Patents
Literature
38 results about "Decreased sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hyponatremia is a low sodium level in the blood. It is generally defined as a sodium concentration of less than 135 mmol/L (135 mEq/L), with severe hyponatremia being below 120 mEql/L. Symptoms can be absent, mild or severe. Mild symptoms include a decreased ability to think, headaches, nausea, and poor balance.
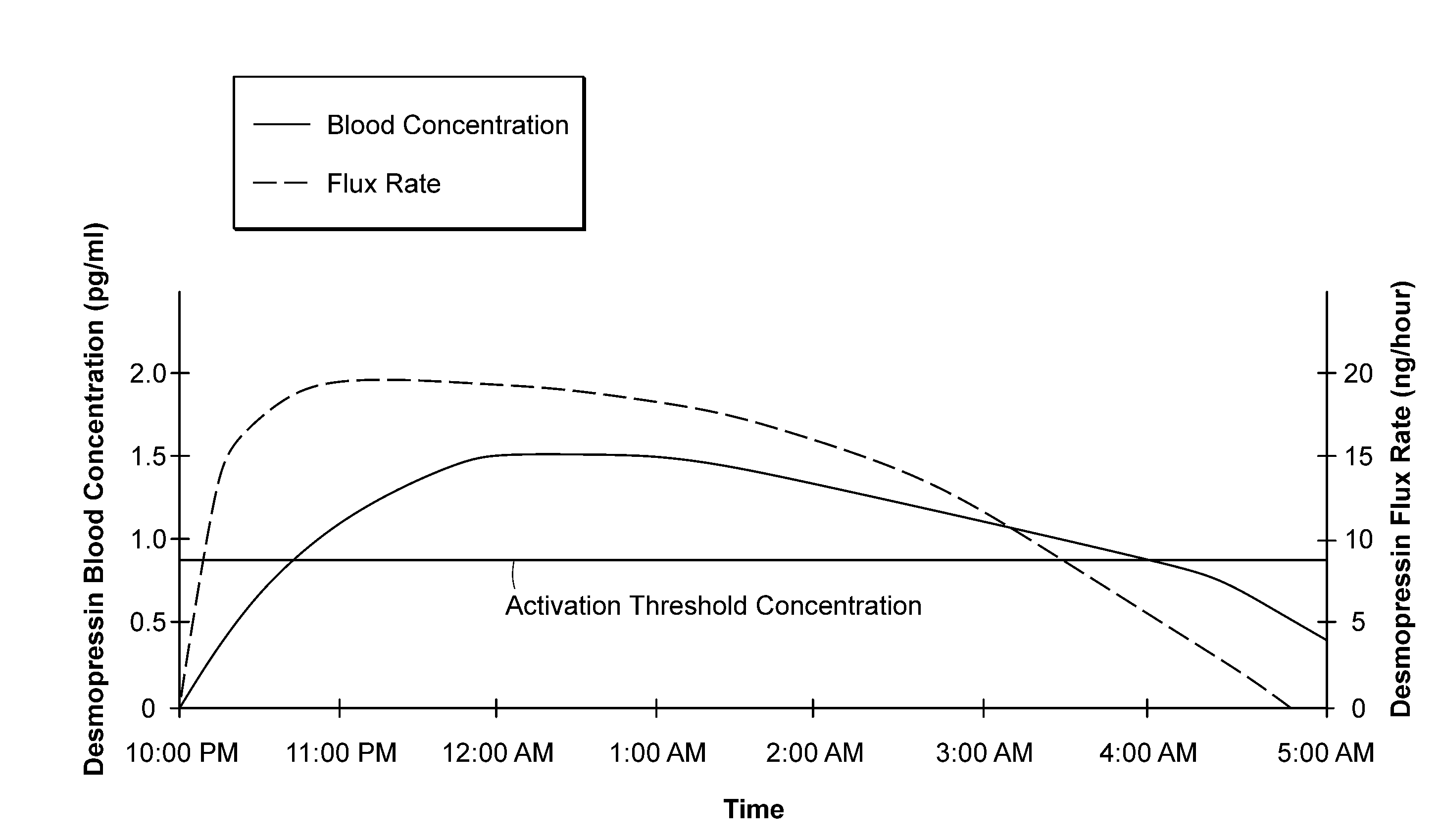
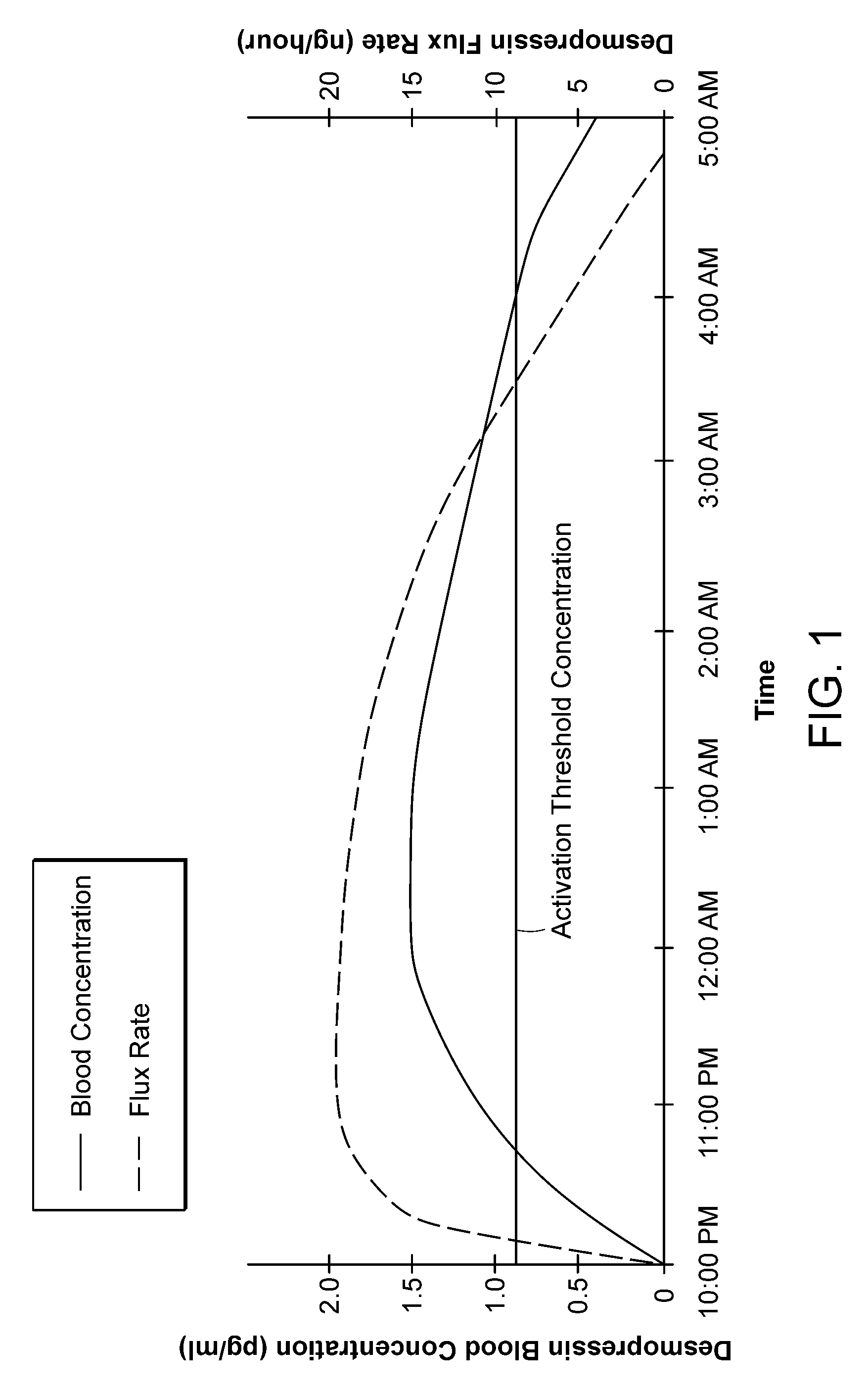
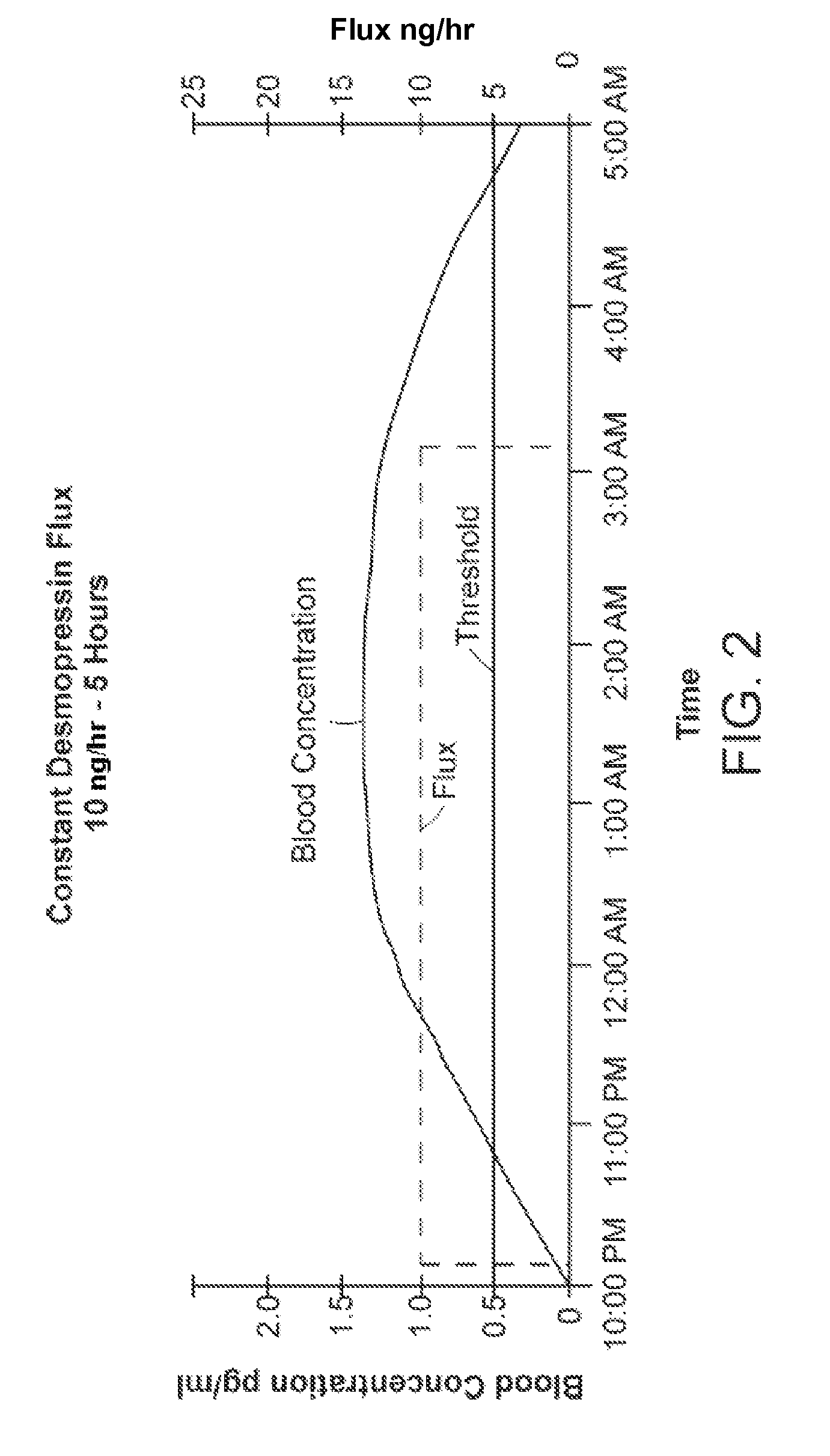
Methods and devices for desmopressin drug delivery
InactiveUS20090042970A1Reduce urine productionRestore normal urine productionBiocidePowder deliveryDecreased sodiumSide effect
Disclosed are devices for urine voiding postponement, and methods for treating conditions such as central diabetes insipidus, enuresis, nocturia, urinary frequency or incontinence. The devices deliver a desmopressin flux through the skin of a patient in a low dose amount just necessary to achieve a desired anti-diuretic effect without undesirable side effects such as hyponatremia. The devices are designed to permit a state of normal urinary production to return quickly after the desmopressin flux is terminated.
Owner:SERENITY PHARMA CORP
Electrolyte Energy Gel
InactiveUS20050095271A1Food ingredient as viscosity modification agentVitamin food ingredientsDecreased sodiumIntestinal Reabsorption
A nutritional composition in gel form containing carbohydrates, electrolytes, anti-oxidants, vitamins and amino acids for increasing available energy during exercise, for replenishing electrolytes that are depleted during exercise, for promoting fluid retention during exercise, for preventing hyponatremia during exercise and for facilitating intestinal reabsorption of fluids during exercise. The primary electrolyte ion is sodium (Na.sup.+) compounds is in an amount such that the sodium content of the gel is in a ratio in the range of 0.4 to 1.2 parts of the sodium to 100.0 parts of the carbohydrates.
Owner:CRANK SPORTS
Methods
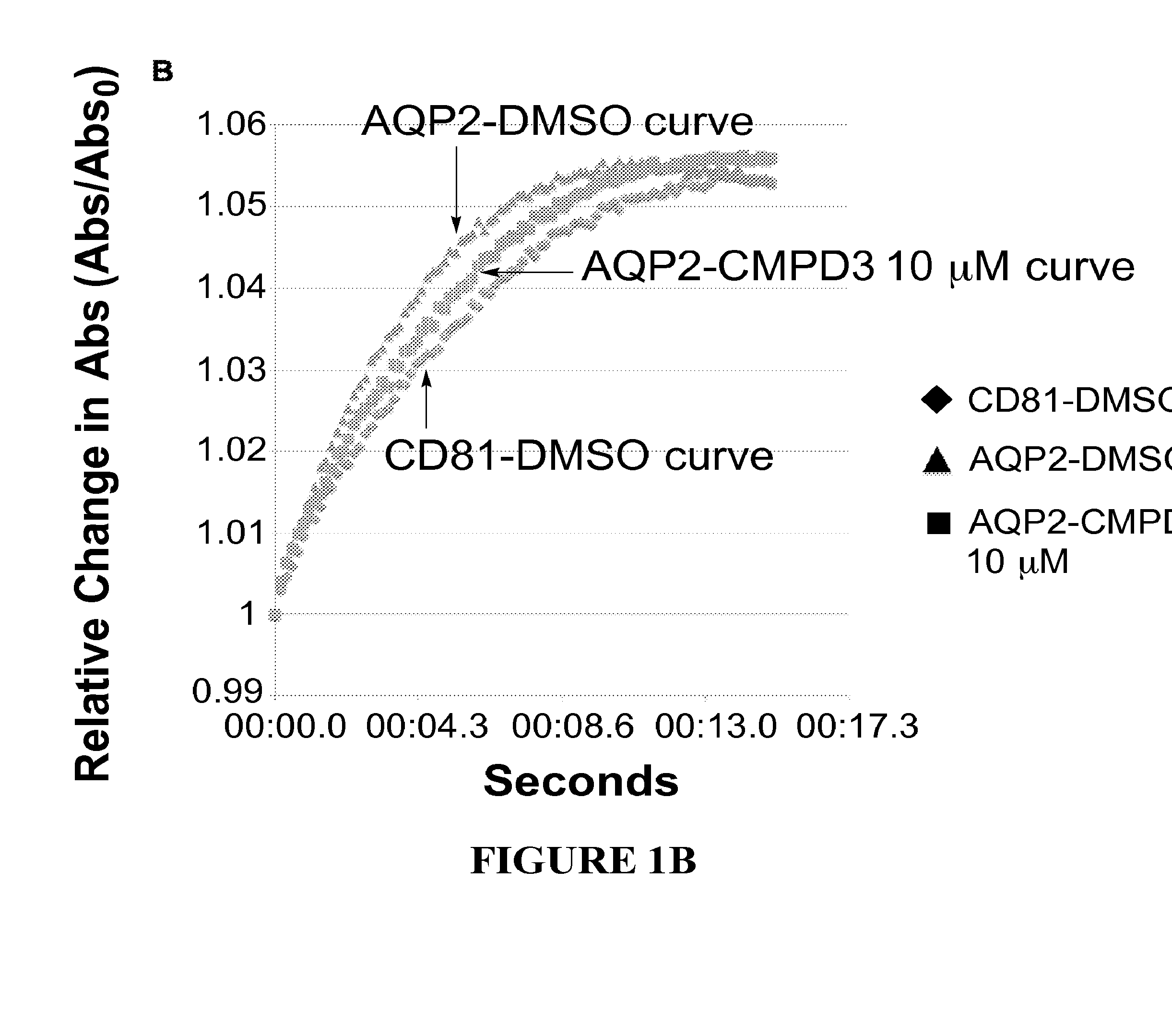
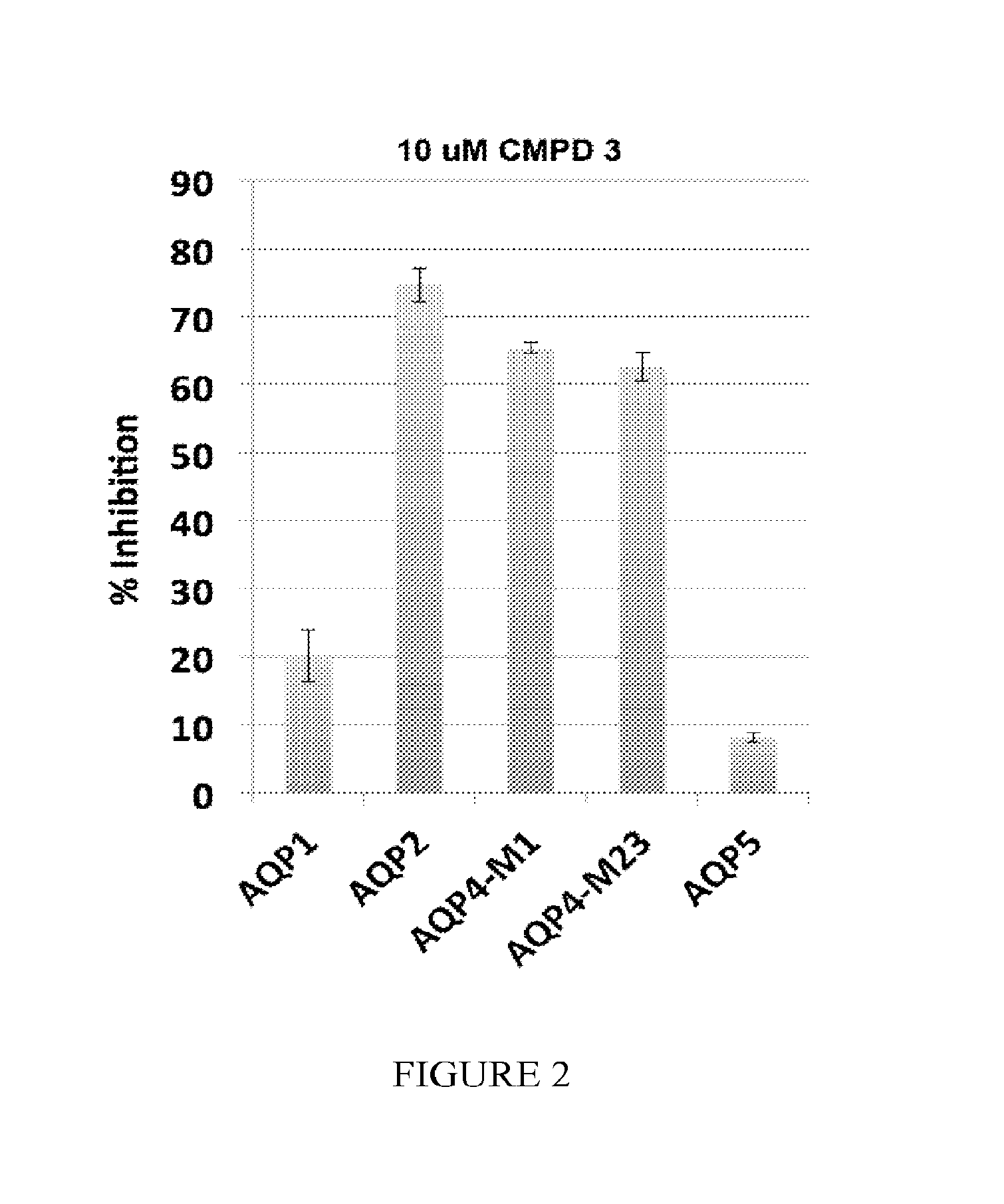
The present invention relates to the use of selective aquaporin inhibitors, e.g., of aquaporin-4 or aquaporin-2, e.g., certain phenylbenzamide compounds, for the prophylaxis, treatment and control of aquaporin-mediated conditions, e.g., diseases of water imbalance, for example edema (particularly edema of the brain and spinal cord, e.g., following trauma or ischemic stroke, as well as the edema associated with glioma, meningitis, acute mountain sickness, epileptic seizures, infections, metabolic disorders, hypoxia, water intoxication, hepatic failure, hepatic encephalopathy, diabetic ketoacidosis, abscess, eclampsia, Creutzfeldt-Jakob disease, and lupus cerebritis, as well as edema consequent to microgravity and / or radiation exposure, as well as edema consequent to invasive central nervous system procedures, e.g., neurosurgery, endovascular clot removal, spinal tap, aneurysm repair, or deep brain stimulation, as well as retinal edema), as well as hyponatremia and excess fluid retention, and diseases such as epilepsy, retinal ischemia and other diseases of the eye associated with abnormalities in intraocular pressure and / or tissue hydration, myocardial ischemia, myocardial ischemia / reperfusion injury, myocardial infarction, myocardial hypoxia, congestive heart failure, sepsis, and neuromyelitis optica, as well as migraines, as well as to novel assays for identifying aquaporin inhibitors.
