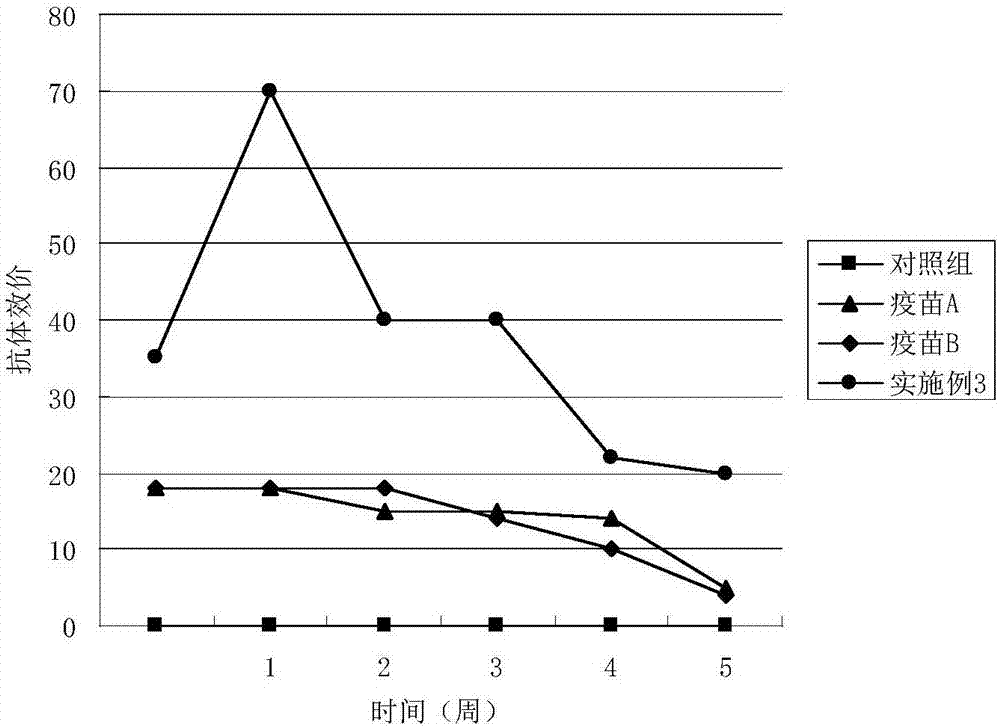
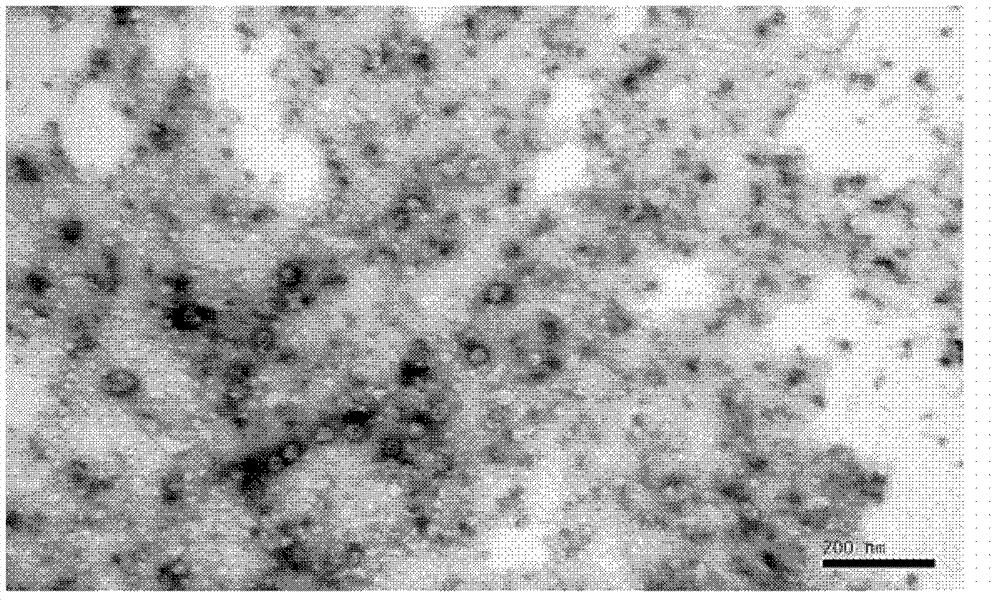
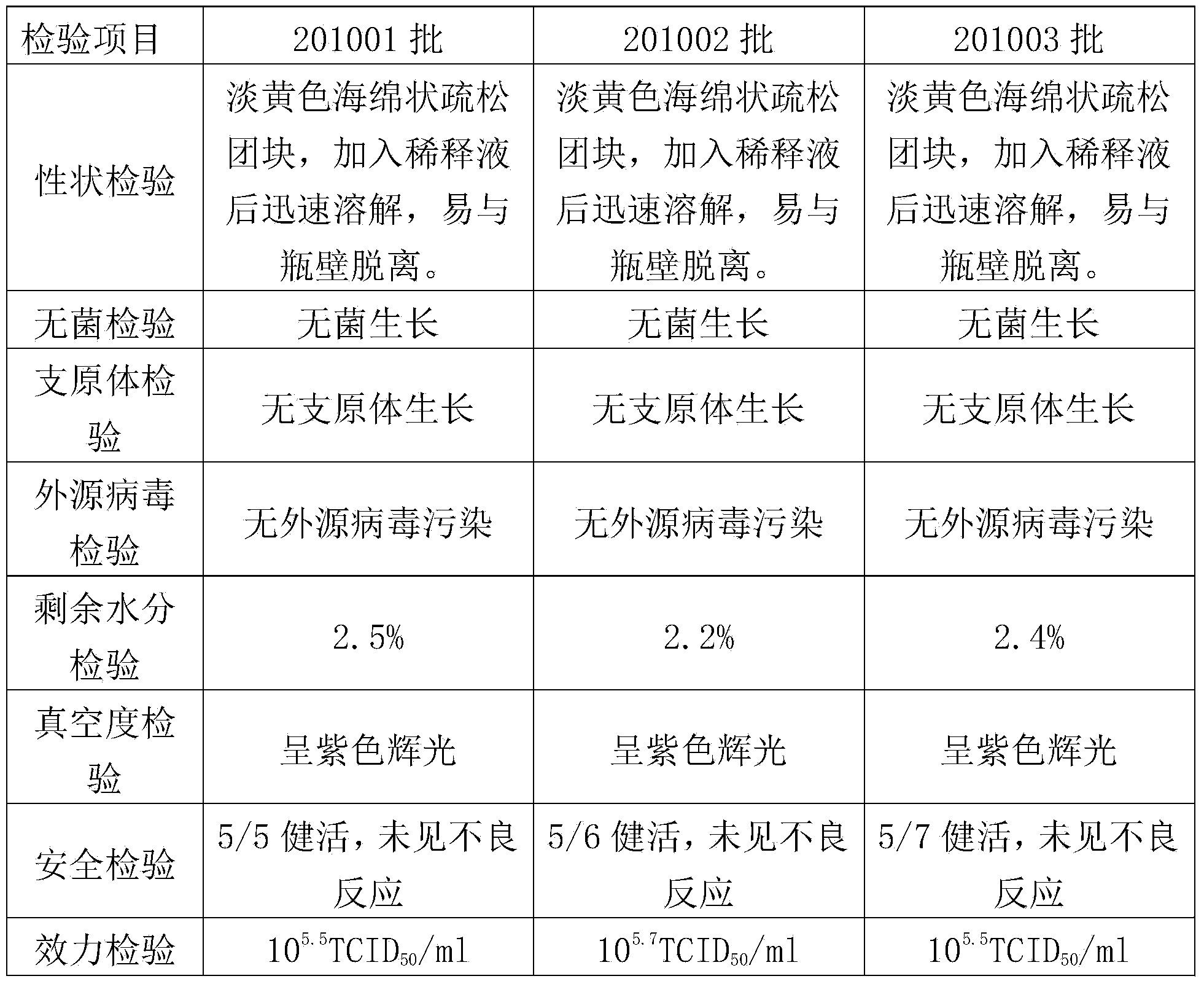
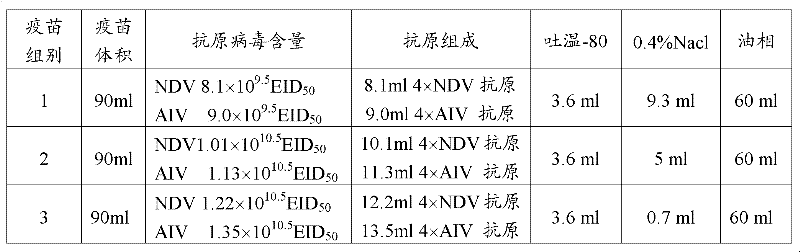
Patents
Literature
46results about How to "Small dose of immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
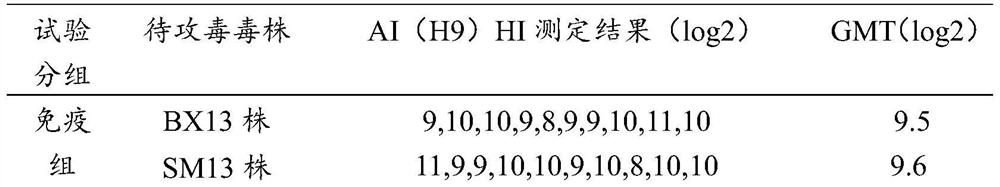
New castle disease and H9 subtype bird flu bivalent vaccine
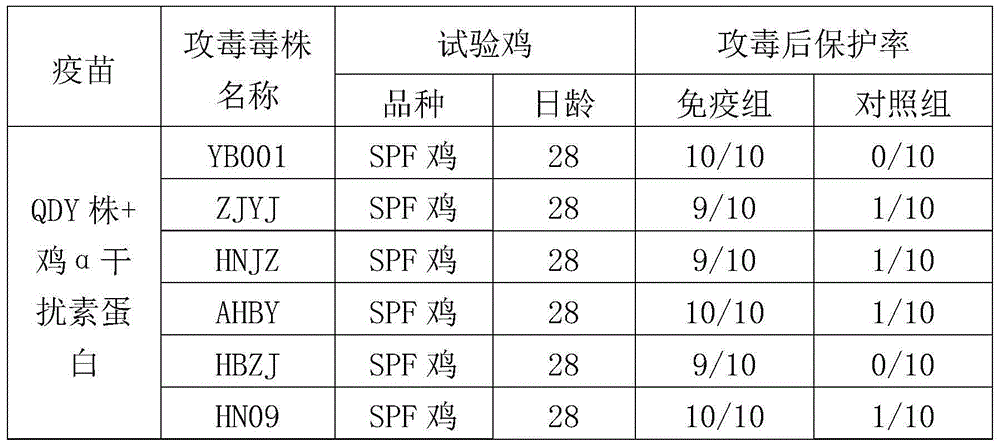
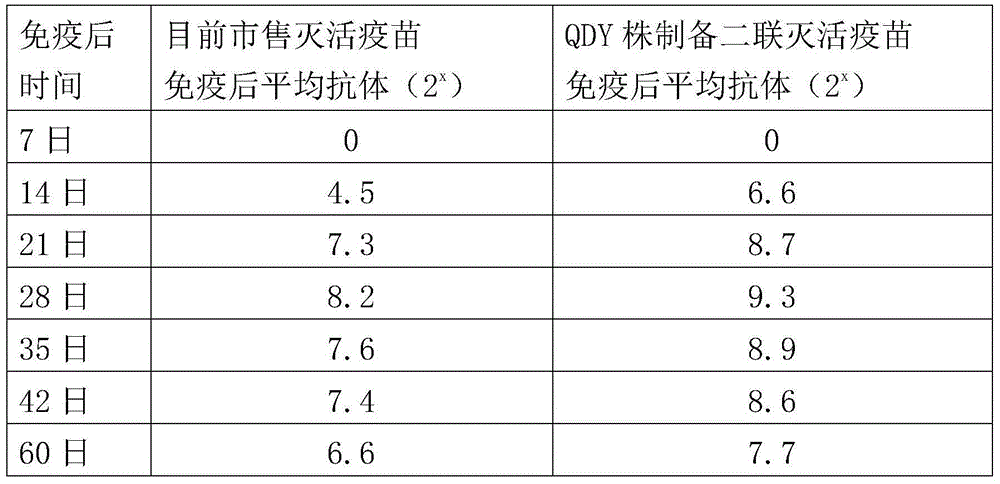
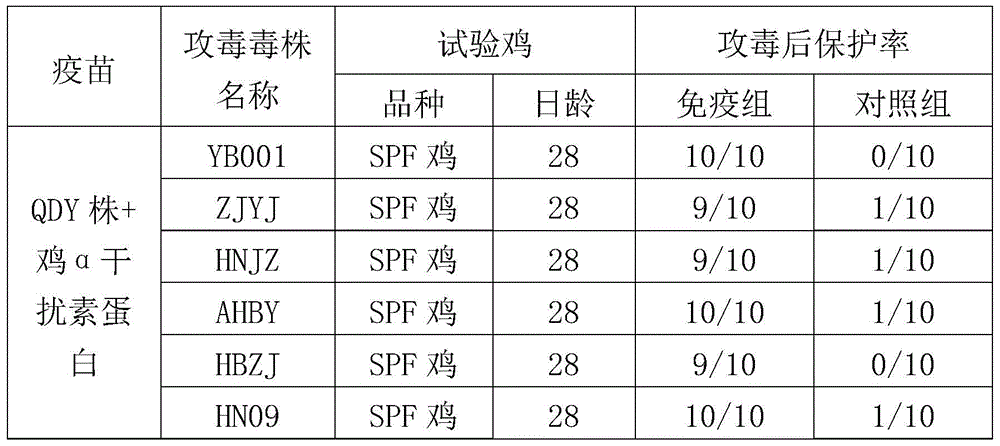
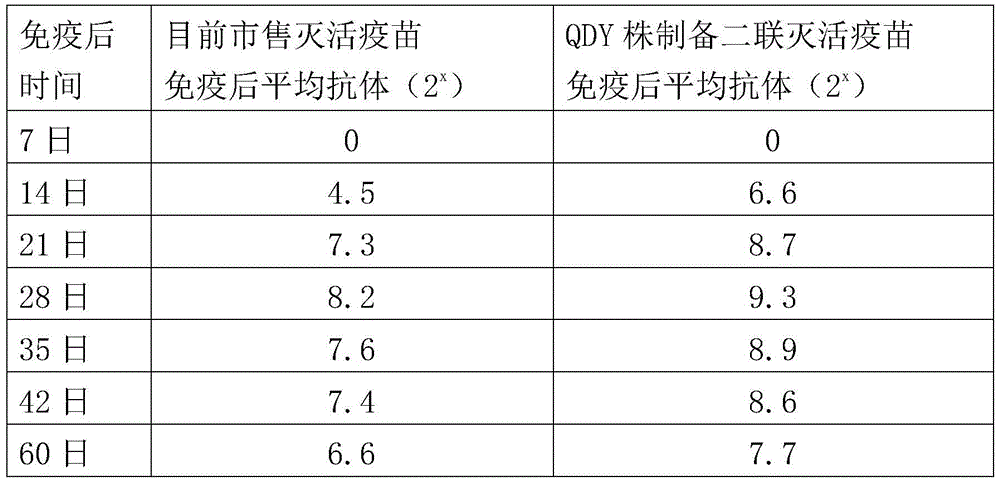
ActiveCN104922663AImproving immunogenicitySmall dose of immunizationViral antigen ingredientsAntiviralsDiseaseOil adjuvant
The invention aims at providing a new castle disease and H9 subtype bird flu bivalent vaccine. The new castle disease and H9 subtype bird flu bivalent vaccine contains antigens and adjuvant. The antigens are inactivated H9 subtype bird flu viruses and new castle disease viruses. The H9 subtype bird flu viruses are QDY strains, and the preservation number of the H9 subtype bird flu viruses is CCTCC v201517. The QDY strains of the H9 subtype bird flu viruses and a Lasota strain of the new castle disease viruses are inoculated to chick embryos respectively, and then virus liquid is collected; the virus liquid and the oil adjuvant are mixed and emulsified into the vaccine after the virus liquid is inactivated through a formaldehyde solution. The new castle disease and bird flu bivalent inactivated vaccine is good in immunogenicity, antibody production is fast after immunity, the produced antibody titer is high, the produced antibody holding time is long, the retention period is long, the immunizing dose is small, the selected adjuvant is easy to inject, and two kinds of diseases can be prevented through one-time injection. The vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Porcine epidemic diarrhea virus inactivated vaccine and preparation method thereof
ActiveCN107050447AImproving immunogenicityPassive immunity is goodSsRNA viruses positive-sensePeptide/protein ingredientsEpidemic diarrheaMicroorganism
