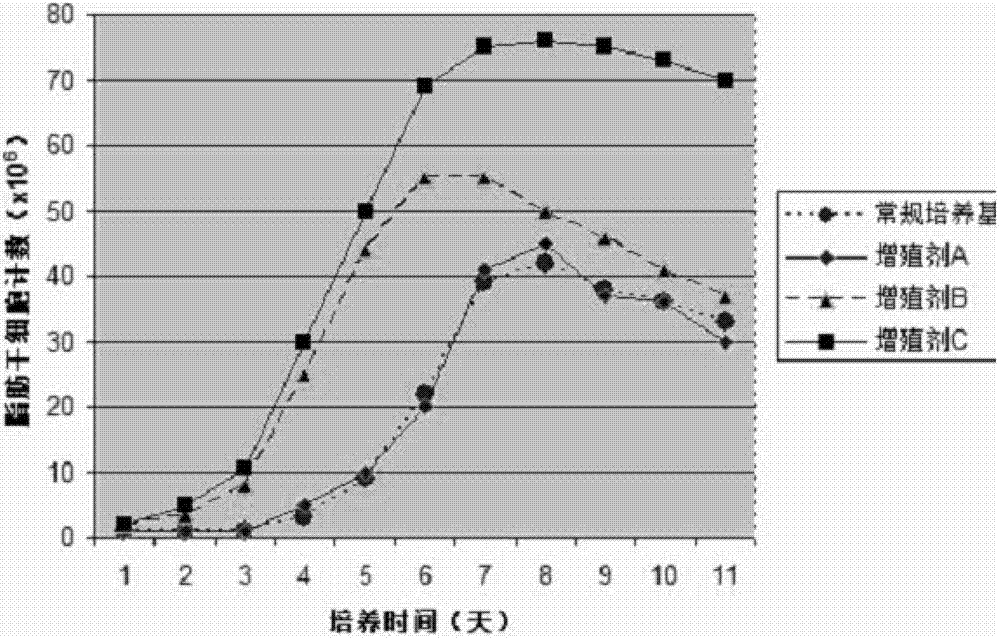
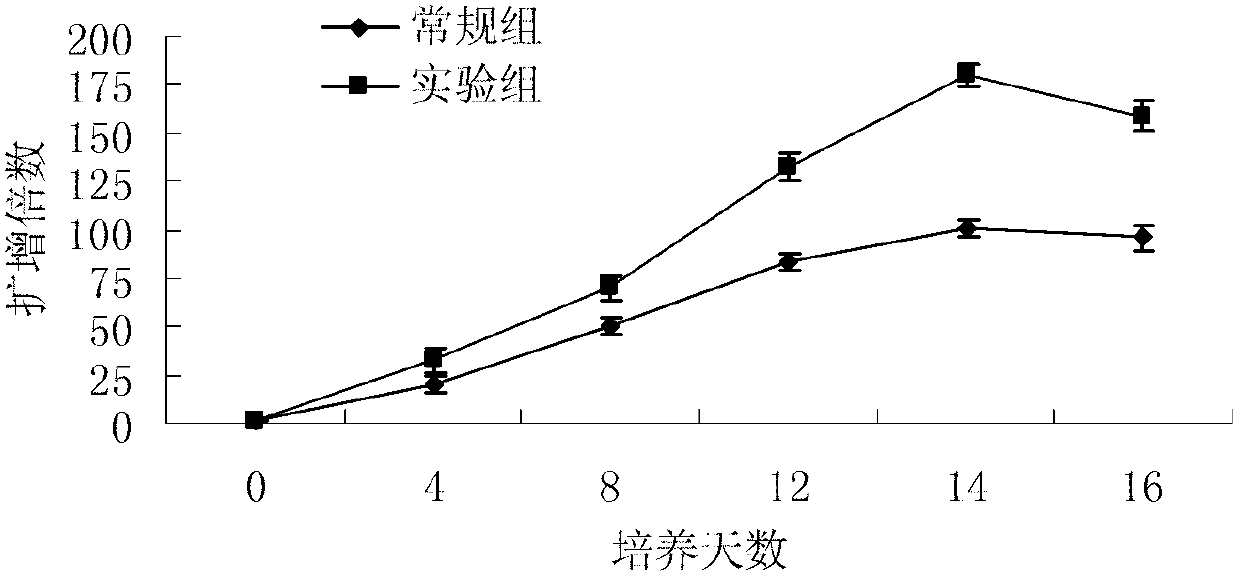
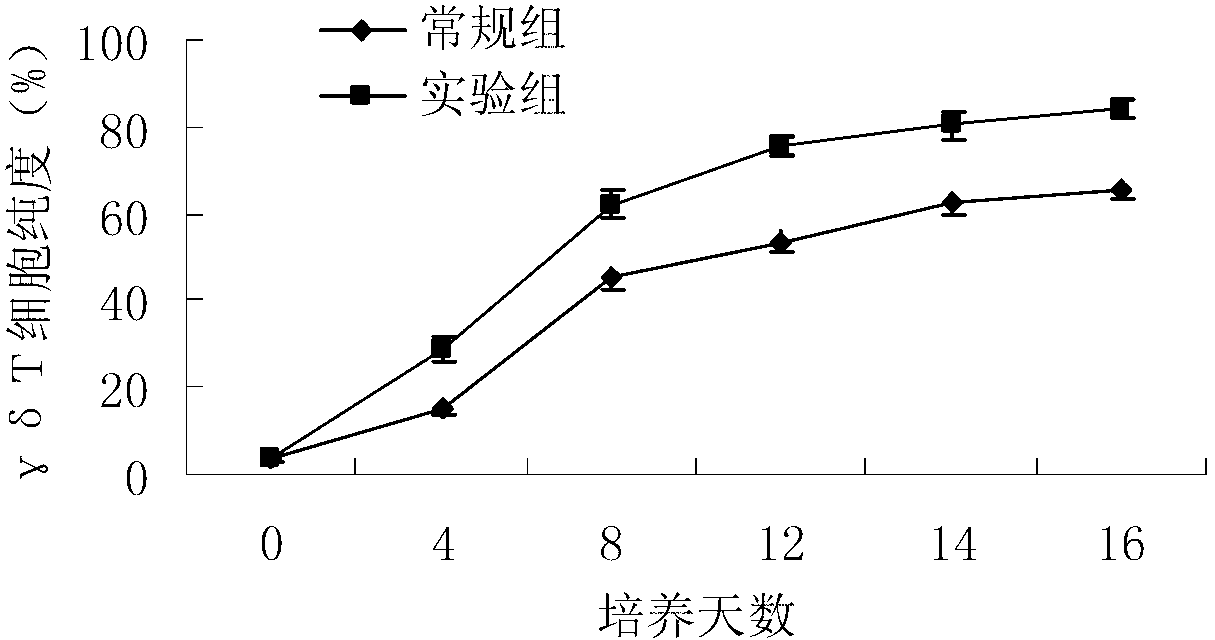
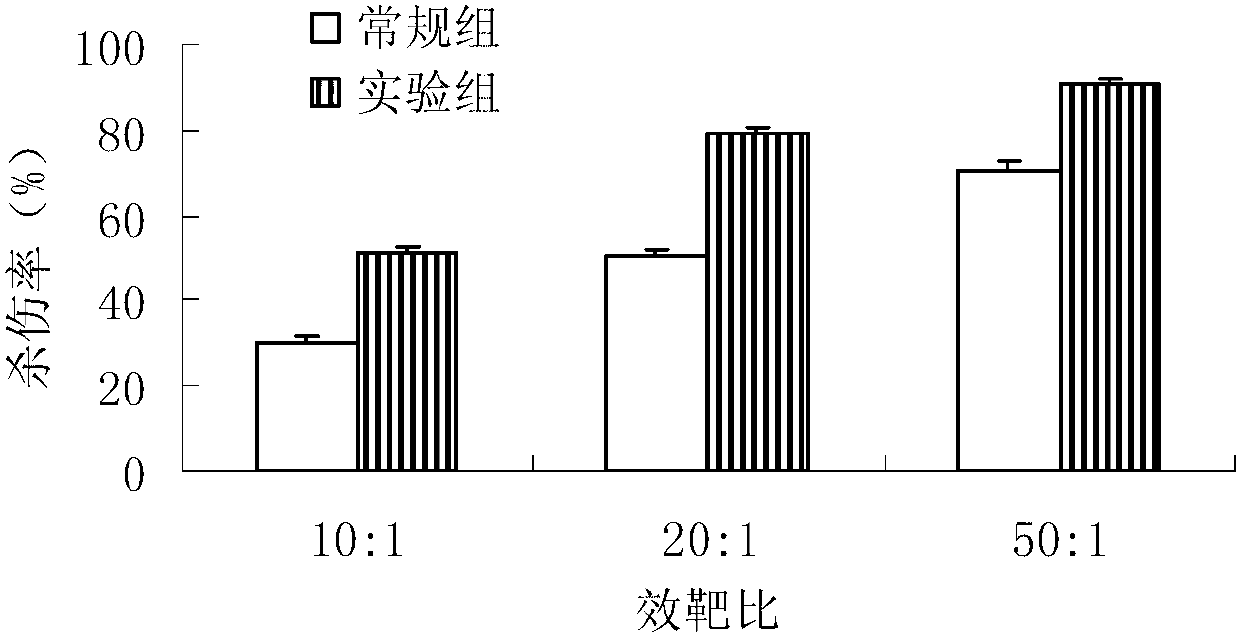
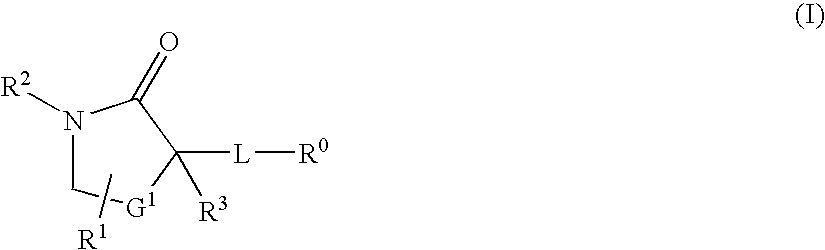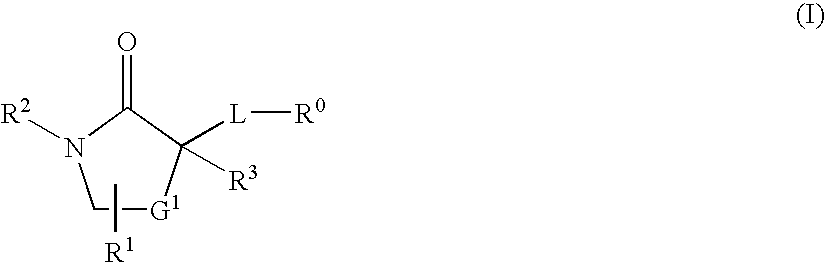Patents
Literature
1085results about How to "Inhibit apoptosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
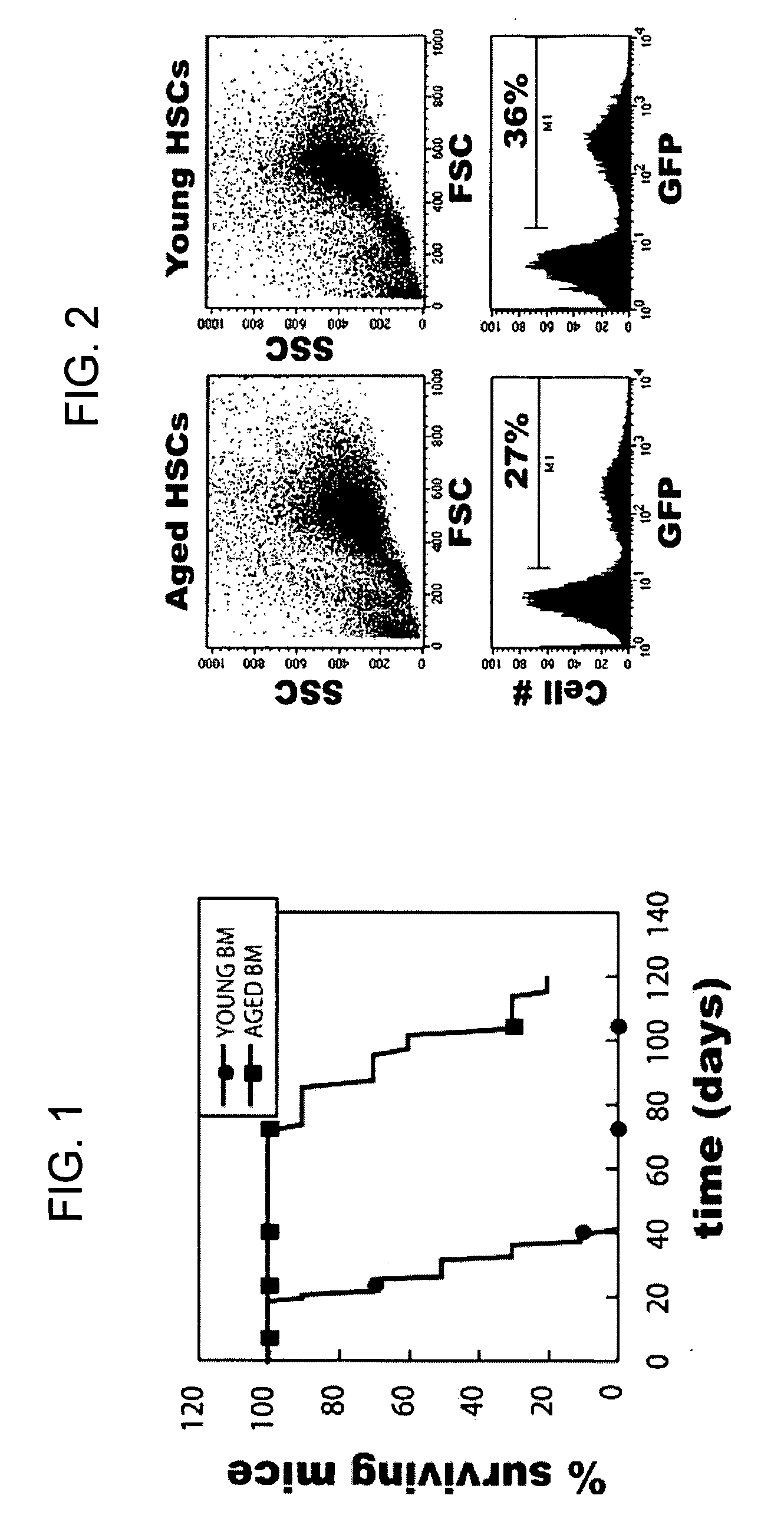
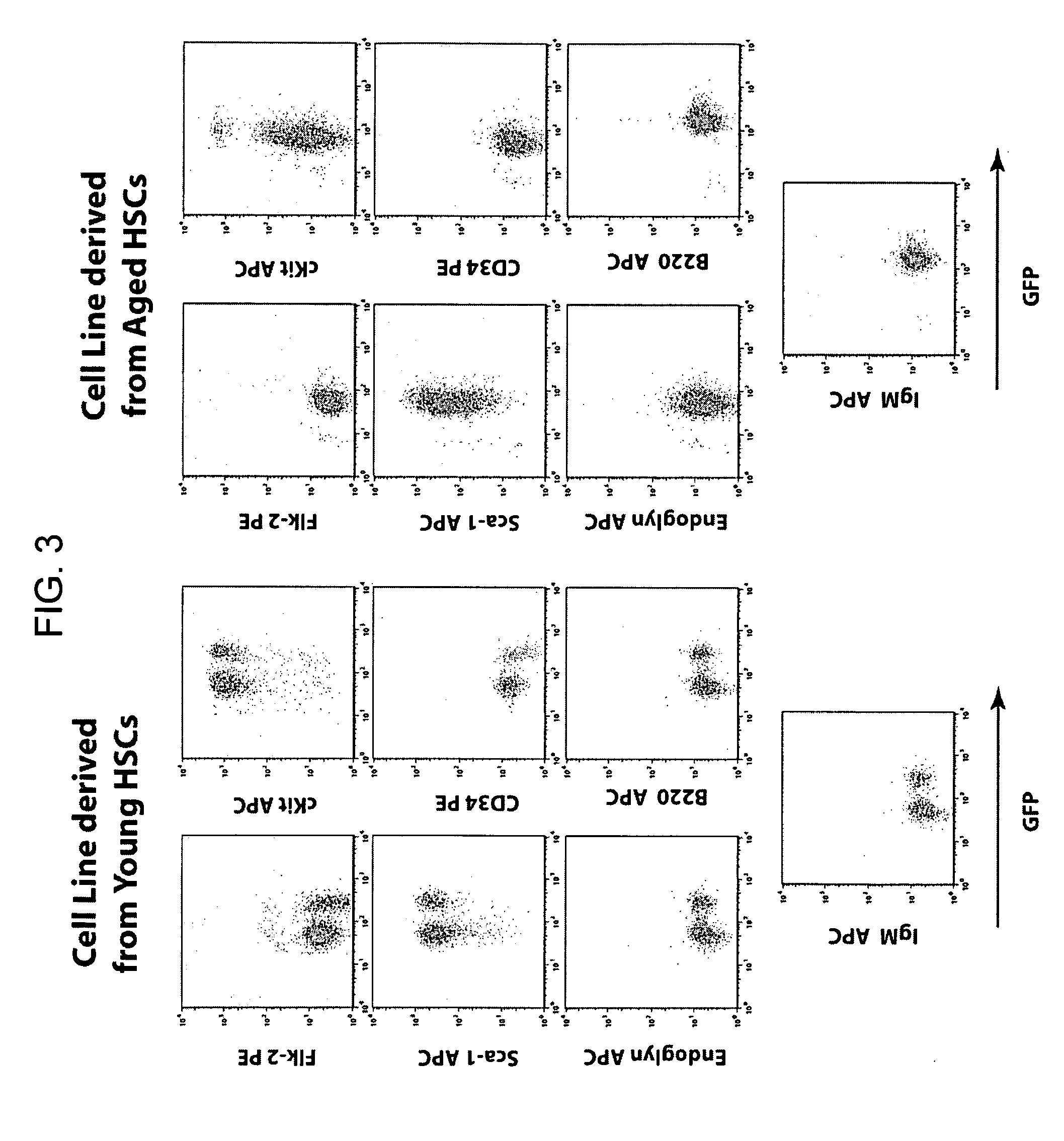
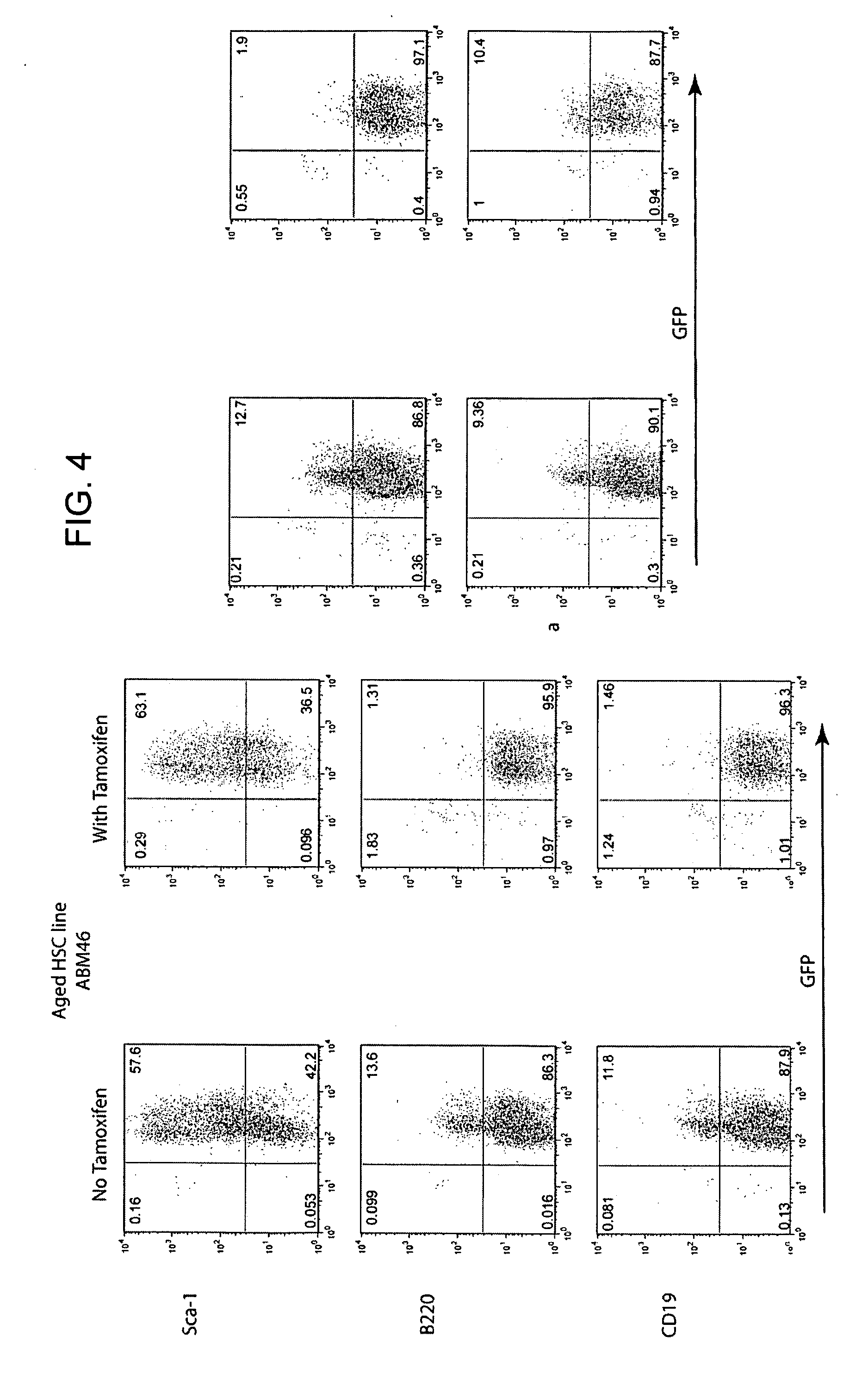
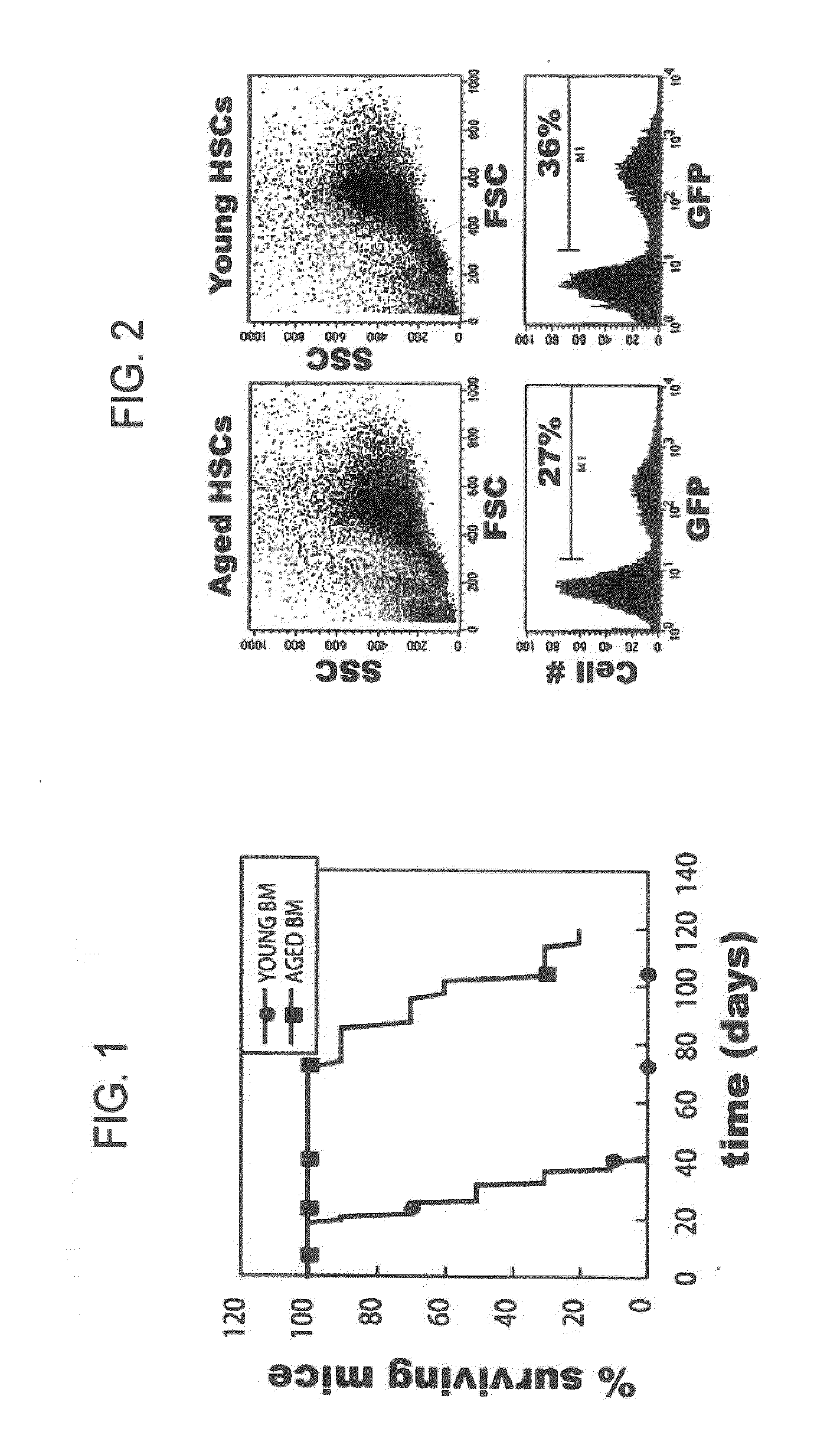
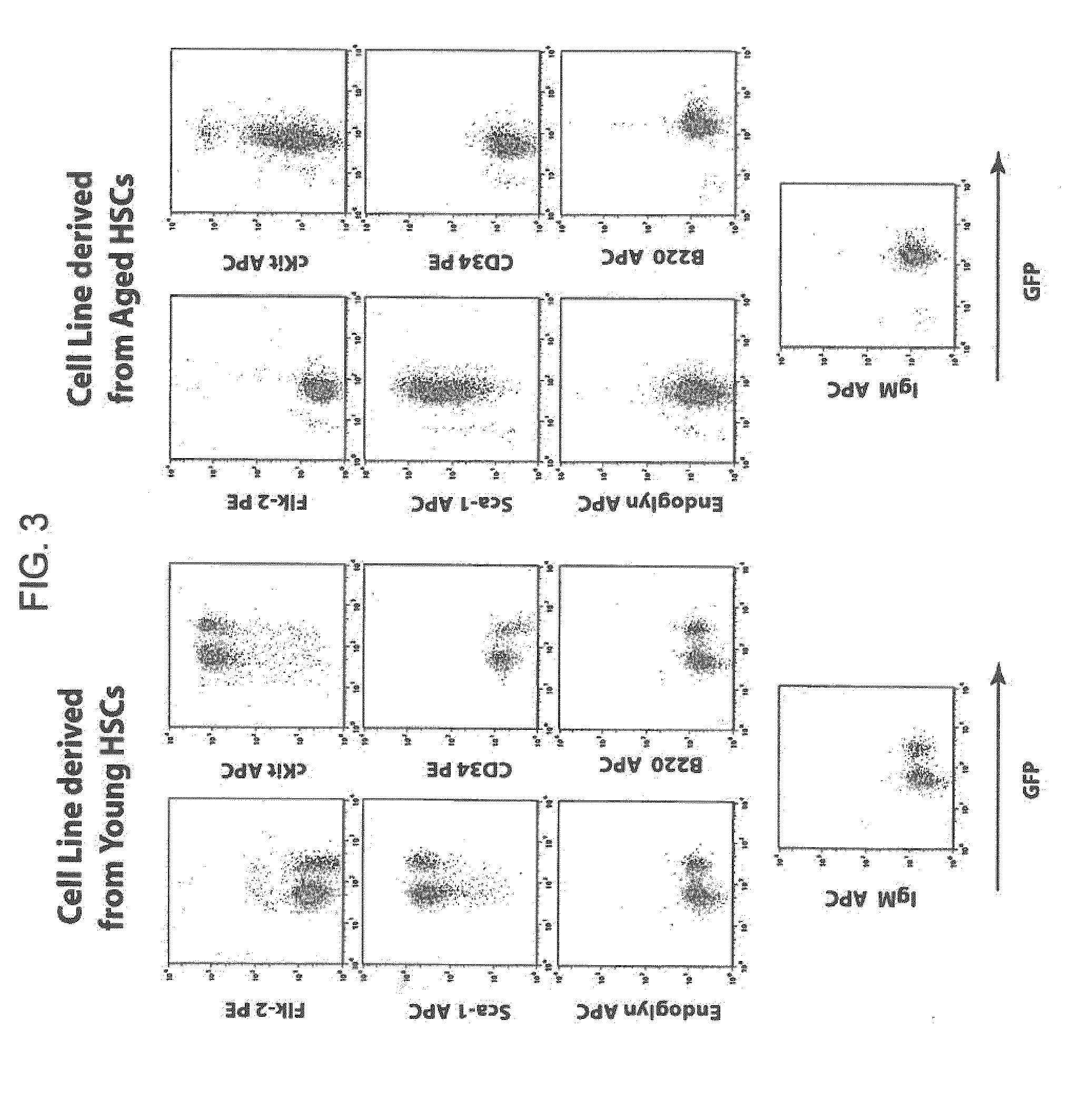
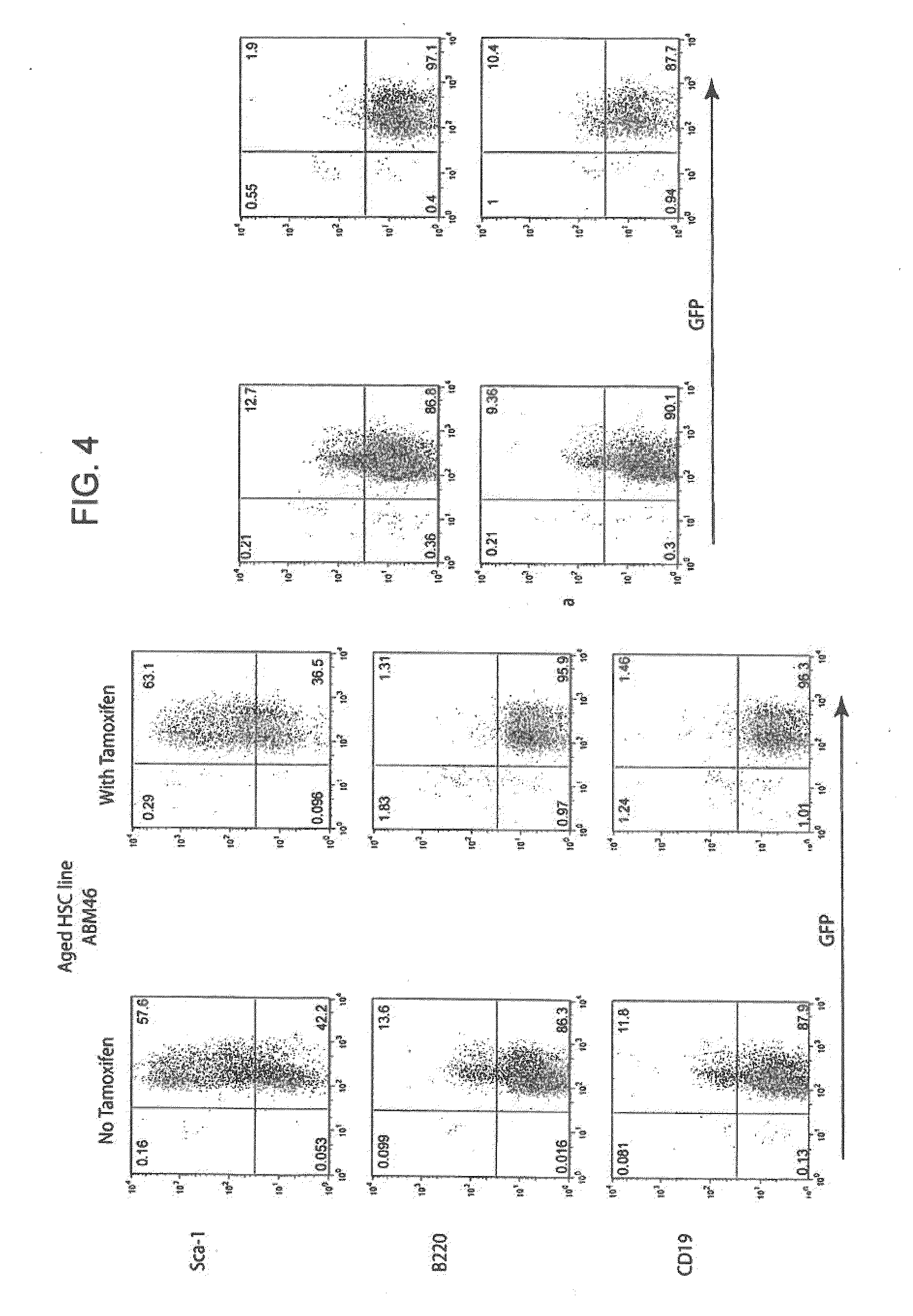
Conditionally immortalized long-term stem cells and methods of making and using such cells
ActiveUS20070116691A1Inhibit apoptosisPromotes proliferationVirusesGenetic material ingredientsMyeloid leukemiaStudy methods
