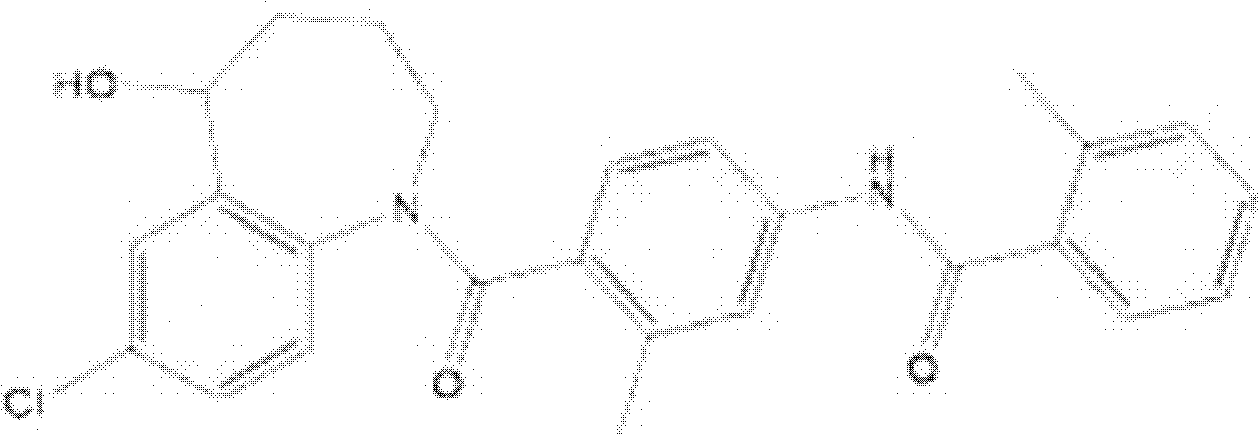
Solid medicine composition of tolvaptan
A technology of tolvaptan and its composition, which is applied in the field of medicine and can solve the problems of increasing costs, low drug inclusion rate, and related substances to be investigated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
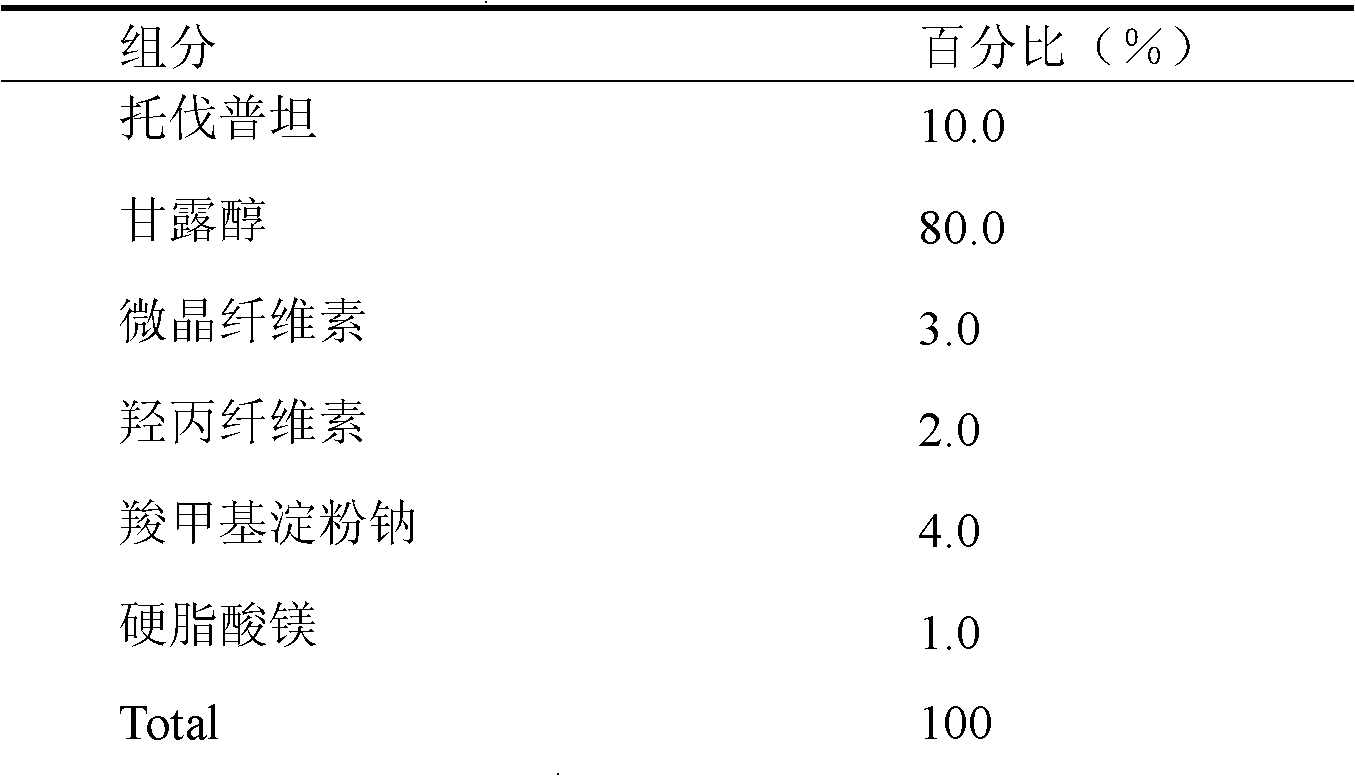
[0024] This embodiment is in the form of a tablet.
