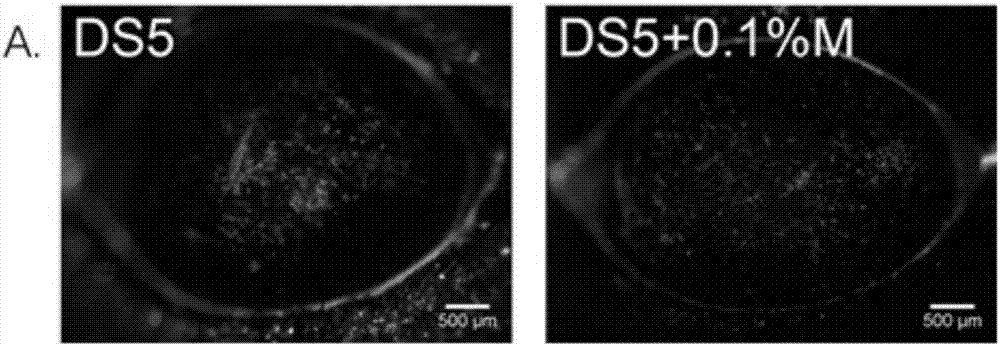
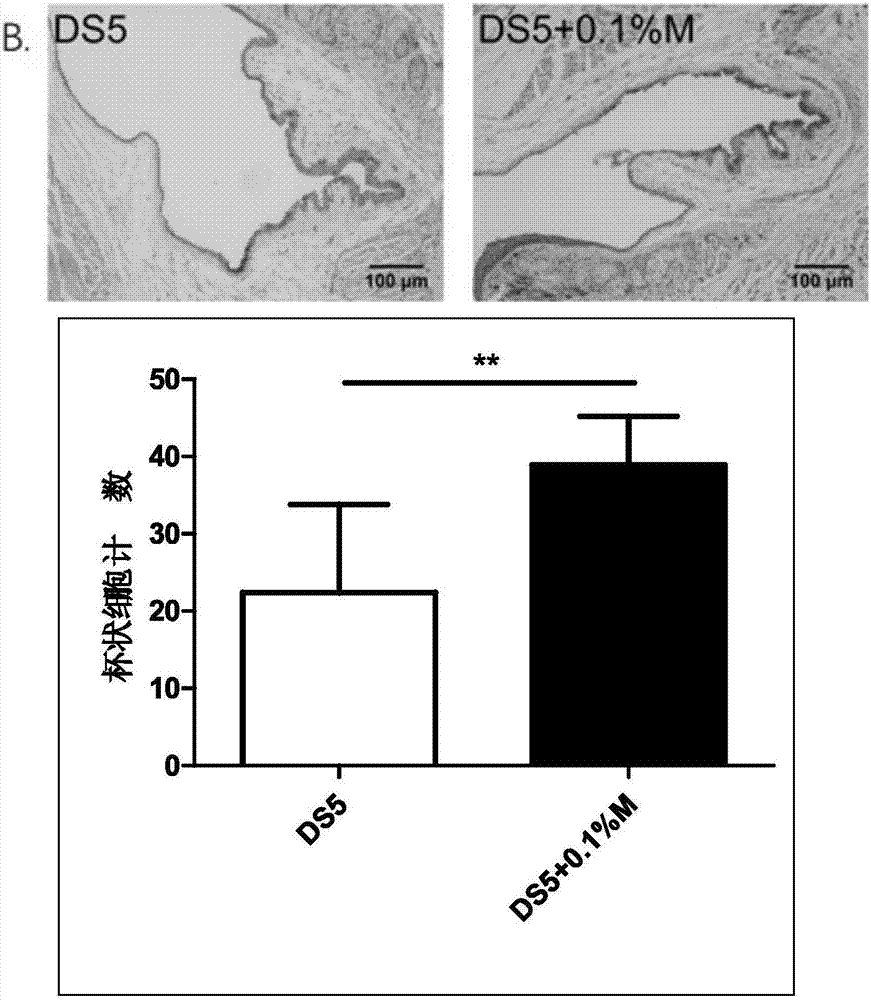
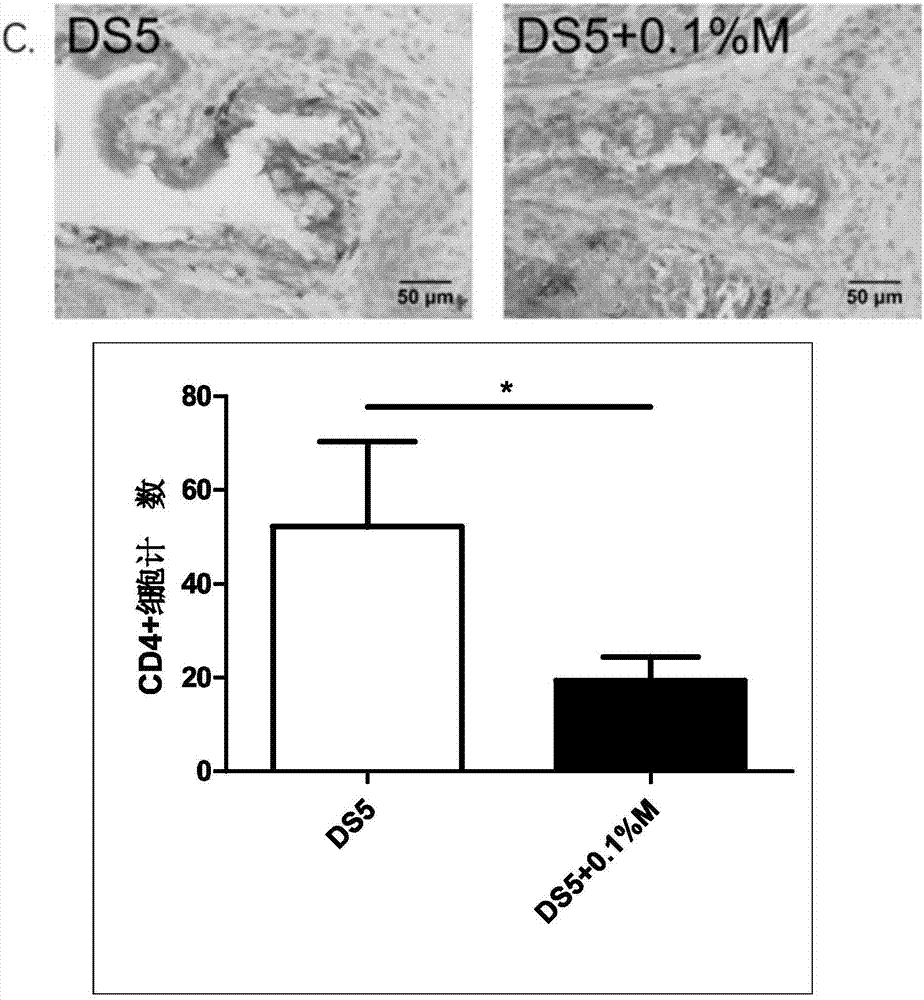
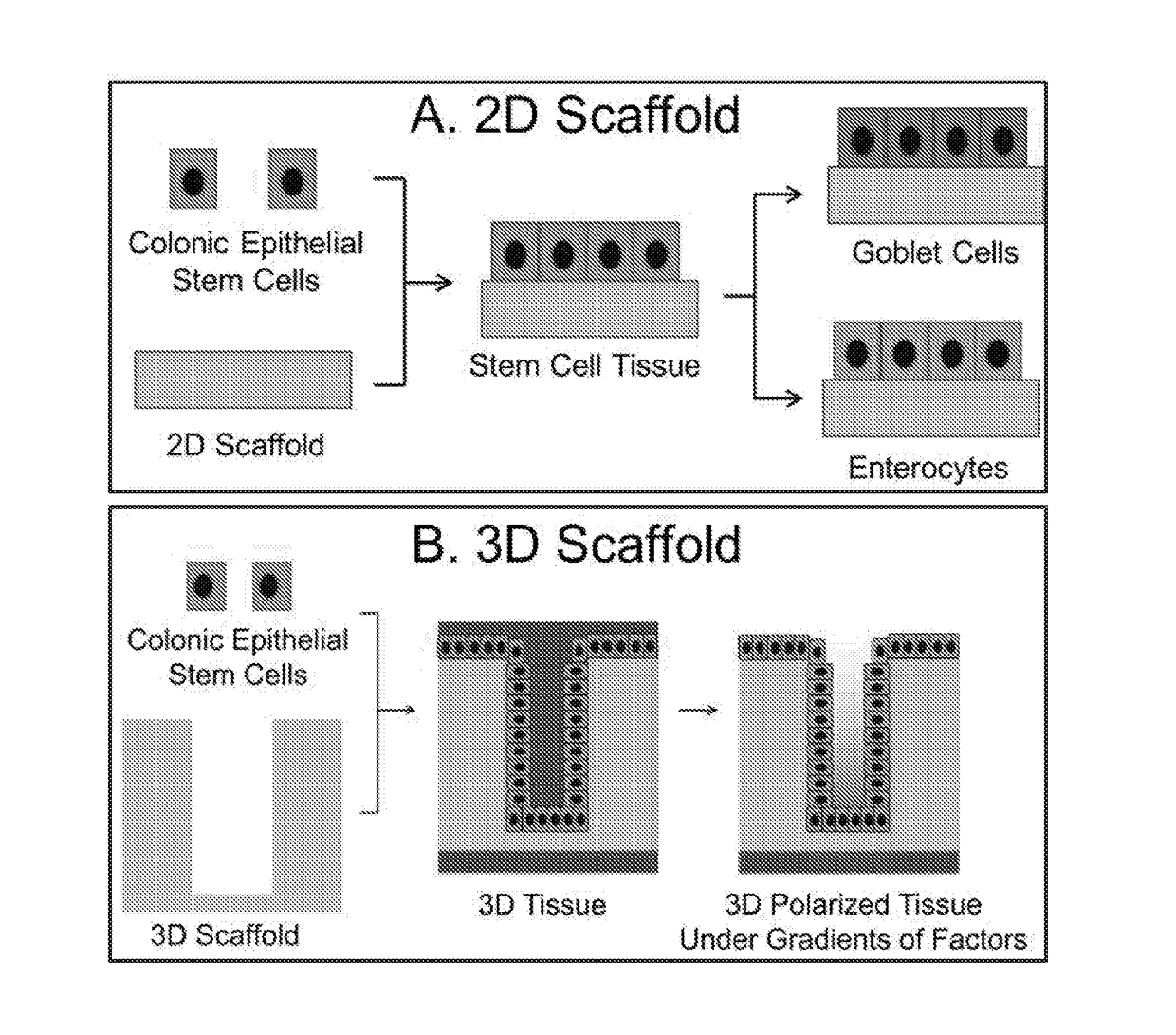
Patents
Literature
194 results about "Goblet cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
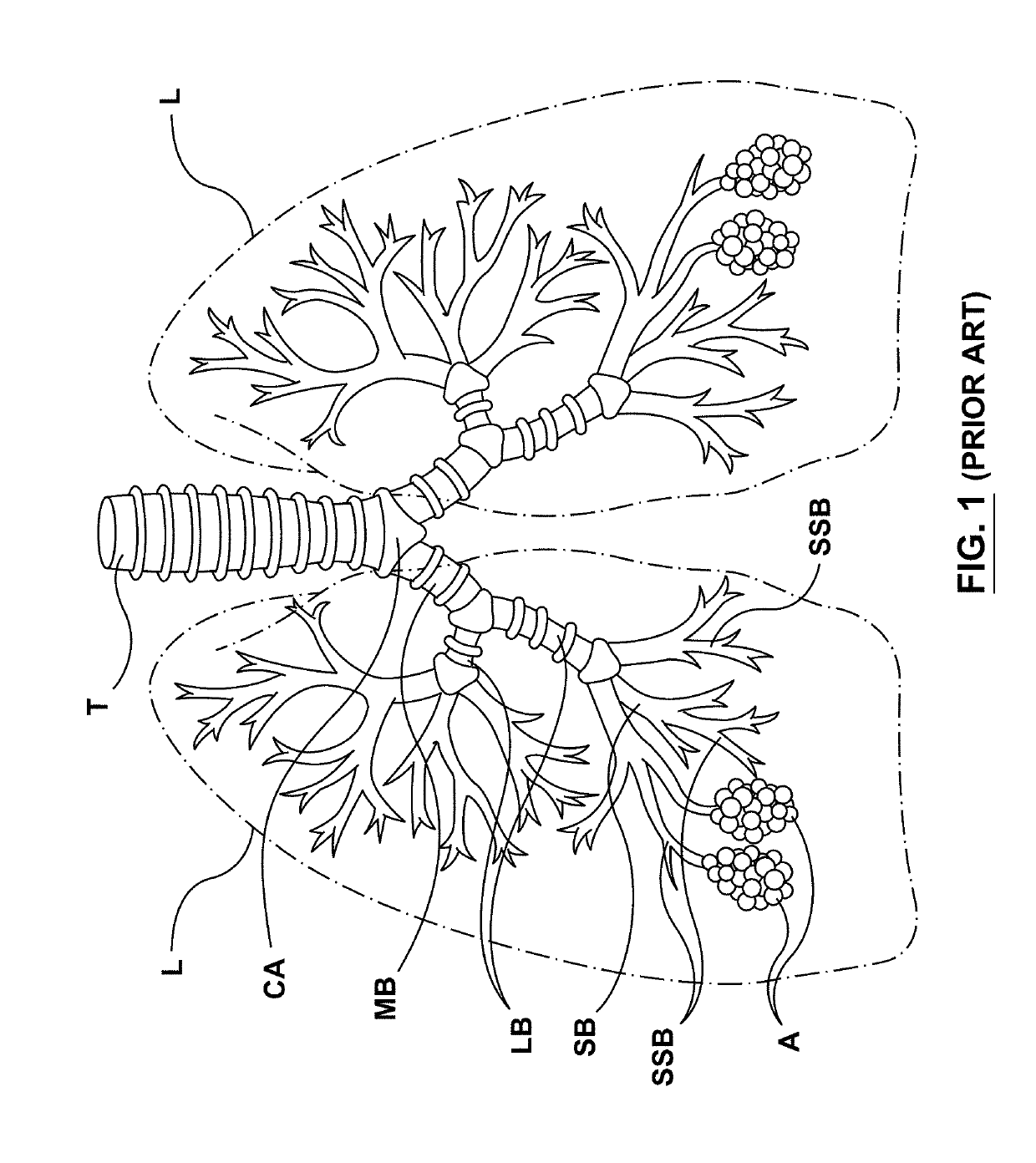
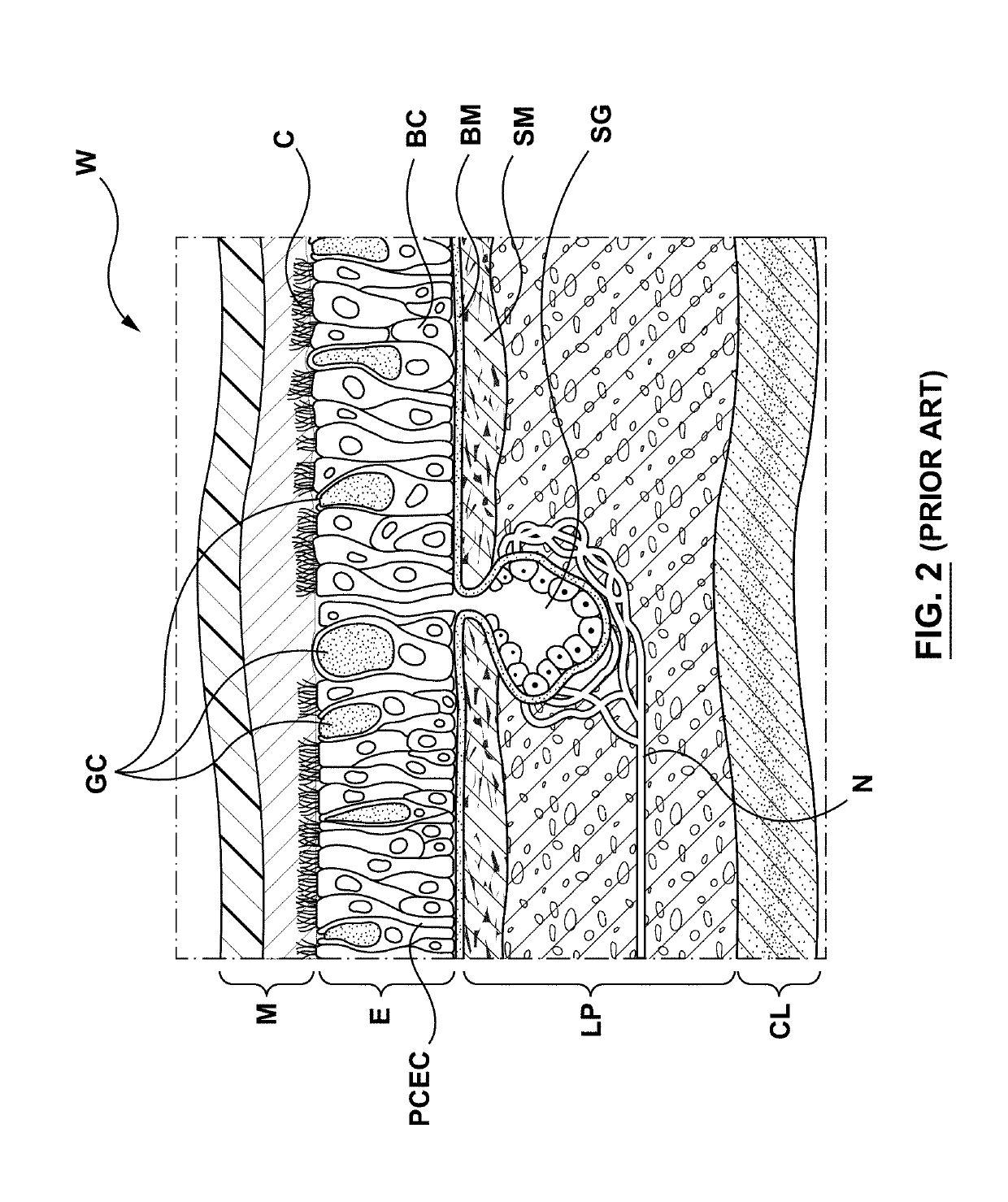
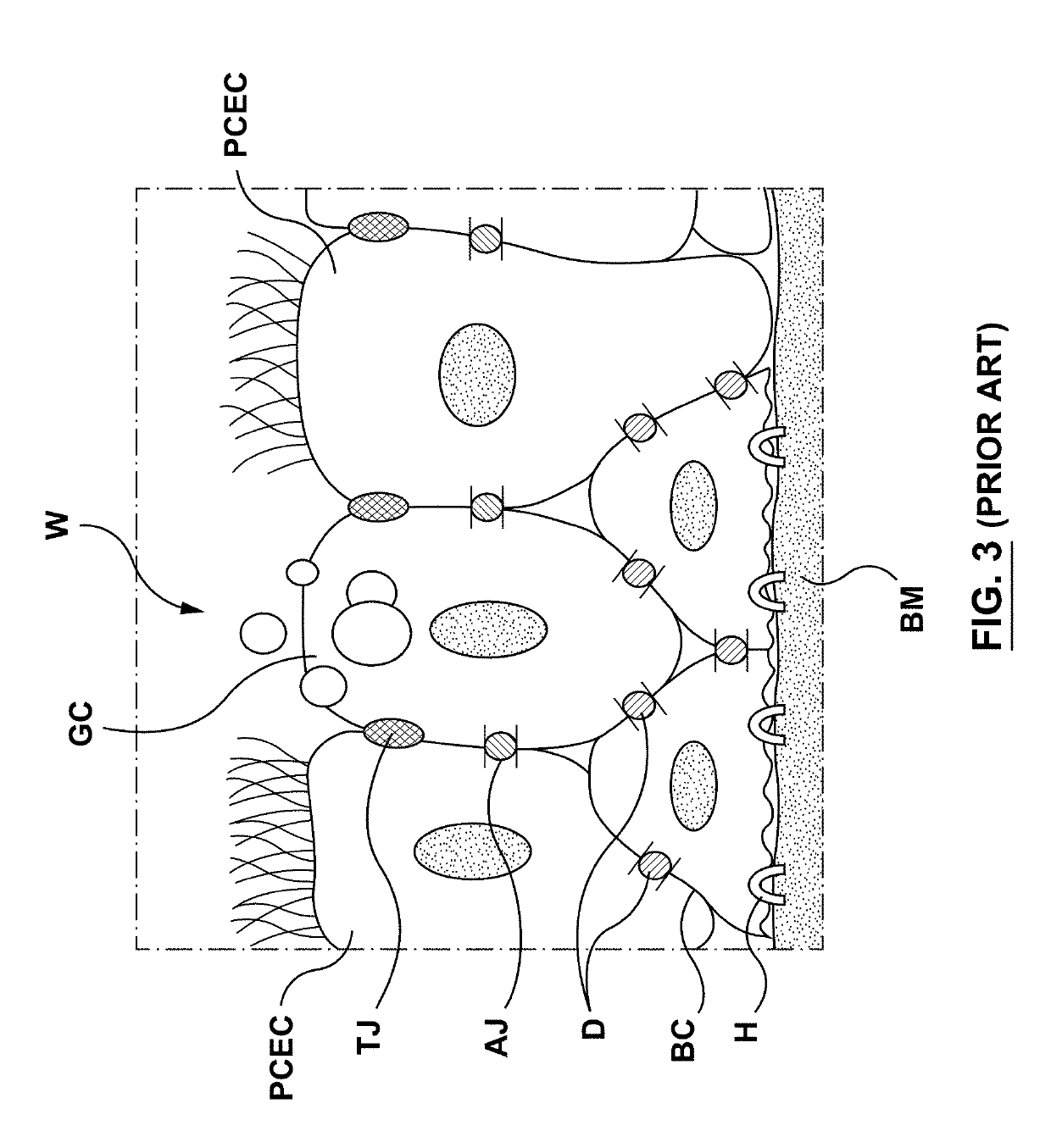
Goblet cells are simple columnar epithelial cells that secrete gel-forming mucins, like mucin MUC5AC. The goblet cells mainly use the merocrine method of secretion, secreting vesicles into a duct, but may use apocrine methods, budding off their secretions, when under stress. The term goblet refers to the cell's goblet-like shape. The apical portion is shaped like a cup, as it is distended by abundant mucus laden granules; its basal portion lacks these granules and is shaped like a stem.
