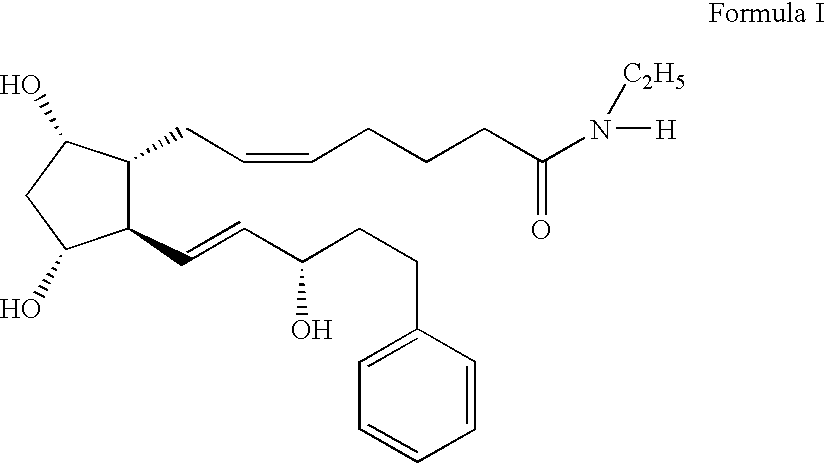
Patents
Literature
884results about How to "Reduced bioavailability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
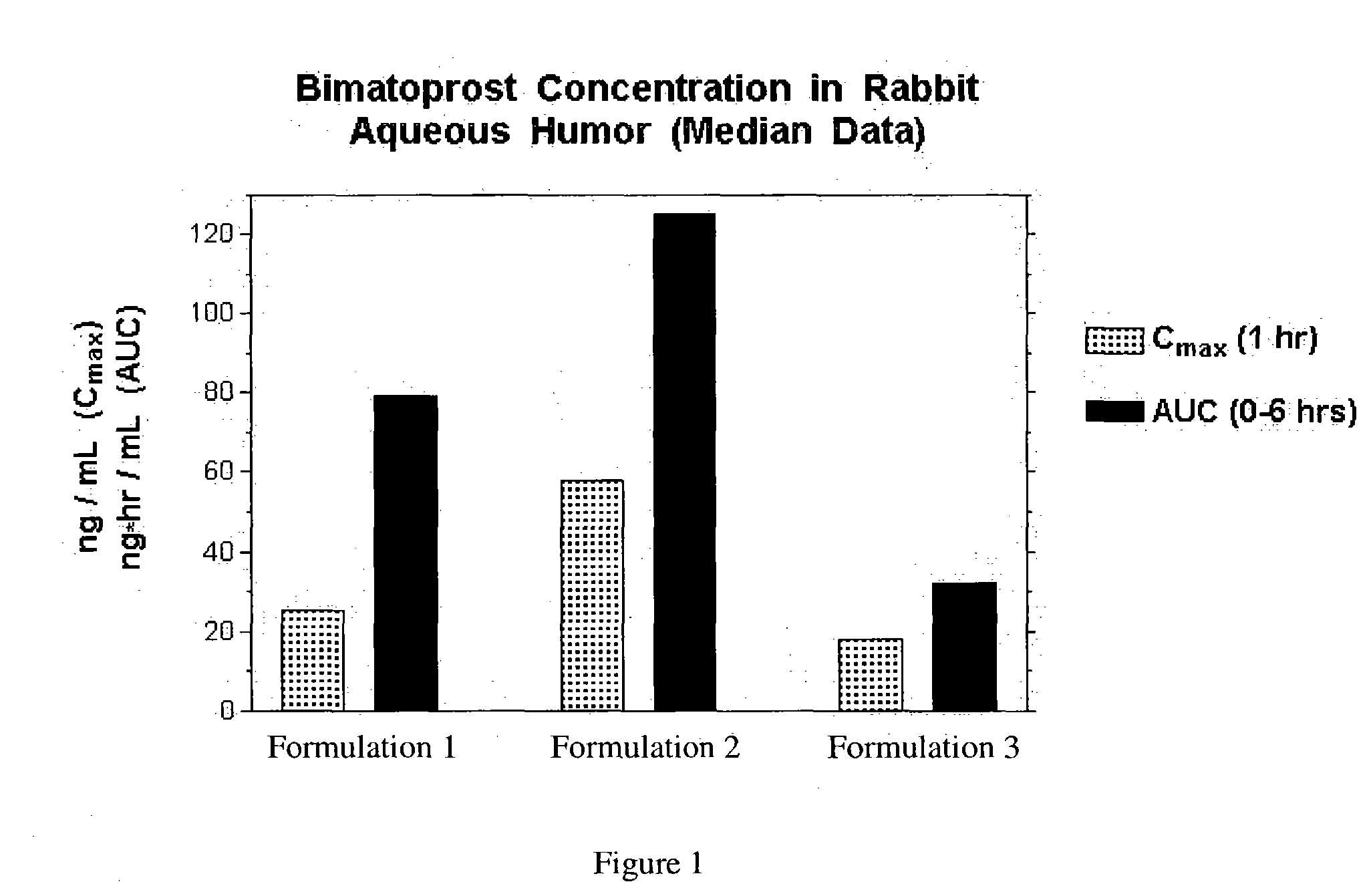
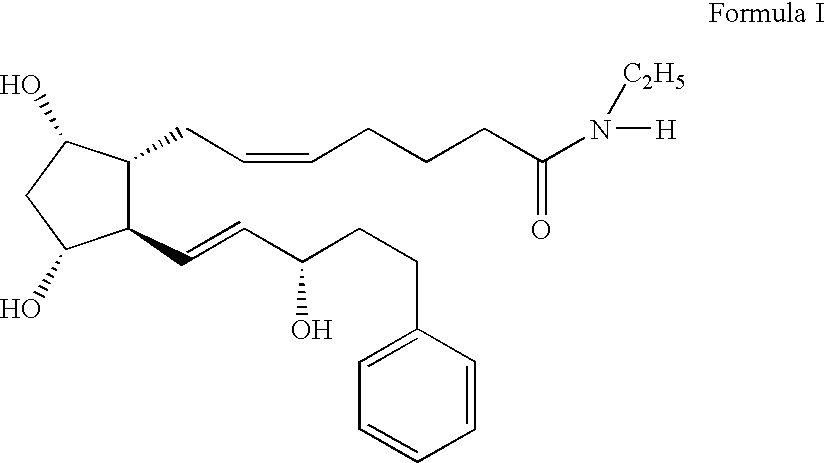
Inhibition of irritating side effects associated with use of a topical ophthalmic medication
InactiveUS20050004074A1Reduce severityEffective deliveryPowder deliveryBiocideCyclodextrin derivativeCyclodextrin Derivatives
This invention relates to a method of reducing an irritating or adverse side effect associated with the topical use of an active ophthalmic drug comprising incorporating an effective amount of a cyclodextrin or cyclodextrin derivative into a formulation to complex the active drug such that the concentration of the free active drug is reduced below a tolerable threshold, and incorporating an effective amount of a viscosity increasing agent in said formulation such that the bioavailability of said drug is high enough to be therapeutically effective, wherein the cyclodextrin or cyclodextrin derivative is not required to solubilize the active drug. Another aspect of this invention relates to topical ophthalmic formulations comprising an active drug, a cyclodextrin or cyclodextrin derivative, and a viscosity-enhancing agent, in effective amounts as stated above.
Owner:ALLERGAN INC
Polymeric drug delivery system for hydrophobic drugs
InactiveUS20050249799A1Low oral bioavailabilityStable against aggregationAntibacterial agentsPowder deliveryHydrophobic polymerImmediate release
An oral delivery system for Class II drugs that have low oral bioavailability due to their insolubility in water and slow dissolution kinetics and method for making such a drug delivery system are disclosed herein. The formulation may be a controlled release or immediate release formulation. The immediate release formulation contains a Class II drug, together with a hydrophobic polymer, preferably a bioadhesive polymer. In one embodiment, the drug and polymer are co-dissolved in a common solvent. The solution is formed into small solid particles by any convenient method, particularly by spray drying. The resulting particles contain drug dispersed as small particles in a polymeric matrix. The particles are stable against aggregation, and can be put into capsules or tableted for administration. The controlled release formulations contain a BCS Class II drug and a bioadhesive polymer. The controlled release formulations may be in the form of a tablet, capsules, mini-tab, microparticulate, or osmotic pump. Enhancement of oral uptake of the drug from use of bioadhesive polymers occurs through (1) increased dissolution kinetics due to stable micronization of the drug, (2) rapid release of the drug from the polymer in the GI tract; and (3) prolonged GI transit due to bioadhesive properties of the polymers. The combination of these effects allows the preparation of a compact, stable dosage form suitable for oral administration of many class II drugs.
Owner:SPHERICS
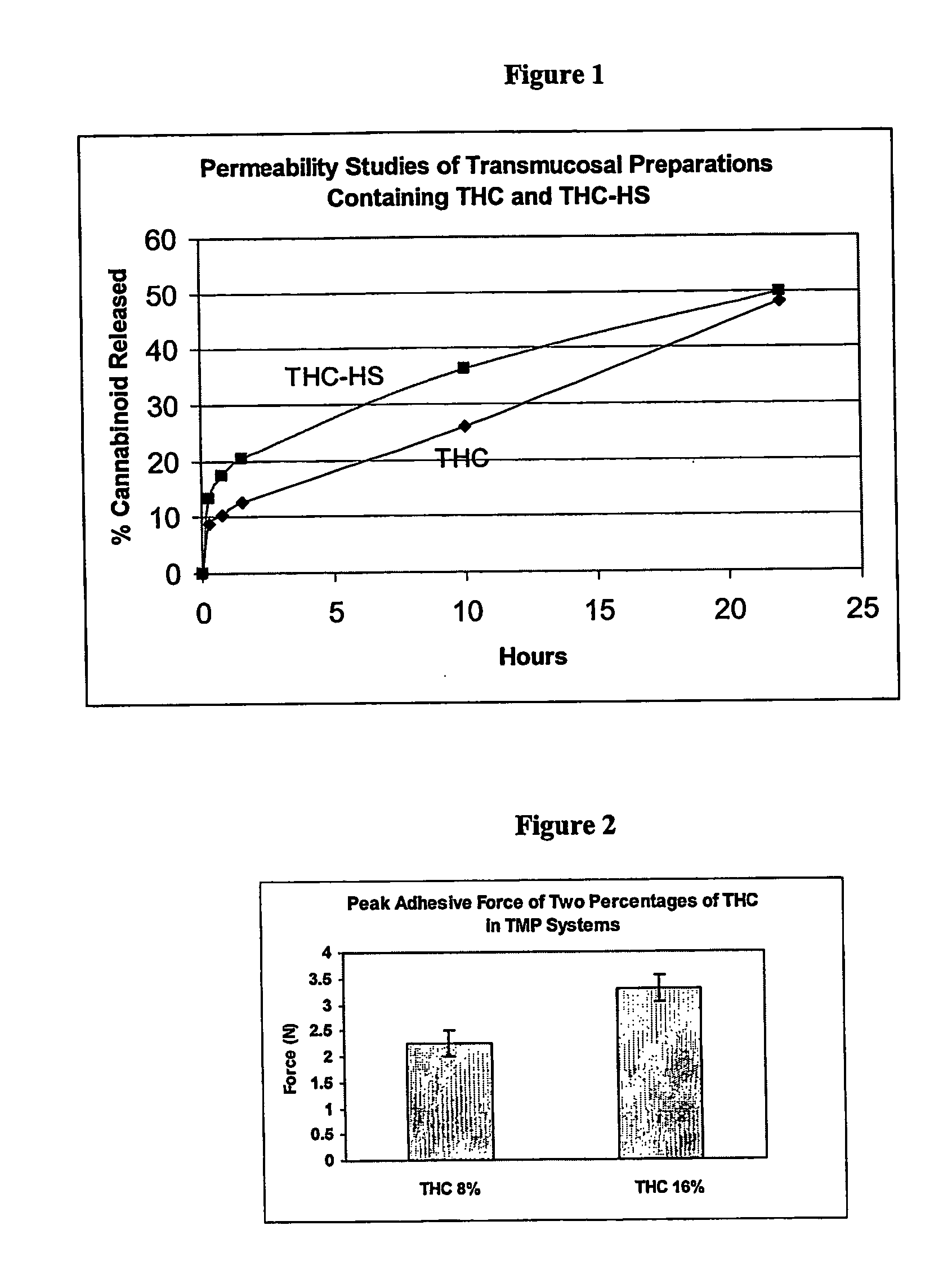
Transmucosal delivery of cannabinoids
InactiveUS20060257463A1Strong tendencyReduced bioavailabilityBiocidePharmaceutical non-active ingredientsCannabisCannabinoid
A method of transmucosally delivering a cannabinoid to a subject in need of such treatment comprising the steps of: administering to the subject a transmucosal preparation containing the cannabinoid wherein said transmucosal preparation is made by incorporating an effective amount of the cannabinoid via hot-melt extrusion technology, hot-melt molding, admixing or a solvent cast technique into a film matrix or a reservoir containing the cannabinoid, and attaching said transmucosal preparation to the mucosa of the subject.
