Method for creating hepatocyte by human embryo stem cell external evoked differentiation
A technique for inducing differentiation of human embryonic stem cells, applied in the field of spheroid structure-embryoid body, which can solve the problems of unfavorable and sufficient contact, and achieve the effect of facilitating morphological observation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
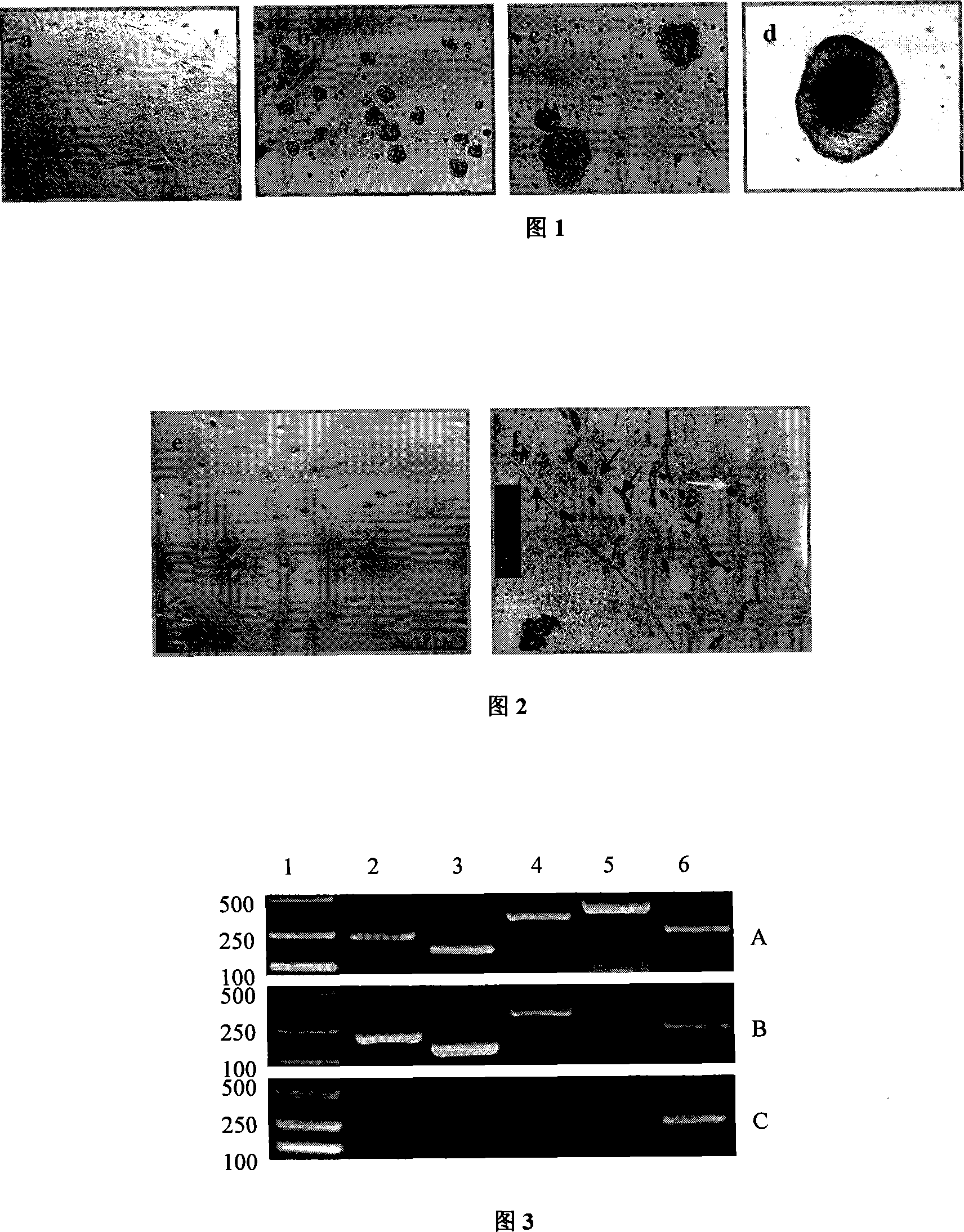
[0029] Embodiment 1, the culture of human ES cell and the formation of EB
[0030] Human ES cell line H9 (purchased from Wicell Company) was inoculated on mouse embryonic fibroblast feeder layer (Kunming mice, serum, 0.25 trypsin-0.02% EDTA and DMEM medium (purchased from Hyclone Company) ( The mitotic activity was inactivated by gamma ray irradiation, seeded on a 0.1% gelatin-coated culture dish), and the culture medium was serum-free human ES cell culture medium: Knockout DMEM medium (purchased from Gibco Company), containing 20% serum Substitute (purchased from Gibco), 0.1mM β-mercaptoethanol (purchased from Gibco), 1mM glutamine (purchased from Gibco), 4ng / ml basic fibroblast growth factor (bFGF, purchased from Chemicon) , 50IU / mL penicillin, 50IU / mL streptomycin, 1% non-essential amino acids (purchased from Gibco company). 37 ° C, 5% CO 2 cultured under the same conditions, and the culture medium was changed every day. The cells grew confluently for about a week, incu...
