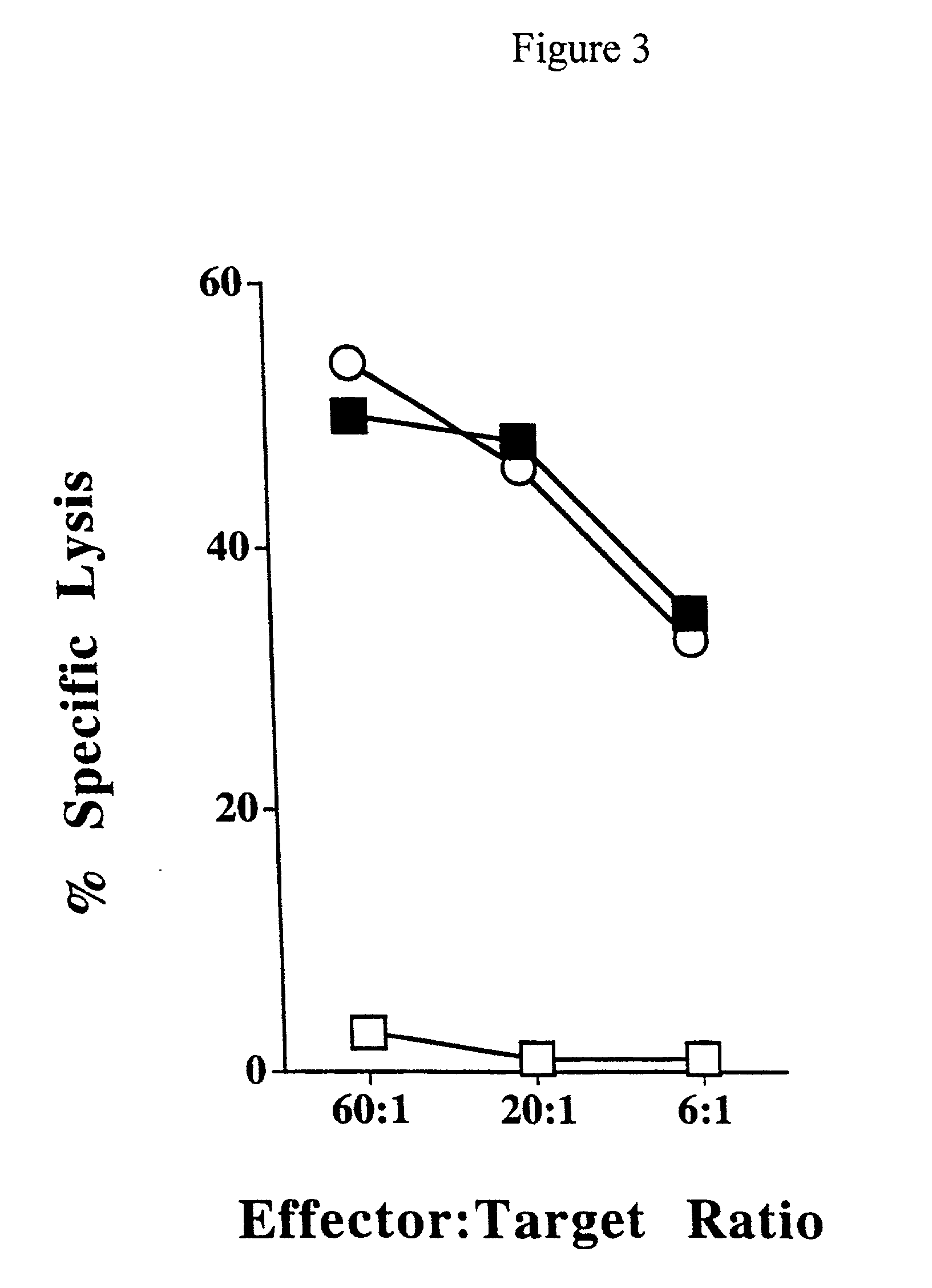
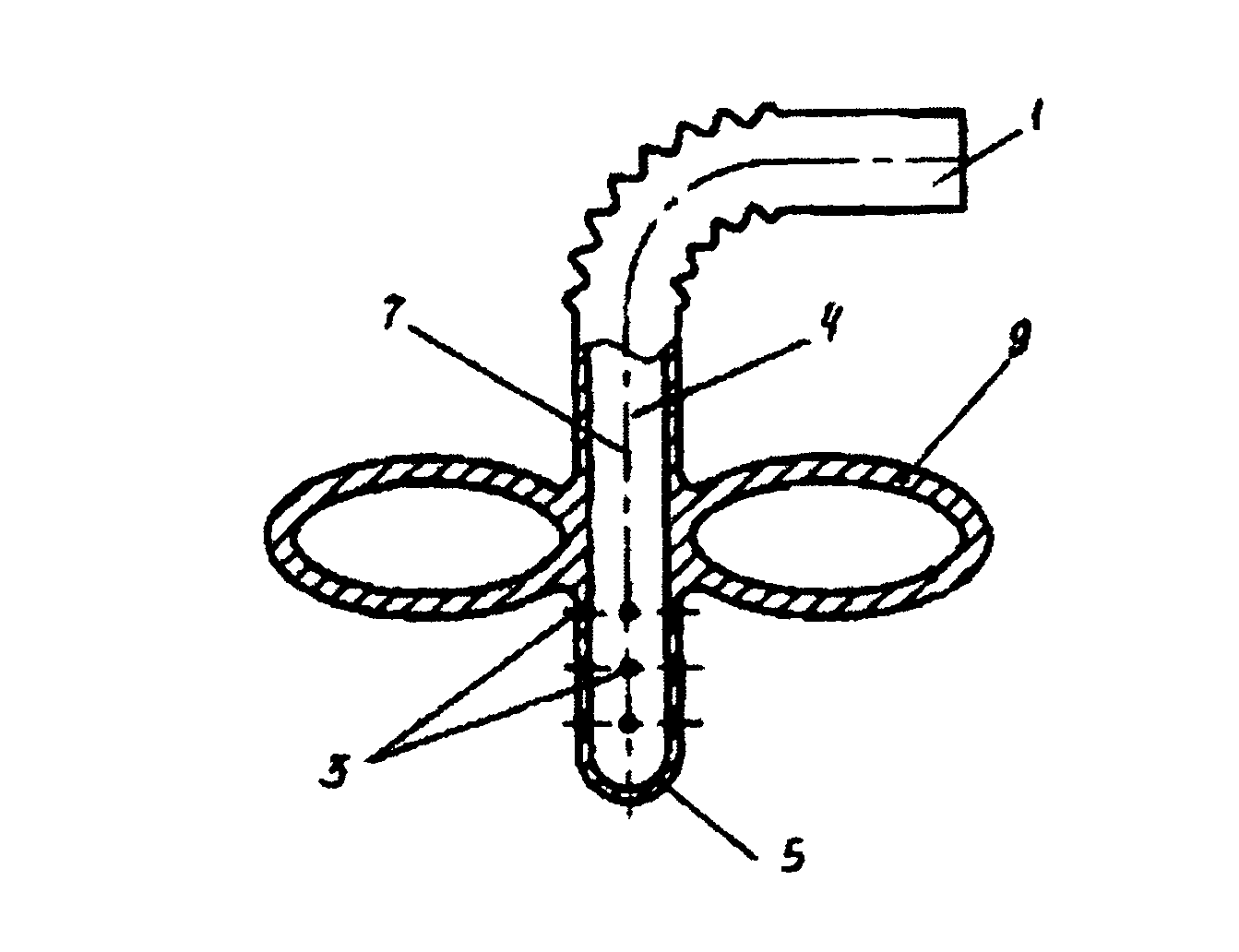
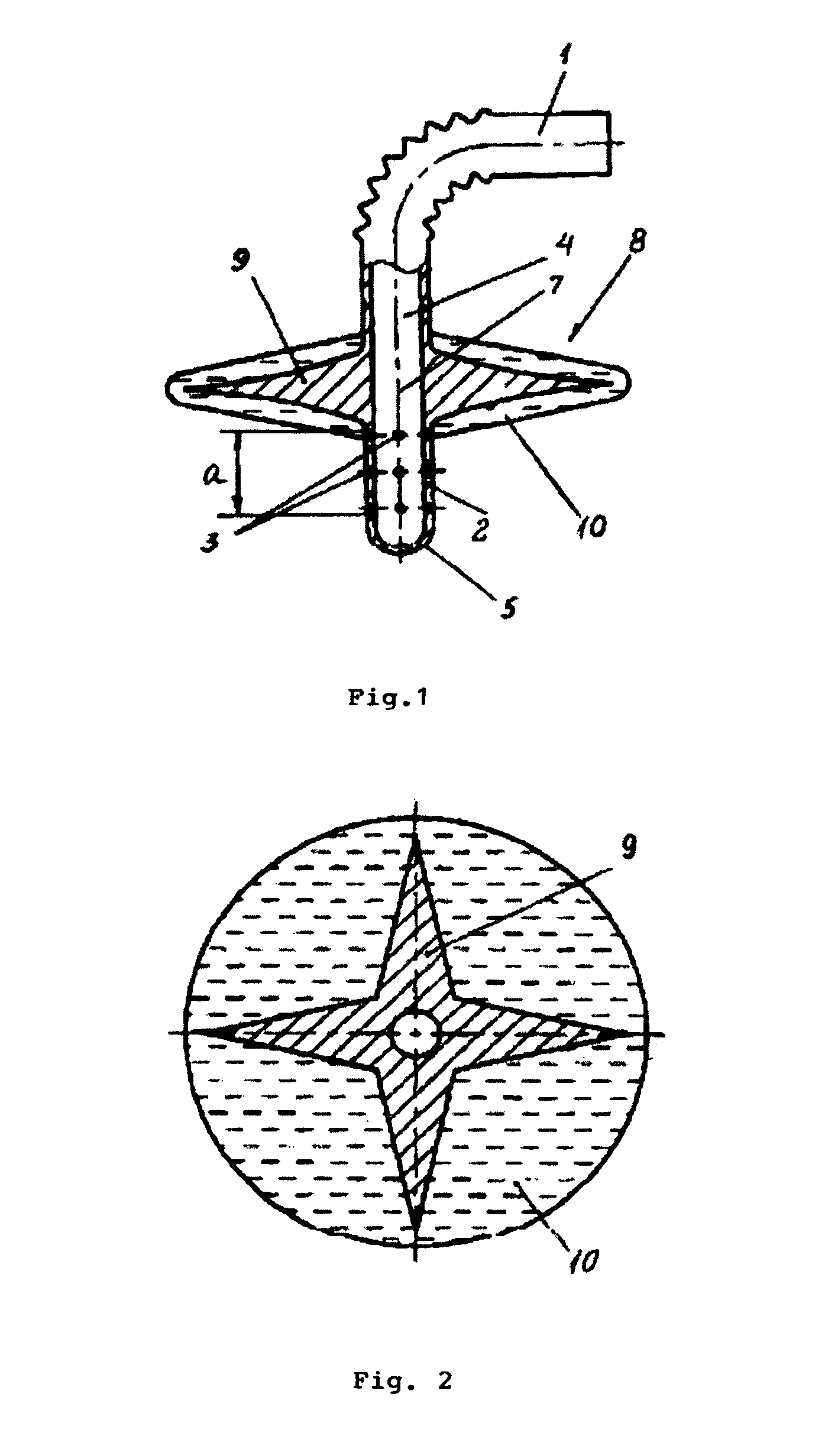
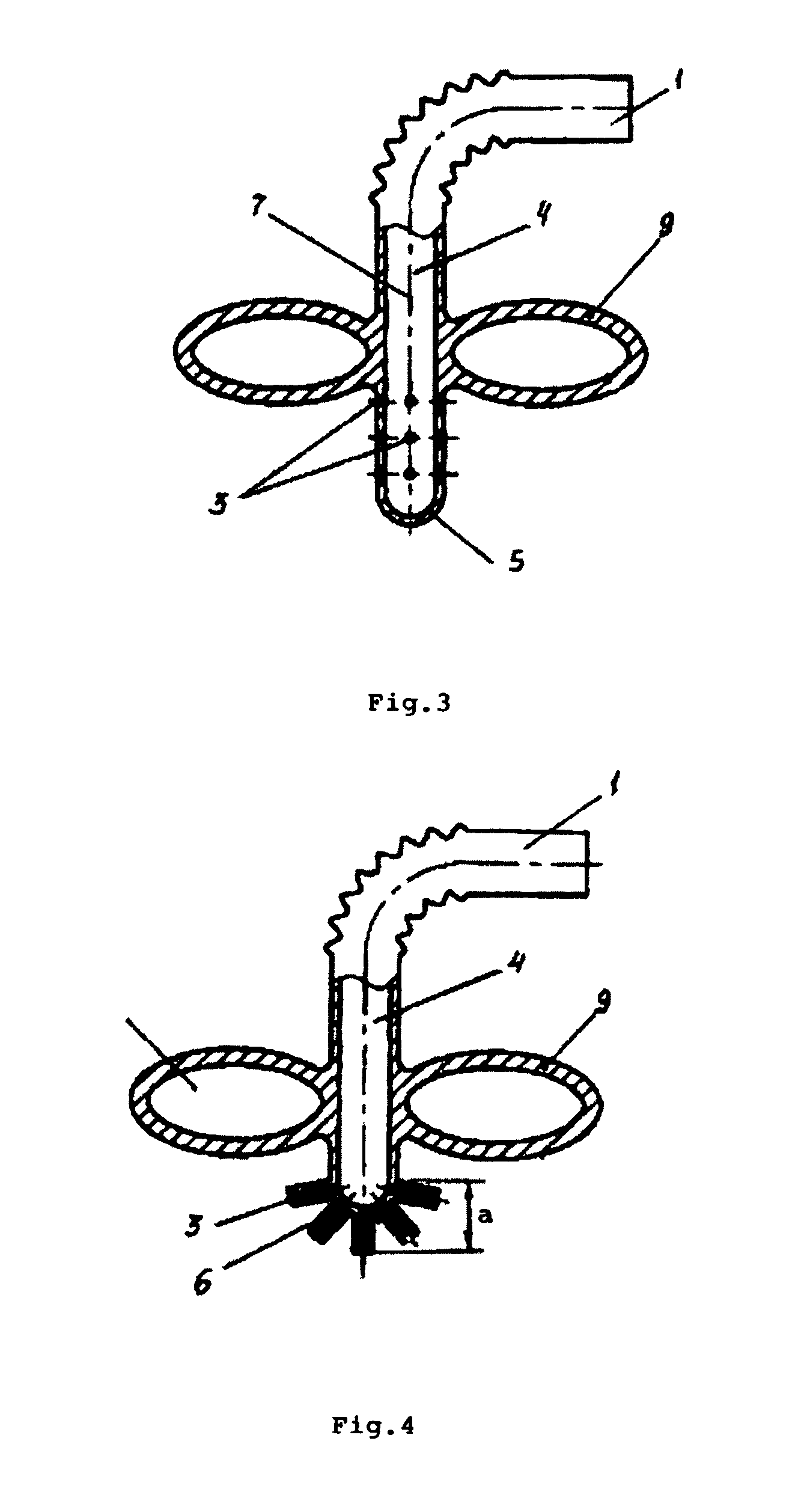
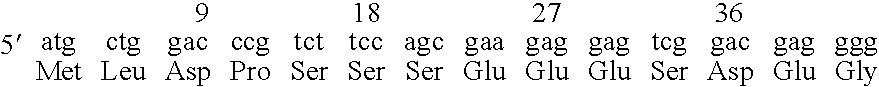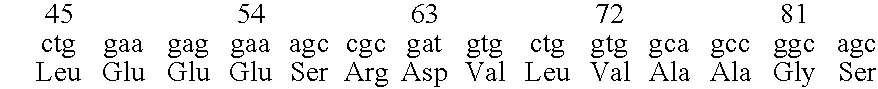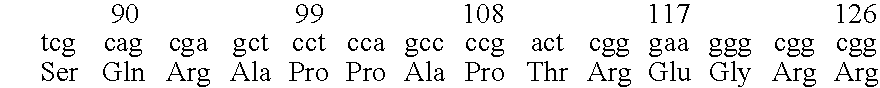Patents
Literature
68 results about "S cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
S cells are cells which release secretin, found in the jejunum and duodenum. They are stimulated by a drop in pH to 4 or below in the small intestine's lumen. The released secretin will increase the secretion of bicarbonate (HCO₃⁻) into the lumen, via the pancreas. This is primarily accomplished by an increase in cyclic AMP that activates CFTR to release chloride anions into the lumen. The luminal Cl⁻ is then involved in a bicarbonate transporter protein exchange, in which the chloride is reabsorbed by the cell and HCO₃⁻ is secreted into the lumen. S cells are also one of the main producers of cyclosamatin.
Neural regeneration peptides and methods for their use in treatment of brain damage
InactiveUS7563862B2High expressionEasy SurvivalPeptide/protein ingredientsGenetic material ingredientsNervous systemInjury brain
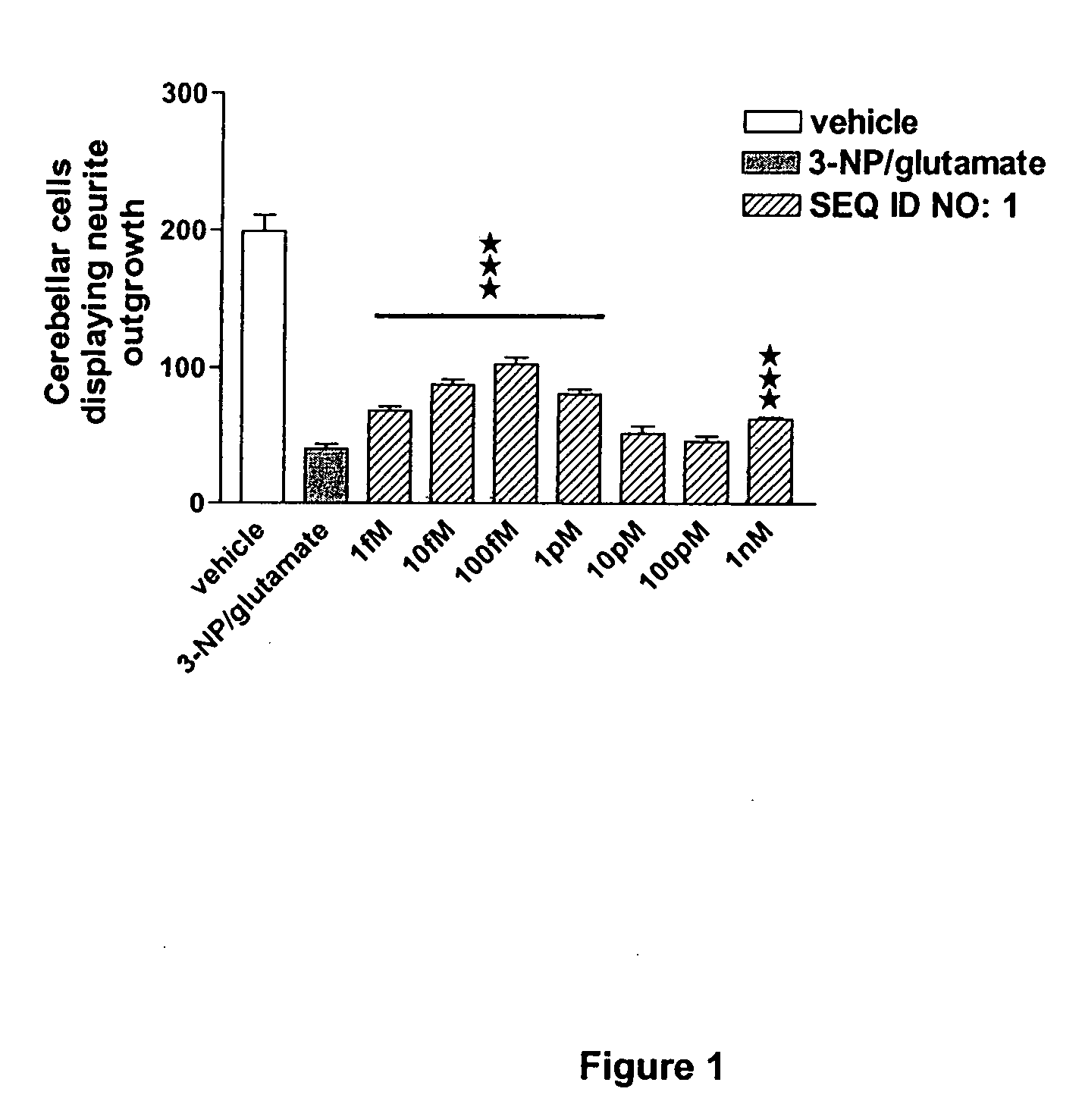
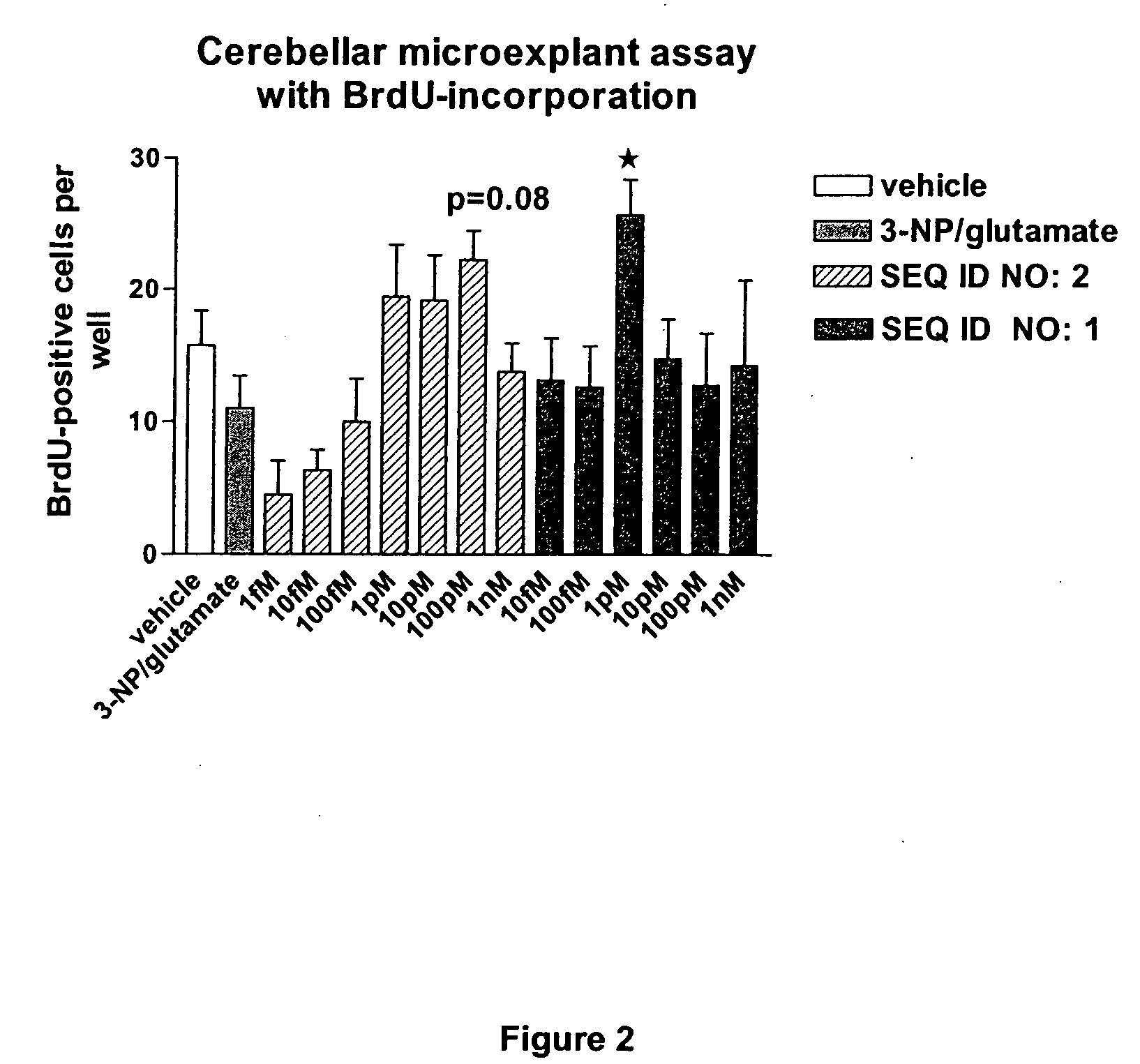
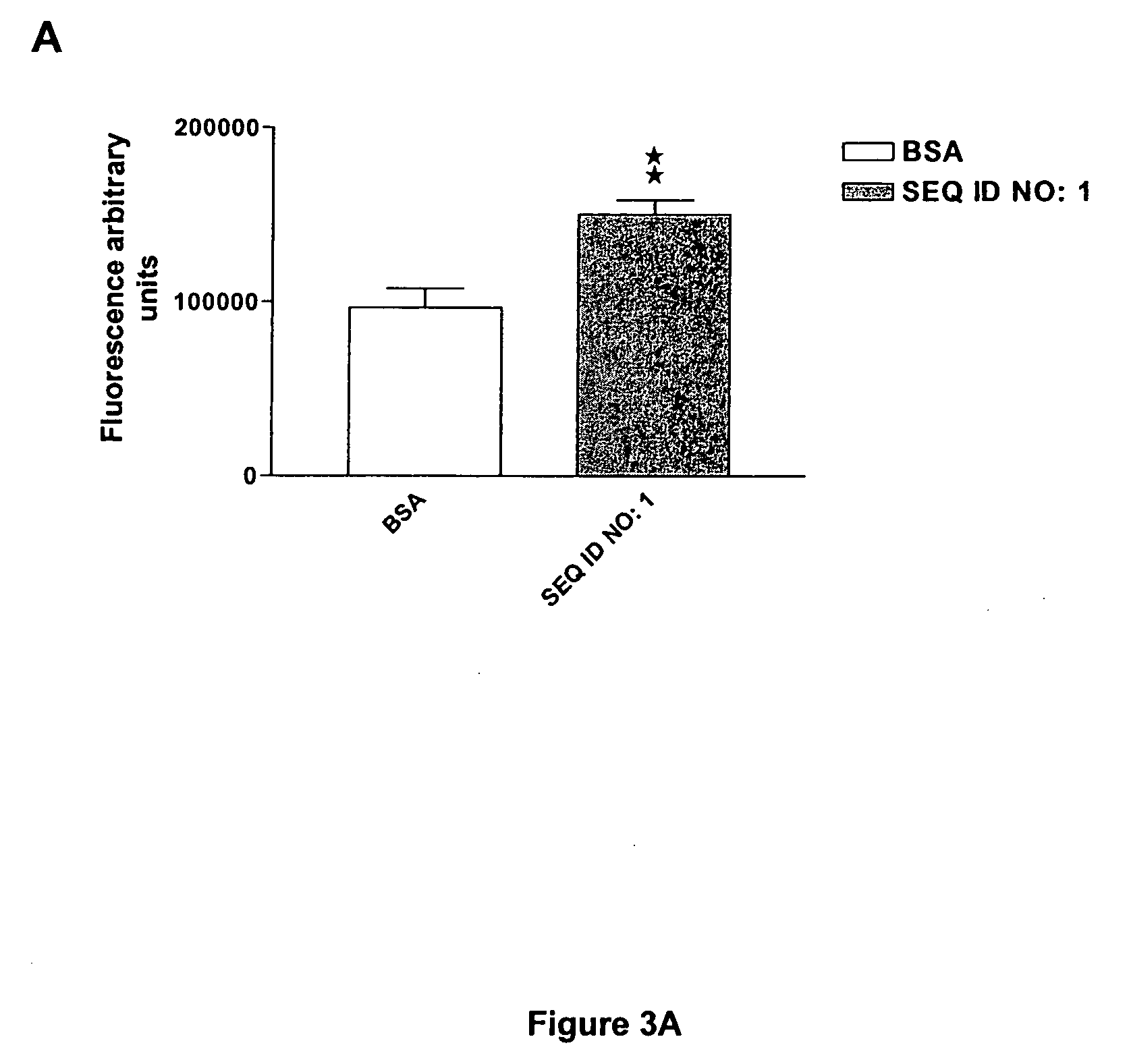
The invention discloses a family of peptides termed NRP compounds or NRPs that can promote neuronal migration, neurite outgrowth, neuronal proliferation, neural differentiation and / or neuronal survival, and provides compositions and methods for the use of NRPs in the treatment of brain injury and neurodegenerative disease. NRP compounds can induce neurons and neuroblasts to proliferate and migrate into areas of damage caused by acute brain injury or chronic neurodegenerative disease, such as exposure to toxins, stroke, trauma, nervous system infections, demyelinating diseases, dementias, and metabolic disorders. NRP compounds may be administered directly to a subject or to a subject's cells by a variety of means including orally, intraperitoneally, intravascularly, and directly into the nervous system of a patient. NRP compounds can be formulated into pharmaceutically acceptable dose forms for therapeutic use. Methods for detecting neural regeneration, neural proliferation, neural differentiation, neurite outgrowth and neural survival can be used to develop other neurally active agents.
