Patents
Literature
116results about How to "Immunogenic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mixture of poly-pneumococcal capsular polysaccharide-protein conjugates and preparation method of mixture
ActiveCN103495161AImproving immunogenicityIncrease productionAntibacterial agentsBacterial antigen ingredientsAdjuvantImmunogenicity
The invention discloses a mixture of poly-pneumococcal capsular polysaccharide-protein conjugates. The mixture contains 13 pneumococcal capsular polysaccharide-protein conjugates and an immunity-enhancement adjuvant, wherein each pneumococcal capsular polysaccharide-protein conjugate is formed by combining corresponding serum-type pneumococcal capsular polysaccharide with a same protein carrier through a covalent bond; the 13 pneumococcal capsular polysaccharides are obtained by purifying and extracting 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F bacteria; the protein carrier is A chain of a diphtherin mutant CRM197 obtained from expression of genetic recombinant escherichia coli. Meanwhile, the invention discloses a preparation method of the mixture. The mixture, compared to conventional bacterial fermentation total-chain diphtherin mutant CRM197, is easy to purify, high in yield and low in cost; experiments prove that the mixture disclosed by the invention is immunogenic, and is applicable to clinical inoculation.
Owner:KANVAX BIOPHARM
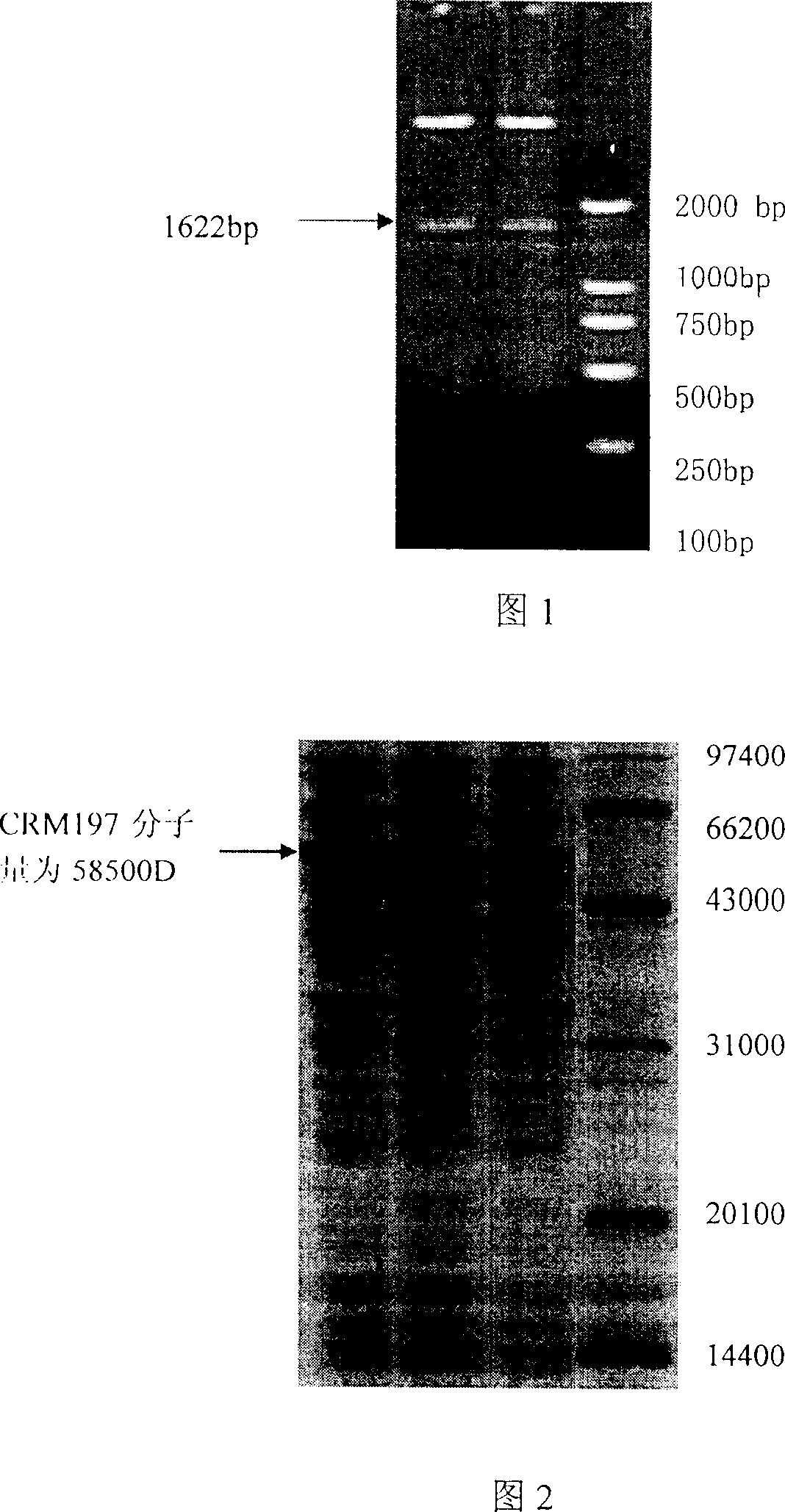
Diphtheria toxin muton CRM197 and its preparation process
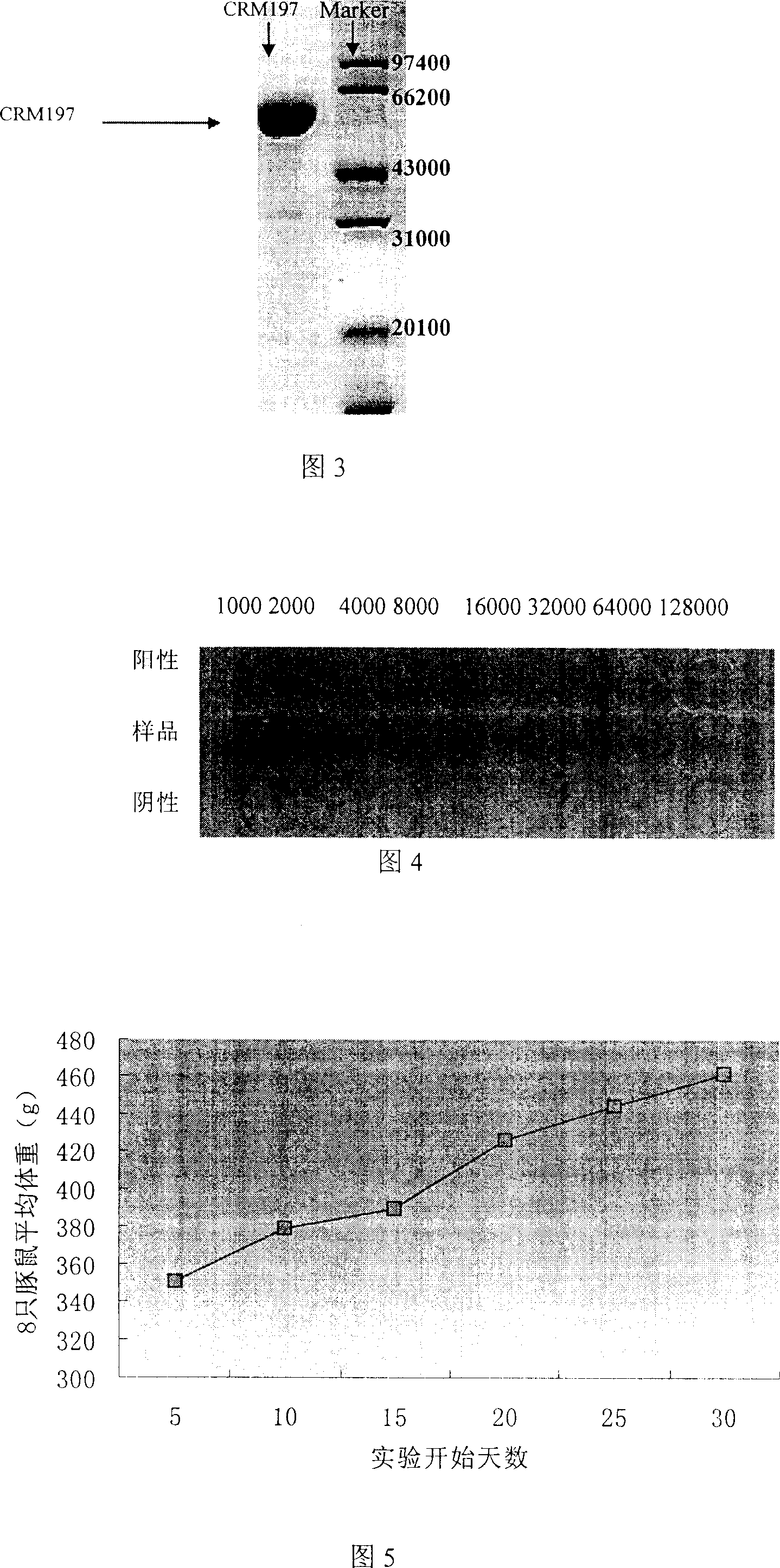
The present invention is diphtheria toxin mutant CRM197 and its preparation process, and belongs to the field of immune protein carrier technology. The diphtheria toxin mutant CRM197 is expressed in colibacillus in the form of inclusion body, and the target product has sequence length of 536 amino acids, the immunogenicity the same as that of diphtheria toxin and no toxicity similar to that of diphtheria toxin. The present invention clones gene coding CRM197, constructs recombinant expression plasmid expressing CRM197 and transforms to colibacillus host for expression in the form of inclusion body. The present invention has target protein accounting for over 24 % of total protein content, high CRM197 purity over 95 % and high target protein recovering rate. The preparation process is simple, low in cost and suitable for industrial production.