Owner:AEROMICS
SGLT-2 inhibitors, methods of making them, and uses thereof
Owner:ALBANY MOLECULAR RESEARCH INC
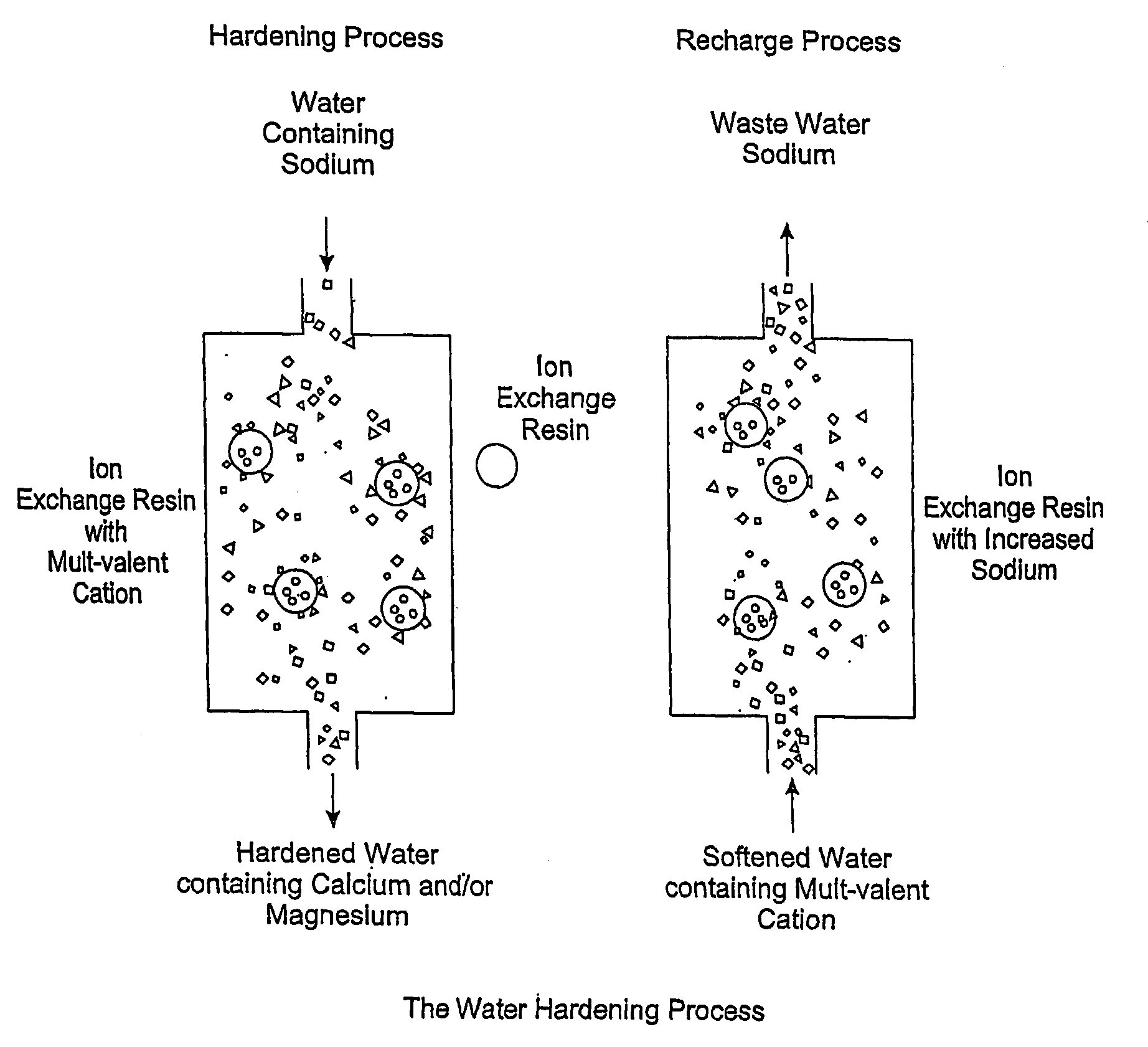
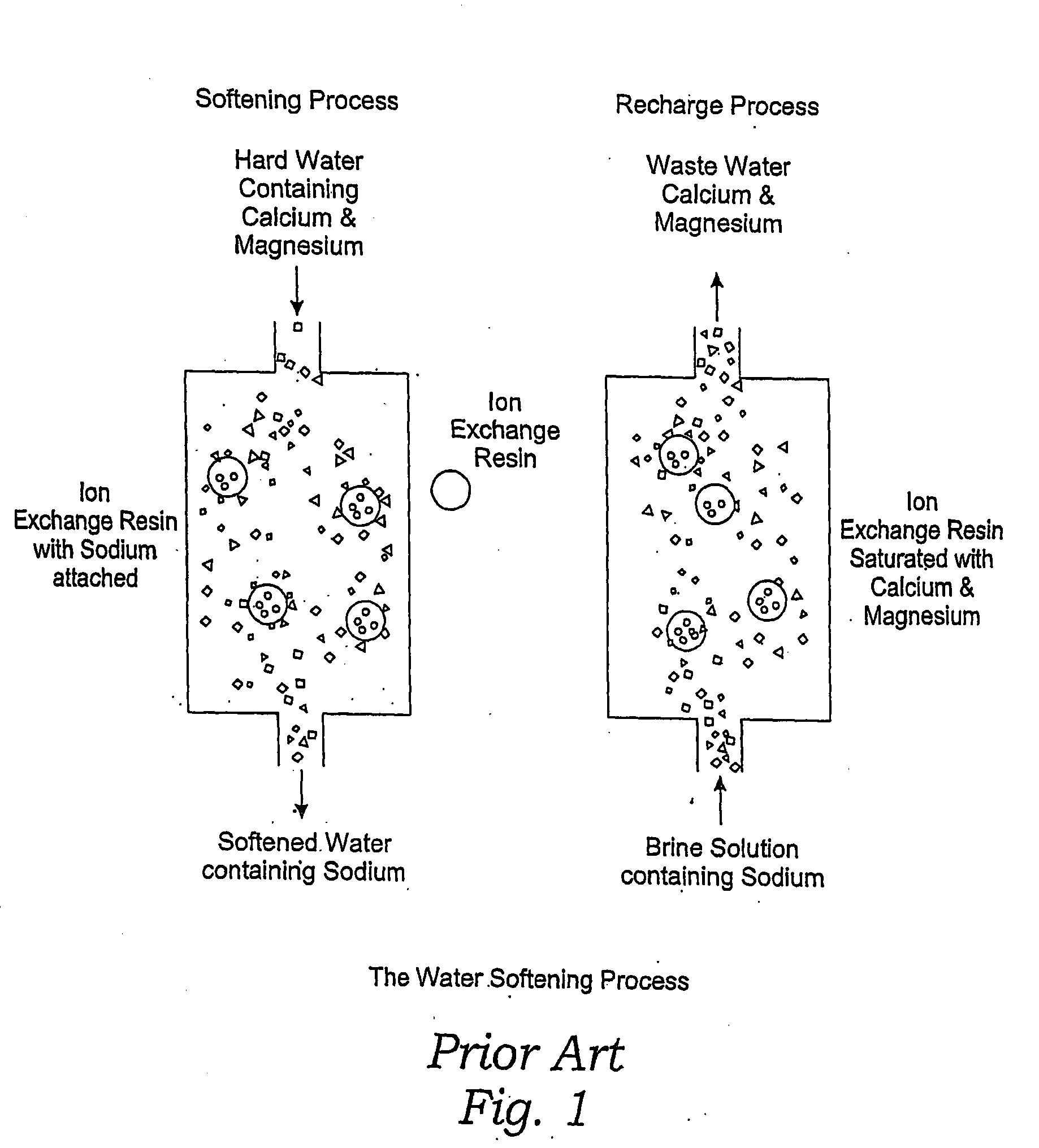
Methods of utilizing waste waters produced by water purification processing
InactiveUS20050210745A1Economically and efficiently processingLow sodium contentSpecific water treatment objectivesScale removal and water softeningDecreased sodiumHigh sodium
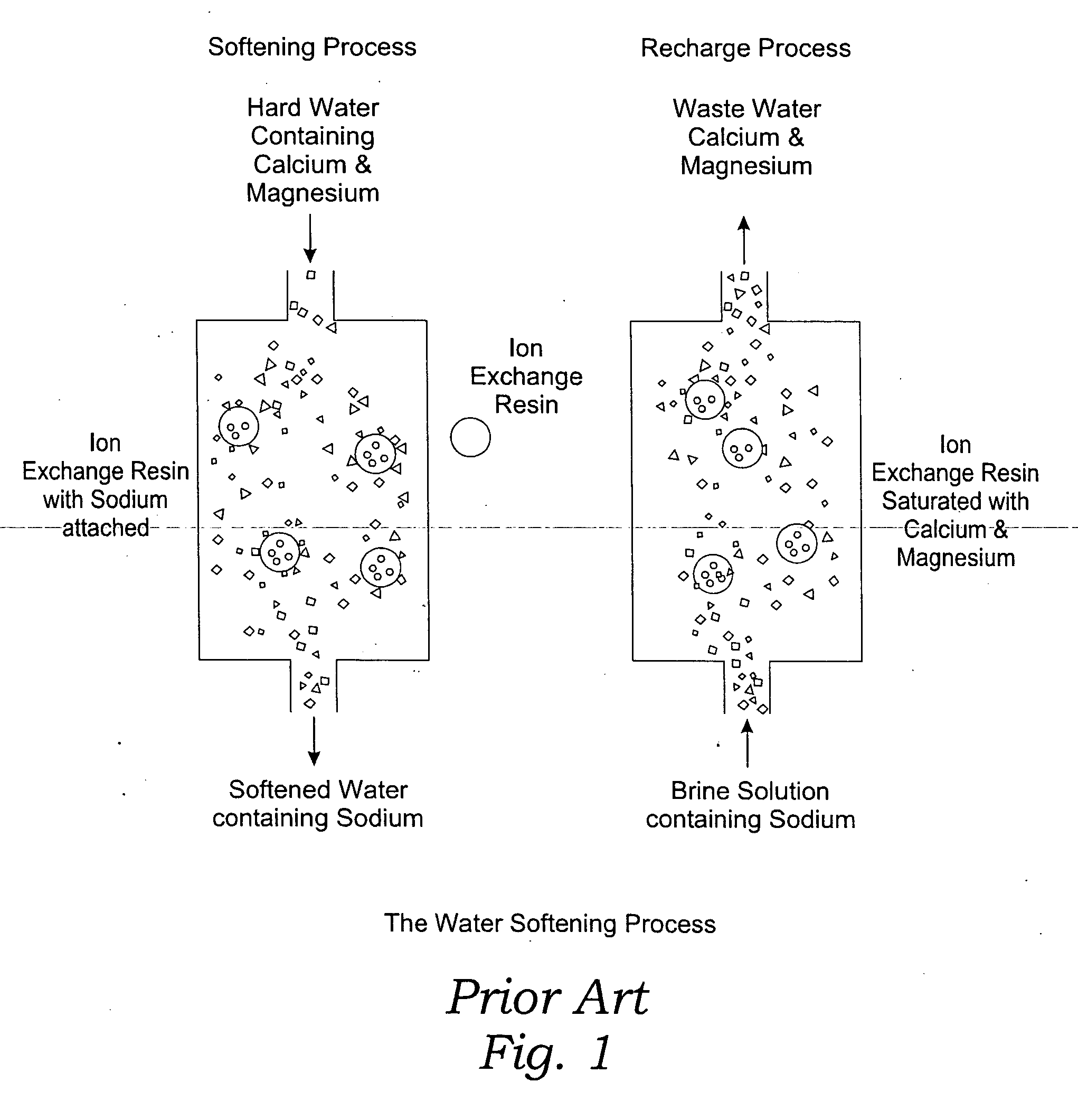
The invention relates to a process for treating unwanted moderately saline waters for producing waters acceptable for treating soil, such as for irrigation. The treated water is also suitable for human and livestock consumption. The process includes passing moderately saline waters having 0.05% or more by weight and less than 1.00% by weight of the salts of Na, K, Ca, Mg, Fe, Cl, SO4, or CO3 or combinations thereof through an ion exchange resin. The ion exchange resin is pre-treated to be saturated with multivalent cations. Preferred multivalent cations include calcium (Ca2+) or magnesium (Mg2+) ions, or combinations thereof. After passing through the ion exchange resin, the effluent has decreased sodium cations and increased calcium and / or magnesium cations compared to the pre-treated moderately saline water. As the moderately saline waters passes through the ion exchange resin, the sodium content of the resin rises as the multivalent cation content lowers until the resin is unacceptable for further water treatment in accordance with the present invention. To regenerate the ion exchange resin, the resin is flushed with a brine solution having more than 1.00% by weight of the salts of Na, K, Ca, Mg, Fe, Cl, SO4, or CO3. Preferably, the brine is particularly high in calcium and / or magnesium content and low in sodium. The brine solution is flushed through the ion exchange resin until the ion exchange resin is sufficiently saturated with multivalent cations to again process moderately saline water having high sodium content.
Owner:GROTT GERALD J
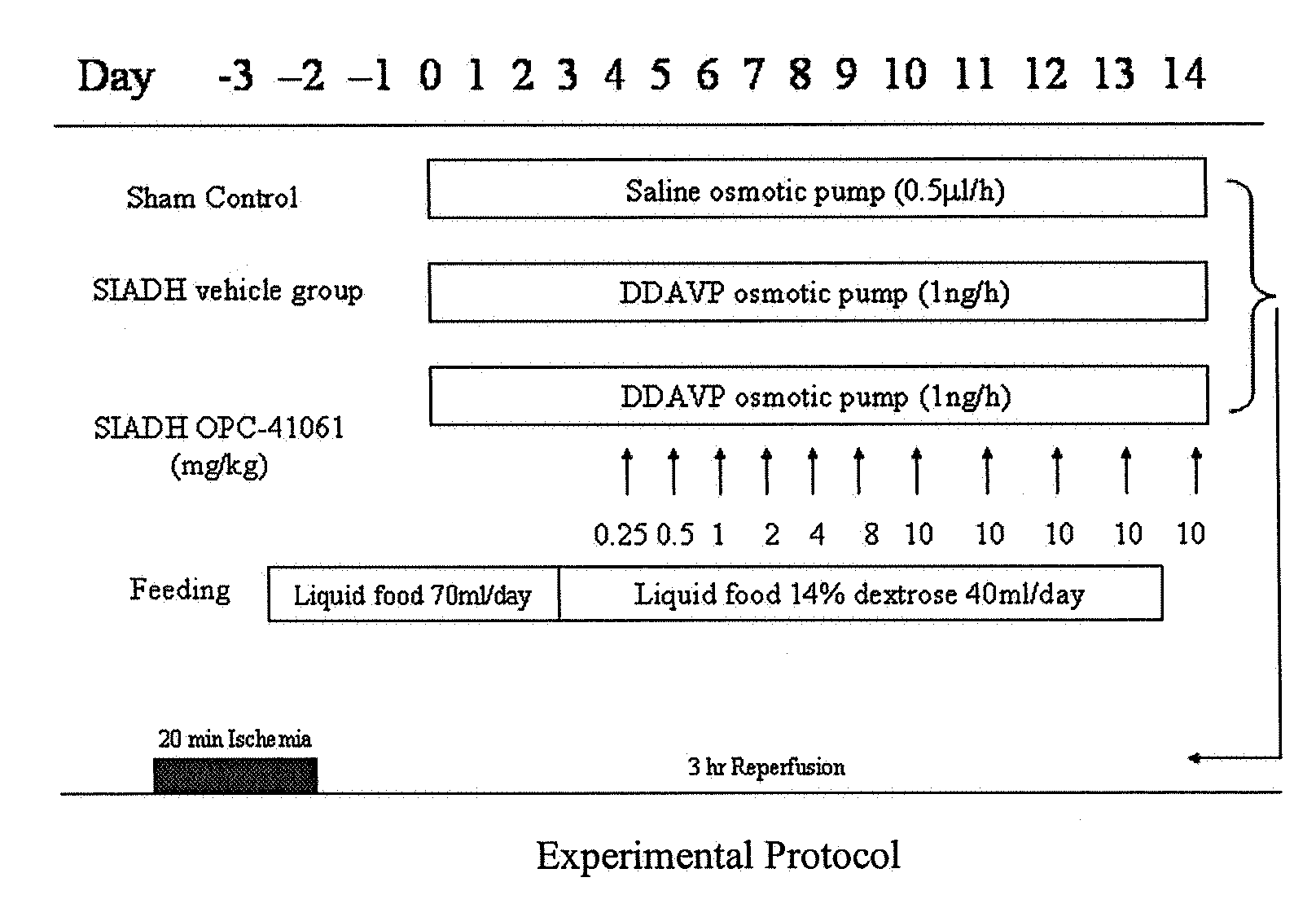
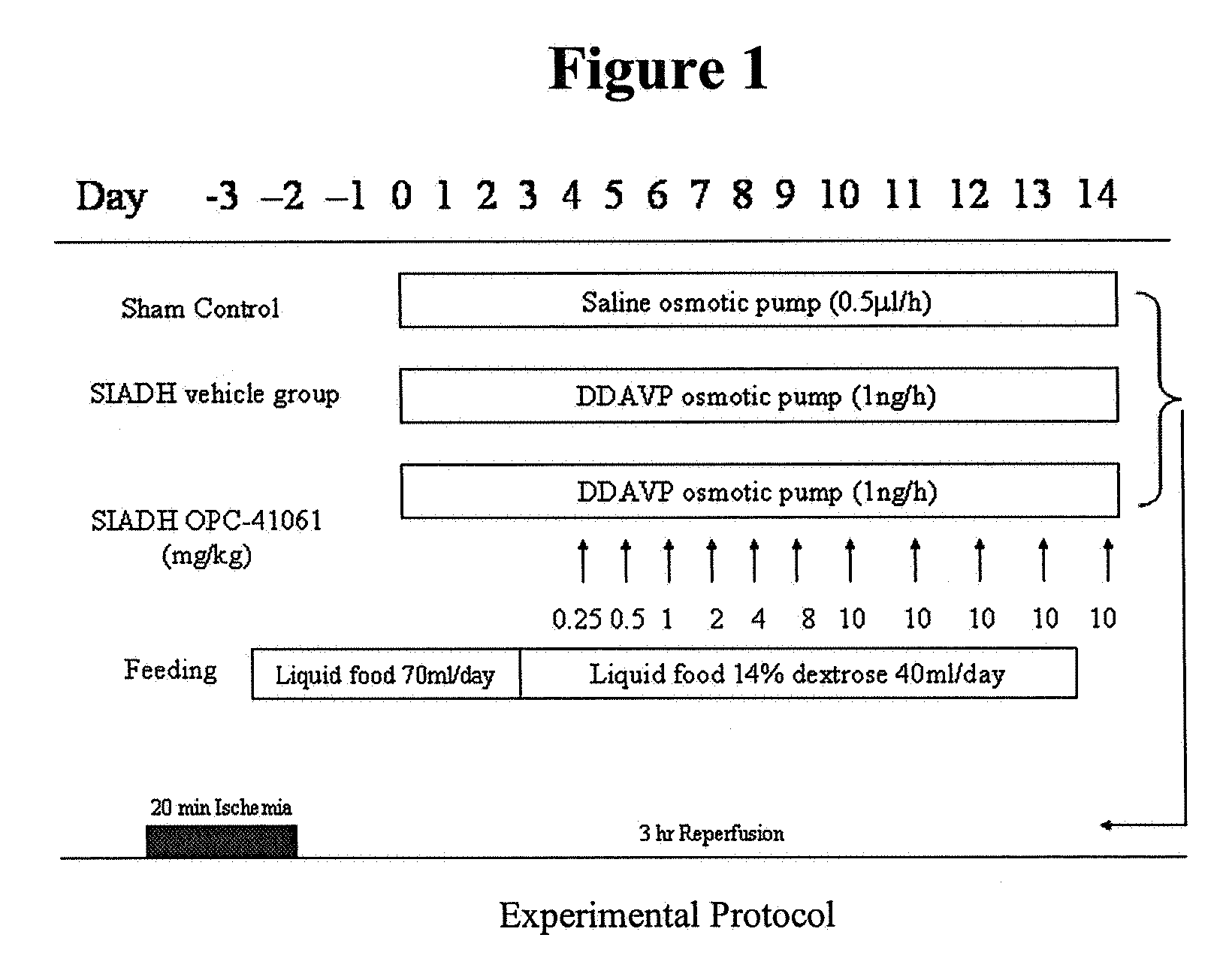
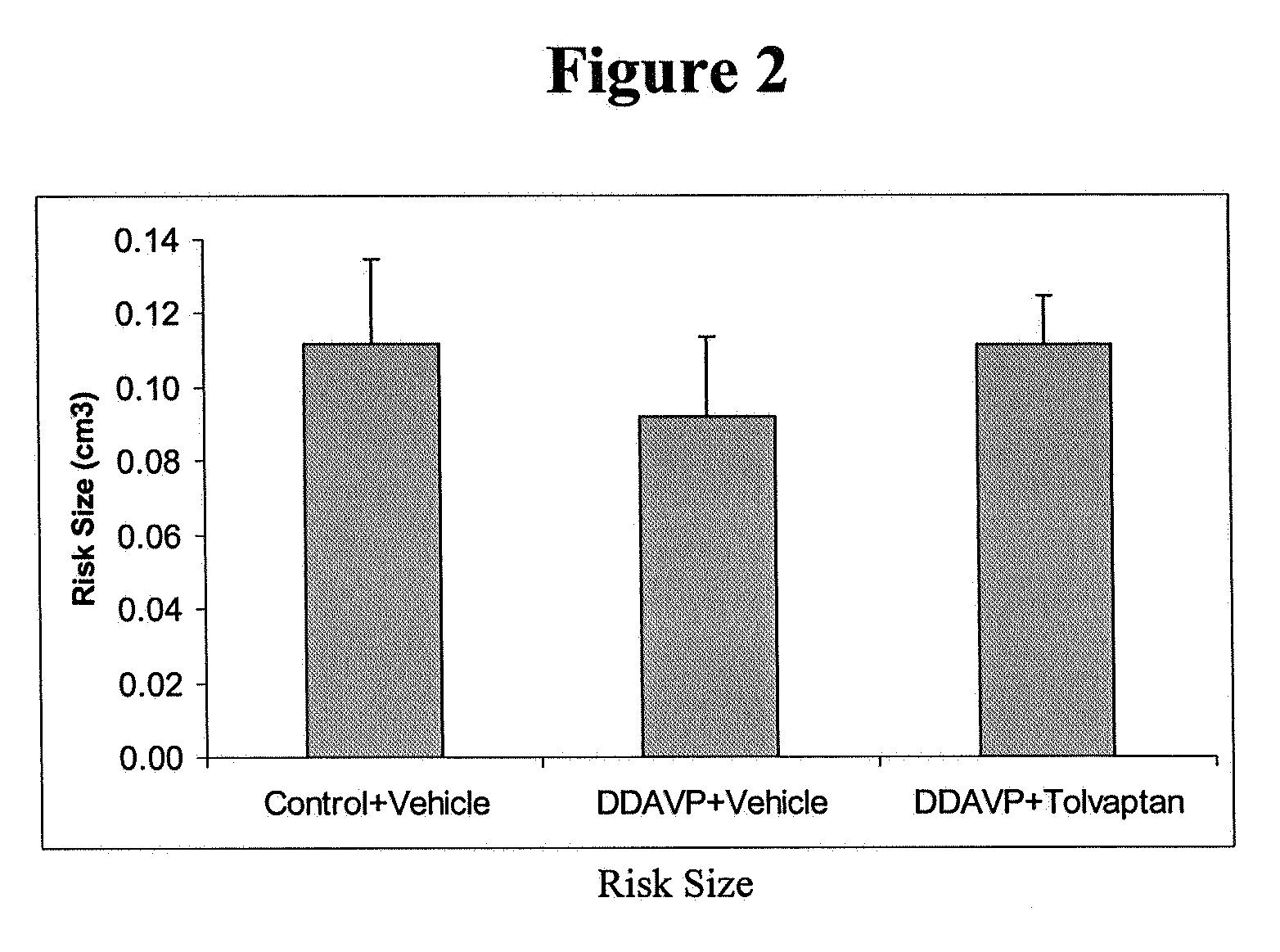
Method for reducing infarction using vasopressin antagonist compounds, and compositions and combinations therefor
InactiveUS20080221084A1Reduce infarctionMyocardial infarctionBiocideAnimal repellantsDiseaseDecreased sodium
The present invention relates to a method for reducing infarction comprising administering to a patient ion need thereof a therapeutically effective amount of a composition comprising as an active ingredient a vasopressin antagonist compound and to a composition useful therefor. The present invention also relates to a method for reducing infarction comprising administering to a patient in need thereof a therapeutically effective amount of a combination of a vasopressin antagonist compound and a beta-blocker and to combinations useful therefor. The methods, compositions and combinations of the present invention can be used for reducing infarction in the heart (myocardial infarction) and the brain (stroke). The methods, compositions and combinations of the present invention can also be used for the treatment and / or prevention of hypertension, edema, ascites, heart failure, renal function disorder, vasopressin inappropriate secretion syndrome (SIADH), hepatocirrhosis, hyponatremia, hypokalemia, polycystic kidney disease, diabetes, or circulation disorder.
