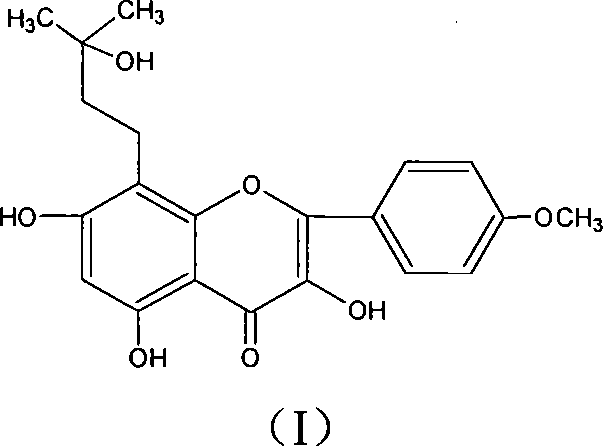
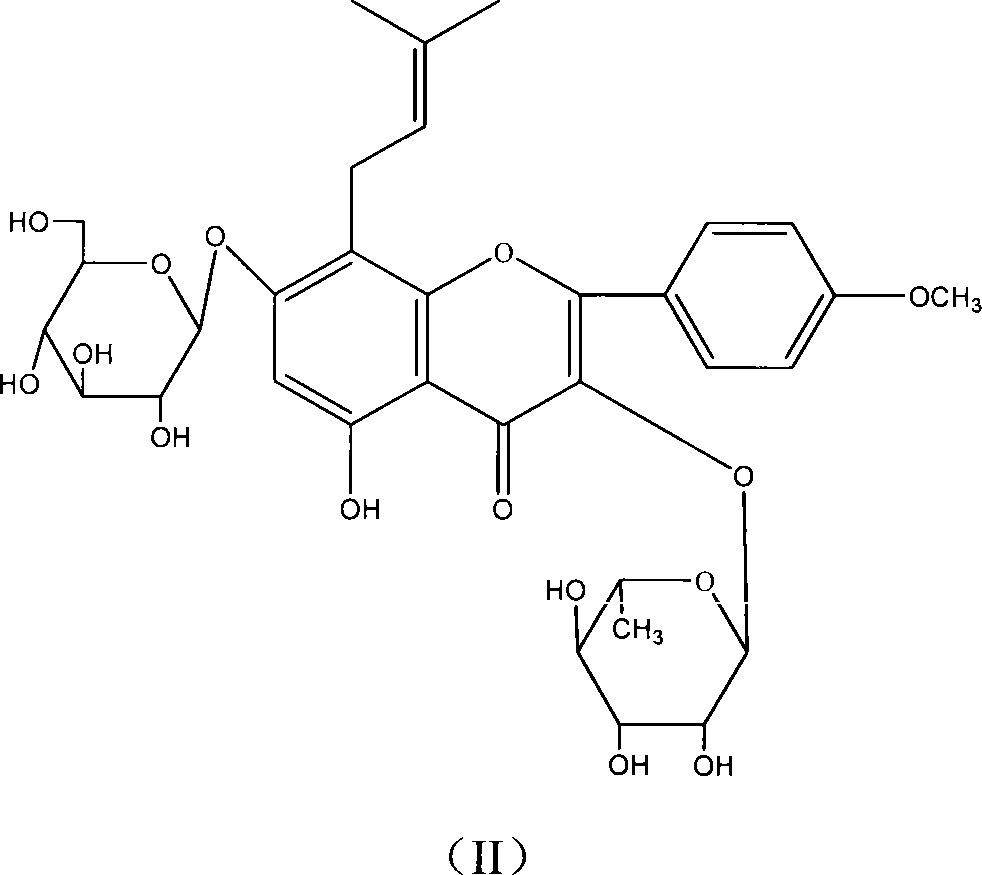
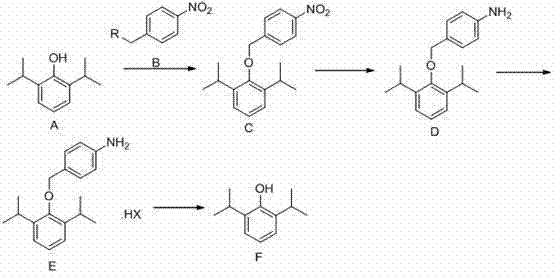
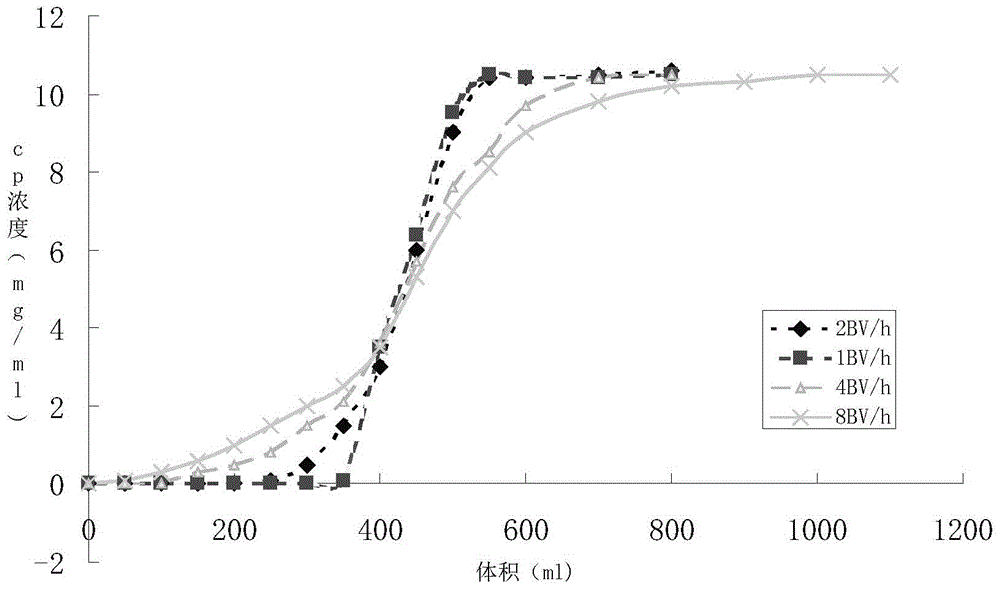
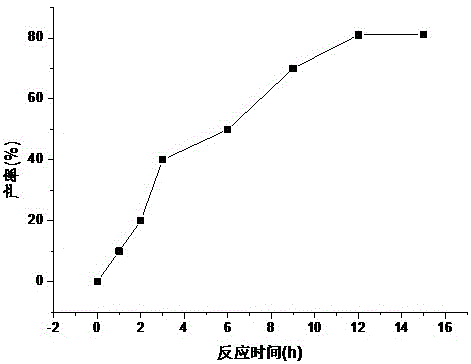
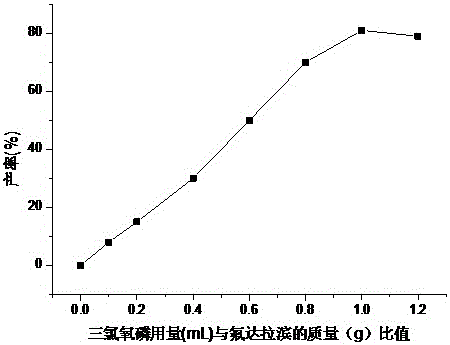
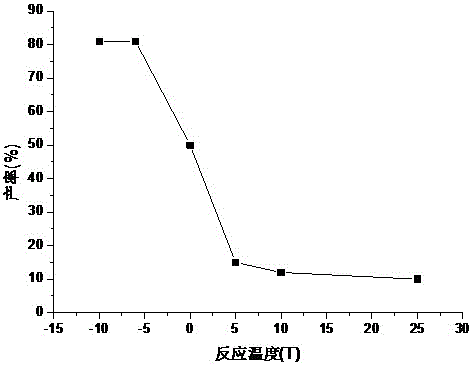
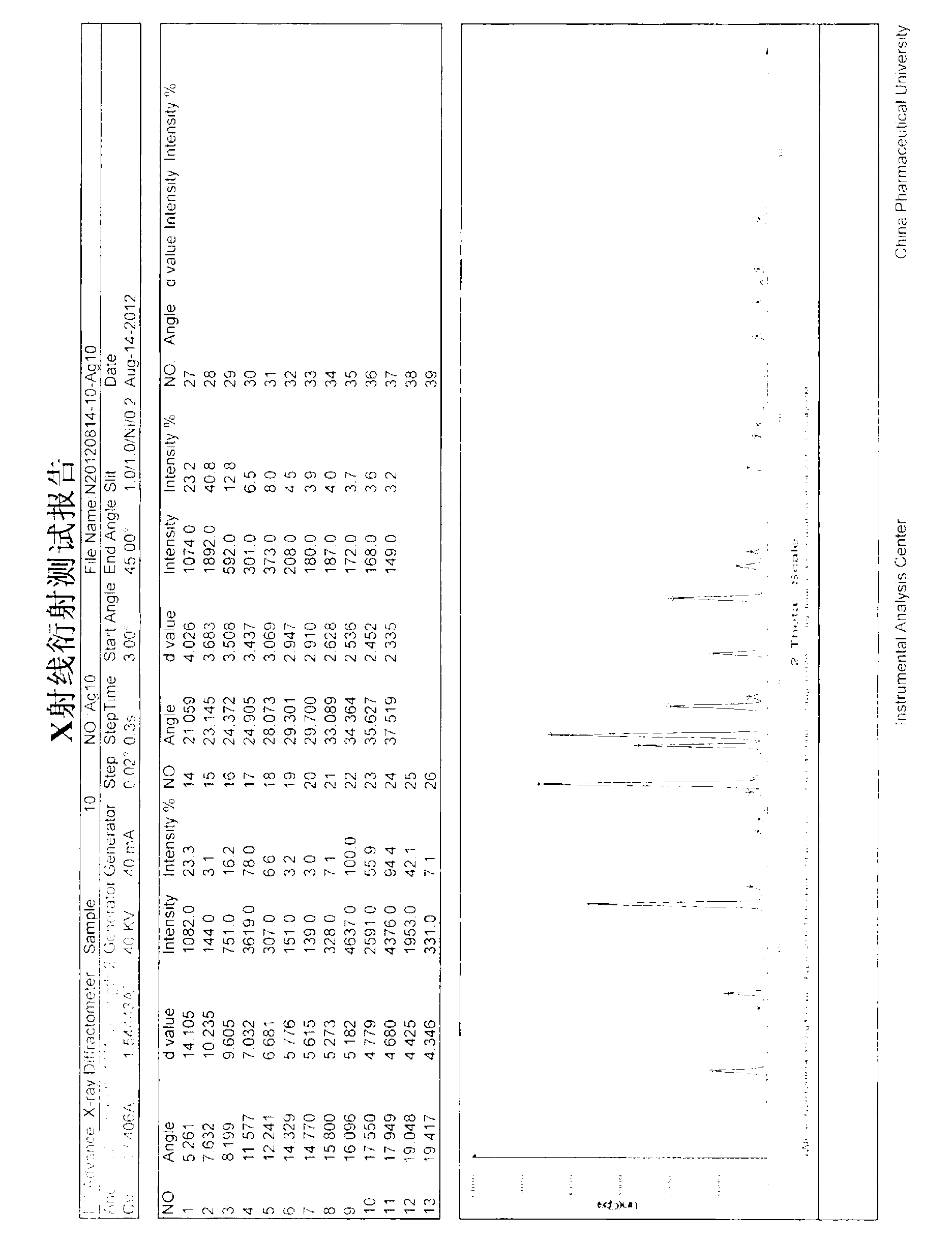
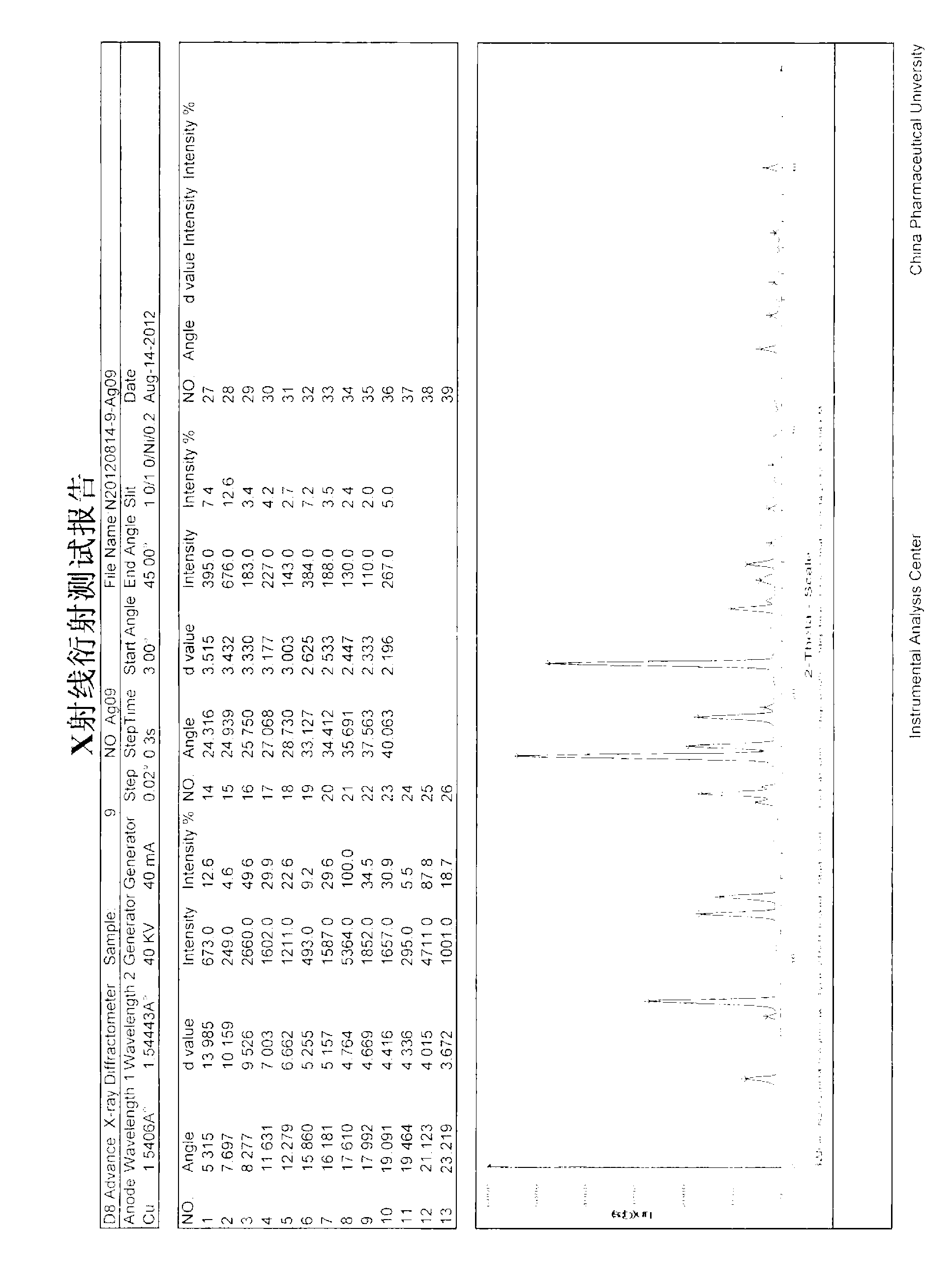
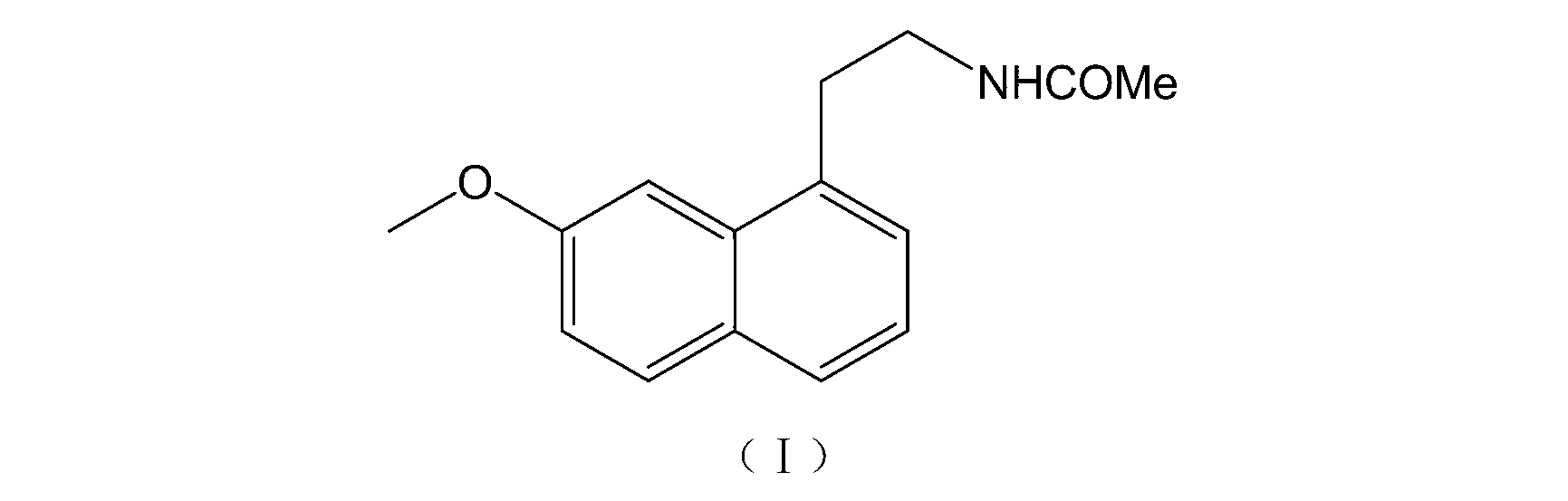
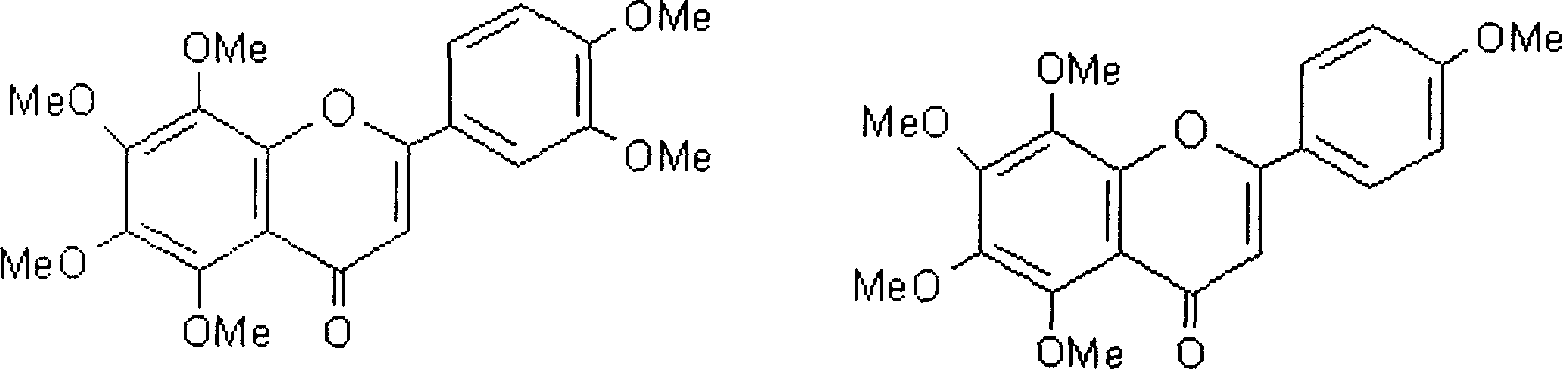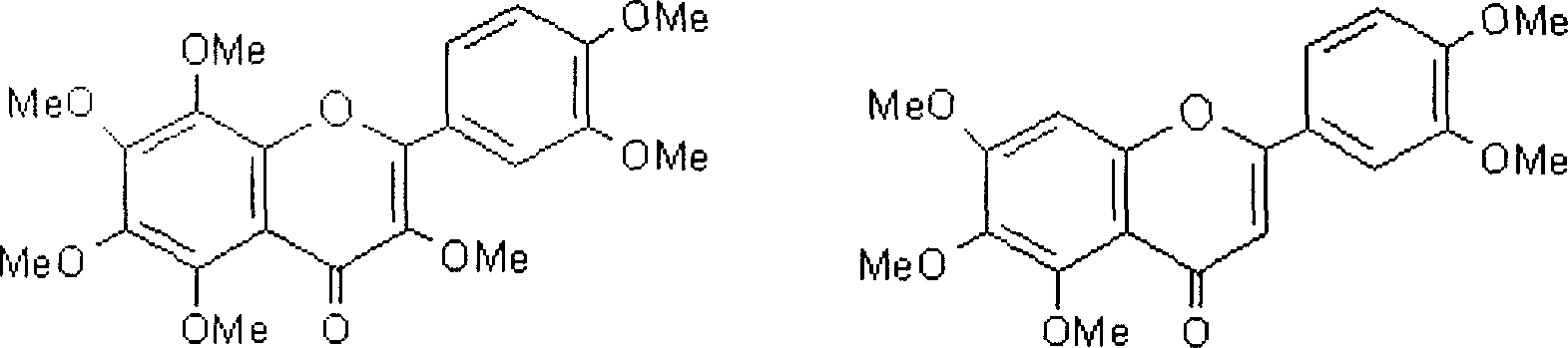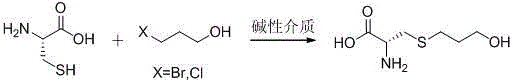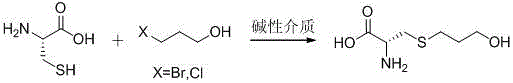Patents
Literature
56results about How to "Meet pharmaceutical standards" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation of icaritin
ActiveCN101302548AHigh purityEase of industrial productionSugar derivativesSugar derivatives preparationAlgluceraseIcariin
The invention relates to a method for preparing icaritin and is characterized in that: the icaritin is prepared by icariin which is subject to the enzymolysis reaction by beta-glycosidase. The method adopts the beta-glycosidase to perform the enzymolysis reaction for the icariin, the beta-glycosidase has complete deglycolysis and high yield, a self-designed method for extracting the icariin from Herba Epimedii is adopted, the whole technical process has simple and convenient operation, the treatment is convenient after the reaction, and the purity of the product fully meets the pharmaceutical standard.
Owner:BEIJING SHENOGEN BIOMEDICAL
Method for purifying orlistat
