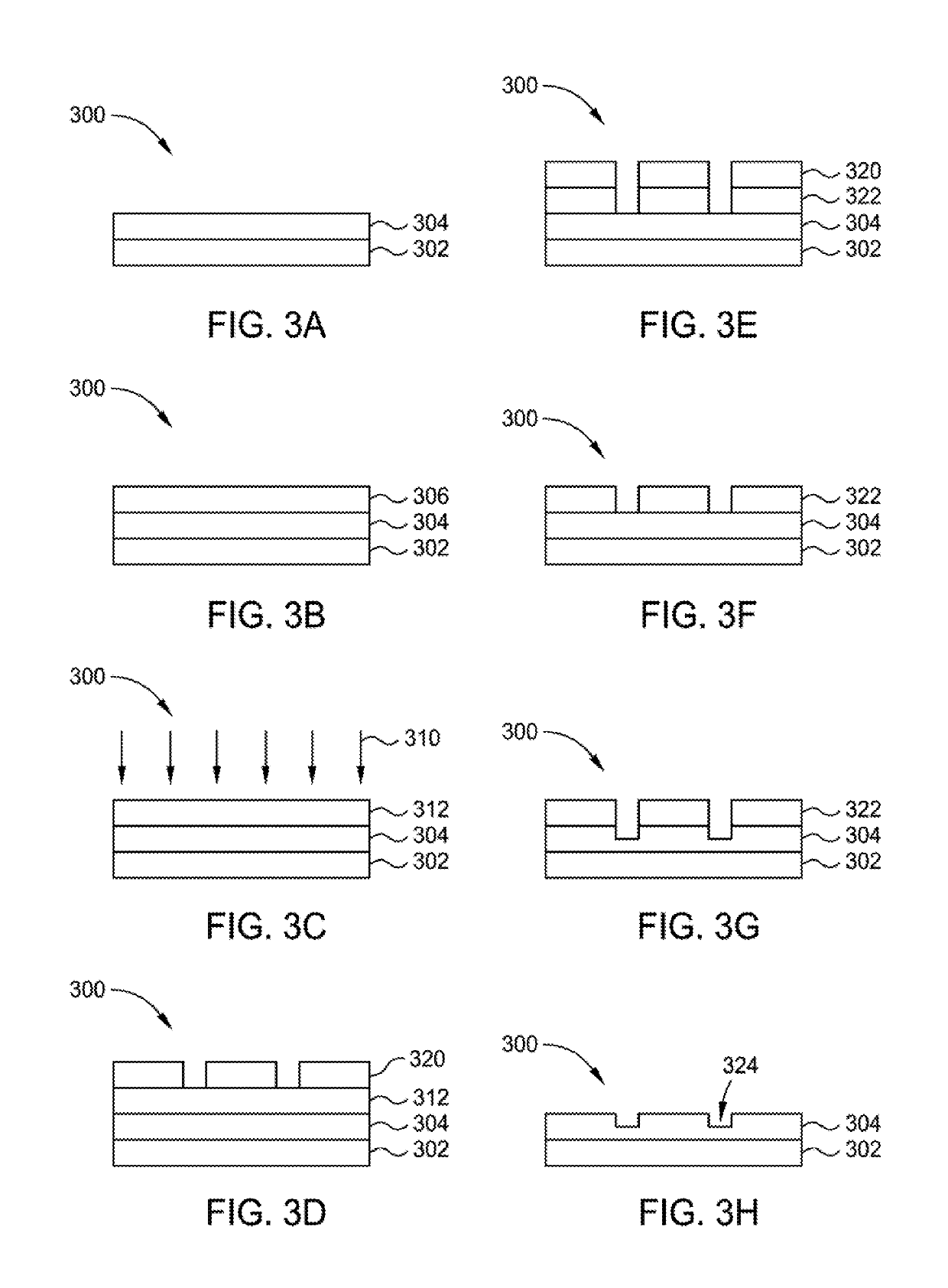
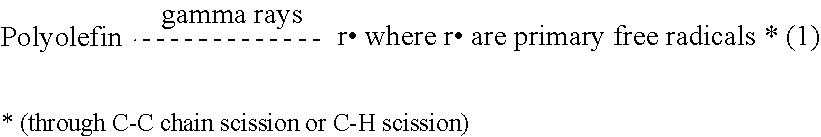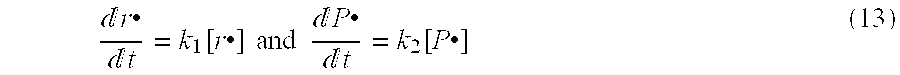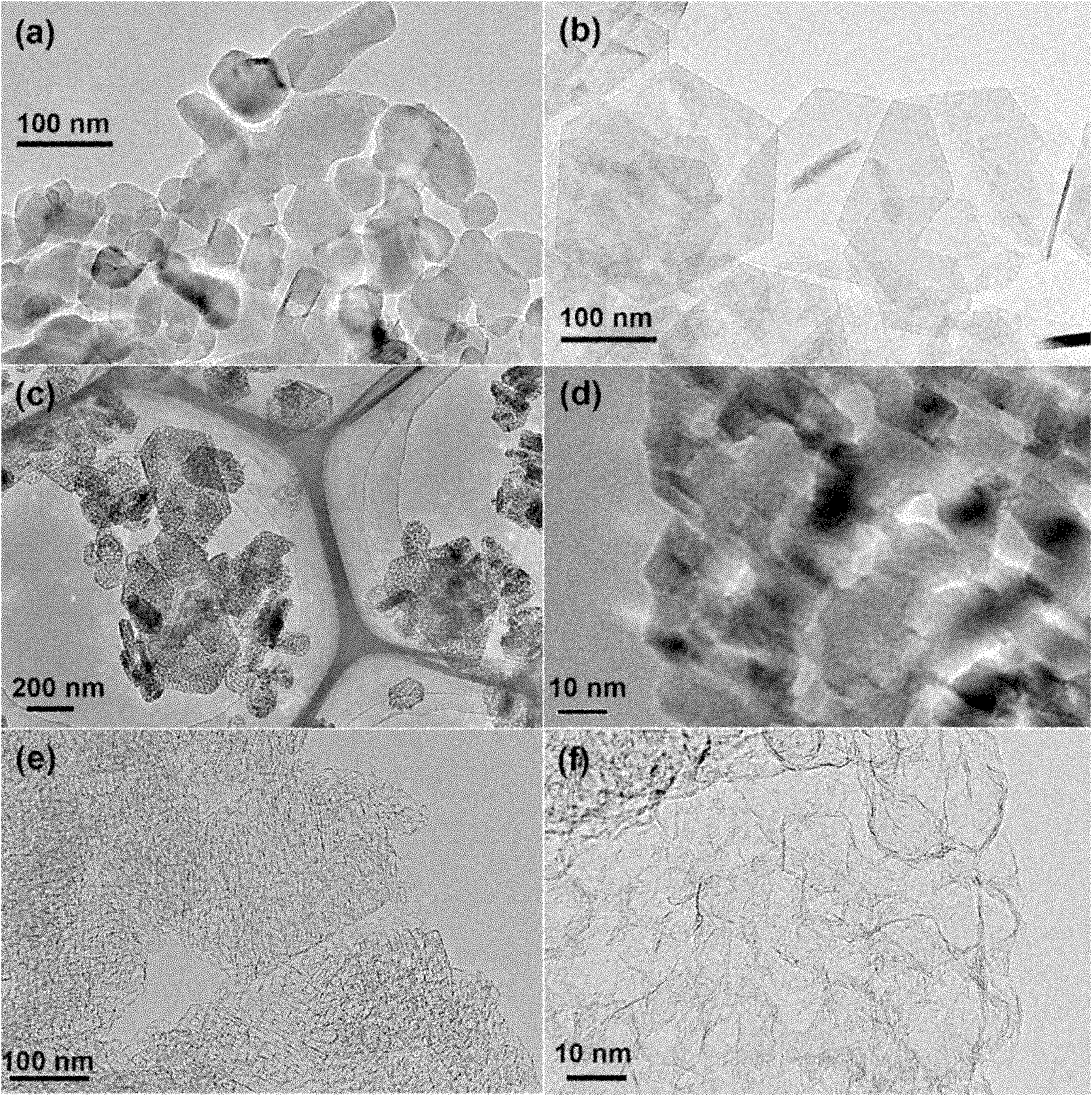Patents
Literature
2818 results about "Helium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Helium (from Greek: ἥλιος, romanized: Helios, lit. 'Sun') is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas, the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the elements. Helium is the second lightest and second most abundant element in the observable universe (hydrogen is the lightest and most abundant). It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and in Jupiter. This is due to the very high nuclear binding energy (per nucleon) of helium-4, with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. Most helium in the universe is helium-4, the vast majority of which was formed during the Big Bang. Large amounts of new helium are being created by nuclear fusion of hydrogen in stars.