Owner:OTSUKA PHARM CO LTD
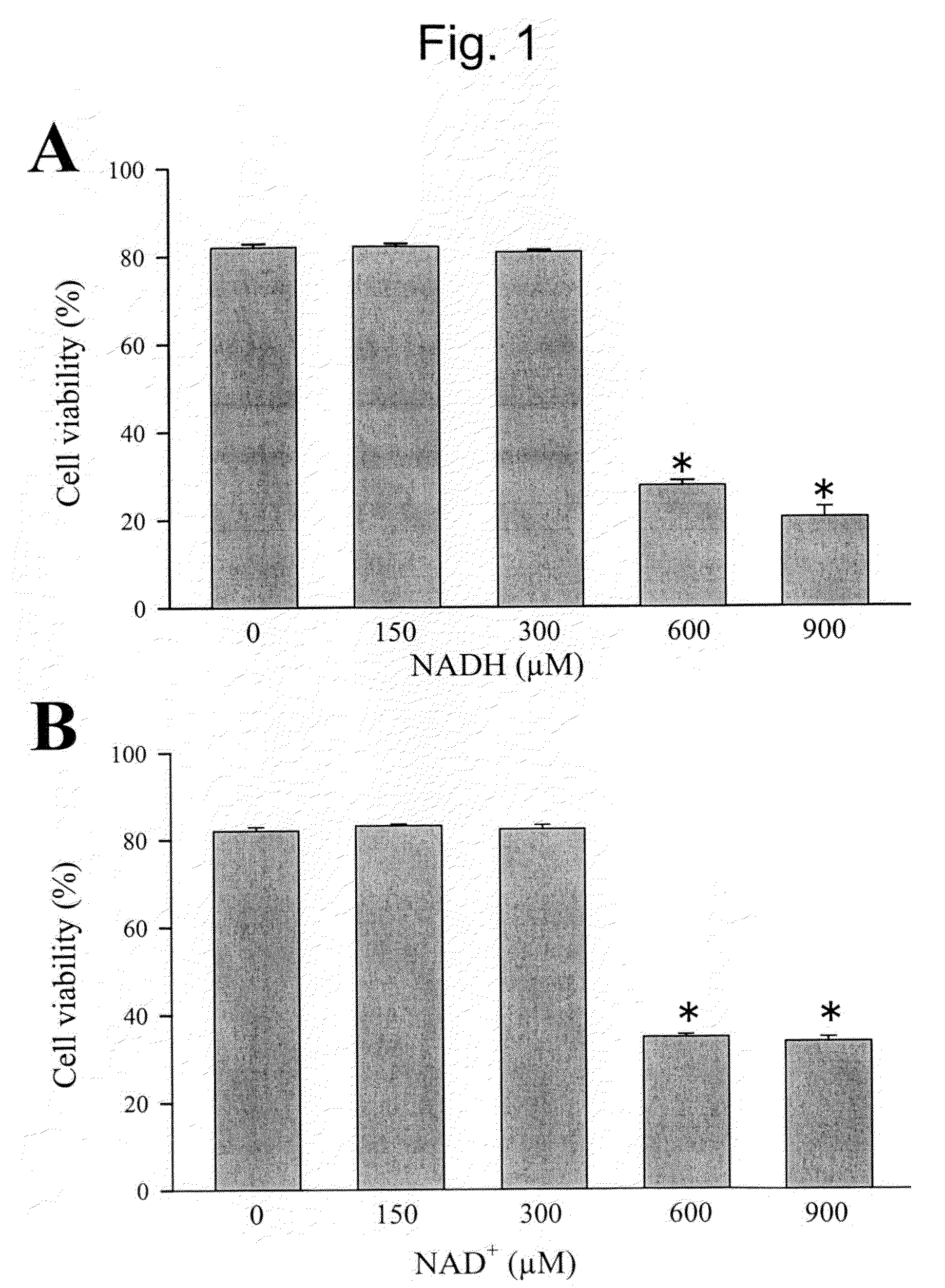
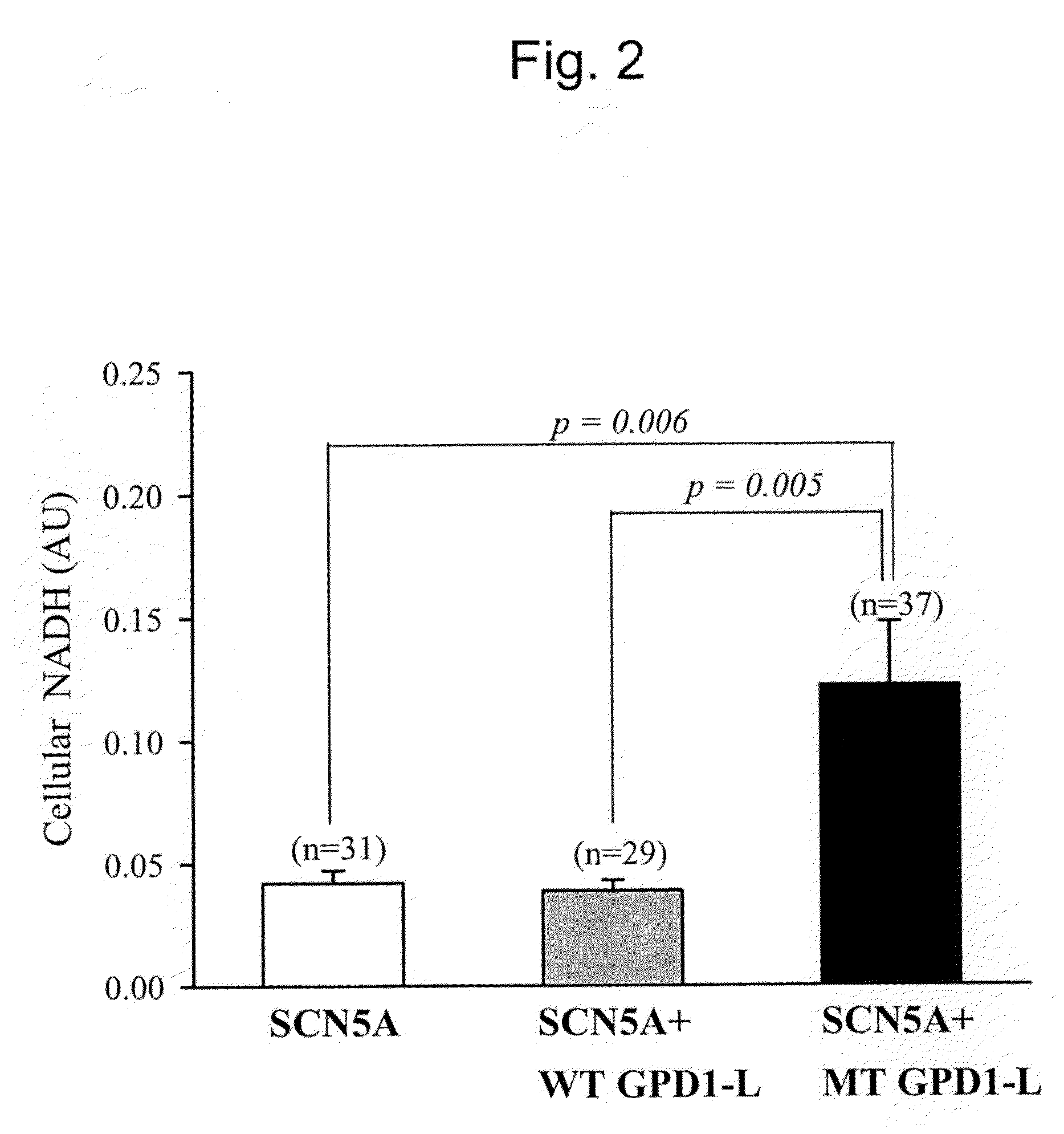
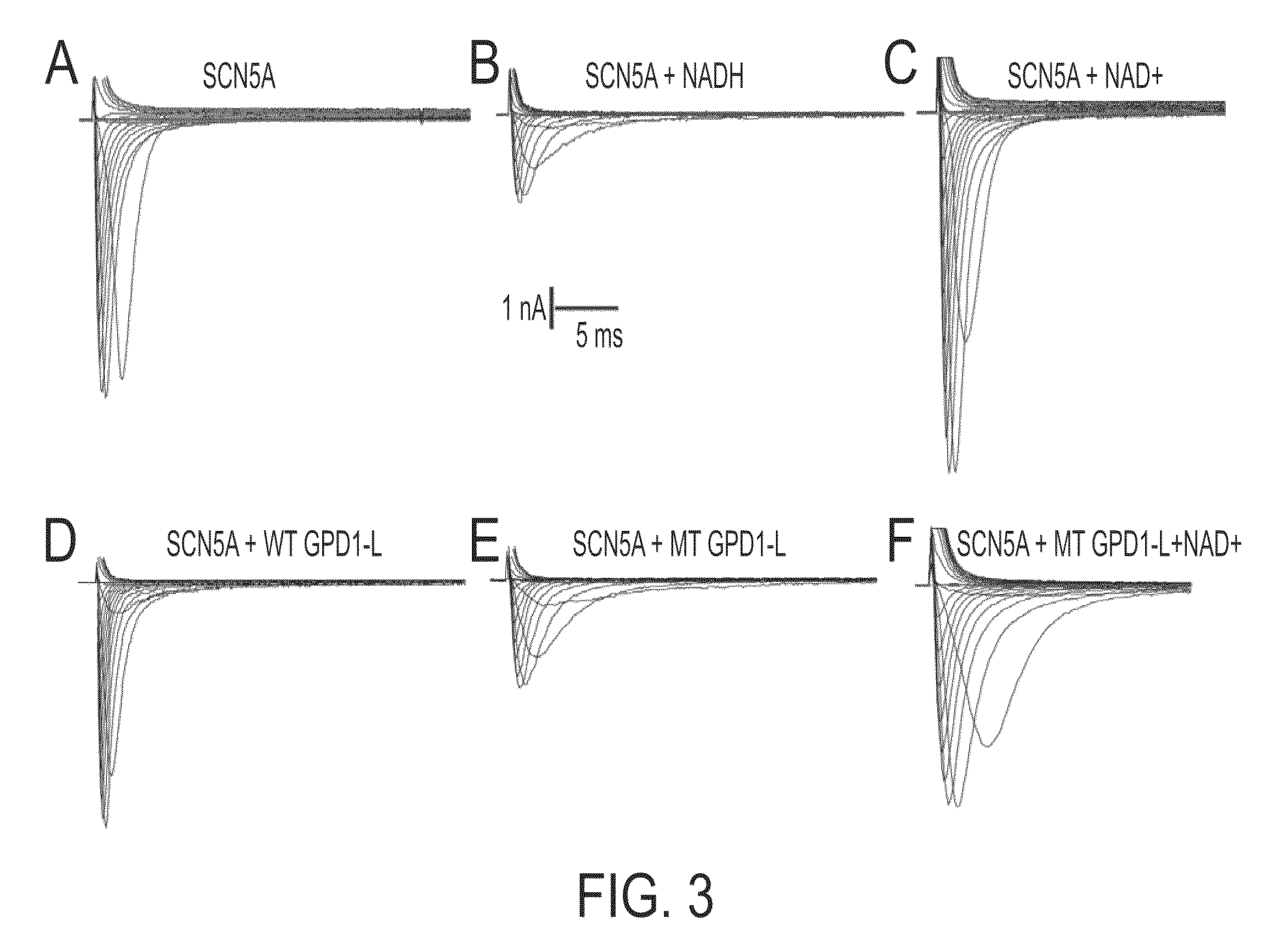
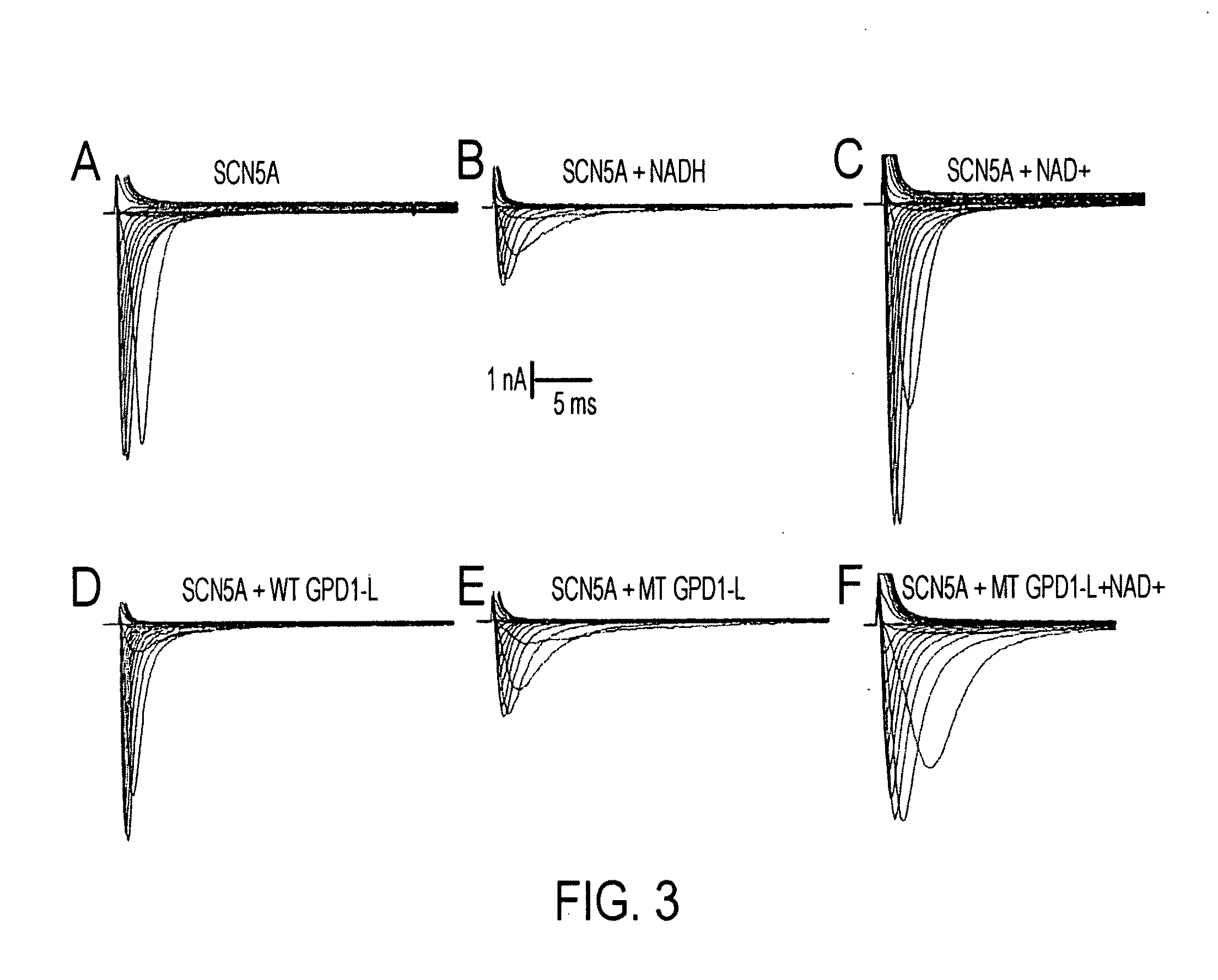
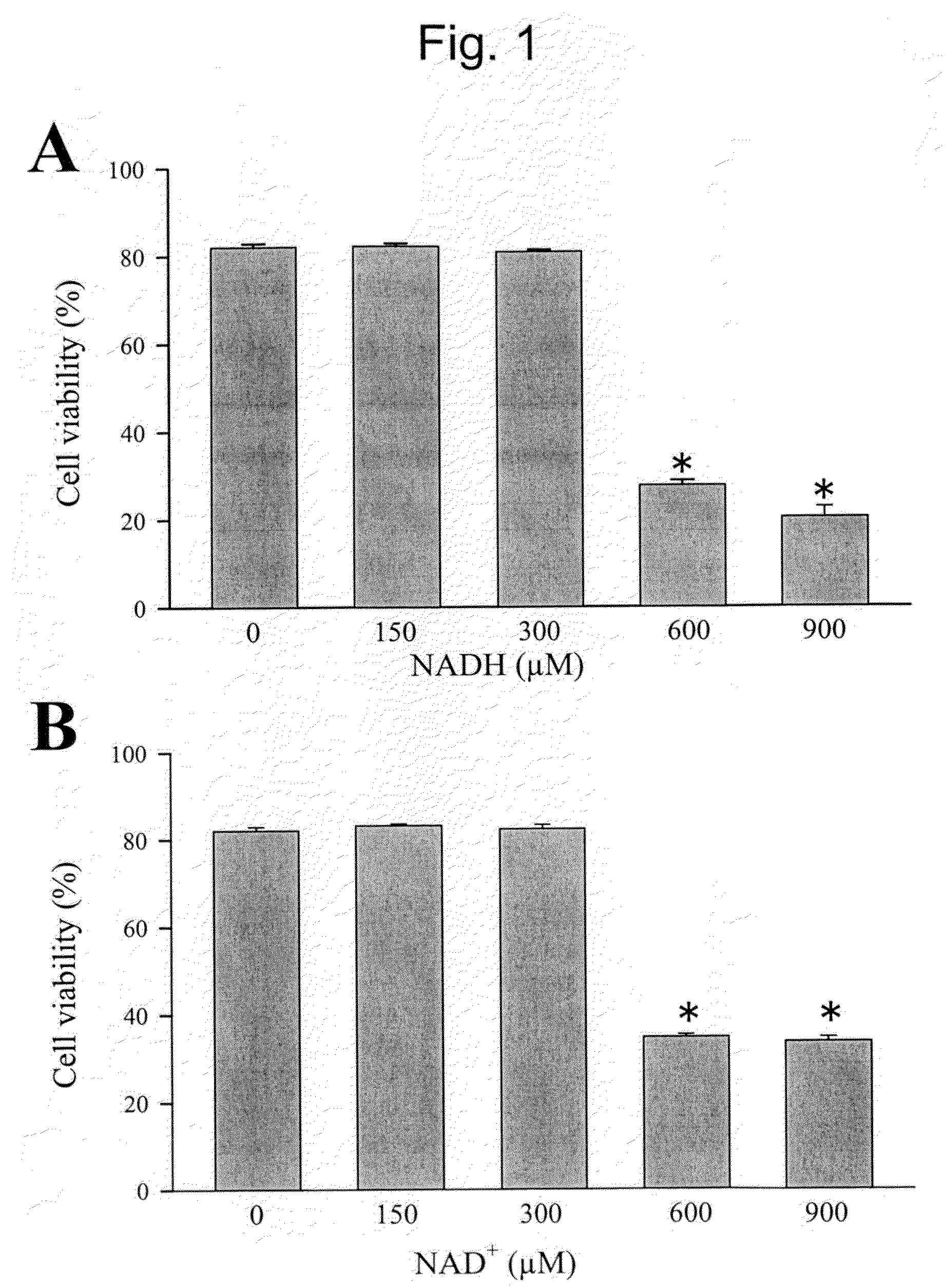
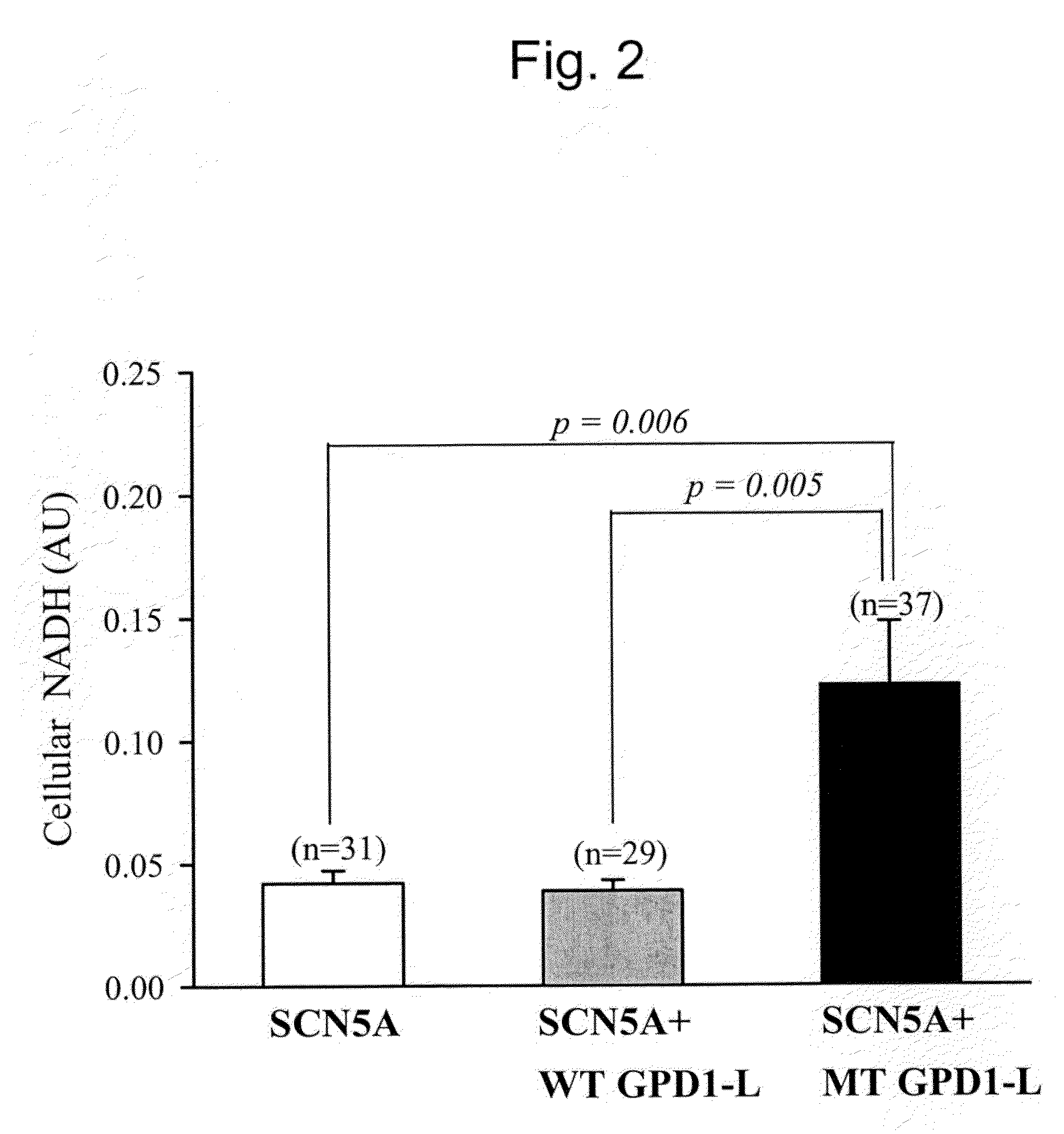
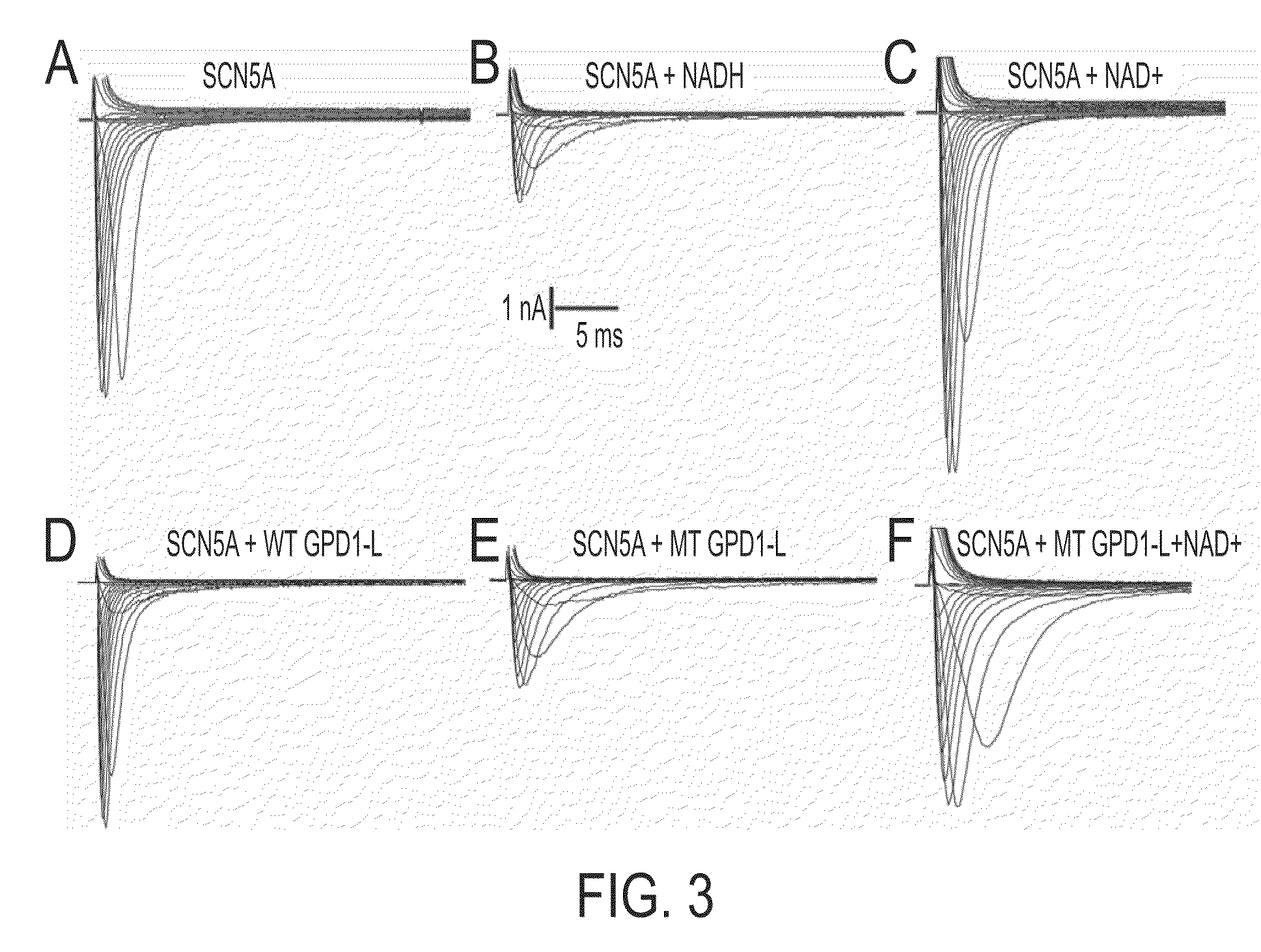
Modulation of sodium channels by nicotinamide adenine dinucleotide
InactiveUS8003324B2Lower average currentIncrease currentBiocideSugar derivativesDecreased sodiumNicotinamide adenine dinucleotide
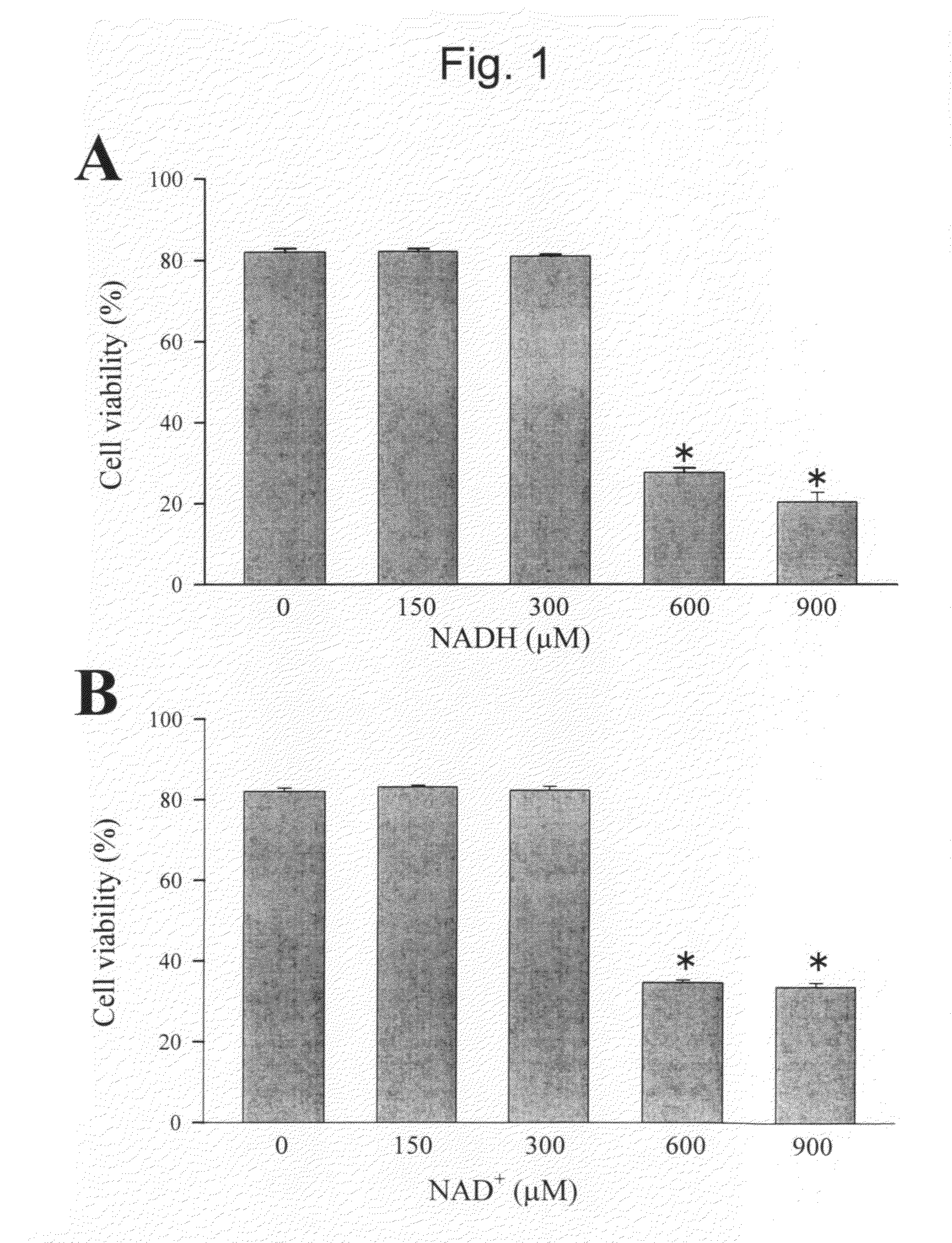
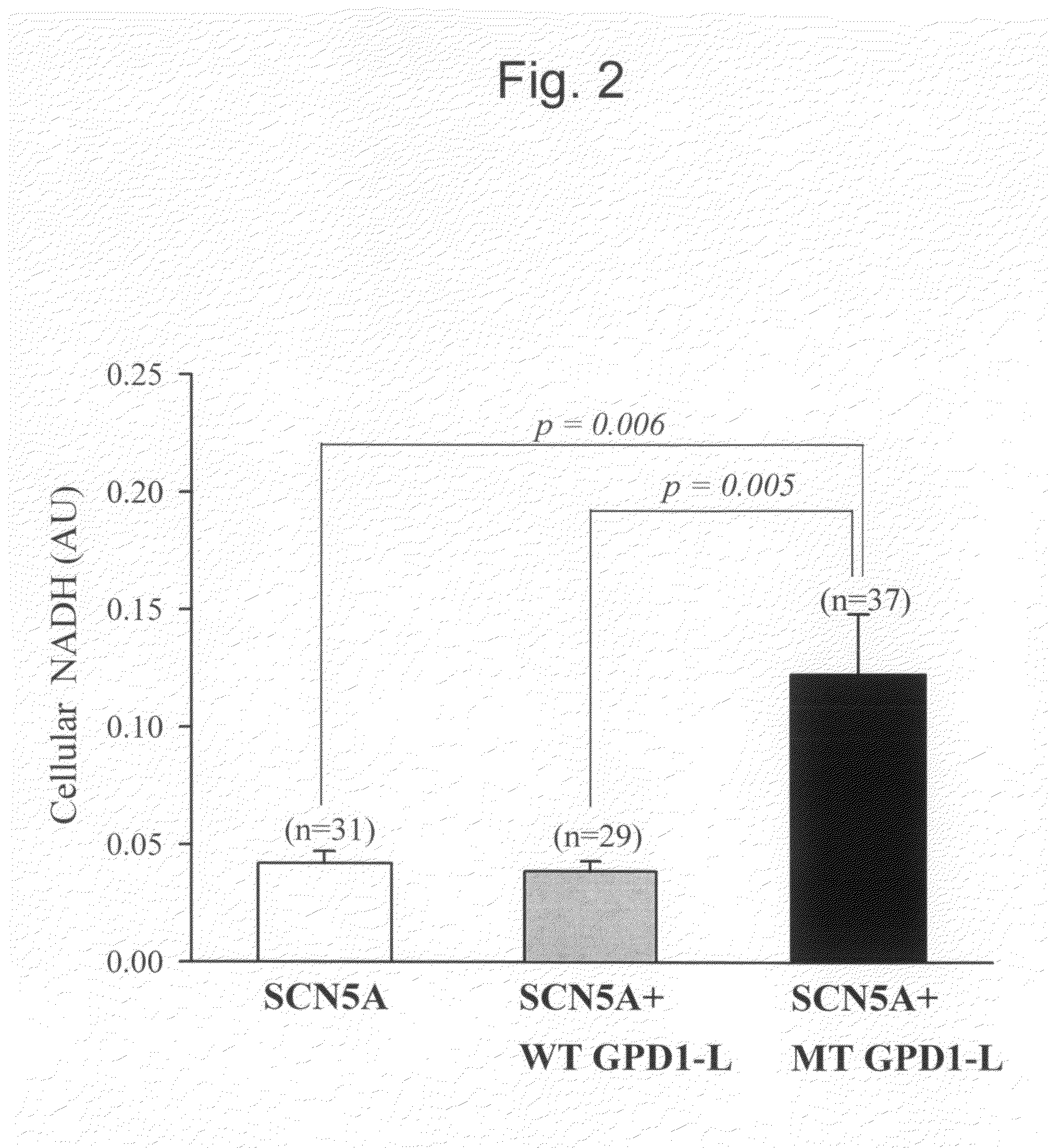
The present invention relates to the use of oxidized nicotinamide adenine dinucleotide (NAD+) or of its reduced form, NADH, as sodium channel modulators. The present invention also relates to the use of compositions containing NAD+ or NADH to treat conditions associated with sodium channel current, such as arrhythmia. NAD+ is found to increase sodium channel current, while NADH is found to decrease sodium channel current. Thus, conditions that are associated with decreased sodium channel current can be treated with NAD+, while conditions that is associated with increased sodium channel current can be treated with NADH.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
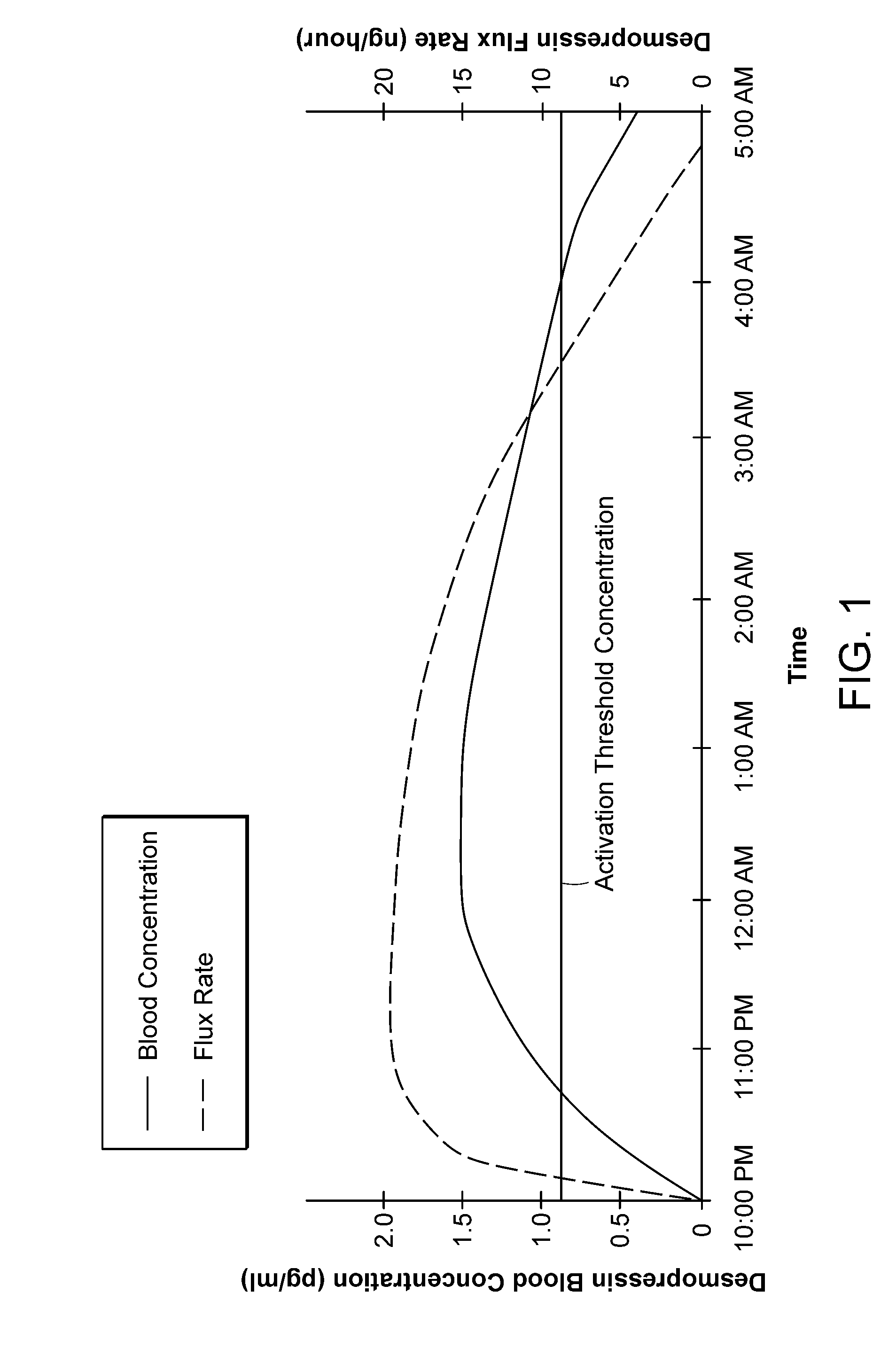
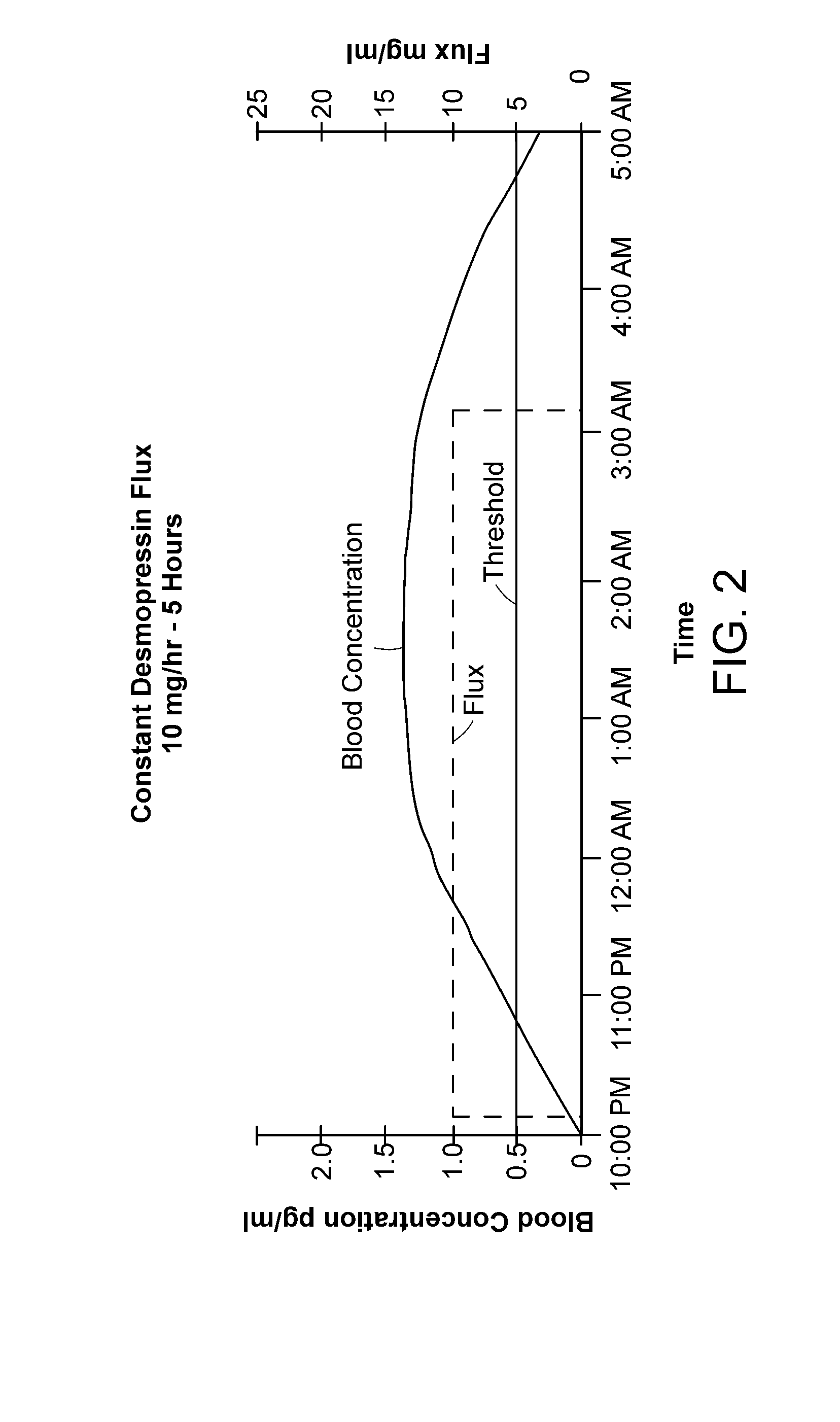
Methods and devices for desmopressin drug delivery
InactiveUS8399410B2Prevent adverse side effectsReduce productionElectrotherapyMicroneedlesDecreased sodiumSide effect
