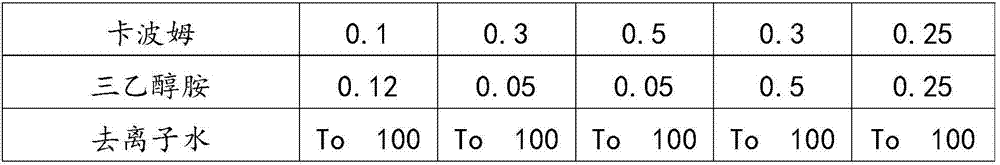
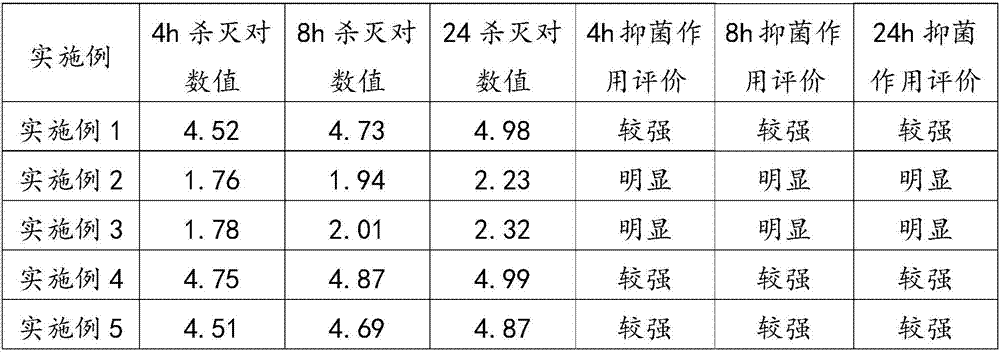
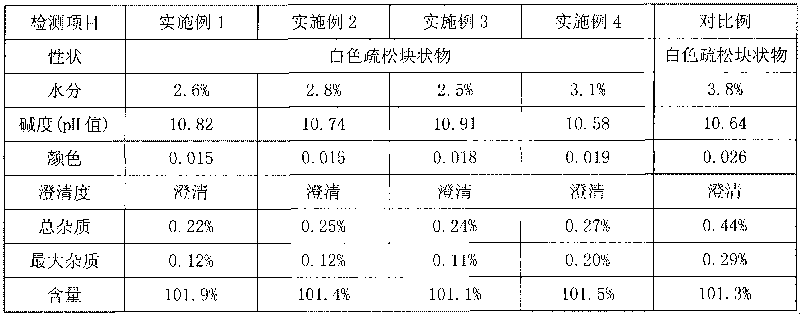
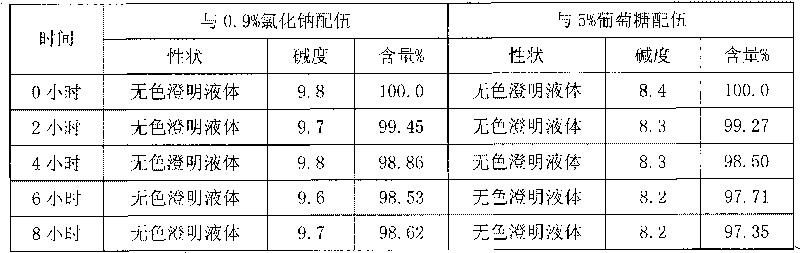
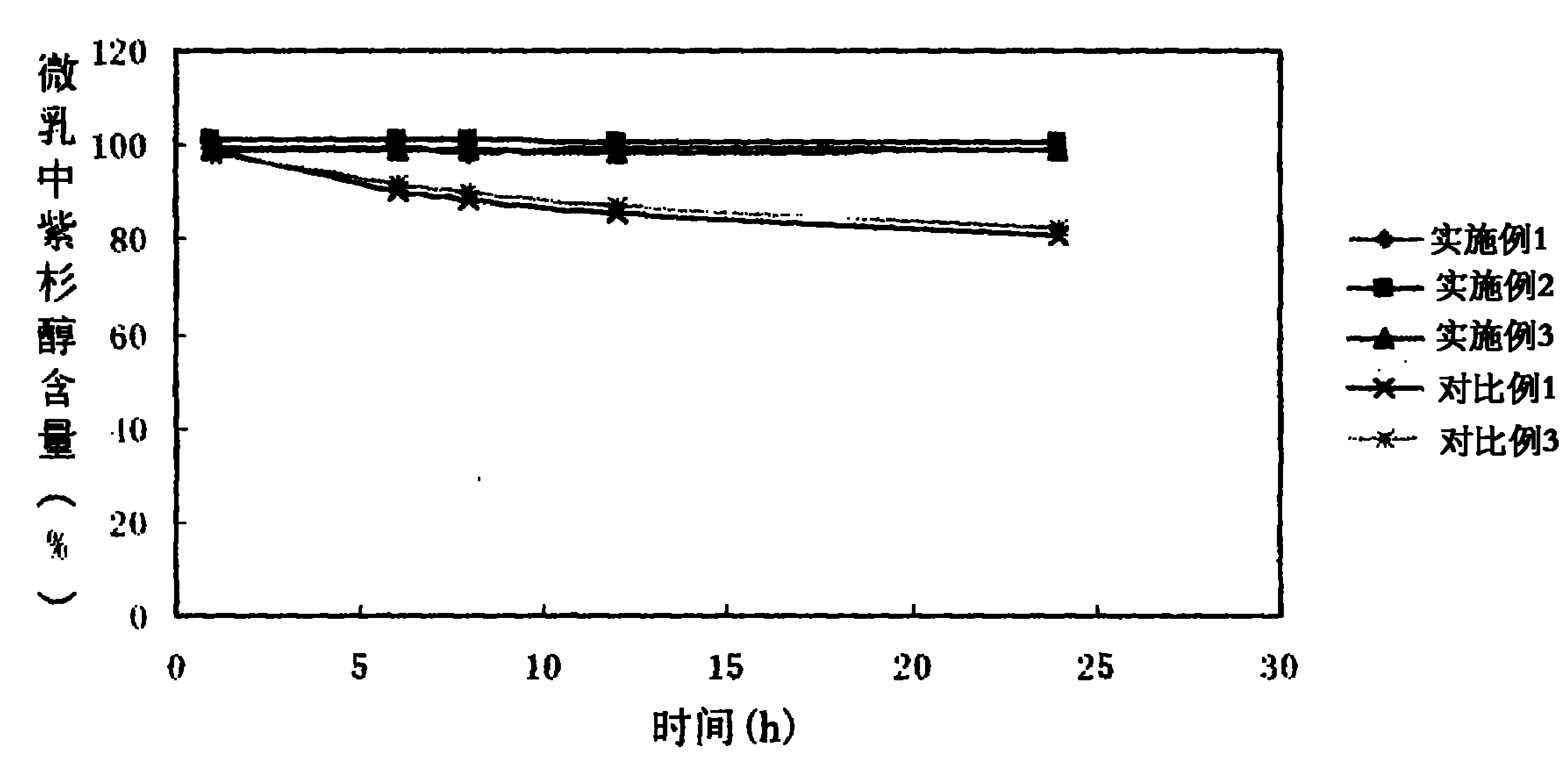
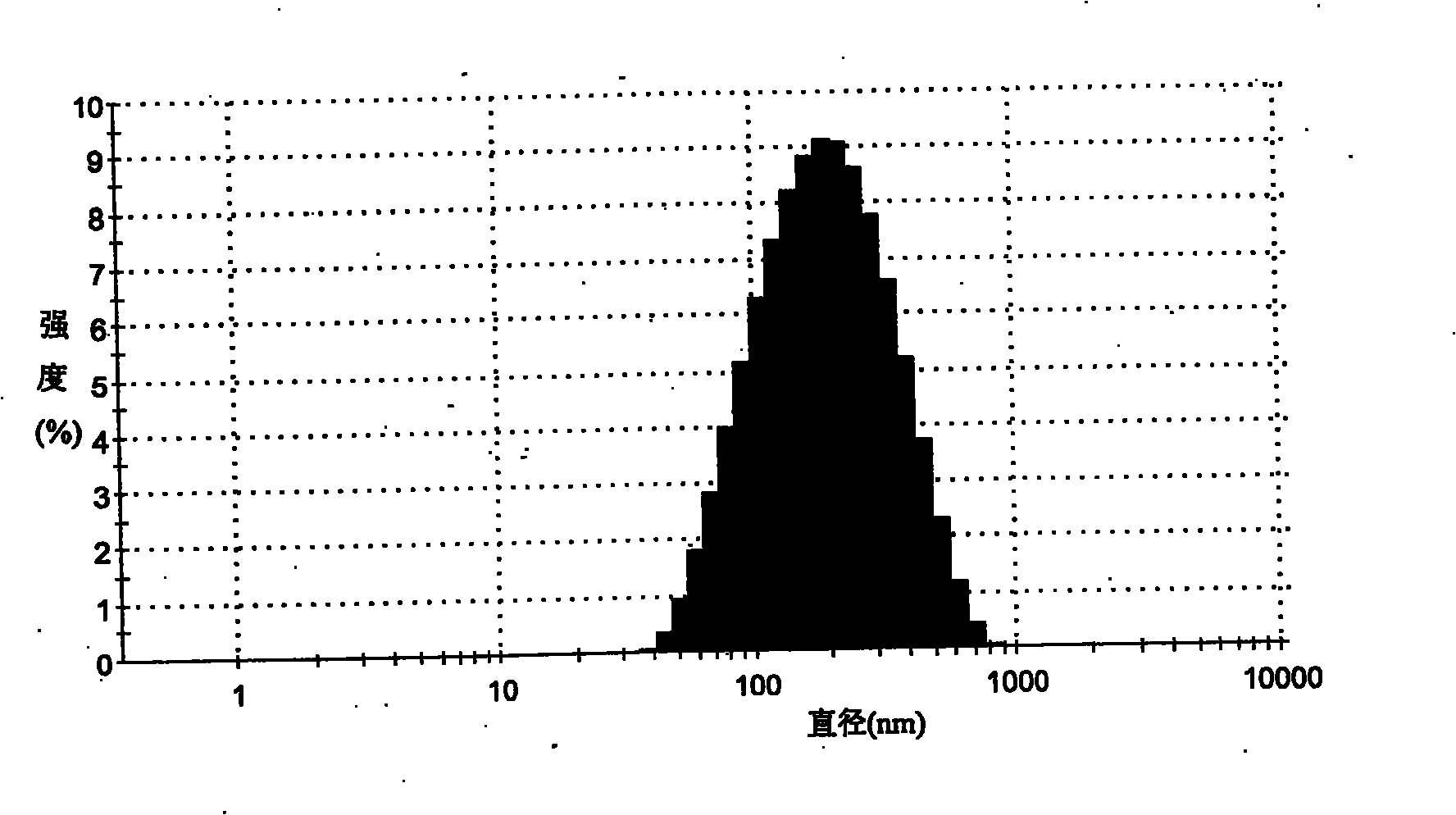
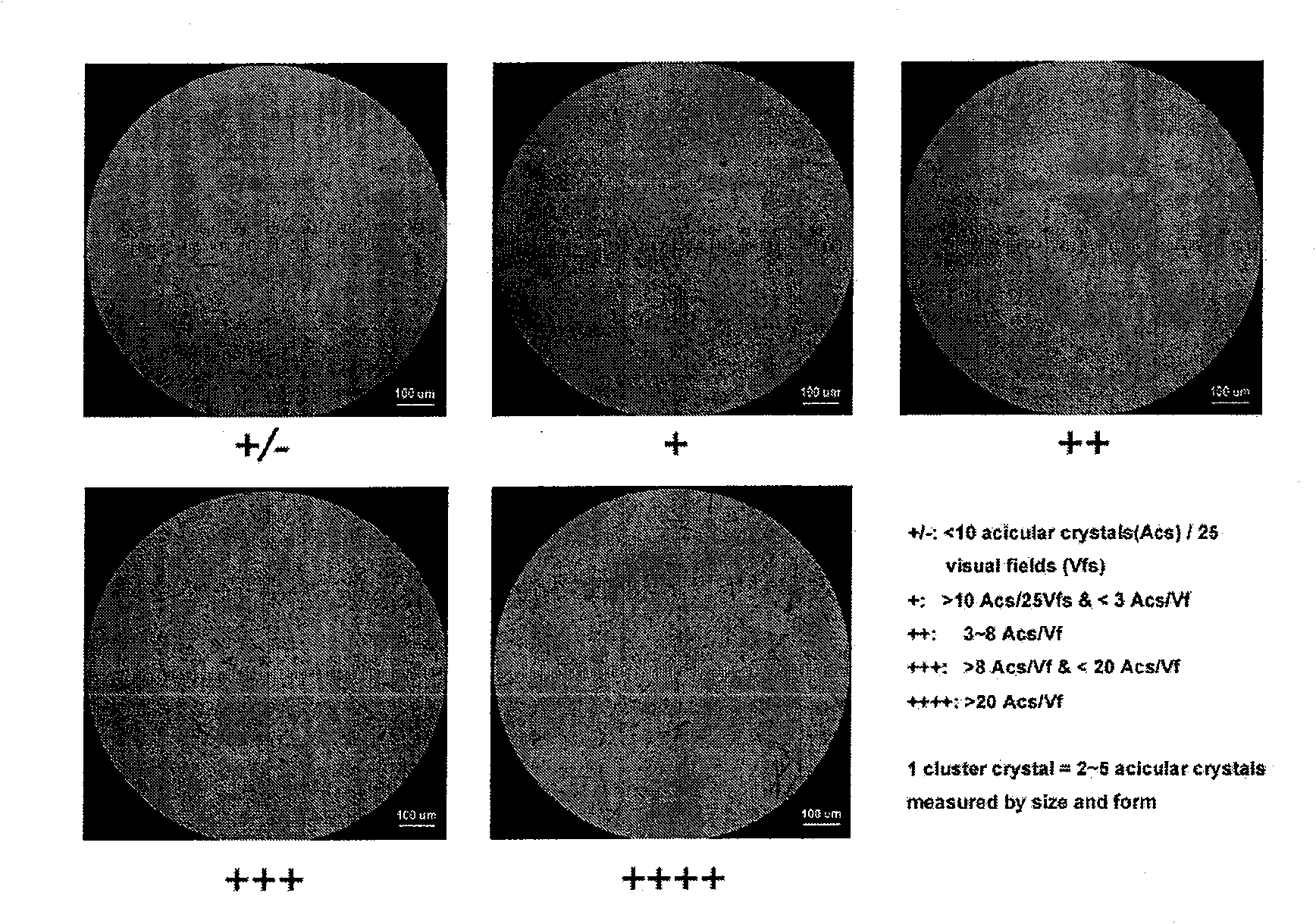
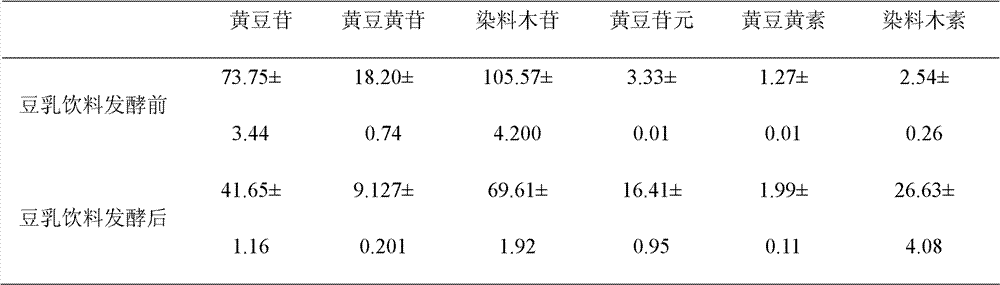
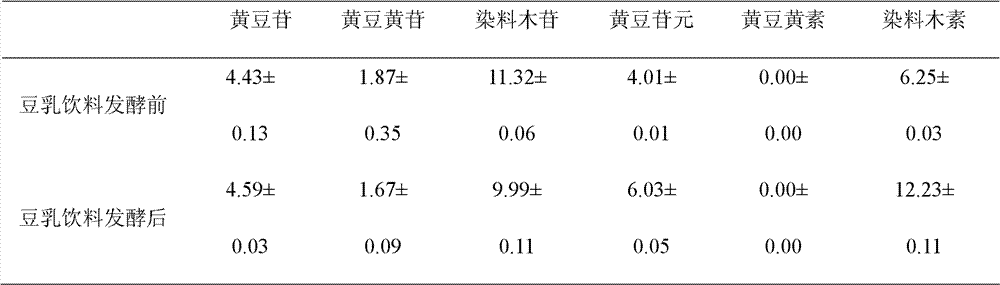
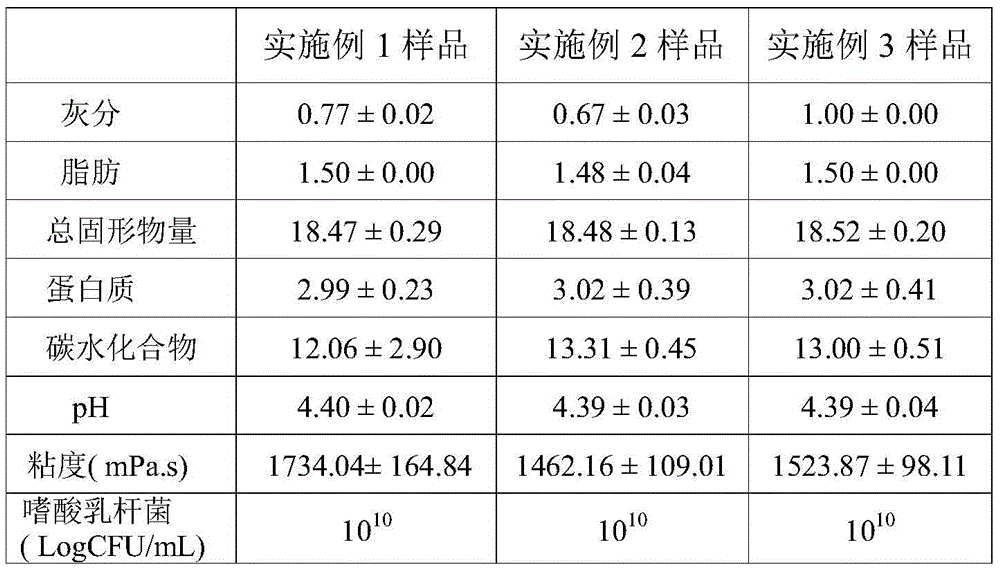
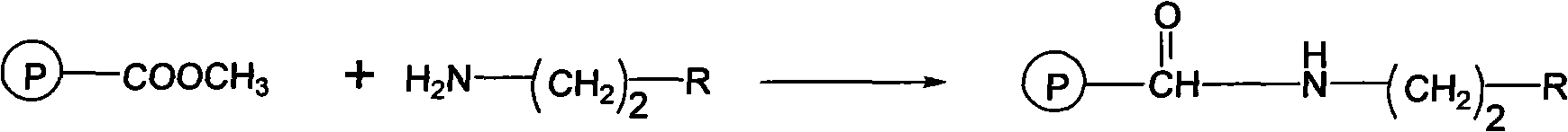Patents
Literature
327results about How to "Reduce allergic reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
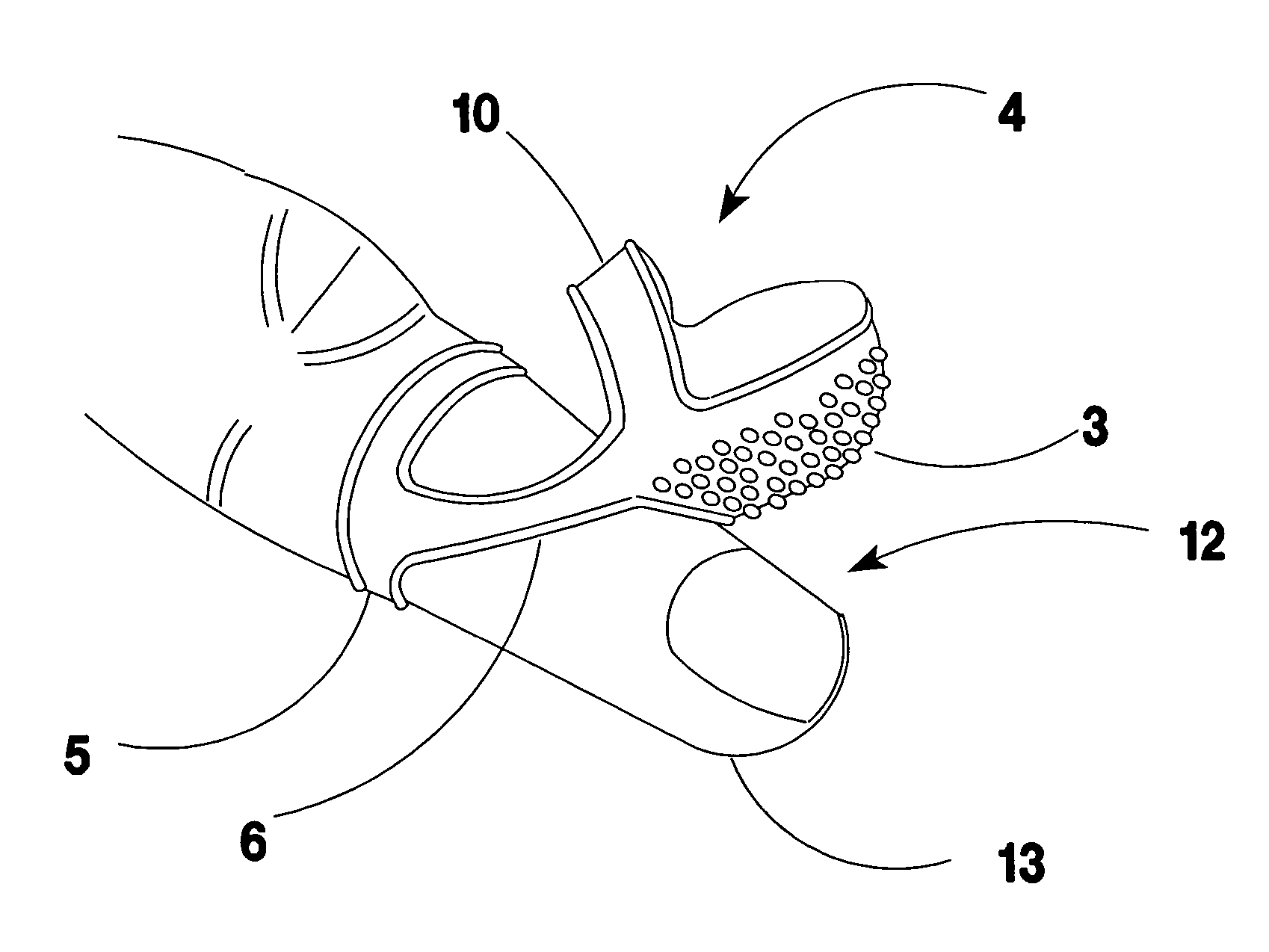
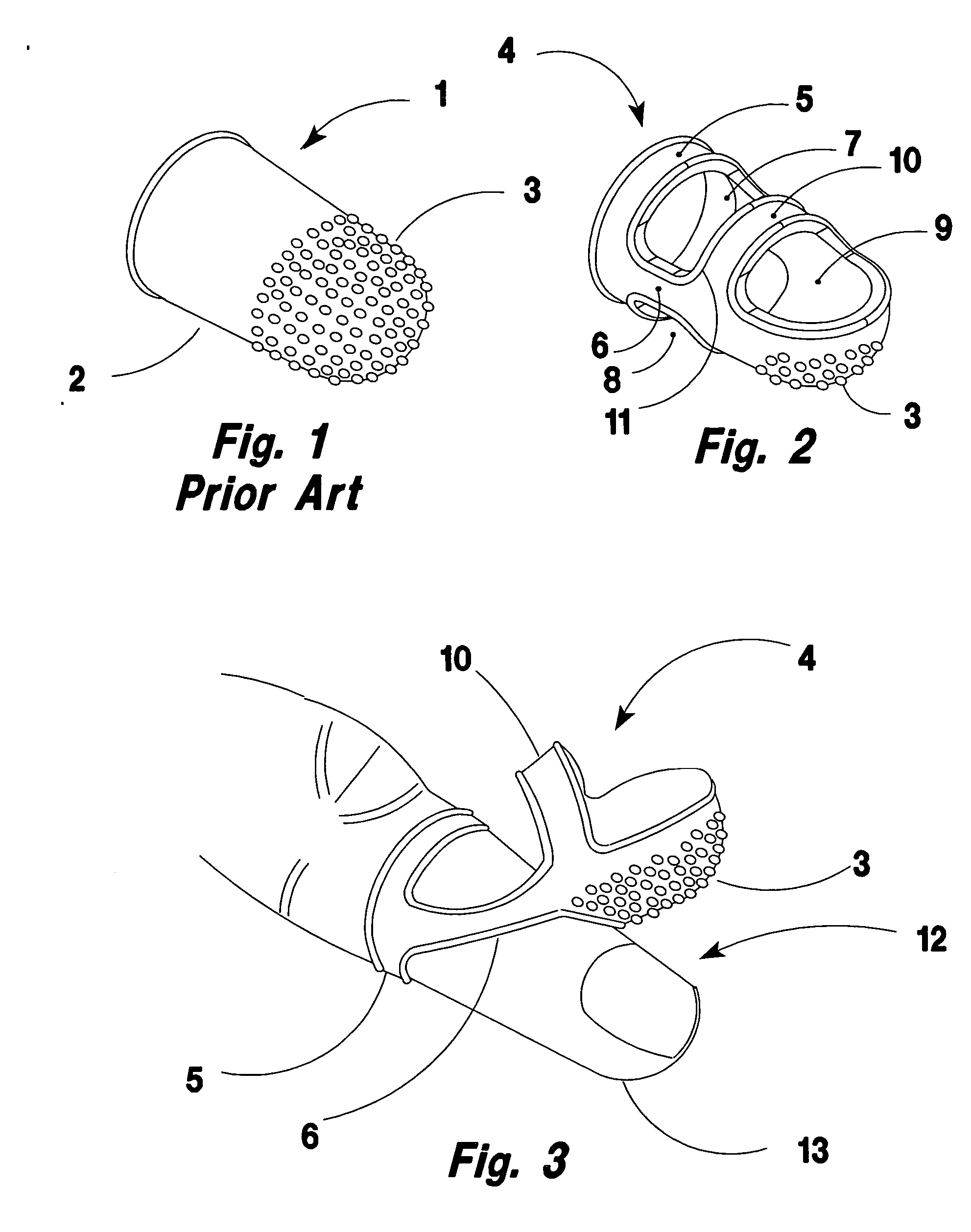