Disclosed are methods for conditionally immortalizing stem cells, including adult and embryonic stem cells, the cells produced by such methods, therapeutic and laboratory or research methods of using such cells, and methods to identify compounds related to cell differentiation and development or to treat diseases, using such cells. A mouse model of acute myeloid leukemia (AML) and cells and methods related to such mouse model are also described.
Owner:UNIV OF COLORADO THE REGENTS OF +1
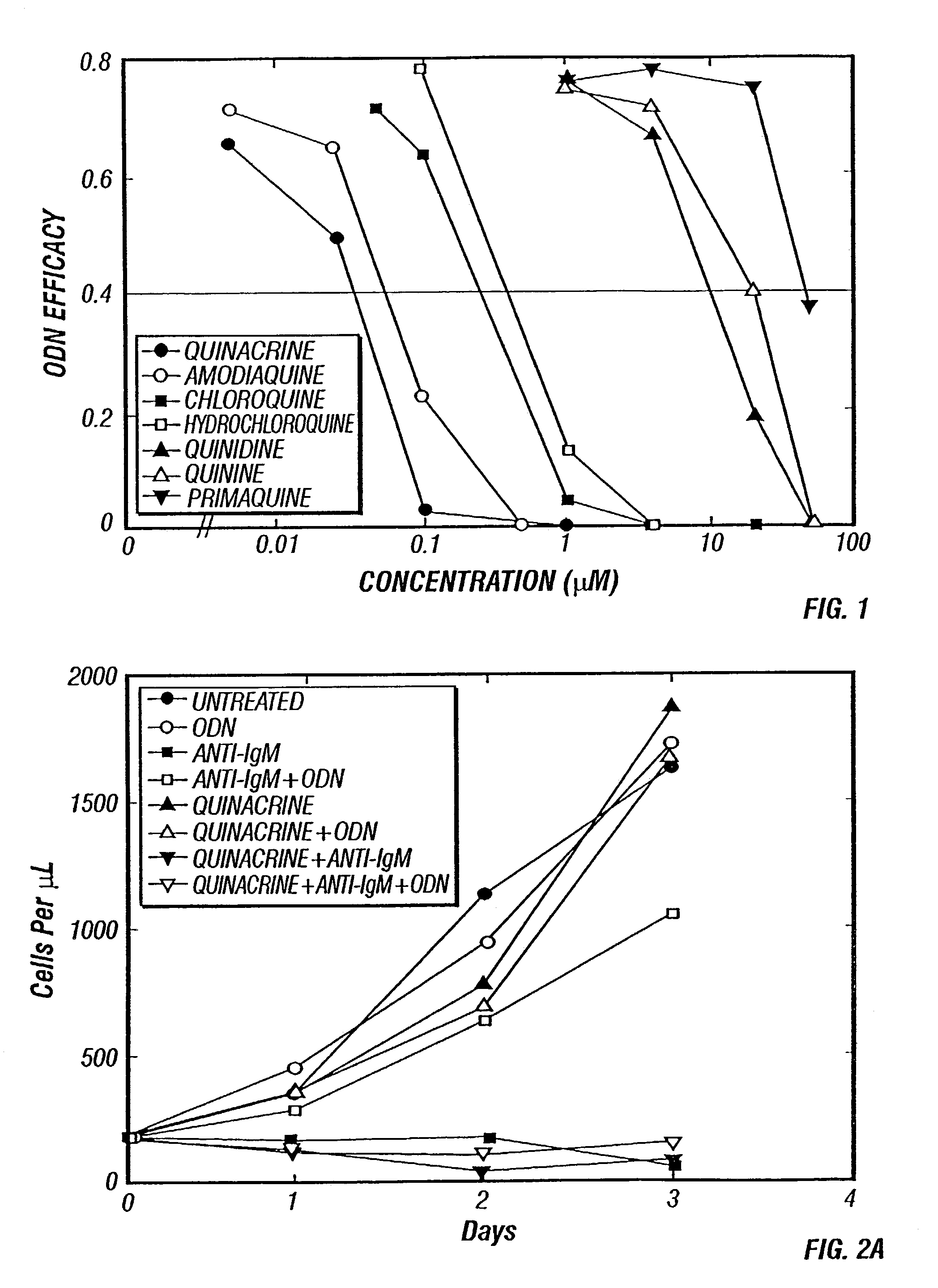
Methods for inhibiting immunostimulatory DNA associated responses
InactiveUS7354711B2Inhibit apoptosisBiocideMicrobiological testing/measurement4-Aminoquinoline9-Aminoacridine
The invention features methods for inhibiting immunostimulatory DNA-associated immune responses, through the administration of specific compounds of the general class of 9-aminoacridines and 4-aminoquiniolines. The invention also features methods of screening compounds useful in inhibiting immunostimulatory DNA-induced immune responses.
