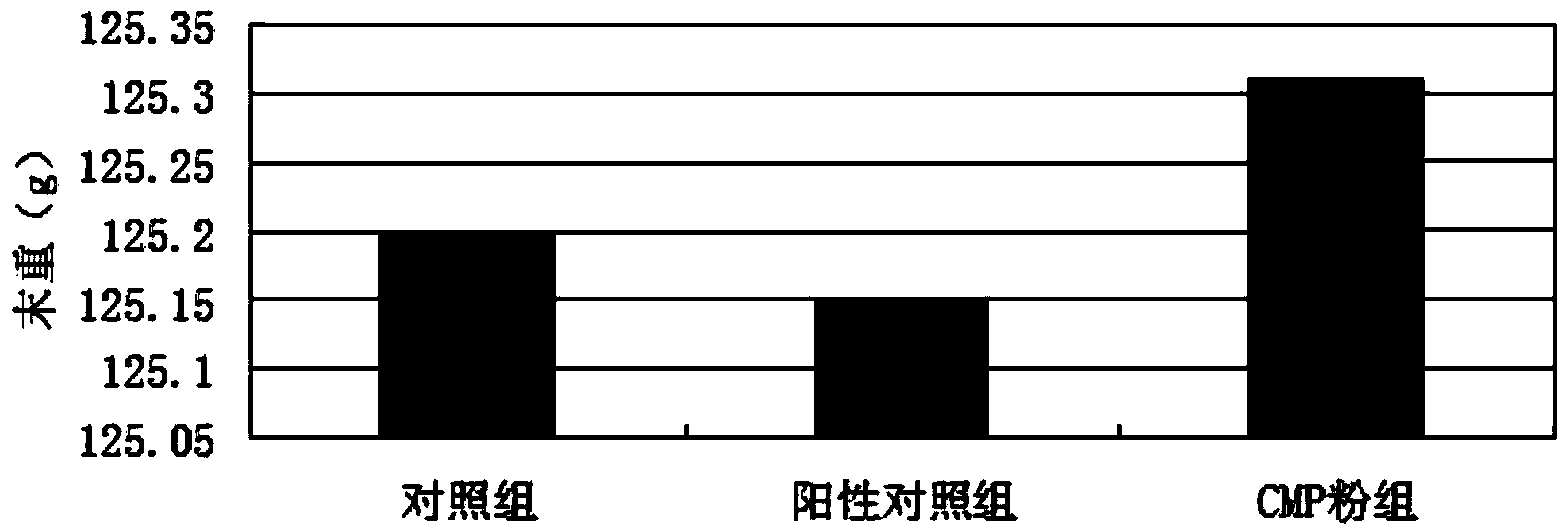
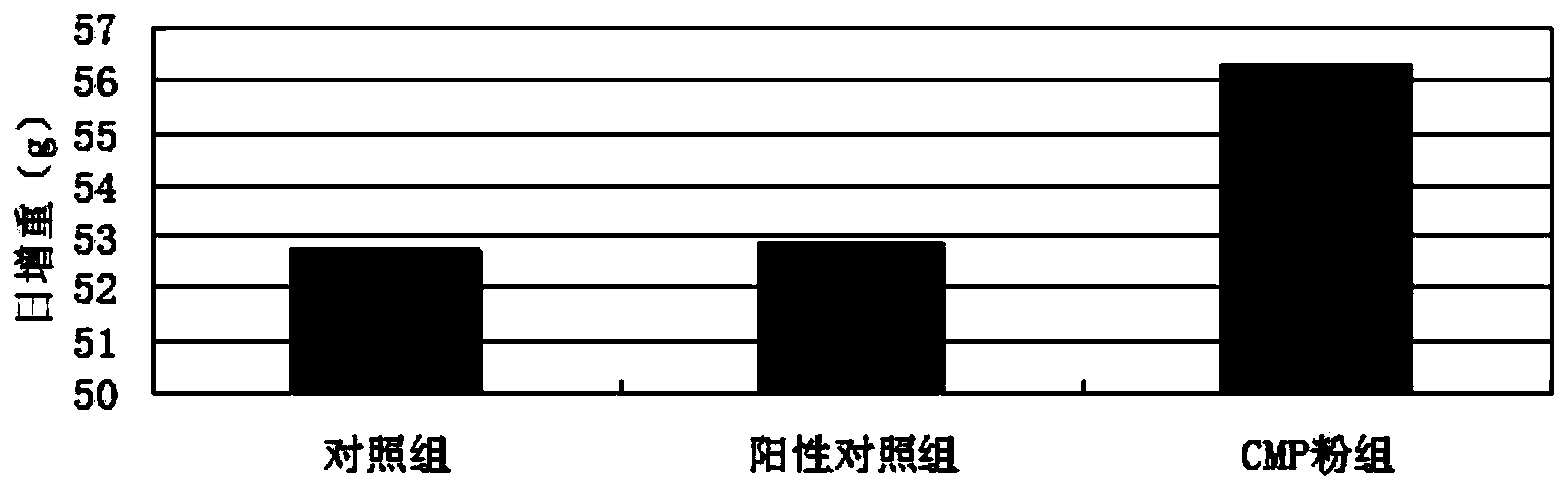
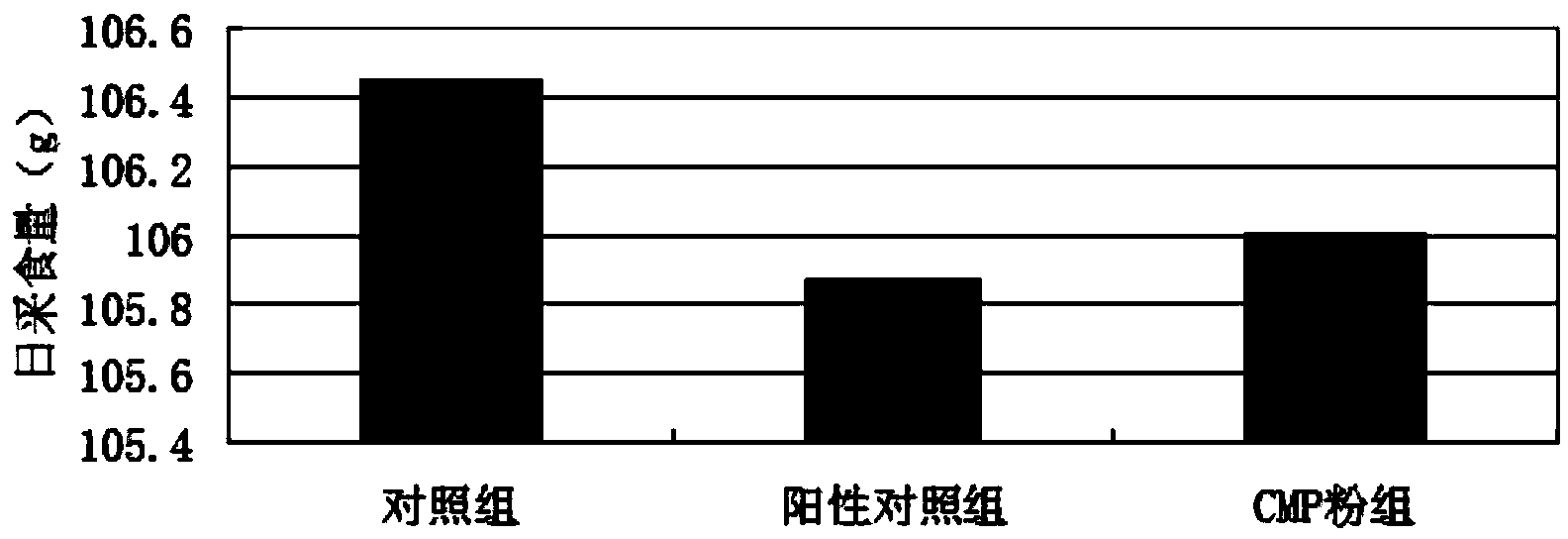
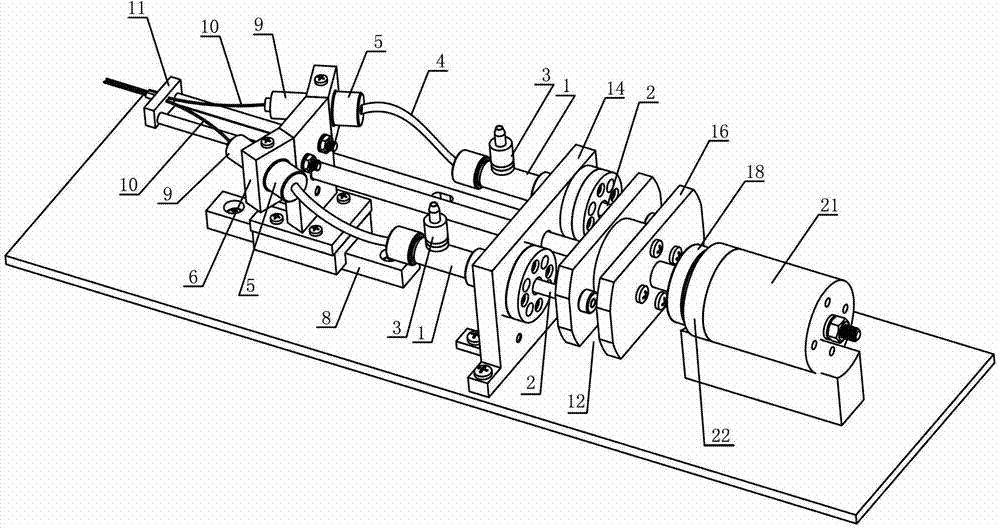
Patents
Literature
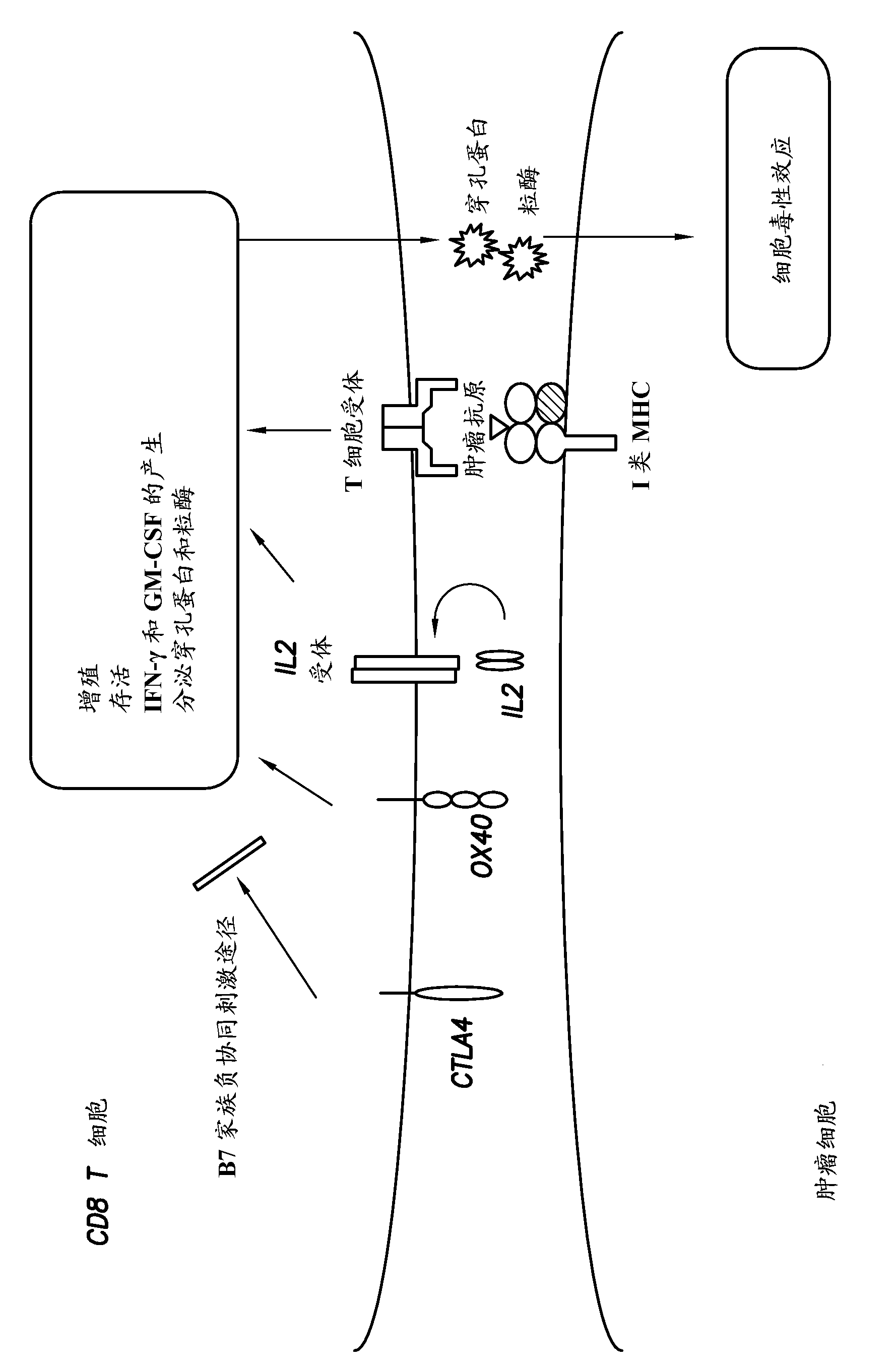
208results about How to "Improve immune efficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
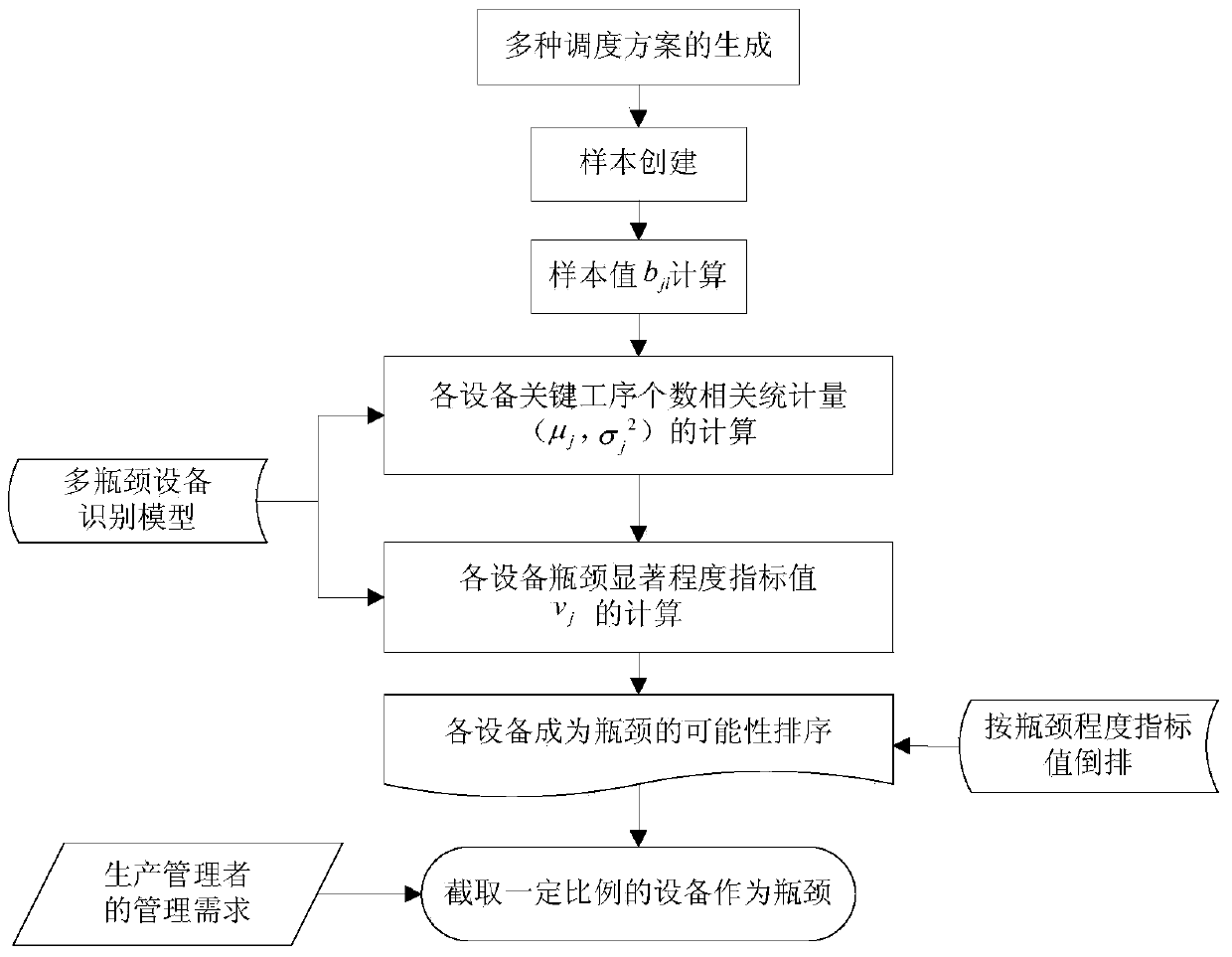
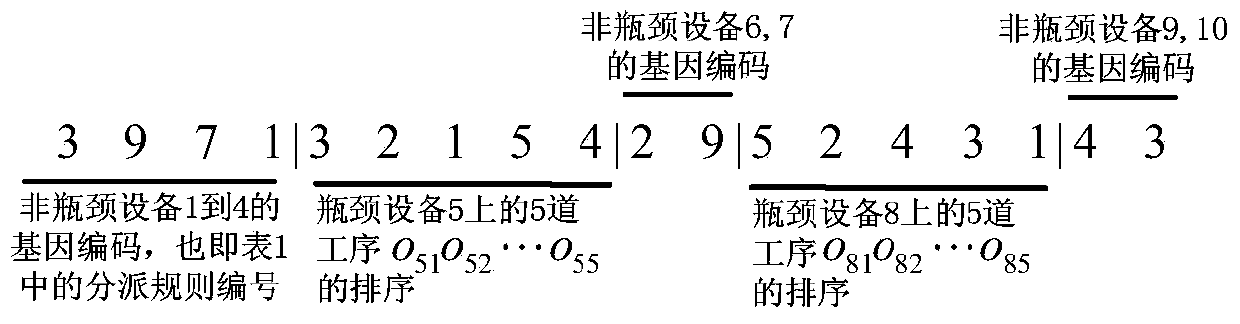
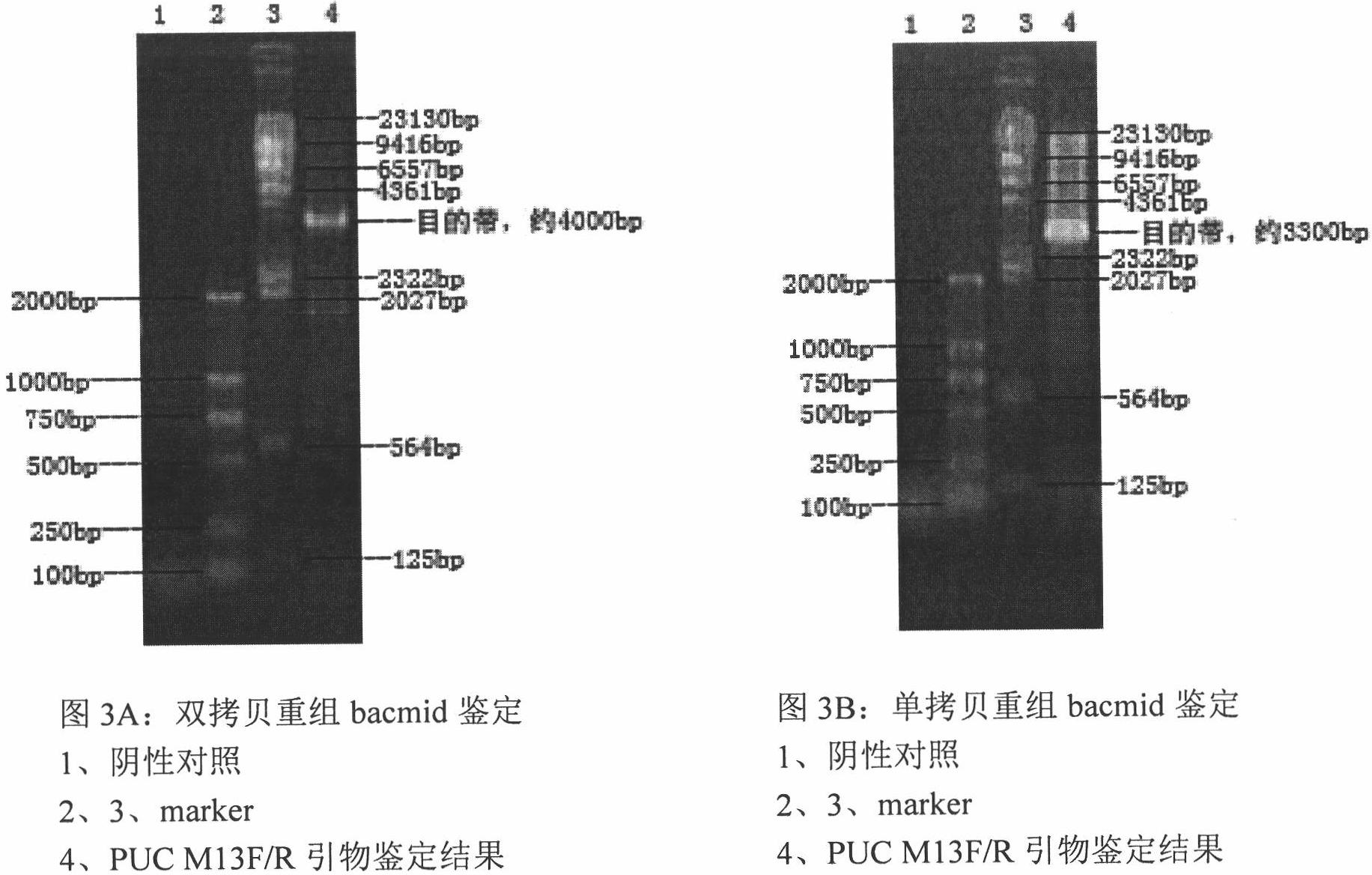
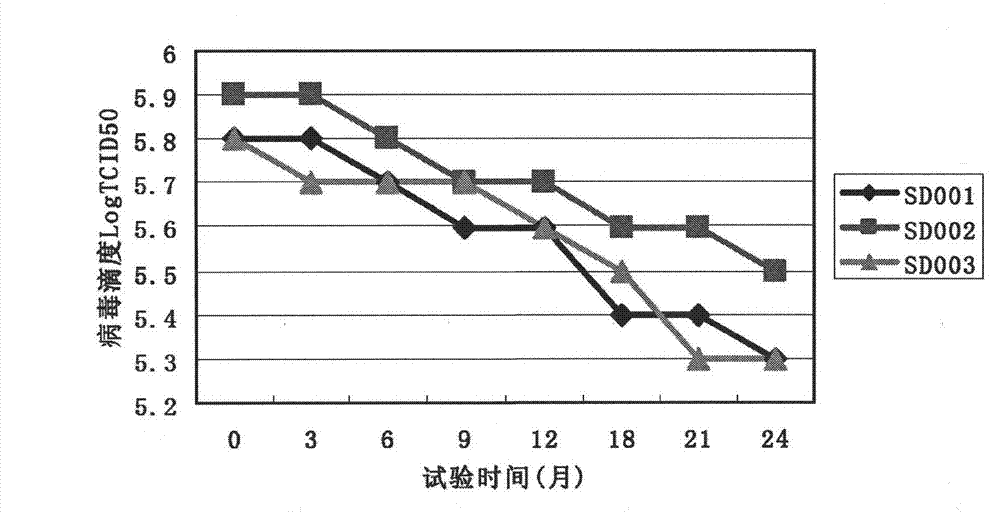
Large-scale operation workshop scheduling method based on bottleneck equipment decomposition
InactiveCN103530702AShortened gene lengthImprove the speed of genetic immune operationGenetic modelsForecastingDecompositionData acquisition
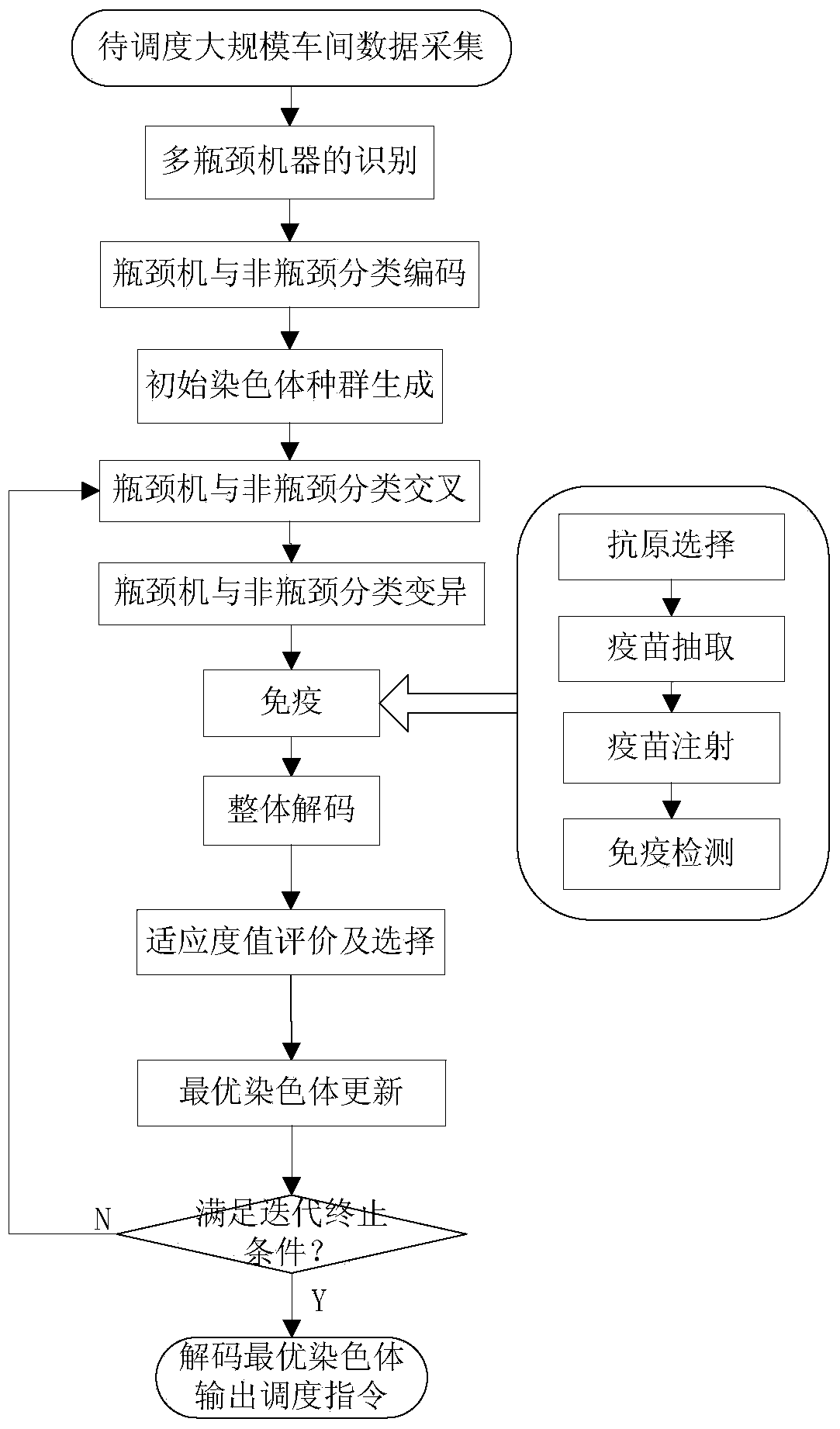
The invention discloses a large-scale operation workshop scheduling method based on bottleneck equipment decomposition. The large-scale operation workshop scheduling method based on the bottleneck equipment decomposition comprises the following steps of (1) acquiring data and modeling; (2) carrying out recognition on bottleneck equipment based on a key path method; (3) sorting and encoding the bottleneck equipment and non-bottleneck equipment; (4) generating an initial chromosome population; (5) carrying out cross and mutation operations on the chromosome population; (6) inoculating an immune operator to the chromosome population; (7) carrying out decoding and fitness value calculation operations on chromosomes; (8) updating an optimal chromosome and an optimal fitness value of an algorithm; (9) judging whether a method ending rule is achieved or not, starting a step (10) if the method ending rule is achieved, and otherwise, jumping to the step (5) to carry out the next iteration; (10) finding out the optimal chromosome from the step (9) to decode, and obtaining a scheduling command to schedule. According to the large-scale operation workshop scheduling method based on the bottleneck equipment decomposition, which is disclosed by the invention, a satisfactory scheduling scheme can be obtained in a shorter time, the production efficiency of an operation workshop can be improved, and the large-scale operation workshop scheduling method based on the bottleneck equipment decomposition can be applied to scheduling management and optimization of the production process of the workshop.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