The invention provides a porcine epidemic diarrhea virus inactivated vaccine. The porcine epidemic diarrhea virus inactivated vaccine contains the inactivated porcine epidemic diarrhea virus and an adjuvant, wherein the adjuvant is prepared from the following components in percentage by weight: 5% of squalane, 1% of oleic acid, 1% of Tween 80, 92% of 0.005M sodium citrate buffer solution, and 1% of beta-glucan, the porcine epidemic diarrhea virus is prepared by inactivating the porcine epidemic diarrhea virus strain PEDV-KB2013-4, is assigned with the microbial accession number of CGMCC No.12663, has the classification name of Porcine Epidemic Diarrhea Virus (PEDV), and is preserved in the China General Microbiological Culture Collection Center (CGMCC) on Aug 23, 2016, and the preservation address is the Institute of Microbiology of Chinese Academy of Sciences located in 3, Courtyard 1, West Beichen Road, Chaoyang District, Beijing.
Owner:陕西诺威利华生物科技有限公司 +1
Preparing method of porcine pseudorabies virus subunit vaccine, vaccine composition and application
ActiveCN106267182AEasy to prepareShort timeAntiviralsAntibody medical ingredientsImmune effectsAdjuvant
The invention relates to a preparing method of porcine pseudorabies virus subunit vaccine composition. The preparing method includes the steps that 1, a gB protein fragment gene and a gD protein gene are cloned and amplified respectively; 2, the amplified gB protein fragment gene and the amplified gD protein gene are used for constructing plasmid of tandem expression gB protein and gD protein; 3, gB+gD recombinant protein is expressed through the obtained plasmid of tandem expression gB protein and gD protein and purified, adjuvant is added, and emulsification is carried out. The preparing method is simple, porcine pseudorabies virus gB and gD protein can be prepared in quantity, shorter time is spent, the expression amount is large, the production cost is greatly reduced, which is beneficial for large-scale production. The virus subunit vaccine containing the prepared gB and gD protein is good in immune effect and small in immune amount and capable of effectively preventing diseases related to the porcine pseudorabies virus and related infectious diseases caused by the porcine pseudorabies virus.
