Patents
Literature
763results about "DsRNA viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
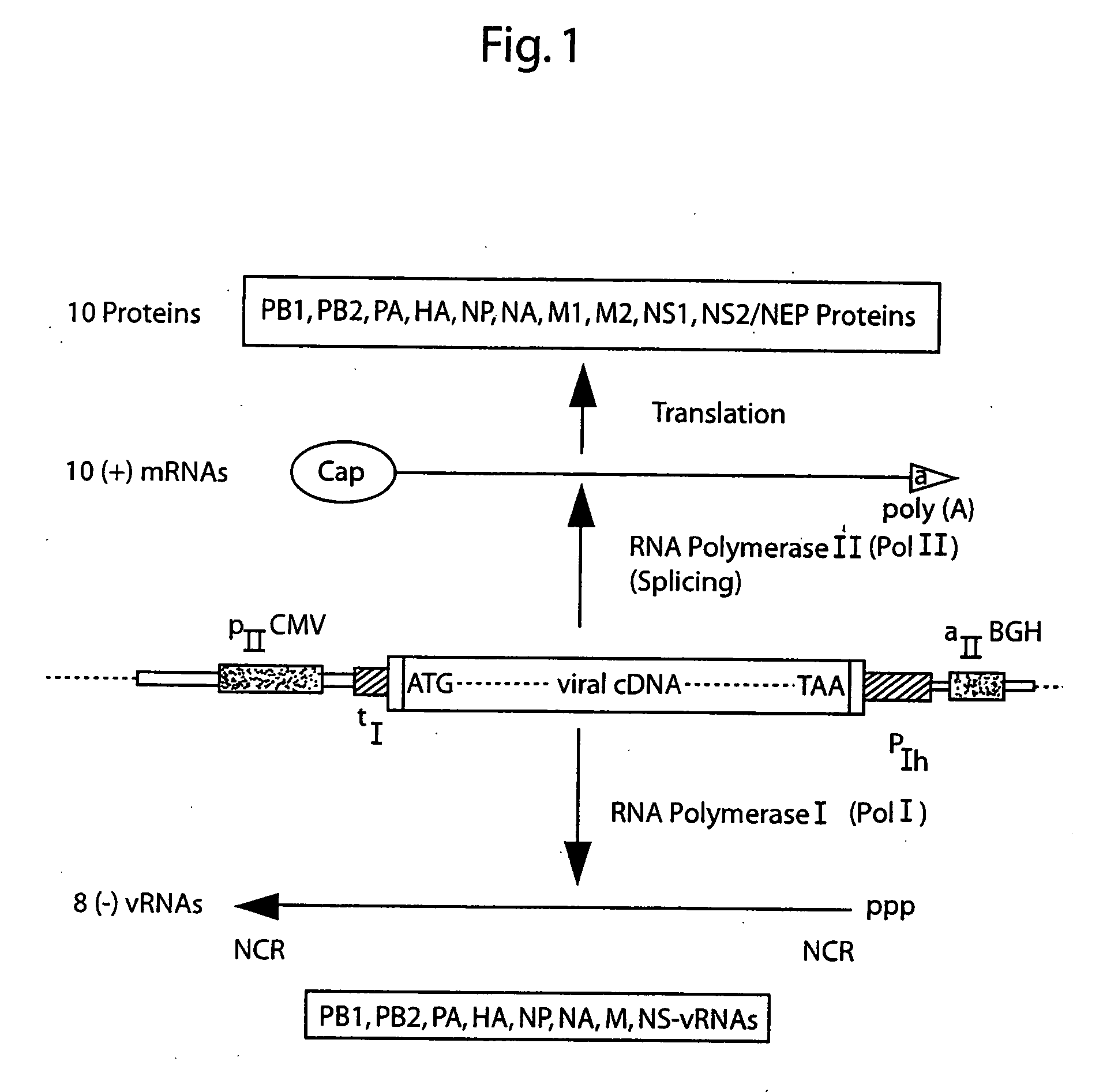
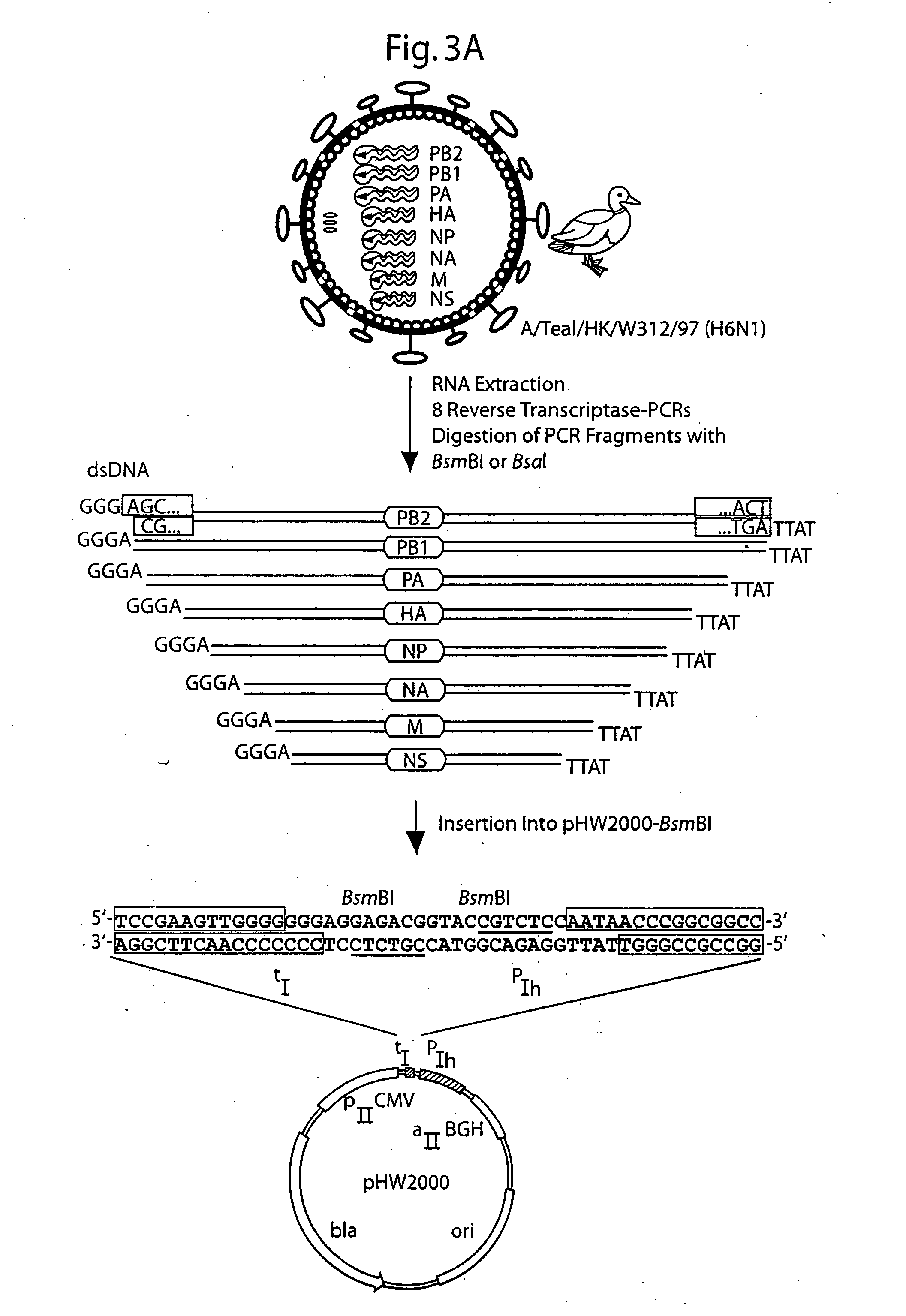
DNA transfection system for the generation of infectious influenza virus
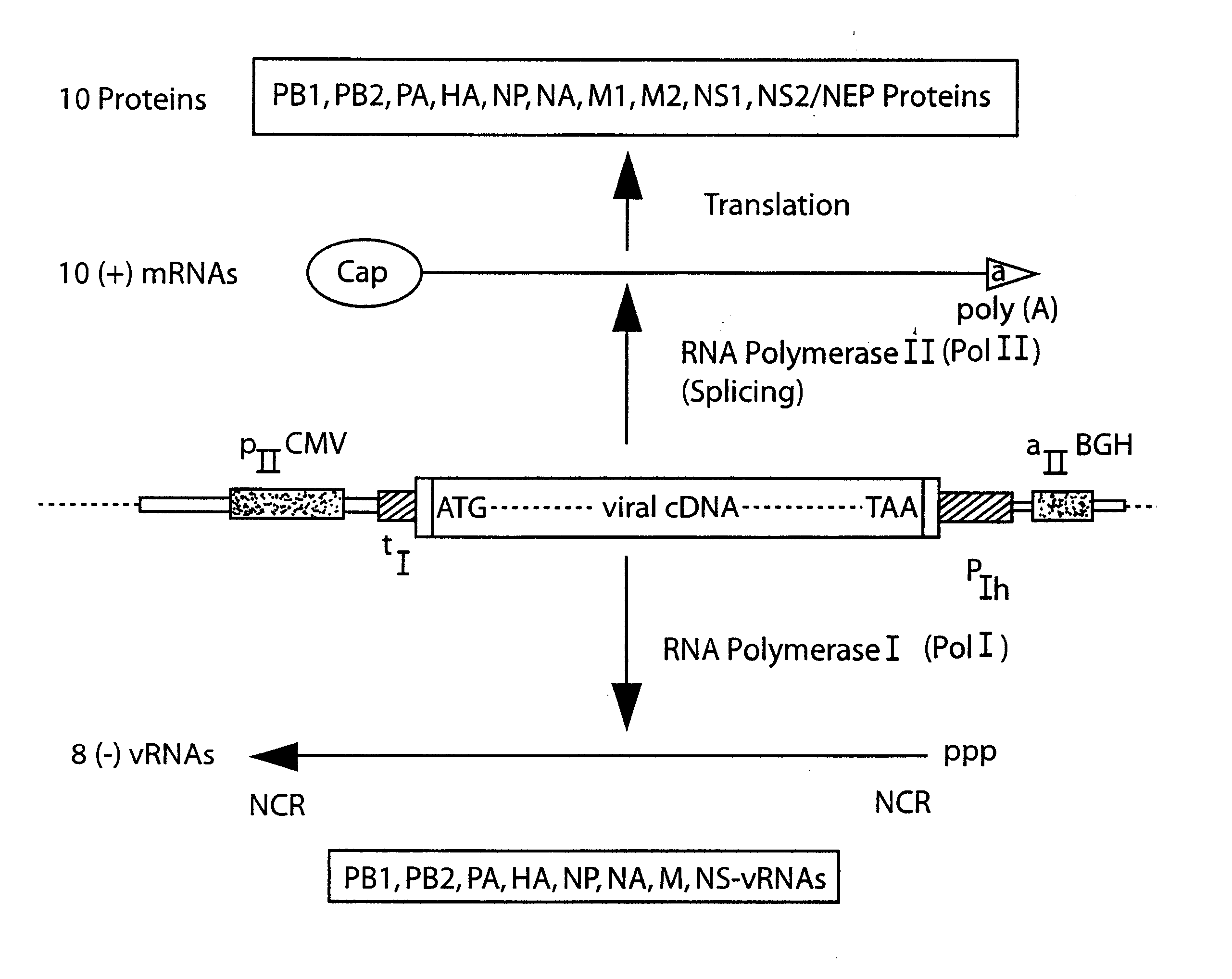
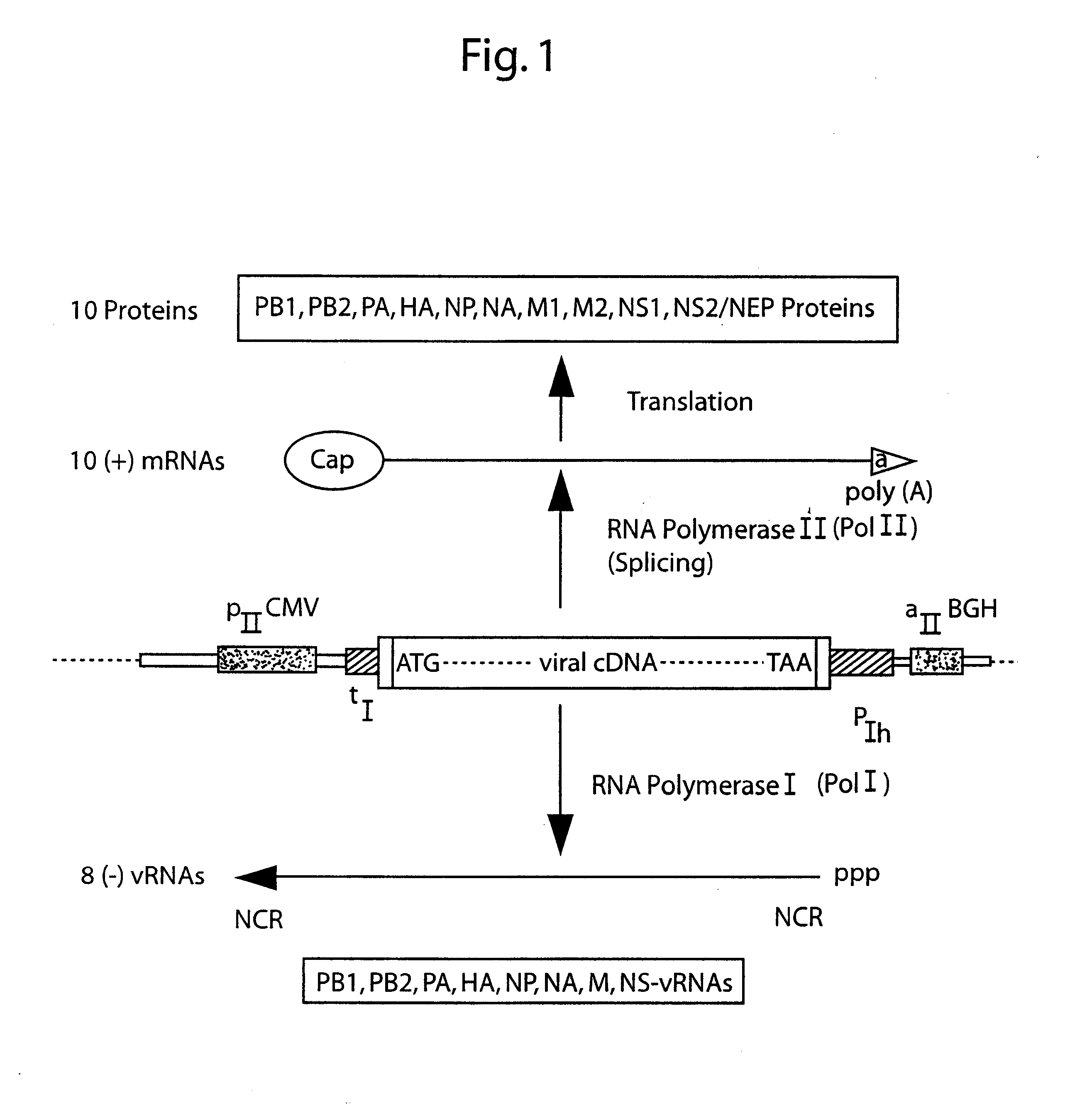
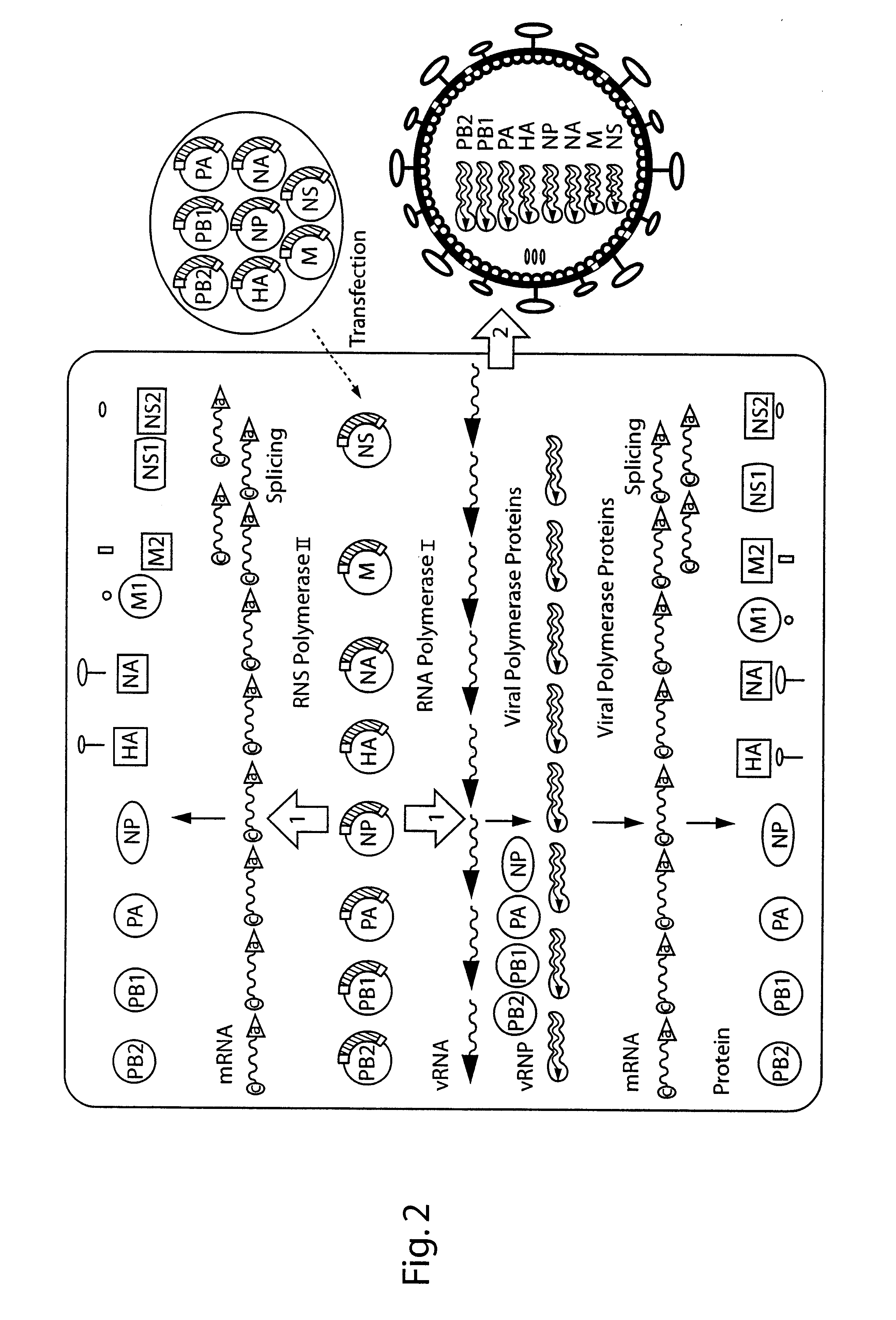
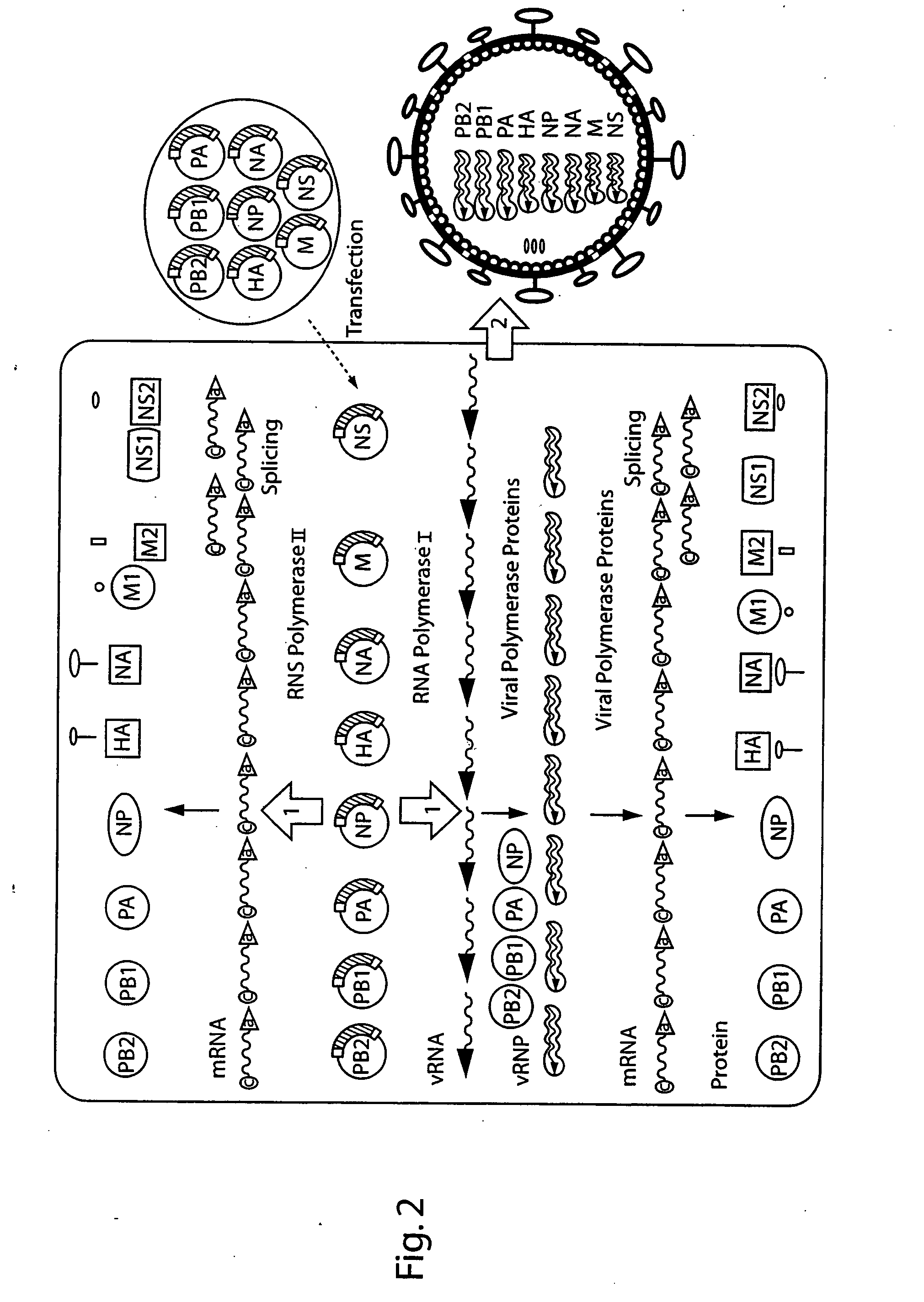
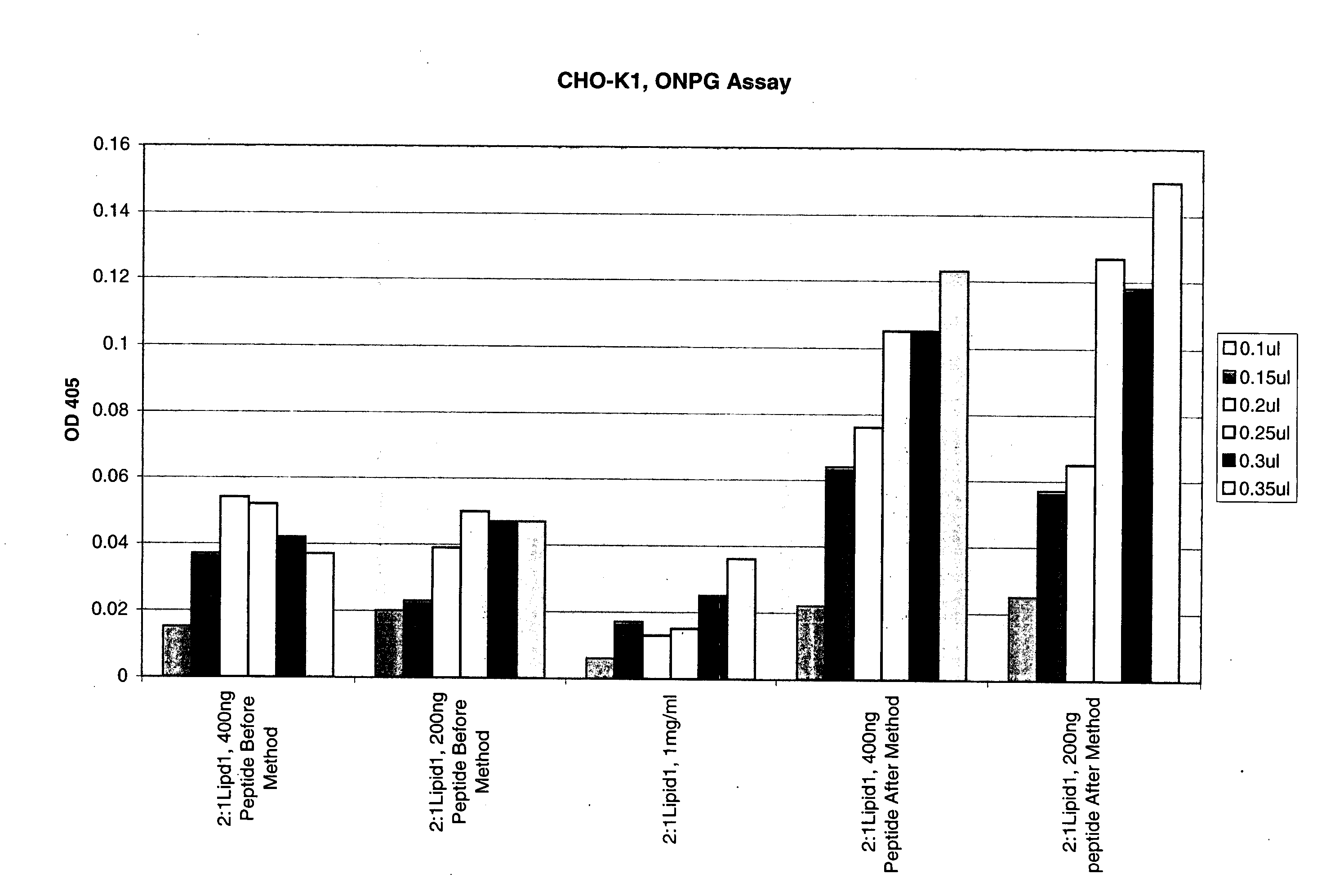
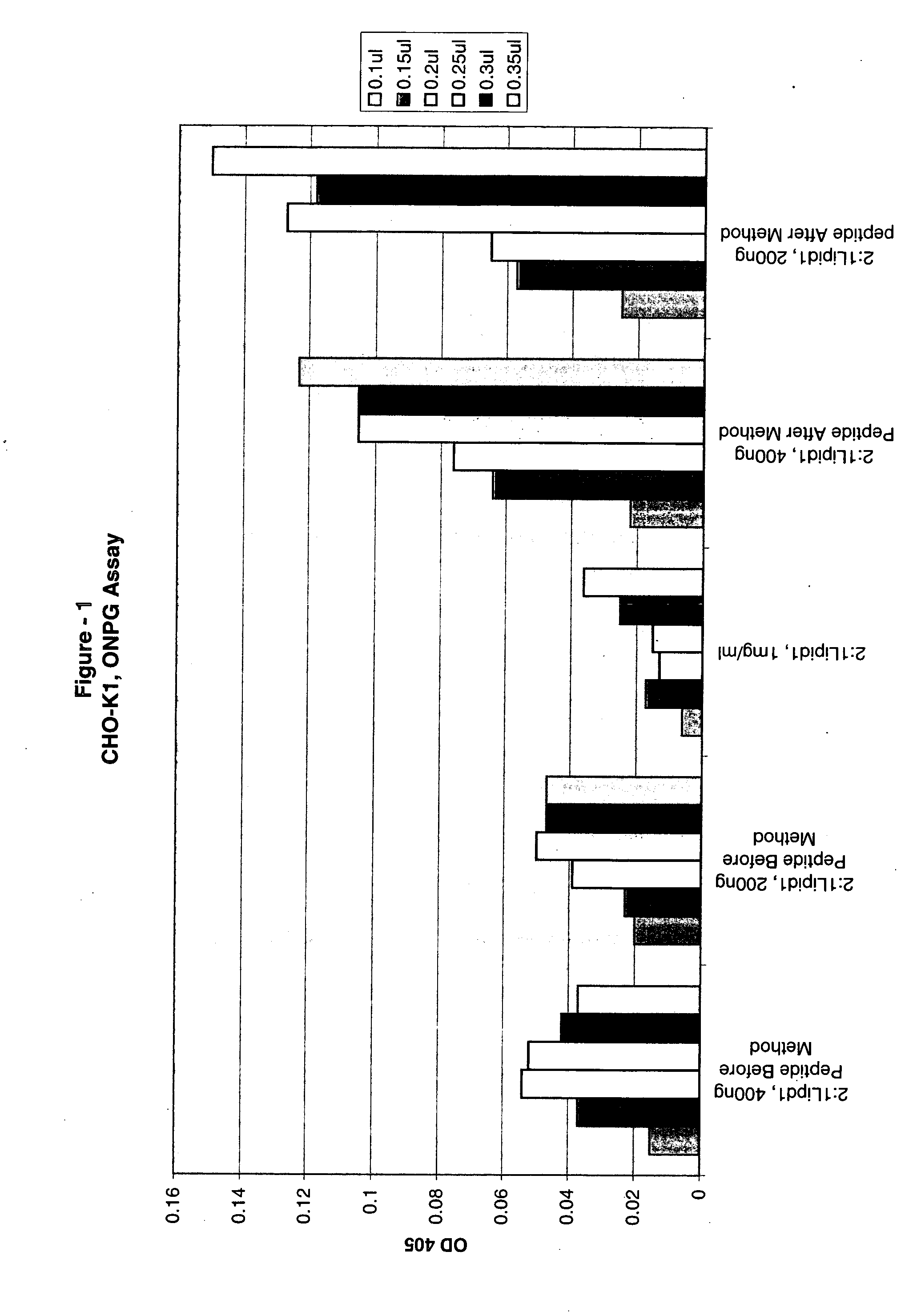
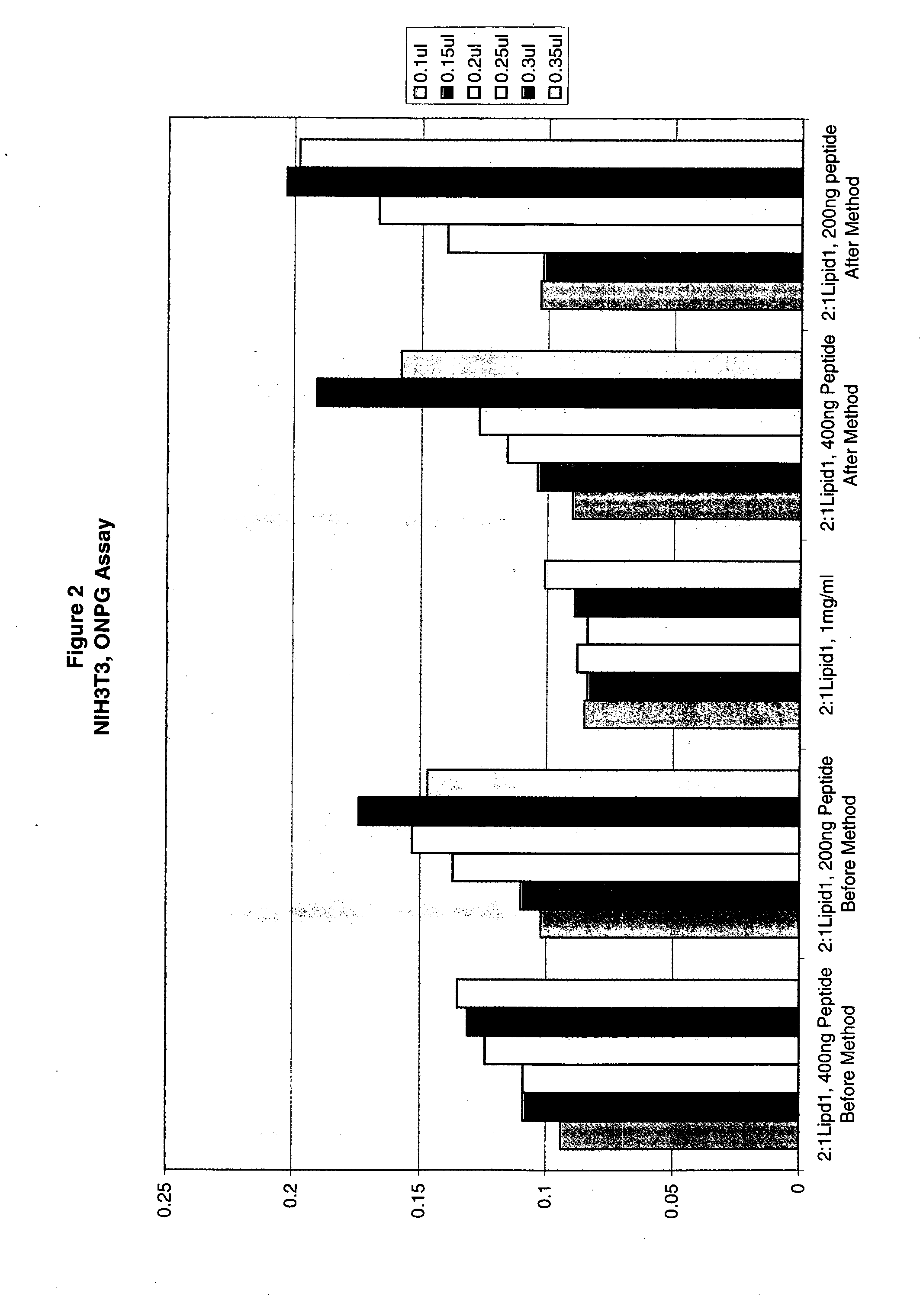
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
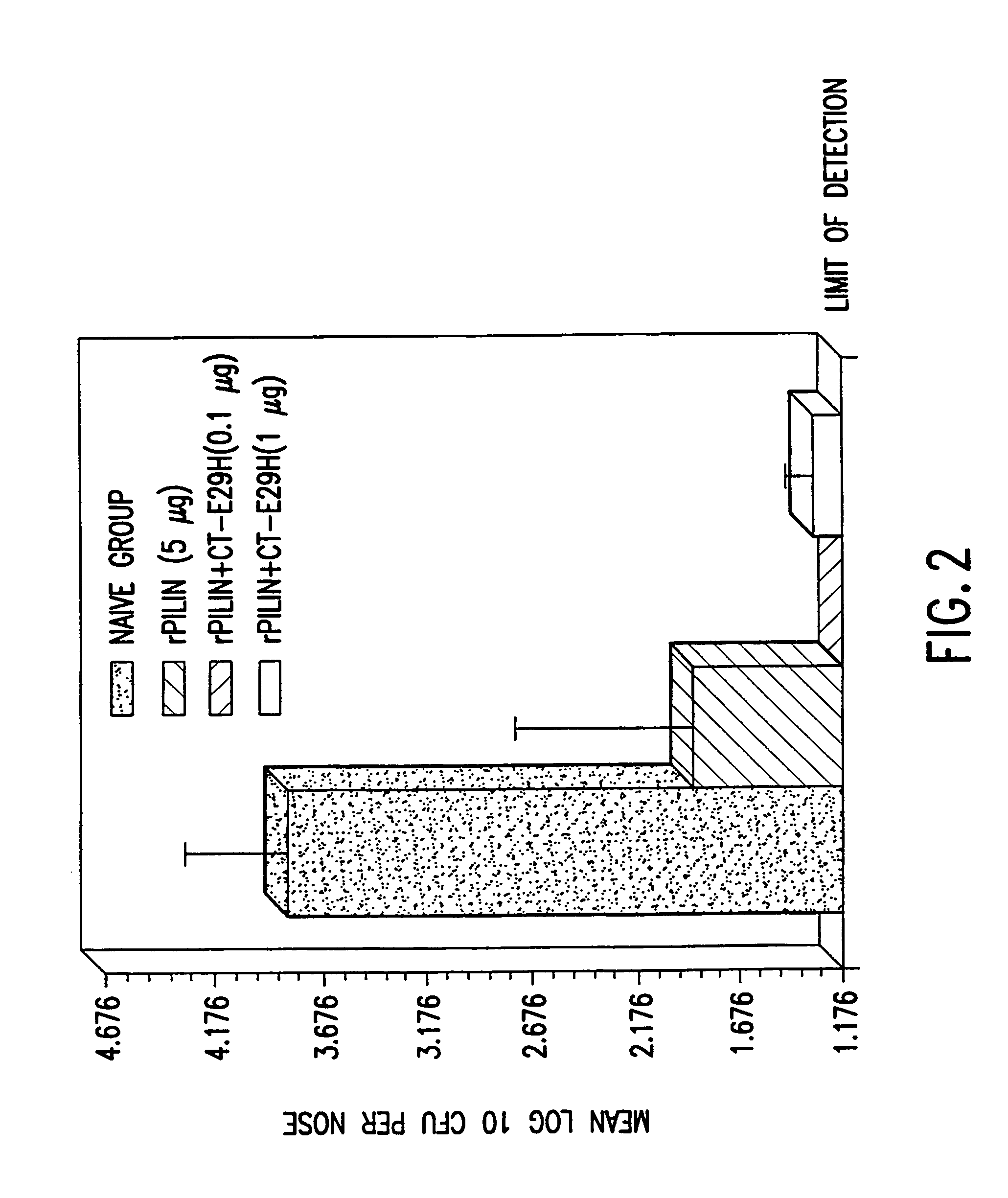
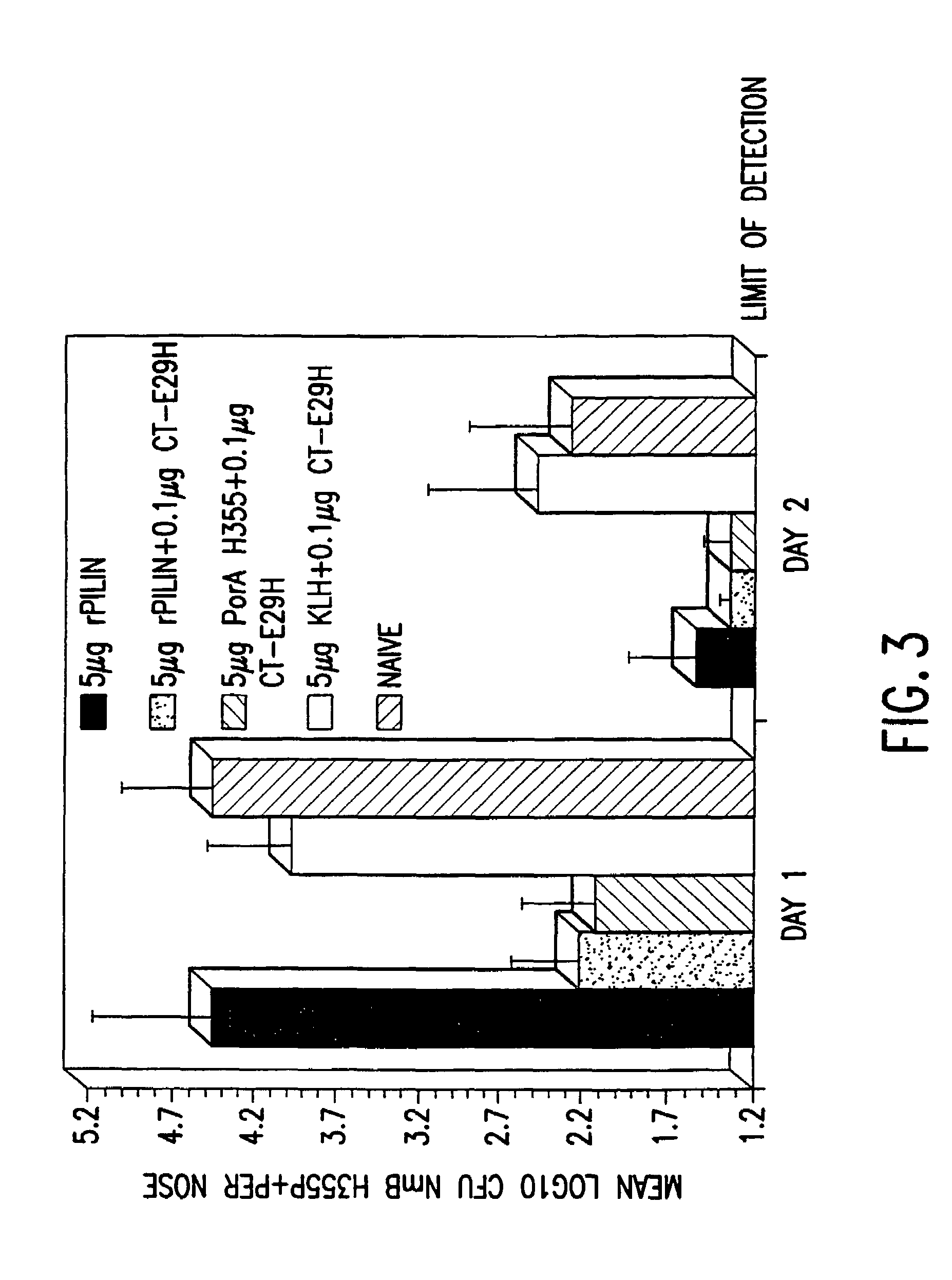
Mutant cholera holotoxin as an adjuvant
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