The present invention discloses orlistat purifying method, which includes dissolving coarse orlistate product in medium polarity solvent, filtering, eliminating impurity, crystallization, dissolving in non-polar solvent, recrystallization, and repeating some steps until reaching the medicinal purity standard. The said method is suitable for purifying coarse orlistate product with purity of 50-85 %, and has the advantages of high yield, short purification period, simple purification process and being suitable for industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Method for preparing hydrated icaritin
The invention relates to a preparation method of hydrated icariin which belongs to the synthesis field of natural compounds. The preparation method of the hydrated icariin is characterized in that the hydrated icariin is obtained from icariin after two steps of the hydrolysis reaction of hydrochloride and cellulose. The desugarization hydrolysis is complete. The chemical structure of barrenwort aglycone can be changed, which changes the barrenwort aglycone into the hydrated icariin. The yield is high. The treatment after the reaction is convenient. And the purity of products fully complies with the medical standards.
Owner:BEIJING SHENOGEN BIOMEDICAL
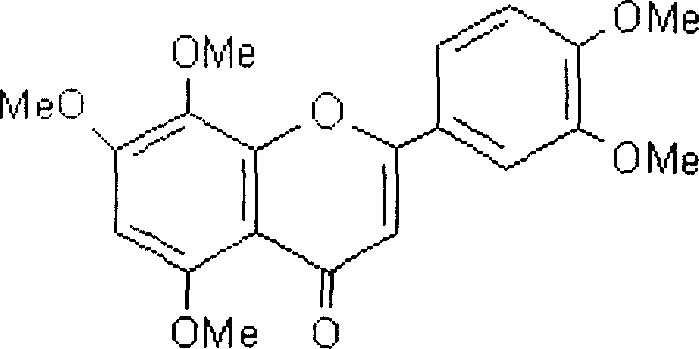
Polymethoxylated flavones and its preparation method
InactiveCN1836657AImprove solubilityImprove securityOrganic active ingredientsMetabolism disorderSolubilityIntramuscular injection
The polymethoxyl flavone injection includes polymethoxyl flavone, alcohol and surfactant, and the polymethoxyl flavone is mixture of two or more of nobiletin, tangeretin, sinensetin, heptamethoxyl flavone and isotangeretin. The present invention solves the solubility and stability problem of polymethoxyl flavone injection, and the polymethoxyl flavone injection has high safety, coincides with medicine standard and suitable for intramuscular injection and intravenous injection. The present invention also discloses the preparation process of the polymethoxyl flavone injection.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Edible ink and preparation method thereof