Owner:PU LIKE BIO ENG
Coxsackievirus A16-type virus strain and use thereof
ActiveCN103087994AGenetic stabilityStable titerSerum immunoglobulinsTransferasesSequence analysisDisease
The invention provides a coxsackievirus A16-type virus strain and a use thereof. The coxsackievirus A16-type virus strain has the preservation number of CGMCC No. 5371. The full-length sequence analysis and the mass spectrometry analysis on a VP1 protein produced by the coxsackievirus A16-type virus strain prove that the oxsackievirus A16-type virus strain is a good CA16 virus strain which is not polluted by allothigenes and has good immunogenicity. The CA16 virus strain can efficiently proliferate in Vero cells and has virus titer of 7.41g CCID 50 / ml. The CA16 virus strain or a vaccine prepared from the CA16 virus strain can be used for preventing diseases caused by CA16 viruses, and has the characteristics of stable titer, good immunogenicity and less immunizing dose.
Owner:SINOVAC BIOTECH
Porcine epidemic diarrhea virus and application thereof
ActiveCN103820399APassive immunity is goodProduced fastInactivation/attenuationImmunoglobulins against virusesMicroorganismMating
The invention provides a porcine epidemic diarrhea virus strain SD10 which is preserved in General Microbiology Center of China Committee for Culture Collection of Microorganisms, School of Chinese Academy of Sciences Committee, at Beichen West Road, No. 1 yard, No. 3, Chaoyang District, Beijing, on November 8, 2013, and the preservation number is CGMCC No. 8503. Compared with the conventional viruses, the porcine epidemic diarrhea virus strain SD10 screened by the invention is low in toxicity, and good in immunogenicity; the prepared vaccine can quick generate antibodies after immunizing; the generated antibodies are high in titer and long in duration; the vaccine is long in storage life, and small in immunity dose, and can enable piglets of a pregnant sow to get better ectophylaxination if being injected to the pregnant sow before mating, so that the piglets can generate strong immune and can resist virulent attacks, and the piglet survival is improved.
Owner:YEBIO BIOENG OF QINGDAO
Porcine epidemic diarrhea virus variant inactivated vaccine and application thereof
InactiveCN106729691AImproving immunogenicityPassive immunity is goodSsRNA viruses positive-senseViral antigen ingredientsEpidemic diarrheaNeutralizing antibody
The invention provides a porcine epidemic diarrhea virus variant inactivated vaccine which is prepared after inactivating a diarrhea virus variant PEDV / CH / BJ / 2014. The vaccine is excellent in immunogenicity; after a sow is inoculated with the vaccine before parturition, a neutralizing antibody is rapidly produced, the antibody titer is high, the maintenance time is long, a piglet of the inoculated sow litter acquires better passive immunity, the piglet is effectively protected against the attack of the epidemic diarrhea virus, and the survival rate of the piglet is increased.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD
Combined inactivated vaccine of Newcastle disease and H9 subtype avian influenza and preparation method thereof
ActiveCN102302775ASmall dose of immunizationWeakened immunityViral antigen ingredientsAntiviralsAntigenImmune effects
The invention provides a combined concentrated and inactivated vaccine of Newcastle disease and H9 subtype avian influenza, which contains an inactivated Newcastle disease virus LaSota strain, an inactivated H9 subtype avian influenza virus HL strain and an adjuvant. The invention further provides a preparation method of the combined concentrated and inactivated vaccine. According to the combinedconcentrated and inactivated vaccine provided by the invention, the immunizing dose of the vaccine is reduced to one third of a traditional vaccine, namely, the immunizing dose of the vaccine is reduced from 0.3 ml to 0.1 ml per chick which is 1-5 weeks old, by means of increasing the antigen content in unit volume of the vaccine so that the stress reaction of vaccine immunity to an organism is reduced. Furthermore, the vaccine provided by the invention is applied to chicken with a small age in days so that the application range of the vaccine is widened. In addition, the immunity effect of the vaccine provided by the invention is not reduced on the premise of reducing the dose of the vaccine. Therefore, the combined vaccine of the Newcastle disease and the avian influenza, provided by the invention, has the advantages of small dose of the vaccine, better immune effect, lower cost and longer immune protection period.
Owner:PU LIKE BIO ENG
Koi herpesvirus ORF25 nucleic acid vaccine
InactiveCN102973951AEasy to produceWill not spreadGenetic material ingredientsVirus peptidesAgricultural scienceImmunogenicity
The invention discloses a koi herpesvirus ORF25 nucleic acid vaccine, which has good immunogenicity. According to the koi herpesvirus ORF25 nucleic acid vaccine, ORF25 gene is cloned to an eukaryotic expression vector pIRES-neo to obtain recombined plasmid. The koi herpesvirus ORF25 nucleic acid vaccine has effective prevention effect to koi herpes.
Owner:JILIN AGRICULTURAL UNIV
Nucleotide sequence, fiber2 protein, expression method of fiber2 protein and duck type 3 adenovirus and duck tembusu virus bivalent inactivated vaccine
PendingCN113846112AAvoid stressHigh protein contentSsRNA viruses positive-senseViral antigen ingredientsCell cultureMolecular biology
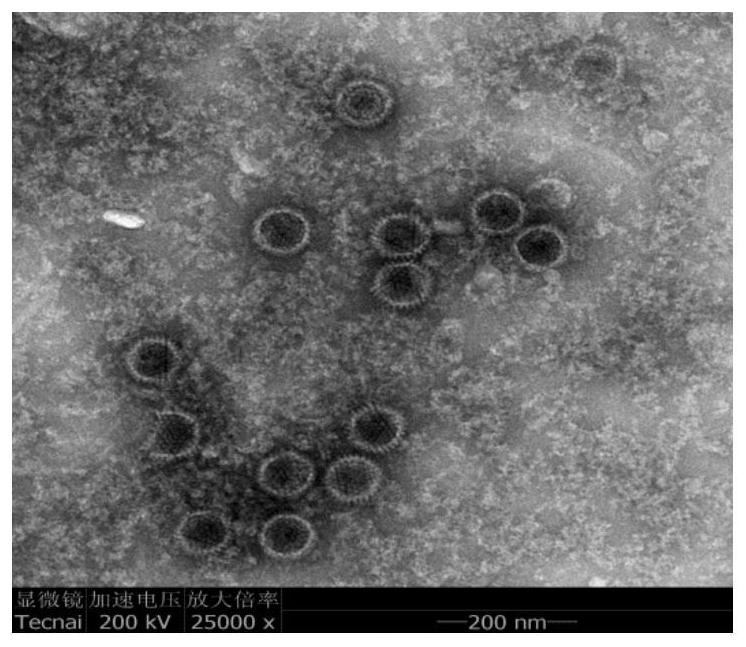
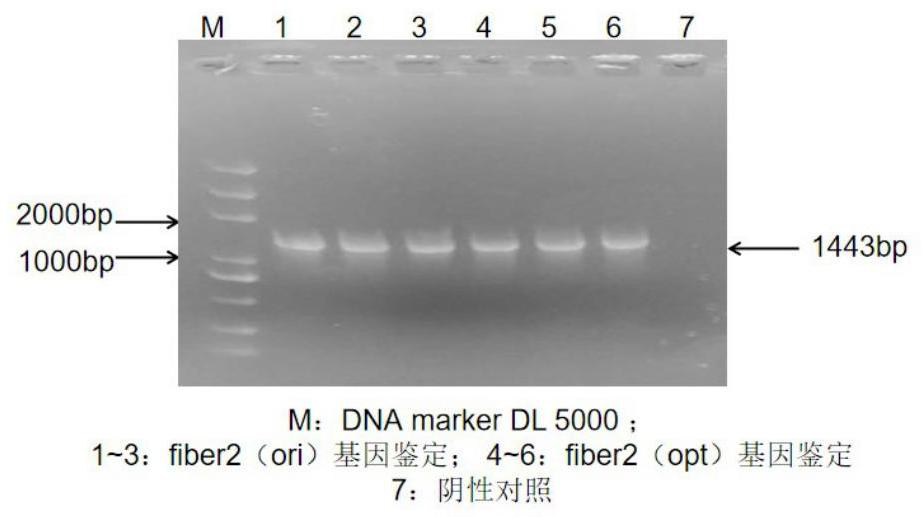
The invention provides a nucleotide sequence, a fiber2 protein, an expression method of the fiber2 protein and a duck type 3 adenovirus and duck tembusu virus bivalent inactivated vaccine. The nucleotide sequence is used for coding the duck type 3 adenovirus antigenic fragment fiber2 protein, the nucleotide sequence is shown as SEQ NO:2, and the amino acid sequence of the duck type 3 adenovirus antigenic fragment fiber2 protein is shown as SEQ NO:3; a fiber2 protein gene segment and a pET-32a vector segment are connected through T4DNA ligase to obtain a pET-32a-fiber2 recombinant expression vector, then the pET-32a-fiber2 recombinant expression vector is converted into an expression strain E.coli BL-21 (DE3) competent cell for cultivation, and the fiber2 protein and the duck type 3 adenovirus and duck tembusu virus bivalent inactivated vaccine are obtained from culture; used antigens of the bivalent inactivated vaccine comprise the duck type 3 adenovirus antigenic fragment fiber2 protein and inactivated duck tembusu viruses; and the volume ratio of the two antigens in the bivalent inactivated vaccine is 1:1, the duck tembusu viruses are duck tembusu virus DF2 strains, and the preservation number of the duck tembusu virus DF2 strain is CCTCC NO: V201635.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Recombinant rabies virus carrying interleukin 6 gene and application thereof
InactiveCN106967691AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsRabies virus strainEukaryotic plasmids
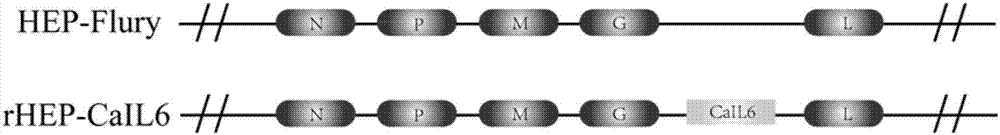
The invention discloses a recombinant rabies virus rHEP-CaIL6 carrying an immune enhancement factor interleukin (IL) 6 gene and application thereof. The recombinant virus takes rabies virus HEP-Flury strain as the skeleton, the IL6 gene of canine is inserted to a position between the G and L gene of HEP-Flury to obtain a recombinant plasmid pHEP-CaIL6, and finally saving screening is carried out to obtain the recombinant rabies virus strain rHEP-CaIL6. The recombinant virus carries the immune enhancement factor, can enhance the immune response and induce the production of higher rabies virus neutralizing antibody, thus better protecting the body from resisting the attack of lethal rabies virus, also can produce a neutralizing antibody with protective ability at a low dose, and lowers the cost of canine vaccines. Moreover, the IL6 gene is recombined into the rabies virus, thus achieving stable expression of IL6 protein, also avoiding the overexpression thereof, and overcoming the defect that excessive IL6 can cause pathological injury.
