Fluoro-acrylamide derivative
A technology of drugs and compounds, applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
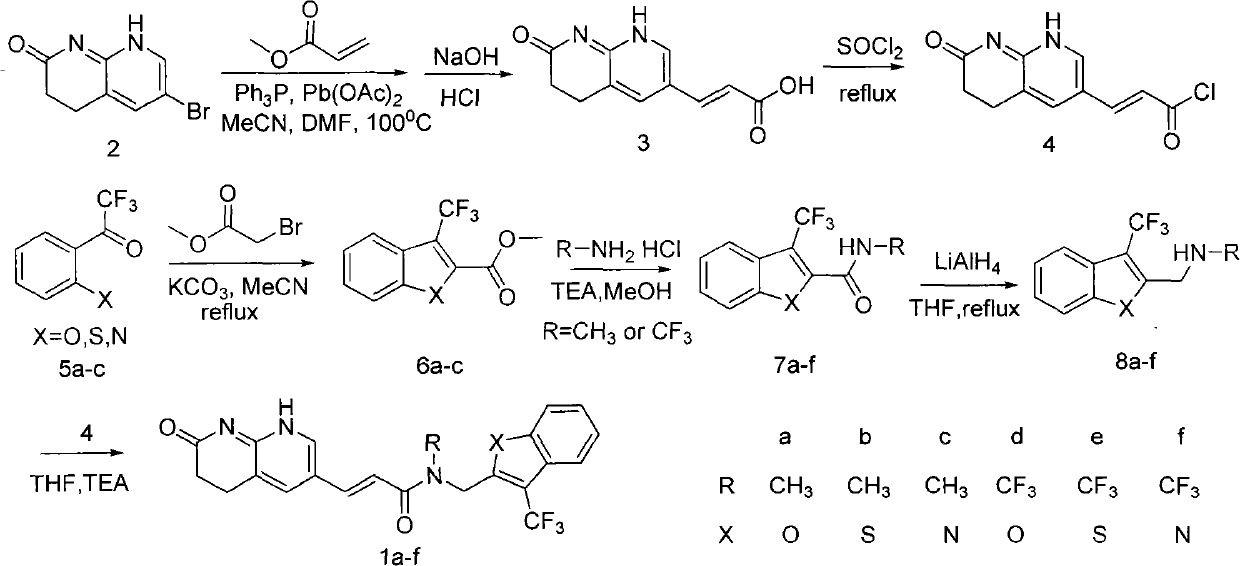
[0006] Embodiment 1: the synthesis of compound 1a
[0007]
[0008] 1) Synthesis of compound 3:
[0009] Dissolve 45.4 grams of raw material 2, 20.6 grams of methyl acrylate and 1.26 grams of triphenylphosphine in 100.0 mL of DMF and 200.0 mL of acetonitrile, add 0.66 grams of palladium acetate, and replace the reaction system with nitrogen three times. Under nitrogen atmosphere, 100 °C overnight. Stop the reaction, cool to room temperature, and filter with suction. Add 400.0 mL of 10.0% sodium hydroxide solution to the filtrate to remove the solvent under reduced pressure. After stirring for 3 hours, adjust the pH to about 3.0 with concentrated hydrochloric acid. A large amount of solids are precipitated. Filter with suction. The filter cake was recrystallized with ethanol, and dried under vacuum at 50° C. for 5 hours to obtain 26.5 g of compound 3 with a yield of 61%. HNMR (400Hz, CDCl 3 ): 11.01(s, 1H), 8.91(s, 1H), 8.37(s, 1H), 8.02(s, 1H), 7.61(d, J=13.6Hz, 1H), 6.4...