Methods of testing for bronchial asthma or chronic obstructive pulmonary disease
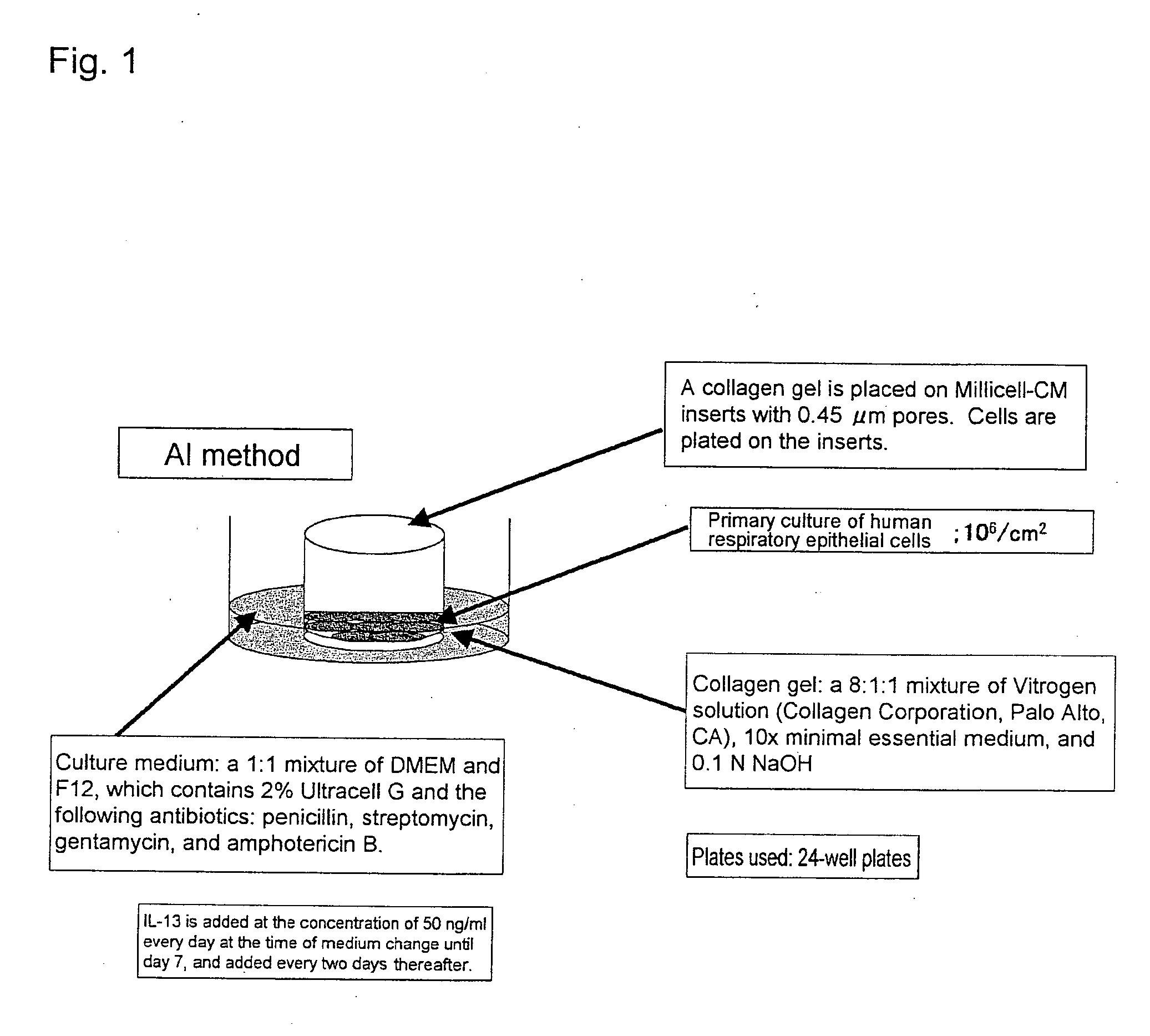
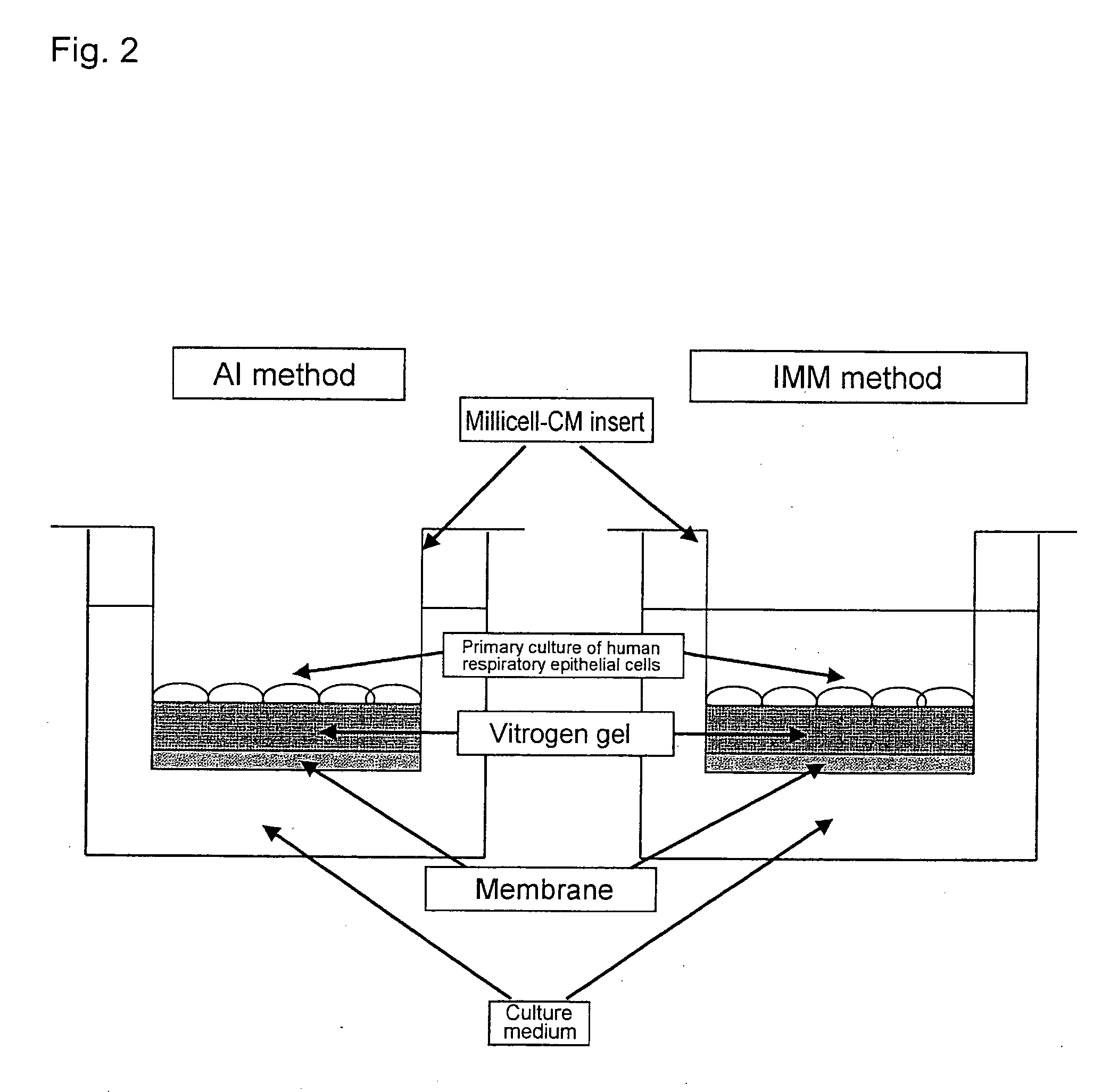
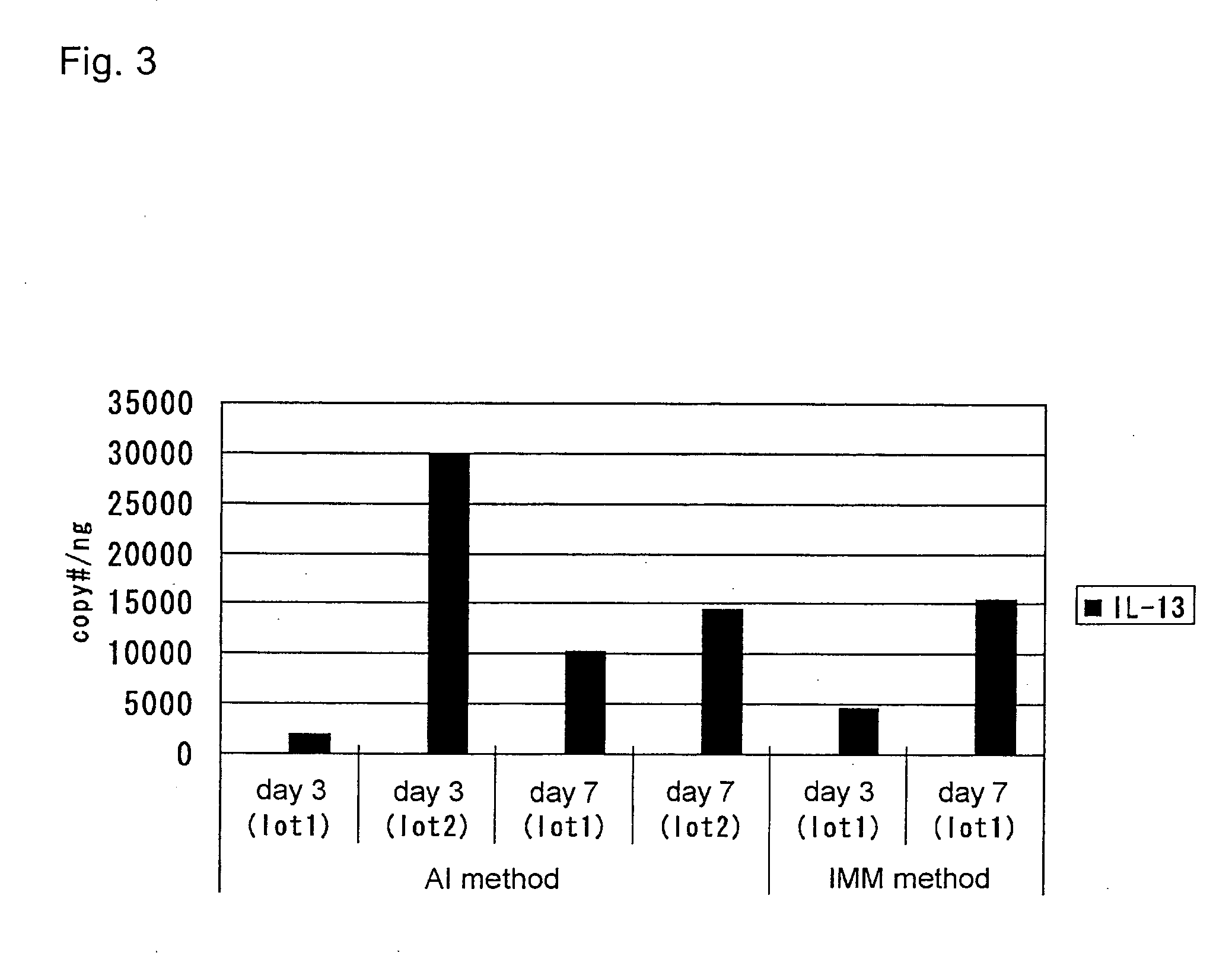
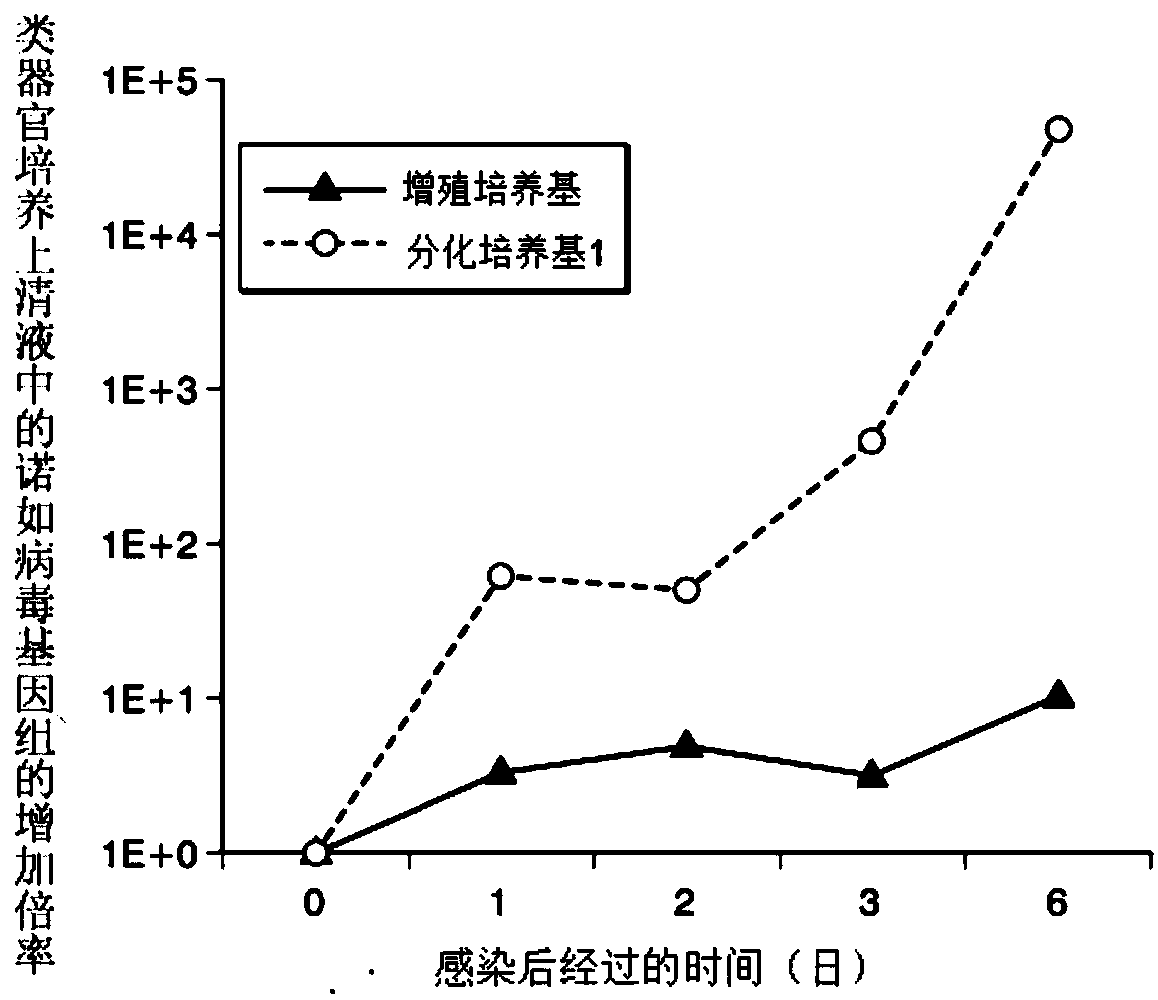
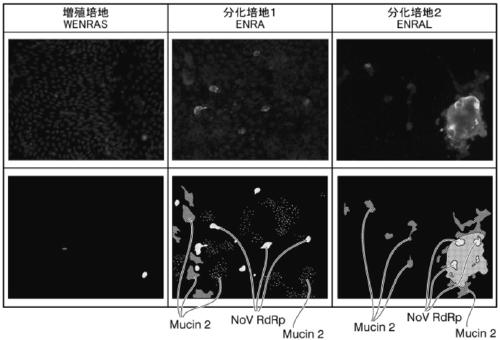
An objective of the present invention is to provide a method of testing for bronchial asthma or chronic obstructive pulmonary disease, a method of screening for candidate compounds for treating bronchial asthma or chronic obstructive pulmonary disease, and a pharmaceutical agent for treating bronchial asthma or chronic obstructive pulmonary disease. The present invention identified genes whose expression levels varied between respiratory epithelial cells that had been stimulated by IL-13 to induce the goblet cell differentiation, and unstimulated respiratory epithelial cells. The respiratory epithelial cells were cultured according to the air interface method. The genes were revealed to be useful as markers for testing for bronchial asthma or chronic obstructive pulmonary disease and screening for therapeutic agents for such diseases. Specifically, the present invention provides methods of testing for bronchial asthma or chronic obstructive pulmonary disease and methods of screening for compounds to treat the diseases based on the comparison of the expression levels of marker genes identified as described above.
Owner:GENOX RES
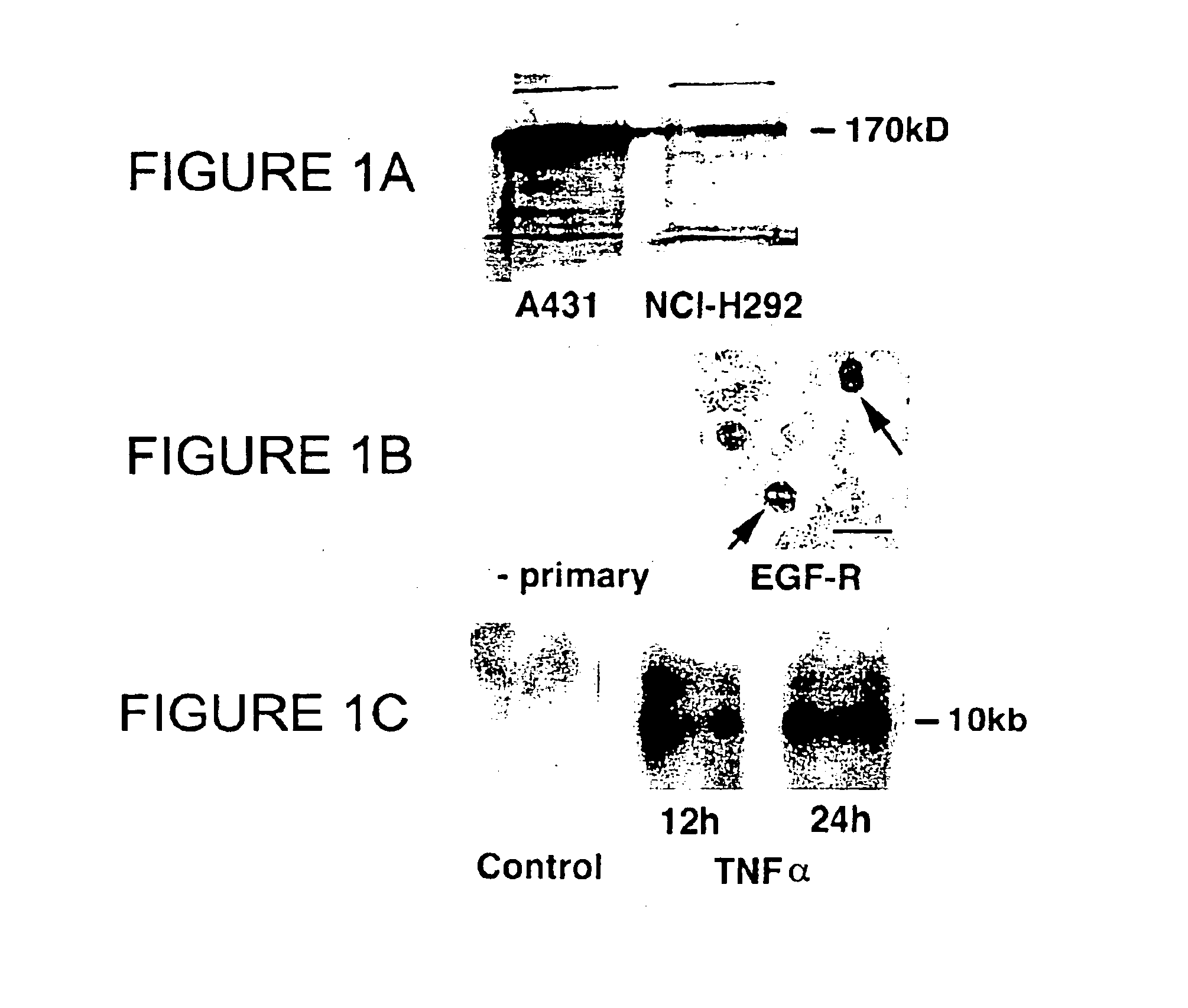
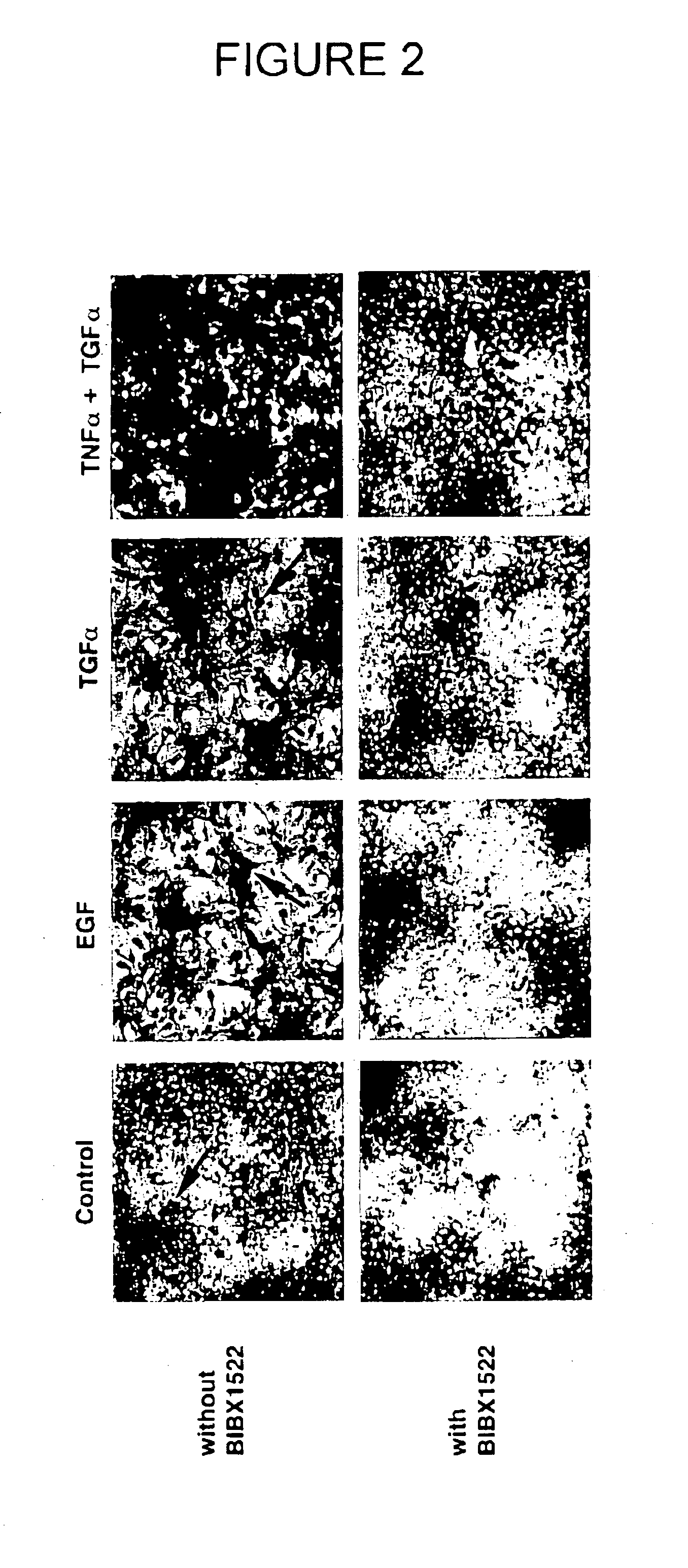
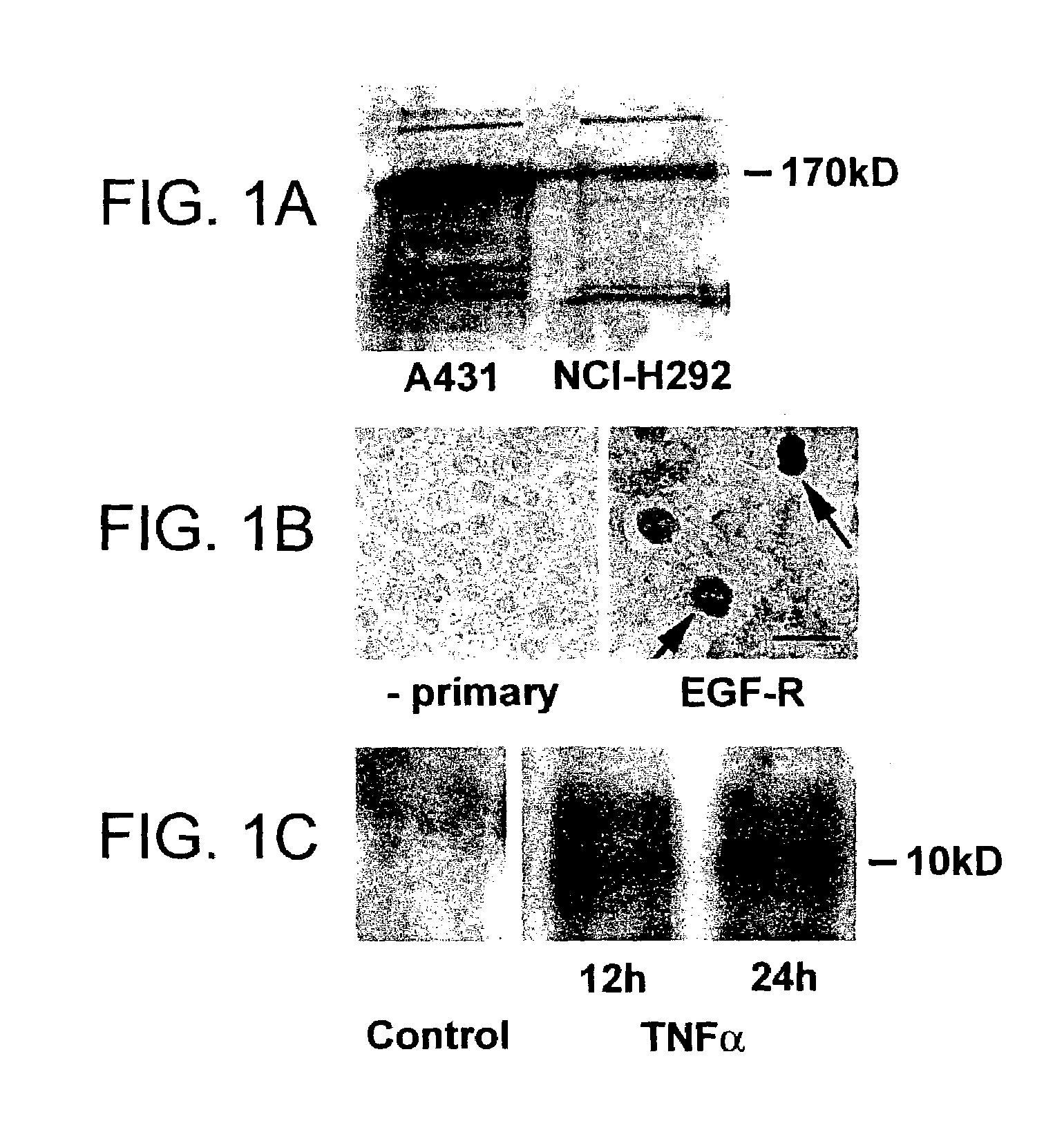
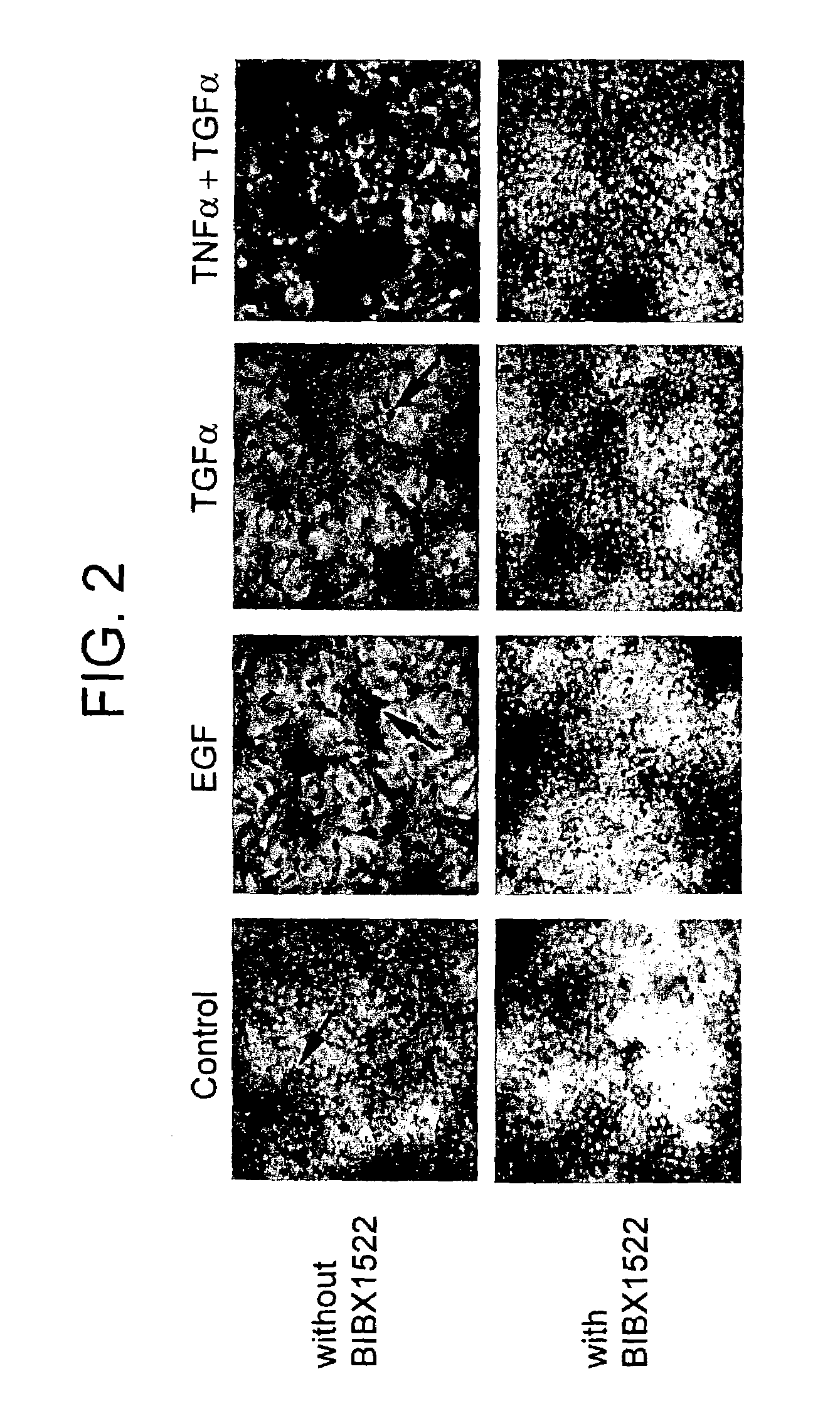
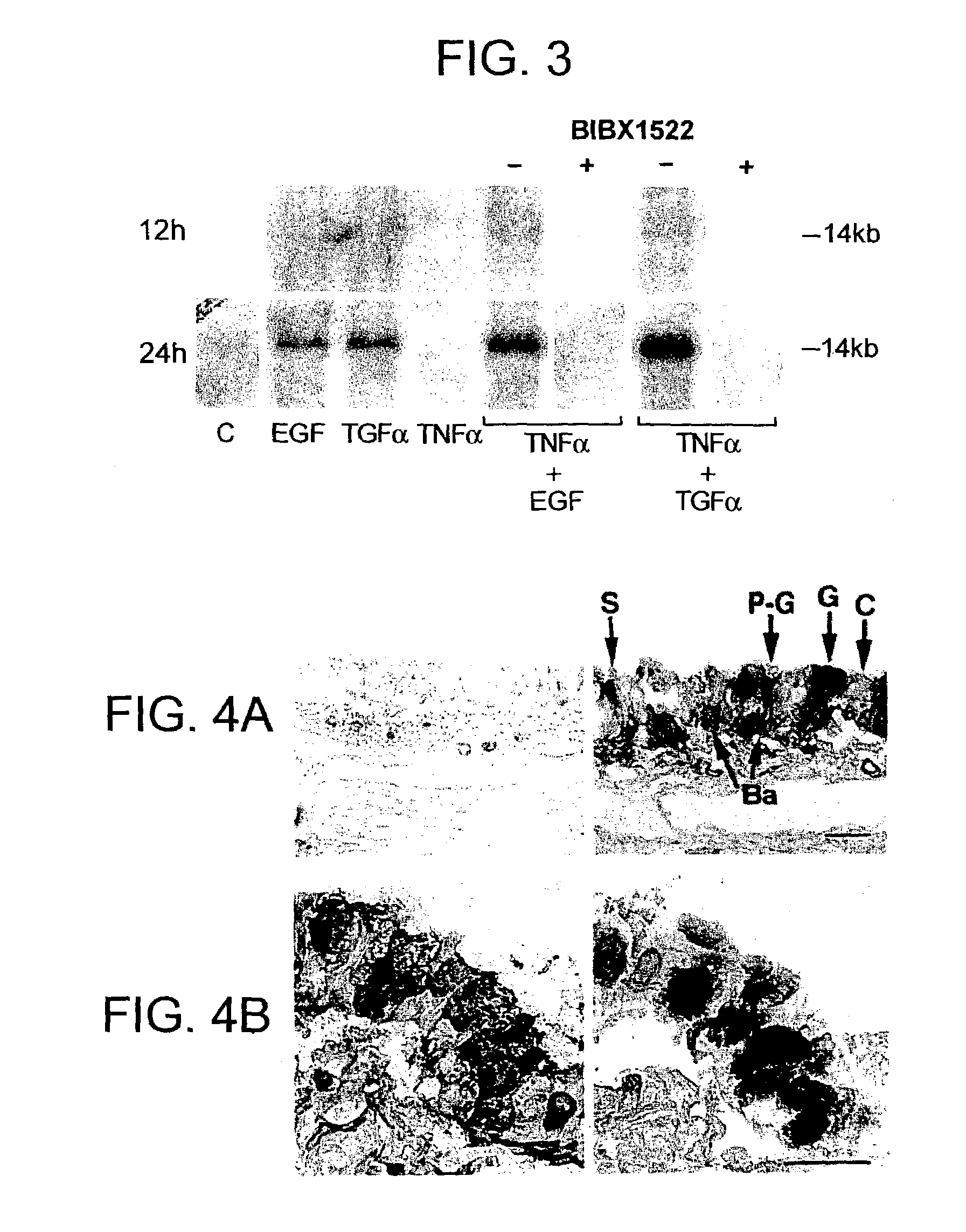
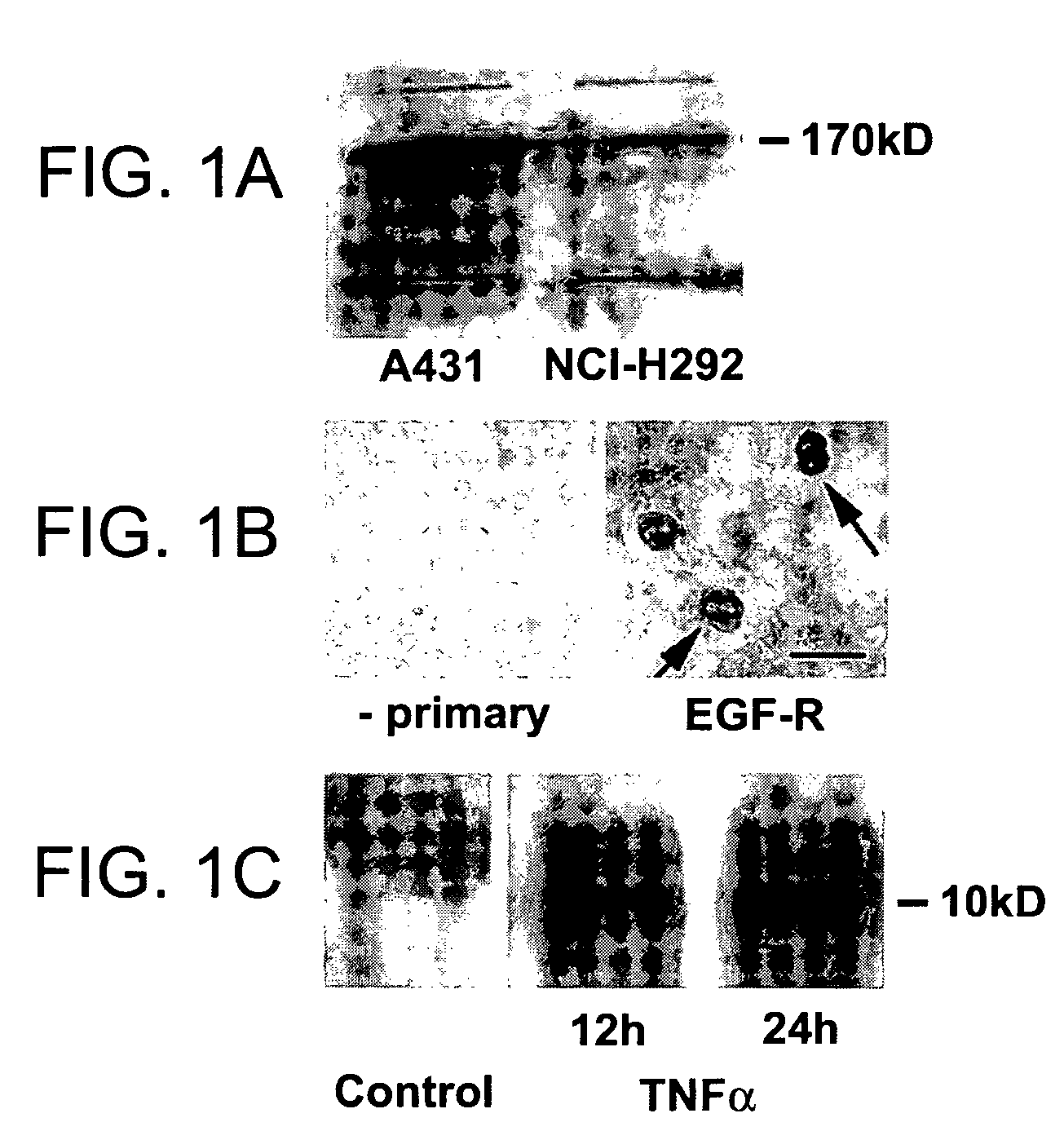
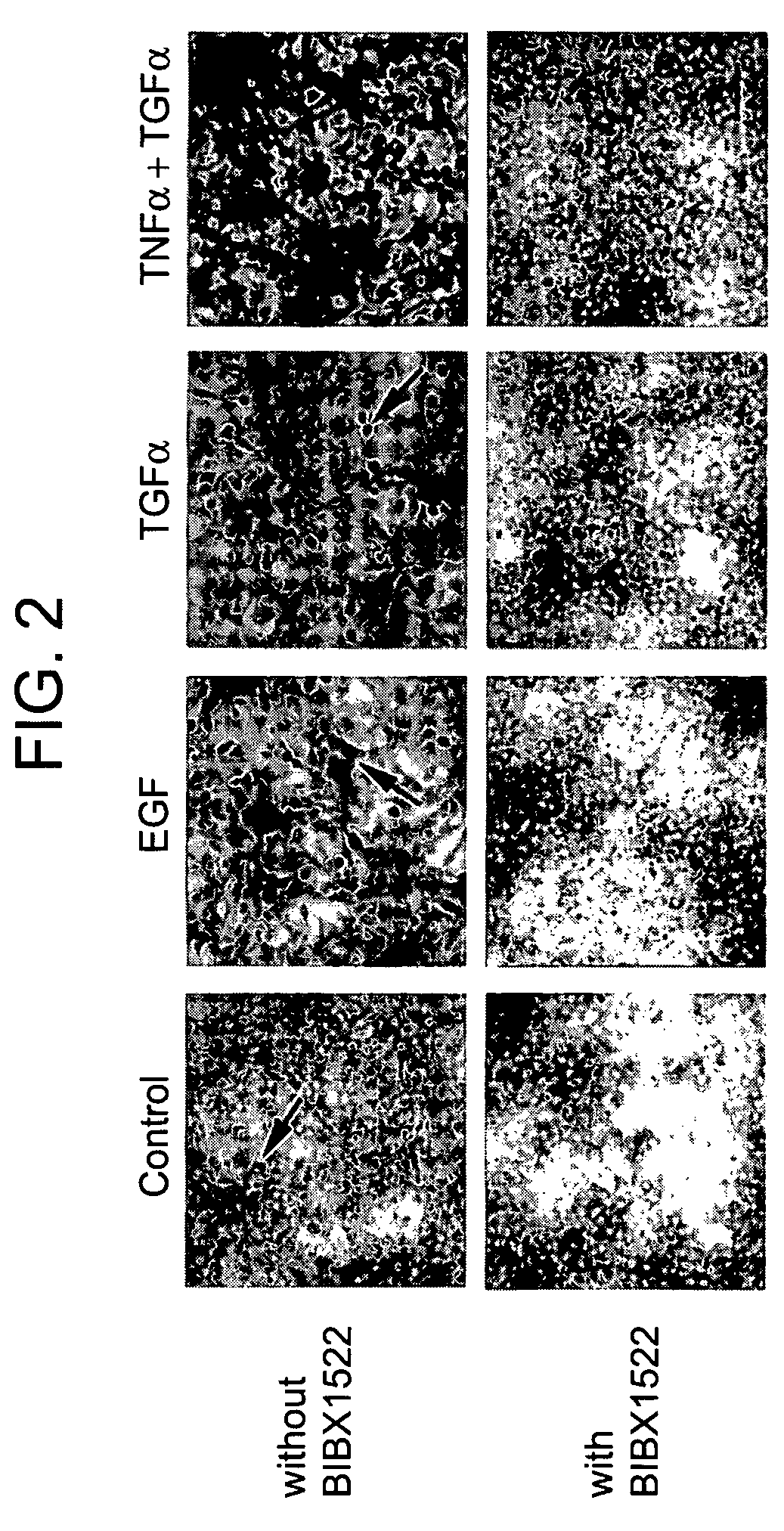
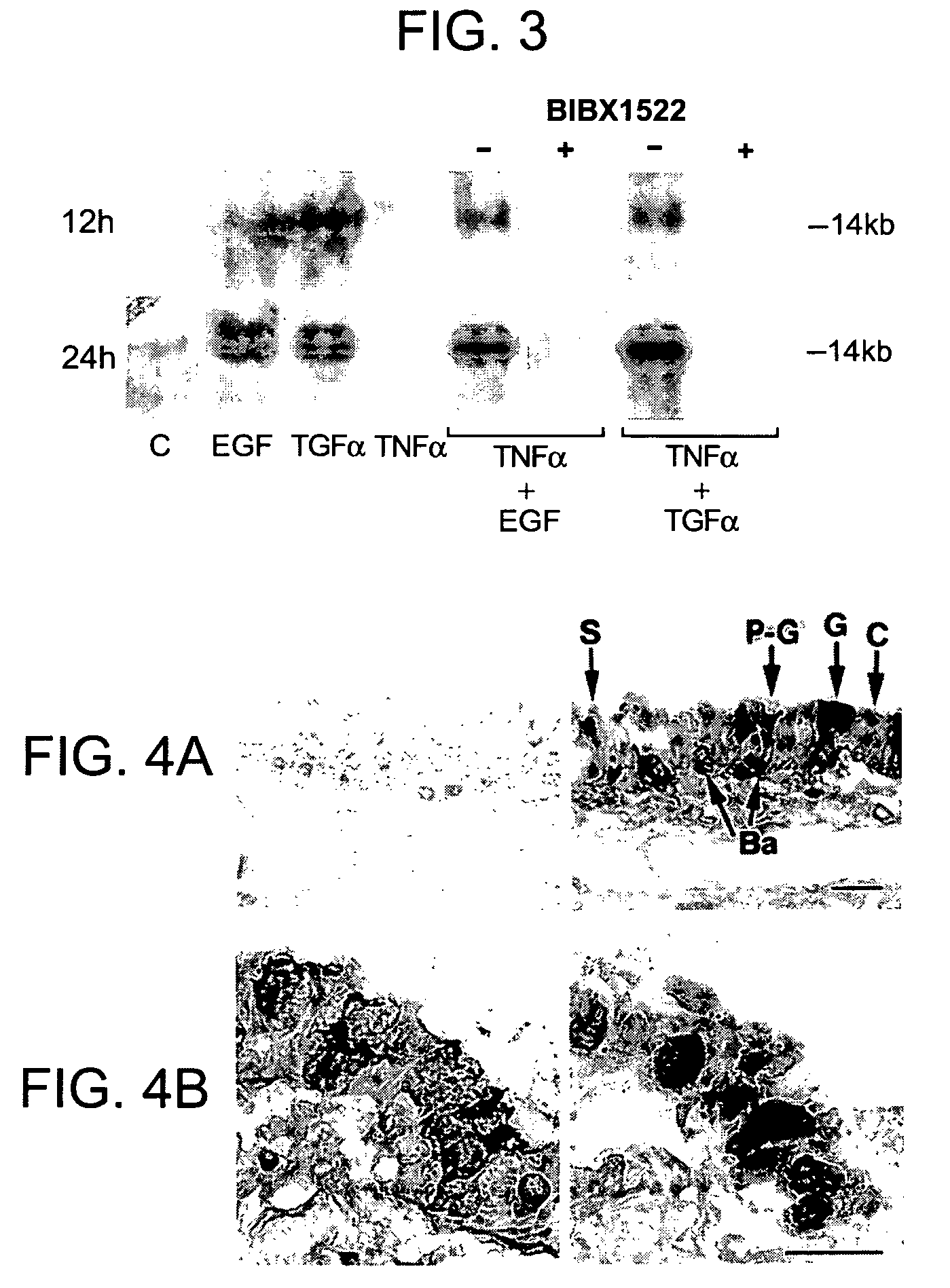
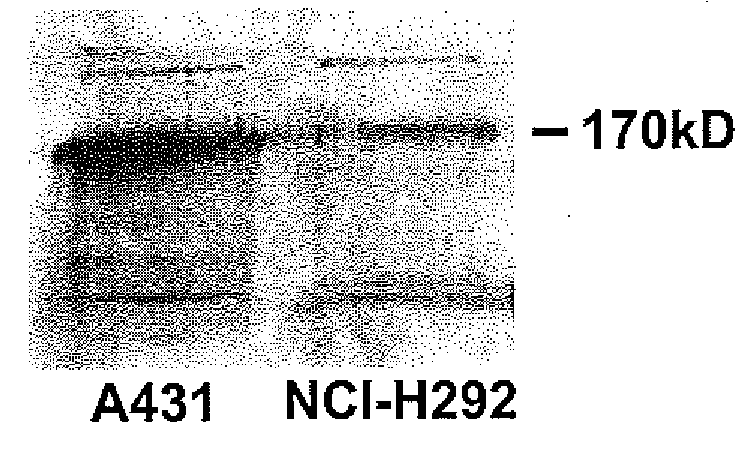
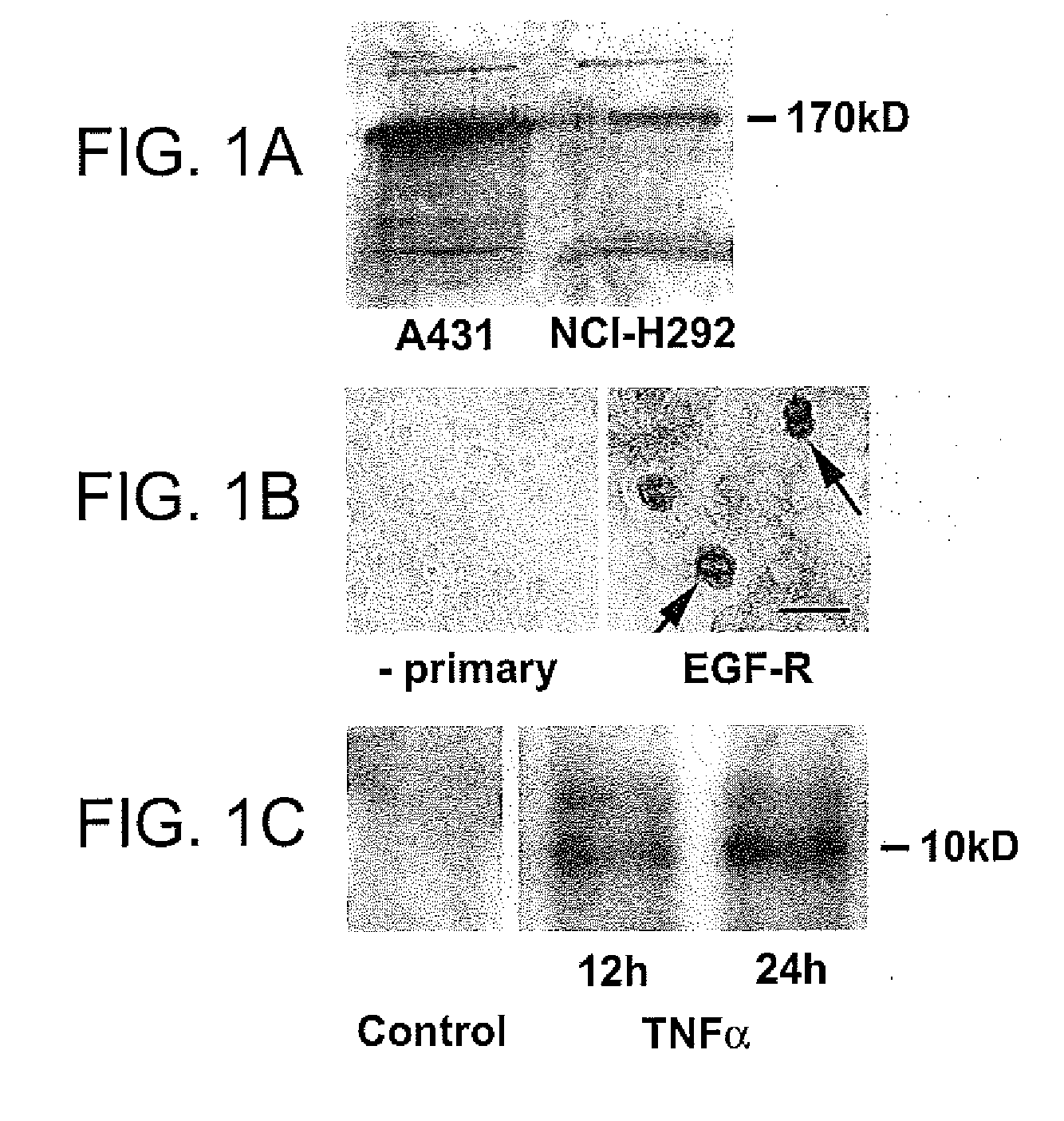
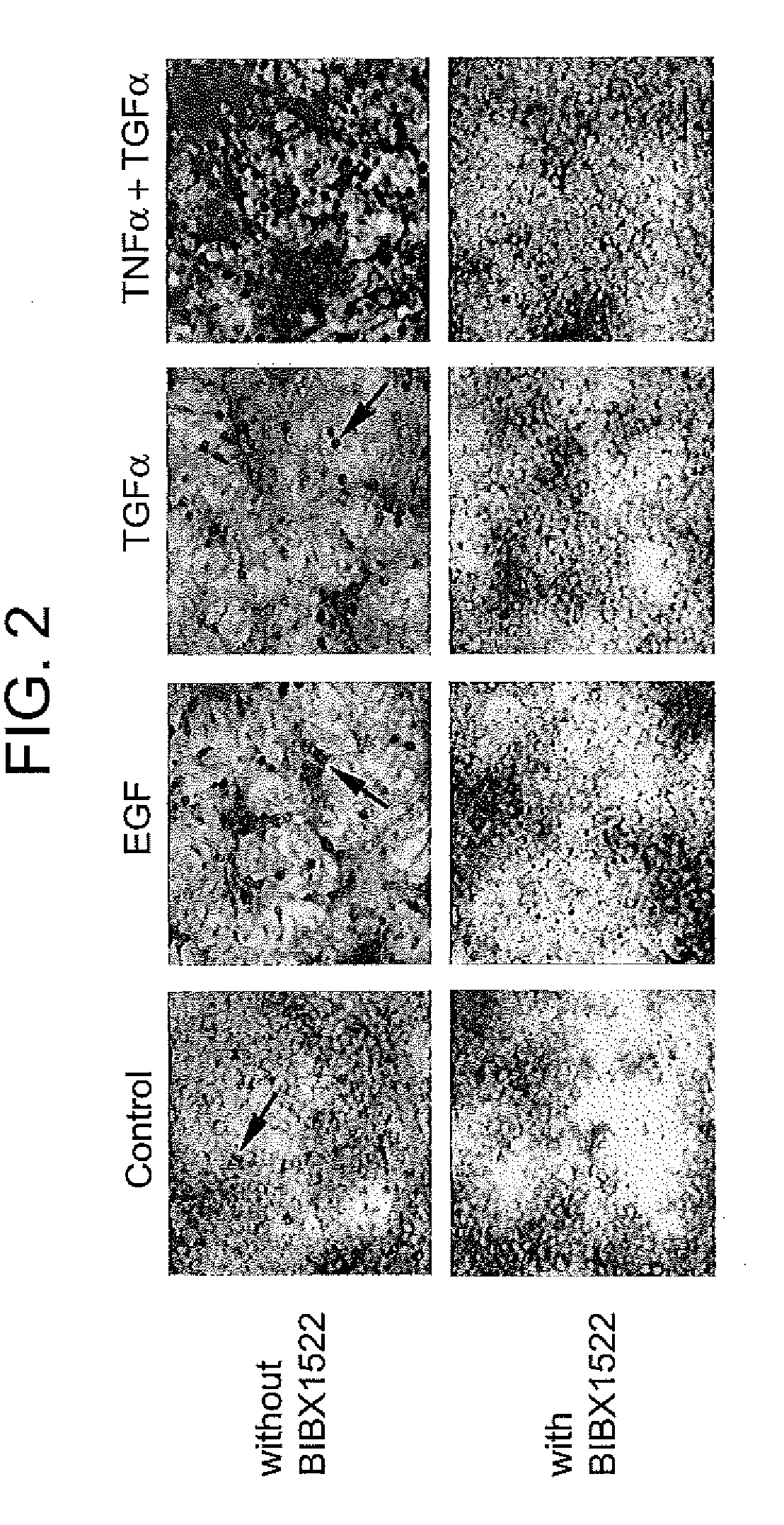
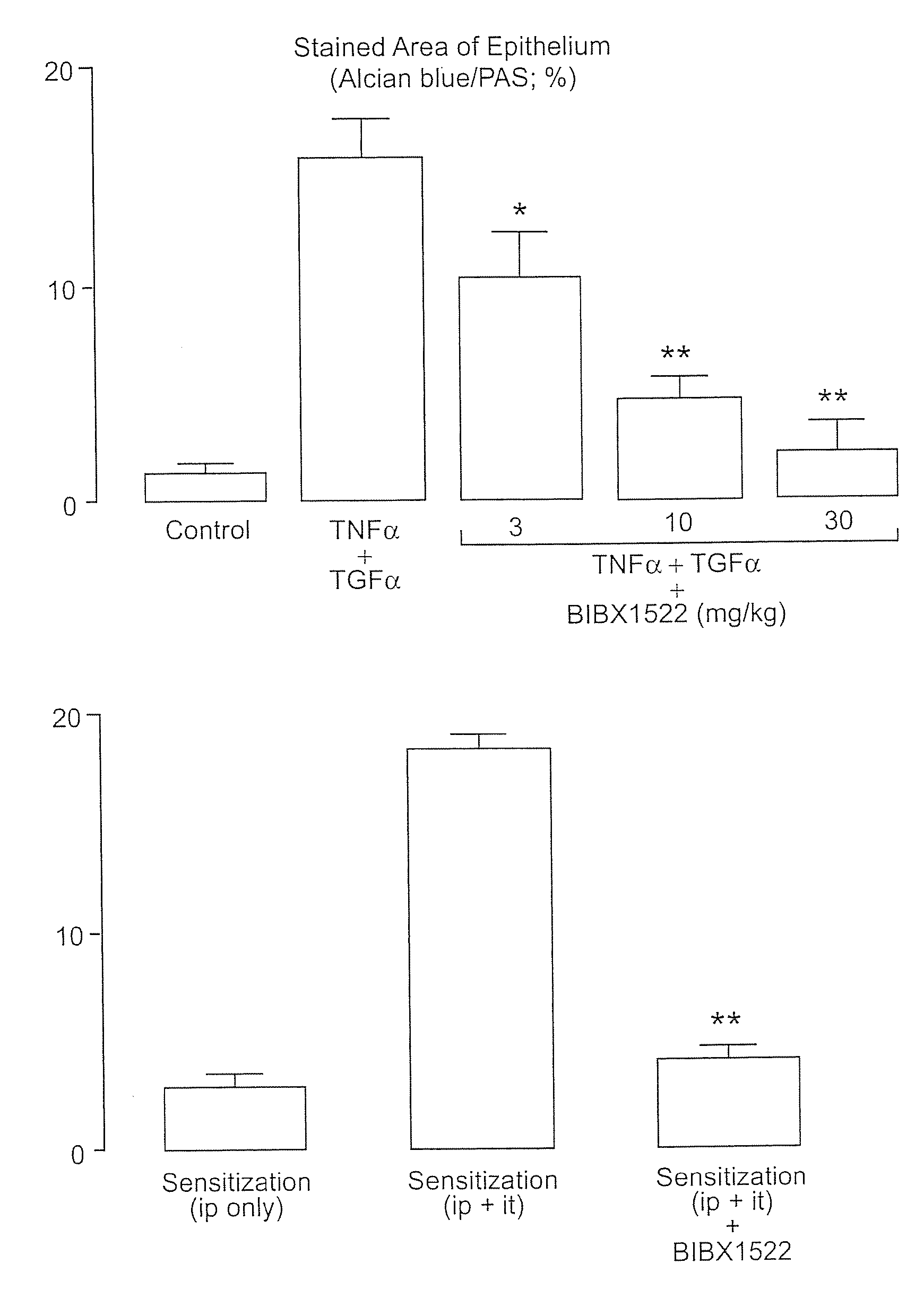
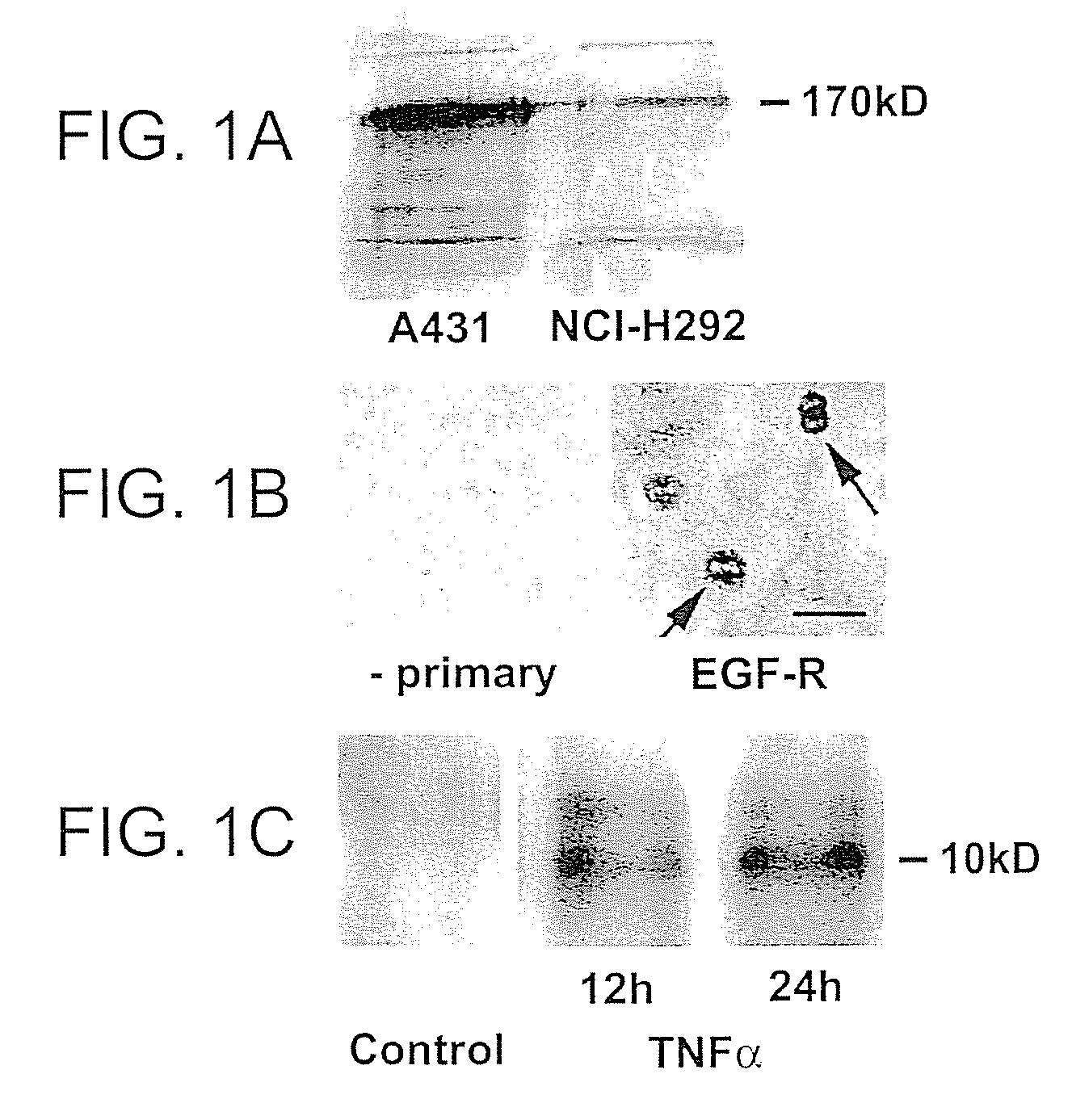
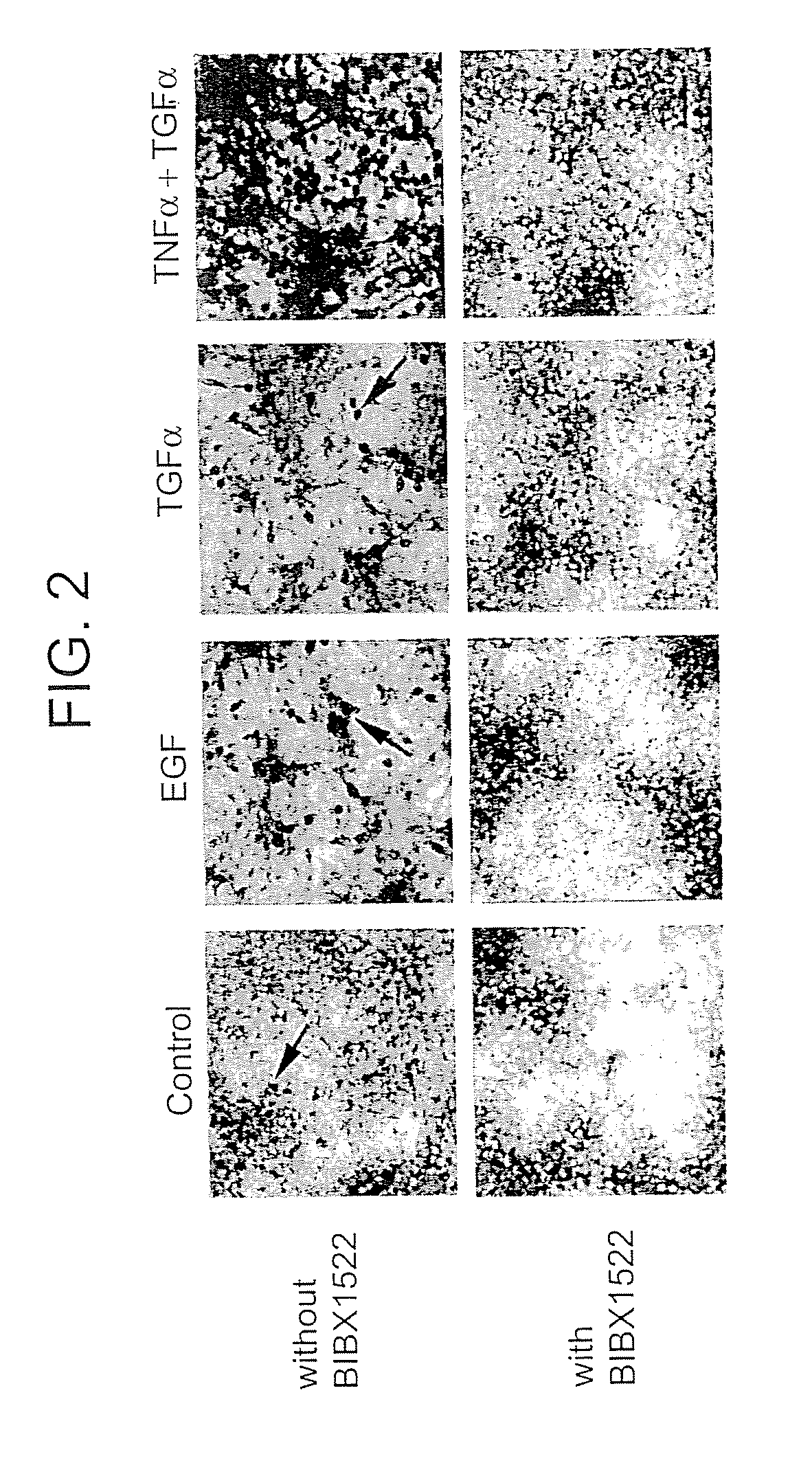
Preventing airway mucus production by administration of EGF-R antagonists
InactiveUS6846799B1Preventing excessive formationReduce formationOrganic active ingredientsBiocideGoblet cellMucus production
Hypersecretion of mucus in the lungs is inhibited by the administration of an epidermal growth factor receptor (EGF-R) antagonist. The EGF-R antagonist may be in the form of a small organic molecule, an antibody, or portion of an antibody that binds to and blocks the EGF receptor. The EGF-R antagonist is preferably administered by injection in an amount sufficient to inhibit formation of goblet cells in pulmonary airways. The degranulation of goblet cells that results in airway mucus production is thereby inhibited. Assays for screening candidate agents that inhibit goblet cell proliferation are also provided.
Owner:RGT UNIV OF CALIFORNIA
Preventing airway mucus production by administration of EGF-R antagonists
InactiveUS7358222B2Preventing excessive formationReduce formationOrganic active ingredientsPeptide/protein ingredientsGoblet cellMucus production
Hypersecretion of mucus in the lungs is inhibited by the administration of an epidermal growth factor receptor (EGF-R) antagonist. The EGF-R antagonist may be in the form of a small organic molecule, an antibody, or portion of an antibody that binds to and blocks the EGF receptor. The EGF-R antagonist is preferably administered by injection in an amount sufficient to inhibit formation of goblet cells in pulmonary airways. The degranulation of goblet cells that results in airway mucus production is thereby inhibited. Assays for screening candidate agents that inhibit goblet cell proliferation are also provided.