Owner:SOUTH CHINA AGRI UNIV
Porcine epidemic diarrhea live vaccine
ActiveCN103933561ALow toxicityImproving immunogenicityDigestive systemAntiviralsAntigenEpidemic diarrhea
The present invention provides a porcine epidemic diarrhea (PED) live vaccine, which contains an antigen and a protection agent, wherein the antigen is a PED virus SD10 strain having the preservation number of CGMCC No.8503. According to the present invention, the used antigen PED virus SD10 strain has characteristics of low toxicity and good immunogenicity, the antibody is rapidly produced after the prepared vaccine is adopted to immunize, the produced antibody has characteristics of high titer, long maintaining time, long storage time and low immune dose, and piglets produced by pregnant sows can achieve good passive immunity through immunization injection before mating, such that the piglets can produce strong immunity and can resist virulent virus attacks so as to improve the piglet survival rate.
Owner:YEBIO BIOENG OF QINGDAO
Optimized porcine circovivus type 2 recombinant adenovirus construction method
InactiveCN106399262AIncrease target gene expressionImprove stabilityVectorsVirus peptidesShuttle vectorAutoimmune responses
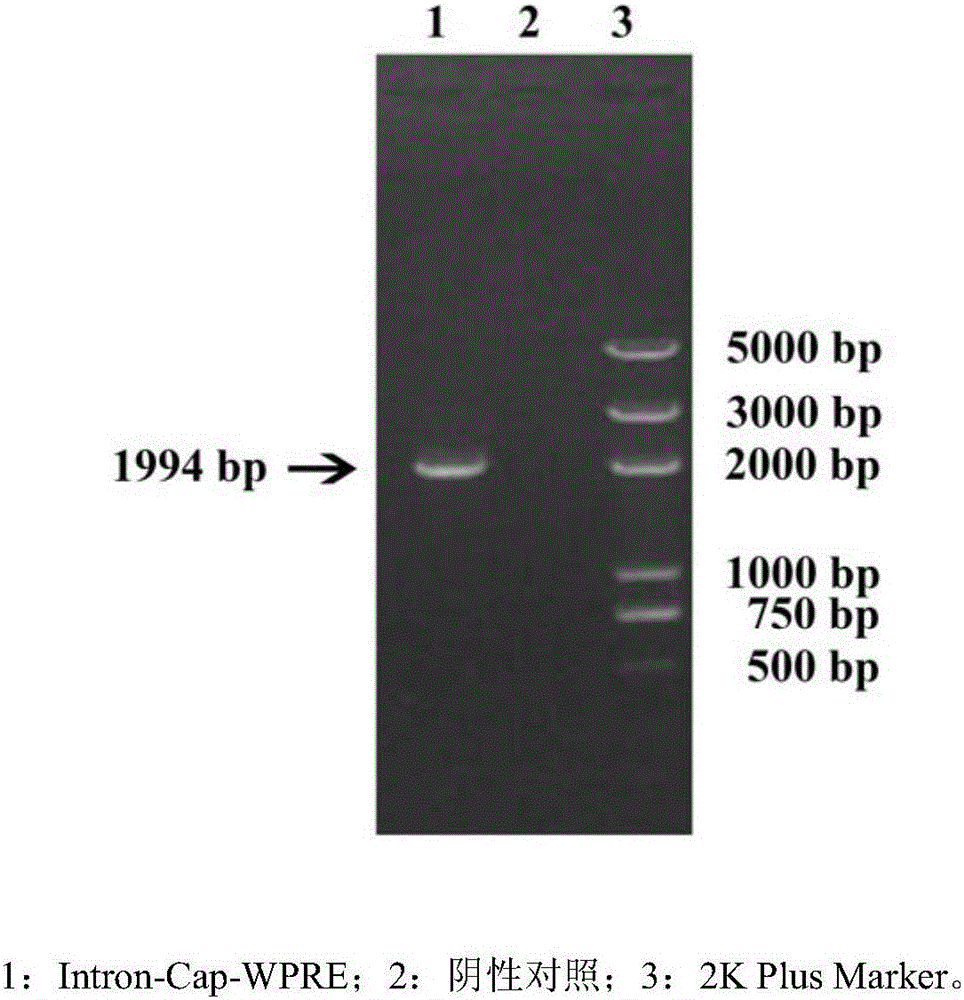
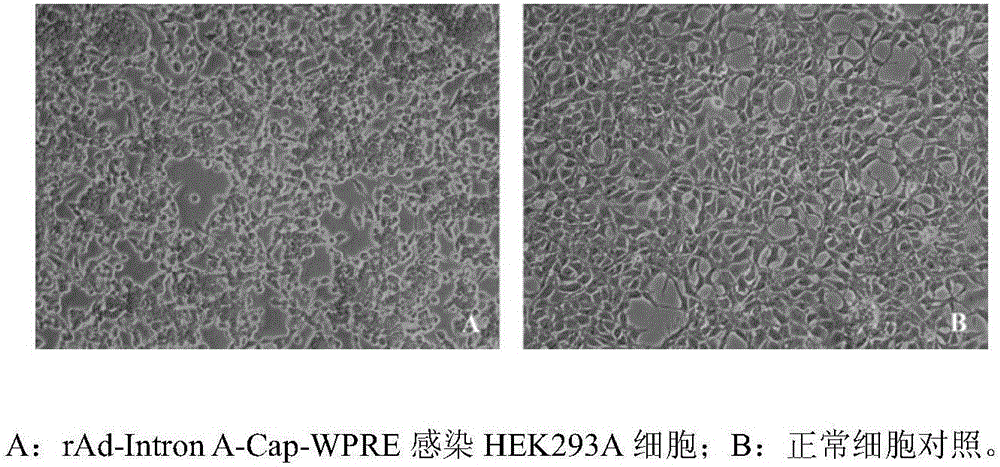
The invention relates to an optimized porcine circovivus type 2 recombinant adenovirus construction method. The construction of recombinant adenovirus is completed by cloning Human cytomegalovirus first intron (Intron A) and woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) into an adenovirus shuttle vector. The construction method provided by the invention has the advantages that a Cap expression quantity is improved, so that adenovirus usage dose and adenovirus protein autoimmune response can be relieved and the preparation efficiency of the porcine circovivus type 2 recombinant adenovirus can be improved.
Owner:NORTHWEST A & F UNIV
Quadruple inactivated vaccine and preparation method thereof
InactiveCN111494617ANo injection stressImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsDiseaseAvian adenovirus
The invention provides a method for preparing a quadruple inactivated vaccine by adopting VP2 protein of a bursal disease virus variant FJ19 (with the preservation number of CGMCC NO: 19381), and a corresponding vaccine. Antigens of the vaccine are a newcastle disease La Sota virus strain, an avian influenza TL18 strain (with the preservation number of CGMCC NO: 19382), a VP2 protein of a bursal disease virus variant FJ19 and an I group 8b type avian adenovirus Hexon protein. The quadruple inactivated vaccine disclosed by the invention is high in VP2 protein and Hexon protein content, high inimmunogenicity and low in formaldehyde and endotoxin content, has a relatively good protection effect on the currently popular variant bursal disease virus FJ19 and the group I 8b type avian adenovirus, is good in safety performance and definite in immune effect, can resist infection of Newcastle disease virus, avian influenza virus, variant bursal disease virus and group I 8b type avian adenovirus to clinical chicken flocks at the same time, is small in immune dose and long in duration, can reach the immune duration of 5 months under the condition of only 0.3 mL once, and at least provides aprotection rate of 70% for the chicken flocks.
