Method for preparing pseudorabies live vaccines from DF1 continuous cells and product prepared by method
A technology for passaged cells and pseudorabies, which is applied in the field of biomedicine, can solve the problems of many cell fragments and impurities, easy to cause allergic reactions, large differences between cell batches, etc., and achieves high virus content, good immune protection, and production technology. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 pseudorabies live vaccine
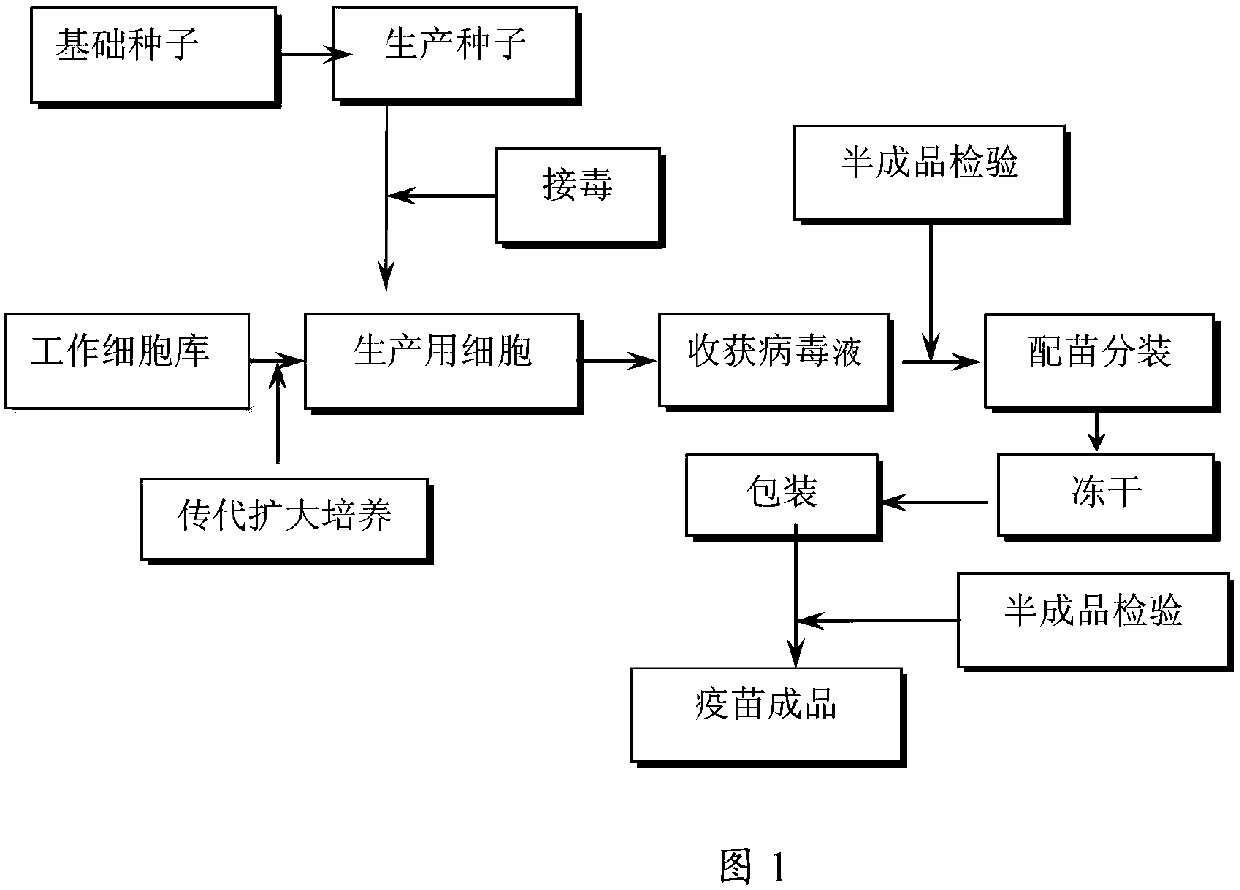
[0031] Such as figure 1 Shown, a kind of method utilizing DF1 passage cell to produce pseudorabies live vaccine comprises the steps:
[0032] (1) Cell selection for seedling production: DF1 passage cells;
[0033] (2) Passage and culture of cells for seedling production: the above-mentioned DF1 passage cells were digested and passaged by EDTA-trypsin cell dispersion liquid, and continued to be cultured with cell growth liquid, and when a good monolayer was formed, they were used for continued passage or virus inoculation;
[0034] In the process of subculture and daily maintenance of cell culture, a lot of expense is required in terms of culture equipment, culture medium and various preparations, and once the cells leave the living body to start primary culture, its various biological characteristics will gradually change and become With the increase of the number of passages and the change of the environment...
