Method for preparing cyclosporin A holoantigen
A complete antigen, cyclosporine technology, applied in the field of biotechnology and pharmacy, can solve the problems of high price, inconvenient patient medication, chronic rejection organ transplantation failure, etc., to save the synthesis process, shorten the photochemical reaction time, and shorten the reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 cyclosporin A complete antigen
[0046] Step 1: Weigh 240mg CsA and 50mg 4-benzoylbenzoic acid (BBa) with an electronic balance, and put them into a quartz cuvette (capacity 14mL), add 10mL tert-butanol with a pipette, and sonicate for 3min to fully dissolve CsA and 4-benzoylbenzoic acid (BBa);
[0047] Step 2: Prepare CsA incomplete antigen (CsA-BBa) by photochemical reaction method: After bubbling nitrogen gas into the cuvette in the above step 1 for 30±10min, turn on the ultraviolet light source and start to illuminate (the distance between the ultraviolet light source and the colorimetric dish 10±2cm), and at the same time unscrew the separating funnel above the cuvette (filled with a certain volume (300±50mL) of tert-butanol) to record the reaction time, so that tert-butanol drips slowly (1h before the drop rate 4 -8 drops / min, 1h 10-15 drops / min after the reaction), during the reaction process, continue to feed nitrogen gas, light ...