The invention relates to edible ink and a preparation method thereof. The ink comprises the following components in percentage by weight: 30 to 70 percent of ethanol, 20 to 45 percent of lac, 10 to 35 percent of edible ferric oxide colorant and 0 to 1 percent of assistant. The ink is prepared by the following steps: adding the lac into the ethanol and constantly stirring till the lac dissolves completely to obtain solution of lac; adding the edible ferric oxide colorant and the assistant into the solution of lac; constantly and uniformly stirring to obtain uniformly mixed solution; filling the uniformly mixed solution into colloid mill; and milling to obtain a finished product. Compared with the prior art, the ink has the advantages of high adhesivity, low corrosivity, no toxicity, no harm, high oil resistance, high acid resistance, and high color fixing performance.
Owner:SHANGHAI YANAN PHARM
Preparation method of high-purity propofol
ActiveCN102731265ASolve the purification problemHigh purityOrganic chemistryOrganic compound preparationBrominePropofol
The invention discloses a preparation method of high-purity propofol. The method comprises the steps shown below, wherein R is selected from chlorine, bromine or sulfonate. The method provided by the invention is advantaged in intelligent idea, simple process, and suitability for industrialized productions. The purity of the prepared propofol is above 99.9%, and the prepared propofol satisfies various medical pharmaceutical standards.