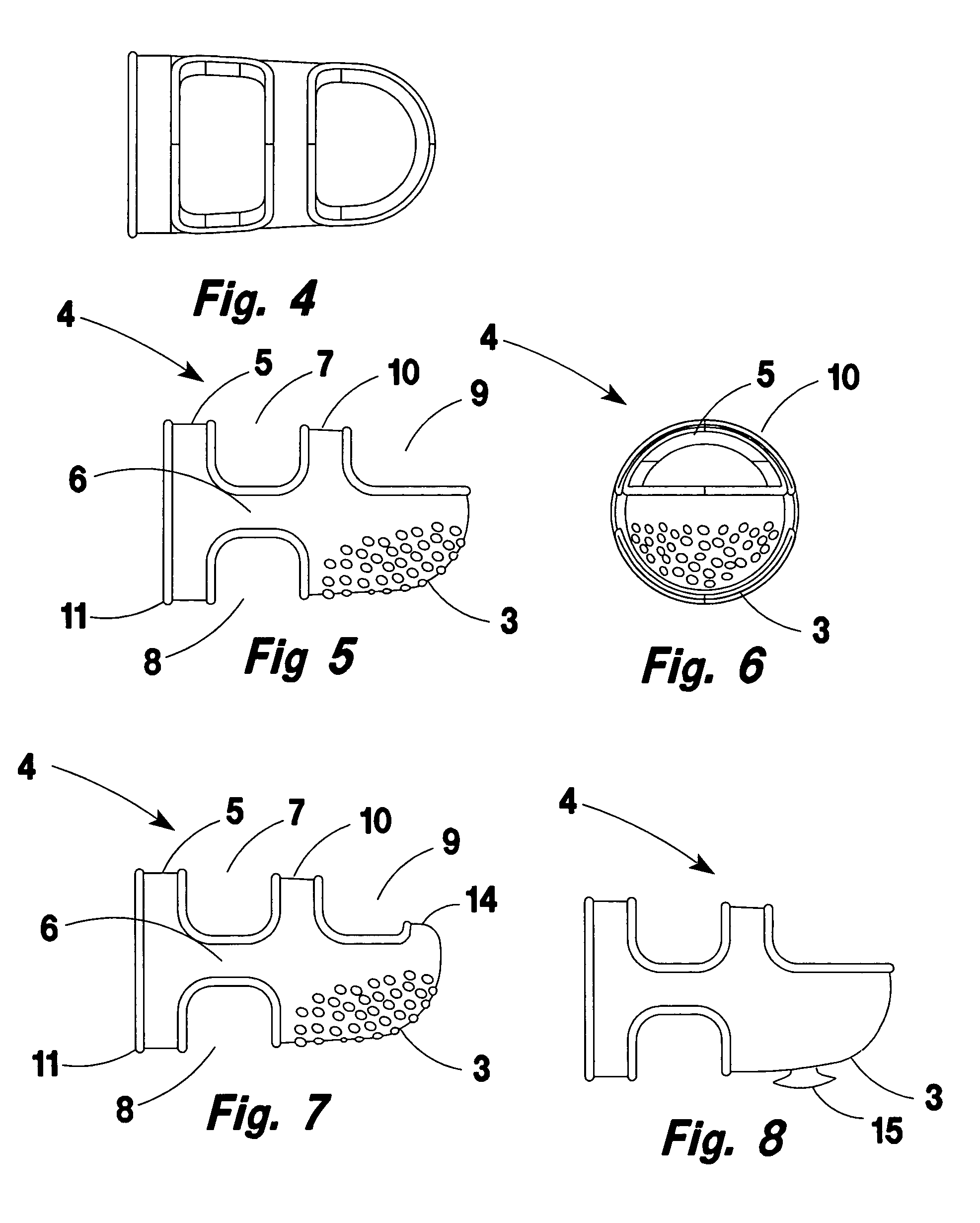
Ventilated and swing away finger cot for handling paper documents
InactiveUS20070118947A1Reduce allergic reactionsSolve the small frictionGarment special featuresBook markersFinger-stallEngineering
A rubber finger cot for handling paper sheets. The cot has an elastic hinge means for swinging the working tip away from the finger tip to a parked position on the top of the finger. Thereby, permitting the finger to be used for tasks requiring tactile feel or the natural shape of the finger tip such as typing and keyboarding while the cot remains installed on the finger.