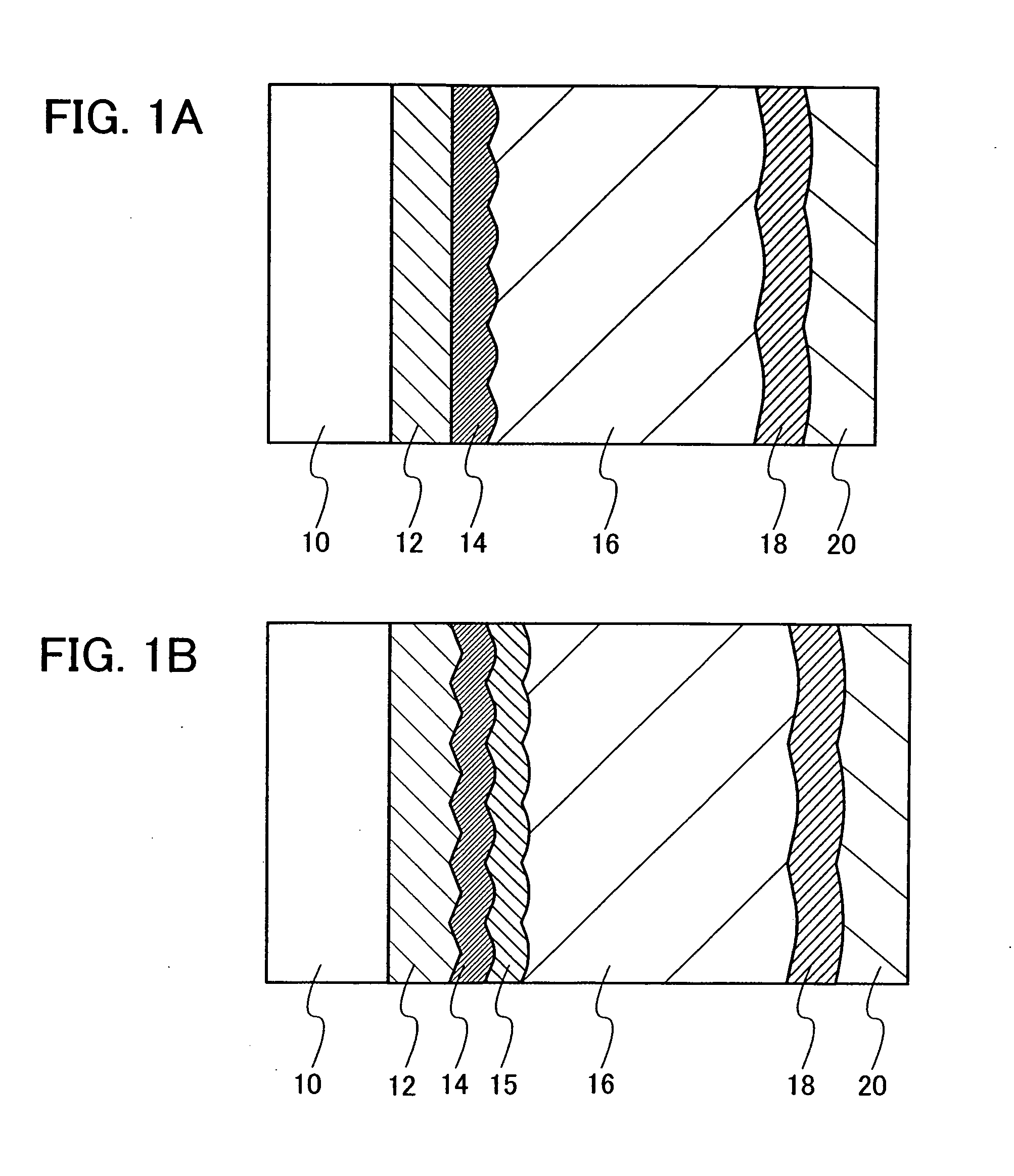
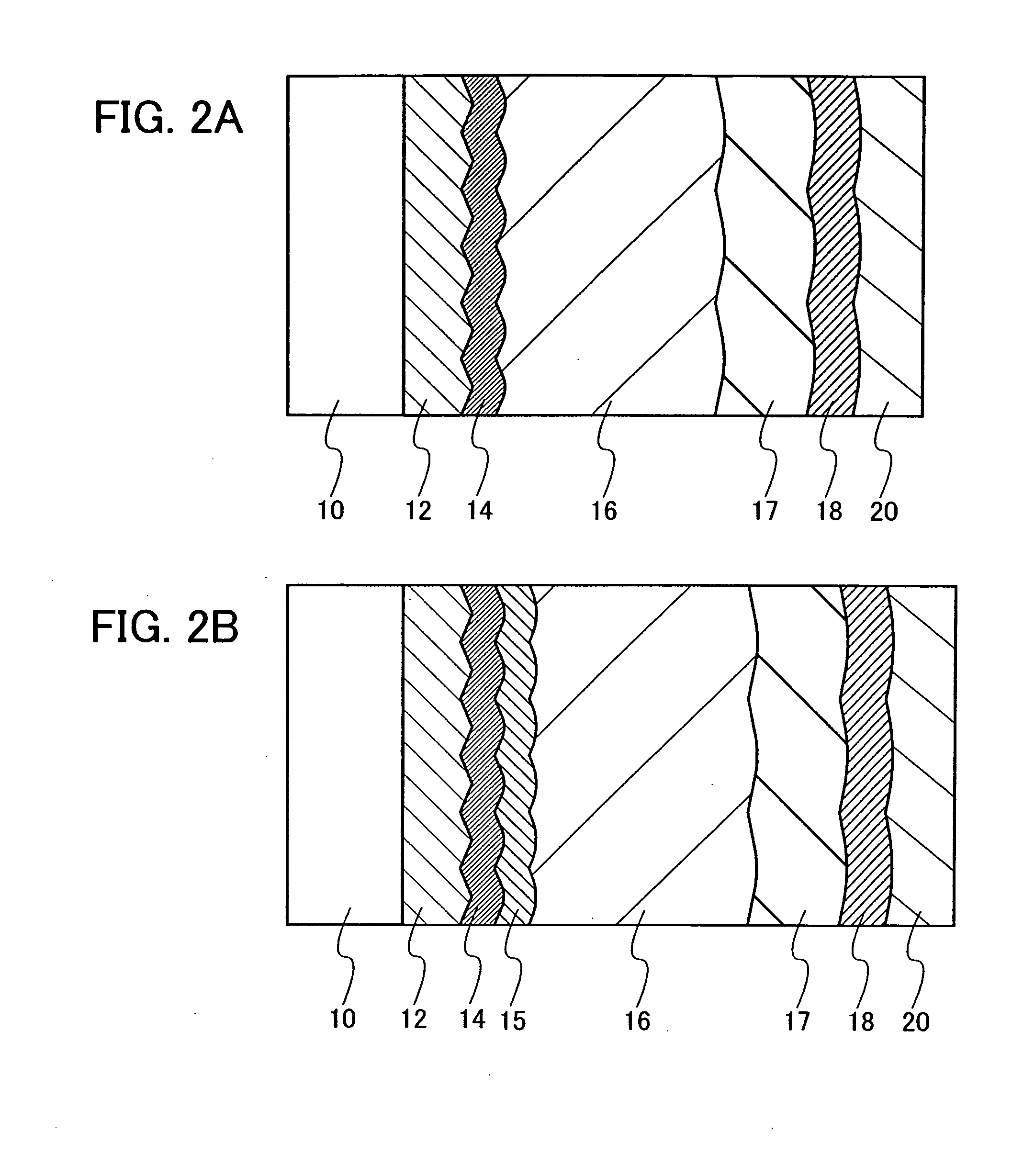
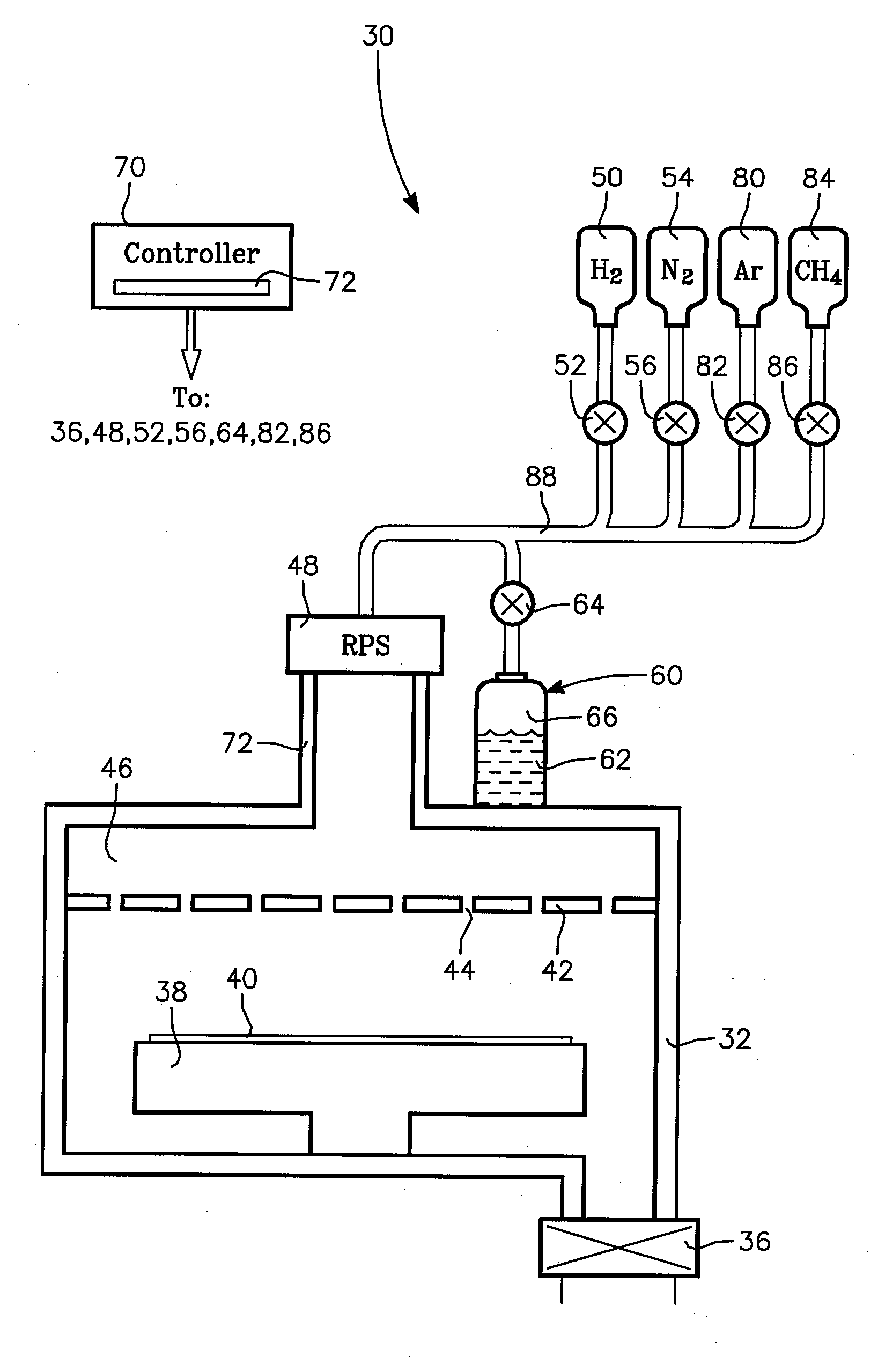
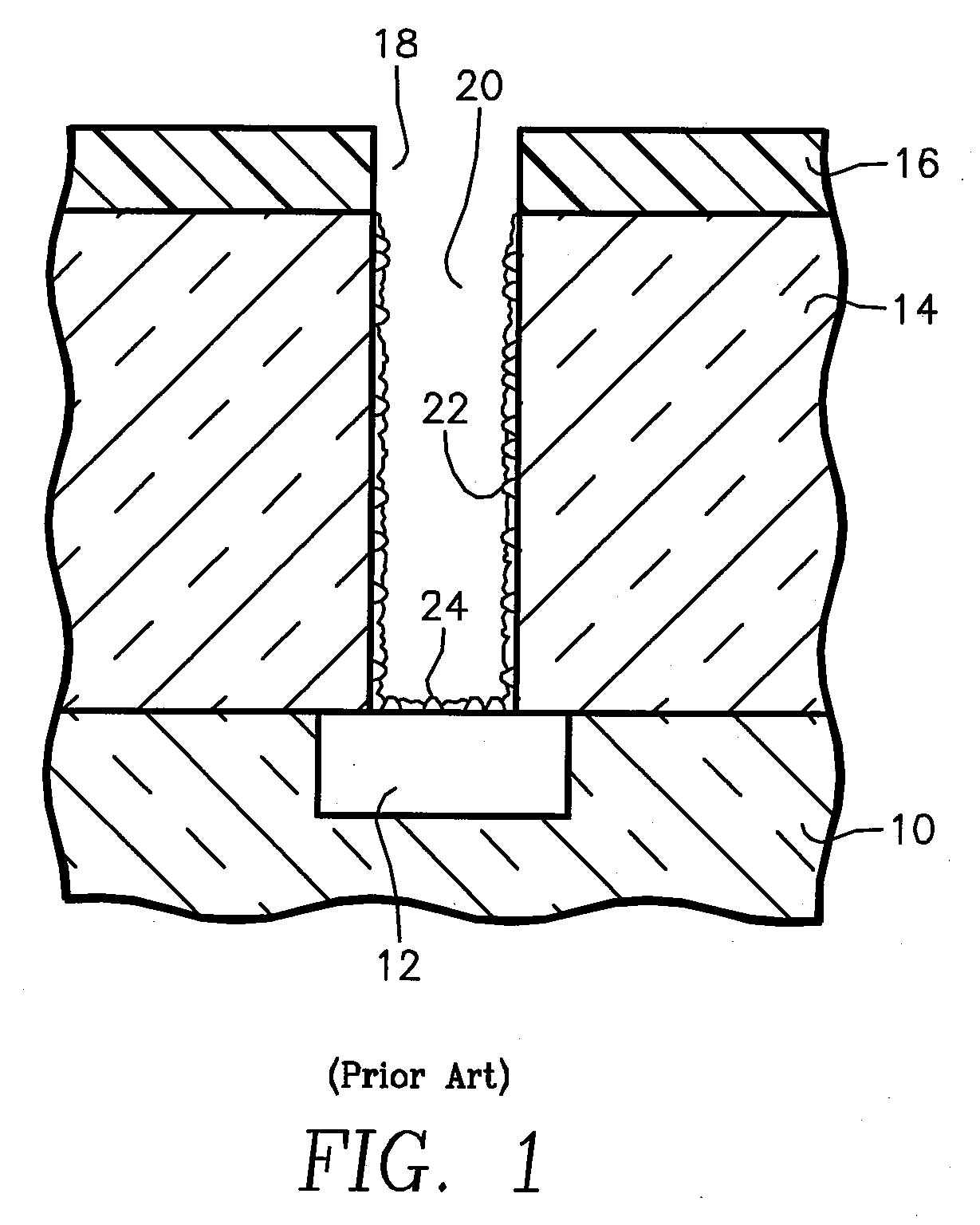
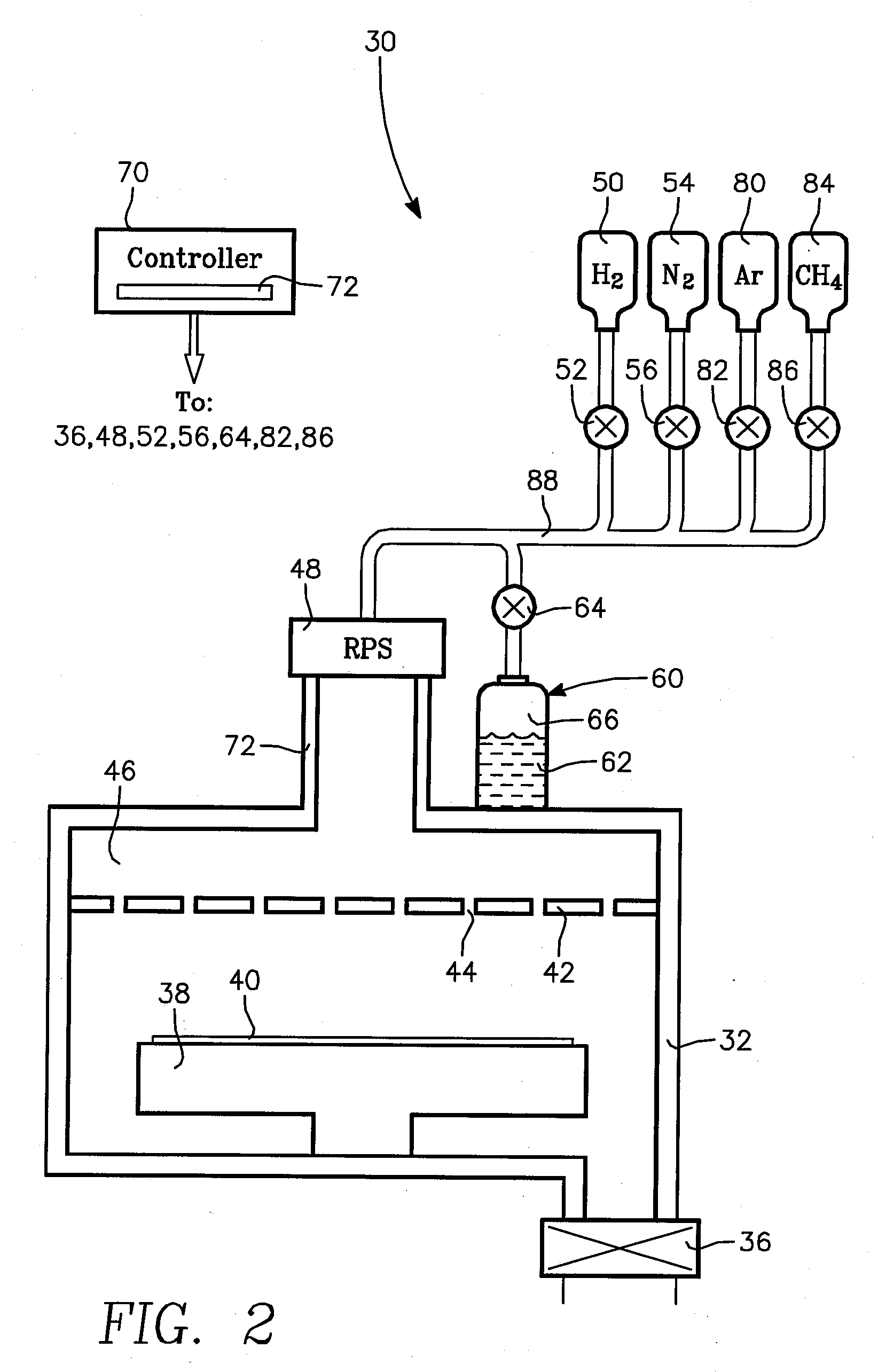
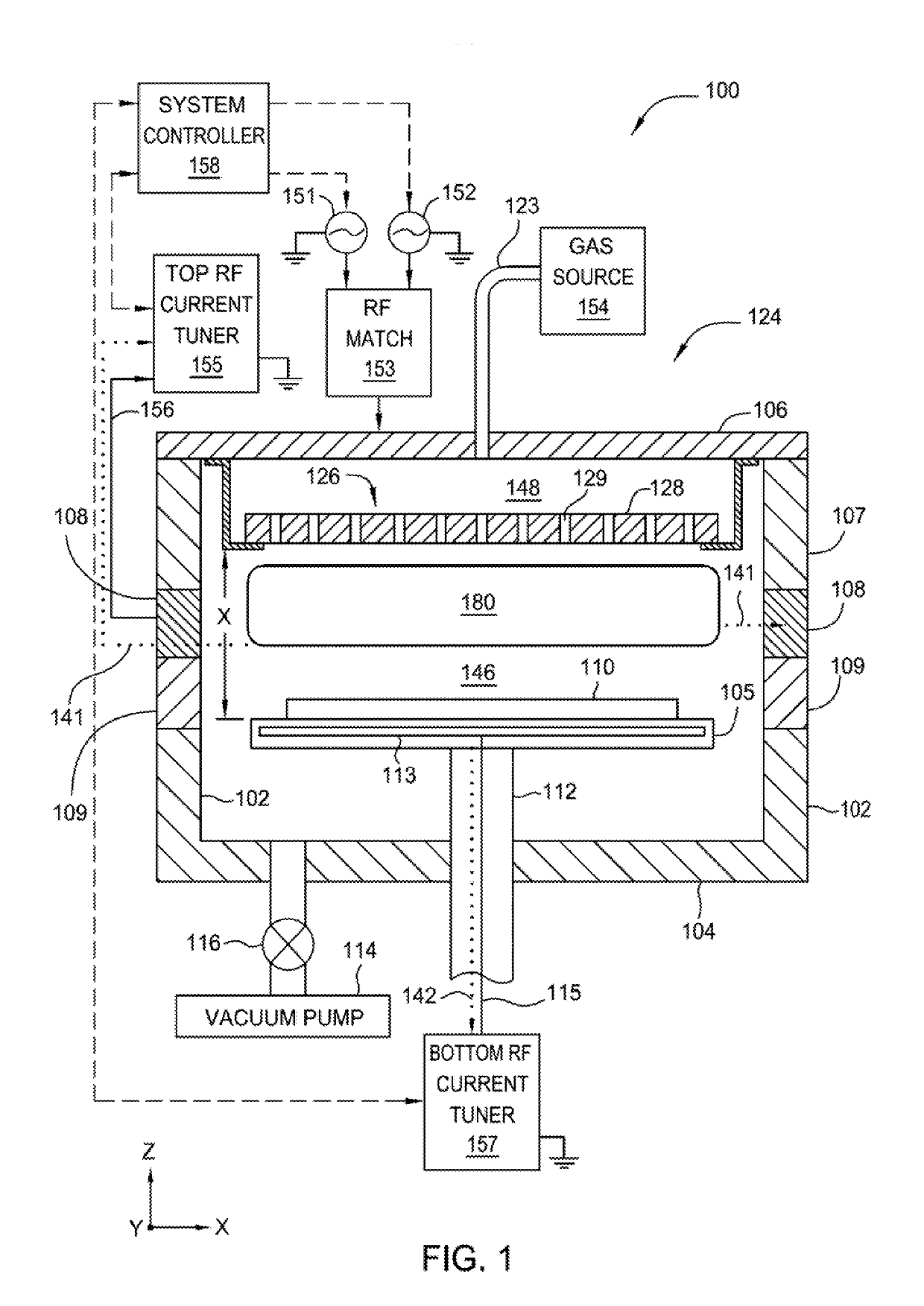
Method for Depositing Conformal Amorphous Carbon Film by Plasma-Enhanced Chemical Vapor Deposition (PECVD)
ActiveUS20100093187A1Good shape retentionHighly conformalSemiconductor/solid-state device manufacturingChemical vapor deposition coatingCarbon layerNitrogen gas
Methods and apparatus for depositing an amorphous carbon layer on a substrate are provided. In one embodiment, a deposition process includes positioning a substrate in a substrate processing chamber, introducing a hydrocarbon source having a carbon to hydrogen atom ratio of greater than 1:2 into the processing chamber, introducing a plasma initiating gas selected from the group consisting of hydrogen, helium, argon, nitrogen, and combinations thereof into the processing chamber, with the hydrocarbon source having a volumetric