Owner:四川国瑞药业有限责任公司
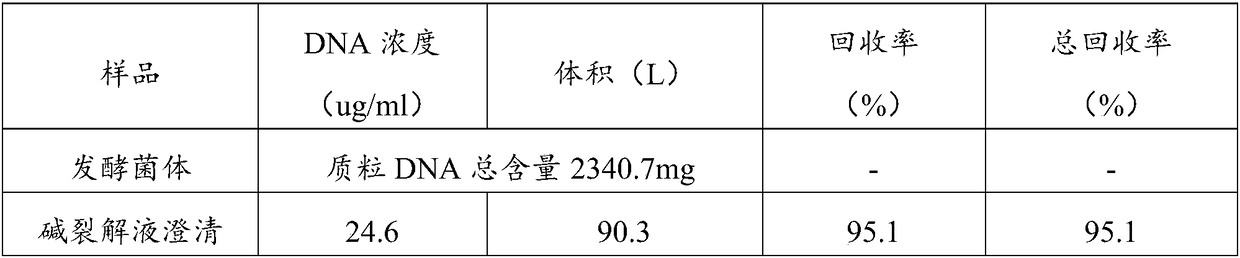
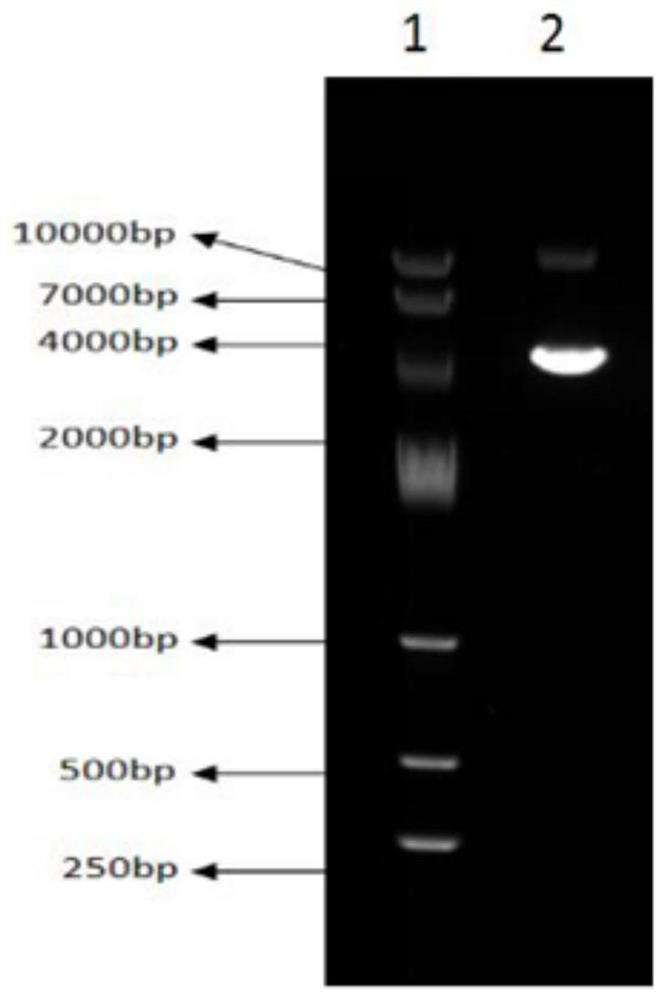
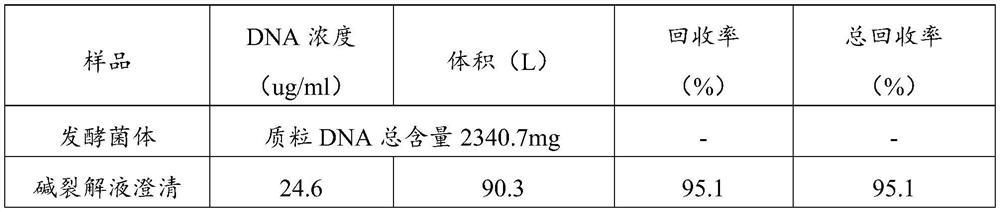
Method for large-scale purification of plasmid DNA
ActiveCN109456963AEasy to operateThe operation method is easy to enlargeDNA preparationDNA/RNA fragmentationUltrafiltrationChemistry
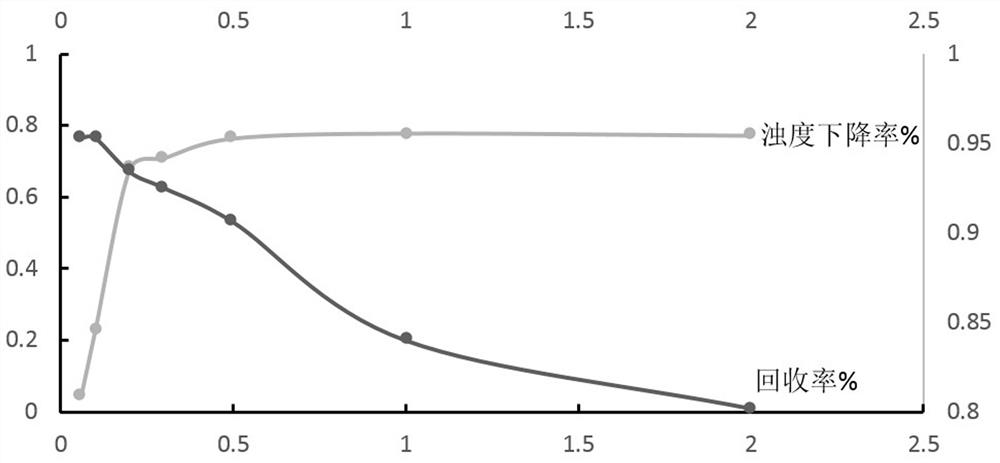
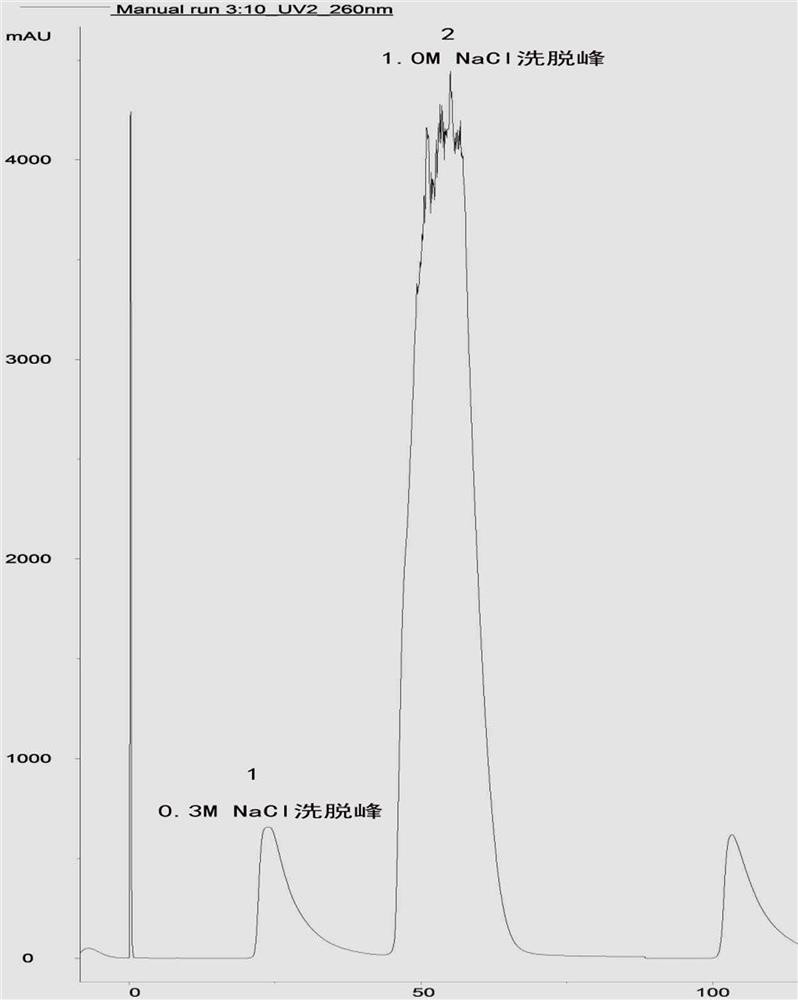
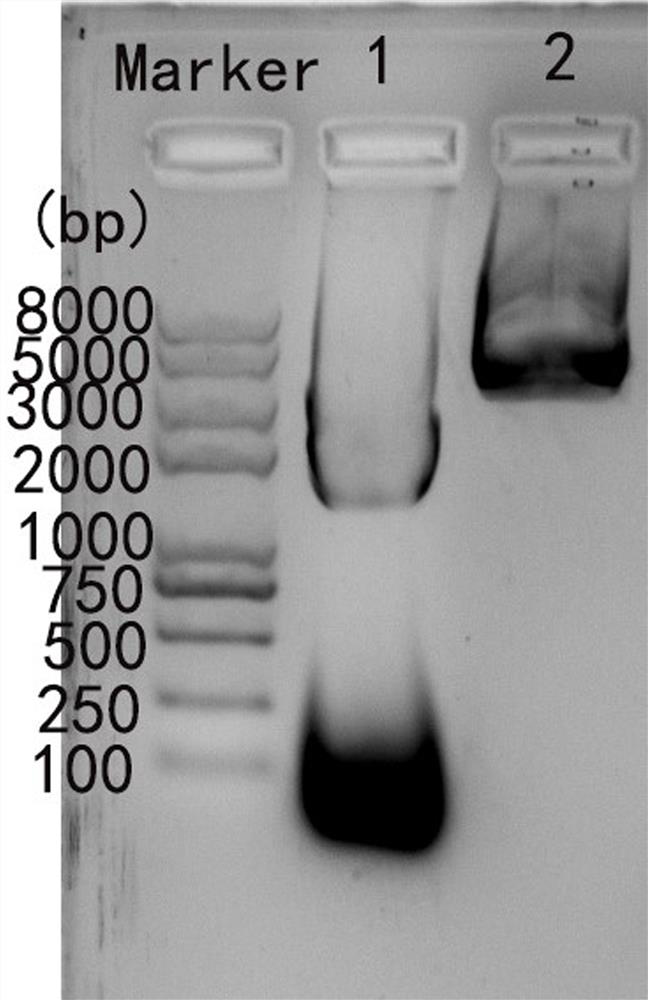
The invention relates to a method for large-scale purification of plasmid DNA, and belongs to the technical field of bioengineering such as DNA vaccines, nucleic acid vaccines and gene therapy. The easy, convenient and rapid separation effect is achieved by adding diatomite into alkali lysate to aid filter and clarification, and then through purification steps such as concentrated ultrafiltration,molecular sieve chromatography, affinity chromatography, anionic exchange chromatography, replacement concentration of final products and degerming filtration of clarification lysate, plasmid DNA products meeting the medicinal standards can be produced. According to the method, the diatomite is used for the first time to aid filter and applied to the field of purification of the plasmid DNA products, and the operation method is simple, convenient and easy to enlarge; and the plasmid DNA purification method provided by the invention is high in operability and good in repeatability, the purified plasmid DNA is high in total recovery rate and meets the medicinal standards, and the requirements of mass industrial production can be met.