Owner:CURONZ HLDG
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
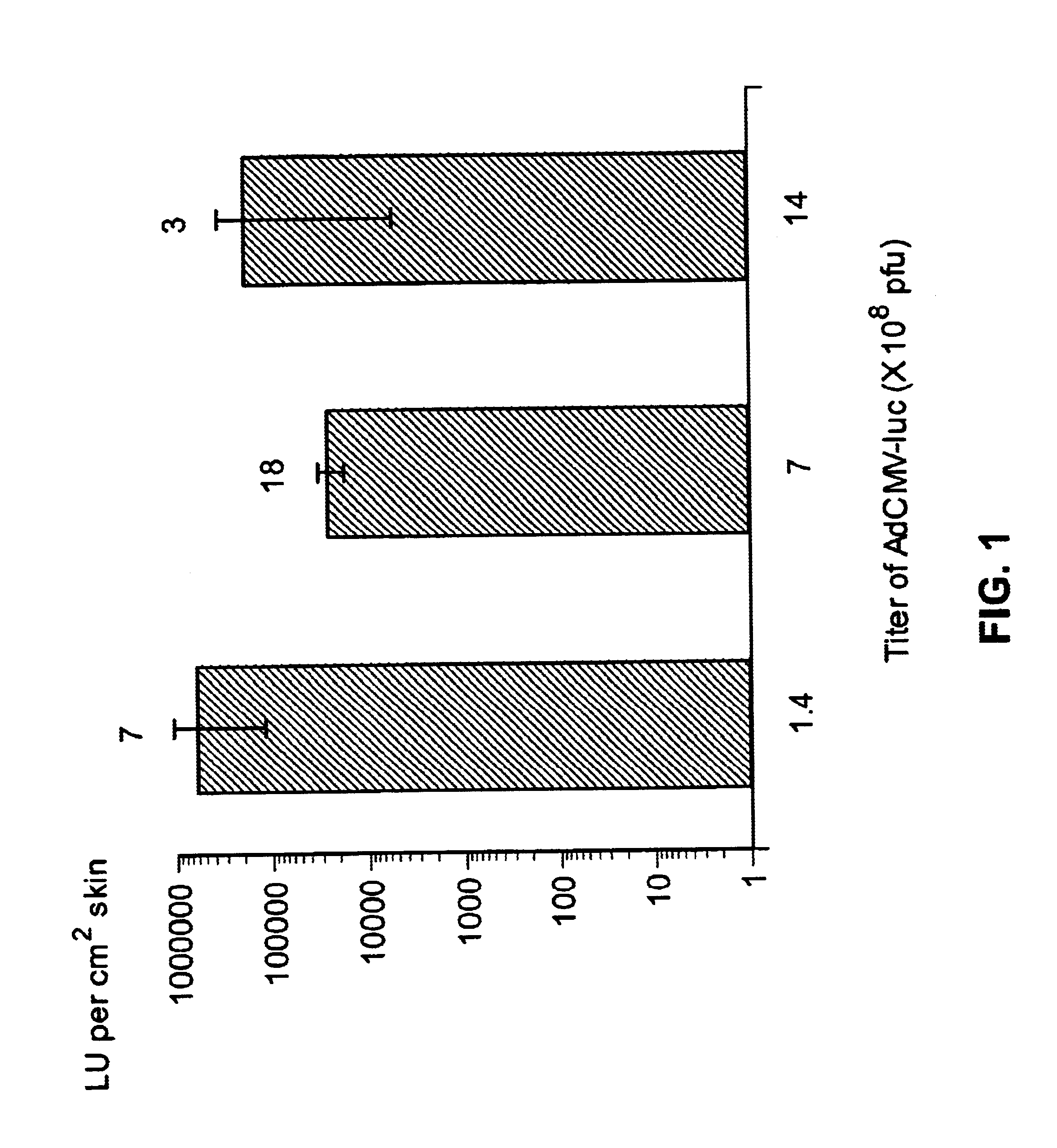
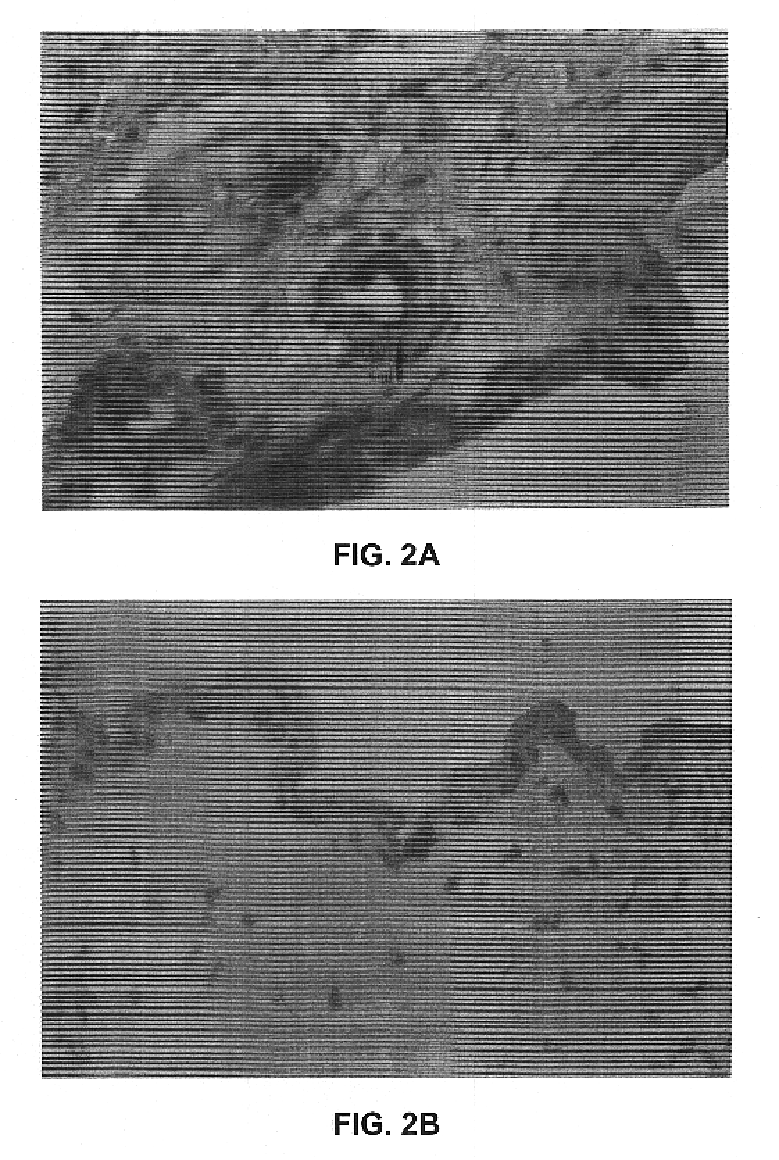
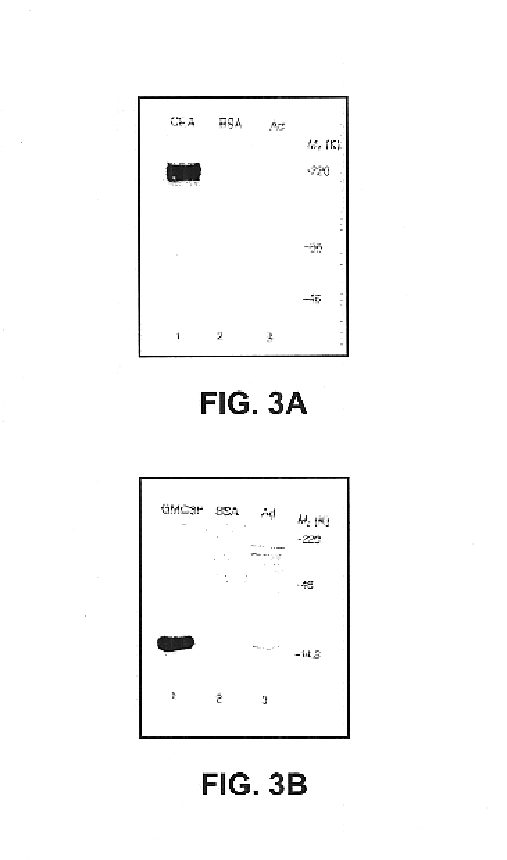
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Generation of human embryonc stem-like cells using intronic RNA
ActiveUS20080293143A1Stable and relatively long-term effectDelivery stabilityOther foreign material introduction processesElectrical/wave energy microorganism treatmentReprogrammingMammal
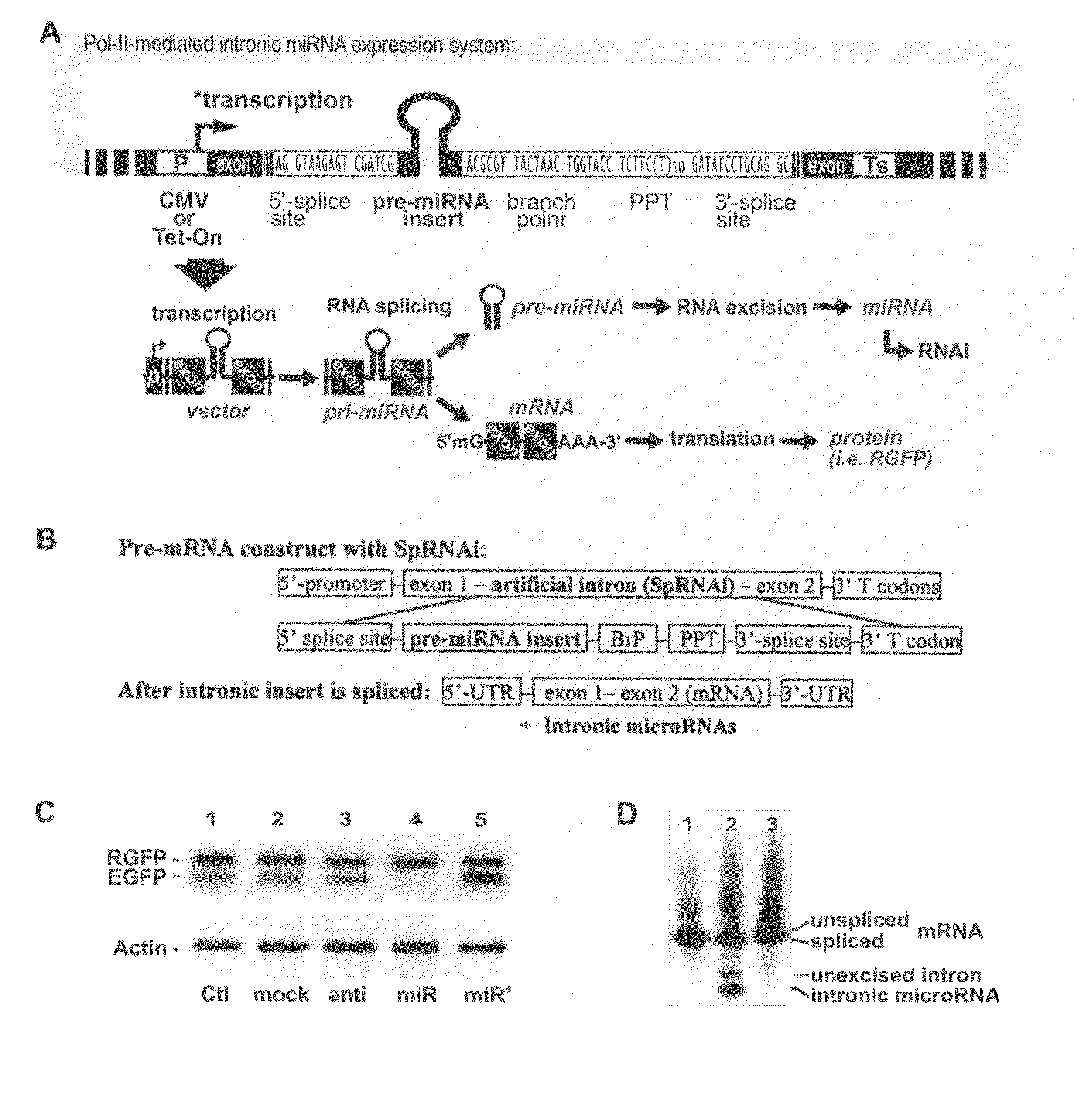
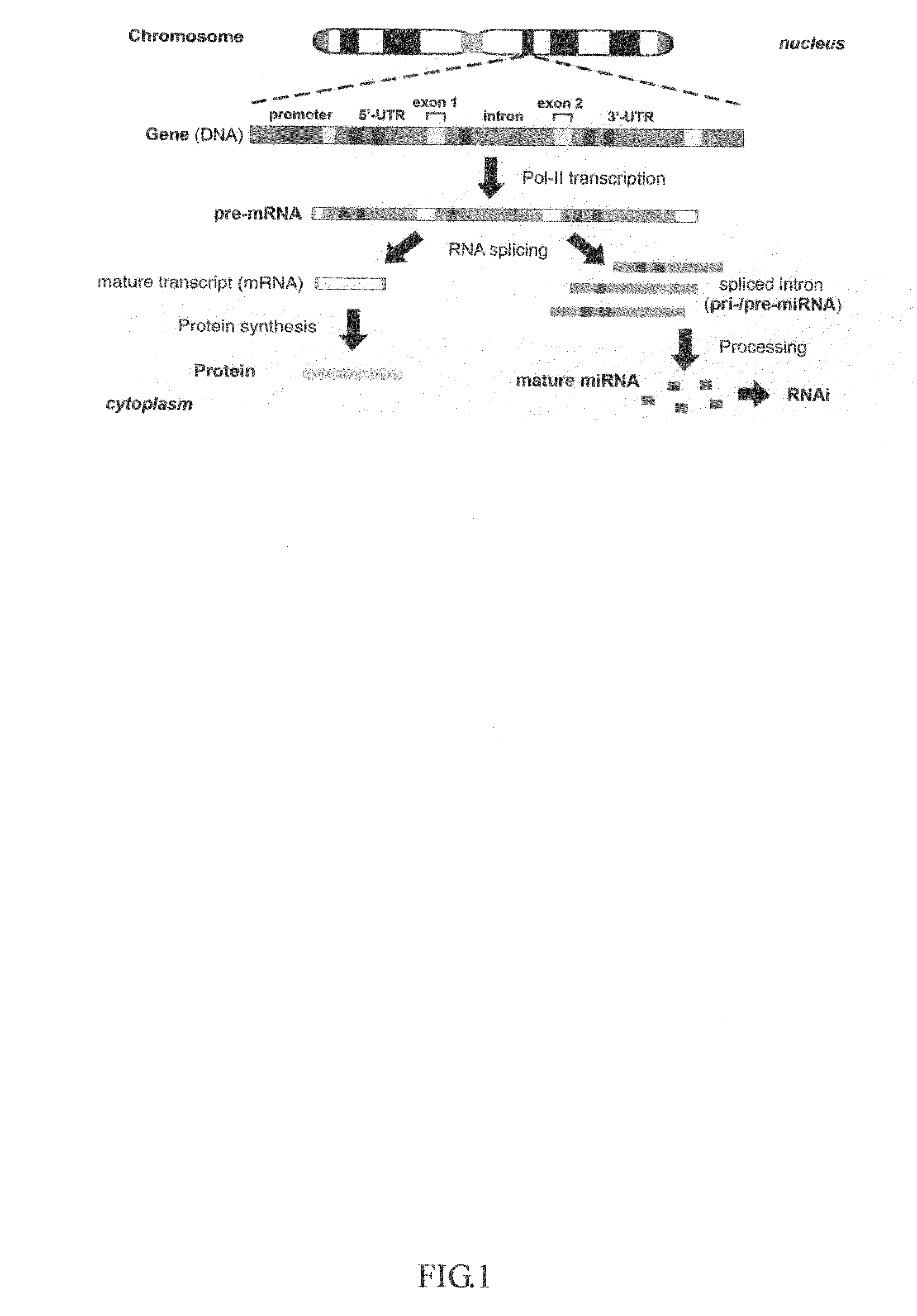
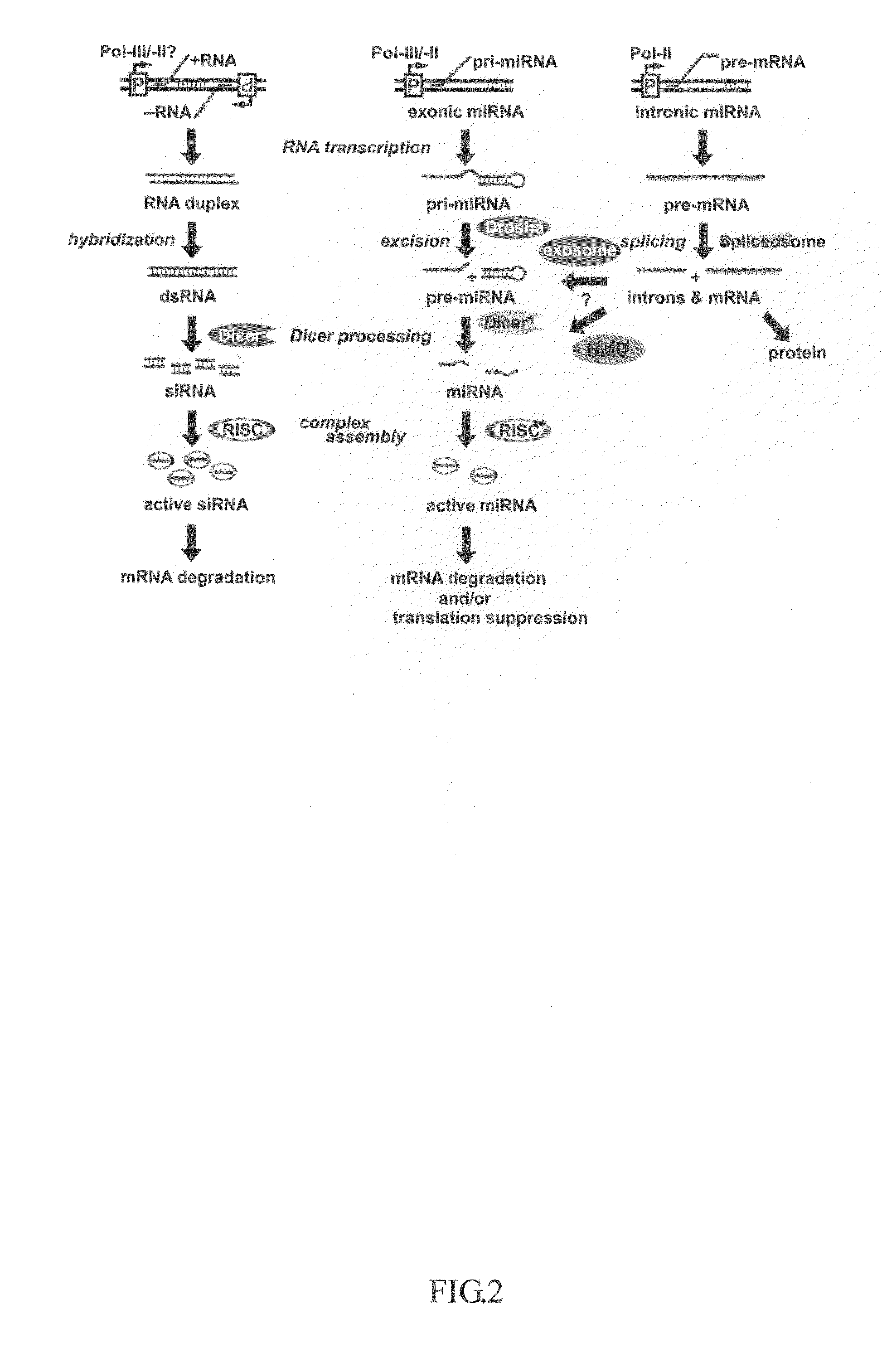
This invention generally relates to a method for developing, generating and selecting human embryonic stem (hES)-like pluripotent cells using transgenic expression of intronic microRNA-like RNA agents. More particularly, the present invention relates to a method and composition for generating a non-naturally occurring intron and its intronic components capable of being processed into mir-302-like RNA molecules in mammalian cells and thus inducing certain specific gene silencing effects on differentiation-related and fate-determinant genes of the cells, resulting in reprogramming the cells into a pluripotent embryonic stem (ES)-cell-like state. The ES-like cells so obtained are strongly express hES cell markers, such as Oct3 / 4, SSEA-3 and SSEA-4, and can be guided into various tissue cell types by treating certain hormones and / or growth factors under a feeder-free cell culture condition in vitro, which may be used for transplantation and gene therapies. Therefore, the present invention offers a simple, effective and safe gene manipulation approach for not only reprogramming somatic cells into ES-like pluripotent cells but also facilitating the maintenance of pluripotent and renewal properties of ES cells under a feeder-free cell culture condition, preventing the tedious retroviral insertion of four large transcription factor genes into one single cell as used in the previous iPS methods.