flow rate to plasma initiating gas volumetric flow rate ratio of 1:2 or greater, generating a plasma in the processing chamber, and forming a conformal amorphous carbon layer on the substrate.
Owner:APPLIED MATERIALS INC
Method for manufacturing photoelectric conversion device
InactiveUS20090029503A1Quality improvementReduce deterioration rateFinal product manufactureSemiconductor/solid-state device manufacturingProduction rateMicrowave
To form a microcrystalline semiconductor with high quality which can be directly formed at equal to or less than 500° C. over a large substrate with high productivity without decreasing a deposition rate. In addition, to provide a photoelectric conversion device which employs the microcrystalline semiconductor as a photoelectric conversion layer. A reactive gas containing helium is supplied to a treatment chamber which is surrounded by a plurality of juxtaposed waveguides and a wall, the pressure in the treatment chamber is maintained at an atmospheric pressure or a subatmospheric pressure, microwave is supplied to a space sandwiched between the juxtaposed waveguides to generate plasma, and a photoelectric conversion layer of a microcrystalline semiconductor is deposited over a substrate which is placed in the treatment chamber.
Owner:SEMICON ENERGY LAB CO LTD
Hydrogen ashing enhanced with water vapor and diluent gas
ActiveUS20080261405A1Decorative surface effectsSemiconductor/solid-state device detailsWater vaporOxygen
An oxygen-free hydrogen plasma ashing process particularly useful for low-k dielectric materials based on hydrogenated silicon oxycarbide materials. The main ashing step includes exposing a previously etched dielectric layer to a plasma of hydrogen and optional nitrogen, a larger amount of water vapor, and a yet larger amount of argon or helium. Especially for porous low-k dielectrics, the main ashing plasma additionally contains a hydrocarbon gas such as methane. The main ashing may be preceded by a short surface treatment by a plasma of a hydrogen-containing reducing gas such as hydrogen and optional nitrogen.