Owner:广州白云山拜迪生物医药有限公司
Preparation method of Fudosteine
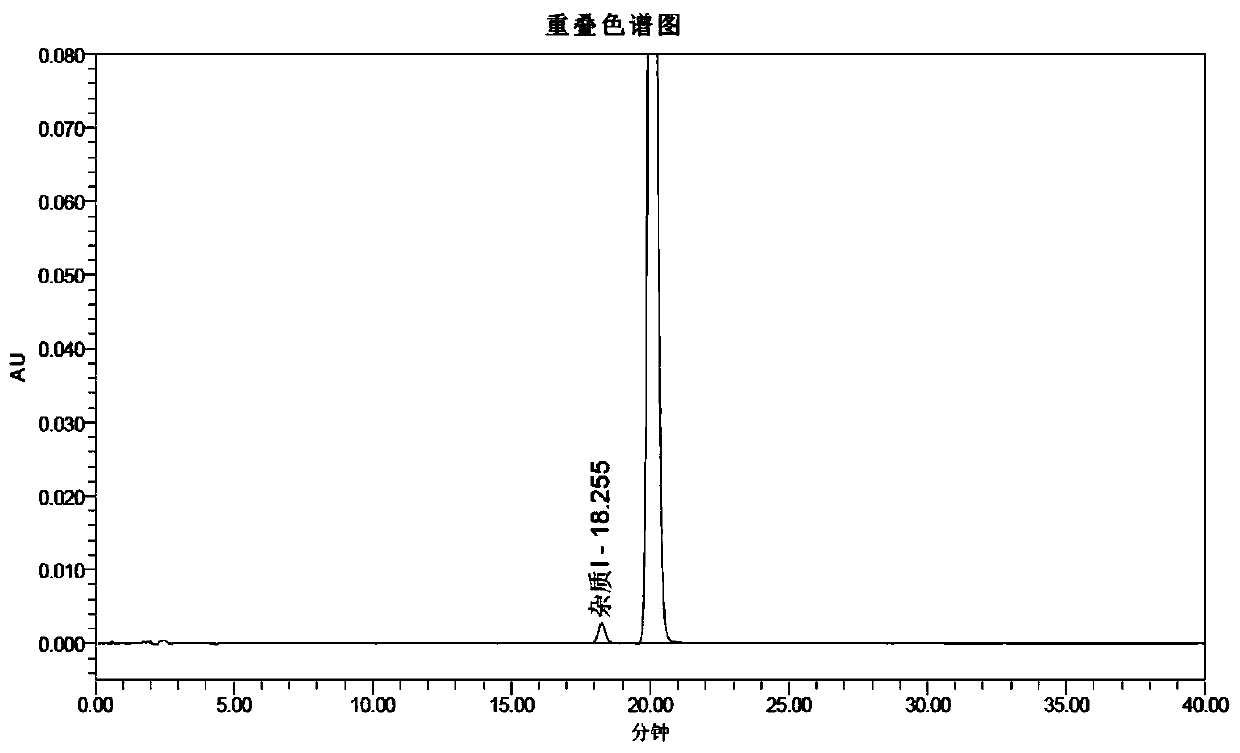
InactiveCN105968035AAvoid generatingQuality improvementOrganic chemistry methodsSulfide preparationFUDOSTEINEAlcohol
The invention discloses a preparation method of Fudosteine, and relates to a preparation method suitable for industrially producing Fudosteine. Water and ethyl alcohol are adopted as reaction solvent, amine substances serves as the catalyst, cooling and crystallization are conducted after reaction, and the product is obtained in the form of sedimentation. The whole process is easy to control, repeatability is high, the yield of Fudosteine is stabilized at 95%, the residual amount is controlled within 0.05%, and the preparation method completely conforms to the medicinal standard and is quite suitable for industrial production.
Owner:WEIHAI DISU PHARMA CO LTD
Preparation method of high-purity etravirine
The invention discloses a preparation method of high-purity etravirine, which comprises the following steps: A. dissolving a crude etravirine product in a solvent at -40-150 DEG C; B. adding a poor solvent into the mixture obtained in the step A at -40-150 DEG C; C. precipitating an etravirine solid from the mixture obtained in the step B at -80-150 DEG C; and D. separating the etravirine solid obtained in the step C to obtain the required high-purity etravirine. The preparation method disclosed by the invention has the advantages of ingenious concept and simple process; and the purity of the prepared etravirine is up to higher than 99.9%, and the prepared etravirine conforms to various medicine standards.
Owner:SICHUAN BAILI PHARM CO LTD
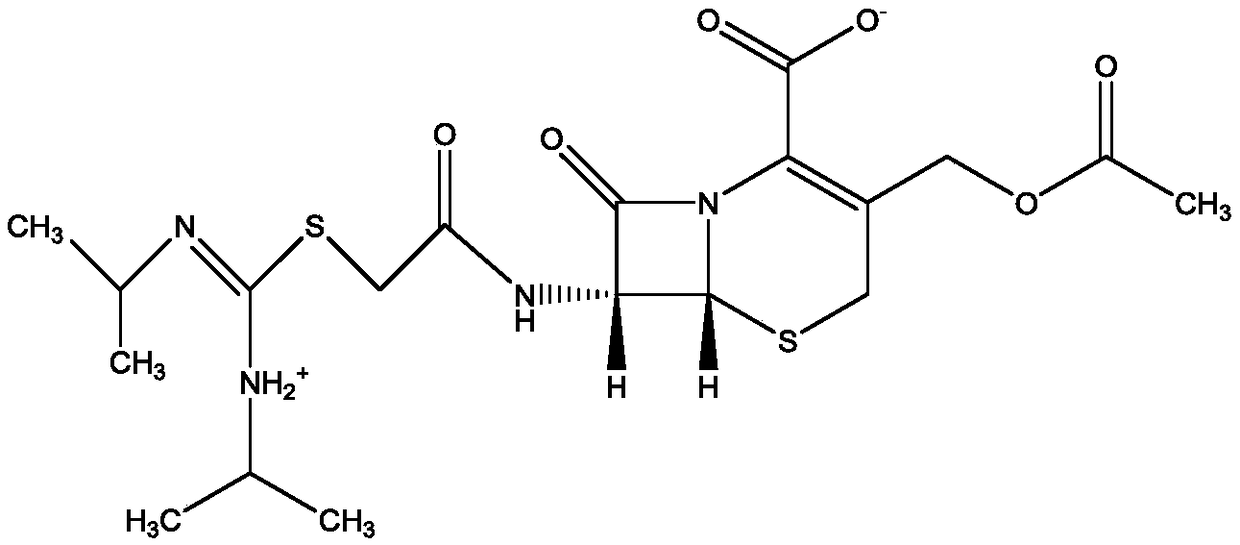
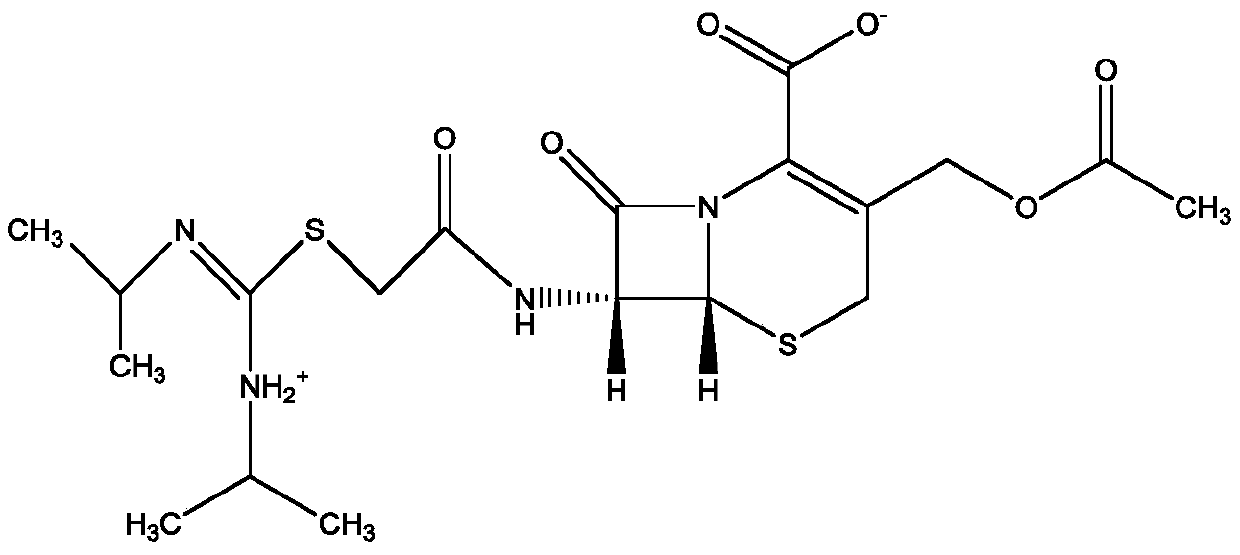
Refining method of cefathiamidine
The invention discloses a refining method of cefathiamidine, belonging to the chemical pharmacy field. The refining method comprises the steps of dissolving a cefathiamidine crude product by virtue ofa dissolving agent, adding a stable adjusting agent, and carrying out secondary decolorization by virtue of an aluminum oxide adsorption column. According to the refining method, the color grade of cefathiamidine can be effectively decreased, obtained cefathiamidine crystal particles are uniform, high in purity and good in stability, the product yield is high, and the preparation method is easy,convenient and controllable and suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
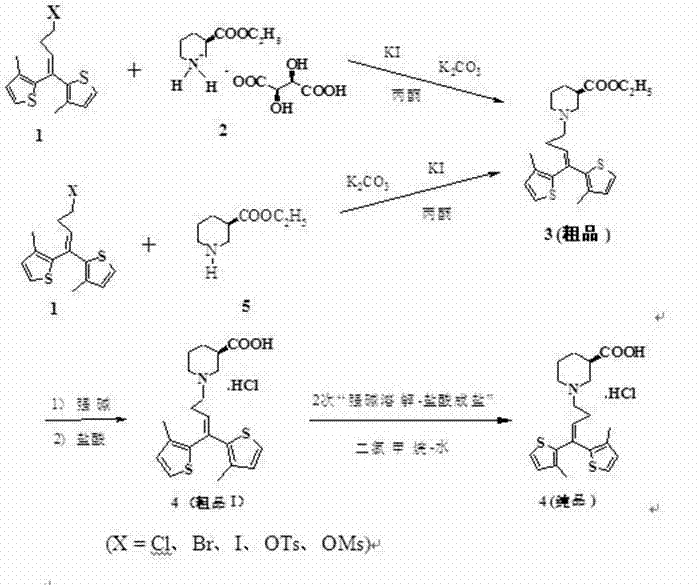
Preparation and purifying method of tiagabine hydrochloride
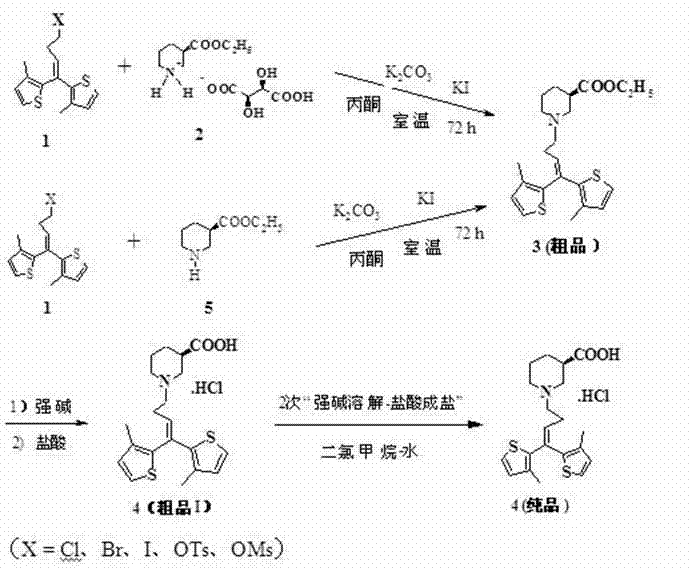
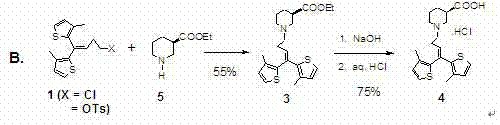
The invention provides a preparation and purifying method of tiagabine hydrochloride. The method comprises the following steps: directly using R-piperidine-ethyl formateXL-tartrate(2), or R-piperidine-3-ethyl formate as raw material, reacting with alkylating agent 1,1-bi(3-methyl-2-thienyl)-4-X-1-butene(1, X is Cl, Br, I, O, Ts and OMs) in the presence of enough potassium carbonate, obtaining a crude product of tiagabine ethyl ester, directly performing alkali hydrolysis, performing the process of alkali solution in dichloromethane-water system and slat formation with hydrochloric acid on the tiagabine hydrochloride crude product for more than twice, removing various impurities in the tiagabine ethyl ester crude product to obtain pure tiagabine hydrochloride (4) with high purity. The process step is shortened, the column chromatography is abolished, the process program is simplified, the cost is greatly lowered, and the product with high purity is obtained.
Owner:FUZHOU NEPTUNUS FUYAO PHARMA
Preparation method for bromhexine hydrochloride
ActiveCN109535010ANo pollution in the processShort synthesis pathOrganic compound preparationAmino-carboxyl compound preparationOrganic synthesisBromhexine hydrochloride
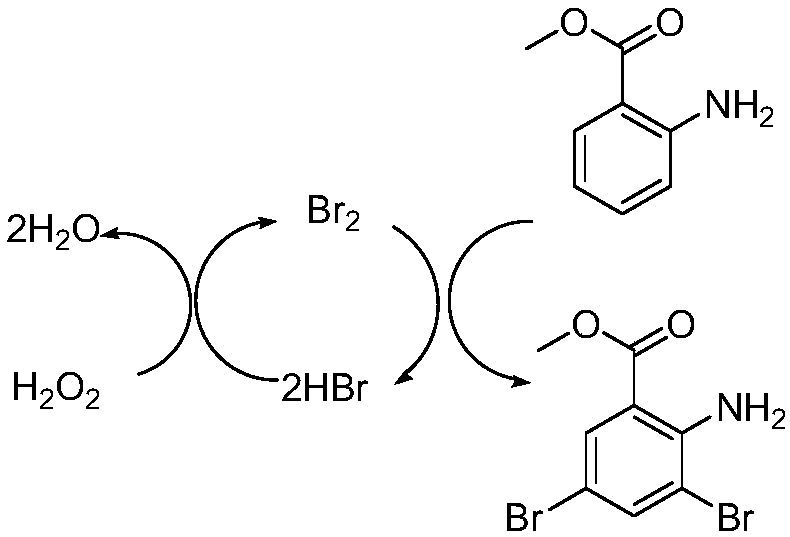