Owner:QILU PHARMA HAINAN
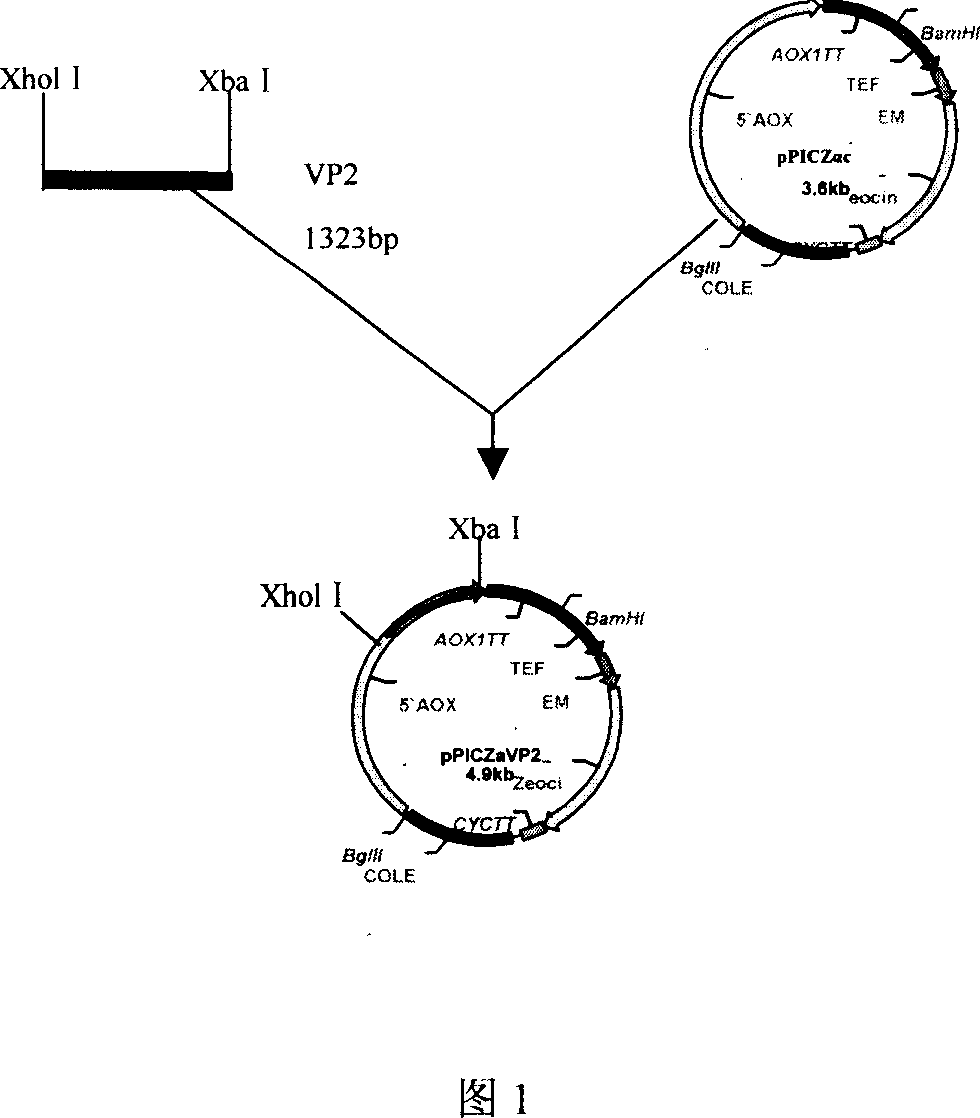
Chicken infectivity bursa of Fabricius virus VP2 cDNA, its expression vector, expressed recombinant protein and application thereof
InactiveCN1990869APrevent proliferationBiologically activeFungiViral antigen ingredientsDiseaseHighly pathogenic
The invention discloses a novel chicken infectious bursal disease virusVP 2c DNA (SEQ ID NO: 1), construction of its expression carrier, expressed recombined VP2 protein and the application of said recombined protein in preparing subunit genetic engineering vaccine against chicken infection bursal disease virus. The chicken infectious bursal disease virusVP 2c DNA can be highly expressed in yeast cell, and expressed recombined protein possesses biological activity and immunogenicity of the chicken infectious bursal disease virus natural protein . The expressed recombined protein can be produced into vaccine, and it is demonstrated through immunity chicken test that: the protein subunit vaccine can effectively induce body to generate spcial humoral immune response, make immunity chicken get 90% protection from deadly attack of highly pathogenic vv IBDV, and effectively prevent virus proliferation in the body.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses)
The invention discloses a multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses), relating to the technical field of virology and immunology. The multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine is characterized in that two CTL epitopes (namely, a NS4B (1793-1801) SMMAFSAAL and a P7 (774-782) AAWYIKGRL) are used for constructing recombinant adenoviruses, then the recombinant adenoviruses are used for infecting human dendritic cells so as to prepare a multi-epitope DC vaccine. Detection results indicate that the multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses) disclosed by the invention has an immunogenicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
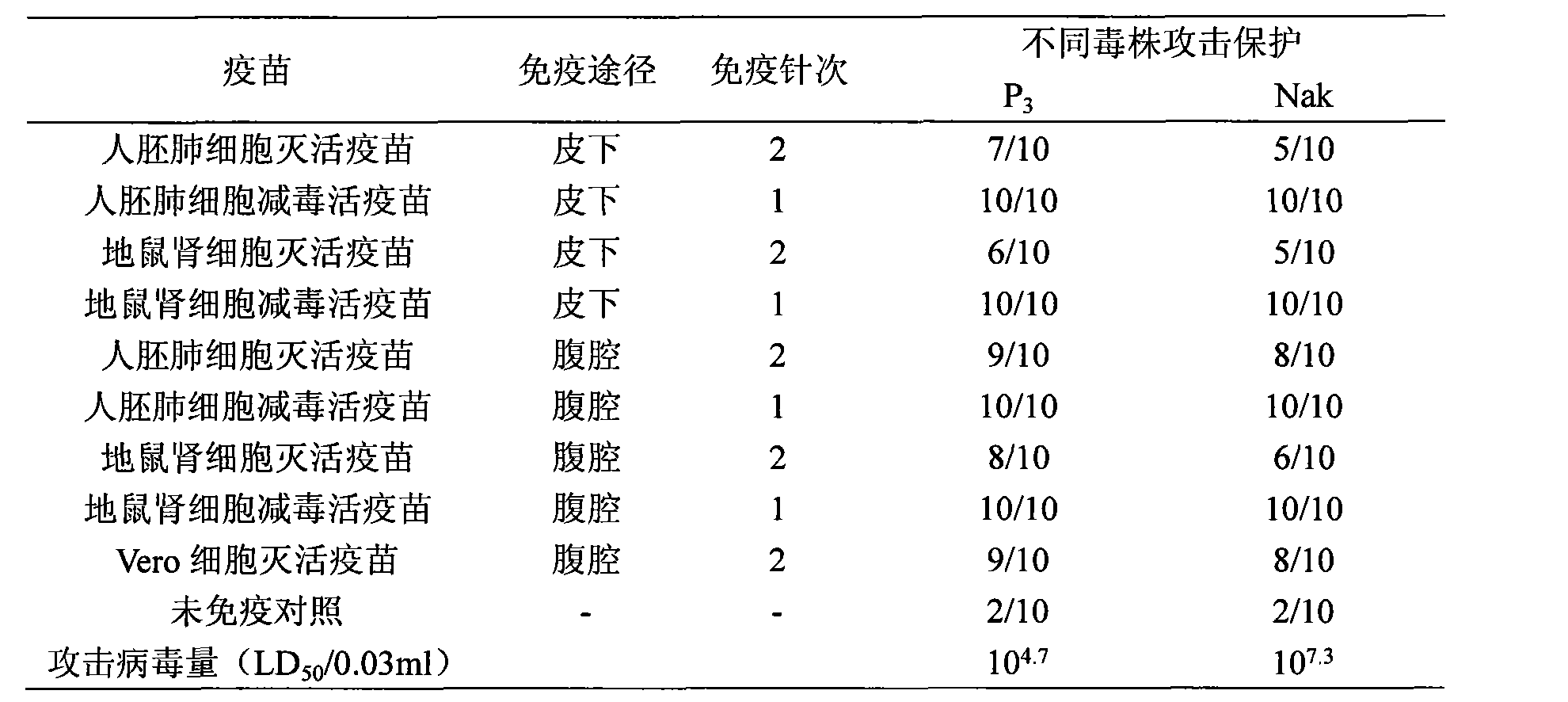
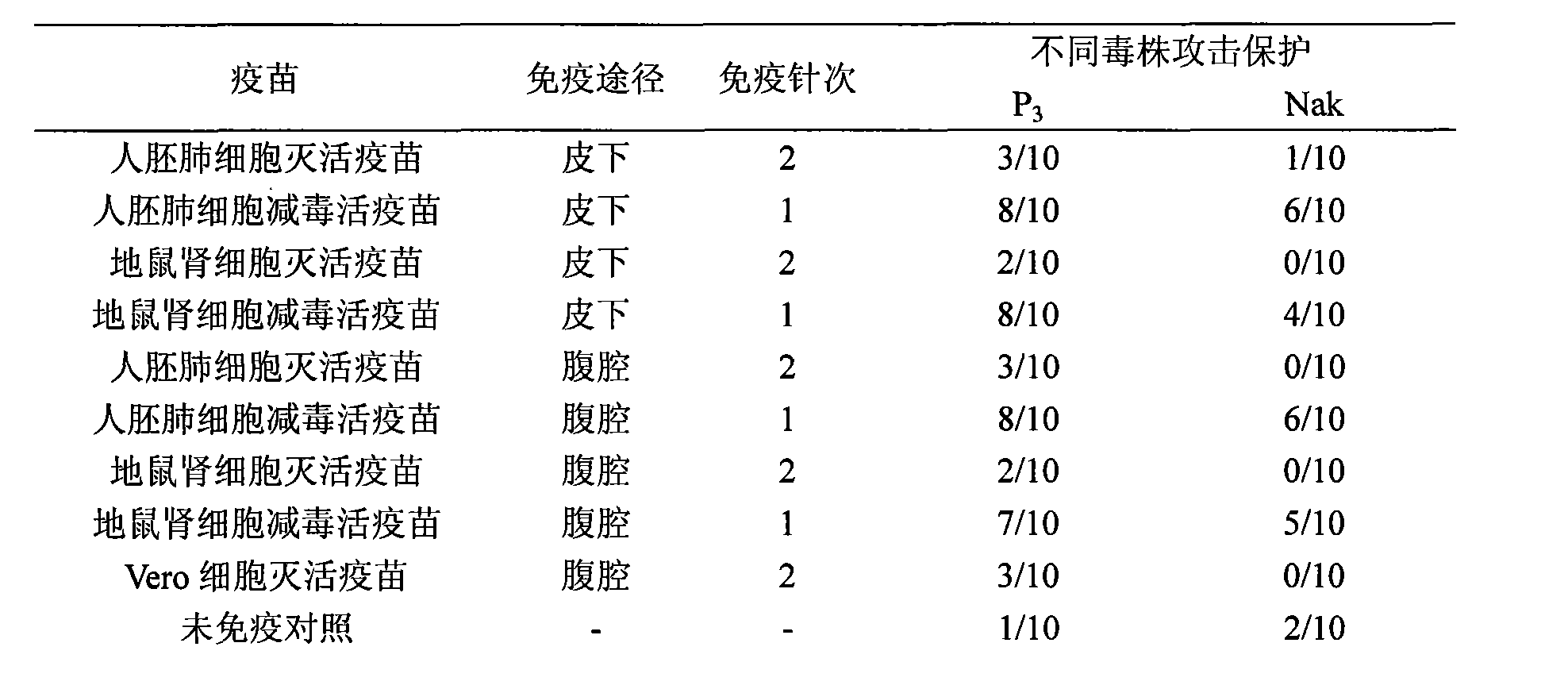
Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and preparation method thereof
ActiveCN101524536AFully identifiedFully standardizedViral antigen ingredientsAntiviralsImmune effectsJapanese encephalitis vaccine
The invention discloses a Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and a preparation method thereof, comprising culture and expansion of the human embryonic lung fibroblasts. The method comprises the following steps: Japanese encephalitis virus strain P3, strain SA14-14-2 or strain Nakayama is naturalized and inoculated to fit the human embryonic lung fibroblasts, and the seeds of Japanese encephalitis viruses are prepared on the human embryonic lung fibroblasts; wherein, an inactivated Japanese encephalitis vaccine also comprises the steps of harvesting viruses, inactivating viruses, concentrating, purifying and the like; an attenuated live vaccine also comprises the steps of harvesting viruses, concentrating, purifying and the like. Due to being prepared by healthy human embryonic lung fibroblasts, the two kinds of Japanese encephalitis vaccines do not contain any adventitious pollution agent and tumorigenicity, has high purity after being purified and has the advantages of good immune effect and high security. The preparation method of the invention is suitable for large-scale industrial production and can meet the preparation processes of Japanese encephalitis vaccines required by domestic and abroad markets.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