Owner:APPLIED MATERIALS INC
Methods of Forming Integrated Circuit Devices Having Ion-Cured Electrically Insulating Layers Therein
ActiveUS20090098706A1Quality improvementDegree of reductionSemiconductor/solid-state device manufacturingAtomic orderEngineering
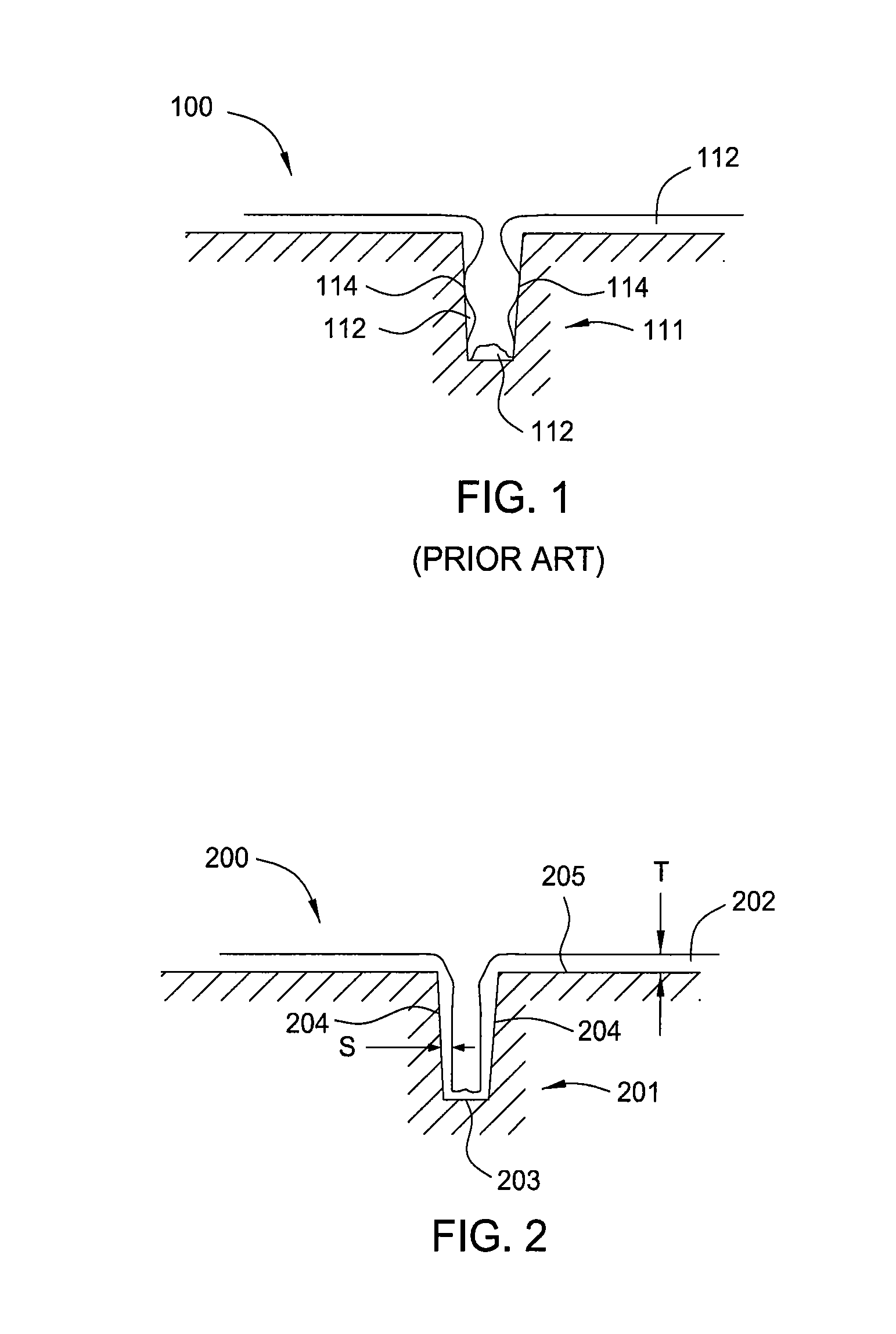
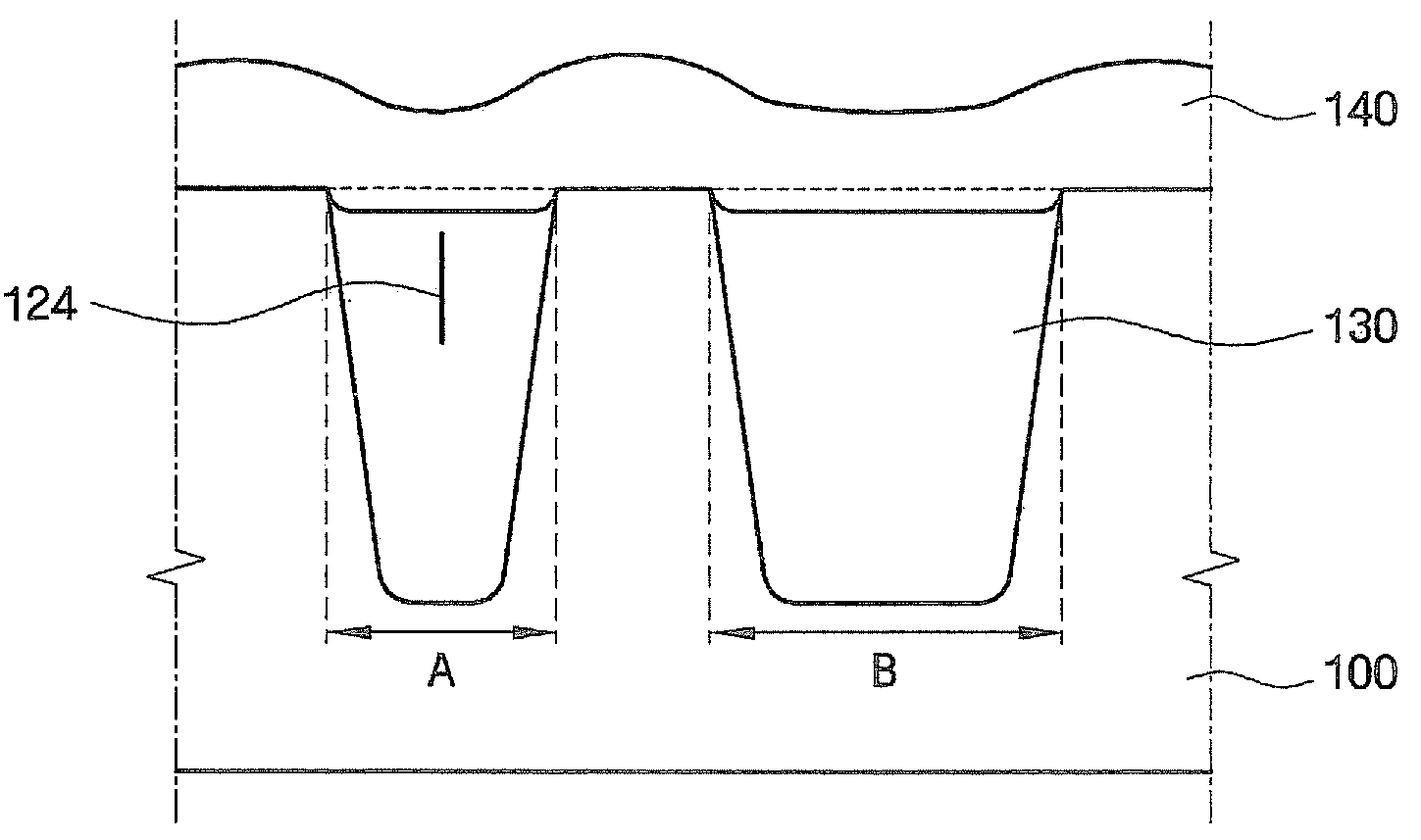
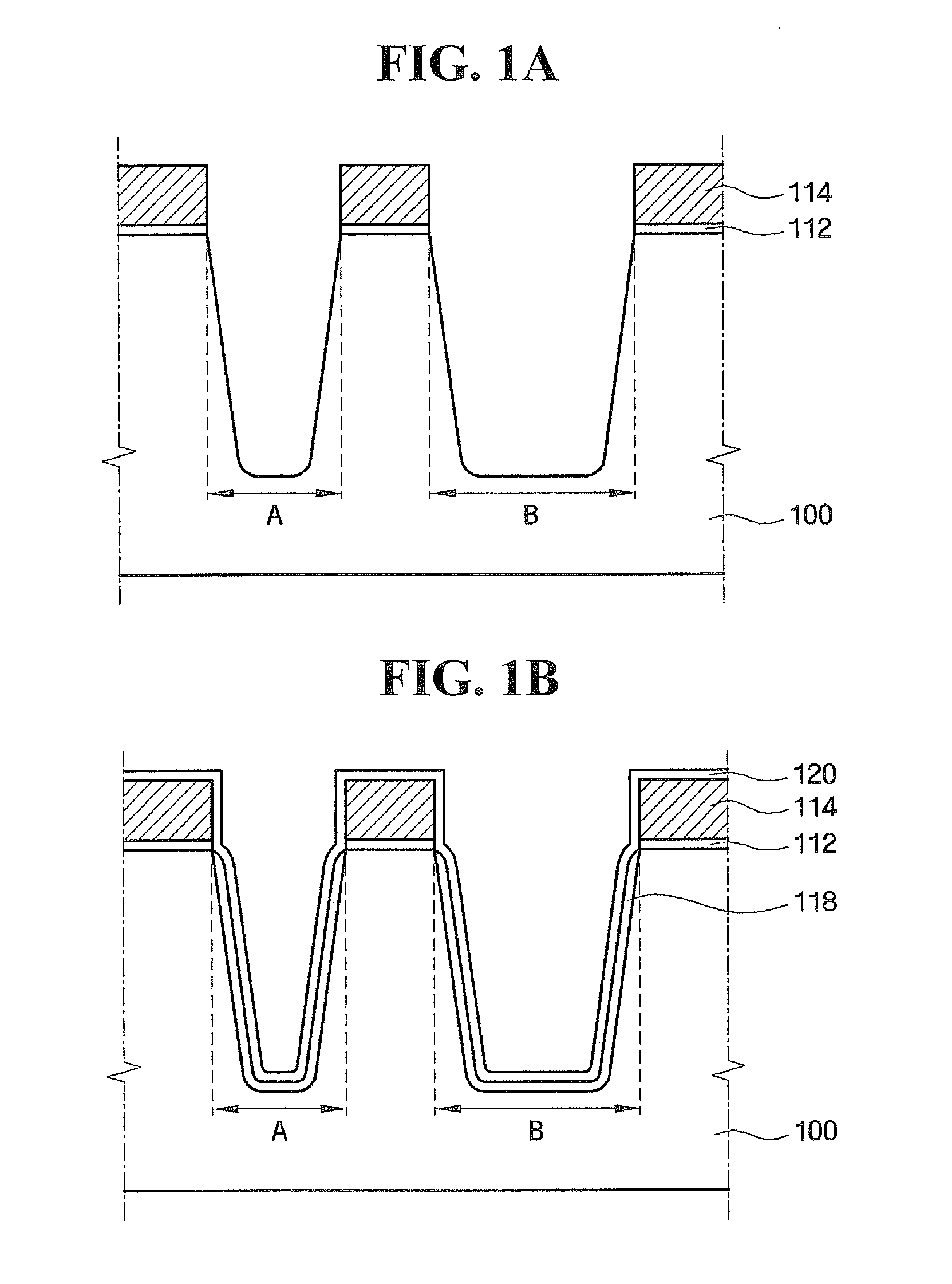
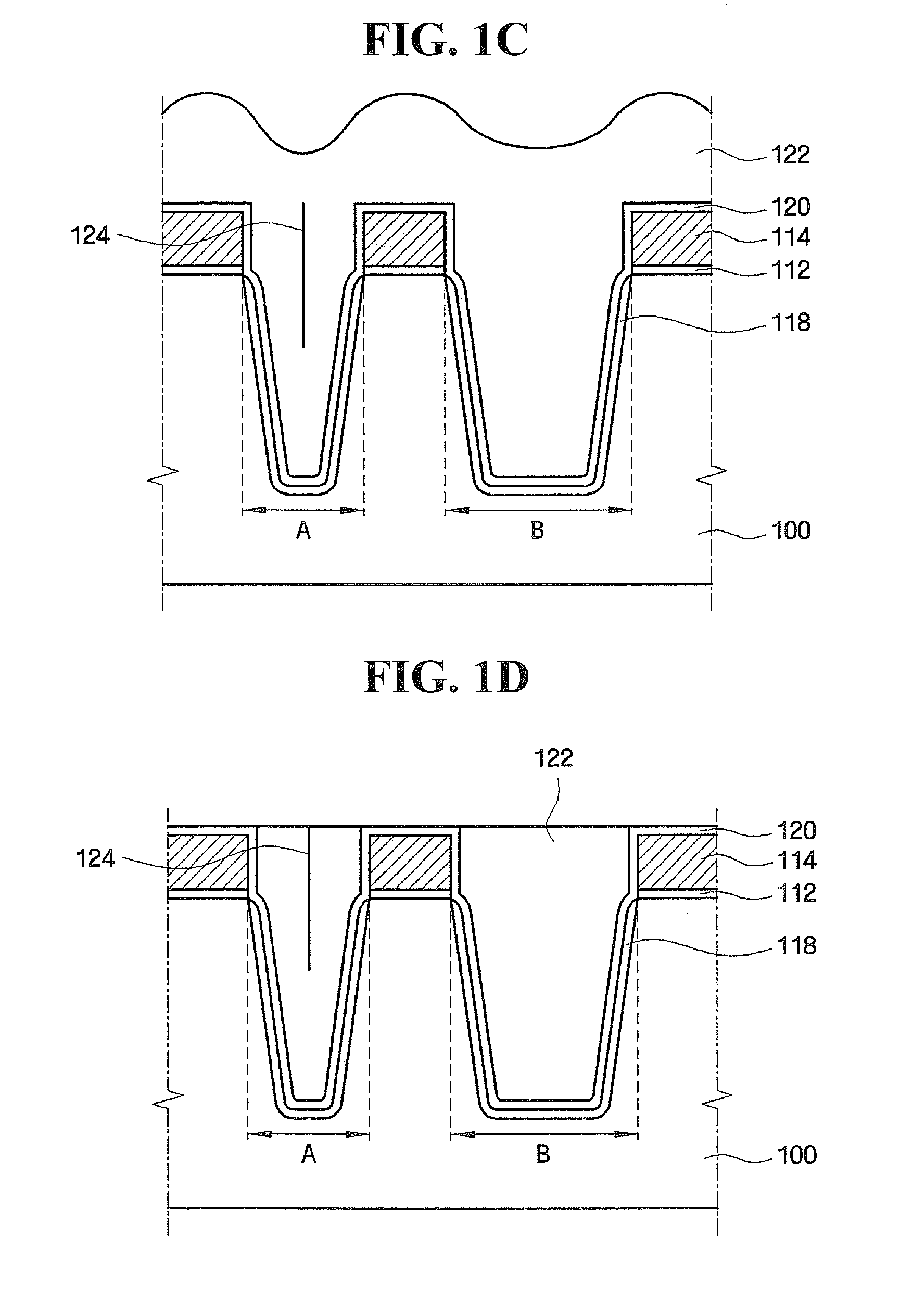
Methods of forming integrated circuit devices include forming a trench in a surface of semiconductor substrate and filling the trench with an electrically insulating region having a seam therein. The trench may be filled by depositing a sufficiently thick electrically insulating layer on sidewalls and a bottom of the trench. Curing ions are then implanted into the electrically insulating region at a sufficient energy and dose to reduce a degree of atomic order therein. The curing ions may be ones selected from a group consisting of nitrogen (N), phosphorus (P), boron (B), arsenic (As), carbon (C), argon (Ar), germanium (Ge), helium (He), neon (Ne) and xenon (Xe). These curing ions may be implanted at an energy of at least about 80 KeV and a dose of at least about 5×1014 ions / cm2. The electrically insulating region is then annealed at a sufficient temperature and for a sufficient duration to increase a degree of atomic order within the electrically insulating region.
