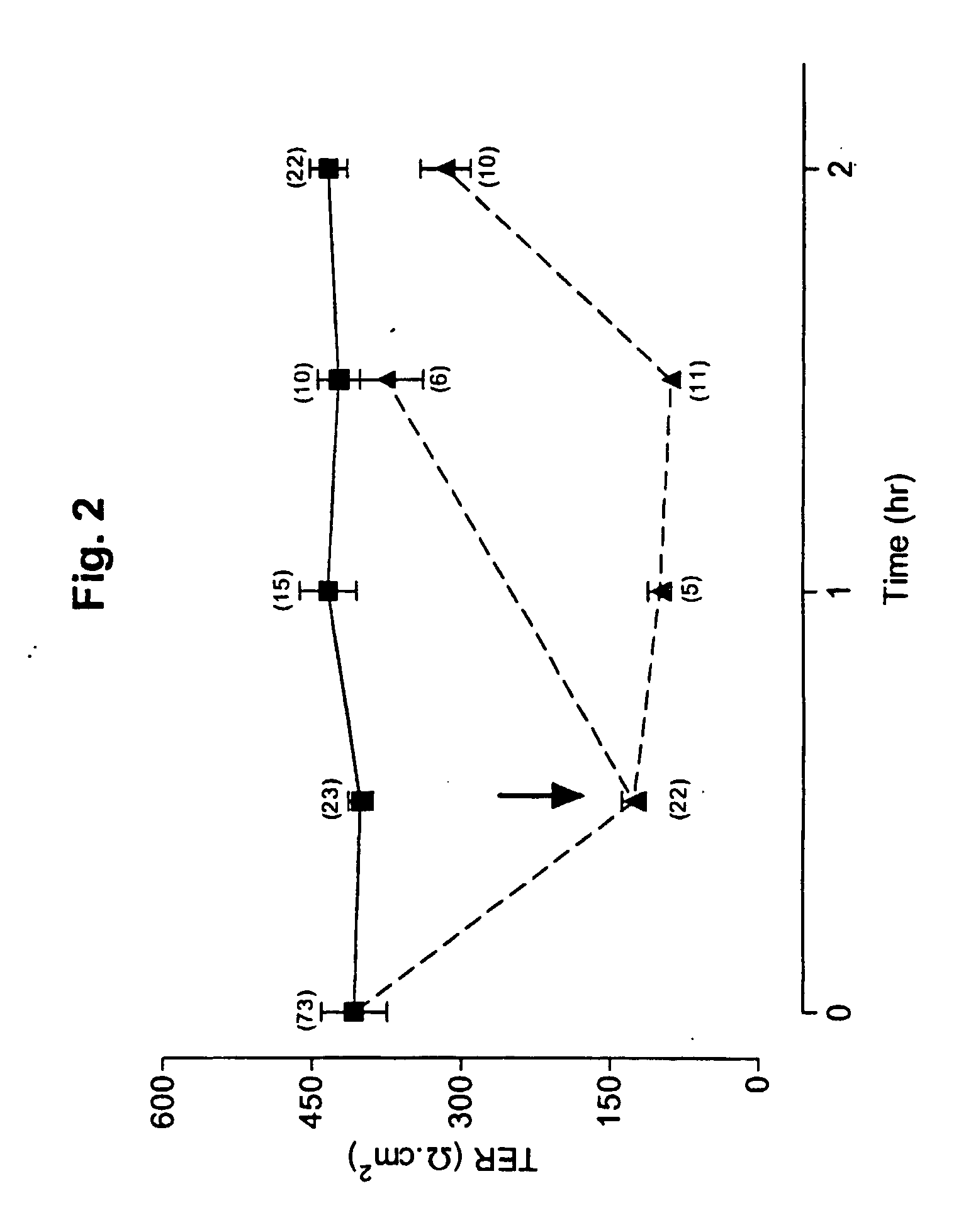
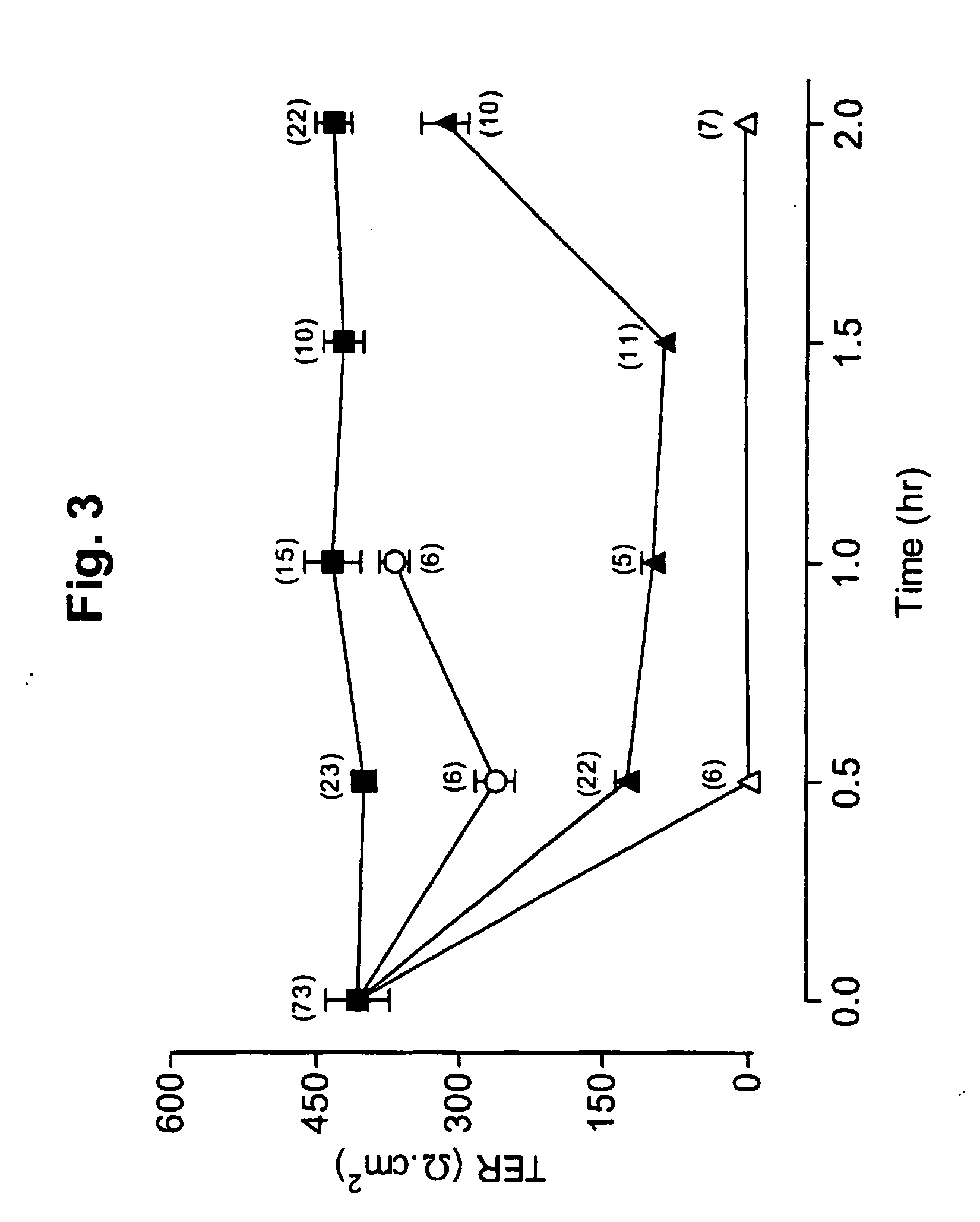
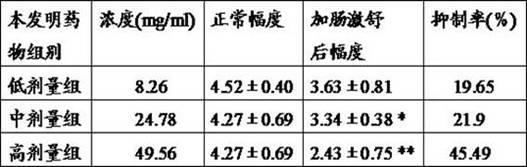
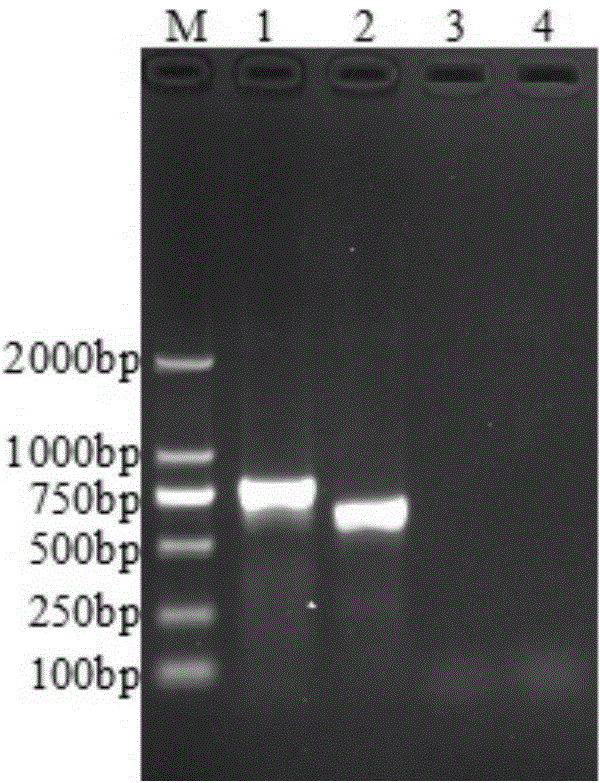
Patents
Literature
242 results about "Rotavirus RNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
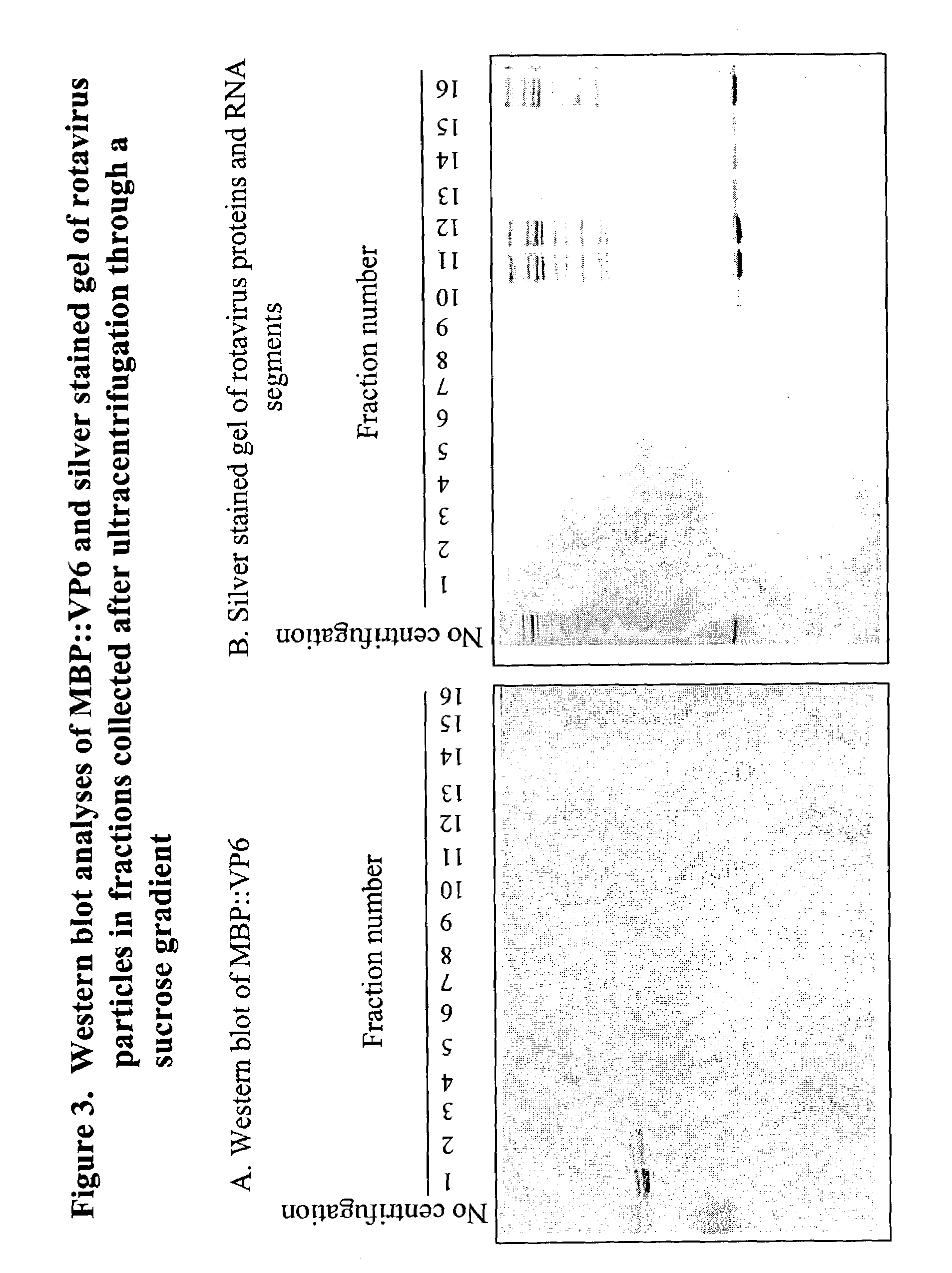