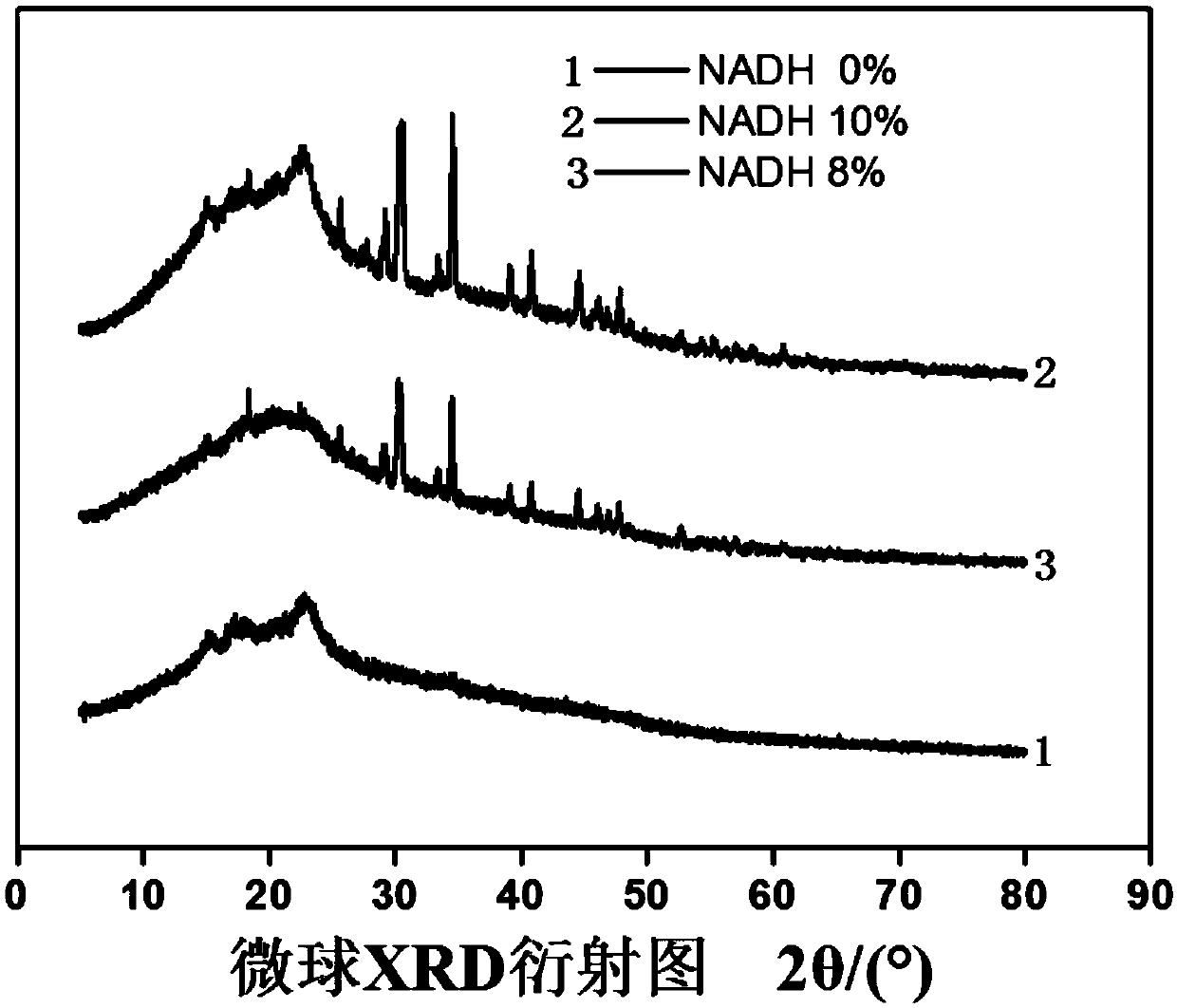
Biological macromolecule nanosphere containing NADH and preparation method and application thereof
InactiveCN109646423ALong storage timeReduce the difficulty of savingPowder deliveryOrganic active ingredientsBiological macromoleculeFunctional food
The invention discloses a biological macromolecule nanosphere containing NADH, which comprises a biological macromolecule carrier and NADH dispersed on the macromolecule carrier. The biological macromolecule carrier is a macromolecule fiber polymer, and the fiber framework of the macromolecule fiber polymer forms a three-dimensional interpenetrating network structure through arrangement modes of disorder arrangement, cross arrangement, crimping arrangement and the like, so that NADH is protected, and the problem that the NADH is easy to decompose when meeting light or oxygen is solved, the storage time of the effective components of the NADH is prolonged and the storage difficulty is reduced. The invention also discloses a preparation process of the nanosphere, which is characterized in that a biological macromolecule material with a moderate chain length is obtained after physical modification, spherical particles are easy to form, a honeycomb-shaped three-dimensional interpenetratingnetwork structure is formed, and NADH small molecules are favorable for being loaded on the inner surface and outer surface of the network structure. The nanospheres can be used for preparing tablets, granules, capsules or soft capsules and other preparations, and can be used as health promoting or pet therapeutic medicine / functional food.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
Construction method for delaying attenuation and increasing expression exogenous antigen salmonella suipestifer carrier through regulation and control of gene
ActiveCN104498418ALarge market applicabilityImmunogenicBacteriaMicroorganism based processesBacteroidesEscherichia coli
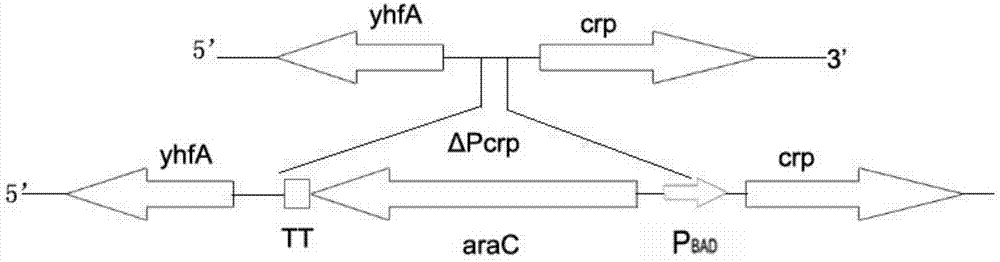
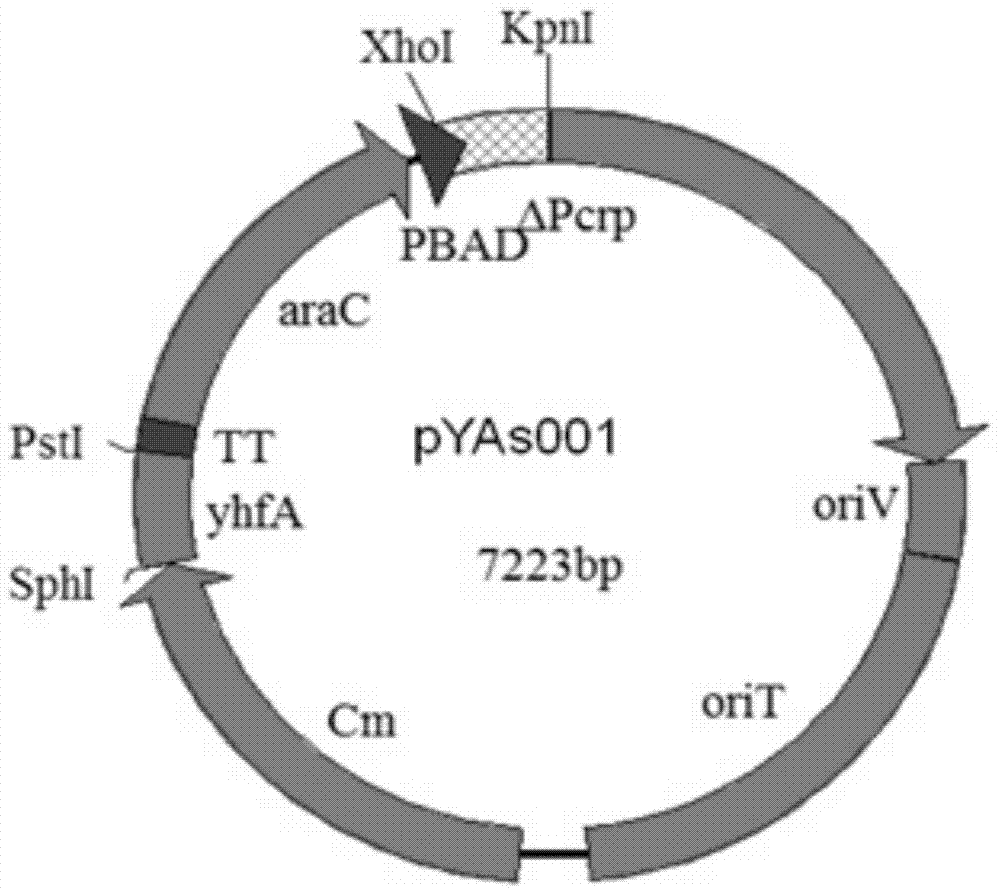
The invention provides a construction method for delaying attenuation and increasing an expression exogenous antigen salmonella suipestifer carrier through regulation and control of gene, which is characterized in that receptor bacterium C78-3 is combined with manA, crp, relA and asd -containing deleted suicide carrier escherichia coli donor bacterium, mutants delta manA, delta Pcrp: : TT ara C PBAD crp, delta relA: : araC PBAD lacI TT and delta asd A are introduced in wild-type salmonella suipestifer C78-3, the introduced salmonella suipestifer after mutation can be called x0011; and four types of mutation enable phenotype identification. The method of the invention makes salmonella suipestifer to become safe and effective vaccine carrier of many exogenous antigens; and provides the delaying attenuation and increasing expression exogenous antigen salmonella suipestifer carrier through regulation and control of gene for various pig diseases, especially many bacteria diseases of pig, and has great market applicability.