Owner:IBM CORP +2
Methods and apparatus for semiconductor etching including an electro static chuck
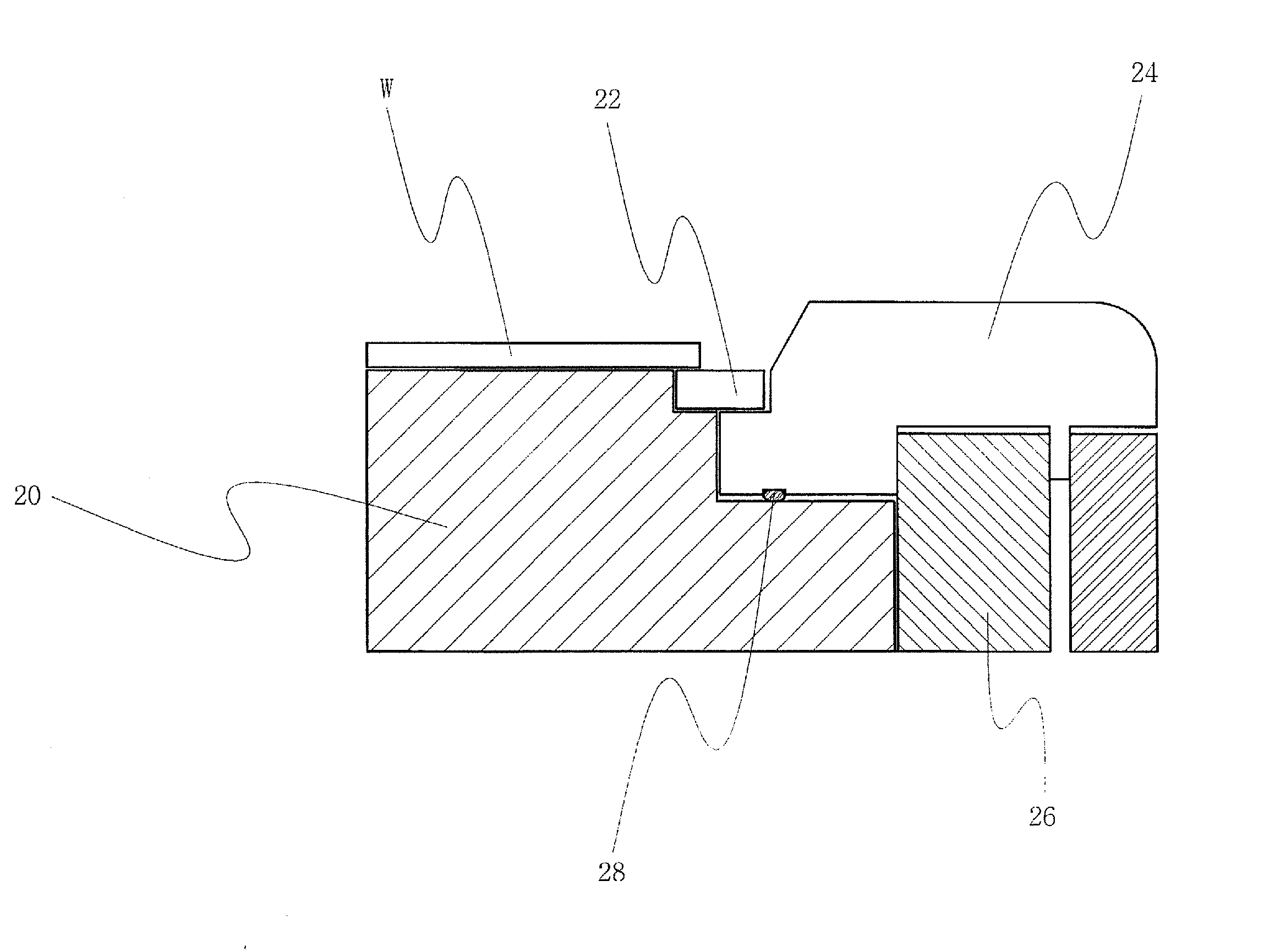
InactiveUS20080194113A1Preventing failure of etching processElectric discharge tubesSemiconductor/solid-state device manufacturingHeliumSemiconductor
There is provided a semiconductor etching apparatus which removes particles remaining on the upper surface of an electro static chuck (ESC) during an etching process, thereby preventing a chucking force from decreasing and minimizing a leak of helium. To prevent a failure of the etching process due to a wafer chucking failure, by preventing polymers from falling down on the upper part of the ESC when a wafer is dechucked or transferred, the semiconductor etching apparatus comprises: an ESC selectively holding a wafer to be entered and positioned inside a chamber, and including a lower electrode part to which RF power is applied; parts positioned at a stepped portion of the ESC and respectively surrounding a side of the ESC; and a gas flow blocking part blocking a gas flow in a vacuum path formed between the ESC and the parts.