Owner:UNIV OF IOWA RES FOUND +1
Death domain containing receptor 4
InactiveUS20040136950A1Increased apoptosisEnhanced signalVirusesPeptide/protein ingredientsAntibodyDisease
The present invention relates to novel Death Domain Containing Receptor-4 (DR4) proteins which are members of the tumor necrosis factor (TNF) receptor family. In particular, isolated nucleic acid molecules are provided encoding the human DR4 proteins. DR4 polypeptides are also provided as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying agonists and antagonists of DR4 activity and methods for using DR4 polynucleotides and polypeptides. The invention also relates to the treatment of diseases associated with reduced or increased levels of apoptosis using antibodies specific for DR4, which may be agonists and / or antagonists of DR4 activity.
Owner:THE RGT OF THE UNIV OF MICHIGAN +1
Method used for in vitro proliferation of NK cells
ActiveCN103756963AIncrease lethalityEasy to synthesizeBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
Owner:SHANGHAI CLAISON BIOTECH
Conditionally immortalized long-term stem cells and methods of making and using such cells
ActiveUS20100297763A1Inhibit apoptosisPromotes proliferationVirus peptidesApoptosis related proteinsDiseaseStudy methods
Disclosed are methods for conditionally immortalizing stem cells, including adult and embryonic stem cells, the cells produced by such methods, therapeutic and laboratory or research methods of using such cells, and methods to identify compounds related to cell differentiation and development or to treat diseases, using such cells. A mouse model of acute myeloid leukemia (AML) and cells and methods related to such mouse model are also described.
Owner:NAT JEWISH HEALTH +1
Novel peptides and combination of peptides for use in immunotherapy against NHL and other cancers
ActiveUS20170296640A1High error ratePromote cell proliferationTumor rejection antigen precursorsTumor specific antigensEpitopeMajor histocompatibility
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Method for in-vitro amplification of NK cells
InactiveCN102994449AEasy to synthesizeIncrease lethalityBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
The invention relates to a method for in-vitro amplification of NK cells, and in particular relates to a method for massive in-vitro amplification of NK cells, wherein the method comprises the following steps of: a, inoculating a peripheral blood mononuclear cell in a CD3McAb and CD226McAb pre-coated culture bottle for coculture; b, adding 1L-2 and 1L-18, coculturing for 72hours to stimulate amplification of NK cells; c, transferring the NK cells, K562 cells after lethal treatment and a serum-free medium containing 1L-2 and 1L-18 in a cell culture bag for coculture; and d, collecting the NK cells. According to the method for in-vitro amplification of the NK cells, two antibodies CD3McAb and CD226McAb are simultaneously coated, so the cell factor synthesis and ADCC effect are promoted, and killing toxicity of the NK cells is remarkably improved; the activation and amplification on the NK cells are achieved just by the 1L-2 and 1L-18 cell factors, so the amplification multiple and cell toxicity of the NK cells are guaranteed, and the cost of cell culture is reduced.
Owner:SHANGHAI CLAISON BIOTECH
Culture medium containing human umbilical cord mesenchymal stem cell exudates and preparation method and applications thereof
The invention relates to a culture medium containing human umbilical cord mesenchymal stem cell exudates and a preparation method and applications thereof. An umbilical cord mesenchymal stem cell finite cell line is established to identify biological characteristics of the umbilical cord mesenchymal stem cell, and the preparation method for the human umbilical cord mesenchymal stem cell exudates is established to verify that the human umbilical cord mesenchymal stem cell (HUCMSC) exudates can promote cell regeneration, improve cell function and suppress cell apoptosis in vitro. A detection of enzyme-linked immunosorbent assay (ELISA) shows that human umbilical cord mesenchymal stem cell exudates contain abundant cell factors which can promote cell proliferation and suppress cell apoptosis, such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF).
Owner:斯坦姆生物科技江苏有限公司
Compositions and methods of promoting wound healing
InactiveUS20100272679A1Promote and accelerate wound healingReduces the formation of scarsChemokinesPeptide/protein ingredientsMedicineBone healing
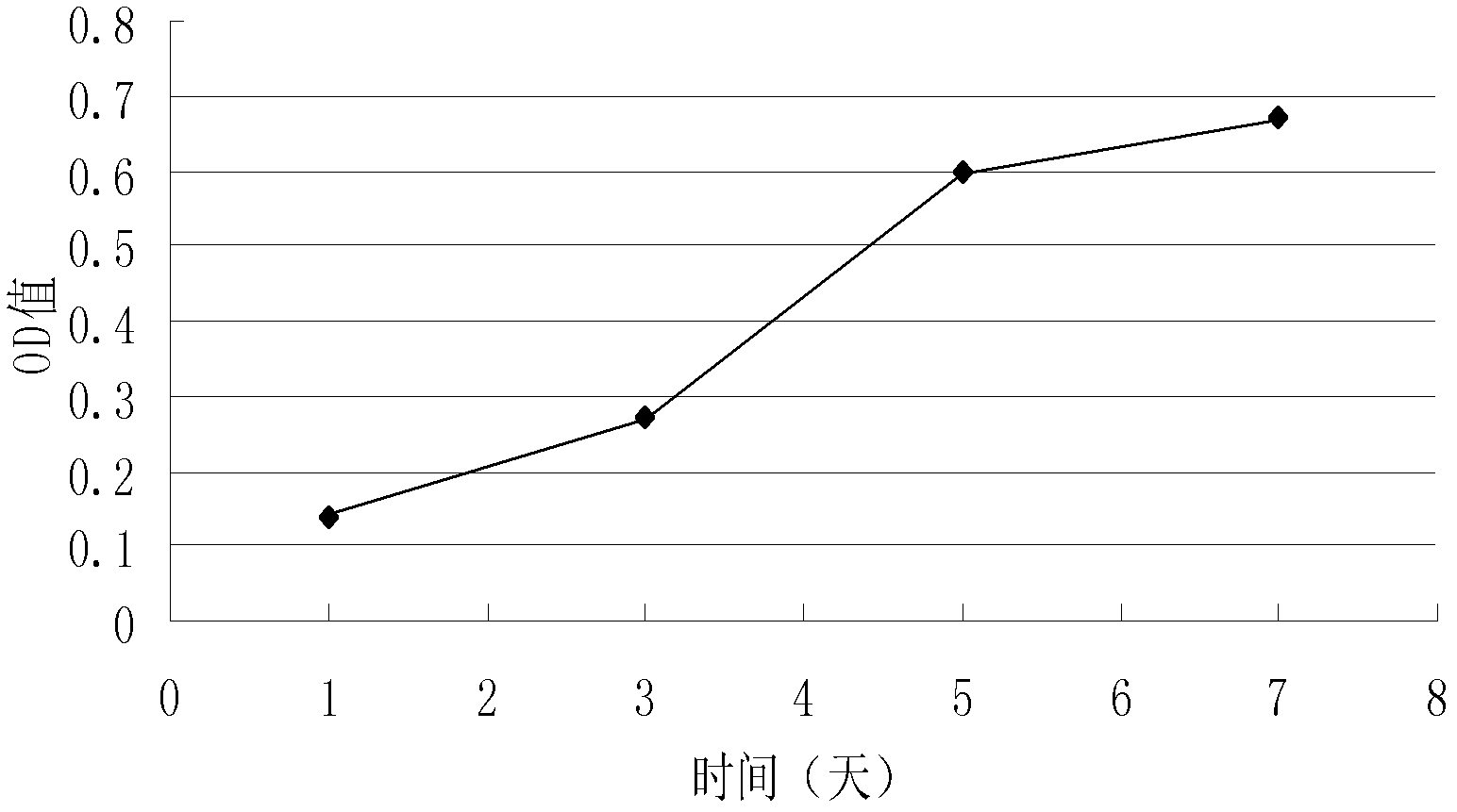
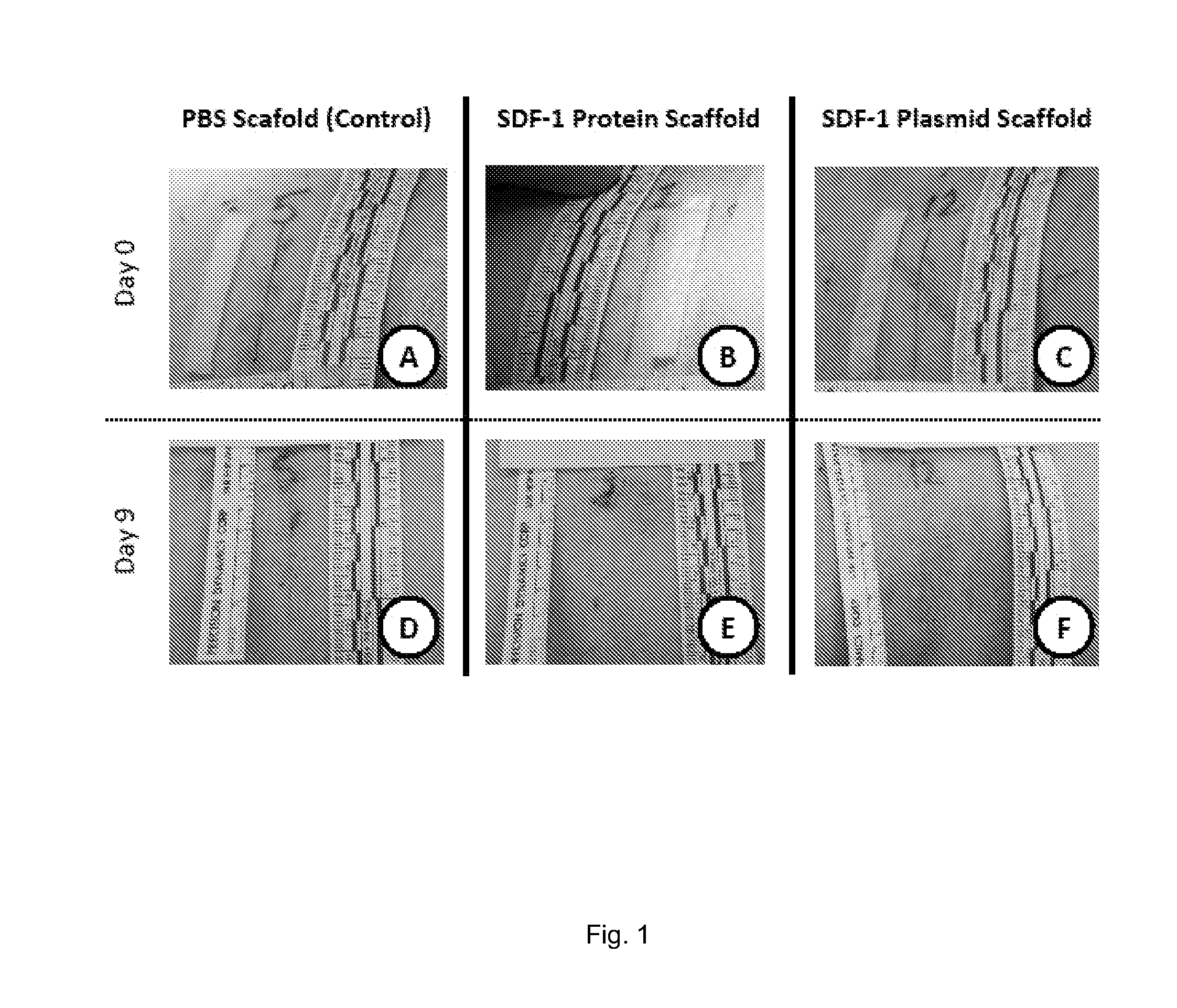
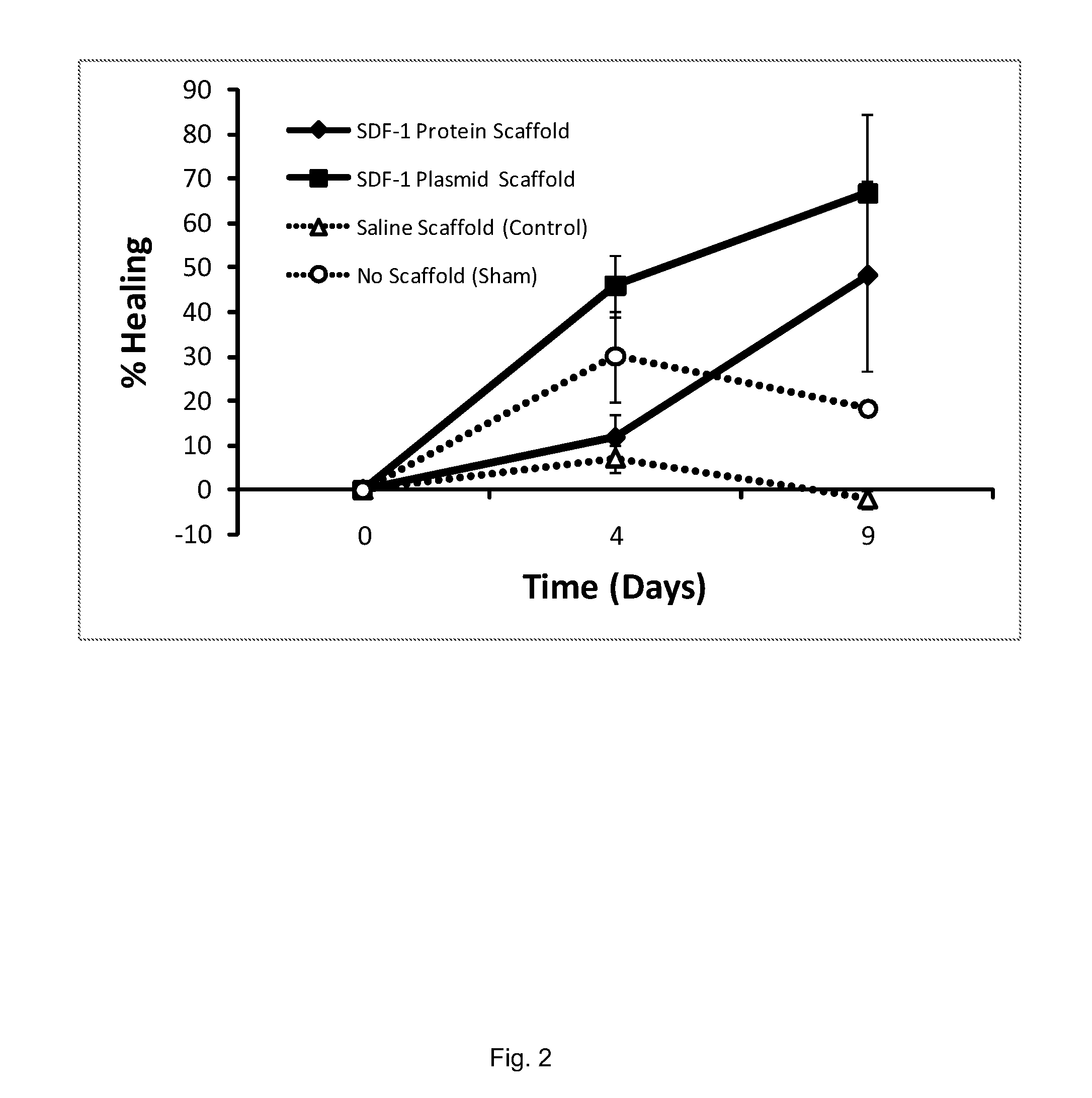
A method of treating a wound in a subject includes administering directly to the wound or an area proximate the wound an amount of SDF-I effective to promote healing of the wound of the subject.
Owner:THE CLEVELAND CLINIC FOUND +1
Mutant human plasminogen kringle5, preparation method and application thereof
The invention discloses a mutant human plasminogen kringle5 gene mK5 and an amplification primer of the mK5 gene, the mutant human plasminogen kringle5 gene modified by adding glutathione-S-transferase before the gene mK5, and a method for preparing protein mK5 recombinant protein of two gene codes, glutathioneS transferase (GST)-mK5 fusion protein and two proteins, wherein the mK5 gene and the GST-mK 5 gene can be applied to preparing medicaments for treating angiogenesis diseases. By the invention, the number of exogenous amino acid in the recombinant K5 protein molecules obtained by a gene engineering method is remarkably reduced, the K5 bioactivity of the obtained mK5 is improved, while the mK5 activity of the GST-mK5 fusion protein is maintained, the stability and water solubility of the protein are improved, the purification steps of an expressed product are simplified, and the purity of the product is improved.
Owner:SUN YAT SEN UNIV
Glucagon Receptor Antagonists, Preparation and Therapeutic Uses
InactiveUS20080125468A1Function increaseInhibit apoptosisBiocideOrganic chemistryDrugDiabetes mellitus
The present invention discloses novel compounds of Formula (I), or pharmaceutically acceptable salts thereof, which have glucagon receptor antagonist or inverse agonist activity, as well as methods for preparing such compounds. In another embodiment, the invention discloses pharmaceutical compositions comprising compounds of Formula (I) as well as methods of using them to treat diabetic and other glucagon related metabolic disorders, and the like.
