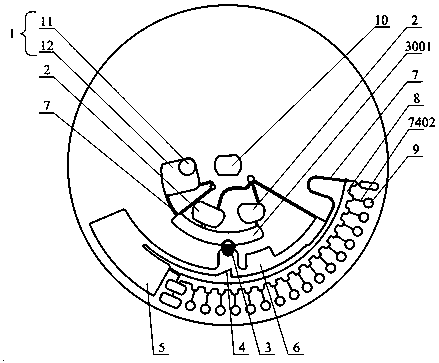
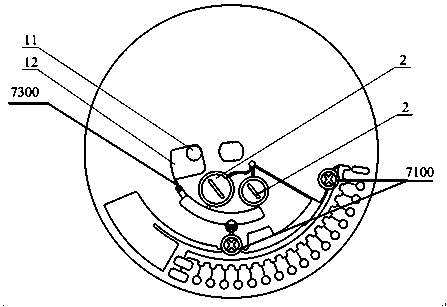
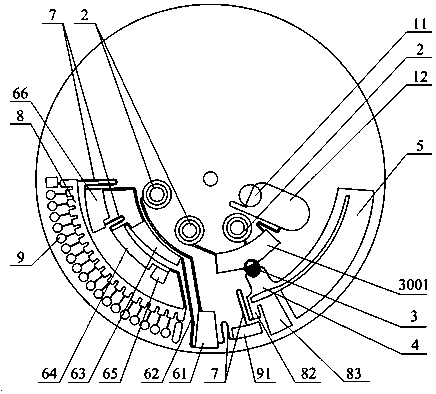
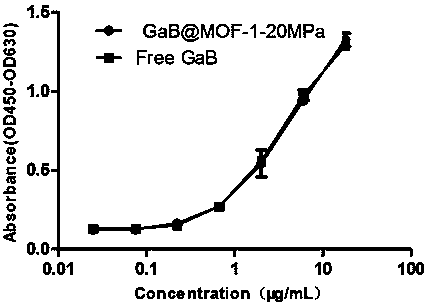
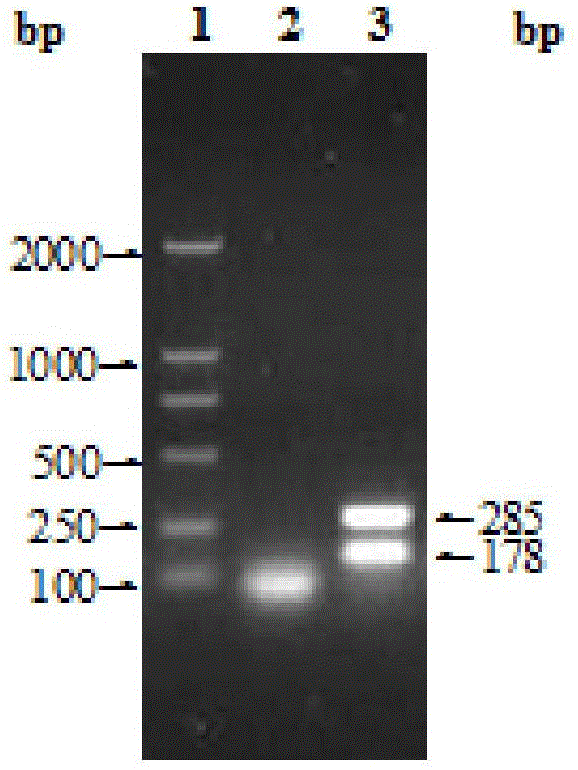
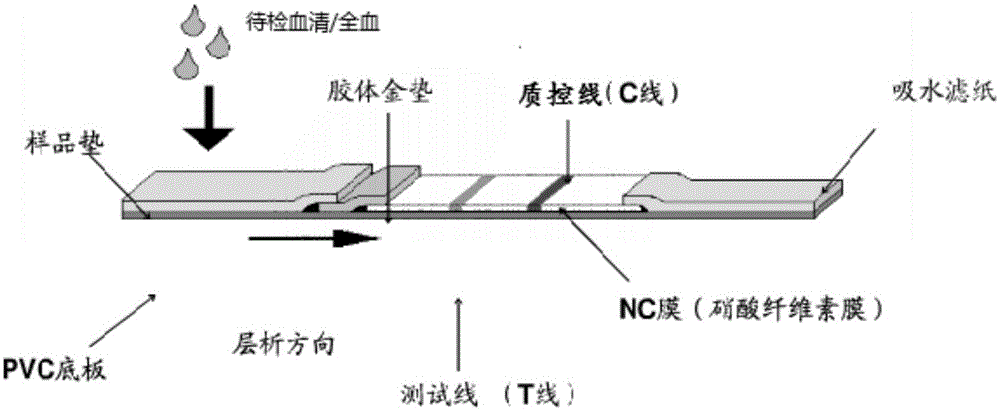
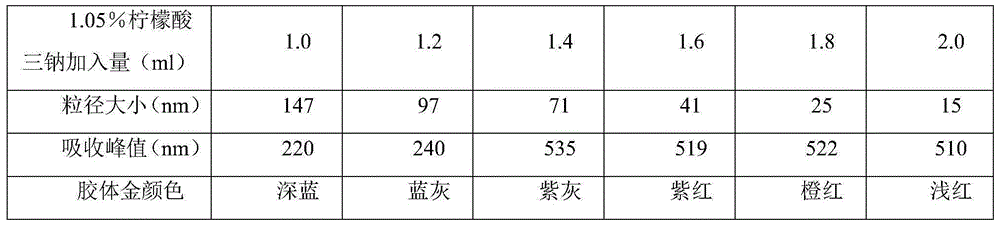
Patents
Literature
67results about How to "Reduce Biosecurity Risks" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
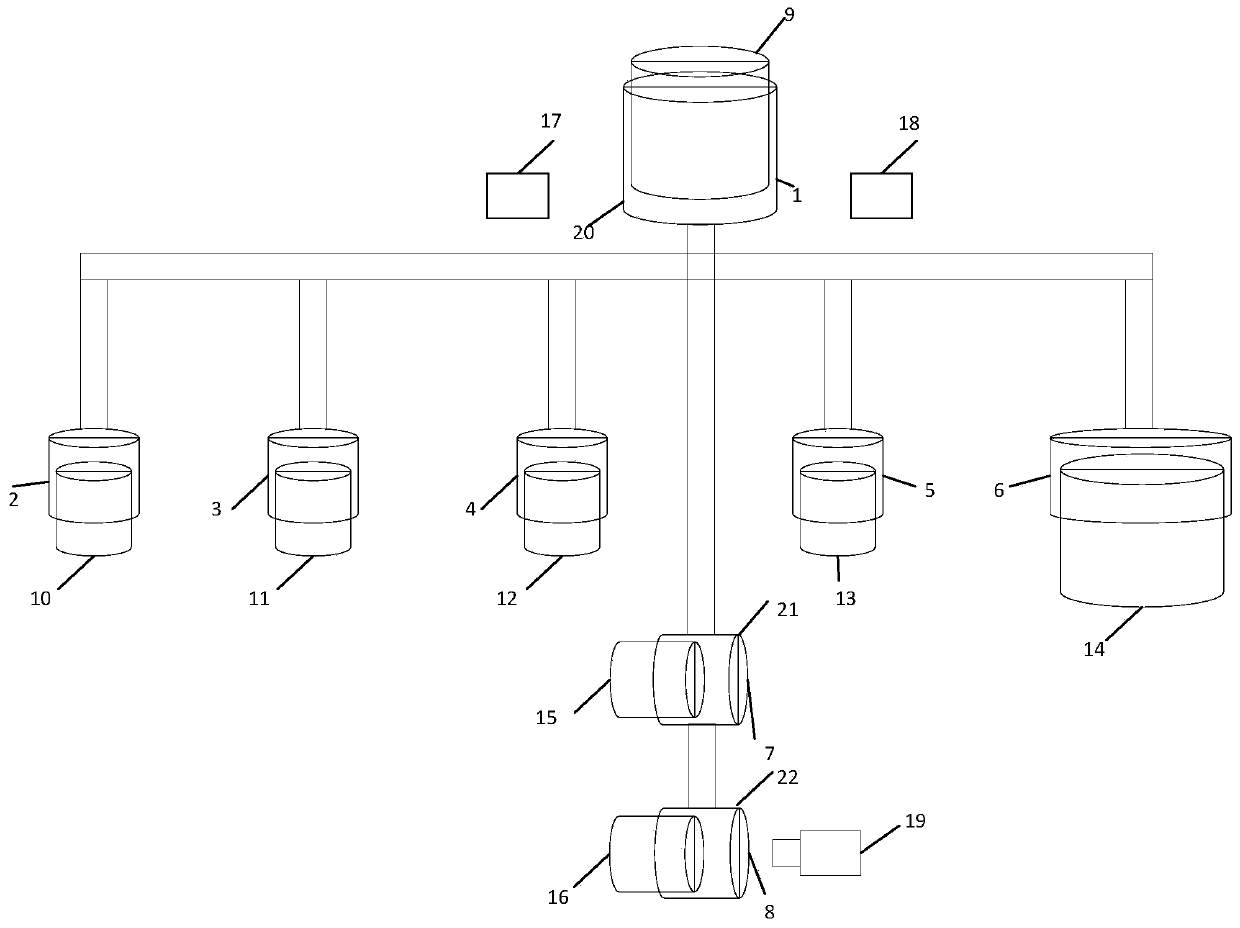
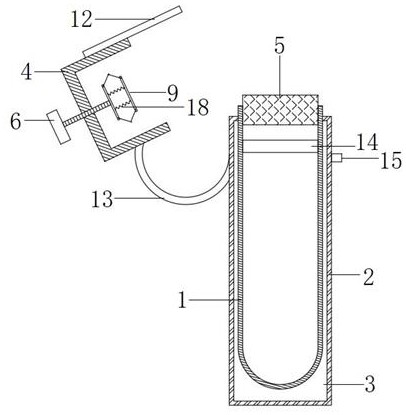
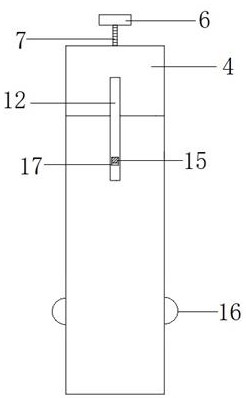
Full-process biological detection device
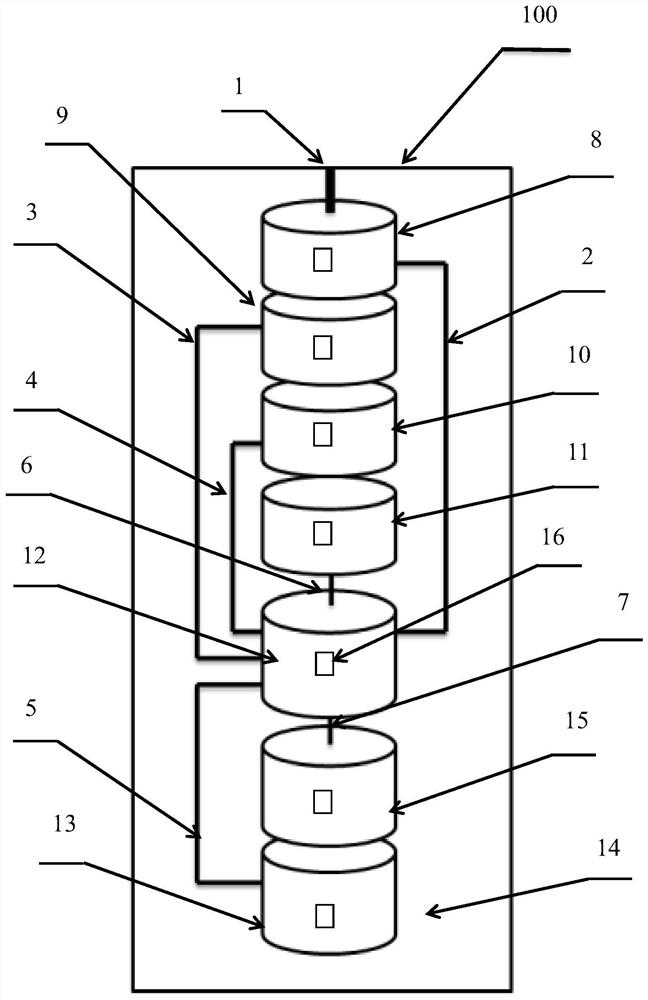
ActiveCN111218395ARealize integrationEasy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsBiochemical engineeringSurface tension force
The invention discloses a full-process biological detection device, belonging to the technical field of biological detection. The a full-process biological detection device comprises at least one reactor, wherein the reactor comprises at least one sample adding unit, at least one controlled liquid release unit, at least one extraction unit, at least one controlled liquid switching unit, at least one liquid storage unit, at least one liquid transfer unit, at least one liquid flow control unit, at least one quantitative liquid dispersion unit and a plurality of reaction tanks, wherein all the above units communicate with each other through runners according to a preset sequence, and when the reactor rotates around a chip rotating shaft, liquid in the reactor can flow to downstream unit fromupstream unit through the runners under the driving of centrifugal force or surface tension or both the centrifugal force and the surface tension. The full-process biological detection device providedby such a scheme has the characteristics of high integration level, no need for additional reagents, simplicity in operation, stability, reliability and the like, realizes full-flow closing of a target during biological detection, and effectively avoids cross contamination between samples and equipment.
Owner:CAPITALBIO CORP
Bovine brucella indirect ELISA antibody detection kit
InactiveCN105067812AHigh purityIncrease digestionColor/spectral properties measurementsEnterocolitisEscherichia coli
The invention relates to a bovine brucella indirect ELISA antibody detection kit. The kit uses the lipopolysaccharide (LPS) of brucella suis S2 acclimated and induced by the application institution in the 1960s as the coating antigen, and improves the sensitivity of detection. The study improves the LPS purification and purity determination method, and endows the developed kit with good specificity. The kit not only can effectively eliminate the interference of conventional Gram negative bacteria to Brucella, but also can get rid of the technical defect that general brucellosis indirect ELISA (enzyme-linked immunosorbent assay) kits cannot distinguish enterocolitis Yersinia O9 and Escherichia coli O157 interference, and improves the specificity of detection. Through improvement and optimization of the main reagent formula, the reaction background of the kit is effectively controlled, and also the time required by detection is shortened.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Porous frame material for storing, transporting and preparing biological products
InactiveCN108456241AGood biocompatibilityLow biological toxicityImmunoglobulins against animals/humansInorganic non-active ingredientsBiomolecular complexBiological organism
