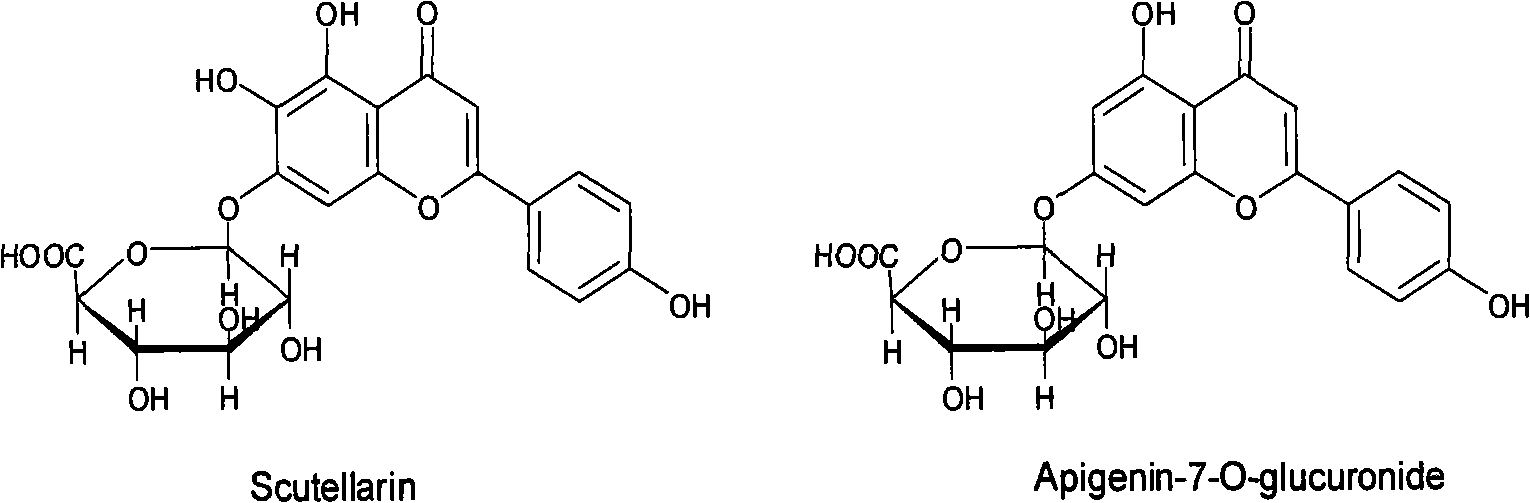
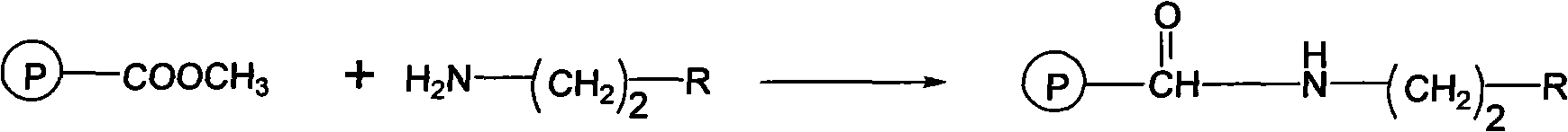Adsorption resin method separation technology of scutellarin in fleabane flower extract
A technology of scutellarin and adsorption resin, which is applied in the synthesis field of highly selective adsorption resin, can solve the problems of single structure, poor adsorption selectivity, and poor selectivity of adsorption separation methods, etc., achieves a simple separation and purification process, and improves adsorption selectivity , Improve the effect of storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of amide resin is realized through the following steps:
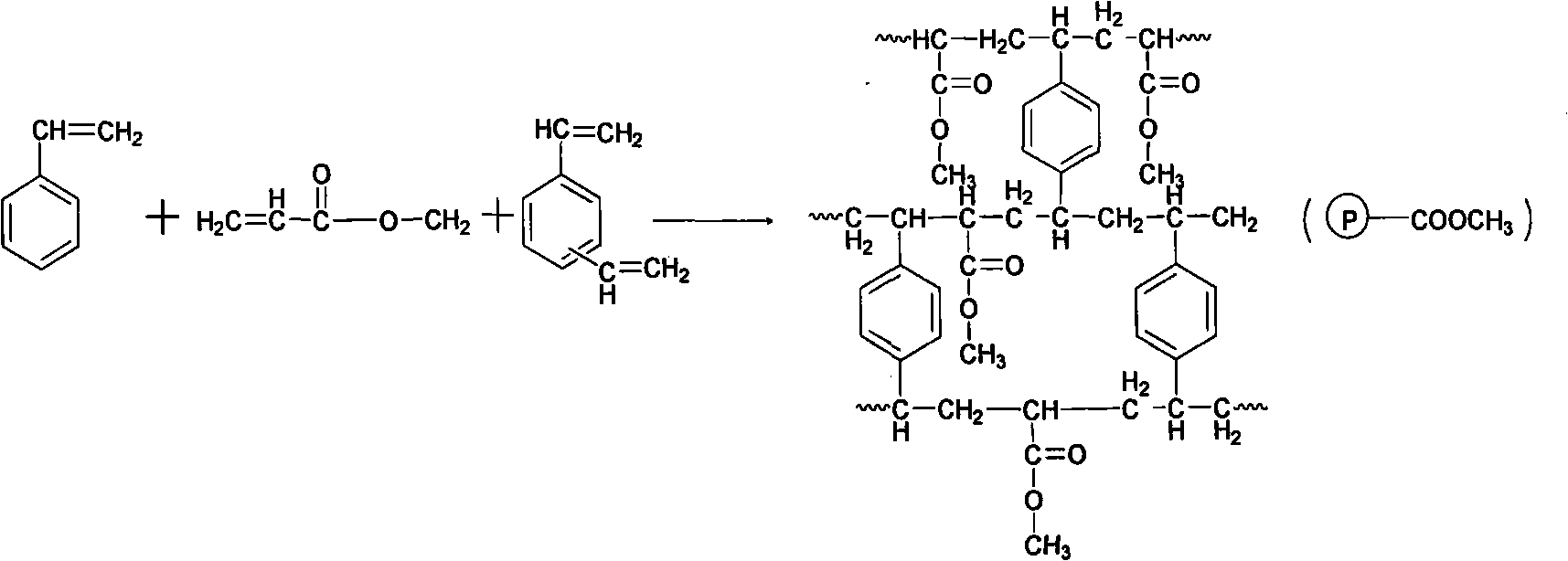
[0030]Step 1: Dissolve 4.5g of polyvinyl alcohol (PVA) and 22.5g of sodium chloride (NaCl) in water in a 1L three-necked bottle to make 500mL of aqueous solution, and heat it to 45°C. In addition, 24g of methyl acrylate, 12g of styrene, 24g of divinylbenzene, 60g of toluene, 30g of n-heptane, and 1.2g of azobisisobutyronitrile (AIBN) were evenly mixed, then added to a three-necked flask, started to stir, and at the same time, the reaction The temperature of the system was raised to 65°C for 5 hours, and the temperature was further raised to 80°C for 4 hours. Stop the reaction, filter, wash, and dry to obtain the initial adsorption resin.
[0031] The second step: add N,N-dimethylformamide (DMF) 150mL to fully swell the obtained 54.8g resin, add 20g of NH 2 (CH 2 ) 2 NH 2 , after mixing evenly, raise the temperature to 95°C, fully react for 10 hours, stop the reaction, filter and wash t...
Embodiment 2