Owner:ELI LILLY & CO
LPA receptor agonists and antagonists and methods of use
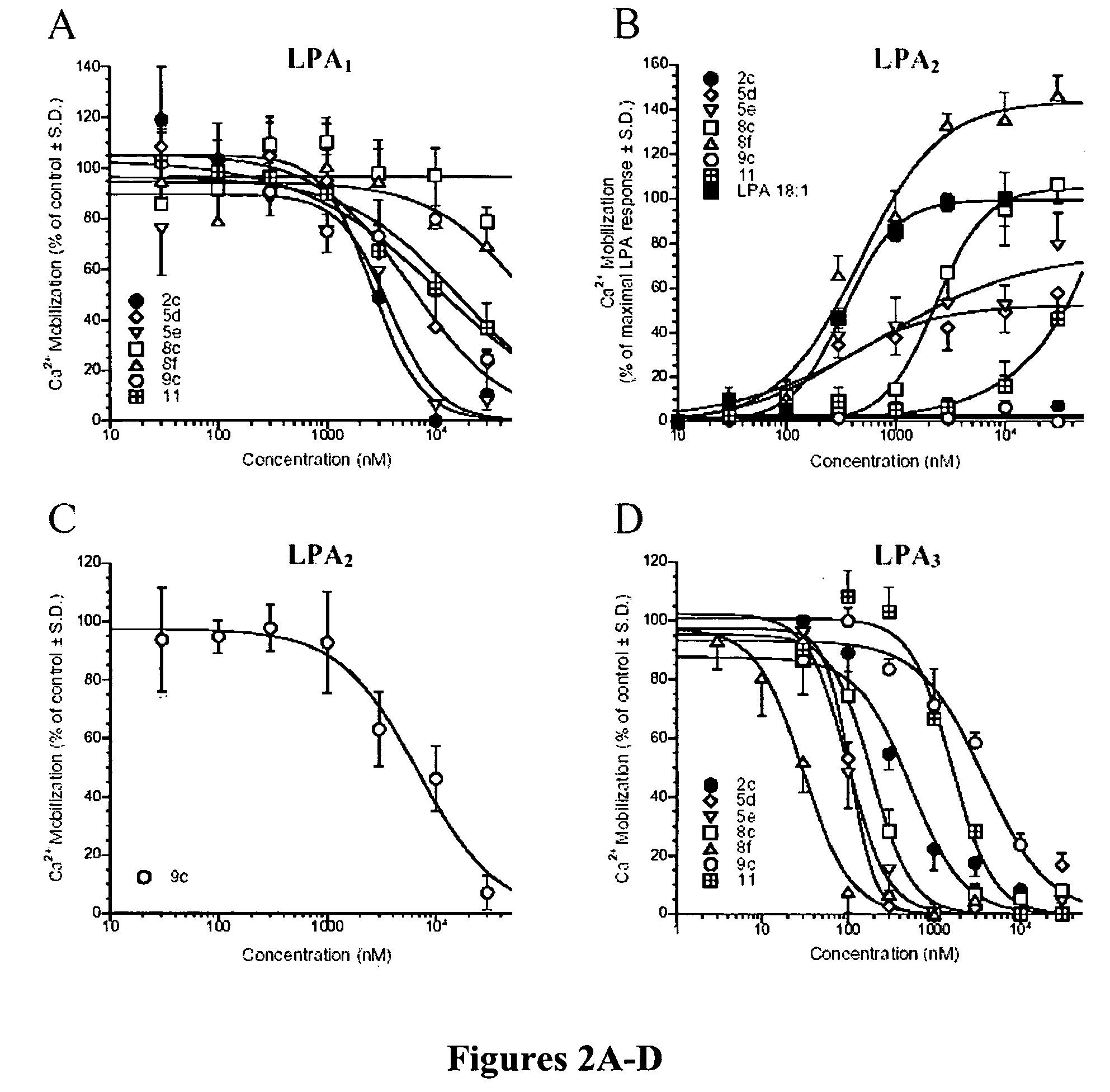
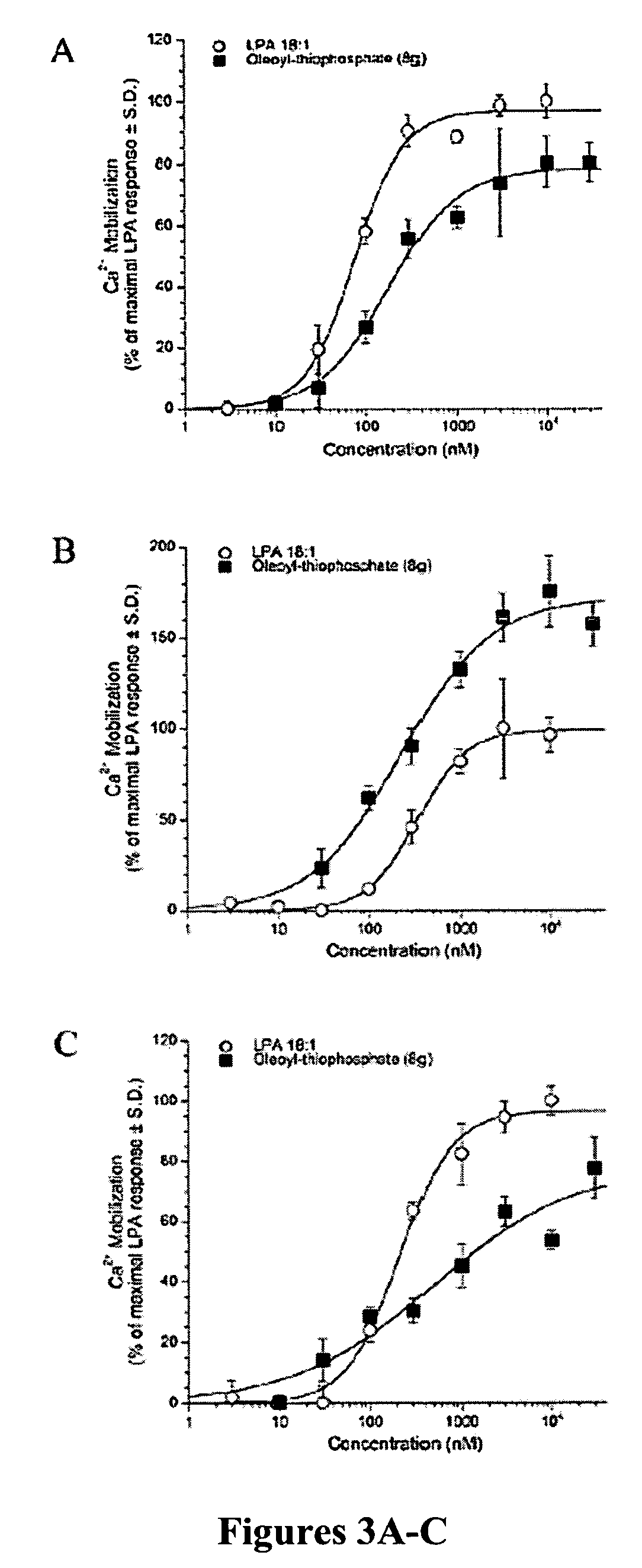
ActiveUS20060009507A1Prevent apoptosisPreserve organ or tissue functionBiocideDigestive systemDrugLPA receptor activity
The present invention relates to compounds according to formula (I) as disclosed herein as well as pharmaceutical compositions which include those compounds. Also disclosed are methods of using such compounds, which have activity as agonists or as antagonists of LPA receptors; such methods including inhibiting LPA activity on an LPA receptor, modulating LPA receptor activity, treating cancer, enhancing cell proliferation, treating a wound, treating apoptosis or preserving or restoring function in a cell, tissue, or organ, culturing cells, preserving organ or tissue function, and treating a dermatological condition.
Owner:UNIV OF TENNESSEE RES FOUND
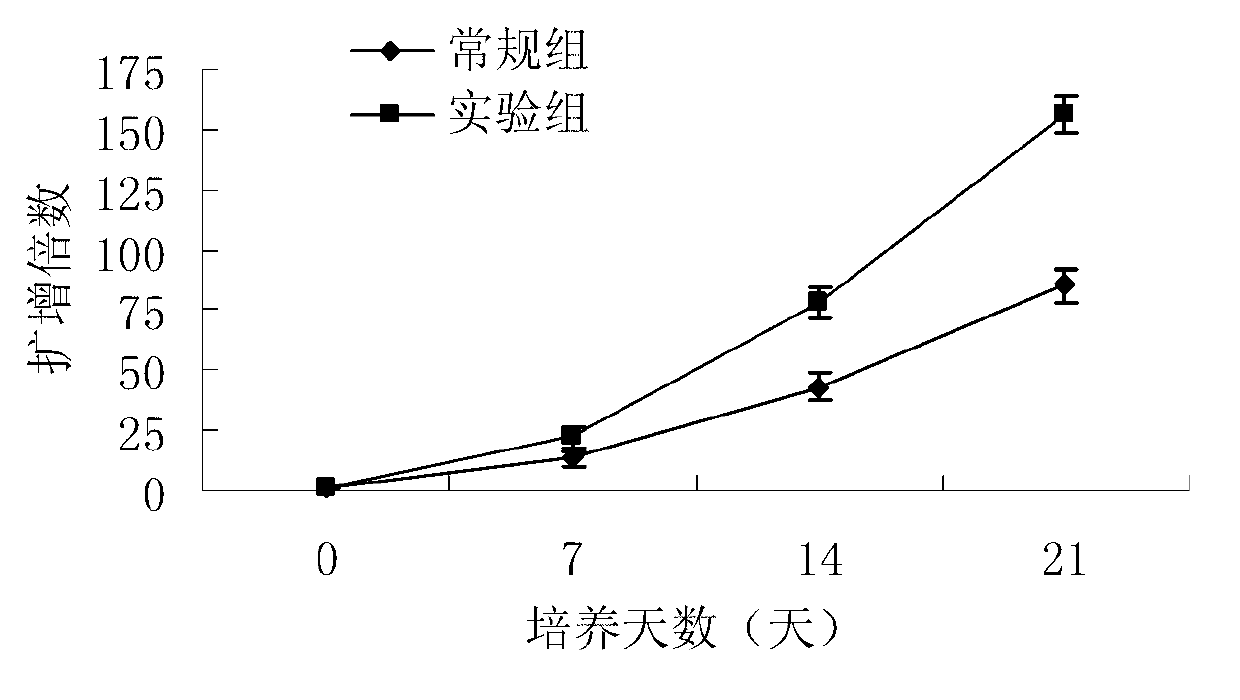
Proliferation accelerator for human adipose-derived stem cells and application method thereof
ActiveCN102757936AInhibit apoptosisConducive to survivalSkeletal/connective tissue cellsL-glutamineCell culture
The invention discloses a proliferation accelerator for human adipose-derived stem cells and an application method thereof. The proliferation accelerator for the human adipose-derived stem cells comprises insulin, hydrocortisone, parathyroid hormone, estradiol, testosterone, a platelet-derived growth factor-AB, an epidermal growth factor, a basic fibroblast growth factor, L-glutamine, 2-mercaptoethanol, a leukemia inhibitory factor, L-ascorbic acid, biotin, dexamethasone, an IGF-I (Insulin-like Growth Factor-I) and a SCF (Stem Cell Factor). The proliferation accelerator for the human adipose-derived stem cells, disclosed by the invention, is applied to proliferation and subculture of the adipose-derived stem cells, can be used to not only specifically promote the growth sped and the proliferation quantity of the adipose-derived stem cells to increase in the form of geometric progression, but also suppress early differentiation and maturity of the adipose-derived stem cells so as to prevent apoptosis or premature senility of the stem cells during culturing and keep the vigorous and active differentiation potentials of the adipose-derived stem cells. The proliferation accelerator for the human adipose-derived stem cells, disclosed by the invention, has good application prospect in the field of culture of the adipose-derived stem cells.
Owner:JIANGSU RE STEM BIOTECH
Nutrient gel for sterilizing and healing wound surface and preparation method of nutrient gel
InactiveCN104399082APromote healingPromote recoveryAntibacterial agentsOrganic active ingredientsNutrition supportSide effect
The invention discloses nutrient gel for sterilizing and healing a wound surface and belongs to the technical field of anti-bacteria gel. The nutrient gel for sterilizing and healing the wound surface is prepared from hydroxyethyl cellulose, chitosan and derivatives thereof, guanidine polymers, glycerinum and compound amino acid. The hydroxyethyl cellulose serves as a gel substrate, the gel which is used for sterilizing and healing the wound surface and contains the guanidine polymers, the chitosan and derivatives thereof and the compound amino acid is prepared through a simple process, and the nutrient gel has good skin compliance, is non-irritant, has sterilization and anti-inflammation functions, provides a certain nutrition support for the wound surface, effectively promotes healing and recovery of the wound surface, has no side effects and is safe and environment-friendly.
Owner:HENAN HUIBO MEDICAL CO LTD
Method for in-vitro amplification of gamma-delta-T cells
InactiveCN102994448AFully stimulatedInhibit apoptosisBlood/immune system cellsPeripheral blood mononuclear cellMicrobiology
The invention relates to a method for culturing gamma-delta-T cells, and in particular relates to a method for in-vitro amplification of gamma-delta-T cells, wherein the method comprises the following operating steps of: pre-coating a T75 culture bottle by a TCR-gamma-delta resisting antibody and CD28McAb for later use use; isolating the peripheral blood mononuclear cell (PBMC) of a patient; regulating the PBMC concentration to 1*10<6> 6 / ml by a serum-free culture medium which contains 5% of autologous plasma, and transferring PBMC cell suspension into the T75 culture bottle; adding an initial culture medium which contains proper concentrations of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; culturing in a saturated humid environment containing 5% of CO2 at 37 DEG C; depending on growth situation of the cell, changing the culture medium every 2-3days, to control the cell concentration at about 2.5*10<6> / ml; meanwhile, compensating full doses of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; and continuously culturing for 12-16days, to obtain a great amount of gamma-delta-T cells which are comparatively high in purity.
Owner:SHANGHAI CLAISON BIOTECH
Methods for treating and preventing gi syndrome and graft versus host disease
ActiveUS20100239572A1Regulate hepatocyte apoptosisProtected hepatocytesAntipyreticAnalgesicsAutoimmune diseaseT lymphocyte
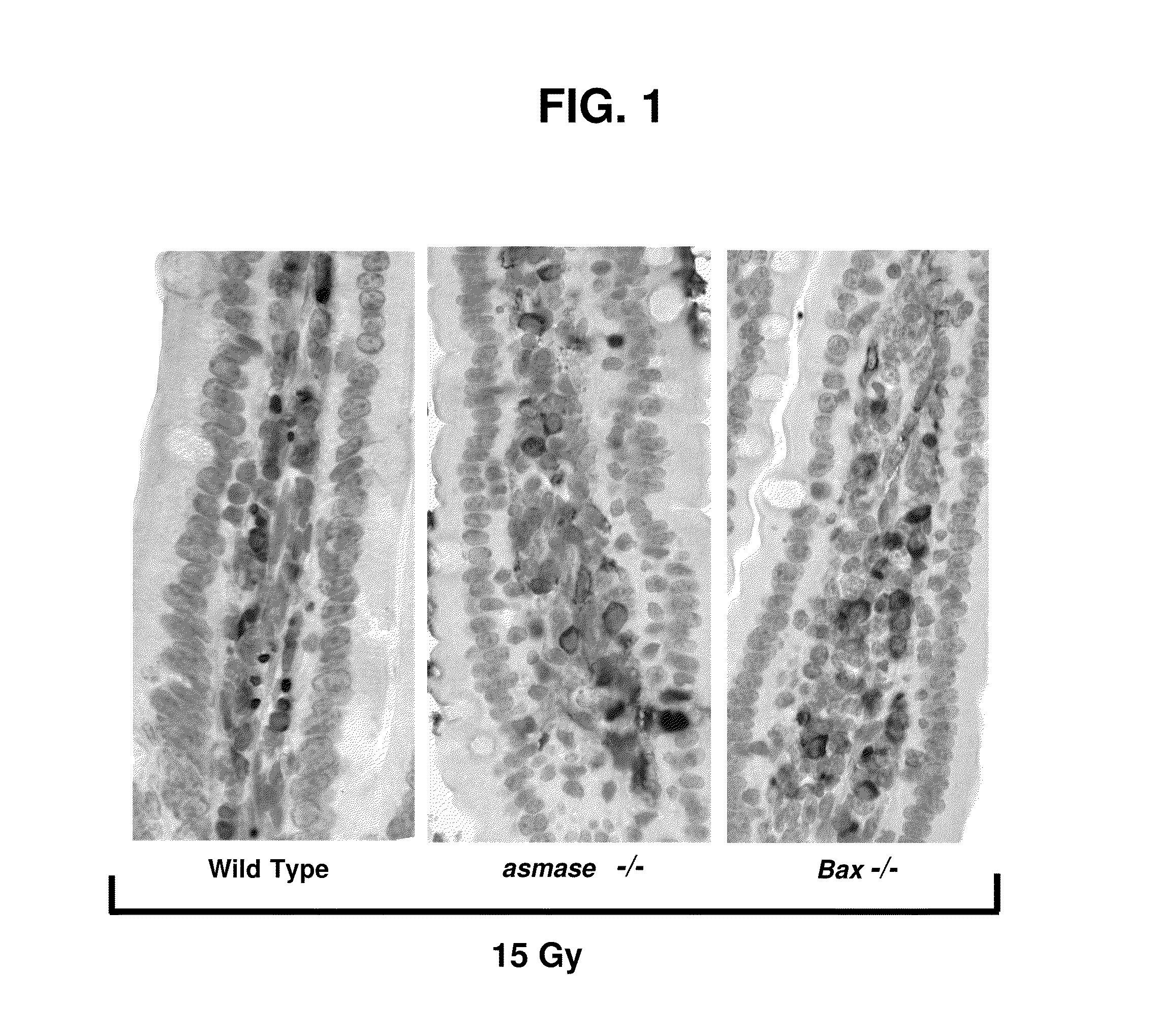
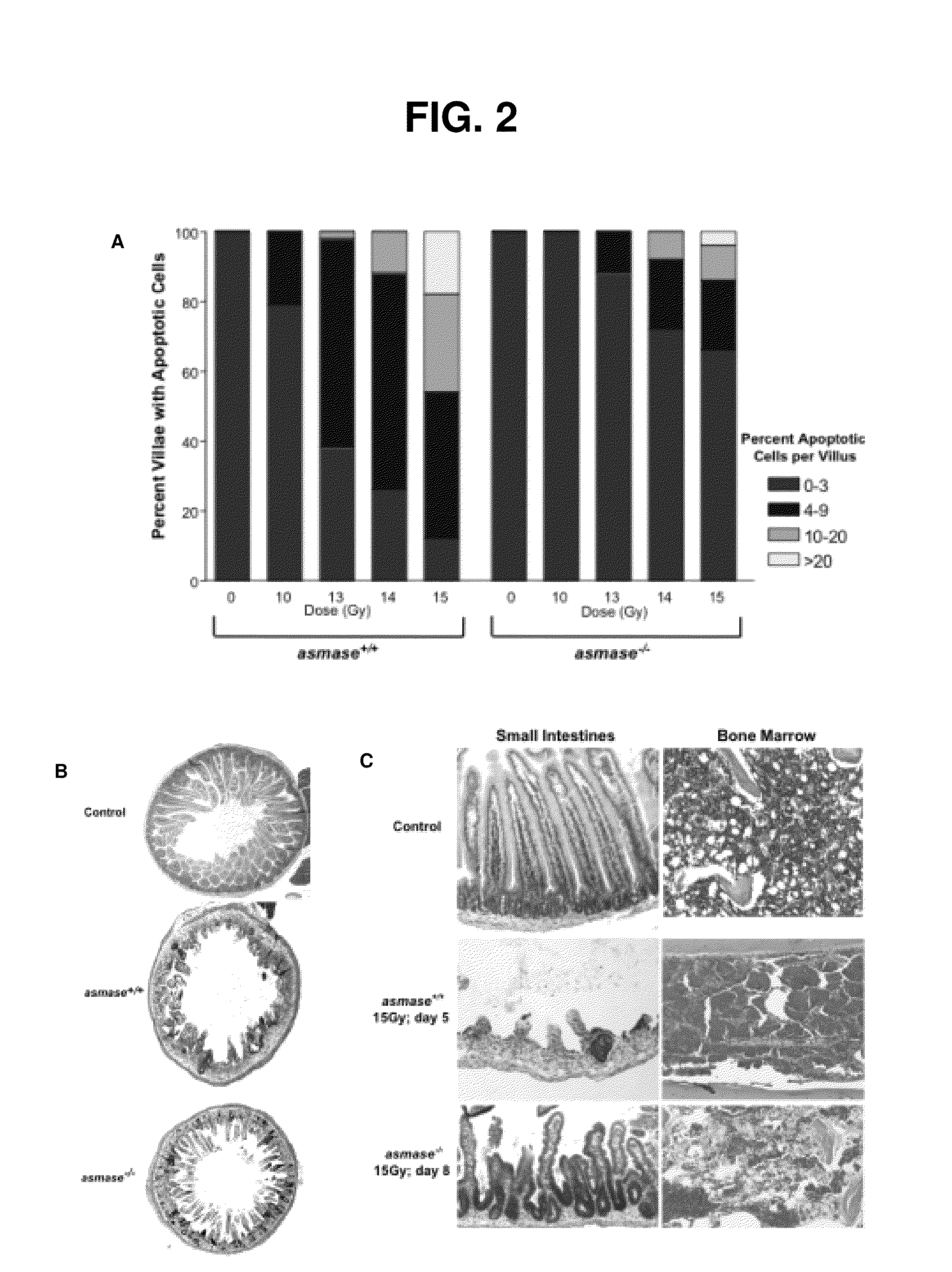
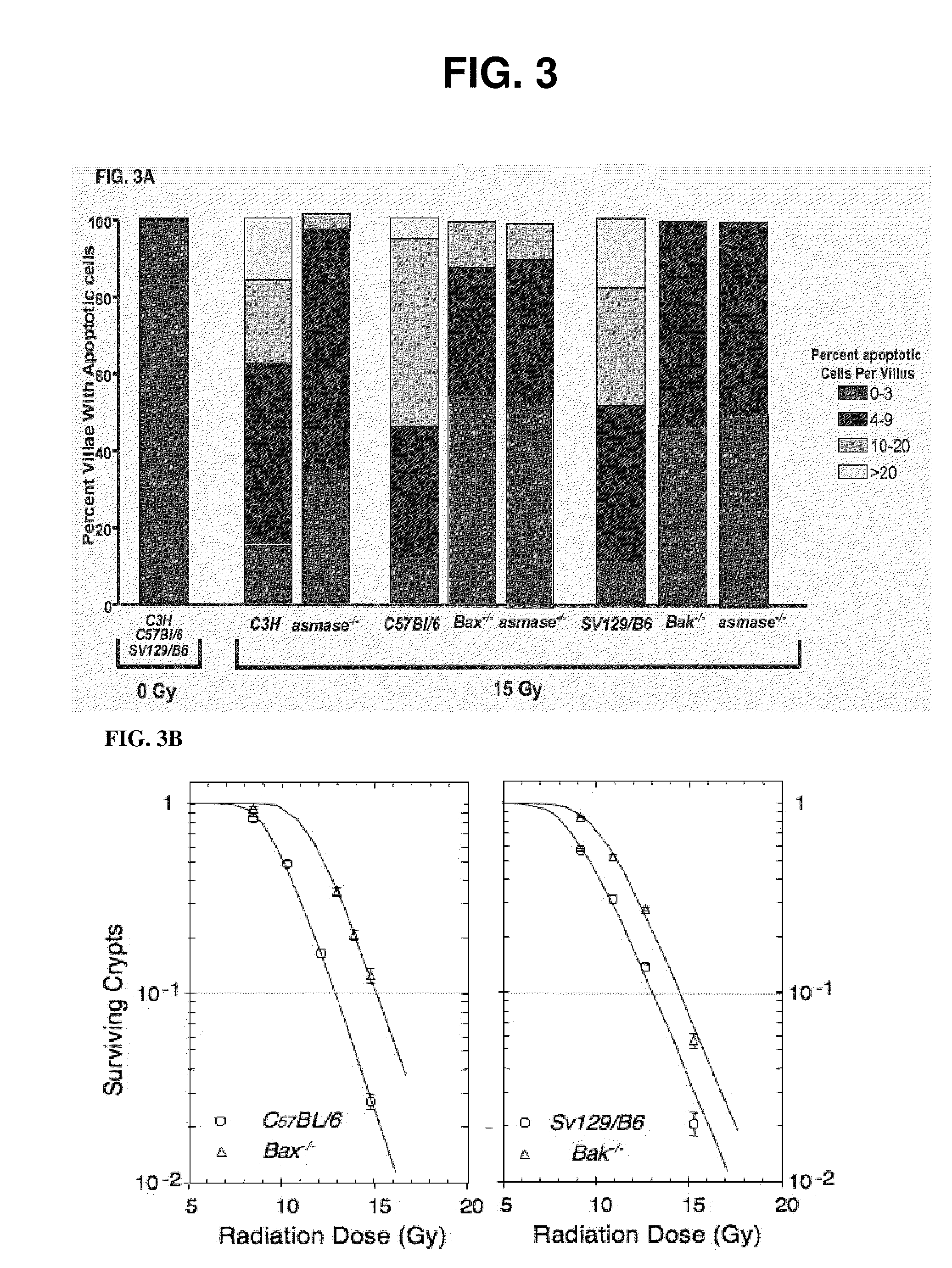
We have discovered that administering anti-ceramide antibody treats and prevents an array of diseases mediated by cytolytic T lymphocyte (CTLs)-induced killing and by damage to endothelial microvasculture, including radiation-induced GI syndrome, Graft vs. Host diseases, inflammatory diseases and autoimmune diseases. We have also discovered new anti-ceramide monoclonal antibodies, that have therapeutic use preferably in humanized form to treat or prevent these diseases.