In order to solve the problems that some biological products are poor in stability, cannot be stored at normal temperature, cannot be well stored easily and are not convenient to use in emergency circumstances such as a disaster, the invention relates to a porous frame material creatively applied to biomolecule storage, and the stability and other performances are improved. The porous frame material and biomolecules are used for forming a porous frame material / biomolecule compound, the biomolecule is wrapped, and therefore the protection effect is achieved. On the premise of keeping the biomolecule activity, the system can achieve efficient separation and recycling of porous materials and biomolecules. While a biological product protecting system is developed, various performances are comprehensively detected and evaluated, the technical purposes of synthesis, storage, release and the like are solved, the good technical effect is achieved, the biomolecules can be effectively protected,and the material is used for storing a biological agent and preparing novel preparations.
Owner:NANKAI UNIV
Bovine Brucella colloidal gold antibody detection test paper strip
InactiveCN105137073AHigh purityReduce non-specific reactionsBiological material analysisBrucella antibodyBioinformatics
The present invention relates to a rapid, efficient and accurate bovine Brucella colloidal gold diagnostic method. According to the present invention, Brucella strain S2 lipopolysaccharide (LPS) is adopted as a testing line coating antigen so as to improve the detection sensitivity; the LPS purification and purity determination method is improved, such that the developed kit has good specificity; the monoclonal antibody on the Fc terminal of the mouse anti-bovine IgG is used to label the colloidal gold to prepare the colloidal gold pad of the colloidal gold antibody detection test paper strip so as to further improve the detection specificity; the bovine Brucella colloidal gold antibody detection test paper strip can be used for detecting the Brucella antibody in the bovine serum, and has characteristics of strong specificity, high sensitivity, convenience, rapidness, and the like; and the complex detection equipment is not required for the Brucella detection, and the bovine Brucella colloidal gold antibody detection test paper strip is especially suitable for cattle farm breeding staffs and grassroots veterinarians.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Silver-carrying antibacterial hydrocolloid dressing and preparation method thereof
InactiveCN106362195AGood antibacterial effectReduce concentrationAbsorbent padsBandagesWound healingAntioxidant
The invention discloses silver-carrying antibacterial hydrocolloid dressing and a preparation method thereof. The silver-carrying antibacterial hydrocolloid dressing is sequentially provided with a backing, a hydrocolloid functional dressing core and an isolation layer from top to bottom; the hydrocolloid functional dressing core is prepared from the following raw materials including sodium carboxymethylcellulose, an SIS thermoplastic elastomer, liquid paraffin, tackifying resin, an antioxidant, dioctyl adipate as a plasticizer, a bacteriostatic agent and a collaborative bacteriostatic agent. In a preparation process, the silver ion bacteriostatic agent and the plant extract collaborative bacteriostatic agent are added; with addition of the plant extract collaborative bacteriostatic agent, the addition concentration of the silver ion bacteriostatic agent is lowered when the antibacterial activity of the dressing is effectively improved; with addition of the silver ion bacteriostatic agent, the antibacterial wide adaptability and the antibacterial durability are both improved; and as the two bacteriostatic agents are used in a matching manner, a wound can be effectively prevented from being infected, and the silver-carrying antibacterial hydrocolloid dressing also has the advantages of blocking bacteria, absorbing exudate, avoiding wound adhesion, supplying an ideal wet healing environment for a wound, promoting healing of the wound and reducing formation of scars.