Owner:LED PROD
Mask solution and mask containing same
InactiveCN107184486AReduce water evaporationGood moisturizing effectCosmetic preparationsToilet preparationsMethacrylateBetaine
The invention discloses a mask solution. The mask solution contains a moisturizer, wherein the moisturizer is prepared from sodium hyaluronate, trehalose, sodium polyglutamate, betaine, tremella polysaccharide and glyceryl polymethacrylate. The mask solution disclosed by the invention is capable of durably and deeply moisturizing the skin, is good in mildness, is also suitable for skin sensitive persons, is natural and non-toxic, is mild and non-irritant, cannot damage the skin after long-term use, has an obvious effect in improving the skin, and is beneficial for enabling the skin to restore elasticity and be glossy.
Owner:曾万祥
Subsensitive lactalbumin hydrolysate and preparation method thereof
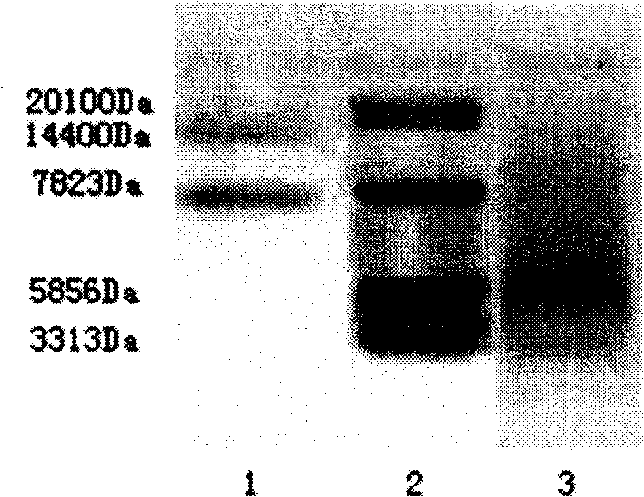
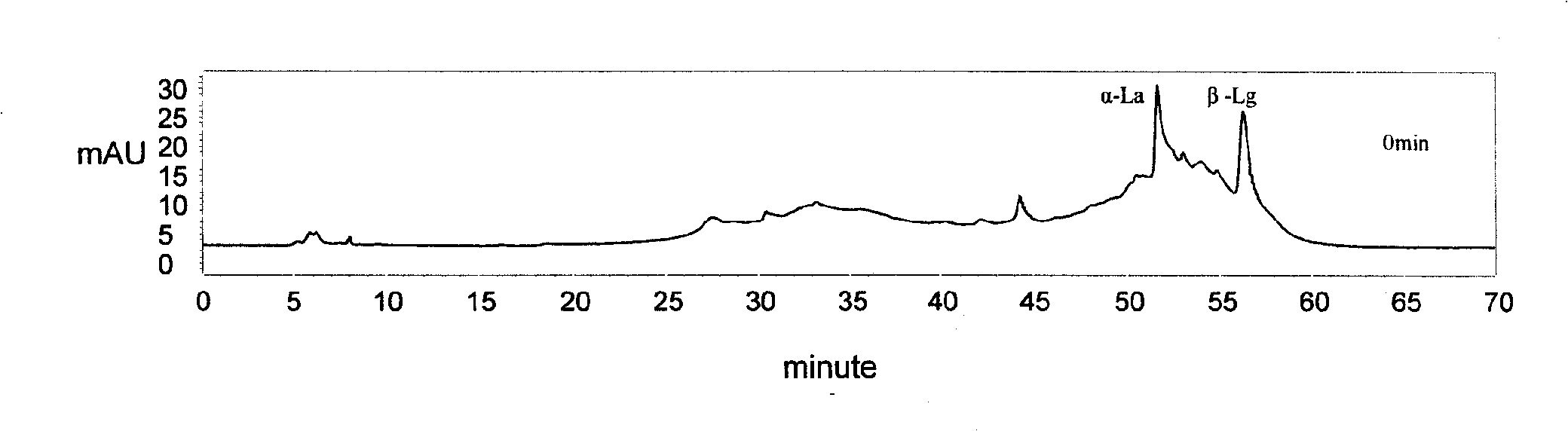
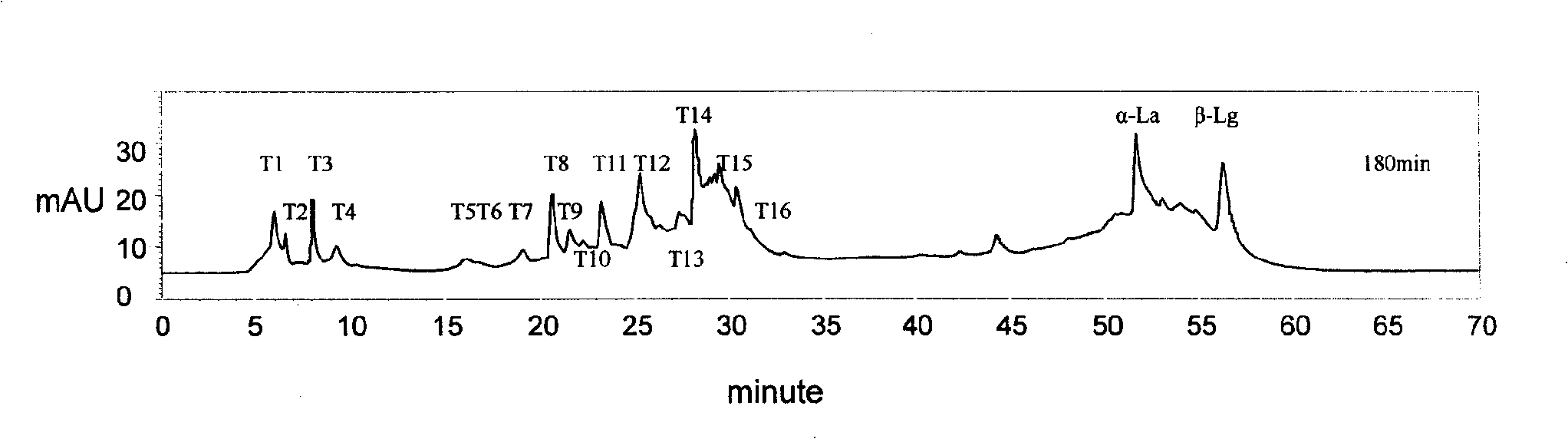
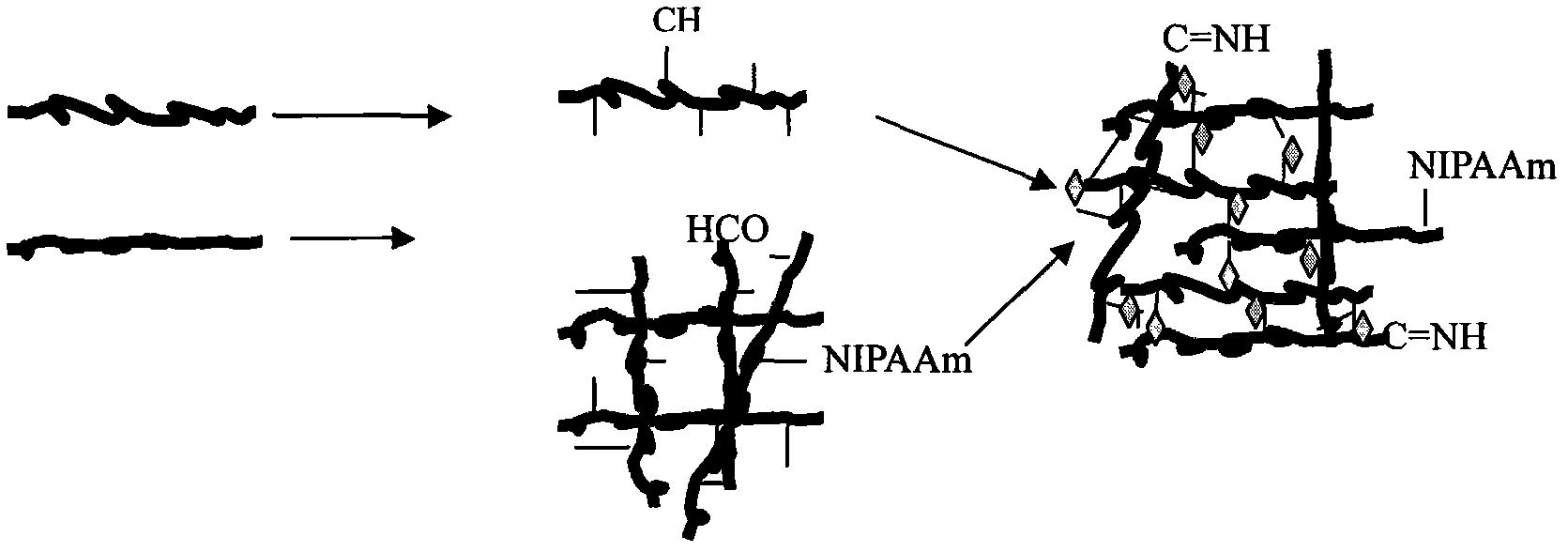
The invention discloses a muting sensitive lactalbumin hydrolysate and a preparation method thereof. The method mainly comprises the following steps: dissolving condensed whey protein in the water, then carrying out denaturation under high temperature, carrying out hydrolysis by using the protamex composed of alkali protease, papain and flavourzyme, treating the lactalbumin hydrating solution with desalinization and drying to obtain the lactalbumin hydrolysate of the invention. The lactalbumin hydrolysate of the invention has high suppression ratio of Beta-lactoglobulin and Alpha- lactalbumin, can reduce or eliminate cow milk allergic reaction; furthermore, the lactalbumin hydrolysate can also produce other active materials, thus also having the functions of promoting immunity, being easy to digest and absorb and reducing blood pressure; and the lactalbumin hydrolysate has high security and no side effect, bitter taste or other bad flavors.
Owner:CHINA AGRI UNIV
Injectable temperature sensitive gel used for filling and repairing damaged tissues
The invention relates to an injectable temperature sensitive gel used for filling and repairing damaged tissues. The gel is characterized in that: medical-grade hyaluronic acid and chitosan are used as gel raw materials; the gel swelling ratio is 2-500; and a temperature sensitive temperature is 32-37 DEG C. The gel provided by the invention has the advantages that the injection temperature sensitive gel has physical and chemical properties of a general gel, also has injectable and temperature sensitive properties and a good fluidity at a room temperature and is easy for injection; the formability in organisms is good and the degradation is slow so that the general gel can not be compared; a cross-linking agent is not used for the gel so that harms of the cross-linking agent to a human body are avoided; a gel system can prolong a persistence time of the gel in a body, and also can continuously stimulate the body to produce reactions so that the tissue in-situ regeneration and repair is promoted and the filling quality is increased; and the gel simultaneously has the advantages of reducing bacterial invansion, anticoagulation and low allergic reaction and the like.