Owner:YANGZHOU UNIV
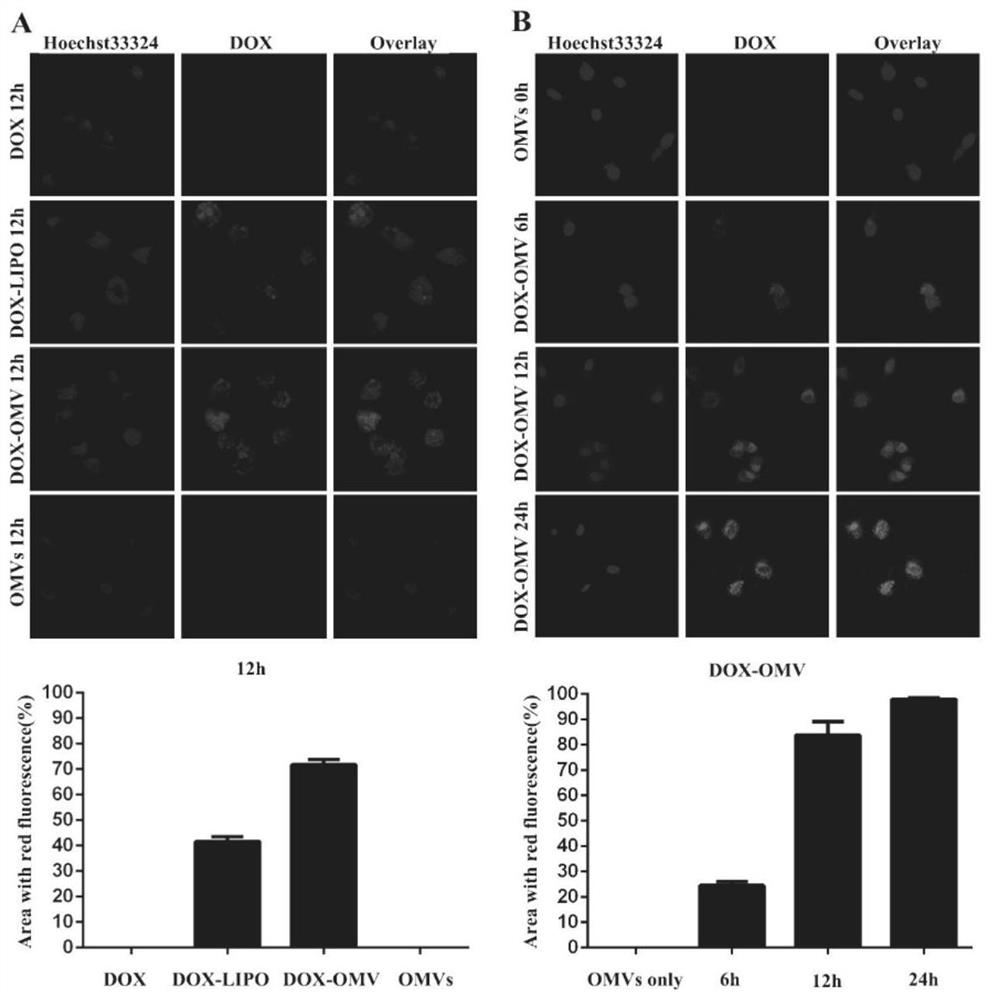
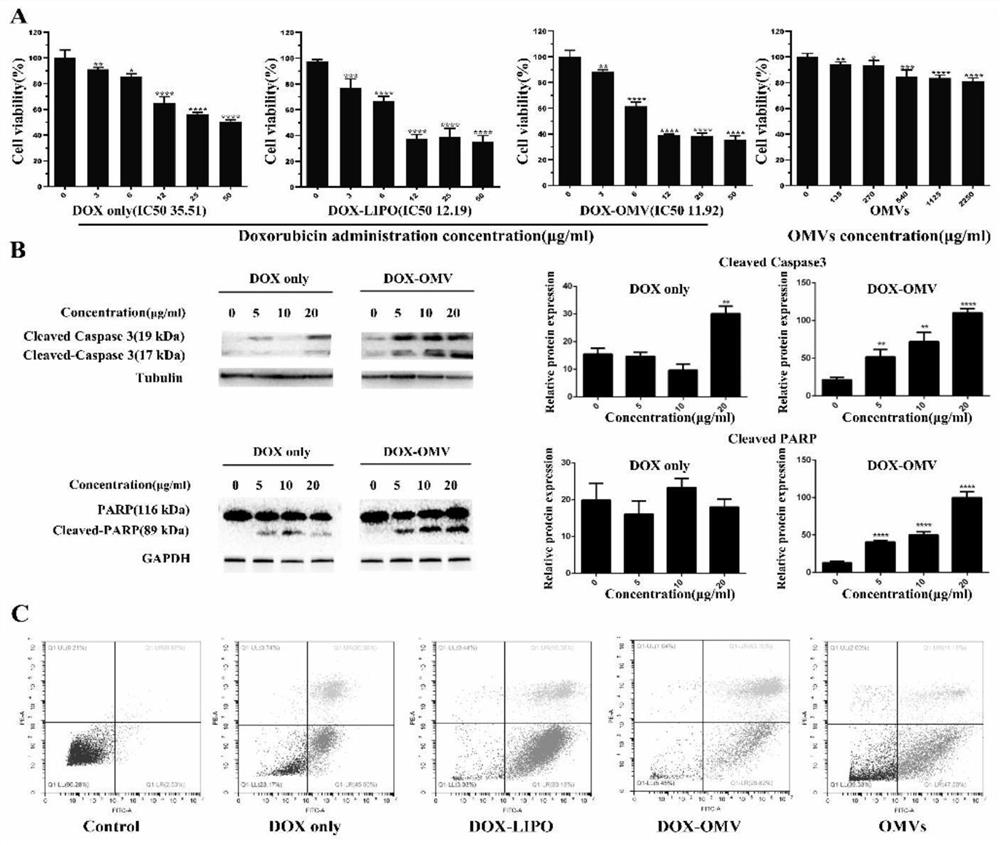
Adriamycin nanoparticles entrapped by bacterial outer membrane vesicles and application of adriamycin nanoparticles
InactiveCN112891318AExtended half-lifeEnhanced anti-non-small cell lung cancer efficacyOrganic active ingredientsPharmaceutical non-active ingredientsK pneumoniaeTumor chemotherapy
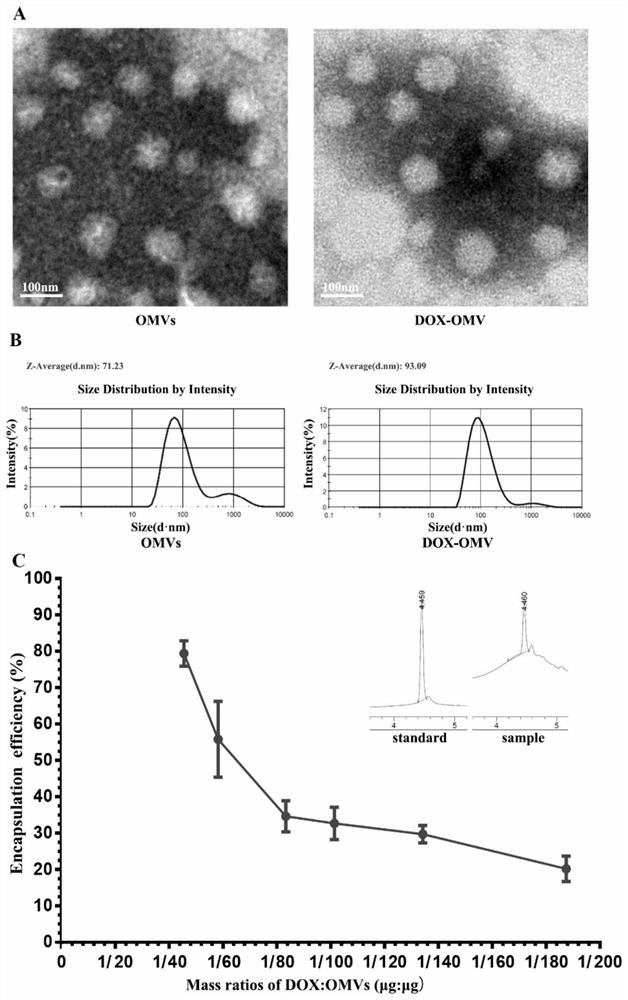
The invention belongs to the technical field of medicines, and relates to drug-loaded nanoparticles, in particular to adriamycin nanoparticles entrapped by bacterial outer membrane vesicles and an application of the adriamycin nanoparticles. The invention provides adriamycin nanoparticles DOX-OMV which are entrapped by bacterial outer membrane vesicles and are prepared by adopting attenuated klebsiella pneumoniae sourced bacterial OMVs (outer membrane vesicles) to entrap a tumor chemotherapeutic drug DOX (doxorubicin). The test research on the effect of resisting non-small cell lung cancer shows that the DOX-OMV nanoparticles can play a lung cancer cell targeting role and an anti-tumor immune induction effect at the same time, and the half-life period of the drug is prolonged, so that the non-small cell lung cancer resisting curative effect of the chemotherapeutic drug adriamycin is remarkably enhanced, and the DOX-OMV nanoparticles have good safety. The prepared DOX-OMV nanoparticles can be used for preparing drugs for resisting non-small cell lung cancer.
Owner:FUDAN UNIV
Method for preparing inactivated vaccine of encephalitis B used for human being
InactiveCN1966075AGood for quality controlThe preparation method is scientific and reasonableViral antigen ingredientsAntiviralsSepharoseAcute viral encephalitis
The invention relates to an encephalitis-B inactivated vaccine, and relative production, wherein it uses human diploid cell KMB17 to cultivate encephalitis B virus DL4, collects clear virus liquid, inactivates via 1:2000 formaldehyde, dialyses, concentrates, purifies via Sepharose 4FF post, and adsorbs via aluminum hydroxide. The inventive vaccine has immunogen, with high safety, while it can prevent encephalitis B.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Preparation method and application of paclitaxel albumin nanoparticles
InactiveCN108524452AImprove drug solubilityGood biocompatibilityOrganic active ingredientsPowder deliveryAlbumin nanoparticlesPharmaceutical formulation
The invention relates to a preparation method and application of paclitaxel albumin nanoparticles and belongs to the field of medicinal preparations. The preparation method includes: weighing paclitaxel active pharmaceutical ingredient, and dissolving in a proper amount of organic solvent to obtain an oil phase; weighing albumin, dissolving in water, stirring, heating to enable the albumin to be fully dissolved to obtain an albumin water solution, adjusting pH of the albumin water solution to 6.0, and adding the organic solvent to obtain an aqueous phase; under action of high-speed shearing, dropwise adding the oil phase into the aqueous phase to obtain an oil-in-water emulsion; transferring the oil-in-water emulsion into a high-pressure microjet nano dispersing instrument for high-pressure homogenizing, and removing the organic solvent from a system after homogenizing is completed through rotary evaporation to obtain a water solution of the paclitaxel albumin nanoparticles. Particle size of the paclitaxel albumin nanoparticles can be controlled by changing proportion of the oil phase and is 100-200nm. The preparation method is simple, controllable and suitable for industrial production.