Owner:HENAN HUIBO MEDICAL CO LTD
SARS-CoV-2 inactivated vaccine and preparation method of vaccine
ActiveCN111569058AReduce Biosecurity RisksLow impurity contentSsRNA viruses positive-senseViral antigen ingredientsVirus inactivationTGE VACCINE
The invention relates to a SARS-CoV-2 inactivated vaccine and a preparation method of the vaccine. The preparation method comprises the following steps: step (1) recovery and large-scale culture of Vero cells; step (2) inoculation of working seeds, batch of virus seeds, virus culture and one-time harvest virus liquid, namely, the virus harvest liquid; step (3) obtaining an inactivated virus concentrated solution after a first virus inactivation, concentration and a second virus inactivation; step (4) obtaining a vaccine stock solution after purifying the inactivated virus concentrated solution; and step (5) preparation of semi-finished product and sub-package after the stock solution is verified to be qualified.
Owner:中国生物技术股份有限公司 +1
Colloidal gold test paper bar for antibody detection of sheep Brucella
The invention relates to a colloidal gold test paper bar for antibody detection of sheep Brucella. In the invention, lipopolysaccharide (LPS) extracted from a Brucella vaccine strain (S2) is taken as an envelope antigen of the colloidal gold test paper bar and improves the sensitivity of detection. Monoclonal-antibody-labeled colloidal gold of a mouse anti-sheep IgGFc terminal is prepared into a gold colloid pad of the colloidal gold test paper bar for antibody detection, and nonspecific adsorption of antibody protein by SPA in the tradition is changed. The monoclonal-antibody-labeled colloidal gold is taken as a colloidal gold labeled protein, so that specificity of detection is further improved. A set of serum for controlling quality of the test paper bar is prepared, and specificity and sensitivity of the test paper bar is ensured. The test paper bar can be used for on-site sampling, is convenient and rapid to use, and is quite suitable for application to a basic farm or a basic veterinarian.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Clostridium perfringens Beta toxin recombination subunit vaccine and production method thereof
ActiveCN109078178AEfficient expressionEfficient soluble expressionAntibacterial agentsBacterial antigen ingredientsClostridium perfringens beta toxinVaccine Production
The invention relates to a clostridium perfringens Beta toxin recombination subunit vaccine and a production method thereof. The prepared clostridium perfringens Beta toxin recombination subunit vaccine is produced by using a recombination clostridium perfringens Beta toxin protein which is processed by codon optimization and contains 4 amino acid mutations, namely integrity and spatial conformation of a natural toxin protein are reserved in the greatest degree, so immunogenicity of the natural toxin protein is kept, and a biological potential safety hazard caused by the single amino acid mutation is avoided. The vaccine further has the advantages of simple preparation process, low immunizing dose, good vaccine efficacy and the like. Compared with a current commercial clostridium perfringens natural toxin inactivated vaccine in China, a bio-safety risk in a vaccine production process is greatly reduced. The vaccine is a perfect candidate vaccine for updating a current C-type clostridium perfringens toxin vaccine in China. In addition, while a mixed vaccine is prepared by the vaccine with other antigens, the mixed vaccine can be prepared without increasing a using dose of the mixedvaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Novel coronavirus SARS-CoV-2 replicon as well as construction method and application thereof
InactiveCN112301043AReduce Biosecurity RisksImprove screening efficiencySsRNA viruses positive-senseVirus peptidesEngineeringHigh-throughput screening
The invention discloses a novel coronavirus SARS-CoV-2 replicon as well as a construction method and application thereof, and belongs to the technical field of virology and molecular biology. The novel coronavirus SARS-CoV-2 replicon is an SARS-CoV-2-GFP replicon with a single reporter gene or an SARS-CoV-2-GFP-Luc replicon with double reporter genes. The invention also discloses the constructionmethod and application of the novel coronavirus SARS-CoV-2 replicon. According to the novel coronavirus SARS-CoV-2 replicon disclosed by the invention, infectious virus particles are not generated, sothat the biological safety risk in the process of screening antiviral drugs by utilizing the infectious virus particles is effectively reduced. Besides, a reporter gene carried by the replicon can allow high-throughput screening to be performed, so that the screening efficiency of anti-novel coronavirus drugs is greatly improved.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
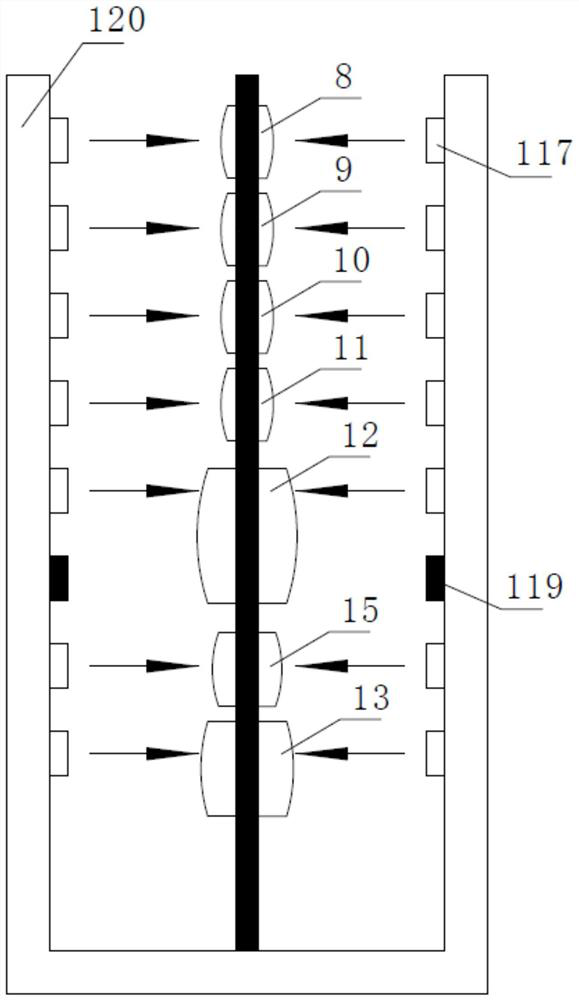
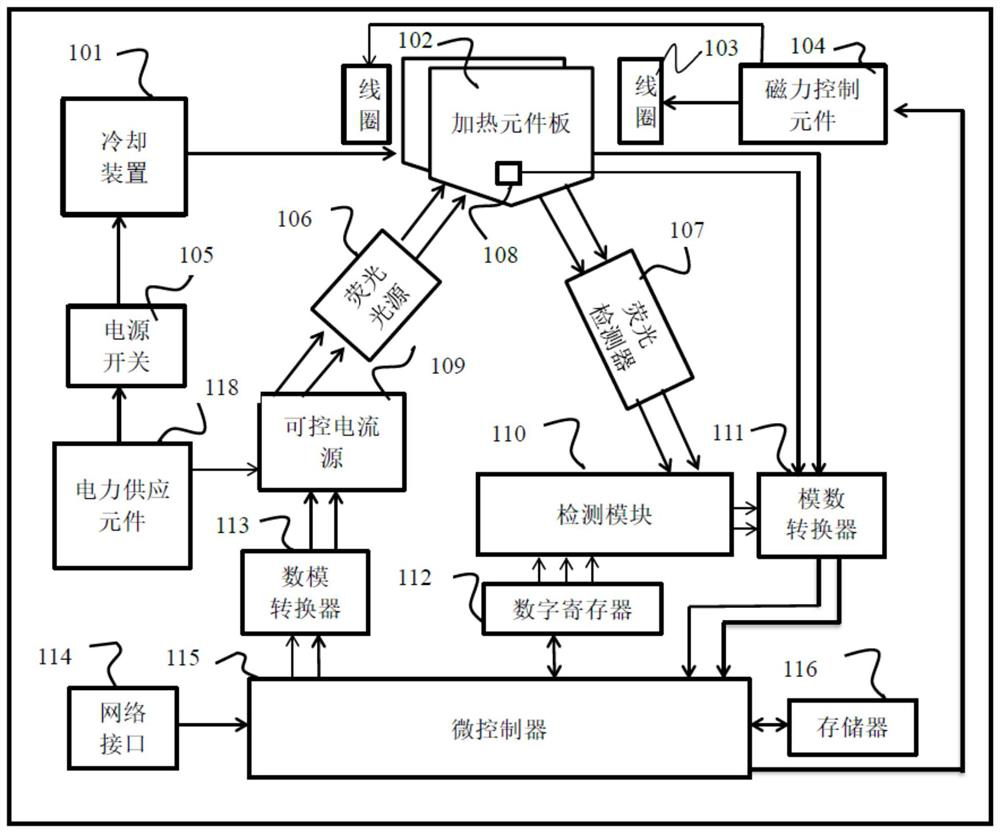
Closed full-automatic nucleic acid extraction and detection system based on CRISPR technology
PendingCN111575176AAvoid Nucleic Acid Cross ContaminationReduce Biosecurity RisksBioreactor/fermenter combinationsBiological substance pretreatmentsBiophysicsCRISPR
The invention discloses a closed full-automatic nucleic acid extraction and detection system based on a CRISPR technology. The closed full-automatic nucleic acid extraction and detection system comprises a bag type biological chip and a control detector, wherein the bag type biological chip comprises a nucleic acid extraction cavity, a No.1 cleaning liquid cavity, a No.2 cleaning liquid cavity, aNo.3 cleaning liquid cavity, an eluent cavity, a waste liquid cavity, an amplification cavity and a CRISPR detection cavity which are respectively used for storing various liquids required by nucleicacid extraction and detection, and the cavities are connected through micro-channels; and the control detector comprises a push rod controller, a controllable alternating electromagnet and a fluorescence detector, wherein the push rod controller is used for controlling a flow direction of liquid, the controllable alternating electromagnet is used for stirring and adsorbing magnetic beads, and thefluorescence detector is used for CRISPR luminescence detection of nucleic acid. According to the closed full-automatic nucleic acid extraction and detection system based on the CRISPR technology, nucleic acid cross contamination during detection can be avoided, in addition, the bag type biological chip and the control detector are relatively separated, a detection sample can be rapidly replaced,and practical detection application is facilitated.