Owner:冯淑芹
Methods for reducing allergies caused by environmental allergens
ActiveUS20100143266A1Reduce and minimize and prevent allergyReducing or preventing allergic responses to an environmental allergenAerosol deliveryImmunoglobulins against animals/humansMast cellEnvironmental health
Compositions suitable for reducing symptoms of an allergic response to environmental allergens comprising molecules that specifically inhibit the ability of the allergen to bind to mast cells in an animal predisposed to having an allergic response to the allergen and methods for reducing such symptoms comprising contacting a source of the environmental allergen with such compositions. Kits, packages, medicaments, and means of communicating about the compositions and methods are also provided.
Owner:SOC DES PROD NESTLE SA
Formula powder for phenylketonuria children and preparation method thereof
ActiveCN104012659AAdequate nutrient absorptionEnsure physiological needsMilk preparationNucleotideHypersensitive response
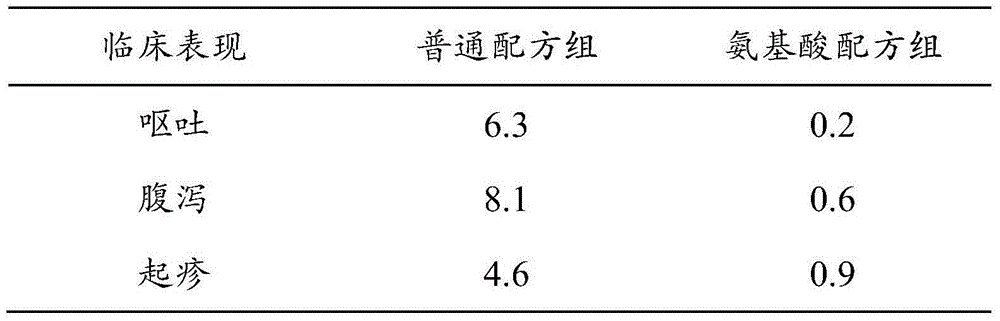
The invention provides formula powder for phenylketonuria children and a preparation method thereof. The formula powder comprises solid glucose syrup, amino acid, fatty acid, nucleotide, fish oil, dietary fiber, composite vitamins, composite mineral substances and an emulsifier, and is prepared by using a wet-method preparation process of materials. According to the formula powder, special free amino acid is adopted, a proper amount of free L-tyrosine is screened and optimized, and phenylalanine is removed, so that the physiological needs of sick children are met, and at the same time hypersensitivity of phenylketonuria infants is effectively prevented; in the formula, the components have the mutual synergistic effect, the powder is approximate to breast milk in absorption, taste and the like, children can absorb sufficient nutrition after taking the powder, and meanwhile anaphylaxis such as diarrhea can be effectively reduced; and the formula powder is simple in preparation method and applicable to requirements of large-scale industrial production.
Owner:中恩(天津)医药科技有限公司
Film coating agent for wound surface treatment and preparation method of film coating agent
ActiveCN104257639AGood antibacterial effectEasy to useOrganic active ingredientsDermatological disorderMedicinePlasticizer
The invention discloses a film coating agent for wound surface treatment and a preparation method of the film coating agent. The film coating agent comprises chitosan, a film-forming material, a plasticizer and a solvent, wherein the plasticizer is glycerin with the amount accounting for 2-15% of the total weight of the film coating agent. The film coating agent has the advantages that the film coating agent is better in antibacterial effect, more convenient to use and better in waterproofness, is not limited by shapes and sizes of skin wound surfaces, does not affect normal limb movement and the like.
Owner:KUNMING YUANRUI PHARMA
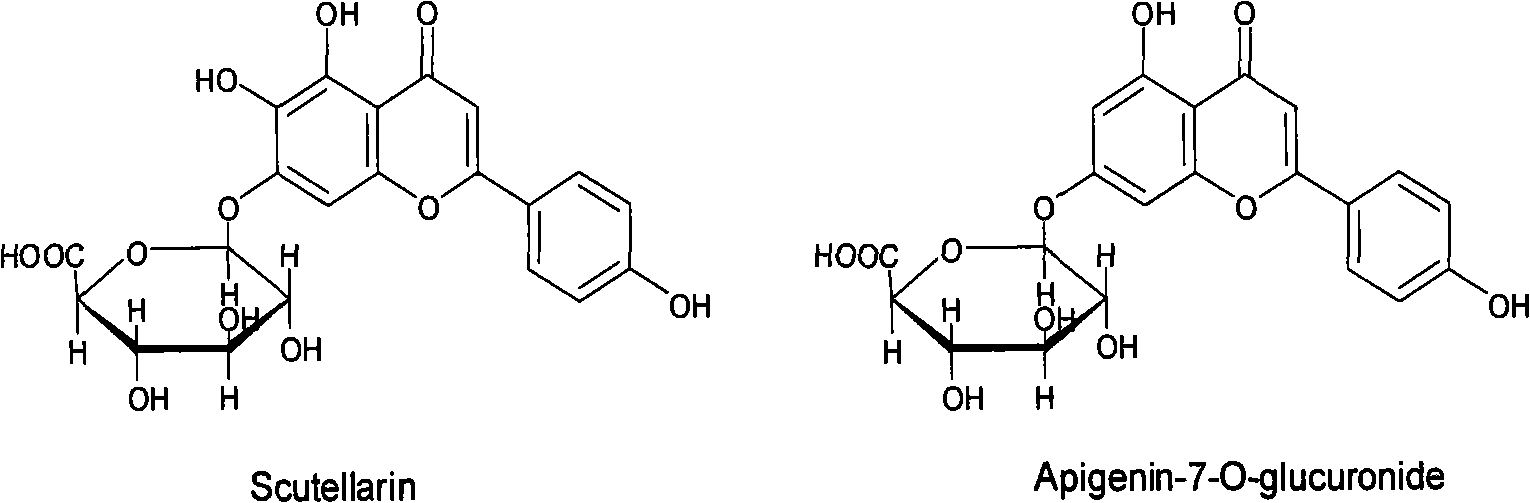
Adsorption resin method separation technology of scutellarin in fleabane flower extract
InactiveCN101580527ATo achieve the purpose of removing other impuritiesHigh purityIon-exchange process apparatusSugar derivativesPurification methodsSeparation technology
The invention discloses an adsorption resin method separation technology of scutellarin in a fleabane flower extract. Based on scutellarin and fleabane flower essence A which have very similar structures, the difference of intermolecular hydrogen bonding capacities is formed, an amide functional group capable of forming hydrogen bonds is introduced on a macroporous adsorption resin skeleton, highly selective adsorption is carried out on the scutellarin by the synergic action of dewatering and the hydrogen bonds, a commercially available extract is used as a raw material, and a specimen with the content of the scutellarin more than 98% and the content of the fleabane flower essence A less than 0.5% is prepared by a one-step continuous technology of adsorption and desorption. The invention avoids using a poisonous organic solvent with low boiling point and does not need the assistance of other purification methods, so that the invention has simple operation and is environment-friendly, resin can be recycled simultaneously, the production cost is greatly reduced, and the invention is suitable for large-scale industrial production. The obtained specimen can meet the requirements for further enhancing the quality standard of a breviscapine preparation, reducing clinical side reactions and the like, and the invention has favorable application prospect.
Owner:NANKAI UNIV
Protecting and fixing device for wound
ActiveCN102429769AAvoid tearingAvoid crackingEnemata/irrigatorsSurgical needlesInfusion catheterRelative displacement
The invention discloses a protecting and fixing device for wound, comprising at least one protecting and fixing layer with openings, wherein the protecting and fixing layer with openings is longitudinally provided with an opening for communicating the upper end face and the lower end face; the opening can enclose the wound therein so as to make the protecting and fixing layer with openings limit the wound tension and increase the mobile corrugation of the wounded skin. The invention can protect and fix the operation wound, the trauma wound or the catheter infusion wound into an opening which can limit the tension increase of the wound and the mobile corrugation of the surface skin of the wound and can prevent the wound from being torn and cracked and also avoid the damage for the wound due to the mobile corrugation and the surgical dressing of the wounded skin as well as the relative displacement frictions of the infusion catheter. Therefore, the invention fills the blank of current technology, which can enable the covered dressings to penetratively contact the secretion so as to absorb the exudate of the wound. Besides, the invention can prevent the foreign material contact anaphylaxis due to the wound contacting by compressing the dressing and the direct bonding of the catheter infusion wound and can prevent the re-injury of the wound caused by the synechia between the dressing and the wound secretion.
Owner:唐二虎 +2
Low-serum culture medium for efficiently culturing mycoplasma hyopneumoniae and preparation method thereof
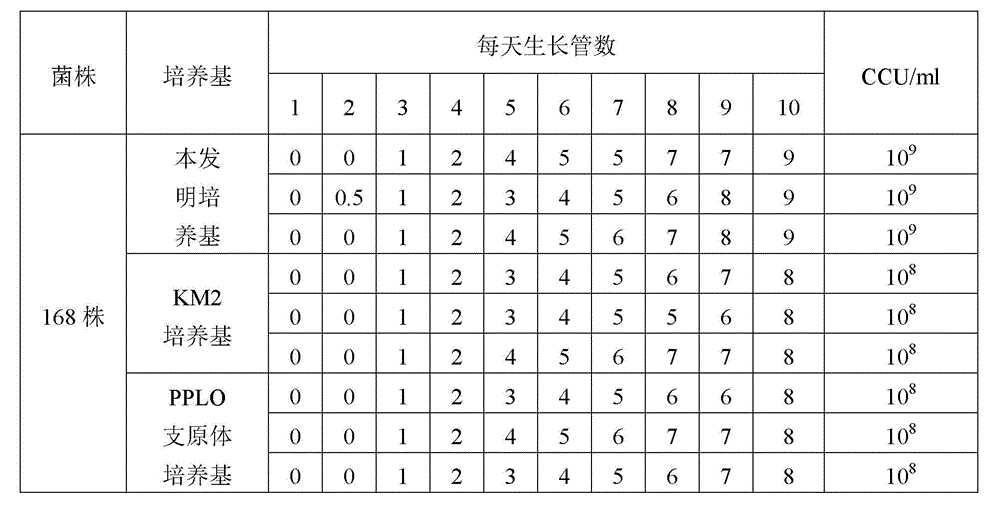
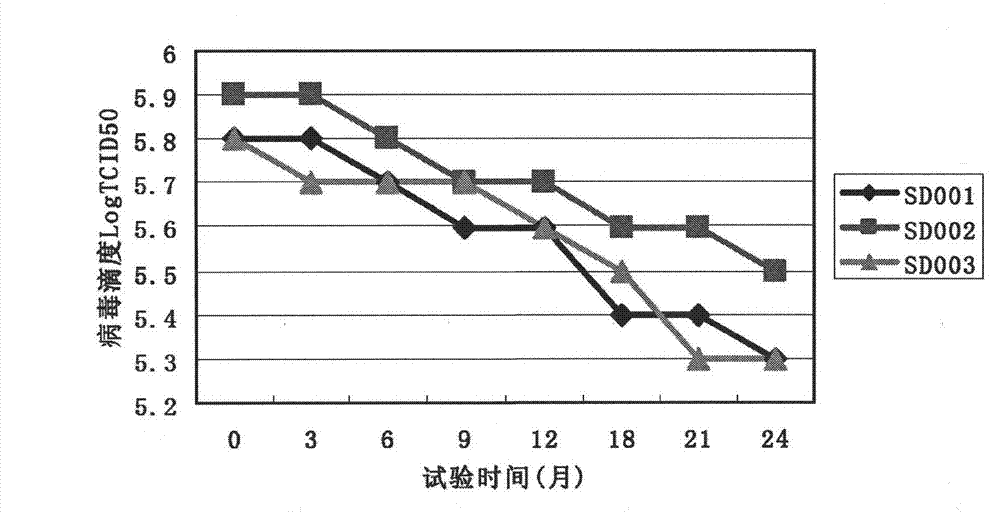
ActiveCN103060220AIncrease the titer of live bacteriaReduce allergic reactionsAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureOrganism
The invention relates to an efficient mycoplasma hyopneumoniae culture medium and a preparation method thereof, and belongs to the technical field of veterinary biology. The efficient mycoplasma hyopneumoniae culture medium comprises an A liquid and a B liquid mainly consisting of MEM, yeast leaching powder, tryptone, glucose, inorganic salt and the like. The prepared culture medium of the invention has the main advantages of low serum content which is only 10%-15%, while the serum content in common culture medium is 20% even more. The culture medium prepared by the low serum relieves the pig allergic to the stress reaction, meanwhile gives consideration to the biosafety of animals. Besides the valence of the semi-finished bacterial solution prepared by the method is up to 109CCU / ml, which is much higher than the culture medium prepared by the common technology.
Owner:兆丰华生物科技(南京)有限公司 +1
Melittin complex nanometer granule for oral dosing and preparation method thereof
InactiveCN101406691AReduce contentReduce allergic reactionsPowder deliveryPeptide/protein ingredientsSolubilityMicroparticle
The invention belongs to the technical field of medicines and discloses melittin complex nanoparticles for oral administration and a preparation method thereof. Water-soluble melittin and an amphiphilic substance are dissolved into a hydrosolvent A to form a hydrophobic protein ion pairing complex; the complex and a suitable polymer material are dissolved into a nonaqueous solvent B; an emulsion solvent diffusion method in a liquid phase is adopted to wrap the complex into the polymer material; and microparticles with the particle diameter between 10 and 1000nm are formed after the solvent is volatilized. The hydrosolvent A is distilled water, double distilled water, deionized water, physiological saline, or a phosphate buffer solution or an acetate buffer solution or a Tris buffer solution with the pH value of between 1 and 11; and the nonaqueous solvent B is a single or mixed solvent of alcohol, acetone, ethyl acetate, methylene dichloride, chloroform and dimethyl sulfoxide. The complex has high fat solubility, and the encapsulation rate of the nanoparticles is more than 90 percent. The preparation process is mild and can assure the biological activity of medicines. The preparation method is suitable to prepare oral administration preparations.