Owner:MELLO BIOTECH +1
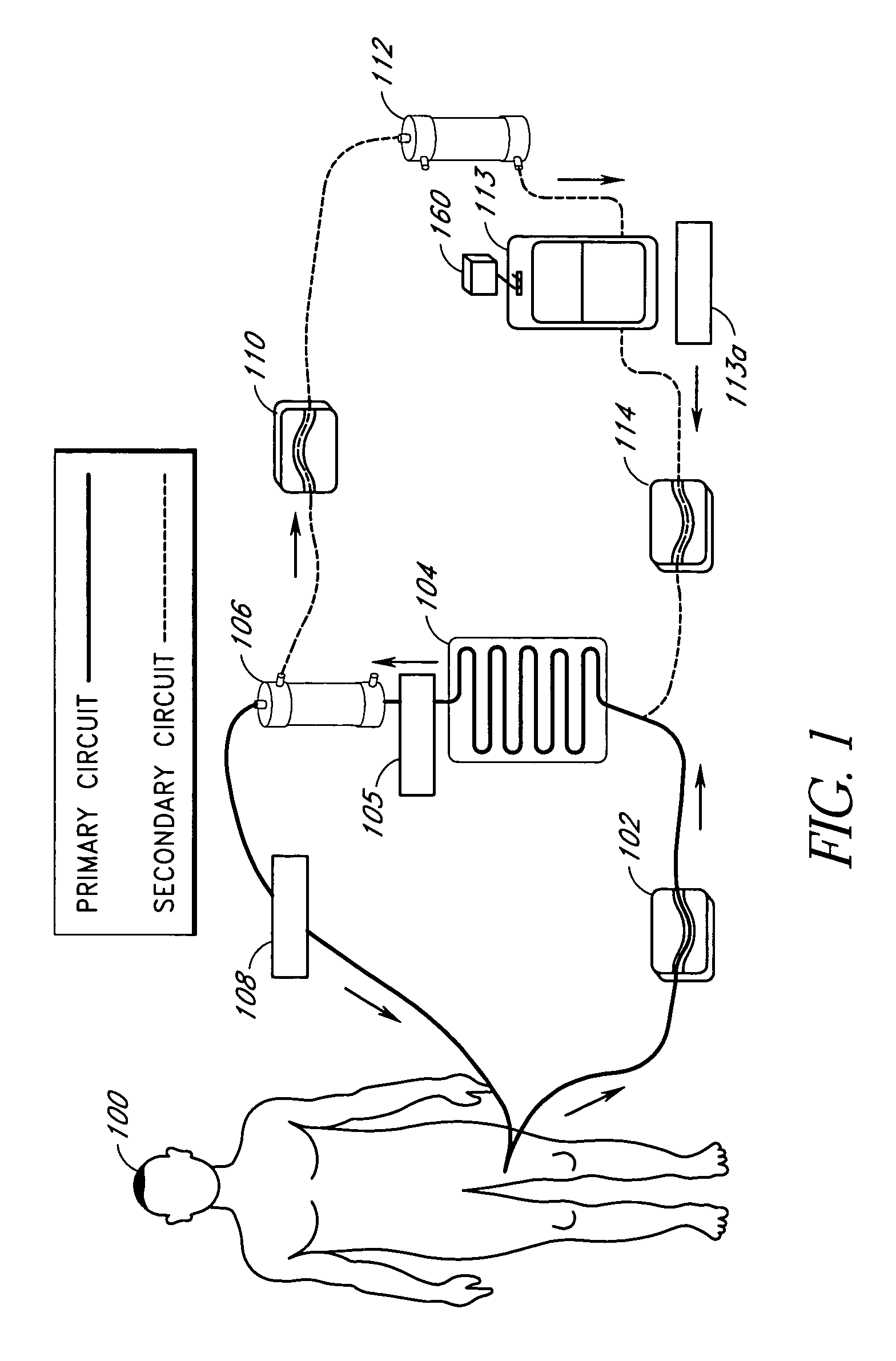
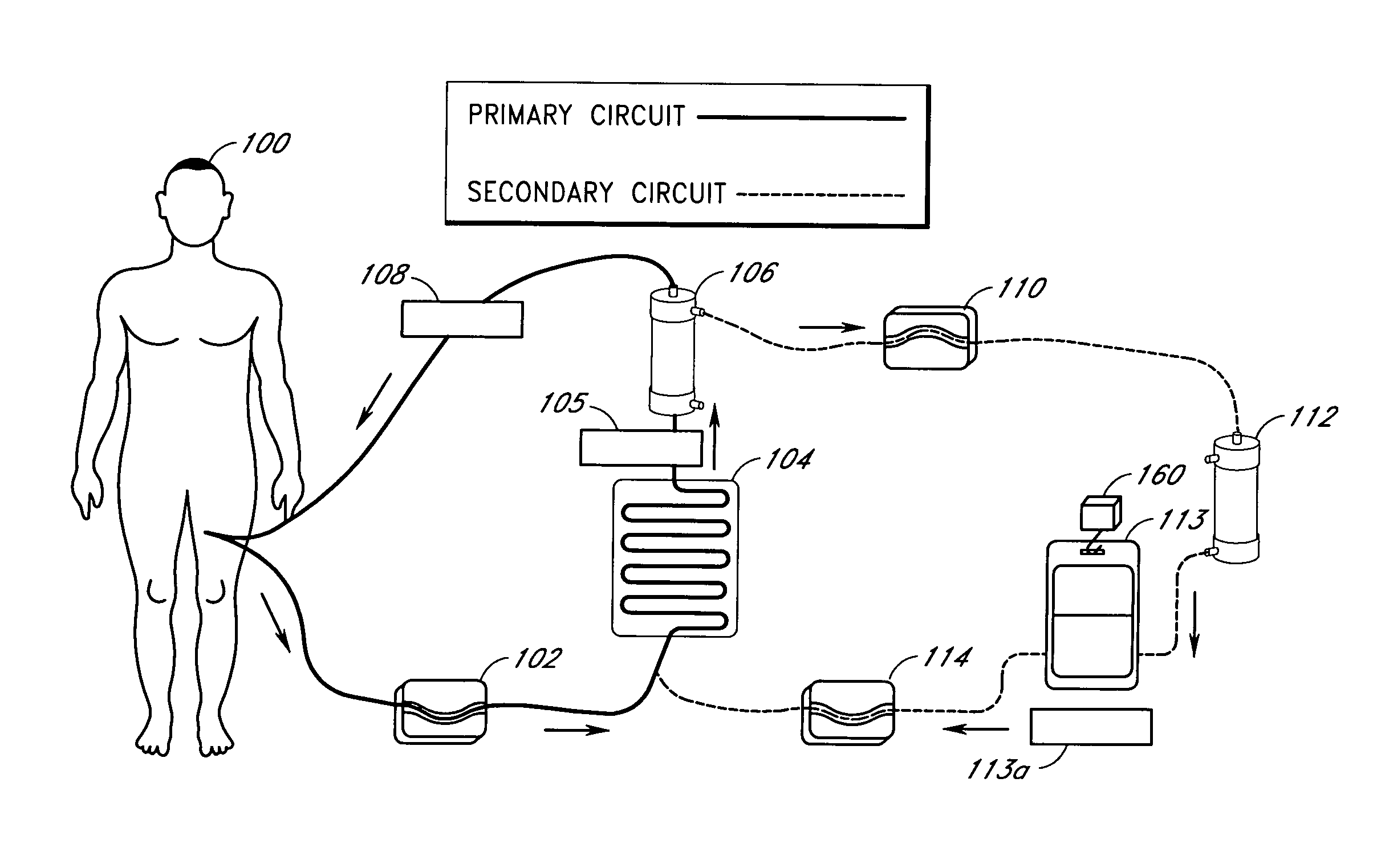
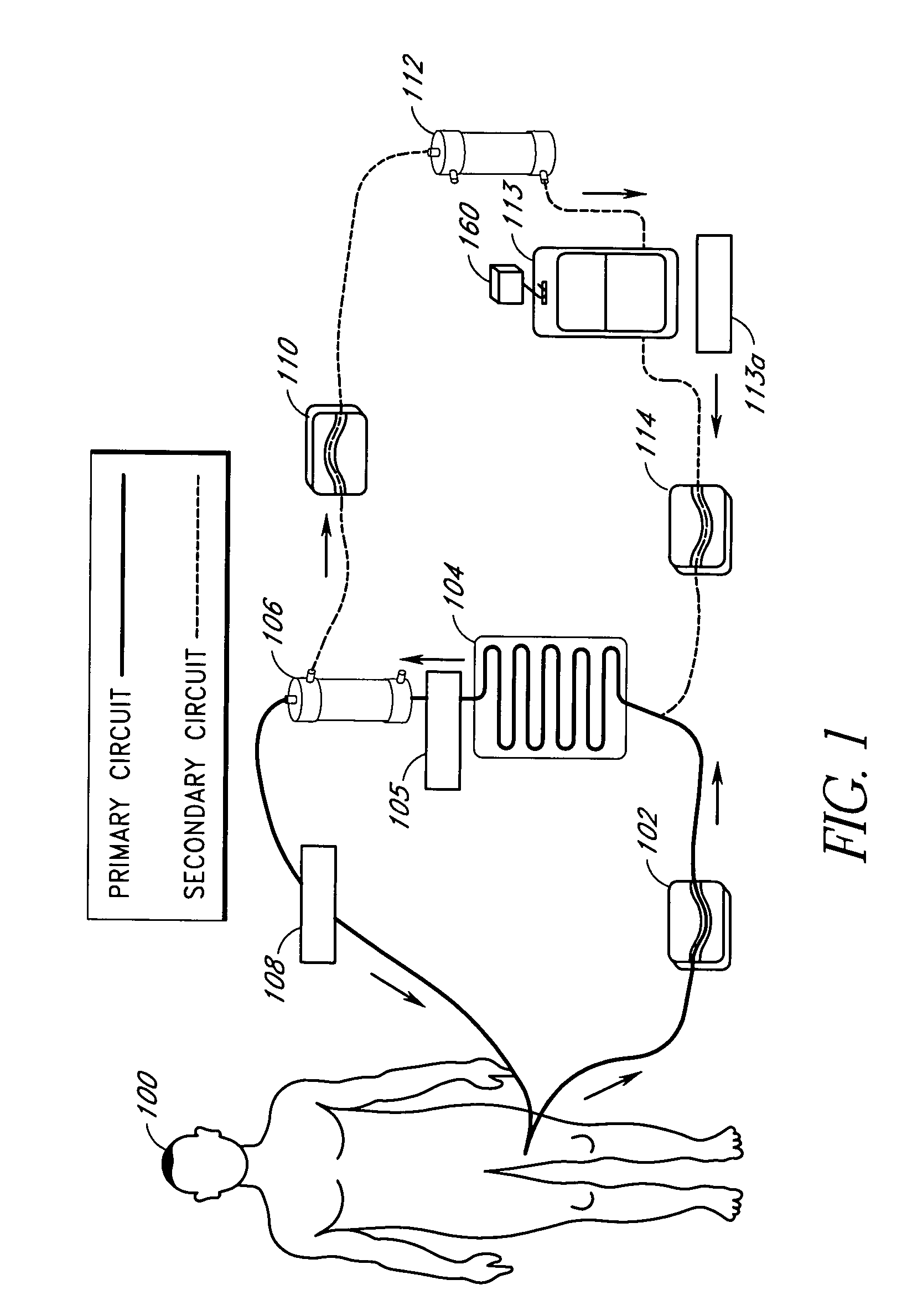
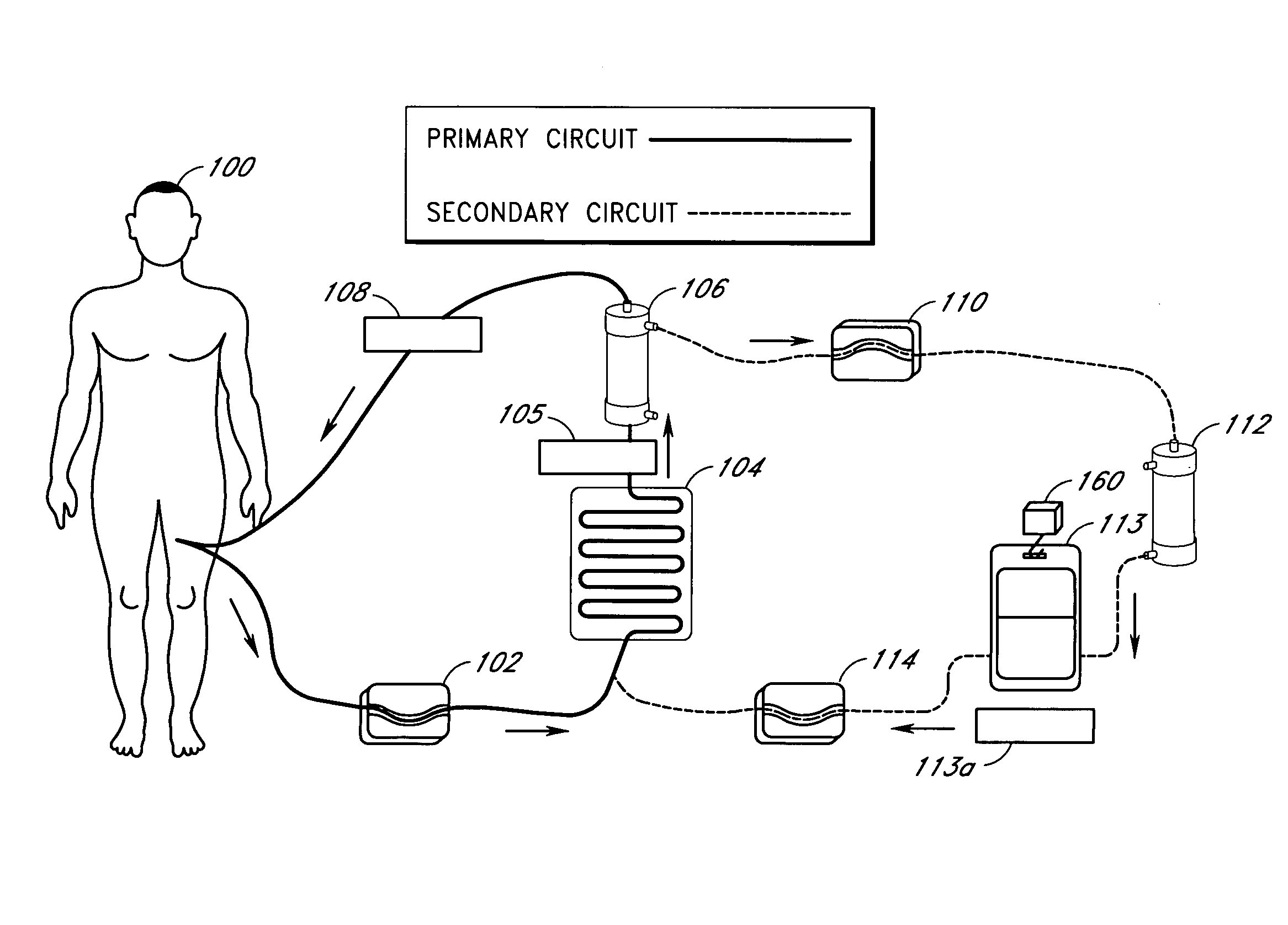
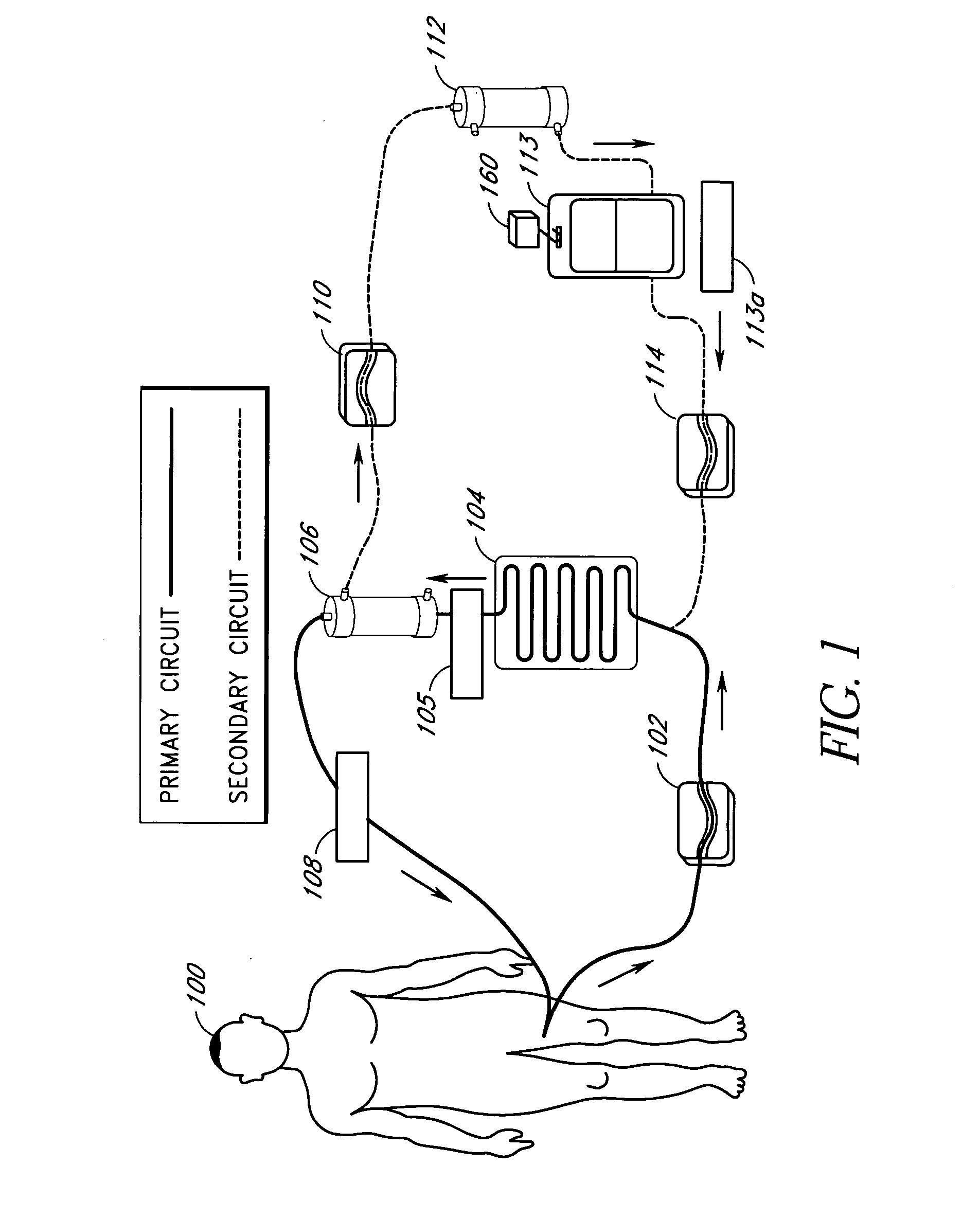
Septicemia prevention and treatment system
InactiveUS6193681B1Electrolysis componentsOther blood circulation devicesStaphylococcus cohniiFiltration
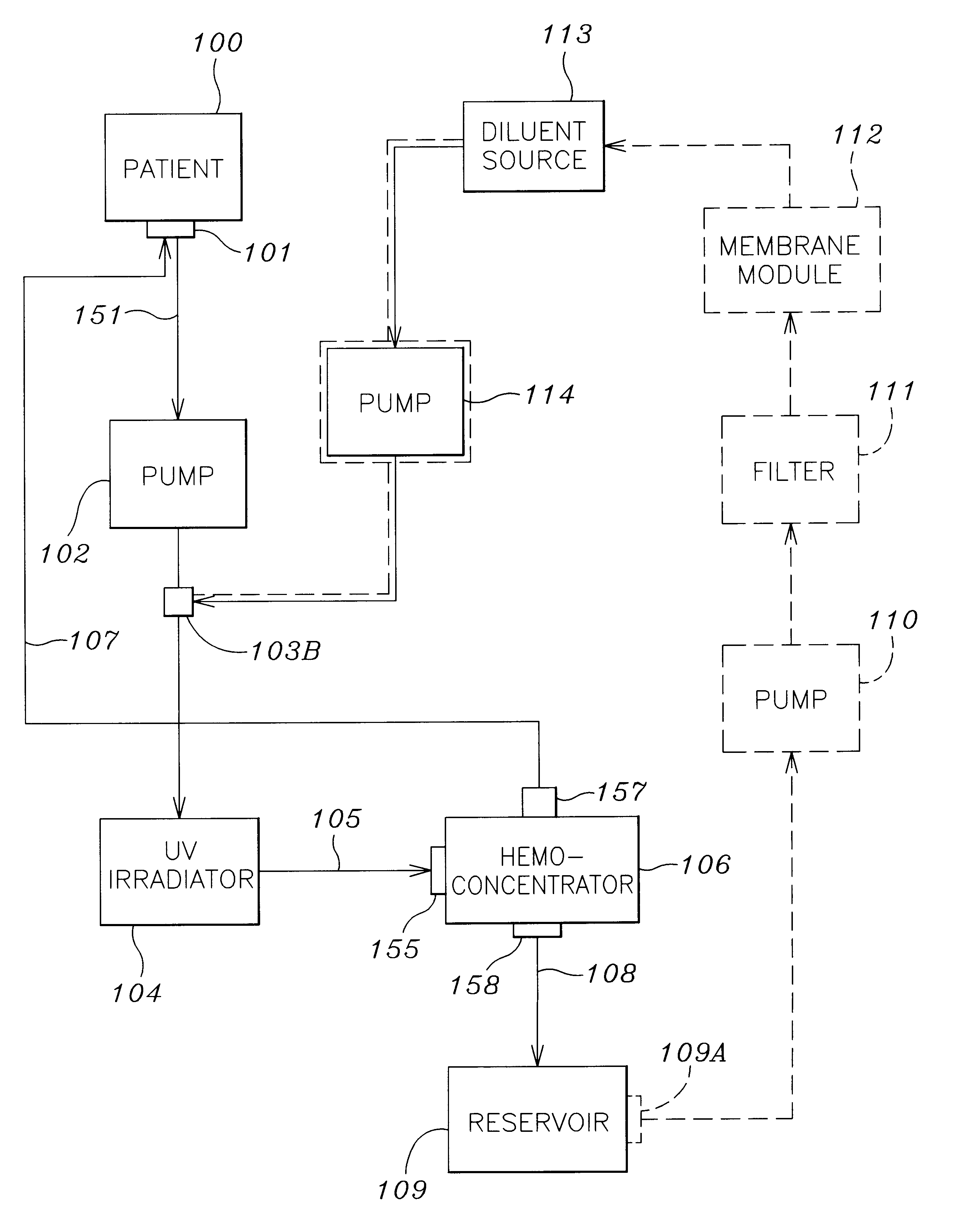
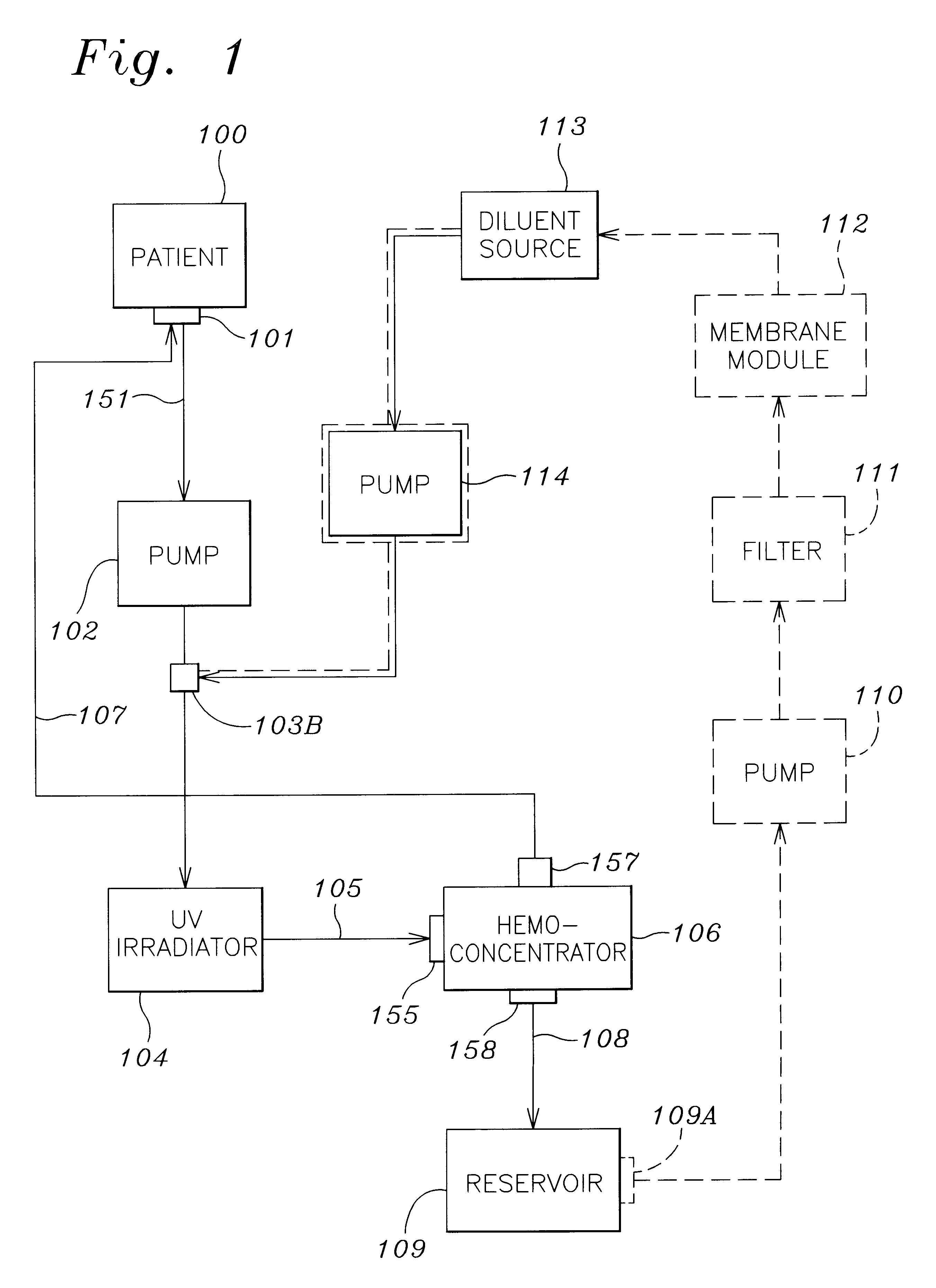
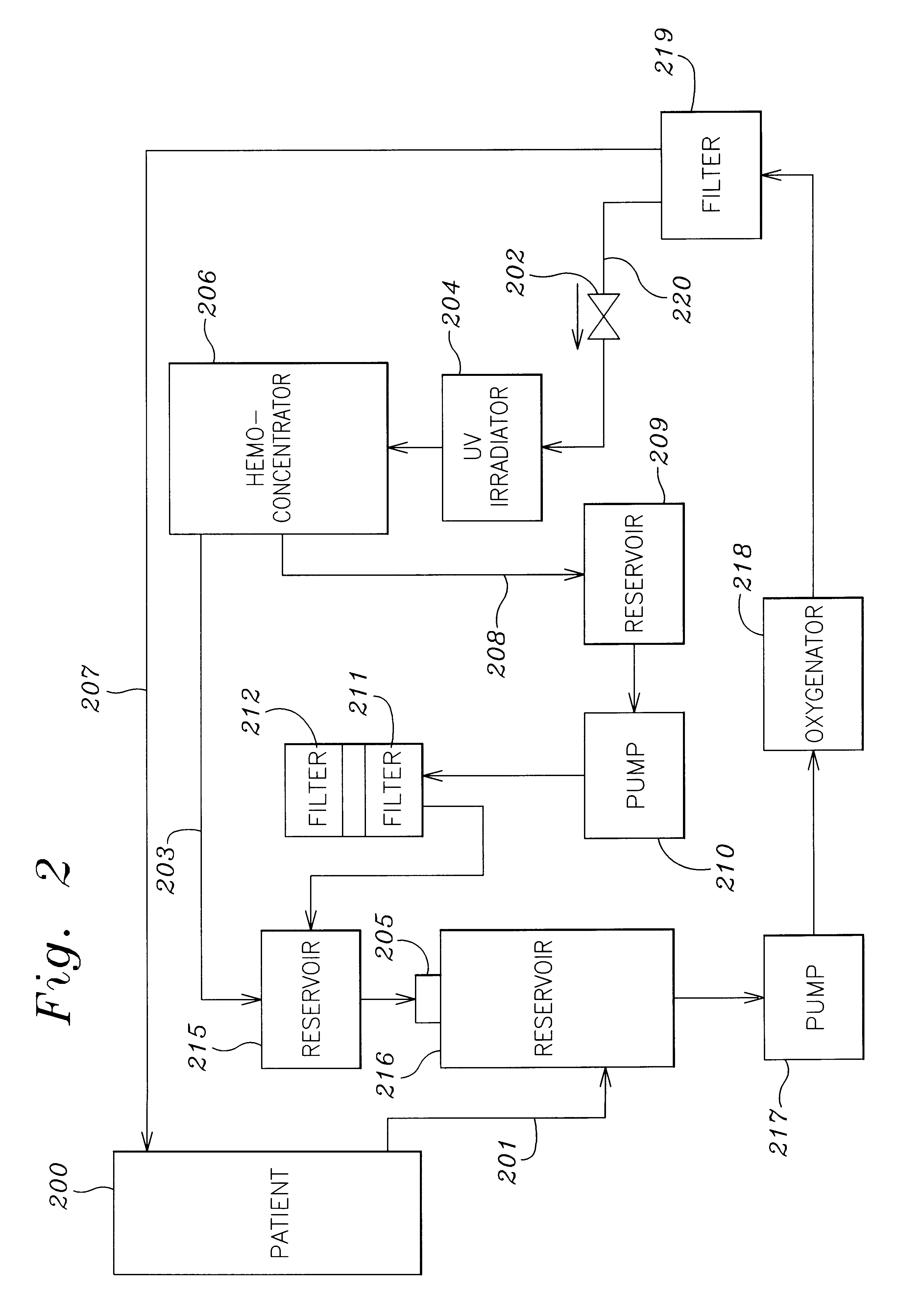
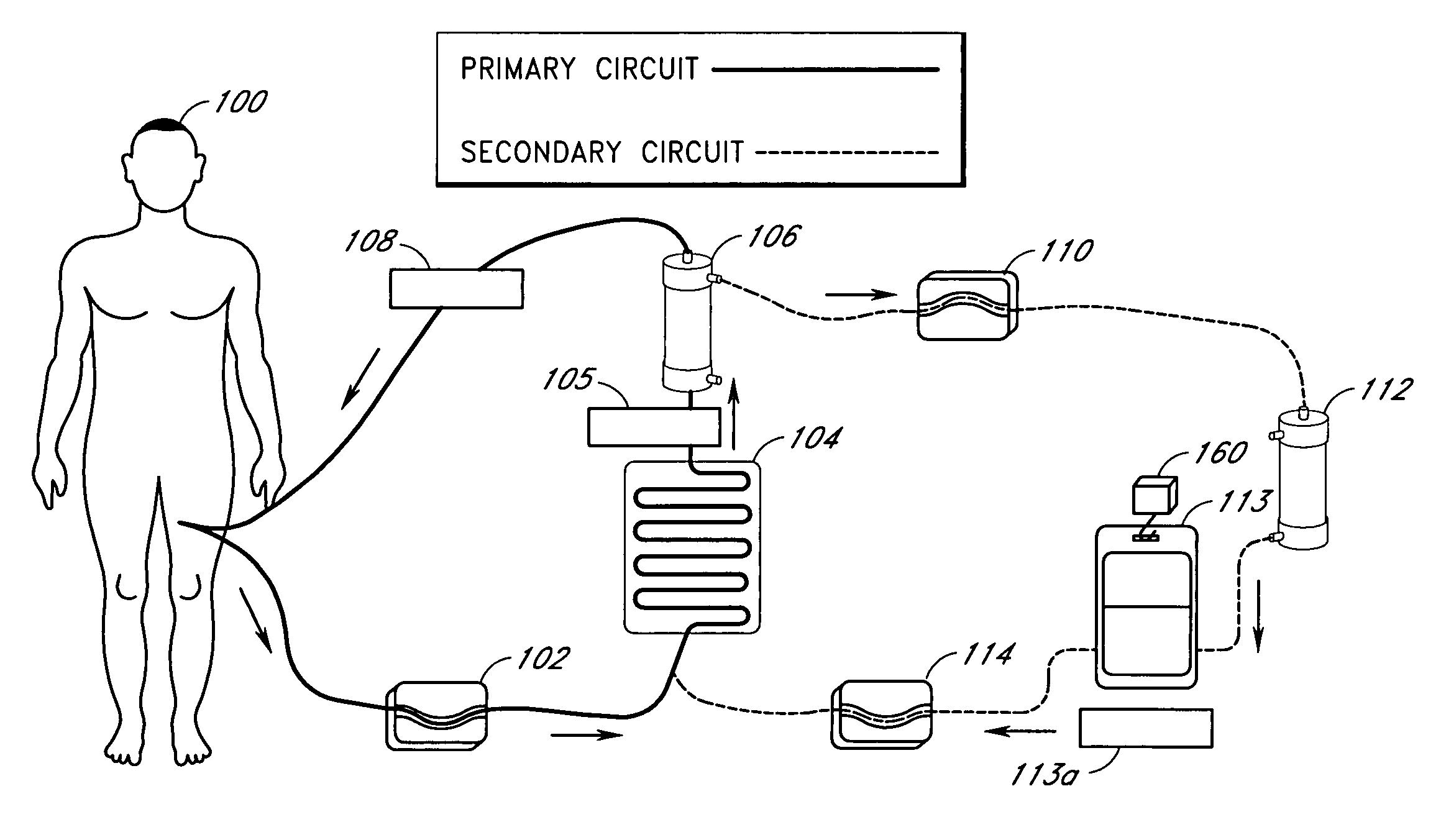
A method and apparatus for preventing and treating septicemia in patient blood. The extracorporeal system includes an anti-microbial device to kill at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 90% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms as well as the patient's cells and include endotoxins from gram negative bacteria, exotoxins from gram negative and gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, complement proteins C3a and C5a, and brandykinin.
