Transmucosal delivery of cannabinoids
a cannabinoid and transmucosal technology, applied in the direction of biocide, plant/algae/fungi/lichens ingredients, bandages, etc., can solve the problems of rapid reduction of the concentration level of the chemical in the bloodstream, rapid metabolization in the body, etc., to limit the bioavailability of the chemical, high lipophilicity, and strong binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example # 1
EXAMPLE #1
Transmucosal Device for THC Delivery via the Oral Mucosa
[0043]
InnerOuterIngredientsmatrix (%)backing layerHydroxypropylcellulose75.9037.00(Klucel ®-EF)Polycarbophil (Noveon ® AA-1)5.00Polyethylene glycol 4008.00Tartaric Acid3.00BHT0.10Tetrahydrocannabinol (THC)8.00Ethyl cellulose60.00Eudragit ® E-1002.00Polyethylene glycol 33501.00
[0044] Example #1 above contains the solubilizer PEG 400, which may also function as a penetration enhancer. An additional example (Example #1a) may include the above formula with the bile salt penetration enhancer, sodium deoxycholate, at the 5% level.
example # 2
EXAMPLE #2, #3, #4
[0045]
Drug / Chemical (% w / w)#2#3#4Hydroxypropyl cellulose (Avg MW: 80,000)15.511.53.5Polyethylene Oxide (Avg MW: 200,000)80.480.480.4Vitamin E succinate0.10.10.1Tetrahydrocannabinol Hemiglutarate4816(THC Pro-drug)
[0046] Example #2-#4 were prepared via a solvent cast technique. The Tetrahydrocannabinol Hemiglutarrate (THC-HG) was dissolved in ethanol (10% w / w of THC-HG). The HPC and PEO were then admixed with the Vitamin E succinate via solvation. Then the THC-HG solution was slowly added to the polymeric dispersion. The resulting dispersion was added to a film forming mold and the solvent was evaporated off. The resulting transmucosal preparation was homogenously dispersed with the cannabinoid pro-drug.
[0047] Example #5 and #6 were prepared using hot-melt extrusion techniques. The formulas are listed below. The PEO, PVP and Vitamin E TPGS were dry blended in a V-blender. The THC and the THC-HS were solubilized in the PEG 400 and immediately sprayed into the dry ble...
examples # 5 & # 6
EXAMPLES #5 & #6
[0048]
Drug / Chemical (% w / w)#5#6Polyethylene Oxide (Avg MW: 1,000,000)68.068.0Polyvinylpyrrolidone (Kollidon)10.010.0Polyethylene glycol (PEG 400)11.011.0Vitamin E TPGS3.03.0Tetrahydrocannabinol (THC)8.0—Tetrahydrocannabinol Hemisuccinate—8.0(THC-HS)
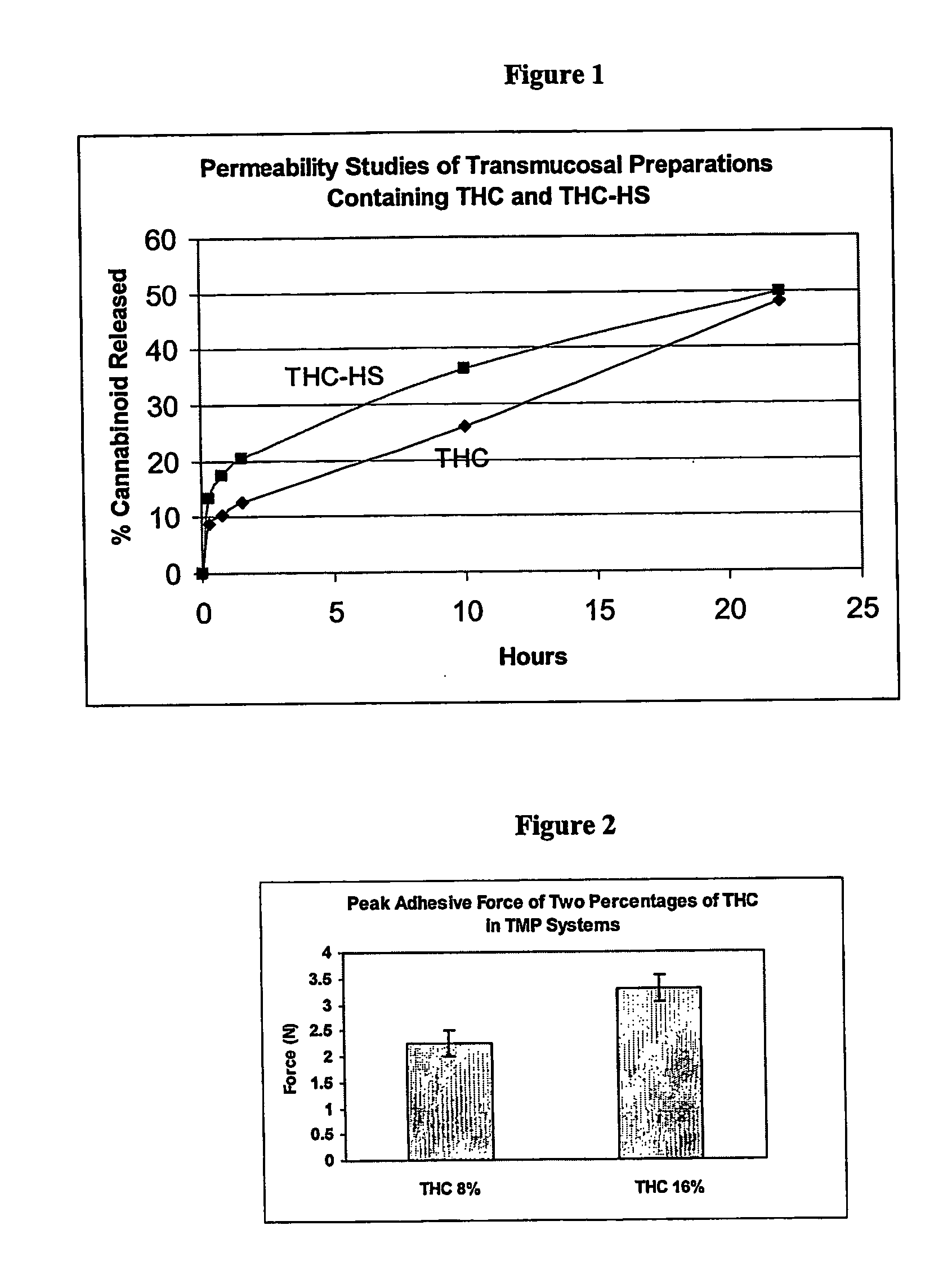
[0049] Diffusion studies of the transmucosal preparation films in Examples #5 and #6 were performed using a PermeGear, Model V9, 9 Cell System. Modified Franz cells were employed using thinly-excised rabbit mucosa as the diffusion membrane. Diffusion media was a diffusion buffer system of Brij® 3.0% (pH=7.2) which was determined by previous testing. FIG. 1 illustrates the results of these studies. As can be seen by the illustration, the THC-HS exhibited a more immediate release with controlled diffusion for over 22 hours. Example #5 (THC) demonstrated a slower release with approximately 50% theoretical drug released at 22 hours. Both formulations have clinical applications for different therapeutic objectives.
[0050] It ha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com