Owner:UNIVERSITY OF MISSISSIPPI
Iron-based bio-char material, preparation process thereof, and application thereof in soil pollution treatment
ActiveCN104388094AImprove performanceReduced bioavailabilityTransportation and packagingContaminated soil reclamationCarbonizationSoil heavy metals
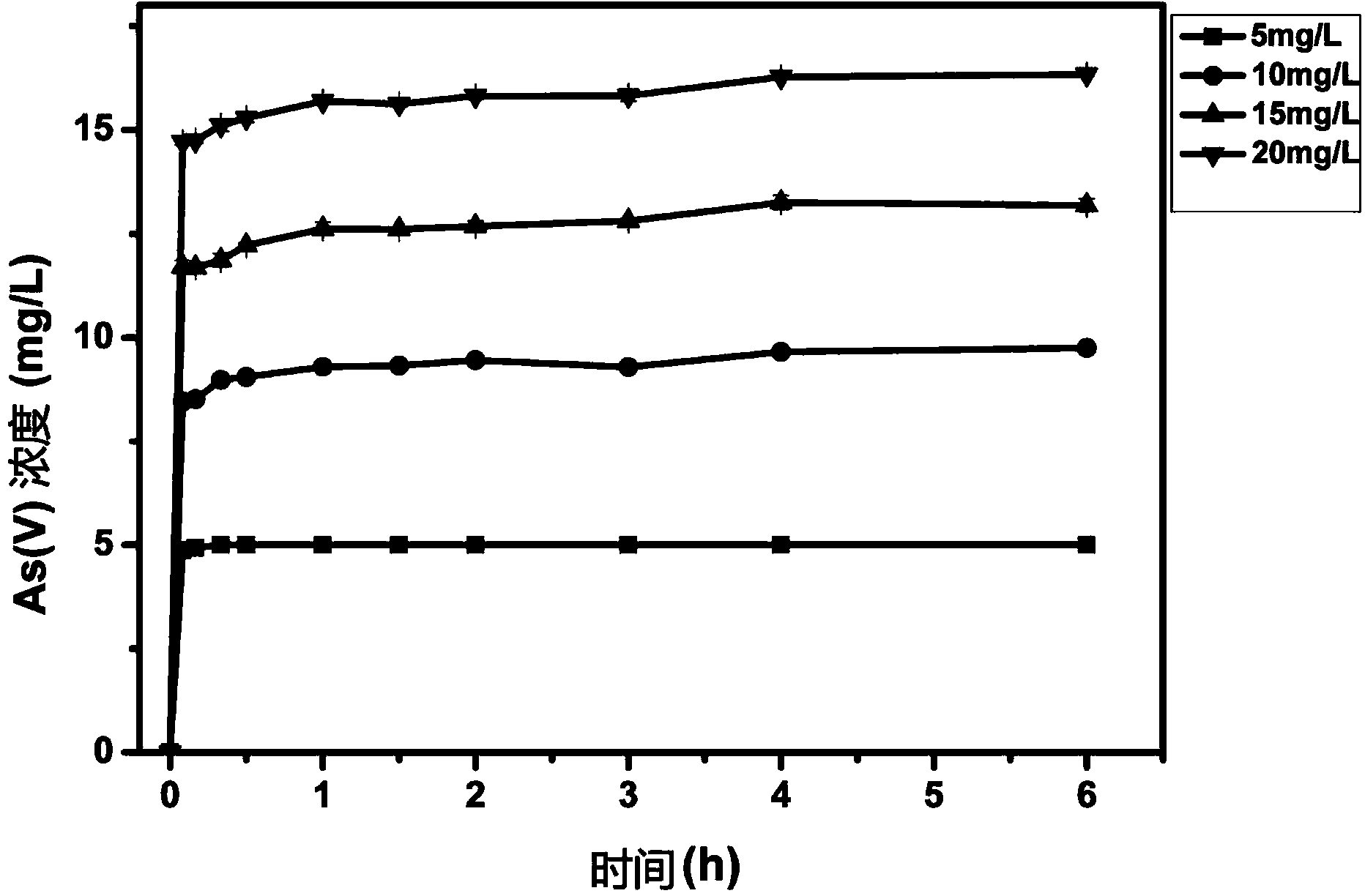
The invention relates to the technical field of soil heavy metal remediation, and specifically discloses a method for preparing an iron-based bio-char material, a prepared iron-based bio-char material, and a method for applying the iron-based bio-char material in treating soil heavy metal pollution. According to the material, biomass is adopted as a raw material; a high-temperature carbonization method is adopted; during the bio-char preparation process, an iron-containing compound is added, such that iron is doped according to a certain ratio, and the iron-based bio-char material with special structure and function is formed. The material has the advantages of simple preparation process, low production cost, and short production period. The obtained iron-based bio-char material has a unique effect in repairing arsenic-cadmium composite polluted soil. With the material, bio-availability of arsenic and cadmium in soil can be effectively reduced, arsenic and cadmium contents in agricultural products planted in the arsenic-cadmium composite polluted soil can be greatly reduced, and no toxic or side effect is caused on crops. The material is safe to apply, and can be used in a large scale in treatment of arsenic-cadmium composite polluted soil.
Owner:GUANGDONG INST OF ECO ENVIRONMENT & SOIL SCI
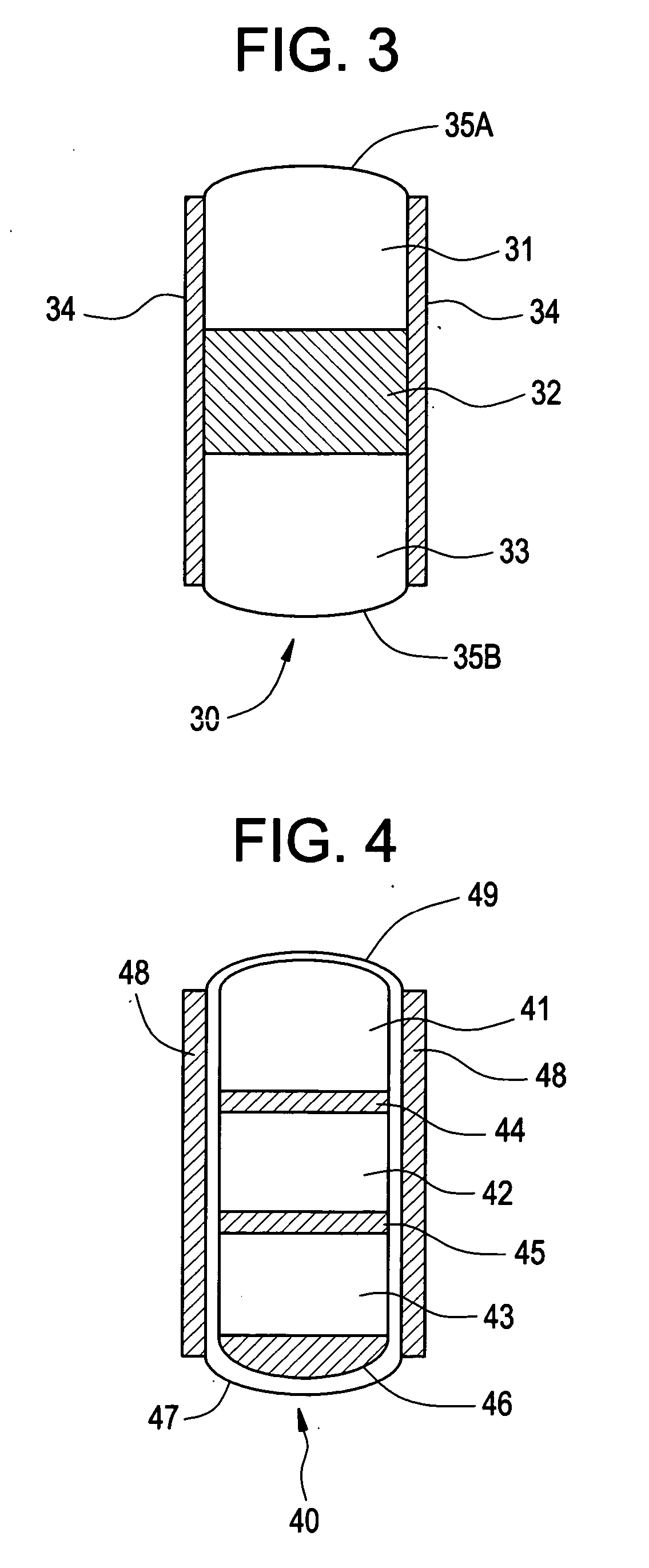
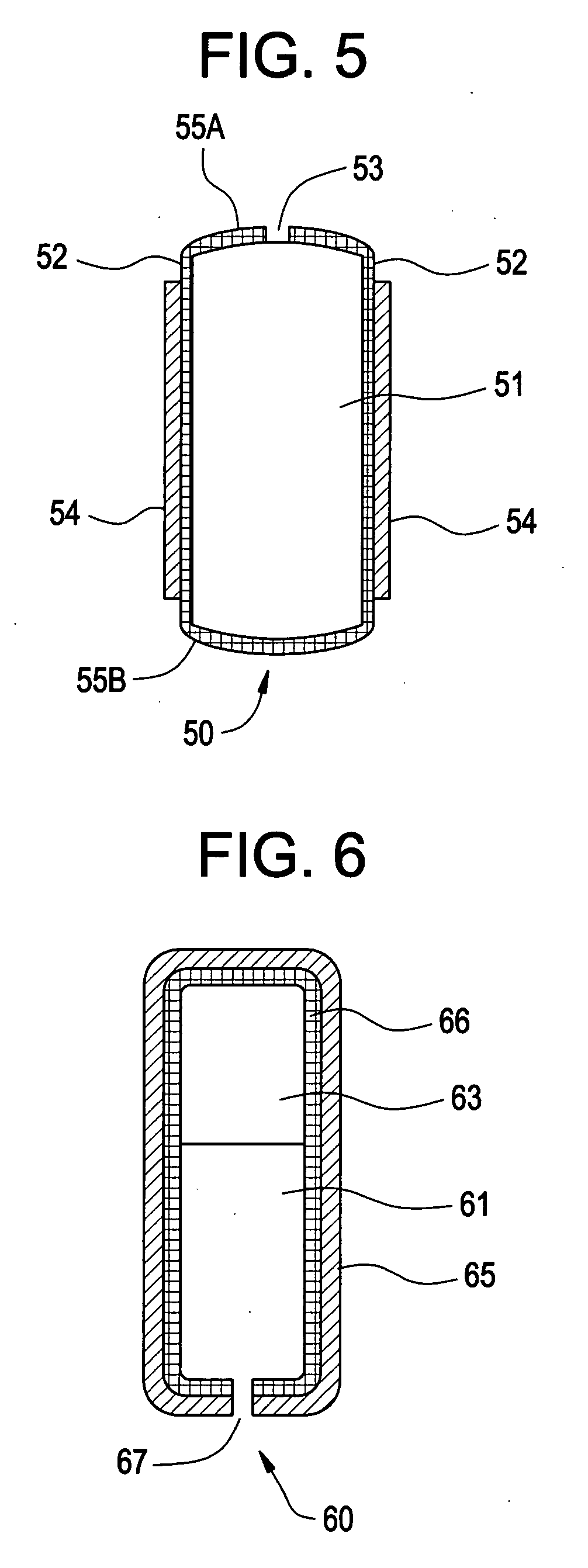
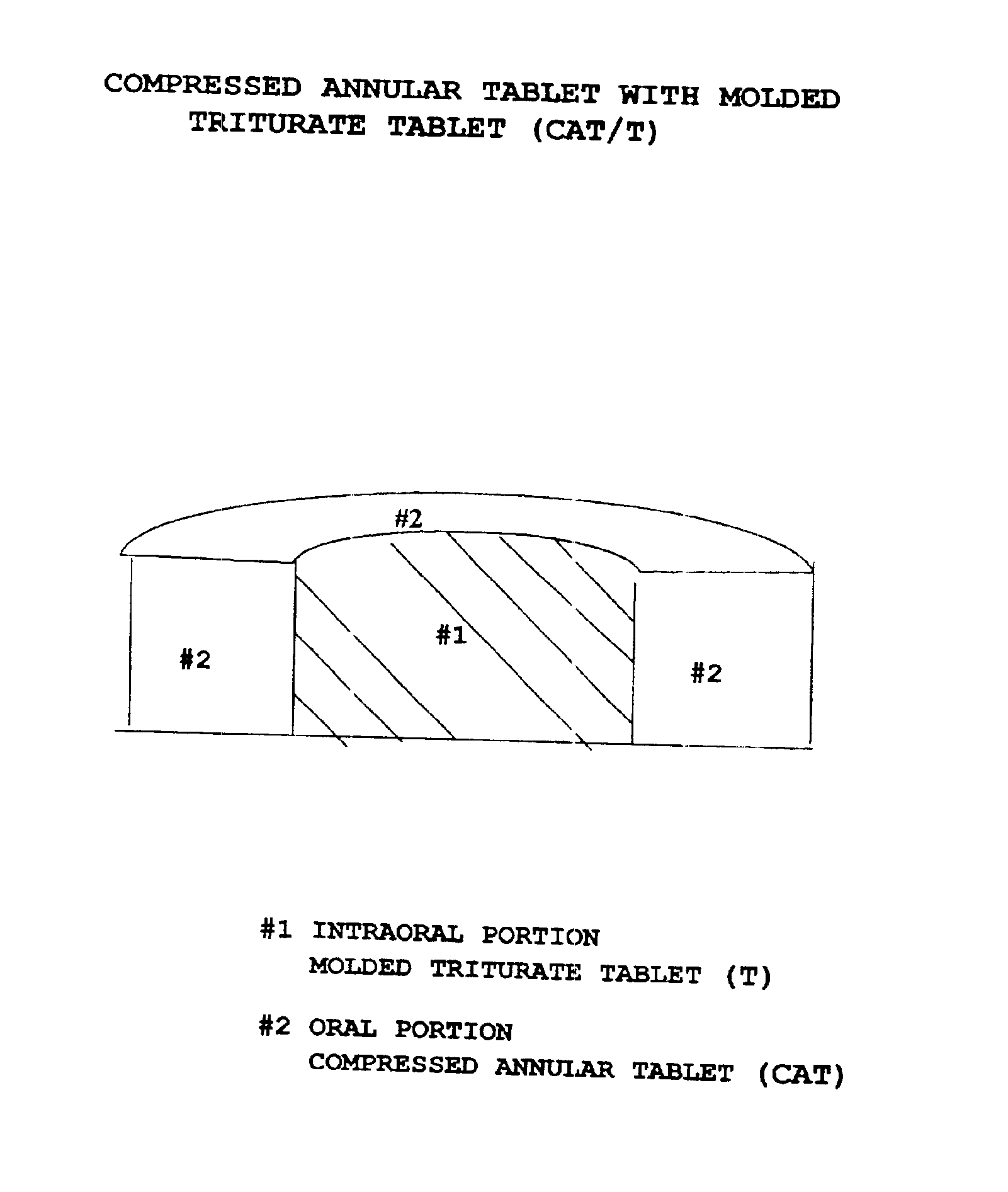
Pharmaceutical composition for compressed annular tablet with molded triturate tablet for both intraoral and oral administration
InactiveUS6863901B2Easily administrable to a patientMaximize the effect of treatmentNervous disorderSkeletal disorderOral medicationPharmaceutical drug
New pharmaceutical compositions in unit dosage form are disclosed for both intraoral and oral administration to a patient, said unit dosage form configured to be placed intraorally of said patient, which comprises:(a) as a first portion, at least one discrete molded triturate tablet comprising a therapeutically effective amount of at least one pharmaceutically active ingredient capable of intraoral administration; and(b) as a second portion located around the said first portion, a therapeutically effective amount of at least one pharmaceutically active ingredient capable of oral administration and which is releasable and orally ingestible by the patient after the molded triturate tablet has disintegrated or has dissolved intraorally.