Owner:HEMAVATION
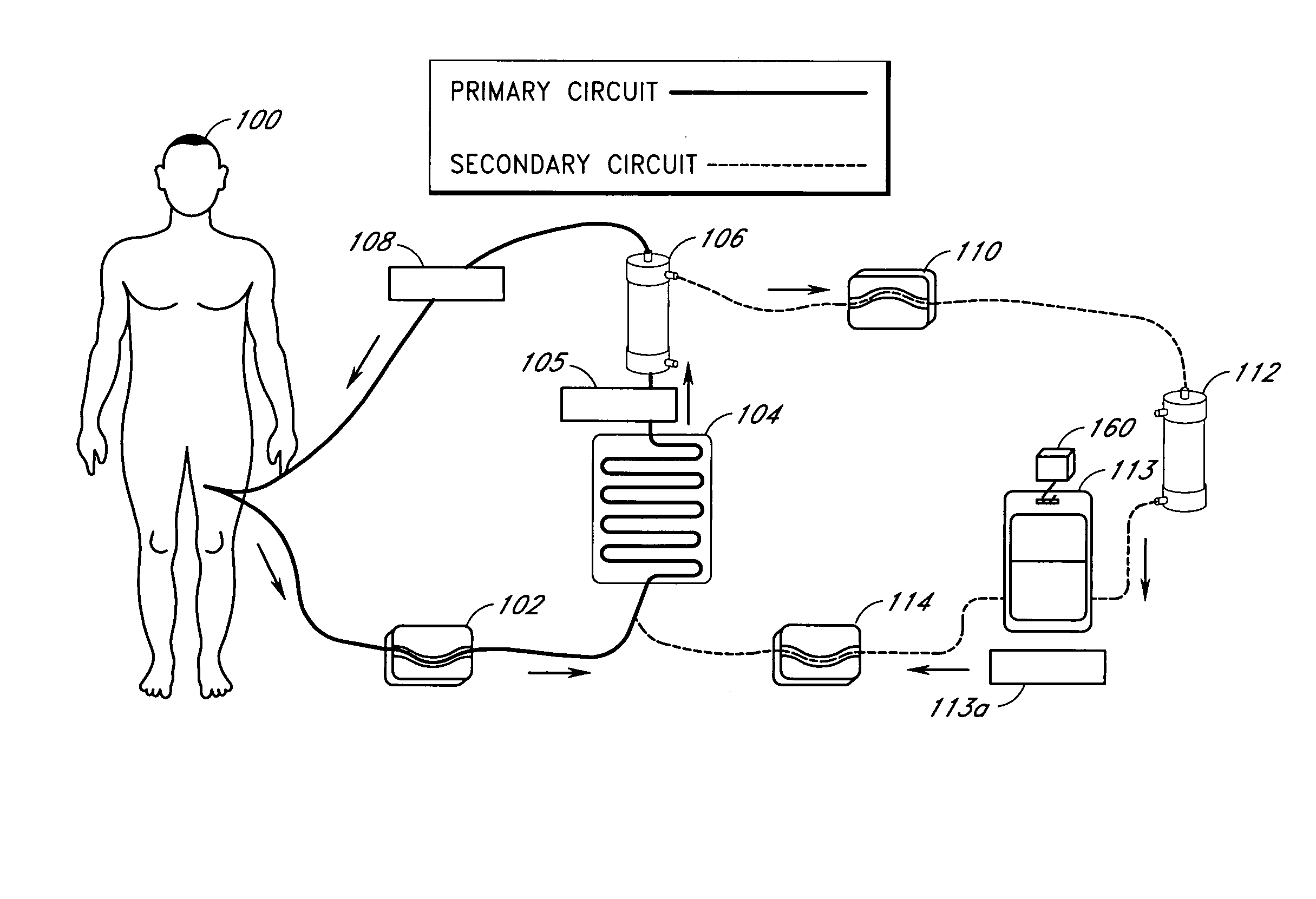
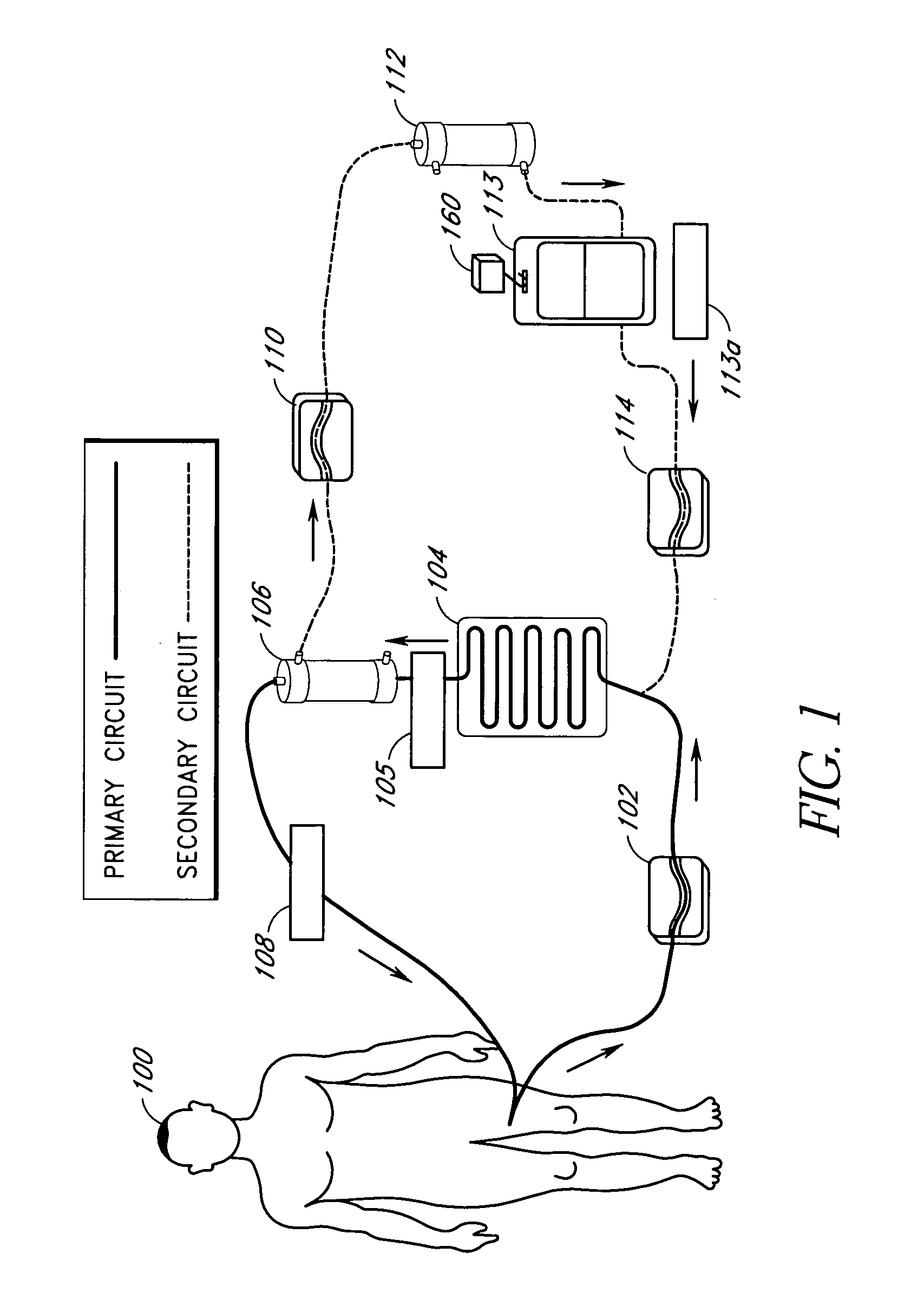
Device and method for reducing inflammatory mediators in blood
InactiveUS7201730B2Reducing free radicals in a patient's bloodReduce concentrationSemi-permeable membranesSolvent extractionInterleukin 6Staphylococcus cohnii
A method and apparatus for preventing and treating septicemia in patient blood is provided. The extracorporeal system includes an antimicrobial device to inactivate at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 50–75% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms, as well as by the patient's cells. These molecules include endotoxins from Gram negative bacteria, exotoxins from Gram negative and Gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, interleukin 6, complement proteins C3a and C5a, and bradykinin.
