Therapeutic uses of keratinocyte growth factor-2
A technology of cell and cell proliferation, which is applied in the field of protection or treatment of bladder and prostate, and can solve problems such as damage to the mucosal surface and avulsion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
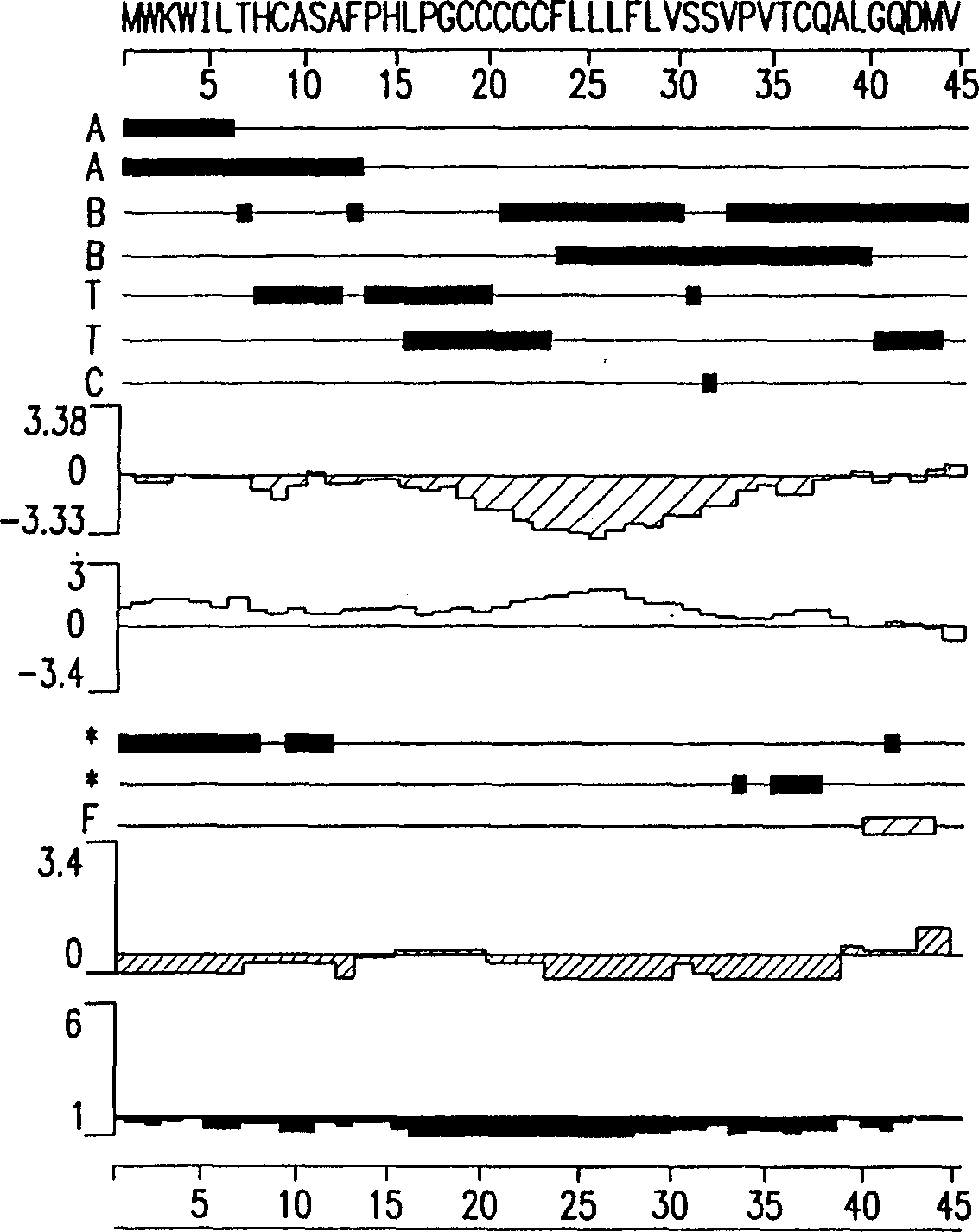
[0147] Epitope-bearing peptides and polypeptides of the invention can be produced by any conventional method for preparing peptides or polypeptides, including recombinant methods using nucleic acid molecules of the invention. For example, short epitope-bearing amino acid sequences can be fused to larger polypeptides that can be used as vectors during recombinant production and purification, and to generate anti-peptide antibodies during immunization. Epitope-bearing peptides can also be synthesized using known chemical synthesis methods. For example, Houghten described a simple method for the synthesis of large numbers of peptides, as 10-20 mg of 248 different peptides representing single amino acid variants of a segment of the HA1 polypeptide were prepared and characterized (by ELISA-type binding studies) within 4 weeks. 13-residue peptides, Houghten, R.A (1985), A general method for rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody int...
Embodiment 24
[0250] In Example 24, the effect of KGF-2 on the proliferation rate of normal rat salivary and lacrimal gland cells was studied. There are three major salivary glands in mammals: the parotid, sublingual, and submandibular salivary glands. A single intravenous injection of KGF-2 induced an approximately 40-fold increase in the proliferation of serous cells in the parotid gland, an 18-fold increase in the proliferation of serous and mucous cells in the submandibular gland, and a 10-fold increase in the proliferation of serous cells in the lacrimal gland. KGF-2-induced increased salivary and lacrimal cell proliferation was reversible.
[0251] KGF-2 significantly increased the proliferation of normal serous and mucus secreting cells in normal rat salivary and lacrimal glands, but did not appear to elicit a proliferative response in ductal cells (see Example 24). This effect on the salivary and lacrimal glands was reversed after removal treatment, suggesting that KGF-2 is safe an...
Embodiment 1
[0325] Bacterial expression and purification of embodiment 1KGF-2
[0326]The DNA sequence ATCC #75977 encoding KGF-2 was first amplified with PCR oligonucleotide primers corresponding to the 5' and 3' sequences of the processed KGF-2 cDNA (including the signal peptide sequence). The 5' oligonucleotide primer contained the sequence 5'CCCCACATGTGGAAATGGATACTGACACATTGTGCC3' (SEQ ID NO. 3), which contained an AflIII restriction enzyme site including and followed by the KGF-2 coding sequence starting from the putative start codon. 30 nucleotides of the sequence. The 3' sequence 5'CCCAAGCTTCCACAAACGTTGCCTTCCTCTATGAG3' (SEQ ID NO. 4) contains the complement of the HindIII site followed by 26 nucleotides of KGF-2. These restriction enzyme sites are compatible with those on the bacterial expression vector pQE-60 (Qiagen, Inc. Chatsworth, CA). pQE-60 encodes antibiotic resistance (AMpr), a bacterial origin of replication (ori), IPTG-regulated promoter operator (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com