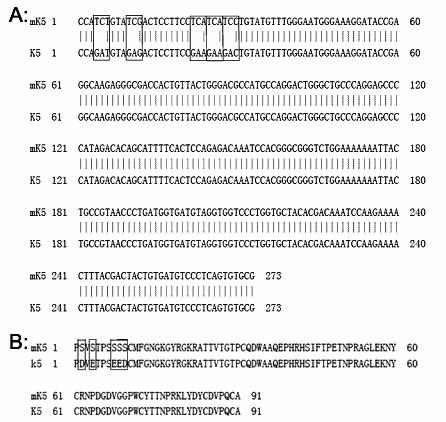
Mutant human plasminogen kringle5, preparation method and application thereof
A human plasminogen and mutant technology, applied in the biological field, can solve problems such as complicated process, low expression yield, and few exogenous amino acids, and achieve the effects of simplifying purification steps, improving biological activity, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: be used for expressing the construction of the mK5 recombinant plasmid with GST mark
[0076] The nucleotide sequence of mK5 is as follows figure 2 shown. Synthetic primers were designed based on the K5 sequence, and the primers contained the nucleotide coding sequence of the N-terminal five neutral amino acid serines and restriction endonuclease enzyme cutting sites. Upstream primer: 5'-ACT GAATTC CCA TCT GTA TCG ACT CCT TCC TCA TCA TCC TGT ATG TTT GG-3', including the amino acid coding sequence of BamHI site and neutral amino acid (Ser) substitution mutation; downstream primer: 5'-TGCTGC CTCGAG TCA CGC ACA CTG AGG GAC ATC ACA GTA, which includes the stop codon followed by an XhoI site. Using human hepatocyte cDNA as a template, mK5 gene was obtained by PCR method. The PCR reaction conditions were: 95°C pre-denaturation for 5 minutes, 95°C for 45 seconds, 55°C for 60 seconds, 72°C for 60 seconds, a total of 30 cycles, and 72°C extension for 10 ...
Embodiment 2
[0078] Example 2: Expression and purification of GST-mK5 fusion protein and simple mK5 protein
[0079] Take the successfully transformed BL21 (DE3) strain for amplification, when the bacterial solution OD 600 When the concentration reaches about 0.6-0.8, add IPTG to a final concentration of 0.6 mmol / L, shake the bacteria at 25 °C and 220 rpm for 6 hours, and collect the bacteria by centrifugation. After the bacterial pellet was rinsed with distilled water, centrifuged, the pellet was resuspended in phosphate buffered saline (PBS) and then sonicated, centrifuged at 10,000 rpm for 30 min at 4°C, and the supernatant was filtered with a 0.45 μm filter membrane at 4°C for affinity chromatography. According to the operation manual of the product, pass the treated supernatant filtrate through the Glutathione Sepharose 4B affinity chromatography column, wash the affinity column with 300 ml washing buffer, add 20ml Elution buffer to incubate the Glutathione Sepharose 4B affinity chr...
Embodiment 3
[0081] Example 3: Identification of GST-K5 fusion protein and pure mK5 protein
[0082] The GST-mK5 protein and mK5 protein were analyzed by SDS-PAGE electrophoresis and identified by Western-blot.
[0083]1. SDS-PAGE analysis: According to the method described in the literature (Sambrook et al, Molecular Cloning: A laboratory Manual, Cold Spring Harbour, 1989), take 20 μl of the above-mentioned eluted GST-mK5 protein and pure mK5 protein respectively, Add 4μl 6× loading buffer (7.2ml glycerol + 2.076g SDS + 1ml β-mercaptoethanol + 7ml 1M Tris PH6.8 + 0.012% (w / v) bromophenol blue, distilled water to 20ml) Afterwards, carry out boiling water bath 10min. The processed samples and 10 μl of commercial protein molecular weight standards were sequentially added to a 12% polyacrylamide gel for electrophoresis. After electrophoresis, stain the gel in Coomassie Brilliant Blue G250 solution (recipe: 0.25g G250, 90ml methanol:water = 1:1, 10ml glacial acetic acid) for 4 hours, and o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com