[0033] Step 1: Dissolve 23g of polyvinyl alcohol (PVA) and 32g of sodium chloride (NaCl) in water in a 2L three-necked bottle to make 900mL of aqueous solution, and heat it to 35°C. In addition, mix 60g of methyl acrylate, 18g of styrene, 42g of divinylbenzene, 90g of toluene, 50g of liquid wax, and 1.2g of benzoyl peroxide (BPO) evenly, then add them into a three-necked bottle, start stirring, and raise the temperature of the reaction system at the same time. React at 78°C for 6 hours, then continue to raise the temperature to 90°C for 4 hours. Stop the reaction, filter and wash to obtain the initial adsorption resin.
[0034] Step 2: After adding 400mL of diethylbenzene to fully swell the obtained 108.7g resin, add 40g of NH 2 (CH 2 ) 2 NHCOCH 3 , after mixing evenly, heat up to 110°C, fully react for 8 hours, stop the reaction, filter and wash to obtain a macroporous adsorption resin with amide functional groups, which is amide resin.
Embodiment 3
[0036] Step 1: Dissolve 100g of polyvinyl alcohol (PVA) and 100g of sodium chloride (NaCl) in water in a 5L three-necked bottle to make 2000mL of aqueous solution, and heat it to 40°C. In addition, 75g methyl acrylate, 62.5g styrene, 112.5g divinylbenzene, 150g toluene, 150g of 200 # After gasoline and 7.5g of azobisisobutyronitrile (AIBN) were mixed evenly, they were added into a three-necked flask, and stirring was started. At the same time, the temperature of the reaction system was raised to 65°C for 4 hours, and then the temperature was raised to 80°C for 5 hours. Stop the reaction, filter and wash to obtain the initial adsorption resin.
[0037] Step 2: After adding 700mL of acetophenone to fully swell the obtained 225.9g resin, add 90g of NH 2 (CH 2 ) 2 NHCOCH 2 CH 3 , after mixing evenly, heat up to 120°C, fully react for 12 hours, stop the reaction, filter and wash to obtain a macroporous adsorption resin with amide functional groups, which is amide resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com