Kit for treating gastrointestinal tract
a technology for the gastrointestinal tract and a kit is applied in the field of viral vaccines, therapeutics, and diagnostics, which can solve the problems of reducing antigen and immunogenity, affecting the normal regulation of host cell gene regulation, and affecting the detection and treatment of human papillomavirus infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
ESTABLISHMENT OF SERUM-FREE SF-9 INSECT CELL LINE
[0200] A new insect cell line designated Sf-9S was derived from the parent S. frugiperda Sf-9 cell line (ATCC CRL-1771) by several rounds of selective processes based on serum-independent growth and enhanced expression of secreted recombinant proteins from baculovirus vectors. Specifically, Sf-9 cells were cultivated to passage 38 in Grace's insect media (Life Technologies, Grand Island, N.Y. 14072) supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, N.Y. 14072) as monolayer cultures in T-75 flasks (Corning, Inc., Corning, N.Y.). The master cell bank of Sf-9 cells was stored at passage 38 in serum-containing media at -70.degree. C. and in liquid nitrogen. A working cell bank was established from a single cryovial of the Sf-9 master cell bank and cultivated in serum-containing insect media for an additional five (5) passages.
[0201] Initially, cell clones capable of growing in commercial serum-free media as suspen...
example 2
ESTABLISHMENT OF TRANSFORMED SF-9S CELL LINE
[0203] In a second selection process, one of the serum-free cell clones developed in Example 1 was chosen to select cell clones that may produce enhanced levels of recombinant extracellular proteins and VLPs from several viruses including rotaviruses and human papillomaviruses by successive rounds of clonal selection of cells infected with recombinant baculoviruses and expressing extracellular self-assembled VLPs.
[0204] This process involved the plating of cell aliquots (200 .mu.l) from a cell suspension (one cell per 200 .mu.l) of the parent cell clone (#23) in serum-free media onto 96-well dishes at a ratio of 200 .mu.l per well. From wells containing a single cell in the original seeding, cells were grown to confluency and subcultured into six replica-plates (96-well). The first round of selection was performed when a total cell density of 2-4.times.10.sup.3 cells / well was obtained; the cells were infected with recombinant baculoviruses...
example 3
CLONING CODON-OPTIMIZED HPV-16 L1 GENES AND ESTABLISHMENT OF RECOMBINANT BACULOVIRUS STOCKS
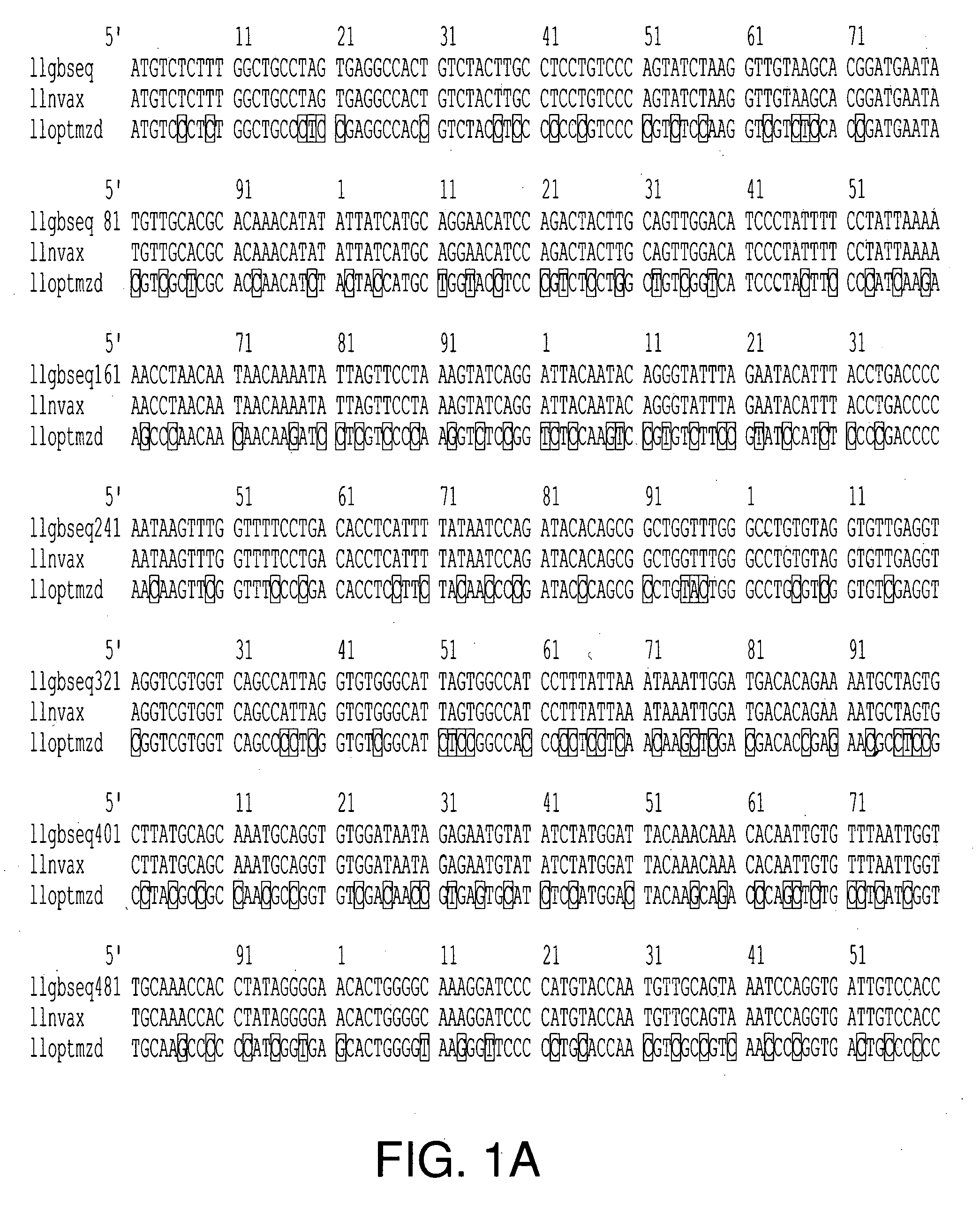
[0207] A HPV-16 L1 prototype (GenBank Accession No. K02718) and modified in U.S. Pat. No. 5,985,610, was optimized for codon usage in insect cells of the Lepidopteran family. The HPV-16 L1 gene was optimized (FIG. 1A) in this embodiment of the present invention for codon usage based on the following criteria: (1) abundance of aminoacyl-tRNAs for a particular codon in Lepidopteran species of insect cells for a given amino acid as described by Levin and Whittome (2000); (2) maintenance of GC-AT ratio in L1 gene sequence at approximately 1:1; (3) minimal introduction of palindromic or stem-loop DNA structures, and (4) minimal introduction of transcription and post-transcription repressor element sequences.
[0208] The optimized gene sequence was synthesized in vitro as overlapping oligonucleotides, cloned into a subcloning plasmid vector, and then cloned into a bacmid transfer vector (i.e., Luckow ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| wet weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com