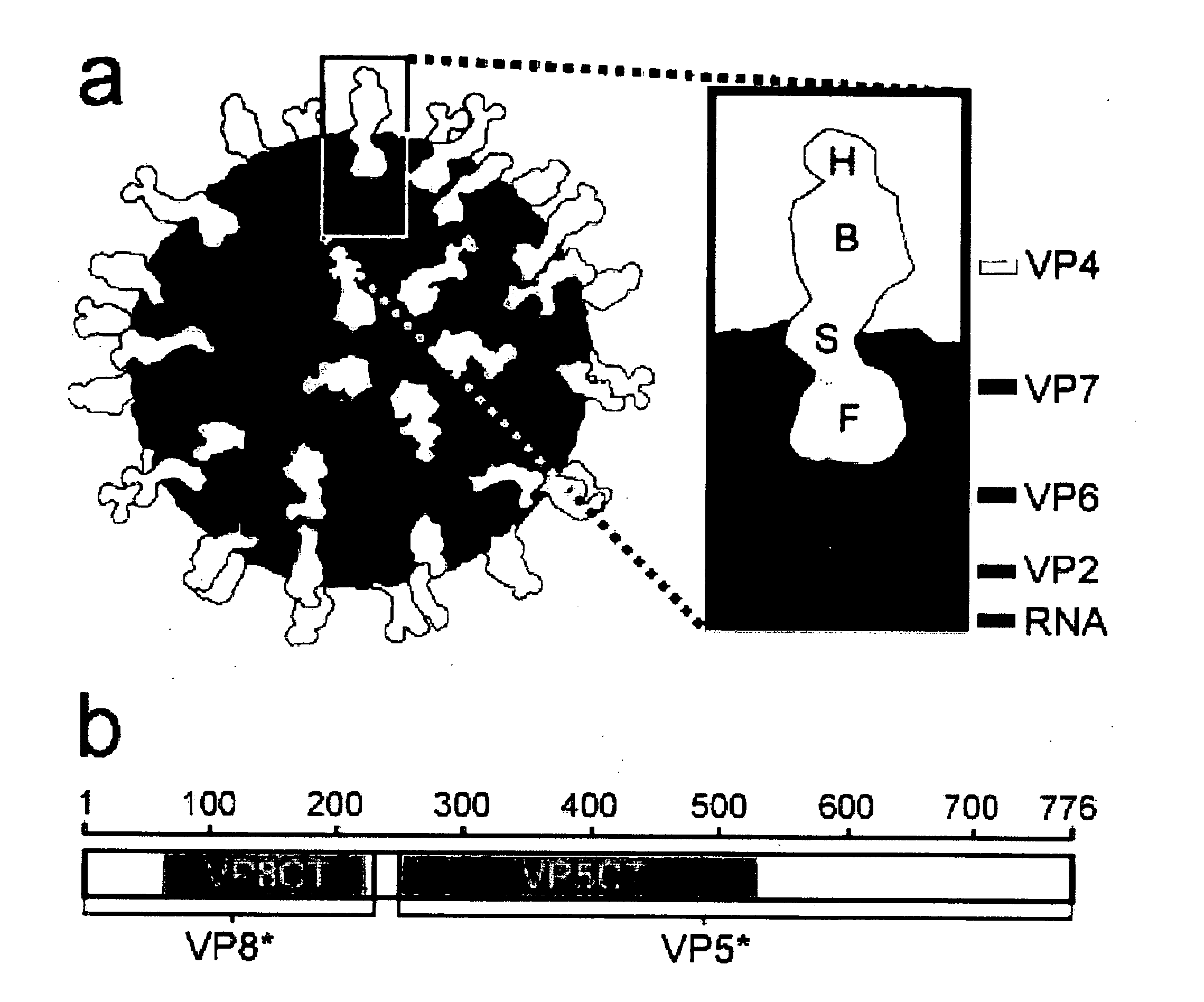
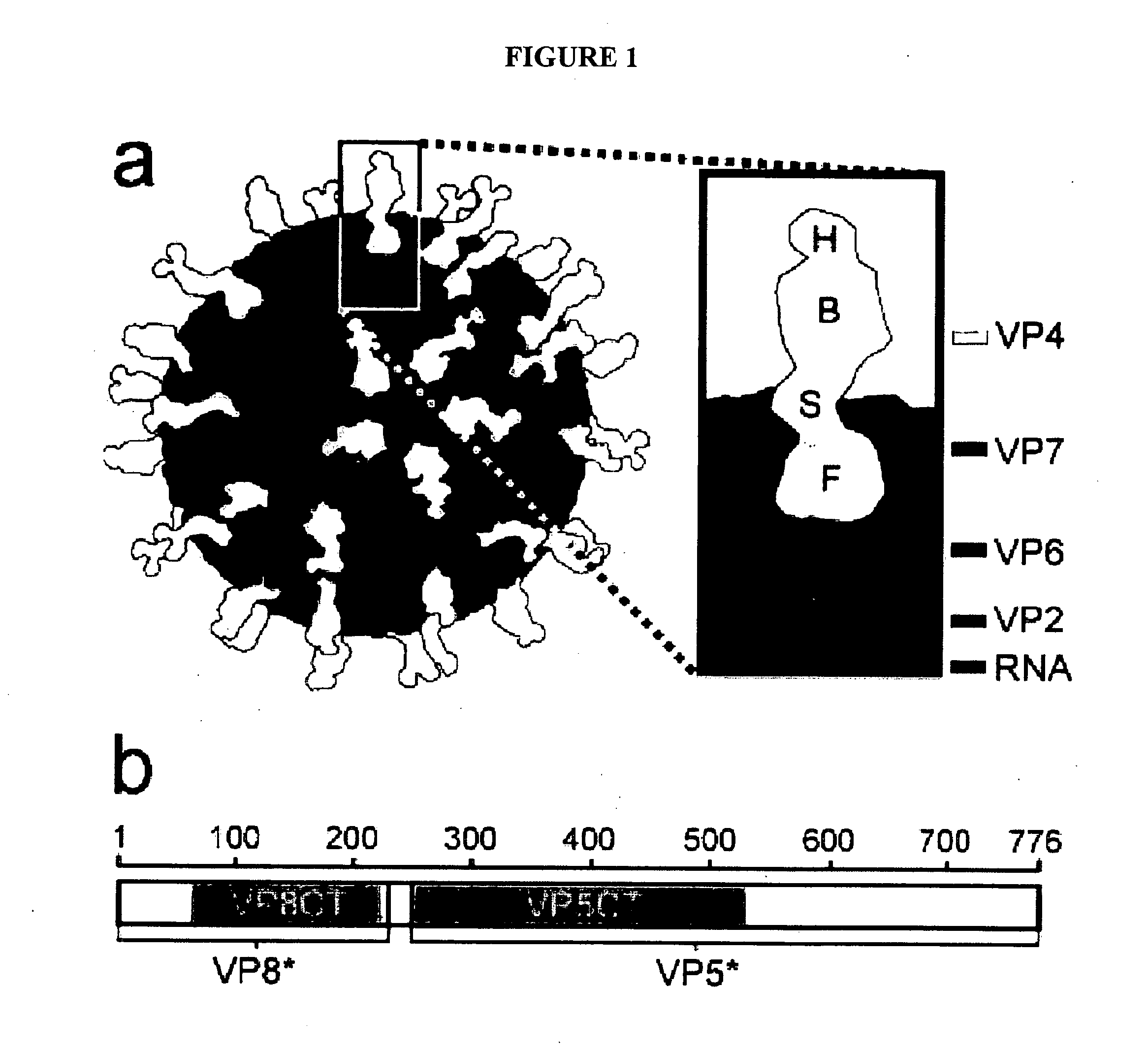
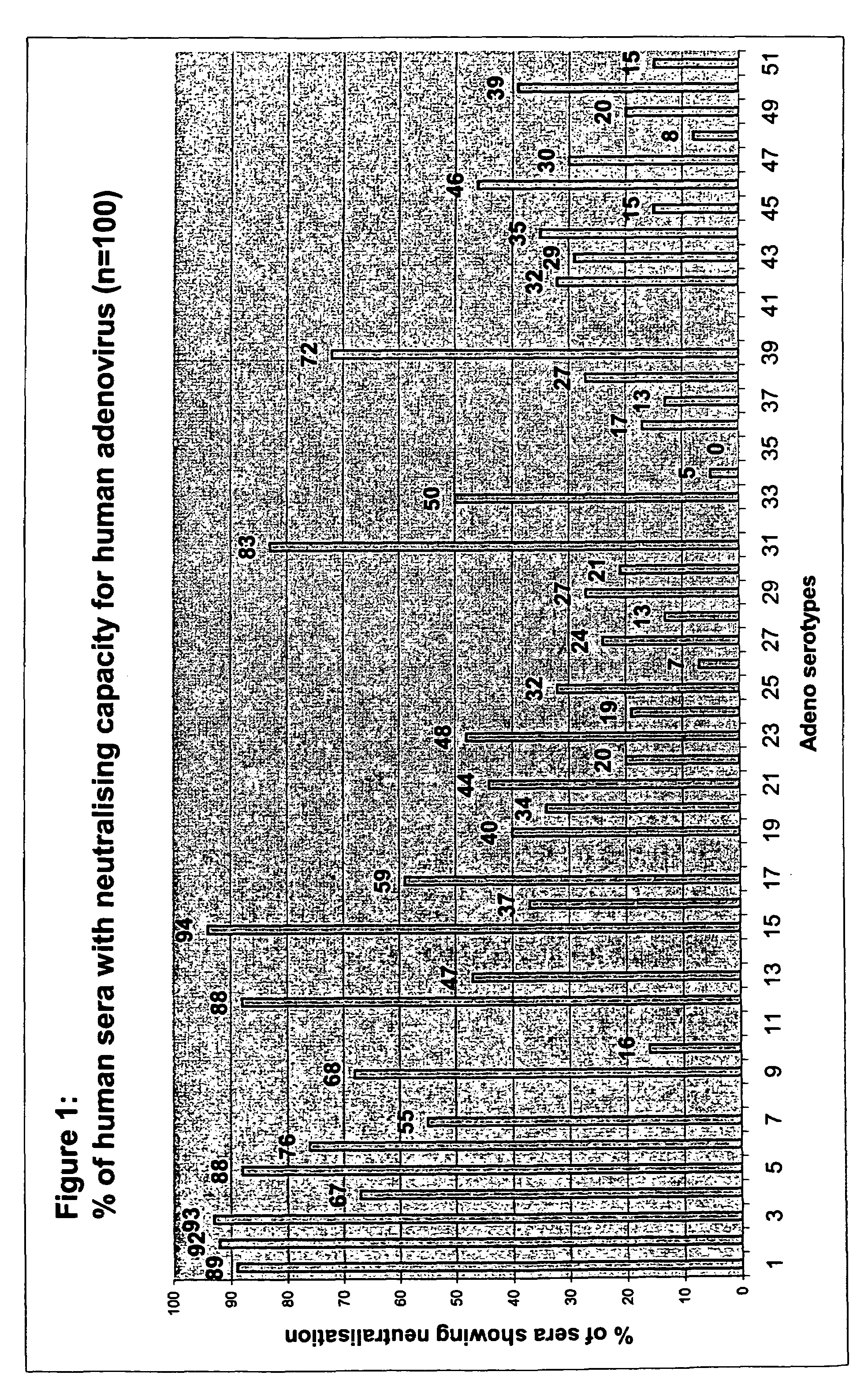
The genome of rotavirus consists of 11 unique double helix molecules of RNA (dsRNA) which are 18,555 nucleotides in total. Each helix, or segment, is a gene, numbered 1 to 11 by decreasing size.