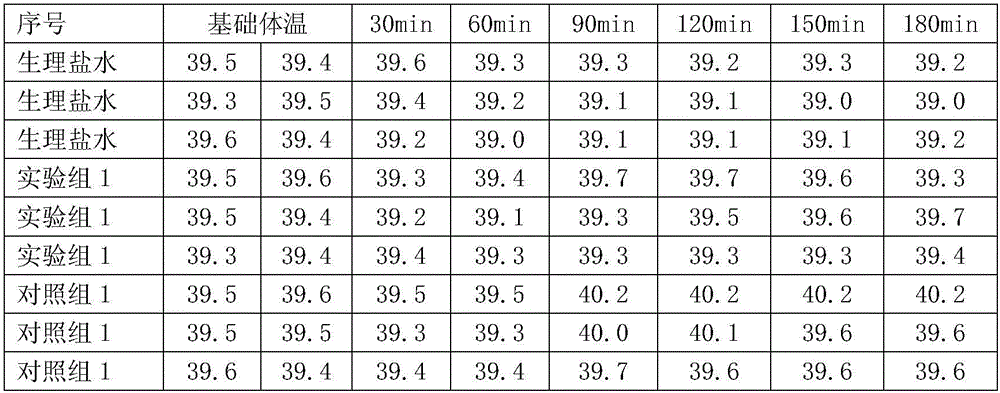
Method for producing swine fever live vaccine with cell line
ActiveCN101181637AGuaranteed to be pureEnsure safetyAntiviralsAntibody medical ingredientsQuality controlSeedling
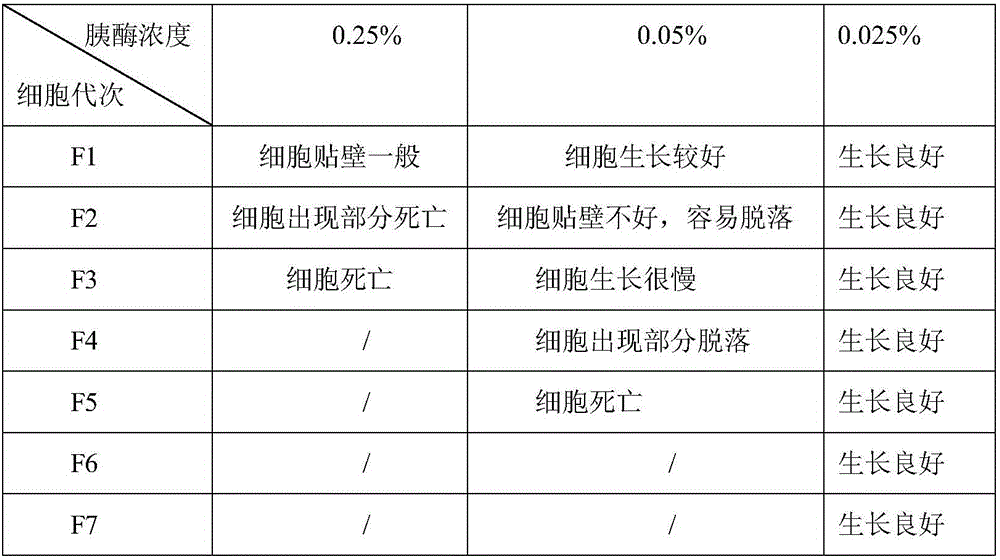
The invention discloses a method for producing a live swine fever vaccine by using a cell line. The present invention comprises the following technical steps: (1) selecting a cell line as the cells for making seedlings; (2) subculture and cultivation of cells for making seedlings; (3) breeding of cytotoxic species; (4) breeding of venom for making seedlings; 5) Mixing seedlings, subpackaging and freeze-drying. The invention has the advantages of simple and stable production process, easy operation, high virus content, small difference between batches, easy quality control, and can significantly improve the yield and quality of vaccines. The live swine fever vaccine produced by the invention has good safety and high immune efficacy, and has complete immune protection against the virulent attack of swine fever.
Owner:CHINA INST OF VETERINARY DRUG CONTROL +1
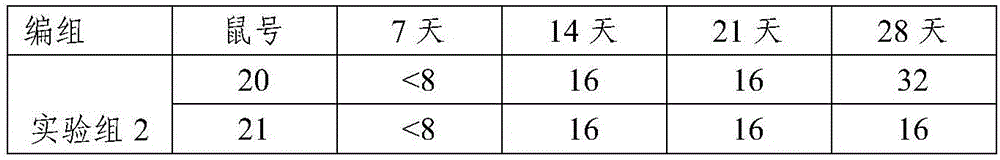
H9 subtype avian influenza virus isolate and vaccine composition prepared thereby
ActiveCN103789272AGood effectImprove immune efficiencyMicroorganism based processesAntiviralsHemagglutininAvian influenza virus
The invention discloses an avian influenza virus isolate belonging to an H9 subtype. An amino acid sequence of an HA1 structural domain of hemagglutinin has the following characteristic sites: 69-bit P, 180-bit A, 221-bit N and 236-bit R; the vaccine composition prepared by the H9 subtype avian influenza virus isolate with the following characteristic sites has good immune efficiency, and is superior to the vaccine prepared by the strain in the prior art in effect, cross protection can be provided for a popular wild strain, significant cross immunogen features are displayed, and the avian influenza virus isolate has a good application prospect in the aspect of preventing and treating poultry cross immune protection.
Owner:PU LIKE BIO ENG +1
Cationic liposome and preparation method thereof
