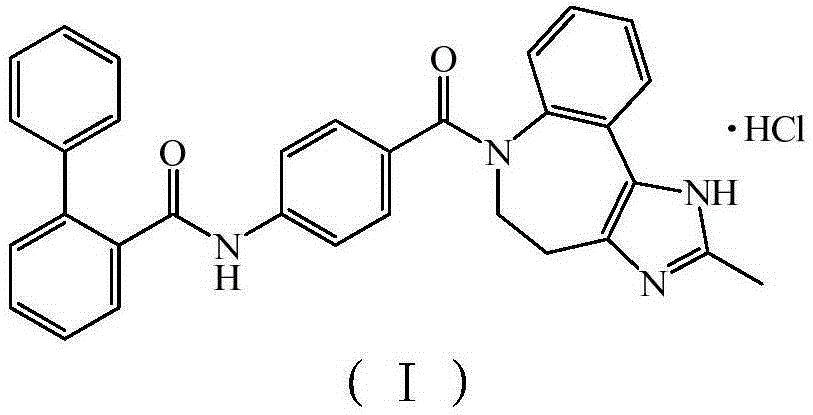
New crystal form of conivaptan hydrochloride and preparation method thereof
A conivaptan hydrochloride and crystal form technology, applied in the field of medicinal chemistry, can solve the problems of environmental protection, poor economy, difficult recovery of solvents, and low crystal form purity, and achieve high solubility and bioavailability, low production cost, The effect of meeting the medicinal requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Preferably, the preparation method of the new crystal form of conivaptan hydrochloride specifically includes
[0037] Step a) dissolving the crude conivaptan hydrochloride in anhydrous methanol and heating to dissolve, decolorizing with activated carbon, filtering, and evaporating the filtrate to dryness;
[0038] Step b) Add acetonitrile, reflux, cool, filter, and dry the filtrate to obtain.
[0039] Wherein, the ratio of the mass of the crude conivaptan hydrochloride to the volume of the anhydrous methanol in step a) is preferably 1 g: 10 mL-15 mL, more preferably 1 g: 10 mL. That is, 10 mL to 15 mL of anhydrous methanol is preferably added to 1 g of crude conivaptan hydrochloride, more preferably 10 mL of anhydrous methanol is added.
[0040]Step a) of the preparation method of the new crystal form of conivaptan hydrochloride according to the present invention: a) the crude conivaptan hydrochloride is dissolved in anhydrous methanol and then heated to dissolve the c...
Embodiment 1
[0052] Embodiment 1: Preparation of new crystal form of conivaptan hydrochloride
[0053] Dissolve 1 g of crude conivaptan hydrochloride in 12 mL of anhydrous methanol, heat it in a water bath to 35-40°C to dissolve it, add 0.06 g of activated carbon into it, heat and stir for 20 minutes to decolorize, filter, and evaporate the filtrate to dryness under reduced pressure at 35-40°C; Add 25mL of acetonitrile, raise the temperature and reflux for 5 hours under rapid stirring, cool to about 15°C, stir for 1 hour and filter, and dry the filter cake at 60°C with an oil pump under reduced pressure to constant weight to obtain 0.694g of white solid powder with a yield of 69.4%. After HPLC analysis, the total amount of related substances in the obtained product was 0.065%, and the maximum single impurity was 0.046% (area normalization method).