DNA transfection system for the generation of infectious influenza virus
InactiveUS20050186563A1Improve effectivenessElicit protective immunitySsRNA viruses negative-senseFungiDual promoterSingle-Stranded RNA
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuraminidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Viral purification methods
The present invention is directed to an improved method of purifying virus, particularly reovirus. Infectious virus can be extracted from a cell culture with a detergent to produce high titers of virus, and the virus can then be purified by simple steps such as filtration and column chromatography. Viruses and compositions comprising the viruses prepared according to the present invention are also provided.
Owner:ONCOLYTICS BIOTECH
Novel reagents for transfection of eukaryotic cells
ActiveUS20090023215A1Improve efficiencyPeptide/protein ingredientsVirus peptidesDendrimerTransfection
Compositions and methods for improved delivery of macromolecules into eukaryotic cells are provided. Fusogenic peptides from fusion proteins of non-enveloped viruses enhance the efficiency of transfection of eukaryotic cells mediated by transfection agents such as cationic lipids, polycationic polymers such as PEI and dendrimers. These fusogenic peptides are used as part of a transfection complex that efficiently delivers a macromolecule, for example, a nucleic acid, into a eukaryotic cell. Novel cationic lipids and compositions of cationic lipids also are provided that may be used for the introduction of macromolecules such as nucleic acids, proteins and peptides into a variety of cells and tissues. The lipids can be used alone, in combination with other lipids and / or in combination with fusogenic peptides to prepare transfection complexes.
Owner:MOLECULAR TRANSFER
DNA encoding ovine adenovirus (OAV287) and its use as a viral vector
A genome of an ovine adenovirus designated OAV287 is isolated from sheep and sequenced. Portions of the genome not essential for maintenance or viability of the virus can be deleted or altered. A nucleotide sequence encoding a non-adenoviral polypeptide can be incorporated into the genome. The a full-length clone of the genome can be provided as part of a plasmid or viral vector. Cells can be transformed with a vector of the invention such that they express an exogenous protein.