Embodiment 2
[0033] Example 2, EB digested into single cells
[0034] Under the microscope, use the mouth pipette technique to select EBs that have been cultured for 7 days and have a relatively uniform size, and place them in two bacteria dishes containing phosphate buffered saline (PBS) to wash twice, and then transfer (use the mouth pipette to transfer under the microscope) Digest in a bacteria dish pre-added with 0.25% trypsin (Amresco), 37°C, 5% CO 2 Incubate under the conditions for about 5 minutes until all single cells can be formed by gently pipetting, then add medium containing 10% serum (HYCLONE) to stop digestion for 1 minute, transfer the single cell suspension to a 10ml centrifuge tube with a dropper, 1000rpm , centrifuge for 5 minutes, and discard the supernatant. Example 3, Induced Differentiation of Single Cells to Hepatocytes
Embodiment 3
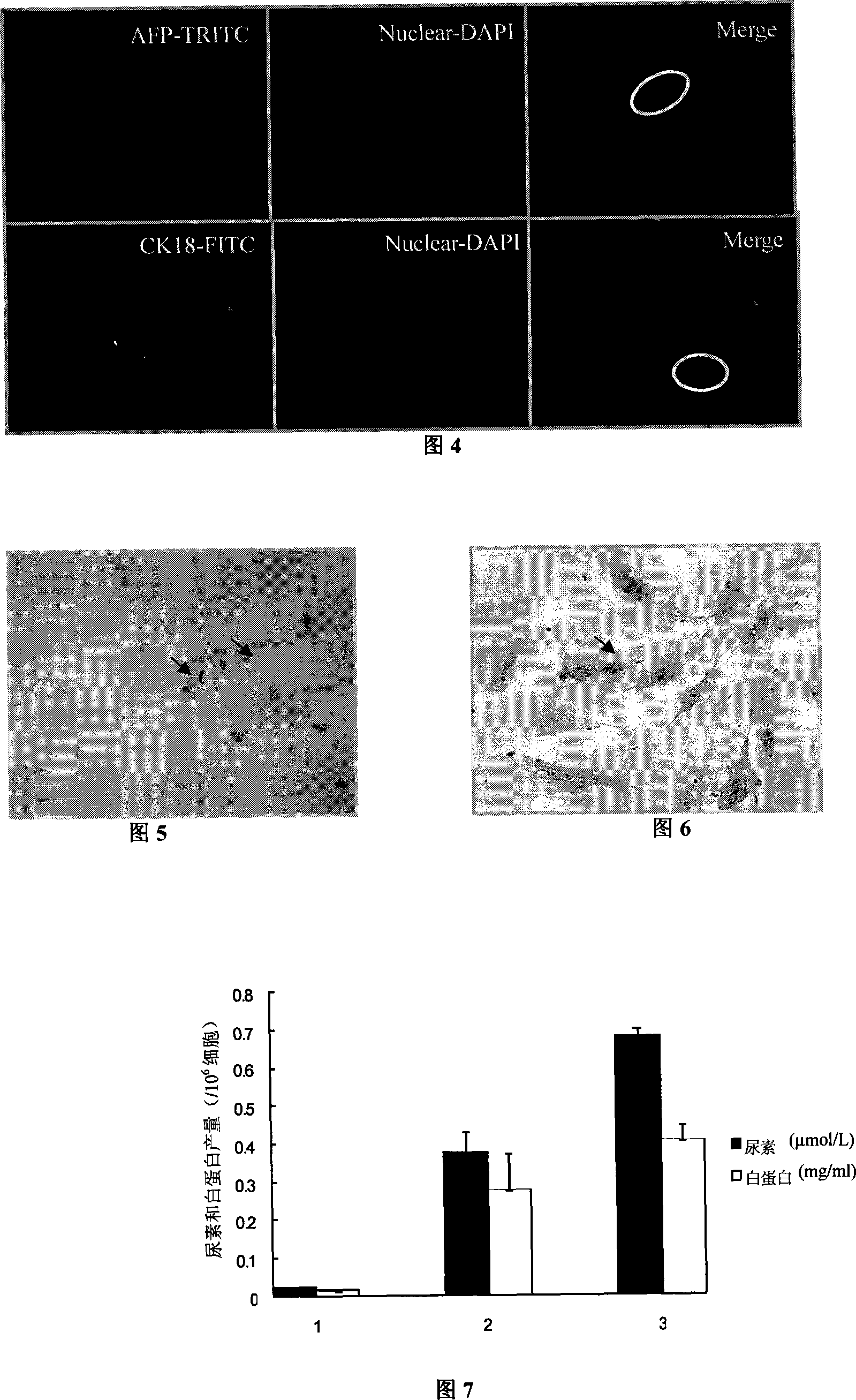
[0035] The pellet obtained by centrifugation in Example 2 was resuspended in the hepatocyte-inducing conditioned medium, and the components of the medium were IMDM medium (Sigma), containing 20% fetal bovine serum (Gibco), 50 nM dexamethasone (Sigma), 0.0625 U / ml human insulin (Lilly France S.A.S). Then inoculated onto culture plates pre-coated with type I collagen. 37°C, 5% CO 2 Cultured under conditioned conditions, the culture medium was changed every two days, and the directional induction and differentiation effect of conditioned cultured was observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com