Disclosed are devices for urine voiding postponement, and methods for treating conditions such as central diabetes insipidus, enuresis, nocturia, urinary frequency or incontinence. The devices deliver a desmopressin flux through the skin of a patient in a low dose amount just necessary to achieve a desired anti-diuretic effect without undesirable side effects such as hyponatremia. The devices are designed to permit a state of normal urinary production to return quickly after the desmopressin flux is terminated.
Owner:SERENITY PHARMA CORP
Cell volume-regulated human kinase h-sgk
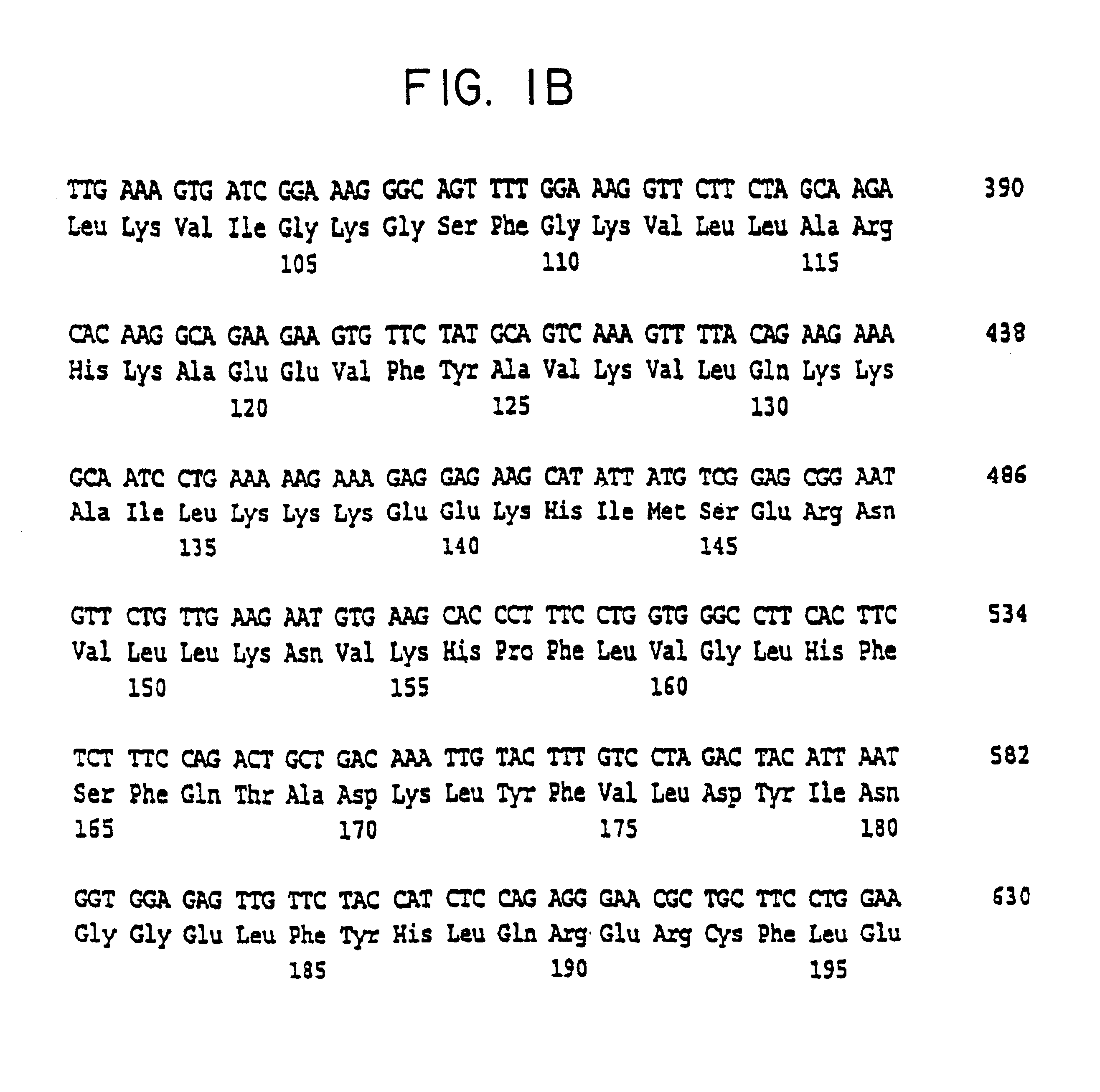
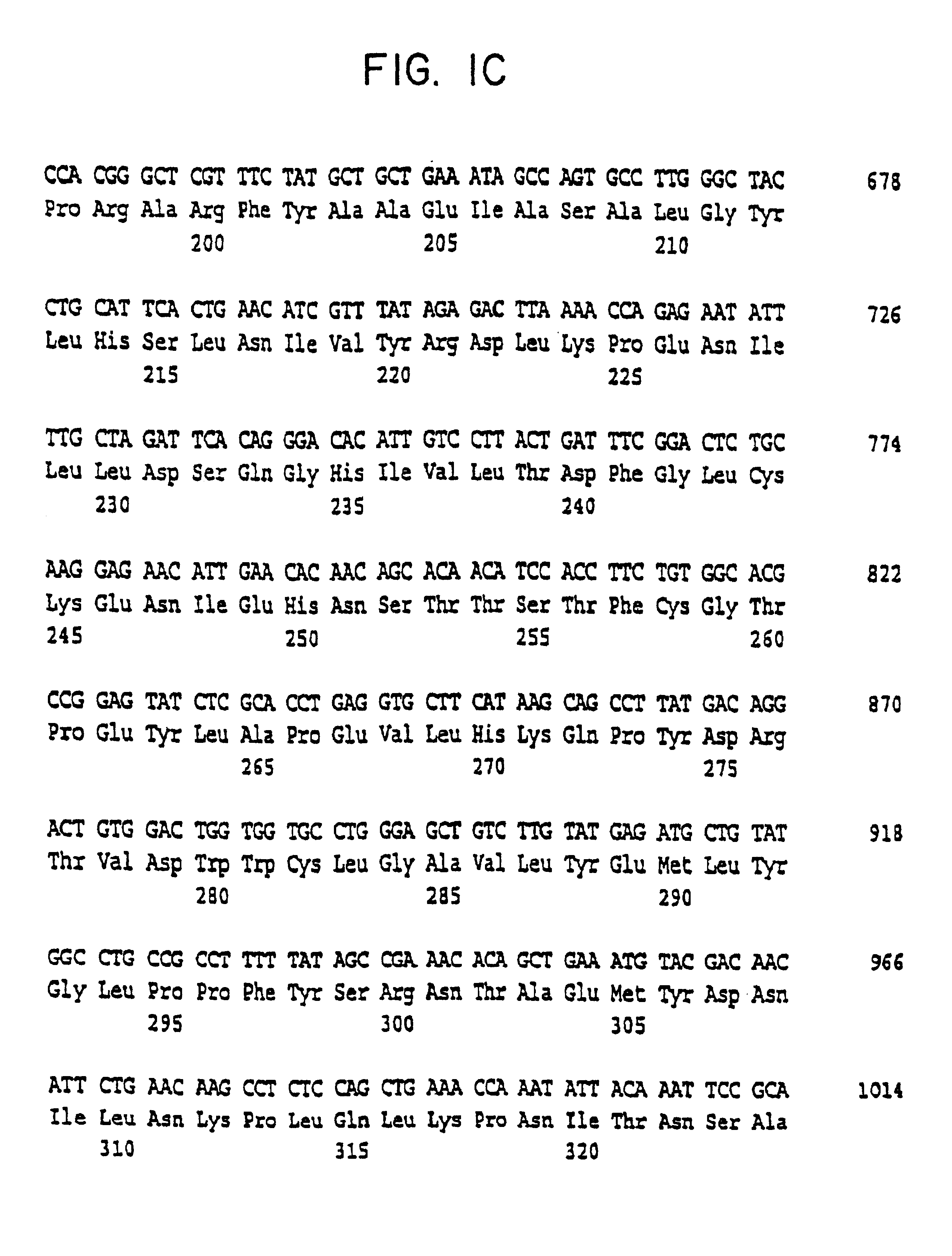
InactiveUS6326181B1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDecreased sodiumHypernatremia
The present invention relates to the cloning and characterization of a human serine / threonine kinase (h-sgk: serum and glucocorticoid dependent kinase). The invention furthermore relates to reagents for diagnosing conditions associated with a change in cell volume and / or in "macromolecular crowding" in the body, such as, for example, hypernatremia, hyponatremia, diabetes mellitus, renal failure, hypercatabolism, hepatic encephalopathy, inflammation and microbial or viral infections. The present invention additionally relates to pharmaceuticals comprising the h-sgk, nucleic acids which code for the h-sgk, or receptors, in particular antibodies, which specifically bind to the h-sgk.
