molecular weight 5000) to a three-necked three-necked st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com