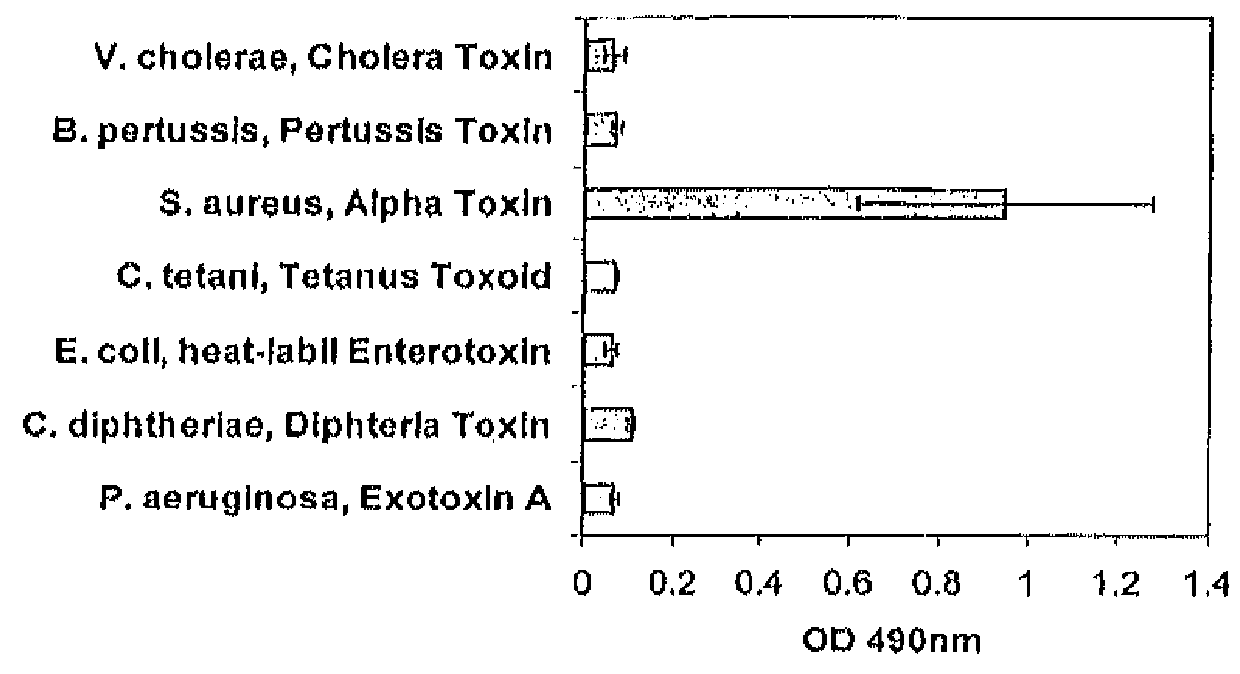
Patents
Literature
54 results about "Alpha-toxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Use of alpha-toxin for treating and preventing staphylococcus infections
InactiveUS20090053235A1Low toxicityAntibacterial agentsBacterial antigen ingredientsBacteroidesAlpha-toxin
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Use of alpha-toxin for treating and preventing staphylococcus infections
InactiveUS20080131457A1Low toxicityAntibacterial agentsBacterial antigen ingredientsBacteroidesAlpha-toxin
Vaccines comprising an S. aureus alpha-toxin antigen and a pharmaceutically acceptable carrier are provided, and are useful for treating and preventing infections. The S. aureus alpha-toxin antigen may contain at least two alterations that reduce its toxicity and / or may be conjugated to or co-administered with another bacterial antigen. The vaccines may comprise one or more other bacterial antigens. Antibody compositions comprising antibodies to alpha-toxin and optionally one or more other bacterial antigens also are provided, and are useful for treating and preventing infections.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
C. perfringens alpha toxoid vaccine
Owner:SCHERING PLOUGH LTD +2
Antibodies that specifically bind Staphylococcus aureus alpha toxin and methods of use
ActiveUS9527905B2Avoid managementAvoid adjustmentAntibacterial agentsAntipyreticStaphylococcus cohniiAlpha-toxin
Herein provided are compositions, methods of manufacture and methods of use pertaining to anti-alpha toxin antibodies and fragments.
Owner:MEDIMMUNE LLC
Antibodies that specifically bind staphylococcus aureus alpha toxin and methods of use
ActiveUS20140072577A1Prevents skin infection conditionAvoid adjustmentAntibacterial agentsAntipyreticStaphylococcus cohniiAlpha-toxin
Herein provided are compositions, methods of manufacture and methods of use pertaining to anti-alpha toxin antibodies and fragments.
Owner:MEDIMMUNE LLC
Fast detection method for clostridium perfringens, detection primer group and detection kit
InactiveCN102134590AAccurate detectionStrong specificityMicrobiological testing/measurementAlpha-toxinBacillus perfringens
The invention discloses a detection method for clostridium perfringens, a primer group and a detection kit for same. The primer group is based on the Loop-Mediated Isothermal Amplification (LAMP), and is obtained through analysis, design and artificial synthesis on the special alpha toxin (C. perfringens alpha toxin, CPa) gene sequence of the clostridium perfringens, the contained nucleotide sequence is shown in SEQ No.1-4, and the primer group has considerably high specificity to the clostridium perfringens. The fast detection method for the clostridium perfringens carries out LAMP reaction on the DNA of the clostridium perfringens in a sample by utilizing the detection primer group, and whether the clostridium perfringens is contained in the sample or not is judged by identifying reaction products. The invention also designs the fast detection kit for the clostridium perfringens according to the detection method, so that fast, simple, accurate and efficient clostridium perfringens detection and identification can be carried out on the sample.
Owner:浙江省质量技术监督检测研究院
Method to produce a tampon that inhibits exoprotein production from gram positive bacteria
InactiveUS7056891B2Inhibit productionImprove abilitiesBiocideCarbohydrate active ingredientsBacteroidesAlpha-toxin
A method to inhibit the production of exoproteins from Gram positive bacteria, such as harmful proteins produced by Staphylococcus species, is described. The method is particularly useful to inhibit the production of TSST-1, alpha-toxin and / or enterotoxins A, B and C from Staphylococcus aureus bacteria. The method is based on exposing Gram positive bacteria to alkyl polyglycoside incorporated into an absorbent product. Alternate methods include bringing Gram positive bacteria into contact with the alkyl polyglycoside in other forms, e.g., when formulated with a pharmaceutically acceptable carrier or incorporated in or on a non-absorbent substrate. Typically, the alkyl polyglycoside has a hydrophilic / lipophilic balance (HLB) of at least about 10 and an alkyl group with an average of 8 to 14 carbon atoms.