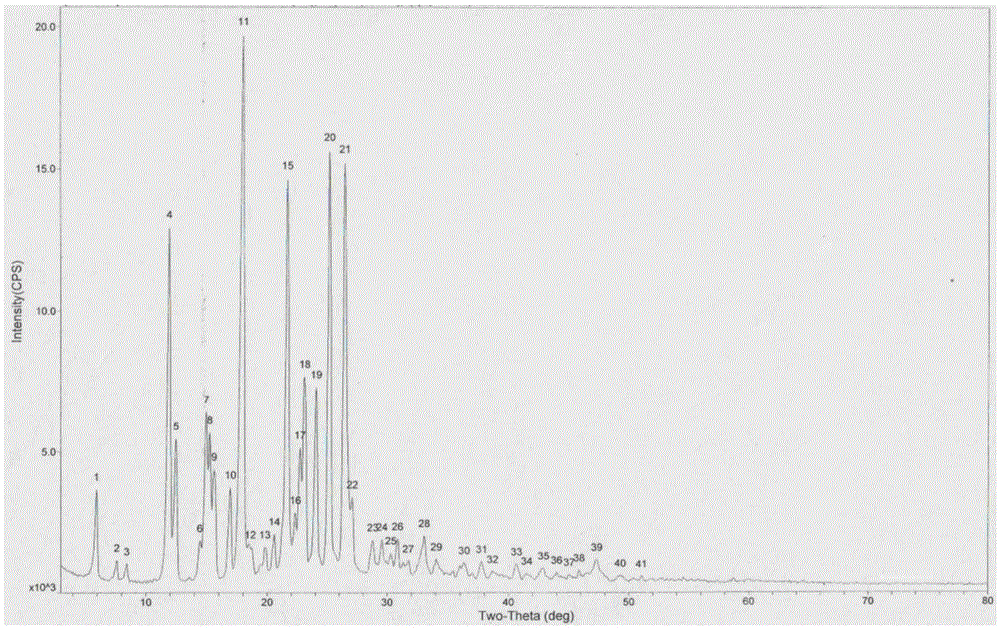
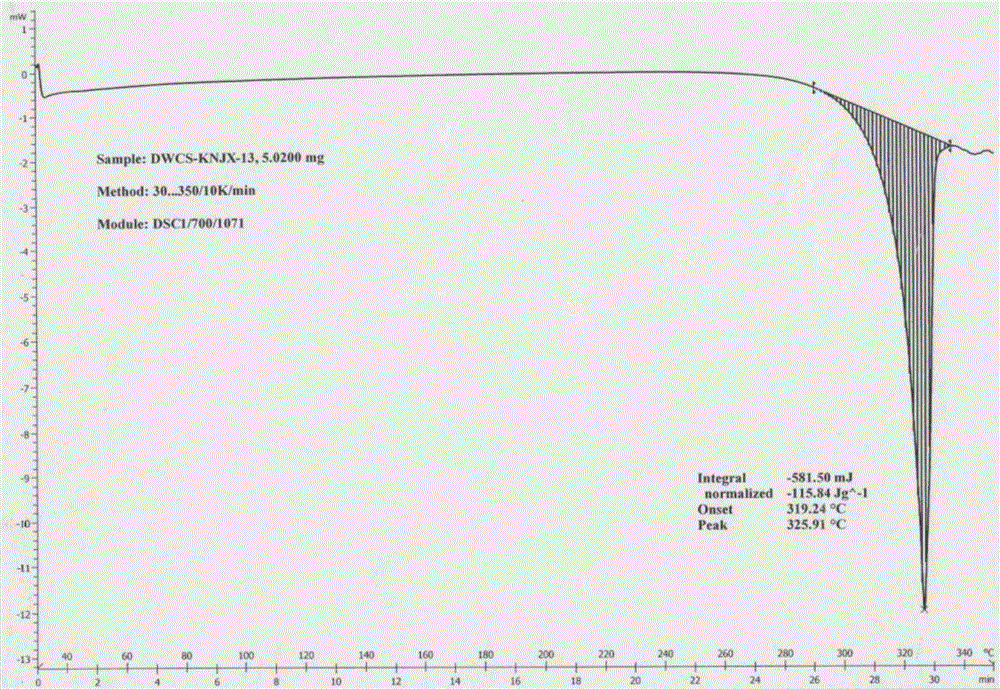
[0054] Adopt Japan RigakuD / max-2550 powder X-ray diffractometer, under the test parameter is: copper target, tube flow 150mA, tube pressure 40...
Embodiment 2
[0057] Embodiment 2: Preparation of new crystal form of conivaptan hydrochloride
[0058] Dissolve 20g of crude conivaptan hydrochloride in 200mL of anhydrous methanol, heat it in a water bath to 35-40°C to dissolve, add 1.0g of activated carbon into it, heat and stir for 30min to decolorize, filter, and evaporate the filtrate to dryness under reduced pressure at 35-40°C; Add 400mL of acetonitrile, raise the temperature and reflux for 5 hours under rapid stirring, cool to about 15°C, stir for 1 hour and filter, and dry the filter cake under reduced pressure at 60°C with an oil pump to constant weight to obtain 14.672g of white solid powder with a yield of 73.36%. After HPLC analysis, the total amount of related substances in the obtained product was 0.067%, and the maximum single impurity was 0.042% (area normalization method).
[0059] The product was analyzed by a Japanese Rigaku D / max-2550 powder X-ray diffractometer and a Swiss Mettler DSC1 thermal analyzer. The results sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com