Owner:THE UNIV OF TEXAS SYST +1
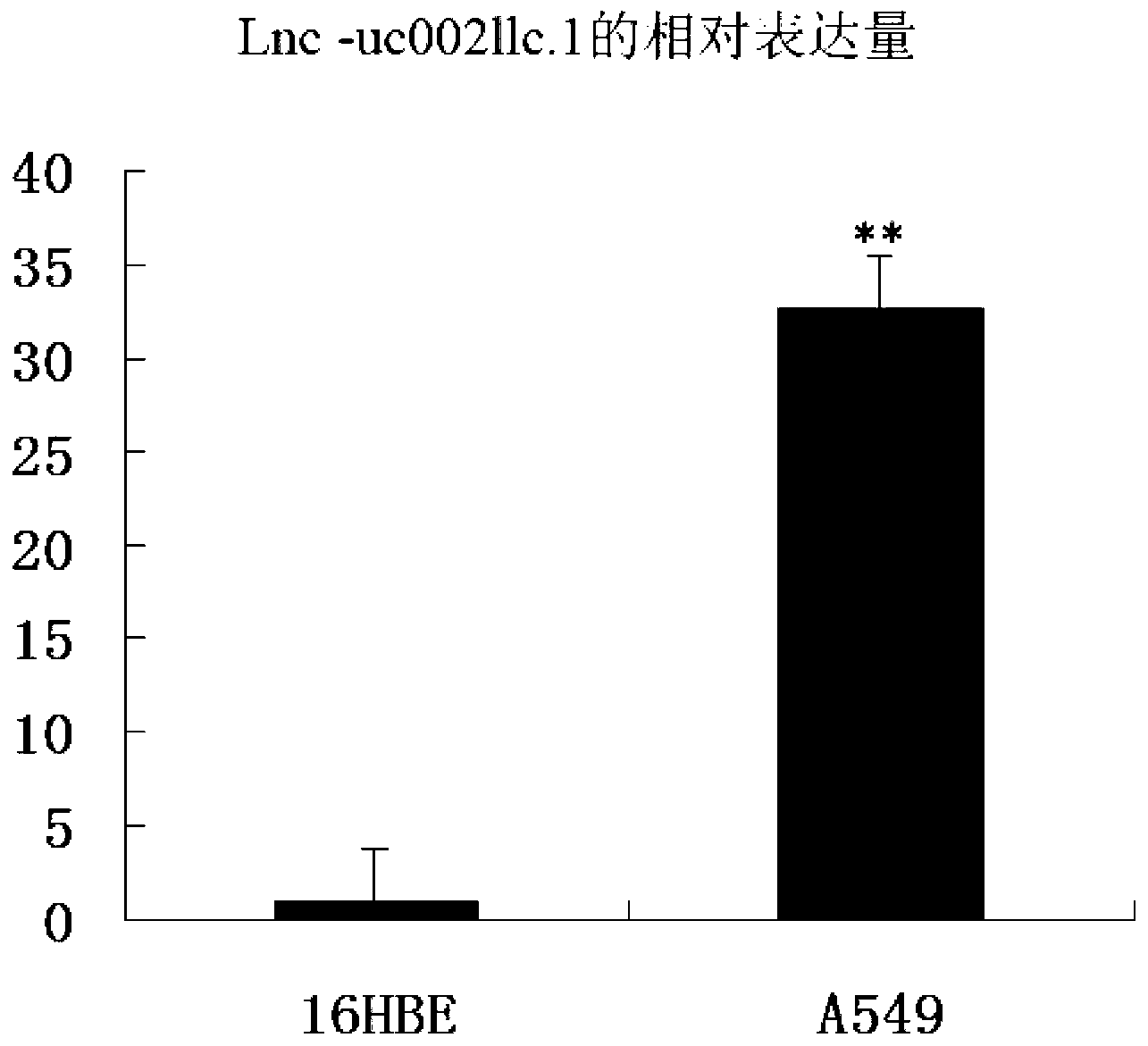
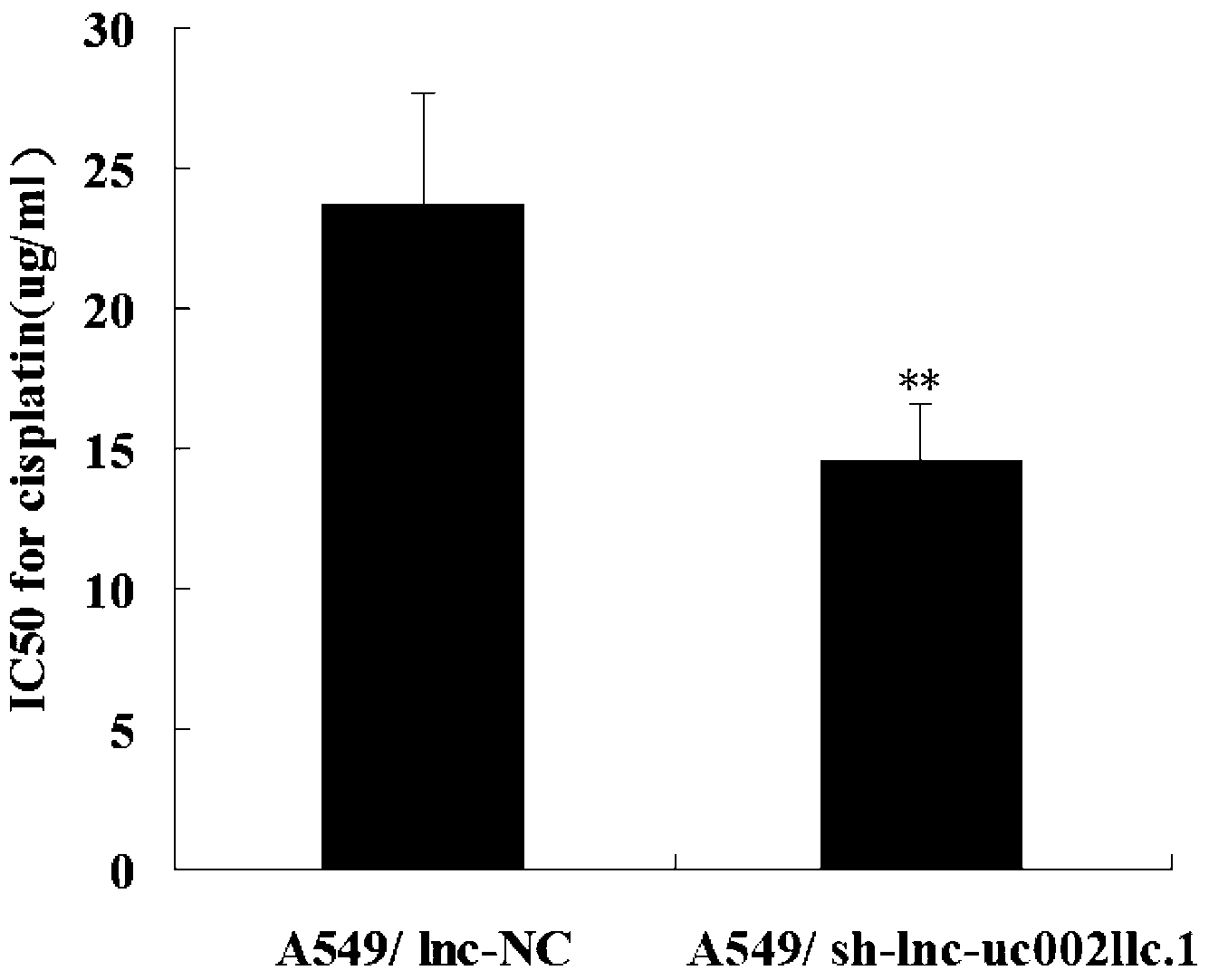
Application of long-chain non-coding RNA in preparation of non-small cell lung cancer treatment drugs
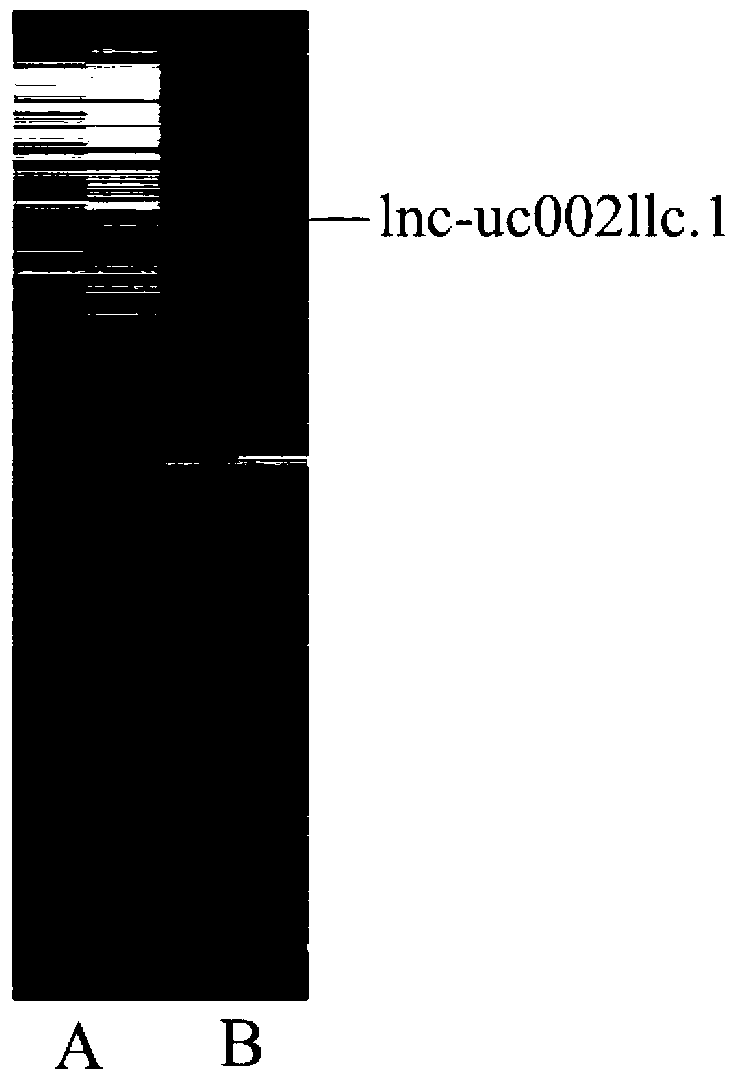
ActiveCN103316359APromote apoptosisInhibit apoptosisGenetic material ingredientsAntineoplastic agentsApoptosisNon-coding RNA
The invention belongs to the genetic engineering field, and especially relates to an application of long-chain non-coding RNA in the preparation of non-small cell lung cancer treatment drugs. The change of the lnc-uc002llc.1 expression has influences on the apoptosis, proliferation, the drug susceptibility and the like of non-small cell lung cancer cells, so the reduction of the lnc-uc002llc.1 expression can realize the apoptosis promotion, proliferation inhibition and chemotherapy drug susceptibility enhancement of the non-small cell lung cancer cells.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
Firming and repairing fair mask product and preparation method thereof
InactiveCN104971016AAccelerated agingAnti agingCosmetic preparationsToilet preparationsBaical Skullcap RootGlycerol
The invention discloses a firming and repairing fair mask product which is prepared by soaking mask paper with the liquid prepared from the following raw materials in parts by weight: 0.1-3 parts of hyaluronic acid oligomer, 0.1-2.5 parts of hyaluronic acid, 0.7-30 parts of synthetic egg white powder, 1-7 parts of an aloe extract, 0.7-9 parts of propylene glycol, 5-50 parts of 1,2-butylene glycol, 5-30 parts of glycerol, 40-80 parts of a ginseng extract, 10-50 parts of a milkvetch root extract, 20-50 parts of a baical skullcap root extract, 20-80 parts of a small cucumber extract, 0.1-1 part of a licorice extract, 0.3-2 parts of arbutin, 1-10 parts of superoxide dismutase, 1-10 parts of a coenzyme q10, 0.1-5 parts of beta-glucan, and the balance of water which is added until the total amount is 1000 parts. The mask product can reduce skin injuries, repair damaged cells, delay skin wrinkling, relieve the generation of facial dark stains and replenish and lock water, and is antiallergic.
Owner:GUILIN HONGXU BIOTECH CO LTD
Smart contact lenses and smart glasses
ActiveUS20180036974A1Progression can be easily and rapidlyImprove stabilityPeptide/protein ingredientsGenetic material ingredientsSmartglassesSmart glass
The present invention provides a smart contact lens including a sensor capable of non-invasively sensing an eye disease in real time and a drug reservoir, and smart glasses for controlling the smart contact lens.