Owner:WALDEGGER SIEGFRIED +1
Sglt-2 inhibitors, methods of making them, and uses thereof
InactiveUS20110237527A1Improve the level ofHigh levelSaccharide with heterocyclic radicalsBiocideDiseaseDecreased sodium
The present invention relates to compounds which are inhibitors of sodium dependent glucose co-transporter-2 (SGLT-2). These compounds are used in the treatment of various disorders, including diabetes, impaired glucose tolerance, insulin resistance, retinopathy, nephropathy, neuropathy, cataracts, hyperglycemia, hyperinsulinemia, hyperchlolesterolemia, elevated blood level of free fatty acids or glycerol, hyperlipidemia, hypertriglyceridemia, obesity, wound healing, tissue ischemia, atherosclerosis, and hypertension. These compounds and compositions are also useful for treating and preventing kidney stones, hyperuricemia, gout, and hyponatremia. Methods of making these compounds are also described in the present invention.
Owner:ALBANY MOLECULAR RESEARCH INC
Modulation of sodium channels by nicotinamide adenine dinucleotide
InactiveUS20110288044A1Lower average currentIncrease currentBiocideCarbohydrate active ingredientsDecreased sodiumNicotinamide adenine dinucleotide
The present invention relates to the use of oxidized nicotinamide adenine dinucleotide (NAD+) or of its reduced form, NADH, as sodium channel modulators. The present invention also relates to the use of compositions containing NAD+ or NADH to treat conditions associated with sodium channel current, such as arrhythmia. NAD+ is found to increase sodium channel current, while NADH is found to decrease sodium channel current. Thus, conditions that are associated with decreased sodium channel current can be treated with NAD+, while conditions that is associated with increased sodium channel current can be treated with NADH.
Owner:U S DEPT OF VETERANS AFFAIRS TECH TRANSFER PROGRAM OFFICE OF RES & DEV +1
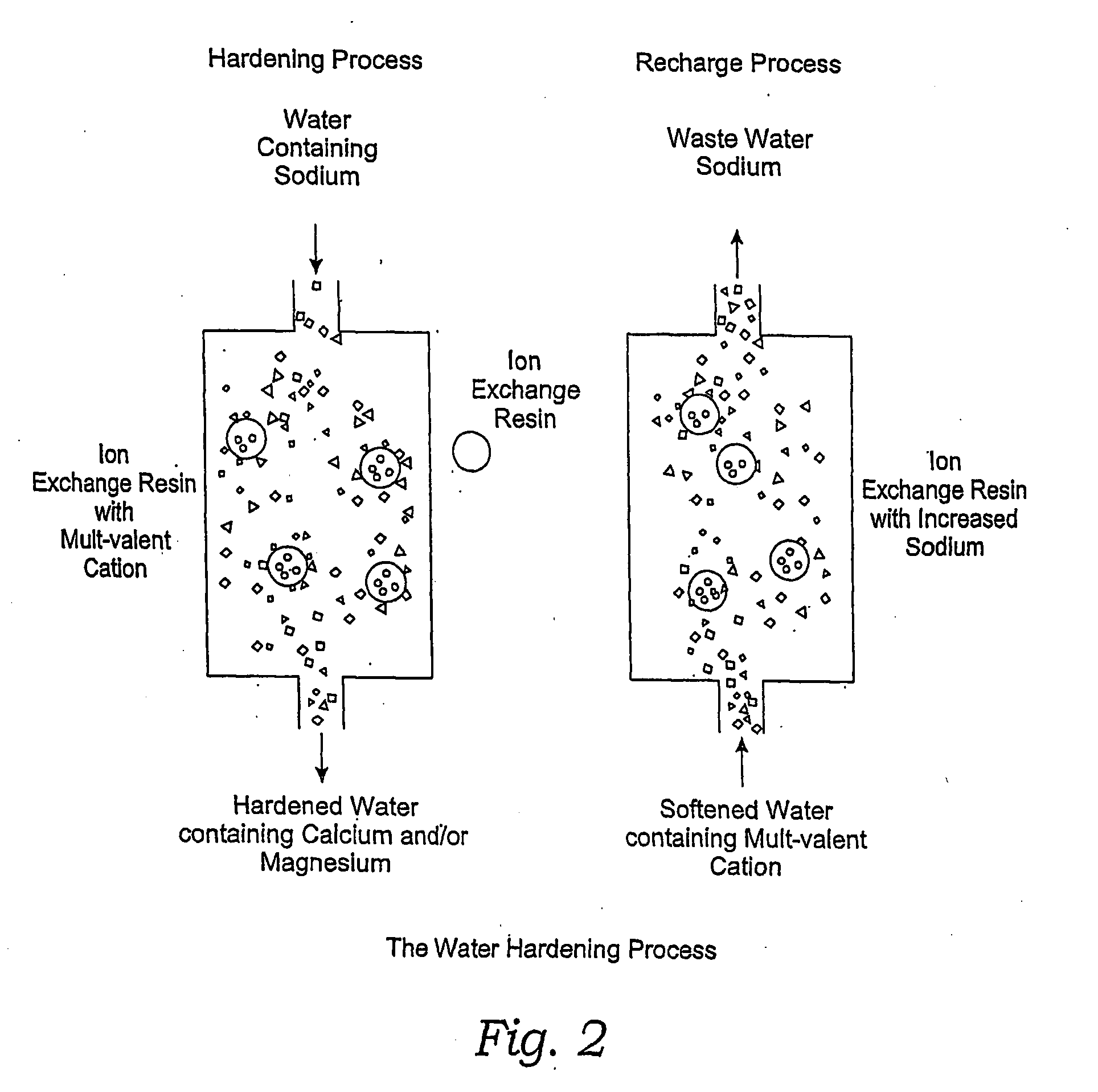
Methods of Utilizing Waste Waters Produced by Water Purification Processing
InactiveUS20080164213A1Economically and efficiently processingReduce contentIon-exchanger regenerationSpecific water treatment objectivesDecreased sodiumMAGNESIUM CATION
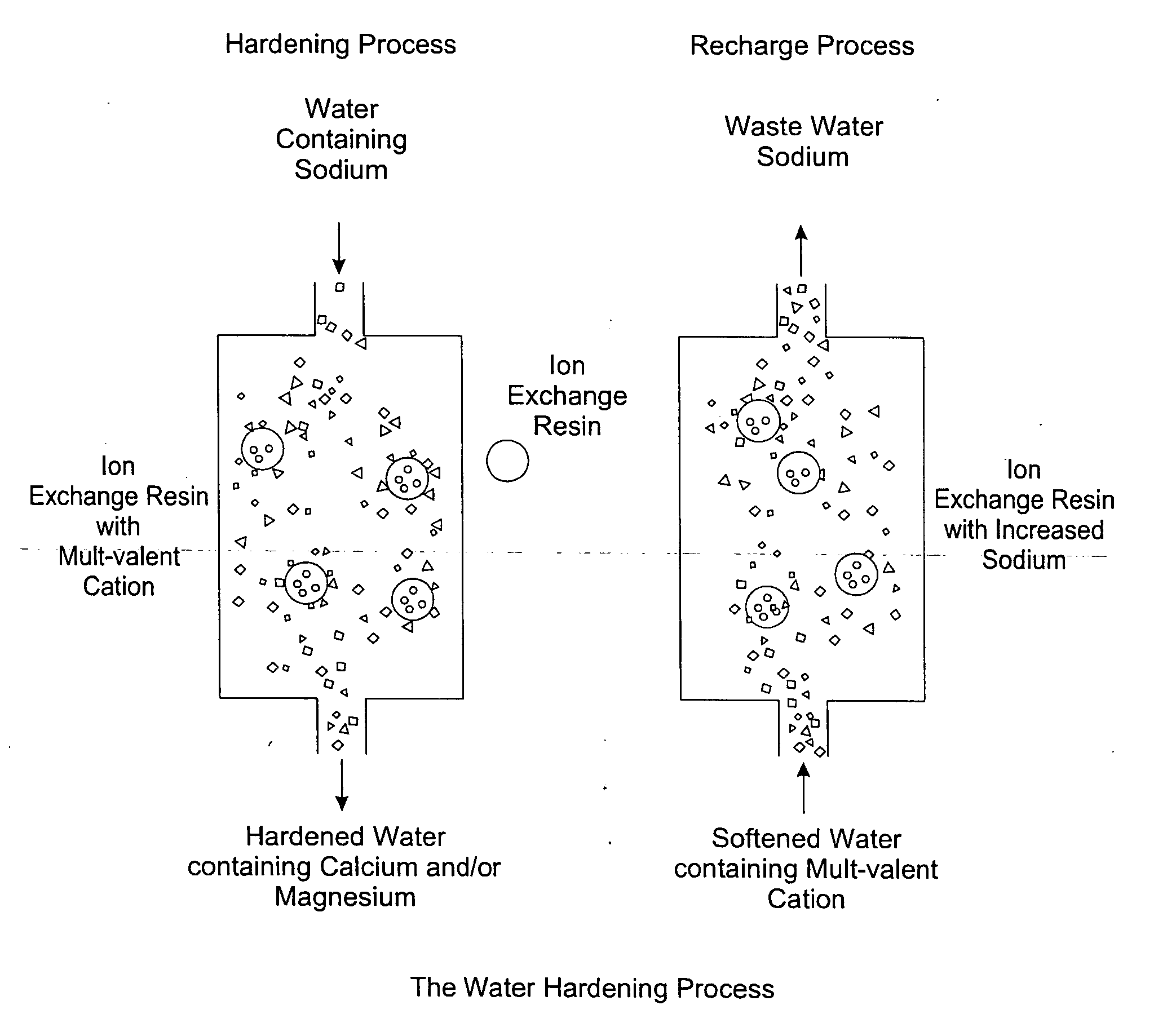
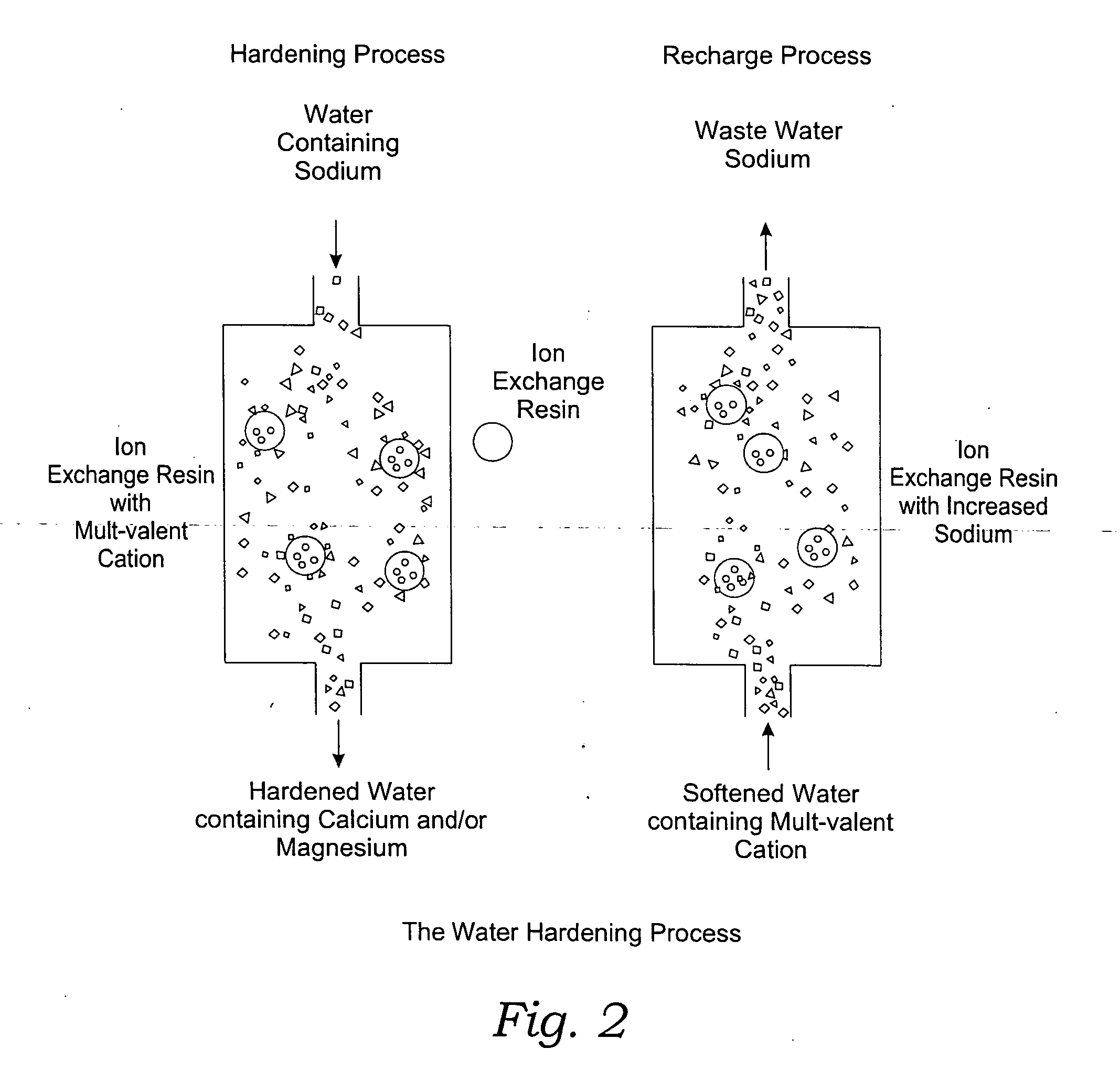
The invention relates to treating unwanted moderately saline water for producing useful water. The treated water is also suitable for human and livestock consumption. The process includes passing moderately saline waters having 0.05% or more weight and less than 1.00% by weight of the salts of Na, Ca, Mg, Fe, Cl, SO4, or CO3 or combinations thereof through an ion exchange media (FIG. 2). The ion exchange media is pre-treated to be saturated with multivalent cations such as calcium (Ca2+) or magnesium (Mg2+) ions (FIG. 2). After passing through the ion exchange media, the effluent has decreased sodium cations and increased calcium and / or magnesium cations compared to the pre-treated moderately saline water (FIG. 2). As the moderately saline waters pass through the ion exchange media, the sodium content of the media rises as the multivalent cation content lowers until the media is unacceptable for further water treatment and must be regenerated.
Owner:ECYCLING
Cell volume-regulated human kinase h-sgk
InactiveUS6855520B2Prevent overcorrectionIncreased osmolarityPeptide/protein ingredientsTransferasesDecreased sodiumHypernatremia
The present invention relates to the cloning and characterization of a human serine / threonine kinase (h-sgk: serum and glucocorticoid dependent kinase). The invention furthermore relates to reagents for diagnosing conditions associated with a change in cell volume and / or in “macromolecular crowding” in the body, such as, for example, hypernatremia, hyponatremia, diabetes mellitus, renal failure, hypercatabolism, hepatic encephalopathy, inflammation and microbial or viral infections. The present invention additionally relates to pharmaceuticals comprising the h-sgk, nucleic acids which code for the h-sgk, or receptors, in particular antibodies, which specifically bind to the h-sgk.
Owner:LANG FLORIAN +1
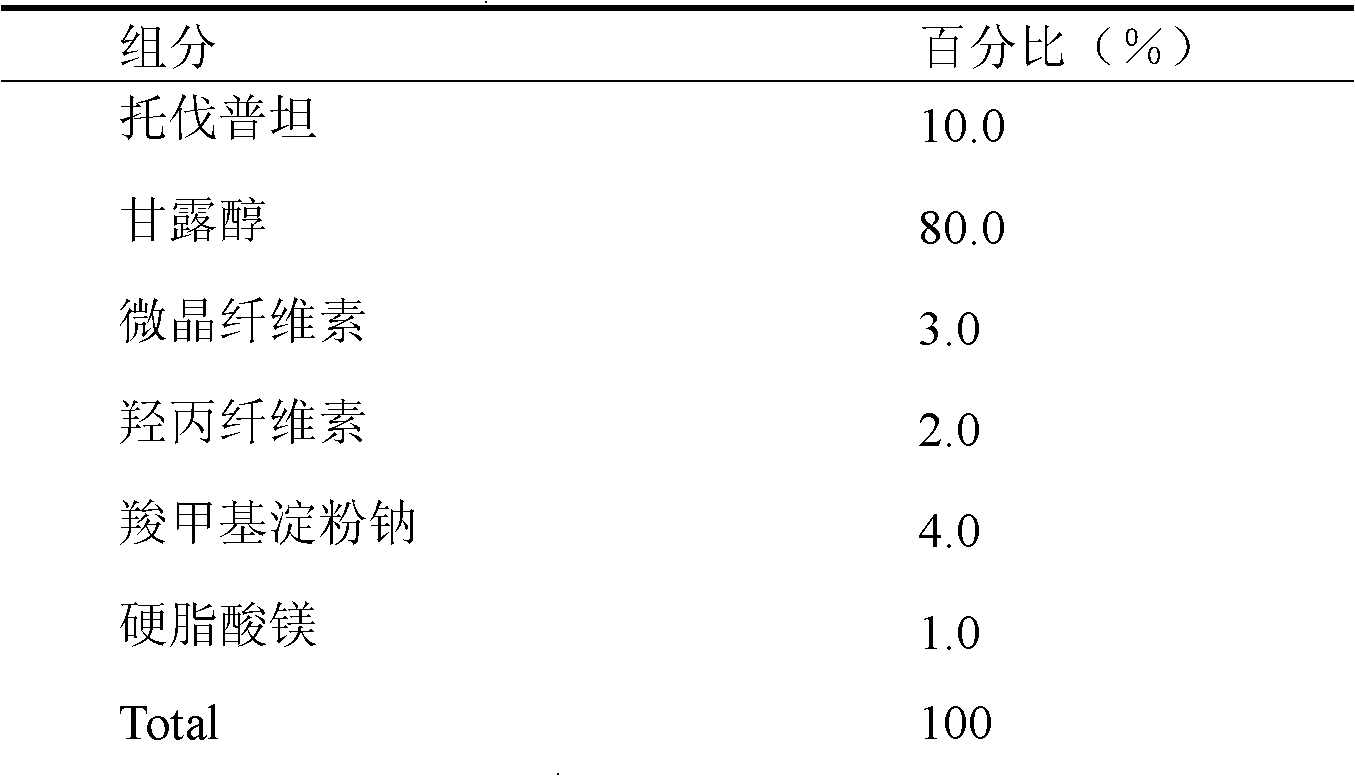
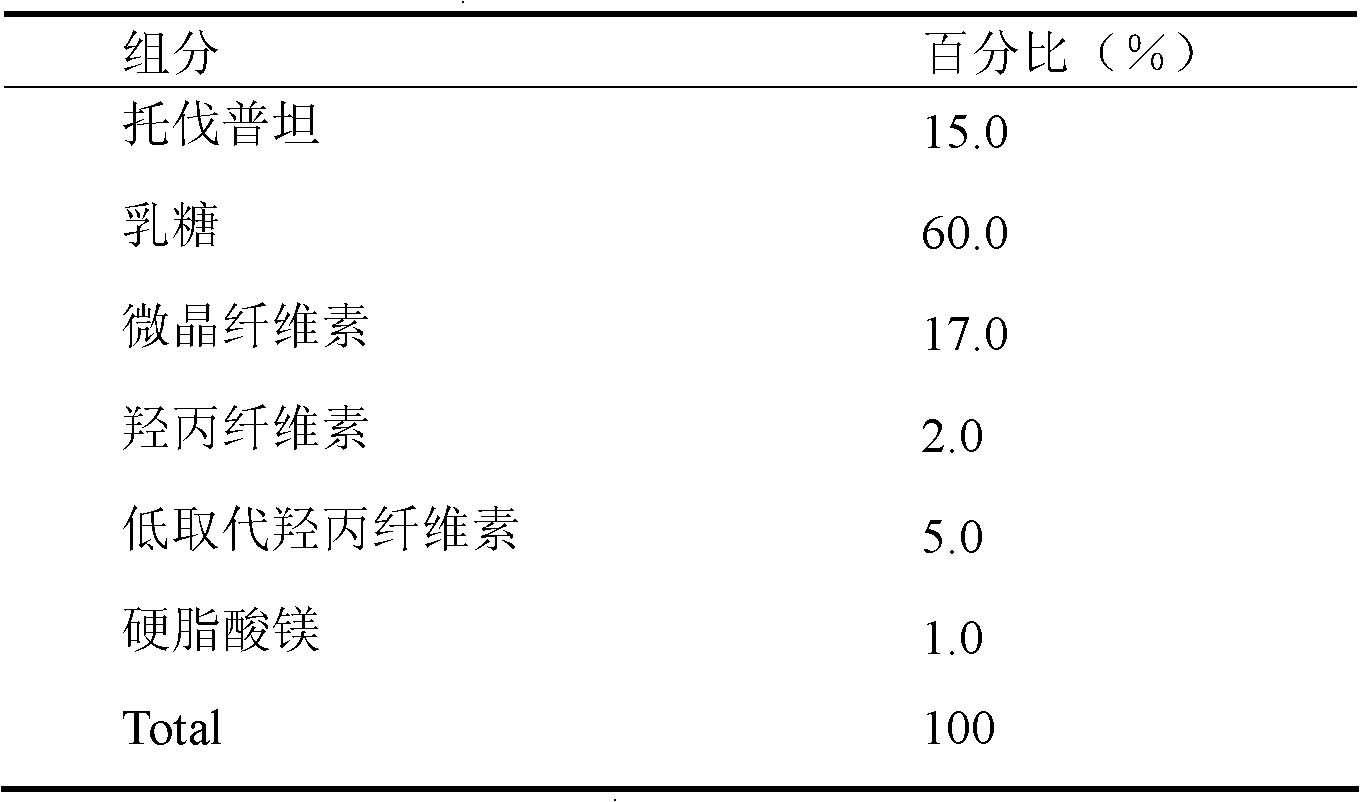
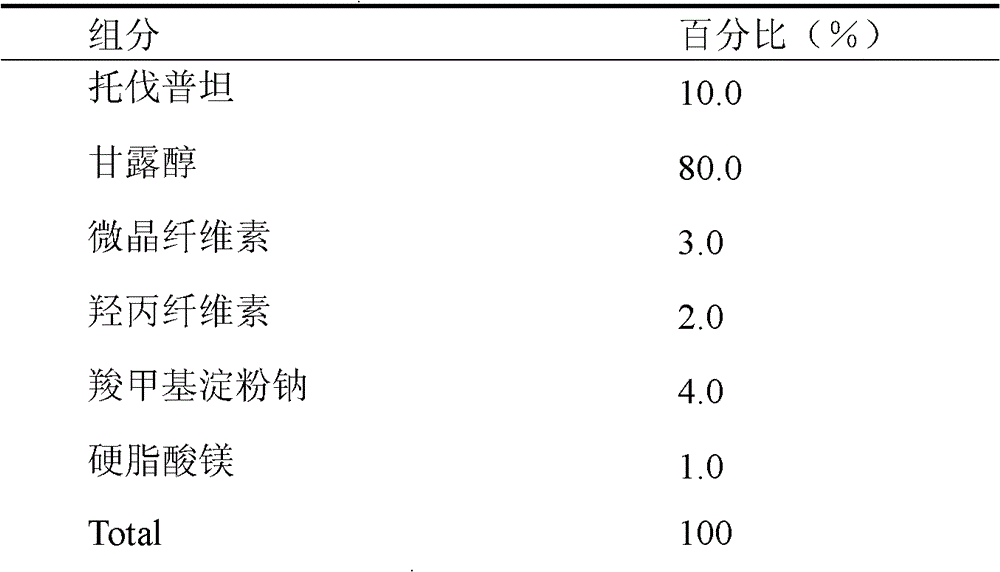
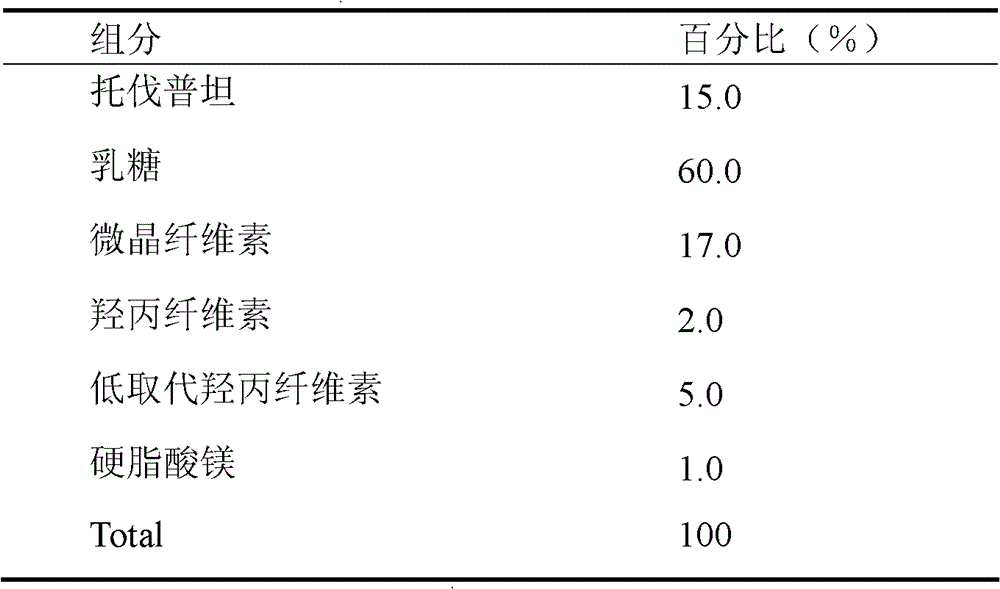
Solid medicine composition of tolvaptan
The invention discloses a solid medicine composition of tolvaptan. The solid medicine composition of tolvaptan comprises granular or unformed powder of tolvaptan, sugar and a sugar alcohol diluent. The preparation method comprises the following steps of: highly dispersing the active component tolvaptan into the diluent to obtain solid dispersoid; and preparing into oral solid preparation with pharmaceutically acceptable auxiliary materials. The solid medicine composition is clinically used for treating hyponatremia.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
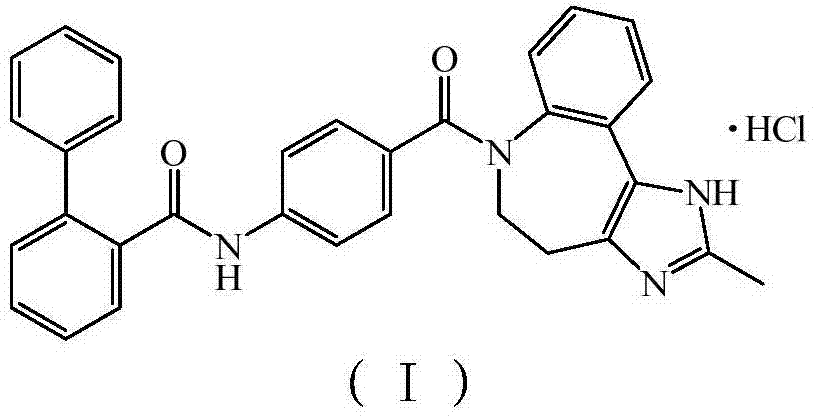
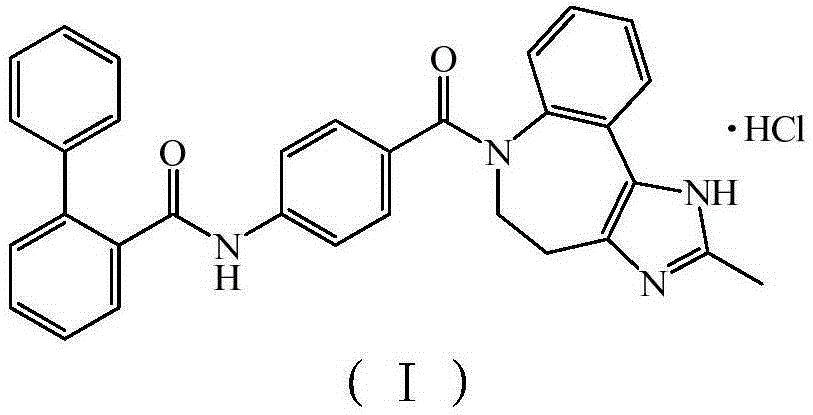
Conivaptan-hydrochloride novel crystal form and preparation method thereof
ActiveCN103497195AImprove stabilityImprove bioavailabilityOrganic chemistryMetabolism disorderSolubilityDecreased sodium
The invention relates to the field of medicinal chemistry, and discloses a conivaptan-hydrochloride novel crystal form and a preparation method of the conivaptan-hydrochloride novel crystal form. The conivaptan-hydrochloride novel crystal form is single in crystal form, high in purity, good in stability, high in solubility and bioavailability, capable of restraining arginine vasopressin V1a and V2 receptors, and capable of being used for preparing medicine for treating hyponatremia. The preparation method of the conivaptan-hydrochloride novel crystal form is simple to operate, avoids complex and fussy crystallization processing conditions, enables solvents to be recycled, is low in production cost, and is suitable for industrial production. Products meet medicinal standards and good in stability.