Owner:SHENYANG PHARMA UNIVERSITY
Natural green tea health edible salt
The invention relates to salt, in particular to a natural green-tea health-care edible salt. The health caring and seasoning health-care salt is made by adding green tea, vegetable juice, trace element, vitamin, Chinese herbal medicine products and other beneficial components based on edible iodized salt.
Owner:黄涛
Omeprazole sodium freeze-dried powder injection and preparation method thereof
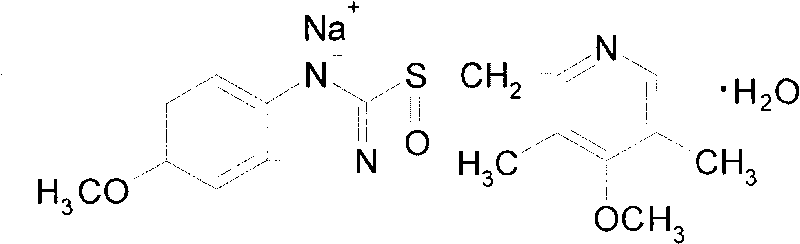
ActiveCN101703483AReduce the risk of adverse reactionsComply with the requirements of human intravenous injectionPowder deliveryOrganic active ingredientsOmeprazole SodiumFiltration
The invention discloses an omeprazole sodium freeze-dried powder injection and a preparation method thereof. The omeprazole sodium freeze-dried powder injection contains an active ingredient, namely, omeprazole sodium monohydrate, and auxiliary materials, namely, calcium disodium edetate and sodium hydroxide. The preparation method of the omeprazole sodium freeze-dried powder injection is characterized by comprising the following steps: weighing the calcium disodium edetate of prescription amount and dissolving the calcium disodium edetate in water for injection, stirring, dissolving, and regulating pH value to 10.0-12.0 by using 10% of sodium hydroxide solution; weighing omeprazole sodium of the prescription amount and adding the omeprazole sodium in the mixture, stirring at room temperature for dissolution, supplementing and adding the water for injection to full amount; adding active carbon, stirring at room temperature for decoloration and endotoxin removal, conducting rough filtration to remove carbon firstly, and then conducting refining filtration by using a filter membrane of 0.22 Mum; taking refining filtrate to test intermediate, conducting encapsulation after meeting requirements; and freeze-drying and unboxing, thus obtaining the omeprazole sodium freeze-dried powder injection. The freeze-drying technology of the omeprazole sodium freeze-dried powder injection takes temperature below minus 40 DEG C as pre-freezing temperature; after pre-freezing for at least two hours, sublimation is started, wherein the sublimation temperature is 5-12 DEG C, the sublimation time is over 14 hours; and then drying is conducted for over 2 hours at the temperature of 20-35 DEG C. Unboxing is carried out after a stopper is added and a cover is put in place, thus obtaining the finished product of the omeprazole sodium freeze-dried powder injection.
Owner:HAINAN LEVTEC PHARMA
Method for producing pseudorabies living vaccines by using subculture cell source and product thereof
ActiveCN101695573AImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesPig kidneyAntibiotic Y
The invention provides a method for producing pseudorabies living vaccines by using a subculture cell source and a pseudorabies living vaccine product thereof. The method comprises the following steps: culturing pseudorabies virus low-virulent strains by using subculture cells; harvesting the strains to obtain cell culture venom; and then adding a stabilizing agent and an antibiotic into the cellculture venom, and freezing and vacuum-drying the mixture to obtain the pseudorabies living vaccines of the subculture cell source. The subculture cells are subculture cells ST of pig testicle or subculture cells PK15 or IBRS-2 of pig kidney. The method for producing the pseudorabies living vaccines by using the subculture cell source has the advantages of simple and stable production process, easy operation, high virus content, little batch difference and controllable quality, can remarkably improve the yield and quality of the vaccines and reduce the anaphylactic reaction and the like. The pseudorabies living vaccines obtained by using the production method of the invention have good safety and high immune efficacy, and have better immune protection effect on pseudorabies virulent attack.
Owner:广东永顺生物制药股份有限公司
Self-emulsifying preparation of taxane compound and preparation method thereof
ActiveCN101829052ASimple production processEase of industrial productionOrganic active ingredientsEmulsion deliveryPhospholipidSurface-active agents
The invention provides a self-emulsifying medical composition for delivering taxane medicaments and a preparation method thereof. The self-emulsifying medical composition is transparent liquid, comprises an effective dose of taxane compound, an oil component, a surface active agent and alcohol and preferentially comprises lactic acid, wherein the surface active agent consists of phospholipid and polyoxyethylenated castor oil. After the self-emulsifying composition is added to a glucose injection, the composition can self-emulsify into micro emulsion the mean grain size of which is 50-300nm, and the micro emulsion can stabilize for more than 12 hours at room temperature. The preferential taxane compound in the invention is taxol.