Owner:PHI BIOMED
Suppression of B-cell apoptosis in transgenic animals expressing humanized immunoglobulin
ActiveUS20070033661A1Enhancing expression of exogenousHigh expressionImmunoglobulins against animals/humansTissue cultureApoptosisIntravenous gammaglobulin
The invention provides a novel approach to increase immunoglobulin expression in non-human transgenic animals. For instance, the invention provides a method to increase humanized immunoglobulin production in animals genetically engineered to express one or several human or humanized immunoglobulin transloci. This can be done by overexpressing the apoptosis inhibitor, i.e. a rabbit bcl-2, whose expression is driven by a B-cell specific promoter specifically in the B-cell of the animal, thereby enhancing the survival of B-cells. This invention further relates to a method for selectively enhancing the survival of exogenous B-cells, that is B-cells expressing any immunoglobulin transgene locus, over the survival of endogenous B-cells that do not express the transgene locus. Selectivity is achieved by expressing the apoptosis-inhibitor only within exogenous B-cells, that is, by coupling exogenous immunoglobulin expression with apoptosis inhibitor expression. This latter method allows for increased expression and production of the transgene encoded product(s) over the endogenously produced immunoglobulin of the transgenic animal. The invention also provides a novel apoptosis-inhibitor, rabbit bcl-2.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Non-cytotoxic oriP replicon
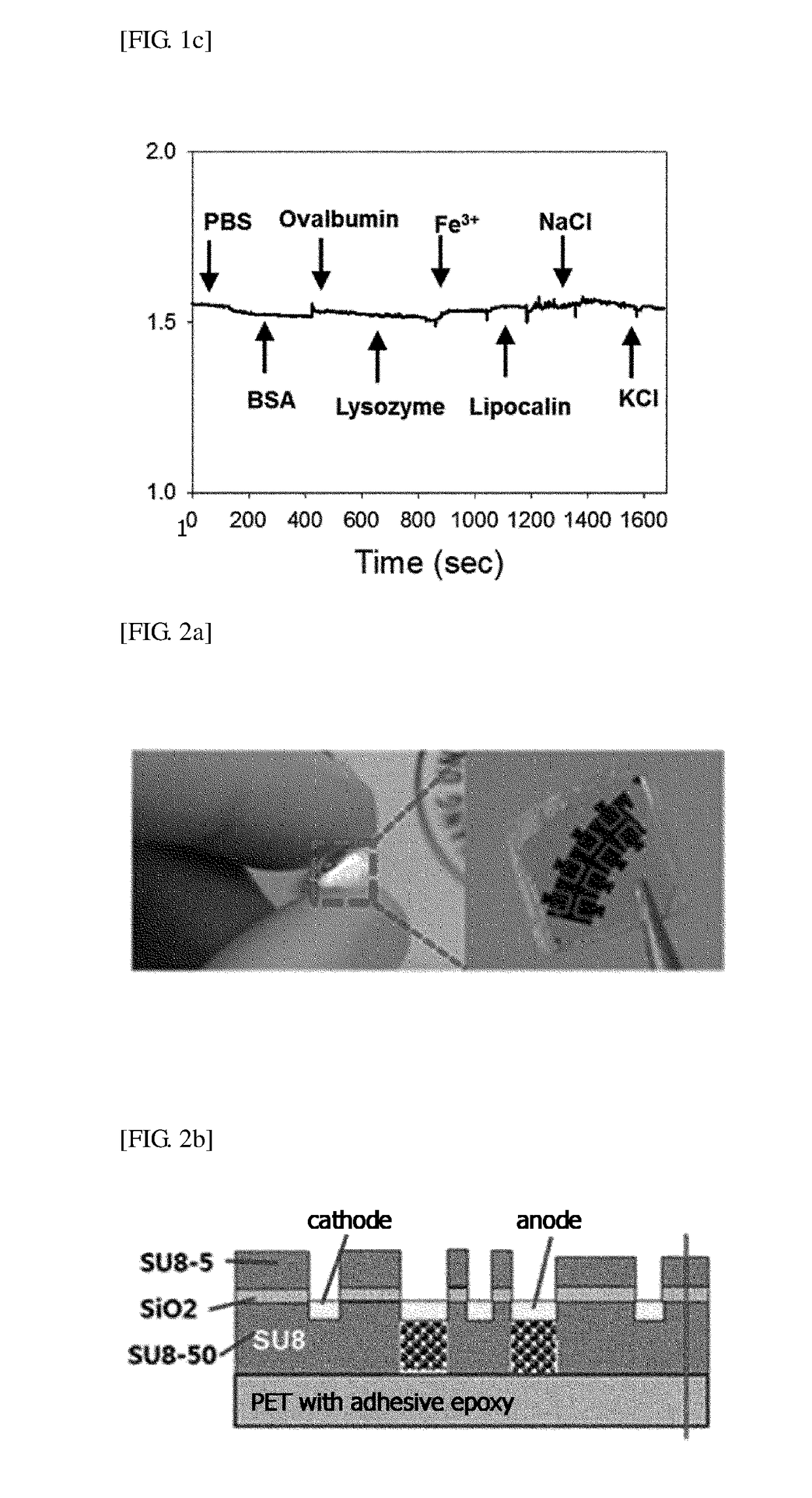
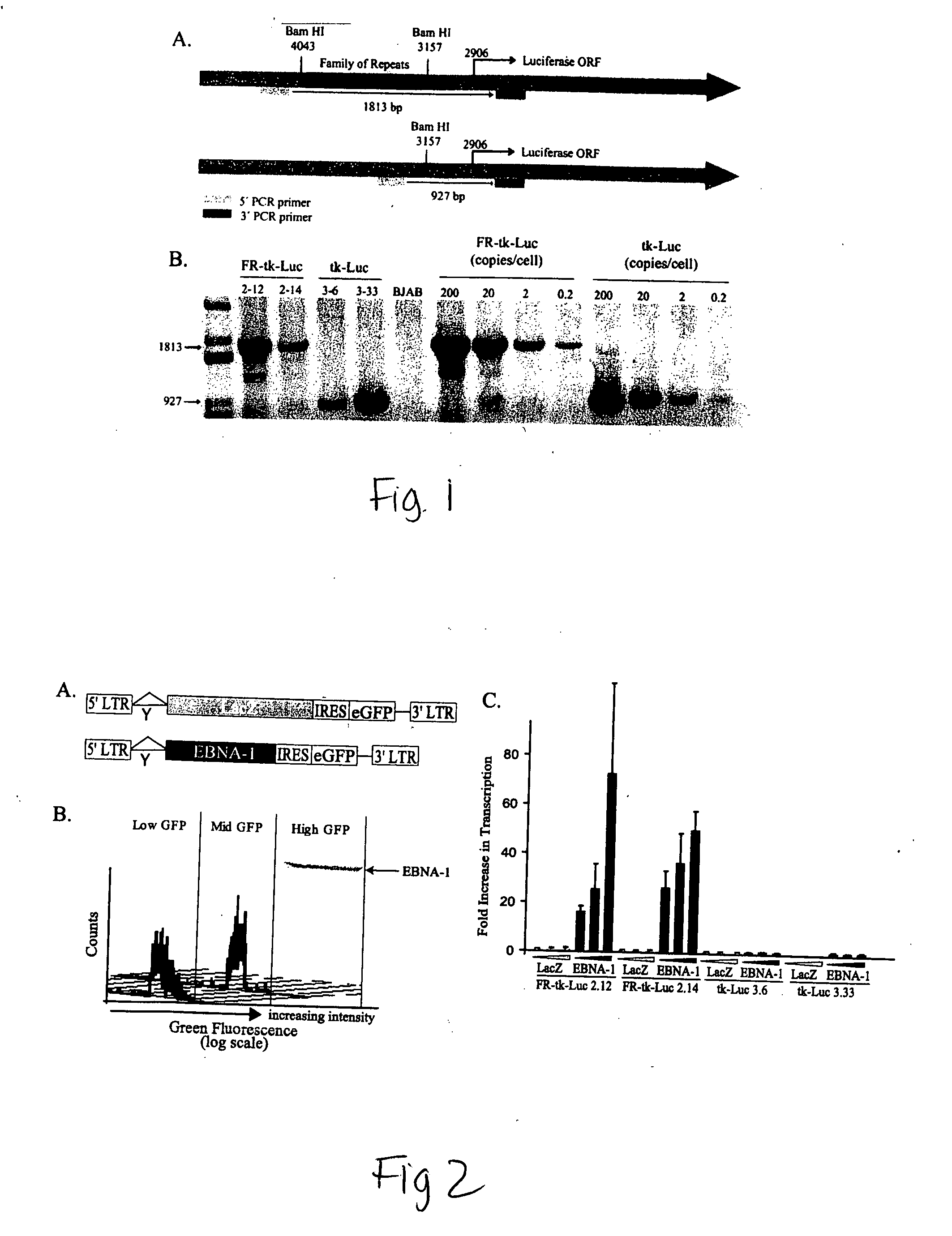
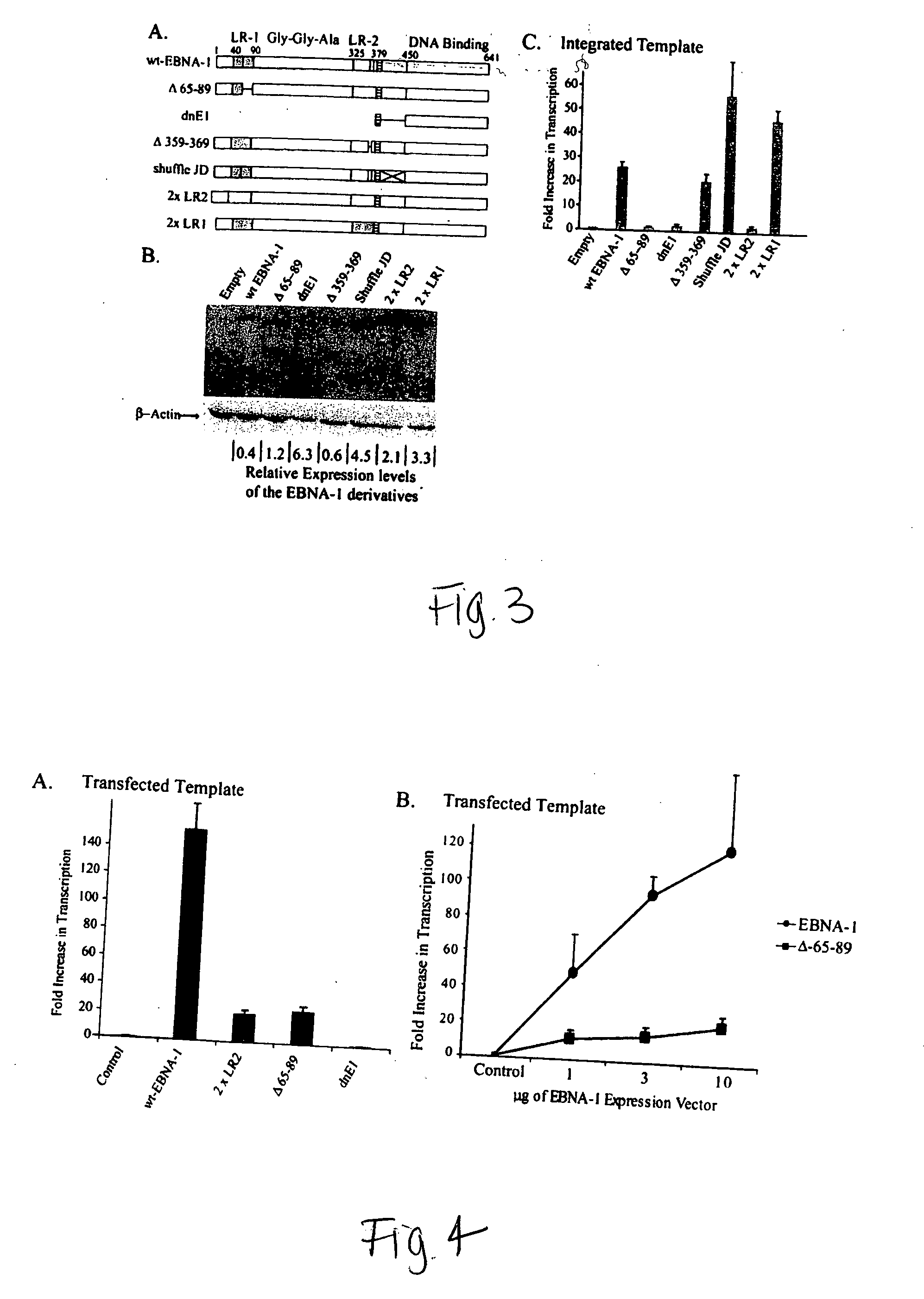
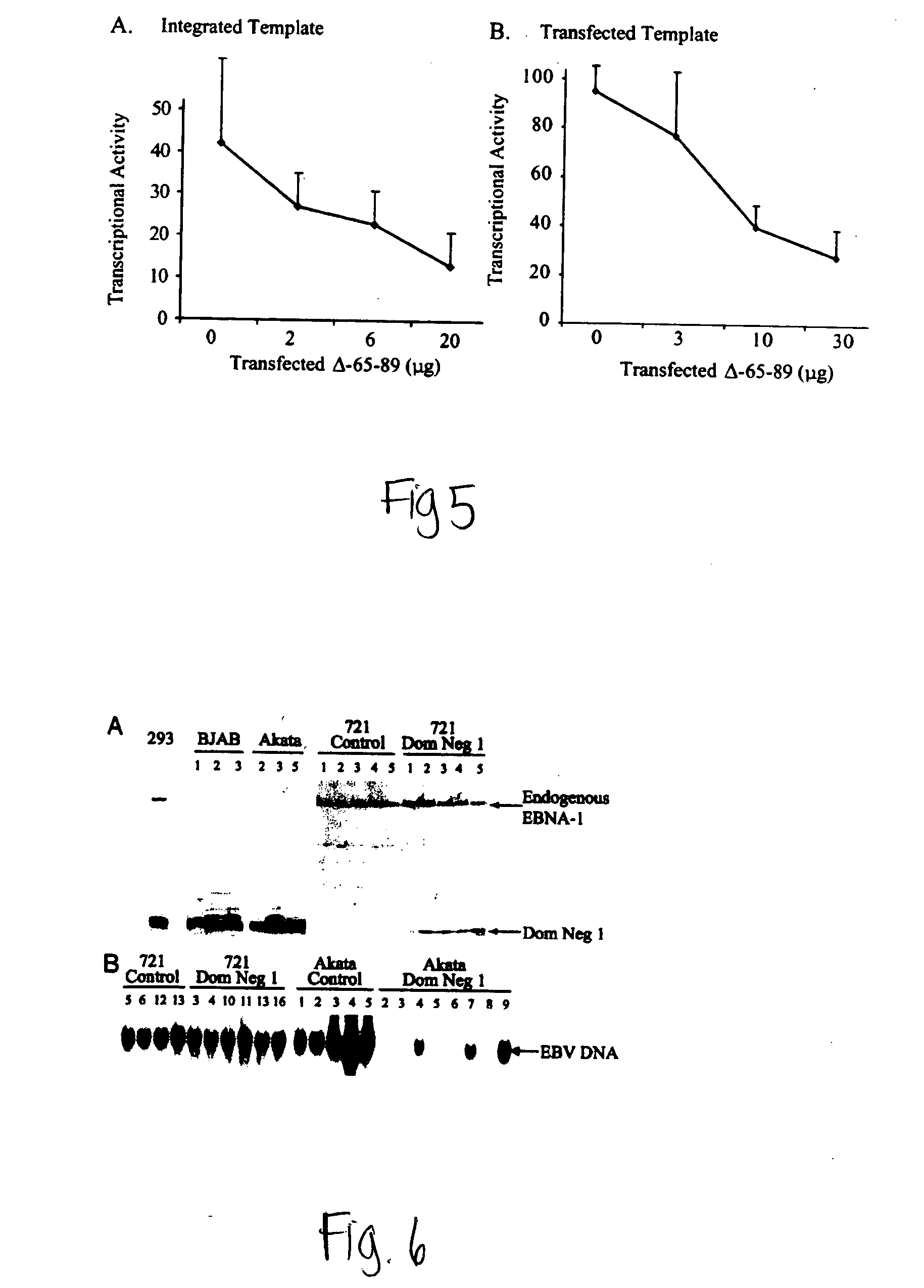
ActiveUS20050260564A1Increase transcriptionReduced survivalGenetic material ingredientsVirus peptidesCytotoxicityCancer research
The invention provides a vector encoding a derivative of EBNA-1 that is not cytotoxic when expressed efficiently in cells, which supports extrachromosomal replication, maintenance and transcription from extrachromosomal oriP containing vectors but does not substantially activate transcription from host cell genes. Also provided is a vector having oriP and encoding a derivative of EBNA-1. The vectors of the invention may be employed in vitro and in gene therapy.
Owner:WISCONSIN ALUMNI RES FOUND
Method for separating and culturing primary chicken hepatocytes
InactiveCN103525756AExtend incubation timeWell differentiatedArtificial cell constructsVertebrate cellsBioproductsEthylene Glycol Tetraacetic Acid
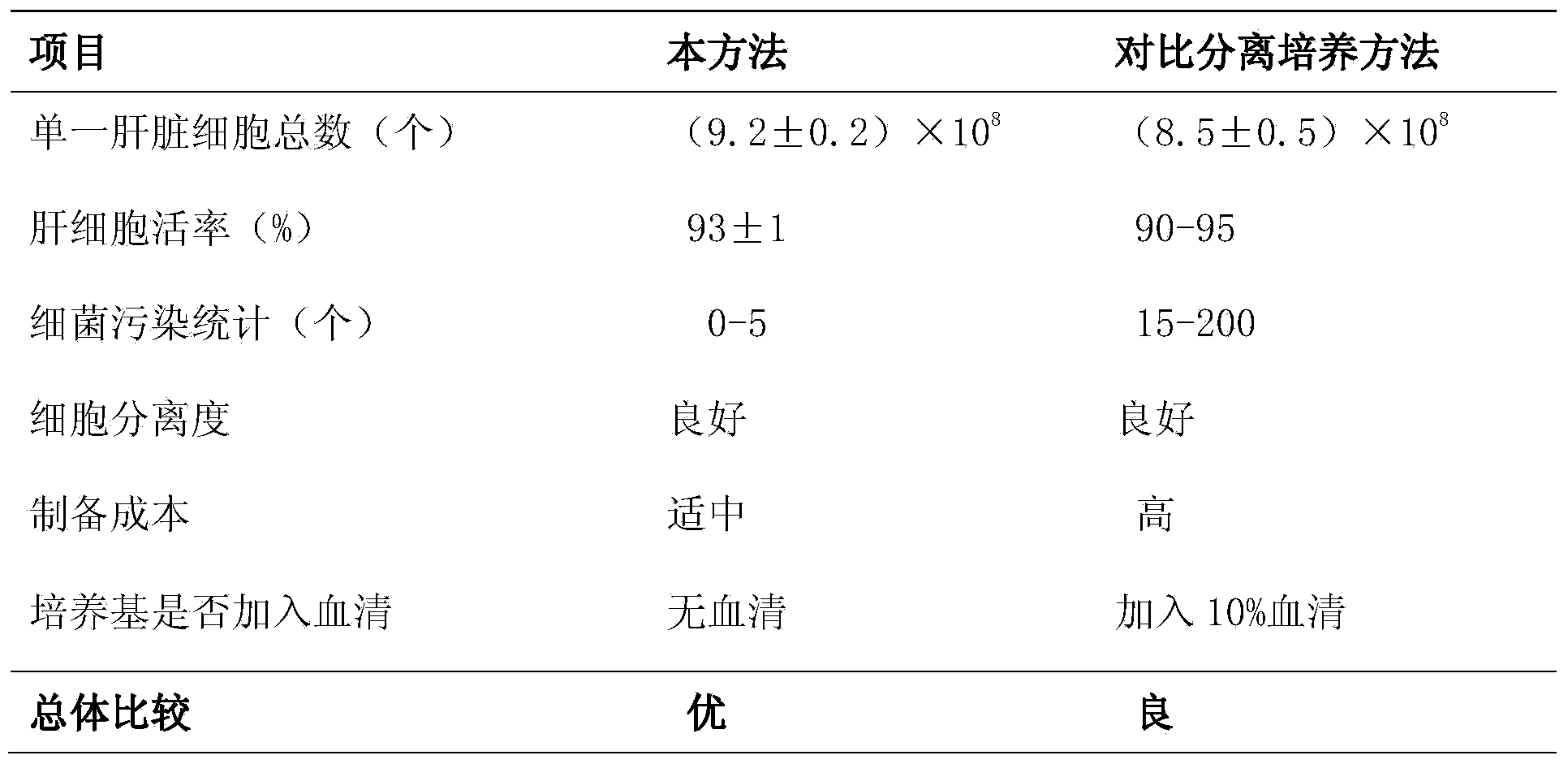
The invention discloses a method for separating and culturing primary chicken hepatocytes. According to the method, EGTA (Ethylene Glycol Tetraacetic Acid) with low hepatotoxicity is added into a perfusate A to loosen the junction between hepatocytes, so that the dispersion degree of the to-be-separated hepatocytes is increased; double resistance is added into the perfusate A, a perfusate B and D-Hanks flushing fluid, so that the pollution is further prevented; and circulating perfusion digestion is adopted during digestion perfusion, so that the perfusion cost is greatly reduced. A serum-free cultural method of the chicken hepatocytes is established; compared with a traditional serum cultural method, the method disclosed by the invention has the same effect on the aspects of cell quantity, activity, function and the like, so that the problems caused by the existence of serum are solved and a foundation is laid for the substance metabolism study and biological product preparation on the basis of the chicken hepatocyte culture.