Owner:KIMBERLY-CLARK WORLDWIDE INC
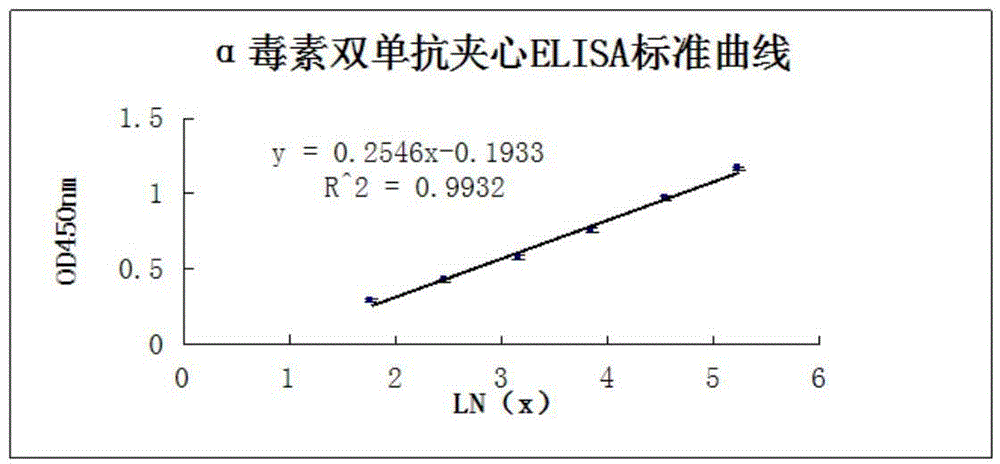
Clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method
ActiveCN103941004AStrong specificityStrong responsivenessMaterial analysisAlpha-toxinClostridium perfringens toxoid
The invention relates to a clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method. According to the clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method, alpha toxin protein obtained via prokaryotic expression is taken as an immunogen; monoclonal antibodies obtained via hybridoma technique are taken as detection antibodies and capture antibodies; reaction conditions are optimized via experiments; alpha toxin protein samples with a series of concentration are used for construction of a standard curve; the double-antibody sandwich ELIS method is established; and indexes of the double-antibody sandwich ELIS are verified. The clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method specifically comprises following steps: (1) prokaryotic expression of alpha toxin; (2) preparation of anti-alpha toxin monoclonal antibodies; (3) establishment of the double-antibody sandwich ELIS method; (4) establishment of the standard curve; and (5) performance evaluation on the double-antibody sandwich ELIS method. The clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method is excellent in specificity, and high in sensitivity and stability, is fast and convenient, and can be used for effective quantitative determination of alpha toxin in A-E type clostridium perfringens cultural supernatants; and the high-efficient detection method is provided for alpha toxin determination.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Recombinant attenuated clostridium organisms and vaccine