Owner:扬州优邦生物药品有限公司
Bivalent inactivated vaccine for porcine parvovirus and porcine epizootic diarrhea
ActiveCN105879023AImproving immunogenicityPassive immunity is goodSsRNA viruses positive-senseViral antigen ingredientsSingle injectionCanine parvovirus
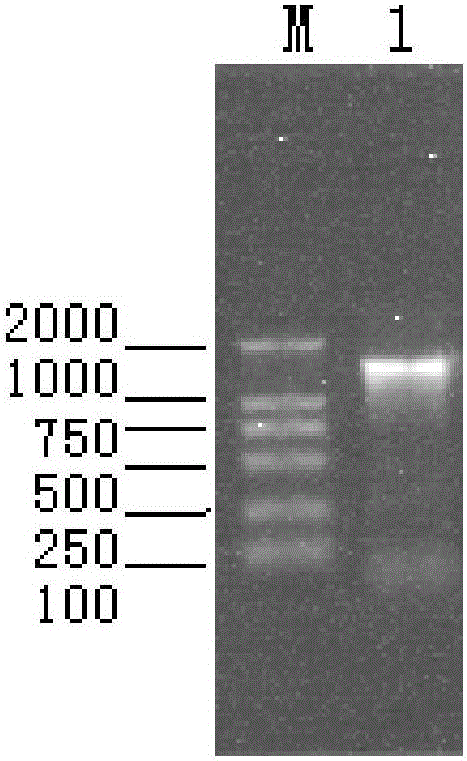
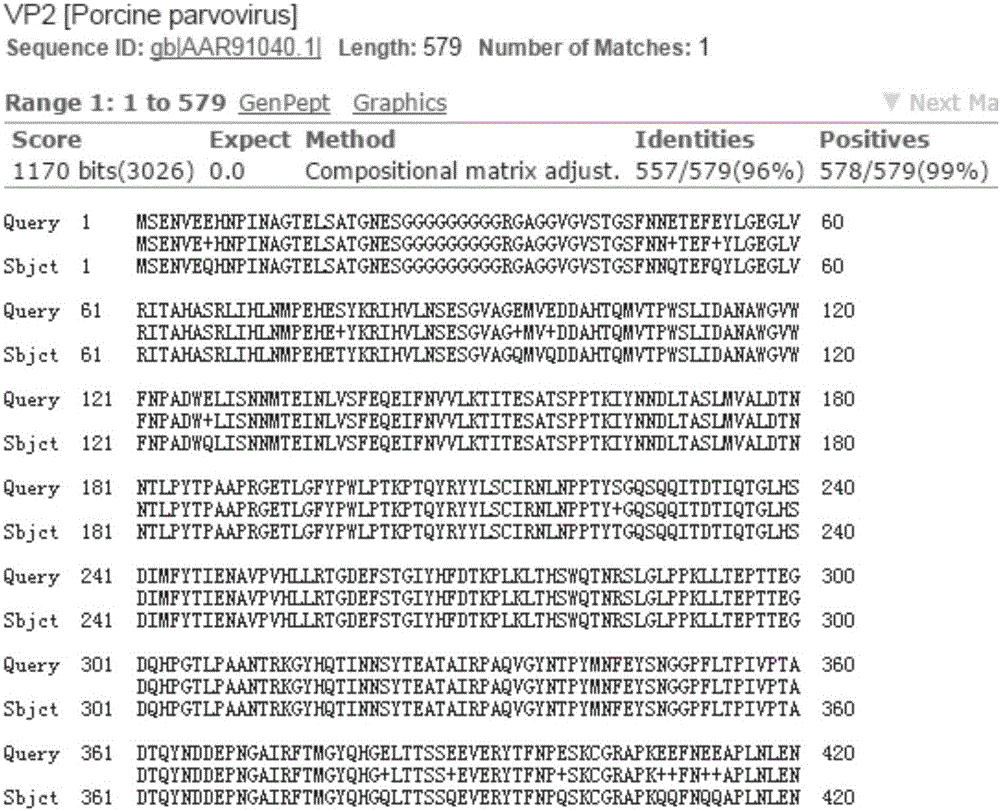
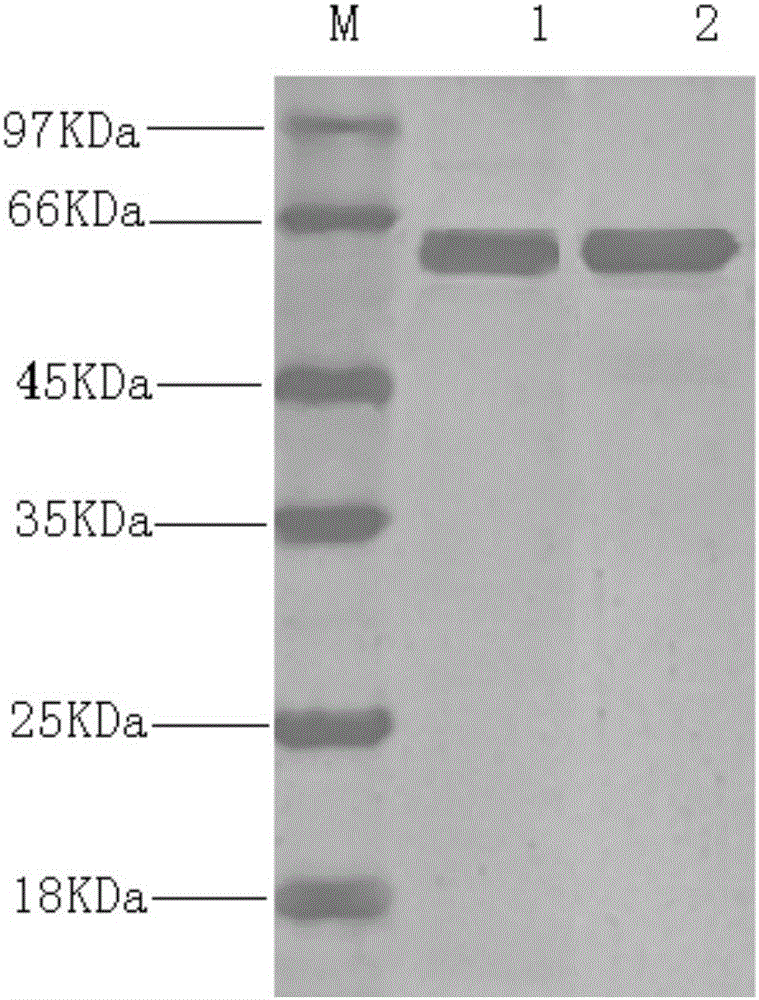
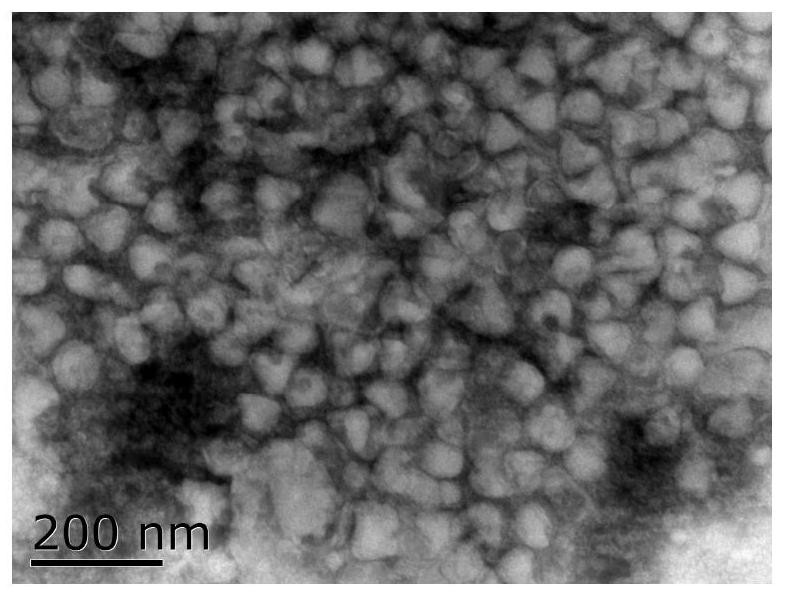
The invention provides bivalent inactivated vaccine for porcine parvovirus and porcine epizootic diarrhea. The bivalent inactivated vaccine comprises antigen and a vaccine adjuvant, wherein the antigen is a porcine epidemic diarrhea virus with the preservation number being CGMCC No. 8503 and porcine parvovirus VP2 protein with the preservation number being CCTCC M 2016098, wherein the porcine parvovirus VP2 protein is expressed by an X33-VP2 strain; the strain was preserved in China Center for Type Culture Collection on 9, March, 2016. The bivalent inactivated vaccine for porcine parvovirus and porcine epizootic diarrhea is good in immunogenicity, an immunized antibody is generated quickly, the generated antibody is high in titer and long in holding time and retention period, the immunizing dose is small, the selected vaccine adjuvant is easy to inject, and the two types of diseases can be prevented and treated through single injection; if immune injection is conducted before mating, piglets born by a pregnant sow can obtain good passive immunity, the piglets can generate high immunity and can resist to attack of powerful viruses, and the survival rate of the piglets is raised.
Owner:YEBIO BIOENG OF QINGDAO
Veterinary bovine ephemeral fever inactivated vaccine and large-scale production method thereof
PendingCN113117068AHigh virus contentImprove immune efficiencySsRNA viruses negative-senseViral antigen ingredientsVaccine PotencyVaccine Production
The invention discloses a veterinary bovine ephemeral fever inactivated vaccine and a large-scale production method thereof, and belongs to the technical field of vaccine production. The method provided by the invention comprises the steps of performing cell culture by utilizing a suspension culture process, performing virus suspension culture, performing virus antigen solution clarification, performing concentration and purification, performing inactivation and performing emulsification vaccine preparation, and the method can realize mass proliferation of the bovine ephemeral fever virus, improves the content and yield of the virus antigen, and has a high automation degree; The synergistic effect of all the steps can jointly improve the quality and stability of the vaccine, and the technical problems that the yield of the bovine ephemeral fever virus produced by cell culture through a traditional spinner bottle or microcarrier process is low, the process is tedious, the difference between virus antigen batches is large, the vaccine efficacy is low, the vaccine validity period is short and the like are solved.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Nontoxic clostridium perfringens and clostridium septicum fusion protein vaccine and production method thereof
PendingCN110051834ASmall dose of immunizationImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsClostridium septicumVaccine Production
The invention relates to a nontoxic clostridium perfringens and clostridium septicum fusion protein vaccine and a production method thereof. The fusion protein vaccine prepared by the method adopts aclostridium perfringens epsilon toxin which is subjected to codon optimization and contains three amino acid mutations, and spoilage which contains four amino acid mutations and eleven amino acid deletions, namely not only kept the integrity and spatial conformation of the natural toxin are to the maximum extent, but also the biological safety hazard caused by single amino acid mutation is avoided. The vaccine also has the advantages of simple preparation process, excellent vaccine efficacy and the like, greatly reduces the biological safety risk in the vaccine production process. Compared with the current commercialized sheep fast-epidemic and sheep enterotoxemia inactivated vaccines in China, the vaccine is an ideal candidate vaccine for upgrading existing clostridium perfringens and clostridium septicum vaccines in China; in addition, when combined vaccines are prepared by the vaccine together with other antigens, the combined vaccine can be prepared without increasing the use doseof the combined vaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
All-round nuclease Benzonase ELISA detection kit
PendingCN113777307AStrong specificityHigh sensitivityBiological material analysisAntiendomysial antibodiesAssay
The invention provides an all-round nuclease Benzonase ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit. The Benzonase ELISA detection kit comprises: a rabbit-derived Benzonase polyclonal antibody; a rabbit-derived Benzonase polyclonal antibody marked by biotin; an HRP labeled by avidin; a standard substance Benzonase; a color developing solution, a stop solution, a confining solution and a washing solution. The lower detection limit of the detection kit is 24 pg / ml, the quantitative detection range is 0.047-3 ng / ml, Benzonase of different manufacturers can be detected, and an imported Benzonase detection kit can be replaced. Meanwhile, methodological system evaluation is carried out on a constructed ELISA detection system, and the kit is good in specificity and high in detection accuracy.