Embodiment 2
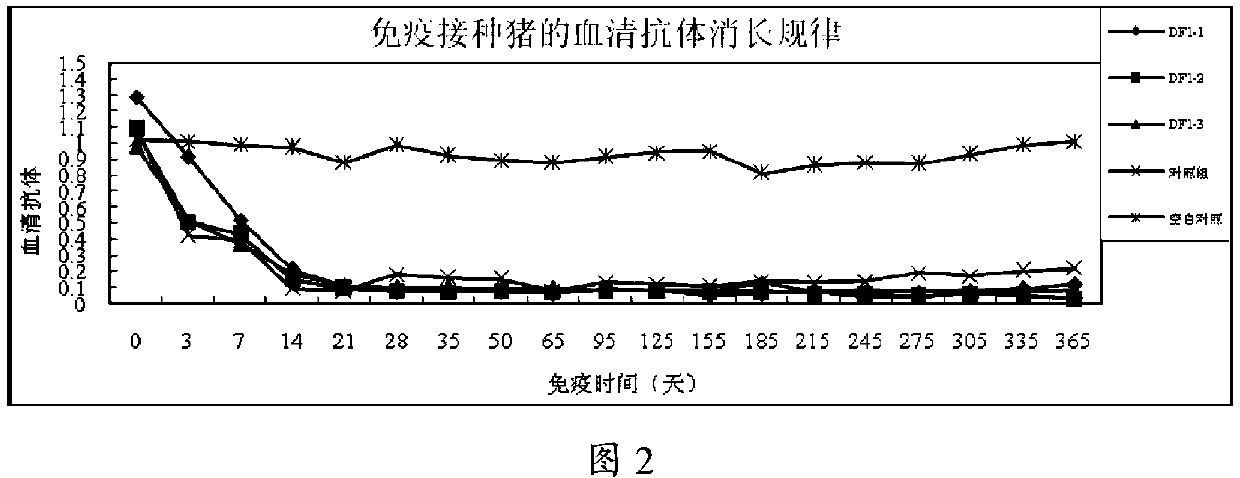
[0048] Example 2 The safety test of pseudorabies DF1 passage cell live vaccine to pigs
[0049] 1 Materials and methods
[0050] 1.1 Experimental vaccines
[0051] Three batches of live cell vaccines for pseudorabies DF1 developed in the laboratory, the batch numbers are DF201301, DF201302, and DF201303.
[0052] 1.2 Experimental animals
[0053] Healthy pigs that were negative for neutralizing antibodies to pseudorabies virus were obtained from the experimental animal farm of Guangdong Yongshun Biopharmaceutical Co., Ltd.
[0054] 1.3 Safety test on pigs
[0055] 1.3.1 Safety trial of a single-dose immunization
[0056] Select healthy pigs aged 20-25 days and 50-55 days, and immunize pigs of different ages with 3 batches of laboratory products in a single dose. Each batch of vaccines is used to immunize 5 pigs, and each pig is intramuscularly injected with 1 head After inoculation, the temperature was measured and observed once a day in the morning and afternoon for 14 d...
Embodiment 2
[0076] Embodiment 2 pseudorabies DF1 subculture cell live vaccine toxin production test
[0077] 1 material
[0078] 1.1 Virus seeds were provided by Guangdong Yongshun Biopharmaceutical Co., Ltd.
[0079] 1.2 The cells were provided by Guangdong Yongshun Biopharmaceutical Co., Ltd.
[0080] 2 methods
[0081] Resuscitate the cells from the working cell bank and passage the cells, take the F10, F15, F20, F25, F30 generation cells that have formed a good monolayer, discard the nutrient solution, and inoculate with 0.1%~3% (ml / ml) production poison Keep the seed maintenance solution at 36-37°C to continue culturing, observe twice a day after inoculation, harvest the virus liquid when the CPE reaches 50%-100%, and store the harvested venom at -20°C or below. The cytotoxins harvested from the working cells of each passage were taken to measure the virus content respectively. And carry out two repeated experiments.
[0082] 3 results
[0083] The cells recovered from the work...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com