Owner:RGT UNIV OF CALIFORNIA
Methods, apparatuses, and systems for the treatment of pulmonary disorders
ActiveUS20190231425A1Reduce in quantityElectrotherapySurgical instruments for heatingDiseaseAcute bronchitis
Apparatuses, systems and methods are provided for treating pulmonary tissues via delivery of energy, generally characterized by high voltage pulses, to target tissue using a pulmonary tissue modification system (e.g., an energy delivery catheter system). Example pulmonary tissues include, without limitation, the epithelium (the goblet cells, ciliated pseudostratified columnar epithelial cells, and basal cells), lamina propria, submucosa, submucosal glands, basement membrane, smooth muscle, cartilage, nerves, pathogens resident near or within the tissue, or a combination of any of these. The system may be used to treat a variety of pulmonary diseases or disorders such as or associated with COPD (e.g., chronic bronchitis, emphysema), asthma, interstitial pulmonary fibrosis, cystic fibrosis, bronchiectasis, primary ciliary dyskinesia (PCD), acute bronchitis and / or other pulmonary diseases or disorders.
Owner:GALVANIZE THERAPEUTICS INC
Preventing airway mucus production by administration of EGF-R antagonists
InactiveUS7354894B2Preventing excessive formationReduce formationOrganic active ingredientsBiocideGoblet cellMucus production
Hypersecretion of mucus in the lungs is inhibited by the administration of an epidermal growth factor receptor (EGF-R) antagonist. The EGF-R antagonist may be in the form of a small organic molecule, an antibody, or portion of an antibody that binds to and blocks the EGF receptor. The EGF-R antagonist is preferably administered by injection in an amount sufficient to inhibit formation of goblet cells in pulmonary airways. The degranulation of goblet cells that results in airway mucus production is thereby inhibited. Assays for screening candidate agents that inhibit goblet cell proliferation are also provided.
Owner:RGT UNIV OF CALIFORNIA
Preventing airway mucus production by administration of egf-r antagonists
InactiveUS20080175797A1Preventing excessive formationReduce formationBiocideOrganic active ingredientsGoblet cellMucus production
Owner:RGT UNIV OF CALIFORNIA
Preventing airway mucus production by administration of EGF-r antagonists
InactiveUS20070270330A1Preventing excessive formationReduce formationPowder deliveryOrganic active ingredientsGoblet cellMucus production
Hypersecretion of mucus in the lungs is inhibited by the administration of an epidermal growth factor receptor (EGF-R) antagonist. The EGF-R antagonist may be in the form of a small organic molecule, an antibody, or portion of an antibody that binds to and blocks the EGF receptor. The ESGF-R antagonist is preferably administered by injection in an amount sufficient to inhibit formation of goblet cells in pulmonary airways. The degranulation of goblet cells that results in airway mucus production is thereby inhibited. Assays for screening candidate agents that inhibit goblet cell proliferation are also provided.
Owner:RGT UNIV OF CALIFORNIA
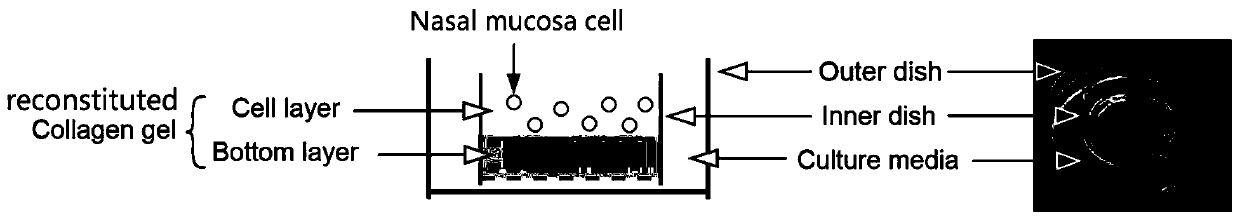
Nasal mucosa organoid culture medium and culture method