Owner:HEMAVATION
Cell transfection apparatus and method
InactiveUS6190380B1Transfection of cells in vivo relatively quicklyReduce amountCatheterSurgical instruments for heatingMicrowaveGenetic Materials
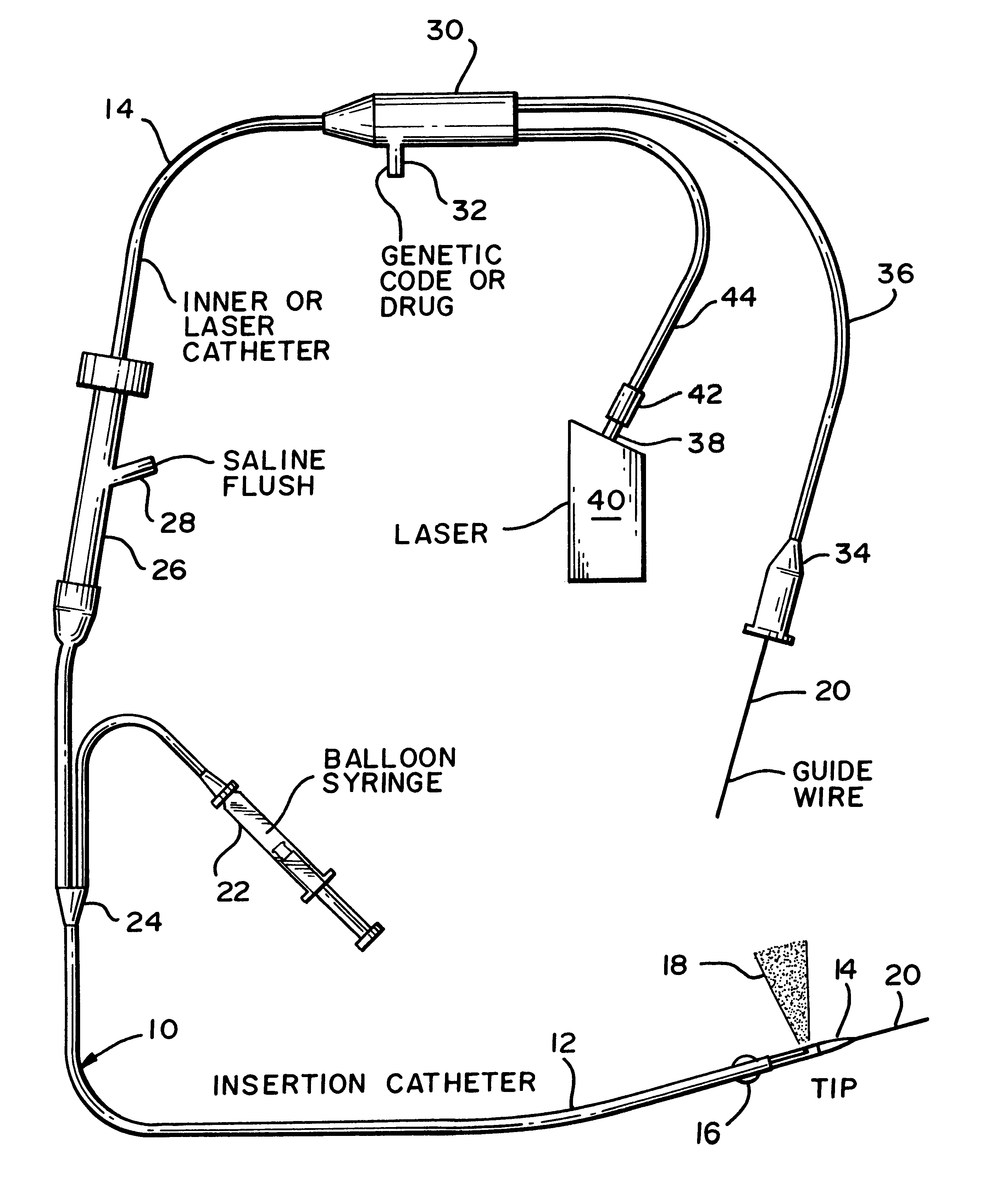
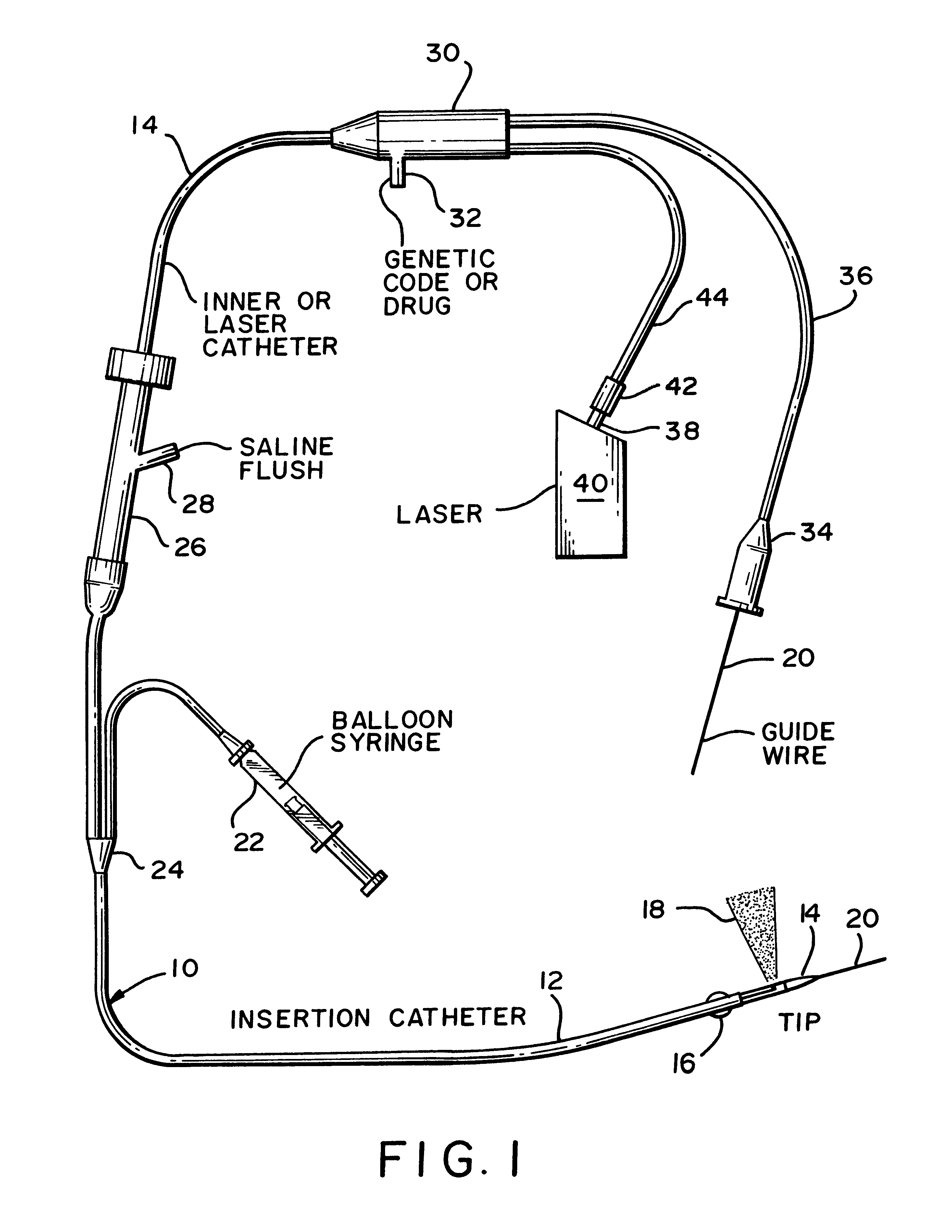
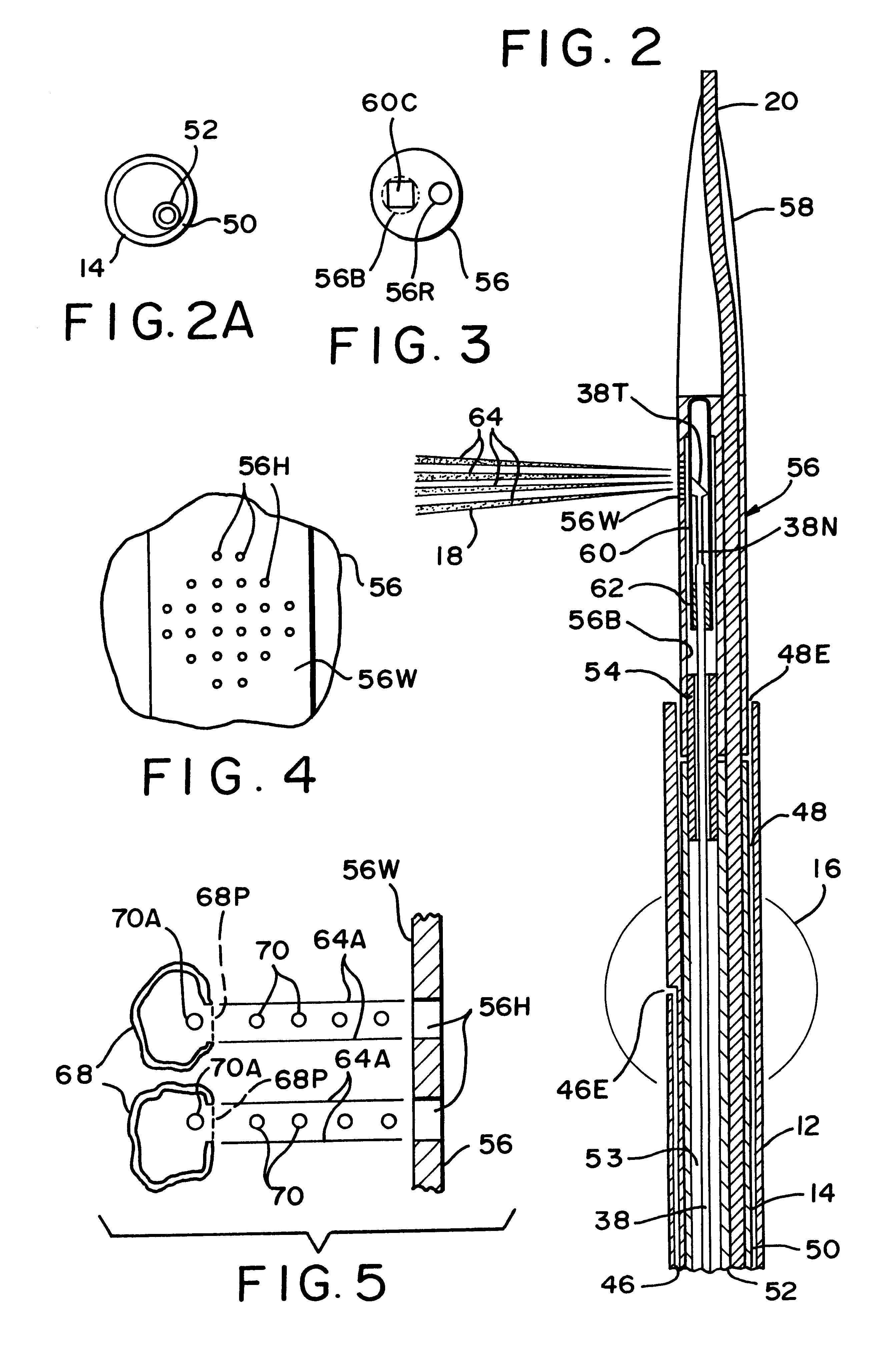
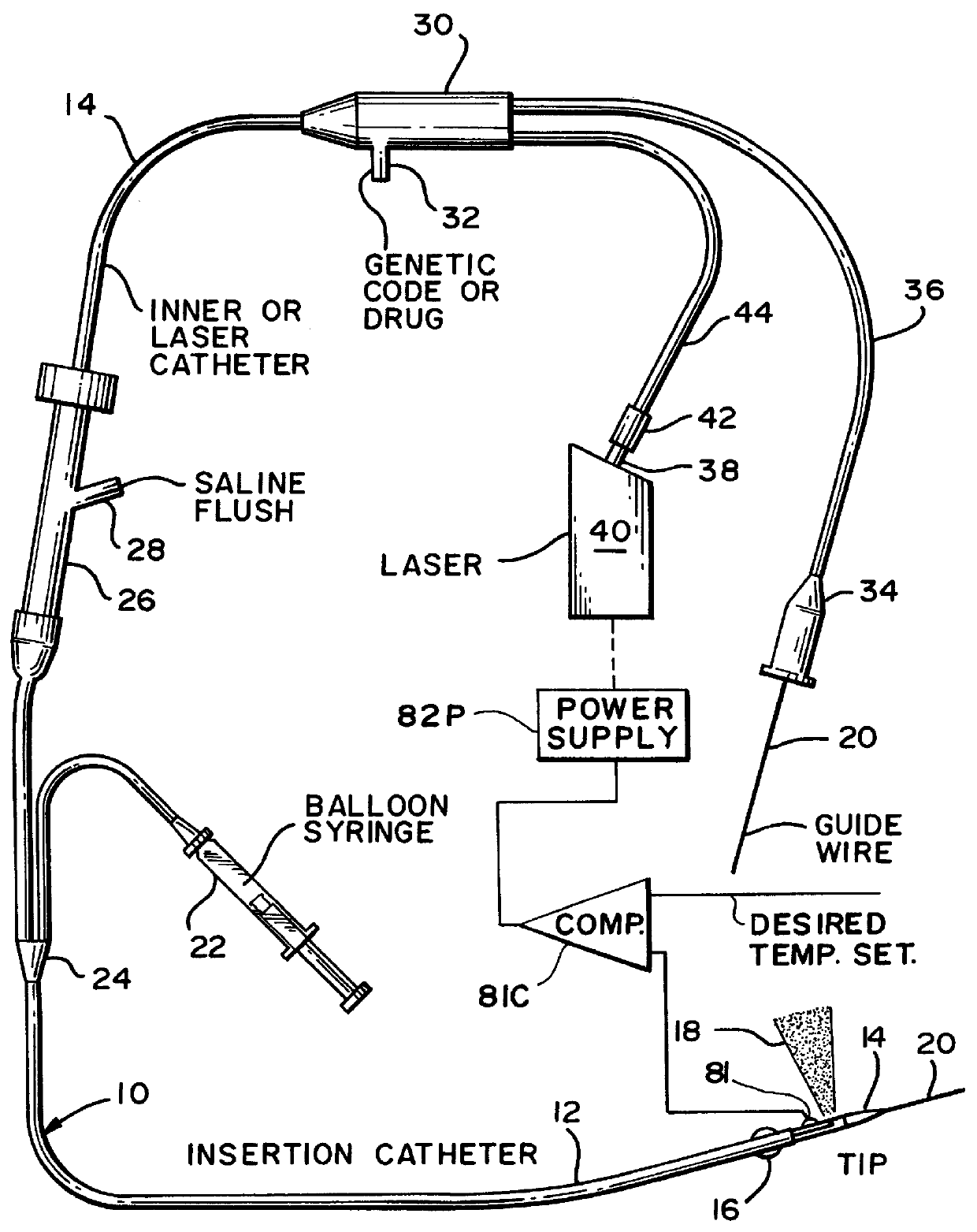
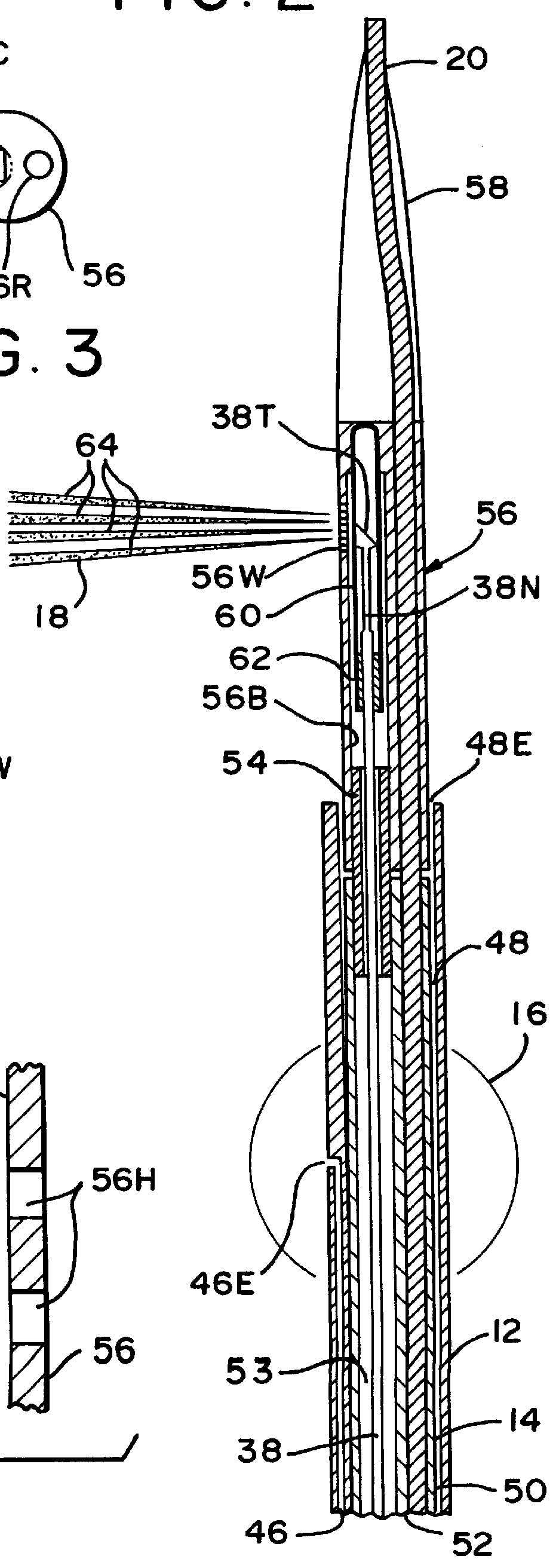
An apparatus and associated method provides for the application of a cell treatment agent, such as genetic material or drugs to be inserted within the cells of a patient in vivo. The apparatus may be a catheter arrangement with various embodiments for applying heat to a patient's cells in vivo in order to improve transfection efficiency or application efficiency. Laser beams may be applied directly to the cells. Alternately, the cells may be heated by electrical heating, chemical heating, radio frequency heating, microwave heating, infrared heating, ultrasound heating, or indirect laser heating. Further, the treatment agent may be heated prior to its application to the patient such that the treatment agent heats the cells of the patient.
Owner:ABELA GEORGE S
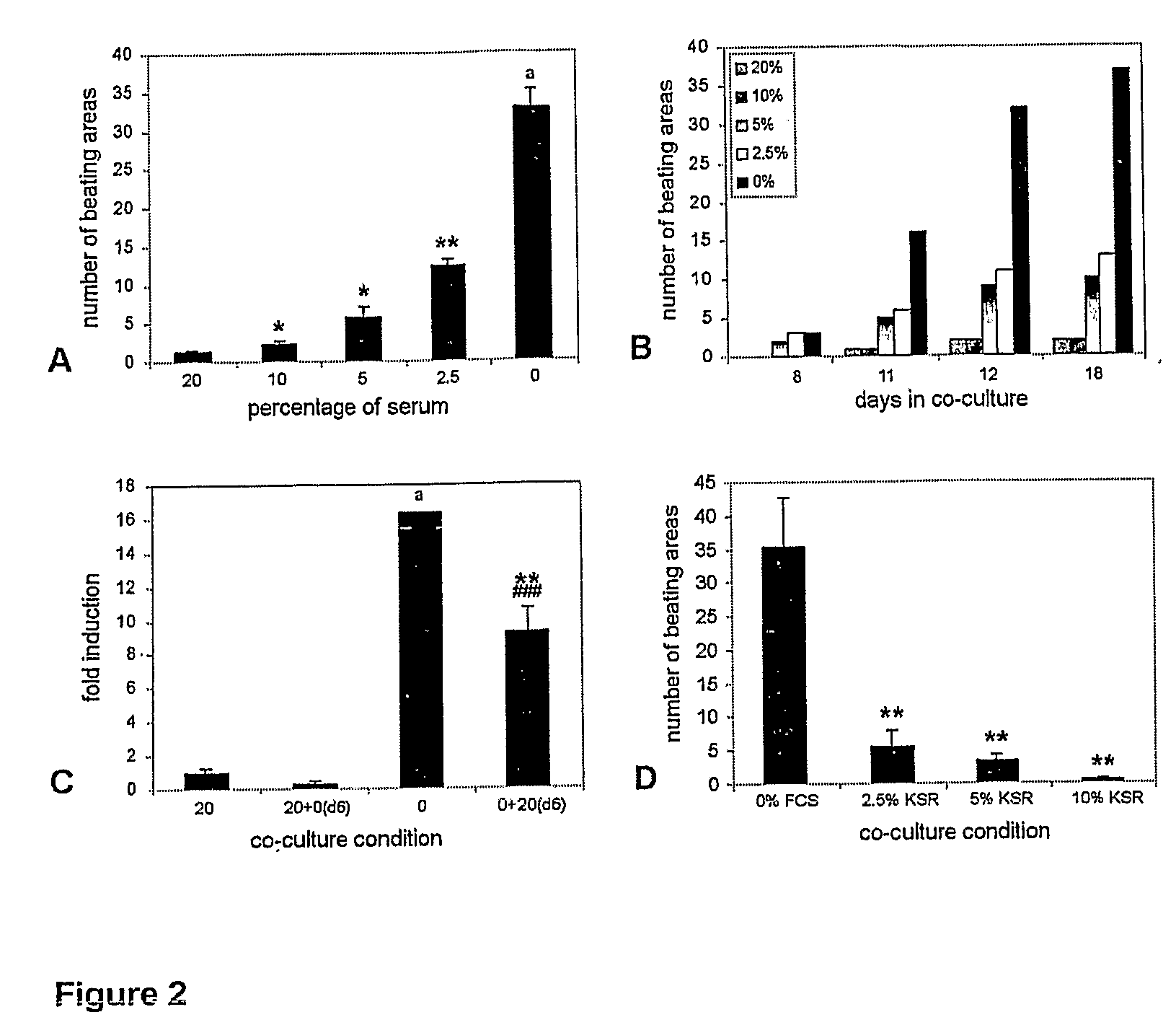
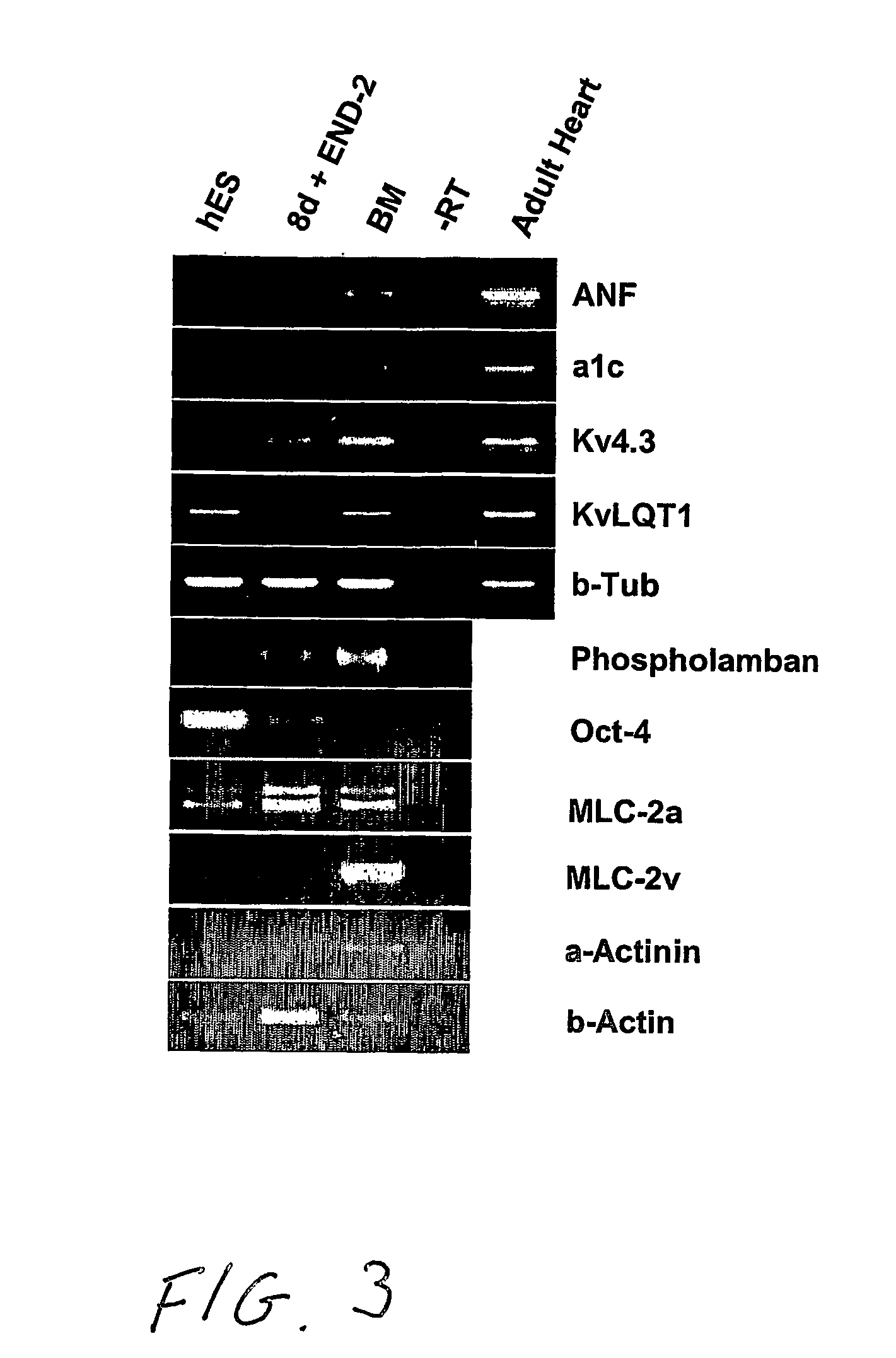
Differentiation of Human Embryonic Stem Cells and Cardiomyocytes and Cardiomyocyte Progenitors Derived Therefrom
The present invention provides a method to improve current culturing methods for the differentiation of cardiomyocytes from hES cells. The method includes culturing the hES cells in the presence of ascorbic acid or a derivative thereof. Preferably the culturing is conducted in serum free conditions. The invention also includes isolated cardiomyocytes and cardiac progenitors differentiated by the methods as well as the use of these cells in methods of treating and preventing cardiac diseases and conditions. Culture media and extracellular media are also provided which include ascorbic acid for the differentiation of hES cells to cardiomyocytes.
Owner:ES CELL INT
Neural regeneration peptides and methods for their use in treatment of brain damage
ActiveUS20050131212A1High expressionEasy SurvivalPeptide/protein ingredientsGenetic material ingredientsNervous systemInjury brain
The invention discloses a family of peptides termed NRP compounds or NRPs that can promote neuronal migration, neurite outgrowth, neuronal proliferation, neural differentiation and / or neuronal survival, and provides compositions and methods for the use of NRPs in the treatment of brain injury and neurodegenerative disease. NRP compounds can induce neurons and neuroblasts to proliferate and migrate into areas of damage caused by acute brain injury or chronic neurodegenerative disease, such as exposure to toxins, stroke, trauma, nervous system infections, demyelinating diseases, dementias, and metabolic disorders. NRP compounds may be administered directly to a subject or to a subject's cells by a variety of means including orally, intraperitoneally, intravascularly, and directly into the nervous system of a patient. NRP compounds can be formulated into pharmaceutically acceptable dose forms for therapeutic use. Methods for detecting neural regeneration, neural proliferation, neural differentiation, neurite outgrowth and neural survival can be used to develop other neurally active agents.