The present invention discloses attenuated Clostridium perfringens organisms that express a substantially nontoxic alpha-toxin. The expressed alpha-toxin is a deletion mutein that relative to the alpha-toxin of the mature alpha-toxin of Clostridium perfringens strain 13, is missing at least nine consecutive amino acid residues including His68. The present invention also discloses attenuated organisms that encode the muteins, as well as the use of such attenuated organisms as vaccines.
Owner:INTERVET INC
Monoclonal antibody blocking ELISA (enzyme-linked immuno sorbent assay) testing method of clostridium perfringens alpha toxin
The invention discloses a monoclonal antibody blocking ELISA testing method of a clostridium perfringens alpha toxin, which is used for early testing of the alpha toxin and provides an effective testing tool for early diagnosis, spreading prevention and subsequent research of clostridium perfringens. The method comprises the following two steps: I, establishing the monoclonal antibody blocking ELISA testing method; and II, determining a result judgment criterion. The monoclonal antibody blocking ELISA testing method which has good specificity and stability and high sensitivity, is quick, simple and convenient, and tests the alpha toxin efficiently is established for the first time, and the method is simple to implement and high in testing speed and can achieve quantitative testing and massive testing. In a word, monoclonal antibody blocking ELISA is expected to play an important role in prevention and treatment of clostridium perfringens diseases.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Clostridium septicum alpha toxin recombinant subunit vaccine and production method thereof
ActiveCN107812183AReduce Biosecurity RisksEasy to purifyAntibacterial agentsBacterial antigen ingredientsAntigenAlpha-toxin