ActiveCN111117946AProliferateCapable of multi-lineage differentiationCulture processEpidermal cells/skin cellsEpitheliumGoblet cell
The invention discloses a culture medium and culture method of a nasal mucosa tissue. According to the culture medium and culture method of the nasal mucosa tissue, epithelial-mesenchymal / matrix components are integrated in an organoid culture system based on a gas-liquid interface method for the first time, and by inducing proliferation and differentiation of adult stem cells in a fresh nasal mucosa epithelial tissue, an organoid which is composed of multiple kinds of cells, including ciliated cells, goblet cells, club cells and basal cells, and is close to an internal mucosa in structure andfunction can be obtained, and becomes an in-vitro nasal mucosa model with proliferation and multi-lineage differentiation capability.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Ophthalmologic external preparation, as well as preparation method and application thereof
InactiveCN103040888APlay a lubricating roleInhibit apoptosisSenses disorderHydroxy compound active ingredientsExperimental researchGoblet cell
The invention discloses an ophthalmologic external preparation, as well as a preparation method and an application thereof, belongs to the technical field of pharmaceuticals, and relates to a pharmaceutical active substance-ginsenoside which is prepared to form eye drops, oculentum, ophthalmologic gel, ophthalmologic external hydropathic compressing tissues and ophthalmologic external atomized liquid for treating dry eyes. The addition of the ginsenoside is 0.1-5.0 wt%. The ophthalmologic external preparation also is added with one or a plurality of pharmaceutical active substances which are selected from flos lonicerae, radix arnebiae, herba taraxaci, chrysanthemum and borneol. The pharmacodynamical and experimental research of the preparation shows that the ginsenoside has a function of increasing conjunctival goblet cells and effectively treating the dry eyes; and the preparation is safe and effective, is better than conventional single-thickening agent artificial tears and has significant popularization and application values.
Owner:段亚东
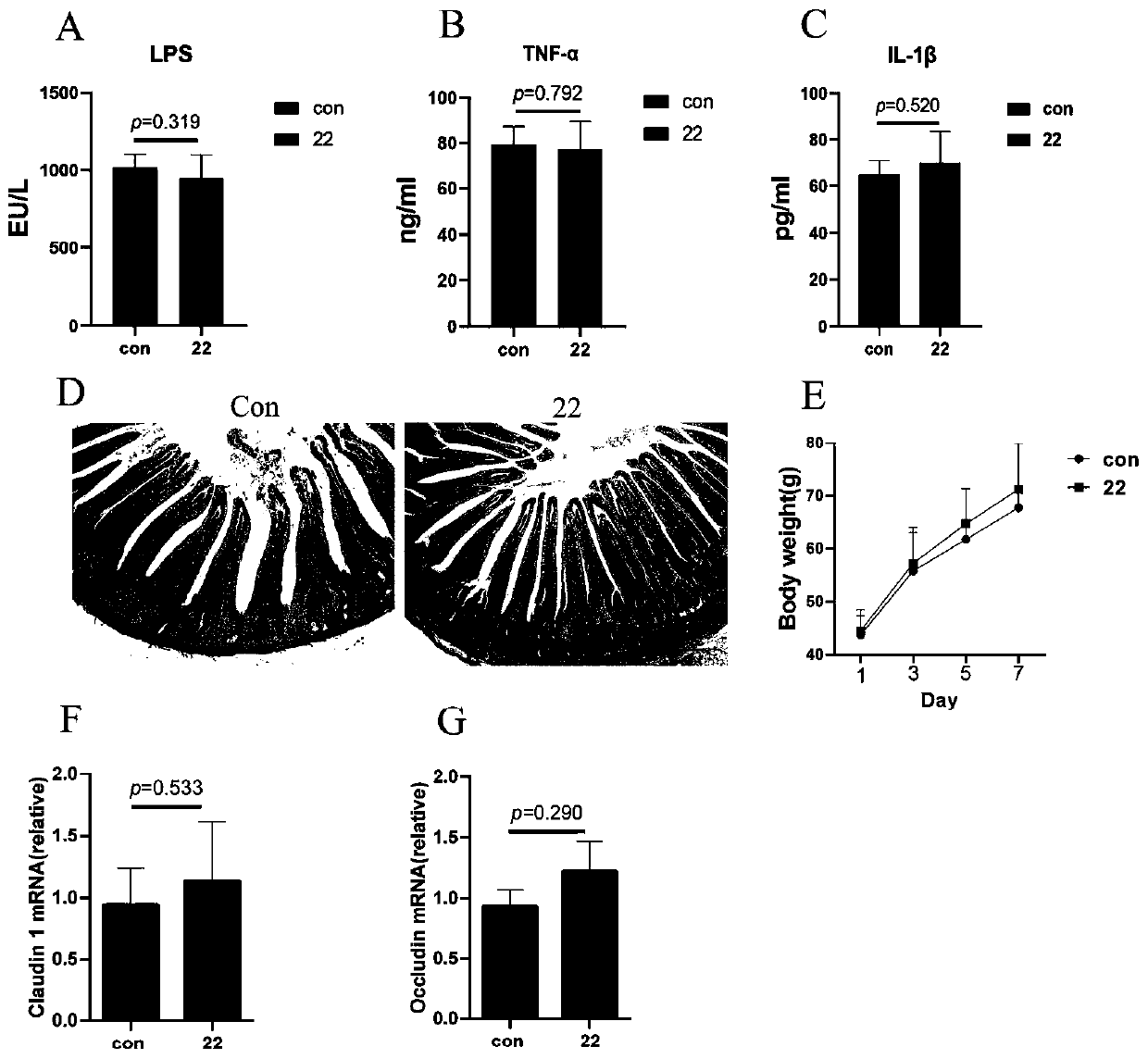
Lactobacillus reuteri 22 and application thereof
ActiveCN110540950AGrowth inhibitionImprove adhesionBacteriaMicroorganism based processesInflammatory factorsBiotechnology
The invention discloses a lactobacillus reuteri 22 and application thereof. The lactobacillus reuteri 22 disclosed by the invention is separated from healthy chicken flocks, is classified and named aslactobacillus reuteri, and has a preservation number of CGMCC No.17932. By detecting the performance of probiotics, the invention finds that the lactobacillus reuteri 22 has good acid resistance, cholate resistance and adhesion performance, and can inhibit growth of pathogenic bacteria. Through Animal assays, in a healthy physiological state, the lactobacillus reuteri 22 does not influence the level of inflammatory factors, but can increase the number of goblet cells and promote the increase of expression levels of related genes such as compact protein, defensin and lysozyme, can increase thelength of intestinal villi and the depth of crypt, and has the potential of maintaining intestinal mucosa barriers. Therefore, the strain is expected to be developed into probiotics for maintaining achicken intestinal mucosa barrier and guaranteeing green and healthy chicken breeding.