Owner:YINGU PHARMA
House dust mite allergen, Der p VII, and uses thereof
InactiveUS6077517AModify lymphokine secretion profileDiminution in allergic symptomFungiBacteriaNucleic acid sequencingBULK ACTIVE INGREDIENT
A novel protein allergen Der p VII of Dennatophagoides pteronyssinus is described. A cDNA clone encoding Der p VII was isolated from a lambda gt11 library of D. pteronyssinus cDNA. The nucleic acid sequence of der p VII encodes a 198 residue mature processed protein having a predicted molecular weight of 22,177 daltons. Der p VII protein may be used as the active ingredient in therapeutic composition for the treatment of sensitivity to house dust mites. The protein may also be used in methods of diagnosing such sensitivity.
Owner:MERCK PATENT GMBH
Method for producing human antibodies with properties of agonist, antagonist, or inverse agonist
ActiveUS20050277173A1The process is simple and effectiveSimple methodAnimal cellsSugar derivativesSynthetic ImmunogensHuman lymphocyte
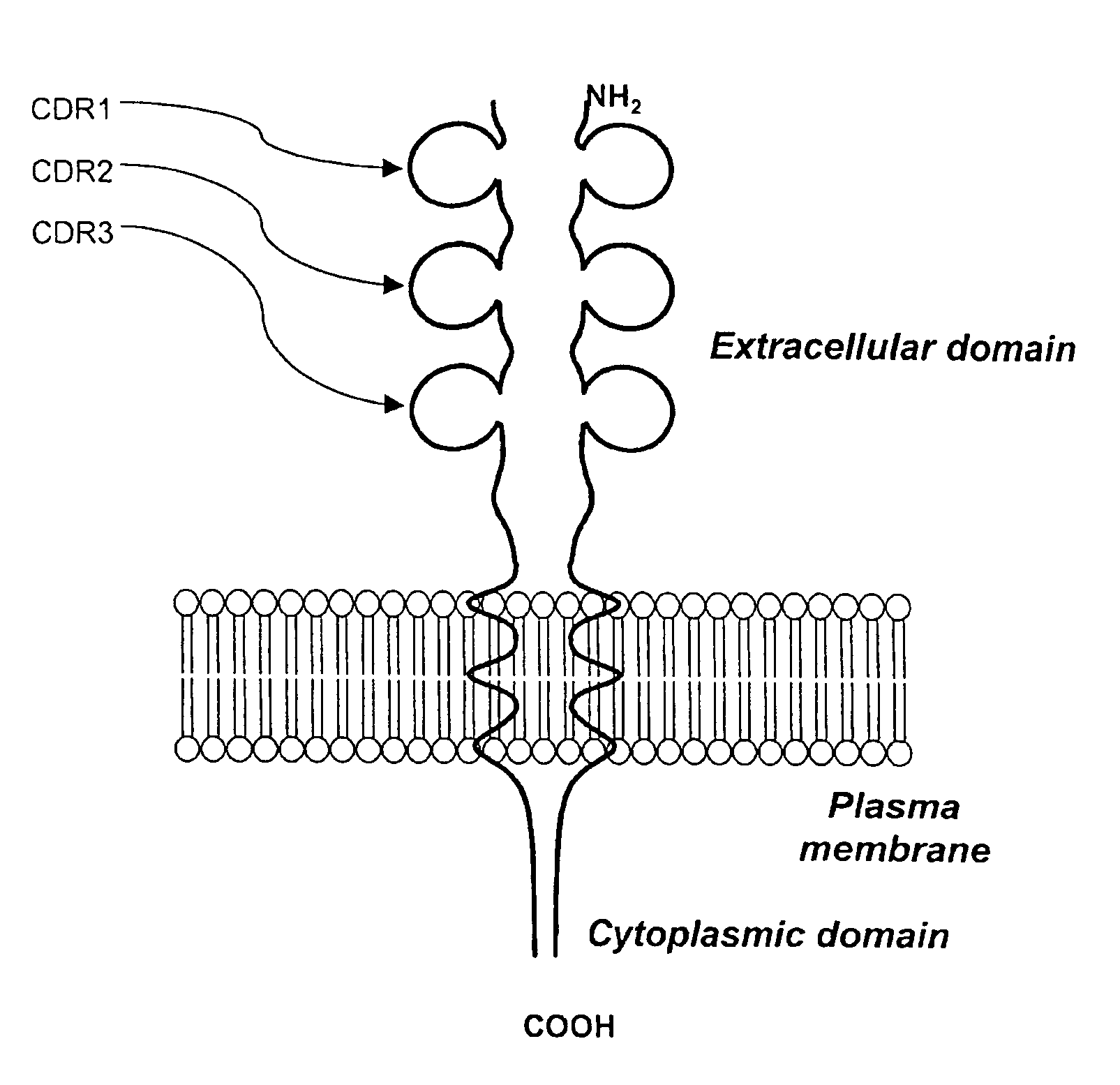
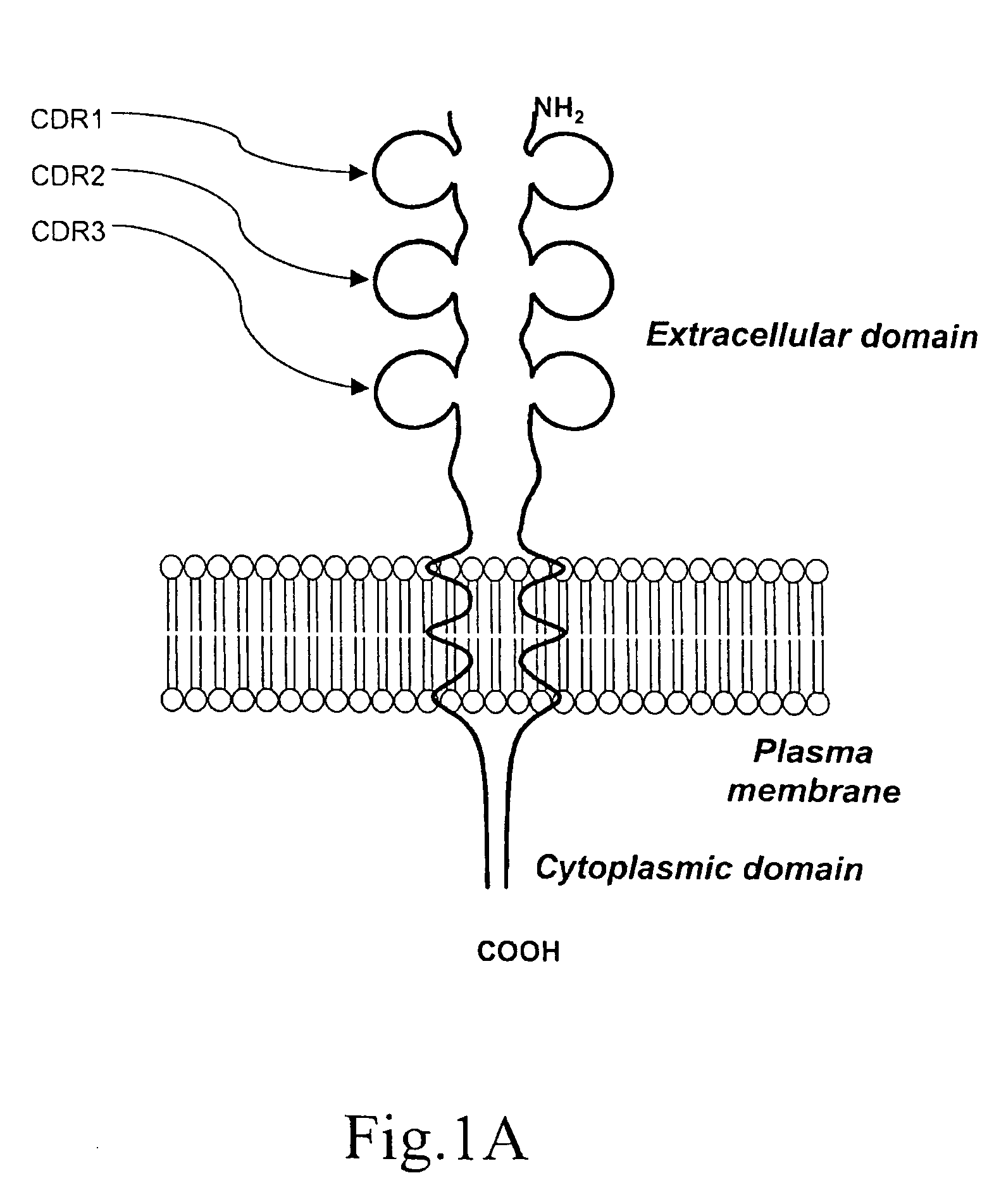
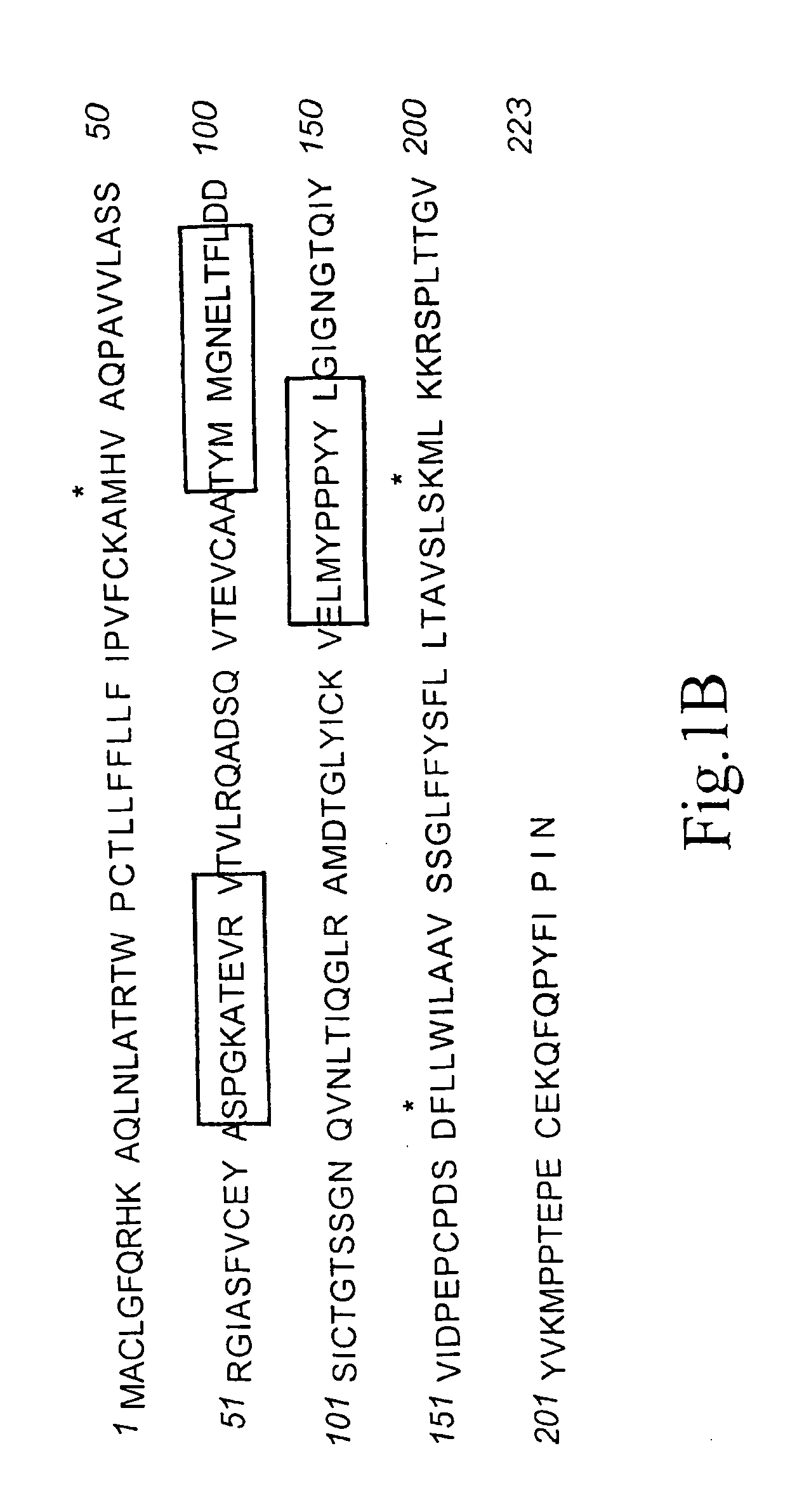
A method for obtaining agonist, antagonist and inverse agonist, to a given physiological receptor is disclosed. For the method, use is made of in silico design synthetic immunogens, which are caused to act in vitro on human lymphocyte-containing cell populations. A preferred receptor is human CD152, particularly the regions of CDRl, CDR2 and CDR3 that elicit antibodies serving as antagonist, inverse agonist and agonist, respectively. Also provided is a method in the treatment of human peripheral lymphocytes for use in the screening of CD152 ligands that yield pharmacological effects.
Owner:HUMORIGIN BIOTECH CORP
Shuang Huanglian liquid preparation and method for measuring content thereof
ActiveCN101474260AReduce allergic reactionsImprove detection efficiencyAntibacterial agentsComponent separationChemistryMeasurement cost
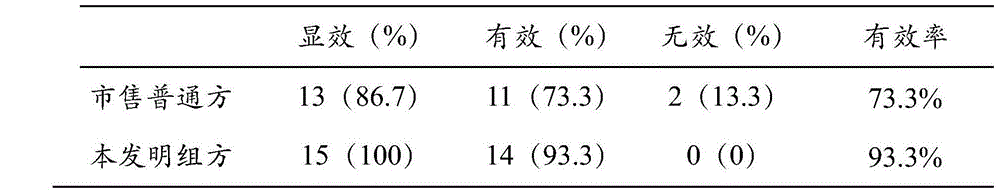
The invention mainly relates to a Shuanghuanglian liquid preparation and a method for measuring the content thereof. The Shuanghuanglian liquid preparation mainly comprises scutelloside, forsythin, chlorogenic acid, and the like, and the adverse reaction thereof is mainly shown in anaphylactic shock, erubescent skin and exanthemata, etc. The invention provides the Shuanghuanglian liquid preparation containing proper chlorogenic acid, scutelloside, forsythin and other effective components, and not only has ideal therapeutic effect, but also reduces the occurrence of the anaphylactic reaction. The invention also provides the method for measuring the content of the Shuanghuanglian liquid preparation; during the application of the measurement method of the invention, only one moving phase is needed to simultaneously measure the content of the five effective components, i.e. the scutelloside, the forsythin, the chlorogenic acid, luteoloside and wogonin. The method not only obviously improves the measurement efficiency, but also effectively reduces the measurement cost.
Owner:HEILONGJIANG ZBD PHARMA +1
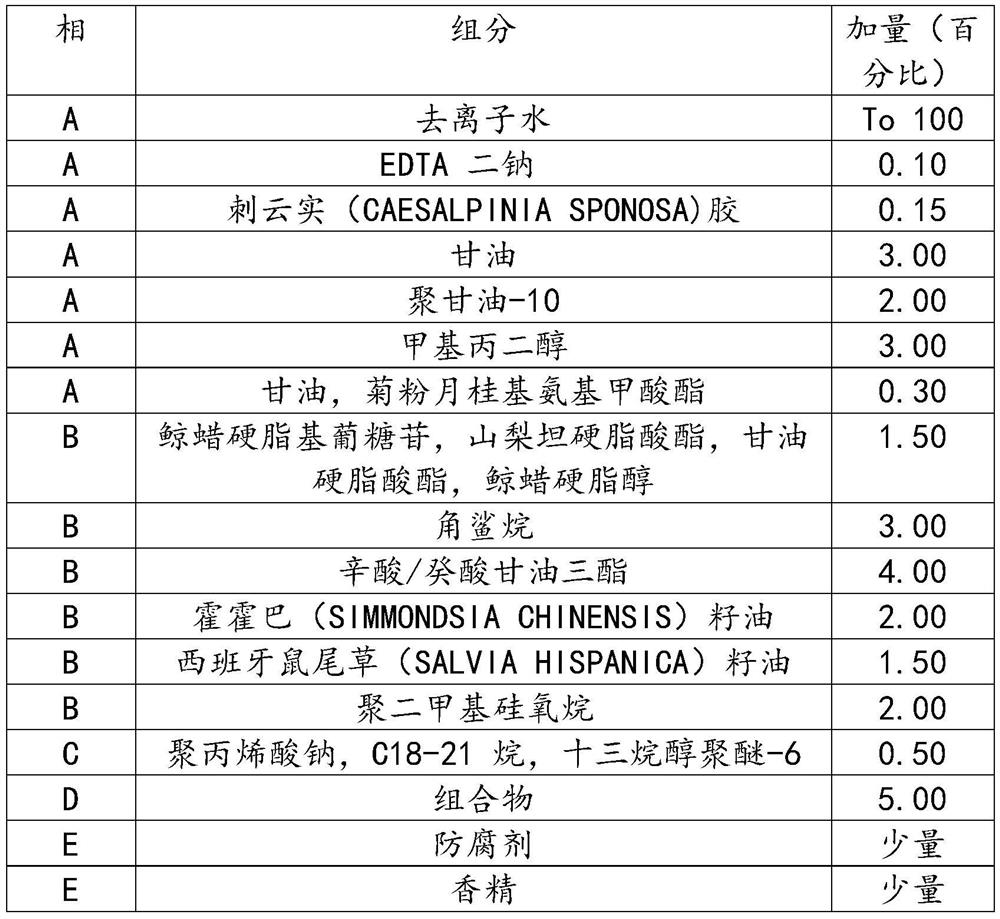
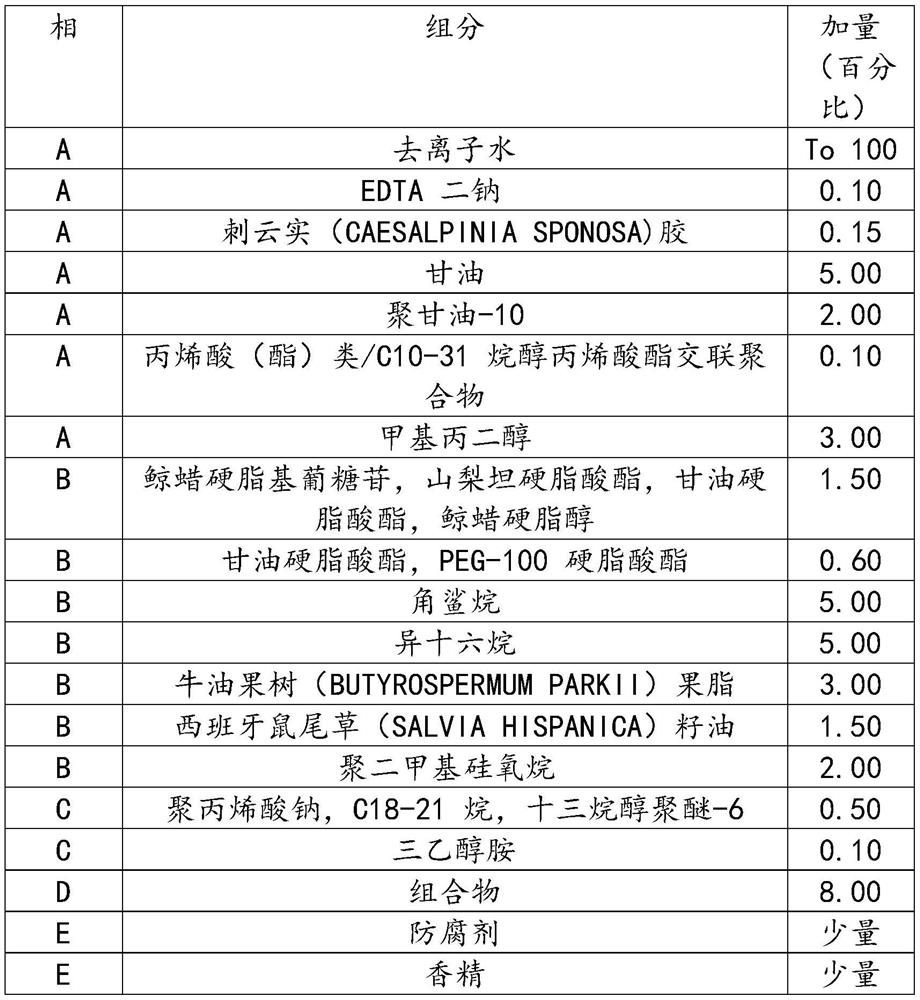
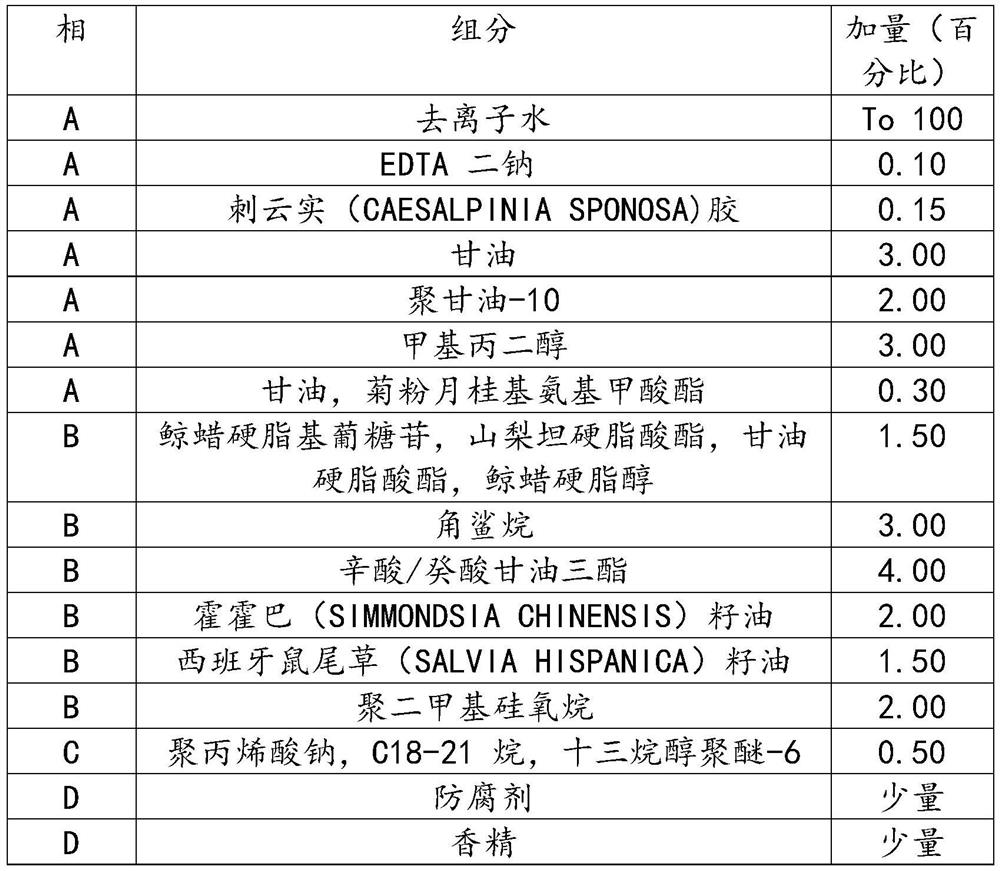
Recombinant human-source collagen mask and preparation method thereof
ActiveCN105213295AIncrease elasticityStay youngCosmetic preparationsConnective tissue peptidesWrinkle skinBetaine
The invention discloses a recombinant human-source collagen mask and a preparation method thereof. The preparation method of the recombinant human-source collagen mask comprises the following steps: under the condition that the recombinant human-source collagen mask is composed of a carrier and a mask substrate solution, after an acrylic (acrylate) / C10-30 alkanol acrylate crosslinked polymer, a tremella extract, a hamamelis extract, ceramide, xanthan gum, a hyaluronic acid, deionized water, glycerin and butanediol are stirred and then completely dispersed, fully swelling the obtained object for 12h, adding methylparaben, betaine, micromolecular recombinant human-source collagen peptide and EDTA disodium into a system, stirring the obtained mixture until the mixture is completely dispersed, heating the obtained object to 50 DEG C, and carrying out heat preservation for 30 minutes under vacuum conditions. The recombinant human-source collagen mask effectively resists aging, removes wrinkles and increases the skin elasticity, and keeps the youthful skin.