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
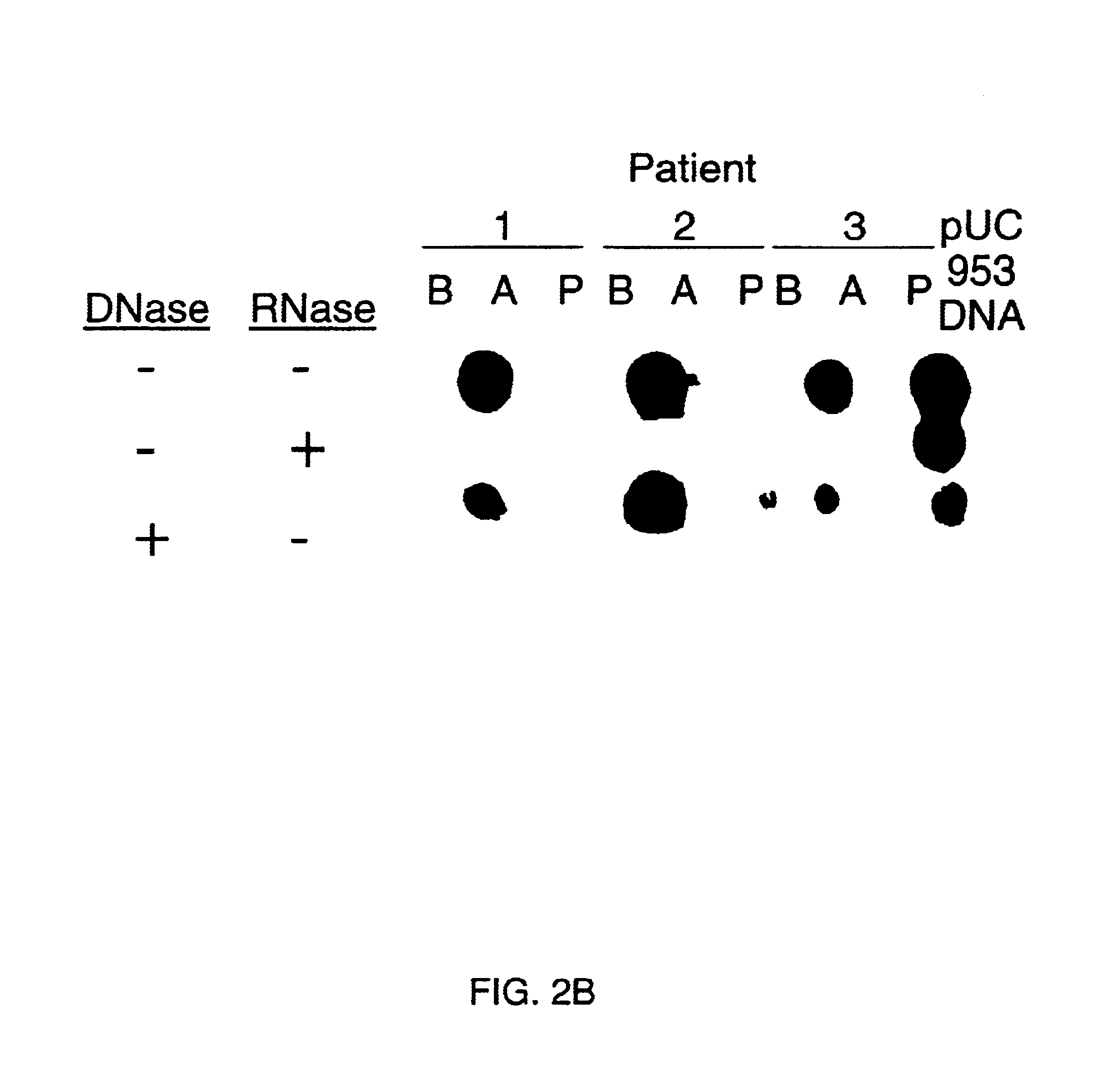
Methods and reagents to detect and characterize norwalk and related viruses
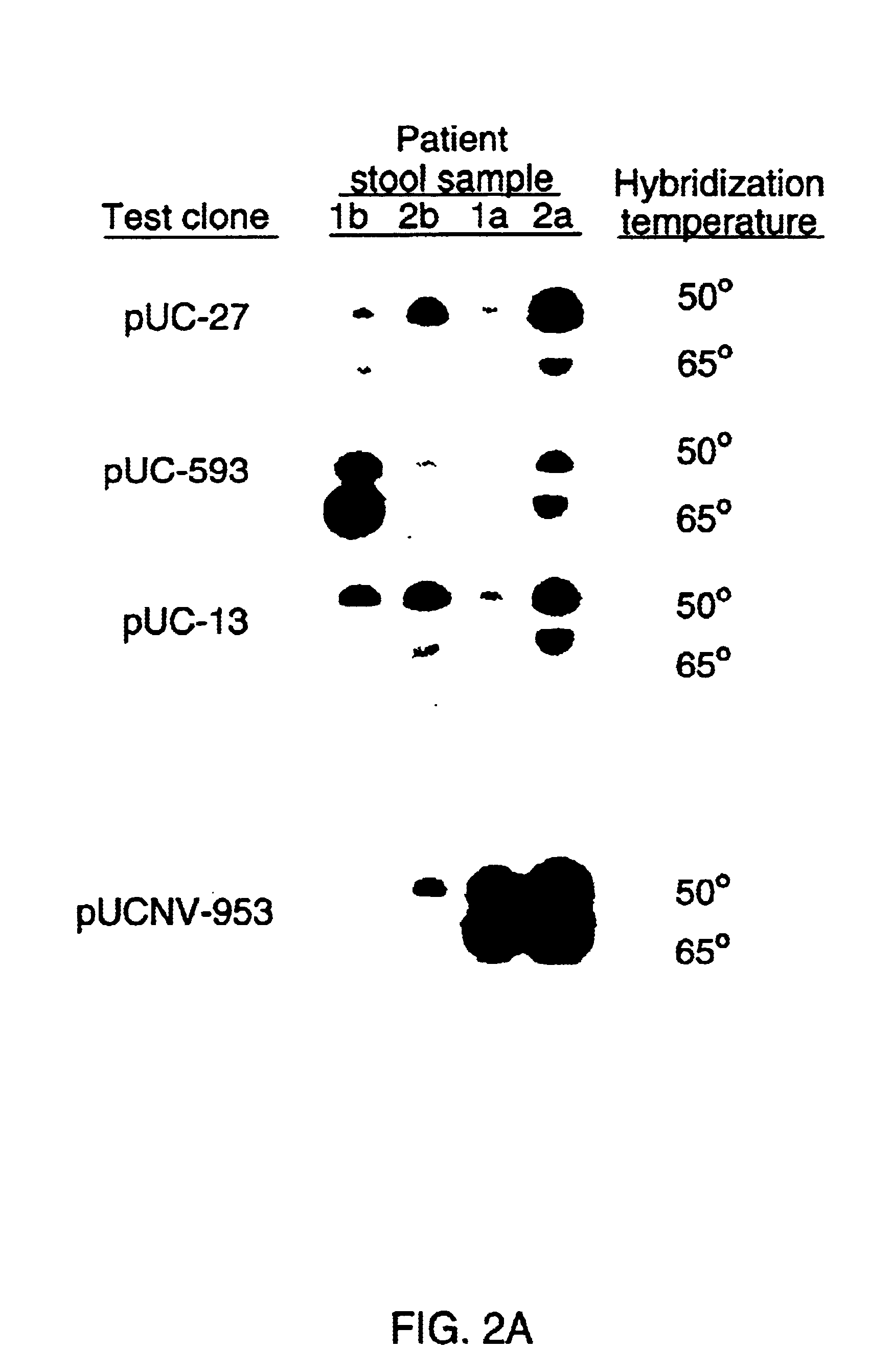
Double-stranded cDNA was synthesized from nucleic acid extracted from Norwalk virus purified from stool specimens of volunteers. One clone was isolated from a cDNA library constructed in a pUC-13 vector after amplification of the cDNA. The specificity of this cDNA (pUCNV-953) was shown by hybridization assays. The cDNA reacted with post (but not pre-) infection stool samples from Norwalk volunteers and with highly purified Norwalk virus, but not with other common enteric viruses such as hepatitis A virus and rotavirus. Finally, the probe detected virus in the same fractions of CsCl gradients in which viral antigen was detected using a specific Norwalk virus radioimmunoassay, and particles were detected by immune electron microscopy. Single-stranded RNA probes derived from the DNA clone after subcloning into an in vitro transcription vector were also used to show that the Norwalk virus contains a ssRNA genome of about 8 kb in size. The original clone was also used to detect additional cDNAs which represent at least 7 kb of nucleic acid of the Norwalk genome. The availability of a Norwalk-specific cDNA and the first partial genome sequence information allow rapid cloning of the entire genome and of establishment of sensitive diagnostic assays. Such assays can be based on detection of Norwalk virus nucleic acid or Norwalk viral antigen using polyclonal or monoclonal antibodies to proteins expressed from the cDNA or to synthetic peptides made based on the knowledge of the genome sequence. Assays using proteins deduced from the Norwlk virus genome and produced in expression systmes can measure antibody responses. Vaccines made by recombinant DNA technology are now feasible.
Owner:BAYLOR COLLEGE OF MEDICINE
Infant milk powder added with biostime and preparation method thereof
ActiveCN101449708AAdvantage maintenanceColonization fastMilk preparationFood preparationTreatment effectVegetable oil
The invention discloses an infant milk power added with synbiotics, comprising the following components: fresh milk 40-68 gram; demineralized whey powder 15-34 gram; granulated sugar3-6 graml; vegetable oil 4-8 gram; compound prebiotics 0.4-20 gram; compound probiotic 1.0*10<5>-1.0*10<11> cfu. The inventive infant milk power can be ysed for building advantages of beneficial bacterium when a newborn infant starts colony valuation. Through adding probiotic, the number of probiotic is advanced to increase in a short time, through adding prebiotics having selectively increasing effect on probiotic, the added probiotic is performed with fast field setting, thereby maintaining advantages of probiotic in intestinal flora. The invention also markedly relieves diarrhoetic symptoms, and has definite treatment effect on diarrhea caused by rotavirus.