Owner:LIAONING UNIVERSITY
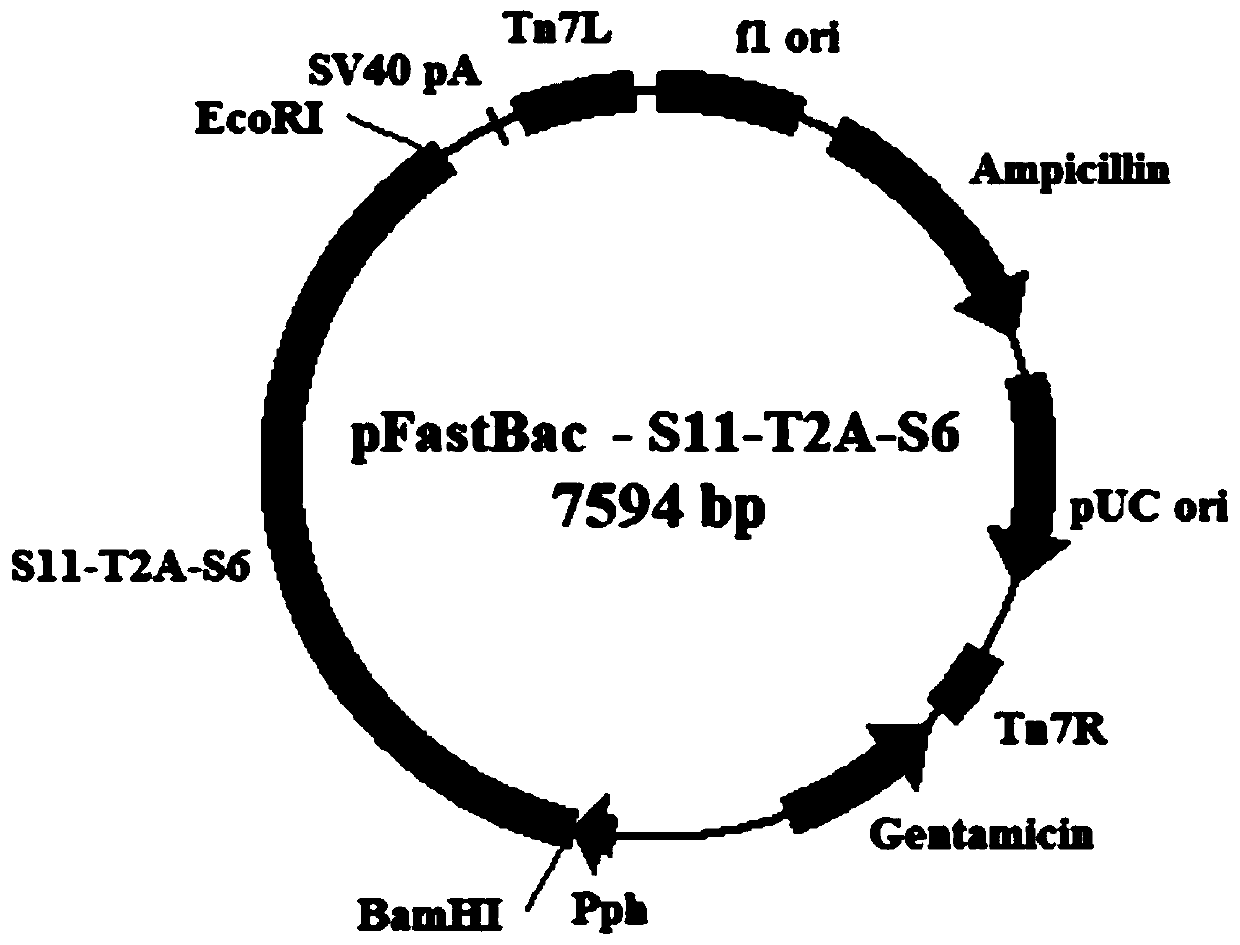
Preparation method and application of recombinant baculovirus co-expressing grass carp reovirus capsid proteins VP4 and VP35
ActiveCN110144334AEfficient expressionHigh immune protection rateViral antigen ingredientsVirus peptidesRestriction Enzyme Cut SiteCapsid
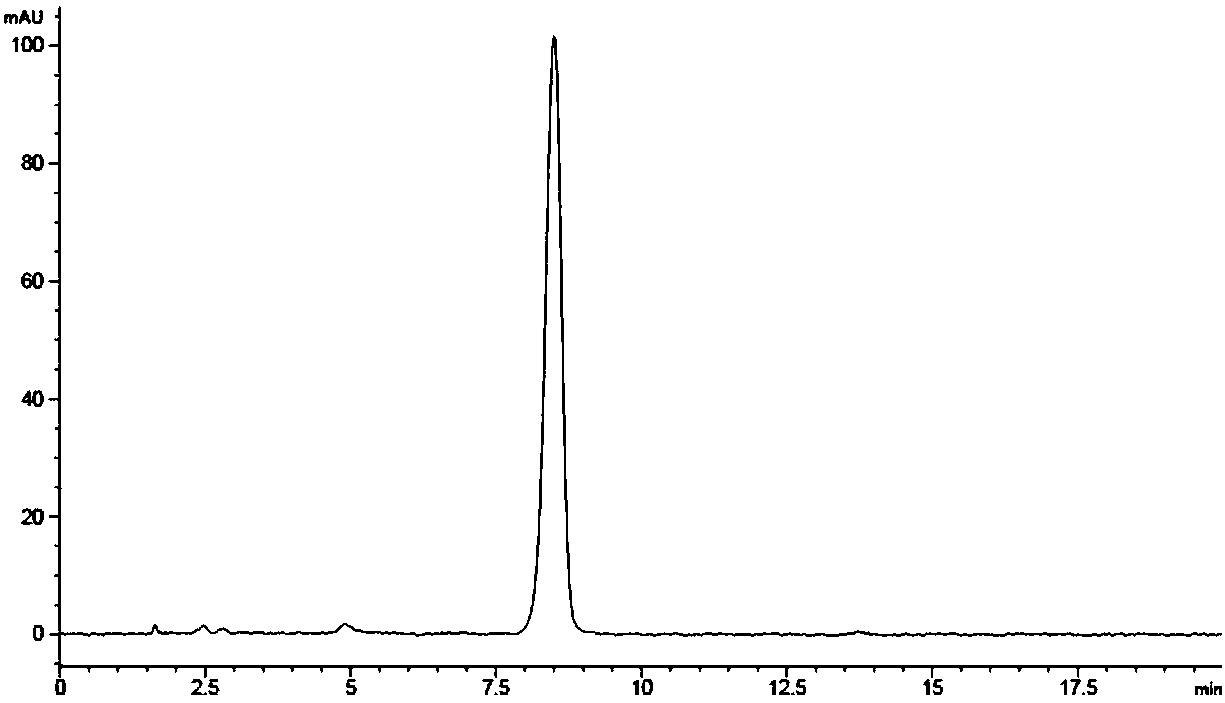
The invention belongs to the technical field of biology, and particularly discloses a preparation method and application of a recombinant baculovirus co-expressing grass carp reovirus capsid proteinsVP4 and VP35. Sequences as shown in SEQ ID NO.1, SEQ ID NO.3 and SEQ ID NO.2 are sequentially inserted among restriction enzyme cutting sites of BamHIand EcoRI pFastBacTMIto obtain recombinant plasmids pFastBac-S11-T2A-S6, and finally obtain the recombinant baculovirus co-expressing grass carp reovirus capsid proteins VP4 and VP35. The recombinant virus has high expression quantity, can be used for preparing proteins applicable to production of grass carp reovirus vaccines and have a wide application prospect.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Preparation method and application of soluble I-type DHV (Duck Hepatitis Virus) 3D protein
ActiveCN104293823AImmunogenicOvercome the defect of easy formation of inclusion bodiesSerum immunoglobulinsVirus peptidesEscherichia coliDuck hepatitis A virus
The invention discloses a preparation method and application of soluble I-type DHV (Duck Hepatitis Virus) 3D protein. The preparation method comprises the steps as follows: firstly, the 9-1376 th nucleotide, shown in SEQ ID NO.3, is connected to the polyclone restriction enzyme cutting site of a pET-32(a)+ carrier; secondly, escherichia coli Rosetta, BL21 or BL21(DE3)PLYS is adopted as a host bacterium; thirdly, after the activation of the host bacterium in an Amp resistant LB culture medium, IPTG is put in additionally until the concentration reaches 0.2-1.0 mmol / L, and then the host bacterium is subjected to inducible expression for 4-12 hours at the temperature of 20-39 DEG C. According to the preparation method provided by the invention, the obtained I-type DHV 3D protein is soluble and has immunogenicity, can stimulate a duck body to generate an antibody for the I-type DHV 3D protein, can be used for detecting the serum sample of I-type DVH (Duck Viral Hepatitis) or a reagent containing the antibody for I-type DHV 3D protein, and has great significance for the diagnosis of the I-type DVH and the researches on vaccine for the I-type DVH.
Owner:SICHUAN AGRI UNIV
One coding gene of rotavirus VP6 protein and its application
InactiveCN1772902AHigh expressionPrevention of Acute DiarrheaViral antigen ingredientsDigestive systemAcute diarrheaRotavirus RNA
The present invention discloses one coding gene of rotavirus VP6 protein and its application, and aims at providing one coding gene of rotavirus VP6 protein with high expression amount in dicotyledonous plant, especially clover, method of producing oral rotavirus vaccine with the coding gene and the obtained oral vaccine. The rotavirus VP6 protein coding gene has one of the following nucleotide sequences: 1. DNA sequence of SEQ ID No. 1 in the sequence list; 2. DNA sequence of SEQ ID No. 2 in the sequence list; 3. the nucleotide sequence capable of hybridizing in high strict condition with the DNA sequence limited by SEQ ID No. 1 or SEQ ID No. 2; and 4. DNA sequence possessing over 80 % homology with the SEQ ID No. 1 or SEQ ID No. 2 limited DNA sequence and high expression amount in dicotyledonous plant. The oral rotavirus vaccine is used in preventing various rotavirus infection caused acute diarrhea of infant and young animal.
Owner:CHINA AGRI UNIV
VZV recombinant gE-flagellin fusion protein as well as preparation method and application thereof
PendingCN108727503AHigh antibody titerGood immune activityViral antigen ingredientsAntibody mimetics/scaffoldsGlycoproteinFlagellin
The invention discloses a VZV recombinant gE-flagellin fusion protein as well as a preparation method and application thereof. The fusion protein at least comprises an N-end conserved region of flagellin protein, varicella zoster virus (VZV) glycoprotein gE and a C-end conserved region of the flagellin protein. Compared with an independently pre-recombinant gE protein, the VZV recombinant gE-flagellin fusion protein provided by the invention can cause higher antibody titer, so that immunological competence is relatively good, cell-mediated immunity and humoral immunity can be motivated, and therefore, the VZV recombinant gE-flagellin fusion protein does not have a potential infection risk, and a good immunological basis is laid for preparing a novel varicella protein vaccine.