ActiveCN102973506AImprove targetingImprove immune efficiencyPharmaceutical non-active ingredientsAntibody medical ingredientsAntigenLipid formation
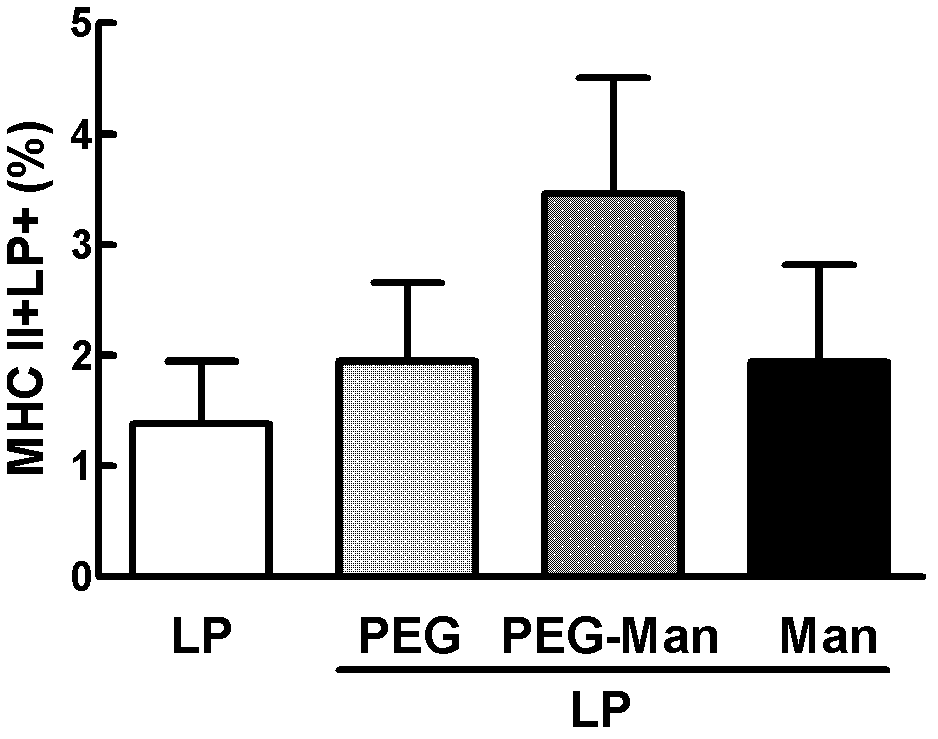
The invention discloses a cationic liposome, comprising cationic lipid, neutral phospholipid, polyethylene glycol derivatived phospholipid and mannose or mannan oligosaccharide, wherein a shell-shaped phospholipid bilayer is formed by phospholipid molecules in the the cationic lipid, the neutral phospholipid and the polyethylene glycol derivatived phospholipid; and mannose- or mannan oligosaccharide- modified polyethylene glycol derivatived phospholipid is formed by connecting the mannose or the mannan oligosaccharide with one end of the polyethylene glycol derivatived phospholipid. By adding the mannose- or mannan oligosaccharide- modified polyethylene glycol derivatived phospholipid, targeting property and enrichment in lymph glands of antigen-presenting cells are increased; the cationic liposome has an immunologic enhancement effect; and the immune efficacy of liposome-encapsulated vaccines can be increased. The invention also provides a preparation method of the cationic liposome.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Method for producing trivalent inactivated vaccine against Haemophilus parasuis infection
ActiveCN102499982AImprove securityImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsImmunogenicityMineral oil
The invention relates to a method for producing a trivalent inactivated vaccine against Haemophilus parasuis infection. The method comprises the following steps of: screening Haemophilus parasuis type 4 strain YBH04, Haemophilus parasuis type 5 strain YBH05 and Haemophilus parasuis type 13 strain YBH13 from predominant prevalence strains, which have good immunogenicity and serve as vaccine strains; inoculating to appropriate culture media for culture respectively; collecting strain suspensions; inactivating with a formaldehyde solution; adding mineral oil adjuvant; and mixing and emulsifying.The vaccine is used for preventing infection of Haemophilus parasuis type 4, Haemophilus parasuis type 5 and Haemophilus parasuis type 13 in pigs, and has the advantages of good safety, high immunization efficacy and long duration of immunity.