Embodiment 2
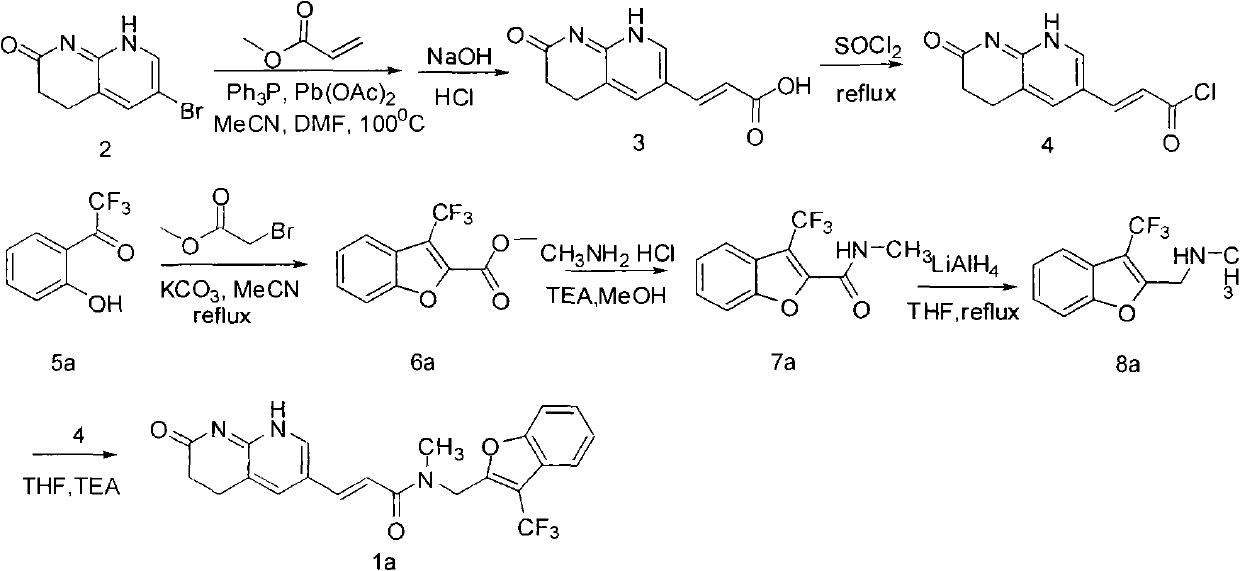
[0020] Embodiment 2: the synthesis of compound 1b
[0021]
[0022] 1) Synthesis of compound 6b:
[0023] 0.2 mol of raw material 5b, 0.24 mol of methyl bromoacetate and 0.6 mol of anhydrous potassium carbonate were refluxed overnight in 200 mL of acetonitrile. Heating was stopped, cooled to room temperature, suction filtered, and the filtrate was distilled under reduced pressure and then recrystallized with ethanol to obtain compound 6b. C 11 h 7 f 3 o 2 S, 37.4 g, 72% yield. MS (m / z): 261.2.
[0024] 2) Synthesis of compound 7b:
[0025] 0.05 mol of compound 6b, 0.1 mol of methylamine hydrochloride and 0.1 mol of triethylamine were added to 100.0 mLd of methanol, and stirred at 60° C. for 5 hours. The reaction was stopped, the reaction liquid was cooled to room temperature, methanol was distilled off under reduced pressure, and 100.0 mL of ice water was added, a large amount of solids were precipitated, filtered with suction to obtain a solid, which was washed wit...
Embodiment 3
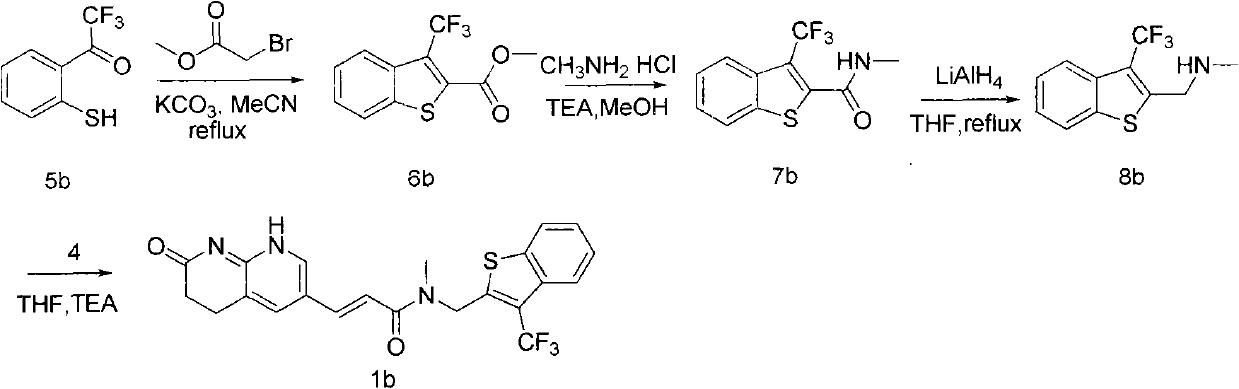
[0030] Embodiment 3: the synthesis of compound 1c:
[0031]
[0032] 1) Synthesis of compound 6c:
[0033] 0.2 mol of starting material 5c, 0.24 mol of methyl bromoacetate and 0.6 mol of anhydrous potassium carbonate were refluxed overnight in 200 mL of acetonitrile. Heating was stopped, cooled to room temperature, suction filtered, and the filtrate was distilled under reduced pressure and then recrystallized with ethanol to obtain compound 6c. C 11 h 8 f 3 o 2 N, 27.2 g, 56% yield. MS (m / z): 244.2.
[0034] 2) Synthesis of compound 7c:
[0035] 0.05 mol of compound 6c, 0.1 mol of methylamine hydrochloride and 0.1 mol of triethylamine were added to 100.0 mLd of methanol, and stirred at 60° C. for 5 hours. The reaction was stopped, the reaction liquid was cooled to room temperature, methanol was distilled off under reduced pressure, and 100.0 mL of ice water was added, a large amount of solids precipitated, filtered by suction to obtain a solid, which was washed with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com