Owner:SHANGHAI INST OF TECHNICAL PHYSICS - CHINESE ACAD OF SCI +1
Clostridium septicum alpha toxin genetic engineering vaccine and production method thereof
ActiveCN109395072AReduce Biosecurity RisksEasy to purifyAntibacterial agentsBacterial antigen ingredientsAlpha-toxinInclusion bodies
The invention provides a prepared clostridium septicum CSA genetic engineering vaccine, which is optimized by a codon and is produced by recombinant clostridium septicum CSA (rCSAM4 delta 11) obtainedby deleting 11 amino acids (212th bit to 222nd bit) on the basis of containing 4 amino acid mutations (C54L, N264A, H269A and W310A); the completeness and spatial conformation of the natural toxin are reserved to the maximum degree, so that the immunogenicity is maintained; the biological safety hazards due to minority amino acid mutation are avoided. Meanwhile, nontoxic rCSAM4 delta 11 can be expressed in a soluble form, so that the influence of the complicated process of the inclusion body denaturation and renaturation on antigen protein immunogenicity is avoided, and the preparation time and the production cost of the vaccine are reduced. In addition, the vaccine also has the advantages of low immunizing dose, good immunizing effect and the like, and belongs to an ideal candidate vaccine for upgrading and updating the existing clostridium septicum toxin vaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
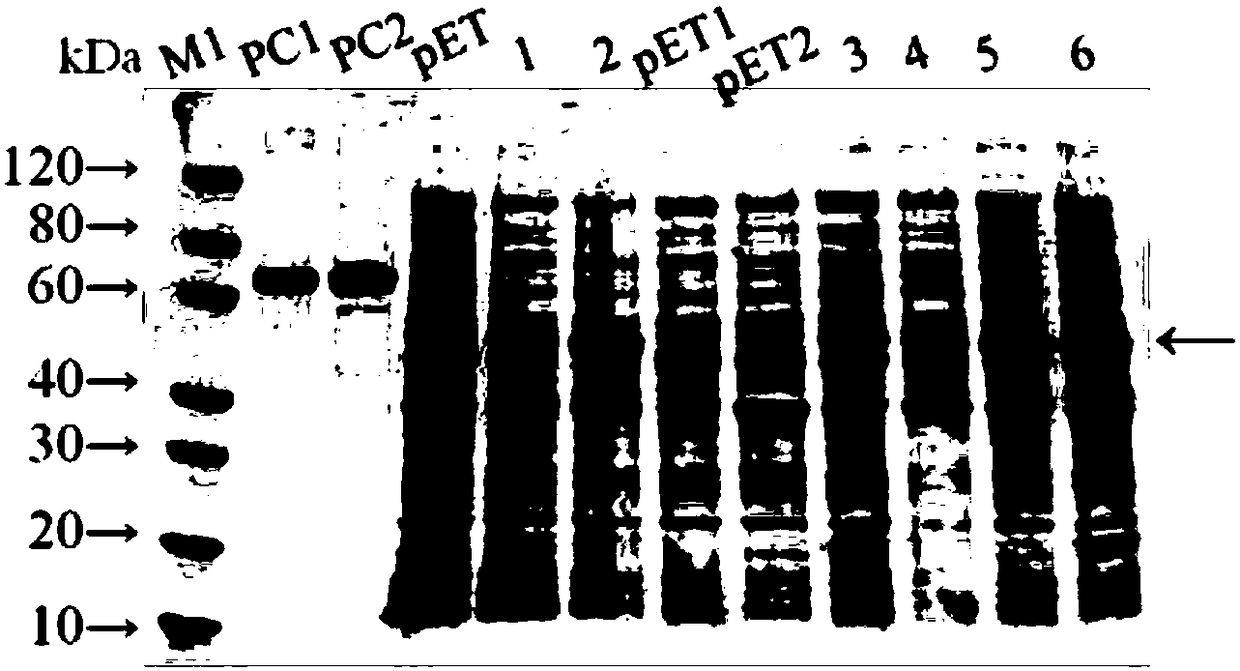
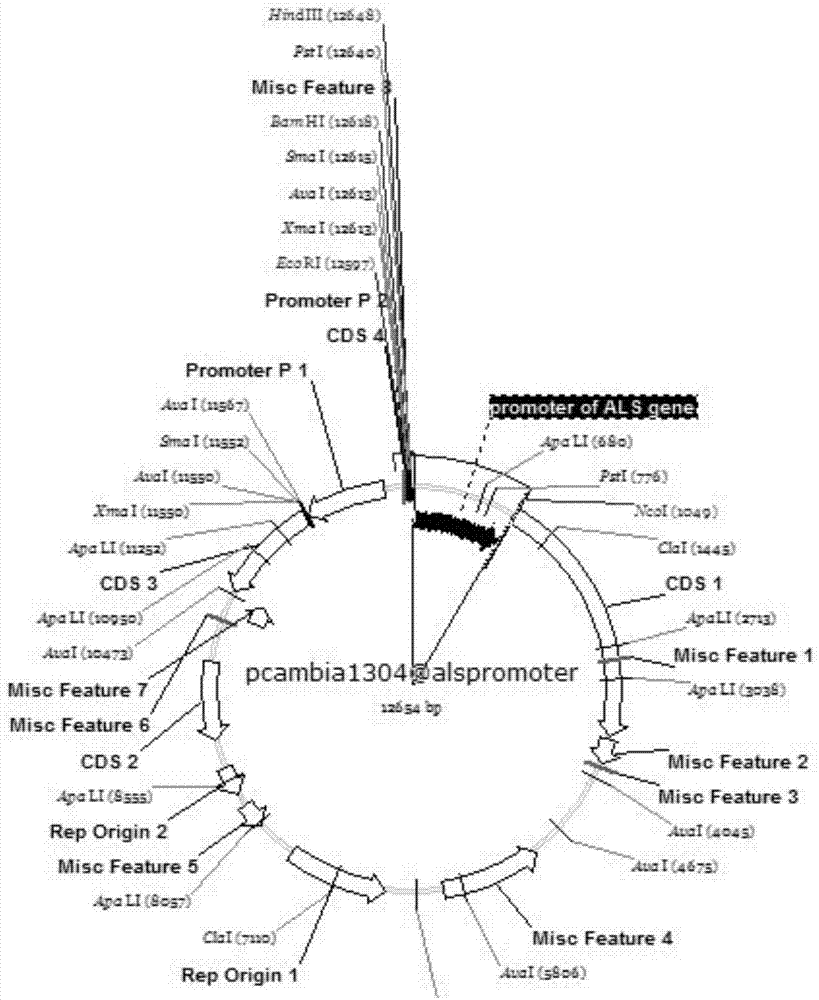
Brassica napus ALS (Acetolactate Synthase) gene promoter and application
InactiveCN107460195AReduce Biosecurity RisksHigh activityTransferasesVector-based foreign material introductionGenetic engineeringAcetolactate synthase
The invention relates to a brassica napus ALS (Acetolactate Synthase) gene promoter and application and belongs to the technical field of biology. The brassica napus ALS gene promoter disclosed by the invention is characterized in that a brassica napus ALS gene promoter sequence is colonized; a promoter element contained in the sequence is predicted by bioinformatics software; the activity of the gene promoter is subjected to qualitative detection and analysis by constructing a plant expression vector; and the results show that the colonized sequence can drive a GUS reporter gene to be expressed in all organs of tobacco seedlings, has high expression activity and characteristics of a constitutive promoter and can be applied to plant genetic engineering research as the strong constitutive expression promoter. The promoter can replace a cauliflower mosaic virus (CaMV) 35S promoter and an agrobaeterittm tumefaciens nopaline gene nos promoter which are normally used in the current plant genetic engineering research to construct a plant transgenic expression vector; and the plant transgenic expression vector is applied to genetic engineering of dicotyledons, so that the biological safety risk of transgenic plants is reduced.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
High immunogenicity rabies virus glycoprotein, its preparation method and application
InactiveCN102964433AAvoid failureImproving immunogenicityAntiviralsDepsipeptidesViral glycoproteinRabies virus strain
The invention discloses a high immunogenicity rabies virus glycoprotein and its preparation method as well as application. The preparation method consists of: performing artificial synthesis to acquire an all dominant neutralization epitope-containing and codon optimized rabies virus glycoprotein gene, then taking a recombinant adenovirus, a recombinant baculovirus or a slow virus as an expression vector, and performing high-efficiency expression in a mammalian cell line, an insect cell line or a silkworm expression system, thus obtaining the rabies virus glycoprotein that has high immunogenicity to domestic epidemic rabies virus strains currently. And the high immunogenicity rabies virus glycoprotein is used for immunoprophylaxis of animal rabies.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
2019-nCoV sampling tube
PendingCN113956960ANot easy to squeeze and breakReduce Biosecurity RisksBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringStructural engineering
The invention relates to a 2019-nCoV sampling tube, which comprises an inner tube of which the upper part is provided with an opening, wherein an outer tube which is integrally formed with the inner tube and is lower than the inner tube is fixedly arranged outside the inner tube in a sleeving mode; a buffer cavity is formed between the inner tube and the outer tube; the opening end of the inner tube is covered with a sealing cover; the sealing cover is provided with a piston through an adjusting mechanism, wherein the piston can be in plug-in sealing fit with the inner tube; the sealing cover is connected with a first connecting belt and a second connecting belt; the first connecting belt is connected with the outer tube; the second connecting belt is provided with a bayonet; and the second connecting belt is clamped with a bulge convexly arranged on the outer tube through the bayonet. The 2019-nCoV sampling tube can be effectively sealed, the sealing cover and the sampling tube are connected through the connecting belts, and cross contamination between samples can be avoided so as to guarantee that a detection result is accurate.