Owner:BRAVOVAX
Energy-saving method for culturing haliotis diversicolor aquatilis
InactiveCN102017913AImprove intestinal environmentImprove immunityBacteriaClimate change adaptationHaliotis diversicolorWater quality
The invention discloses an energy-saving method for culturing haliotis diversicolor aquatilis. The method comprises the following steps of: (1) culturing algae; (2) adding nutrient salt; (3) collecting the spats and distributing eggs, namely, changing water in an algae culturing pool, adding bdellovibrio bacteriovorus plasmid bacterial liquid into the pool until the concentration of bdellovibrio bacteriovorus plasmid in the pool water is between 10 and 107 pfu / mL and distributing 40,000 to 50,000 haliotis diversicolor aquatilis fertilized eggs in each pool during spat distribution; (4) changing water, namely, changing the pool water once every 5 to 30 days and adding the bdellovibrio bacteriovorus plasmid bacterial liquid into the pool water after the water is changed each time until the concentration of the bdellovibrio bacteriovorus plasmid in the pool water is between 10 and 107 pfu / mL; and (5) stripping and collecting. The spat culturing method remarkably promotes the growth of the haliotis diversicolor aquatilis, greatly increases the survival rate of the haliotis diversicolor aquatilis and improves the water quality of a culturing environment. The bdellovibrio bacteriovorus plasmid does not have toxic or side effect on abalone, so that the method is suitable for large-scale culturing of the abalone and provides new culturing technology for promoting the spat culturing and growth of haliotis diversicolor aquatilis.
Owner:SOUTH CHINA UNIV OF TECH
Recombined subunit vaccine of haemaphysalis concinna and preparation method thereof
InactiveCN101757618AGood effectPromotes Anti-Tick ImmunityAntiparasitic agentsAntibody medical ingredientsDiseaseProtective antigen
The invention discloses a recombined subunit vaccine of haemaphysalis concinna and a preparation method thereof. The recombined subunit vaccine is formed by mixing antigenic gene recombined protein of the haemaphysalis concinna and Freund's complete adjuvant (FCA), wherein the content of the antigenic gene recombined protein is 50 mg / ml, that is 50 mL rHc-23 and 950 ml Freund's complete adjuvant are mixed to prepare the recombined subunit vaccine of the haemaphysalis concinna; the amino acid sequence of the antigenic gene recombined protein is shown in the table SEQID NO:1; and the nucleotide sequence of the antigenic gene is shown in the table SEQID NO:2. Through screening and cloning, prokaryotic expression and separation and purification of protective antigen gene of the haemaphysalis concinna and the application effect test of the recombined subunit vaccine, the method shows that an expression product of recombined vector bacteria can be identified by rabbit anti-haemaphysalis concinna positive serum. In an animal immunity test, after rHc-23-FCA is subjected to three immune rabbits, the blood saturation rates of haemaphysalis larva, haemaphysalis middle and haemaphysalis imago are 40.3 percent, 45.6 percent and 41.3 percent respectively; and compared with the blood saturation rates of the haemaphysalis larva, haemaphysalis middle and haemaphysalis imago in a contrast set: 90.1 percent, 94.3 percent and 97.7 percent, the differences are remarkable. The method promotes the development of anti-haemaphysalis immunity, haemaphysalis control and haemaphysalis disease spread work.
Owner:SICHUAN AGRI UNIV
Recombinant feline parvovirus VP2 protein antigen and application thereof in antibody diagnosis and vaccine preparation
ActiveCN113956362AHigh sensitivityImmunogenicViral antigen ingredientsAntibody mimetics/scaffoldsFeline parvovirusCellular antigens
The invention provides a recombinant feline parvovirus VP2 protein antigen and application thereof in vaccine preparation and virus diagnosis. A plurality of B cell antigens of the feline parvovirus VP2 protein are subjected to tandem expression by using a prokaryotic expression vector. The expressed recombinant protein is purified and used as a coating antigen for detecting the feline parvovirus antibody. And compared with a whole virus coating method in parallel, the values of detected positive and negative serum are highly consistent. The antigen treatment method provided by the is convenient, the test time is shortened, and the operation steps are simpler. According to the invention, an indirect ELISA method is established for detecting the antibody level of the feline parvovirus in feline serum, which has the characteristics of good repeatability and high specificity, and can be used for feline parvovirus serology investigation. Therefore, the indirect ELISA detection kit for the feline parvovirus based on the tandem expression of the VP2 protein B cell antigens, provided by the invention, is very suitable for the detection of clinical large samples and is suitable for large-scale popularization.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Composite active matters of mask and preparation method of composite active matters
ActiveCN102895165AEfficient degradationOperational securityCosmetic preparationsToilet preparationsProtein moleculesCulture fluid
The invention relates to a mask process of daily necessities, in particular to an application of residual primary culture fluids of mammalian cells in the preparation of active matters of a mask. According to the invention, the residual cell culture fluids are sources of growth factor type matters at home and abroad, and are inexpensive and high in quality, renewable, safe and reliable; silk fibroins can be effectively degraded at the normal temperature by enzymes contained in the residual cell culture fluids, so that small molecular silk fibroins are obtained, and the operation is simple and safe; then sericin in the silk fibroins has immunogenicity so that anaphylaxis may be caused, and the posterior silk gland-derived silk fibroins are completely free of sericin so that the possibility of anaphylaxis is greatly reduced; and the film-forming property of a silk mask is the difficulty of the preparation, a silk I conformation of protein molecules is gradually transformed to silk II in the process of repeatedly stirring and drying the silk fibroins at a high temperature, so that the silk fibroins are stable in structure and have excellent mechanical properties.
Owner:延荣(海南)企业管理中心(有限合伙)
Mycoplasma bovis enolase and new applications of coding gene thereof
InactiveCN102533940ACtiveImmunogenicMicrobiological testing/measurementMicroorganism based processesDiseaseMycoplasma bovis Antibody
The invention discloses mycoplasma bovis enolase and new applications of a coding gene thereof. Specifically, the invention discloses the mycoplasma bovis enolase and applications of the coding gene of the mycoplasma bovis enolase in preparing reagents for detecting plasminogen and detecting or diagnosing infections caused by mycoplasma bovis. According to the mycoplasma bovis enolase disclosed by the invention, an enolase protein is obtained through prokaryotic expression, and soluble expression is successfully performed on the protein through regulating induction conditions, and further, through recombinant enolase protein and plasminogen ligand experiments and enolase protein and mycoplasma bovis antibody Western blot experiments, the protein is finally proved to have the binding activity and the immunogenicity of the plasminogen, therefore, the protein can be used for detecting the plasminogen and detecting or diagnosing the infections or diseases caused by the mycoplasma bovis. Moreover, a new path is also opened up for researching the adhesion mechanism and the pathogenicity of the mycoplasma bovis and developing vaccines in future.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Pseudorabies virus GE gene major antigen-epitope region recombinant protein preparation and colloidal-gold immunochromatographic strip
InactiveCN110540578ASmall sample sizeEasy to detectVirus peptidesBiological material analysisAntigen epitopeAntigen
The invention provides a pseudorabies virus GE protein and preparation of a colloidal-gold immunochromatographic strip thereof. The pseudorabies virus colloidal-gold immunochromatographic strip is provided with a carrier plate, a sample pad, a colloidal-gold conjugate pad, a laminate membrane, a detection line, a quality-control line and an absorption pad. The expressed recombinant protein existedin a supernatant is easy to purify. The detection method established by taking the recombinant protein, which is purified by affinity chromatography, as a diagnostic antigen is low in cost and simpleand convenient, and especially the diagnostic antigen concentrates major antigen-epitopes of the gE antigen. The detection sensitivity and specificity of the recombinant protein are both higher thanthe intact gE protein.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI +1
CD25 nanometer antibody as well as coding sequence and application thereof
InactiveCN103333248AEfficient expressionImmunogenicBacteriaMicroorganism based processesEpitopeEscherichia coli
The invention discloses a nanometer antibody for CD25 polypeptide molecular antigenic epitope and also discloses a gene sequence for coding the nanometer antibody and a host cell capable of expressing the nanometer antibody. Through the gene sequence and the host cell of the nanometer antibody, the nanometer antibody can be efficiently expressed in escherichia coli and can be applied to the research and development of a CD25 molecular detection reagent.
Owner:SOUTHEAST UNIV
Preparation method of Burkholderia pseudomallei recombined BLF1 protein, product prepared through preparation method and application of Burkholderia pseudomallei recombined BLF1 protein
InactiveCN105505973AMaintain immunogenicityThe purification conditions matchPeptide/protein ingredientsBacteria peptidesImmunogenicityWilms' tumor
The invention discloses a preparation method of Burkholderia pseudomallei recombined BLF1 protein, the product prepared through the preparation method and an application of the Burkholderia pseudomallei recombined BLF1 protein. The preparation method includes the steps that a primer for amplifying a Burkholderia pseudomallei BLF1 gene is designed firstly, a sequence shown in SEQ ID NO.3 of a coding area sequence is obtained through amplification, then the acquired sequence is connected to an expression vector to construct a recombinant expression vector, the recombinant expression vector is converted into host bacteria, and through inducible expression, the Burkholderia pseudomallei recombined BLF1 protein is obtained. The preparation method is simple, the space design conception and immunogenicity of the purified protein can be kept to the maximum degree, the purity of the protein is larger than 95%, it is proved through a mouse experiment that the purified protein has animal toxicity, it is shown that the purified protein has biological activity, the purified protein acts on A549 cells, cell growth can be restrained, and thus the Burkholderia pseudomallei recombined BLF1 protein can be used for preparing anti-tumor medicine and has wide application prospects.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Phase change type nano particle and preparation method and application thereof
ActiveCN109620976AAchieve precise imagingAchieve therapeuticPowder deliveryEnergy modified materialsTreatment effectMelanoma
The invention belongs to the technical field of medicines and relates to a nano particle, in particular to a phase change type nano particle. The phase change type nanoparticle is formed by liposome-encapsulated gold nanorods and liquid fluorocarbon, wherein monoclonal antibodies of melanoma-associated antigens are attached to the surfaces of liposomes. The phase change type nanoparticle has phototransformation properties, thereby having superior development and tumor treatment effects; drug encapsulation of the phase change type nano particle is achieved by using a liposome material, so thatthe immunity of the nanoparticle is reduced, and he biocompatibility of the nanoparticle is improved. The nanoparticle prepared by the technical scheme can be applied to contrast agents and therapeutic drugs of melanoma.