Owner:YEBIO BIOENG OF QINGDAO
Method for preparing live vaccines of hog cholera and product thereof
InactiveCN101879311ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsVaccine ProductionFreeze-drying
The invention discloses a method for preparing live vaccines of hog cholera and a product thereof. The preparation method comprises the following steps of: (1) culturing porcine passage cell lines; (2) inoculating the porcine passage cell lines with live vaccine production seed viruses of the hog cholera to obtain attenuated vaccine strains of the hog cholera; (3) performing virus multiplication on the attenuated vaccine strains of the hog cholera; (4) measuring the virus titer of multiplication virus suspension by adopting an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the virus suspension which is detected to be qualified for vaccine matching and freeze-drying. The preparation method has the advantages of producing the live vaccines of the hog cholera by using the cell lines so as to achieve small quality differences among batches and the characteristics of simple and stable process, easy operation, high yield, low cost, the feasibility and extendibility of industrial production and the like, and measuring the virus titer of the multiplication virus suspension by adopting the immunofluorescence method so as to achieve sensitive, fast, specific and accurate detection, high repeatability and reliable results. The live vaccines of the hog cholera prepared by the method can completely protect pigs from the attacks of violent hog cholera viruses.
Owner:武华
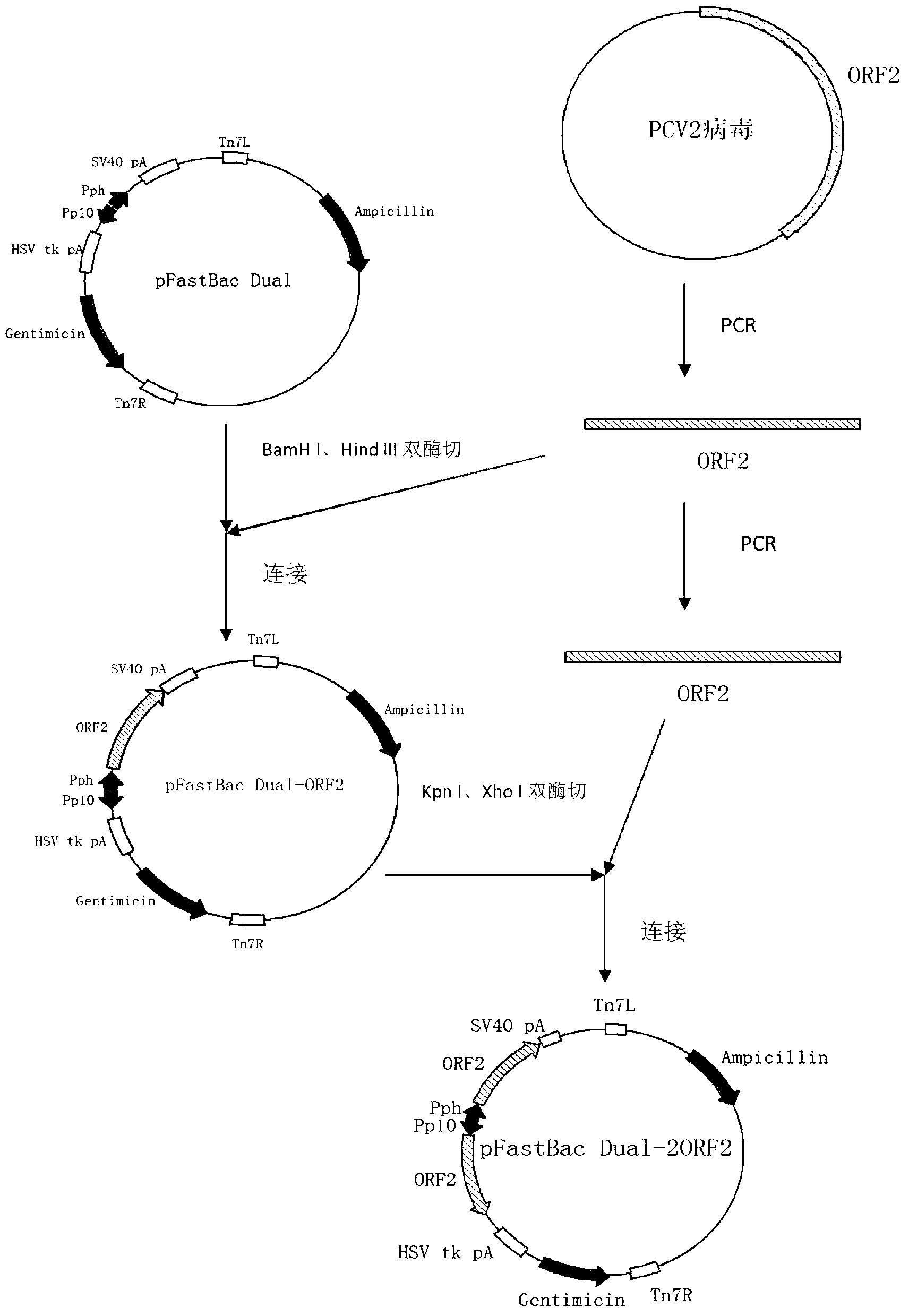
2 type subunit vaccine for porcine circovirus as well as preparation method and application thereof
InactiveCN102517331AQuick responseHigh activityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
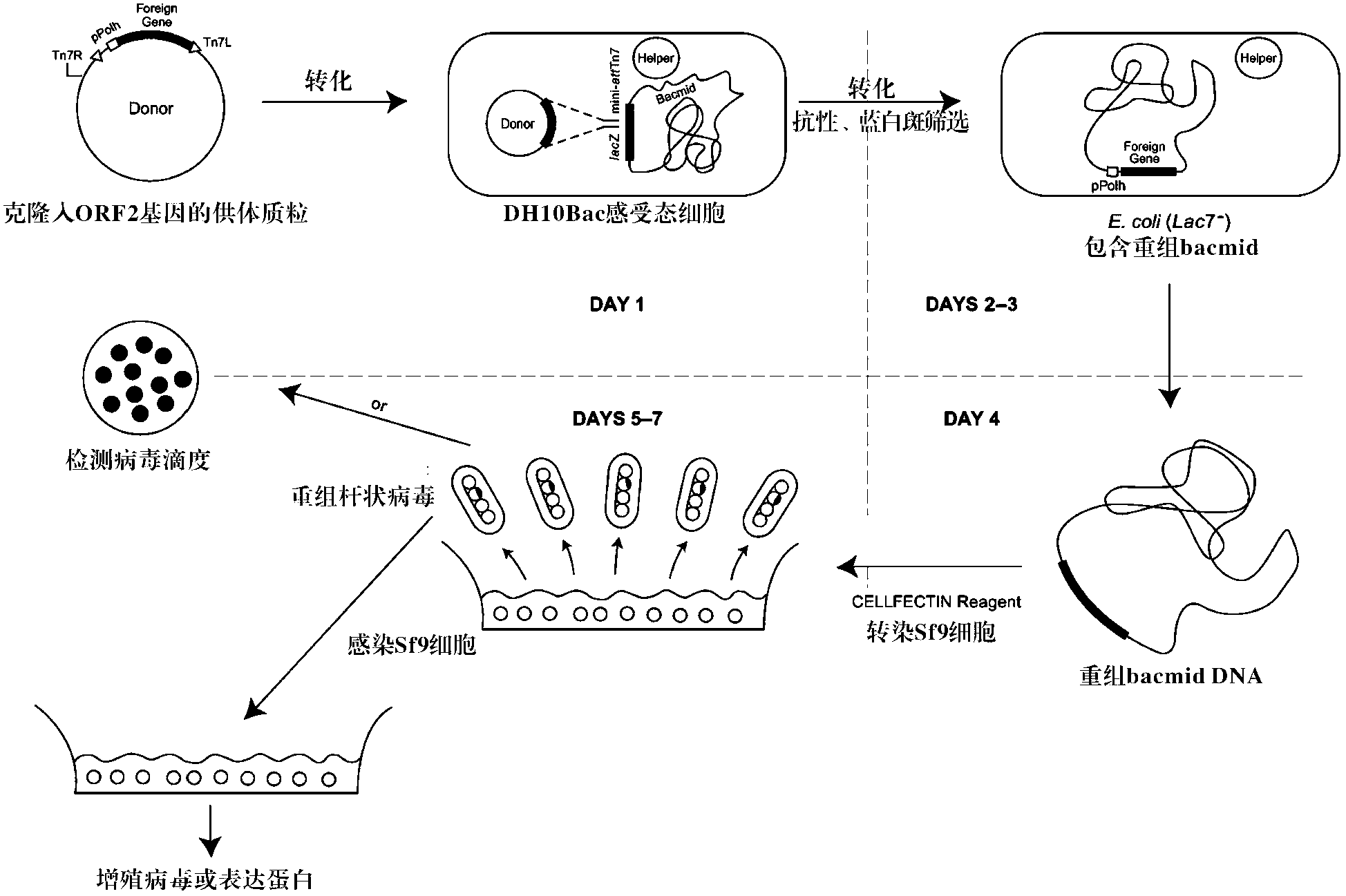
The invention relates to a 2 type subunit vaccine for a porcine circovirus as well as a preparation method and application thereof. A recombinant bacilliform virus contains double promoters (a polyhedrin promoter and a P10 promoter), a coding gene of a Cap protein with double copying can be expressed, and the expression efficiency of the protein is obviously enhanced; moreover, the Cap protein expressed by an inserted foreign gene does not contain an excess sequence, virus-like particles (VLPs) can be effectively formed, and the immunogenicity of an expressed protein is enhanced; furthermore, a produced antigen has high content; and according to the 2 type subunit vaccine for the porcine circovirus, which is disclosed by the invention, the productivity ratio and the quality of a viral protein of the 2 type subunit vaccine for the porcine circovirus are obviously enhanced, and a prepared vaccine composition has the advantages of stable and persistent immune effect, high safety and the like.