Owner:JINAN BAIBO BIOTECH
Quick time-resolved fluorescence immunoassay kit for detection of T cells infected with tuberculosis and detection method of kit
InactiveCN106053783AHigh sensitivityAvoid secondary pollutionBiological material analysisBiological testingTime resolved fluorescence immunoassayAntibody
The invention discloses a quick time-resolved fluorescence immunoassay kit for detection of T cells infected with tuberculosis. The kit is characterized by comprising 1) a test reaction cup; 2) a calibrator reaction cup; 3) a blank control culture tube containing an anticoagulant substance inside; 4) a test culture tube; 5) a positive control culture tube containing an anticoagulant substance and cell agglutinin inside; 6) an experiment buffer solution and 7) a wash concentrate. The invention further discloses a detection method of the quick time-resolved fluorescence immunoassay kit for detection of T cells infected with tuberculosis. The kit adopts a gamma-interferon in-vitro release method, a biotin-avidin method and double-antibody sandwich time-resolved fluorescence immunoassay together, the experimental methods are simple and quick, automatic operation is realized, and the detection system is an open type operation system.
Owner:GUANGZHOU FENGHUA BIOENG
Polycyclic aromatic hydrocarbon degradation gene engineering strain, and construction method and application thereof
ActiveCN106754597AImprove controllabilityReduce Biosecurity RisksBacteriaHydrolasesLaboratory cultureChemistry
The invention relates to a polycyclic aromatic hydrocarbon degradation gene engineering strain. The strain Pseudomonas putida GLEB3 is collected by China General Microbiological Culture Collection Center on September 19th, 2016; the collection number is CGMCC No.13014; and the collection address is Institute of Microbiology, Chinese Academy of Sciences, No.1 Yard 3, Beichenxi Road, Chaoyang District, Beijing City. The controllability of the gene engineering strain can be effectively enhanced in the growth process, and the biosafety risk is lowered in the microbe-reinforced restoration process.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Method for detecting ADCC (antibody-dependent cell-mediated cytotoxicity) activity of anti-HIV (human immunodeficiency virus) antibody
ActiveCN106800603AHigh sensitivityImprove featuresAntibody mimetics/scaffoldsMicrobiological testing/measurementHuman immunodeficiencyGenetic engineering
The invention relates to the fields of molecular virology and genetic engineering. In particular, the invention relates to an HIV (human immunodeficiency virus) envelope protein, a coding sequence thereof, and an HIV pseudovirus comprising the HIV envelope protein; the HIV pseudovirus can be used to detect ADCC (antibody-dependent cell-mediated cytotoxicity) activity of an anti-HIV antibody. The invention also relates to a kit for detecting the ADCC activity of the anti-HIV antibody. The invention further relates to a method for detecting the ADCC activity of the anti-HIV antibody by using the HIV pseudovirus, the method can achieve rapid, simple and high-throughput detection of the ADCC activity of the anti-HIV antibody, and thereby the method is of great significance for protection effect on analysis of vaccine immune response, and development and quality control of a new vaccine.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Sheep aphthosis virus recombinant subunit vaccine, and production method thereof
The invention relates to a sheep aphthosis virus fusion protein rB2LcF1L. An amino acid sequence of the sheep aphthosis virus B2L and F1L fusion protein is SEQ ID No:2; compared with the wild type sheep aphthous virus F1L protein, the sheep aphthosis virus fusion protein rB2LcF1L has amino acid sequences of sites No.1 to 70 deleted to facilitate the expression of the fusion protein and increase the expression level. At the same time, 6 groups of histidine tags are added to the C-terminus of the fusion protein to facilitate purification in subsequent production. In the above amino acid sequence, compared with a B2L amino acid sequence of a conventional isolate, the V / L of the amino acid at a site No.9 is mutated to G, namely ''IPVG or IPLG is mutated to IPGG'', and the LDCF at a site No.35is mutated to LYCF. The above mutation significantly increases the expression level and immunogenicity of the fusion protein.
Owner:YULIN UNIV
Automatic nucleic acid extraction detector and detection method thereof
PendingCN112899141AAvoid cross contaminationQuick changeBioreactor/fermenter combinationsBiological substance pretreatmentsMicrocontrollerNucleic acid detection
The invention provides an automatic nucleic acid extraction detector and a detection method thereof. The automatic nucleic acid extraction detector comprises a chip and a hardware system, wherein the chip comprises a supporting body and a plurality of containing cavities with elastic outer walls, the containing cavities are formed in the supporting body, the containing cavity comprises a sample pool, a cracking pool, a washing solution pool, an LAMP buffer solution pool, an amplification detection pool and a waste liquid pool, and the hardware system comprises a side extrusion device for inserting the chip, a temperature control module for adjusting a temperature of each containing cavity, a magnetic force control element for controlling a movement direction of magnetic beads, a fluorescence detection module for detecting nucleic acid and a microcontroller for regulating and controlling operation of the automatic nucleic acid extraction detector. The nucleic acid detection method comprises steps of chip insertion, lysis, washing, elution, amplification, detection and the like. According to the automatic nucleic acid extraction detector and the detection method, cross contamination during nucleic acid detection is avoided, a detection sample can be rapidly replaced, and automatic detection of nucleic acid is facilitated.
Owner:HAINAN MICROKRYPTON BIOTECHNOLOGY CO LTD
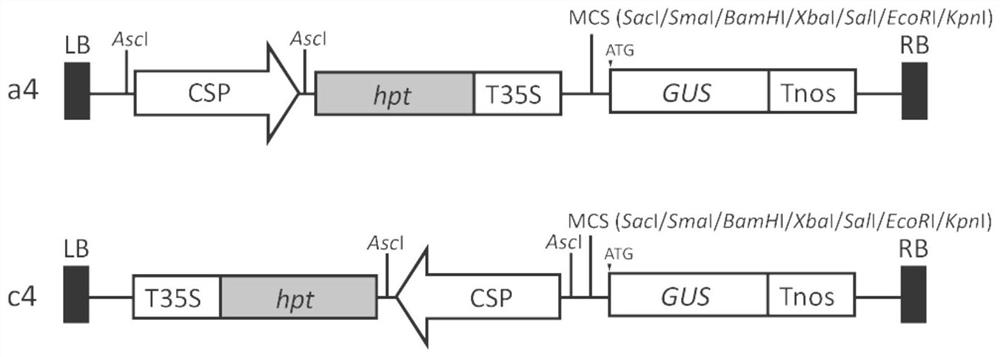
Vector for analyzing expression specificity of plant promoter, preparation method and application thereof
PendingCN113430225AReduce non-specific interactionsReduce Biosecurity RisksGenetic engineeringFermentationBiotechnologyGenetic engineering
The invention relates to the field of genetic engineering, and particularly relates to a vector for analyzing the expression specificity of a plant promoter, a preparation method and application thereof. A T-DNA region of the vector sequentially comprises a screening marker gene expression cassette driven by a callus specific promoter and a reporter gene expression cassette with multiple cloning sites from the 5' end to the 3' end; and the screening marker gene expression cassette driven by the callus specific promoter comprises the callus specific promoter, a screening marker gene coding sequence and a terminator. The vector utilizes the rice callus specific promoter to drive the expression of a screening marker gene, can greatly reduce the non-specific interaction of a target gene promoter, improves the specificity of target gene expression, can effectively reduce the biological safety risk caused by the screening marker gene in transgenic plants, and has important application value in basic theoretical research and molecular breeding.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES +1
Porcine circovirus gene modified attenuated strain as well as construction method and application thereof
PendingCN113355292AEnsure safetyEasy to measureBacteriaViral antigen ingredientsNucleotidePorcine Circoviruses
The invention provides a porcine circovirus gene modified attenuated strain as well as a construction method and application thereof, and relates to the technical field of biological gene engineering. The genome of the attenuated strain is subjected to codon mutation or codon optimization or gene truncation and the like, so that the gene does not encode or encode part of capsid protein (Cap protein) finally, and a fluorescent protein gene is inserted into a nucleotide sequence deleted by gene truncation. On the basis, a genome of the gene modified attenuated strain is transfected into a donor cell capable of expressing capsid protein, and a complete virion capable of infecting a host cell is finally self-assembled by utilizing a replication system of the cell and the genome of the virus.