Owner:NANJING AGRICULTURAL UNIVERSITY
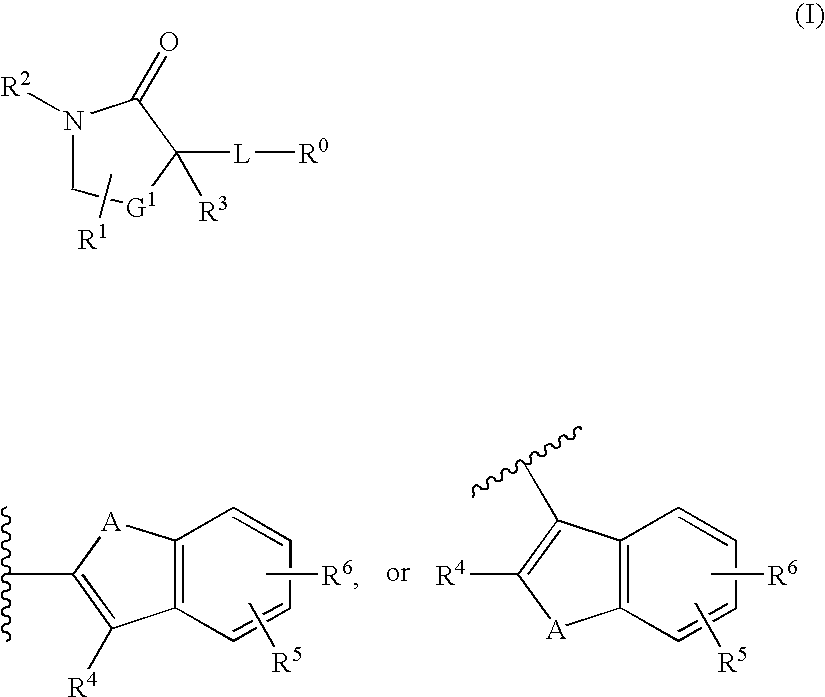
Cycloalkyl Lactam Derivatives as Inhibitors of 11-Beta-Hydroxysteroid Dehydrogenase 1
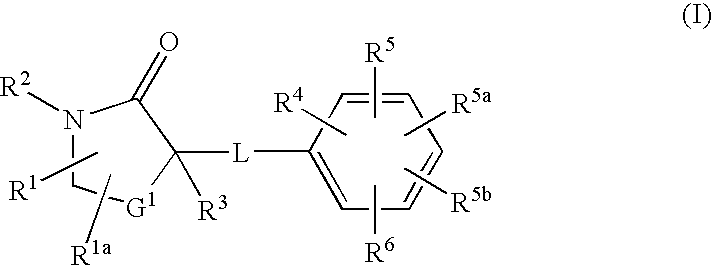
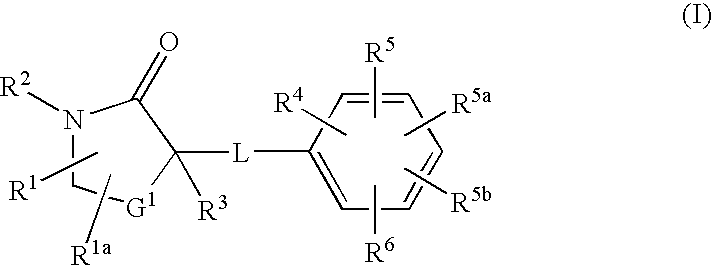
InactiveUS20080275043A1Function increaseInhibit apoptosisBiocideOrganic chemistryAcute hyperglycaemiaDisease
The present invention discloses compounds of Formula I: (I) having 11beta-HSD type 1 antagonist activity, as well as methods for preparing such compounds. In another embodiment, the invention discloses pharmaceutical compositions comprising compounds of Formula I, as well as the use of the Fomula I and compositions as medicaments to treat diabetes, hyperglycemia, obesity, hypertension, hyperlipidemia, Syndrome X, and other conditions associated with hyperglycemia.
Owner:ELI LILLY & CO
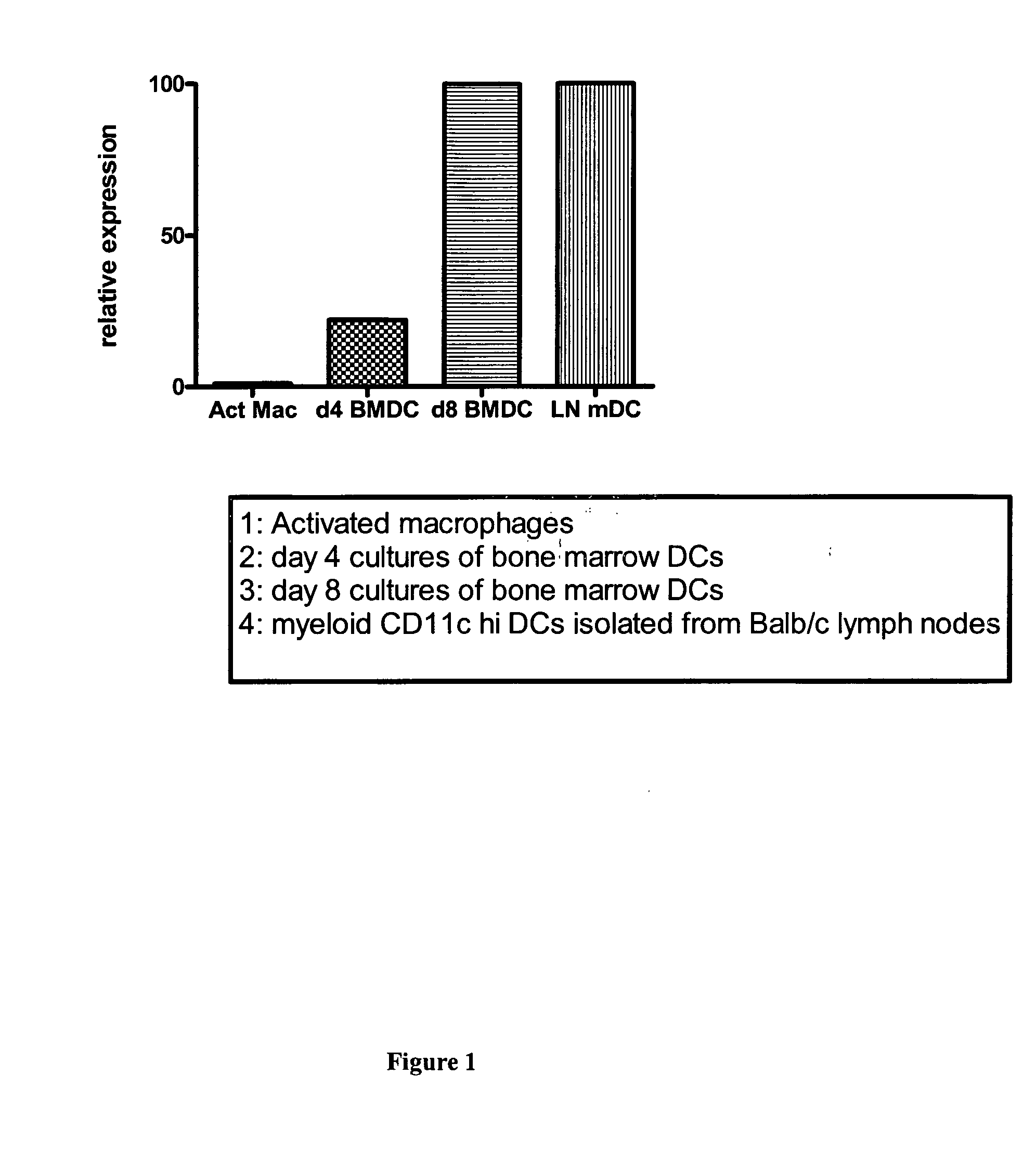
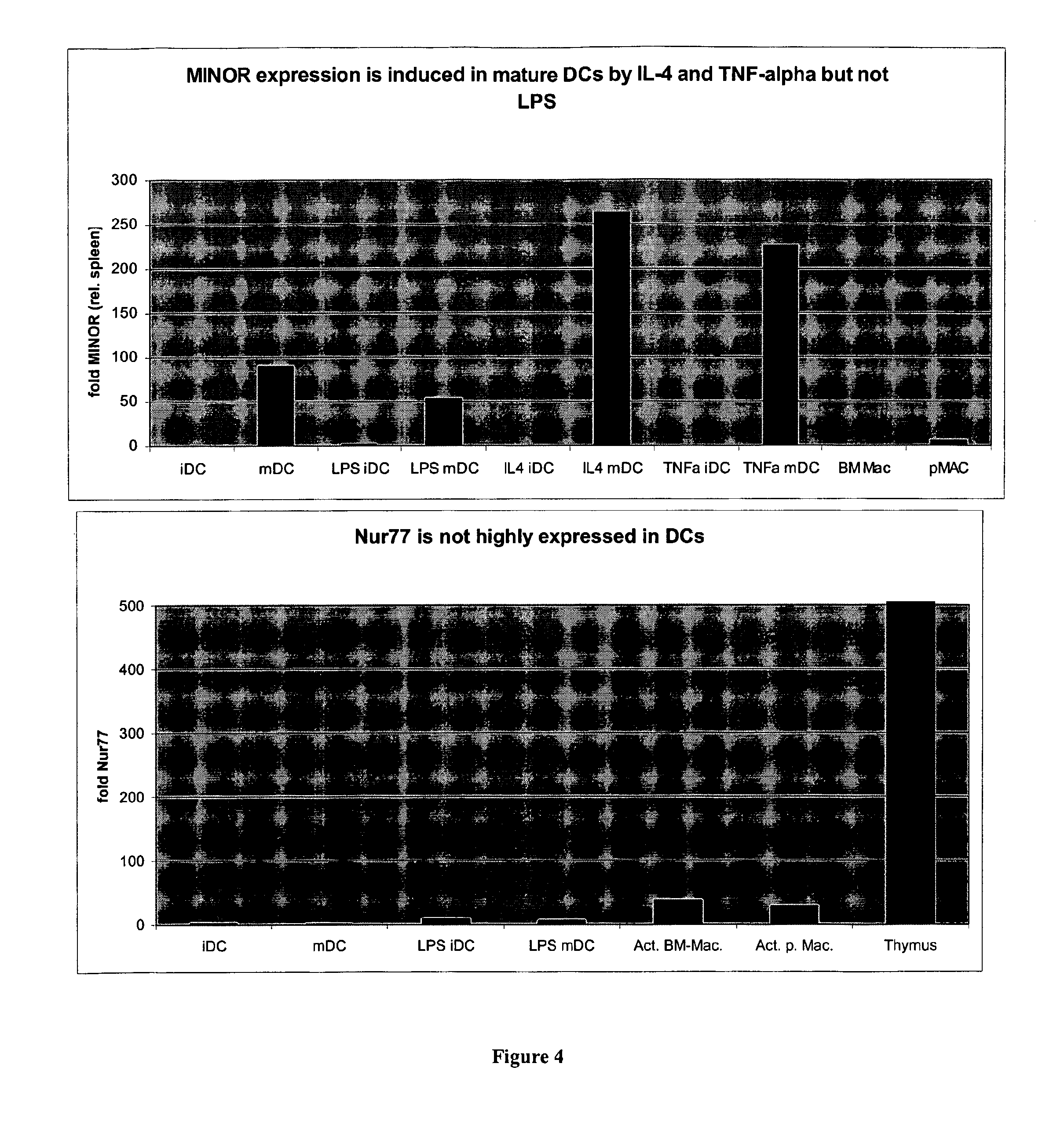
Immune Modulation By Regulating Expression Of The "Minor" Gene In Immune Dendritic Cells
InactiveUS20070196335A1Limiting uncontrolled T cell activationImproving DC-based therapyBiocideGenetic material ingredientsDendritic cellApoptosis
Mitogen induced nuclear orphan receptor (MINOR) is described as inducing apoptosis in dendritic cells (DCs). Downregulation of its expression results in a downregulation of apoptosis. A novel approach of inhibiting DC apoptosis is described employing small interfering RNA (siRNA) that targets MINOR. Improved DC-based vaccines exhibiting longer lifespan of DCs, increased potency of DCs, and enhanced immunogenicity are described.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Glucagon Receptor Antagonists, Preparation and Therapeutic Uses
ActiveUS20070249688A1Function increaseInhibit apoptosisBiocideOrganic chemistryPharmaceutical medicineDiabetes mellitus
The present invention discloses novel compounds of Formula I, or pharmaceutically acceptable salts thereof, which have glucagon receptor antagonist or inverse agonist activity, as well as methods for preparing such compounds. In another embodiment, the invention discloses pharmaceutical compositions comprising compounds of Formula I as well as methods of using them to treat diabetic and other glucagon related metabolic disorders, and the like.
Owner:ELI LILLY & CO
AGE production inhibitor
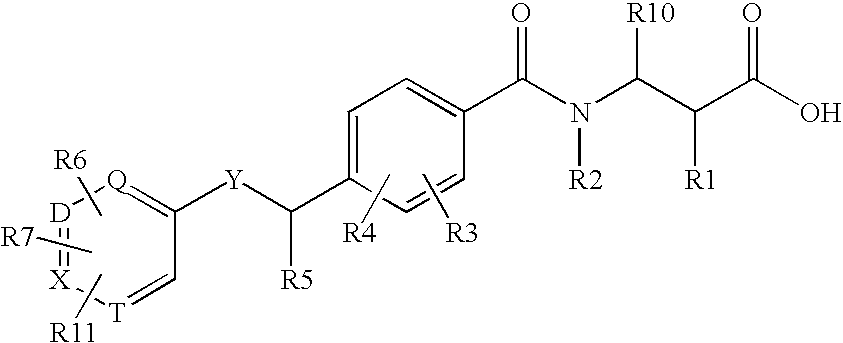
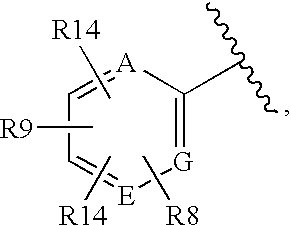
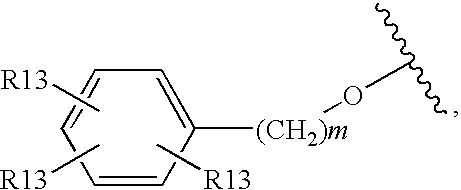
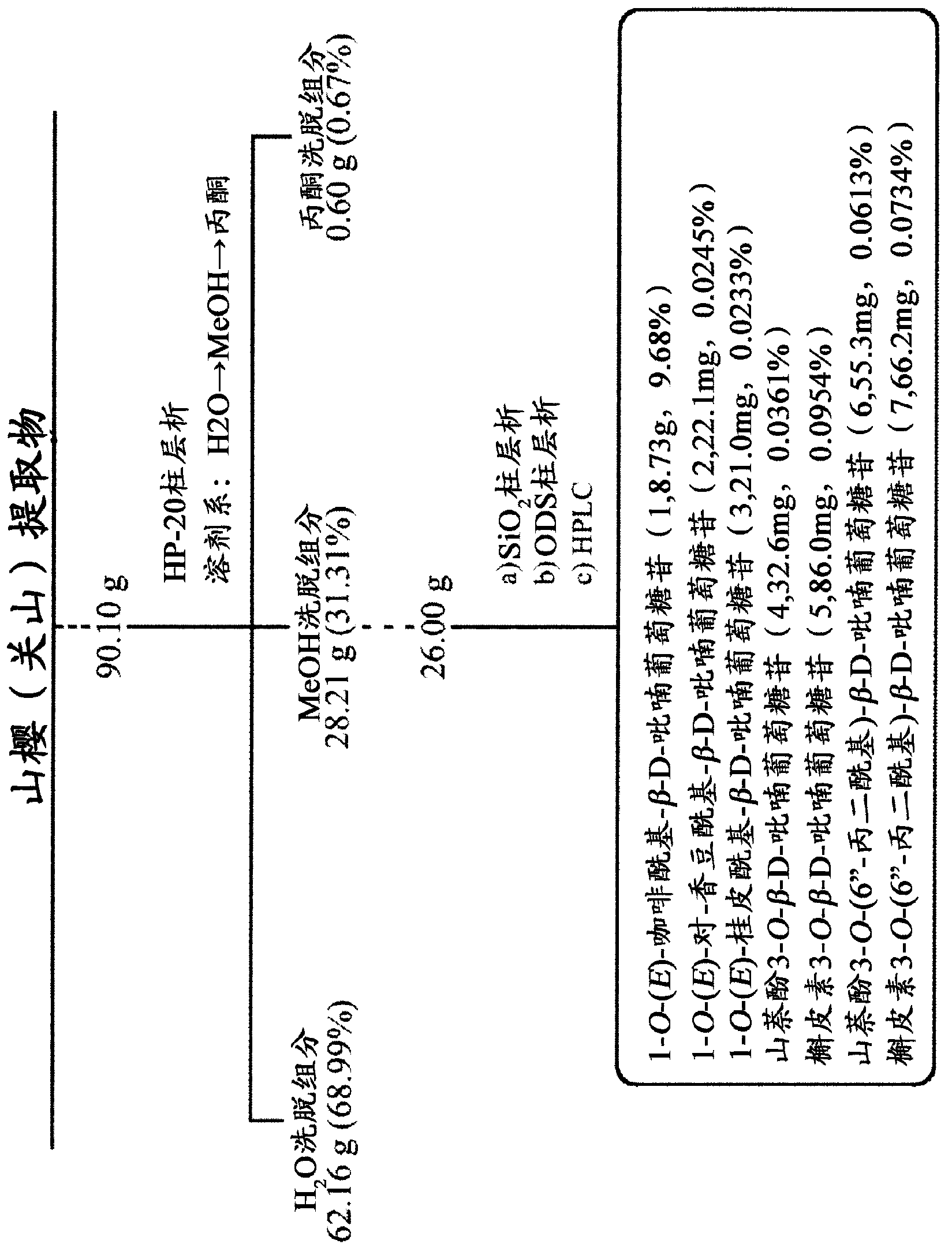
ActiveCN102883733AInhibits AGE productionAvoid side effectsOrganic active ingredientsCosmetic preparationsChemistryAdvanced glycation end-product
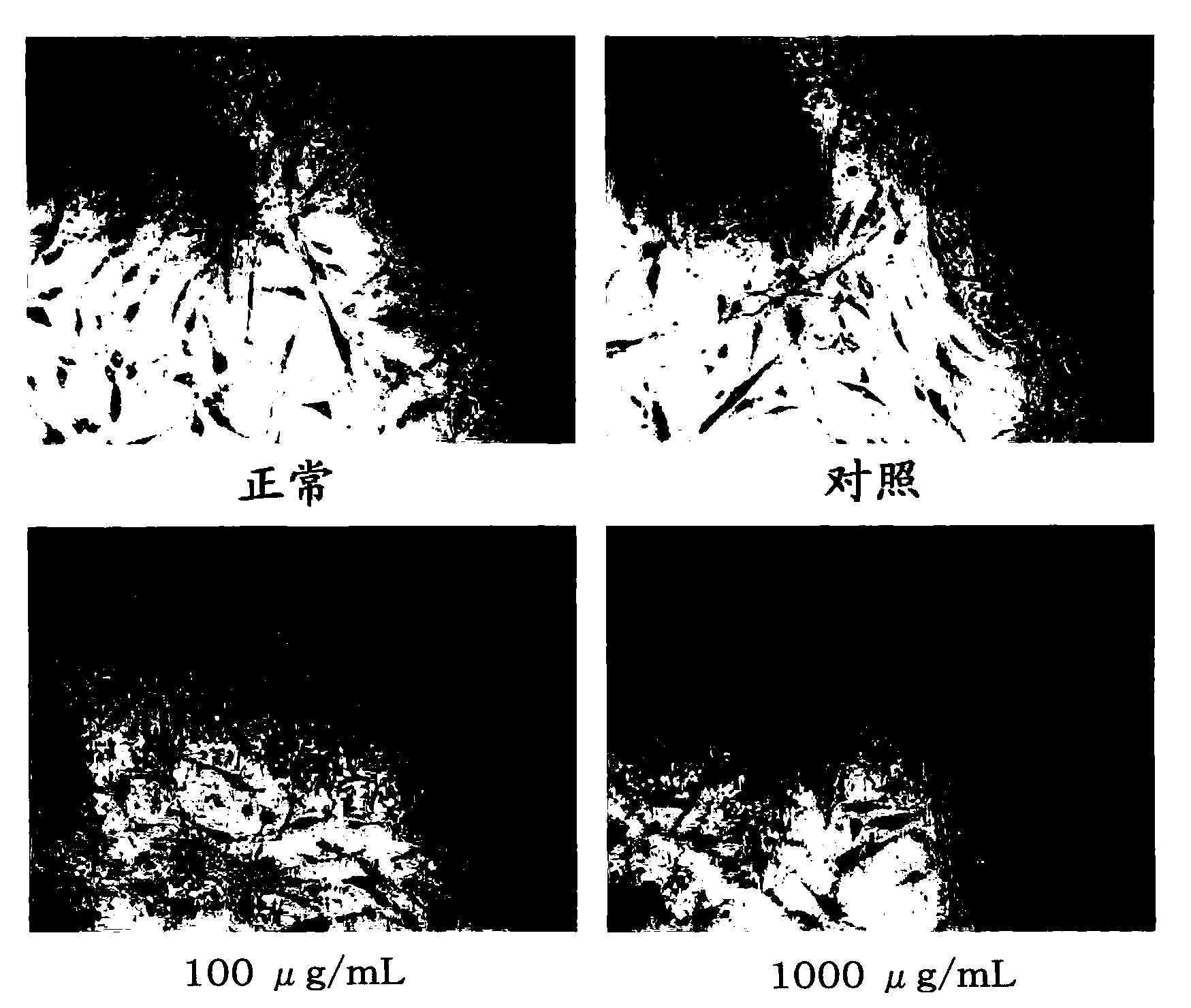
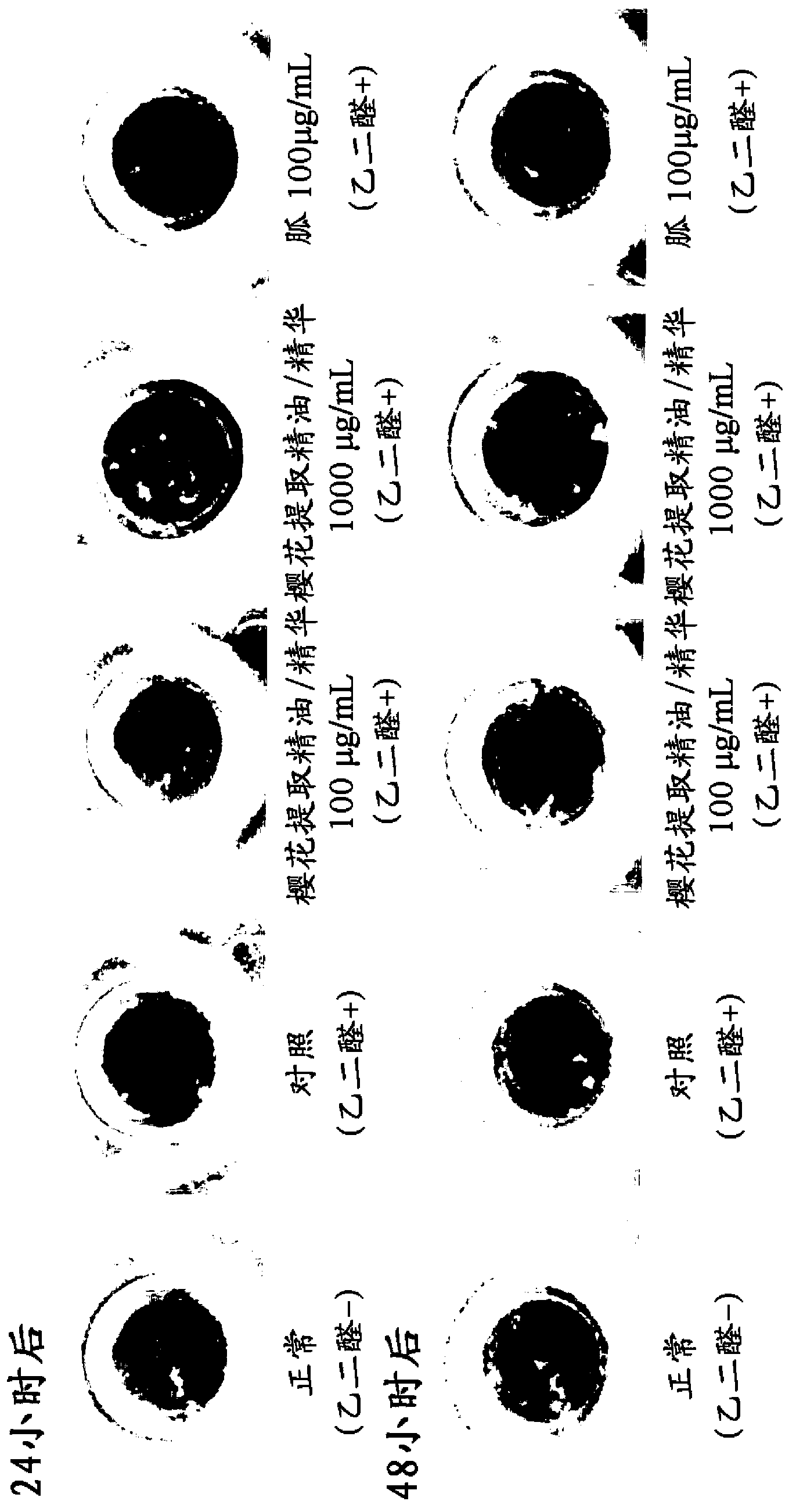
Disclosed is an AGE production inhibitor or the like, which effectively inhibits the production of an advanced glycation end product (AGE), while having improved biological safety. Specifically disclosed is an AGE production inhibitor or the like, which contains an extract of cherry (preferably blossoms or leaves thereof) and / or a processed product of the extract as an active ingredient. The AGE production inhibitor or the like contains, as an active ingredient, at least one compound that is selected from the group consisting of 1-O-(E)-caffeoyl-ss-D-glucopyranoside, 1-O-(E)-coumaroyl-ss-D-glucopyranoside, 1-O-(E)-cinnamoyl-ss-D-glucopyranoside, kaempferol 3-O-ss-D-glucopyranoside, quercetin 3-O-ss-D-glucopyranoside, kaempferol 3-O-(6"-malonyl)-ss-D-glucopyranoside, and quercetin 3-O-(6"-malonyl)-ss-D-glucopyranoside.
Owner:ORIZA YUKA KK
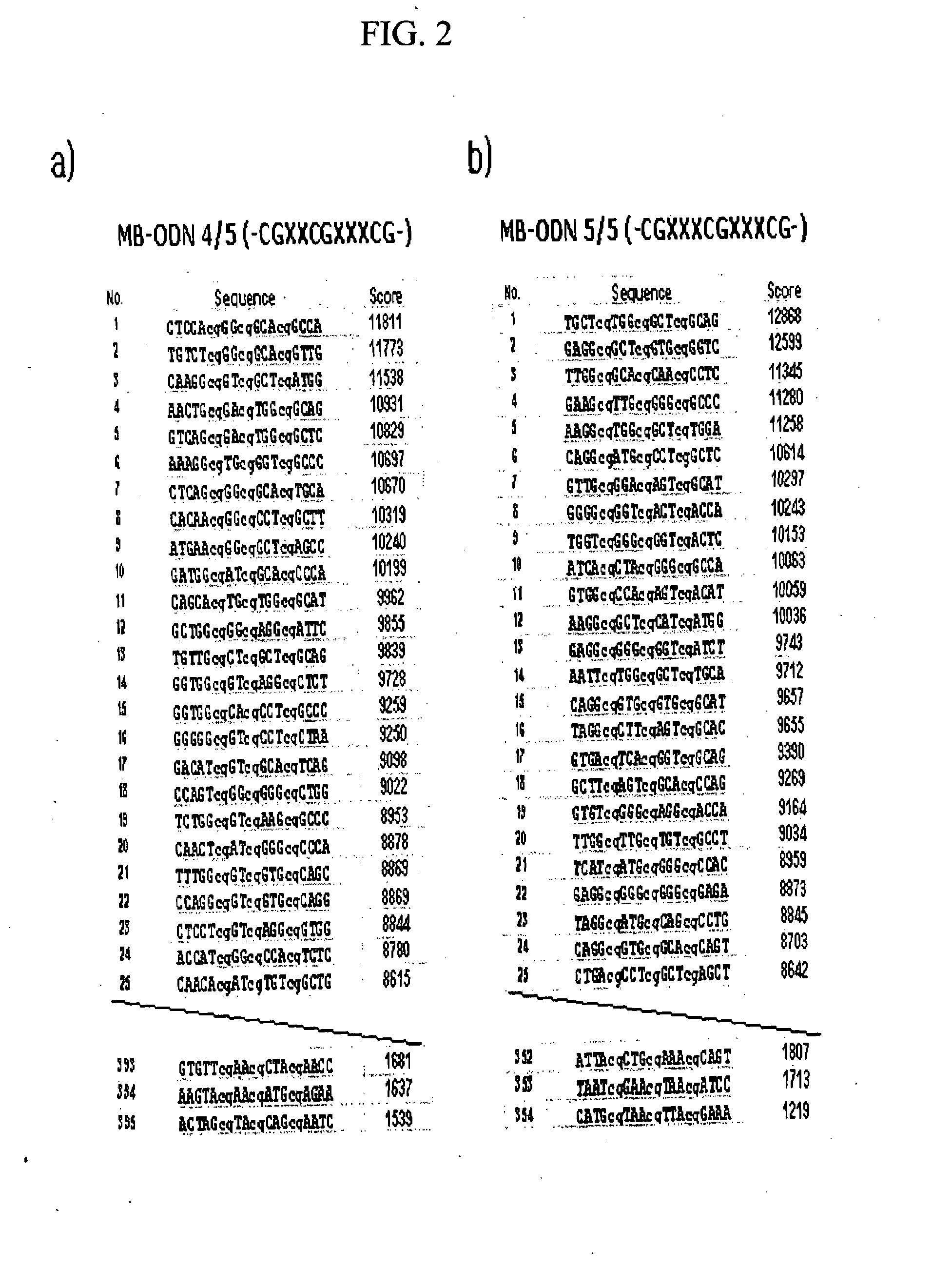
Oligonucleotides Derived From Mycobacterium for Stimulating Immune Function, Treating Immune-Related Diseases, Atopic Dermatitis and/or Protecting Normal Immune Cell
InactiveUS20080249291A1Reduce riskPromote decompositionSugar derivativesGenetic material ingredientsAtopic dermatitisOligonucleotide