Owner:SAMSUNG ELECTRONICS CO LTD
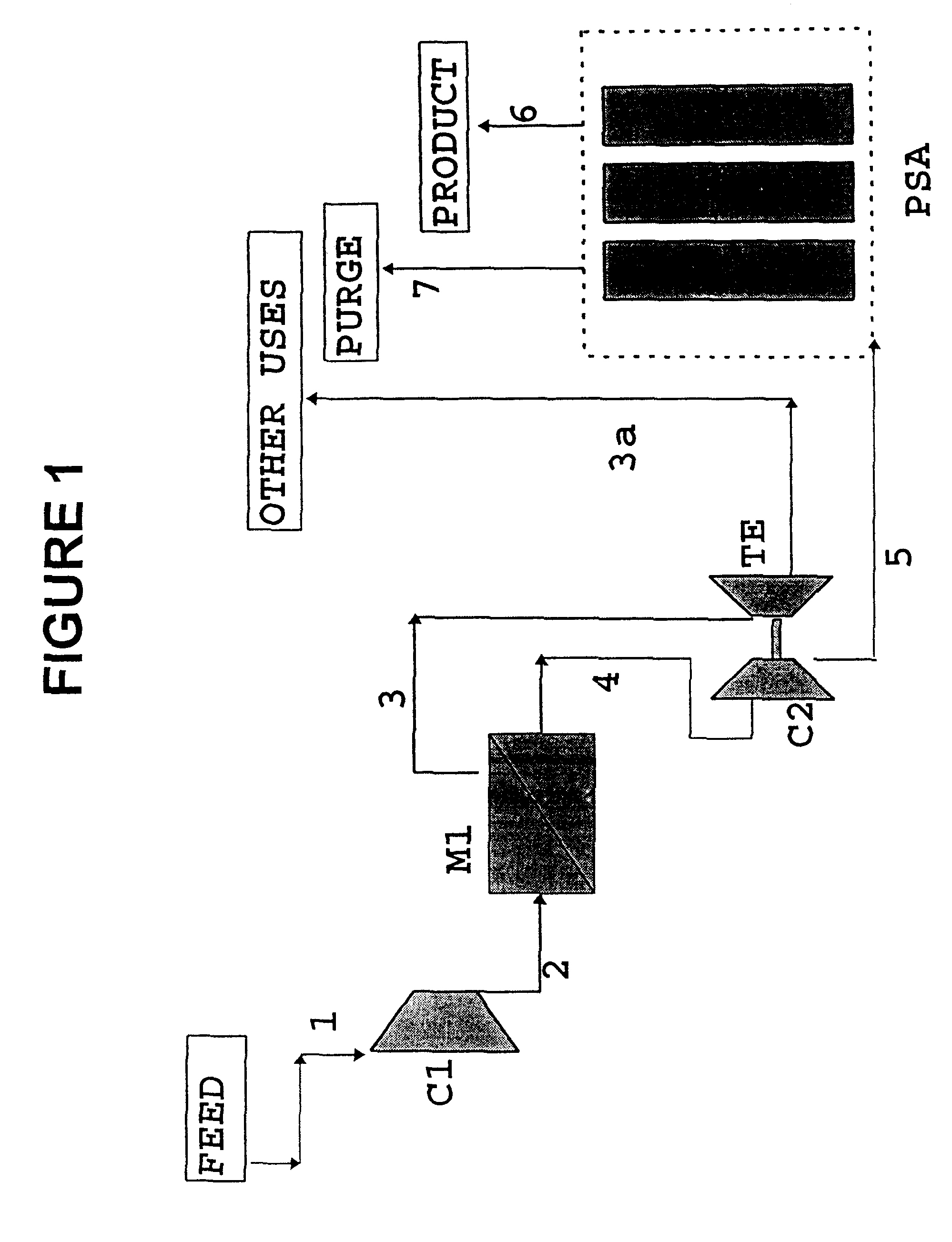
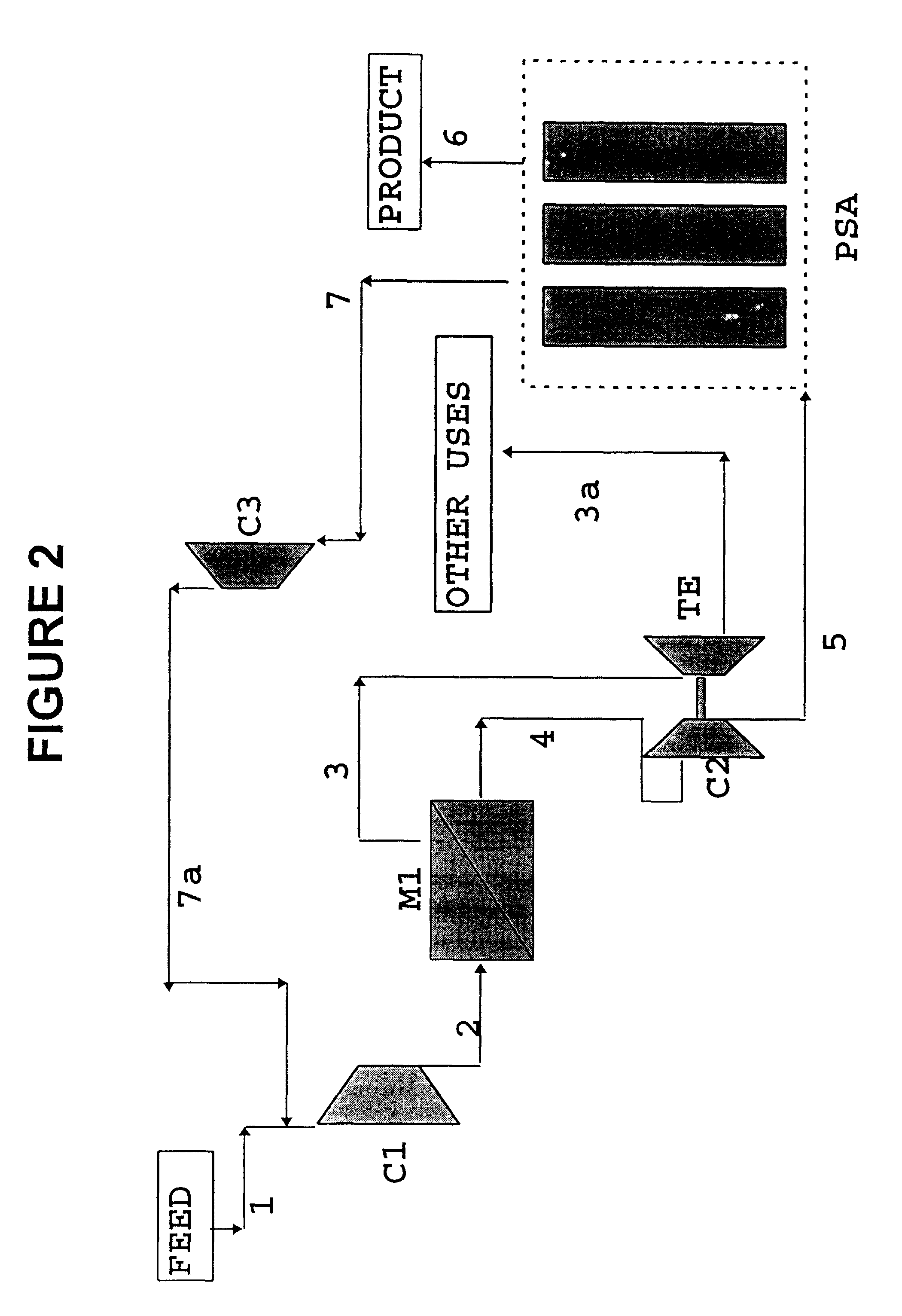
Process for the separation/recovery of gases
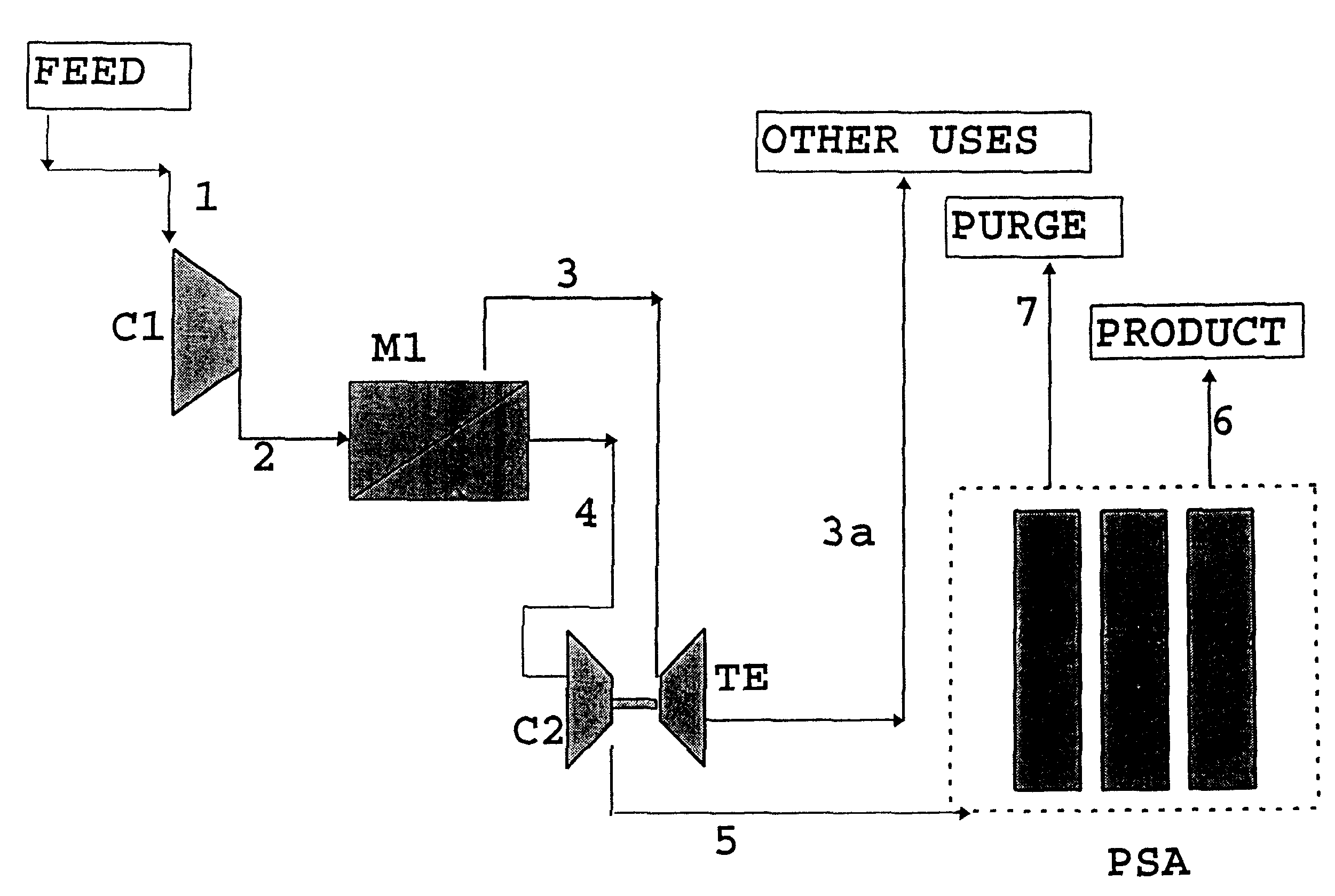
InactiveUS6179900B1Low costHigh purityNitrogen purification/separationGas treatmentHydrogenHigh pressure
A process for the separation / recovery of gases is described where the desired component to be separated from the gaseous mixture is present in low / very low molar amount and / or low / moderate pressure, the process comprising contacting the gaseous mixture with a separation membrane unit containing high permeability / medium selectivity membranes which separate the feed stream into a permeate stream, enriched in the desired component and at a lower pressure and a retentate stream lean in the desired component and at a higher pressure. The retentate stream is made to convey its pressure to the permeate stream which then is directed to an adsorption PSA unit for further purification of the permeate stream so that the effluent stream from the PSA unit which contains the purified component has a purity of up to 99.90%. The process is useful for separating hydrogen from refinery off gases such as FCC gases and can also be applied to other gases such as helium and any other valuable gases present in gaseous streams in low to very low molar contents and low to moderate pressures.
Owner:GKSS FORSCHUNGSZENTRUM GEESTHACHT GMBH +1
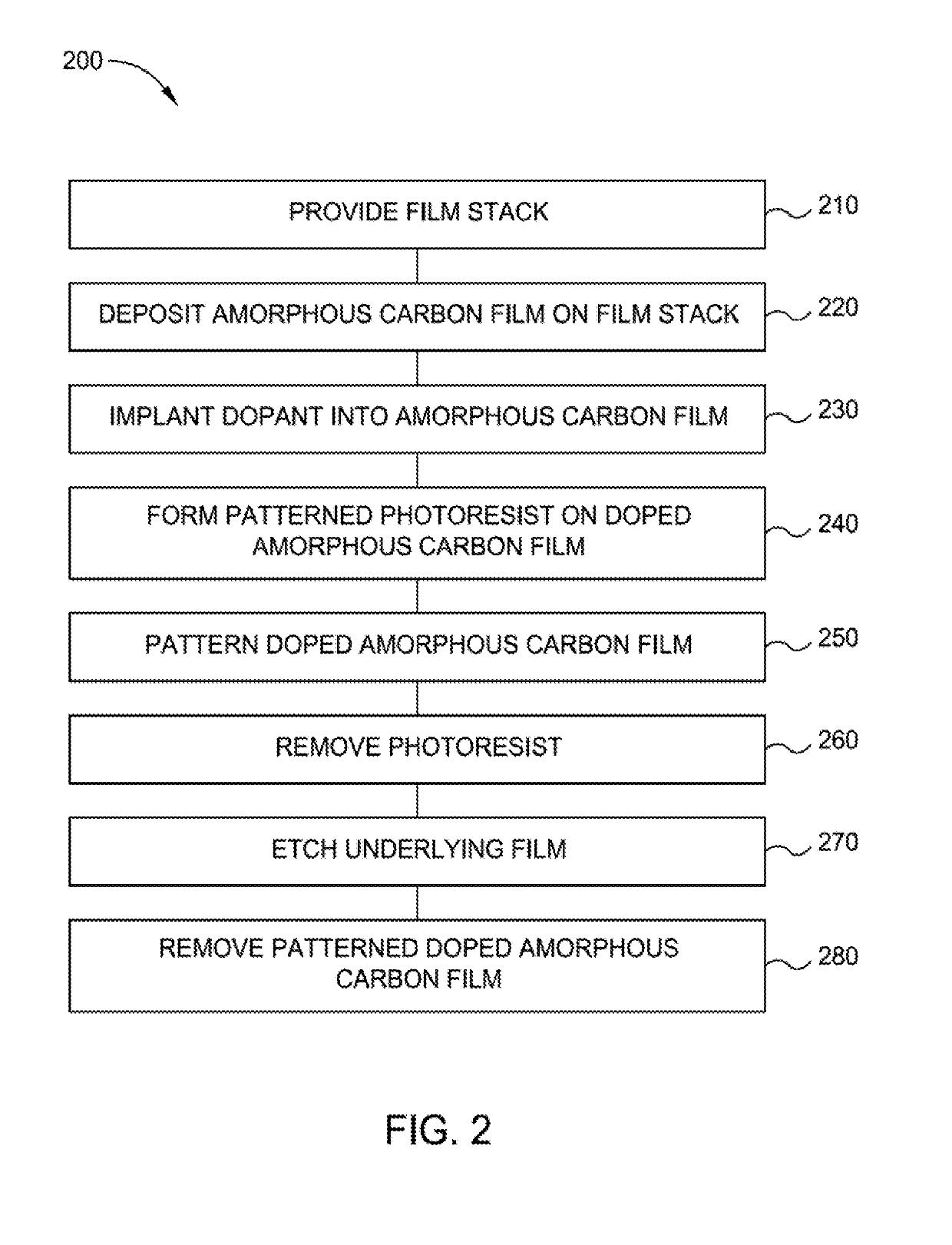
Highly etch selective amorphous carbon film
ActiveUS20190172714A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingKryptonDopant
Implementations described herein generally relate to the fabrication of integrated circuits. More particularly, the implementations described herein provide techniques for deposition of amorphous carbon films on a substrate. In one implementation, a method of forming an amorphous carbon film is provided. The method comprises