Owner:YEASEN BIOTECHNOLOGY (SHANGHAI) CO LTD
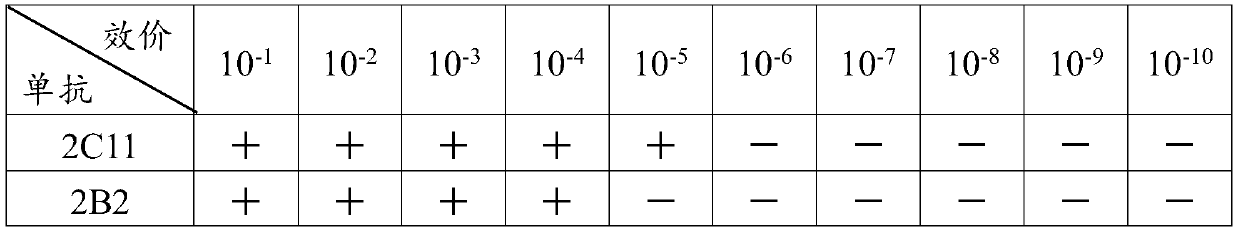
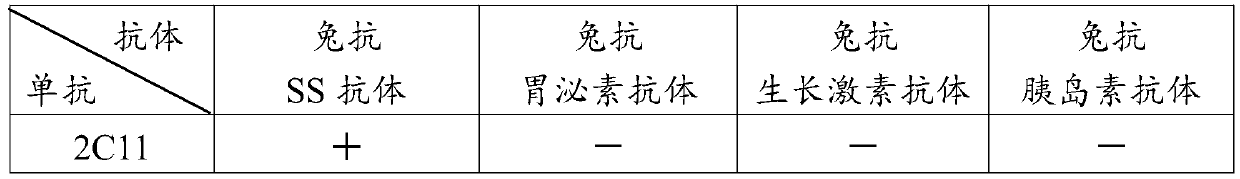
Somatostatin (SS) anti-idiotype monoclonal antibody vaccine and preparation method thereof
InactiveCN110283250APromote growthFacilitated releaseAntibody ingredientsImmunoglobulinsSomatotropic hormoneAntiendomysial antibodies
The invention provides a preparation method of an SS anti-idiotype monoclonal antibody, and belongs to the technical field of bioengineering and vaccine preparation. The method disclosed by the invention comprises the following steps: (1) coupling SS with a macromolecular protein carrier to obtain an SS immunogen complex; (2) carrying out primary immunization on animals with the SS immunogen complex to obtain an SS antibody; (3) carrying out secondary immunization on the animals which are the same or different from the animals in the step (2) with the SS antibody to obtain an SS anti-idiotype monoclonal antibody. The SS anti-idiotypic monoclonal antibody can be prepared by using the method disclosed by the invention. The SS anti-idiotypic monoclonal antibody prepared by the invention can be used as the vaccine to immunize the animals, so that the animals can produce the SS antibodies to immunize and neutralize SS in vivo, thereby promoting the release of growth hormones to promote the growth of the animals.
Owner:西安德轩驰生物科技有限公司 +1
Nucleic acid vaccine for kio herpesvirus
InactiveCN102988973AEasy to produceWill not spreadGenetic material ingredientsAntiviralsHerpesvirus diseasesImmunogenicity
The invention discloses a nucleic acid vaccine for kio hepesvirus. The nucleic acid vaccine has high immunogenicity. The nucleic acid vaccine for the kio hepesvirus is a recombinant plasmid which is obtained by cloning an ORF81 gene into a eucaryon expression vector pIRES-neo. The nucleic acid vaccine for the kio hepesvirus has an effective preventative effect on a kio herpesvirus disease.
Owner:JILIN AGRICULTURAL UNIV
Multi-epitope combined peptide used for treating and preventing human papillomavirus infection and related diseases
InactiveCN108409866AImprove bindingImprove survivabilityViral antigen ingredientsAntibody mimetics/scaffoldsCtl epitopeDisease
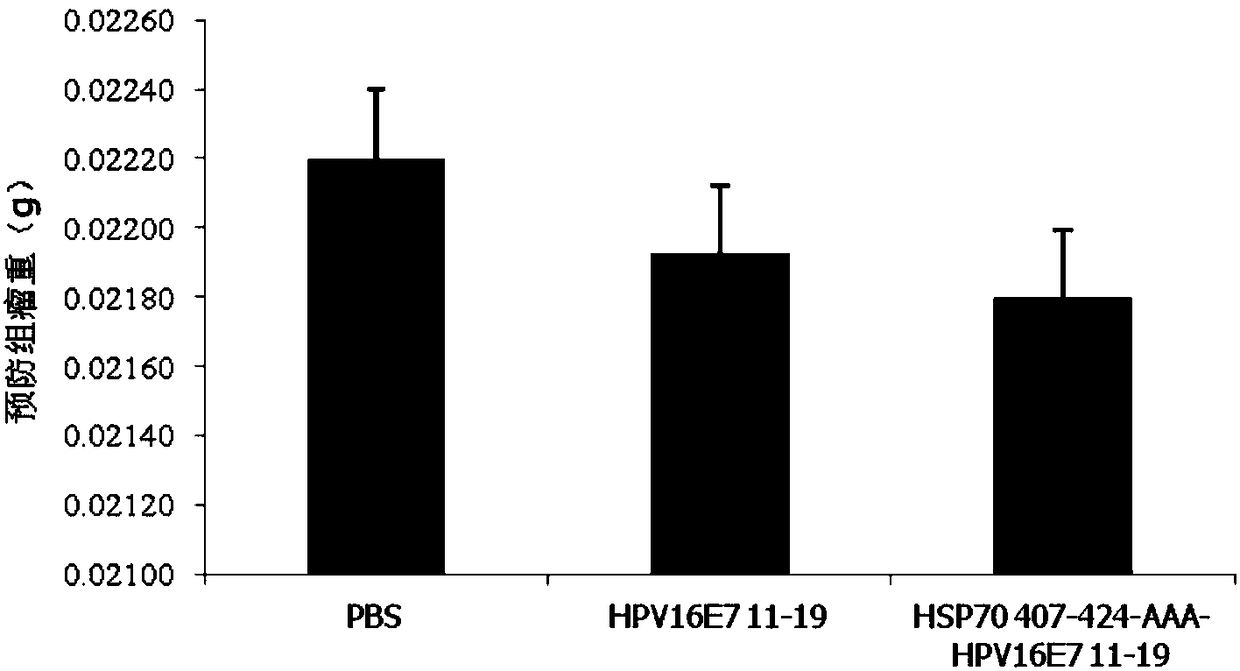
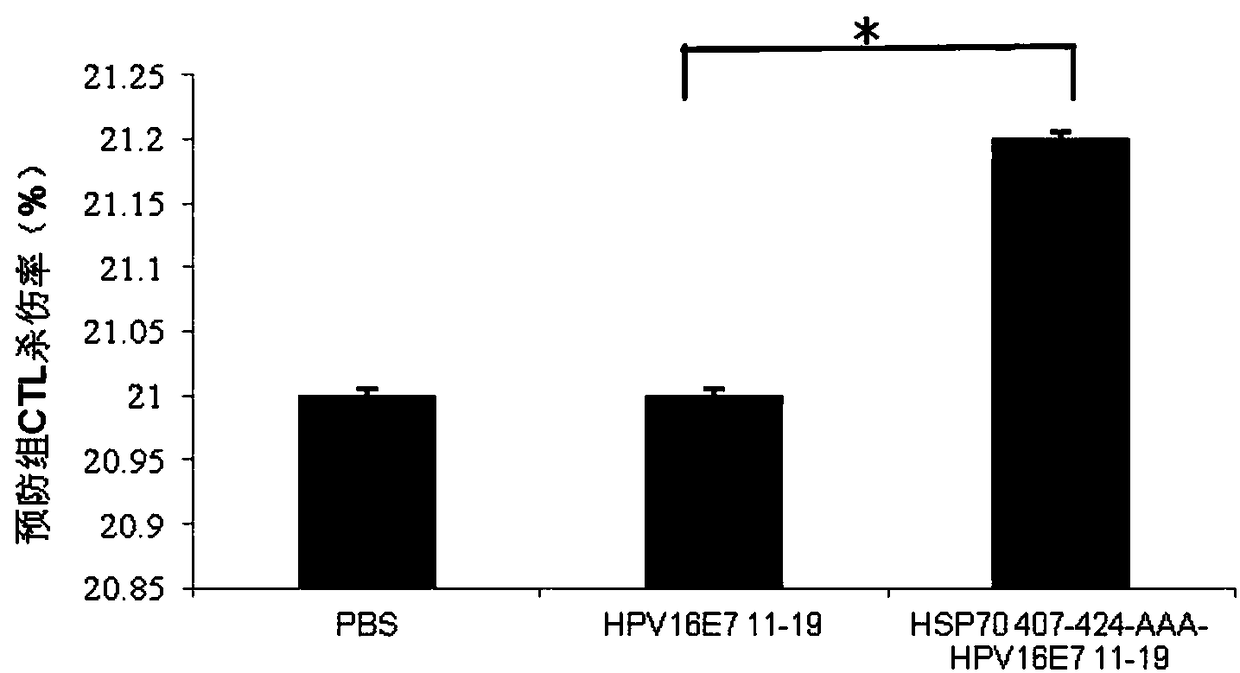
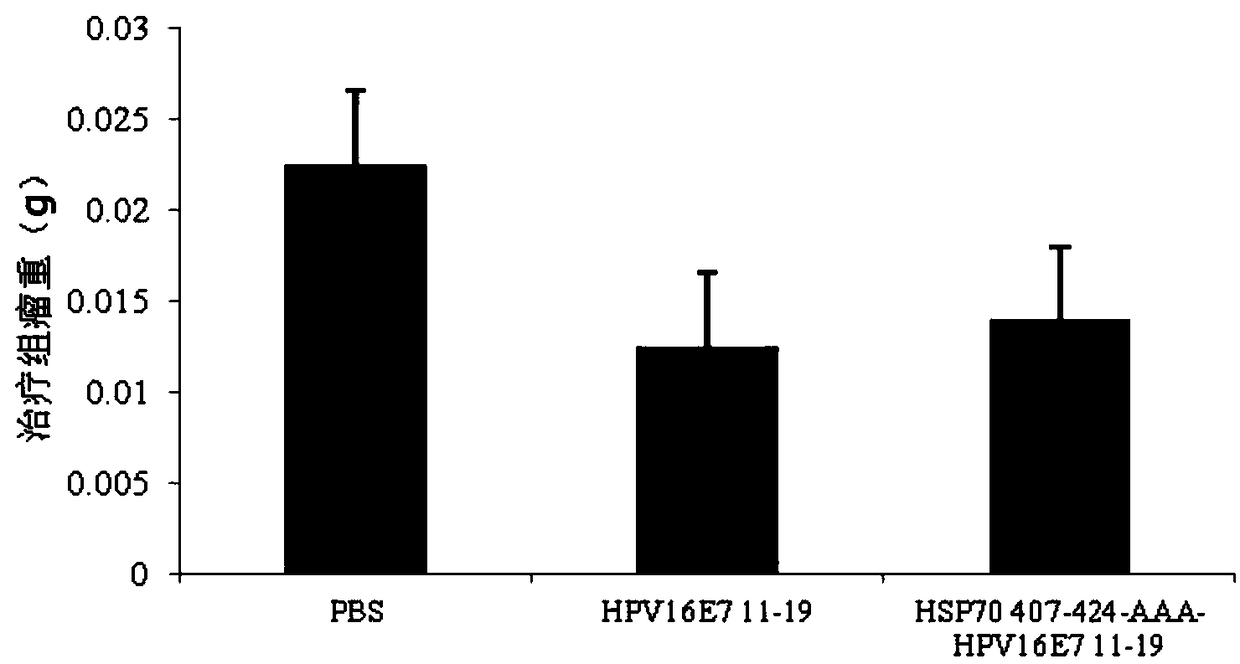
The invention relates to a multi-epitope combined peptide used for treating and preventing human papillomavirus (HPV) infection and related diseases. The multi-epitope combined peptide consists of a binding domain and a hinge domain of carboxyl-terminated peptide of mycobacterium tuberculosis heat shock protein 70 (HSP70), and antigen epitope peptides of T cells of HPV E6 and E7, and the multi-epitope combined peptide is in linear arrangement through the arrangement mode of HSP stimulated epitope peptide-hinge area-HPV CTL epitope. The multi-epitope combined peptide provided by the invention can be used as a vaccine to induce the immune response mediated by specific T lymphocytes through injection of intradermal, hypodermic, focus or mucosal tissues, and can induce effective antiviral effect in tissues and local tissues for treating and preventing HPV infection and related diseases. The multi-epitope combined peptide provided by the invention has the advantages of low use dose and no need of adding artificial excipients.
Owner:HEFEI RUICHENGSHENG BIOTECH CO LTD
Recombinant human papillomavirus vaccine composition and use thereof
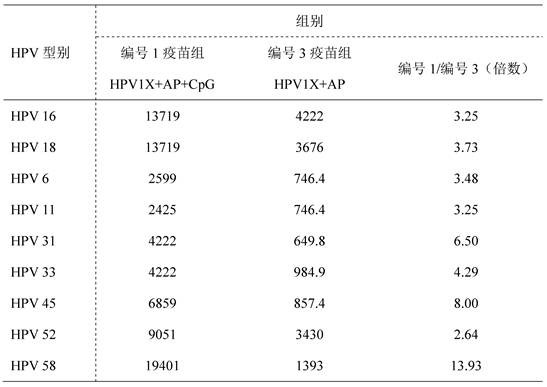
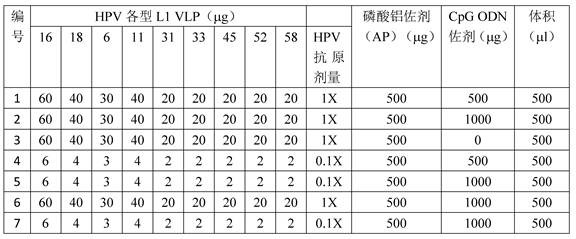