Disclosed are oligonucleotides for manipulating an immune reaction. The oligonucleotides of the present invention may be useful to stimulate the immune function, to treat the immune-related diseases and the atopic dermatitis, or to protect the normal immune cells.
Owner:KWON HYUNG JOO +1
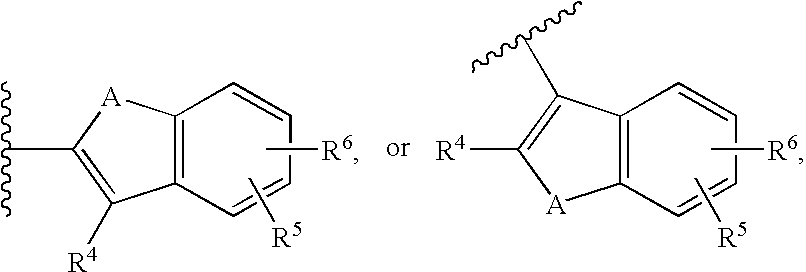
Cycloalkyl Lactam Derivatives As Inhibitors Of 11-Beta-Hydroxysteroid Dehydrogenase 1
InactiveUS20080207691A1Function increaseInhibit apoptosisBiocideOrganic chemistry11-beta-Hydroxysteroid DehydrogenasesSelective inhibition
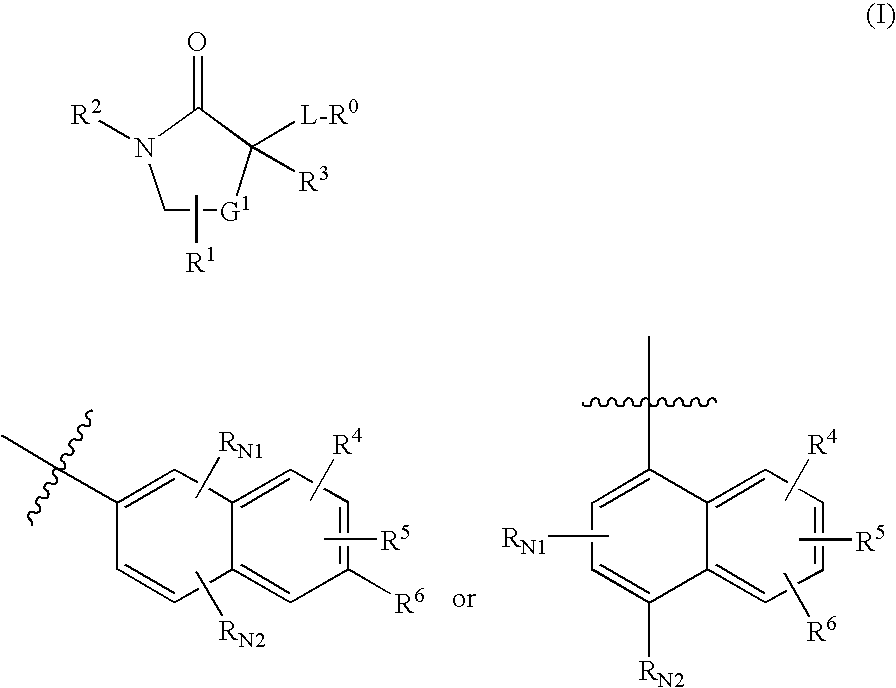
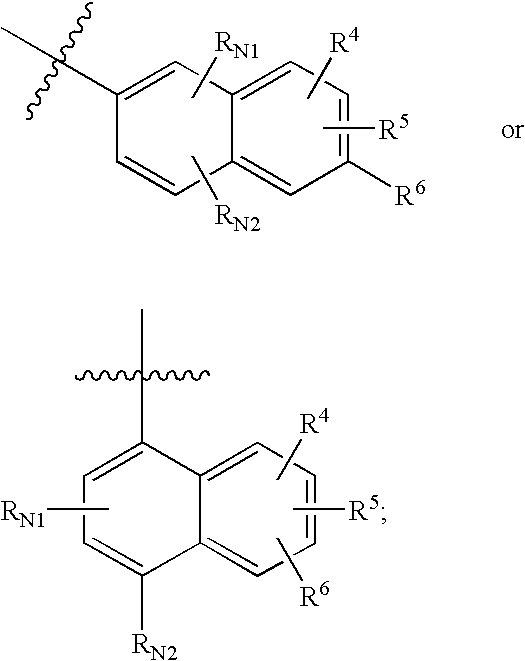
The present invention provides compounds of formula I that are useful as potent and selective inhibitors of 11-beta hydroxysteroid dehydrogenase 1. The present invention further provides a pharmaceutical composition which comprises a compound of Formula I, or a pharmaceutical salt thereof, and a pharmaceutically acceptable carrier, diluent, or excipient. In addition, the present invention provides compositions comprising compounds of formula I for the treatment of metabolic syndrome, diabetes, hyperglycemia, obesity, hypertension, hyperlipidemia, other symptoms associated with hyperglycemia, and related disorders. Formula (I) wherein, Ru0 is (II), or (III) G1 is methylene or ethylene; L is a divalent linking group selected from —(C1-C4) alkylene-, —S—, —CH(OH)—, or —O—; A is methylene, —S—, —O—, or —NH—; and the other substituents are as defined in the claims.
Owner:ELI LILLY & CO
Moisturizing lotion for repairing skin barriers and resisting free radicals
InactiveCN107638331AGood conditionPromote formationCosmetic preparationsToilet preparationsCuticleTopical treatment
The invention discloses moisturizing lotion for repairing skin barriers and resisting free radicals. The moisturizing lotion comprises, in weight percent, 2%-6% of polyhydric alcohols, 0.2%-3% of thickening suspending agents, 1%-5% of natural squalane, 2.5%-4.5% of emulsifying agents, 0.1%-3% of ceramide NP, 0.1%-5% of nicotinamide, 0.1%-2% of lycopene, 0.5%-3% of grape seed extracts, 0.1%-1% of ferulic acid, 0.1%-2% of coenzyme Q10, 0.1%-2% of vitamin E and the balance water. By the aid of the proportion and combination of different components, a novel local treatment formula can be developedaccording to skin lesion relevant to a plurality of cuticles and skin damage and resistance of the free radicals. The components in the formula are necessary components forming skin permeability barriers and have synergistic interaction between antioxidant components, the skin barriers can be repaired, protection films are formed on the surfaces of skins, the moisturizing lotion resists oxidationand the free radicals, damage of external factors to the skins is avoided, transepidermal water loss rate is effectively reduced, and the moisturizing lotion has good moisturizing effects on the skins.
Owner:OPAL COSMETICS HUIZHOU
Cycloalkyl Lactam Derivatives As Inhibitors Of 11-Beta-Hydroxysteroid Dehydrogenase 1
InactiveUS20080214621A1Function increaseInhibit apoptosisBiocideOrganic chemistrySelective inhibition11-beta-Hydroxysteroid Dehydrogenases
The present invention provides compounds of formula I that are useful as potent and selective inhibitors of 11-beta hydroxysteroid dehydrogenase 1. The present invention further provides a pharmaceutical composition which comprises a compound of Formula I, or a pharmaceutical salt thereof, and a pharmaceutically acceptable carrier, diluent, or excipient. In addition, the present invention compositions containing these compounds for the treatment of metabolic syndrome, diabetes, hyperglycemia obesity, hypertension, hyperlipidemia, other symptoms associated with hyperglycemia, and related disorders. Formula I wherein G1 is methylene or ethylene; L is —(C1-C4)alkylene-, —S—, —CH(OH)—, or —O—; R0 is Formula II or Formula III and the other substituents are as defined in the claims.
Owner:ELI LILLY & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com