Owner:WUHAN CHOPPER BIOLOGY
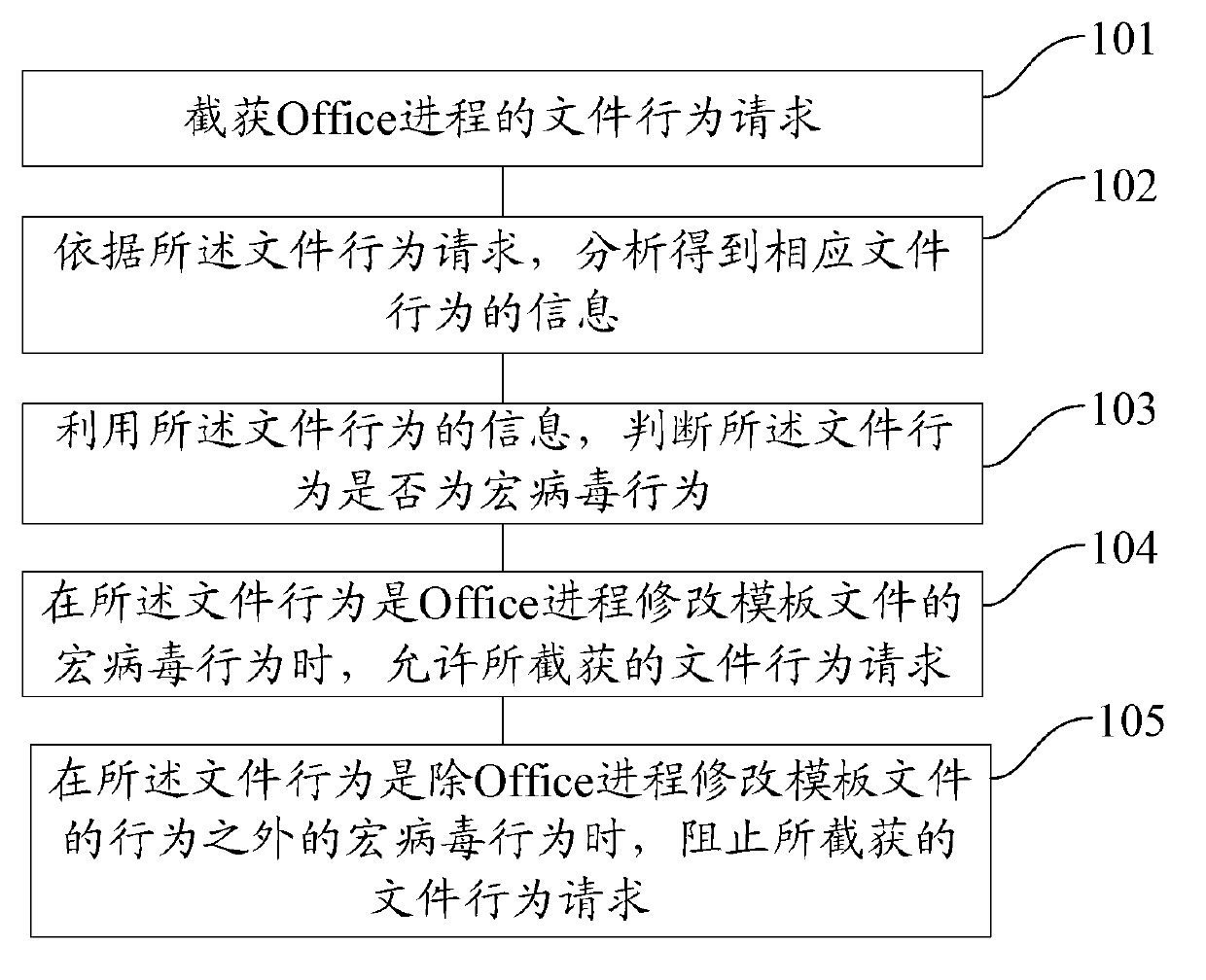
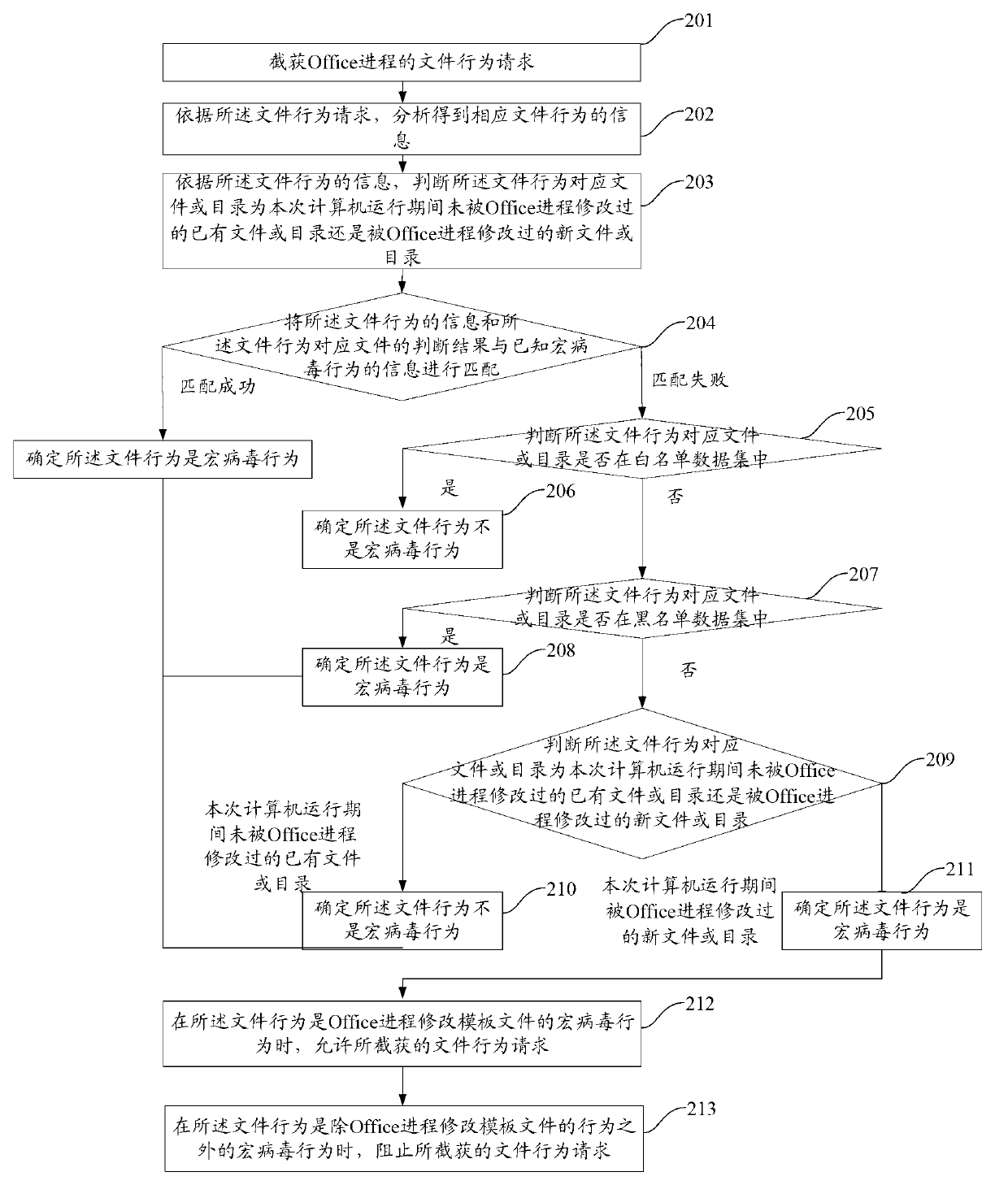
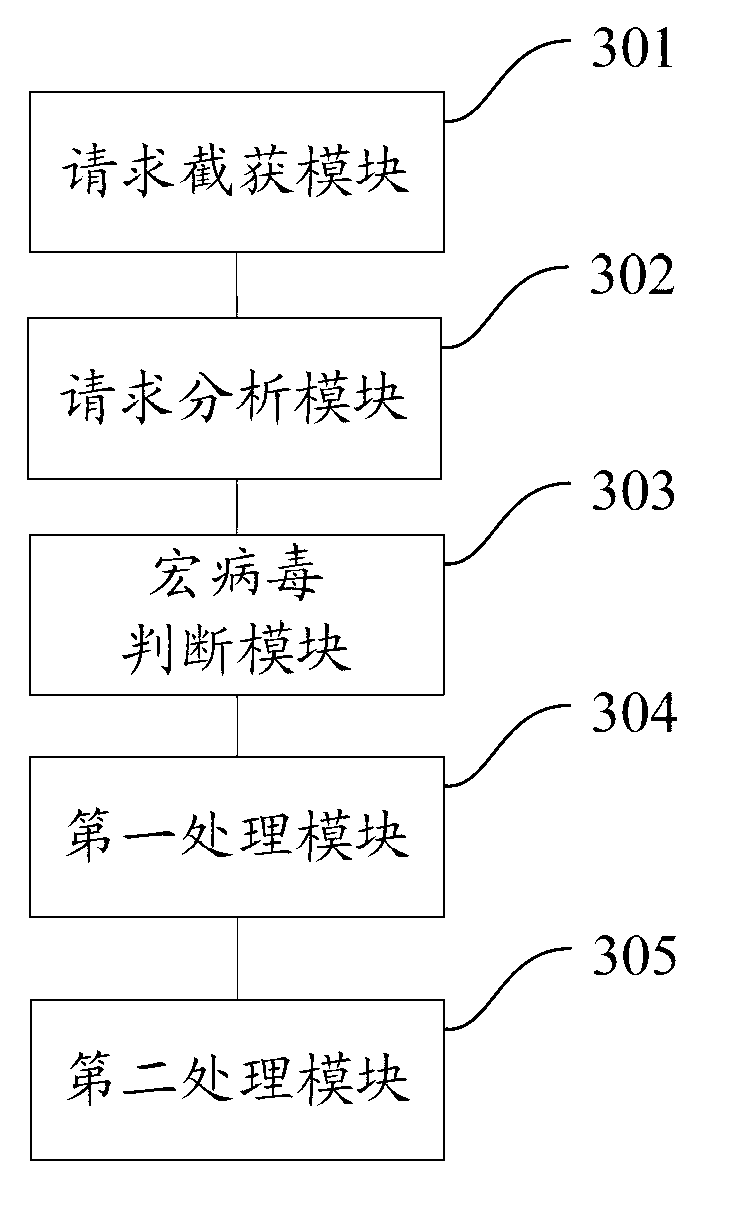
File macro virus immunization method and device
ActiveCN102999726AImprove immune efficiencyAvoid infringementPlatform integrity maintainanceApplication softwareVirus
The invention discloses a file macro virus immunization method and device. The device comprises a request intercepting and capturing module, a request analyzing module, a macro virus judging module, a first processing module and a second processing module, wherein the request intercepting and capturing module is applicable to interception and capture of a file behavior request of an Office progress; the request analyzing module is suitable for analyzing the file behavior request to obtain information of a corresponding file behavior; the macro virus judging module is suitable for judging whether a file behavior is a macro virus behavior or not; the first processing module is suitable for allowing the file behavior request when the Office progress modifies the macro virus behavior of a template file; the second processing module is suitable for stopping the file behavior request in other macro virus behaviors; the request analyzing module is suitable for analyzing parameters of an application program interface (API) to obtain information of the corresponding file behavior; and the information of the file behavior at least comprises one or more of the following information: a file path, a behavior name, a sharing manner and a file attribute. According to the file macro virus immunization method and device disclosed by the invention, an immunization range of the macro viruses can be provided and the immunization efficiency of the macro viruses is improved.
Owner:三六零数字安全科技集团有限公司
Preparation method of carboxymethyl pachyman and novel application of carboxymethyl pachyman
ActiveCN104387482AImprove carboxymethylation efficiencyHigh degree of substitutionOrganic active ingredientsAnimal feeding stuffReaction temperatureFeed additive
The invention provides a preparation method of carboxymethyl pachyman and a novel application of the carboxymethyl pachyman. According to the method, in the process of carrying out carboxymethylation modification on pachymaran, components and ratios of a reaction medium are changed, the reaction temperature is regulated and a catalyst process is adopted, so that the carboxymethylation efficiency of the pachymaran is remarkably improved, the utilization rate of the raw and auxiliary materials is increased, and the cost is saved. Meanwhile, by virtue of the novel application of the carboxymethyl pachyman prepared by the method to preparation of immunopotentiators of livestock and poultry, the immunity to pathogenic microorganisms of the livestock and the poultry can be remarkably enhanced, and the immune efficacy of vaccines can be improved; by virtue of the novel application of the arboxymethyl pachyman prepared by the method to feed additives, the meat performance of the livestock and the poultry can be effectively improved.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Automatic continuous injection unit for simultaneously injecting poultry with two drugs
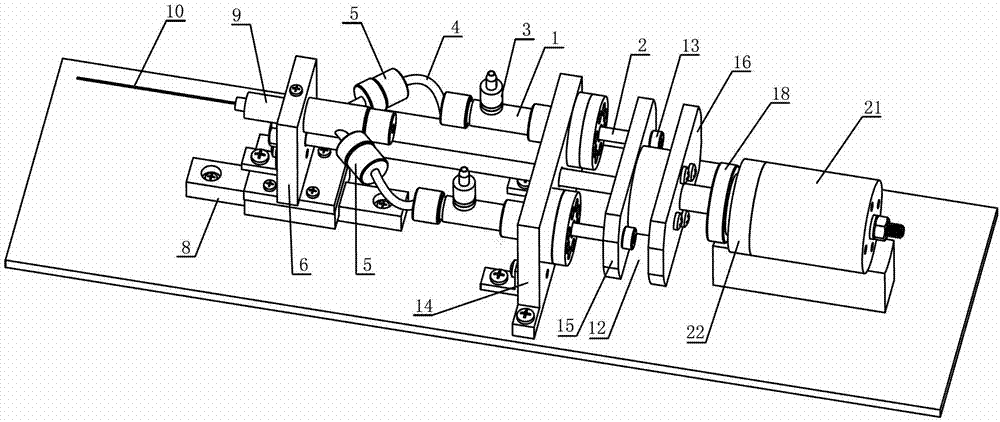
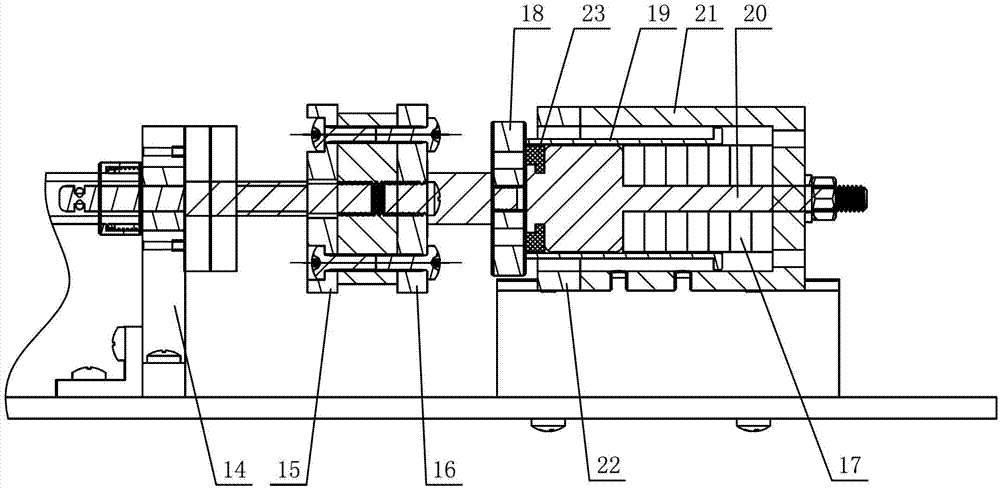
InactiveCN102846407AReduce the number of injectionsLess traumatic injectionsVeterinary instrumentsDrugSyringe
The invention discloses an automatic continuous injection unit for simultaneously injecting poultry with two drugs and belongs to the technical field of poultry syringes. The automatic continuous injection unit comprises two injection systems driven by one push system. The automatic continuous injection unit can be used for injecting two drugs simultaneously, and injection times and injection wounds are reduced for young fowls.