Owner:CHONGQING MEDICAL UNIVERSITY
Preparation and application of Her2-neu antigen positive tumor therapeutic vaccine
The invention provides preparation and application of a Her2-neu antigen positive tumor therapeutic vaccine, and particularly provides a fusion protein. The fusion protein contains (a) a tumor antigen Her2-neu extracellular region element and (b) a heat shock protein element. The invention also provides a dendritic cell sensitized by fusion protein and a corresponding tumor therapeutic vaccine. Test proves Her2-neu antigen specific immunological response can be effectively activated by applying the vaccine in vivo, and the vaccine is effectively applied to Her2-neu positive tumor.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
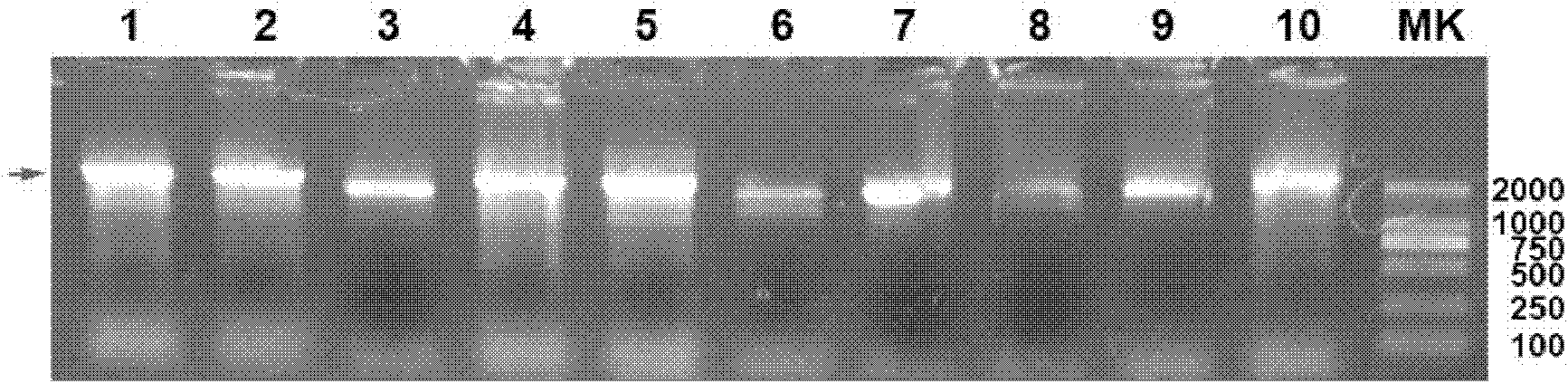
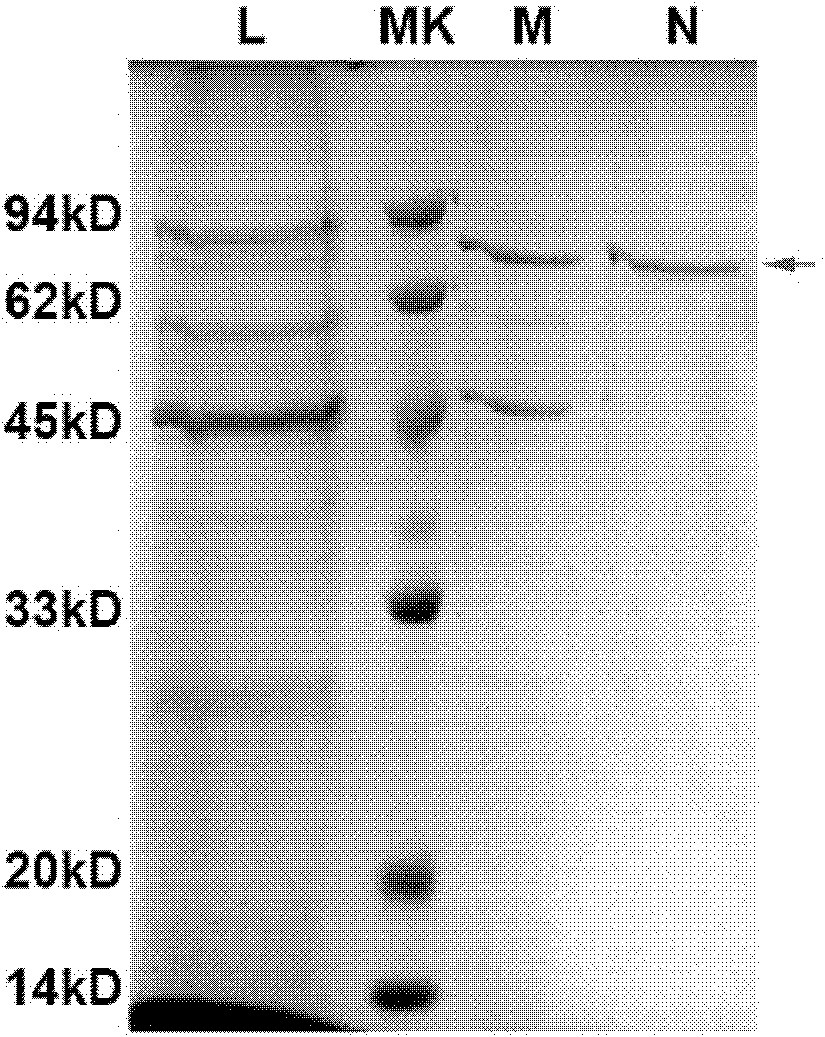
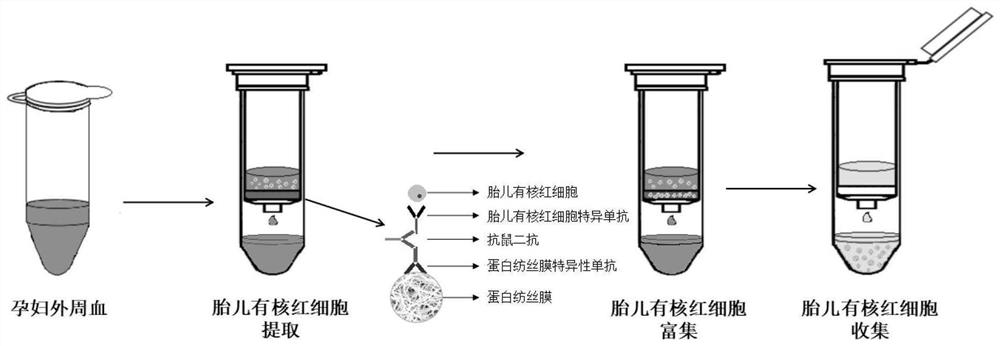
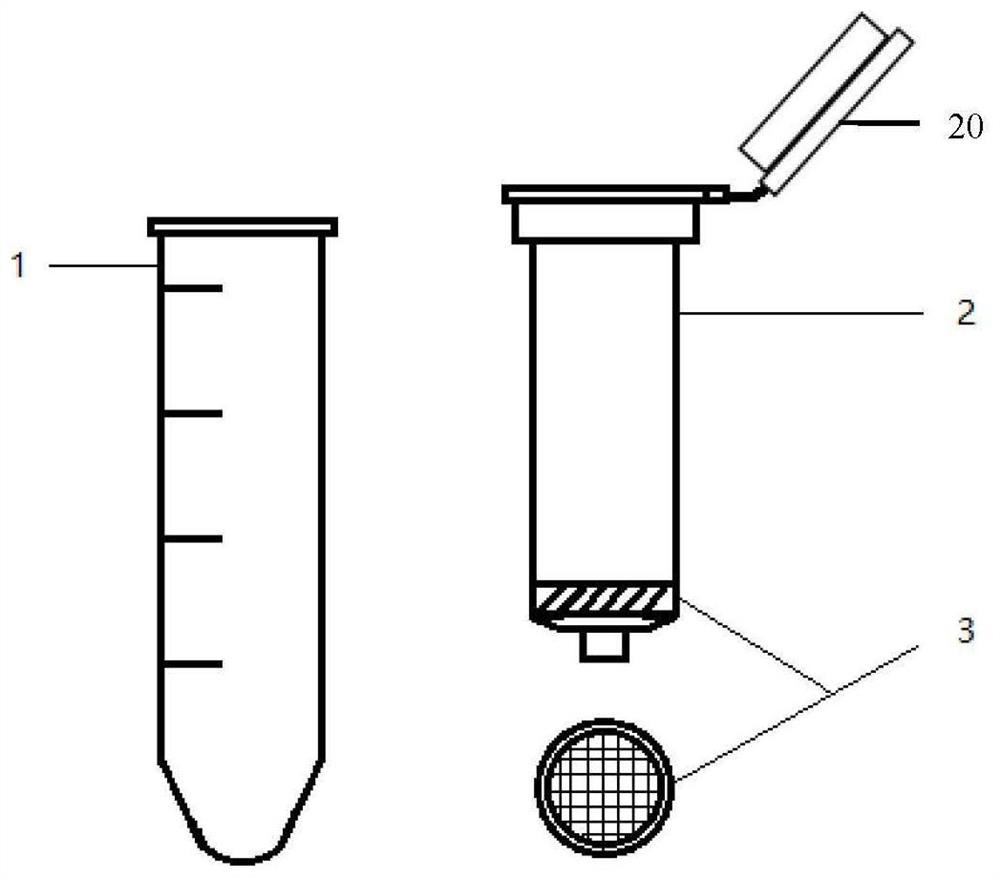
Infantile nucleated red blood cell trap vector, extraction device and method
ActiveCN111893086AImmunogenicImprove hydrophilicityBioreactor/fermenter combinationsCell dissociation methodsAntibodyMolecular biology
The invention discloses an infantile nucleated red blood cell trap vector, an extraction device and a method. The infantile nucleated red blood cell trap vector is obtained by coupling a protein spinning membrane murine monoclonal antibody and a nucleated red blood cell marker murine monoclonal antibody on a protein spinning membrane. The scheme can specifically trap a nucleated red blood cell, has the characteristics of strong detection sensitivity and specificity and overcomes the defects of non-specific binding, disordered coating and low efficiency of a traditional method; the protein spinning membrane has a multi-layer porous structure, has the surface area bound with a reactor far larger than that of an existing vector, and is extremely weakly bound with non-specific ingredients in ablood sample; and furthermore, protein ingredients of the protein spinning membrane have an excellent protective effect on bound bio-active ingredients and have good stability. The extraction deviceis simple in structure and easy in preparation, is not limited by raw material source and has low cost. The extraction method is easy to operate and can avoid loss of cells in the operating process.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
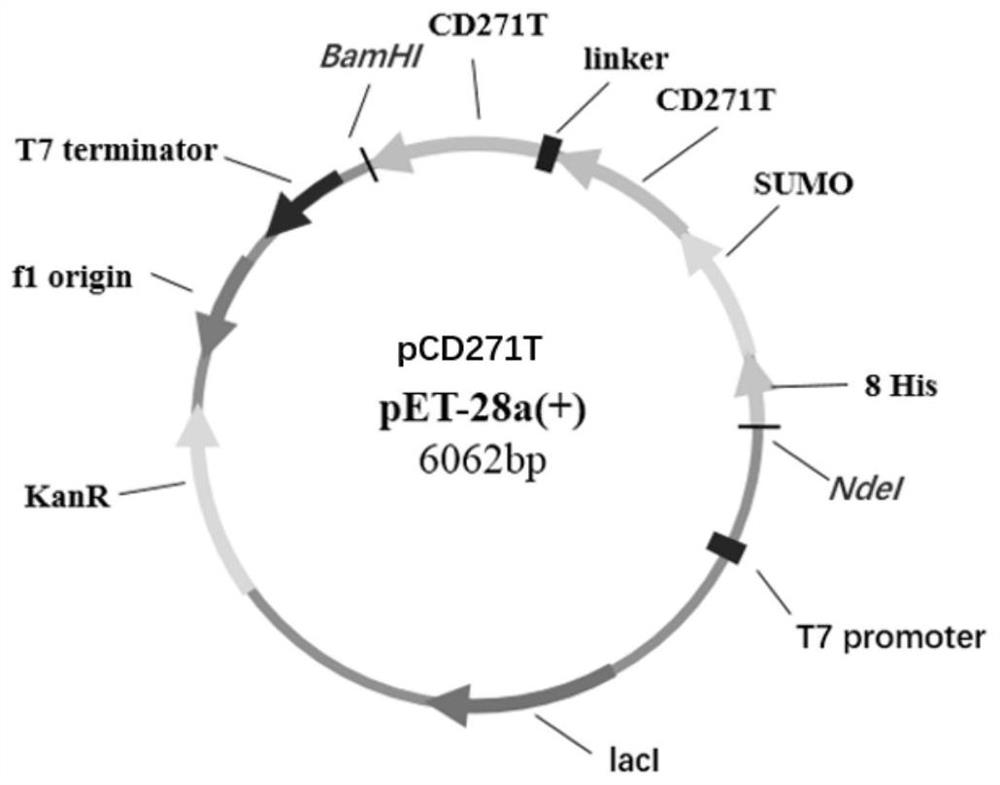
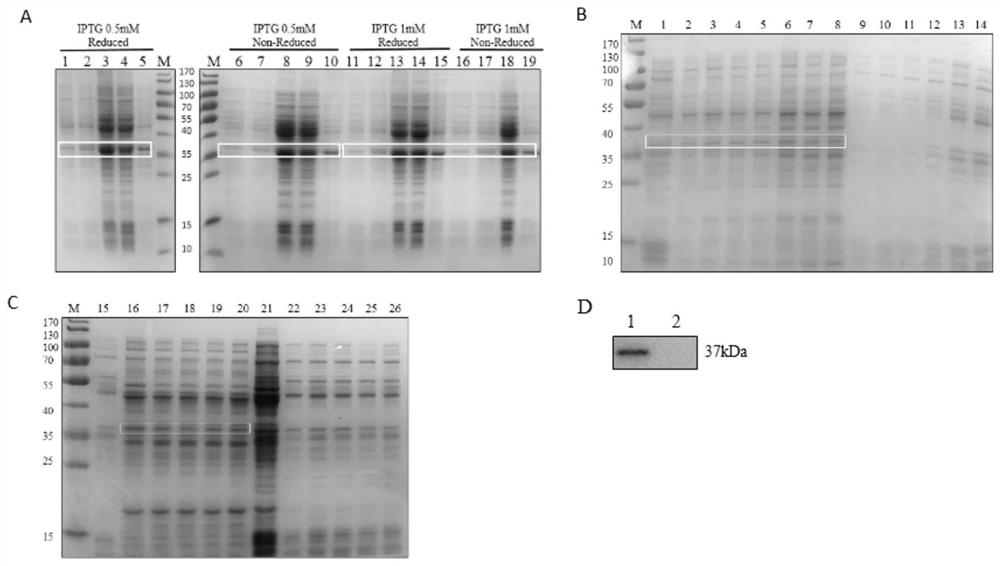
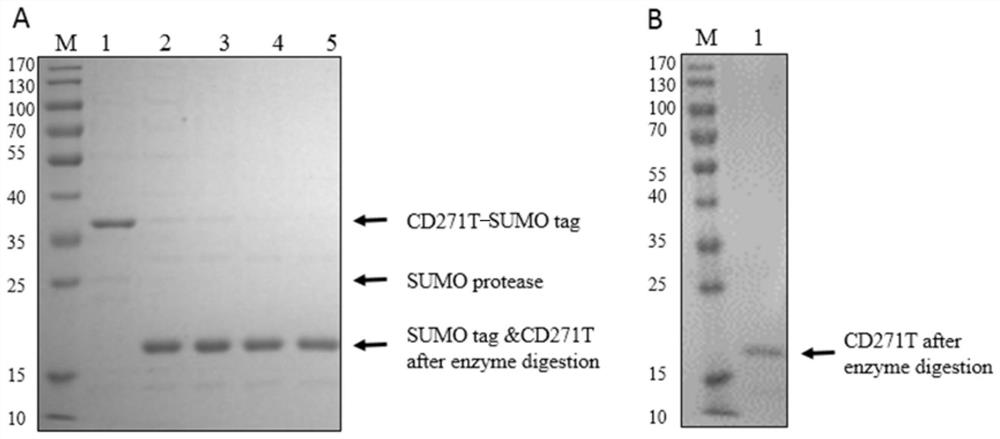
Novel antigen epitope based on CD271 and application thereof
The invention relates to a novel antigen epitope peptide based on CD271 and application of the novel antigen epitope peptide. The antigen epitope peptide is: (1) located in SEQ ID NO.1; or (2) is located in an amino acid sequence having at least 80% identity with the SEQ ID NO.1 sequence in (1); wherein SEQ ID NO.1 contains one or more antigen epitopes, and the length of the amino acid sequence of the antigen epitope peptide is 5-70% of the full length of the SEQ ID NO.1. An antibody, a nucleic acid aptamer, a vaccine, nanoparticles and the like targeting the antigen peptide can be stably combined with natural cells with CD271 protein. The antigen epitope peptide or nucleic acid, fusion protein, carrier or host cell and the like containing the antigen epitope peptide have important application significance in the fields of preparation of CD271 related detection reagents, disease treatment biological drugs, vaccines and the like.
Owner:苏州铂维生物科技有限公司
Recombinant lactobacillus plantarum for expressing newcastle disease virus antigen gene and fermentation process and application of recombinant lactobacillus plantarum
ActiveCN112300976AStrong acid resistanceExcellent growthSsRNA viruses negative-senseBacteriaImmunogenicityOrganism
The invention provides a recombinant lactobacillus plantarum for expressing a newcastle disease virus antigen gene and a fermentation process and application of the recombinant lactobacillus plantarum, and belongs to the technical field of microorganisms. The recombinant lactobacillus plantarum NC8-pSIP409-pgsA' (ata)-HN-DCpep is successfully constructed. Tests prove that the recombinant lactobacillus plantarum can efficiently express the HN antigen protein, and the protein has immunogenicity. Compared with an original starting strain NC8, the recombinant lactobacillus plantarum has better growth, fermentation, storage performance and the like, meanwhile, SPF chicks are immunized with recombinant lactobacillus plantarum freeze-dried bacterial powder, the safety performance is good, and thechicks has no abnormal reaction after large-dose immunization. The immune performance of the mucous membrane of an organism can be remarkably improved by adopting low-dose immunization, the effect ofresisting the NDV-VII toxic strain is achieved, the survival rate of chickens counteracting toxic substances is 71.6%, and therefore good practical application value is achieved.