Owner:HIRSH JANE +1
Spherical Particle and Method for Producing the Same
InactiveUS20100247665A1Improve versatilityImprove wear resistancePretreated surfacesPharmaceutical non-active ingredientsMass ratioAlcohol sugars
A spherical particle of the present invention contains a sugar alcohol and a crystalline cellulose and / or powdered cellulose, wherein the mass ratio between the sugar alcohol and the crystalline cellulose and / or powdered cellulose is within a range from 50:50 to 90:10, the particle size is within a range from 75 to 250 μm, the sphericity is not less than 0.8, and the bulk density is not less than 0.6 g / ml. Further, a method for producing the spherical particle of the present invention includes a granulation step of rolling a sugar alcohol having an average particle size of not more than 40 μm and a crystalline cellulose and / or powdered cellulose having an average particle size of not more than 50 μm while spraying a liquid thereon.
Owner:FREUNT IND
Extended Release Oral Pharmaceutical Compositions of 3-Hydroxy-N-Methylmorphinan and Method of Use
InactiveUS20120065221A1Increased CmaxSufficient bioavailabilityBiocideNervous disorder3-Hydroxy-N-methylmorphinanExtended Release Dosage Form
The present invention is directed to oral, therapeutically effective extended release pharmaceutical compositions of 3-hydroxy-N-methylmorphinan, including delayed onset, extended release dosage forms and the use thereof.
Owner:RELMADA THERAPEUTICS
Chemosensory Receptor Ligand-Based Therapies
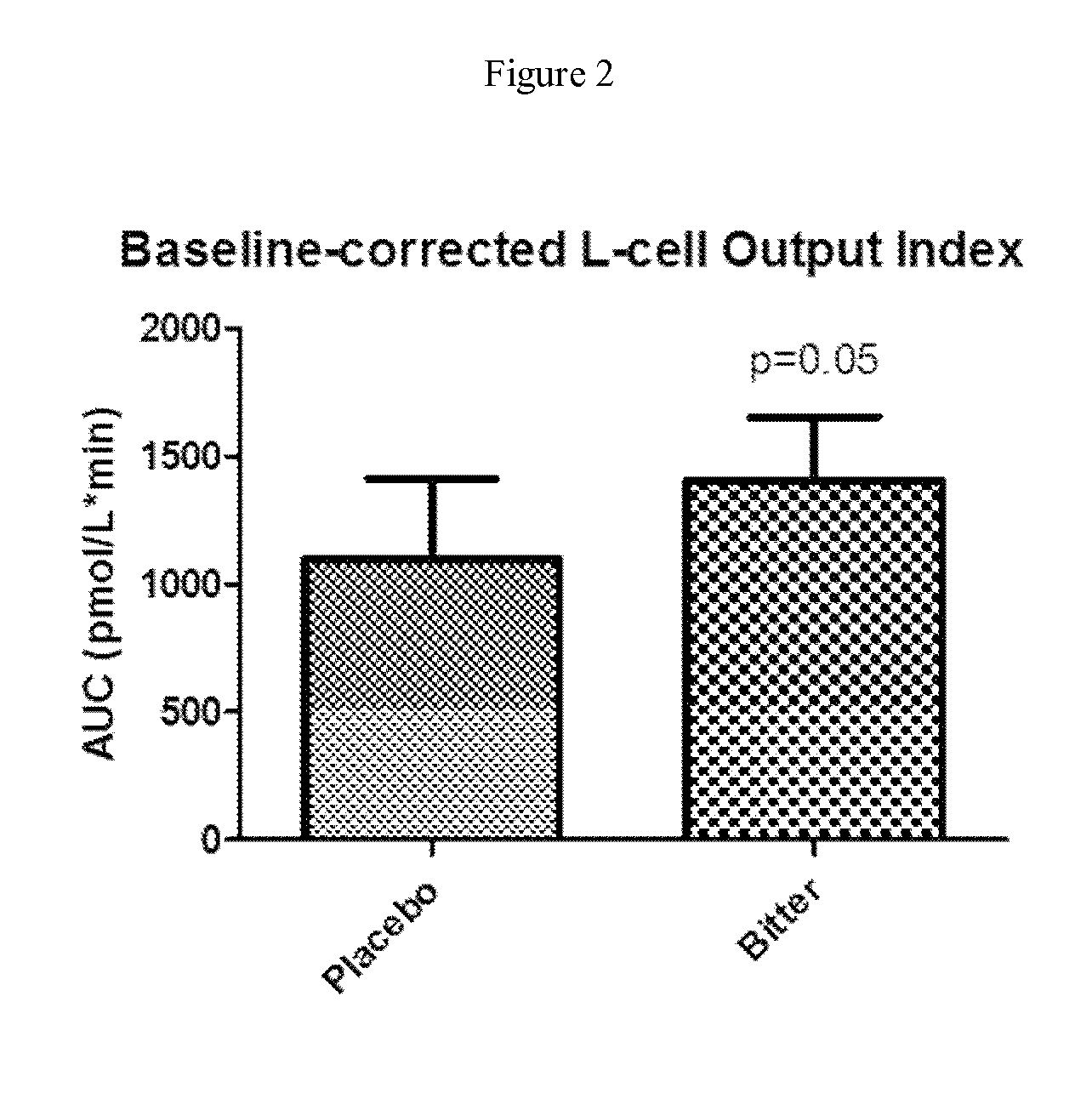
Provided herein are methods for treating conditions associated with a chemosensory receptor, including diabetes, obesity, and other metabolic diseases, disorders or conditions by administrating a composition comprising a chemosensory receptor ligand, such as a bitter receptor ligand. Also provided herein are chemosensory receptor ligand compositions, including bitter receptor ligand compositions, and methods for the preparation thereof for use in the methods of the present invention. Also provided herein are compositions comprising metformin and salts thereof and methods of use.
Owner:ANJI PHARMA US LLC
Crystallization method and bioavailability
ActiveUS20130035315A1Improve bioavailabilityPoor blend/physical mixture uniformityPowder deliveryBiocideDrug deliveryDrug
Owner:THAR PHARMA
Nanoparticulate clopidogrel and aspirin combination formulations
InactiveUS20070003615A1Reduced bioavailabilityHigh dissolution ratePowder deliveryBiocideControl releasePharmaceutical drug
The present invention is directed to compositions comprising a nanoparticulate clopidogrel and aspirin combination, or salts or derivatives thereof, having improved clopidogrel bioavailability. The nanoparticulate clopidogrel particles, and optionally the nanoparticulate aspirin particles, of the composition have an effective average particle size of less than about 2000 nm and are useful in the prevention and treatment of pathologies induced by platelet aggregation. The clopidogrel and aspirin particles may also be formulated as a controlled release polymeric coating or matrix drug delivery system.
Owner:ELAN PHRMA INT LTD
Drug dispensing device with flexible push rod
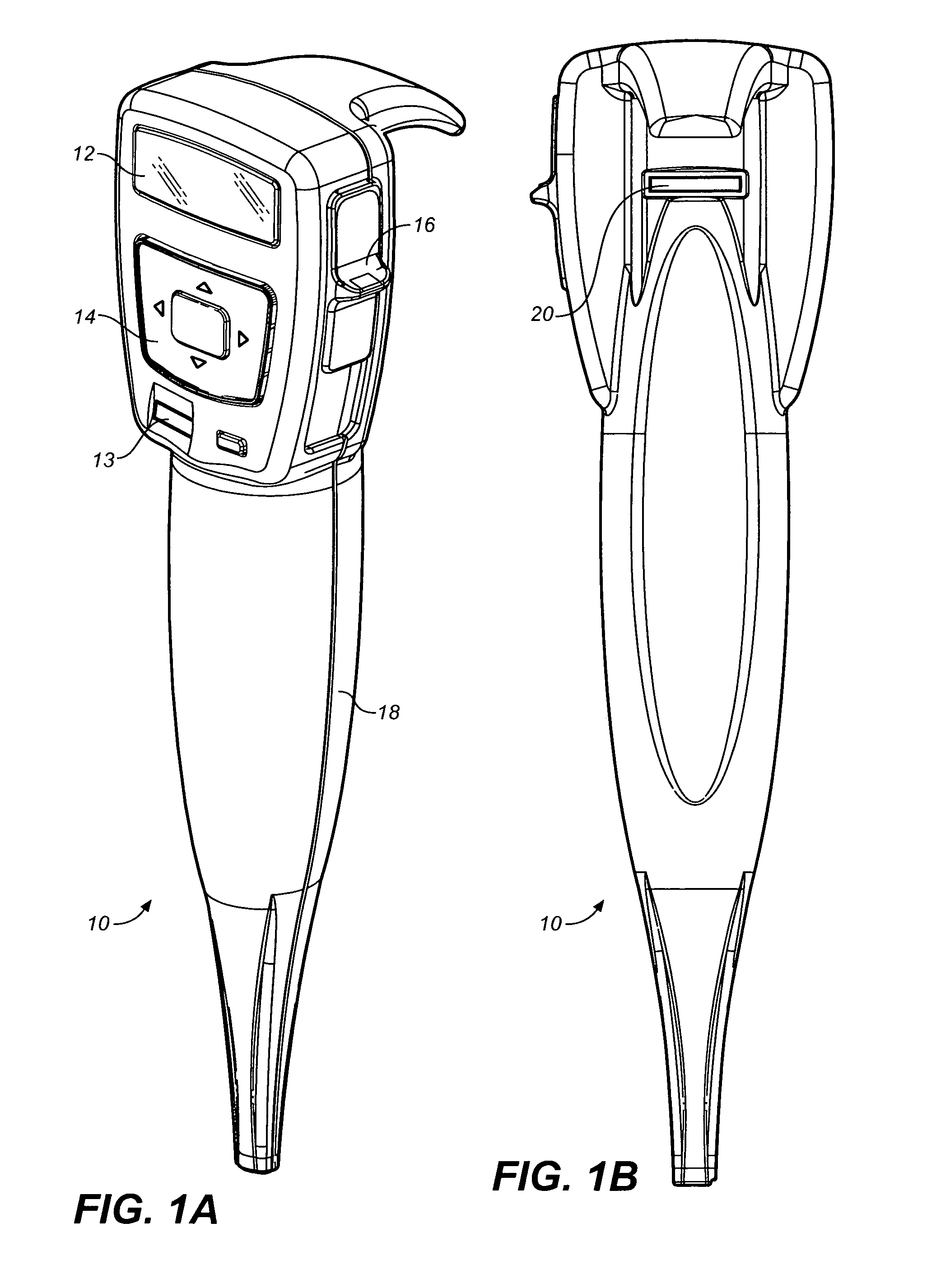
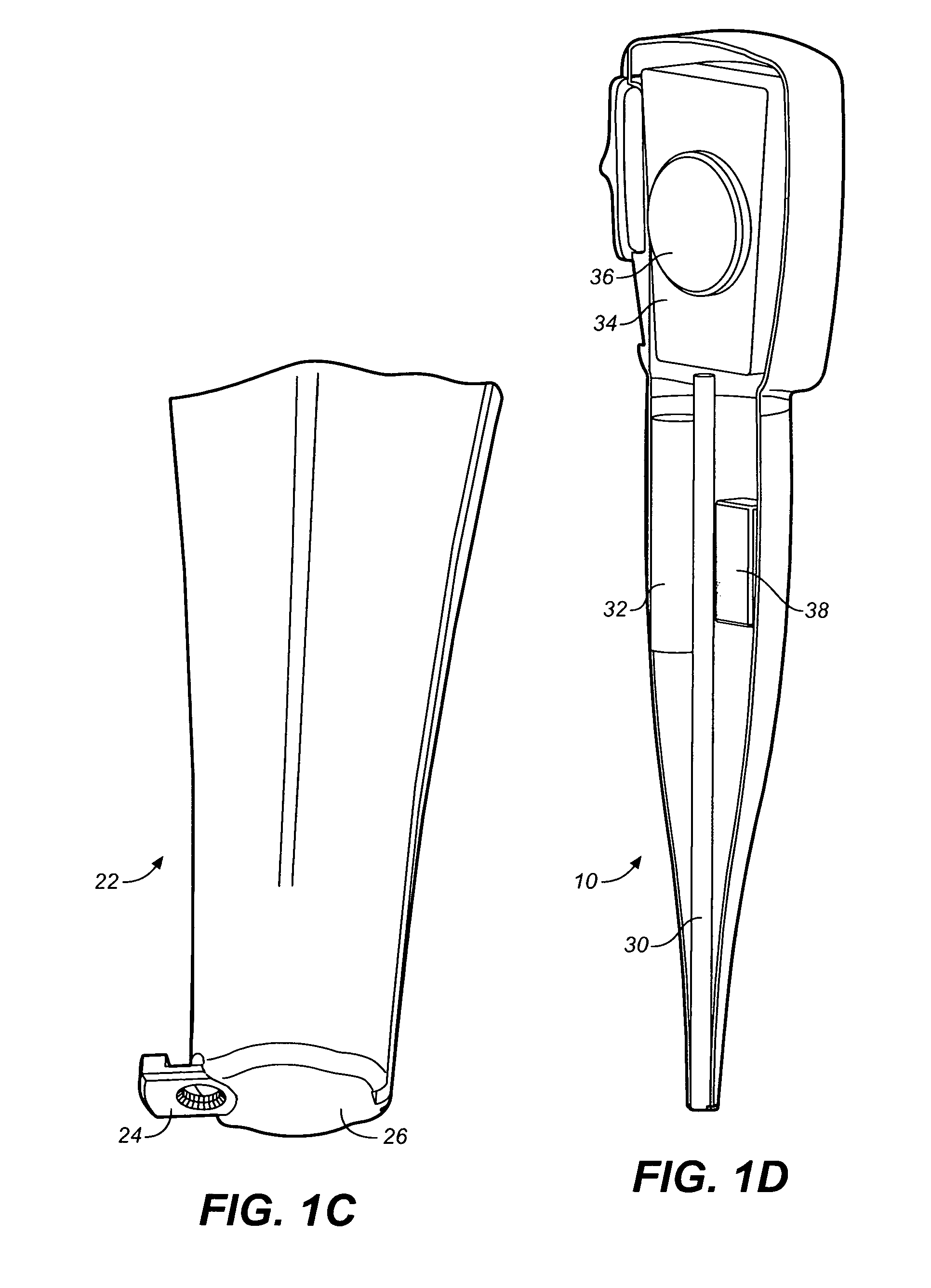
ActiveUS8357114B2Minimizing saliva influxReduced responsePowdered material dispensingDrug and medicationsDrug dispensingNonlinear channel
Drug storage and dispensing devices for dispensing a drug dosage form are disclosed. Devices having a housing defining a nonlinear passageway and a flexible push rod to move within the nonlinear passageway are disclosed. Devices having a flexible push rod coupled to a rotation actuating member at at an end portion thereof are also disclosed.