Owner:HUZHOU ZHONGAN AGRI INTELLIGENT TECH
Method for producing pseudorabies living vaccines by using subculture cell source and product thereof
ActiveCN101695573AImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesPig kidneyAntibiotic Y
The invention provides a method for producing pseudorabies living vaccines by using a subculture cell source and a pseudorabies living vaccine product thereof. The method comprises the following steps: culturing pseudorabies virus low-virulent strains by using subculture cells; harvesting the strains to obtain cell culture venom; and then adding a stabilizing agent and an antibiotic into the cellculture venom, and freezing and vacuum-drying the mixture to obtain the pseudorabies living vaccines of the subculture cell source. The subculture cells are subculture cells ST of pig testicle or subculture cells PK15 or IBRS-2 of pig kidney. The method for producing the pseudorabies living vaccines by using the subculture cell source has the advantages of simple and stable production process, easy operation, high virus content, little batch difference and controllable quality, can remarkably improve the yield and quality of the vaccines and reduce the anaphylactic reaction and the like. The pseudorabies living vaccines obtained by using the production method of the invention have good safety and high immune efficacy, and have better immune protection effect on pseudorabies virulent attack.
Owner:广东永顺生物制药股份有限公司
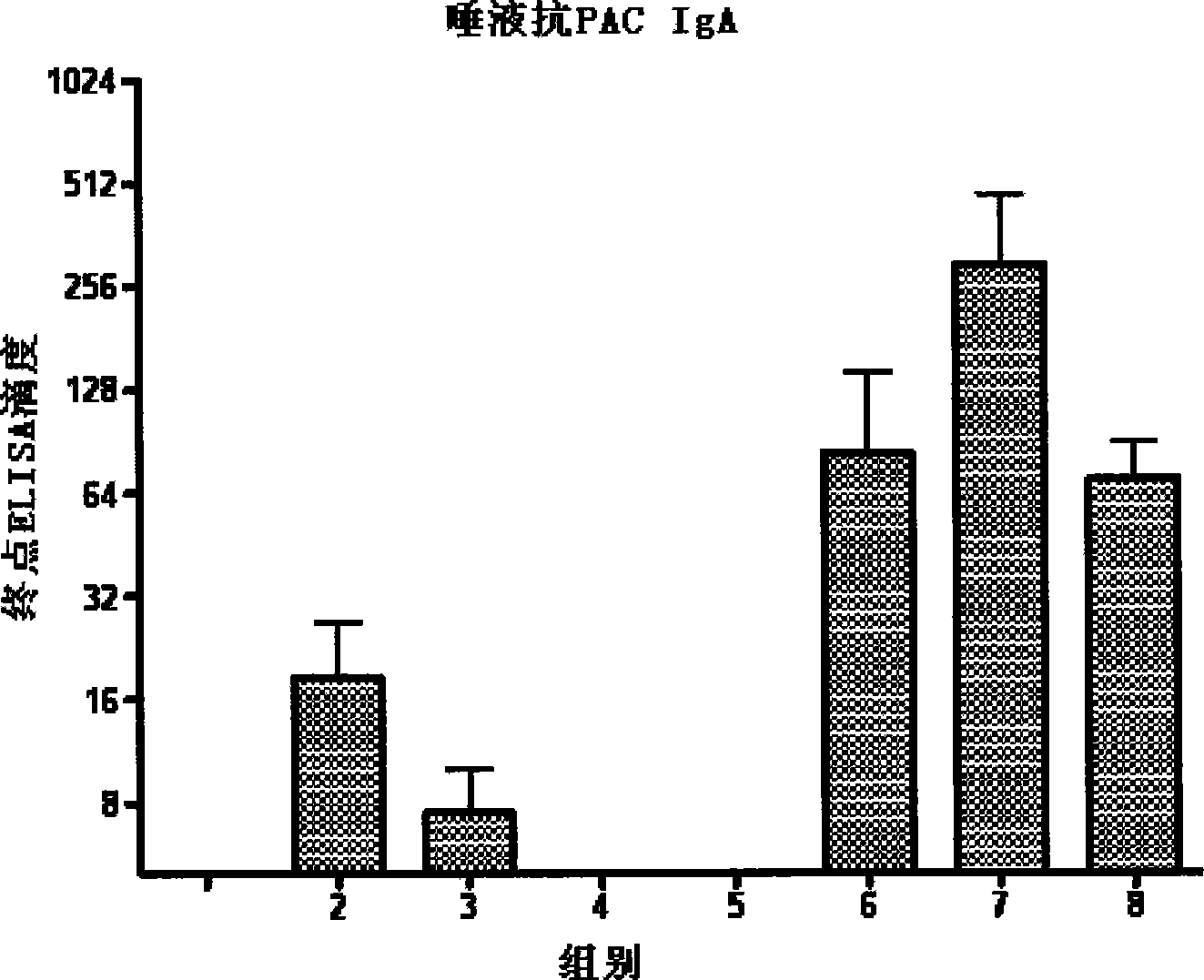
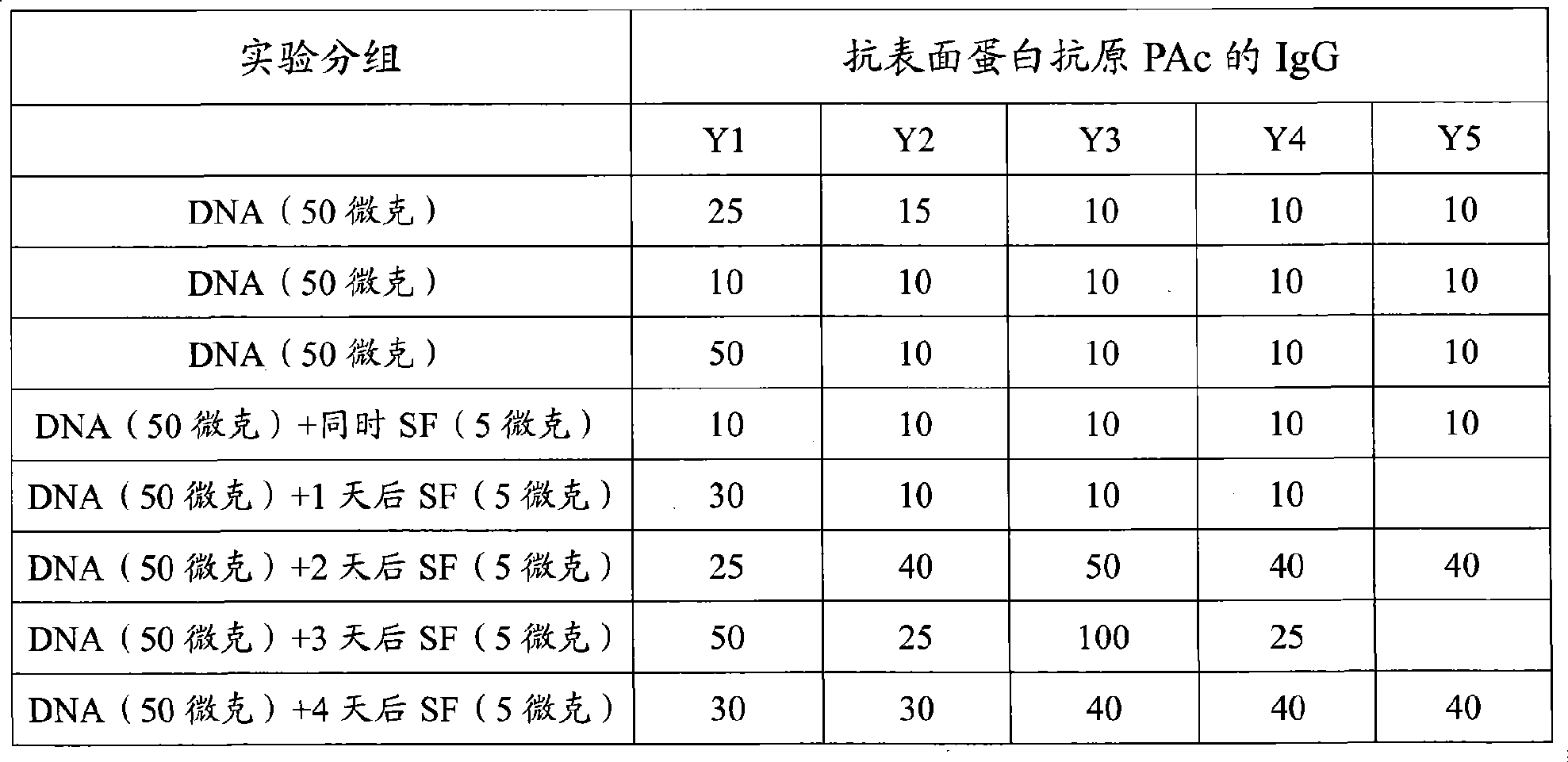
Vaccine reagent kit for preventing carious tooth and method of use thereof
InactiveCN101411872AImproving immunogenicityComprehensive persistent immune responseBacterial antigen ingredientsDigestive systemAdjuvantA-DNA
The invention relates to a vaccine reagent kit for preventing decayed tooth, which comprises antigen and adjuvant, wherein the antigen is a dna recombinant plasmid of at least one surface proteantigen and at least one glucosyltransferase which co-express decayed tooth streptococcus mutans; and the adjuvant is flagellins of one or more than one germ. The vaccine reagent kit for preventing the decayed tooth has strong immunogenicity, can excite comprehensive and lasting immune response, and has strong specificity, even immune effect and simple and convenient operation. The invention also provides an application method for the vaccine reagent kit for preventing the decayed tooth, and the vaccine reagent kit has good immune effect and simple and convenient operation.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Porcine circovirus type 2 subunit vaccine, and preparation method and application thereof
ActiveCN102925486AImprove expression efficiencyImproving immunogenicityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
The invention relates to a porcine circovirus type 2 subunit vaccine, and a preparation method and an application thereof. The recombinant baculovirus contains double promoters (a polyhedrin protein promoter and a P10 promoter), and can express double copies of Cap protein coding genes, such that protein expression efficiency is substantially improved. Also, Cap protein expressed by an inserted exogenous gene does not contain excess sequences, such that virus-like particles (VLPs) can be effectively formed, expressed protein immunogenicity is improved, and the content of produced antigen is high. According to the porcine circovirus type 2 subunit vaccine provided by the invention, protein yield and quality of porcine circovirus type 2 subunit vaccine are substantially improved, and prepared vaccine compositions have the advantages of stable and long-lasting immune effect, high safety, and the like.