Owner:COMMONWEALTH SCI & IND RES ORG
Viruses and virus-like particles for multiple antigen and target display
InactiveUS20060121468A1Improve effectivenessImprove responseSsRNA viruses positive-senseVectorsTissue targetingVaccine Immunogenicity
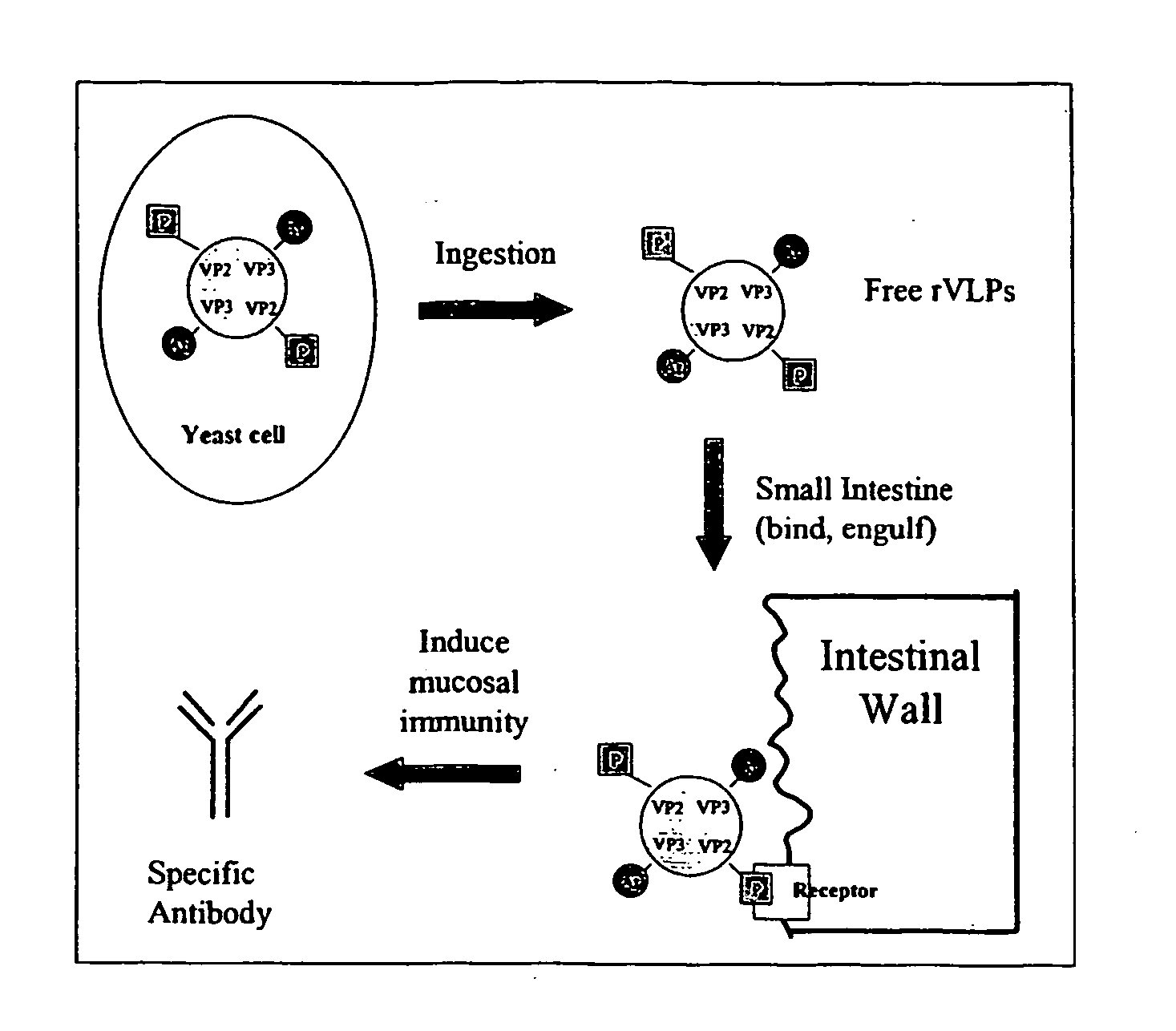
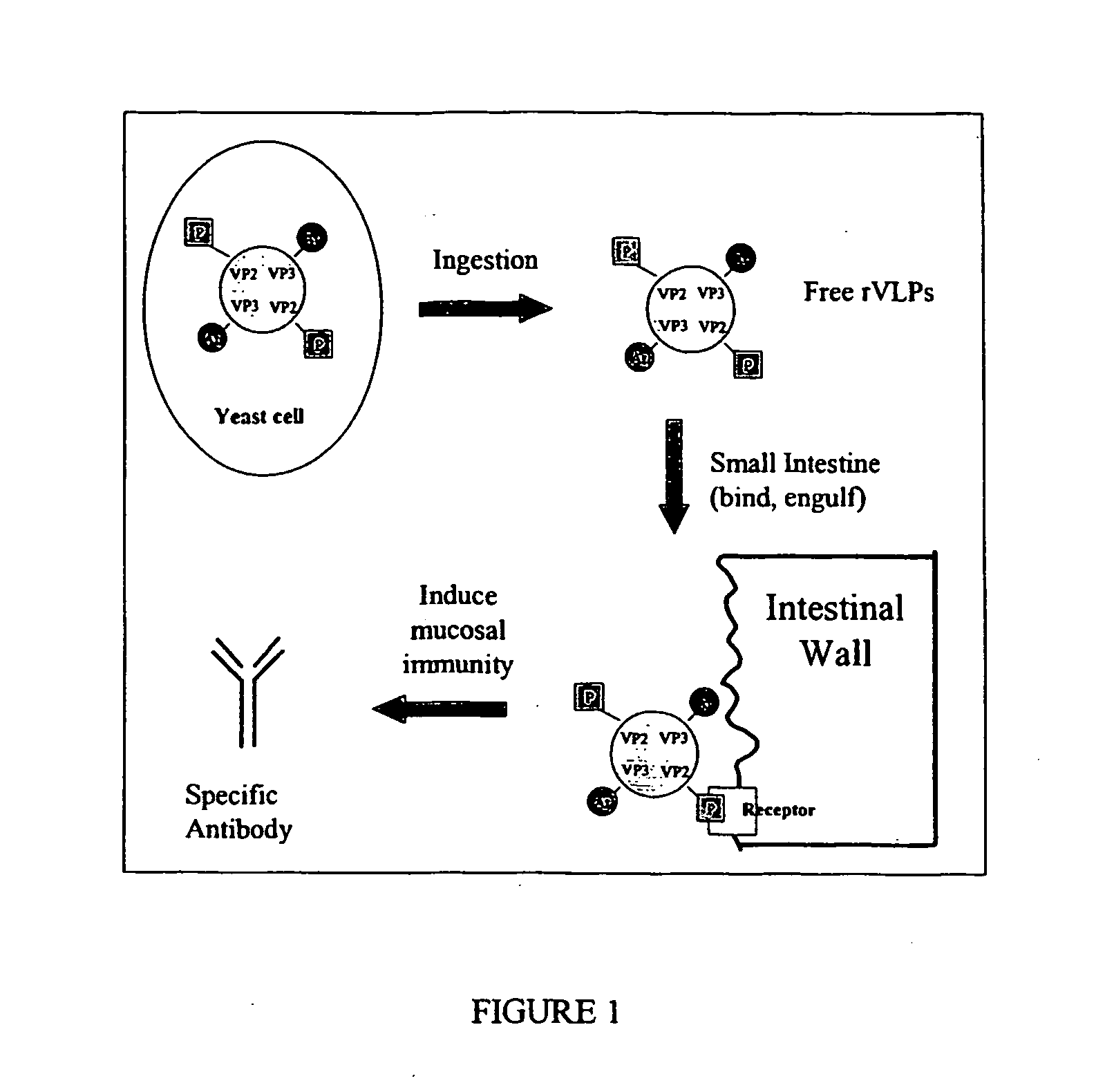
The present invention relates to the display of antigenic or allergenic components along with a tissue-targeting component on viruses or virus-like particles. Capsid protein genes are recombinantly modified to contain the specified components then expressed within a host organism, such as yeasts, bacteria, or algae, and allowed to spontaneously form active virus particles or virus-like particles. The recombinant complexes (virus or virus-like particle) can then be purified or used in situ as a therapeutic tool for disease or allergy prevention. The expression of multivalent and multifunctional components to increase the immunogenicity of the recombinant complexes, especially on oral administration, is provided.
Owner:ADVANCED BIONUTRITION CORP
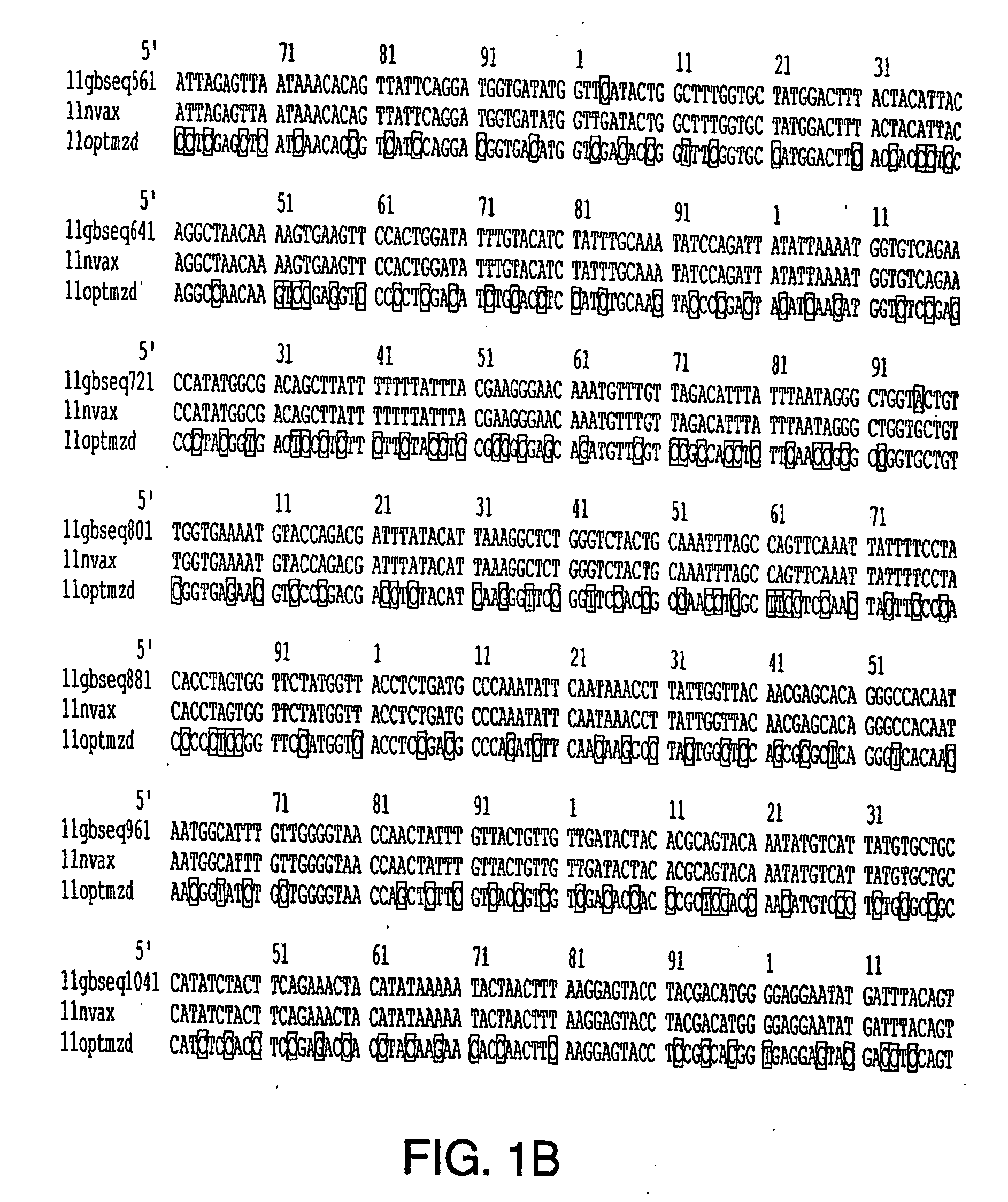
Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
InactiveUS20050118191A1Minimize the numberSequence minimizedAnimal cellsViral antigen ingredientsDiagnostic testTGE VACCINE
Owner:NOVAVAX
Viral purification methods
ActiveUS7223585B2Simple methodViral antigen ingredientsGenetic material ingredientsPurification methodsFiltration
The present invention is directed to an improved method of purifying virus, particularly reovirus. Infectious virus can be extracted from a cell culture with a detergent to produce high titers of virus, and the virus can then be purified by simple steps such as filtration and column chromatography. Viruses and compositions comprising the viruses prepared according to the present invention are also provided.
Owner:ONCOLYTICS BIOTECH
Vaccines
Processes for the production of a stabilised vaccine composition of labile immunogens, wherein a fluid comprising one or more immunogens is sprayed into a reactor containing fluidised particles of a pharmaceutically acceptable water soluble material at a temperature of about 25° C. to about 50° C., such that the immunogen coats and is dried onto the particles under the fluidising conditions, and thereafter collecting from said reactor dried immunogen containing particles having a moisture content between about 0.1% w / w to about 10% w / w are described. Also described are stabilised vaccine compositions of labile immunogens.
Owner:PFIZER INC
Optimization of gene sequences of virus-like particles for expression in insect cells
InactiveUS20040121465A1Improve the level ofMinimize the numberAnimal cellsViral antigen ingredientsPolynucleotideTGE VACCINE
Codon optimized polynucleotides for optimal expression of recombinant proteins in eukaryotic cells are provided. The codon optimized polynucleotides encode a viral capsid protein that self assembles into a virus-like particle. The virus-like particle is expressed extracellularly and exhibits conformational antigenic epitopes capable of raising neutralizing antibodies. Pharmaceutical compositions, vaccines, and diagnostic test kits containing the gene products of the codon-optimized polynucleotides are also provided.
Owner:NOVAVAX
Kit for treating gastrointestinal tract
InactiveUS20040063188A1Viral antigen ingredientsGenetically modified cellsPurification methodsVirus-like particle
Methods for isolation and purification or recombinant gene products are disclosed. In particular, methods for isolation and purification of extracellular and intracellular viral gene products, including virus-like particles, are disclosed herein.
Owner:NOVAVAX
Method of extracting virus from cell culture
InactiveUS7186542B2High virus titersConveniently performedViral antigen ingredientsMicroorganism based processesInfectious virusBiomedical engineering
The present invention is directed to a method of extracting virus, particularly reovirus, from a culture of cells. Infectious virus can be extracted from the culture with a detergent at a convenient temperature such as 25° C. or 37° C. to produce high virus titers. Both ionic and non-ionic detergents can be used in the present invention.