Owner:天康制药股份有限公司
Method for preparing circular DNA in vitro
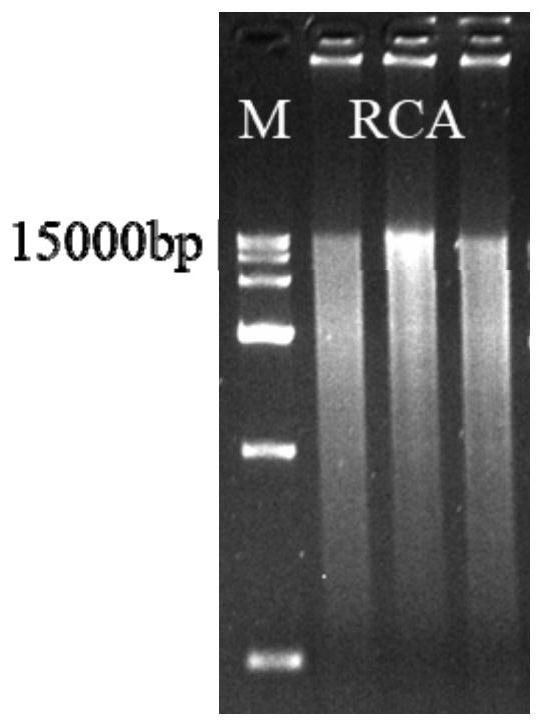
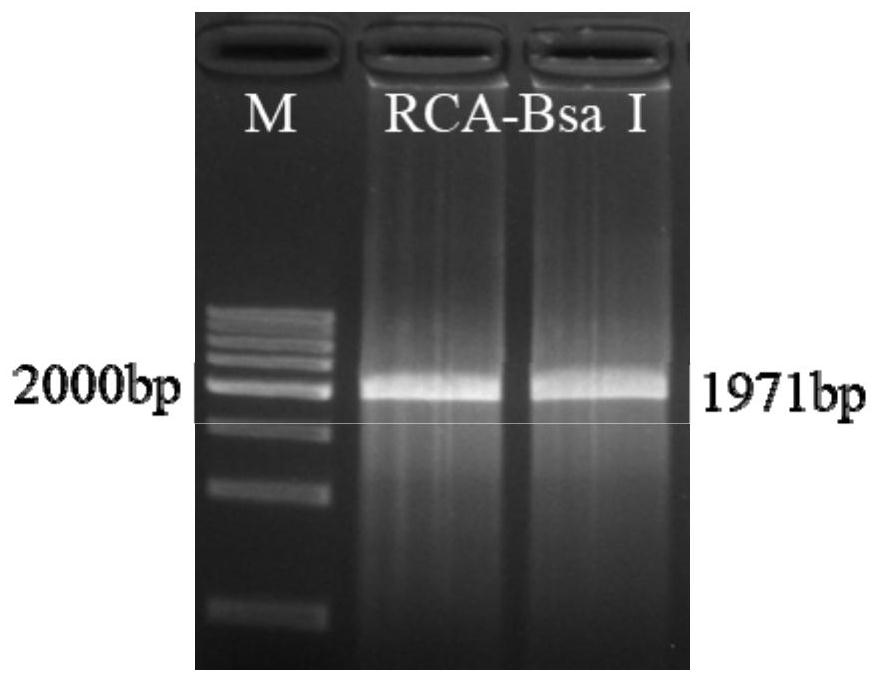
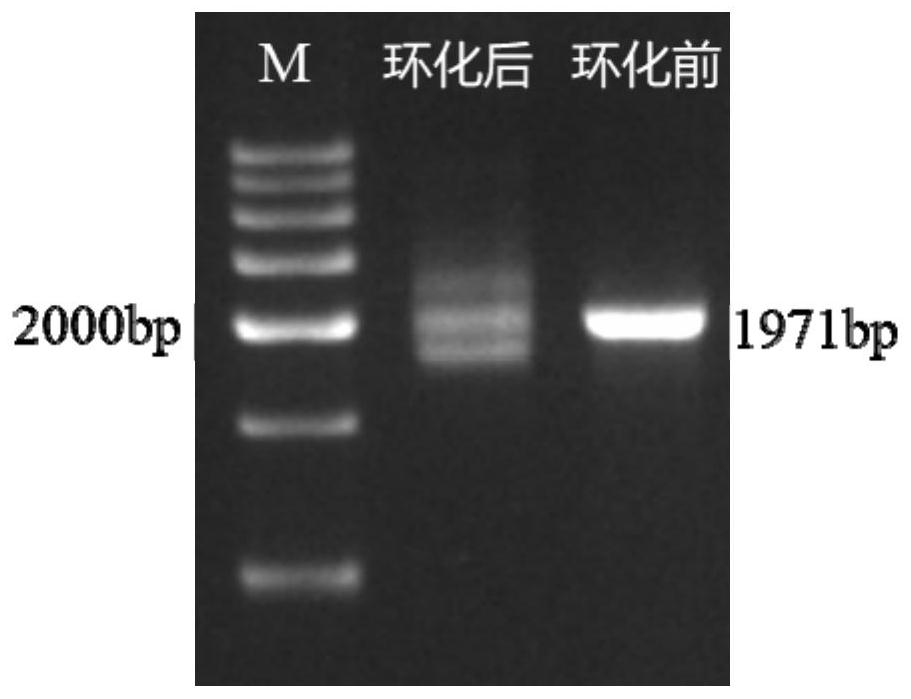
PendingCN114231524ASmall molecular weightAvoid internal processesFermentationDNA preparationEnzyme digestionGibson assembly
The invention discloses a method for preparing circular DNA (Deoxyribose Nucleic Acid) in vitro. The method comprises the following steps: designing a Gibson assembly connection fragment according to a target linear DNA fragment, introducing a recognition site sequence of restriction enzyme Bsa I, mixing the target linear DNA fragment and the Gibson assembly connection fragment, carrying out Gibson assembly, carrying out rolling circle amplification and purification by taking a Gibson assembly product as a template to obtain an RCA product, carrying out Bsa I enzyme digestion on the purified RCA product, and carrying out purification to obtain the RCA. Carrying out agarose gel electrophoresis after enzyme digestion, carrying out gel cutting to recover an enzyme digestion band consistent with the target linear DNA fragment in size, and finally cyclizing and purifying an enzyme digestion product by using T4 DNA ligase to obtain a cyclized product of the target linear DNA fragment. The method can replace traditional plasmids, only an incision enzyme recognition sequence with the length of 11 basic groups is additionally introduced except necessary elements, the molecular weight of circular DNA is reduced to the minimum, meanwhile, the biological safety risk is reduced, and in-vitro cyclization of linear DNA fragments is achieved.