Owner:CURONZ HLDG
Biomarkers for mdm2 inhibitors for use in treating disease
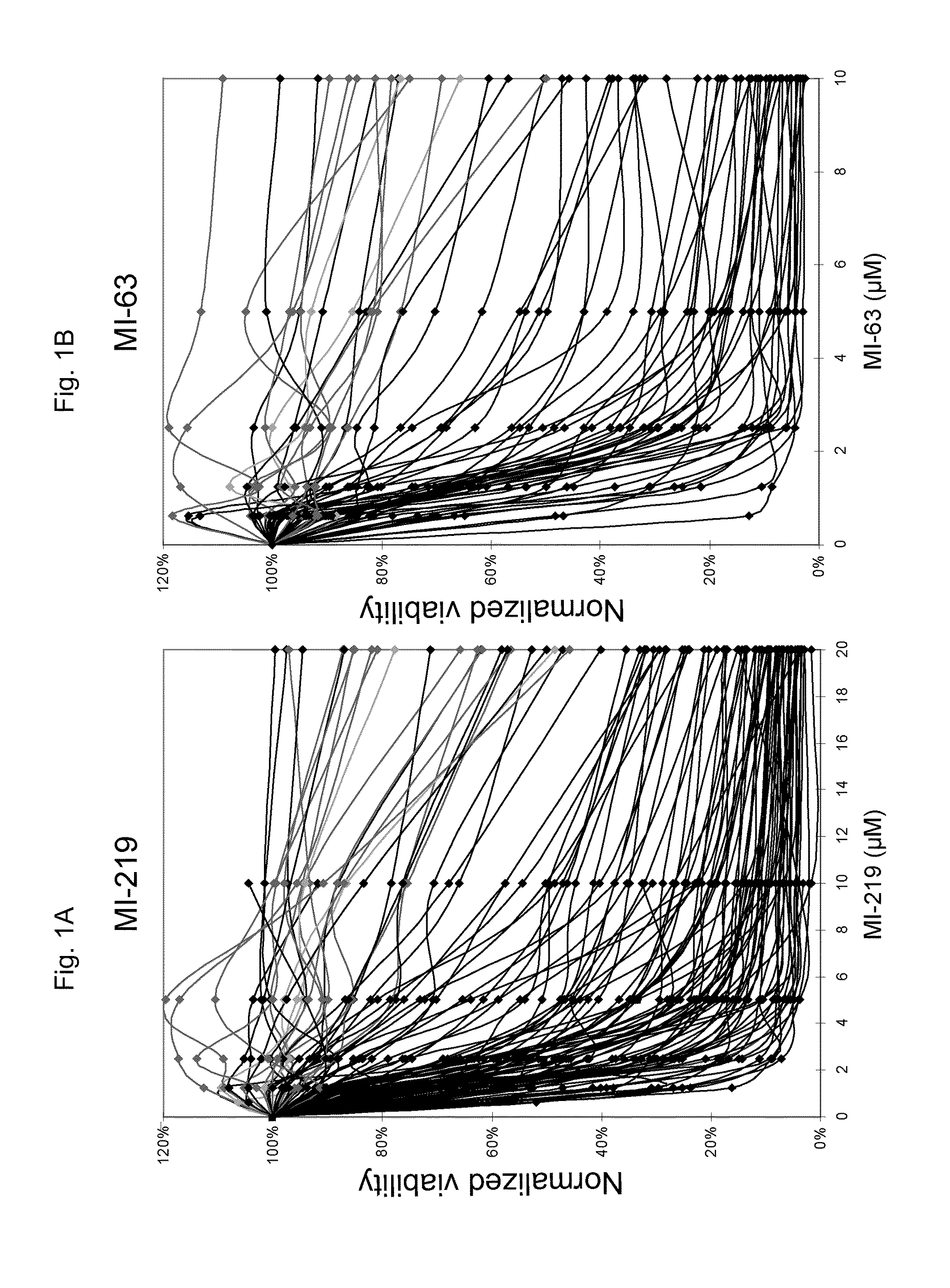
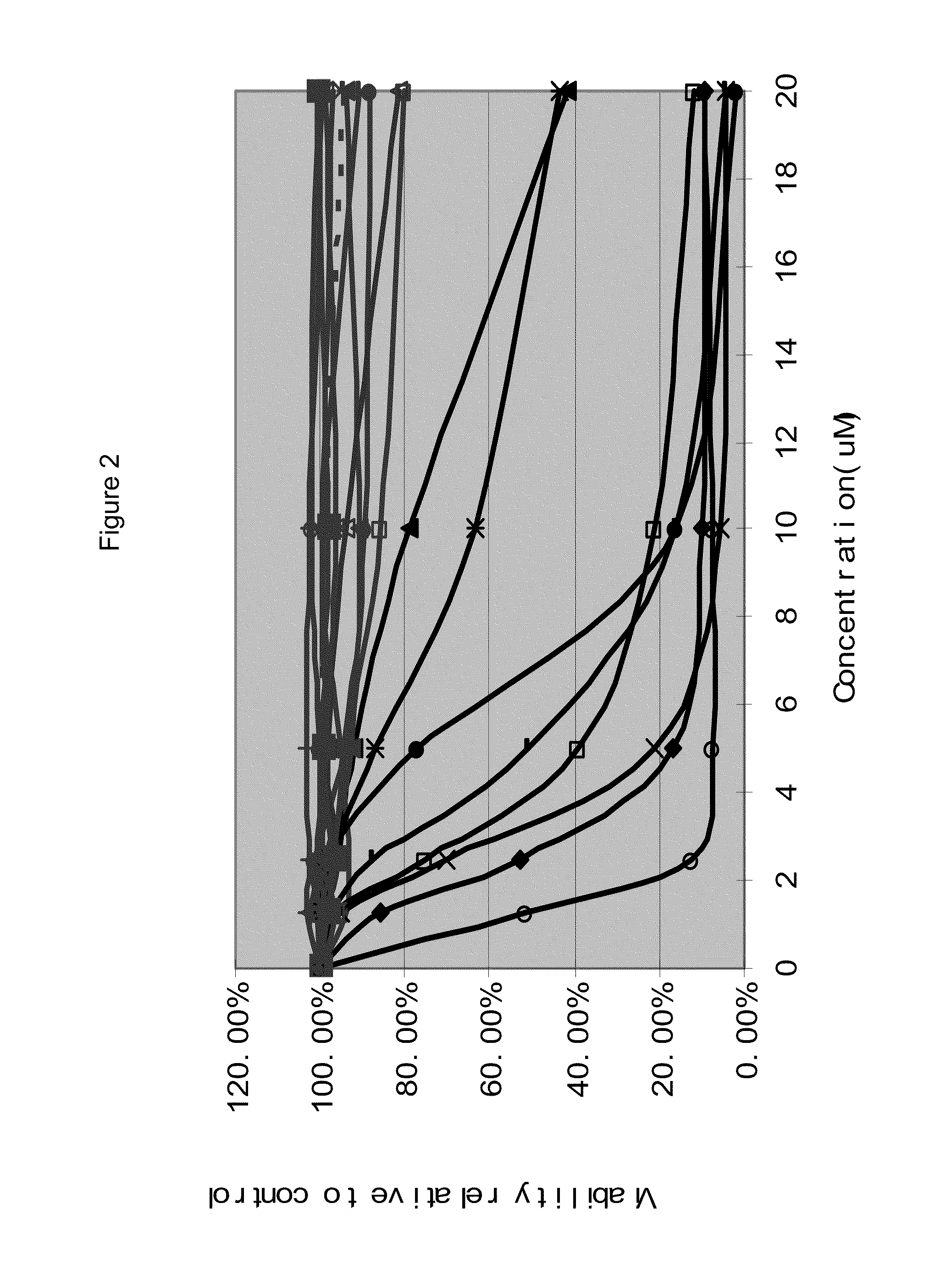
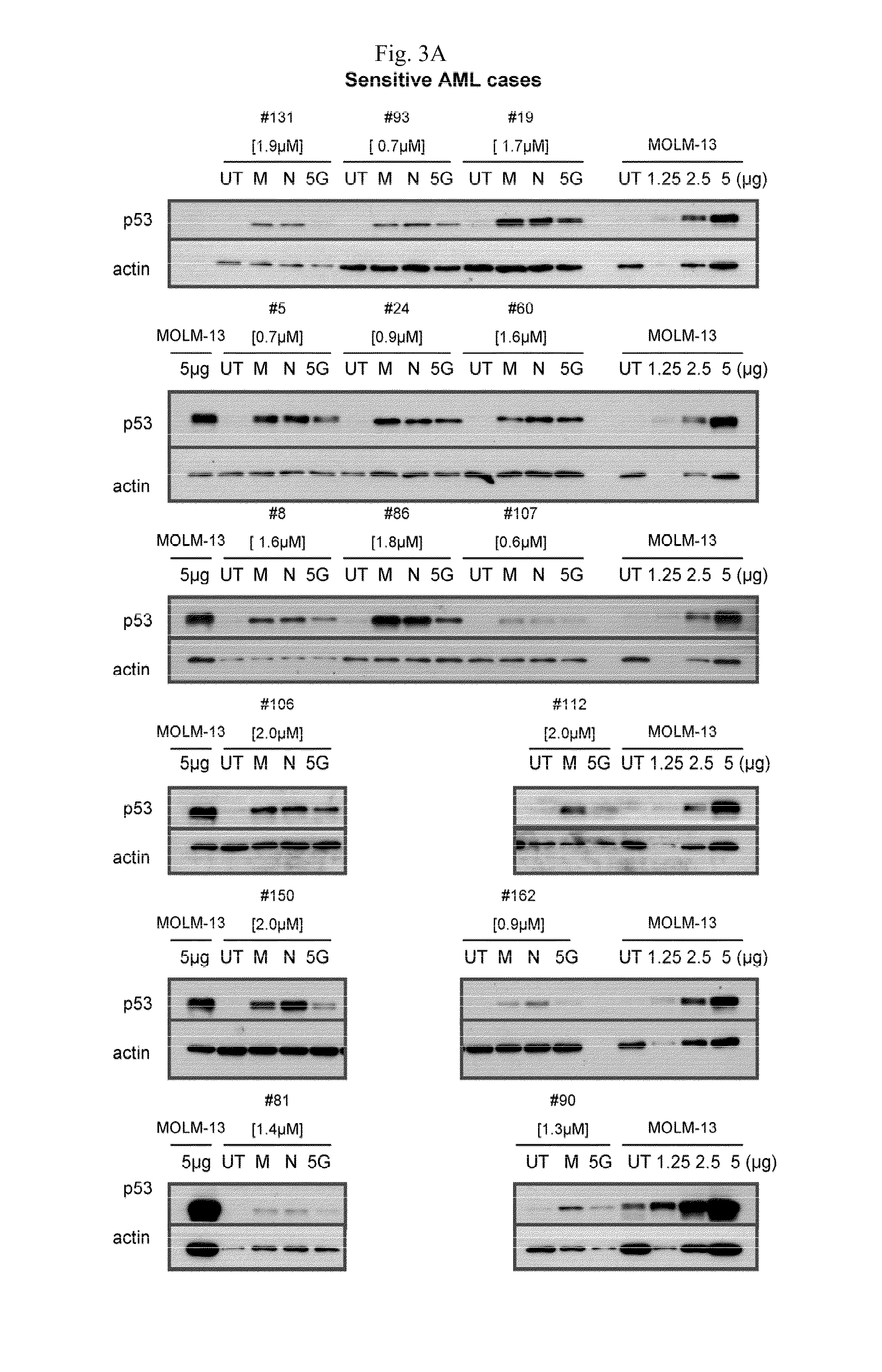
InactiveUS20110251252A1Raise the possibilityBiocideMicrobiological testing/measurementDiseaseFLT3/ITD Mutation
Owner:RGT UNIV OF MICHIGAN
Cell transfection apparatus and method
InactiveUS6071276ATransfection of cells in vivo relatively quicklyReduce amountCatheterSurgical instruments for heatingIn vivoRadio frequency
An apparatus and associated method provides for the application of a cell treatment agent, such as genetic material or drugs to be inserted within the cells of a patient in vivo. The apparatus may be a catheter arrangement with various embodiments for applying heat to a patient's cells in vivo in order to improve transfection efficiency or application efficiency. Laser beams may be applied directly to the cells. Alternately, the cells may be heated by electrical heating, chemical heating, radio frequency heating, microwave heating, infrared heating, ultrasound heating, or indirect laser heating. Further, the treatment agent may be heated prior to its application to the patient such that the treatment agent heats the cells of the patient.
Owner:ABELA GEORGE S
Apparatus and method for down-regulating immune system mediators in blood
InactiveUS7207964B2Reducing free radicals in a patient's bloodReduce concentrationSemi-permeable membranesSolvent extractionInterleukin 6White blood cell
A method and apparatus for preventing and treating septicemia in patient blood is provided. The extracorporeal system includes an antimicrobial device to inactivate at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 50–75% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms, as well as by the patient's cells. These molecules include endotoxins from Gram negative bacteria, exotoxins from Gram negative and Gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, interleukin 6, complement proteins C3a and C5a, and bradykinin.
Owner:HEMAVATION
Differentiation of human embryonic stem cells to cardiomyocytes
InactiveUS20070161107A1Improve compatibilityPromote differentiationArtificial cell constructsSkeletal/connective tissue cellsCardiac muscleCo culturing
A method for inducing cardiomyocyte differentiation of a hES cell, the method comprising co-culturing the hES cell with a cell excreting at least one cardiomyocyte differentiation inducing factor or with an extracellular medium therefrom, under conditions that induce differentiation, cells and cell populations so produced, and uses of the cells.
Owner:MUMMERY CHRISTINE LINDSAY +2
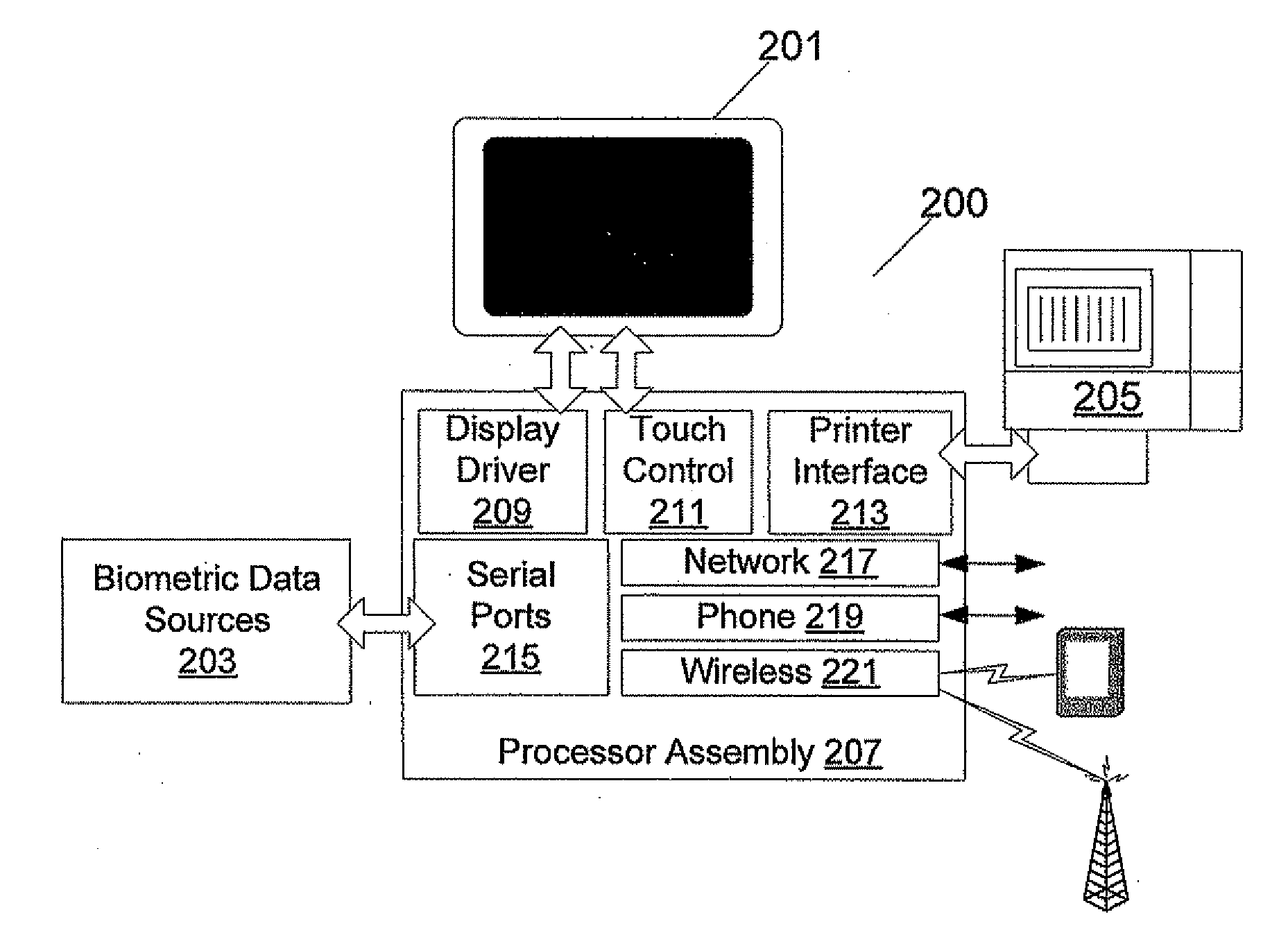
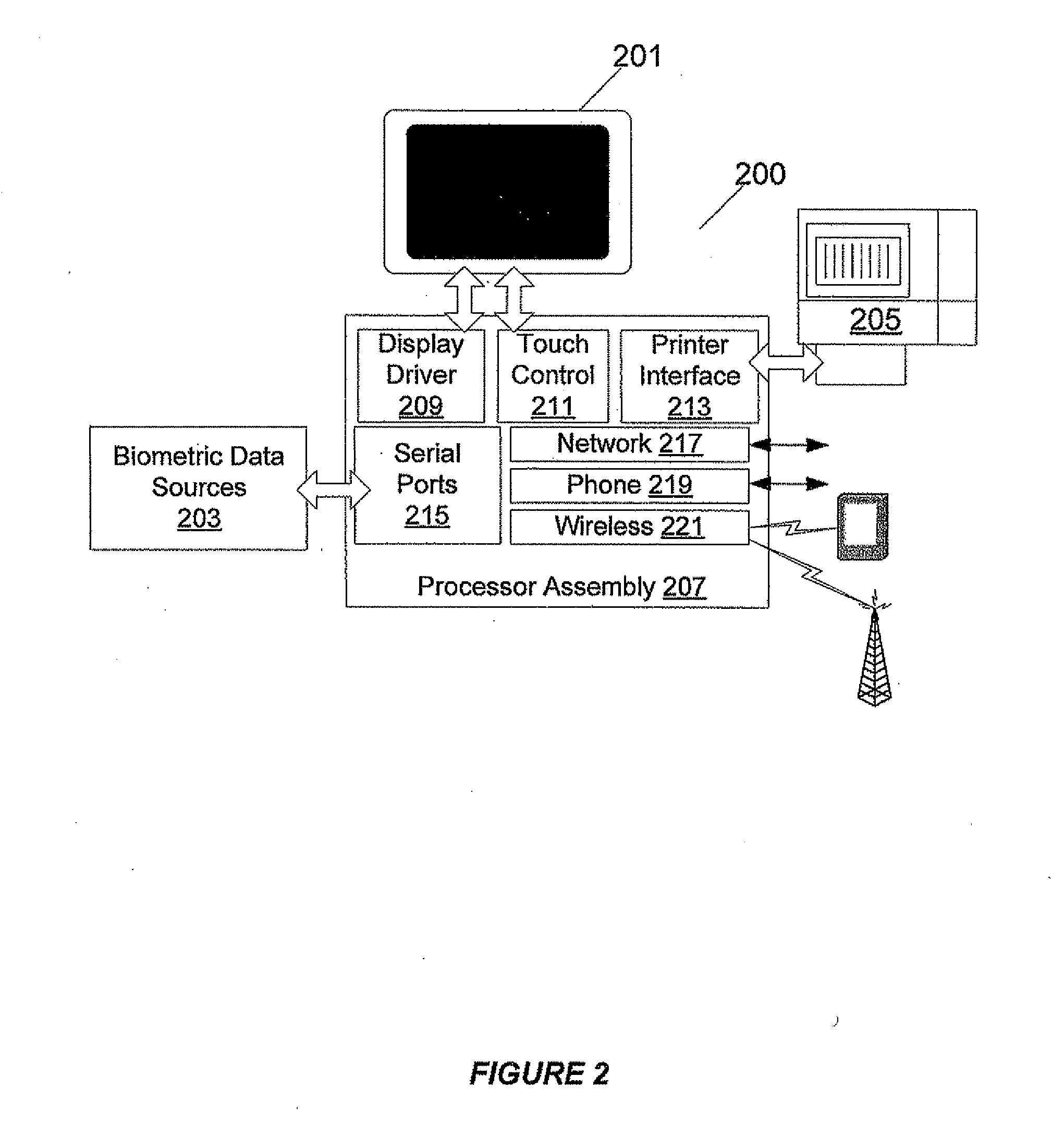
Biometric Network Exchange System
ActiveUS20080114213A1Reduce sanitationMedical communicationData processing applicationsMeasurement deviceEngineering
A biometric measurement device and communications infrastructure implements a system of information exchange and usage. In an embodiment of the invention, a kiosk user's cell phone is used to wirelessly connect via the machine to a call center, doctor, or emergency center. In addition, the kiosk may automatically and wirelessly connect the user's cell phone through the kiosk to a call center representative or emergency service. The kiosk is able to collect and transmit user data, such as for promotional or advertising purposes in a further example.
Owner:BAGAN KENNETH J
Extracorporeal blood treatment system using ultraviolet light and filters
InactiveUS20060210424A1Optimize ultraviolet outputPreventing pressurizationOther blood circulation devicesHaemofiltrationInterleukin 6White blood cell
A method and apparatus for preventing and treating septicemia in patient blood is provided. The extracorporeal system includes an antimicrobial device to inactivate at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 50-75% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms, as well as by the patient's cells. These molecules include endotoxins from Gram negative bacteria, exotoxins from Gram negative and Gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, interleukin 6, complement proteins C3a and C5a, and bradykinin.