The invention relates to a clostridium septicum alpha toxin recombinant subunit vaccine and a production method thereof. According to the production method, 54-site cysteine of wild clostridium septicum alpha mature toxin is mutated into leucine, 264-site asparaginate is mutated into alanine, 269-site histidine is mutated into alanine, and 310-site tryptophan is mutated into alanine, so that an alpha toxin mutant which is nontoxic to animals is obtained; and 6 groups of histidine tags are added to a C end of a mutant protein so as to obtain an antigen for preparing vaccines. The invention simultaneously discloses a recombinant expression vector and a recombinant host cell which contain an encoding gene of a nontoxic alpha toxin mutant of clostridium septicum. The efficacies of the preparedclostridium septicum alpha toxin recombinant subunit vaccine are far higher than the efficacies of existing vaccines, and the bio-safety risk in the production process of the vaccine is greatly reduced. Besides, by virtue of the superiority that a semi-finished product of the vaccine is high in protein concentration, a mixed vaccine can be prepared without increasing the dosage of the vaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Methods of using Anti-alpha toxin antibody
InactiveUS20190077851A1Reduce riskAntibacterial agentsImmunoglobulins against bacteriaAntigenAlpha-toxin
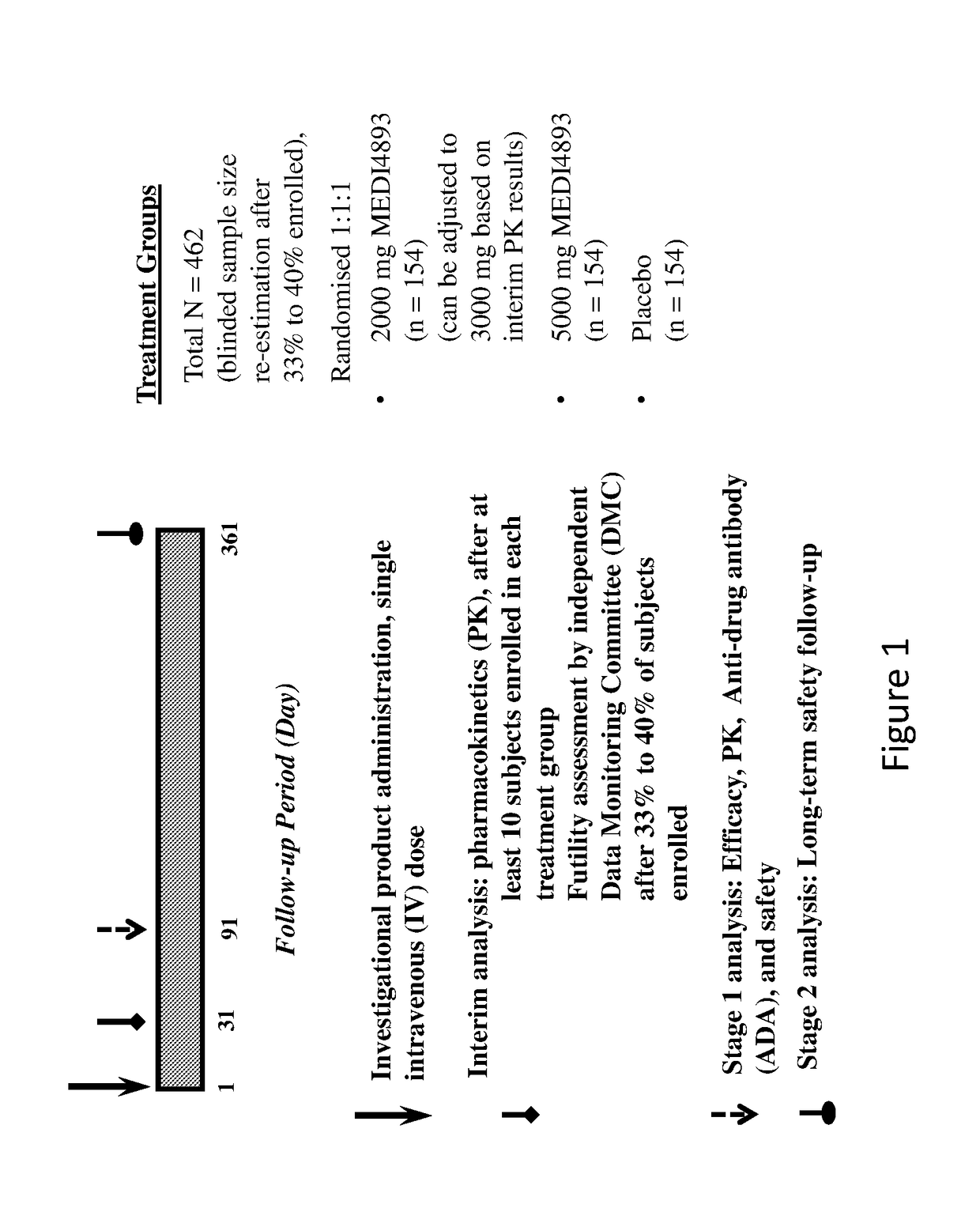
Provided herein are methods of preventing and treating a bacterial infection, e.g., a Staphylococcus aureus infection, in a patient, comprising administering to the patient an effective amount of an anti-alpha toxin antibody or an antigen-binding fragment thereof, such as MEDI4893.
Owner:MEDIMMUNE LLC
Cross-reactive staphylococcus aureus antibody sequences
InactiveUS20160244511A1Additional componentHigh neutralizing potencyAntibacterial agentsSenses disorderComplementarity determining regionStaphylococcus cohnii
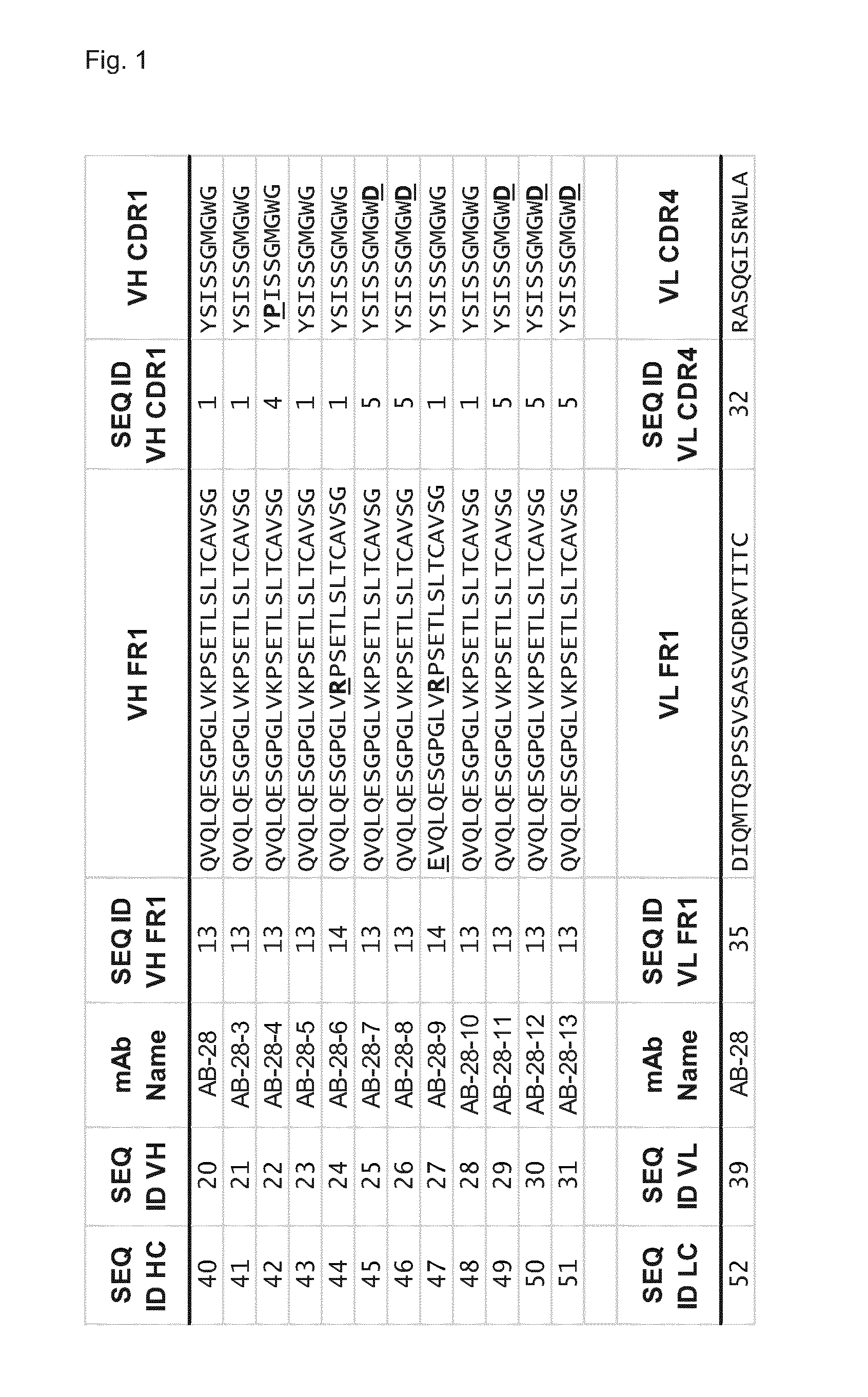
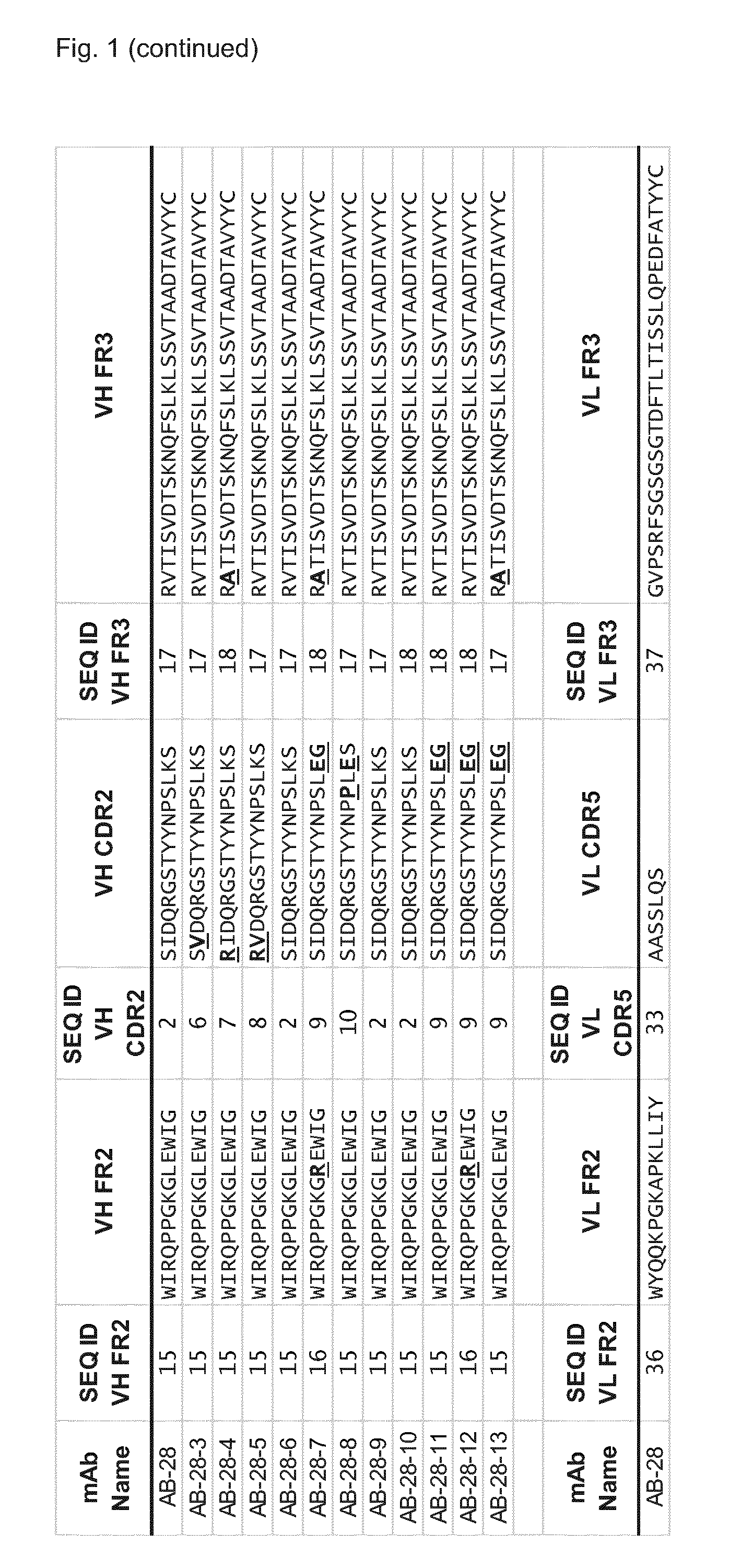
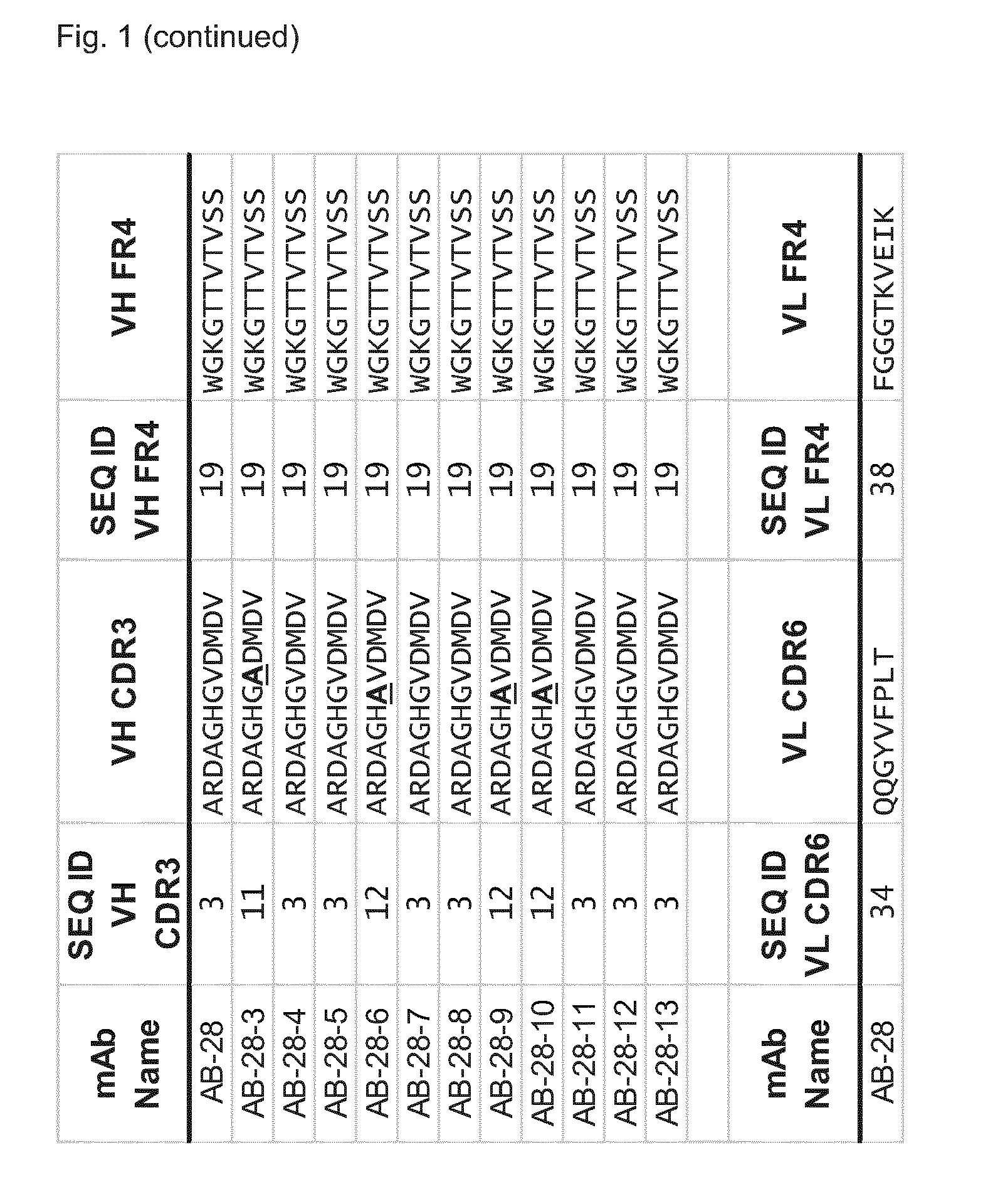