Owner:SHANDONG LONGLIVE BIO TECH CO LTD
Methods for Diagnosing Pervasive Development Disorders, Dysautonomia and Other Neurological Conditions
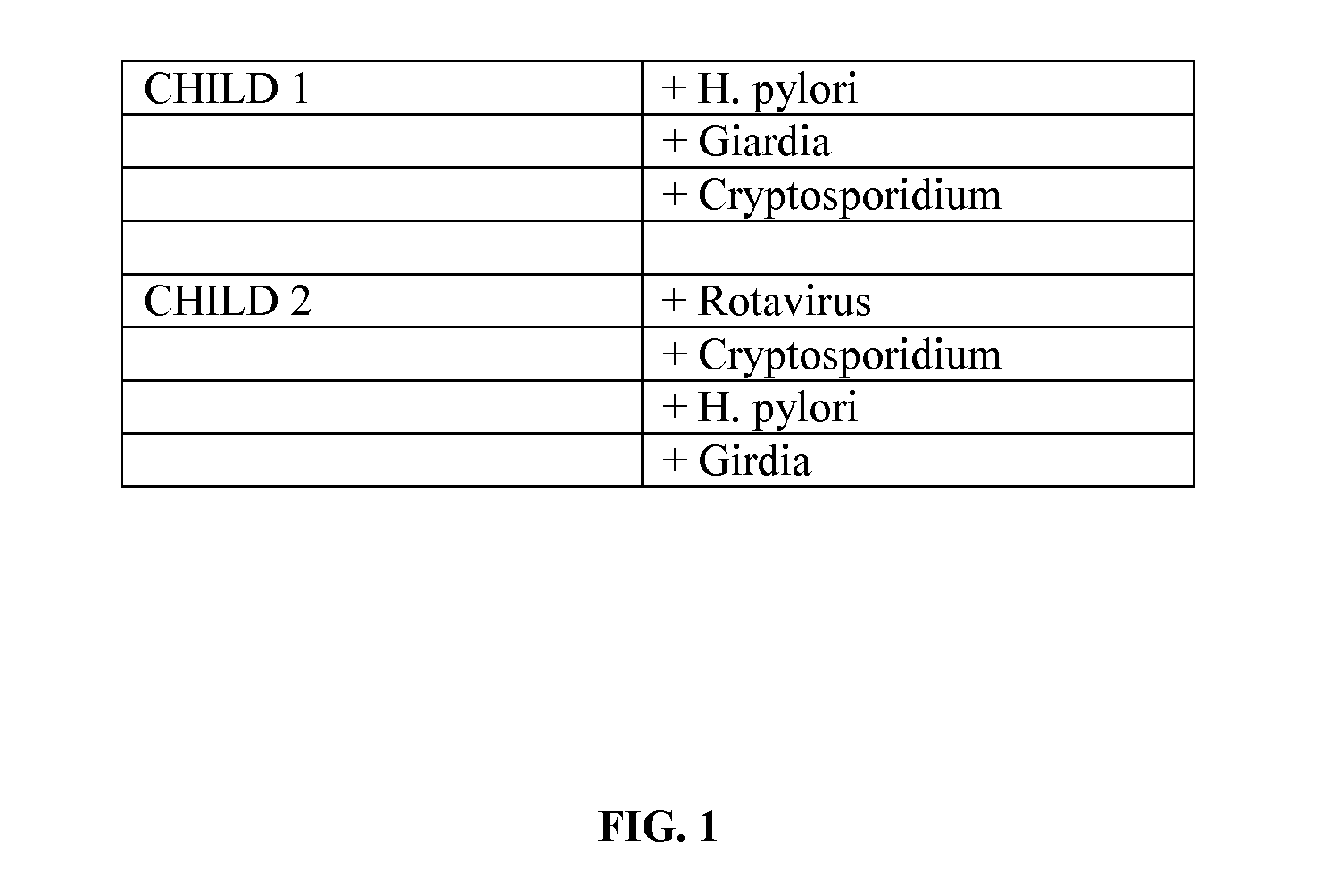
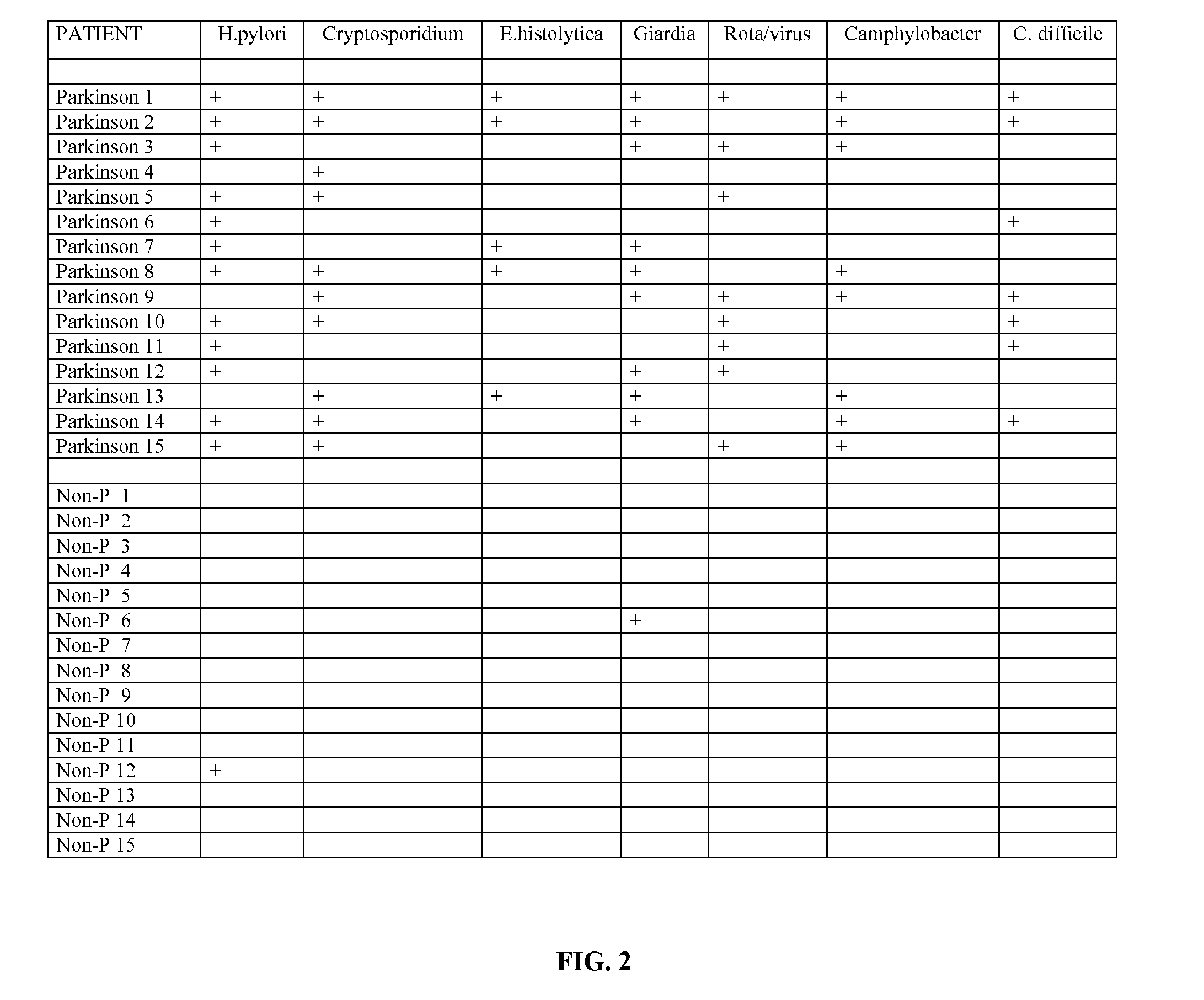
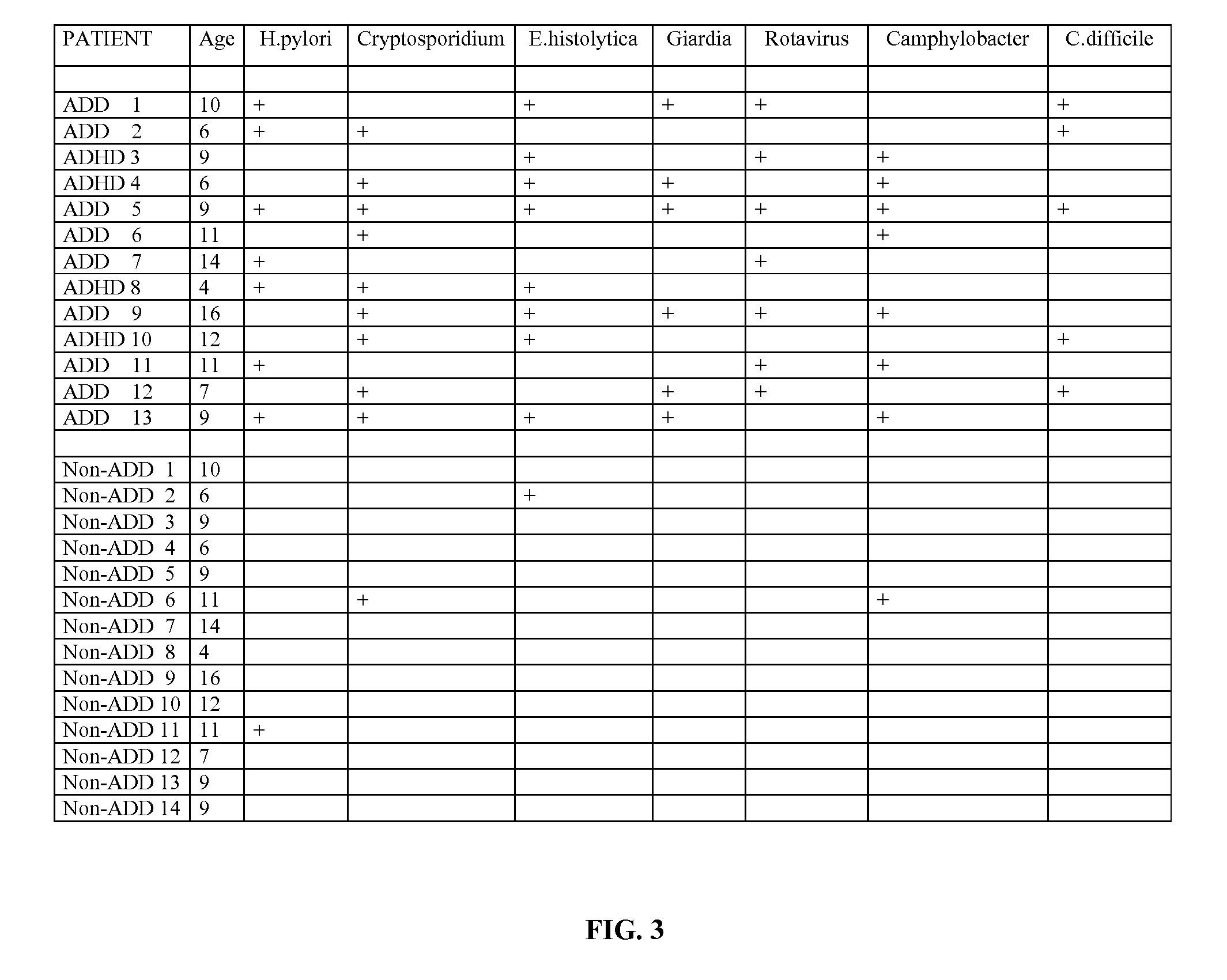
Methods for aiding in the diagnosis of disorders including, but not limited to, PDDs (Pervasive Development Disorders), Dysautonomic disorders, Parkinson's disease and SIDS (Sudden Infant Death Syndrome). In one aspect, a diagnosis method comprises analyzing a stool sample of an individual for the presence of a biological marker (or marker compound) comprising one or more pathogens, which provides an indication of whether the individual has, or can develop, a disorder including, but not limited to, a PDD, Dysautonomia, Parkinsons disease and SIDS. Preferably, the presence of one or more pathogens is determined using a stool immunoassay to determine the presence of antigens in a stool sample, wherein such antigens are associated with one or more pathogens including, but not limited to, Giardia, Cryptosporidium, E. histolytica, C. difficile, Adenovirus, Rotavirus or H. pylori.
Owner:CUREMARK
Therapeutic antimicrobial compositions and methods
InactiveUS20050271711A1Applied to skinImmediate and residual effectivenessAntibacterial agentsBiocideOrganic acidRotavirus RNA
Therapeutic antimicrobial compositions and methods for providing enhanced immediate and residual anti-viral and antibacterial efficacy against rhinovirus, rotavirus, coronovirus, respitory syricytial virus, Gram-positive bacteria, Gram-negative bacteria and combinations thereof. More specifically, therapeutic antimicrobial compositions comprising an organic acid or organic acid mixture and a short-chain anionic surfactant having at least one of a large head group; a branched alkyl chain and an unsaturated alkyl chain, and therapeutic methods of use thereof.
Owner:THE PROCTER & GAMBLE COMPANY
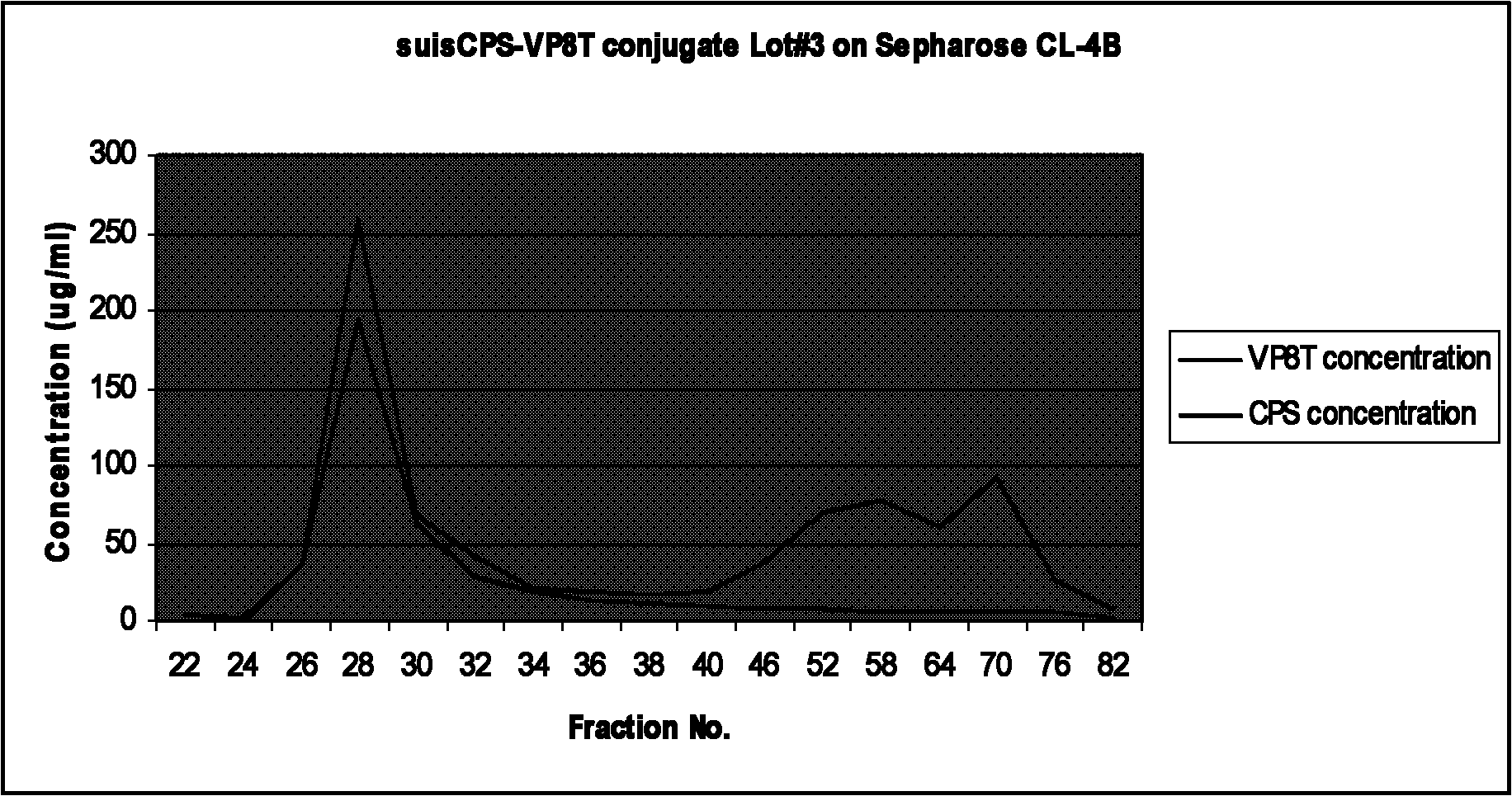
Bacterial polysaccharide-protein conjugate vaccine and preparation method thereof
The invention relates to a bacterial polysaccharide-protein conjugate vaccine with immunogenicity, in particular to a conjugate vaccine which is formed by connecting a recombinant rotavirus protein with a bacterial polysaccharide by using a covalent bond, a nucleotide sequence for coding the recombinant rotavirus protein, a recombinant expression system, a protein expressed by the recombinant expression system, a preparation method of the conjugate vaccine and a pneumococcus polysaccharide-recombinant rotavirus protein conjugate vaccine. The bacterial polysaccharide is connected with a recombinant rotavirus surface protein through a covalent bond. The recombinant rotavirus protein is selected from a partial or complete amino acid sequence of a P-gene rotavirus protein and a partial or complete amino acid sequence of a G-gene rotavirus protein.