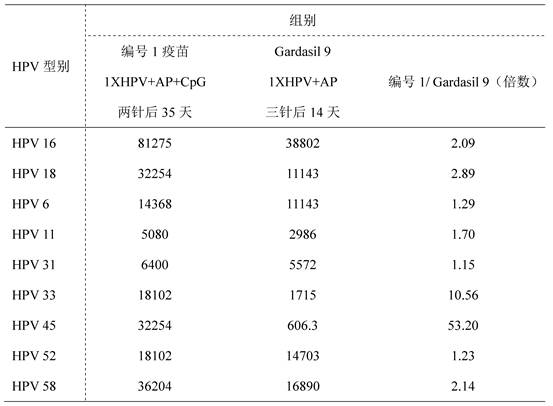
ActiveCN112439059BSmall dose of immunizationImprove immune activityViral antigen ingredientsVirus peptidesImmune effectsAdjuvant
The invention discloses a recombinant human papillomavirus vaccine composition and its application. Compared with the combination of other antigens and adjuvants, the novel vaccine composition provided by the present invention has more beneficial immune effects, reduces the number of immunizations, reduces the dose of antigen immunization, reduces immunization costs, and is beneficial to improve the immunization coverage. Therefore, the vaccine composition of the present invention becomes a new generation of human papillomavirus vaccine.
Owner:IMMUNE PATH BIOTECHNOLOGY SUZHOU CO LTD
A triple subunit vaccine for porcine epidemic diarrhea, porcine transmissible gastroenteritis and porcine D-coronavirus disease
ActiveCN107899008BExtended shelf lifeSmall dose of immunizationSsRNA viruses positive-senseViral antigen ingredientsAstrovirus gastroenteritisTransmissible gastroenteritis virus
Owner:陕西诺威利华生物科技有限公司
Staphylococcus aureus TRAP targeted recombinant protein antigen and application thereof
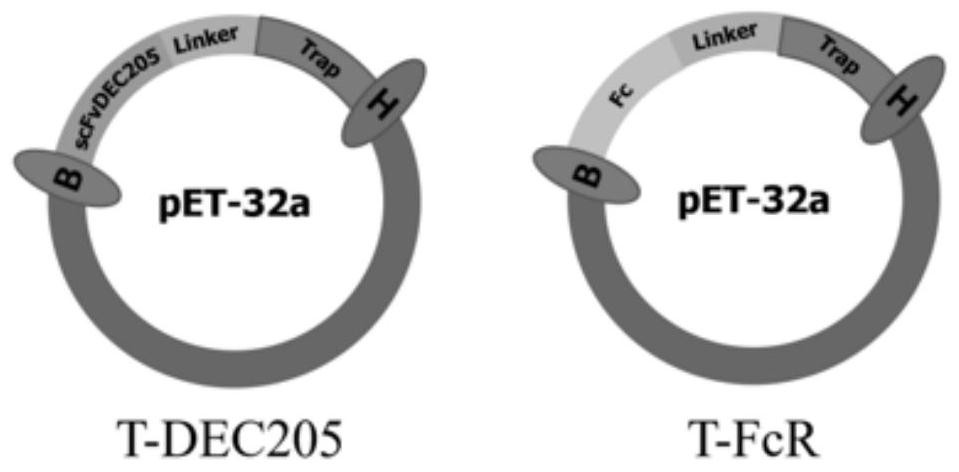
PendingCN114437237AEnhances the level of humoral immune responseSolve the problem of parasitic infectionAntibacterial agentsAntibody mimetics/scaffoldsDendritic cellNucleotide
The invention relates to a staphylococcus aureus TRAP recombinant protein antigen of a targeted dendritic cell DEC205 receptor. The nucleotide sequence of the staphylococcus aureus TRAP recombinant protein antigen is shown as SEQ ID No. 1. The invention further relates to a staphylococcus aureus TRAP recombinant protein antigen of the targeted presentation cell FC receptor, and the nucleotide sequence of the staphylococcus aureus TRAP recombinant protein antigen is shown as SEQ ID No.2. The TRAP protein and the receptor peptide fragment of the targeted antigen presenting cell are recombined together, so that the humoral immune response level of a mouse body is enhanced, the cellular immune response level of the mouse is obviously enhanced, and the problem of intracellular infection parasitism of staphylococcus aureus is solved; according to the method, the immunometering of the vaccine is remarkably reduced, the utilization efficiency of the vaccine is improved, and the immune protection rate of the vaccine is increased to 90% and is remarkably higher than that of untargeted TRAP protein, so that the immune cost is reduced, and the popularization of the vaccine for preventing the cow mastitis is accelerated.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Coxsackievirus A16-type virus strain and use thereof
ActiveCN103087994BGenetic stabilityStable titerSerum immunoglobulinsTransferasesCoxsackievirus a16Titer
The invention provides a coxsackievirus A16-type virus strain and a use thereof. The coxsackievirus A16-type virus strain has the preservation number of CGMCC No. 5371. The full-length sequence analysis and the mass spectrometry analysis on a VP1 protein produced by the coxsackievirus A16-type virus strain prove that the oxsackievirus A16-type virus strain is a good CA16 virus strain which is not polluted by allothigenes and has good immunogenicity. The CA16 virus strain can efficiently proliferate in Vero cells and has virus titer of 7.41g CCID 50 / ml. The CA16 virus strain or a vaccine prepared from the CA16 virus strain can be used for preventing diseases caused by CA16 viruses, and has the characteristics of stable titer, good immunogenicity and less immunizing dose.
