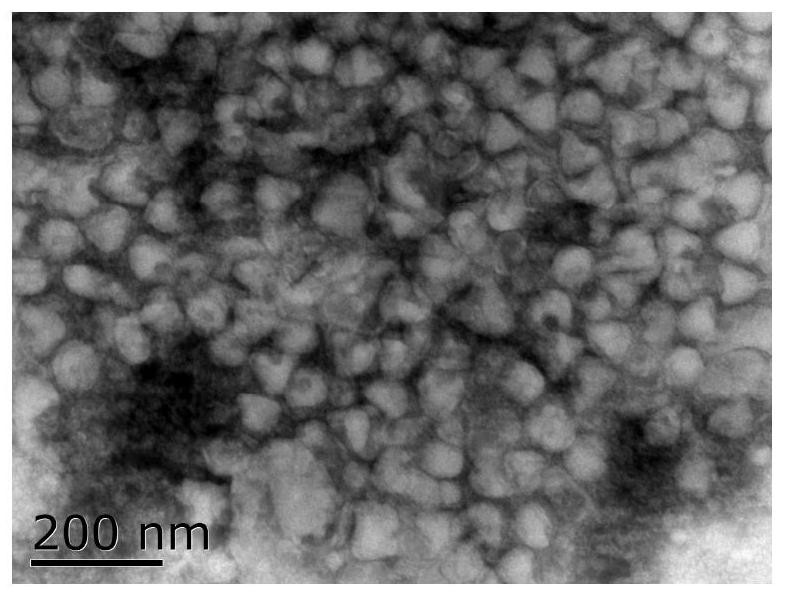
Veterinary bovine ephemeral fever inactivated vaccine and large-scale production method thereof
A bovine epidemic fever and production method technology, which is applied in the field of vaccine preparation in biomedicine, can solve the problems of high labor cost, large batch-to-batch variation, and low vaccine efficacy, and achieve high automation, small immune dose, and toxin production efficiency high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, isolation and identification of bovine epidemic fever virus
[0038] The bovine epidemic fever virus strain used in the embodiment is isolated from a suspected bovine epidemic fever cattle in a farm in Nanyang City, Henan Province. The specific isolation operation is as follows:
[0039] Collect sick cattle anticoagulant blood, utilize bovine epidemic fever (bovine epidemic fever virus) fluorescent quantitative PCR method (with reference to the primers and PCR detection methods disclosed for bovine epidemic fever virus among CN109355431A) detect this anticoagulant blood, if bovine epidemic fever shows Positive, indicating that the anticoagulant blood contains bovine epidemic fever antigen; the anticoagulant blood was frozen and thawed 3 times and inoculated on BHK21-C13 monolayer cells (stored in Jinyu Baoling Biopharmaceutical Co., Ltd.), and the culture medium contained 8 %-10% newborn bovine serum in DMEM solution, cultured at 37°C for 48-72h, and when ...
Embodiment 2
[0075] Embodiment 2, the optimization of bovine epidemic fever virus inoculation and culture conditions
[0076] 2.1. Determination of optimal harvesting time of virus antigen:
[0077] The F6 generation seed virus in Example 1 was passed on for 10 generations to obtain the F16 generation seed virus, which was inoculated in BHK21-C13 suspension cells according to 0.2% (volume percentage content) (the BHK21-C13 suspension cell culture is described in detail in the following embodiment 3) method), the initial seeding density of cells was 2×10 6 cells / ml, cultured at 37°C, and the culture medium was a special medium for suspension (1.15g / L sodium bicarbonate was added to BBSM medium (purchased from Beijing Qingda Tianyi Technology Co., Ltd., product code: MD910), and 3% to 5% (percentage by volume) of newborn bovine serum, adjust the pH value to 7.2 ± 0.5), take samples when culturing 24, 48, 72, 96, and 120h respectively to observe the bovine epidemic fever virus infection of B...
Embodiment 3
[0097] Embodiment 3, the production of bovine epidemic fever inactivated vaccine
[0098] This embodiment utilizes the bovine epidemic fever virus strain that above-mentioned embodiment 1 isolates as seed virus as example, according to the virus inoculation and culture condition determined in above-mentioned embodiment 2, cultivate bovine epidemic fever virus seed virus with the BHK21-C13 cell of suspension culture, Thereby the virus antigen used for producing the bovine epidemic fever inactivated vaccine is prepared. Wherein the existing bovine epidemic fever virus strain can also be used as the seed virus, and the claimed technical effect of the present invention can also be realized. The production of bovine epidemic fever inactivated vaccine specifically includes the following operations.
[0099] 3.1 Large-scale cultivation of BHK21-C13 suspension cells in bioreactors
[0100] 3.1.1. Recovery of BHK21-C13 cells in suspension culture
[0101] Take out a frozen BHK21-C13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com