Owner:普大生物科技(泰州)有限公司
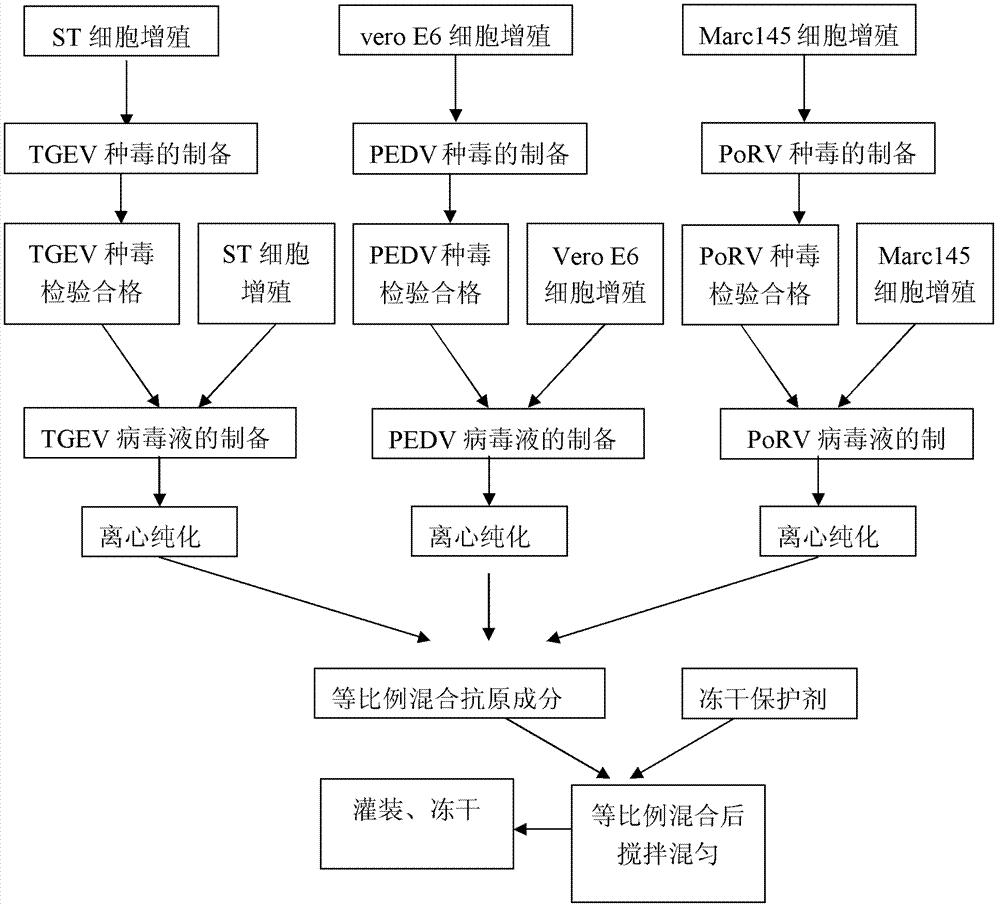
Triple vaccine of pig transmissible gastroenteritis, pig epidemic diarrhea and pig rotavirus
InactiveCN101491673AAvoid pollutionDoes not destroy nutrientsViral antigen ingredientsDigestive systemDiseaseCytopathic effect
The invention provides a method for preparing triple vaccine for preventing porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus. The method comprises the following steps: inoculating a host-cell line with a 90 percent grown monostratum against a porcine transmissible gastroenteritis virus, a porcine epidemic diarrhea virus and a porcine rotavirus respectively, and adding a cell maintenance media into the host-cell lines respectively to be cultured at 37 DEG C; after cytopathic effect reaches over 75 percent, collecting viruses to be stored at 20 DEG C below zero for standby; mixing the viruses according to 10 TCID50 in 1:1:1, and simultaneously adding Freund's complete adjuvant and immunopotentiator into the mixture to inactivate the mixture by formaldehyde at 37 DEG C for 24 hours; and adding an oil adjuvant into the mixture to prepare a vaccine of water-oil-water preparation. The method can be used for preparing the triple vaccine for preventing the porcine transmissible gastroenteritis, the porcine epidemic diarrhea and the porcine rotavirus so as to solve the problem that the diseases do not have an effective medicine to treat currently.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Rotavirus subunit vaccine
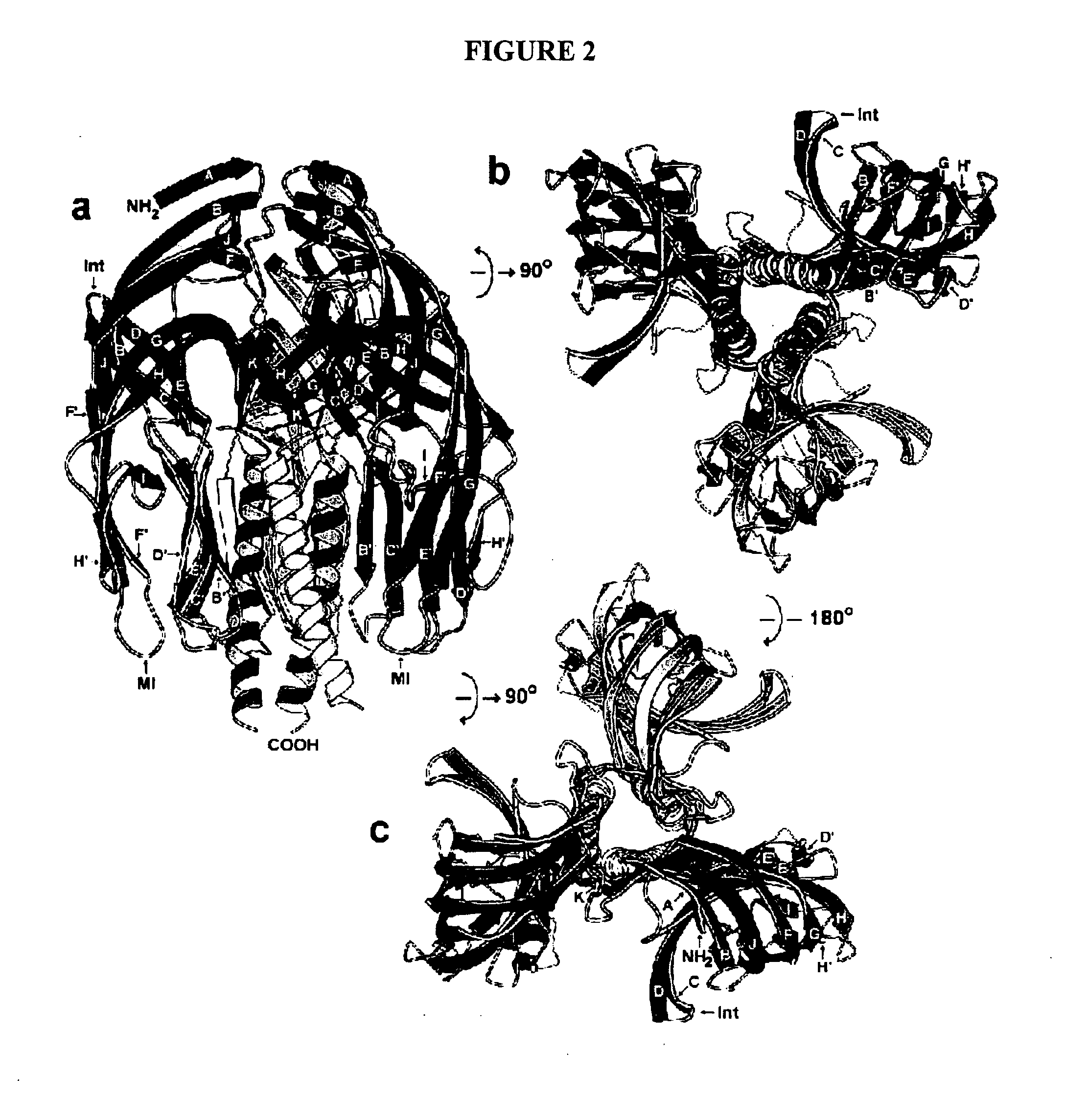
The present invention is directed to the generation and use of recombinant rotavirus fusion proteins as immunogens to produce a protective immune response from immunized individuals. In one embodiment, the present invention contemplates a recombinant rotavirus fusion protein vaccine composition comprising a rotavirus subunit protein or immunogenic fragment thereof, and an adjuvant in combination with the recombinant rotavirus subunit fusion protein. In one aspect of this embodiment, the recombinant rotavirus fusion protein comprises a rotavirus subunit protein and a fusion partner protein in genetic association with the rotavirus subunit protein, wherein the fusion partner protein does not interfere with expression and immunogenicity of the rotavirus subunit protein, the fusion partner protein prevents complex formation by the rotavirus subunit protein, and the fusion partner protein facilitates purification of the recombinant rotavirus fusion protein. In another aspect of this embodiment, the rotavirus subunit protein is selected from the group consisting of VP1, VP2, VP3, VP4, VP6, VP7, NSP1, NSP2, NSP3, NSP4 or NSP5. In yet another aspect of this embodiment, the rotavirus subunit protein is VP6.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Antimicrobial compositions, products and methods employing same
InactiveUS20090202463A1Immediate and residual effectivenessApplied to skinAntibacterial agentsBiocideRotavirus RNARotavirus
Antimicrobial compositions that provide enhanced immediate and residual anti-viral and antibacterial efficacy against rhinovirus, rotavirus, coronovirus, respiratory syncytial virus, Gram-positive bacteria, Gram-negative bacteria and combinations thereof. More specifically, antimicrobial compositions comprising an organic acid or organic acid mixture and a short-chain anionic surfactant having at least one of a large head group; a branched alkyl chain and an unsaturated alkyl chain. Further, products incorporating the antimicrobial compositions of the present invention and methods of using the antimicrobial compositions and products are disclosed herein.