Owner:SINOVAC BIOTECH
A kind of chicken Newcastle disease and h9 subtype avian influenza double combination vaccine
ActiveCN104922663BSmall dose of immunizationImprove securityViral antigen ingredientsAntiviralsDiseaseOil adjuvant
The invention aims at providing a new castle disease and H9 subtype bird flu bivalent vaccine. The new castle disease and H9 subtype bird flu bivalent vaccine contains antigens and adjuvant. The antigens are inactivated H9 subtype bird flu viruses and new castle disease viruses. The H9 subtype bird flu viruses are QDY strains, and the preservation number of the H9 subtype bird flu viruses is CCTCC v201517. The QDY strains of the H9 subtype bird flu viruses and a Lasota strain of the new castle disease viruses are inoculated to chick embryos respectively, and then virus liquid is collected; the virus liquid and the oil adjuvant are mixed and emulsified into the vaccine after the virus liquid is inactivated through a formaldehyde solution. The new castle disease and bird flu bivalent inactivated vaccine is good in immunogenicity, antibody production is fast after immunity, the produced antibody titer is high, the produced antibody holding time is long, the retention period is long, the immunizing dose is small, the selected adjuvant is easy to inject, and two kinds of diseases can be prevented through one-time injection. The vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Coxsackie virus A16-type virus strain and applications thereof
ActiveCN102533671BGenetic stabilityStable titerSerum immunoglobulinsMicroorganism based processesSequence analysisVirus strain
The invention provides a coxsackie virus A16-type virus strain. The collection number of the coxsackie virus A16-type virus strain is CGMCC No.5372, wherein CGMCC refers to China General Microbiological Culture Collection Center. The virus is a 20-face stereoscopic symmetrical sphere under observation through an electron microscope, and the diameter of the virus is 23-30nm. VP1 conserved region sequence analysis and mass spectrum analysis are respectively performed on the virus strain, and a result shows a CA16 virus. The CA16 virus can be efficiently proliferated in Vero cells (African green monkey kidney cells), and the virus titer can reach 6.61g CCID50 / ml. Moreover, the virus strain has no external pollution, better immunogenicity and a good effect.
Owner:SINOVAC BIOTECH
A kind of trivalent genetic engineering subunit vaccine composition for preventing duck infectious serositis and preparation method thereof
ActiveCN114099660BImprove immunitySmall dose of immunizationAntibacterial agentsBacterial antigen ingredientsBiotechnologyImmune effects
The invention discloses a trivalent genetic engineering subunit vaccine composition for preventing duck infectious serositis and a preparation method thereof, belonging to the field of veterinary biological products. The trivalent genetic engineering subunit vaccine composition for preventing duck infectious serositis provided by the present invention includes TbdR1-T3, SIP-T3, CAMP, GldG-T3, OmpA-T3, TrpB and HA-T3 recombinant proteins. The duck infectious serositis trivalent genetic engineering subunit vaccine of the invention has good immunization effect and small immunization dose, and can effectively prevent diseases caused by different serotypes of L. anatipestifer. The preparation method of the invention is simple, can prepare a large amount of antigenic proteins of L. anatipestifer, has short time consumption, high expression amount, greatly reduces the production cost, is beneficial to large-scale production, and fills up the existing commercial duck infectivity. Blanks of trivalent vaccine types 1, 2 and 10 in serositis vaccines.
Owner:扬州优邦生物药品有限公司
A kind of poultry adenovirus, a kind of quadruple vaccine and preparation method thereof
ActiveCN109097340BImproving immunogenicityExtended shelf lifeSsRNA viruses negative-senseViral antigen ingredientsDiseaseAdjuvant
The invention provides a new poultry adenovirus, a quadruple vaccine and a preparation method thereof. The new poultry adenovirus is a poultry adenovirus group I serotype 4 DC strain, which was preserved in China on April 25, 2018 The General Microbiology Center of the Microbiological Culture Collection Management Committee, the preservation number is CGMCC No.15589; the quadruple vaccine is a quadruple vaccine of Newcastle disease, avian influenza, infectious bursa and group I type 4 avian adenovirus DC strain. The quadruple vaccine of the present invention has good immunogenicity, rapid antibody generation after immunization, high antibody titer and long maintenance time, small immunization dose, easy injection of the selected adjuvant, and can prevent and treat four diseases with one injection. The advantages of high efficiency and good safety.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
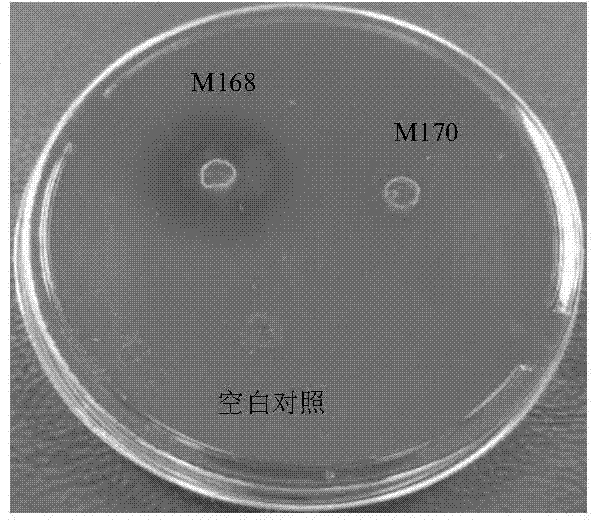
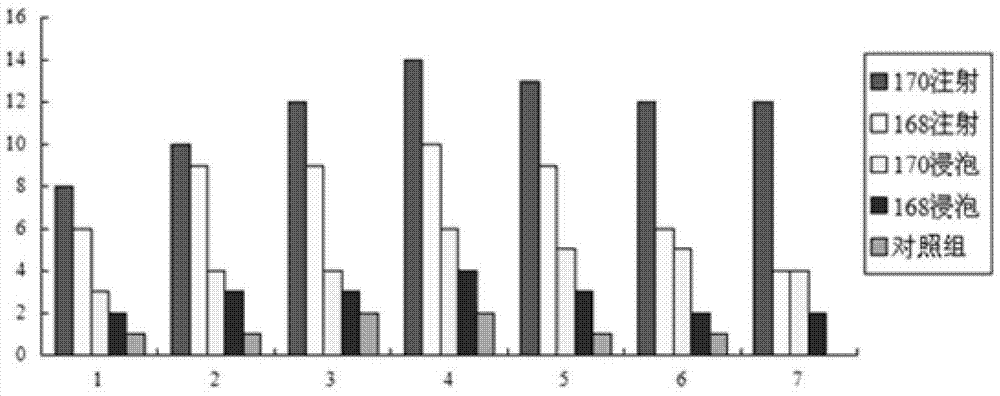
A live attenuated vaccine of fish gill rot pathogen Flavobacter johnii and its construction method
ActiveCN104784684BSmall dose of immunizationHigh potencyAntibacterial agentsBacterial antigen ingredientsDiseaseCarp
The invention discloses a fish gill rot pathogen Flavobacter johnii live attenuated vaccine and a construction method. In the present invention, the wild-type Flavobacterium johniiii is continuously subcultured by streptomycin to induce mutations to weaken its virulence, and the attenuated mutant strain M170 of Flavobacterium johniiii is obtained, and the live vaccine prepared by using the attenuated strain is injected and soaked to immunize grass carp, The immune protection rate of mandarin fish and others can reach more than 73.1%, and the immune protection period can reach more than 8 months. It has the advantages of small immune dose, high potency, high protection rate, and long protection period. Preventing the occurrence of bacterial gill rot diseases such as grass carp and mandarin fish has good application value and broad market prospects.
Owner:湖北宜水生物科技有限公司
A live porcine epidemic diarrhea vaccine
ActiveCN103933561BLow toxicityImproving immunogenicityDigestive systemAntiviralsEpidemic diarrheaImmunogenicity
The present invention provides a porcine epidemic diarrhea (PED) live vaccine, which contains an antigen and a protection agent, wherein the antigen is a PED virus SD10 strain having the preservation number of CGMCC No.8503. According to the present invention, the used antigen PED virus SD10 strain has characteristics of low toxicity and good immunogenicity, the antibody is rapidly produced after the prepared vaccine is adopted to immunize, the produced antibody has characteristics of high titer, long maintaining time, long storage time and low immune dose, and piglets produced by pregnant sows can achieve good passive immunity through immunization injection before mating, such that the piglets can produce strong immunity and can resist virulent virus attacks so as to improve the piglet survival rate.
Owner:YEBIO BIOENG OF QINGDAO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com