Owner:ONCOLYTICS BIOTECH
Method of producing infectious reovirus
InactiveUS7049127B2Simple methodHigh yieldViral antigen ingredientsGenetically modified cellsHEK 293 cellsPurification methods
Owner:ONCOLYTICS BIOTECH
CRISPRs
A composition for treating a lysogenic virus, including a vector encoding isolated nucleic acid encoding two or more gene editors chosen from gene editors that target viral DNA, gene editors that target viral RNA, and combinations thereof. A composition for treating a lytic virus, including a vector encoding isolated nucleic acid encoding at least one gene editor that targets viral DNA and a viral RNA targeting composition. A composition for treating both lysogenic and lytic viruses, including a vector encoding isolated nucleic acid encoding two or more gene editors that target viral RNA. A composition for treating lytic viruses. Methods of treating a lysogenic virus or a lytic virus, by administering the above compositions to an individual having a virus and inactivating the virus.
Owner:EXCISION BIOTHERAPEUTICS INC
Reovirus for the treatment of ral-mediated cellular proliferative disorders
Methods for treating proliferative disorders, by administering reovirus to a ral-mediated proliferative disorder, are disclosed. The reovirus is administered so that it ultimately directly contacts target cancer cells. Proliferative disorders include but are not limited to neoplasms. Human reovirus, non-human mammalian reovirus, and / or avian reovirus can be used. If the reovirus is human reovirus, serotype 1 (e.g., strain Lang), serotype 2 (e.g., strain Jones), serotype 3 (e.g., strain Dearing or strain Abney), other serotypes or strains of reovirus, and recombinant reovirus can be used. Combinations of more than one type and / or strain of reovirus can be used, as can reovirus from different species of animal. Either solid neoplasms or hematopoietic neoplasms can be treated.
Owner:ONCOLYTICS BIOTECH
Sensitization of chemotherapeutic agent resistant neoplastic cells with a virus
The present invention relates to a method of increasing the sensitivity of neoplastic cells to chemotherapeutic agents by using a virus, a method of treating proliferative disorders with a virus and chemotherapeutic agents, and a method for preventing a neoplasm from developing drug resistance to chemotherapeutic agents. The virus is preferably a reovirus.
Owner:ONCOLYTICS BIOTECH
Live attenuated rotavirus vaccine for oral administration
ActiveUS8192747B2Maintain immunogenicityStable over a long shelf-lifeBacterial antigen ingredientsViral antigen ingredientsDiseaseOral medication
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
In Ovo vaccine against infectious bursal disease
The present invention relates to a non pathogenic vaccine comprising a recombinant Infectious Bursal Disease virus that includes a recombinant Segment A, designated as rD78GLSNSΔ, that includes sequences from D78 and GLS strains and wherein the NS protein is not expressed.
Owner:UNIV OF MARYLAND BIOTECH INST
Recombinant vaccine against bluetongue virus
ActiveUS20070280960A1Provide securityPractical and convenientAntibacterial agentsViral antigen ingredientsAntigenAdjuvant
The present invention relates to an immunogenic or vaccine composition to induce an immune response or protective immune response against Orbiviruses, more specifically bluetongue virus (BTV) in an animal susceptible to BTV infection. The composition may include a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector may contain heterologous nucleic acid molecule(s), expresses in vivo in the animal BTV antigen, immunogen or epitope thereof, e.g., BTV VP2; BTV VP2 and VP5; BTV VP2 and VP5 and VP3 and / or VP7. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.AGACAGTGGTCAATTCCAATGGTACTGTTTGACGATAC
Owner:MERIAL LTD +3
Microparticulated vaccines for the oral or nasal vaccination and boostering of animals including fish