Owner:ACEIRX PHARM INC
Nitric oxide donating derivatives for the treatment of cardiovascular disorders
InactiveUS20050080024A1Increases extentIncreases pathological severityBiocideSugar derivativesVascular diseaseEndothelial dysfunction
Compounds comprising nitric oxide derivatives of stilbenes, polyphenols and flavonoids and methods of their use are provided for treating patients suffering from any of hypercholesterolemia, vascular oxidative stress and endothelial dysfunction.
Owner:RESVERLOGIX
Peptide boronic acid inhibitors
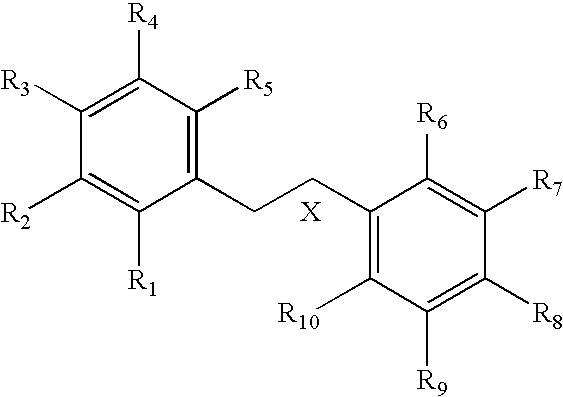
InactiveUS20060084592A1Low variabilityImprove bioavailabilityPeptide/protein ingredientsPeptide sourcesArylSulfur
A pharmaceutically acceptable base addition salt of an organoboronic acid of formula (XXX): wherein: P is hydrogen or an amino-group protecting moiety; R is hydrogen or alkyl; A is 0, 1 or 2; R1, R2 and R3 are independently hydrogen, alkyl, cycloalkyl, aryl or —CH2—R5; R5, in each instance, is one of aryl, aralkyl, alkaryl, cycloalkyl, heterocyclyl, heteroaryl, or —W—R6, where W is a chalcogen and R6 is alkyl; and where the ring portion of any of said aryl, aralkyl, alkaryl, cycloalkyl, heterocyclyl, or heteroaryl in R1, R2, R3 or R5 can be optionally substituted.
Owner:TRIGEN
Charcoal-based microbial soil conditioner and preparation method thereof
ActiveCN104789226AReduce hydrogen ion levelsRaise the pHAgriculture tools and machinesOther chemical processesAureobasidium sp.Bacillus thuringiensis
The invention discloses a charcoal-based microbial soil conditioner. The charcoal-based microbial soil conditioner comprises, by weight, 80-85 parts of charcoal, 10-13 parts of a composite microbial flora and 1-2 parts of an oxidized starch adhesive, and the composite microbial flora comprises photosynthetic bacteria, actinomyces, lactic acid bacteria, microzyme, Bacillus subtilis and Bacillus thuringiensis. The invention also discloses a preparation method of the charcoal-based microbial soil conditioner. The combined effect of charcoal and microbes alleviates soil acidification, reduces the unit weight of soil and increases the permeability and the water content of soil; soil organic matter formation is promoted to improve the validity of mineral elements; and the abundance of microbes at the rhizosphere of crops is cultivated to improve the rhizospheric environment of plants in order to make pathogen microbes difficultly grow and breed, so the insect diseases of crops is reduced, and soil heavy metal pollution is alleviated and restored.
Owner:迪斯科科技集团(宜昌)有限公司
Compositions and dosage forms for gastric delivery of irinotecan and methods of treatment that use it to inhibit cancer cell proliferation
InactiveUS6881420B2Improve oral bioavailabilityReduced bioavailabilityBiocideCapsule deliveryWhole bodyCancer cell proliferation
The present invention provides oral dosage forms and compositions for administering antineoplastic agents, such as irinotecan, etoposide, paclitaxel, doxorubicin and vincristine, whose oral effectiveness is limited by pre-systemic and systemic deactivation in the GI tract. Gelling of the gastric retention vehicle composition, and in the case of solid forms concomitant expansion of the composition, retains the antineoplastic drug in the patient's stomach, minimizing pre-systemic and / or systemic deactivation of the drug.
Owner:TEVA PHARM USA INC
Biguanide Compositions and Methods of Treating Metabolic Disorders
ActiveUS20130095140A1Reduced average systemic bioavailabilityMinimize systemic bioavailabilityBiocideOrganic chemistryDiseaseDiabetes obesity
Provided herein are methods for treating certain conditions, including diabetes, obesity, and other metabolic diseases, disorders or conditions by administrating a composition comprising a biguanide or related heterocyclic compound, e.g., metformin. Also provided herein are biguanide or related heterocyclic compound compositions, and methods for the preparation thereof for use in the methods of the present invention. Also provided herein are compositions comprising metformin and salts thereof and methods of use.
Owner:ANJI PHARMA INC
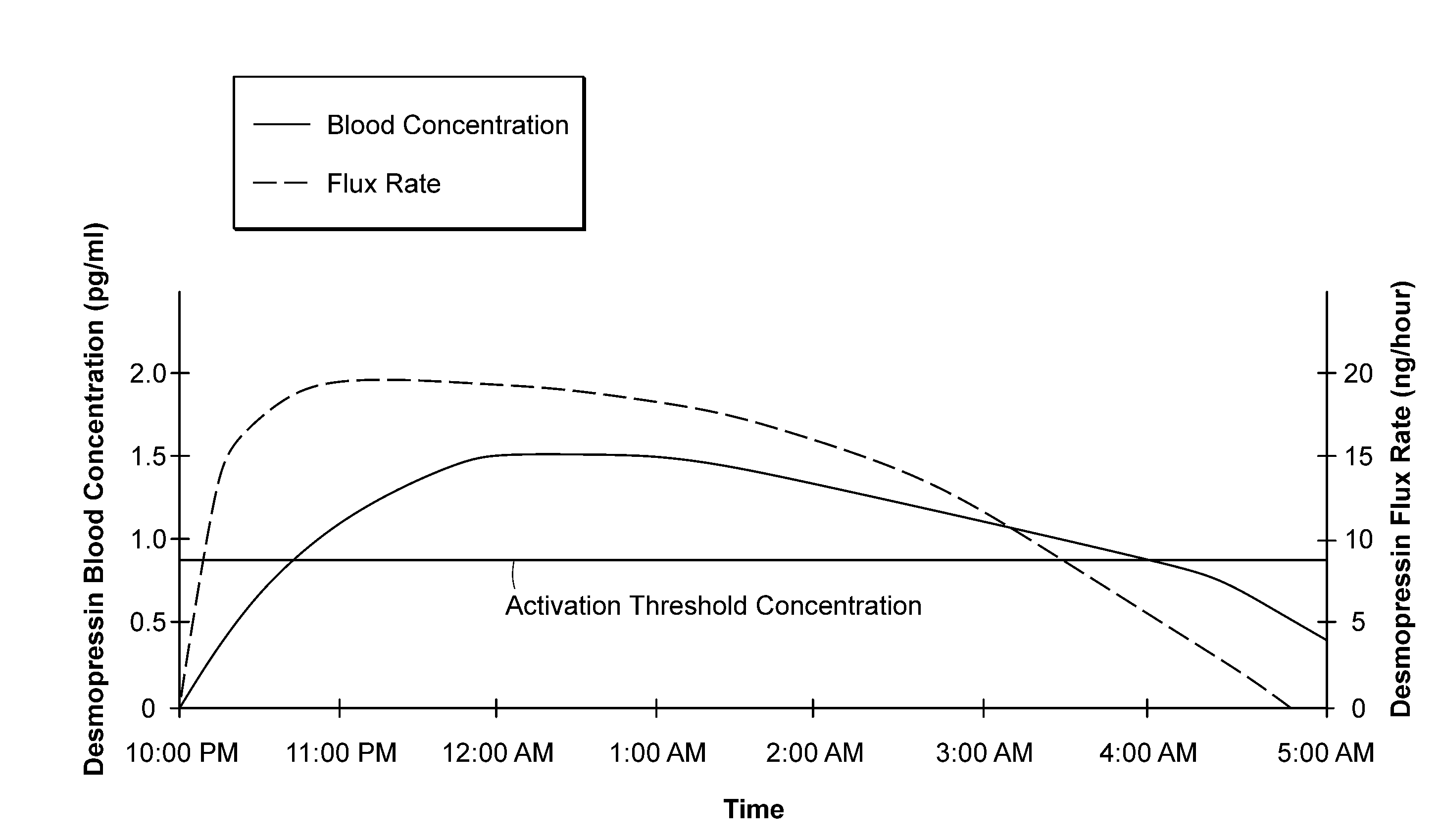
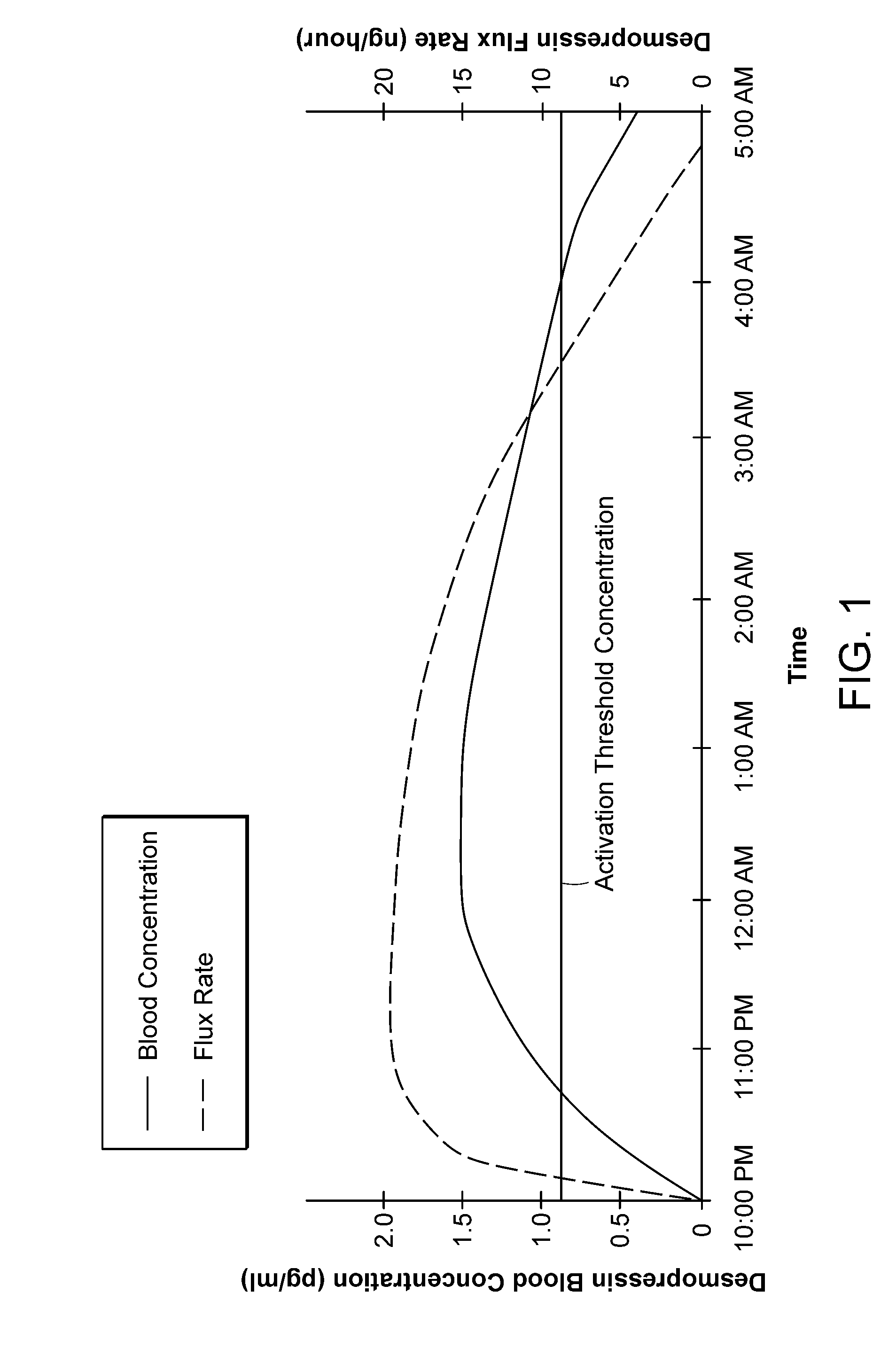
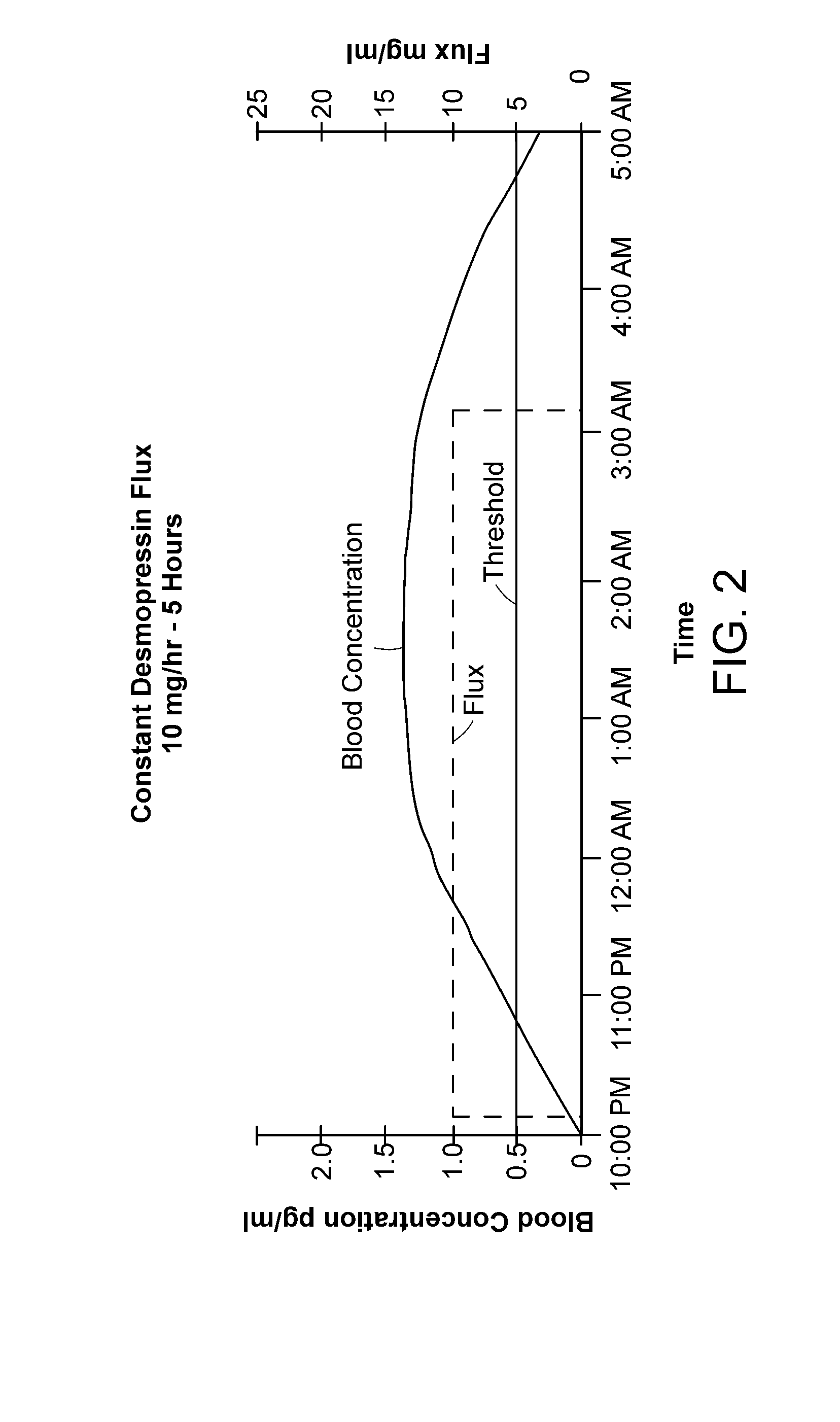
Methods and devices for desmopressin drug delivery
InactiveUS20090042970A1Reduce urine productionRestore normal urine productionBiocidePowder deliveryDecreased sodiumSide effect