Owner:JIANGSU JLAND BIOTECH CO LTD
Muscular amino acid and peptide nucleoside powder injection and its preparation method
InactiveCN1522757AImprove stabilityHigh preparation process yieldPowder deliveryPeptide/protein ingredientsMedicineFreeze-drying
The present invention relates to a sarcopeptidoglucoside powder injection and its preparation method. It is formed from polypeptide, hydroxanthine and excipient. Said invention adopts the modern biological extraction technique to prepare sarcopeptidoglucoside solution, and utilizes the modern low-temp. freeze-drying preparation technique to obtain the invented sterile freeze-dried powder injection. When it is used, it can be dissolved by injection water or infusion fluid, then can be injected. Said invention has good stability, long storage time and high safety, etc.
Owner:黑龙江江世药业有限公司
Probiotics active soybean milk beverage and preparation method thereof
InactiveCN104255930AIncrease the number ofImprove immunityMilk substitutesFood scienceCrystalline fructoseSoya bean
The invention provides a probiotics active soybean milk beverage and a preparation method thereof. The active soybean milk beverage is prepared from the following raw materials in percentage by weight: 1.2%-1.6% of degreased soybean protein powder, 5%-7% of crystal fructose, 5%-10% of soybean polysaccharide and the balance being water; the materials are prepared into a soybean milk base material; and the inoculating amount of a lactobacillus casei fermenting agent and a bifidobacterium fermenting agent is 0.0015%-0.002% of the amount of a finished product, wherein the ratio of the lactobacillus casei fermenting agent to the bifidobacterium fermenting agent ranges from 1:2 to 2:1. An experiment shows that in the active soybean milk beverage prepared by the preparation method, the viable counts of lactobacillus casei and bifidobacteria can reach up to 1.33*10<9>cfu / ml and are more than 6 times as much as the viable counts of commercially available like products. The product can be refrigerated for 42 days at 6-10 DEG C without unacceptable layering and precipitation phenomena; and the whole product has sour and sweet taste, is fresh, cool and delicious and has a unique flavor.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
Soybean milk beverage prepared through using Lactobacillus casei Zhang and Bifidobacterium animalis subsp.lactis V9, and its preparation method
InactiveCN102813004AHigh survival contentPrevent constipationMilk substitutesFood scienceSucroseSaccharum
The invention discloses a soybean milk beverage prepared through using Lactobacillus casei Zhang and Bifidobacterium animalis subsp.lactis V9, and its preparation method. A technical scheme adopted in the invention is that the soybean milk beverage is characterized in that the beverage is composed of 300kg of soybean milk or soybean powder reduction milk, 10-15kg of glucose, 100-120kg of sucrose, 2-4kg of a stabilizing agent, 0.1-0.3kg of an acid solution, 0.1-0.3kg of an edible perfume, 0.015-0.03kg of a Lactobacillus casei Zhang fermentation agent, and 0.015-0.03kg of a Bifidobacterium animalis subsp.lactis V9 fermentation agent, wherein strains of the above fermentation agents are preserved in China General Microbiological Culture Collection Center, and the preservation numbers of the strains are CGMCC No.5469 and CGMCC No.5470 respectively. The invention discloses the preparation method of the beverage.
Owner:北京和美科盛生物技术有限公司
Methods for reducing allergies caused by environmental allergens
ActiveUS8454953B2Reduce and minimize and prevent allergyReducing or preventing allergic responses to an environmental allergenAerosol deliveryImmunoglobulins against animals/humansMast cellEnvironmental health
Compositions suitable for reducing symptoms of an allergic response to environmental allergens comprising molecules that specifically inhibit the ability of the allergen to bind to mast cells in an animal predisposed to having an allergic response to the allergen and methods for reducing such symptoms comprising contacting a source of the environmental allergen with such compositions. Kits, packages, medicaments, and means of communicating about the compositions and methods are also provided.
Owner:SOC DES PROD NESTLE SA
Goat milk kefir produced with polymerized lactalbumin as thickener
InactiveCN105519670ASolving Stable Gel ProblemsImprove stabilityMilk preparationAdditive ingredientLactalbumin
The invention relates to a goat milk kefir produced with polymerized lactalbumin as a thickener, and belongs to the technical field of functional foods. The goat milk kefir is produced through coordinating fresh goat milk as liquid raw milk with sugar, polymerized lactalbumin, low methoxyl pectin and a kefir starter. The goat milk kefir product is produced through the following steps: mixing sugar with low methoxyl pectin in advance, adding the obtained mixture to the liquid raw milk; adding a polymerized lactalbumin solution; stirring and heating the above solution to 80-90DEG C, carrying out heat insulation for 10-20min for sterilization, and carrying out ice bath cooling to room temperature; and adding the kefir starter, stirring above materials until uniformity, and fermenting the obtained mixture at 25-35DEG C for 8-14h. The product has the advantages of high content of proteins, low content of fats, stable curd state, uniform structure, moderate viscosity, unique flavor and various health functions; and a production technology of the goat milk kefir has the advantages of few raw material components, easy operation, energy saving and low cost, and is a simple production processing mode.
Owner:JILIN UNIV
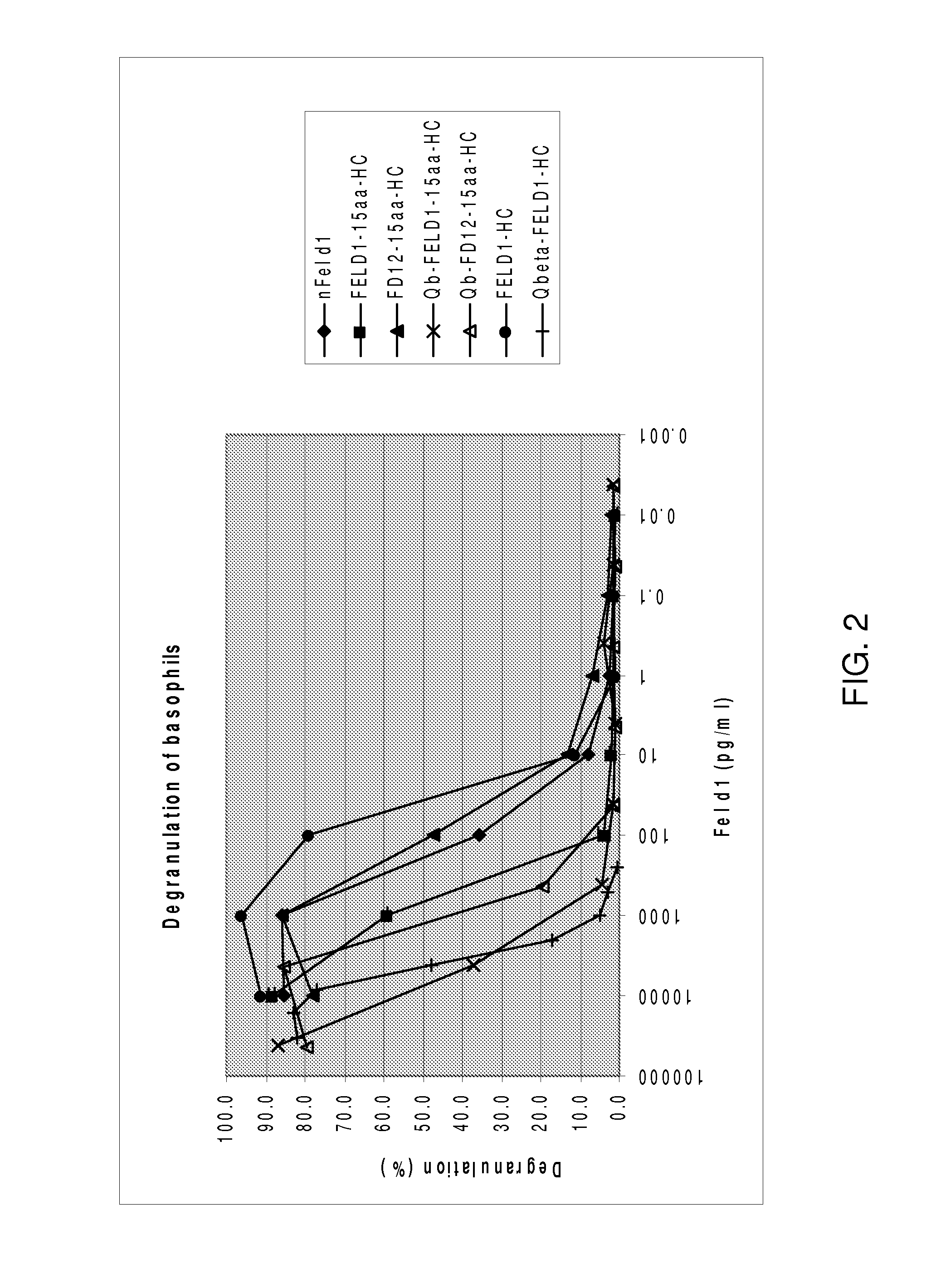
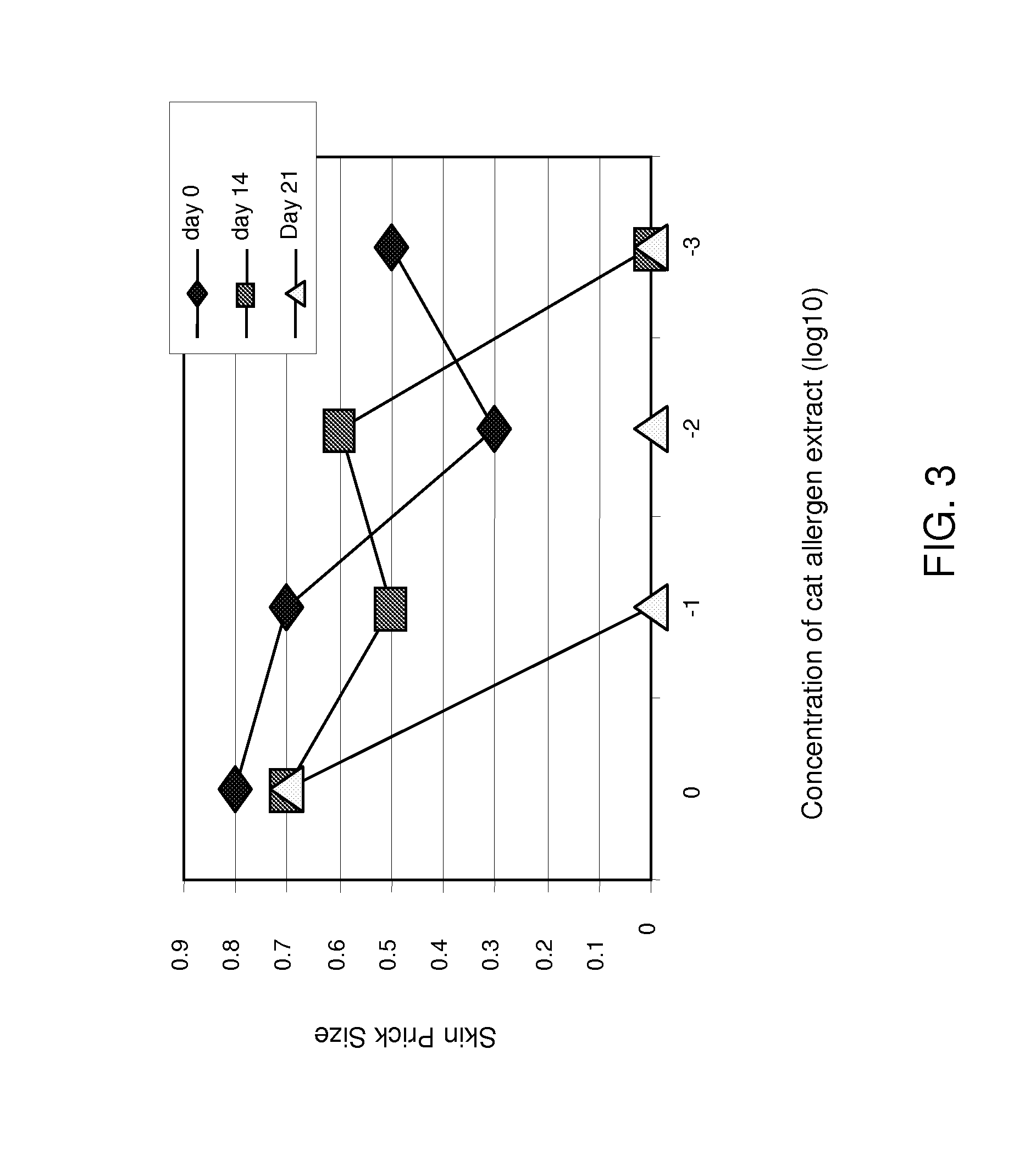
CAT allergen conjugates and uses thereof
InactiveUS7767212B2Improve efficacyReduce riskAntibody mimetics/scaffoldsVirus peptidesMammalAllergy to cats
The present invention is in the fields of medicine, public health, immunology, molecular biology and virology. The invention provides compositions comprising a virus-like particle (VLP) or a virus particle and at least one antigen, particularly at least one feline antigen, and more particularly at least one feline antigen that is a human allergen. In certain embodiments, the antigen is a Fel d1 antigen or a fragment thereof, covalently linked to the VLP. The invention also provides methods for producing the compositions. The compositions of the invention induce efficient immune responses, in particular antibody responses, in mammals, particularly humans. The compositions and methods of the invention are useful in the production of vaccines, in particular for the treatment and / or prevention of allergies to cat dander and other cat antigens and allergens.