Owner:BEIJING COLLAB PHARMA
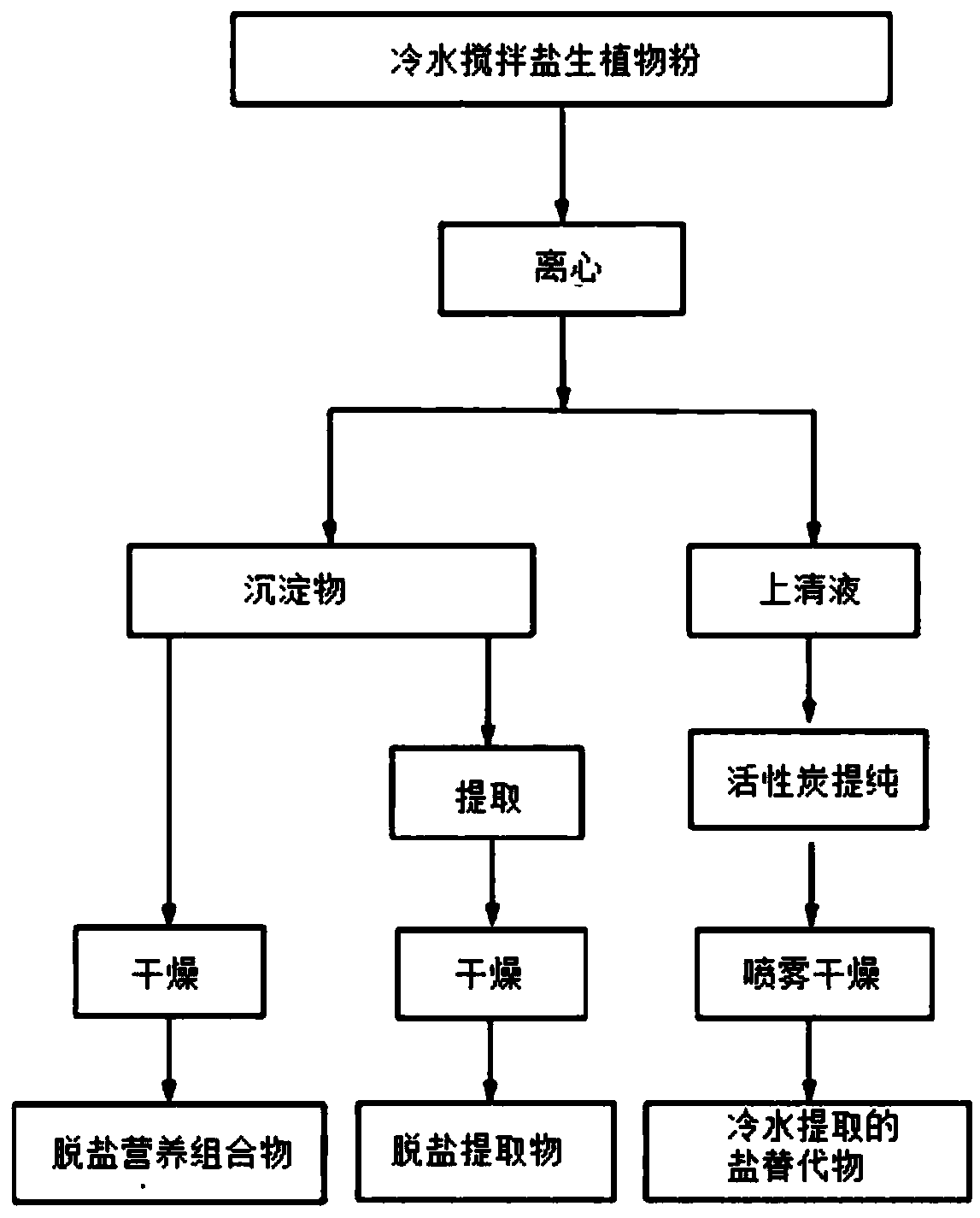
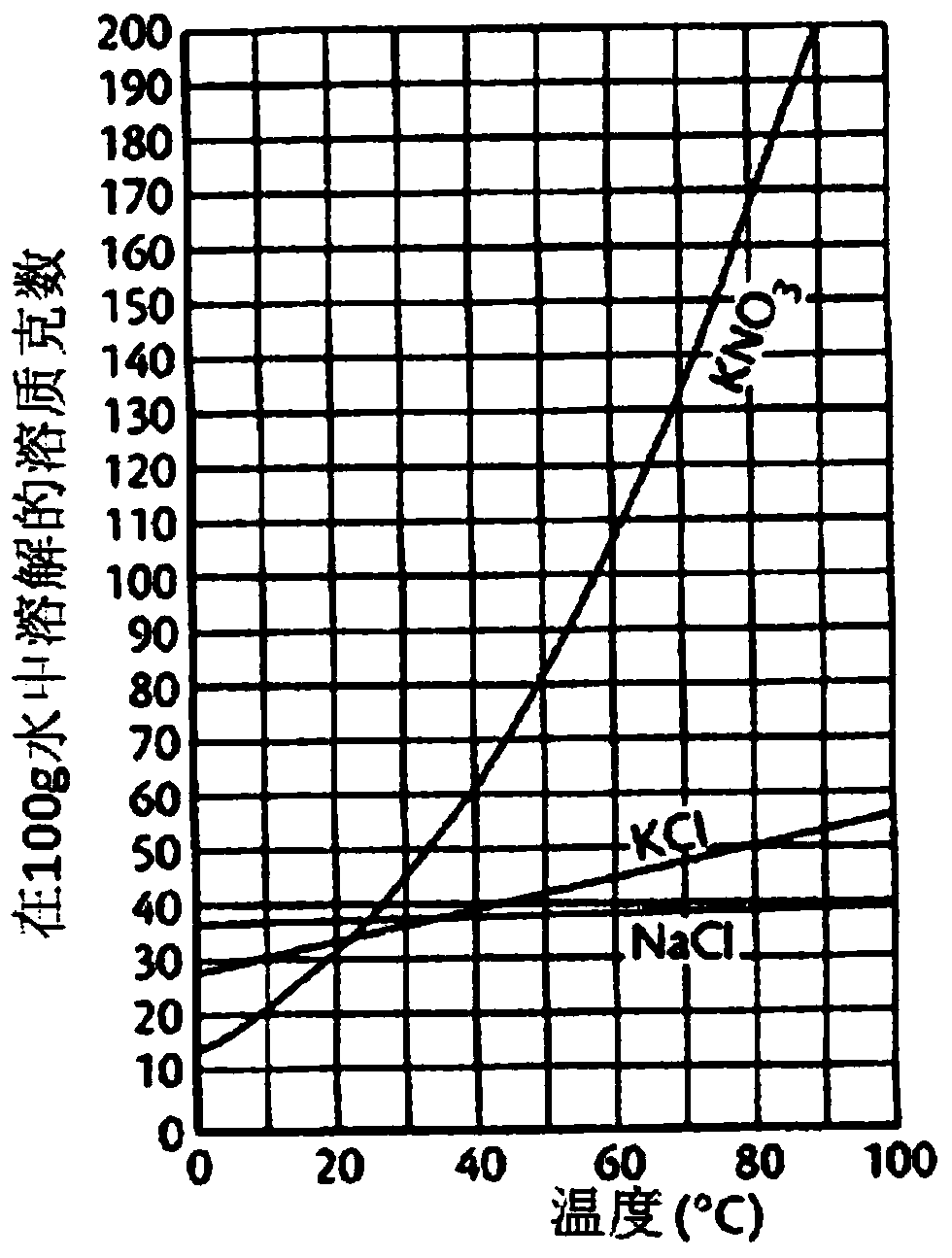
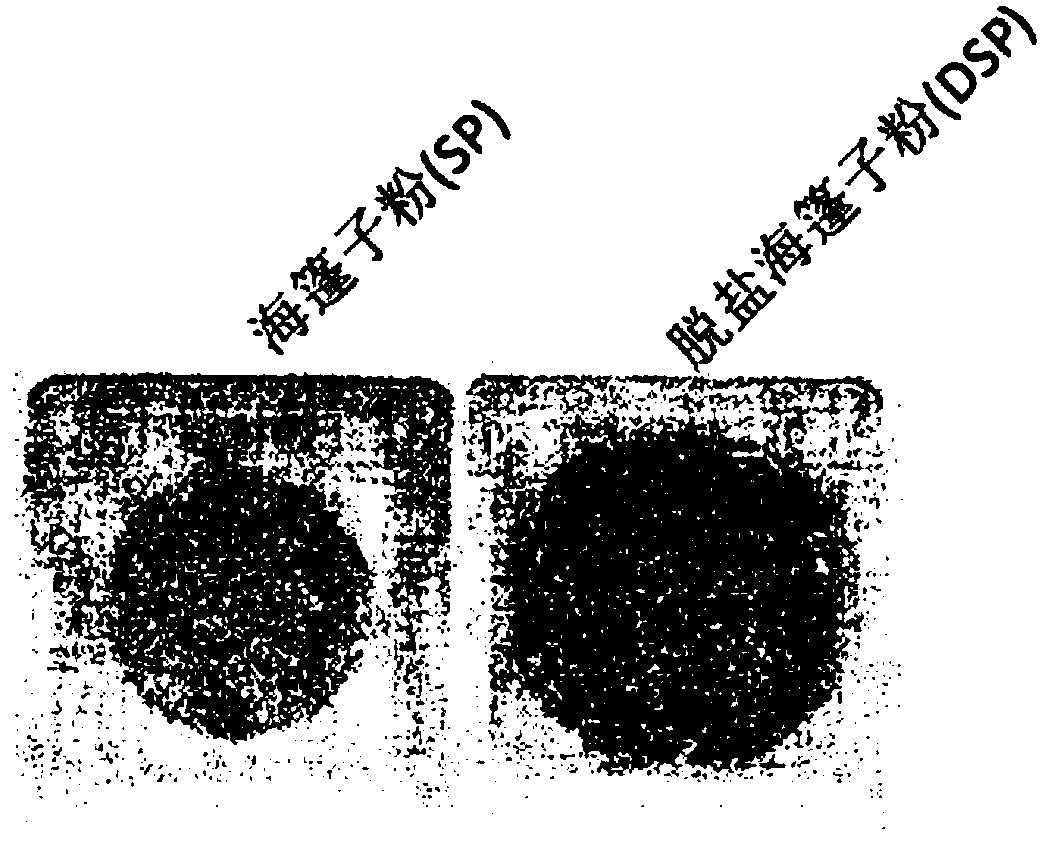
Functionally reinforced desalted nutritional compositions from halophytes and preparation method thereof
ActiveCN109152404AEfficient removalOrganic active ingredientsMetabolism disorderDecreased sodiumHalophyte
Disclosed are a functionally reinforced desalted nutritional composition, a desalted extract and a cold-water-extracted salt substitute, which are derived from halophytes that grow in coastal regionsunder highly saline conditions and thus retain high salt concentrations, as well as the use of the desalted nutritional composition for combatting obesity. More particularly, this invention relates toa functionally reinforced desalted nutritional composition, a desalted extract and a salt substitute cold-water-extracted from halophytes that inhabit extreme environments of high salinity under highsalt stress, the halophytes being desalted through a cold water extraction process at a low temperature based on the difference in water solubility of salts with change in temperature to allow only sodium chloride to be selectively removed, and the composition thus having decreased sodium content as well as having increased content of useful minerals such as potassium, as well as nutrients and physiologically active substances, which are naturally contained in halophytes.
Owner:派图有限公司
Modulation of sodium channels by nicotinamide adenine dinucleotide
ActiveUS20090105181A1Reducing arrhythmic riskReduce arrhythmic riskBiocideSugar derivativesDecreased sodiumNicotinamide adenine dinucleotide
The present invention relates to the use of oxidized nicotinamide adenine dinucleotide (NAD+) or of its reduced form, NADH, as sodium channel modulators. The present invention also relates to the use of compositions containing NAD+ or NADH to treat conditions associated with sodium channel current, such as arrhythmia. NAD+ is found to increase sodium channel current, while NADH is found to decrease sodium channel current. Thus, conditions that are associated with decreased sodium channel current can be treated with NAD+, while conditions that is associated with increased sodium channel current can be treated with NADH.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