Owner:SHENZHEN HUADA GENE INST
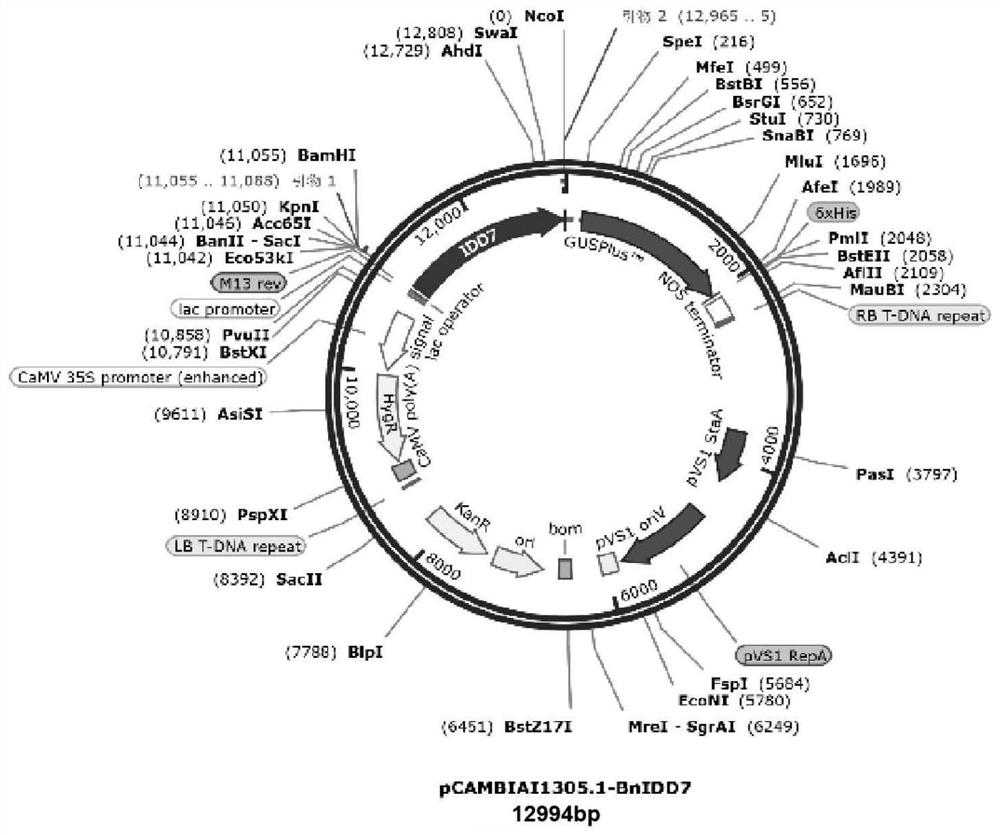
Brassica napus Bna.A08IDD7 gene promoter and application thereof
ActiveCN112011541AGreat application potentialHigh activityPlant peptidesVector-based foreign material introductionCauliflower mosaic virusBiological organism
The invention discloses a brassica napus Bna.A08IDD7 gene promoter and application thereof, the nucleotide sequence of the promoter is shown as SEQ ID NO.5, and the promoter can be strongly expressedin roots, leaves, stems, flowers, siliques, seeds and other tissues of arabidopsis thaliana; therefore, it is indicated that the sequence has very strong expression activity and characteristics of a constitutive promoter, and can be used as a strong constitutive promoter to be applied to plant genetic engineering research. Therefore, the promoter creates excellent germplasm resources and other genetic engineering research aspects; the promoter has good application potential, can replace a cauliflower mosaic virus (CaMV) 35S promoter commonly used in current plant genetic engineering research,constructs a plant transgenic expression vector, is applied to plant genetic engineering, and can widely cultivate transgenic plants with high biosafety.
Owner:SOUTHWEST UNIVERSITY
Non-toxic tetanus toxin and clostridium novyi alpha toxine recombinant fusion protein
ActiveCN110041437AImprove stabilityReduce Biosecurity RisksAntibacterial agentsBacterial antigen ingredientsVaccine antigenToxin protein
The invention relates to non-toxic tetanus toxin and clostridium novyi alpha toxin recombinant fusion protein. The prepared recombinant fusion protein is produced in the manner that The prepared recombinant fusion protein is produced in the manner that through codon optimization, a tetanus toxin C fragment, C terminal of clostridium novyi alpha toxin, and N-end non-toxic epitope of clostridium novyi alpha toxin are subjected to fusing expression, so that the immunogenicity of two kinds of toxin protein can be reserved to the maximum extent, and biology potential safety hazard of natural toxincan be avoided. The recombinant fusion protein can be used for preparing clostridium tetani and clostridium novyi subunit vaccines. Compared with the clostridium tetani and clostridium novyi subunit vaccines commercialized in China at present, the non-toxic tetanus toxin and clostridium novyi alpha toxin recombinant fusion protein has the advantages of being simpler in preparation technology, lower in immunizing dosage, better in vaccine effects and the like, the biology security risk in the production process of the vaccine is greatly reduced, and the non-toxic tetanus toxin and clostridium novyi alpha toxin recombinant fusion protein is an ideal candidate vaccine antigen for upgrading and regenerating two clostridial toxin vaccines. When the non-toxic tetanus toxin and clostridium novyialpha toxin recombinant fusion protein and other antigens are in jointed preparation of a combined vaccine, the using dosage of the combined vaccine does not need to be increased, and the combined vaccine can be prepared.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
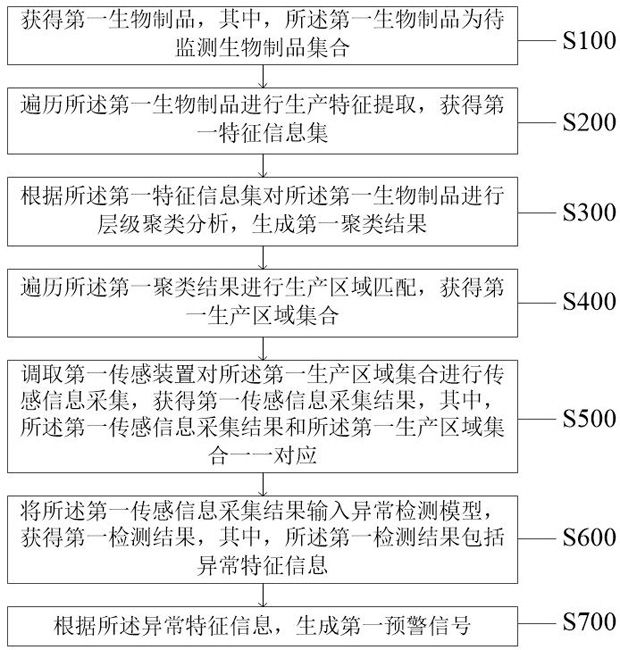
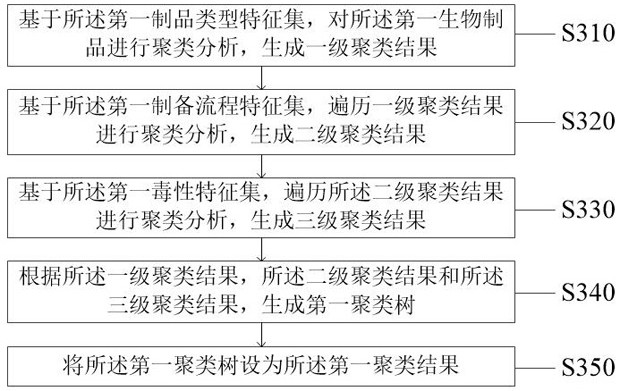
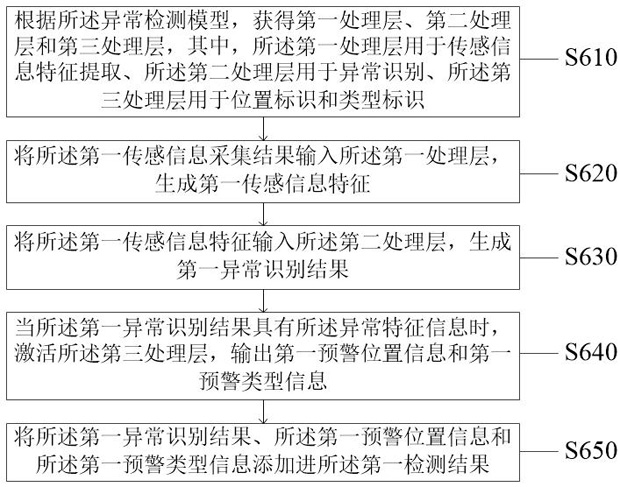
Biological product production early warning method and system
ActiveCN114723334AGuarantee the safety of lifeRealize intelligent identificationDigital data information retrievalCharacter and pattern recognitionPathogenic microorganismData mining
The invention discloses a production early warning method and system for a biological product, and relates to the field of artificial intelligence, in particular to the production early warning method and system for the biological product. The method comprises the steps of obtaining a first feature information set by traversing a first biological product; performing hierarchical clustering analysis to generate a first clustering result, and obtaining a first production area set; collecting first production area set sensing information to obtain a first sensing information collection result, and obtaining a first detection result; and generating a first early warning signal for the abnormal feature information. The problems that in the prior art, when pathogenic microorganism biological products are produced, risks of all production links cannot be found in time, targeted measures cannot be taken in time, and finally production and life safety are affected are solved. Through comprehensive and timely monitoring and analysis of biological safety risks and timely early warning of abnormal conditions, the technical effects of improving the production efficiency of biological products, reducing the biological safety risks and guaranteeing property and life safety are achieved.