Disclosed are devices for urine voiding postponement, and methods for treating conditions such as central diabetes insipidus, enuresis, nocturia, urinary frequency or incontinence. The devices deliver a desmopressin flux through the skin of a patient in a low dose amount just necessary to achieve a desired anti-diuretic effect without undesirable side effects such as hyponatremia. The devices are designed to permit a state of normal urinary production to return quickly after the desmopressin flux is terminated.
Owner:SERENITY PHARMA CORP
Injectable pharmaceutical composition for systematic administration of pharmacologically active ingredients
InactiveUS7309497B2Quick eliminationReduce frequencyOrganic active ingredientsNervous disorderAdditive ingredientWhole body
The invention relates to novel pharmaceutical compositions for the systemic administration of pharmacologically active ingredients. The invention relates in particular to an injectable pharmaceutical composition comprising (a) a pharmacologically active ingredient in a solid phase, (b) a vehicle consisting substantially of polyol fatty acid esters with a degree of esterification of over 80%, and (c) a wetting agent consisting substantially of polyol fatty acid esters with a monoester proportion of over 60%. The inventive composition is used for the systemic administration of numerous pharmacologically active ingredients, whereby the ingredients are released from the pharmaceutical composition over a period of at least 12, preferably at least 24 hours.
Owner:UCB SA
Inhibition of irritating side effects associated with use of a topical ophthalmic medication
InactiveUS6933289B2Reduced bioavailabilityEliminate the effects ofBiocidePowder deliverySide effectOphthalmic drug
This invention relates to a method of reducing an irritating or adverse side effect associated with the topical use of an active ophthalmic drug comprising incorporating an effective amount of a cyclodextrin or cyclodextrin derivative into a formulation to complex the active drug such that the concentration of the free active drug is reduced below a tolerable threshold, and incorporating an effective amount of a viscosity increasing agent in said formulation such that the bioavailability of said drug is high enough to be therapeutically effective, wherein the cyclodextrin or cyclodextrin derivative is not required to solubilize the active drug.Another aspect of this invention relates to topical ophthalmic formulations comprising an active drug, a cyclodextrin or cyclodextrin derivative, and a viscosity-enhancing agent, in effective amounts as stated above.
Owner:ALLERGAN INC
High-strength, low viscosity herbicidal formulations of glyphosate
ActiveUS7316990B2Improve herbicidal activityMinimizing viscosityBiocideDead animal preservationHigh concentrationGlyphosate
This invention relates to a high-strength herbicidal formulation containing high concentrations of glyphosate monomethylamine or dimethylamine salt and one or more surfactants selected to enhance the herbicidal activity of the glyphosate salts. The formulations exhibit significantly lower viscosity at high concentrations.
Owner:CORTEVA AGRISCIENCE LLC
Steroidal quinols and their use for estrogen replacement therapy
ActiveUS7300926B2Optimize allocationImprove permeabilityOrganic active ingredientsSteroidsUterusBioavailability
The present invention relates to novel estrogen-related steroidal quinols and their use as drugs for estrogen replacement therapy. The quinols of the present invention provide improved physicochemical properties, increased bioavailability, and improved distribution into tissues, bone, in the cardiovascular system, and in the CNS (central nervous system) with only a slight estrogenic action or no estrogenic action in the uterus. The compounds are suitable for the production of pharmaceutical agents for use in numerous indications (for example, estrogen replacement therapy, prevention and treatment of osteoporosis).
Owner:UNIV OF FLORIDA RES FOUNDATION INC +2
Solid dose delivery vehicle and methods of making same
InactiveUS20050276759A1Reduce riskAccurate dosePowder deliveryPeptide/protein ingredientsDelivery vehicleDose delivery
The present invention encompasses a solid dose delivery vehicle for ballistic administration of a bioactive material to subcutaneous and intradermal tissue, the delivery vehicle being sized and shaped for penetrating the epidermis. The delivery vehicle further comprises a stabilizing polyol glass loaded with the bioactive material and capable of releasing the bioactive material in situ. The present invention further includes methods of making and using the solid dose delivery vehicle of the invention.