Embodiment 2
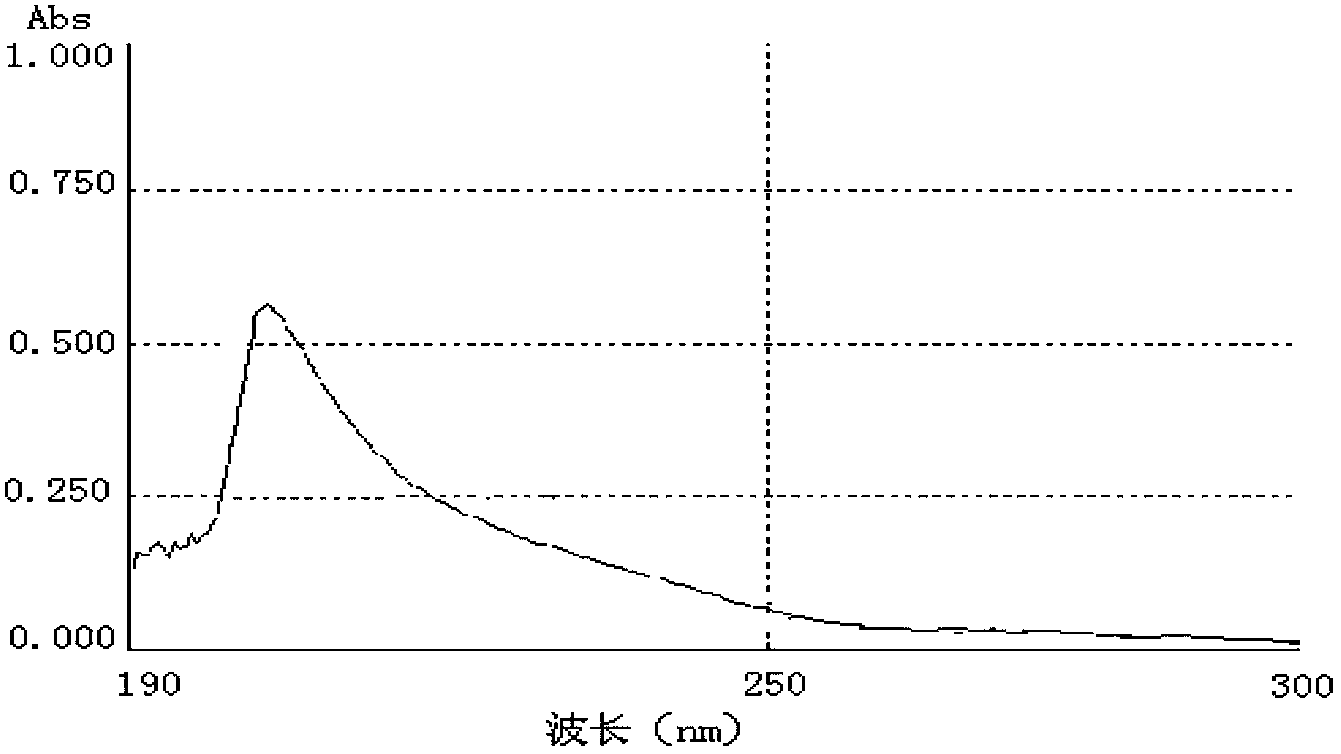
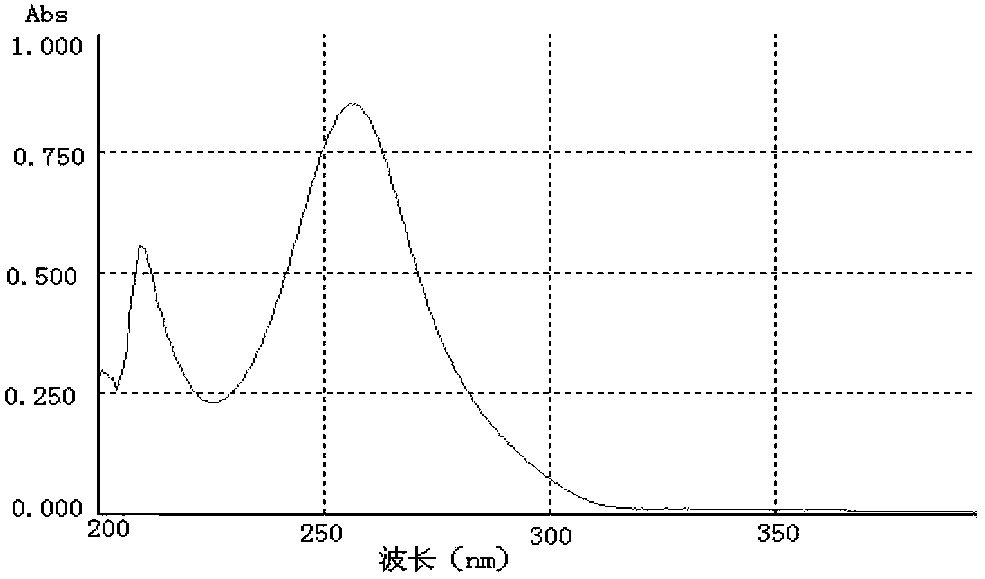
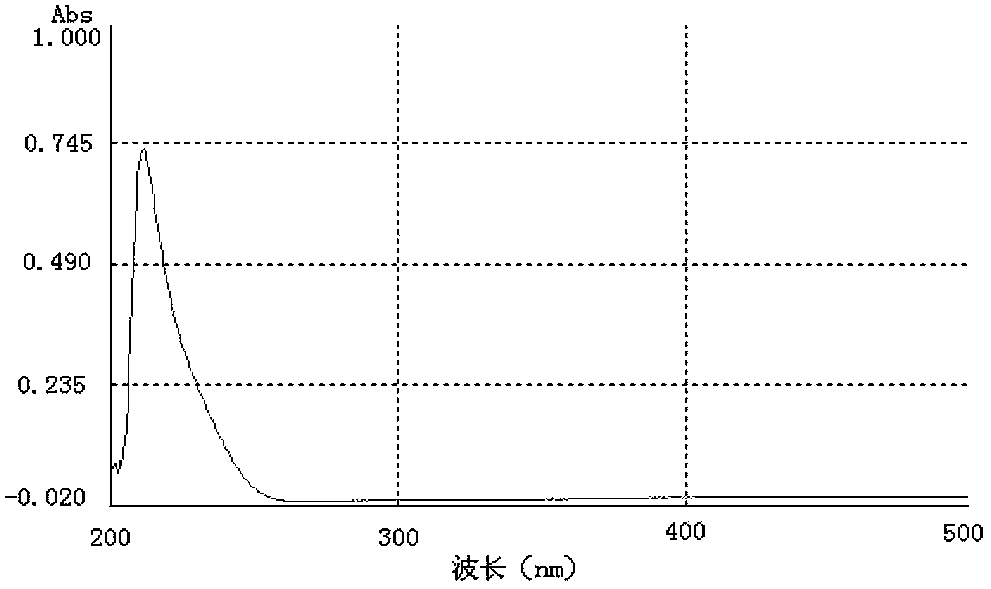
[0065] Identification of the cyclosporine A complete antigen prepared in Example 1:
[0066] 1. Identification of colorless solid powder product 2CsA incomplete antigen (CsA-BBa)
[0067] 1.1 TLC identification results
[0068] Chromatography (thin layer chromatography) of the product 2 in CHCl 3 / CH 3 OH=85 / 15 (volume ratio) binary expansion system, the test results confirmed: the Rf value of product 2 is 0.60, which is close to 0.58 measured in foreign patent literature, and it can be preliminarily judged that product 2 may be an incomplete antigen of CsA ( CsA-BBa).
[0069] 1.2 Melting point measurement results
[0070] The melting points of the reactant CsA, 4-benzoylbenzoic acid (BBa) and the product 2CsA incomplete antigen (CsA-BBa) were respectively measured with a melting point apparatus. The results are shown in Table 1:
[0071] Table 1. Melting points of CsA, BBa and product CsA-BBa
[0072]
[0073] It can be seen from Table 1 that the melting point of pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com