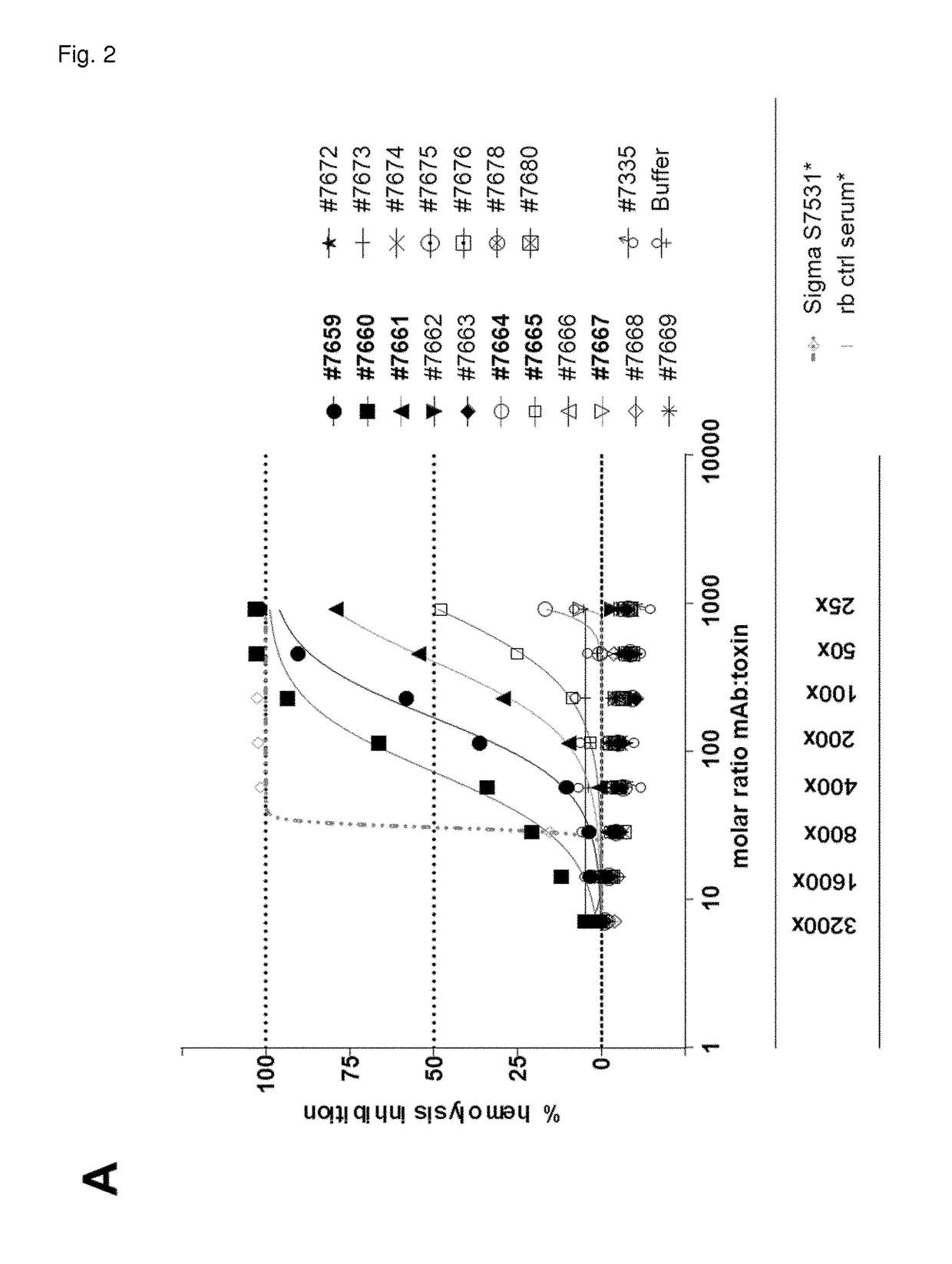
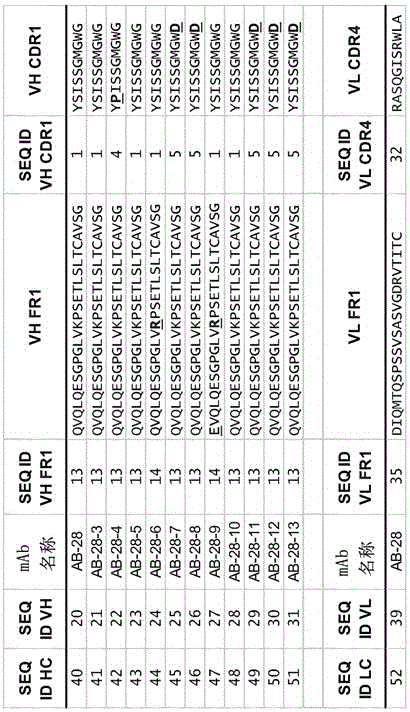
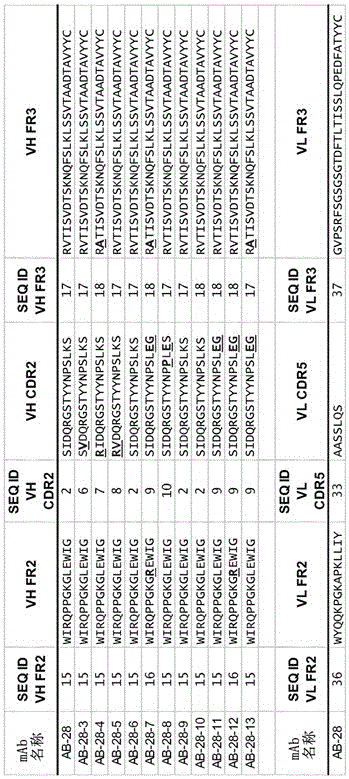
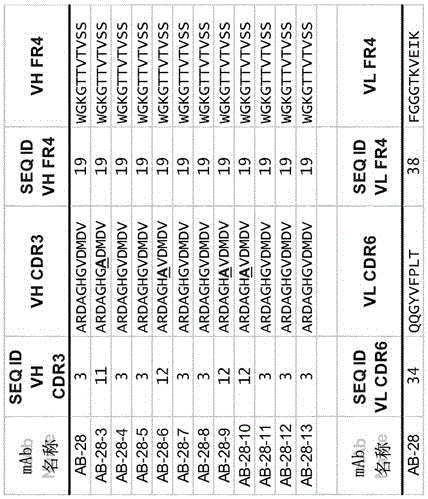
The invention refers to a cross-neutralizing antibody comprising at least one polyspecific binding site that binds to alpha-toxin (Hla) and at least one of the bi-component toxins of Staphylococcus aureus, which antibody comprises at least three complementarity determining regions (CDR1 to CDR3) of the antibody heavy chain variable region (VH), wherein A) the antibody comprises a) a CDR1 comprising or consisting of the amino acid sequence YSISSGMGWG (SEQ ID 1); and b) a CDR2 comprising or consisting of the amino acid sequence SIDQRGSTYYNPSLKS (SEQ ID 2); and c) a CDR3 comprising or consisting of the amino acid sequence ARDAGHGVDMDV (SEQ ID 3); or B) the antibody comprises at least one functionally active CDR variant of a) the parent CDR1 consisting of the amino acid sequence of SEQ ID 1; or b) the parent CDR2 consisting of the amino acid sequence of SEQ ID 2; or c) the parent CDR3 consisting of the amino acid sequence of SEQ ID 3; wherein the functionally active CDR variant comprises at least one point mutation in the parent CDR sequence, and comprises or consists of the amino acid sequence that has at least 60% sequence identity with the parent CDR sequence. It further refers to such cross-neutralizing antibody which is a functionally active variant antibody of a parent antibody that comprises a polyspecific binding site of the VH amino acid sequence of SEQ ID 20, and the VL amino acid sequence of SEQ ID 39, which functionally active variant antibody comprises at least one point mutation in any of the framework regions (FR) or constant domains, or complementarity determining regions (CDR1 to CDR6) in any of SEQ ID 20 or SEQ 39, and has an affinity to bind each of the toxins with a Kd of less than 10−8M, preferably less than 10−9M.