Owner:MALLETT SCOTT R +2
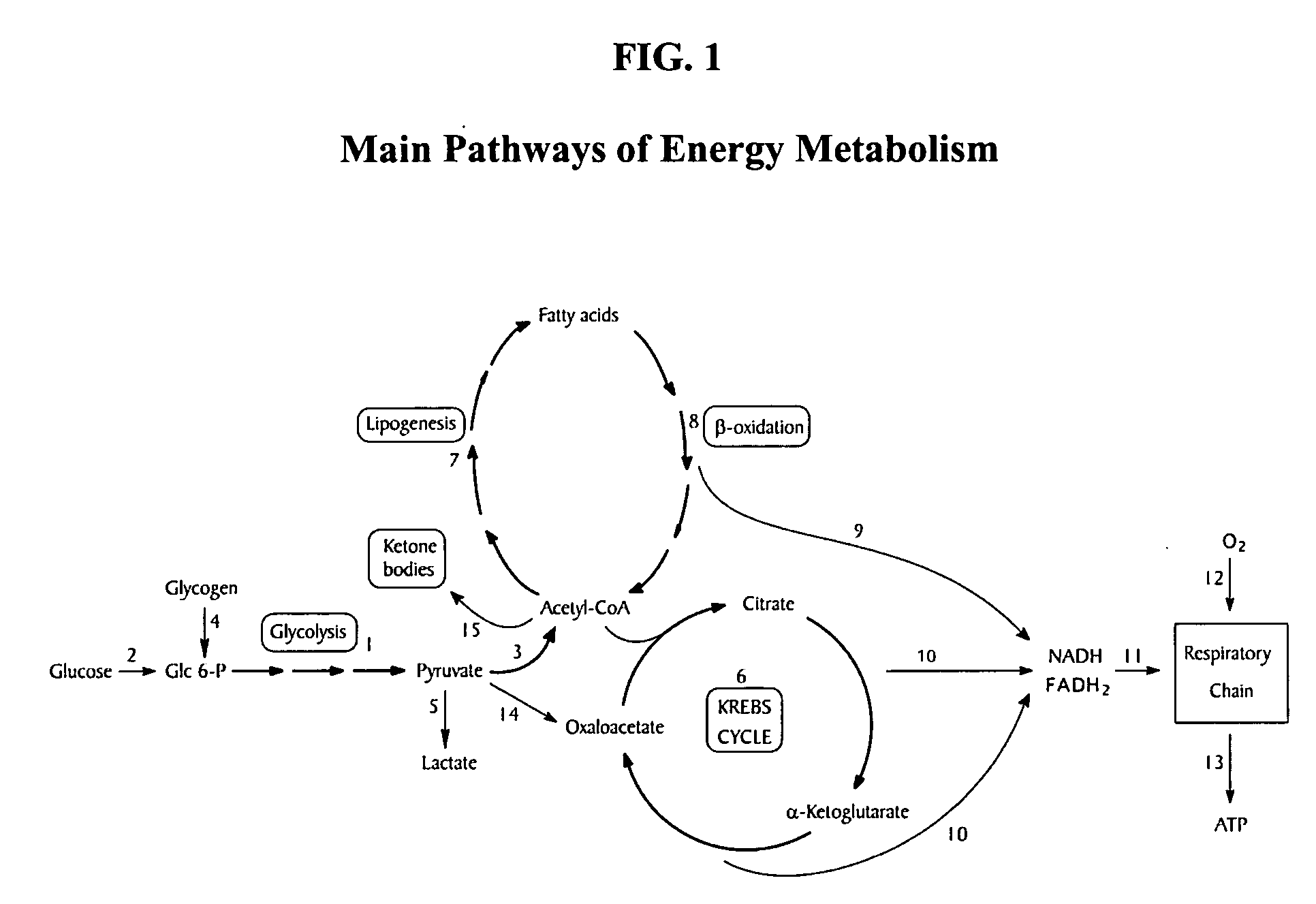
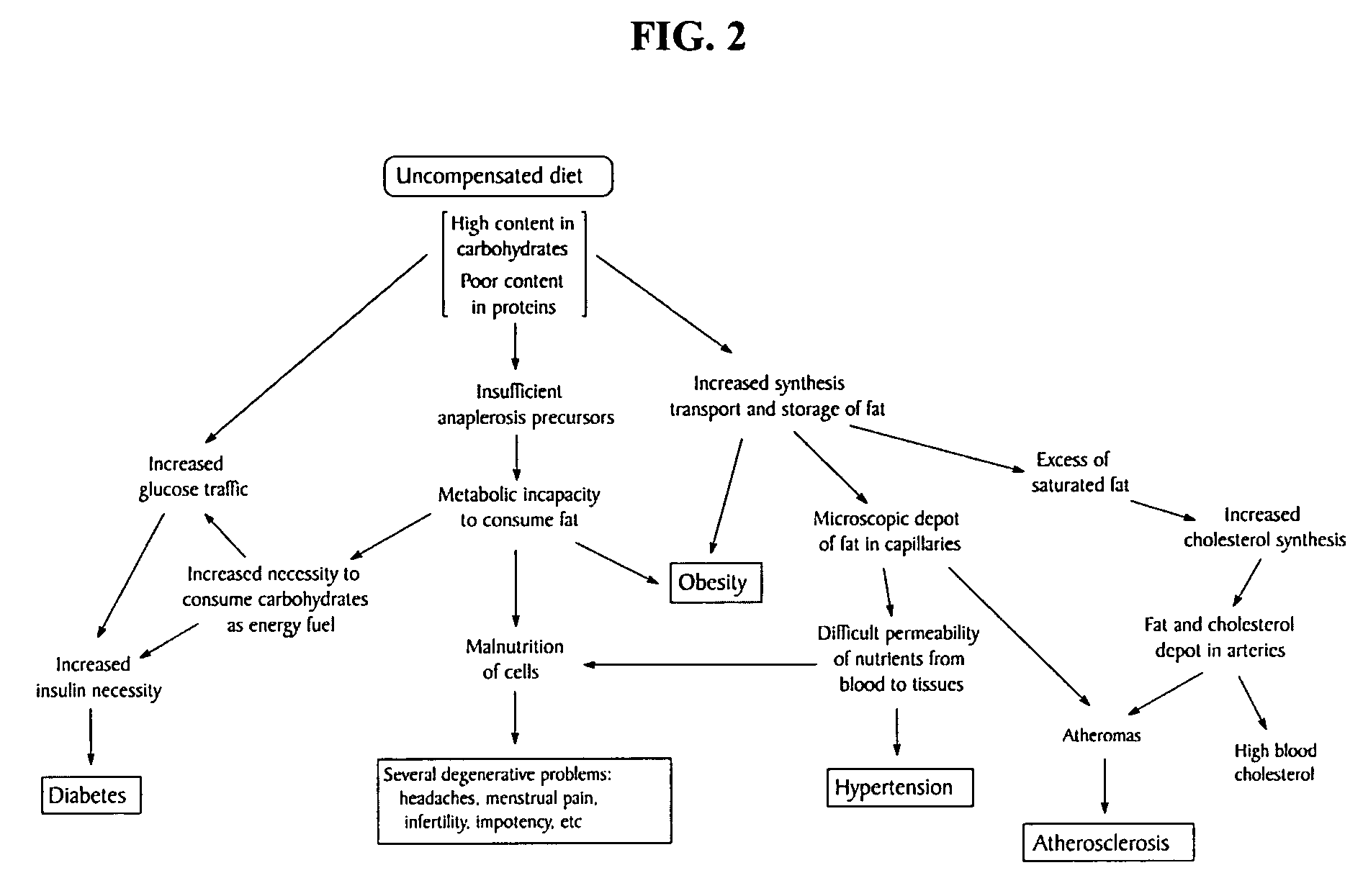
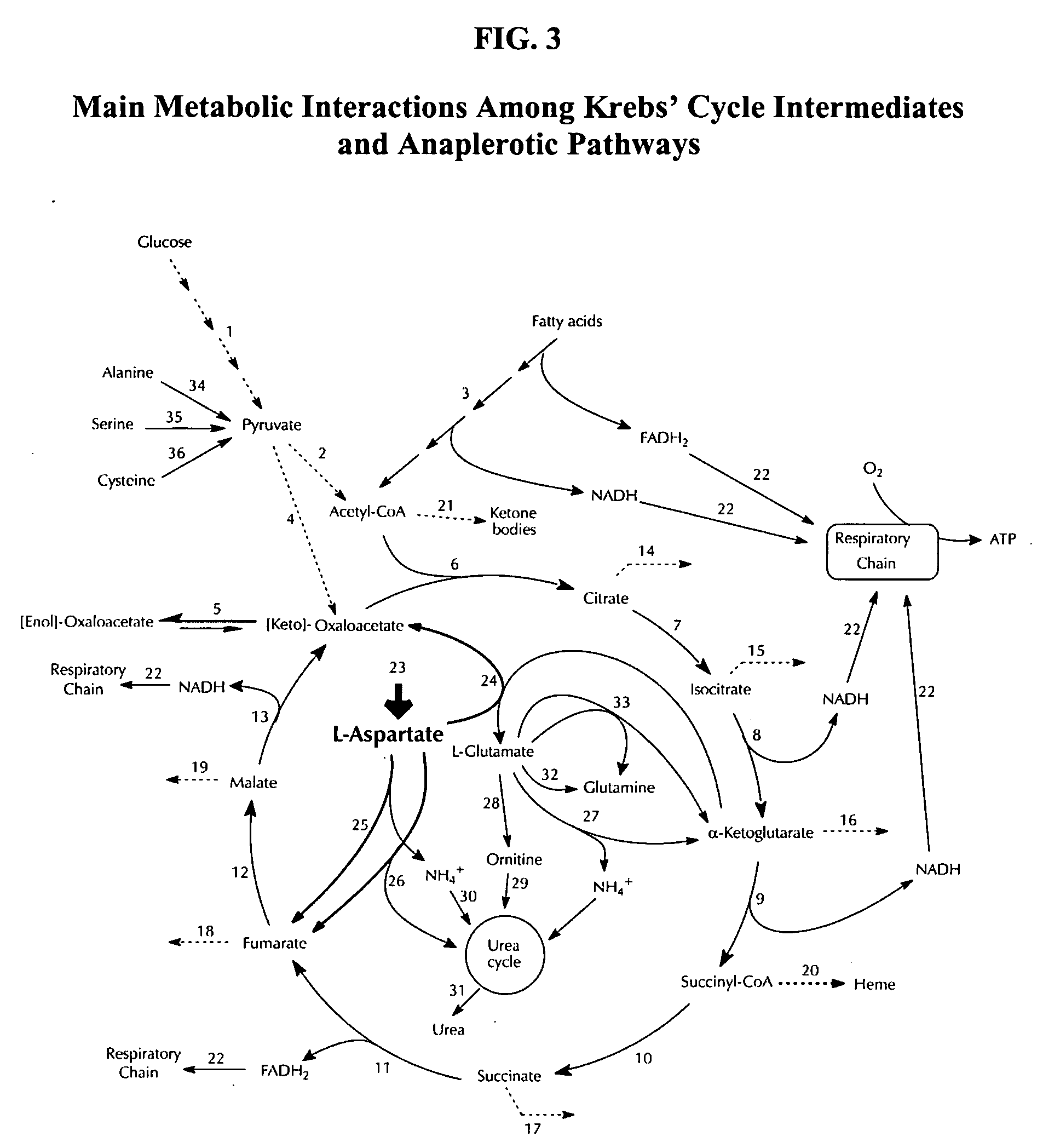
L-aspartic acid for the treatment of assorted health problems
A method of treating or preventing one's health problem that are associated with one's cells having a poor metabolic capability to process fat includes ingesting a therapeutically effective dosage of an anaplerotic precursor. Examples of such health problems include obesity and the related health conditions that are aggravated by obesity, diabetes, hypertension, atherosclerosis, migraines and headaches, menstrual cramps, cholesterol excesses, multiple sclerosis and Alzheimer's disease, anemia, high blood uric acid levels, ketosis, tobacco and other drug addictions, and digestive problems. In a preferred embodiment, this method includes the daily ingestion of L-aspartic acid, or a pharmaceutically acceptable analog thereof, at the rate of 10-14 g / day and restricting from one's diet the intake of starchy foods.
Owner:INST DEL METABOLISMO CELULAR
Anti-Oxidant Synergy Formulation Nanoemulsions to Treat Caner
A uniform microfluidized nanoemulsion is disclosed containing a synergistic combination of two antioxidants and a cell membrane stabilizer phospholipid (i.e., an anti-oxidant synergy formulation; ASF). The microfluidized nanoemulsion improves the combination's cell membrane permeability by at least four-fold over conventional nanoemulsion compositions, which significantly increases the intracellular concentration of typically cell-impermeant antioxidants (i.e., for example, tocopherol) and / or systemic bioavailability. As a nanoemulsion, synergistic combination has greater anticancer efficacy than the same combination applied as a free solution.
Owner:UNIV OF MASSACHUSETTS
Compositions and Methods for Growth of Pluripotent Cells
InactiveUS20090087907A1Low costEasy to presentBioreactor/fermenter combinationsBiological substance pretreatmentsSomatomedinGenotype
A method of propagating embryonic stem (ES) cells in an undifferentiated state, while maintaining both the pluripotency and the cells normal genotype is disclosed. The method comprises using recombinantly produced protein domains to attach human embryonic stem cells to the surface of a bioreactor. The ES cells are supplied with nutrients while they held in place by the recombinantly produced protein domains which may be chosen from Laminin G domain, Fibronectin domain 2, Fibronectin domain 3, Nidogen G2 domain, Nidogen G3 domain, Vitronectin somatomedin B domain, and Vitronectin somatomedin C terminal domain. Useful molecules are characterized by a high binding affinity for hES cells and a molecular weight of about 50 kDa±20%.
Owner:THE UNIV OF QUEENSLAND
Modified Organs and Cells for Xenotransplantation
InactiveUS20070089178A1Maintain integrityMaintaining viabilityBiocideGenetic material ingredientsHuman bodyGenetically engineered
Owner:RBC BIOTECH
Modified organs and cells for xenotransplantation
InactiveUS7166278B2Maintain integrityMaintaining viabilityBiocidePeptide/protein ingredientsHeterograftsEngineered genetic
It has been discovered that there are at least two significant antigens present on the cells of animal species such as pigs that elicit an immune or inflammatory response immediately upon implantation into humans or contact with human serum. The first is an α-galactosyl (Gal) epitope, for example, Galα(1->3)Galβ(1->4)GlcNac (linear B type 2) or Galα(1->3) Galβ(1->4)Glc (linear B type 6). The second is an N-glycolylneuraminic acid (NeuGc) structure. By eliminating these epitopes, preferably by genetically engineering the animal so that the epitope is either not produced or is greatly reduced, or by chemical or enzymatic treatment of the animal's cells to remove the epitopes, it is possible to produce organs, tissues and cells suitable for xenotransplantation into humans. Cells can be rendered even more compatible by genetically engineering the animal to express a human complement regulatory protein (inhibitor), such as CD59, on its cells, or to express an excess of a pig complement regulatory protein.
Owner:RBC BIOTECH
Device and method for reducing inflammatory mediators in blood
InactiveUS20050277863A1Optimize ultraviolet outputPreventing pressurizationOther blood circulation devicesHaemofiltrationInterleukin 6Filtration
A method and apparatus for preventing and treating septicemia in patient blood is provided. The extracorporeal system includes an antimicrobial device to inactivate at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 50-75% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms, as well as by the patient's cells. These molecules include endotoxins from Gram negative bacteria, exotoxins from Gram negative and Gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, interleukin 6, complement proteins C3a and C5a, and bradykinin.
Owner:HEMAVATION
Method for creating hepatocyte by human embryo stem cell external evoked differentiation
InactiveCN101117626AFully contactedEasy to observeArtificial cell constructsEmbryonic cellsDiseaseArtificial liver
The present invention provides a method that embryo of human embryonic stem cells (HES) is directionally induced and divided into liver cells. First, the biological property of high degree of self-regeneration multiplication of HES cell is made use of, the collagenase IV digestion is adopted for transfer of culture and augmentation, thereby obtaining a plurality of stem cells; second, the HES cell is inoculated into a cell utensil with lower adsorbability to form a mature imitated embryonic plant (EB); third, the mature cystic EB is digested with 0.25 tryptic enzyme to 0.02 percent EDTA to a single cell, and then is inoculated to a tissue culture dish which is packed with I-shaped collagen in advance, solution culture is induced under the condition of dexamethasone and human trypsin, and the differentiation towards the liver cell direction is observed and judged. The prevent invention provides the method that the HES is efficiently divided into liver cells, at the same time, the method makes the follow-up judging work more simply and more efficient, and lay a foundation for the stem cellular transplantation or biologic artificial liver curing the disease such as serious hepatitis, hepatic failure and so on.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Therapeutic applications of antigens or epitopes associated with impaired cellular peptide processing, e.g. expressed on rma-s cells transfected with a b7-1 gene
The following observation shows that antigens or epitopes associated with impaired cellular peptide processing, especially MHC class I dependent, especially antigens associated with impaired TAP-function, can be used for immunization against cancer or as a virus vaccine.
Owner:ACCURO IMMUNOLOGY
Drinking straw
ActiveUS8025242B2Low germination rateLess activeDwelling equipmentSpray nozzlesWater basedEngineering
The straw designed for the intake of water based drink from a container. In the lower part of the flexible straw there are holes that are connected to the main tube of the straw. Above the holes and attached to the straw, there is a floating device which allows for the intake of the drink from the container only from fixed depth ranges, preferably from 5-15 mm from the surface of the water. The straw allows for the protection of the organism of the user from negative effects of the drink on the body's cells.
Owner:EFREMKIN PAVEL V +1
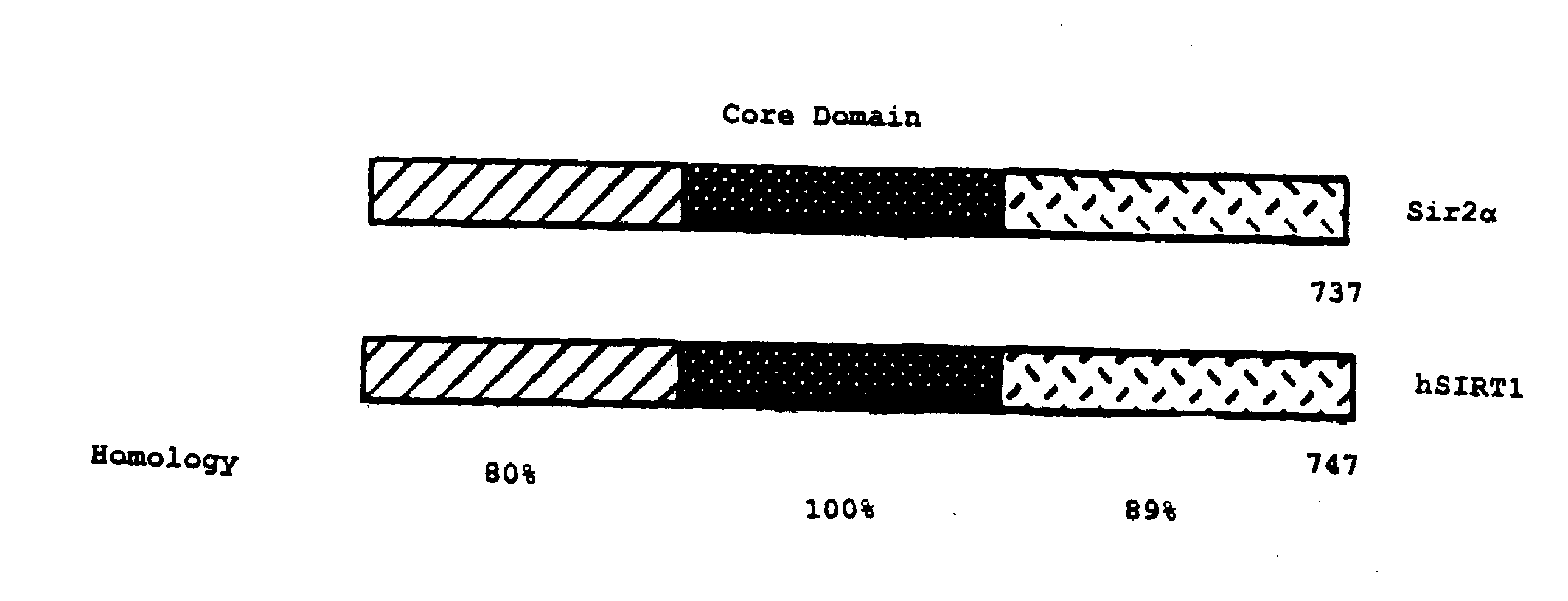
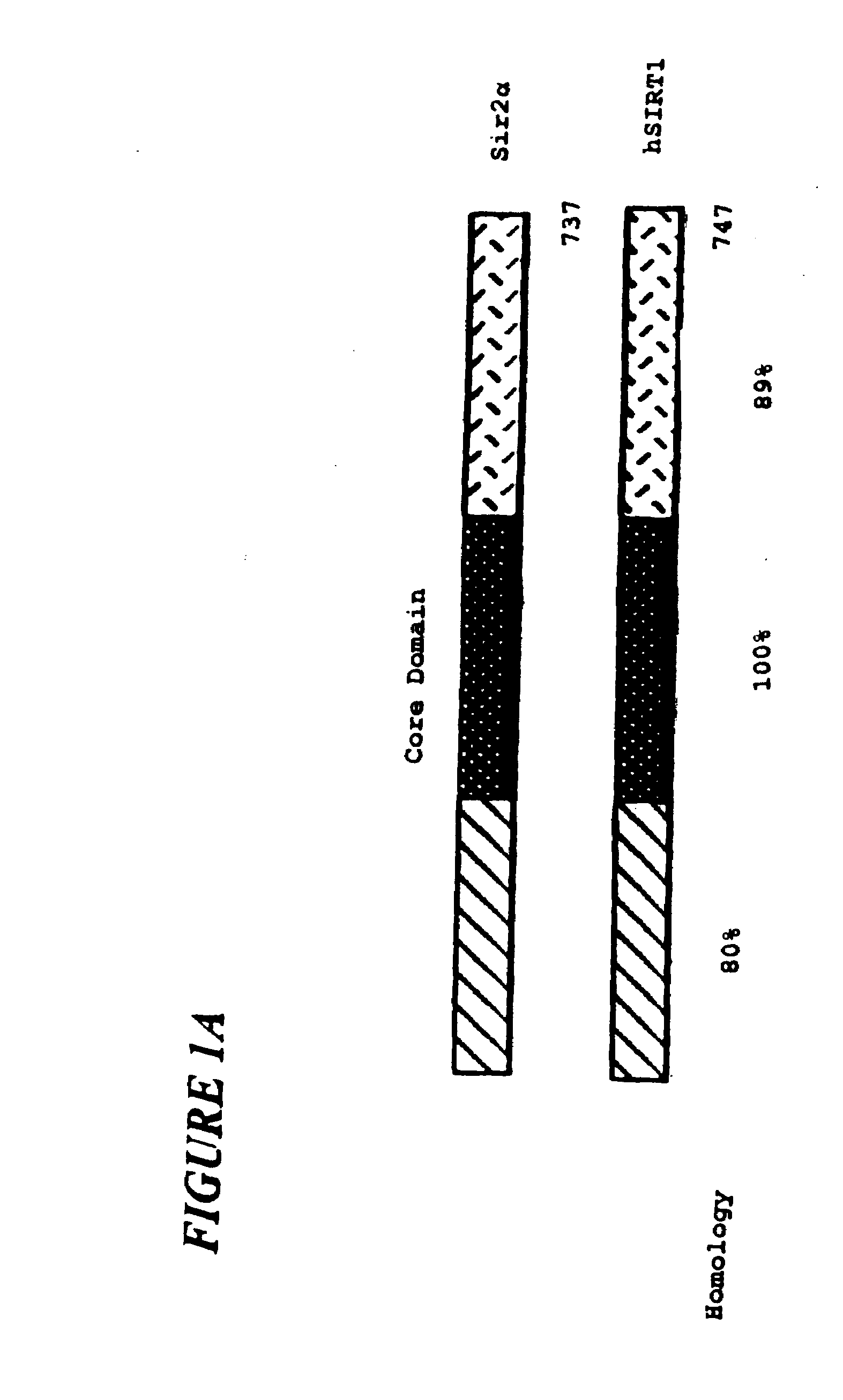
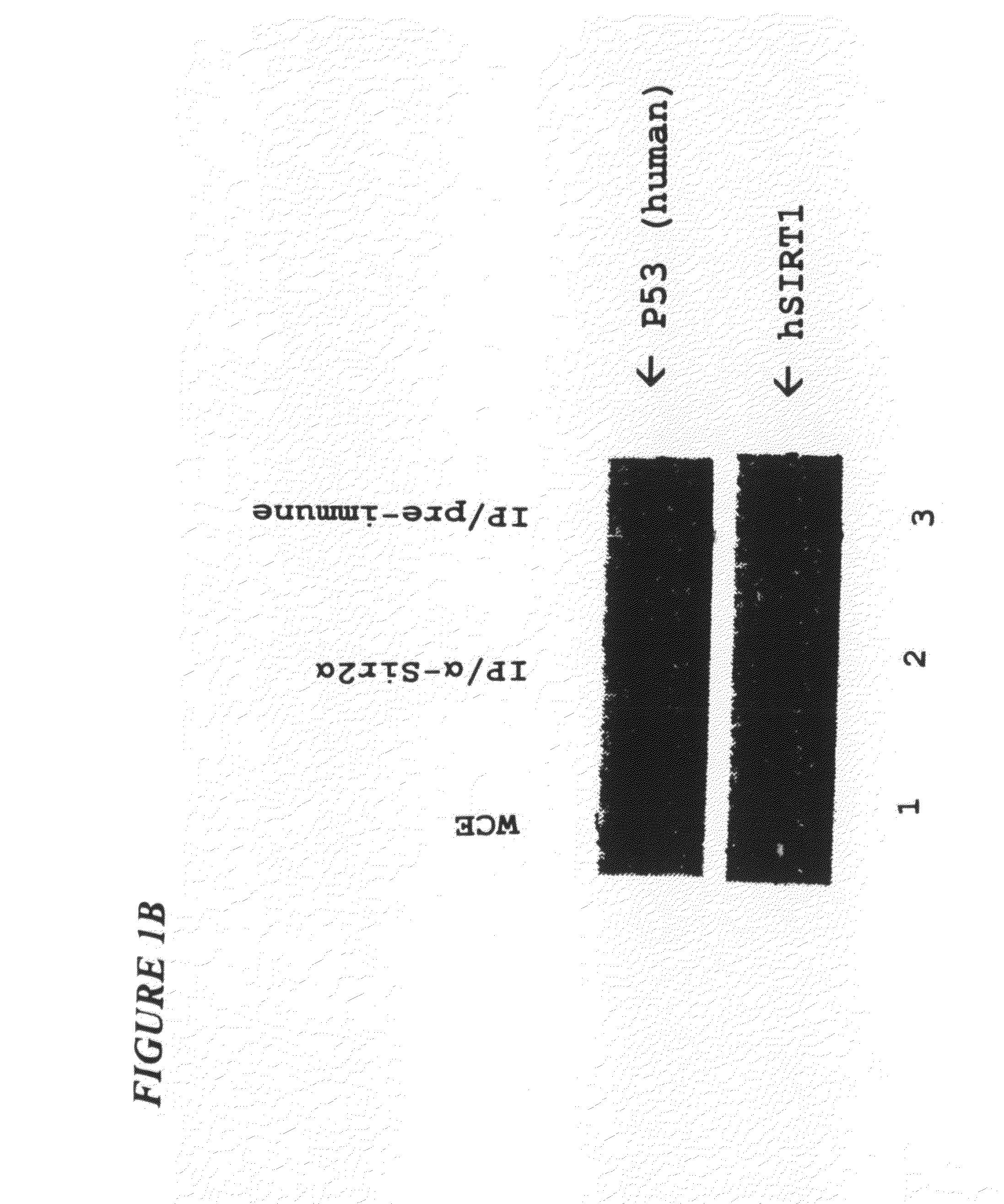
Sir2a-based therapeutic and prophylactic methods
InactiveUS20080119434A1Reduce the amount requiredExtend your lifeGenetic material ingredientsEther/acetal active ingredientsApoptosisPhysical stress
This invention provides methods for treating and for inhibiting the onset of cancer in a subject comprising administering an agent that inhibits the ability of Sir2α to inhibit p53-dependent apoptosis. This invention also provides a related method for inducing the death of a cell. This invention further provides a method for decreasing the amount of damage to a subject's cells caused by physical stress comprising administering agent that increases the amount of Sir2α in the subject's cells and / or the ability of Sir2α to inhibit p53-dependent apoptosis in the subject's cells. This invention further provides related methods for prolonging the life-span of a subject, decreasing the amount of damage to a cell caused by physical stress, and prolonging the life-span of a cell. Finally, this invention provides two articles of manufacture for performing the instant methods.