Owner:WUHAN CHOPPER BIOLOGY
Porcine rotavirus delta VP8* subunit recombinant protein and applications thereof
InactiveCN103304642AStrong immune responseFast titerViral antigen ingredientsVirus peptidesVp4 geneInclusion bodies
The invention relates to a porcine rotavirus delta VP8* subunit recombinant protein and an encoding gene of the protein. The invention further provides a recombinant protein formed after increasing tetanus toxin T cell epitope P2 into the recombinant protein, and an encoding gene. The delta VP8* protein is 64th-site to 223th-site amino acid in the VP8* and can effectively stimulate an organism to produce specific serum antibody, humoral immune response is good, the problem that the VP4 gene can not conduct prokaryotic expression due to overlarge fragment can be overcome, the protein can be expressed as a soluble protein in vitro, so that the problem that the VP8* is expressed as an inclusion body in vitro can also be overcome; the T cell epitope P2 (830th-site to 844th-site amino acid of TT) in the tetanus toxin is induced into the delta VP8* subunit recombinant protein, so that the immunity efficacy of the protein can be greatly improved, the faster and stronger neutralizing antibody titer can be induced, and high-titer rotavirus cross neutralizing antibody can also be induced.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Method for producing pseudorabies attenuated vaccine by using bioreactor and pseudorabies attenuated vaccine product
ActiveCN101695572AImprove immune efficiencyIncrease growth densityMicroorganism based processesAntiviralsVaccine ProductionAntibiotic Y
The invention provides a method for producing a pseudorabies attenuated vaccine by using a bioreactor and a pseudorabies attenuated vaccine product. After being sterilized, the bioreactor and a micro carrier are inoculated with cells for producing the vaccine, and a cell growth medium is added for culture. A maintenance medium containing attenuated strains of pseudorabies viruses are inoculated into the bioreactor to continue culturing the cells. 2 to 3 days after virus inoculation, cell culture virus liquid is obtained and added with a stabilizer and antibiotics, and the cell culture virus liquid is refrigerated and dried under vacuum to obtain the pseudorabies attenuated vaccine. In the method, the cell density and virus concentration are improved greatly, the titer of the vaccine is improved, the side reactions, labor intensity and product cost are reduced, the monitoring performance of vaccine production is improved and uniform and stable product quality is guaranteed. The pseudorabies attenuated vaccine produced by the method has high safety, immune efficacy and good immune and protective effect against the attack by the virulent pseudorabies viruses.
Owner:广东永顺生物制药股份有限公司
Compound-type nano-vaccine and preparation method thereof
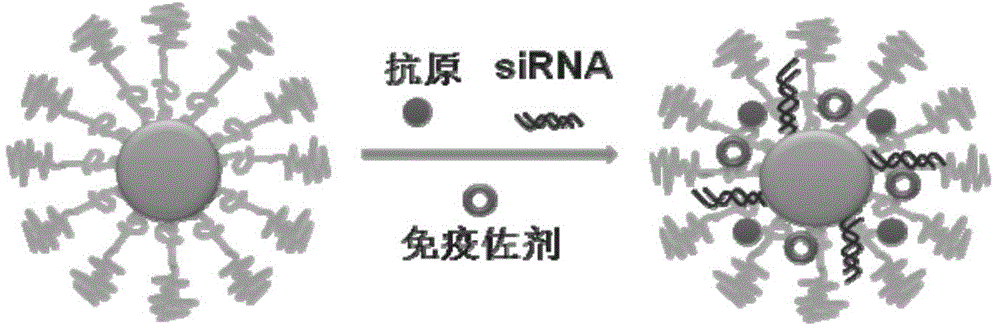
InactiveCN104645349AImprove Uptake PresentationImprove immune efficiencyGenetic material ingredientsMacromolecular non-active ingredientsDendritic cellBiocompatibility Testing
The invention discloses a compound-type nano-vaccine taking an amphiphilic three-block polymer of polyethylene glycol derivative-poly lysine-poly leucine as a nano-carrier bearing a tumor antigen, an immunological adjuvant and siRNA (si Ribonucleic Acid), and a preparation method of the compound-type nano-vaccine. According to the nano-vaccine, taking and presenting of the antigen by antigen-presenting cells can be improved by a nano-micelle bearing the antigen; siRNA is efficiently delivered to TADCs (Tumor-associated Dendritic Cells) to block immunosuppression signals of TADCs, and TADCs are induced and activated by collaboration with the immunological adjuvant, so that a tumor resisting effect of a tumor vaccine is improved; the nano-vaccine has the advantages that the adopted carrier of the nano-vaccine is good in biocompatibility and low in toxicity, and is degradable in a biological body; degradation products are nontoxic and harmless, and can be absorbed or metabolized; and the preparation method of the nano-vaccine is simple and convenient and feasible, good in stability and convenient for popularization.
Owner:SHENZHEN INST OF ADVANCED TECH
New mycoplasma hyopneumoniae strain and vaccine composite of new mycoplasma hyopneumoniae
ActiveCN104450559AImprove immunityDoes not affect weight gainAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaMycoplasma
The invention provides a mycoplasma hyopneumoniae strain HN0613 which has better immunogenicity proved through isolation and identification, also provides a mycoplasma hyopneumoniae antigen prepared by using the mycoplasma hyopneumoniae strain HN0613 and a mycoplasma hyopneumoniae vaccine containing the mycoplasma hyopneumoniae antigen. The invention further provides a porcine circovirus type 2 / mycoplasma hyopneumoniae bicombinant vaccine, containing a PCV2 antigen (inactivated PCV2 antigen or PCV2ORF2 protein), an inactivated mycoplasma hyopneumoniae and vaccine adjuvants. Through injection of the bicombinant vaccine, the aim of preventing PCV and mycoplasma hyopneumoniae can be achieved and the effect of prevention and protection from mycoplasma hyopneumoniae infection can be achieved further.
Owner:PU LIKE BIO ENG +1
Preparation method and application of swine vaccine specific swine spleen transfer factor (TF)
ActiveCN103566370AReduce the use volumeEasy to mix and prepareAntiviralsAntibody ingredientsHigh concentrationDead volume
The invention discloses a preparation method of a swine vaccine specific swine spleen transfer factor (TF). The preparation method comprises the following steps: slaughtering a swine with a positive swine vaccine antibody to harvest the swine spleen; preserving the swine spleen at low temperature; unfreezing the swine spleen; removing fasciae; mincing the swine spleen; homogenizing the pulp; filling the homogenized pulp into a bottle and storing the pulp; repeatedly freezing and thawing the pulp; centrifuging the pulp; carrying out microfiltration; carrying out ultrafiltration; carrying out inactivation; regulating the pH value; regulating the osmotic pressure; removing bacteria; and detecting the quality. The preparation method has the advantages that the pH value of water for injection is regulated to 4-6 in the pulp homogenizing step, thus being beneficial to increasing of the yields of ribose and polypeptide; a box type membrane coating is adopted for tangential flow filtration, so that the dead volumes of system residues are small and linear amplification production is easy to achieve; a 1-3KD box type membrane coating is adopted to carry out tangential flow nanofiltration on a TF crude product, thus preparing the high-concentration TF and improving the using effects; phenol red is used as an acid-base indicator, thus being convenient for clients to observe the pH value of the TF; the TF uses beta-propiolactone for inactivation instead of traditional formaldehyde and an osmotic pressure regulation process is added, thus being beneficial to combined immunization of the TF and vaccines.
Owner:派生特(福州)生物科技有限公司 +2
Combined live vaccine against porcine reproductive and respiratory syndrome and pseudorabies, and preparation method thereof
ActiveCN102727884AImprove immune efficiencyIncrease productionViral antigen ingredientsGenetic material ingredientsPseudorabiesImmunosuppression
The invention provides a combined live vaccine for preventing porcine reproductive and respiratory syndrome and pseudorabies, and a preparation method and application thereof. According to the invention, no immunosuppression occurs between two vaccines of the combined live vaccine; compared with each single vaccine, the combined live vaccine has no obvious difference in security, immunogenicity, immunity duration and immuno-protective effects and has remarkable immuno-protective effects on preventing porcine reproductive and respiratory syndrome and pseudorabies.
Owner:华威特(江苏)生物制药有限公司
Compound adjuvant for animal vaccine and application of compound adjuvant
ActiveCN105688207AImprove immune efficiencyGood immune protectionAntibody medical ingredientsSide effectVegetable oil
The invention relates to a vaccine adjuvant and particularly discloses a compound adjuvant for an animal vaccine. The compound adjuvant comprises raw materials in parts by weight as follows: 10-70 parts of vegetable oil for injection, 0.1-20 parts of an emulsifier, 1-10 parts of an additive, 0.01-10 parts of polymers and 0.01-5 parts of an immunopotentiator, wherein the polymers are selected from natural polymers and / or synthetic polymers. According to the animal vaccine prepared from the compound adjuvant, a synergistic effect is realized through compounding of the vegetable oil for injection, the polymers and the immunopotentiator, the immune efficacy of the animal vaccine is improved effectively, and immune protection of the animal vaccine is facilitated. Side effects of the vaccine adjuvant are reduced obviously in the aspect of animal safety compared with a mineral oil adjuvant.
Owner:CHINA ANIMAL HUSBANDRY IND
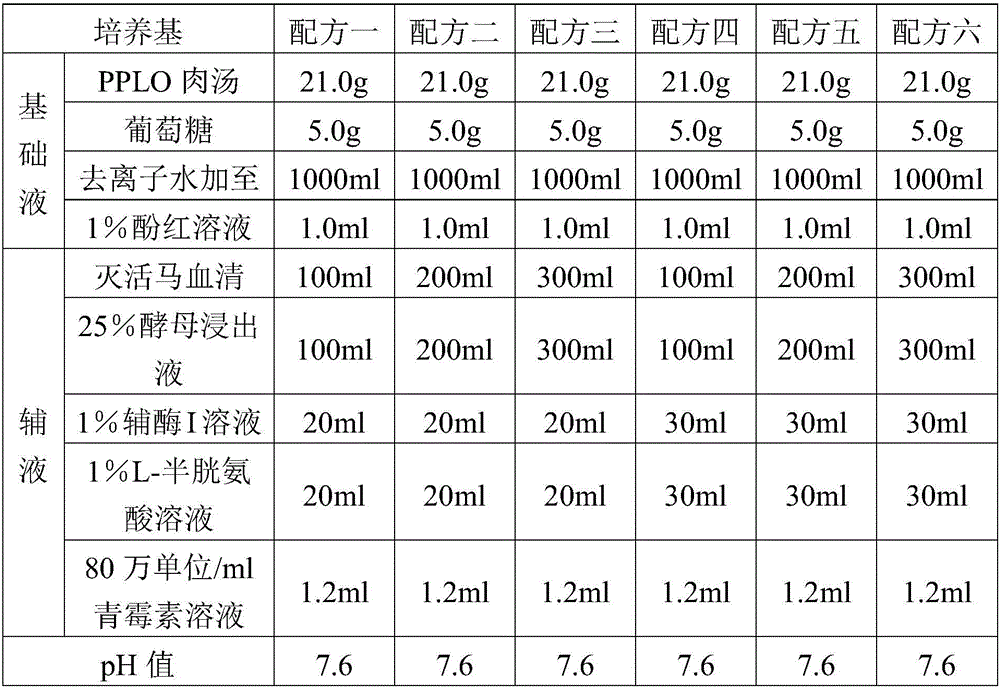
Fluid medium for culturing mycoplasma synoviae (MS)
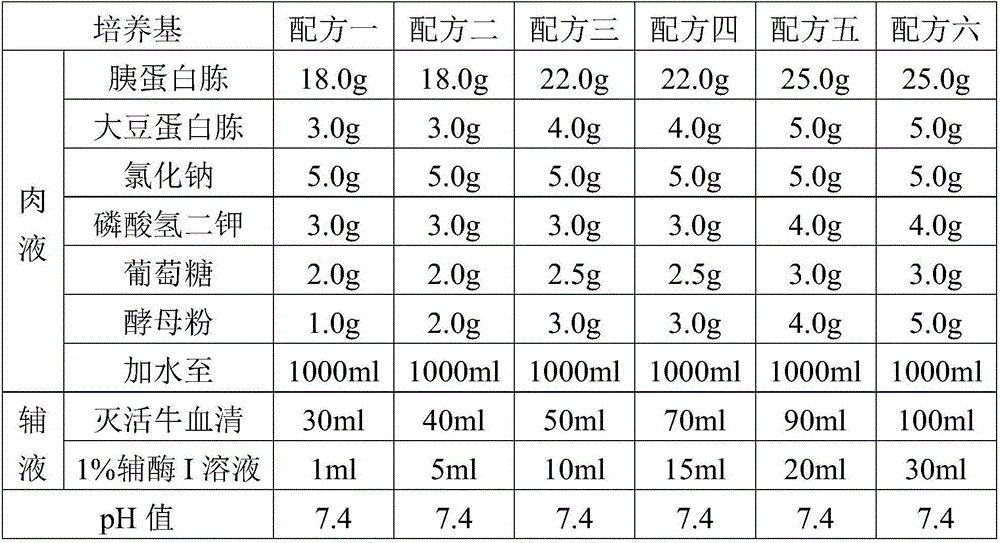
PendingCN105695362AGood securityAnd immune potencyBacteriaMicroorganism based processesMycoplasma cultureYeast
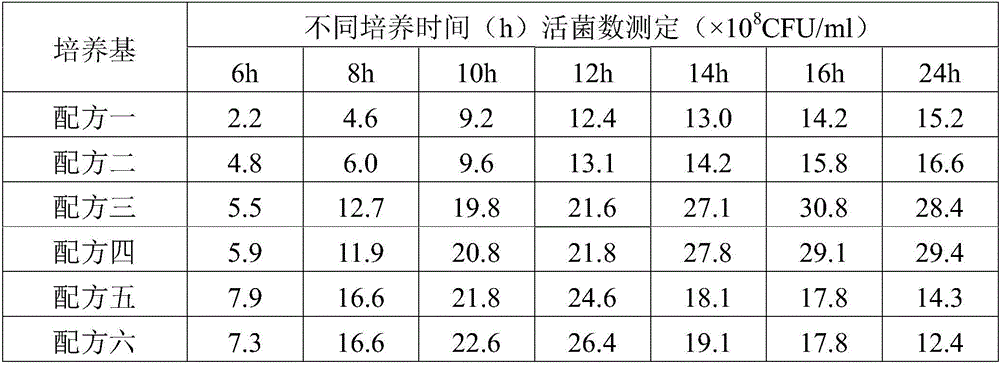
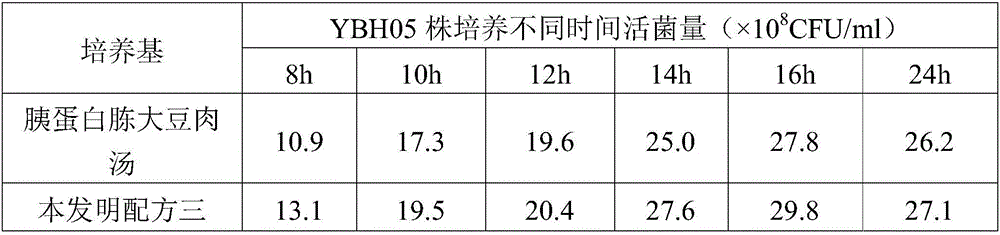
The invention provides a fluid medium for culturing mycoplasma synoviae (MS), being prepared from the following components: pleuropneumonia-like organism (PPLO) broth, glucose, inactivated horse serum, yeast leachate, a phenol red solution, a coenzyme I solution and an L-cysteine solution; the pH value of the fluid medium is 7.6-7.8. The fluid medium is beneficial to the growth of the MS by supplementing various nutritional ingredients needed by microorganisms and effectively controlling the pH value of the fluid medium by sodium hydroxide; viable counts are not less than 109CCU / ml; an inactivated vaccine prepared by a bacteria solution of the MS is good in safety and immunization efficacy.