Owner:KUROS BIOSCIENCES AG
Combined live vaccine against porcine reproductive and respiratory syndrome and pseudorabies, and preparation method thereof
ActiveCN102727884AImprove immune efficiencyIncrease productionViral antigen ingredientsGenetic material ingredientsPseudorabiesImmunosuppression
The invention provides a combined live vaccine for preventing porcine reproductive and respiratory syndrome and pseudorabies, and a preparation method and application thereof. According to the invention, no immunosuppression occurs between two vaccines of the combined live vaccine; compared with each single vaccine, the combined live vaccine has no obvious difference in security, immunogenicity, immunity duration and immuno-protective effects and has remarkable immuno-protective effects on preventing porcine reproductive and respiratory syndrome and pseudorabies.
Owner:华威特(江苏)生物制药有限公司
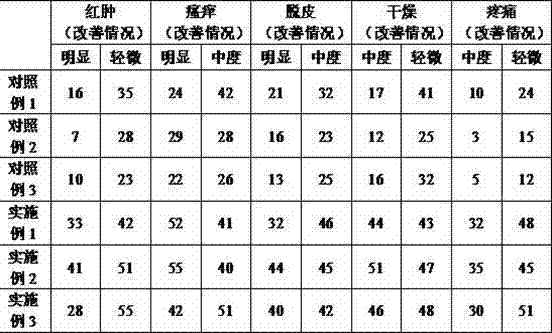
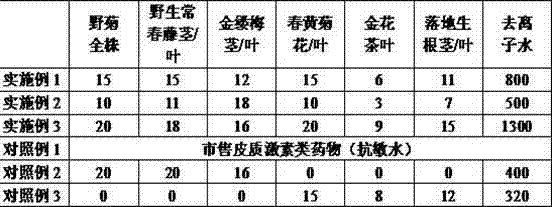
Skin allergy relieving liquid and preparation method and application for skin allergy relieving liquid
ActiveCN104721098ATo promote metabolismImprove microenvironmentCosmetic preparationsToilet preparationsFiltrationUltrafiltration
The invention discloses skin allergy relieving liquid and a preparation method and application for the skin allergy relieving liquid, and belongs to the field of cosmetics. The skin allergy relieving liquid is made from six types of plant materials including total chrysanthemum indicum plants, wild hedera helix stems / leaves, witch hazel stems / leaves, chamomile flowers / leaves, golden camellia leaves and kalanchoe pinnata stems / leaves. The preparation method includes steps of cleaning, grinding, extraction by the aid of hot water, adsorption by the aid of kaolin, still standing, pressure filtration, ultra filtration and the like so as to obtain refined complex extract. The skin allergy relieving liquid, the preparation method and the application have the advantages that the skin allergy relieving liquid is a pure-plant preparation and is unique in formulation, synergistic effects can be realized by beneficial factors in the six types of plant materials, accordingly, immune functions of human bodies can be effectively regulated, the microvascular circulation of the skins of the human bodies can be improved, inflammation diminishing and itching relieving effects can be realized, the metabolism of the skins of the human bodies can be promoted, allergic sources can be inhibited and eliminated, and allergic reaction can be relieved; the skin allergy relieving liquid is simple in production process, high in operability and convenient to use, not only can be used as spray, but also can be used as emulsion to be directly applied to the skins of the human bodies.
Owner:广东颜芝堡生物科技有限公司
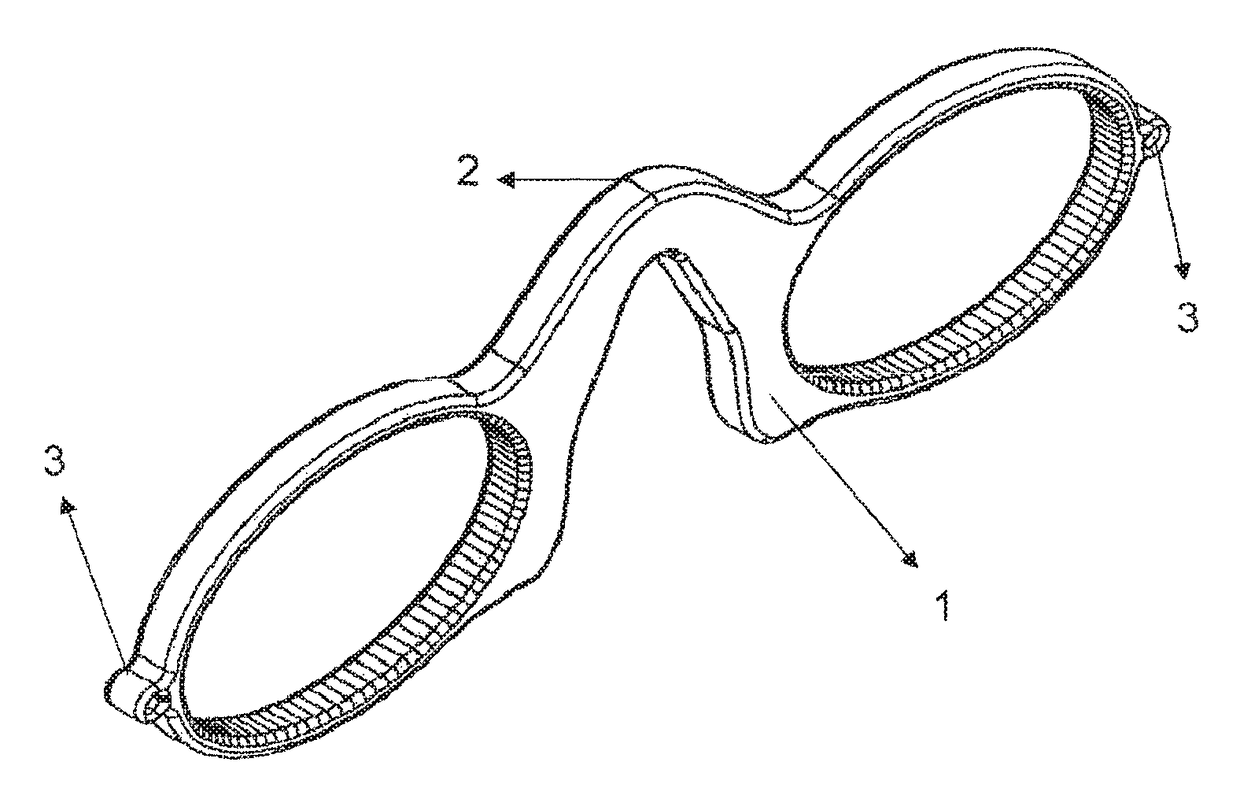
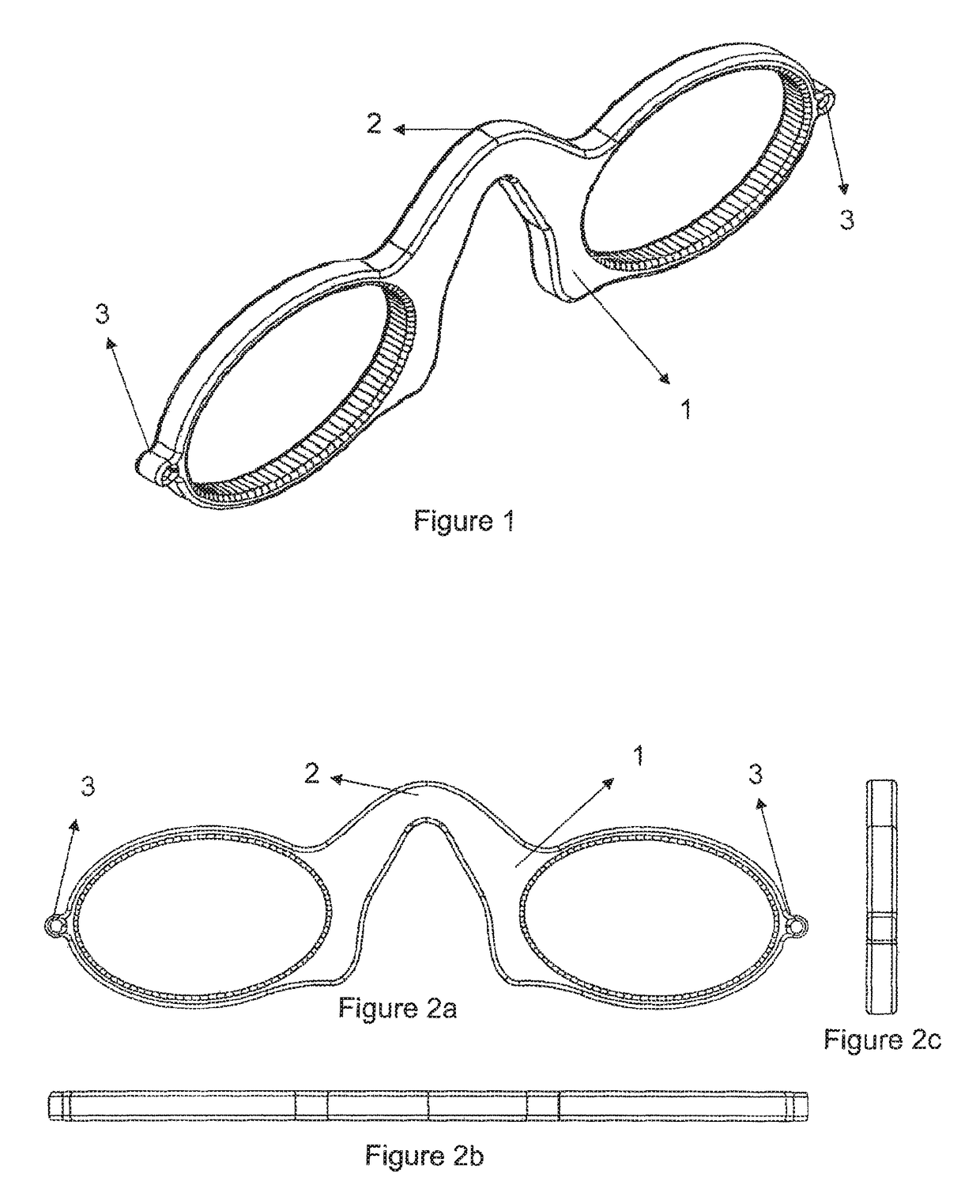
Flexible, silicone eyeglass frame without legs
The silicone eyeglass frame (1) according to the invention consists of a highly flexible and mobile siloxane structure. It is capable of being folded without being broken and of being directed to particular surfaces. Thanks to the material providing the frame to stand on the nose, the need for legs extending to the ears in the eyeglass frame (1) is eliminated.
Owner:VEDERE ENDUSTRI URUNLERI VE DIS TIC LTD STI
Methods for reducing allergies caused by environmental allergens
ActiveUS20130236475A1Reduce and minimize and prevent allergyReducing and preventing allergic responseAntibody mimetics/scaffoldsImmunoglobulins against animals/humansMast cellEnvironmental health
Compositions suitable for reducing symptoms of an allergic response to environmental allergens comprising molecules that specifically inhibit the ability of the allergen to bind to mast cells in an animal predisposed to having an allergic response to the allergen and methods for reducing such symptoms comprising contacting a source of the environmental allergen with such compositions. Kits, packages, medicaments, and means of communicating about the compositions and methods are also provided.
Owner:SOC DES PROD NESTLE SA
Composition with anti-allergy, anti-inflammatory and soothing effects as well as preparation method and application thereof
InactiveCN112957308AQuality improvementGood varietyCosmetic preparationsAntipyreticLactobacillusGlycoside
The invention discloses a composition with anti-allergy, anti-inflammatory and soothing effects as well as a preparation method and application thereof, and the composition comprises the following components in percentage by mass: 5-30% of lactobacillus / soybean milk fermentation product filtrate, 5-25% of kava extract, 0.5-5% of dipotassium glycyrrhizinate, 5-25% of oat kernel extract, 1-5% of glycosylglycerol and the balance of solvent. The composition provided by the invention achieves the effects of anti allergy, anti-inflammatory and soothing from the aspects of skin micro-ecological barrier recovery, skin physical barrier recovery, nerve calming and soothing, inflammation inhibition and the like.
Owner:广东嘉梦化妆品有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com