ActiveUS20120040010A1Stabilizes sensitive bioactiveImprove thermal stabilitySsRNA viruses negative-sensePowder deliveryVaccinationAquatic animal
The invention relates to a composition and a method for manufacturing semi-dry or dry particles containing a mucoadhesive polymer and a bioactive agent such as, but not limited to, an Immunogenic Substance (e.g., a vaccine), that allows the oral or nasal administration and delivery of the bioactive agent essentially unaltered to mucosal surfaces in the animal, including an aquatic animal.
Owner:INTERVET INC
Combination of transplantation and oncolytic virus treatment
InactiveUS20050214266A1Assess effectGuaranteed accuracyBiocideGenetic material ingredientsInfectious virusPre treatment
Oncolytic viruses can be used to purge cellular compositions to remove undesired neoplastic cells before the cellular compositions are used for transplantation. The present invention relates to the use of a virus to pre-treat a subject prior to delivery into the subject a transplant that has been purged with the same virus. This pre-treatment serves to elicit an immune response in the subject against the virus, thereby protecting the subject from infections by the virus after receiving the transplant, which likely contains infectious viruses.
Owner:ONCOLYTICS BIOTECH
Methods and compositions for modulating the immune system of animals
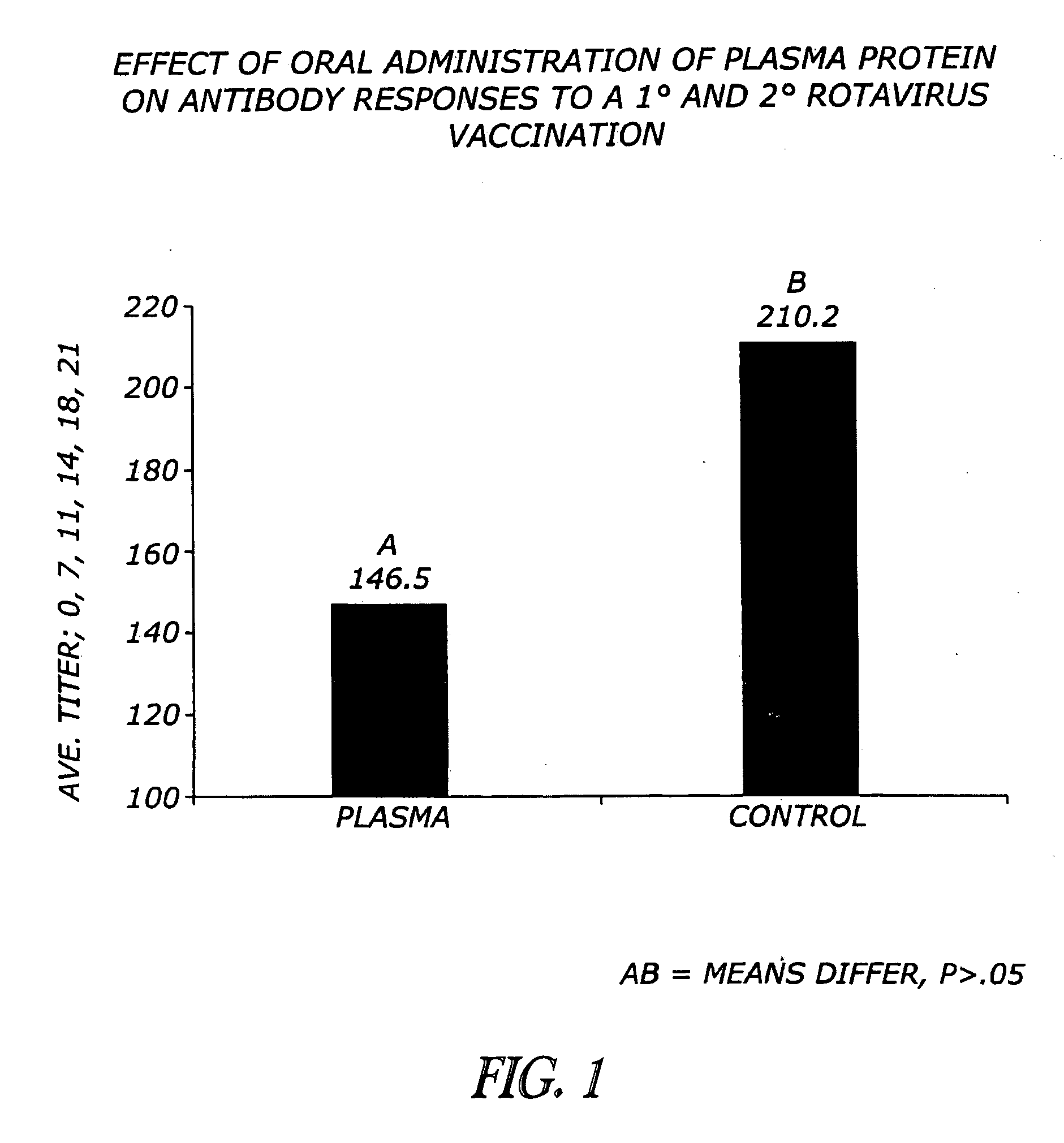
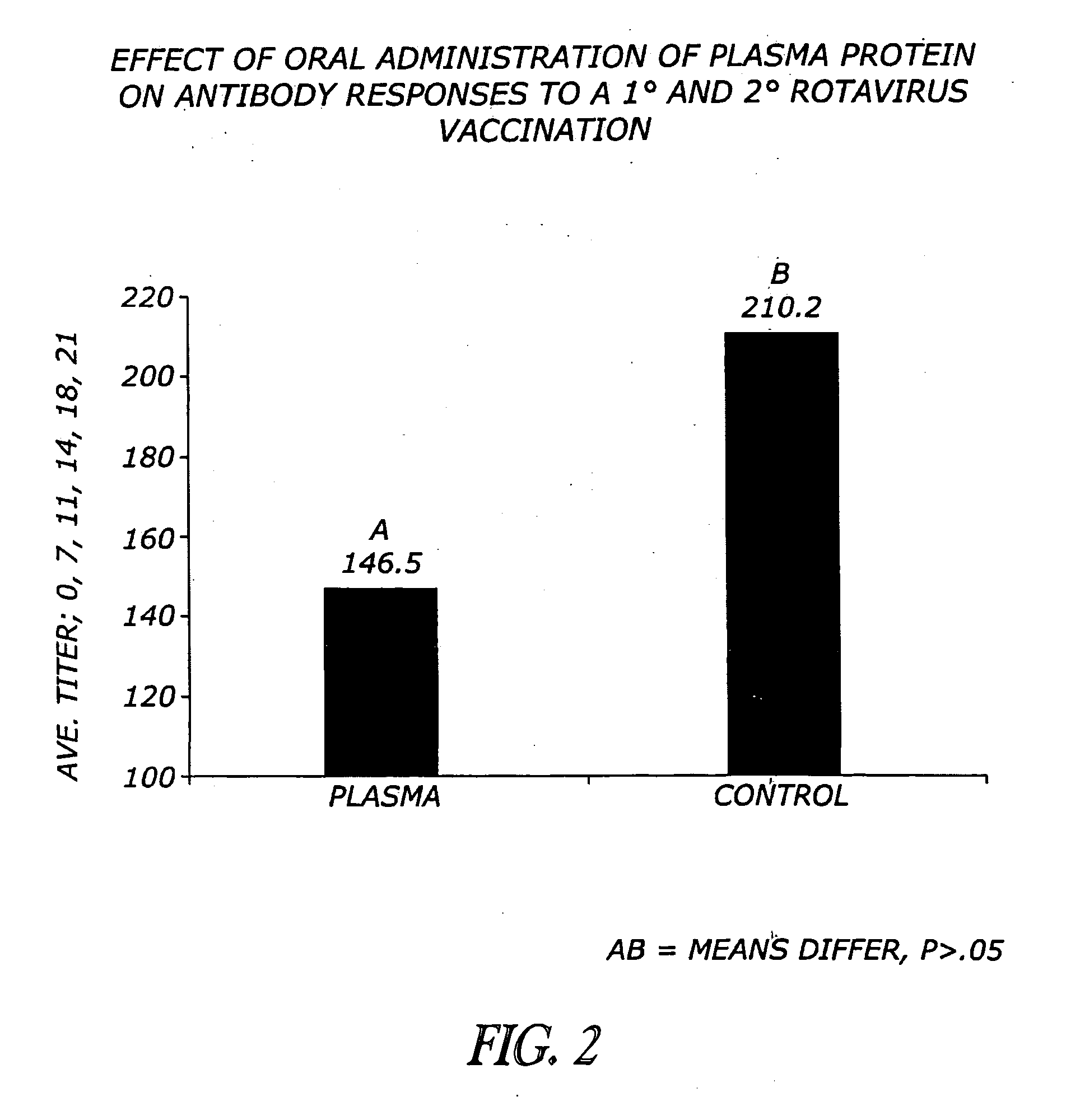
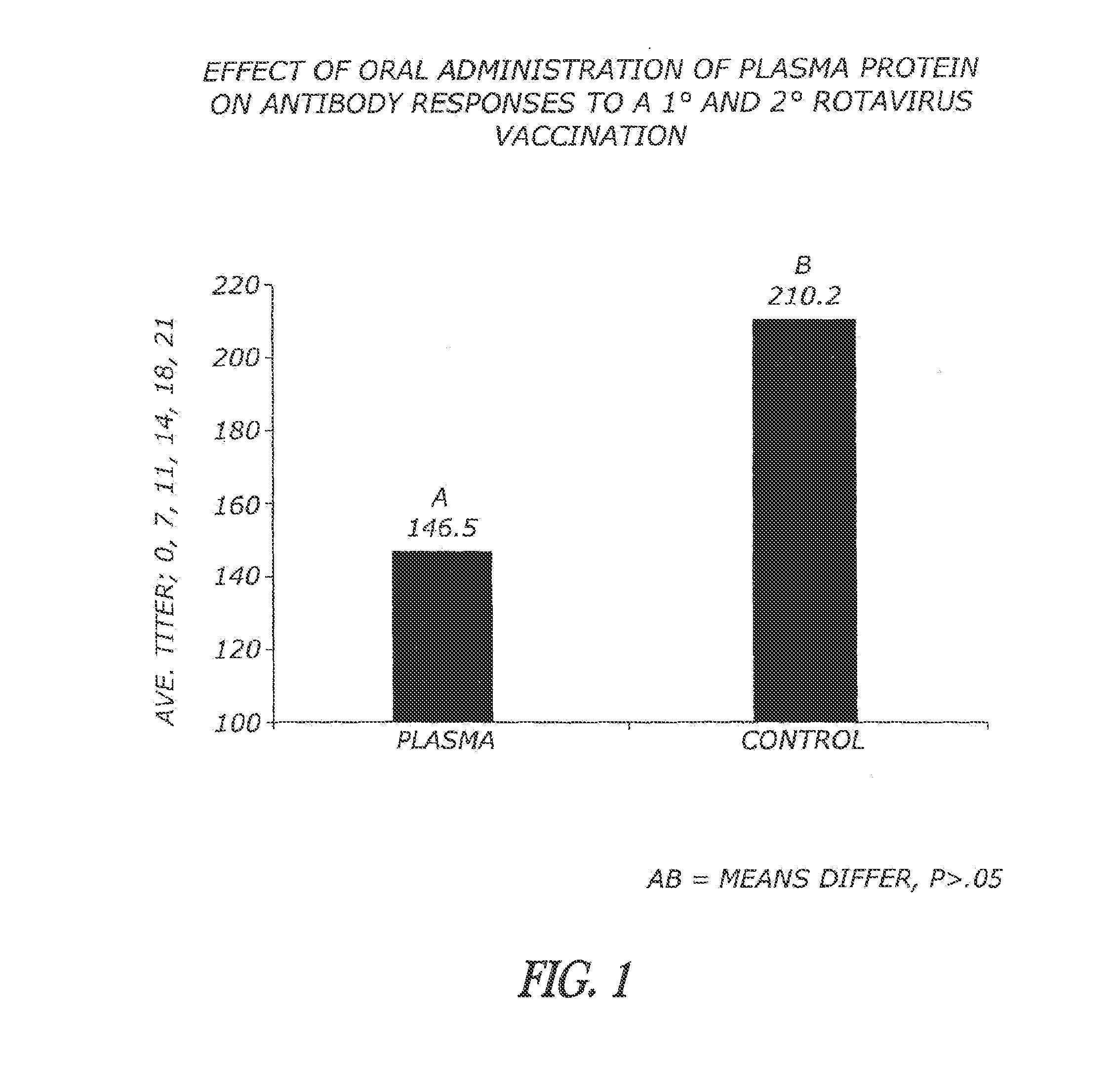
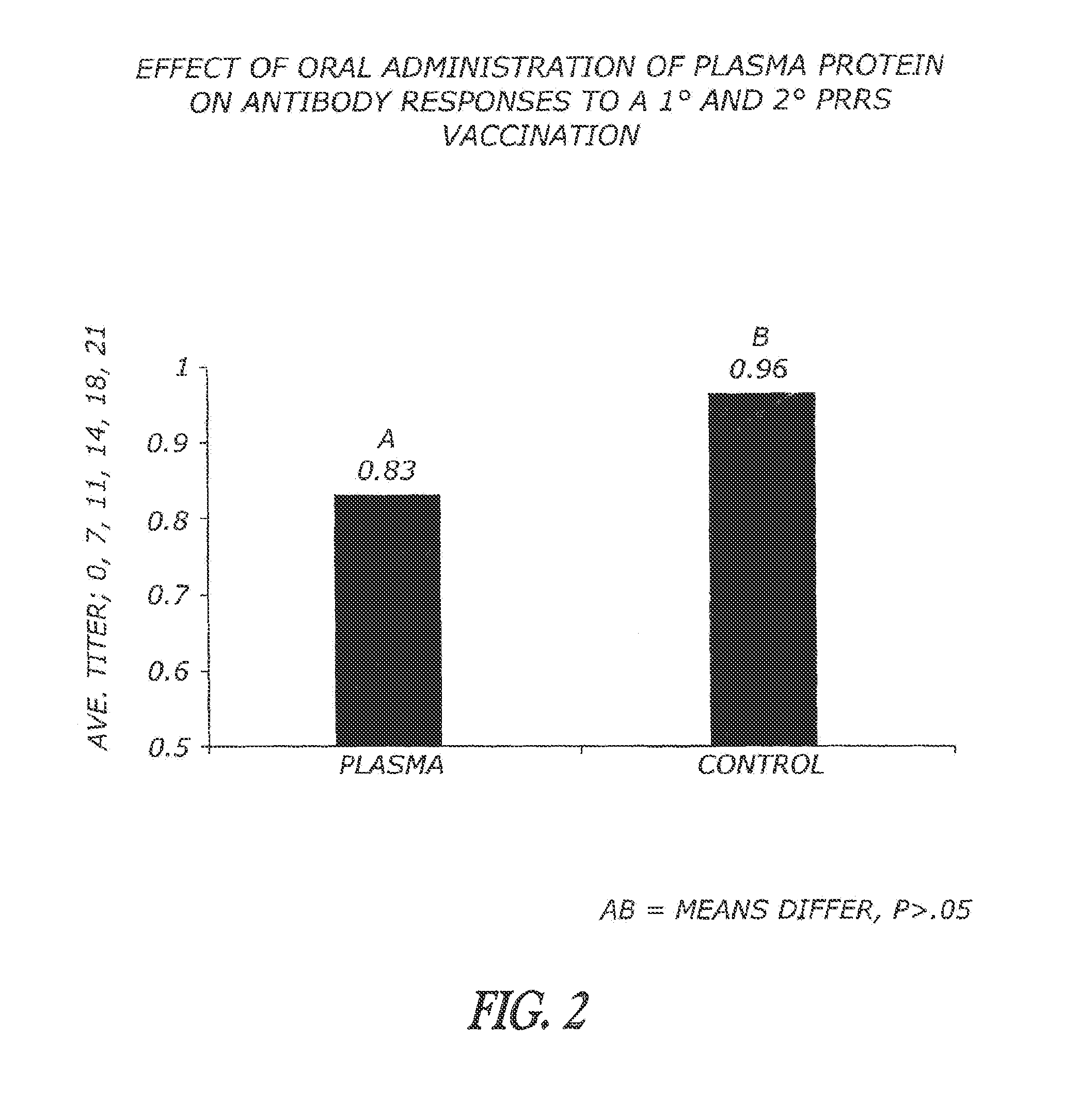
Methods and compositions are disclosed for modulating the immune system of animals. Applicant has identified that oral administration of immunoglobulins purified from animal blood can modulate serum IgG levels for treatment of immune dysfunction disorders, potentiation of vaccination protocols, and improvement of overall health and weight gain in animals, including humans.
Owner:THE LAURIDSEN GROUP
Methods and compositions of treatment for modulating the immune system of animals
InactiveUS20100215667A1Good coagulationEasy to appreciateEgg immunoglobulinsSenses disorderSpray dried plasmaDisease
Methods and compositions are disclosed for the dietary modulation of the immune system and gut microbial response in animals. Applicant has identified that oral administration of a supplemental spray dried plasma purified from animal serum can modulate serum IgG levels for treatment in such things as diminished immune capacity, intestinal microbial balance, autoimmune disorders, potentiation of vaccination protocols, and improvement of overall health and weight gain in animals, including humans.
Owner:THE LAURIDSEN GROUP
Viral Purification Methods
Owner:ONCOLYTICS BIOTECH
Antigenic class of avian reoviruses
InactiveUS6951650B1Effectively affords protection in poultryEffective protectionViral antigen ingredientsDigestive systemFowlDisease
The present invention provides a new antigenic class of avian reoviruses which are involved in enteric disease conditions in poultry. Moreover, the invention provides a vaccine for use in the protection of poultry against such disease conditions derived from the new type of avian reoviruses.
Owner:INTERVET INT BV
Recombinant HVT vectors expressing antigens of avian pathogens and uses thereof
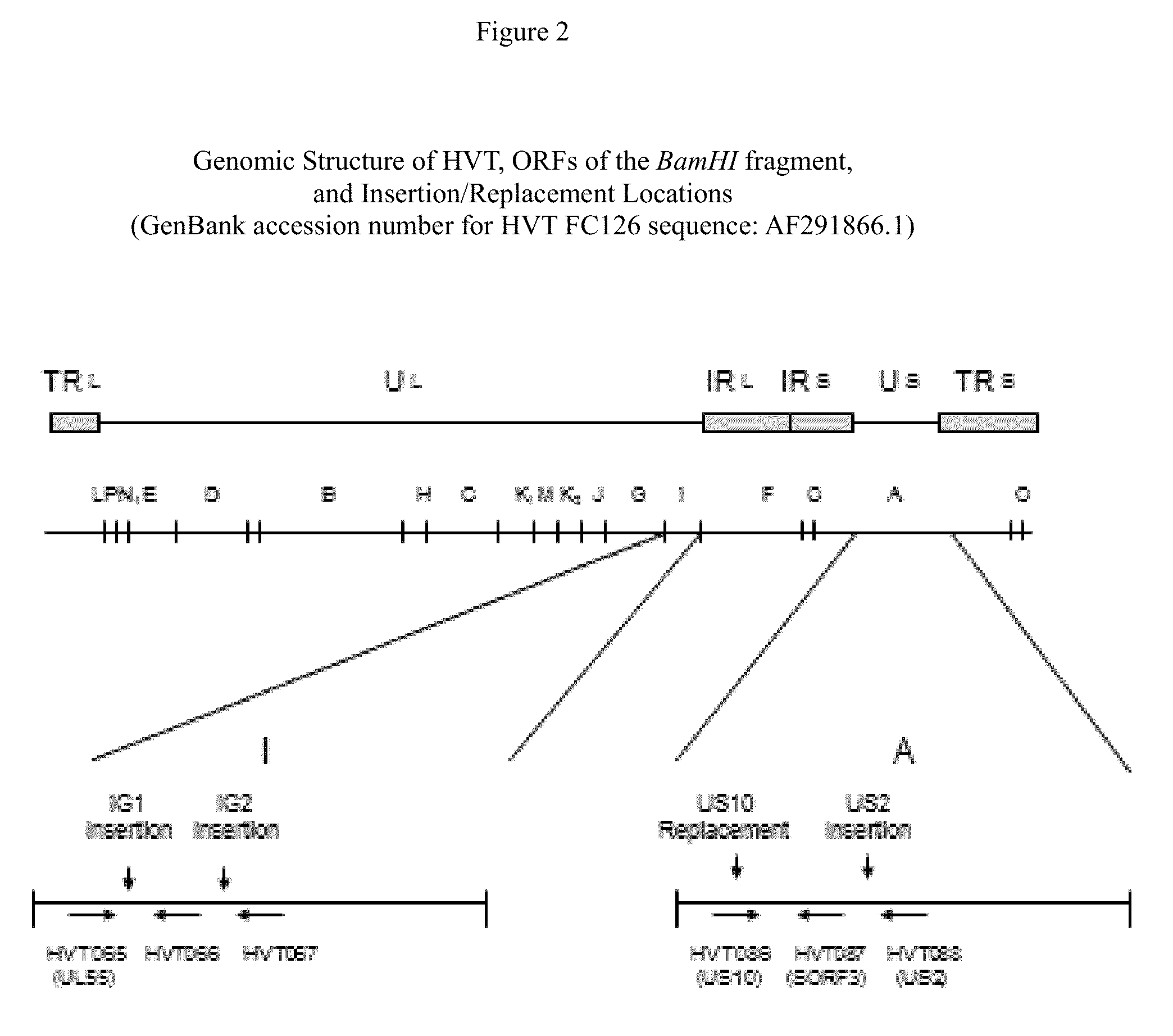
ActiveUS9114108B2Effective protectionSsRNA viruses negative-senseViral antigen ingredientsAntigenFowl
The present invention provides recombinant herpesvirus of turkeys (HVT) vectors that contain and express antigens of avian pathogens, compositions comprising the recombinant HVT vectors, polyvalent vaccines comprising the recombinant HVT vectors and one or more wild type viruses or recombinant vectors. The present invention further provides methods of vaccination against a variety of avian pathogens and method of producing the recombinant HVT vectors.