[0025]
[0026] Preparation process: weigh tolvaptan and mannitol according to the prescription ratio, mix them, grind them with a ball mill, and pass through a 200-mesh sieve. Weigh microcrystalline cellulose and hydroxypropyl cellulose according to the prescription quantity, mix them uniformly by equal-volume incremental method, add water-based soft materials, granulate with a 20-mesh sieve, dry, and measure moisture; Sodium Methyl Starch, Magnesium Stearate. Φ6mm shallow concave stamping sheet.
Embodiment 2
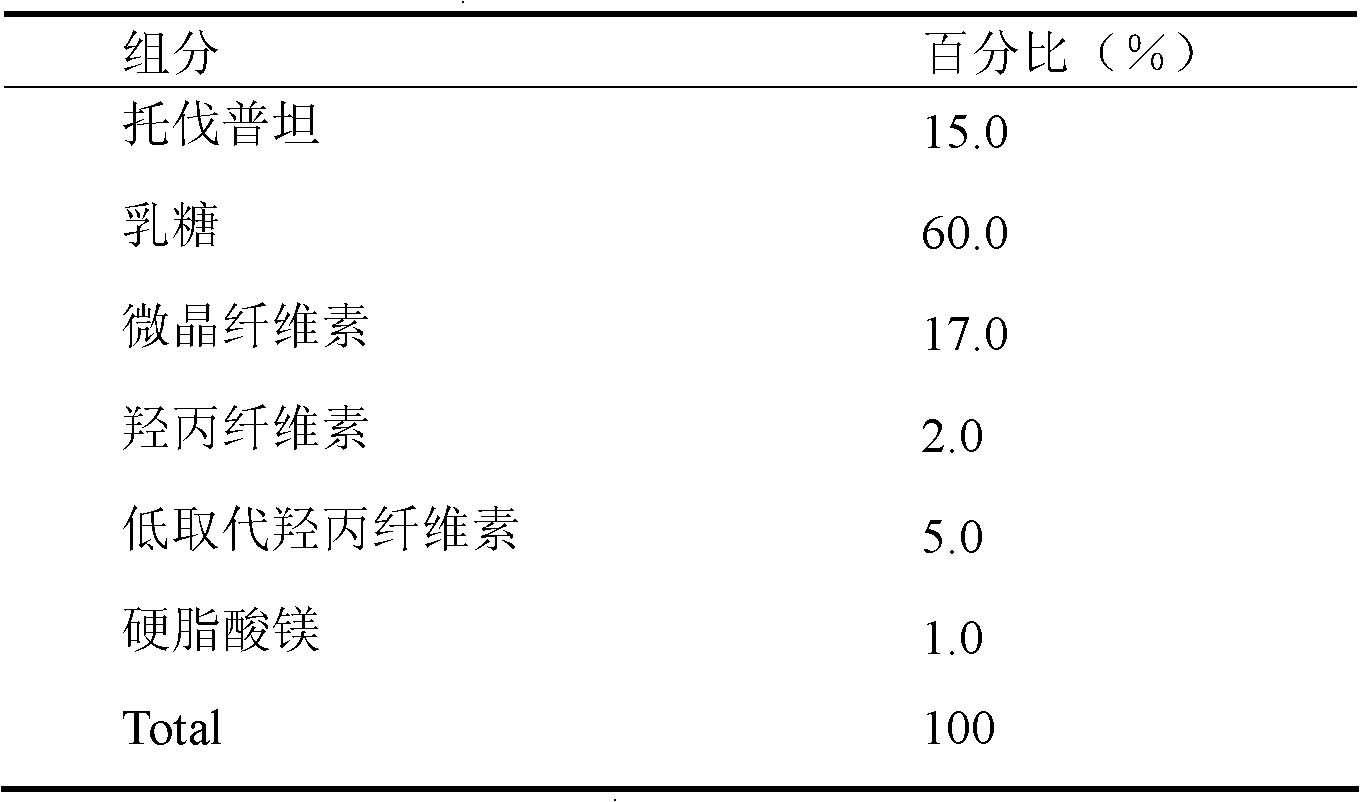
[0028] This embodiment is in the form of a tablet.
[0029]
[0030] Preparation process: Weigh tolvaptan and lactose according to the prescription ratio, mix them, grind them with a ball mill, and pass through a 200-mesh sieve. Weigh microcrystalline cellulose and hydroxypropyl cellulose according to the prescription amount and mix them uniformly by equal-volume incremental method, then add soft material made of 50% ethanol, granulate with 20 mesh sieve, dry, measure moisture; granulate with 18 mesh sieve, add low Substitute for hypromellose and magnesium stearate. Φ6mm shallow concave stamping sheet.
Embodiment 3
[0032] This embodiment is in the form of a capsule.
[0033]
[0034] Preparation process: Weigh tolvaptan and lactose according to the prescription ratio, mix them, grind them with a ball mill, and pass through a 200-mesh sieve. Weigh the pregelatinized starch, sodium carboxymethyl starch, and talcum powder according to the prescription and mix them evenly. Pack No. 3 capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com