Owner:PROCTER & GAMBLE CO
Kit and oligonucleotide sequences for detecting rotavirus A
InactiveCN101671746AHigh sensitivityHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesMagnesium pyrophosphateRotavirus RNA
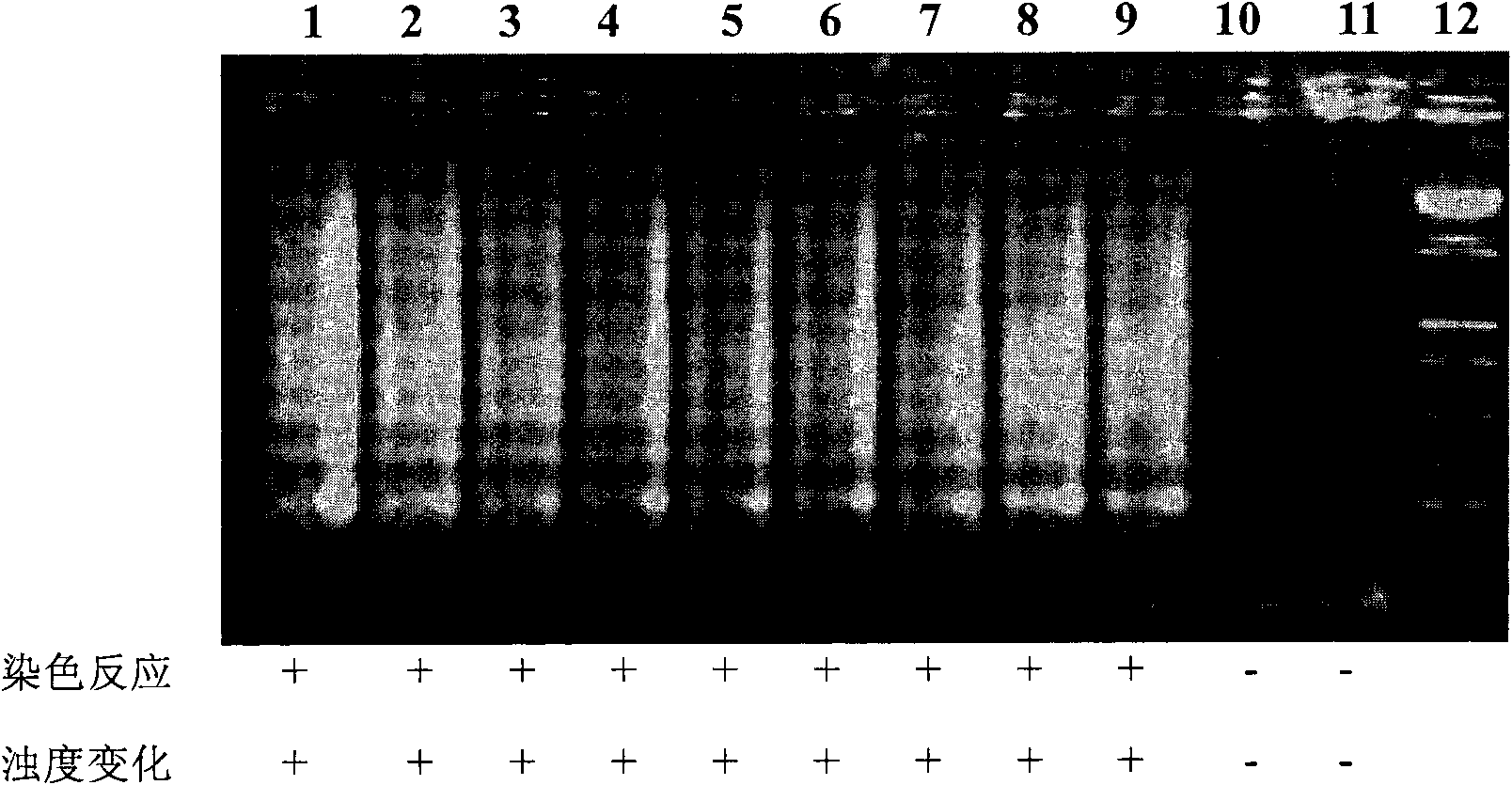
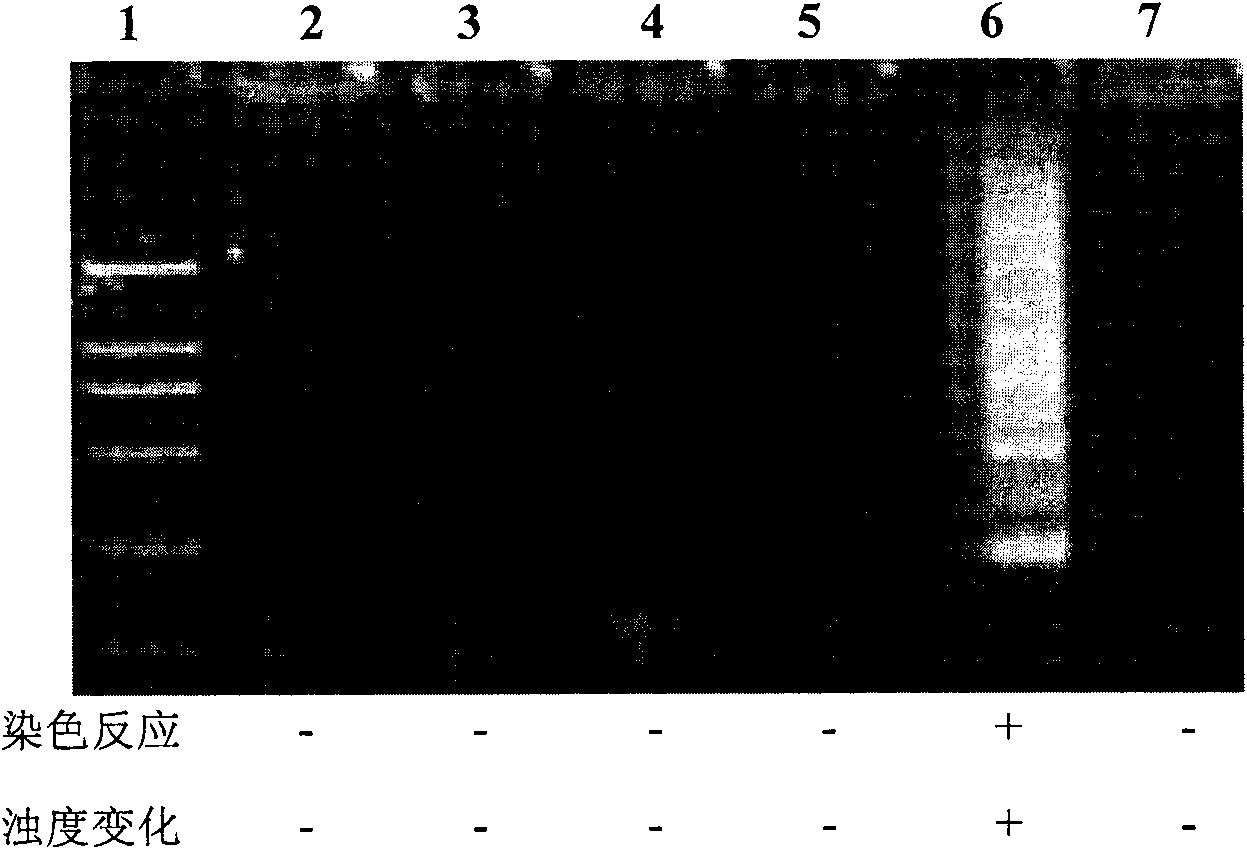
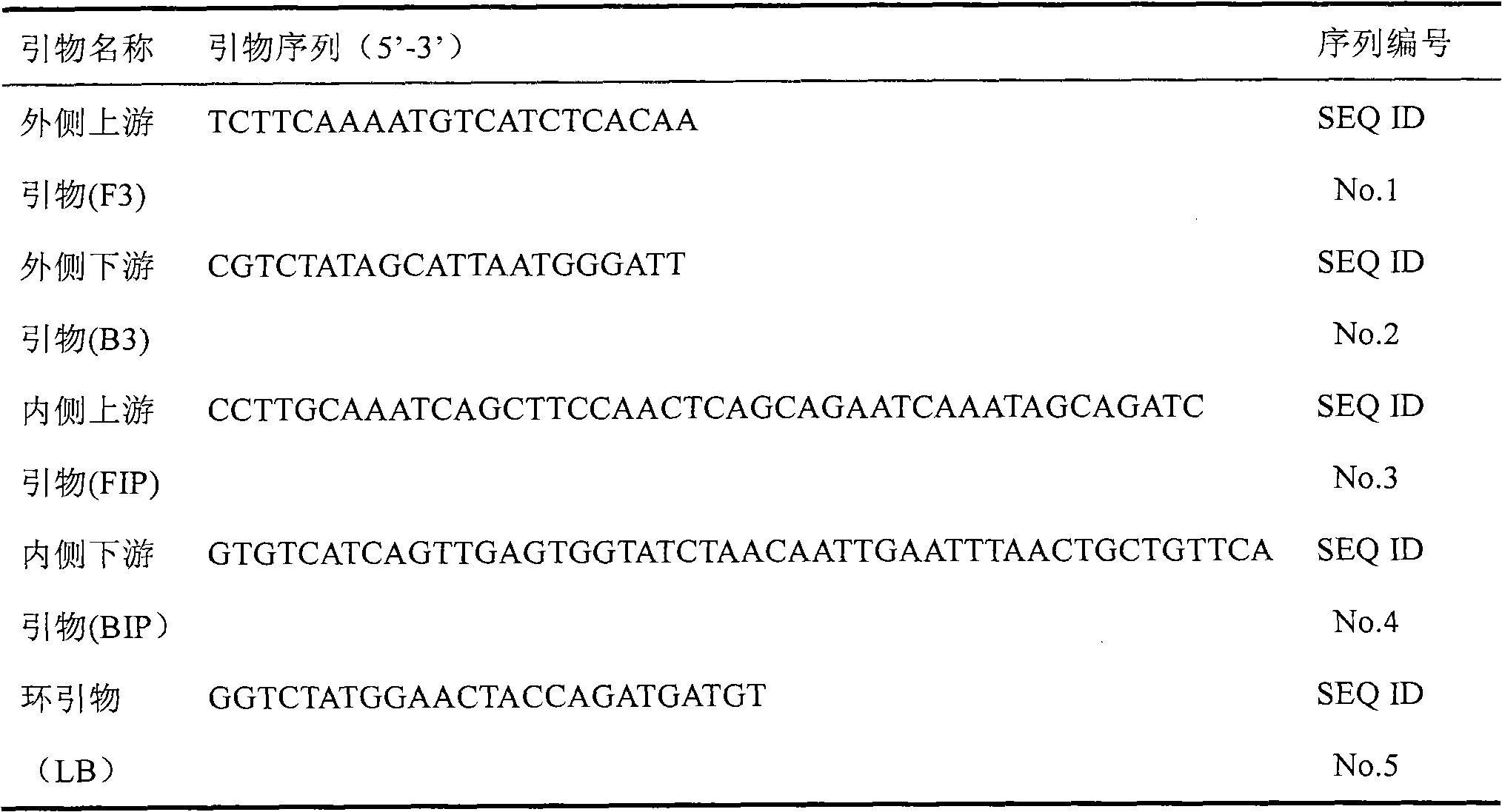
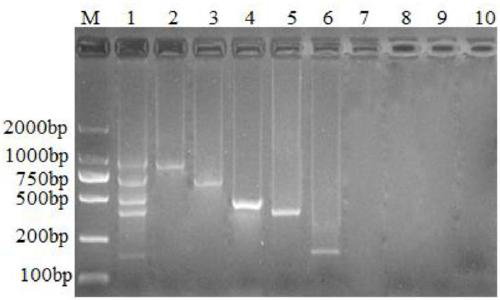
The invention discloses a kit and oligonucleotide sequences for detecting rotavirus A, in particular to a group of oligonucleotides which are used for detecting rotavirus A and have the oligonucleotide sequences shown from the sequence table SEQ ID No.1 to the sequence table SEQ ID No.5, the kit containing the oligonucleotides and a detection method thereof. The kit of the invention has high sensitivity, strong specificity, low cost and simple and convenient operation. During detection, when lots of nucleic acid is synthesized, a by-product magnesium pyrophosphate precipitate is generated, which has high specificity. Whether the products are amplified or not can be judged only by observing the turbidity of the products with naked eyes.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Method for simultaneously detecting multiple RT-PCR of GETV, PEDV, TGEV, PDCoV and PoRV
InactiveCN108950083AStrong specificityImprove efficiencyMicrobiological testing/measurementMicroorganism based processesRotavirus RNANucleotide
The invention discloses a multiple RT-PCR primer group for simultaneously detecting porcine gatahvirus (GETV), porcine epidemic diarrhea virus (PEDV), porcine deltacoronavirus (PDCoV) and porcine A rotaviruses (PoRV), which has a nucleotide sequence as shown in SEQ ID NO:1-SEQ ID NO:10. The invention further discloses a multiple RT-PCR detection method for detecting GETV, PEDV, TGEV, PDCoV and PoRV from a sample in one time by utilizing the multiple RT-PCR primer group. Compared with an existing conventional RT-PCR, the detection method has strong specificity and high sensitivity, can realizesimultaneous identification of five viruses including GETV, PEDV, TGEV, PDCoV and PoRV, and has accurate detection result and high detection efficiency.
Owner:HENAN AGRICULTURAL UNIVERSITY
Rotavirus antigens
ActiveUS20070276130A1Improve efficacyImprove securityVirus peptidesDepsipeptidesAntigenRotavirus RNA
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Primer, probe and kit for detecting rotavirus and Norovirus liquid phase chips
InactiveCN102154528AAccurate detectionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationRotavirus RNAFluorescence
The invention provides a primer, a probe and a kit for detecting rotavirus and Norovirus liquid phase chips. The primer comprises three pairs of primers of a specific amplification A type rotavirus sequence, a GI type Norovirus sequence and a GII type Norovirus sequence. The probe comprises three specificity detection probes of A type rotavirus, GI type Norovirus and GII type Norovirus. The kit comprises the three pair of primers and a specificity detection microsphere mixture prepared by coupling the three specificity detection probes and a fluorescence coding microsphere. The experiment proves that the primer and the probe disclosed by the invention are characterized in that a method of combining multiple PCR (polymerase chain reaction) with liquid phase chip detection can simultaneously and accurately detect the A type rotavirus, the GI type Norovirus and the GII type Norovirus. The primer, the probe and the kit have the advantages of strong specificity and high sensitivity.