The invention belongs to the field of organic synthesis and provides a preparation method for bromhexine hydrochloride. According to the invention, 2-aminobenzoic ester compounds are taken as raw materials and take part in bromination reaction, reduction reaction, condensation reaction and salt forming reaction, so as to acquire bromhexine hydrochloride. The preparation method has the advantages of short synthesis route, low cost, stable intermediate, no pollution to environment, high purity, high yield, reaching officinal standard and suitability for industrial expanded production.
Owner:GUANGZHOU YIPINHONG PHARMA +5
Preparation method of fodor stanozolol suitable for industrialized production
ActiveCN105777595AAvoid generatingQuality improvementSulfide preparationStanozololBiochemical engineering
The invention relates to a preparation method of fodor stanozolol suitable for industrialized production. According to the preparation method disclosed by the invention, water-ethanol is used as a reaction solvent, amine substances are used as a catalyst, after the reaction is completed, temperature reduction and crystallization are performed, and products are separated in a precipitation form. The whole process is easy to control and high in repeatability, the yield of the fodor stanozolol is stabilized to 95%, and the residue quantity is controlled within 0.05%, so that medical standards are completely met, and the preparation method is very suitable for industrialized production.
Owner:迪嘉药业集团股份有限公司
Hemodialysis medicinal component sodium diacetate preparation method
InactiveCN104774144AEasy to transportEasy to storeOrganic compound preparationCarboxylic acid salt preparationSodium acetateAcetic acid
The present invention discloses a hemodialysis medicinal component sodium diacetate preparation method, which comprises: (1) adding a raw material drug sodium acetate to a reaction kettle, heating while stirring, and adding food grade glacial acetic acid to the reaction kettle in a dropwise manner to react, wherein a molar ratio of the medical sodium acetate to the food grade glacial acetic acid is 1:(1.1-2.0); and (2) after completing the reaction, heating to a temperature of 110-120 DEG C, carrying out reflux for 1-2 h, filtering while hot, cooling to a room temperature to obtain a crystal, and rapidly drying the crystal to obtain the medicinal sodium diacetate. According to the present invention, the prepared sodium diacetate meets the medicinal standard, the product is the solid, has characteristics of easy transportation, easy storage and risk reducing, and can be used for hemodialysis powder preparation.
Owner:JIANGSU QINFEN PHARMA
Method for extracting gelatin from grass carp fishskin
The invention relates to a method for extracting gelatin from grass carp fishskin, and belongs to the technical field of gelatin extracting. The method comprises the steps that 1, after the grass carp fishskin is crushed into paste, the paste is mixed with a 5% citric acid aqueous solution, dispersing under stirring is conducted under the ultrasonic action, and solid substances are filtered out; 2, the solid substances obtained in the step 1 are mixed with petroleum ether, the solid substances are filtered out after being soaked, and reduced pressure evaporation is conducted to remove the solvent; 3, the solid substances obtained in the step 2 are mixed with an alkaline solution, and extracting under heating is conducted to obtain an extracting solution; 4, wheat husk powder is added into the extracting solution, stirring is conducted for adsorption, after the powder is filtered, filtrate is centrifuged, and supernatant liquid is taken; 5, the supernatant liquid is concentrated through a nanofiltration membrane, and spray drying is conducted on a nano-filtrated concentrated solution to obtain the fishskin gelatin. According to the method, the high-purity high-yield gelatin product can be extracted from the fishskin and meets the medicinal standards.