Owner:NANJING AGRICULTURAL UNIVERSITY
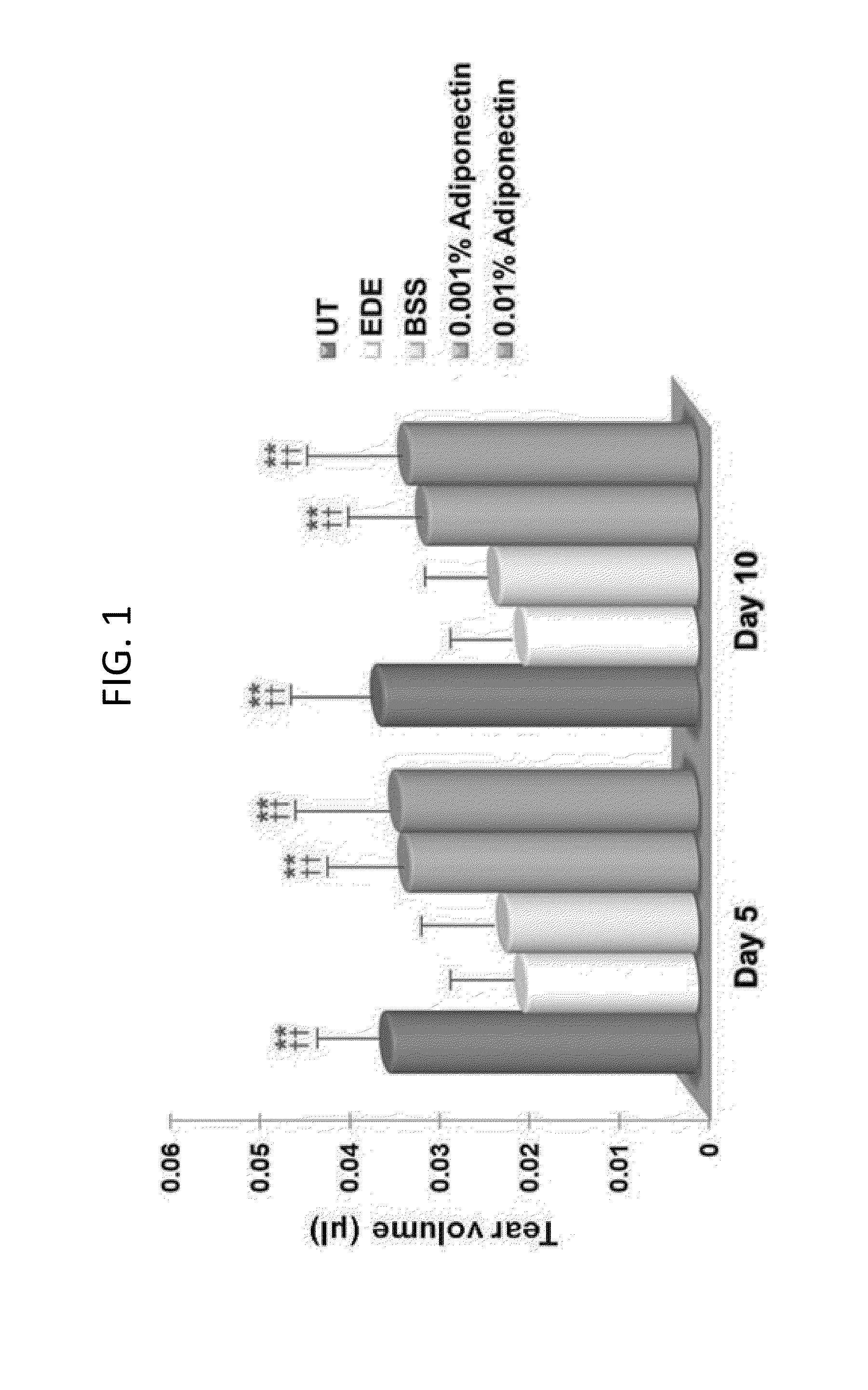
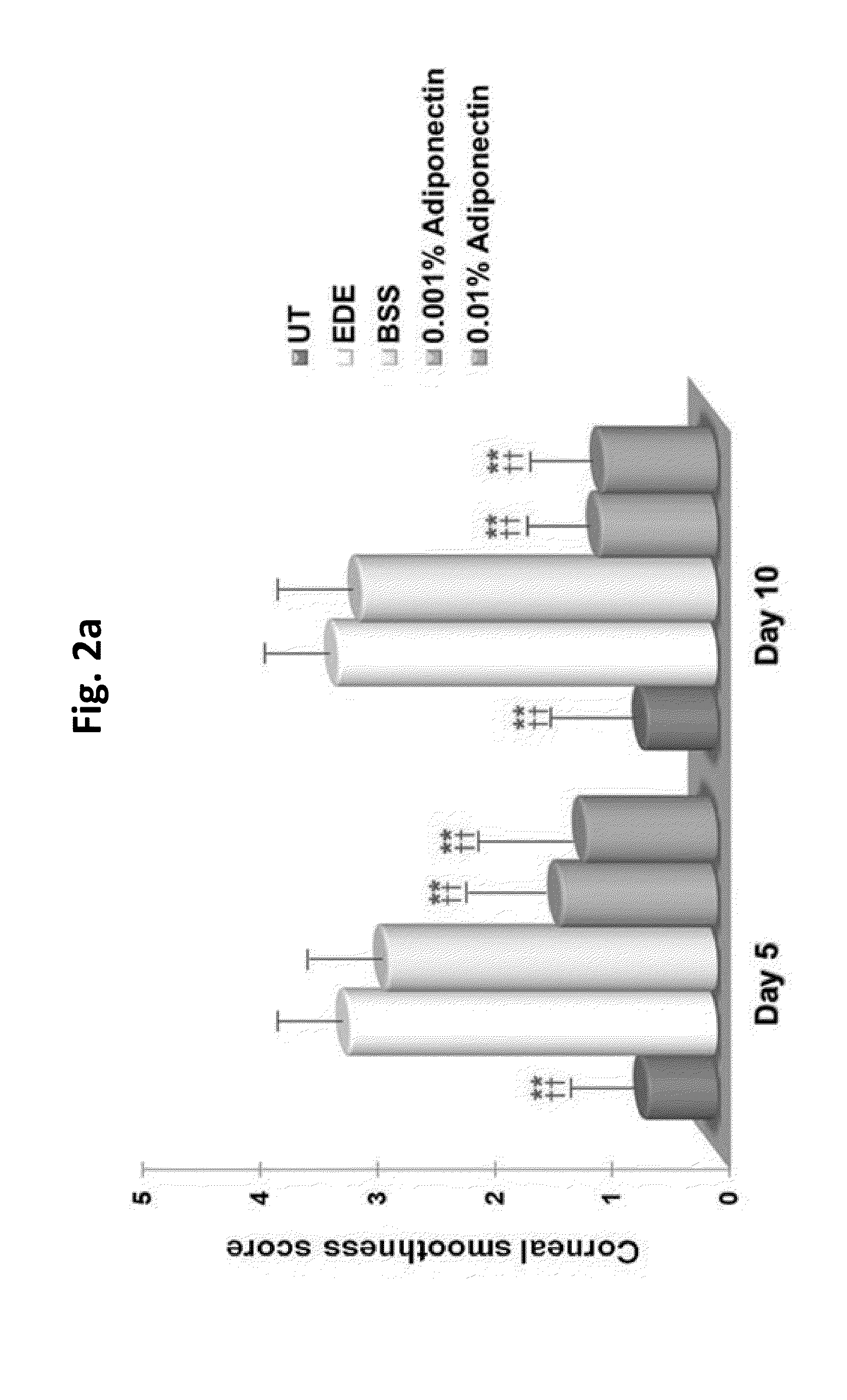
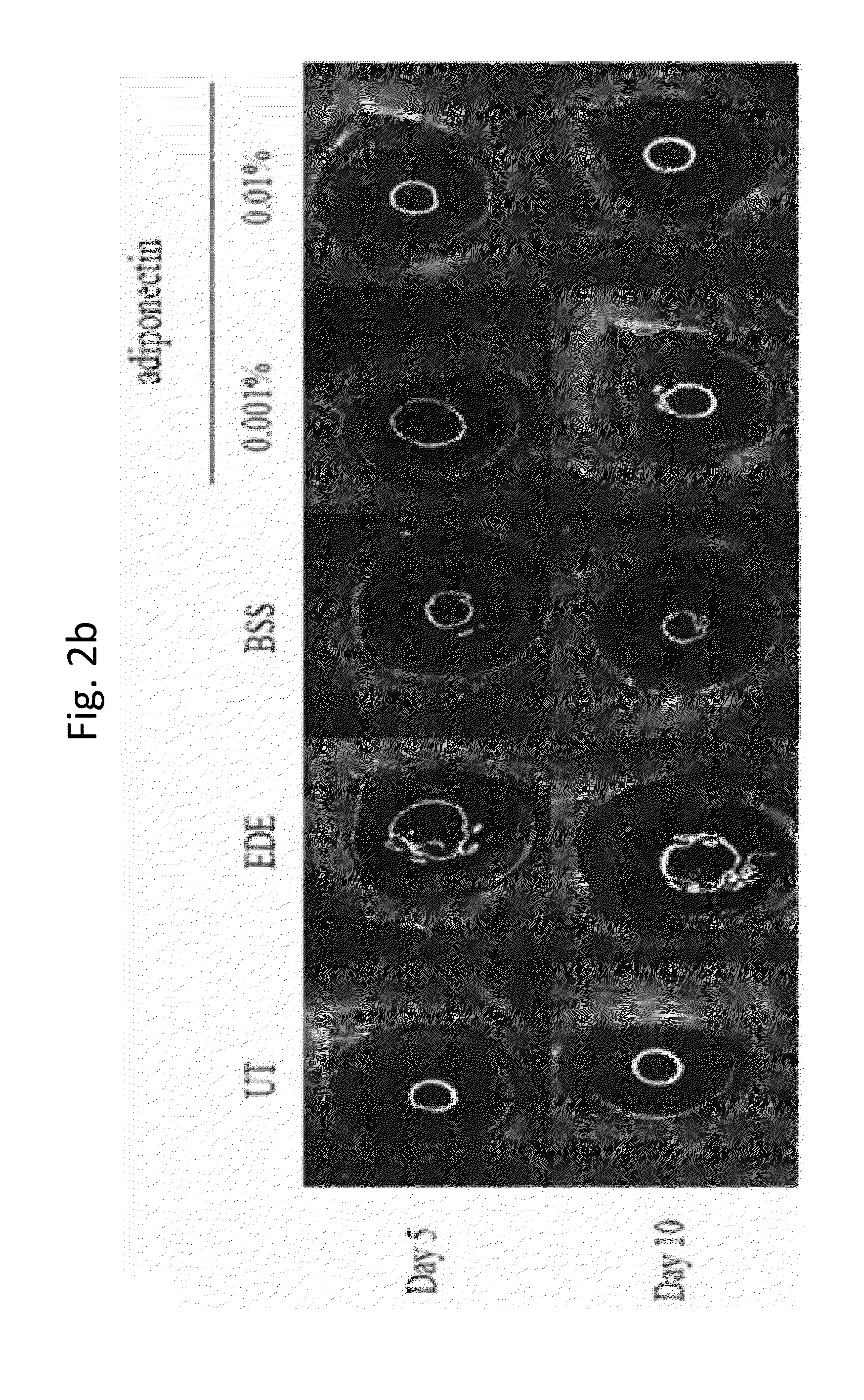
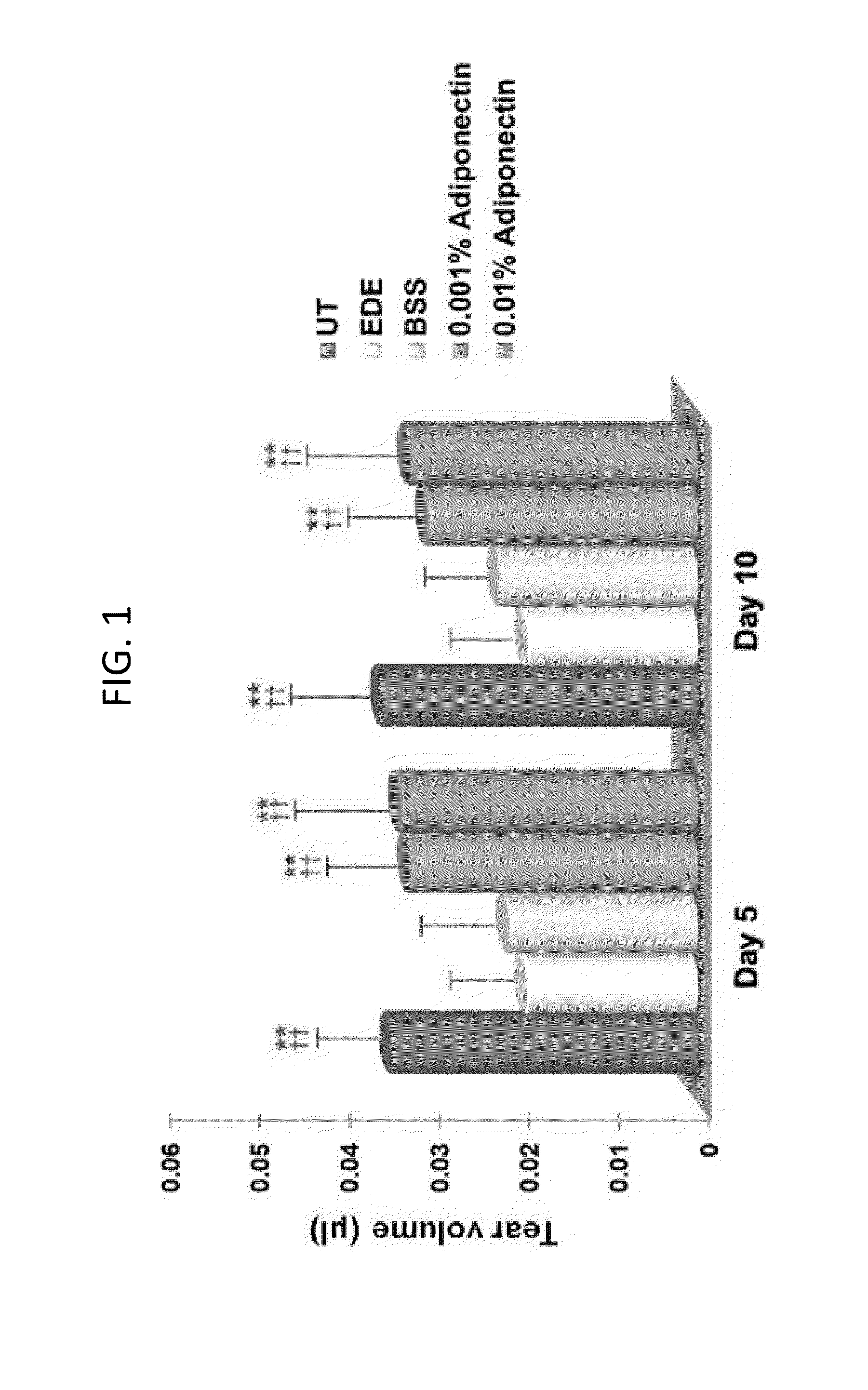
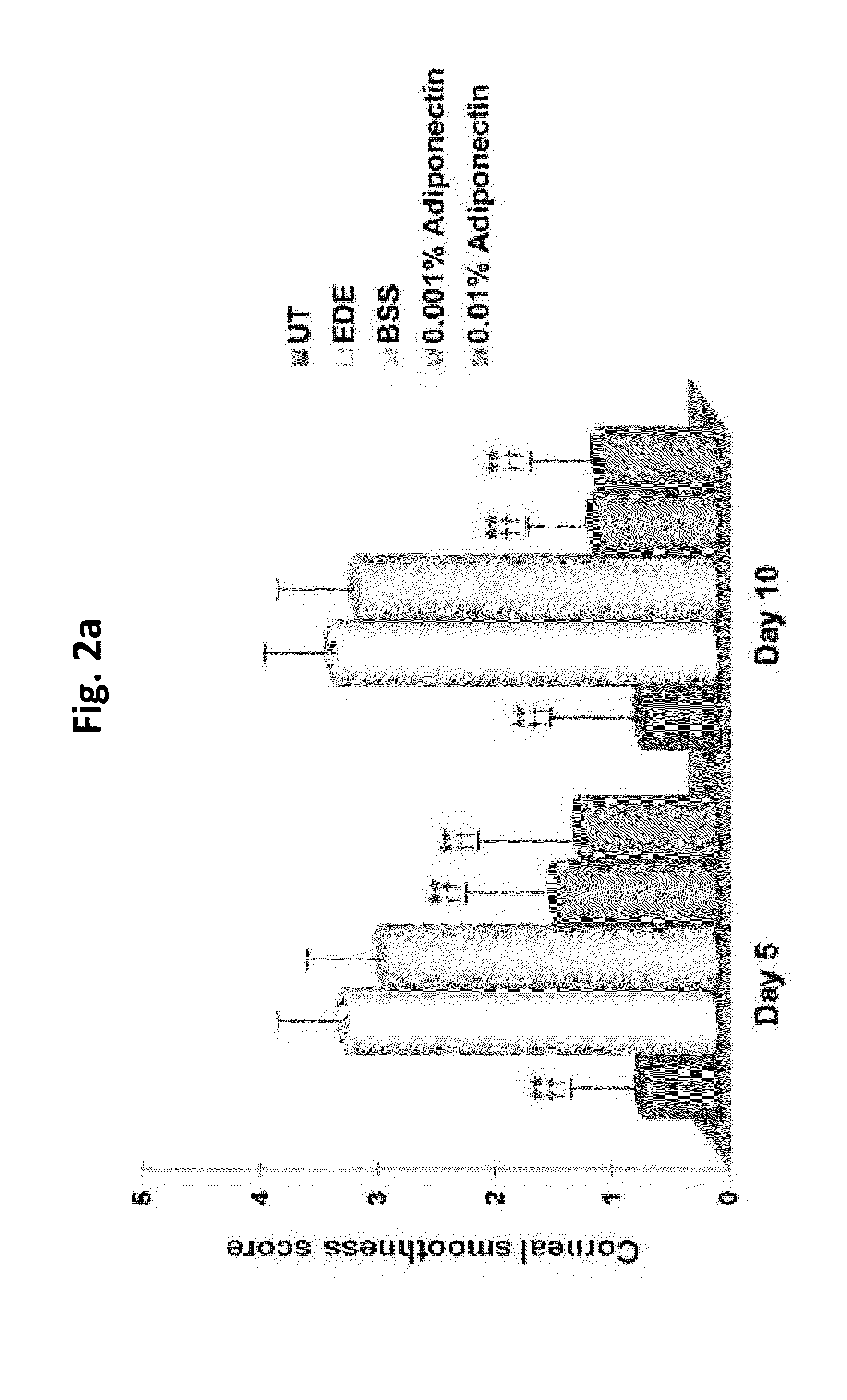
Methods for preventing or treating eye diseases using adiponectin
ActiveUS20140037712A1Stimulate tear productionAlleviating ocular surface irregularityHormone peptidesSenses disorderDiseaseSide effect
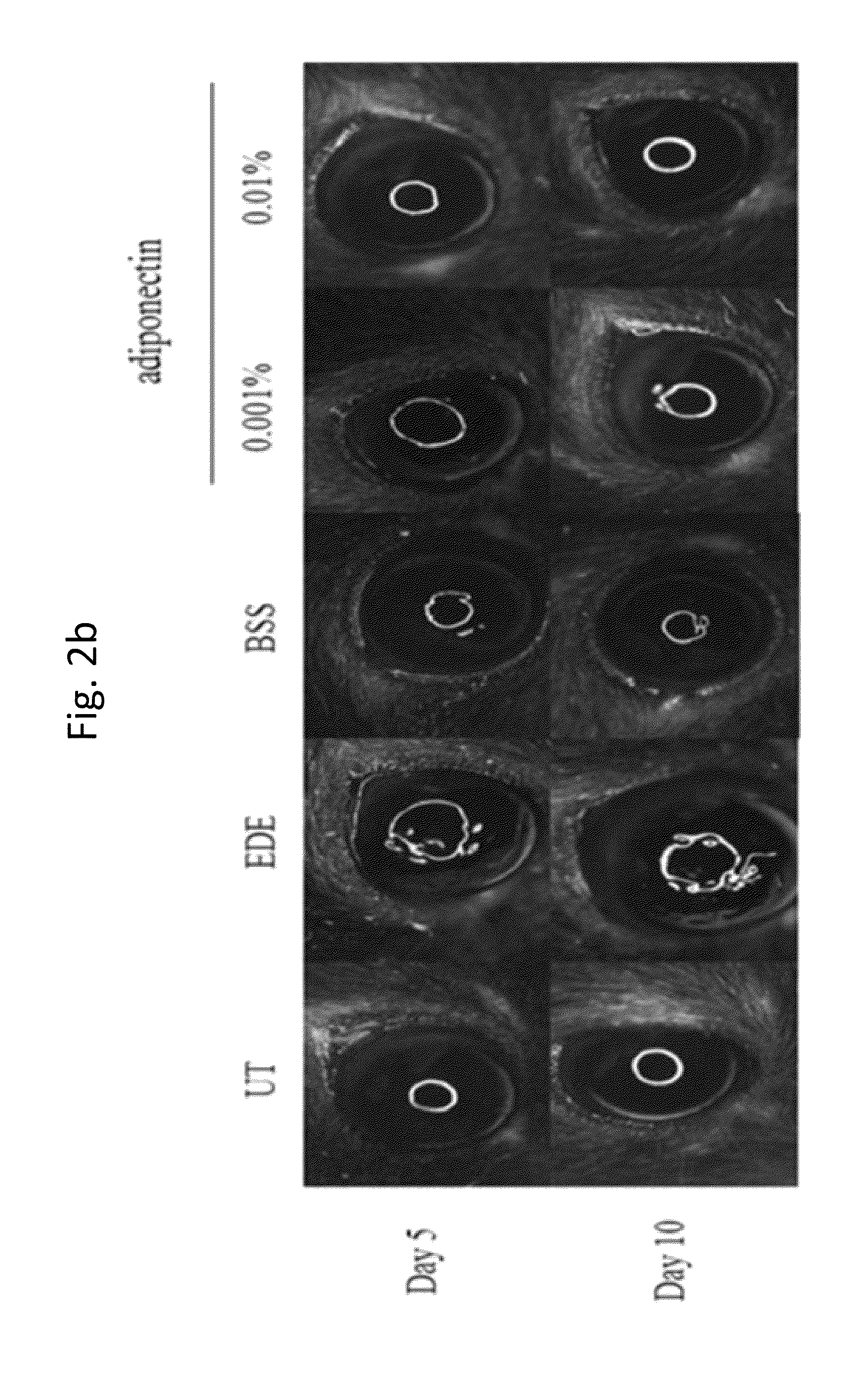
A composition for preventing or treating an eye disease includes adiponectin as an active ingredient. Adiponectin as an active ingredient is eventually revealed to show prevention or therapeutic efficacies for eye diseases such as dry eye (syndrome), inflammatory eye disease and side effects due to the use of contact lenses by promoting tear secretion, alleviating ocular surface irregularities, decreasing inflammatory cytokines on the ocular surface and lacrimal gland, and increasing conjunctival goblet cell density. In addition, the composition having eye contact lubrication effects may be used as cleaners or lubricants for preventing non-bacterial inflammation due to wearing contact lenses.
Owner:IND FOUND OF CHONNAM NAT UNIV
Eye drop for treating eye dryness
ActiveCN107049938AProtect epithelial barrier functionRecovery quantityOrganic active ingredientsSenses disorderConjunctivaEye dryness
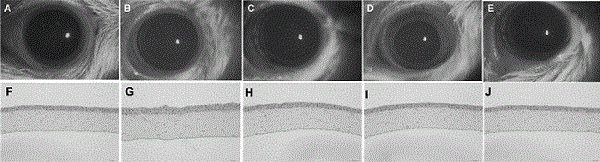
The invention discloses an eye drop for treating eye dryness. The pH value of the eye drop is 5.0-7.0, the eye drop comprises effective components of 5-hydroxy-1-beta-D-furanose-1H-imidazole-5-carboxamide, and specifically the eye drop consists of the following components in percentage by weight: 0.05-0.1% of 5-hydroxy-1-beta-D-furanose-1H-imidazole-5-carboxamide, a proper amount of a pH value adjusting agent, 0.01-3% of an iso-osmotic agent, 0.003-0.5% of a bacteriostatic agent, 0.001-0.5% of a stabilizer, 0.01-0.5% of a tackifier, 2-5% of a solubilizer and the balance of water. Through partial treatment of the eye drop, epithelial barrier functions of dry eye mouse cornea can be protected, the number of goblet cells of cornea can be recovered, and relatively good anti-inflammatory and immunity inhibition effects can be achieved.
Owner:EYE MEDICAL XIAMEN BIOTECHNOLOGY CO LTD
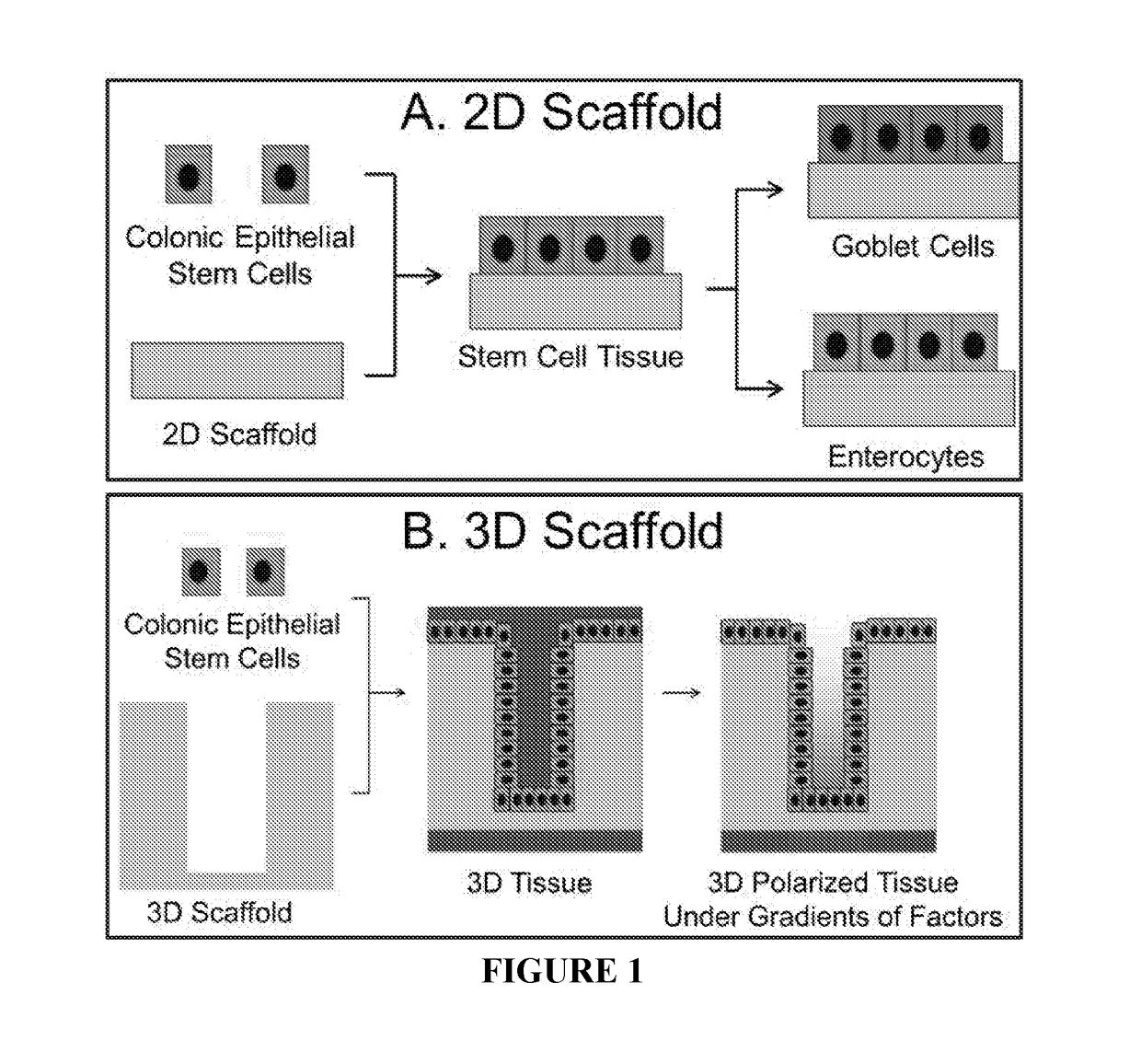
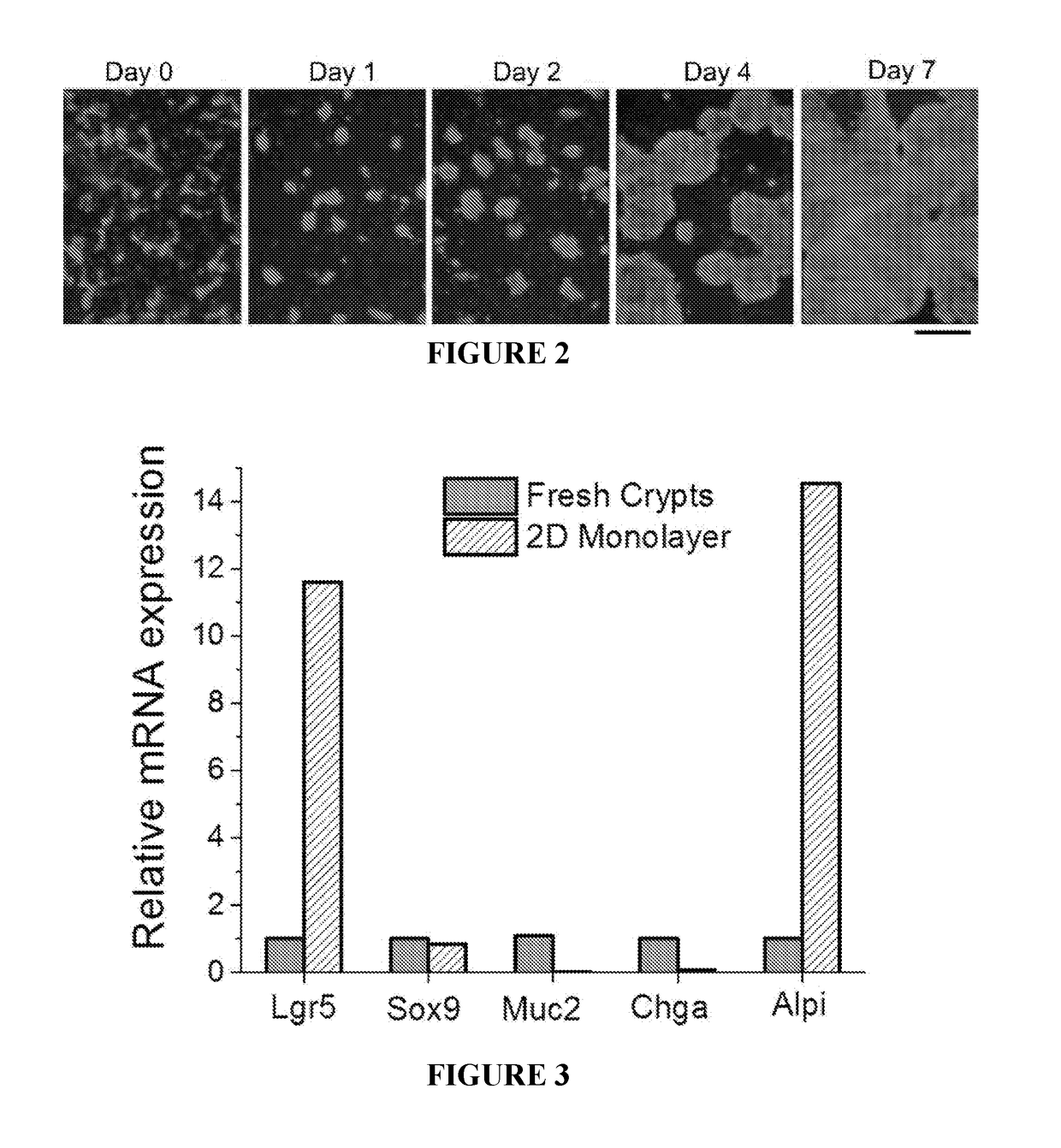
Methods to generate gastrointestinal epithelial tissue constructs
ActiveUS20180002672A1Maintain their viabilityCompound screeningApoptosis detectionProgenitorGoblet cell
A method of making a live cell construct is carried out by: (a) providing a non-cellular support having a top surface and a bottom surface, (b) contacting live undifferentiated cells to the non-cellular support, and then (c) propagating a gastrointestinal epithelial cell monolayer on said top surface. In some embodiments, the live cells in the monolayer include: (i) undifferentiated cells (e.g., stem or progenitor cells); and (ii) optionally, but in some embodiments preferably, differentiated cells (e.g., enterocytes, Paneth cells, enteroendocrine cells, tuft cells, microcells, intra-epithelial lymphocytes, and / or goblet cells). Constructs formed by such methods and methods of using the same (e.g., in high through-put screening) are also described.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Methods for preventing or treating eye diseases using adiponectin
ActiveUS8815795B2Significantly effectivePromote productionHormone peptidesSenses disorderDiseaseSide effect
A composition for preventing or treating an eye disease includes adiponectin as an active ingredient. Adiponectin as an active ingredient is eventually revealed to show prevention or therapeutic efficacies for eye diseases such as dry eye (syndrome), inflammatory eye disease and side effects due to the use of contact lenses by promoting tear secretion, alleviating ocular surface irregularities, decreasing inflammatory cytokines on the ocular surface and lacrimal gland, and increasing conjunctival goblet cell density. In addition, the composition having eye contact lubrication effects may be used as cleaners or lubricants for preventing non-bacterial inflammation due to wearing contact lenses.