Owner:THE TRUSTEES OF COLUBIA UNIV
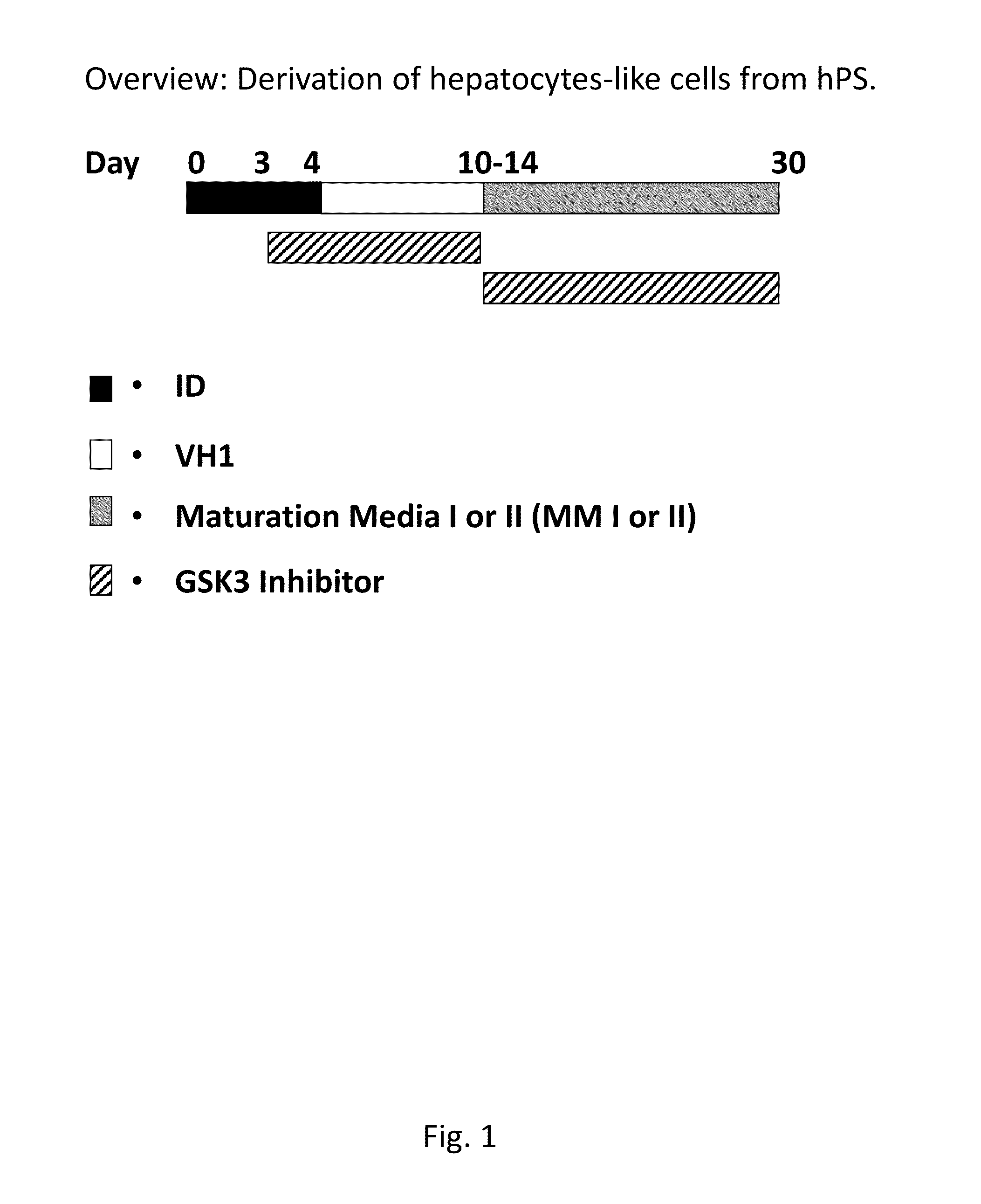
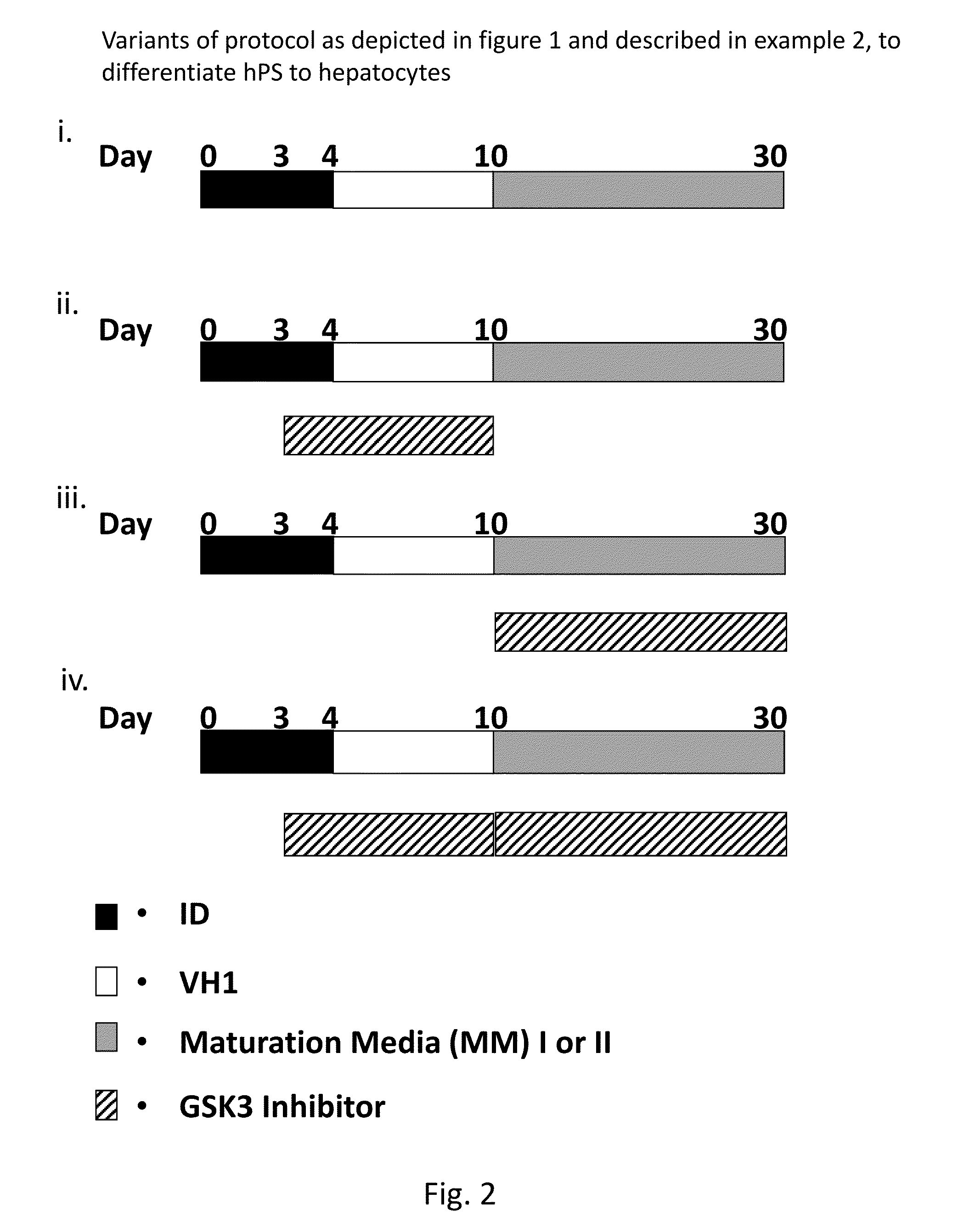
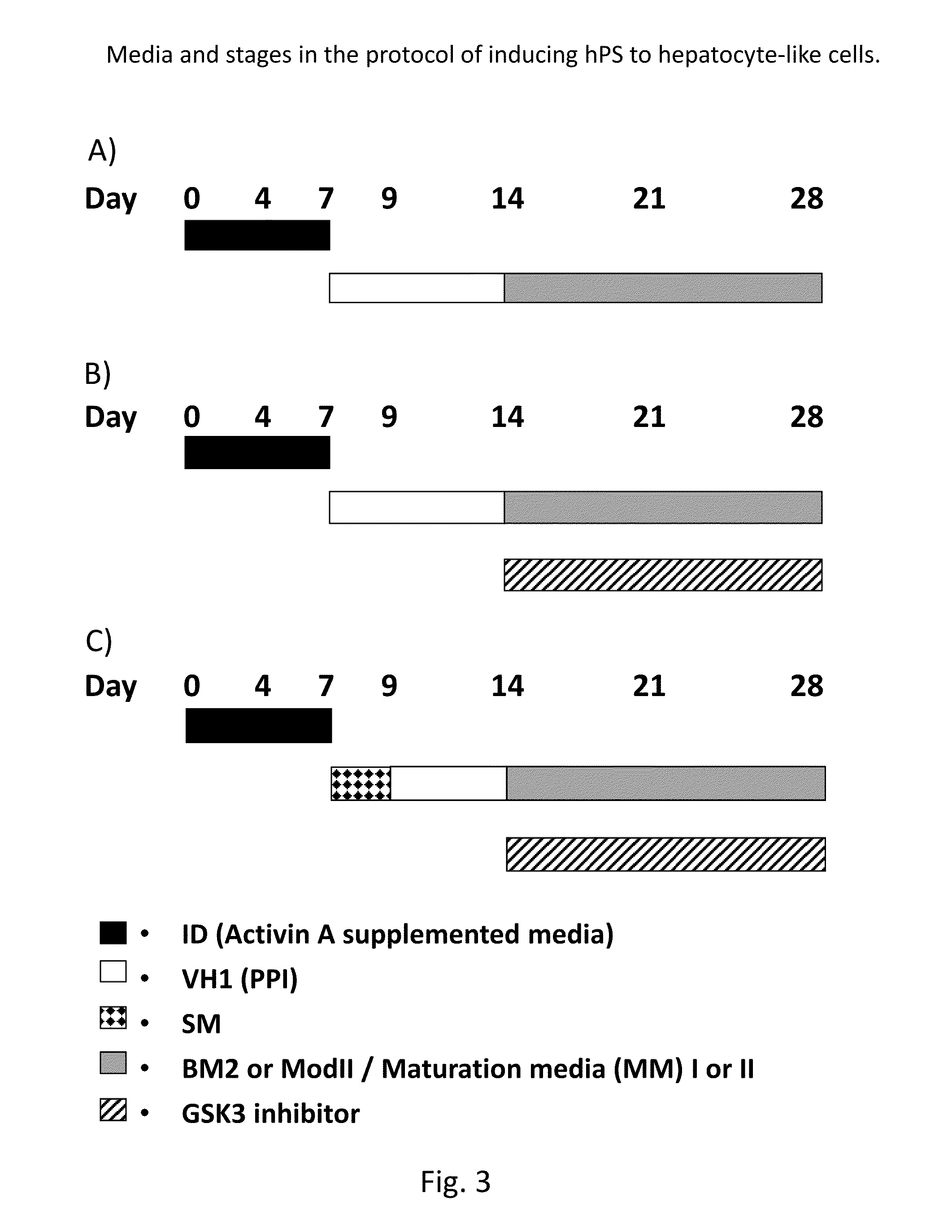
Directed differentiation and maturation of pluripotent cells into hepatocyte like cells by modulation of Wnt-signalling pathway
ActiveUS9394522B2Stable expressionHighly suitableHepatocytesDigestive systemGerm layerDevelopmental stage
Provided are improved methods using Glycogen synthase kinase 3 (GSK3) inhibitors by which endodermal cells, notably endodermal cells derived from human pluripotent stem cells (hPS), such as but not limited to hiPS-cells and hES-cells may be differentiated into hepatocyte like cells. The specific modulation of wingless integration gene (WNT)-signalling pathway and use of GSK3 inhibitors achieve direct differentiation and maturation of hepatocytes derived from human pluripotent stem (hPS) cells. GSK-3 inhibitors, when added to the growth medium at certain developmental stages, leads to more mature and functional features for the hepatocyte like cells as well as more pure and homogenous populations of hepatocyte like cells. Provided are also hepatocyte like cells obtained by these methods as well as compositions comprising them.
Owner:TAKARA BIO EURO
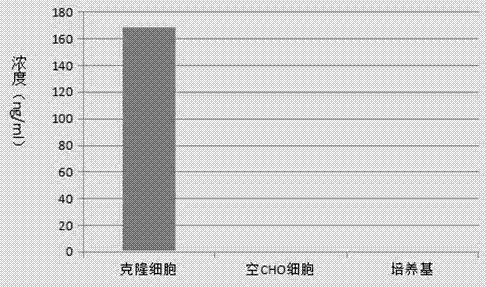
CHO cell strain capable of stably and efficiently expressing recombinant human BMP7 (bone morphogenetic protein-7) and medical application
ActiveCN107164333AGuaranteed stabilityGood compatibilityBone-inducing factorOsteogenic factorBone morphogenetic protein 6Cultured cell
The invention provides a CHO cell strain capable of stably and efficiently expressing recombinant human BMP7 (bone morphogenetic protein-7) and a medical application. A bmp7 gene sequence is constructed by modifying a bmp7 gene of a mouse, a CHO cell serving as a host is also derived from the mouse, the genetic compatibility and the translation efficiency are greatly improved, the defect that gene sequence templates of BMP7 protein expressed by a CHO system are all derived from human bmp7 genes in the prior art is overcome, and the situation that human-sourced gene sequences are difficult to transcribe and translate efficiently by CHO cells due to codon preference is avoided; meanwhile, the adopted CHO-S cells are suspension cultured cells which have been adapted to a serum-free culture medium, the cell strain does not need to be subjected to suspension acclimation, and the engineering CHO cell strain can keep the stability conveniently. The adopted technical means has a remarkable efficiency improvement function in key steps of BMP expression as follows: transcription, translation, folding, conditioned cleavage, secretion, pairing of disulfide bonds and glycoform modification of glycosylation sites.
Owner:长春生物制品研究所有限责任公司
Irradiation and filter device for treatment of blood
InactiveUS7229427B2Reducing free radicals in a patient's bloodReduce concentrationOther blood circulation devicesHaemofiltrationInterleukin 6Filtration
A method and apparatus for preventing and treating septicemia in patient blood is provided. The extracorporeal system includes an antimicrobial device to inactivate at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 50–75% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms, as well as by the patient's cells. These molecules include endotoxins from Gram negative bacteria, exotoxins from Gram negative and Gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, interleukin 6, complement proteins C3a and C5a, and bradykinin.
Owner:HEMAVATION
Neural regeneration peptides and methods for their use
ActiveUS20100130410A1Prevent degenerationAvoid deathBiocideNervous disorderNeuronal migrationCell type
Embodiments of this invention include novel peptides that can promote survival of neurons and other cell types. Other embodiments of this invention include the methods for the use of peptides to promote neuronal migration, neurite outgrowth, neuronal proliferation, neural differentiation, neuronal survival and / or trophoblast proliferation, trophoblast migration and trophoblase survival. NRP compounds may be administered directly to a subject or to a subject's cells by a variety of means including orally, intraperitoneally, intravascularly or indirectly via a replicable vehicle. NRP compounds can be formulated into pharmaceutically acceptable dosage forms for therapeutic use. Kits containing pre-determined doses of an NRP can be used to conveniently store, prepare and administer an NRP to a subject in need thereof.
Owner:CURONZ HLDG
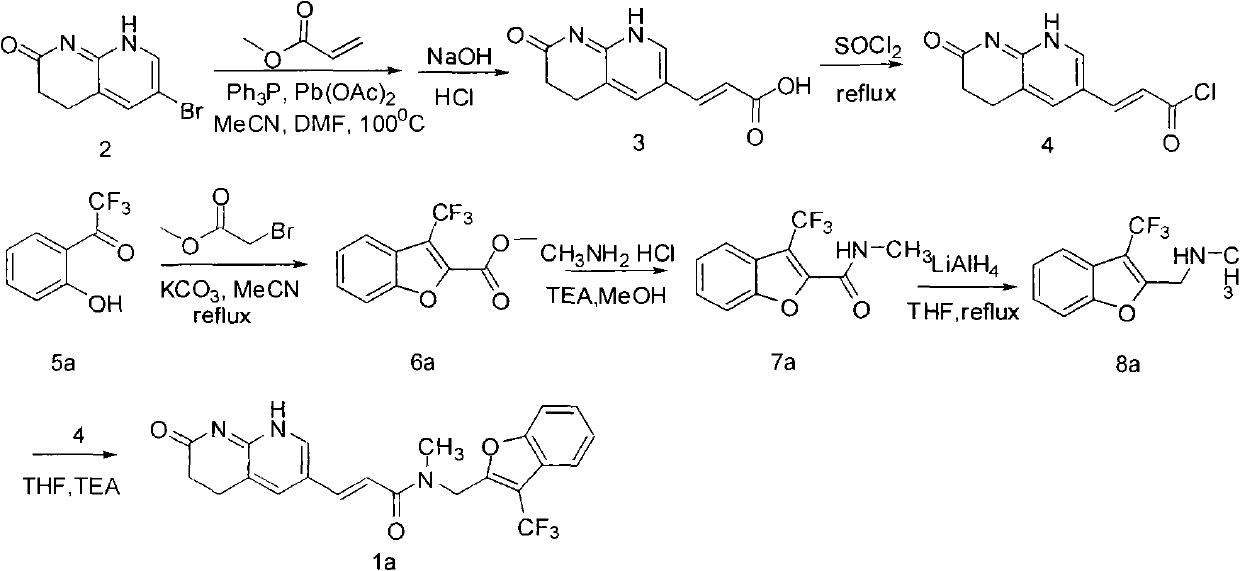
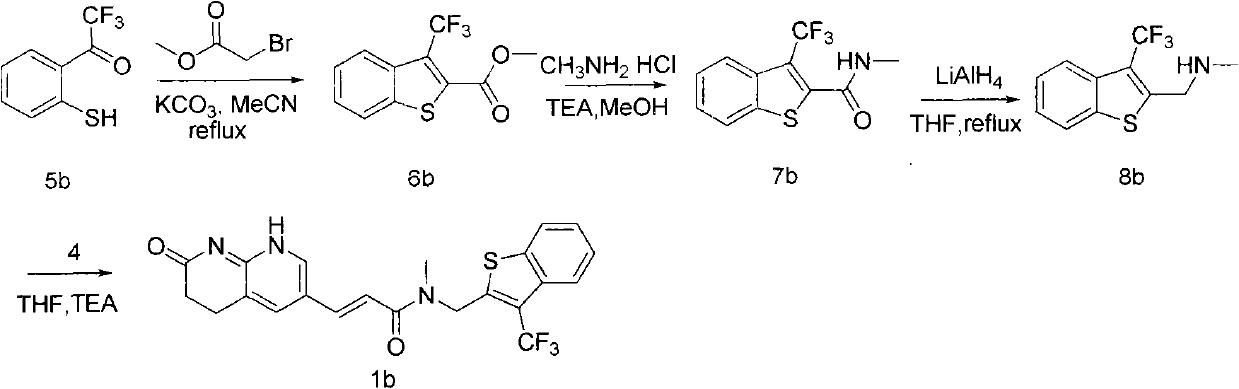
Fluoro-acrylamide derivative
InactiveCN102675311AAntibacterial agentsOrganic active ingredientsStaphylococcus cohniiMethicillin sensitive
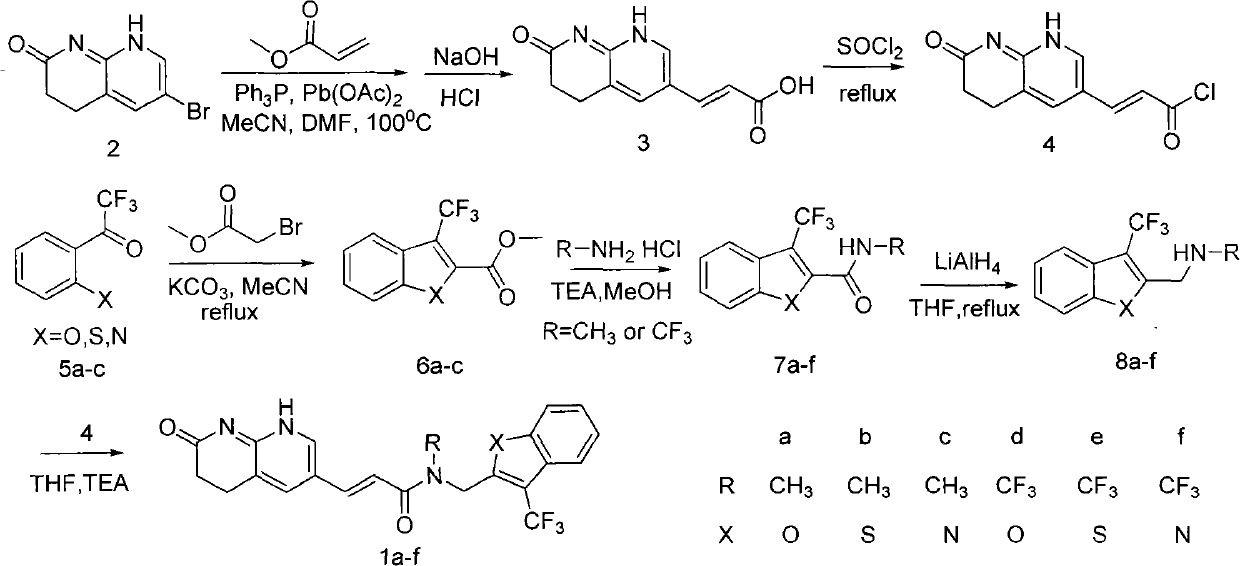
The invention relates to a new compound with remarkable antibacterial and antineoplastic activity, which has the following structure (shown in formula I), and has good antibacterial function for methicillin-resistant staphylococcus aureus (MRSA) and methicillin sensitive staphylococcus aureus (MSSA) as well as good antineoplastic function for human cervical cancer Hela cells, human liver cancer BEL-7402 cells, human mucinous epidermoid lung cancer A549 cells, human breast cancer MCF-7 / s cells and human glioma U251 cells. (In the formula I,) R=-CH3 or CF3, X=O, S or N, and R1=-Cl, -F, -CH3, -CF3, -OCH3 and -OCF3.
Owner:苏春华 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com