Owner:FOSHAN WIN COSMETIC
Process for preparing anhydrous alcohol for medical use
InactiveCN1699317AEasy to operateMeet pharmaceutical standardsOrganic compound preparationHydroxy compound preparationChromium trioxideDistillation
The invention relates to a process for preparing anhydrous alcohol for medical use which comprises the steps of, (1) loading 95% of ethanol into reaction pot, charging oxidizing agent by the amount of 1-10g of oxidizing agent for 10L of 95% ethanol, wherein the oxidizing agent is any one agent selected from hypermanganate, manganese dioxide, bichromate, chromate, chromium trioxide, (2) thermal insulating 10-50 minutes at 25-55 deg. C, then charging sodium metabisulfite till the color of the oxidizing agent disappears, (3) charging calcium oxide into reaction pot by the amount of 1.5-2.5kg of calcium oxide for 10L of 95% ethanol, steam heating and backwashing 3-10 hours, (4) cooling the reaction pot and distilling, arranging a cloth bag containing calcium chloride above the reaction pot, thermally distilling, abandoning the first distillation liquid, gathering the distillate at 78.5 deg C.
Owner:杨振华
Preparation method of high-purity propofol
ActiveCN102731265BSolve the purification problemImprove stabilityOrganic chemistryOrganic compound preparationBrominePropofol
The invention discloses a preparation method of high-purity propofol. The method comprises the steps shown below, wherein R is selected from chlorine, bromine or sulfonate. The method provided by the invention is advantaged in intelligent idea, simple process, and suitability for industrialized productions. The purity of the prepared propofol is above 99.9%, and the prepared propofol satisfies various medical pharmaceutical standards.
Owner:四川国瑞药业有限责任公司
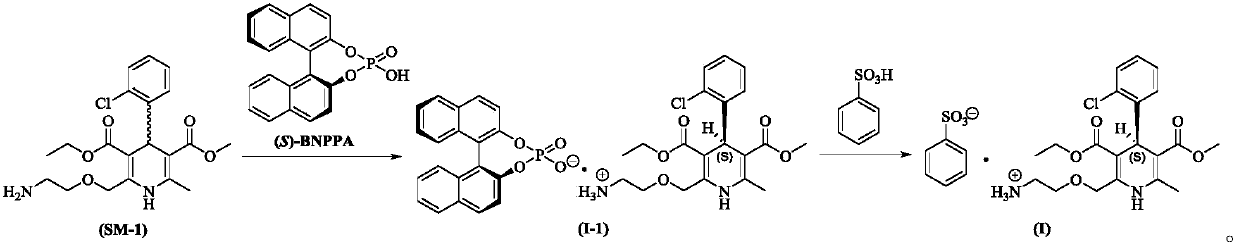
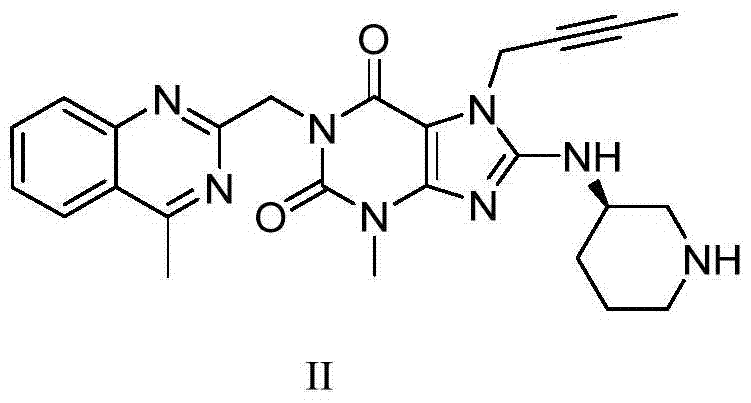
Preparation method of amino intermediate
ActiveCN110746378AMaximum impurity content reductionGuaranteed Maximum Impurity ContentOrganic chemistryEthanesulfonic acidCombinatorial chemistry
The invention relates to a preparation method of an amino intermediate. The invention relates to the preparation method of the amino intermediate represented by a formula (I), and the method comprisesthat a compound of a formula (III) is subjected to operations such as condensation and reduction to obtain the target compound. Compared with the existing preparation method, the content of an impurity 1 in the formula (I) compound obtained by the method is significantly reduced, a process guarantee for industrial preparation of high-purity nintedanib ethanesulfonate salt is provided, and therebythe safety of drug use is ensured.
Owner:JIANGSU HANSOH PHARMA CO LTD
Industrial purification method of plasmid DNA and plasmid DNA
The invention discloses an industrial purification method of plasmid DNA and plasmid DNA. The purification method comprises the following steps of, adding sodium caprylate into a plasmid DNA ultrafiltration concentrated solution, uniformly mixing, standing at normal temperature for a period of time, then standing at low temperature for a certain period of time, and then clarifying; and then sequentially carrying out ion exchange chromatography, hydrophobic chromatography, ultrafiltration desalination concentration and filtration sterilization to finish purification. According to the purification method, the sodium caprylate is applied to purification of plasmid DNA products under the low-temperature condition, operation is easy, convenient, amplifiable, operation steps are reduced, meanwhile, the total recovery rate of purified plasmids is increased, and the medicinal standard is reached; in addition, the use of ammonium sulfate is greatly reduced, and the influence on operators and environment is reduced; and the purification method is good in purification effect, the use of an ammonium sulfate reagent is greatly reduced in the purification process, the repeatability is high, theoperation is simple, and the industrial purification production requirements can be met.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
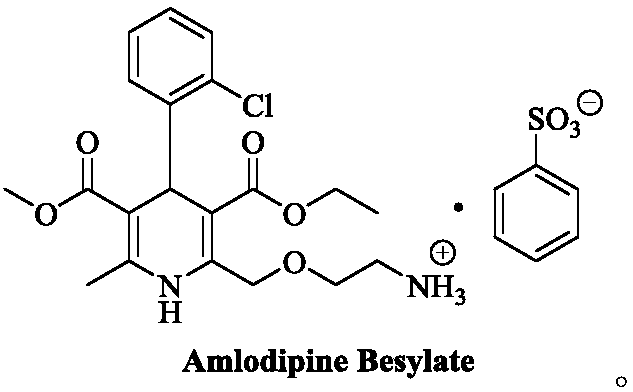
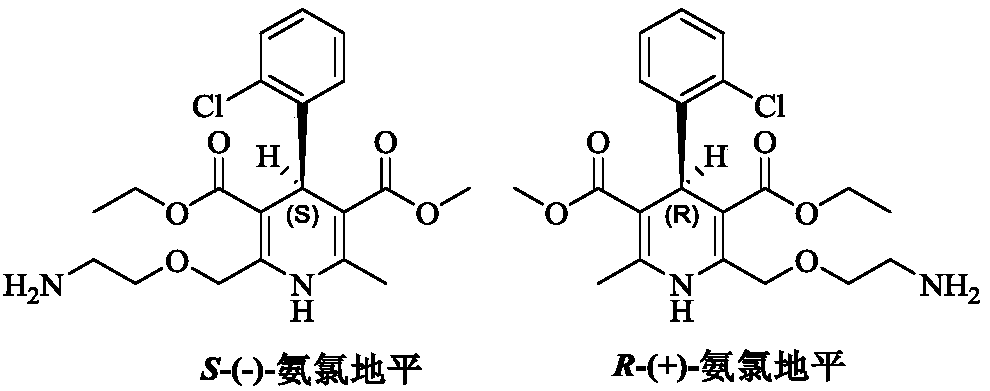
Preparation method of levamlodipine besylate
ActiveCN111377851AHigh reaction yieldHigh purityOrganic chemistry methodsSulfonic acids salts preparationProcess engineeringEnvironmental geology
The invention belongs to the technical field of medicine synthesis, and provides a production method of levamlodipine besylate, which comprises the following steps: using (R, S)-amlodipine besylate asa raw material to react with a resolving agent, and directly reacting with benzenesulfonic acid salt conversion to obtain the levamlodipine besylate, wherein the yield and purity are very good, the used reaction solvent is economic and environment-friendly, and the resolving agent can be further recycled. The method is simple and convenient to operate and does not need special equipment, so thatthe method has a better industrial application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method for creatine phosphate sodium
ActiveCN105132489AHigh purityLow costOn/in organic carrierFermentationCreatine kinaseSulfate radicals
The invention discloses a preparation method for creatine phosphate sodium. The method comprises the specific steps that creatine jubase is fixed to carrier resin to obtain fixed creatine jubase, creatine and ATP are subjected to a reaction under the catalysis of the fixed creatine jubase at the temperature of 5-40 DEG C and the pH value of 7-11 on the basis that the mole ratio of the creatine to the ATP is 2:1-10:1 and the molar concentration of magnesium ions is 0.5-5 times that of the ATP till the concentration of creatine phosphate sodium is constant, and a reaction solution is collected; then the reaction solution is pretreated with the ethyl alcohol with the volume fraction being 40-70%, then, a pretreated creatine phosphate sodium mother solution is loaded, the creatine is eluted, then the creatine phosphate sodium is eluted, an eluent is collected, and crystallization and drying are performed to obtain the creatine phosphate sodium. The purity of the creatine phosphate sodium obtained through the preparation method is high and can reach 99.8%, the creatine phosphate sodium does not contain sulfate radicals or barium ions, the safety is high, and the medical standards are met.