Owner:IND FOUND OF CHONNAM NAT UNIV
Application of novel eye drops in treatment of dry eyes
InactiveCN104436159AExtended burst timePromote secretionSenses disorderPeptide/protein ingredientsStainingWhite blood cell
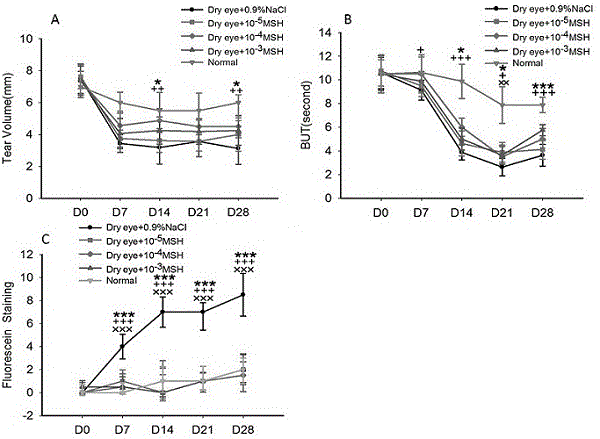
The invention discloses an application of novel eye drops in treatment of dry eyes. The novel eye drops contain alpha-MSH; the mass concentration of the alpha-MSH is 10<-4> to 10<-3>mg / ml; the novel eye drops are used for treating the dye eyes; and the optimized mass concentration of the alpha-MSH is 10<-4>mg / ml. By using the novel eye drops, experiment analysis on the breakup time of a tear film (BUT), schirmer test, fluorescein staining, grade and tear ferns is carried out on rats; an overall eyeball of each rat is extracted to be subjected to HE and periodic acid-Schiff (PAS) staining; and the fresh cornea of each rat is extracted to be subjected to real-time quantitative PCR detection of expression levels of interleukin-1beta and tumor necrosis factor-alpha m RNA in corneal tissues, so that the experiments prove that the eye drops containing alpha-MSH are capable of promoting schirmer in rats, prolonging the breakup time of the tear film, stabilizing the tear film, promoting corneal epithelium damage repair and goblet cell quantity repair, and relieving ocular surface inflammation, and are favorable for relieving lesions of the dry eyes.
Owner:天津医科大学眼科医院
Feed additive capable of improving intestinal function of grass carps and application of feed additive
InactiveCN105028978APromote growthImprove antioxidant capacityAnimal feeding stuffBiotechnologyAnimal science
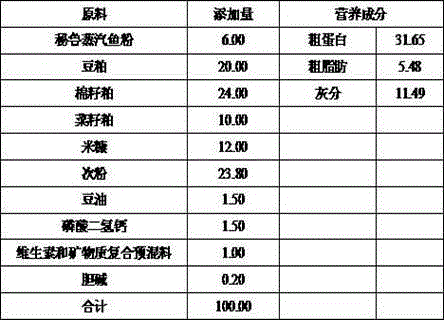
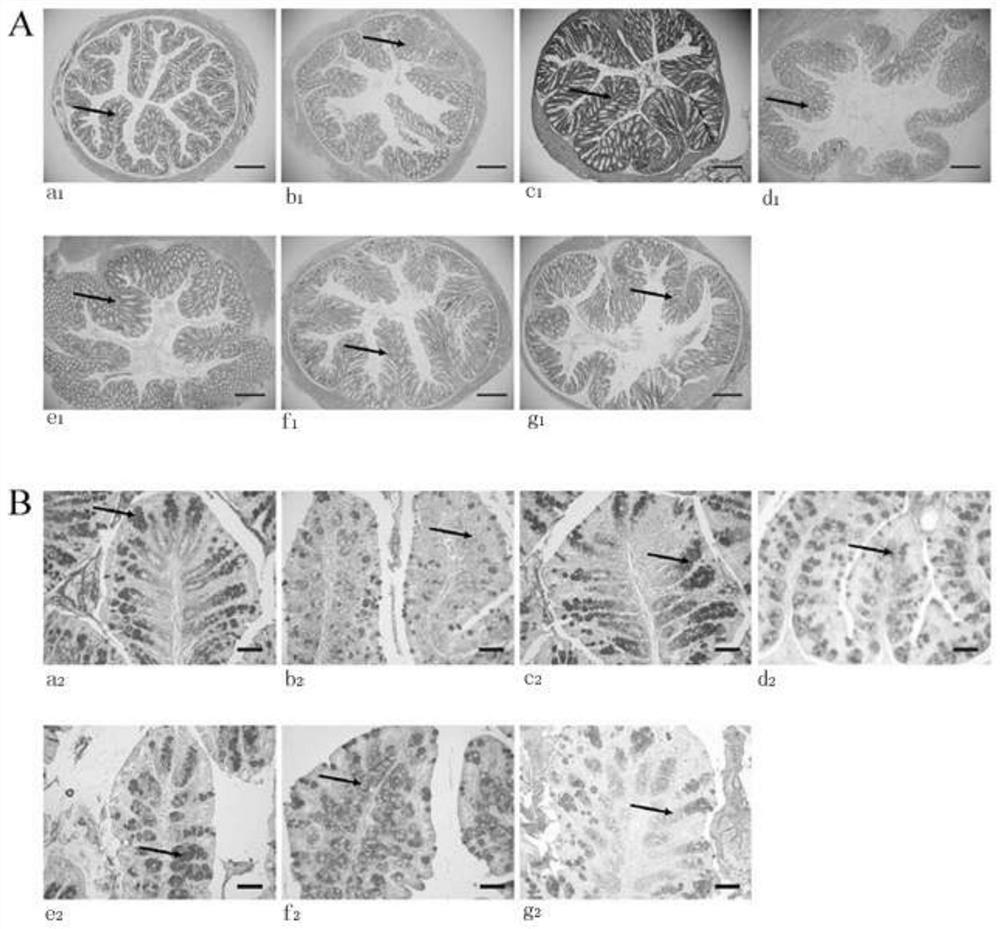
The invention discloses a feed additive capable of improving an intestinal function of grass carps and application of the feed additive. An effective component of the additive is glycylglutamine; and the adding amount in grass carp feed is 0.25-0.75%. With the adoption of the additive disclosed by the invention, the growth and fat metabolism of the grass carps can be effectively facilitated, and the oxidation resistance of the grass carps is improved; a shape structure of small intestines of the grass carps can be improved; villus height, crypt depth, mucous membrane thickness, the quantity of goblet cells and the quantity of lymphocytes of the intestines are increased; and the secretion of an intestinal digestive enzyme can be accelerated, and the feed additive has active improvement effect on the intestinal function of the grass carps.
Owner:YANCHENG TIANBANG FEED SCI & TECH CO LTD +1
Therapeutic uses of keratinocyte growth factor-2
The present invention relates to the administration of Keratinocyte Growth Factor-2 (KGF-2) to stimulate proliferation of platelets and to increase levels of fibrinogen, albumin, globulin and total serum protein. Further, the present invention relates to administering KGF-2 to protect or treat the bladder and prostate. Moreover, the present invention relates to administering KGF-2 to stimulate growth of nasal, oral, and esophageal mucosa, lacrimal glands, salivary glands and Goblet cells.
Owner:HUMAN GENOME SCI INC
Lactobacillus rhamnosus, microbial agent and food product
ActiveCN111154682AImprove protectionReduce integrity breachesMilk preparationBacteriaBiotechnologyInflammatory factors
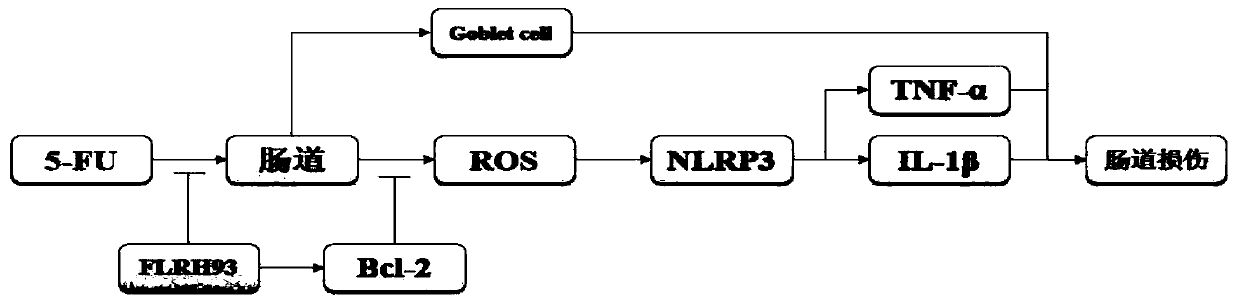
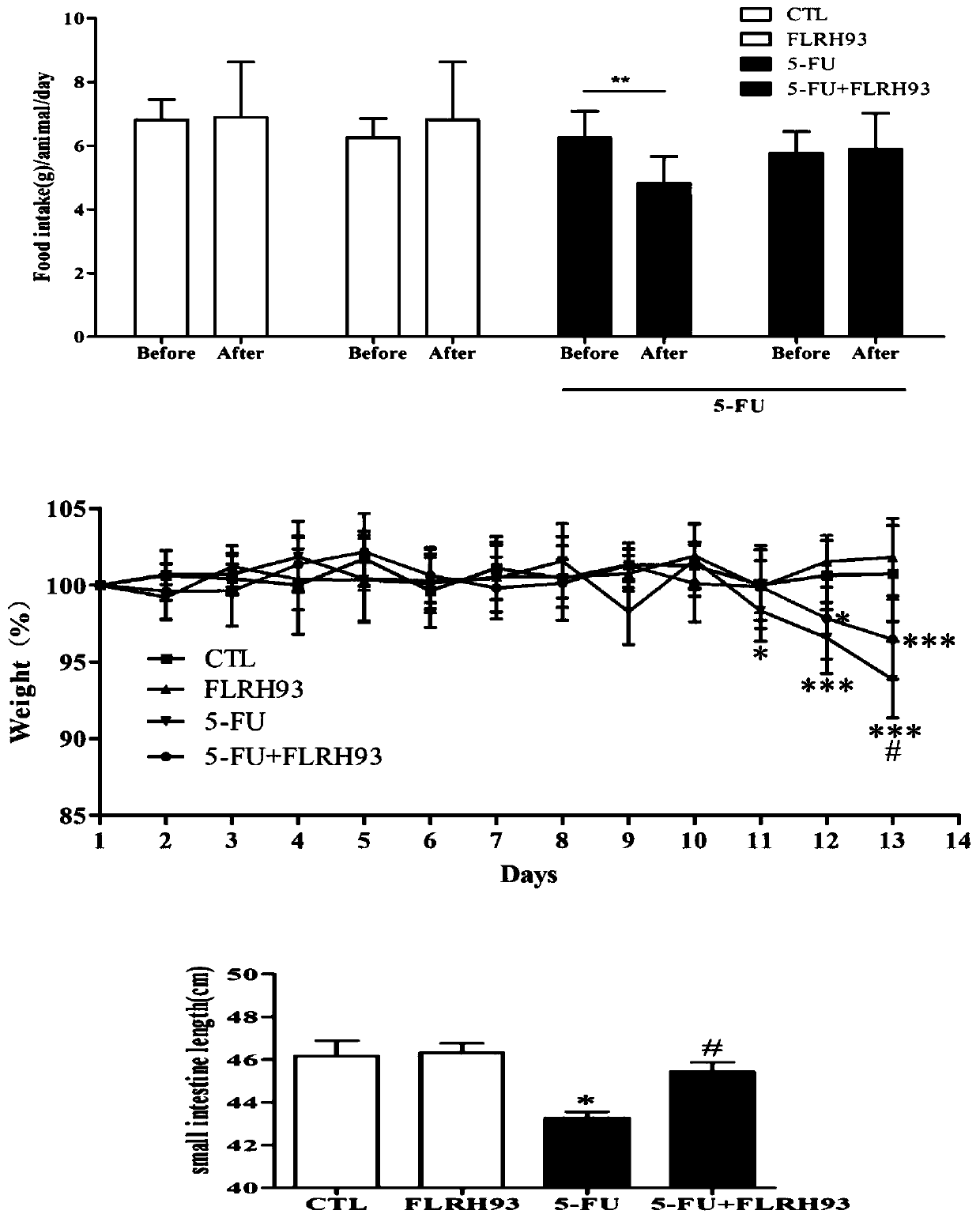
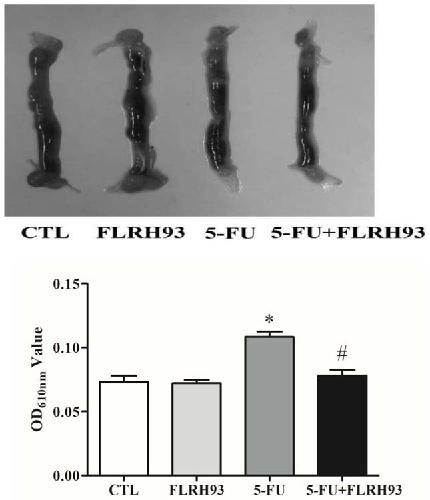
The invention discloses lactobacillus rhamnosus FLRH93. The lactobacillus rhamnosus FLRH93 is preserved in the Guangdong Microbial Culture Collection Center, and has a preservation number of GDMCC No:60956. Animal damage intervention experiment results show that the lactobacillus rhamnosus FLRH93 can obviously improve the intestinal injury caused by drugs; tissue staining sections show that the strain maintains the integrity of a small intestine structure, reduces the loss of goblet cells after drug treatment, and can regulate and control the secretion of Bcl-2 and NLRP3, thereby reducing generation of inflammatory factors IL1-beta and TNF-alpha. The invention belongs to the technical field of microorganisms. The strain provided by the invention can improve the survival rate of mice withdrug injury and can also remarkably promote healing of wound skin.
Owner:深圳市沁帆科技有限公司
Composition for treating inflammatory bowel disease
InactiveCN105362338AImprove blood supplyIncrease oxygen supplyOrganic active ingredientsDigestive systemSalvia miltiorrhizaGoblet cell
The invention discloses a composition for treating an inflammatory bowel disease and belongs to the field of medicines. The composition comprises ganoderma lucidum, salvia miltiorrhiza, poria cocos, threonine, serine and cysteine. The ganoderma lucidum, the salvia miltiorrhiza and the poria cocos have the effects of enhancing blood supply and oxygen supply for a human body. The threonine, the serine and the cysteine are the constituent parts of mucoprotein. The composition provides nutrients, oxygen and raw materials for development, update and repair of goblet cells and composition of an intestinal tract surface mucous layer barrier by goblet cell secretory mucoprotein. The composition has the obvious effects of eliminating inflammation, repairing intestinal tissues and establishing the normal intestinal tract surface mucous layer barrier of the human body and is an effective formula for treatment of the inflammatory bowel disease.
Owner:何松庆
Compositions and methods for treatment of lung dysfunction
Described are compositions and methods for the treatment, prevention, or amelioration of a symptom of an airway disorder. In certain aspects, the airway disorder may be one characterized by one or more conditions, such as goblet cell metaplasia, lung tissue inflammation, increased airway hyperresponsiveness, mucus hyperplasia, decreased airway resistance, and increased production of pro-inflammatory cytokines. The compositions and methods may be useful for the treatment of an airway disorder such as asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), allergic disorders, pulmonary inflammatory diseases, pulmonary fibrosis, and / or interstitial lung diseases.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Composition, for preventing or treating dry eye syndrome, containing polyethylene glycol and flavonoid nanocomposite as active ingredient
ActiveUS20190374531A1Improve bioavailabilityIncrease productionPowder deliveryAerosol deliveryOphthalmologyGoblet cell
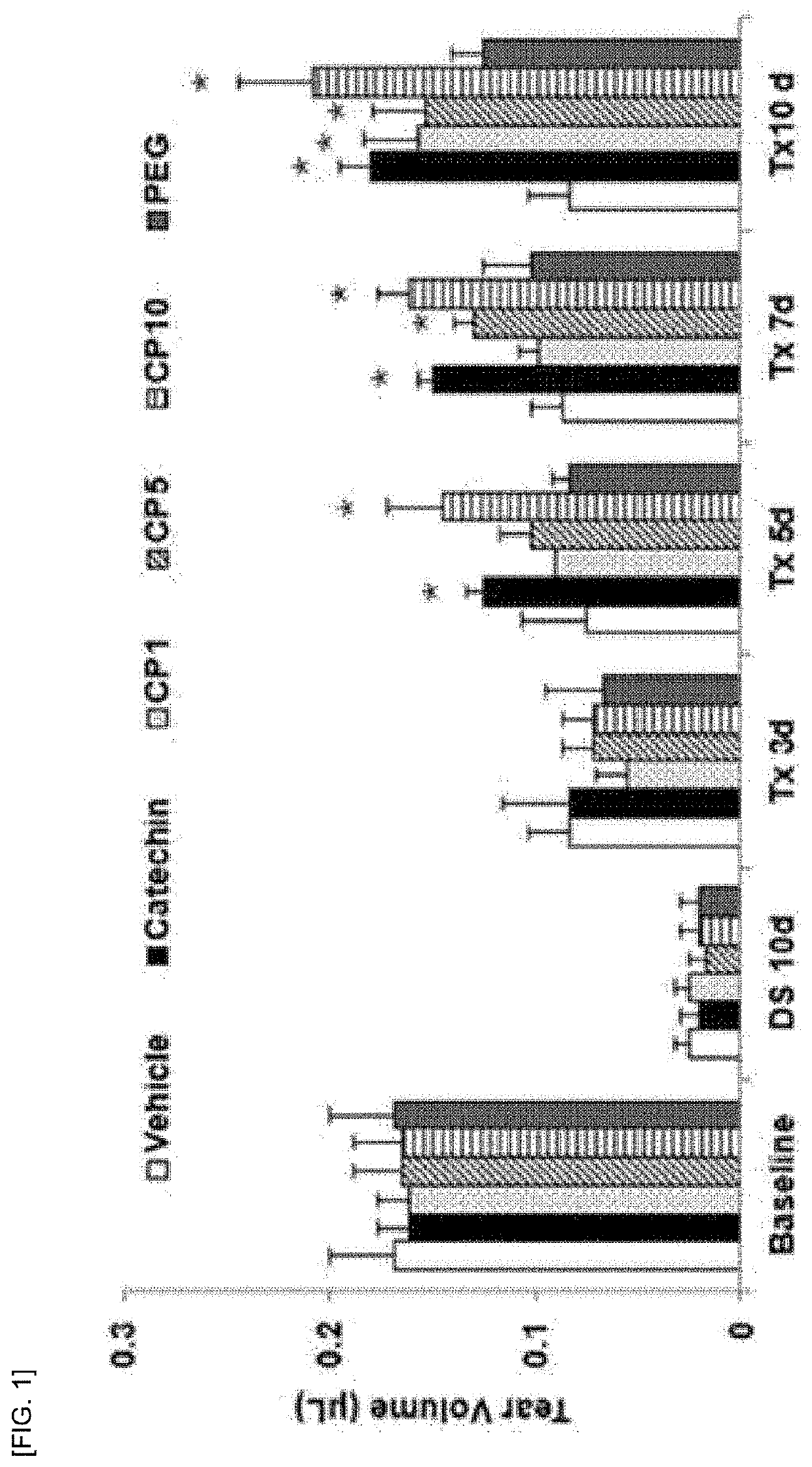
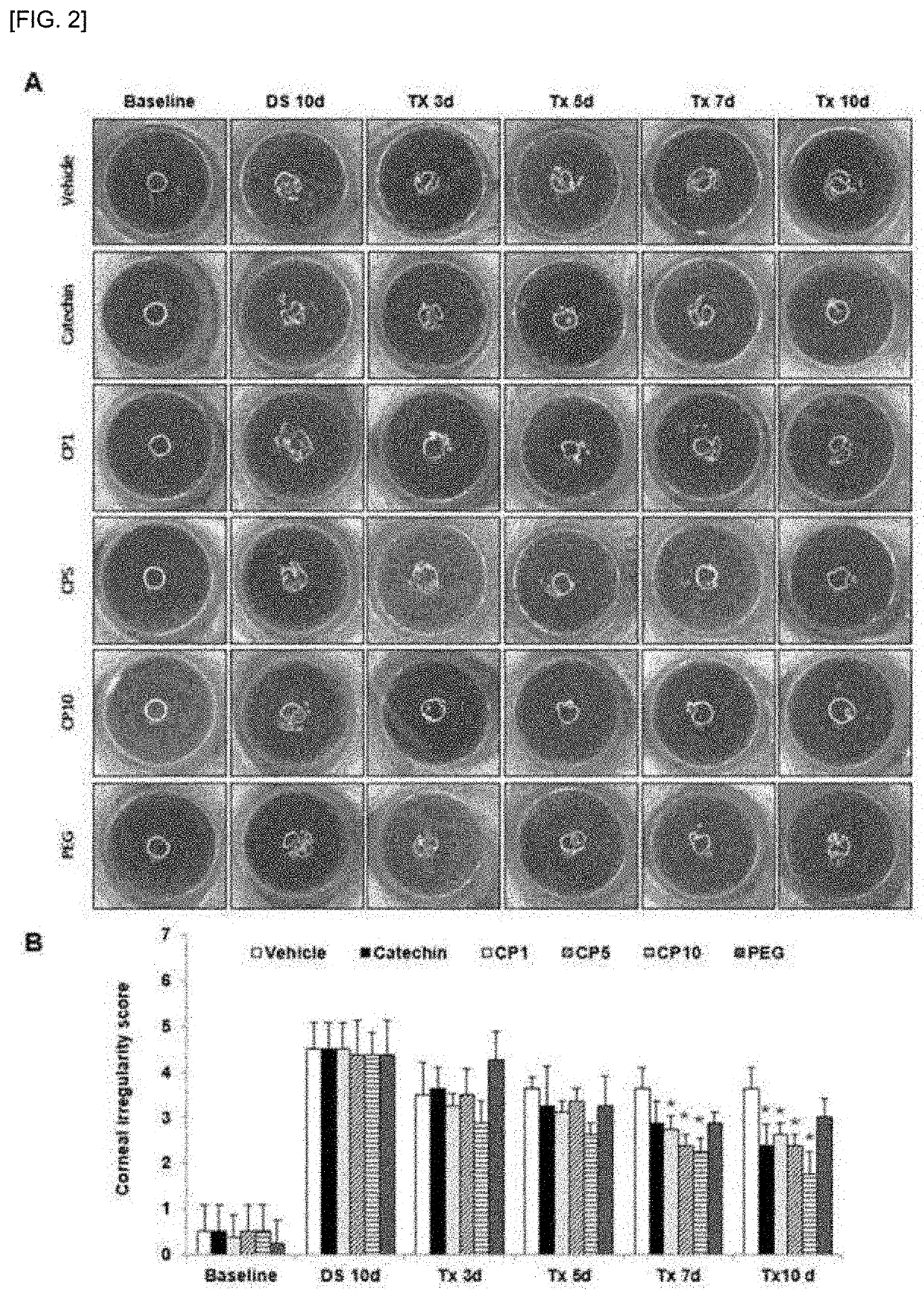
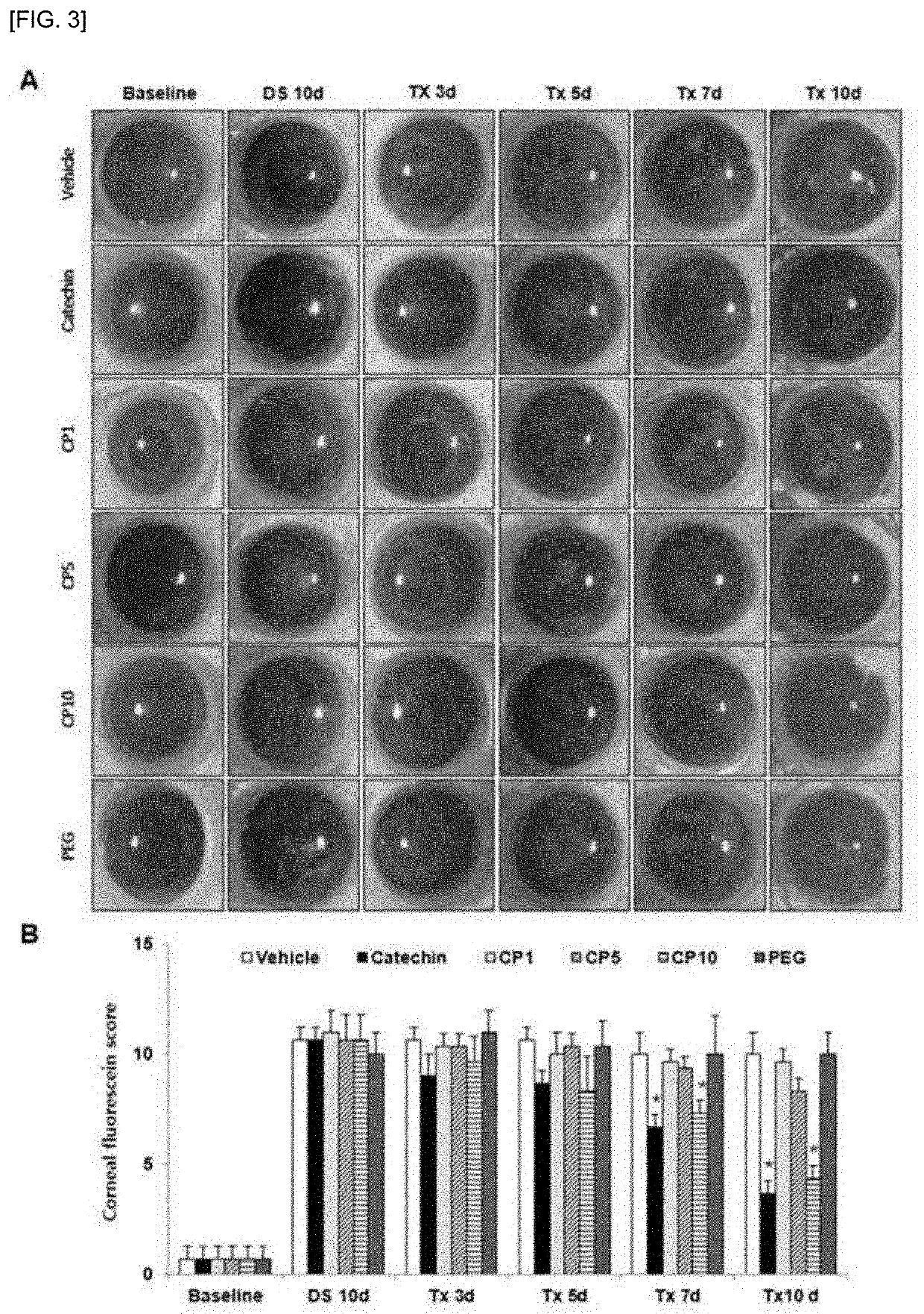
A composition for preventing or treating dry eye syndrome includes nanocomposite of a polyethylene glycol and a flavonoid as an active ingredient, and in the present invention, a catechin / PEG nanocomposite having increased catechin bioavailability is prepared by using catechins which are an antioxidant and polyethylene glycol which is a hydrophilic polymer used in a drug delivery system, and an increase in tear generation, stabilization of corneal epithelial cells, an increase in conjunctival goblet cells and enhanced anti-inflammatory effects accordance to a PEG dosage by the catechin / PEG nanocomposite are confirmed in a mouse model with dry eye syndrome.