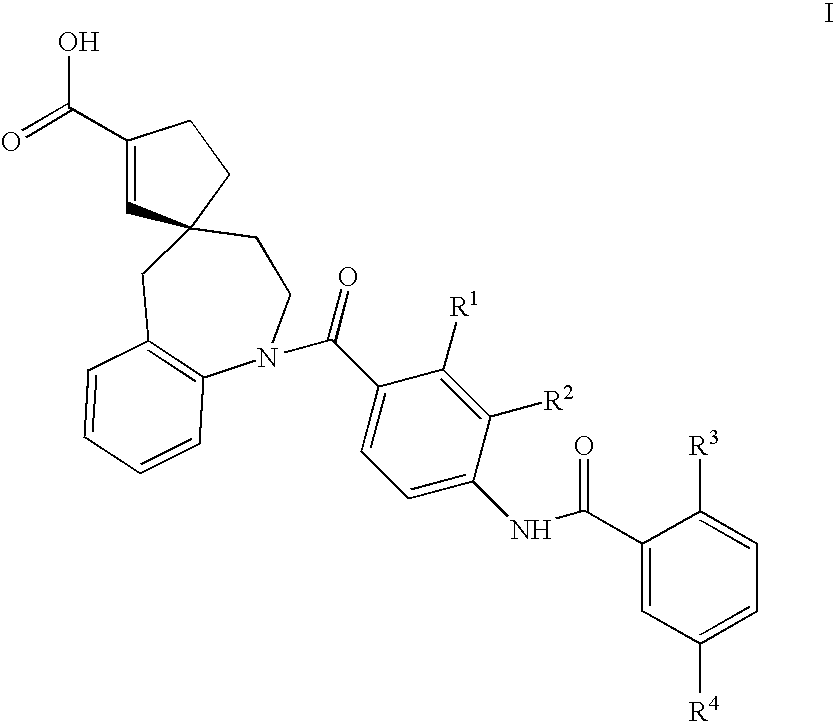
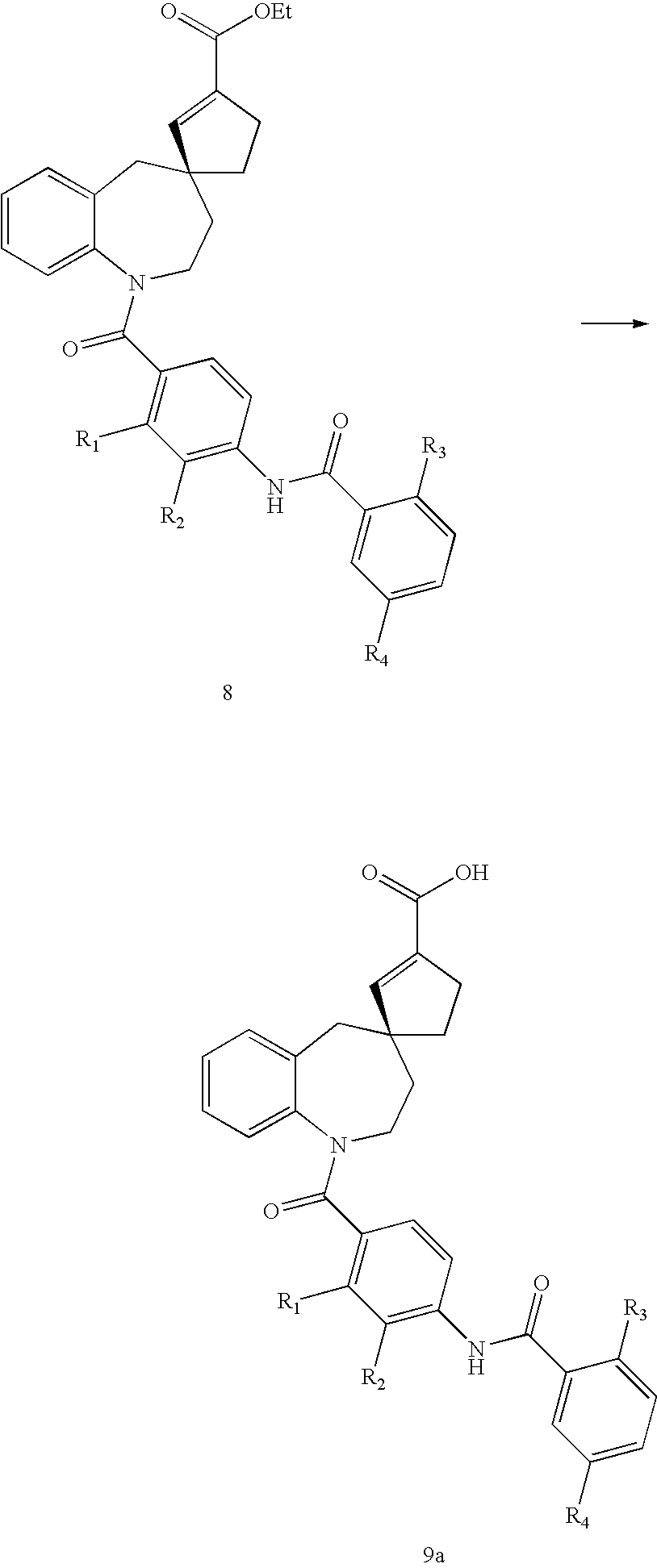
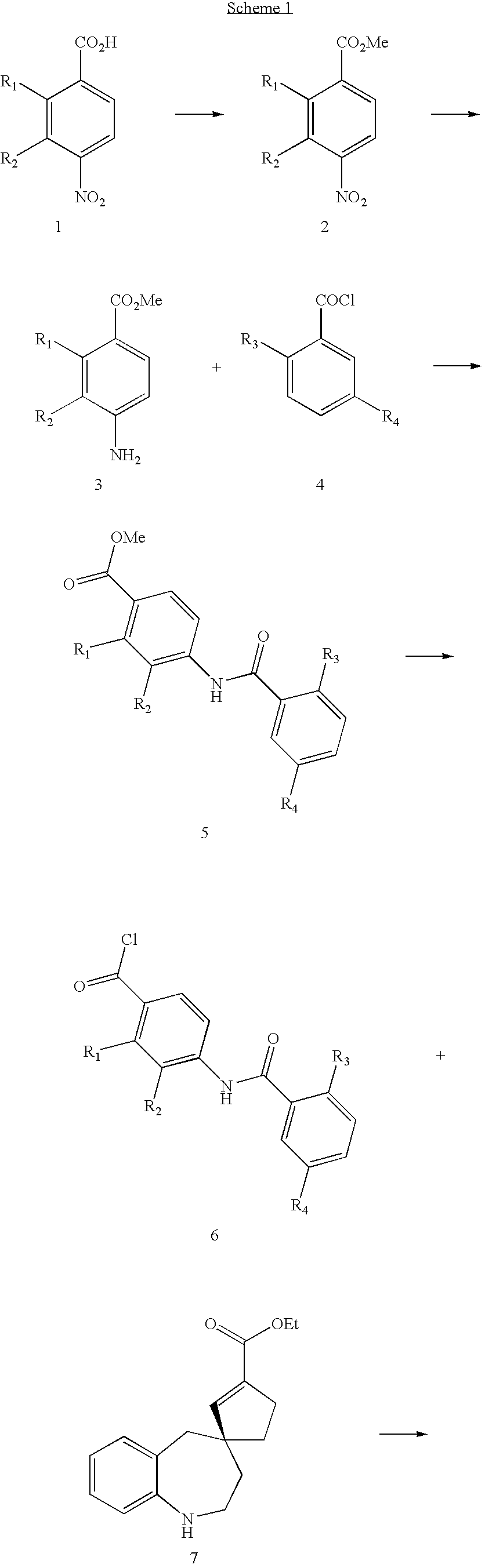
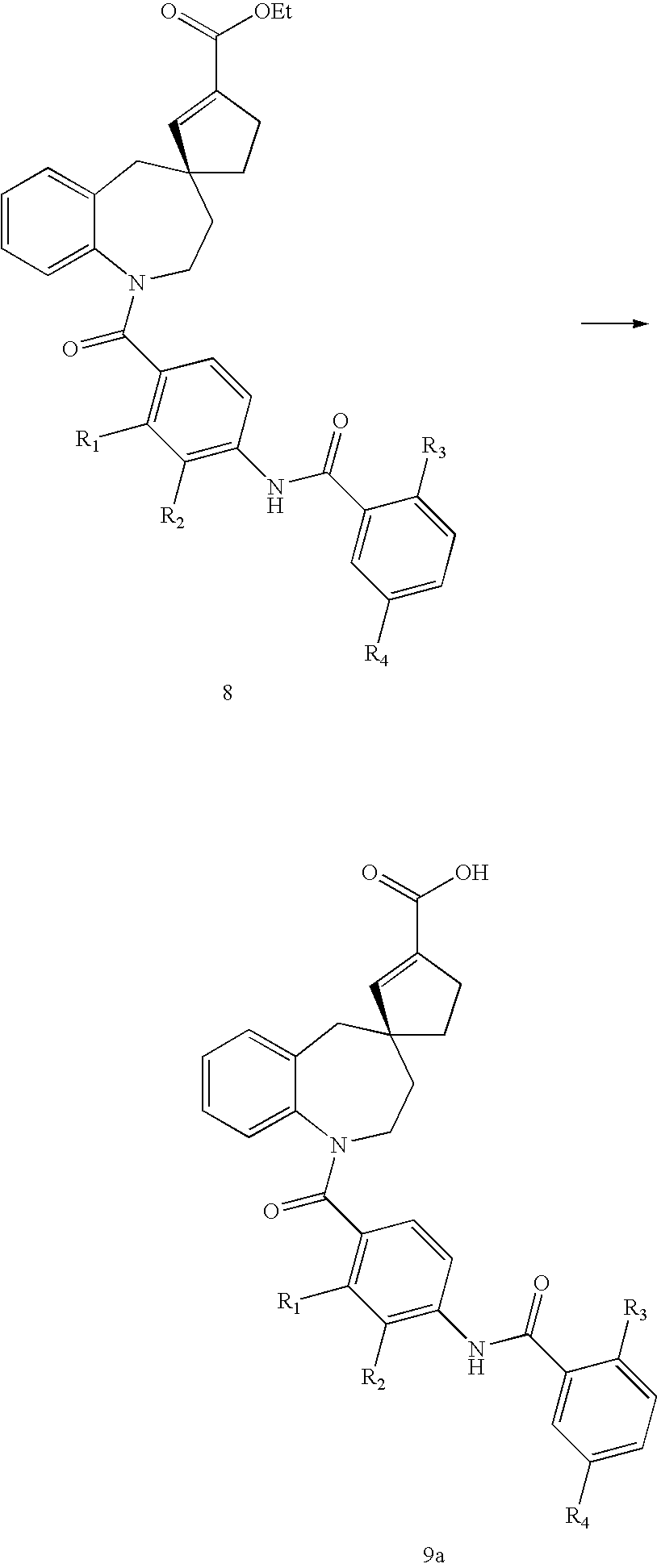
Substituted spirobenzazepines
Owner:JANSSEN PHARMA NV
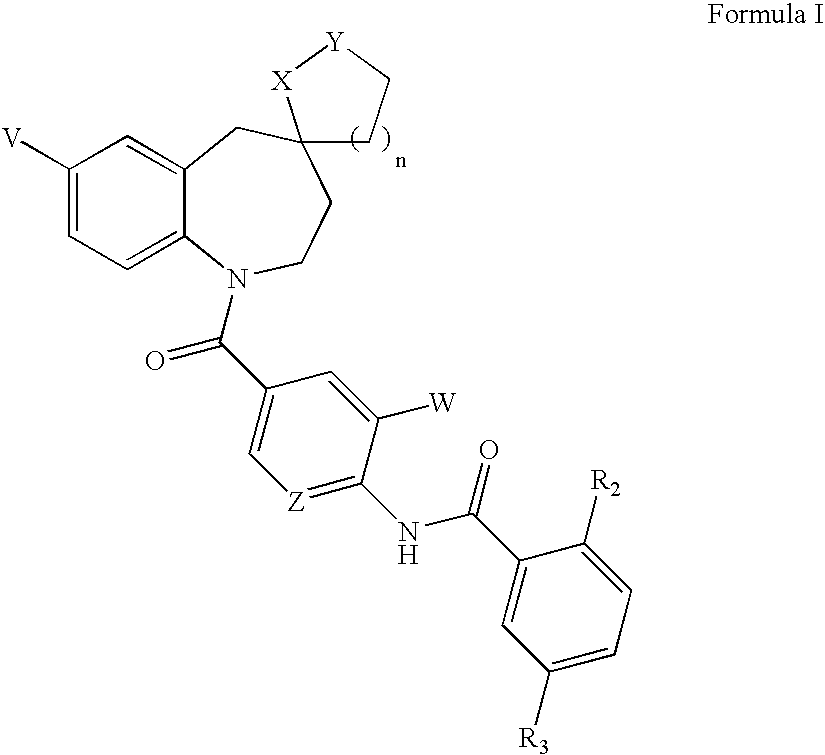
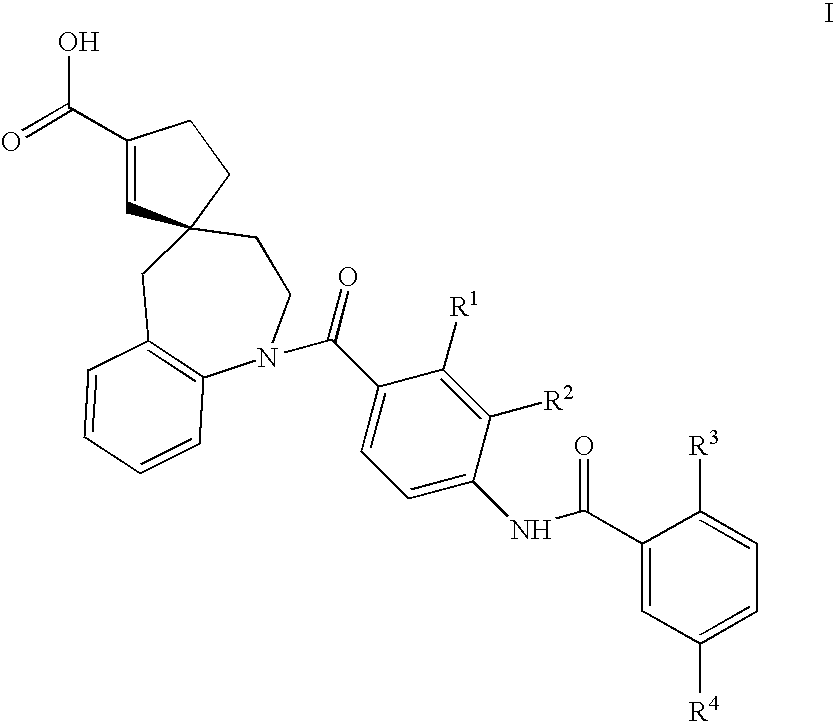
Substituted spiroheterocycles
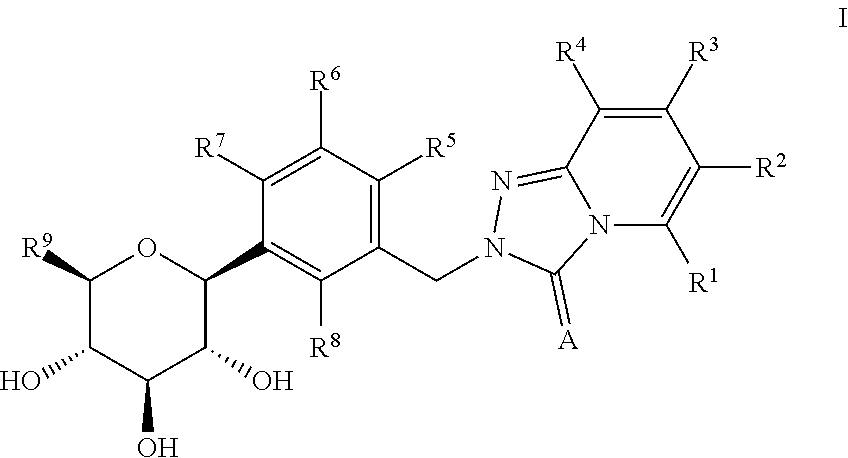
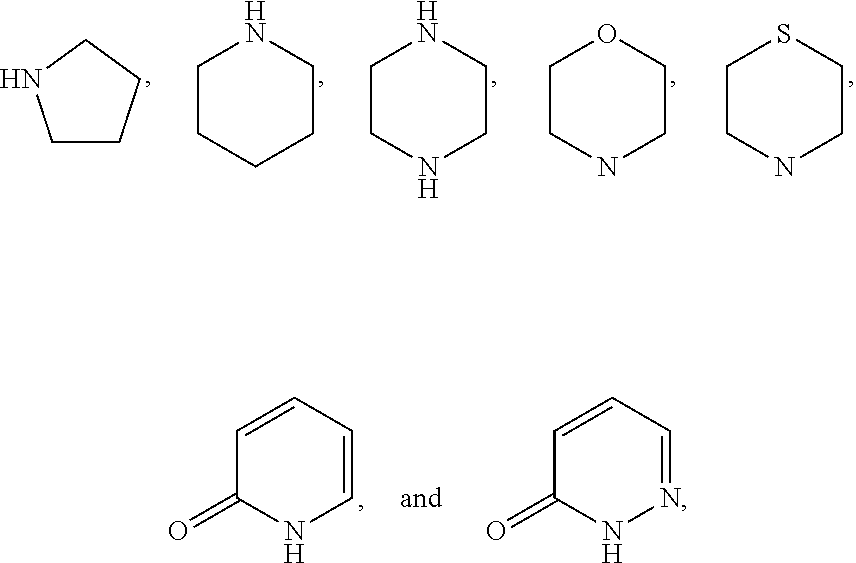
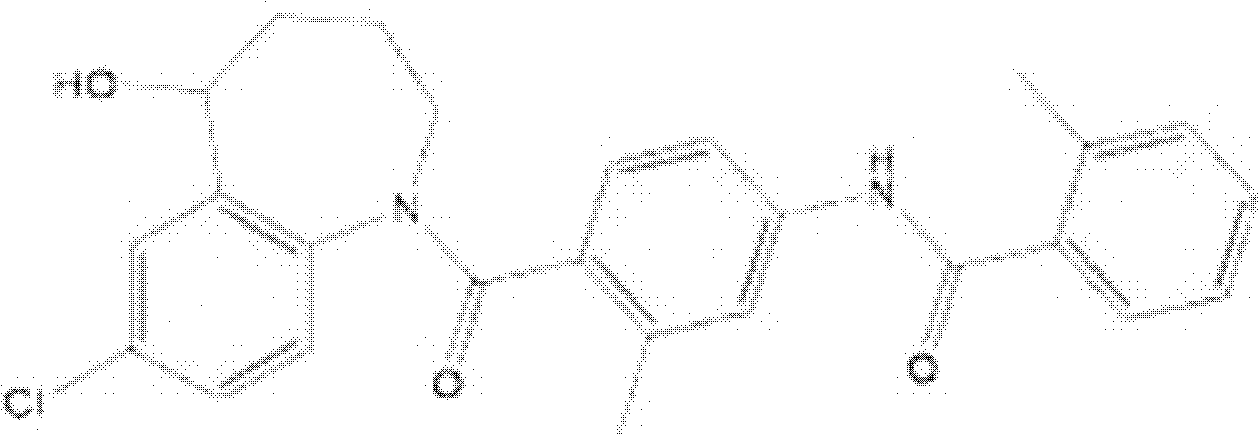
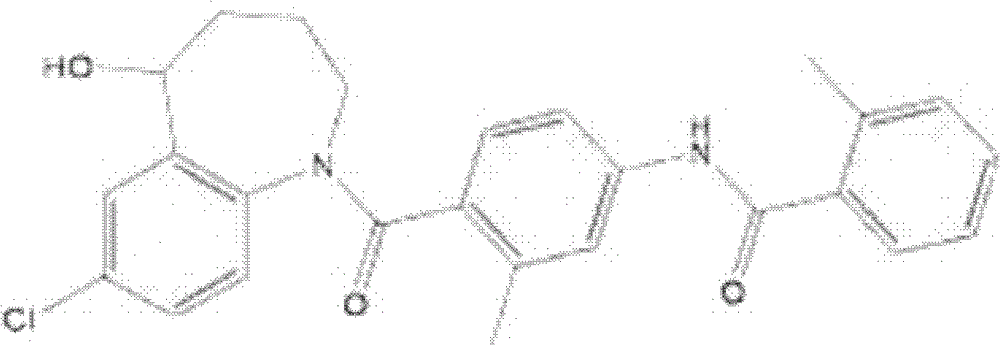
The invention is directed to nonpeptide substituted spiroheterobenzazepine of Formula I, which are useful as vasopressin receptor antagonists for treating conditions associated with vasopressin receptor activity such as those involving increased vascular resistance and cardiac insufficiency, including congestive heart failure, hyponatremia, and hypertension. Pharmaceutical compositions comprising a compound of Formula I and methods of treating conditions such as hypertension, congestive heart failure, cardiac insufficiency, coronary vasospasm, cardiac ischemia, liver cirrhosis, hyponatremia, renal vasospasm, renal insufficiency, renal failure, diabetic nephropathy, cerebral edema, cerebral ischemia, stroke, thrombosis, or water retention are also disclosed.
Owner:JANSSEN PHARMA NV
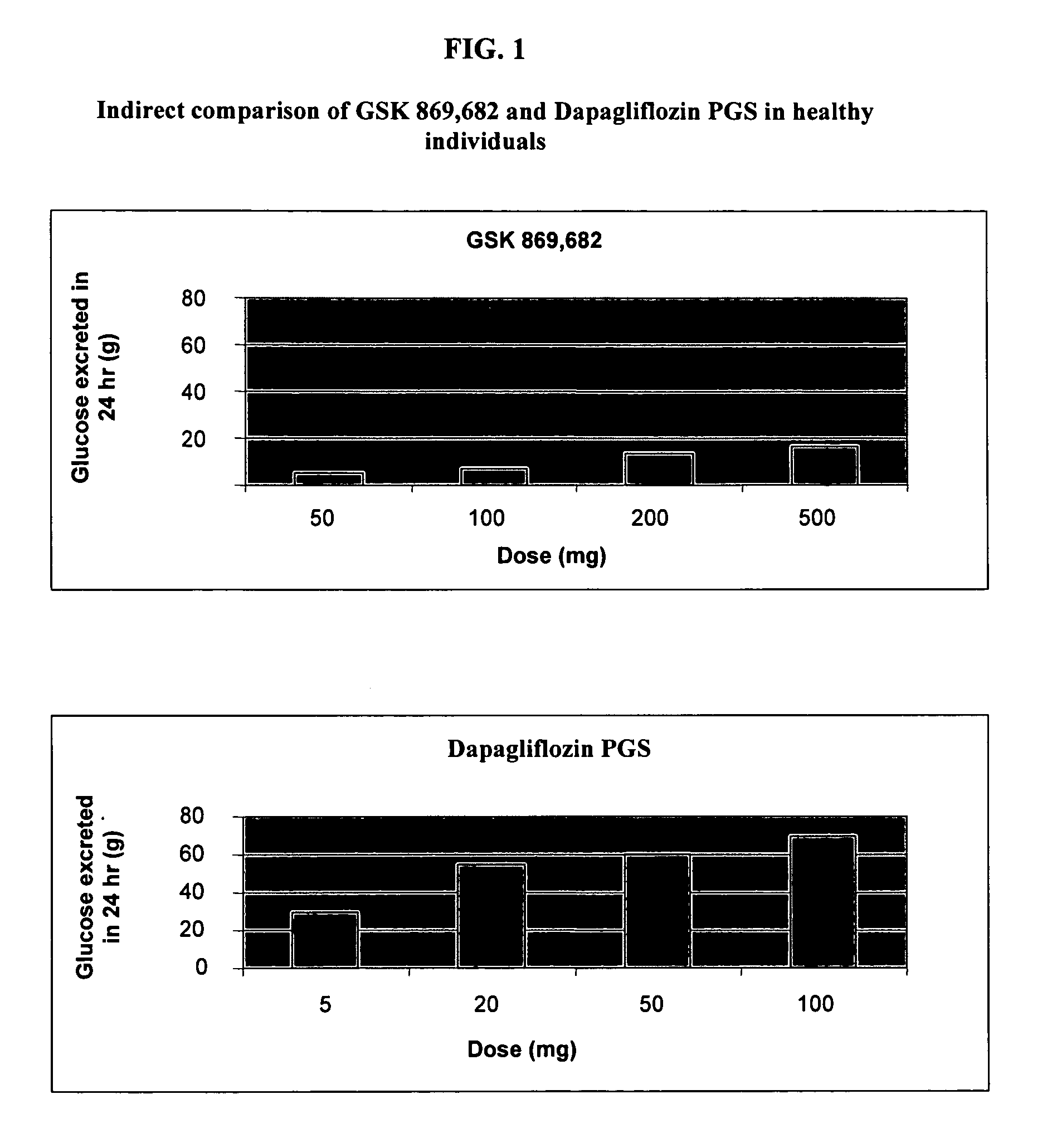
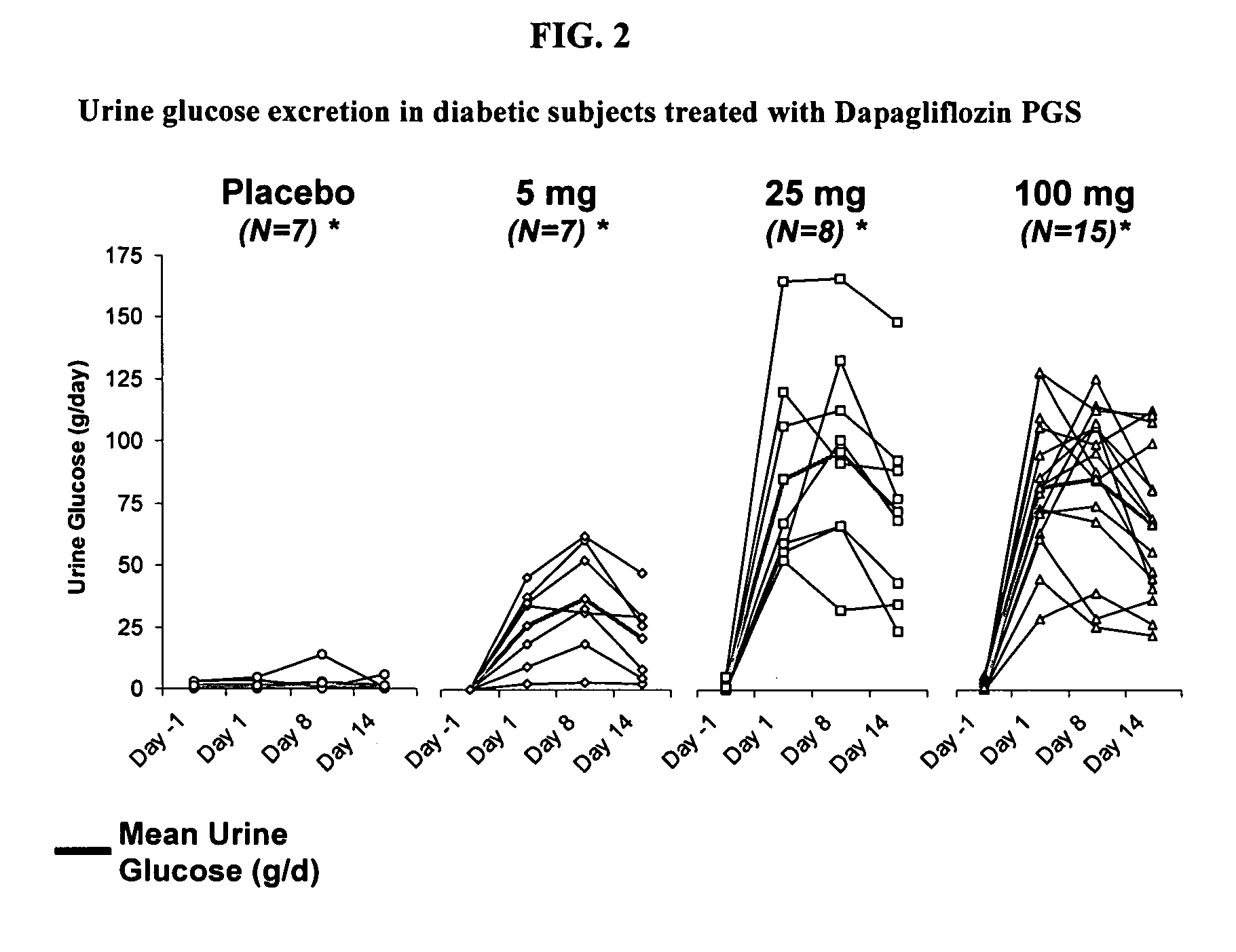
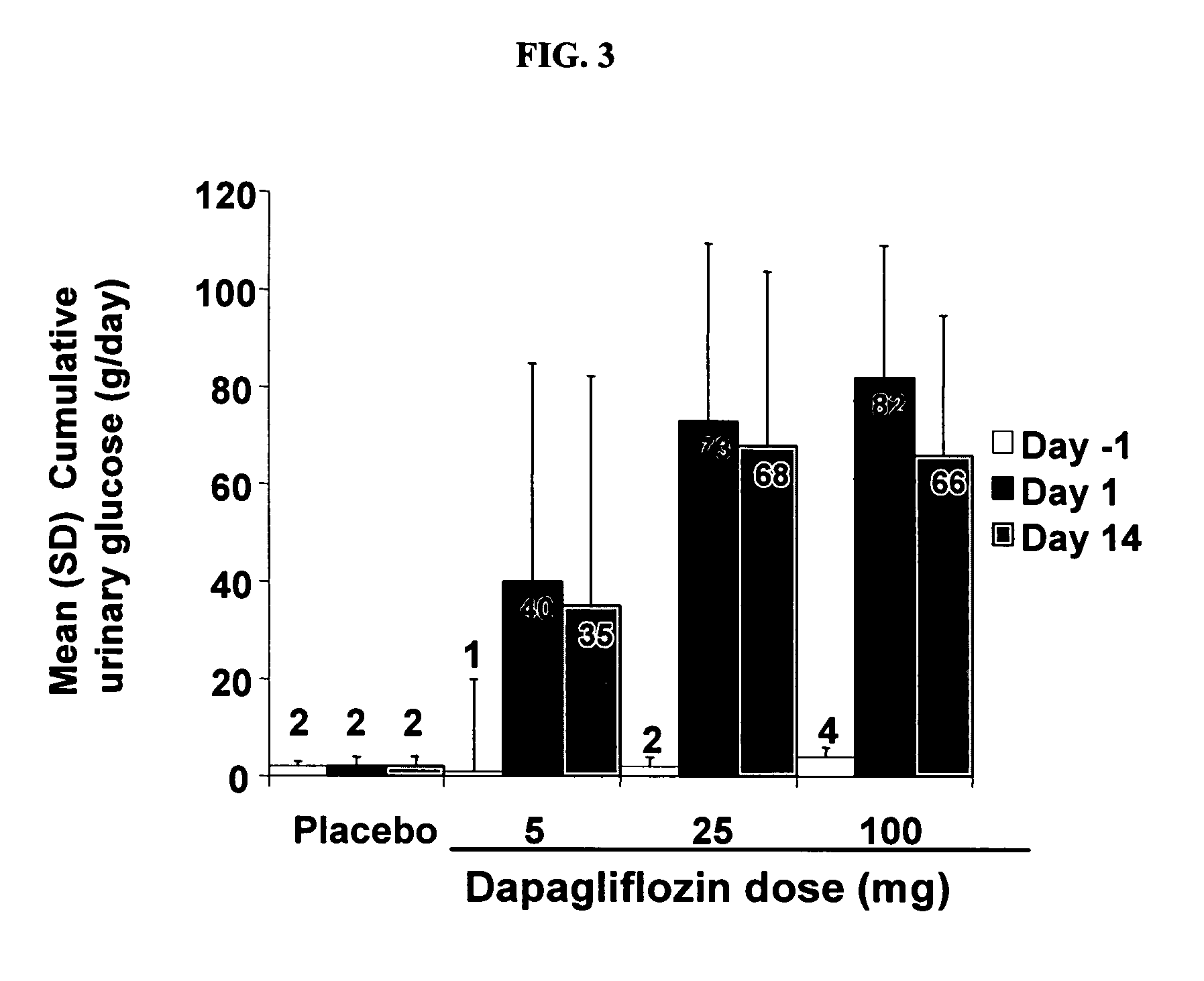
Method for treating hyponatremia employing an SGLT2 inhibitor and composition containing same
Methods are provided for treating hyponatremia, employing an SGLT2 inhibitor alone, or in combination with a supply of carbohydrate, and / or in combination with a diuretic agent. Additionally, compositions comprising an SGLT2 inhibitor, optionally with a supply of carbohydrate, and / or a combination of an SGLT2 inhibitor and a diuretic agent are provided in the instant invention and are provided for use in the inventive methods.