Owner:ARSANIS BIOSCI
Bovine type-A clostridium perfringens subunit vaccine and preparation method and application thereof
ActiveCN107596361AStrong immune responseOvercoming the problem of incomplete inactivationAntibacterial agentsBacterial antigen ingredientsAntigenAlpha-toxin
The invention provides a bovine type-A clostridium perfringens subunit vaccine and a preparation method and an application thereof. The subunit vaccine comprises a bovine type-A clostridium perfringens alpha-toxin protein truncation body and a pharmacologically acceptable adjuvant. The bovine type-A clostridium perfringens alpha-toxin protein truncation body is an antigen determinant amino acid sequence from 255th to 372nd amino acids of alpha-toxin protein. The subunit vaccine has the following advantages that 1) safety problems due to uncompleted inactivation are not existed; 2) the qualityis controllable, and the difference between the batches is not existed; 3) the production equipment and space requirement is low, the expression level is high, and the cost is low; and 4) toxin dispersion risk is not existed, and the safety of operators can be guaranteed.
Owner:NOVO BIOTECH CORP
Multi-PCR detection kit capable of simultaneously detecting clostridium perfringens, clostridium hemolyticumbovis and B-type clostridium novyi and application of multi-PCR detection kit
ActiveCN106148548AImprove featuresIncreased sensitivityMicrobiological testing/measurementAlpha-toxinBacillus perfringens
The invention relates to a multi-PCR detection kit capable of simultaneously detecting clostridium perfringens, clostridium hemolyticumbovis and B-type clostridium novyi and application of the multi-PCR detection kit, and belongs to the technical field of PCR detection kits. The kit comprises primers PrimerF1 and PrimerR1 which are designed by taking alpha toxin group of the clostridium perfringens as templates, and primers PrimerF2, PrimerR2 and PrimerR3 which are designed by taking flagellin genes FliA(C) and FliA (H) of the clostridium hemolyticumbovis and flagellin gene FliA of the B-type clostridium novyi as templates. The kit is convenient to operate, low in cost and high in specificity, and time and labor are saved.
Owner:QINGHAI ACAD OF ANIMAL SCI & VETERINARY MEDICINE
Human monoclonal antibody against S. aureus derived alpha-toxin and its use in treating or preventing abscess formation
ActiveUS9249215B2Improve the immunityPromote secretionAntibacterial agentsPeptide/protein ingredientsAlpha-toxinMonoclonal antibody
The present invention includes a human monoclonal antibody specific for the alpha-toxin of S. aureus, a hybridoma producing it, nucleic acids encoding it, and host cells transfected therewith. Further, the present invention includes methods for producing the monoclonal antibody. In addition, the present invention includes pharmaceutical compositions comprising at least one antibody or at least one nucleic acid encoding the antibody. Further, the present invention includes the use of the monoclonal antibody for treating or preventing abscess formation.
Owner:ARIDIS PHARMA INC
Method of producing a tampon capable of inhibiting exoprotein production from gram positive bacteria
InactiveUS20040053856A1Inhibit productionReduce toxin productionBiocideCarbohydrate active ingredientsBacteroidesGlycoside
A method of inhibiting the production of exoproteins from Gram positive bacteria, such as harmful proteins produced by Staphylococcus species, is described. The method is particularly useful for inhibiting the production of TSST-1, alpha-toxin and / or enterotoxins A, B and C from S. aureus bacteria. The method may be based on exposing Gram positive bacteria to alkyl polyglycoside incorporated into an absorbent product. Alternatively, the methods can include causing Gram positive bacteria to come into contact with the alkyl polyglycoside in other forms, e.g., when formulated with a pharmaceutically acceptable carrier or incorporated in or on a non-absorbent substrate. Typically, the alkyl polyglycoside has an HLB of at least about 10 and an alkyl group with an average of 8 to 14 carbon atoms.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Clostridium septicum alpha toxin genetic engineering vaccine and production method thereof
ActiveCN109395072AReduce Biosecurity RisksEasy to purifyAntibacterial agentsBacterial antigen ingredientsAlpha-toxinInclusion bodies