Owner:INJE UNIV IND ACADEMIC COOP FOUND
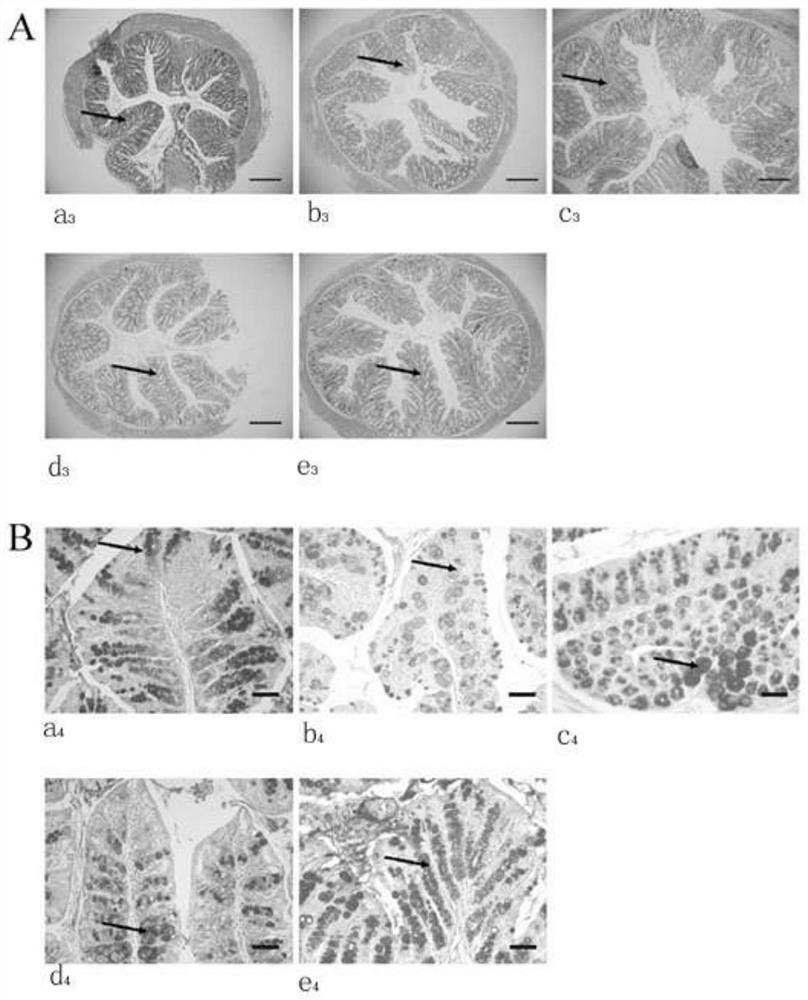
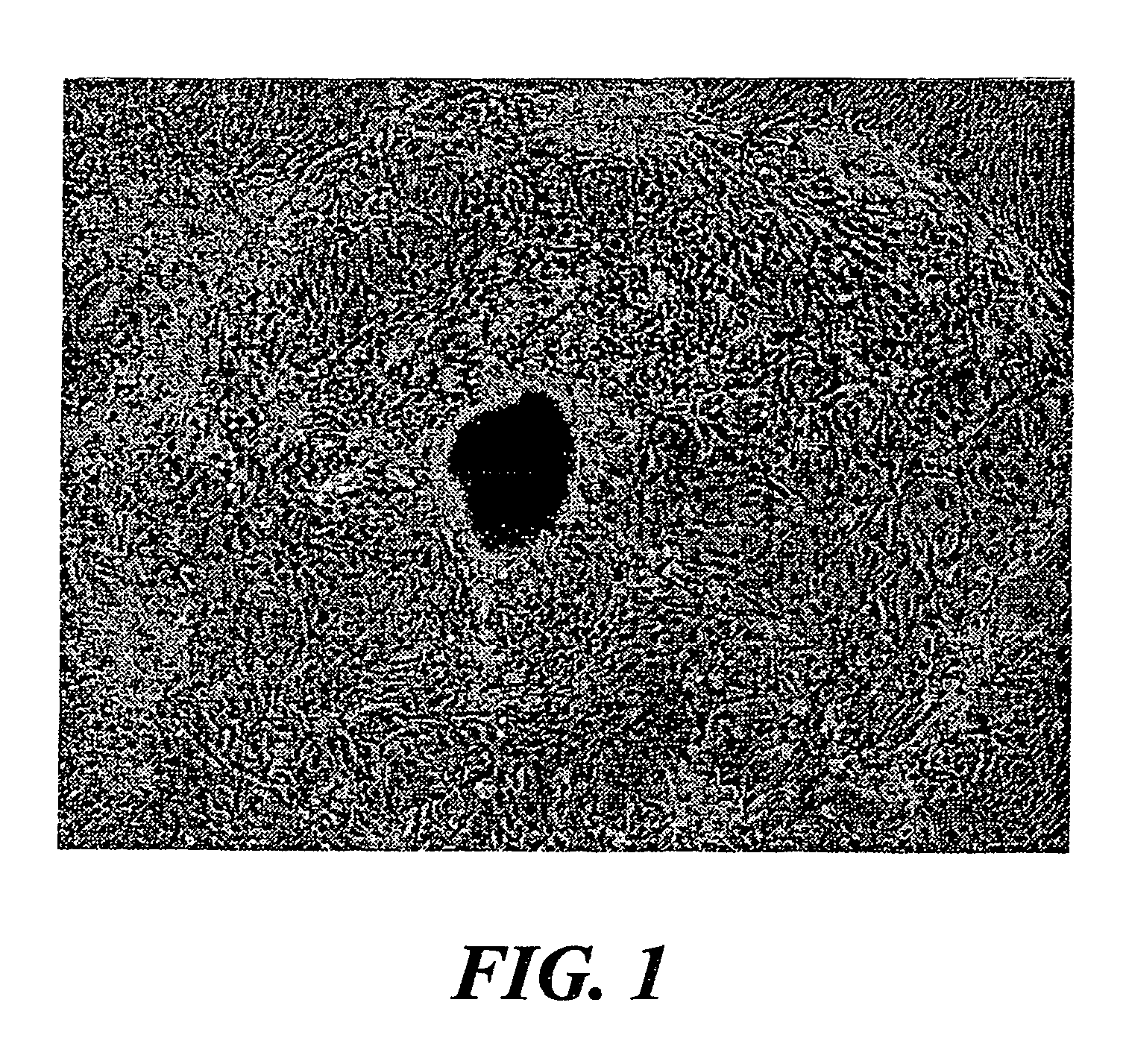
Culture of goblet cells
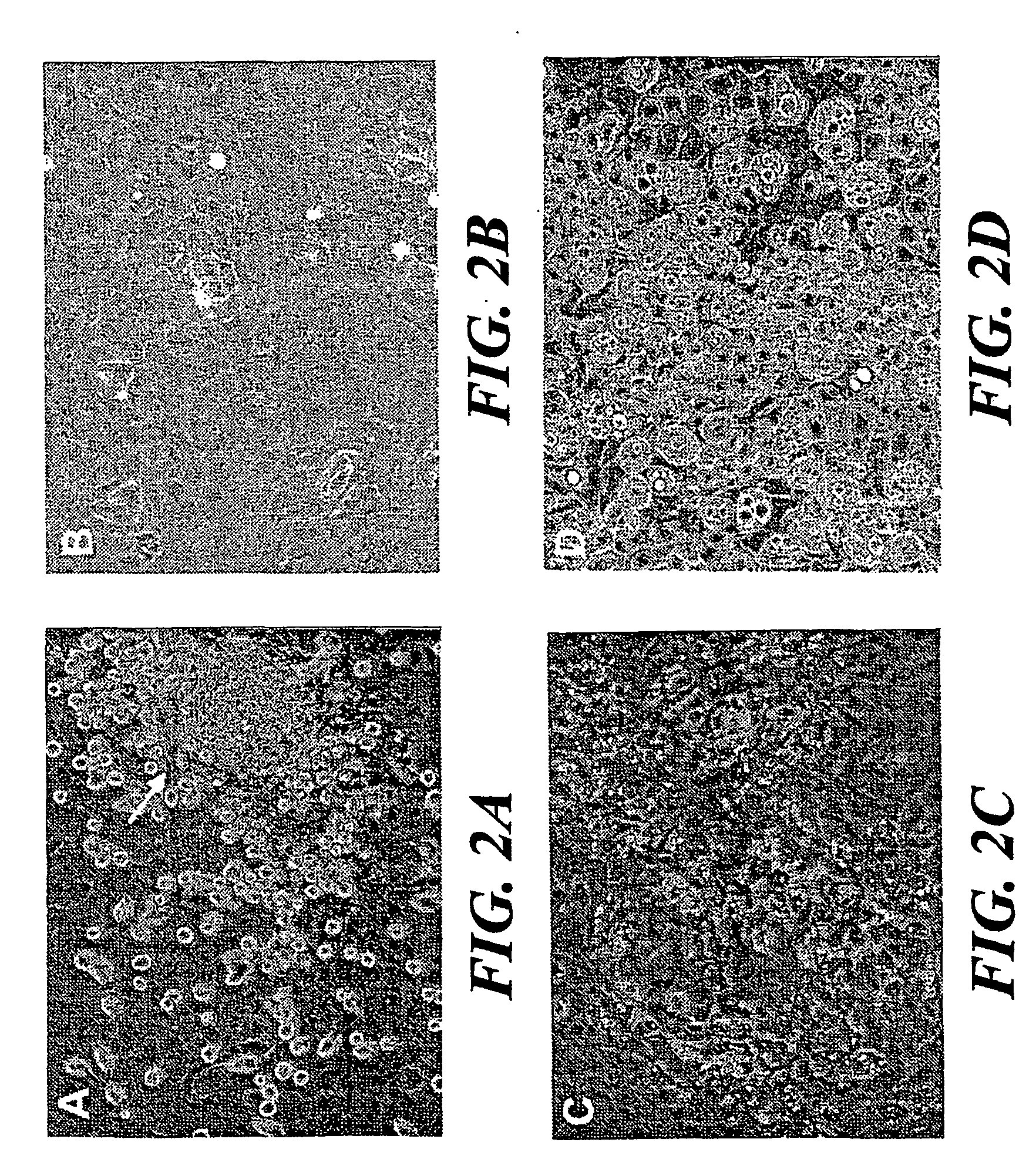
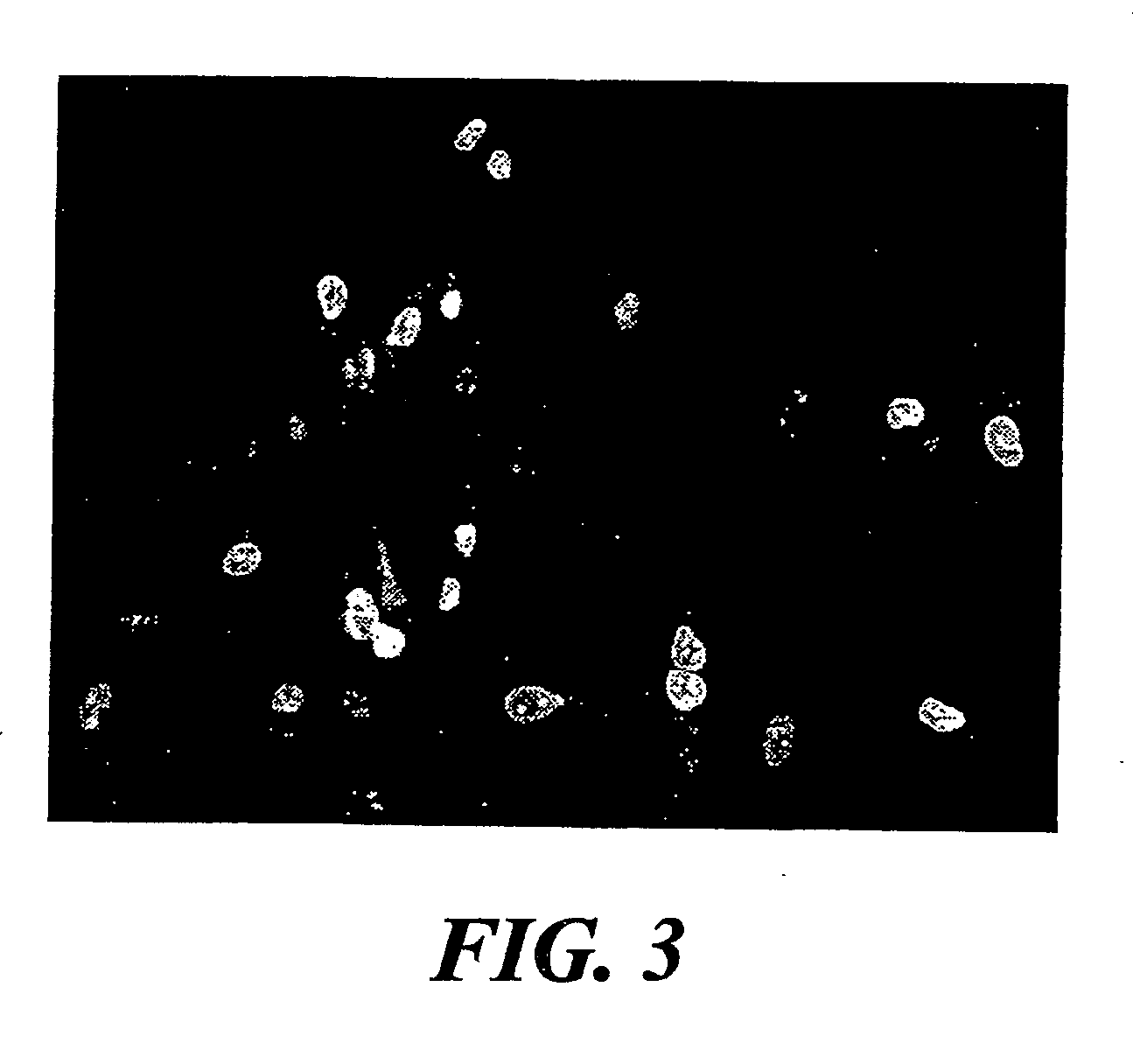
The invention encompasses isolation, culture and characterization of goblet cells in vitro form mammalian conjuctiva. Goblet cells can be cultured from conjunctiva of such mammals as, e.g., humans, rats, mice, rabbits and the like. In another aspect of the invention, the culture of goblet cells has a concentration of pure goblet cells of 10% or greater.
Owner:THE SCHEPENS EYE RES INST
A preparation method of a recombinant eye conjunctival epithelial patch containing goblet cells
ActiveCN102266587APromote proliferationHigh densityVertebrate cellsArtificial cell constructsConjunctival EpitheliumGoblet cell
The invention relates to a preparation method of a recombinant conjunctival epithelial diaphragm containing goblet cells, which induces differentiation of goblet cells by a gamma-secretase inhibitor. The preparation method provided by the invention takes autologous conjunctiva or allogenic conjunctiva as a raw material for culturing and preparing the recombinant conjunctival epithelial diaphragm containing goblet cells. Compared with the original preparation methods of conjunctival epithelial diaphragms, the culture method provided by the invention can promote proliferation of conjunctival epithelial cells at the early stage, so that the formed conjunctival epithelial multiple layers are more uniform; an amniotic membrane nested type culture mold is used as a culture support, the culture surface of the amniotic membrane is flat, the differentiated goblet cells exist among the conjunctival epitheliums, have functions and are convenient in clinical application, and the amniotic membrane is not liable to rupture; and the density and ability to secrete mucoprotein of the goblet cells in the recombinant conjunctival epithelial diaphragm can also be increased, thus more meeting the clinical demand.
Owner:SHANDONG EYE INST
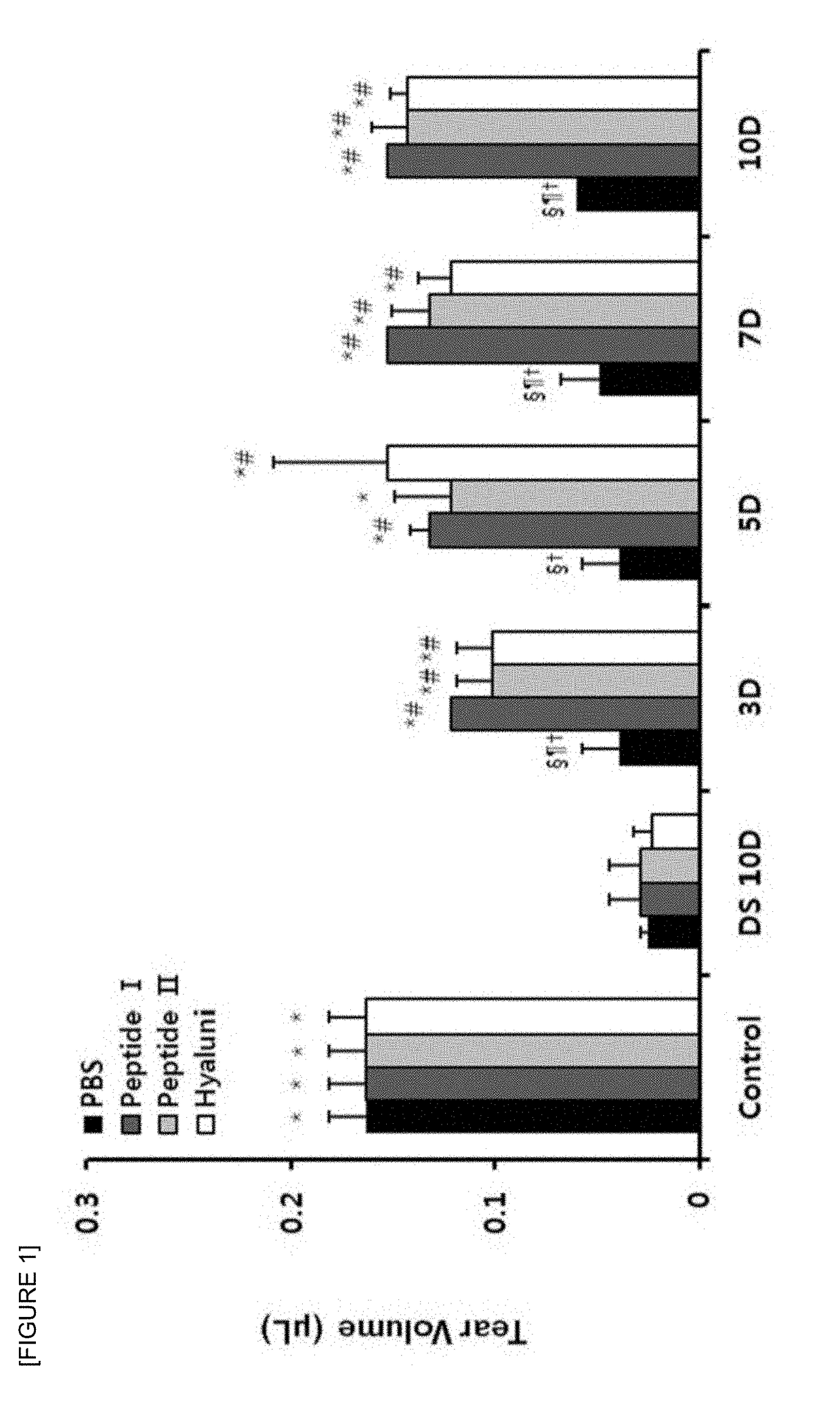
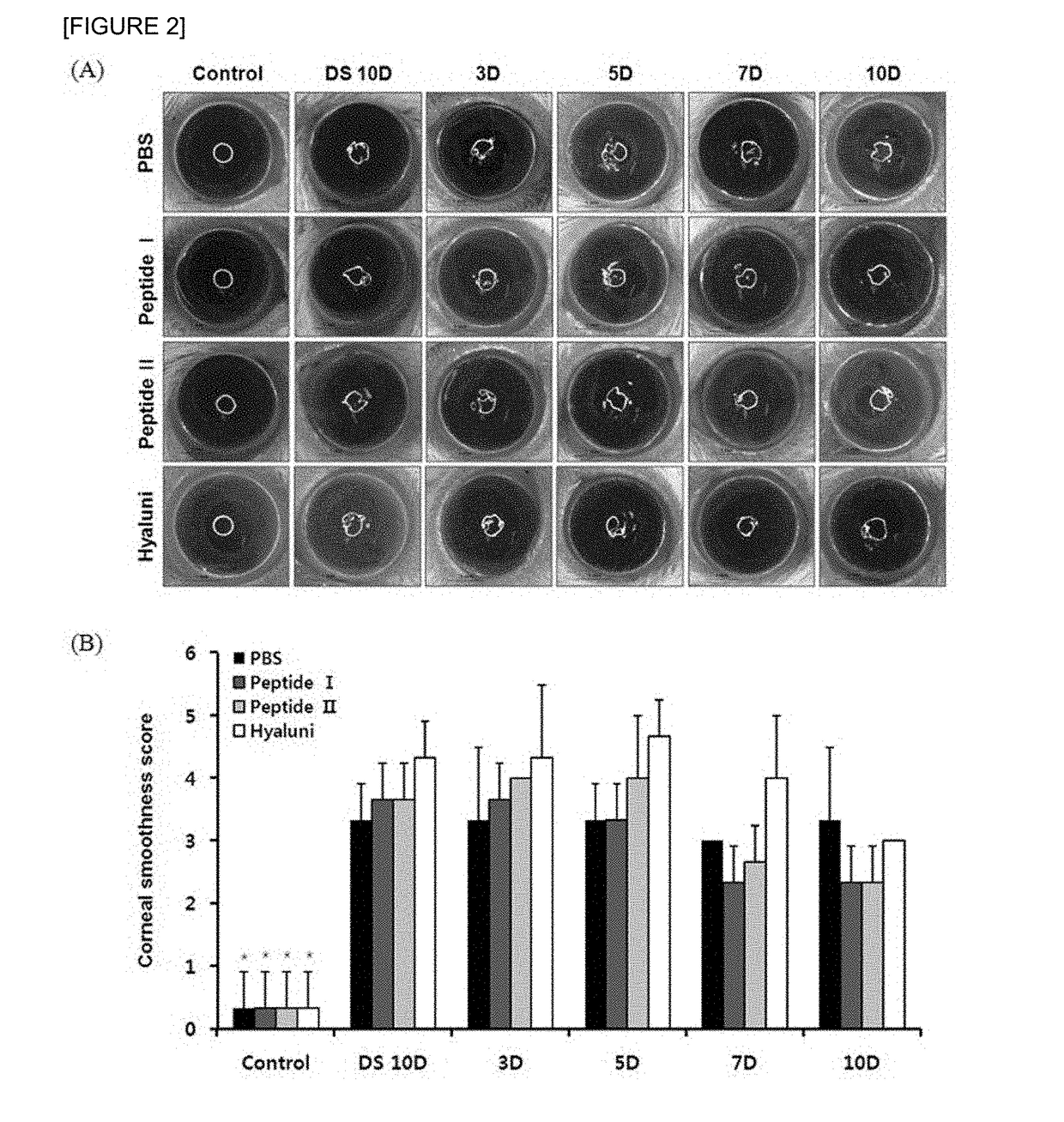
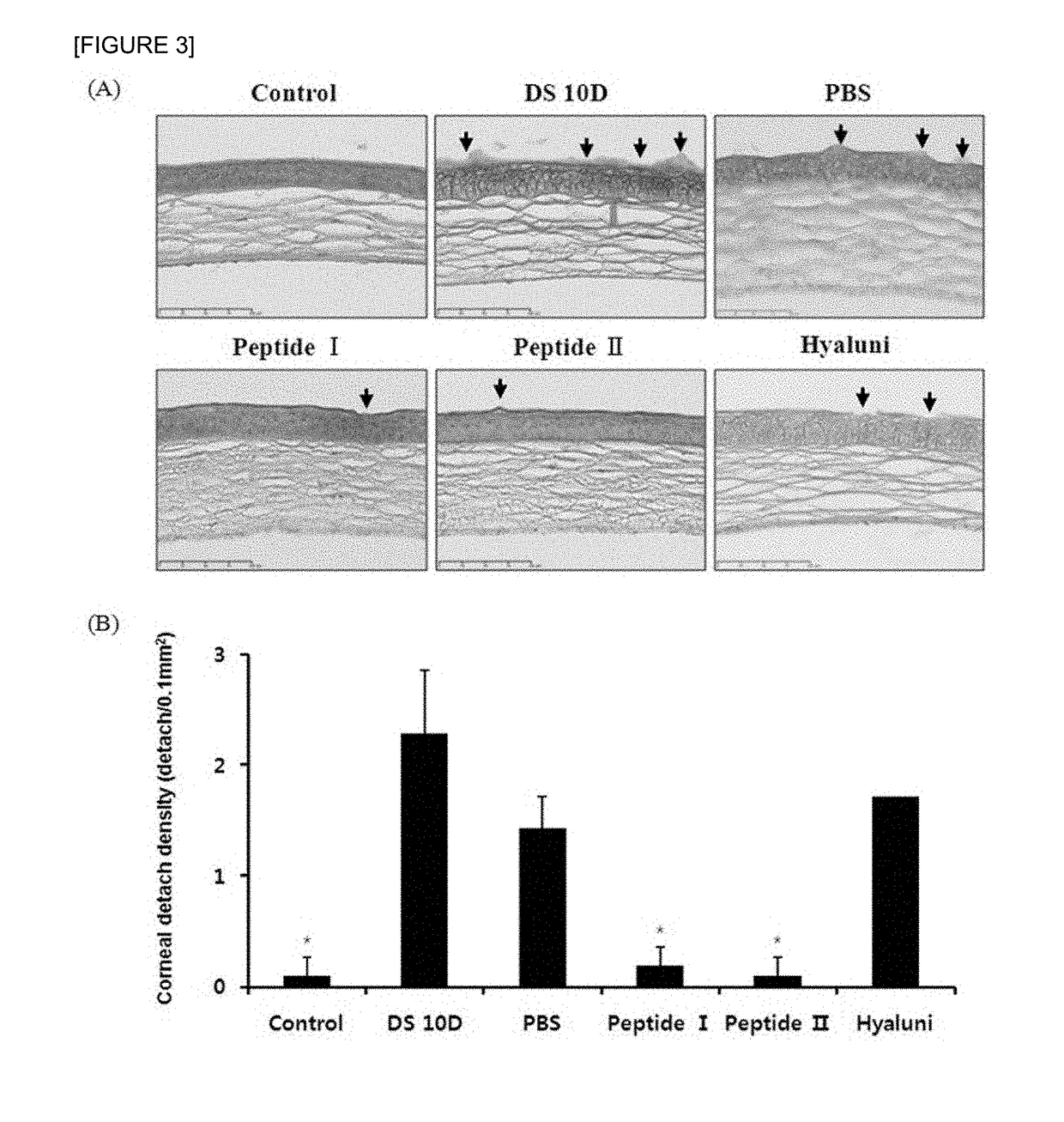
Pharmaceutical composition for preventing or treating dry eyes
ActiveUS20190002528A1Increase tear productionImprovement of corneal surface smoothnessSenses disorderConnective tissue peptidesInflammatory factorsGoblet cell
The present invention relates to a pharmaceutical composition for preventing or treating dry eye, the pharmaceutical composition including, as an active component, a novel peptide is disclosed, wherein it is confirmed that the peptide has effects on improving tear production and corneal surface smoothness for dry eyes induced by desiccation stress and suppressing detachment of corneal epithelial cells, reduction in conjunctival goblet cells, and generation of inflammatory factors, thereby applying a composition including the peptide as an active component to the pharmaceutical composition for preventing or treating dry eye.