Owner:ASTRAZENECA AB
Dairy product drink and preparation method thereof
The invention provides a dairy product drink and a preparation method thereof and relates to the field of food processing. The dairy product drink contains 10-20 parts by weight of pure milk and 101 parts by weight of yoghourt. The preparation method comprises the following preparation steps: sterilizing, cooling, removing milk skin, inoculating, fermenting, demulsifying, re-fermenting, mixing, sterilizing, cooling and encapsulating. The dairy product drink and the preparation method thereof provided by the invention have the benefits that the original-flavored dairy product drink is made by using common pure milk, does not contain harmful additive and can eliminate multifaceted health problems caused by improper use of a food additive; the dairy product drink is suitable for a wide range of people, has the efficacies of enhancing immunity, nourishing stomach, realizing high protein blood fat removal and blood pressure reduction, strengthening brain, calming nerves, relieving restlessness, improving eyesight, moistening intestine, beautifying and caring skin, and can relieve tension fatigue, dry eye soreness, fatigue, eye fatigue, chronic fatigue syndrome, osteoporosis in the aged, hyponatremia in the aged, postmenopausal osteoporosis and other symptoms.
Owner:BURQIN COUNTY BAHAR BEVERAGE CO LTD
Application of scutellaria baicalensis in preparing medicament for treating ascites due to cirrhosis
InactiveCN105193930APromote excretionImprove liver and kidney functionDigestive systemBlood disorderDecreased sodiumHypoproteinemia
An application of scutellaria baicalensis in preparing a medicament for treating ascites due to cirrhosis relates to a novel application of the scutellaria baicalensis. The invention aims at providing a novel application of the scutellaria baicalensis, and particularly relates to the application of the scutellaria baicalensis in a medicament for treating ascites due to cirrhosis, a medicament for treating liver function damage, a medicament for treating hepatic fibrosis, a medicament for treating liver tissue damage, a medicament for treating hypoproteinemia and a medicament or treating cholestasis. Compared with a modern therapy method, the application of the scutellaria baicalensis has the advantage that the complications, such as hepatic encephalopathy, renal function damage, hyponatremia, hypokalemia or hyperkalemia and the like are avoided, also has the advantages of low treatment cost, simple use method, ideal treatment effect and the like and is conductive to clinical practical application. The scutellaria baicalensis is used for treating ascites due to cirrhosis.
Owner:刘树民
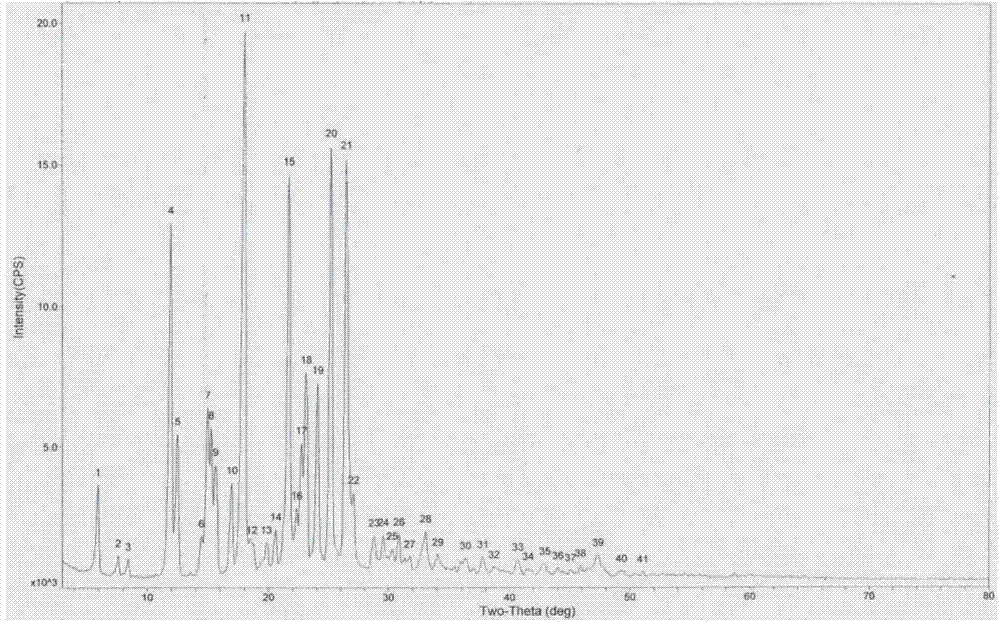
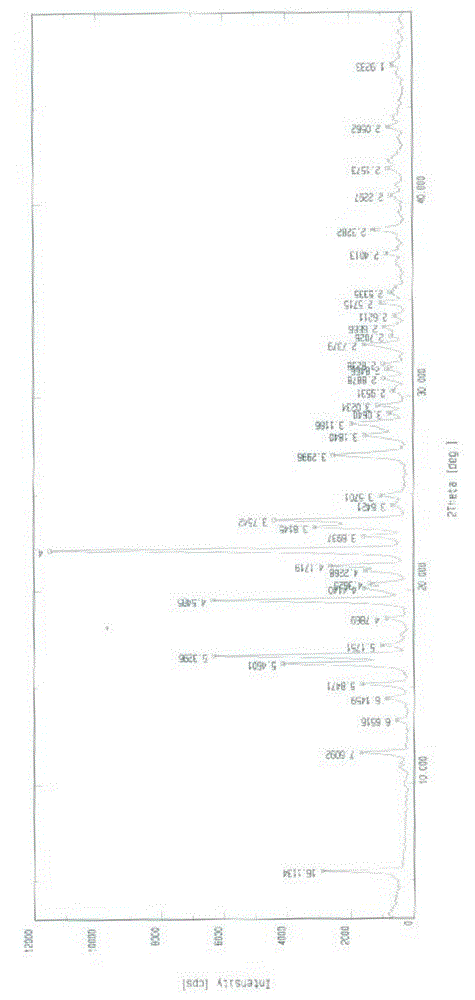
Lixivaptan crystal form I and preparation method and use thereof
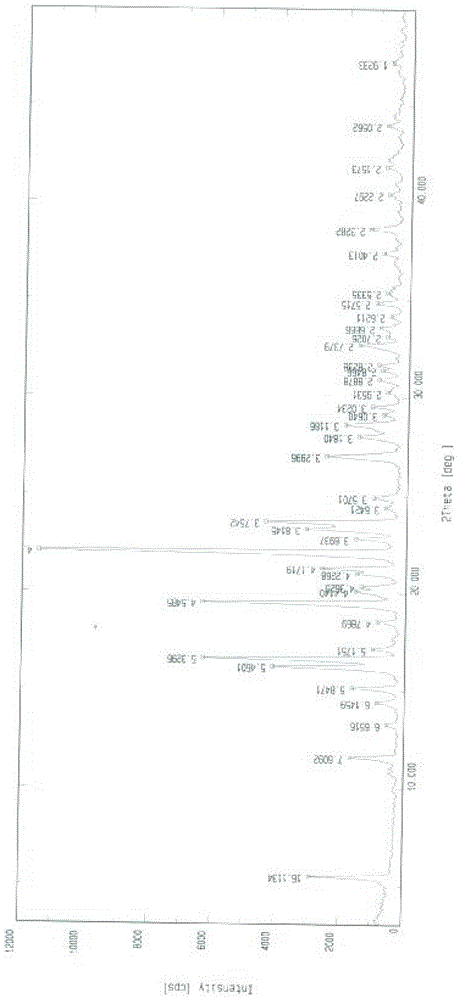
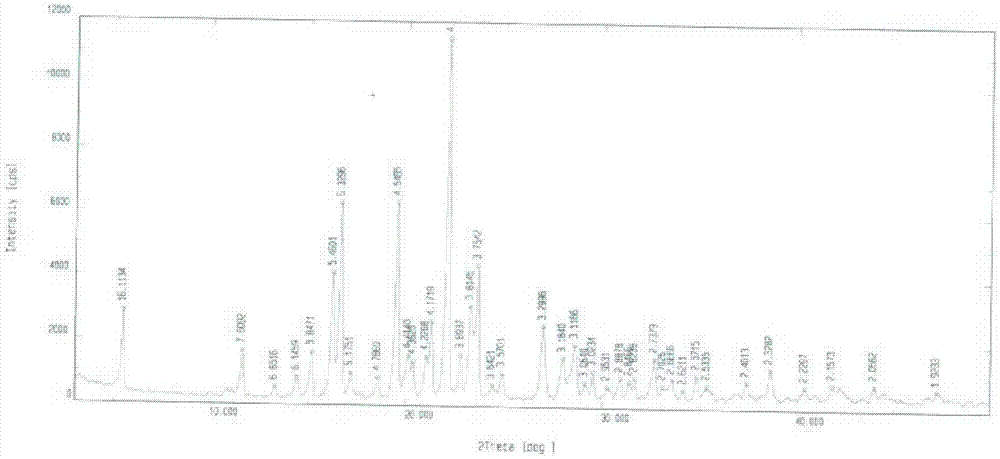
The invention belongs to the field of anti heart failure drugs, and more specifically, relates to lixivaptan crystal form I and a preparation method and use of a pharmaceutical composition containing the lixivaptan crystal form I in preparation of drugs for treating hyponatremia. The lixivaptan crystal form I is prepared from 2-chloro-4-nitro benzoic acid as a starting material by esterification, hydrogenation reduction, acylation, hydrolysis, acyl chlorination and other reaction steps, and the prepared lixivaptan purity is 97.5%. The lixivaptan crystal form I and its powder are characterized by X ray diffraction diagrams, and the lixivaptan crystal form I is important for obtaining of compounds with high purity, very determined crystal form and good reproducibility due to good prospects for development and pharmaceutical value of the lixivaptan crystal form I.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
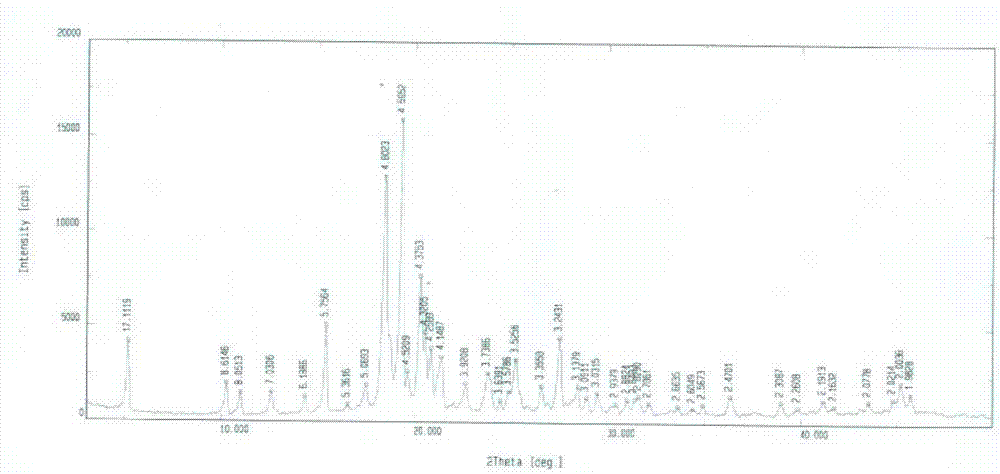
Lixivaptan crystal form II, preparation method and use thereof
The invention belongs to the field of anti-heart failure drugs, and more particularly relates to a lixivaptan crystal form II and a preparation method thereof, a drug composition containing the lixivaptan crystal form II, and a use of the lixivaptan crystal form II in preparation of hyponatremia treatment drugs. According to the present invention, 2-chloro-4-nitrobenzoic acid is adopted as a starting raw material, and esterification, hydrogenation reduction, acylation, hydrolysis, chlorination and other reaction steps are performed to prepare the lixivaptan crystal form II, wherein the purity of the obtained lixivaptan is 97.5%, and the lixivaptan crystal form II is characterized by the powder X-ray diffraction pattern; and due to the good development prospects and the pharmaceutical values of the compound, it is important to obtain the compound with characteristics of high purity, determined crystal form and good reproducibility.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
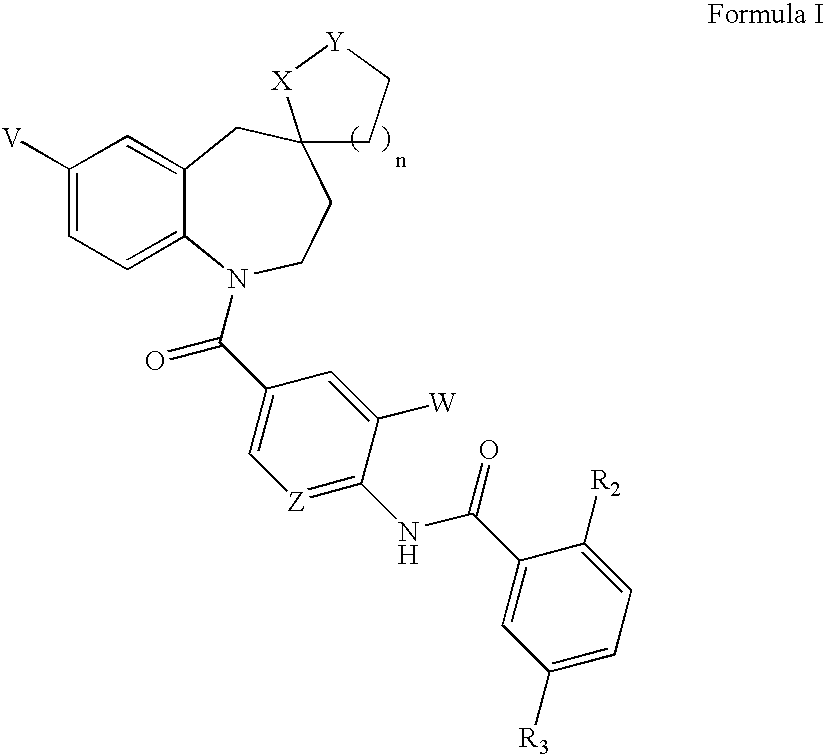
Substituted spirobenzazepines
The invention is directed to nonpeptide substituted benzazepines of Formula I, which are useful as vasopressin receptor antagonists for treating conditions associated with vasopressin receptor activity such as those involving increased vascular resistance and cardiac insufficiency, including congestive heart failure, hyponatremia, and hypertension, among others disclosed. Pharmaceutical compositions comprising a compound of Formula I and methods of treating conditions such as hypertension, congestive heart failure, cardiac insufficiency, coronary vasospasm, cardiac ischemia, liver cirrhosis, hyponatremia, renal vasospasm, renal failure, diabetic nephropathy, cerebral edema, cerebral ischemia, stroke, thrombosis, or water retention are also disclosed.
Owner:JANSSEN PHARMA NV
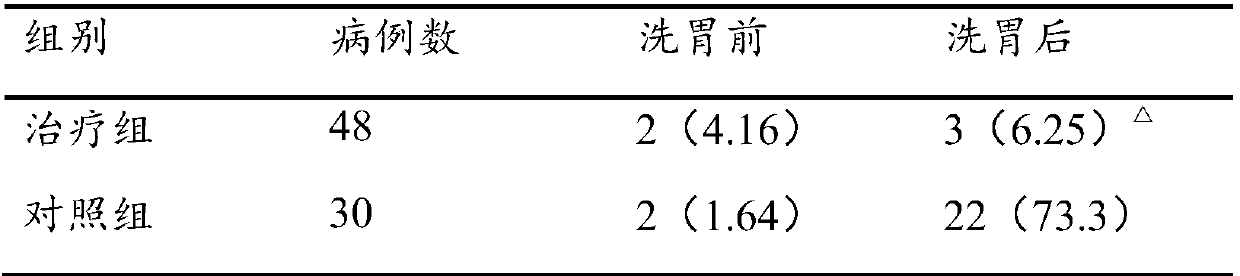
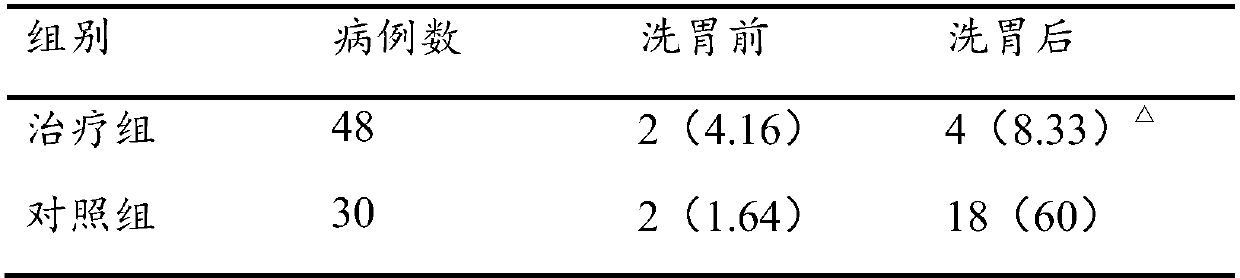
Stomach cleaning combined reagent used for gastroenterology department and preparation method thereof
InactiveCN107929702AImprove antibacterial propertiesPromote secretionDigestive systemBird material medical ingredientsDecreased sodiumAdditive ingredient
The invention discloses a stomach cleaning combined reagent used for the gastroenterology department. The stomach cleaning combined reagent comprises the following ingredients in parts by weight: 10-20 parts of panax notoginseng, 3-9 parts of radix astragali, 5-15 parts of radix codonopsis, 10-15 parts of Mangnolia officinalis, 8-12 parts of radix glycyrrhizae, 5-15 parts of rhizoma corydalis, 12-20 parts of cistanche salsa, 10-15 parts of bitter gourds, 3-8 parts of honeysuckles, 5-10 parts of endothelium corneum gigeriae galli, 8-10 parts of chrysanthemums, 5-10 parts of fructus forsythia, 3-7 parts of royal jelly, 2-8 parts of honey, 5-10 parts of rhizoma cyperi, 5-15 parts of fresh ginger, 10-15 parts of barleys, 50-100 parts of normal saline and 50-100 parts of 10% potassium chloride.Compared with the prior art, the stomach cleaning combined reagent has the beneficial effects that the incidence rate of complications, that is the incidence rate of hyponatremia and hypokalemia in astomach cleaning process can be reduced; meanwhile, the stomach cleaning combined reagent has an effect of nursing a stomach and is beneficial to later recovery of the stomach.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Traditional Chinese medicine for treating hyperuricemia complicated with joint swelling
InactiveCN106924608AEasy dischargeCompatibility scienceSkeletal disorderPlant ingredientsDecreased sodiumMedicinal herbs
The invention relates to the technical field of medicines, and in particular relates to a traditional Chinese medicine for treating hyperuricemia complicated with joint swelling. The traditional Chinese medicine is prepared from the following medicinal materials in parts by weight: 20-30 parts of folium isatidis, 20-30 parts of dandelion, 20-30 parts of notopterygium root, 15-25 parts of herba potentillae discoloris cum radice, 15-25 parts of cortex moutan, 13-17 parts of rhizoma anemarrhenae, 13-17 parts of herba centellae, 10-20 parts of indigo naturalis, 10-20 parts of herba houttuyniae, 10-20 parts of fructus trichosanthis, 15-19 parts of radix paeoniae rubra, 12-18 parts of rhizoma atractylodis, 11-17 parts of rhizoma coptidis, 10-14 parts of rhizoma smilacis glabrae, 10-14 parts of rhizoma polygoni cuspidati, 10-14 parts of radix notoginseng, 10-14 parts of black soya beans, 11-17 parts of rhizoma cyperi, 10-18 parts of radix saposhnikoviae, 10-22 parts of herba artemisiae annuae, 5-9 parts of herba plantaginis, and 3-7 parts of licorice root. For the traditional Chinese medicine for treating hyperuricemia complicated with joint swelling, through scientific mixing, the ingredients achieve a synergic effect, so that the traditional Chinese medicine can promote the discharge of uric acid, supplement the elements including sodium, potassium, calcium, zinc and the like, and prevent the side reactions including hyponatremia, hypokalemia, hypocalcemia and the like, and also has the efficacies of relieving swelling and pain, regulating Qi and blood, clearing damp and dissolving turbidity, and treating arthritis.
Owner:QINGDAO ZHITONG SIHAI FURNITURE DESIGN RES & DEV CO LTD
A kind of solid pharmaceutical composition of tolvaptan
The invention discloses a solid medicine composition of tolvaptan. The solid medicine composition of tolvaptan comprises granular or unformed powder of tolvaptan, sugar and a sugar alcohol diluent. The preparation method comprises the following steps of: highly dispersing the active component tolvaptan into the diluent to obtain solid dispersoid; and preparing into oral solid preparation with pharmaceutically acceptable auxiliary materials. The solid medicine composition is clinically used for treating hyponatremia.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Lixiptan crystal form Ⅱ and its preparation method and use
The invention belongs to the field of anti-heart failure drugs, and more particularly relates to a lixivaptan crystal form II and a preparation method thereof, a drug composition containing the lixivaptan crystal form II, and a use of the lixivaptan crystal form II in preparation of hyponatremia treatment drugs. According to the present invention, 2-chloro-4-nitrobenzoic acid is adopted as a starting raw material, and esterification, hydrogenation reduction, acylation, hydrolysis, chlorination and other reaction steps are performed to prepare the lixivaptan crystal form II, wherein the purity of the obtained lixivaptan is 97.5%, and the lixivaptan crystal form II is characterized by the powder X-ray diffraction pattern; and due to the good development prospects and the pharmaceutical values of the compound, it is important to obtain the compound with characteristics of high purity, determined crystal form and good reproducibility.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
New crystal form of conivaptan hydrochloride and preparation method thereof
ActiveCN103497195BHigh purityImprove stabilityOrganic chemistryMetabolism disorderDecreased sodiumSolubility
The invention relates to the field of medicinal chemistry, and discloses a conivaptan-hydrochloride novel crystal form and a preparation method of the conivaptan-hydrochloride novel crystal form. The conivaptan-hydrochloride novel crystal form is single in crystal form, high in purity, good in stability, high in solubility and bioavailability, capable of restraining arginine vasopressin V1a and V2 receptors, and capable of being used for preparing medicine for treating hyponatremia. The preparation method of the conivaptan-hydrochloride novel crystal form is simple to operate, avoids complex and fussy crystallization processing conditions, enables solvents to be recycled, is low in production cost, and is suitable for industrial production. Products meet medicinal standards and good in stability.
Owner:BEIJING COLLAB PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com