Owner:张家港长三角生物安全研究中心
Nontoxic clostridium perfringens and clostridium septicum fusion protein vaccine and production method thereof
PendingCN110051834ASmall dose of immunizationImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsClostridium septicumVaccine Production
The invention relates to a nontoxic clostridium perfringens and clostridium septicum fusion protein vaccine and a production method thereof. The fusion protein vaccine prepared by the method adopts aclostridium perfringens epsilon toxin which is subjected to codon optimization and contains three amino acid mutations, and spoilage which contains four amino acid mutations and eleven amino acid deletions, namely not only kept the integrity and spatial conformation of the natural toxin are to the maximum extent, but also the biological safety hazard caused by single amino acid mutation is avoided. The vaccine also has the advantages of simple preparation process, excellent vaccine efficacy and the like, greatly reduces the biological safety risk in the vaccine production process. Compared with the current commercialized sheep fast-epidemic and sheep enterotoxemia inactivated vaccines in China, the vaccine is an ideal candidate vaccine for upgrading existing clostridium perfringens and clostridium septicum vaccines in China; in addition, when combined vaccines are prepared by the vaccine together with other antigens, the combined vaccine can be prepared without increasing the use doseof the combined vaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Non-toxic clostridium perfringens beta-toxin genetic engineering vaccine and production method thereof
InactiveCN109701007AHigh expressionIncreased expression of solubleAntibacterial agentsBacterial antigen ingredientsVaccine ProductionClostridium perfringens beta toxin
The present invention relates to a non-toxic clostridium perfringens beta-toxin genetic engineering vaccine. The prepared clostridium perfringens beta-toxin recombinant subunit vaccine is produced bycodon-optimized production and multiple-amino-acid-mutation- containing recombinant clostridium perfringens beta-toxin proteins, thus maximally retains integrity and spatial conformation of natural toxin proteins, keeps immunogenicity, and also avoids biosafety hazards brought by single amino acid mutations. The vaccine also has advantages of simple preparation technology, low immune dose, excellent vaccine efficacy, etc., greatly reduces biosafety risks in vaccine production processes compared with current commercial clostridium perfringens natural toxin inactivated vaccines in China, and isan ideal candidate vaccine for upgrading of current type B and type C clostridium perfringens toxin vaccines in China; and when the vaccine and other antigens are commonly used to prepare a combined vaccine, dose of the combined vaccine cannot be increased and the combined vaccine can be prepared.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Production method of recombinant strain expressing ETX-CSA at same time and vaccine related to recombinant strain
PendingCN109112152AImprove immunityIncreased serum titerAntibacterial agentsBacterial antigen ingredientsCombined VaccinesTiter
The invention discloses a production method of a recombinant strain expressing ETX-CSA at the same time and a vaccine related to the recombinant strain. A construction method of the recombinant straincomprises the following steps of introducing encoding gene of a fusion protein ETX-CSA into escherichia coli to obtain the recombinant strain expressing the fusion protein, wherein an amino acid sequence of linking peptide is 297th-311st sites of SEQ ID No.1. According to the method provided by the invention, two kinds of toxin proteins can be acquired at the same time by fermenting one strain only, and the strain can prevent two diseases after being prepared into a vaccine; after the strain and a C type clostridium perfringens toxin are prepared into a combined vaccine, the combined vaccinecan generate excellent immune effect, and titers of rabbit serums in three components (S, C, D) can be increased to 3, 4 and 10 times of a norm standard respectively. The method provided by the invention is good in stability, short in consumed time and low in cost, wherein the costs of the S component and the D component are lowered to be 1 / 100 of the traditional technology, and the titer cost ofthe three components (S, C, D) can be increased to be 344, 4 and 993 times of a traditional technology respectively.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Preserving solution for sputum and preparation method
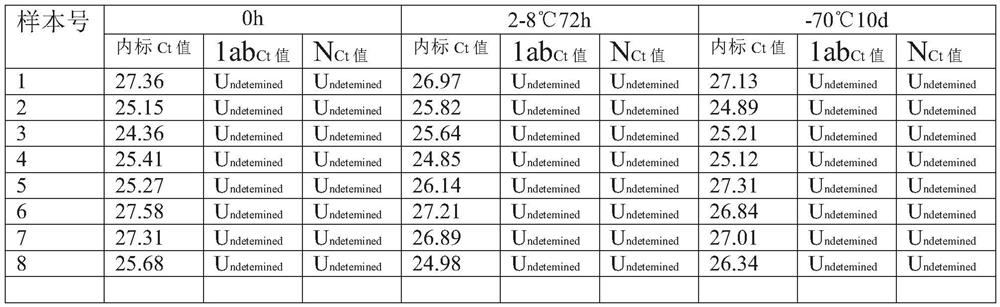
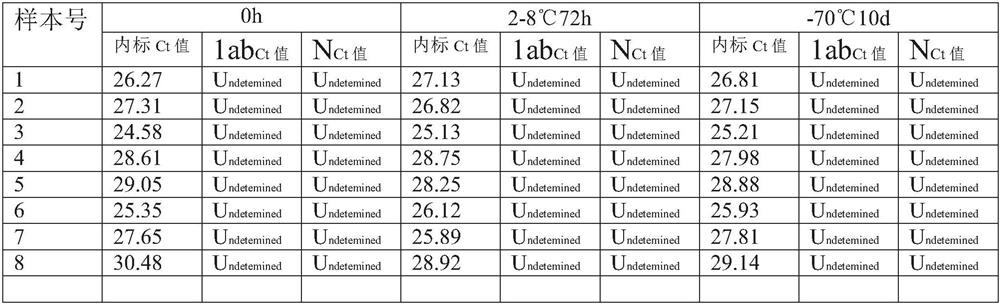
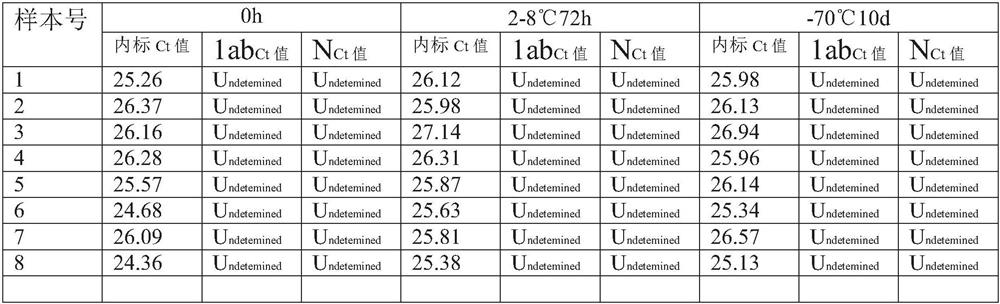
PendingCN111944876AWith digestion/liquefaction functionEffective for digestion/liquefactionMicrobiological testing/measurementMicroorganism based processesBiotechnologyPhosphate
The invention relates to the technical field of biology, in particular to a preserving solution for sputum and a preparation method. The preserving solution of each 1000ML comprises the following components: 0.5-3.5g of dithiothreitol, 5.0-30g of tris(hydroxymethyl)methyl aminomethane, 0.1-10.5g of sodium chloride, 0.01-2.0g of phosphorus trichloride, 0.01-2.5g of disodium hydrogen phosphate, 0.01-2.5g of potassium dihydrogen phosphate, 0.2-3.5g of guanidinium isothiocyanate and the balance nucleic acid-free water, and the pH value is 5.5-8.5. The preserving solution prepared by the inventioncan be stored and applied at room temperature, has a digesting / liquefying function, can effectively digest / liquefy specimens (sputum), does not need to digest the specimens before detection, and meanwhile, has an inactivation effect on specimen (sputum) viruses, so that the biosafety risk of specimen preservation, transportation, processing and detection is lowered; after being collected, sputum specimens collected by using the preserving solution can be stored for 72 hours at 2-8 DEG C, and can be stored for 10 days or more at ultra-low temperature (-70 DEG C); and moreover, the components ofthe preserving solution are easy to obtain, and the preparation cost is low.
Owner:长沙健路医学检验所有限公司
Goat contagious pleuropneumonia subunit vaccine as well as preparation method and application thereof
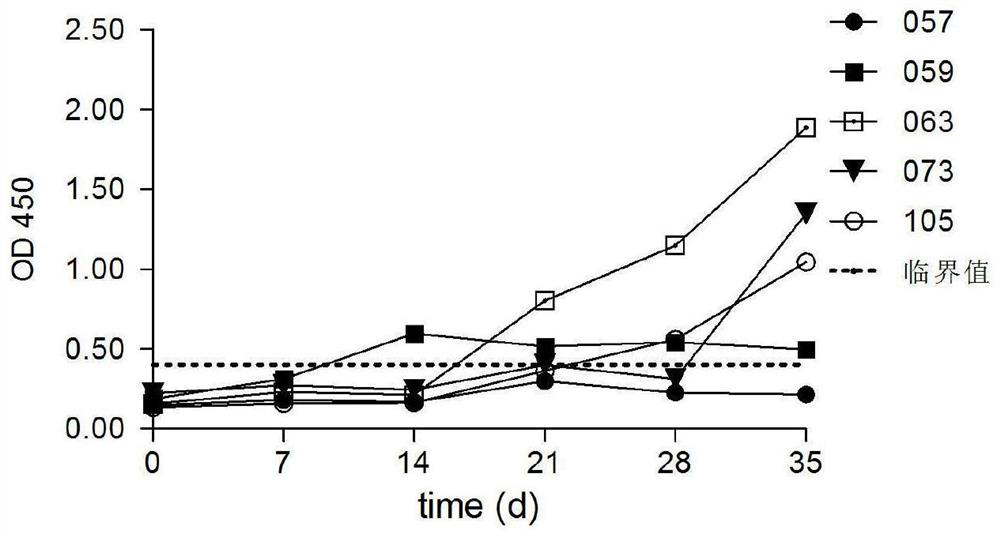
ActiveCN112870341AReduce spreadReduce the risk of contaminationAntibacterial agentsBacterial antigen ingredientsImmune effectsAdjuvant
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com