Cationic liposome and preparation method thereof
A cationic liposome and cationic technology, applied in liposome delivery, pharmaceutical formulations, antibody medical components, etc., can solve problems such as retention, reduced immune efficacy of liposome vaccines, and inability to effectively enter lymphatic organs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Provide the preparation method of the above-mentioned cationic liposome of one embodiment below, comprise the steps:
[0054] S10, providing polyethylene glycol derivatized phospholipids, linking mannose or mannoside to the polyethylene glycol derivatized phospholipids to obtain polyethylene glycol derivatized phospholipids modified with mannose or mannan oligosaccharides.
[0055] Polyethylene glycol derivatized phospholipids can be purchased directly, obtained by linking one end of polyethylene glycol (PEG) to phospholipids and amination of the other end.
[0056] In this embodiment, one end of polyethylene glycol is connected with distearoylphosphatidylethanolamine (DSPE), which is denoted as DSPE-PEG. In other embodiments, one end of polyethylene glycol can also be selected to be connected to other types of phospholipids, which can be any one of the above-mentioned cationic lipids or neutral phospholipids.
[0057] Mannose or mannan oligosaccharides are linked to t...
Embodiment 1
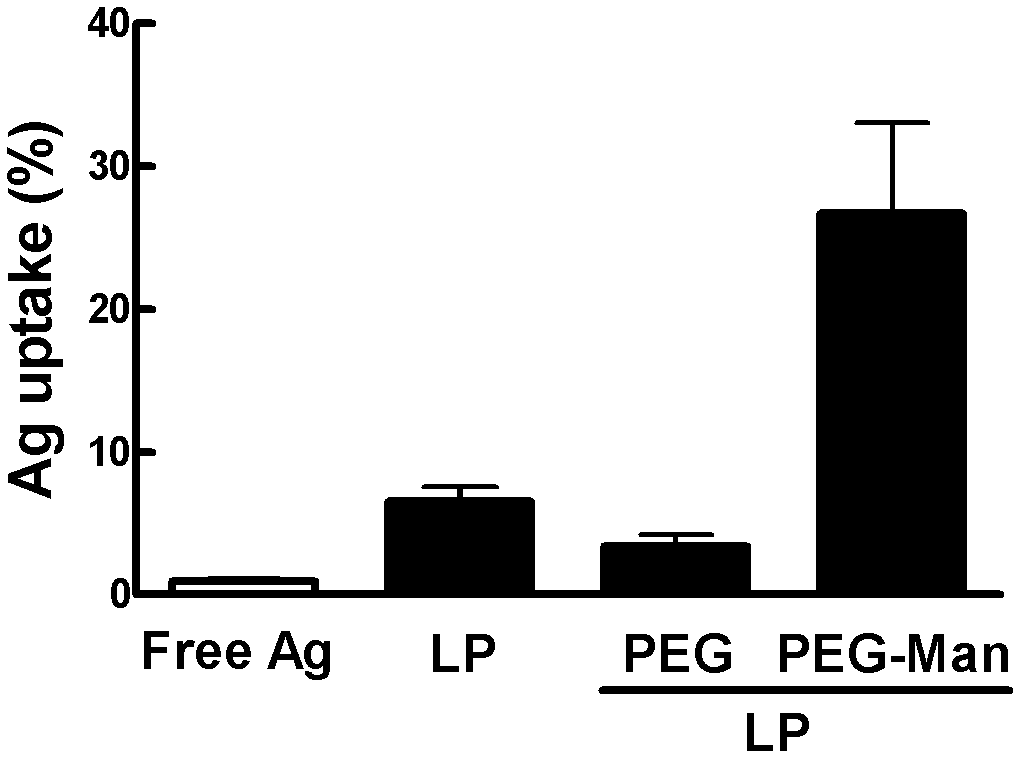
[0066] D-mannose was linked to DSPE-PEG2000 through aldehyde-amino condensation to obtain D-mannose-modified DSPE-PEG2000. DOTAP, DOPC and D-mannose-modified DSPE-PEG2000 were respectively dissolved in a mixed solvent of chloroform and methanol with a volume ratio of 2:1, and then the D-mannose-modified The ratio of the moles of DSPE-PEG2000 to the total moles of DOTAP and DOPC is 1:50, and the three are mixed to obtain a mixed solution, which is then placed in a round-bottomed flask. Rotate and dry the mixture with a steady nitrogen flow to form a uniform film. After vacuum drying overnight, add PBS buffer containing antigen protein BSA-FITC and place it at 4°C for hydration, and then ultrasonic water bath for 10min The polycarbonate membrane was extruded twice to obtain cationic liposomes coated with BSA-FITC, denoted as LP-PEG-Man.
Embodiment 2
[0068]Fucose was linked to DSPE-PEG2000 through aldehyde-amino condensation to obtain fucose-modified DSPE-PEG2000. DDAB, DOPC and fucose-modified DSPE-PEG2000 were respectively dissolved in a mixed solvent of chloroform and methanol with a volume ratio of 2:1, and then according to the molar ratio of DDAB and DOPC at 7:3, the fucose-modified DSPE-PEG2000 The ratio of the number of moles of PEG2000 to the total number of moles of DDAB and DOPC is 2:25, and the three are mixed to obtain a mixed solution, which is then placed in a round-bottomed flask. Rotate and dry the mixture with a stable helium flow to form a uniform film. After vacuum drying overnight, add PBS buffer containing antigenic protein BSA-FITC the next day and place it at 4°C for hydration, then ultrasonic water bath for 10min The polycarbonate membrane was extruded twice to obtain cationic liposomes coated with BSA-FITC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com