Owner:YEBIO BIOENG OF QINGDAO
Serum-5 type haemophilus lus paradis vaccine strain and application thereof
InactiveCN104450557ABiologically stableStrong pathogenicityAntibacterial agentsBacteriaSerum igeHaemophilus
The invention relates to a serum-5 type haemophilus lus paradis vaccine strain. The classified name of the vaccine strain is haemophilus lus paradis, the strain name is JSYZ10 and the vaccine strain is preserved in the China Center for Type Culture Collection on June 15, 2014 with the preservation number of CCTCC NO:M 2014260. The invention further relates to an application of the serum-5 type haemophilus lus paradis vaccine strain in preparation of a haemophilus lus paradis inactivated vaccine. The serum-5 type haemophilus lus paradis JSYZ10 strain is stable in biology, has a strong pathogenicity to a piglet, and has a good immunogenicity when being inactivated and vaccinated on the piglet. A univalent vaccine prepared from the vaccine strain serving as a vaccine candidate strain has good safety, can produce a relatively high antibody on the piglet, has long duration and good immune potency, and can be used for resisting attack of homotype wild strains. After a pig group is immunized, the morbidity and death rate are remarkably reduced, the economic loss of a piggery is reduced, and the immunizing effect of the vaccine strain is the same as or superior to that of existing commercial vaccines on the market.
Owner:扬州优邦生物药品有限公司
Method for producing avian adenovirus inactivated vaccine through LMH clone line
InactiveCN106798918AGood immune effectReduce manufacturing costViral antigen ingredientsInactivation/attenuationAntigenEmulsion
The invention relates to a method for producing avian adenovirus inactivated vaccine through an LMH clone line. The method comprises the following steps: firstly, preparation continuous cell line LMH cell; secondly, virus-seed reproduction; thirdly, virus collection, concentration and purification; fourthly, virus content determination; fifthly, inactivation and emulsification of virus, and forming emulsion inactivated vaccine. Compared with the traditional technical method for producing avian adenovirus through cell culture, the method has the advantages that high-quality inactivated vaccine with higher antigen titer through the optimization of the concentration of digestive LMH cell pancreatin-EDTA, the culture time of LMH cell and the inoculation concentration and harvest time of virus, injection immunization is performed at different doses, and all the vaccine protection rates reach 100 percent, so that the immune efficiency of the avian adenovirus type IV inactivated vaccine produced through the method is high, and the vaccine has complete immune protection effect on avian adenovirus type IV.
Owner:广州博恒生物科技有限公司
Liquid medium used for culturing haemophilus parasuis
InactiveCN106282075APromote growthImprove securityBacteriaMicroorganism based processesLiquid mediumAdditive ingredient
The invention provides a liquid medium used for culturing haemophilus parasuis. The liquid medium comprises the following components: tryptone, soya peptone, sodium chloride, dipotassium phosphate, glucose, yeast powder, bovine serum and a coenzyme I, and the liquid medium has a pH value of 7.2-7.4. The liquid medium has the beneficial effects that the liquid medium is beneficial to growth of haemophilus parasuis by supplementing the nutritional ingredients required by various microorganisms and effectively controlling the pH value in the medium via sodium hydroxide; the viable counts are not lower than 10<9>CFU / ml; and inactivated vaccines are prepared from haemophilus parasuis liquid and have good safety and immune efficacy.
Owner:YEBIO BIOENG OF QINGDAO
Lateral flow immune test strip based on ordered micro-nano structure
PendingCN111751525AAvoid cross reactionPrecise electromagnetic field confinement effectMaterial analysisAnalyteNanoparticle
The invention discloses a lateral flow immune test strip based on an ordered micro-nano structure. The lateral flow immune test strip is used for detecting a target in an analyte. According to the test strip, a base is used as a substrate, a sample pad, a conjugate pad, a chromatography pad, an ordered micro-nano structure detection pad and an absorption pad are sequentially distributed on the substrate from left to right, the sample pad is a target loading area, nanoparticles capable of being coupled with a target are combined on the conjugate pad, the ordered micro-nano structure detection pad is arranged on the chromatography pad, and a detection area and a quality control area are distributed on the ordered micro-nano structure detection pad; the sample pad and the conjugate pad are overlapped together, the conjugate pad and the chromatography pad are overlapped together, the ordered micro-nano structure detection pad is fixed on the chromatography pad, and the chromatography pad and the absorption pad are overlapped together; in practical application, when a sample reaches a detection area, multi-target detection can be realized by collecting signals; the test strip has the advantages of short detection time, simplicity and convenience in operation, low cost, simultaneous detection of multiple targets and the like, and is expected to be widely applied to the field of rapiddetection.
Owner:SOUTHEAST UNIV
Tumor targeted delivery of immunomodulators by nanoplymers
InactiveCN103002919AEnhanced immune-stimulating potencyDoes not cause systemic immune responseAntibody ingredientsPharmaceutical non-active ingredientsAntitumor immunityTumor target
Nanoconstructs having three components: (1) biodegradable nanopolymers and nanoparticles, (2) immunodrugs such as CpG, and a (3) tumor binding device, which are actively targeted to tumor cells such as melanoma cells through receptor-mediated uptake and methods of using the same are described. Antitumor immunity is further enhanced by combination of PG-CpG nanoconstructs with agonists of positive costimulatory signals and inhibitors of negative immune regulatory signals.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Thermal-stability foot-and-mouth disease O type recombinant virus as well as preparation method and application thereof
InactiveCN109810954AImprove thermal stabilityGood genetic stabilityAntiviralsViruses/bacteriophagesAntigenStructural protein
The invention provides thermal-stability foot-and-mouth disease O type recombinant virus as well as a preparation method and application thereof and belongs to the technical field of biological products of veterinary drugs. The virus takes an O / rV virus strain as a basic virus strain and a 98th site of structural protein VP2 is mutated into phenylalanine. According to the preparation method of therecombinant virus, point mutation S93F and point mutation Y98F are introduced into the VP2 at the same time; however, the Y98F is stably kept in a cell passage process. The FMDV O / rV-3 containing VP2Y98F has good thermal stability and a good immune effect. A thermal mutation resisting strain O / rV containing the VP2 Y98F, which is constructed by the invention, can be used as a high-quality inactivated vaccine for producing seed virus; the content of effective antigens in the inactivated vaccine is remarkably improved, the immune effect of the inactivated vaccine is increased and the inactivated vaccine is used for effectively preventing and controlling foot-and-mouth diseases of China.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Application of yeast dextran injection for improving immune efficiency of foot-and-mouth disease vaccine of dairy cow
InactiveCN102048686AIncreased resistance to FMD infectionEnhance humoral immunityOrganic active ingredientsPharmaceutical delivery mechanismYeastBiotechnology
The invention discloses an application of a yeast dextran injection for improving the immune efficiency of a foot-and-mouth disease vaccine of a dairy cow. Simultaneously, the invention also discloses an application of the yeast dextran injection for enhancing the cellular immune function of the dairy cow, wherein the yeast dextran injection is a 2%-6% carboxymethyl yeast dextran water solution. The yeast dextran injection prepared by the invention is safe, does not have adverse reaction, can effectively enhance the cellular immune function of the dairy cow organism and improve the antibody level of the dairy cow inoculated with an foot-and-mouth disease inactivated vaccine, and can enhance the disease resistance of animals.
Owner:天津市农业科学院
Attenuated vector bacterium of salmonella choleraesuls and construction method of attenuated vector bacterium
InactiveCN106754594AReduce usageHas attenuated safety featuresBacteriaMicroorganism based processesBacteroidesAntibiotic Y
The invention provides an attenuated vector bacterium of salmonella choleraesuls and a construction method of the attenuated vector bacterium and belongs to the technical field of animal bacterium genetic engineering. The attenuated vector bacterium is C78-3 salmonella choleraesuls without deltamanA, deltacrp::TT araC PBAD, deltarelA:: araC PBAD lacI TT, deltasoB and deltaasdA genes and is named as rSC0016. The method for taking a suicide vector without a resistance marker as a salmonella choleraesuls constructing vector is adopted and the utilization of the vector marked with antibiotics can be avoided; the vector can be safely used in clinical practice; furthermore, a balanced lethal system carries an exogenous antigen and can guarantee that only vaccine strains carrying the exogenous antigen can be used for producing vaccines and enter a host and survive, so that the immunity efficiency of the vaccines is improved.
Owner:YANGZHOU UNIV
Blood serum 5 type haemophilus parasuis and application thereof
InactiveCN104388340ABiological performance is stableStrong pathogenicityAntibacterial agentsBacteriaBlood serumWild strain
The invention provides a blood serum 5 type haemophilus parasuis strain JX1002. The blood serum 5 type haemophilus parasuis strain JX1002 is collected in China Center for Type Culture Collection (CCTCC), and the collection number is CCTCC M 2014127. The strain provided by the invention is stable in biological performance, has very strong pathogenicity to piglets, and has very good immunogenicity when the strain is inoculated to the piglets after inactivation. A monovalent vaccine prepared by using the strain as a vaccine candidate strain is good in safety, can be used for generating relatively high antibodies to the piglets, is long in duration, can be used for resisting the attack of wild strains with the same type, and has very good immune efficiency. An inactivated vaccine can be used for effectively reducing the occurrence of diseases and reducing the economic loss of a pig farm in clinical use, and the immune effect of the inactivated vaccine can reach or exceed that of existing commercial vaccines in markets.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com