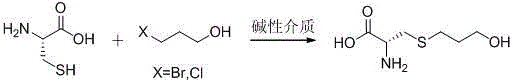Preparation method of Fudosteine
A technology of fudosteine and ethanol, which is applied in the field of preparation of antitussive and expectorant drug fudosteine, can solve the problems of product loss, cumbersome steps, and high product residue, and achieve the effect of improving quality and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] At room temperature, dissolve 100g of L-cysteine in a mixed solvent of a certain amount of water and ethanol (300g:750g), add 96g of allyl alcohol, then add 0.05ml of n-propylamine, and stir to dissolve. The temperature was raised to 40°C, and the reaction was stirred for 4.0h. After the reaction was completed, the temperature was lowered to 0° C., stirred and crystallized for 2 hours, and a white solid was obtained by suction filtration. Blast drying at 45°C to constant weight yielded 140.5 g of white solid powder with a content of 99.1%, and the residue was determined to be 0.01%. Yield 94.9%.
Embodiment 2
[0022] At room temperature, dissolve 100g of L-cysteine in a mixed solvent of a certain amount of water and ethanol (300g:750g), add 96g of allyl alcohol, then add 0.05ml of isopropylamine, and stir to dissolve. The temperature was raised to 40°C, and the reaction was stirred for 4.0h. After the reaction was completed, the temperature was lowered to 0° C., stirred and crystallized for 2 hours, and a white solid was obtained by suction filtration. Air-dried at 45°C to constant weight to obtain 144.6 g of white solid powder, which was analyzed by HPLC with a content of 99.2% and a residue of 0.01%. The yield is 97.7%.
Embodiment 3
[0024] At room temperature, dissolve 100g of L-cysteine in a mixed solvent of a certain amount of water and ethanol (300g:750g), add 96g of allyl alcohol, then add 0.05ml of triethylamine, and stir to dissolve. The temperature was raised to 40°C, and the reaction was stirred for 4.0h. After the reaction was completed, the temperature was lowered to 0° C., stirred and crystallized for 2 hours, and a white solid was obtained by suction filtration. Blast drying at 45°C to constant weight yielded 145.9 g of white solid powder with a content of 99.0%, and the residue was determined to be 0.01%. The yield is 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com