The invention provides a prepared clostridium septicum CSA genetic engineering vaccine, which is optimized by a codon and is produced by recombinant clostridium septicum CSA (rCSAM4 delta 11) obtainedby deleting 11 amino acids (212th bit to 222nd bit) on the basis of containing 4 amino acid mutations (C54L, N264A, H269A and W310A); the completeness and spatial conformation of the natural toxin are reserved to the maximum degree, so that the immunogenicity is maintained; the biological safety hazards due to minority amino acid mutation are avoided. Meanwhile, nontoxic rCSAM4 delta 11 can be expressed in a soluble form, so that the influence of the complicated process of the inclusion body denaturation and renaturation on antigen protein immunogenicity is avoided, and the preparation time and the production cost of the vaccine are reduced. In addition, the vaccine also has the advantages of low immunizing dose, good immunizing effect and the like, and belongs to an ideal candidate vaccine for upgrading and updating the existing clostridium septicum toxin vaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Clostridium perfringen alpha toxin genetic engineering vaccine and application thereof
InactiveCN103861094AImprove immunityTargeted optimizationAntibacterial agentsBacterial antigen ingredientsDiseaseAlpha-toxin
The invention relates to a clostridium perfringen alpha toxin genetic engineering vaccine and application thereof. The clostridium perfringen alpha toxin genetic engineering vaccine is prepared by designing a specific primer, implementing polymerase chain reaction (PCR) amplification on A-type clostridium perfringen DNA to obtain an alpha toxin gene segment, cloning the gene segment onto a pMD18-T Vector, selecting a positive recon and linking the positive recon onto a PET-28 alpha (+) plasmid; introducing the recombinant plasmid to a receptor strain to successfully construct a recombinant strain BL21 (DE3)-alpha; emulsifying the prepared alpha toxin protein and an aluminum hydroxide adjuvant at a volume ratio of 9:1, and adding 0.01% thiomersal to obtain the clostridium perfringen alpha toxin genetic engineering vaccine. The clostridium perfringen alpha toxin genetic engineering vaccine is easy for realization of industrial production, simple to operate and good in safety, and is capable of effectively preventing such diseases as animal necrotic enteritis, enterotoxemia, and human and animal traumatic gas gangrene caused by the A-type clostridium perfringens.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Envision immunohistochemical detection method of Clostridum Perfringens alpha toxin
InactiveCN106093404AStrong specificityIncreased sensitivityBiological material analysisAlpha-toxinMonoclonal antibody
The invention relates to the field of animal bacteriology, and provides an Envision immunohistochemical detection method of Clostridum Perfringens alpha toxin. The Envision immunohistochemical detection with the advantages of high specificity, good repeatability, low background and realization of visual and simple detection of the alpha toxin is established on the basis of a mouse anti-alpha toxin monoclonal antibody. The method can be used for detecting the Clostridum Perfringens alpha toxin in tissues of animals except mice, and provides an effective and reliable way for detection of the distribution of the toxin in the animal bodies.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
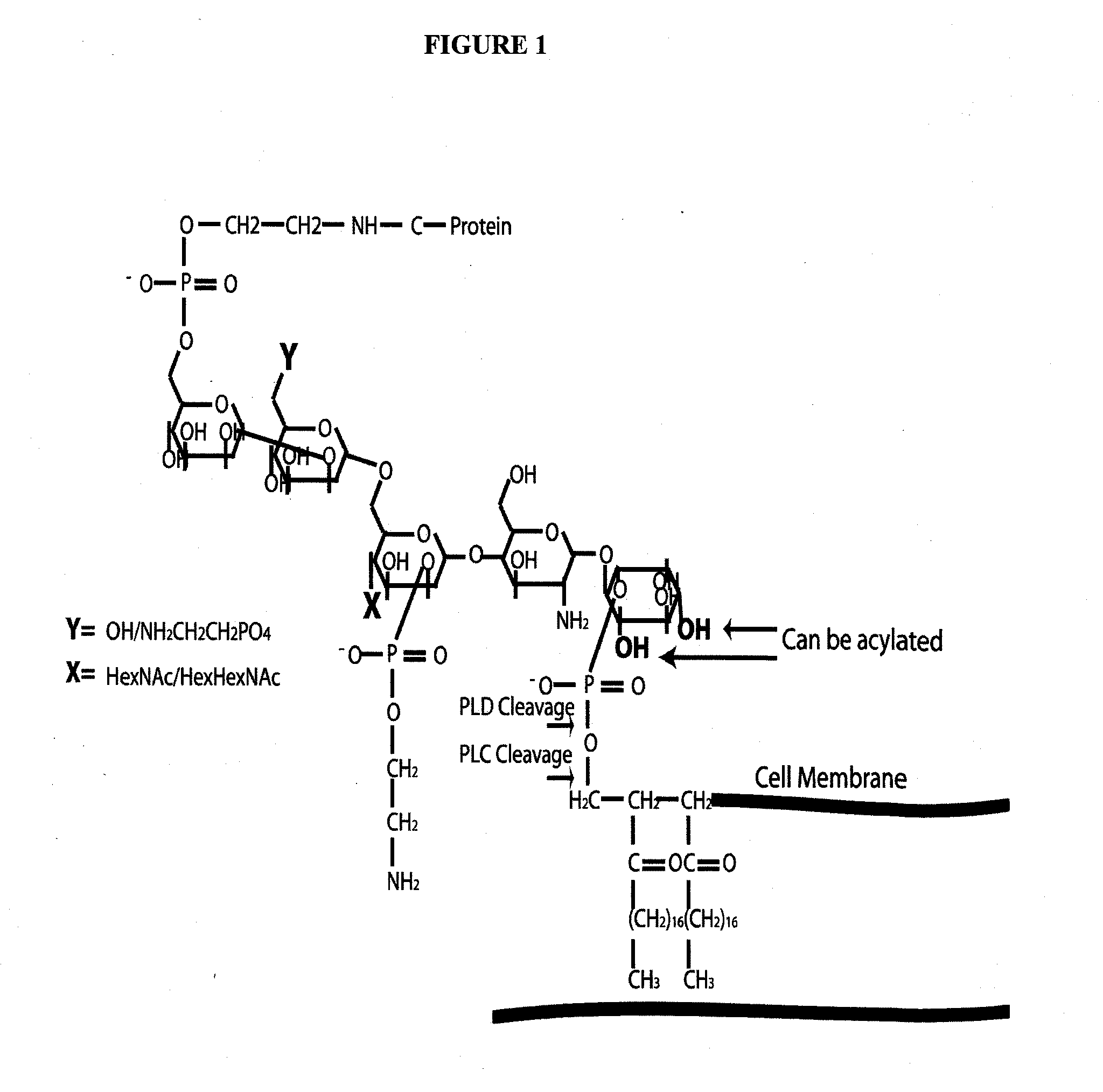
Alpha toxin detection of gpi anchored proteins
InactiveUS20140315212A1Cell receptors/surface-antigens/surface-determinantsMicrobiological testing/measurementDiseaseProstate cancer
The present invention relates to a method for the purification, concentration and identification of glycosylphosphatidylinositol anchored proteins (GPI-APs) from a biological sample (cells, tissues and / or blood / serum) in a patient or subject, including a human patient or subject. A new method to separate GPI-anchored glycoproteins, a class of glycoproteins found in all animal cells and fluids including serum, from other glycoproteins and proteins for the purpose of identifying potential biomarkers for various diseases, including cancer, especially breast cancer, vaginal cancer, endometrial cancer, uterine cancer, cervical cancer, pancreatic cancer and prostate cancer. The method uses the alpha-toxin from Clostridium septicum to separate GPI-anchored glycoproteins for identification and optionally quantification. The GPI-APs so obtained may be used to raise antibodies for inclusion in an immunosorbent assay for the diagnosis or the monitoring of therapy of cancer in a patient.