Owner:QUADRANT DRUG DELIVERY
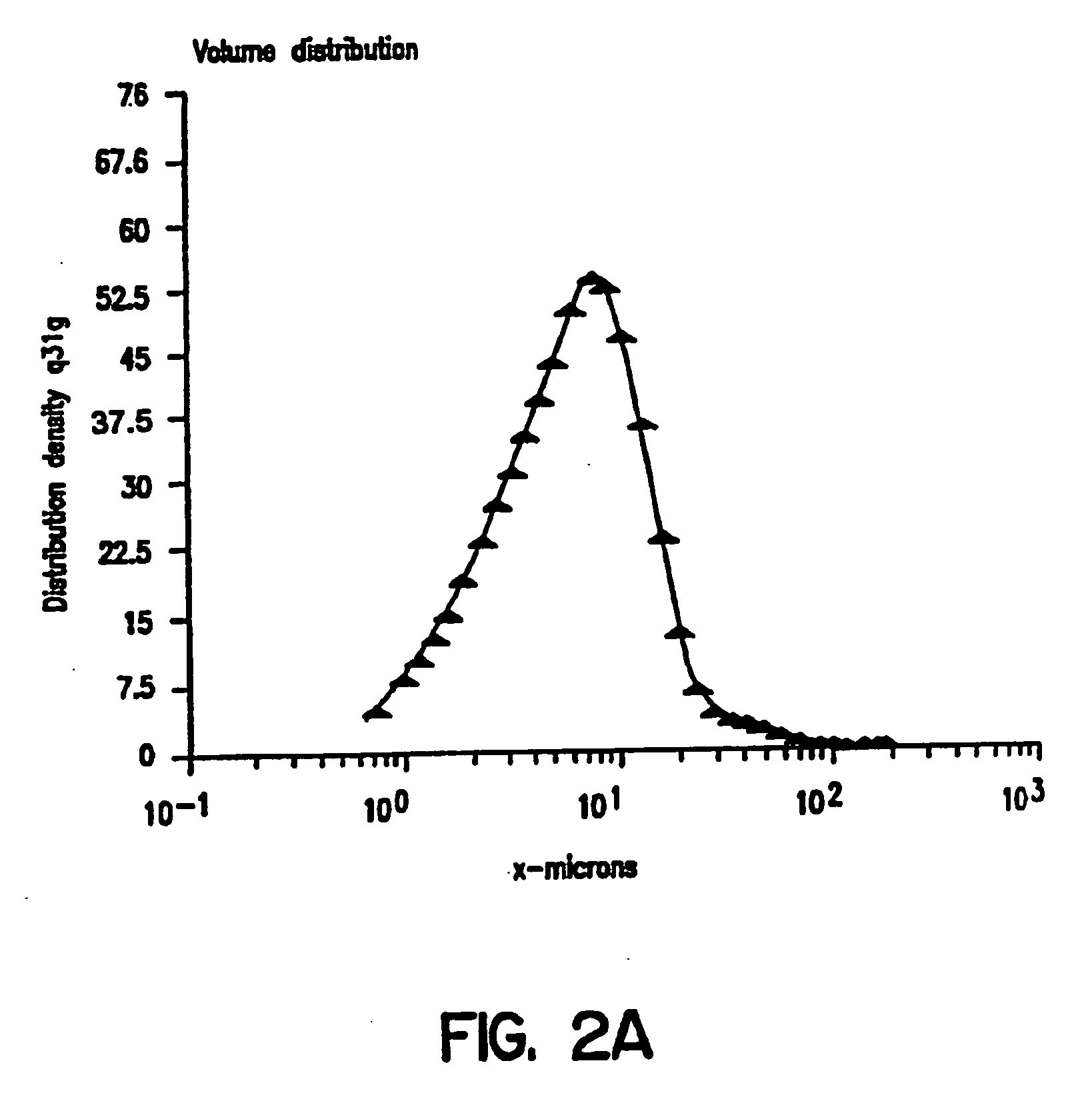
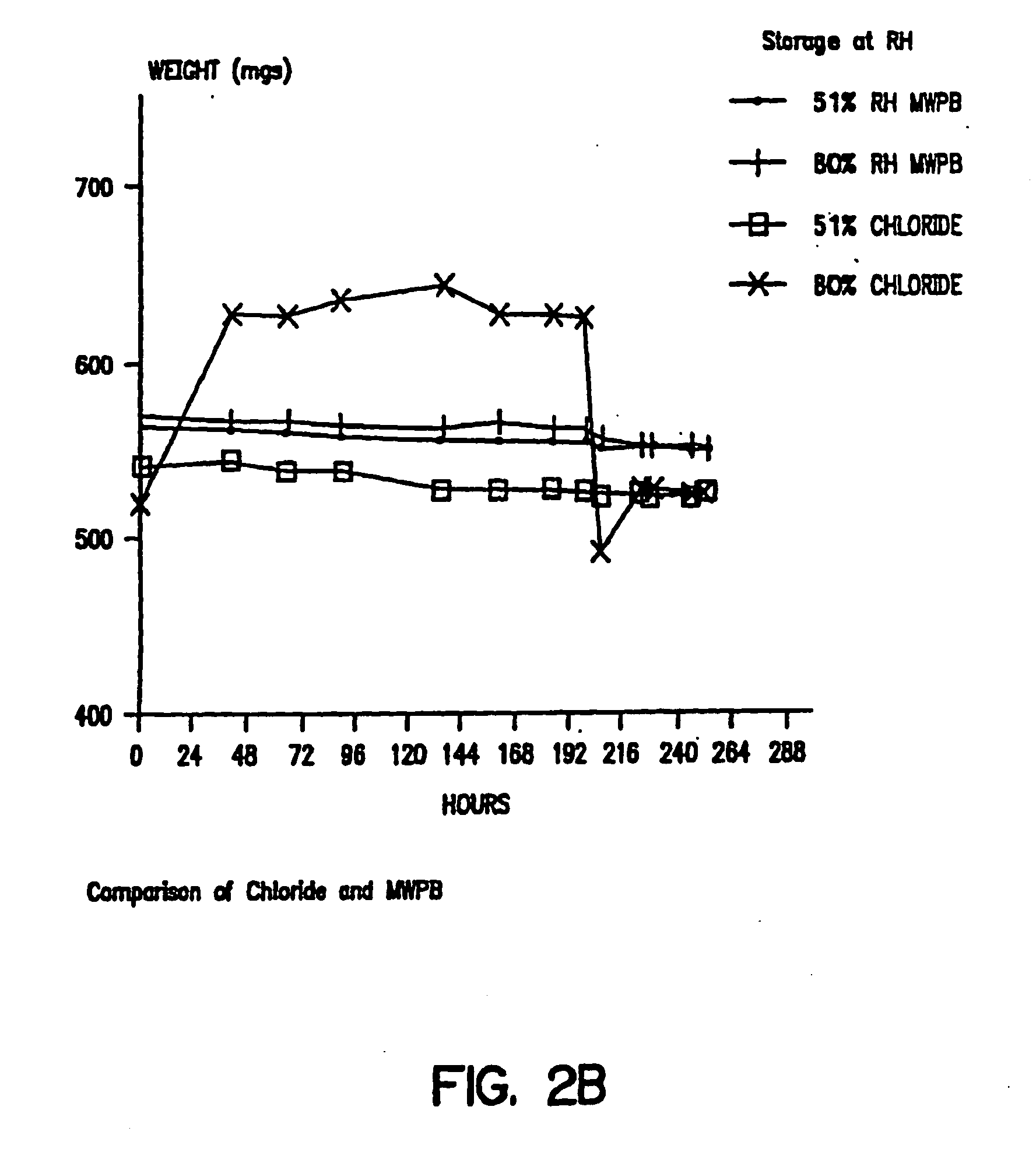
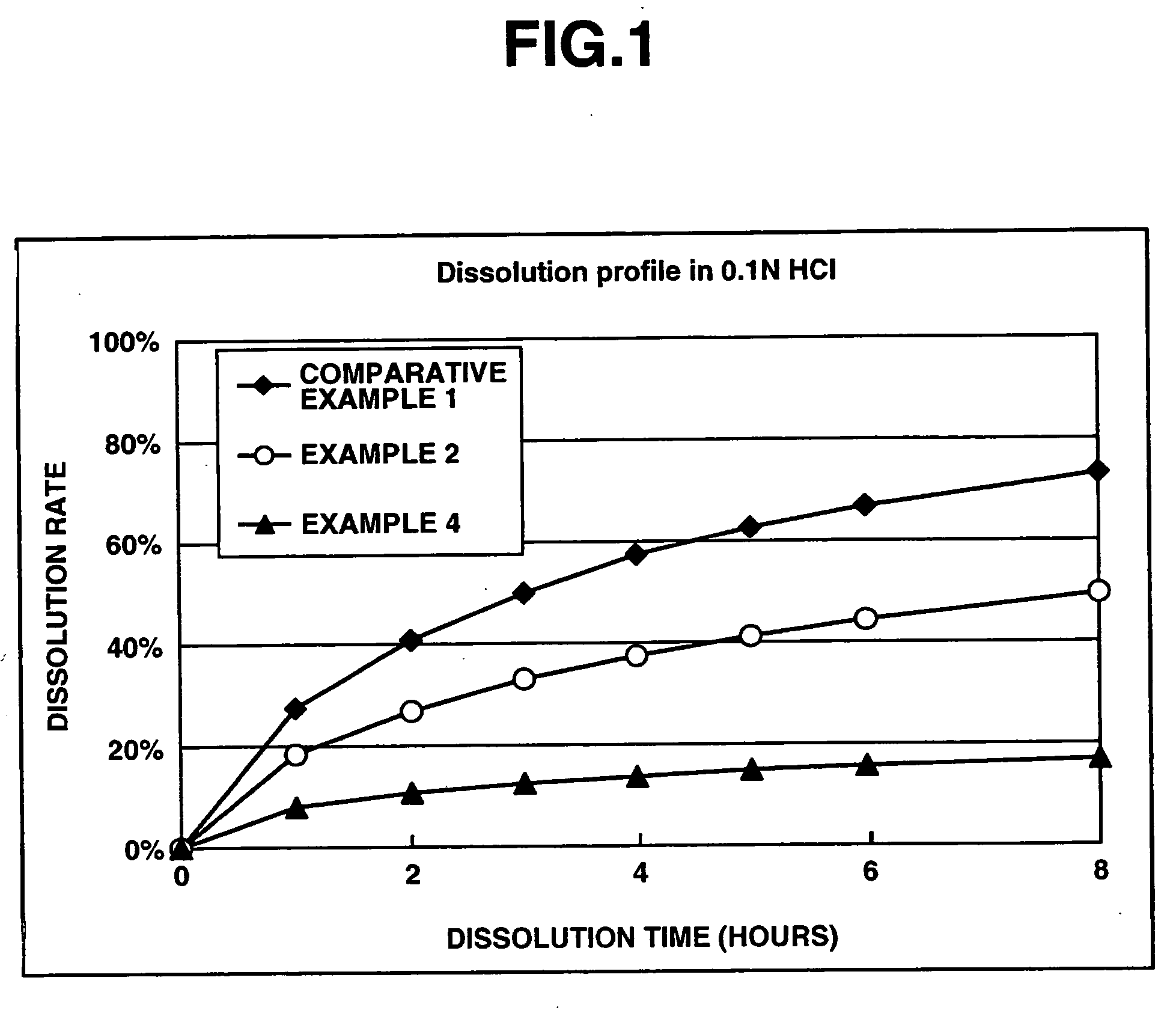
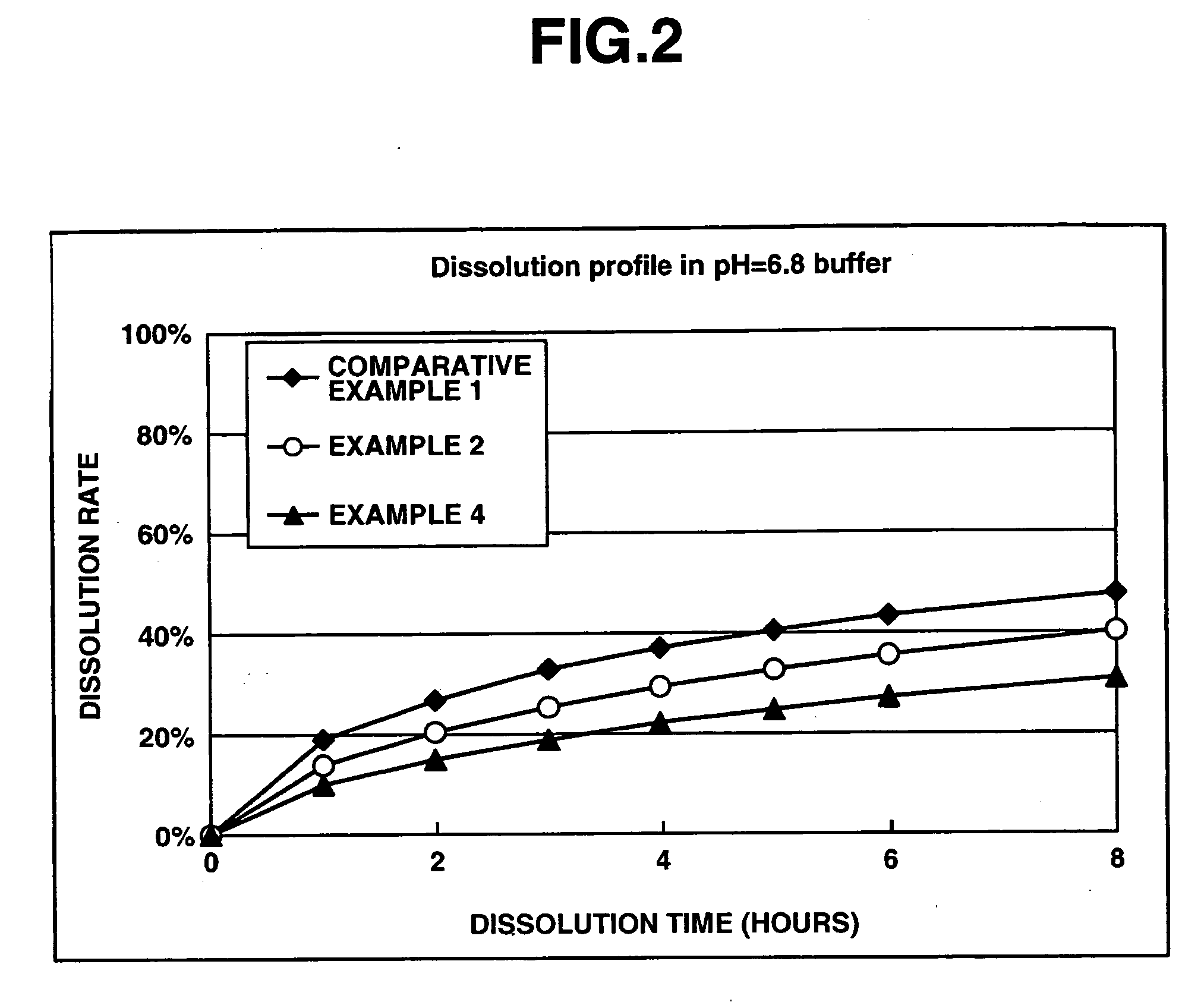
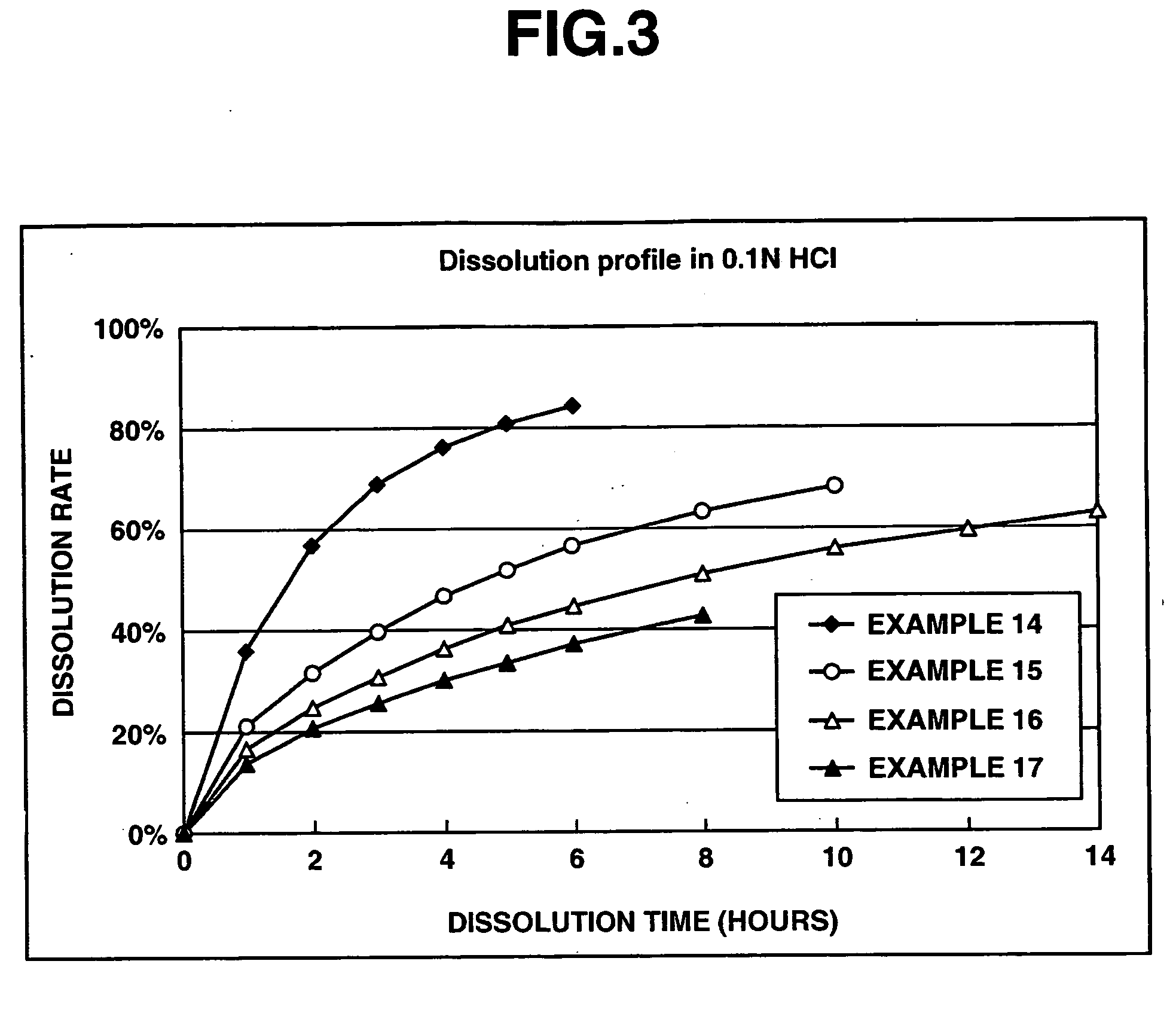
Matrix type sustained-release preparation containing basic drug or salt thereof
A matrix type sustained-release preparation and a manufacturing method therefor are provided wherein dissolution with low pH dependence of a basic drug or a salt thereof at the early stage of dissolution can be ensured in a dissolution test, and wherein as the dissolution test proceeds, a ratio of a dissolution rate of the basic drug or the salt thereof in an acidic test solution to a dissolution rate of the basic drug or the salt thereof in a neutral test solution (dissolution rate in the acidic test solution / dissolution rate in the neutral test solution) decreases with dissolution time at the late stage of dissolution, as compared to the early stage of dissolution. According to the present invention, the matrix type sustained-release preparation contains a basic drug or a salt thereof and at least one enteric polymer, in which solubility of the basic drug or the salt thereof in a 0.1 N hydrochloric acid solution and a neutral aqueous solution, pH 6.0 is higher than in a basic aqueous solution, pH 8.0.