Owner:MERIAL INC
Rotavirus subunit vaccine
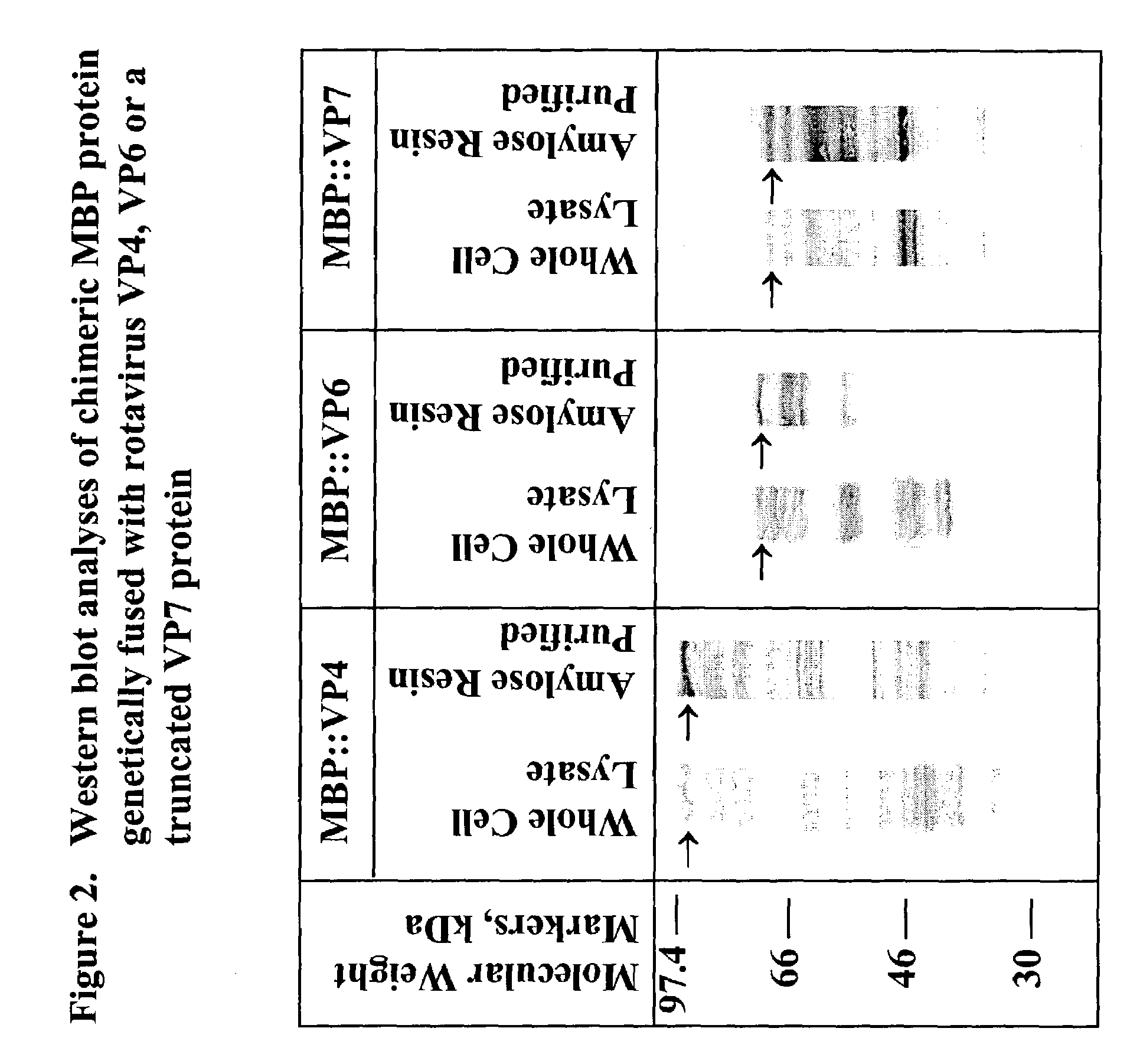
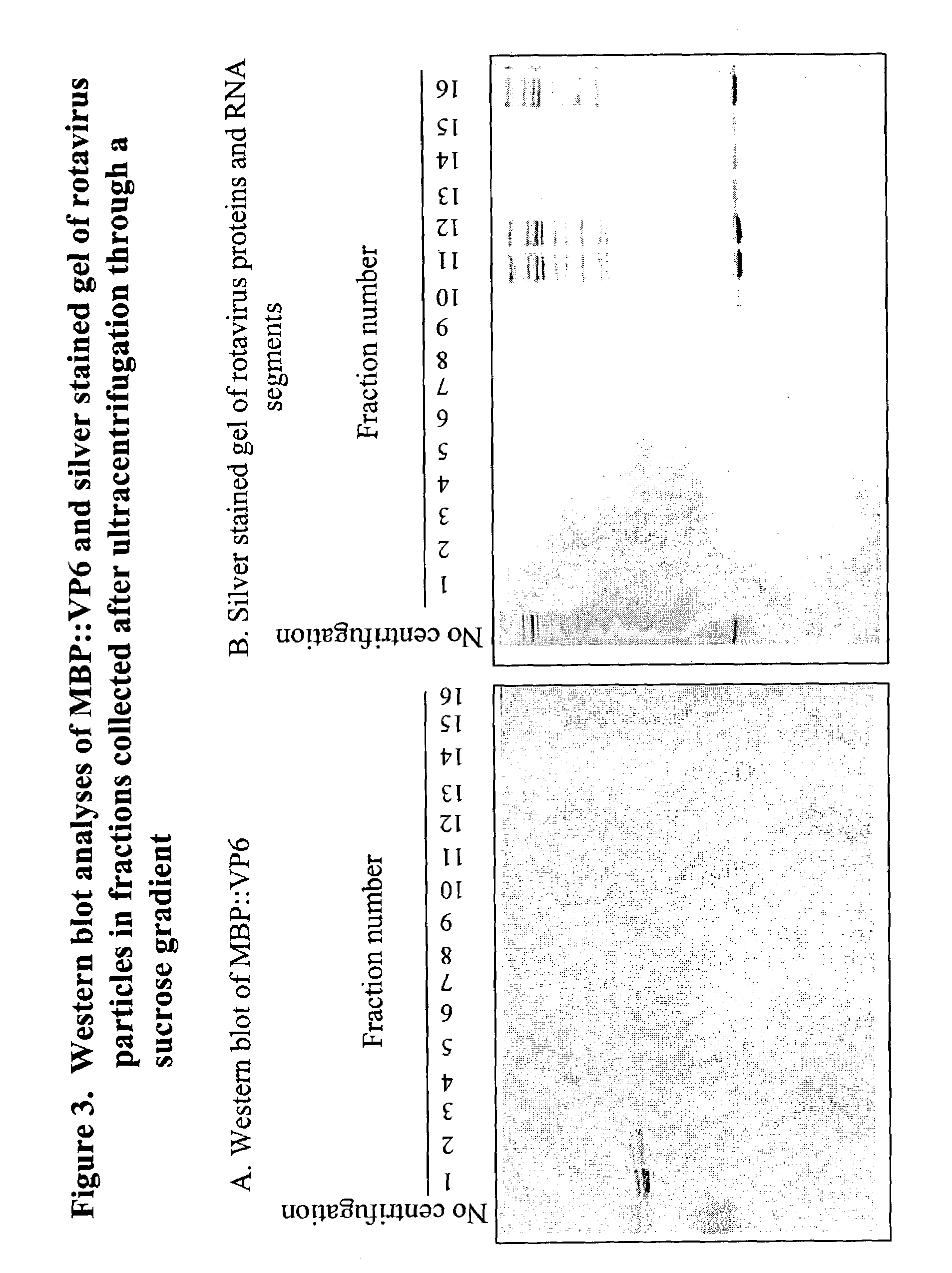
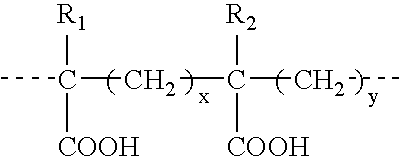
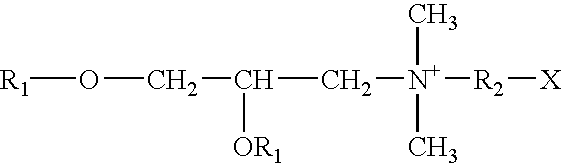
The present invention is directed to the generation and use of recombinant rotavirus fusion proteins as immunogens to produce a protective immune response from immunized individuals. In one embodiment, the present invention contemplates a recombinant rotavirus fusion protein vaccine composition comprising a rotavirus subunit protein or immunogenic fragment thereof, and an adjuvant in combination with the recombinant rotavirus subunit fusion protein. In one aspect of this embodiment, the recombinant rotavirus fusion protein comprises a rotavirus subunit protein and a fusion partner protein in genetic association with the rotavirus subunit protein, wherein the fusion partner protein does not interfere with expression and immunogenicity of the rotavirus subunit protein, the fusion partner protein prevents complex formation by the rotavirus subunit protein, and the fusion partner protein facilitates purification of the recombinant rotavirus fusion protein. In another aspect of this embodiment, the rotavirus subunit protein is selected from the group consisting of VP1, VP2, VP3, VP4, VP6, VP7, NSP1, NSP2, NSP3, NSP4 or NSP5. In yet another aspect of this embodiment, the rotavirus subunit protein is VP6.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Methods of characterizing infectious bursal disease virus
InactiveUS20050214316A1Sugar derivativesViral antigen ingredientsInfectious bursal disease virus IBDVInfectious bursitis
Characterization of infectious bursal disease virus (“IBDV”) for use in vaccine identification and production that provides rapid selection of a specific vaccine strain with an IBDV sequence most related to the IBDV of interest or identification of a novel strain of IBDV.
Owner:UNIV OF GEORGIA RES FOUND INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com