Owner:JILIN AGRICULTURAL UNIV +1
Method for preparing cyclosporin A holoantigen
InactiveCN103421187ASave synthesis processShorten photochemical reaction timeChemical reactionHigh pressure
The invention provides a method for preparing cyclosporin A (CsA) holoantigen. According to the method, a high pressure mercury lamp is taken as a UV light source of a photochemical reaction, tert-butyl alcohol is taken as a reaction solvent, an intermediate CsA incomplete antigen is obtained by performing an addition reaction on 4-benzoylbenzoic acid and CsA under conditions of the photochemical reaction; the CsA incomplete antigen is dissolved in dimethyl formamide, and reacted with poly-L-lysine in water under the effect of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride for synthesis of cyclosporin A holoantigen. The method is simple and feasibile and easy to operate, helps to shorten the photochemical reaction time of producing CsA incomplete antigen and substantially save the synthetic process of cyclosporin A holoantigen. ELISA reaction experiments and animal (mice) immunization experiments verify that the CsA holoantigen synthesized by the method has good specificity and immunogenicity, and the method is applicable to synthesis of CsA holoantigen.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
A kind of bone implant filling material and preparation method thereof
ActiveCN103800944BFast degradationControl to adjust release speedPeptide/protein ingredientsProsthesisBiocompatibility TestingSlow Release Formulation
The invention provides a bone-grafting filling material and a preparation method thereof. The method comprises the following steps: mixing and crushing biphasic calcium phosphates, calcium sulphate and iron oxide into grains of 0.1-1mm, burning at 800 DEG C for 3 hours, taking out to soak in 1M of (NH4)2HPO4 solution for 24 hours; drying two days, and burning at the temperature of 1100 DEG C for 1-2 days; slowly cooling, rinsing and baking, so as to obtain a skeleton structure; spraying a slow release formulation on a layer of collagen surface; spraying a layer of collagen on the surface of the slow release formulation to obtain a compound protein layer; baking the composite protein layer at 30 DEG C, carrying out superfine grinding, so as to obtain protein particles which are 50-100nm in particle size; finally, adding the skeleton structure and the protein particles to absolute ethyl alcohol; placing under the conditions at 1-5 DEG C and -0.01 to -0.05MPa for 2-3 days, so as to obtain the bone-grafting filling material. The bone-grafting filling material disclosed by the invention has biodegradability and biocompatibility, is free of or low in immunogenicity, strong in bone conductibility, bone inductivity, mechanical tolerance and the like, and kinds of performance requirements required by the bone filling material are completely met.
Owner:徐州博创建设发展集团有限公司
Antigen protein and kit for detecting diphtheria antibody and preparation method
InactiveCN109100517AAvoid security risksIncrease productionBiological material analysisBiological testingProtein LAntigenic protein
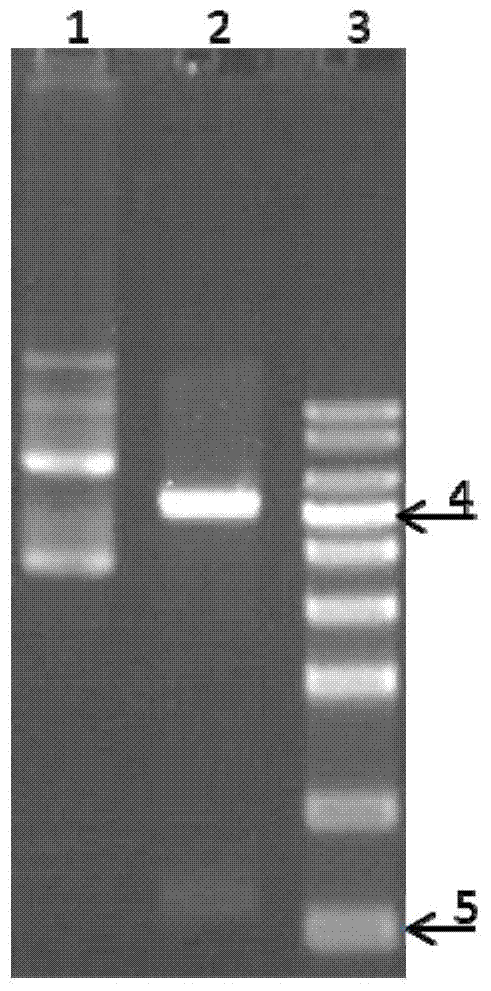
The invention relates to a method for preparing an antigen protein for detecting a diphtheria antibody. The method is characterized by comprising the steps as follows: S1: infecting a baculovirus expression vector carrying a CRM197 protein expression cassette into insect cells so as to obtain infected insect cells; S2: carrying out cultivation and proliferation on the infected insect cells; and S3: carrying out crushing on the proliferated insect cells, and extracting the antigen protein in the insect cells. The invention further relates to an antigen protein prepared by the method and furtherrelates to a kit comprising the antigen protein. An expressed CRM197 protein is safe, non-toxic, high in yield and easy to separate and purify, meanwhile, has very high affinity with the diphtheria antibody, and is good in specificity.
Owner:WUHAN LIFE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com