Owner:EISIA R&D MANAGEMENT CO LTD
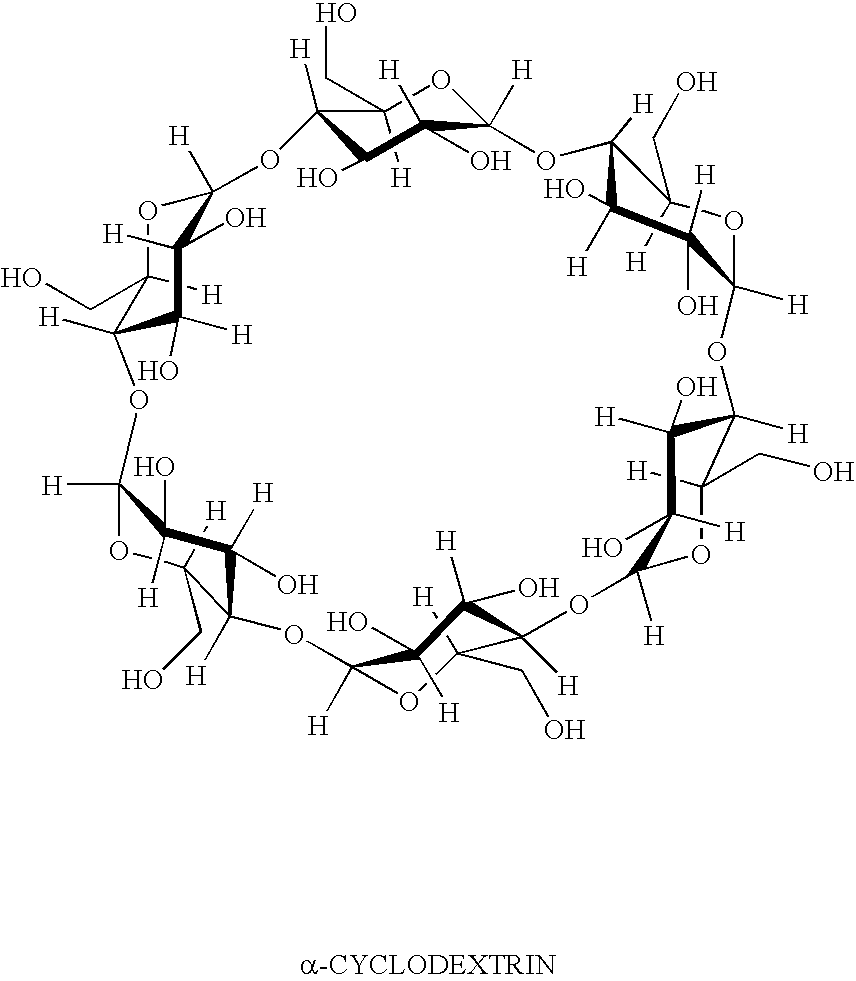
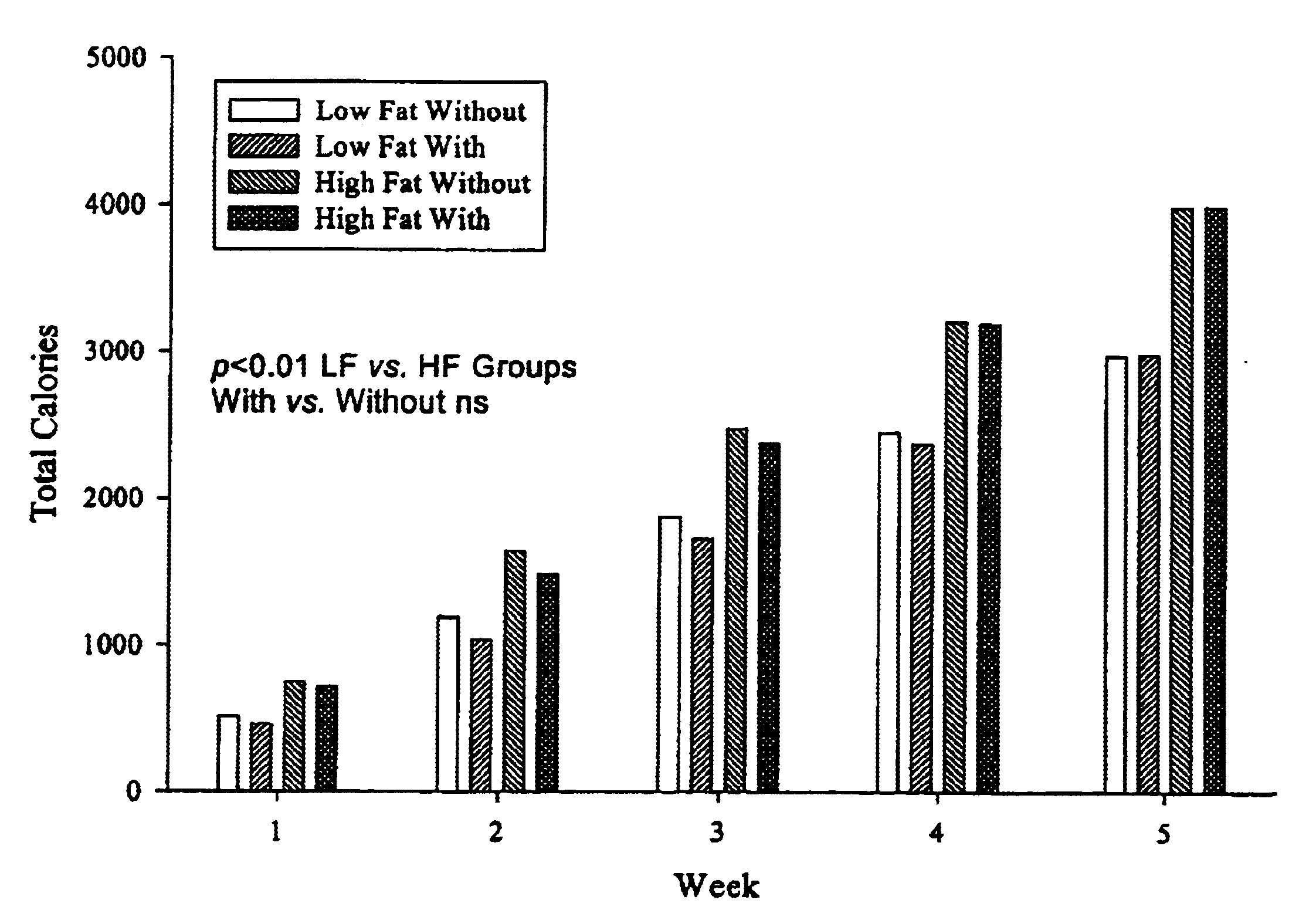
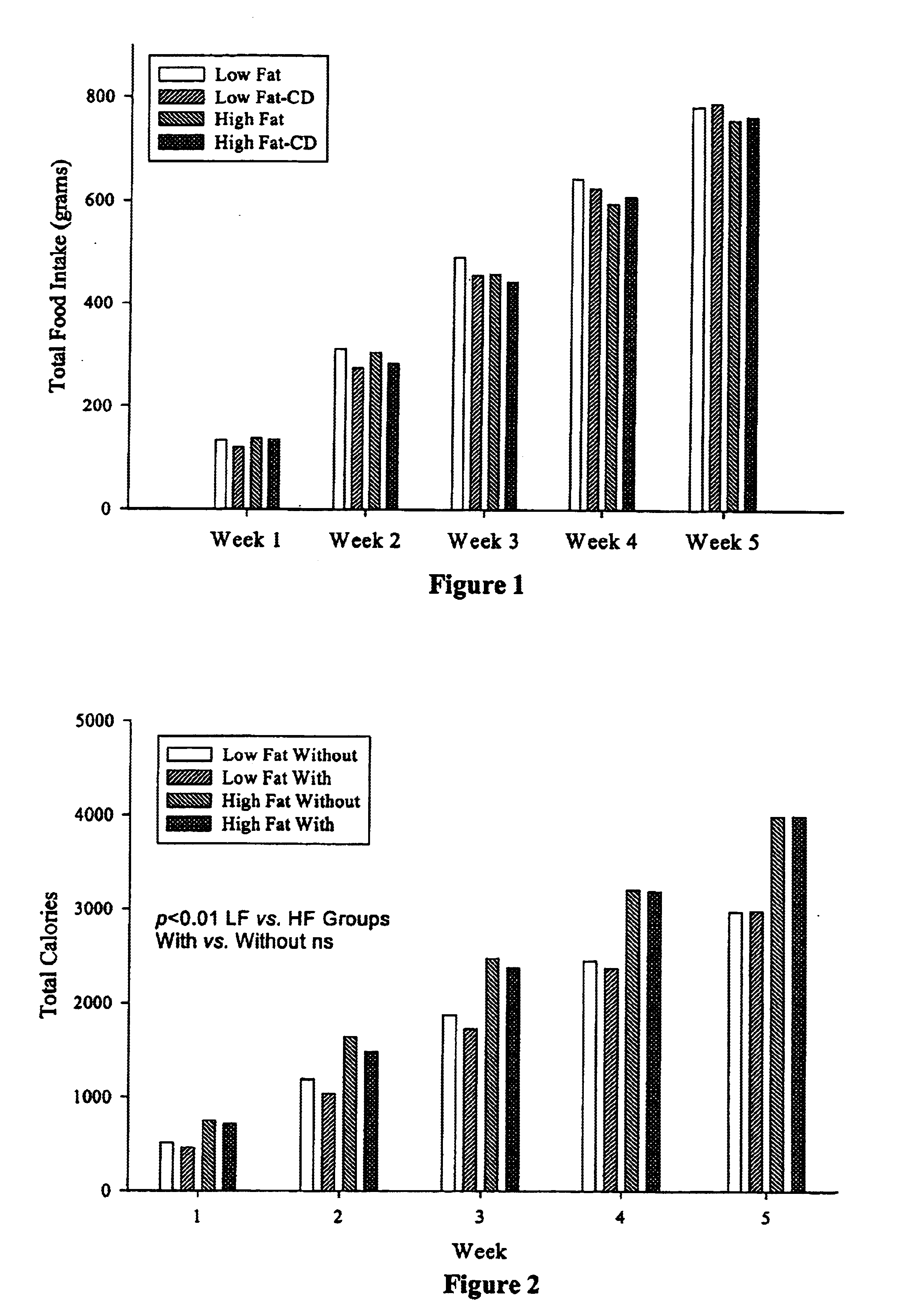
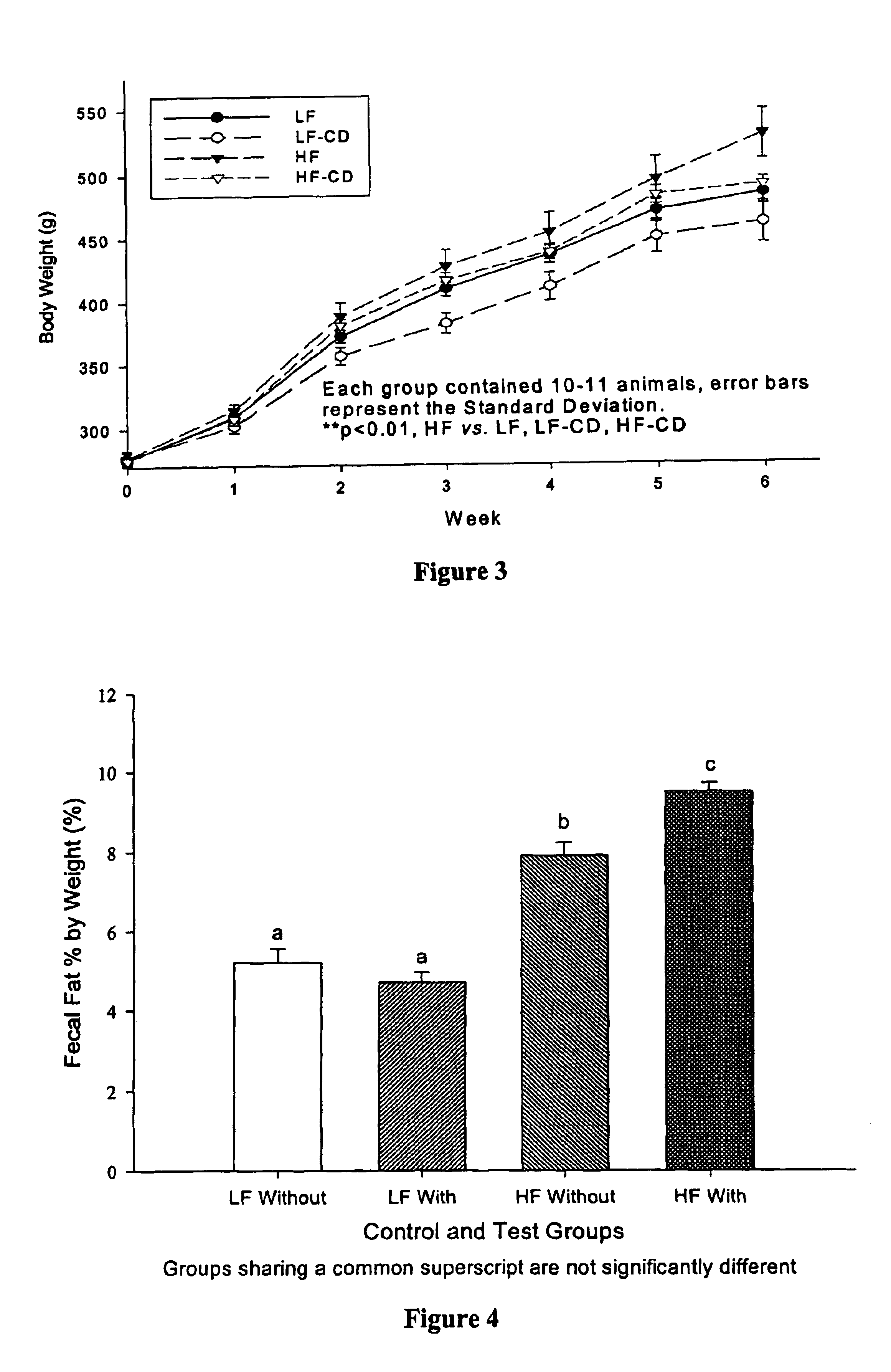
Compositions comprising dietary fat complexer and methods for their use
InactiveUS6890549B2Decrease in levelTotal calories lowPowder deliveryOrganic active ingredientsHigh densityBioavailability
This invention relates to fat containing consumable food products comprising α-cyclodextrin. The food products have reduced levels of bioavailable fat but have substantially the same fat, cholesterol and caloric content as a like food without α-cyclodextrin. The invention also relates to methods for reducing the bioavailability of fats in fat containing food products without reducing caloric intake as determined by bomb calorimetry and to methods for increasing high density lipoproteins in a subject and reducing or controlling weight by administering the food products of this invention.
Owner:SOHO FLORDIS INT
Method for trapping and fixing soil persistent organic pollutant using biological carbon
InactiveCN101380639AReduced bioavailabilityRaw materials are easy to getContaminated soil reclamationBiomassEnvironmental chemistry
The invention disclose a method for intercepting and fixing the persistent organic contaminants of soil through bio-carbon which includes the following steps: 1) a biomass material is carbonized for 0.5 to 6 hours under the temperature of 250 to 550 DEG C and the condition of oxygen limitation and passes through the screen of 10 to 200 meshes, thus preparing the bio-carbon; 2) the prepared bio-carbon is added into the soil polluted by the persistent organic matters according to the ratio of 1 to 200tons / hektare and the soil within 0 to 30cm of a surface layer is ploughed for leading the soil to be fully mixed with the bio-carbon; 3) after being aged, the bio-carbon fiercely absorbs the persistent organic matters, thereby greatly reducing the biological effectiveness thereof and being capable of producing safe farm products in the oil polluted by the organic mattes. The bio-carbon adopted by the treatment method has the advantages of easy obtaining, low cost, environmental friendlessness, non-secondary pollution, simple preparation technique, good treatment effect and long time effectiveness; simultaneously the bio-carbon contains a plurality of carbon and other nutrition elements, thus being capable of providing abundant carbon sources and nutrition for the growing of soil crops and having the effect for enriching the soil.
Owner:ZHEJIANG UNIV
In situ restoring agent for heavy metal polluted soil
ActiveCN1631561AGuarantee continuous developmentSimple handling of equipmentContaminated soil reclamationMicroorganismCoal
The invention concerns the repairing dose to repair the soil polluted by heavy metal, which belongs to the technique field of repairing soil polluted by heavy metal. The repairing dose mainly includes: Na unit soil, haibao stone, stick stone, coal powder and microorganism germ root. Every composition' rate by weight is: Na unit soil15--40%, haipao stone 10--50%, stick stone 10--30%, coal powder 5--30%, microorganism germ root 0--40%. The repairing dose in the invention has the advantages of low cost of material, simple working equipment, good effect and no second pollution.
Owner:CHINA UNIV OF MINING & TECH (BEIJING)
Novel Salts of Fumaric Acid Monoalkylesters and Their Pharmaceutical Use