Owner:何雅青 +2
Employment of rotavirus proteins, derived proteins and peptides for the modulation of tisssue permeability
InactiveUS20060251663A1Improve drug deliveryImprove permeabilityOrganic active ingredientsVirusesCancer cellRotavirus RNA
The present invetnion refers to the use of rotavirus proteins VP4, VP8 and their derived fusion proteins and peptides, for enhancing the delivery of pharmaceutical agents through the paracellular pathway. These rotavirus proteins and derived peptides may additionally be employed to reduce unwanted cellular adhesion that can occur between cancerous cells, or between normal cells as a result of surgery, injury, chemotherapy, disease, inflammation or other pathological conditions.
Owner:CENT DE INVESTIGACION & DE ESTUDIOS AVANZADOS DEL INST POLITECNICO NACIONAL
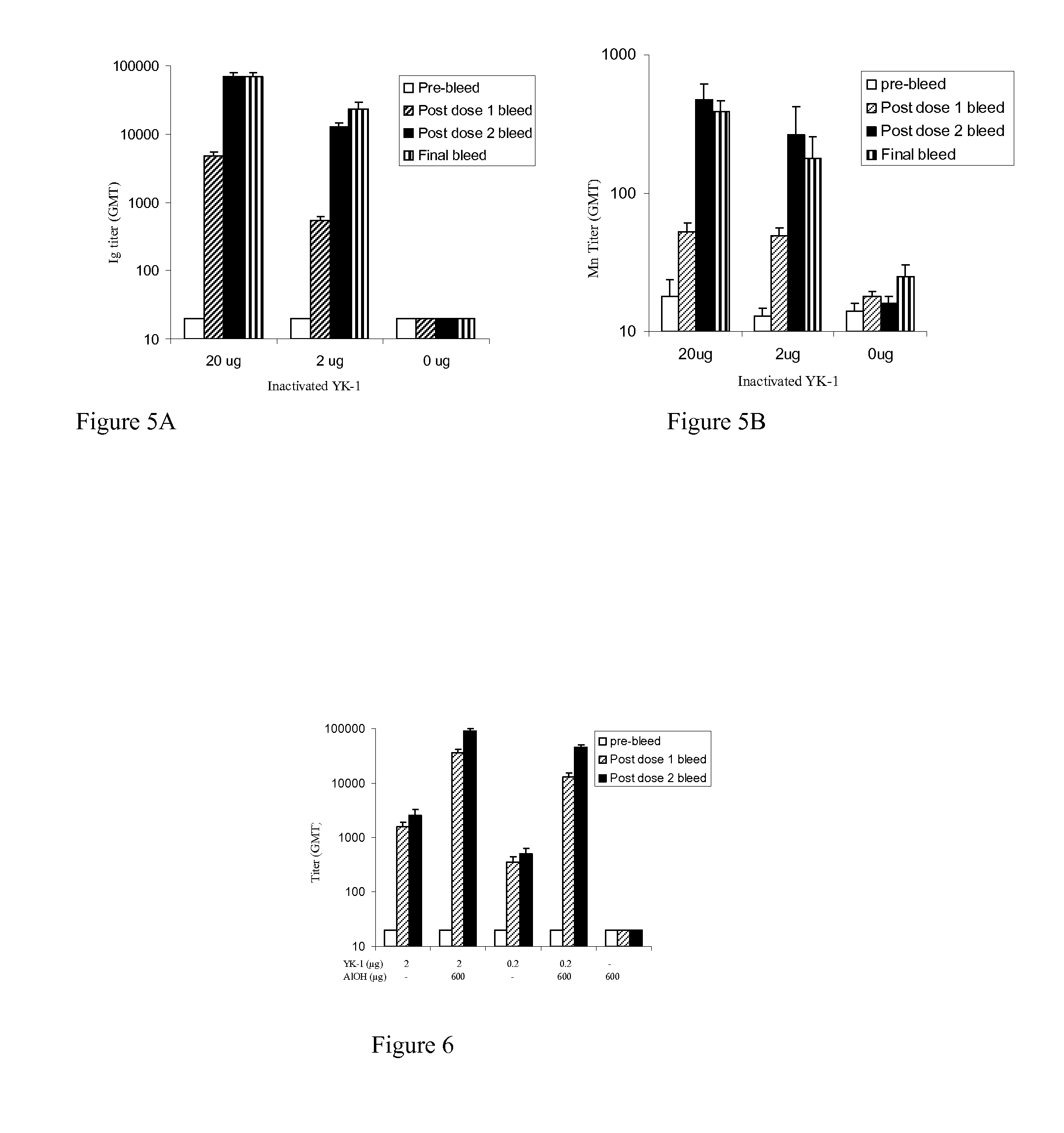
Prepn process of inactivated rotavirus vaccine
ActiveCN101020053APrevent infectious diseasesViral antigen ingredientsAntiviralsRotavirus RNAResearch Object
The present invention discloses preparation process of inactivated rotavirus vaccine. Rotavirus strains G1, G2, G3 and G4 are made to adapt human diploid cell line and propagate on human diploid cell line to obtain the producing strain of inactivated rotavirus vaccine, with the human diploid cell line being KMB17. The human diploid cell line used as the cell matrix for producing inactivated rotavirus vaccine is safer compared with available cell matrixes. Therefore, by means of the established technological platform, it is possible to develop inactivated rotavirus vaccine with other serotypes of rotavirus as the target.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Lactobacillus reuteri useful as probiotics
The present invention relates to a Lactobacillus reuteri variant from animal sources that inhibits rotavirus infection and other pathogenic microorganisms, as well as being tolerant of gastric and bile acids. Also, this invention relates to a prophylactic and therapeutic composition comprising the same for contributing in many probiotic ways to the host's general health and preventing and treating diseases or conditions associated with rotavirus and other enteric pathogens.
Owner:PROBIONIC
Chinese medicine preparation for treating infant rotavirus enteritis and preparation method thereof
InactiveCN102198262AEasy to manufactureLow costDigestive systemAntiviralsRotavirus RNAManufacturing technology
The invention discloses a Chinese medicine preparation for treating infant rotavirus enteritis. The Chinese medicine preparation comprises the following raw materials in parts by weight: 10-20 parts of Chinese pulsatilla root, 10-20 parts of purslane, 10-20 parts of yam, 10-20 parts of baical skullcap root, 10-20 parts of wrinkled gianthyssop herb, 10-20 parts of radix glehniae, 10-20 parts of bighead atractylodes rhizome, 10-20 parts of Indian buead, 10-20 parts of burnet, 10-20 parts of longstamen onion bulb, 10-20 parts of kudzu vine root, 10-20 parts of coptis, 10-20 parts of dried tangerine peel, 10-20 parts of nutmeg, 10-20 parts of pomegranate rind, 10-20 parts of malt, 10-20 parts of grifola, 10-20 parts of ginger and 10-20 parts of endothelium corneum gigeriae galli. The preparation has the effects of relieving pain, regulating vital energy, tonifying the spleen, nourishing the spleen, the lung and the kidney, tonifying middle-Jiao and Qi, ascending yang and stopping diarrhea, and is mainly used for treating diarrhea caused by invasion of exogenous evil and weakness of spleen and stomach. The Chinese medicine preparation has the advantages of simple manufacture technology, small toxic side effect, convenience for administration, easiness for manufacture and low cost. The preparation can directly act on the focus, requires short curative time and avoids relapse after rehabilitation.
Owner:南通市星期七旅游开发有限公司
Molecular kit for rapidly identifying three types of piglet virus diarrhea and application of molecular kit
ActiveCN104611466ANo mutual interferenceLow minimum detectable concentrationMicrobiological testing/measurementMicroorganism based processesRotavirus RNAAstrovirus gastroenteritis
The invention discloses a kit for detecting pig epidemic diarrhea viruses, pig transmissible gastroenteritis viruses and pig rotaviruses. The kit comprises primer pairs shown by SEQ ID NO:1-2, SEQ ID NO:3-4 and SEQ ID NO:5-6 for respectively performing specific amplification on the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses. The invention also discloses applications of the primer pairs shown by the SEQ ID NO:1-2, the SEQ ID NO:3-4 and the SEQ ID NO:5-6 in preparation of reagents for detecting the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses. The detection kit disclosed by the invention can be used for accurately and effectively detecting the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses, and is strong in specificity, high in sensitivity, short in time consumption, rapid in detection and good in application prospect.
Owner:SICHUAN AGRI UNIV
Formulations for preservation of rotavirus
This invention provides formulations and methods for stabilizing viruses in liquid and dried formulations. In particular, formulations are provided including Zn2+ cations that stabilize the viability of Rotaviruses. Methods of vaccination include neutralization of gastric contents and administration of the vaccine formulations of the invention.
Owner:ARIDIS PHARMA INC
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604AImprove immune efficiencyFast titerBacteriaViral antigen ingredientsCross neutralizationRotavirus RNA
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Preparation of tetravalent wheel shaped virus inactivated vaccine and application
ActiveCN1686540APrevent infectious diseasesViral antigen ingredientsAntiviralsBacteroidesRotavirus RNA
A deactivated tetravalent rotavirus vaccine for preventing the infantile rotavirus infections diseases is prepared from the calf kidney cells digested and dispersed by pancreatin or cultured Vero cells through inoculating rotaviruses G1, G2, G3 and G4, culturing in non-serum culture liquid D-MEM, concentrating, purifying, deactivating, mixing and adding aluminium hydroxide.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Isoflavone compound with anti-virus activity as well as preparation method and application thereof
ActiveCN104761526ASimple structureGood anti-rotavirus activityOrganic chemistryAntiviralsAnti virusChromatographic separation
The invention discloses an isoflavone compound shown in a formula (I) in the specification as well as a preparation method and application thereof. The preparation method comprises the following steps: by taking a total tobacco plant as a raw material and taking a mixed solvent of methyl alcohol-water, ethyl alcohol-water or acetone-water as an extracting solvent, performing extraction, combining extracting solutions, filtering, and performing reduced pressure concentration on a filtrate to prepare an extract; performing silica-gel column chromatography on the extract by using silica-gel dry-process column packing; performing gradient elution by using a mixed solvent of trichloromethane-acetone; and further performing high-pressure liquid chromatographic separation and purification on an 8:2 part of an eluent to prepare the required isoflavone compound. Activity tests show that the compound disclosed by the invention has a very good inhibition effect on rotavirus; and the compound disclosed by the invention is simple in structure and good in compound activity, and can be used as an anti-rotavirus lead compound.
Owner:CHINA TOBACCO YUNNAN IND
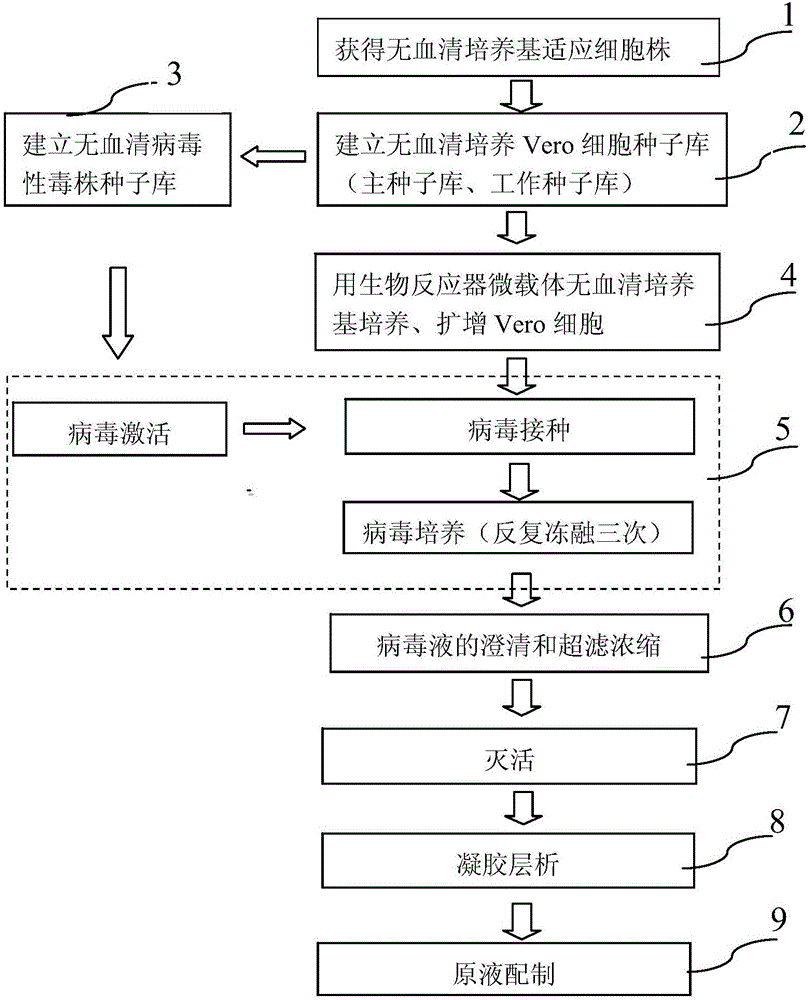
Method for preparing rotavirus vaccine stock solution by using serum-free Vero cells and serum-free rotavirus vaccine product
InactiveCN106676076ARemove inhibitionHigh titerViral antigen ingredientsMicroorganism based processesRotavirus RNAFiltration
The invention provides a method for preparing a rotavirus vaccine stock solution by using serum-free Vero cells. The method comprises the following steps: culturing Vero cells by using a serum-free culture medium, thus obtaining serum-free culture medium adapted cell strains; establishing a serum-free Vero cell seed bank by utilizing the obtained serum-free culture medium adapted cell strains; establishing a serum-free rotavirus strain working seed bank by utilizing the obtained serum-free culture medium adapted cell strains; carrying out reviving, culturing, passage and amplification on cells in a Vero cell working seed bank by utilizing the serum-free culture medium, using the cells in the Vero cell working seed bank as basic cells cultured in a bioreactor, and carrying out continuous perfusion culture on high-density Vero cells by applying the bioreactor and a microcarrier and using the serum-free culture medium after cell amplification; after inoculating virus seeds in the rotavirus strain working seed bank, carrying out bioreactor-microcarrier serum-free culture, obtaining a virus solution when virus is amplified to the summit, obtaining liquid virus titer, and carrying out clarification and ultra-filtration concentration, thus obtaining a serum-free rotavirus stock solution for human.