Owner:UNIV OF GEORGIA RES FOUND INC
Use of alpha-toxin for treating and preventing staphylococcus infections
InactiveUS20150165015A1Low toxicitySerum immunoglobulinsImmunoglobulins against bacteriaBacteroidesAlpha-toxin
Vaccines comprising an S. aureus alpha-toxin antigen and a pharmaceutically acceptable carrier are provided, and are useful for treating and preventing infections. The S. aureus alpha-toxin antigen may contain at least two alterations that reduce its toxicity and / or may be conjugated to or co-administered with another bacterial antigen. The vaccines may comprise one or more other bacterial antigens. Antibody compositions comprising antibodies to alpha-toxin and optionally one or more other bacterial antigens also are provided, and are useful for treating and preventing infections.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Clostridium perfringen alpha toxin genetic engineering vaccine and preparation method thereof
ActiveCN109602898APreserve immunogenicityReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsAlpha-toxinWild type
The invention provides a clostridium perfringen alpha toxin genetic engineering vaccine and a preparation method thereof. Compared with wild type alpha toxins, clostridium perfringen alpha toxin recombination protein is characterized in that the 176th site histidine of an amino acid sequence is mutated to obtain asparagine. The clostridium perfringen alpha toxin genetic engineering vaccine is prepared from detoxifcation clostridium perfringen alpha toxin recombination protein of self-induction secretory expression. The vaccine has the advantages of being safer, better in immune efficacy, simpler in technology, lower in cost and the like, and can effectively solve the problems that in the prior art, the production technology of the vaccine is complexer, and the cost is higher.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Recombinant epsilon toxin and alpha toxin fusion protein vaccine of non-toxic clostridium perfringens and production method of fusion protein vaccine
ActiveCN109745554AHigh expressionNo virulenceAntibacterial agentsBacterial antigen ingredientsDiseaseEscherichia coli
The invention relates to a recombinant epsilon toxin and alpha toxin fusion protein vaccine of non-toxic clostridium perfringens and a production method of the vaccine. According to an ETX mutant of non-toxic clostridium perfringens and C end fusion protein rETXm3CPAC of CPA, the non-toxic ETX mutant ETXm3 is in serial connection with a C end (CPAC) of CPA, soluble expression is achieved in escherichia coli BL21(DE3), the spatial conformation of natural toxin protein can be reserved to the highest extent, and accordingly the immunogenicity of escherichia coli can be maintained; the influence of a complex technology of inclusion body denaturation and renaturation on immunogenicity of the antigen protein is also avoided, the time of preparing the vaccine is shortened, and the production costis reduced. The C end of rETXm3CPAC contains six histidine (6*His) labels, and convenience is provided for protein purification; the obtained toxin fusion protein is completely free of toxicity in amouse body and has good safety, immunogenicity and immune protection performance in a rabbit model. The vaccine also has the advantages that the preparation technology is good, the efficacy of the vaccine is excellent, and A-type and D-type clostridium perfringens diseases are prevented at the same time.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Cross-reactive Staphylococcus aureus antibody
InactiveUS9914767B2Antibacterial agentsImmunoglobulins against bacteriaAlpha-toxinStaphylococcus cohnii
The subject relates to a cross-neutralizing antibody comprising at least one polyspecific binding site that binds to alpha-toxin (Hla) and at least one of the bi-component toxins of Staphylococcus aureus, its medical and diagnostic use, method of producing the antibody, including an isolated nucleotide sequence, plasmids and host cells as used in the production of the antibody; and further an isolated conformational epitope recognized by a specific cross-neutralizing antibody.
Owner:ARSANIS BIOSCI
Cross-reactive staphylococcus aureus antibody
InactiveUS20150086539A1Improved cross-reactiveImproved cross-neutralizing potencyAntibacterial agentsLibrary screeningStaphylococcus cohniiAlpha-toxin
The subject relates to a cross-neutralizing antibody comprising at least one polyspecific binding site that binds to alpha-toxin (Hla) and at least one of the bi-component toxins of Staphylococcus aureus, its medical and diagnostic use, method of producing the antibody, including an isolated nucleotide sequence, plasmids and host cells as used in the production of the antibody; and further an isolated conformational epitope recognized by a specific cross-neutralizing antibody.
Owner:ARSANIS BIOSCI
Recombinant attenuated clostridium organisms and vaccine
The present invention discloses attenuated Clostridium perfringens organisms that express a substantially nontoxic alpha-toxin. The expressed alpha-toxin is a deletion mutein that relative to the alpha-toxin of the mature alpha-toxin of Clostridium perfringens strain 13, is missing at least nine consecutive amino acid residues including His68. The present invention also discloses attenuated organisms that encode the muteins, as well as the use of such attenuated organisms as vaccines.
Owner:INTERVET INC
C. perfringens alpha toxoid vaccine
Owner:SCHERING PLOUGH LTD +2
Cross-reactive staphylococcus aureus antibody sequences
InactiveCN105873946AAntibacterial agentsSenses disorderComplementarity determining regionStaphylococcus cohnii
The invention refers to a cross-neutralizing antibody comprising at least one polyspecific binding site that binds to alpha-toxin (Hla) and at least one of the bi- component toxins of Staphylococcus aureus, which antibody comprises at least three complementary determining regions (CDR1 to CDR3) of the antibody heavy chain variable region (VH), wherein A) the antibody comprises a) a CDR1 comprising or consisting of the amino acid sequence YSISSGMGWG (SEQ ID 1); and b) a CDR2 comprising or consisting of the amino acid sequence SIDQRGSTYYNPSLKS (SEQ ID 2); and c) a CDR3 comprising or consisting of the amino acid sequence ARDAGHGVDMDV (SEQ ID 3); or B) the antibody comprises at least one functionally active CDR variant of a) the parent CDR1 consisting of the amino acid sequence of SEQ ID 1; or b) the parent CDR2 consisting of the amino acid sequence of SEQ ID 2; or c) the parent CDR3 consisting of the amino acid sequence of SEQ ID 3; wherein the functionally active CDR variant comprises at least one point mutation in the parent CDR sequence, and comprises or consists of the amino acid sequence that has at least 60% sequence identity with the parent CDR sequence. It further refers to such cross-neutralizing antibody which is a functionally active variant antibody of a parent antibody that comprises a polyspecific binding site of the VH amino acid sequence of SEQ ID 20, and the VL amino acid sequence of SEQ ID 39, which functionally active variant antibody comprises at least one point mutation in any of the framework regions (FR) or constant domains, or complementarity determining regions (CDR1 to CDR6) in any of SEQ ID 20 or SEQ 39, and has an affinity to bind each of the toxins with a Kd of less than 10-8M, preferably less than 10-9M.
Owner:ARSANIS BIOSCI
Clostridium perfringens type A inactivated anatoxin vaccine for cattle and preparation method of clostridium perfringens type A inactivated anatoxin vaccine
ActiveCN109954135AHigh recovery rateHigh levels of toxin productionAntibacterial agentsBacterial antigen ingredientsAlpha-toxinBacillus perfringens
The invention discloses a clostridium perfringens type A inactivated anatoxin vaccine for cattle and a preparation method of the clostridium perfringens type A inactivated anatoxin vaccine. A cattle-source clostridium perfringens type A bacterial strain which is high in toxin production level, good in immunogenicity and stable in culture characteristics is used for preparing the clostridium perfringens type A inactivated anatoxin vaccine for cattle. Through optimization of a formula of a culture medium and culture condition, the culture stability and the titer of a basic antigen are improved,and alpha toxin liquid high in MLD level is obtained; in a safe, convenient and efficient alpha toxin concentrating manner, the alpha toxin recovery rate is increased, and the culture cost is reduced;and a high-content anatoxin antigen is used for preparing the vaccine, so that a clostridium perfringens type A anatoxin vaccine which is good in safety, high in potency and low in immunity dosage isobtained.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com