InactiveUS20080227847A1Promote absorptionLow water solubilityBiocideOrganic chemistryPsoriasisAmino acid
The present invention relates to novel amino acid salts of fumaric acid monoalkylesters. The salts are suitable for use as active substances in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders.
Owner:ADITECH PHARMA AG
Pharmaceutical compositions and methods to achieve and maintain a targeted and stable copper status and prevent and treat copper-related central nervous system diseases
InactiveUS20070207191A1Reduce needEasy maintenanceOrganic active ingredientsBiocideDiseasePharmaceutical drug
Owner:PIPEX THERAPEUTICS
Iron-based biochar material, preparation therefor and use thereof in soil pollution control
ActiveUS20170282229A1Reduce cadmium-arsenic combined pollutionMaintain stable propertiesMixing methodsTransportation and packagingCarbonizationSoil heavy metals
The present invention belongs to the technical field of soil heavy metal remediation, specifically discloses a method for preparing the iron-based biochar material, the iron-based biochar material prepared there from and a method for controlling the heavy metal pollution in soil using the iron-based biochar material. For the iron-based biochar material of the present invention, by using a method of high-temperature carbonization, a biomass is used as a raw material and an iron-containing compound is add in the process of preparing biochar, wherein iron is incorporated in a specific ratio, to form the iron-based biochar material with a special structure and function. The material has a simple preparation process, low cost and a short production period; the prepared iron-based biochar material has an unique effect on the arsenic-cadmium combined pollution soil remediation, can effectively reduce the bioavailability of arsenic and cadmium in the soil, significantly reduces the arsenic and cadmium contents in the agricultural products planted in the arsenic-cadmium combined pollution soil, and has no toxic and side effects on the crops, is safe to apply and can be applied to the control of arsenic-cadmium combined pollution soil in a large scale.
Owner:GUANGDONG INST OF ECO ENVIRONMENT & SOIL SCI
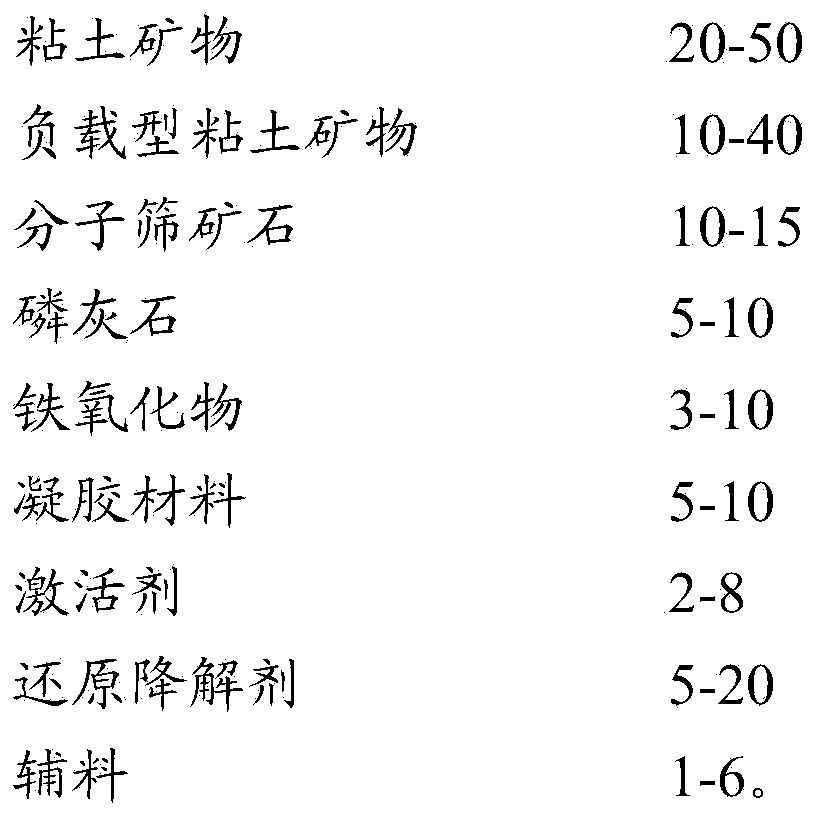
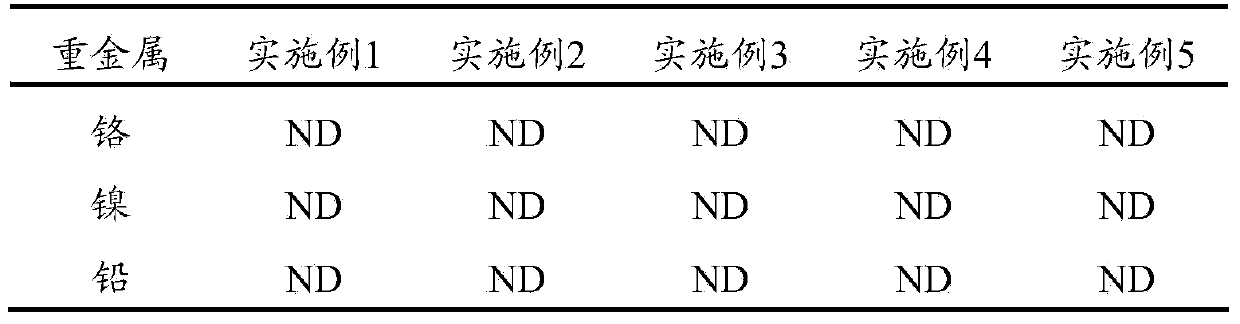
Reductive degradable stabilizer of polluted soil, bottom mud and sludge as well as preparation method and using method of stabilizer
ActiveCN103436265AAchieve partial reductionReduce sizeContaminated soil reclamationOrganic fertilisersHealth riskSludge
The invention relates to a reductive degradable stabilizer for heavy metal and / or organic matter polluted soil as well as a preparation method and use of the stabilizer. The reductive degradable stabilizer can be used for quickly and efficiently stabilizing the heavy metals and the organic matters in the polluted soil by virtue of a synergistic effect of a plurality of active ingredients, and can also be used for further lowering the content of the organic matters, so that the purpose of lowering leaching toxicity of pollutants and health risk is realized; therefore, the reductive degradable stabilizer is a novel and efficient stabilizer which has the advantages of being wide in treatment substrate, capable of reducing the total amount of the organic matters, good in stabilizing effect, convenient to operate and the like, and has a wide range of application in the governance fields including polluted soil, bottom mud, sludge and the like.
Owner:付融冰
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com