Owner:EYEBIO KOREA
Application of tetrahydrocurcumin in improving allergic asthma
ActiveCN108771664AAlleviate itchingInhibitionKetone active ingredientsRespiratory disorderDiseaseGoblet cell
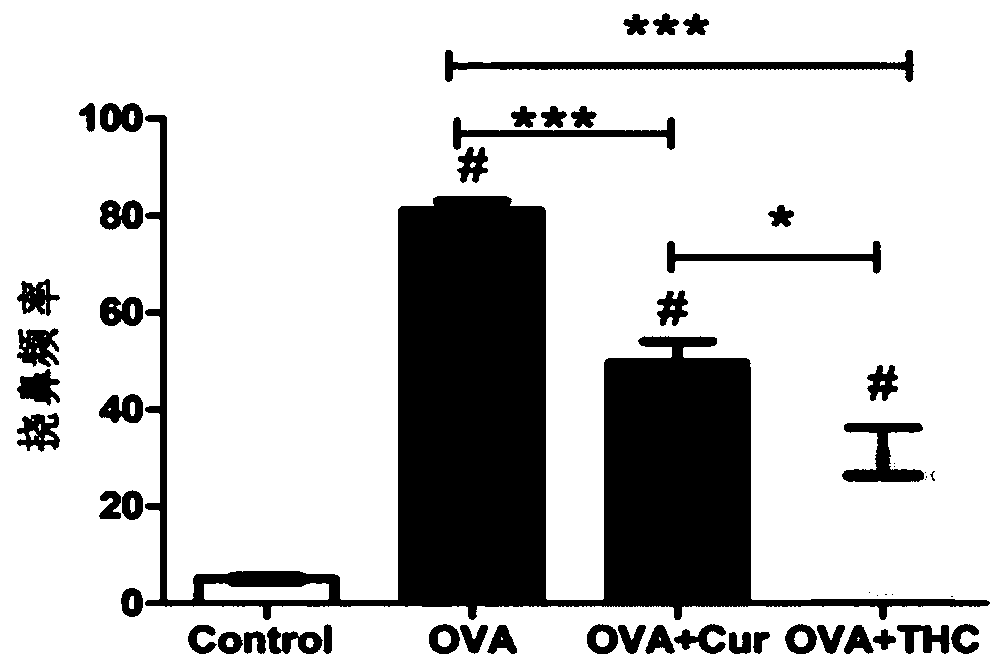
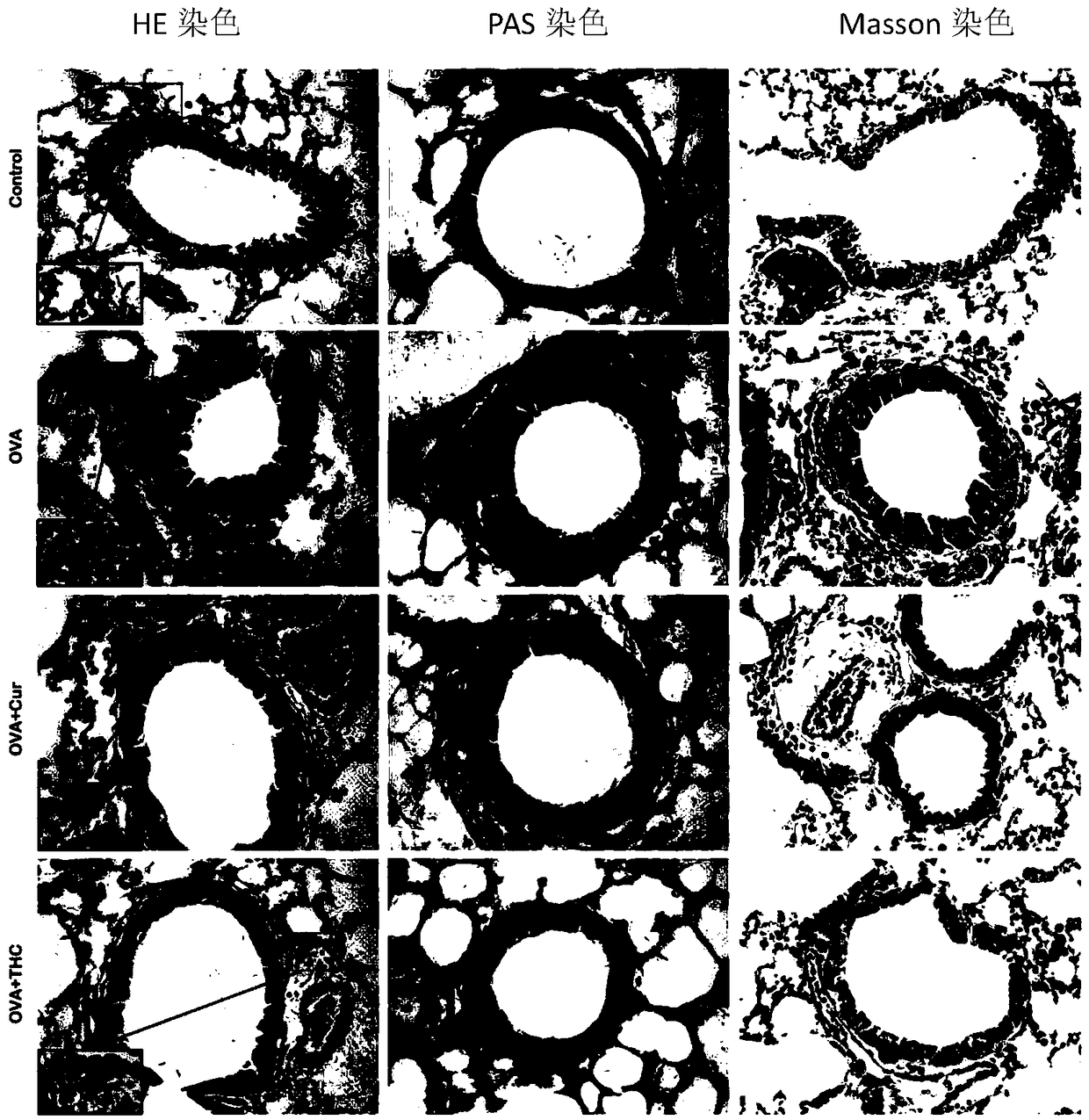
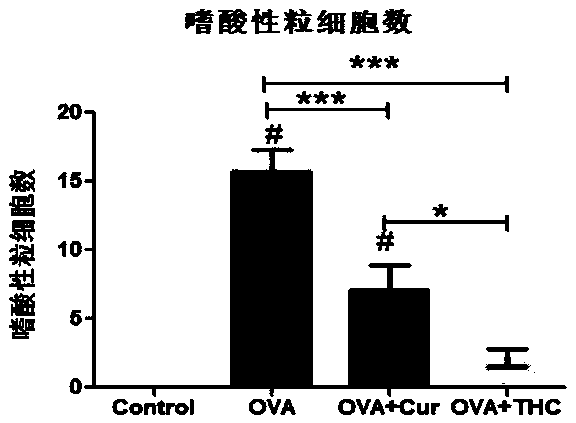
The invention discloses application of tetrahydrocurcumin in treating allergic asthma. The therapeutic action of the tetrahydrocurcumin (THC) on allergic asthma is firstly found and proved; from the perspective of immunity, the THC can be beneficial to alleviating nose allergy and itching, inhibiting lung eosinophilic granulocyte infiltration, reducing lung goblet cell grume generation, reducing lung collagen deposition, and inhibiting Th2 cell factor production, can provide beneficial information for clinic disease evaluation, and has a favorable application prospect in asthma treatment.
Owner:SUN YAT SEN UNIV
2d organoid for infection and culture of human diarrhea virus, and use of said 2d organoid
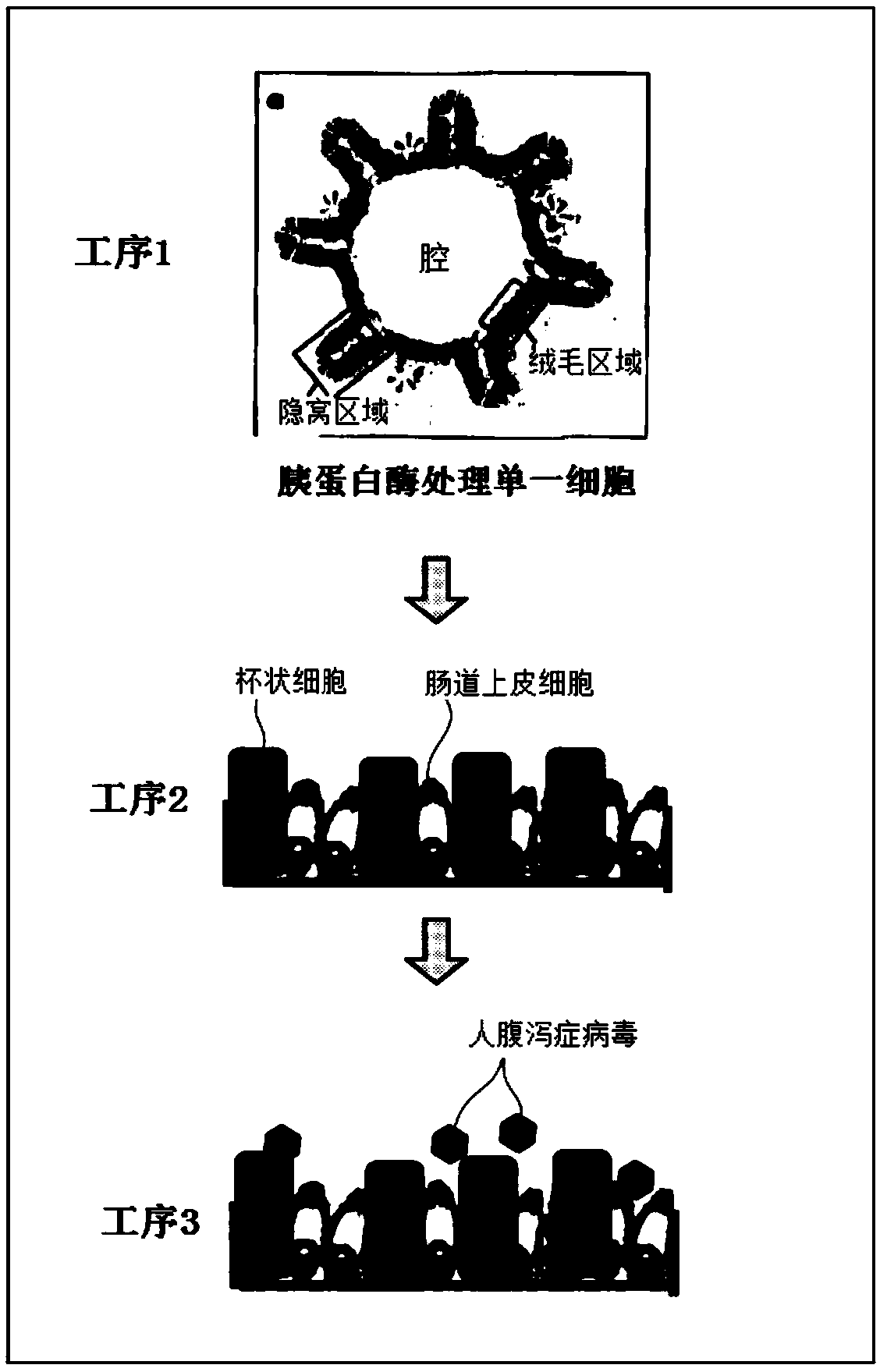
PendingCN109661460ASsRNA viruses positive-senseGastrointestinal cellsCell-Extracellular MatrixGoblet cell
A 2D organoid for infection and culture of human diarrhea virus epithelial cell stem cell, which is obtained by Step 1, in which: human intestinal epithelial stem cells, human intestinal epithelial cells, or a tissue that includes at least one of these cells is subjected to three-dimensional culturing in the extracellular matrix; and a 3D organoid is obtained, and Step 2, in which: the 3D organoids obtained in Step 1 are dispersed to prepare single cells; the single cells are subjected to monolayer culturing in the extracellular matrix; and a 2D organoid is obtained in which the epithelial cells, which include differentiated trophoblastic cells and caliciform cell, and which constitute part of the human intestinal cavity, have a single-layer structure.
Owner:KEIO UNIV
Dendrobium officinale composition as well as preparation method and application thereof
PendingCN114404525AGood control effectIncrease abundanceDigestive systemNatural extract food ingredientsUlcerative colitisGoblet cell
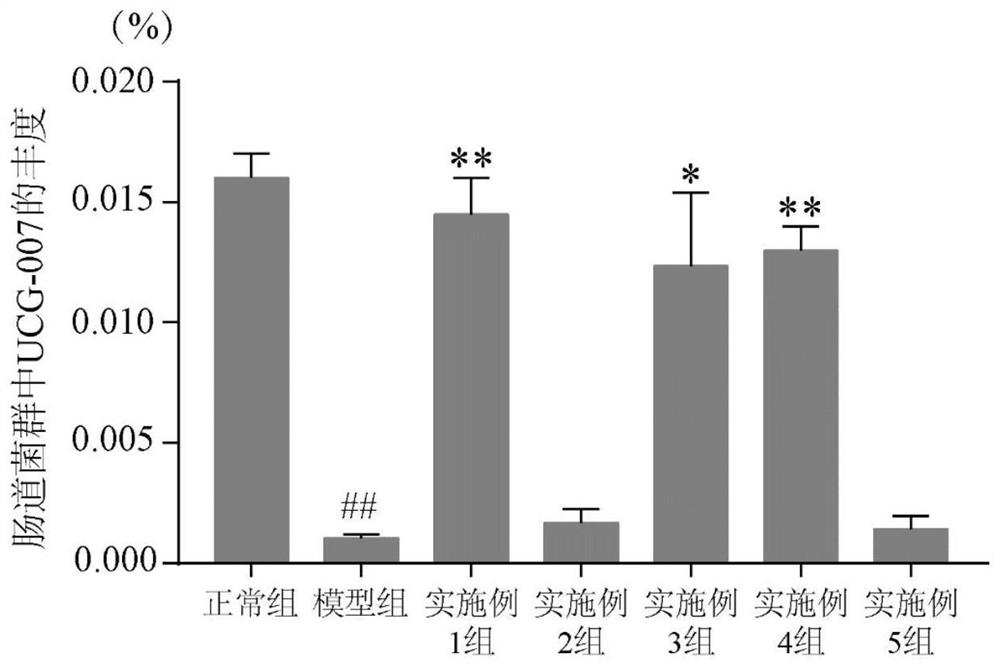
The invention discloses a dendrobium officinale composition and a preparation method and application thereof.The dendrobium officinale composition is prepared from, by weight, 10-50 parts of dendrobium officinale and 5-30 parts of radix asparagi, the medicine components are mixed and smashed, a certain amount of water is added, the medicine components are soaked for a period of time and subjected to reflux extraction, an extracting solution is filtered, filtrate is subjected to vacuum concentration, and a traditional Chinese medicine composition extract is obtained. The composition disclosed by the invention has a relatively good prevention and treatment effect on ulcerative colitis, and can be used for remarkably reducing the disease activity score (DAI score) of a mouse with ulcerative colitis, increasing the colon length, improving the histological lesions such as colonic mucosa injury, inflammatory cell infiltration, goblet cell and mucus reduction and the like, and preventing and treating the ulcerative colitis. One of the ways for preventing and treating ulcerative colitis is to increase the abundance of UCG-007 bacteria in intestinal flora. The traditional Chinese medicine composition has the characteristics of simple formula and exact curative effect, and has better effect, lower cost and good market development and application prospects compared with single use of dendrobium officinale.
Owner:ZHEJIANG UNIV OF TECH
Culture of goblet cells
The invention encompasses isolation, culture and characterization of goblet cells in vitro form mammalian conjuctiva. Goblet cells can be cultured from conjunctiva of such mammals as, e.g., humans, rats, mice, rabbits and the like. In another aspect of the invention, the culture of goblet cells has a concentration of pure goblet cells of 10% or greater.
Owner:THE SCHEPENS EYE RES INST
Traditional Chinese medicinal composition for treating xerophthalmia
InactiveCN103585452AReduce releaseInhibit apoptosisSenses disorderPlant ingredientsTreatment effectGoblet cell
The invention provides a traditional Chinese medicinal composition for treating xerophthalmia, and belongs to the technical field of xerophthalmia treatment medicines. The traditional Chinese medicinal composition comprises 5-15g of radix bupleuri, 8-16g of Chinese angelica, 10-20g of white peony root, 10-20g of Poris cocos, 5-15g of Rhizoma Atractylodis Macrocephalae, 4-12g of mint, 10-20g of Radix Codonopsis, 5-15g of Radix Ophiopogonis, 7-17g of Chinese magnoliavine, 10-20g of Rhizome of Perny False Fairybells and 5-15g of Divaricate Saposhnikovia Root. The action mechanism of the traditional Chinese medicinal composition is that each of all the medicines composing the composition has obvious inflammation prevention and immunity adjustment effects. The composition can decrease the content of relevant immune inflammatory cytokines in tears and adjust the sex hormone disorder. The traditional Chinese medicinal composition reduces the release of proinflammatory cytokines and prevents the apoptosis of goblet cells by adjusting the level of the sex hormone and intervening the inflammatory cytokines, performs pharmacological effects and has a substantial treatment effect.
Owner:谢立科
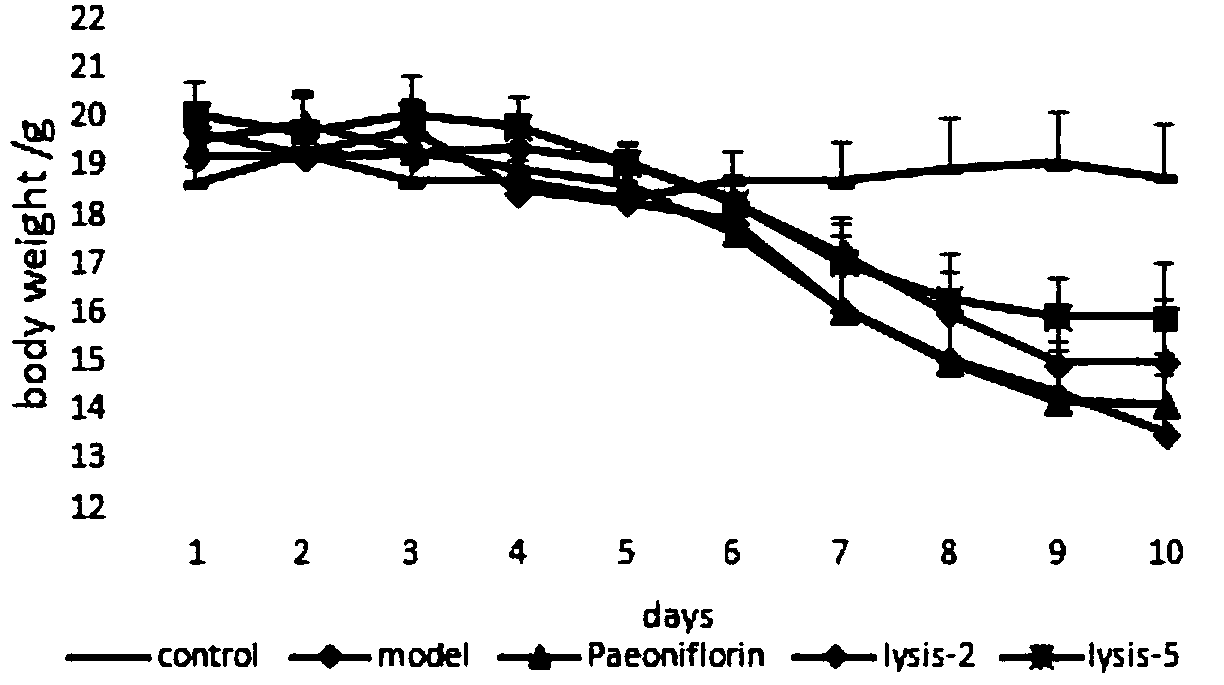
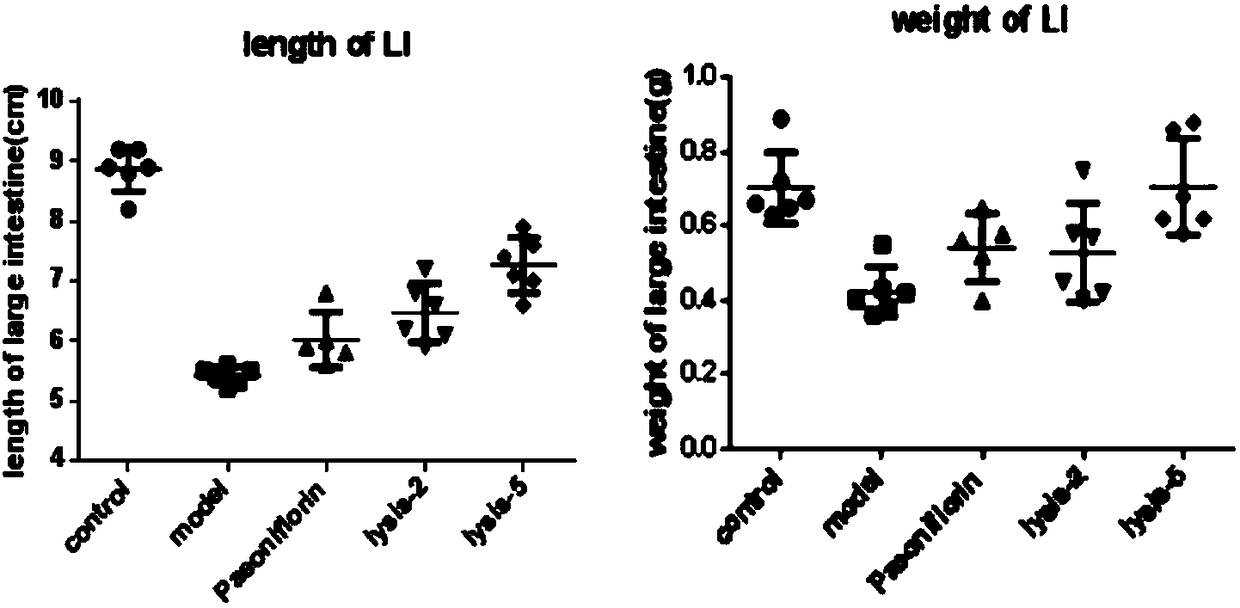
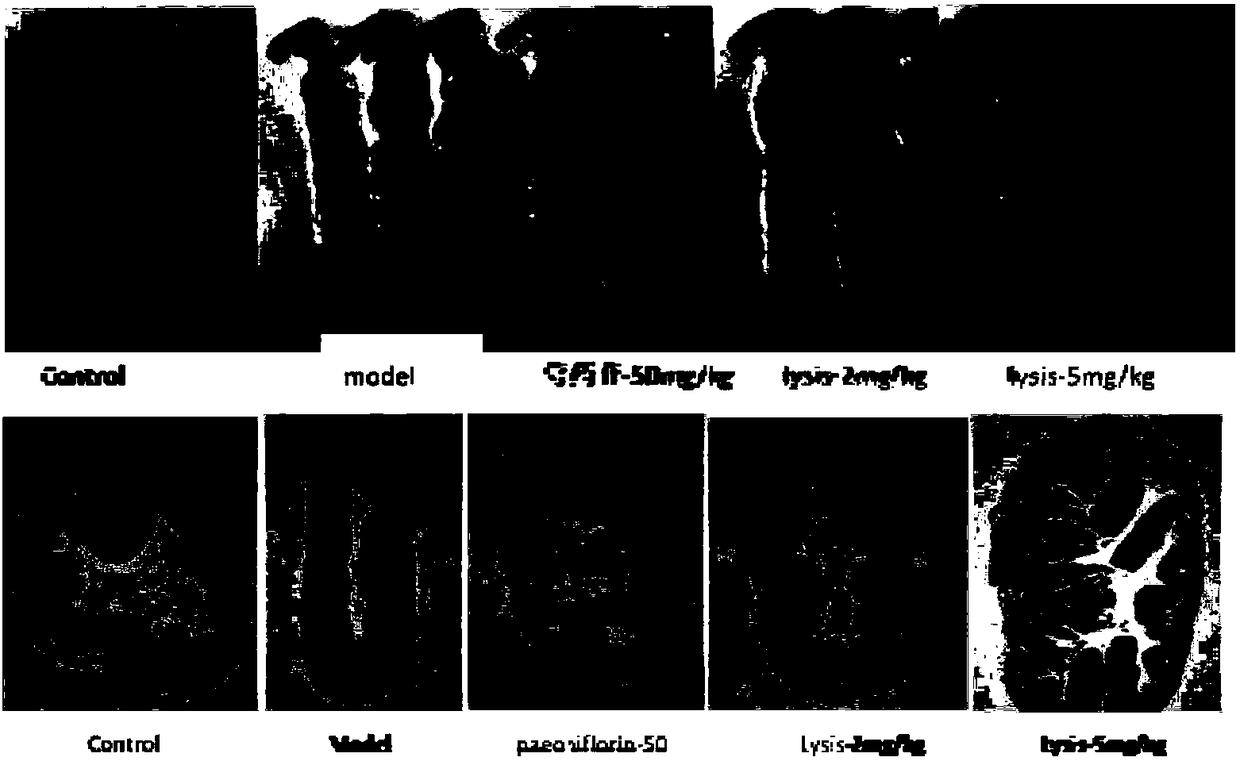
Application of paeoniflorin metabolism pigment I in preparing medicine for treating colitis
ActiveCN108451949AProve the effect of the treatmentEffectively play a therapeutic roleOrganic active ingredientsDigestive systemHyperaemiaTreatment effect
The invention researches the anti-inflammatory activity of DSS (Dextran Sulfate Sodium) inducted colitis model for paeoniflorin and paeoniflorin metabolism pigment I. An experiment result indicates that the paeoniflorin metabolism pigment I has a good treatment effect on ulcerative colitis. Compared with a control group and a paeoniflorin group, a C57BL / 6 mouse which orally takes the paeoniflorinmetabolism pigment I, the paeoniflorin metabolism pigment I is characterized in that a colon tissue damage degree is lightened, the mouse colon tissue damage degree of the low and high dosage medication administration team of the paeoniflorin metabolism pigment I is lightened, a tissue structure is clear in level, the infiltration of inherent layer inflammatory cells is reduced, the amount of cup-shaped cells is relatively increased, muscularis mucosa does not contain tissue edema and hyperaemia, and tissue forms are obviously recovered. The paeoniflorin metabolism pigment I is proved to havea good treatment effect on the ulcerative colitis.
Owner:SHANDONG ANALYSIS & TEST CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com