Owner:AB&B BIO TECH CO LTD JS
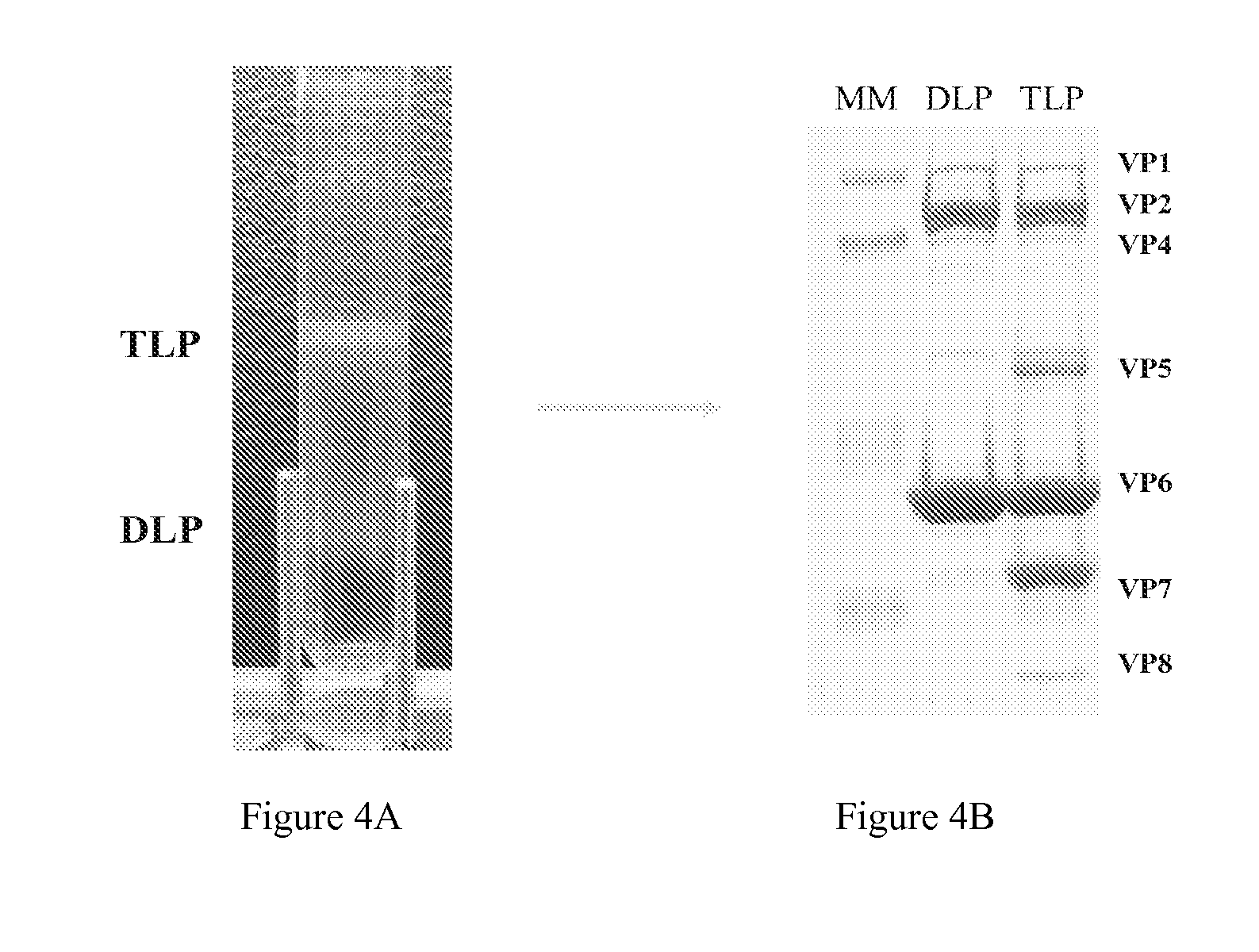
Human rotavirus vaccine strains and diagnostics
A vaccine composition and method of vaccination are provided useful for immunizing a subject against a rotavirus. The vaccines include rotavirus strains CDC-9 and CDC-66, fragments thereof, homologues thereof, or combinations thereof. Inventive vaccines may include a fragment of CDC-9, CDC-66, homologues thereof, or combinations thereof. Methods of inducing an immunological response are provided by administering an inventive vaccine.
Owner:THE GOVERNMENT OF THE US SEC THE DEPT OF HEALTH & HUMAN SERVICES CENT FOR DISEASE CONTROL & PREVENTION
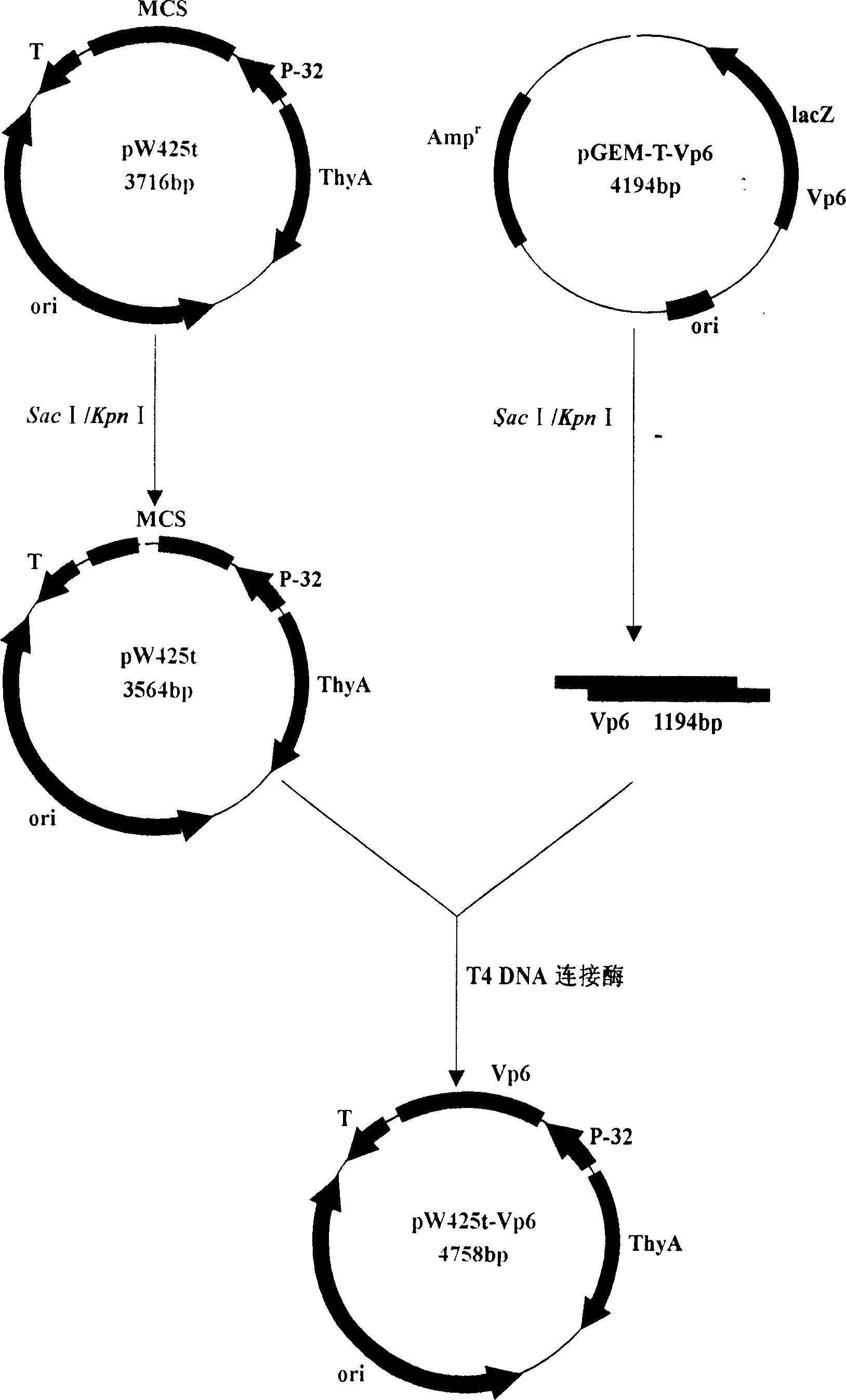
Recombination and expression for non-antibiotic expression vector of rotavirus Vp6 gene and lactic acid bacteria
InactiveCN1952156AFight reinfectionImprove immunityVirus peptidesFermentationEscherichia coliRotavirus RNA
The invention belongs to the field of biological gene, disclosing the recombination and expression of a rotavirus Vp6 gene and Bacterium acidi lactic nonresistance expression vector and it is characterized in: digesting the cloning vector and vector taking thyA gene as a selection pressure using Sac I and KpnI, purifying and recovering, linking the products with T4 DNA ligase, transforming to thyA gene-deficient E. coli competence E. coli X13, culturing in the medium, and screening to obtain prokaryotic recombinant expression plasmid through growing functional redeem, inducing positive bacteria for expression using threonine to obtain high expression of the recombinant plasmid. Molecular weight of fusion protein is about 44.88 KD. Beneficial effects are: VP6 protein is RV-group-specific antigen, locating at the virus endoconch, occupying 51%of the virus particles. As VP6 mainly stimulates organism to generate mucosal immune antibody sIgA, it plays a very important role in the mucosal immune, thus advanced development of rotavirus Vp6 mediated gene oral mucosal immune genetically engineered vaccine has important significance.
Owner:JILIN AGRICULTURAL UNIV +3
Diarrhea virus detection kit and method
ActiveCN103215379AImprove detection efficiencySimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceRotavirus RNAFluorophore
The invention is applicable to the technical field of biotechnology and medical detection and provides a diarrhea virus detection kit and method. The detection kit comprises PCR (polymerase chain reaction) liquid which comprises a PCR buffer solution, MgCl2, dNTP, DNA polymerase, primer pairs, probe sequences and Homo-tag, wherein the primer pairs and the probe sequences respectively aim at type I, type II and type IV of sapovirus, type I and type II of norovirus, rotavirus, adenovirus and astrovirus, the 5' ends of the probe sequences are connected with fluorophore, and the 3' ends of the probe sequences are connected with quencher. The detection kit provided by the invention can detect seven viruses (subtype) related to diarrhea at one time, and compared with a detection method in the prior art, the detection method provided by the invention has the advantages that operation is simple and convenient, a result is quick to read, and false positive is avoided.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
Complementing cell lines
A packaging cell line that complements recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells that are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 that expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell lines can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. Also, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, and measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Reagent kit for detecting rotavirus nucleic acid
ActiveCN101660001AStrong specificityHigh sensitivityMicrobiological testing/measurementRotavirus RNAFluorescence
The invention relates to a reagent kit for detecting rotavirus nucleic acid, in particular to a reagent kit for detecting rotavirus nucleic acid by using a fluorescence PCR technique. The reagent kitcan be used for qualitatively detecting the rotavirus nucleic acid. Due to the characteristics of high sensitivity, high specificity, simple operation and high detecting speed, the reagent kit can bewidely used for the auxiliary diagnosis of rotavirus infections.
Owner:DAAN GENE CO LTD
Attenuated human rotavirus vaccine
InactiveUS7150984B2Enhance immune responseMaximum efficacyViral antigen ingredientsInactivation/attenuationRotavirus RNACold adapted
The present invention provides vaccine compositions of attenuated human rotavirus. More particularly, the attenuated human rotavirus is produced by cold passage and thus contains attenuating mutations which produce virus having a cold-adapted (ca) and temperature sensitive (ts) phenotype. The attenuated strains are used in methods for stimulating the immune system of an individual to induce protection against human rotavirus by administration of the ca attenuated rotavirus.
Owner:GOVERNMENT UNITED STATES OF AMERICA THE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com