Owner:XINAN PHARMA
Fludarabine phosphate preparation method
InactiveCN105859812AResidue reductionMild reaction conditionsSugar derivativesSugar derivatives preparationOrganic solventTriethylamine phosphate
The invention discloses a fludarabine phosphate preparation method. The method comprises the specific steps that fludarabine and triethyl phosphate are added into a reaction container, the reaction container is placed into a subzero 6 DEG C low-temperature reaction bath, phosphorus oxychloride is added on the stirring condition, water and dichloromethane are added into the reaction container after the reaction is performed for 12 h, standing is performed, then, extraction is performed to obtain a water phase and an organic phase, the pH value of the water phase is adjusted to 2-3, recrystallization is performed to obtain white focculus, and filtering and vacuum drying are performed to obtain a target product of fludarabine phosphate with the purity being 99.95%. The method has the advantages that reaction conditions are mild, operation is easy, the product is easy to separate and purify, the yield is high, the product is environmentally friendly, high in purity, small in organic solvent residual quantity and capable of meeting the medicinal standard, and the method is suitable for industrial production.
Owner:HENAN NORMAL UNIV
Preparation method of dextran
InactiveCN105907830AEasy to operateGuaranteed quality and qualityMicroorganism based processesOn/in organic carrierPenicillium chrysogenumWeight distribution
The invention discloses a preparation method of dextran and belongs to the technical field of preparation of the dextran. The preparation method of the dextran comprises the following steps of: mixing cultured leuconostoc mesenteroides thalli, penicillium chrysogenum thalli and sodium alginate; dropwise adding the mixture into calcium chloride and granulating; then filtering and drying to obtain immobilized particles; putting the immobilized particles into a fermentation tank filled with a sugarcane solution; and after fermenting, raising the temperature and stirring and inactivating so as to prepare the dextran. An embodiment shows that the method disclosed by the invention is novel and unique; the fermentation process is simple and feasible and subsequent processing is not needed; in a preparation process, a lot of chlorides are not generated, the process is simplified and the quality of the dextran is guaranteed; the weight-average molecular weight of the finally prepared dextran is 5000D-7000D, the molecular weight distribution is smaller than 2.0 and the pharmaceutical criteria are completely met.
Owner:高大元
Preparation method of high-purity benidipine hydrochloride
InactiveCN112812054AEasy to operateHigh purityOrganic chemistryPhosphoric Acid EstersCarboxylic acid
The invention relates to a preparation method of high-purity benidipine hydrochloride, and solves the technical problems. The preparation method comprises the following steps: reacting 2, 6-dimethyl-4-(m-nitrophenyl)-1, 4-dihydropyridine-5-carboxylic acid methyl ester-3-carboxylic acid with chlorophosphate under an alkaline condition to generate mixed anhydride; enabling the mixed acid anhydride to react with the N-benzyl-3-hydroxypiperidine to generate benidipine; cooling the reaction liquid containing benidipine to 40-50 DEG C, and decolorizing with activated carbon; dissolving the decolored benidipine crude product with dichloromethane, washing with a NaOH solution, water, hydrochloric acid and water in sequence, then drying, and spin-drying the solvent to obtain a benidipine hydrochloride crude product; and dissolving a benidipine hydrochloride crude product in a mixed solution of ethanol and acetone, refluxing, stirring, and separating out a benidipine hydrochloride pure product. The method can be widely applied to the field of medicine preparation.
Owner:山东华素制药有限公司
Novel agomelatine crystal form L and preparation method thereof
InactiveCN102838504ASimple and fast operationEasy to scale up for industrial productionOrganic active ingredientsNervous disorderChemical synthesisRoom temperature
The invention belongs to the technical field of pharmaceutical chemical synthesis and relates to a novel agomelatine crystal form L and a preparation method thereof. The preparation method of the novel Agomelatine crystal form L comprises the following steps of: adding an agomelatine crude product into a solvent and heating and dissolving the agomelatine crude product; then cooling to the room temperature, naturally volatilizing to be dry; and finally obtaining the product through vacuum drying.
Owner:FUJIAN COSUNTER PHARMA CO LTD
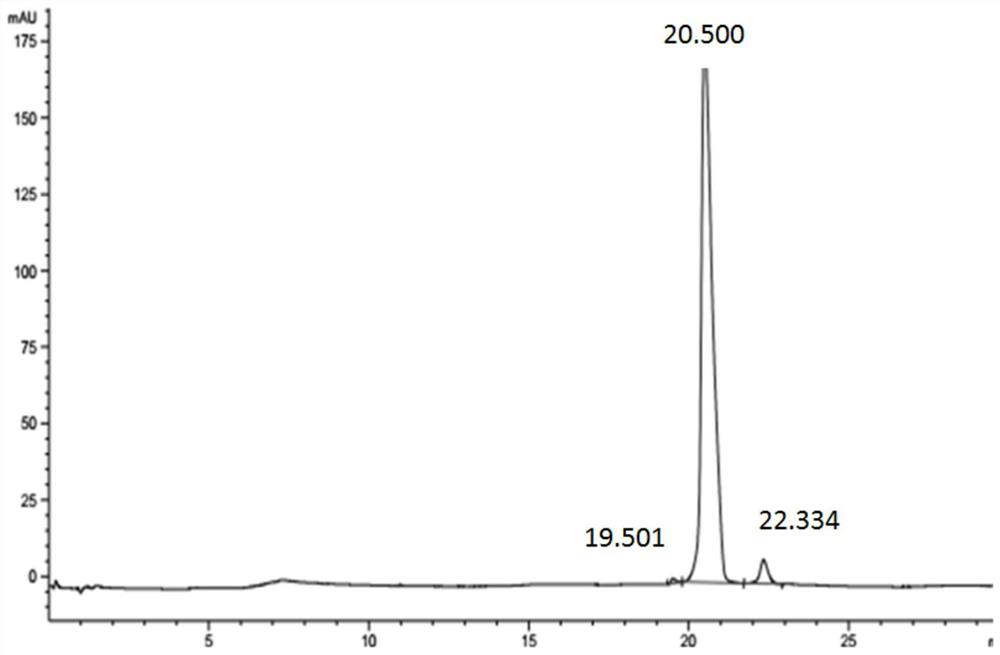
Linagliptin purification method
InactiveCN105712995AEfficient removalMeet pharmaceutical standardsOrganic chemistryPurification methodsSolvent
The invention provides a linagliptin purification method. The purification method comprises the following steps: mixing a coarse product of linagliptin and organic diacid in a solvent to carry out salt-forming reactions; separating salts generated through crystallization; and carrying out free alkali extraction and condensation to obtain purified linagliptin. The single purity content measured by HPLC is less than 0.1%.
Owner:ZHEJIANG JINGXIN PHARMA +2
A kind of purification method of linagliptin
InactiveCN105712995BEfficient removalMeet pharmaceutical standardsOrganic chemistryPurification methodsOrganic solvent
The invention provides a method for purifying linagliptin. The steps include salting the crude product of linagliptin and an organic diacid in a solvent, separating the salt produced by crystallization, and performing alkali free extraction and concentration to obtain purified linagliptin, whose single hetero HPLC is less than 0.1%.
Owner:ZHEJIANG JINGXIN PHARMA +2
A method for purifying plasmid dna on a large scale
ActiveCN109456963BGood repeatabilityImprove overall recoveryDNA preparationDNA/RNA fragmentationBiotechnologyMolecular sieve
Owner:广州白云山拜迪生物医药有限公司
A kind of refining method of cefathiamidine
ActiveCN108948048BLow color levelUniform particlesOrganic chemistryPhysical chemistryChemical engineering
The invention discloses a method for refining cefathiamidine, belonging to the field of chemical pharmacy, comprising dissolving crude cefathiamidine with a dissolving agent, adding a stabilizing regulator, and using an alumina adsorption column for secondary decolorization; the invention can effectively reduce the The color grade of cefathiamidine, the obtained cefathiamidine crystal particles are uniform, the purity is high, the stability is good, and the product yield is high, the preparation method is simple and easy to control, and it is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Levodropropizine-guaifenesin capsule and preparation method thereof
ActiveCN110934872ASolve instabilitySimple preparation processOrganic active ingredientsCapsule deliveryDropropizineDrugs preparations
The invention relates to a levodropropizine-guaifenesin capsule and a preparation method thereof. The capsule comprises the following components, by total weight: 40 to 80 percent of levodropropizineand guaifenesin as active ingredients, 10 to 60 percent of a filling agent, 0 to 10 percent of a disintegrating agent, 0 to 10 percent of a bonding agent and 0.2 to 10 percent of a lubricating agent.The weight ratio of the active ingredients to the filling agent is 1: 1.5 to 4: 1. According to the invention, a problem that the two active ingredients are easy to discolor when meeting light and moisture during mixing is solved by lots of prescription screening and raw material-auxiliary material compatibility studies. The prepared levodropropizine-guaifenesin capsule has advantages of good disintegration, high dissolution rate and high stability and meets the drug quality standard.
Owner:太阳升(亳州)生物医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com