depositing an amorphous carbon film on an underlayer positioned on a susceptor in a first processing region. The method further comprises implanting a dopant or inert species into the amorphous carbon film in a second processing region. The dopant or inert species is selected from carbon, boron, nitrogen, silicon, phosphorous, argon, helium, neon, krypton, xenon or combinations thereof. The method further comprises patterning the doped amorphous carbon film. The method further comprises etching the underlayer.
Owner:APPLIED MATERIALS INC
Non-oxidizing polymeric medical implant
InactiveUS20050059750A1Improve antioxidant capacityPrevent further oxidationSurgeryPharmaceutical containersCross-linkNitrogen gas
A medical implant made of polymeric material having an increased oxidation resistance is formed by a method including the steps of placing a resin powder in a sealed container. A substantial portion of the oxygen is removed from the sealed contained by either a vacuum, an oxygen absorbent or by flushing with inert gas. The container is then repressurized with a gas such as nitrogen, argon, helium or neon so that long term storage may be possible. On use, the resin in transferred to a forming device which both melts and forms the resin in an oxygen reduced atmosphere to produce a polymeric raw material such as a rod or bar stock. The medical implant is then formed from this raw material annealed and sealed in an airtight package in an oxygen reduced atmosphere. The implant is then radiation sterilized and thereafter annealed in the package for a predetermined time and temperature sufficient to form cross-links between any free radicals in neighboring polymeric chains.
Owner:HOWMEDICA OSTEONICS CORP
Sputter deposition and etching of metallization seed layer for overhang and sidewall improvement
ActiveUS20060030151A1High selectivitySolid-state devicesSemiconductor/solid-state device manufacturingEtchingElectrochemistry
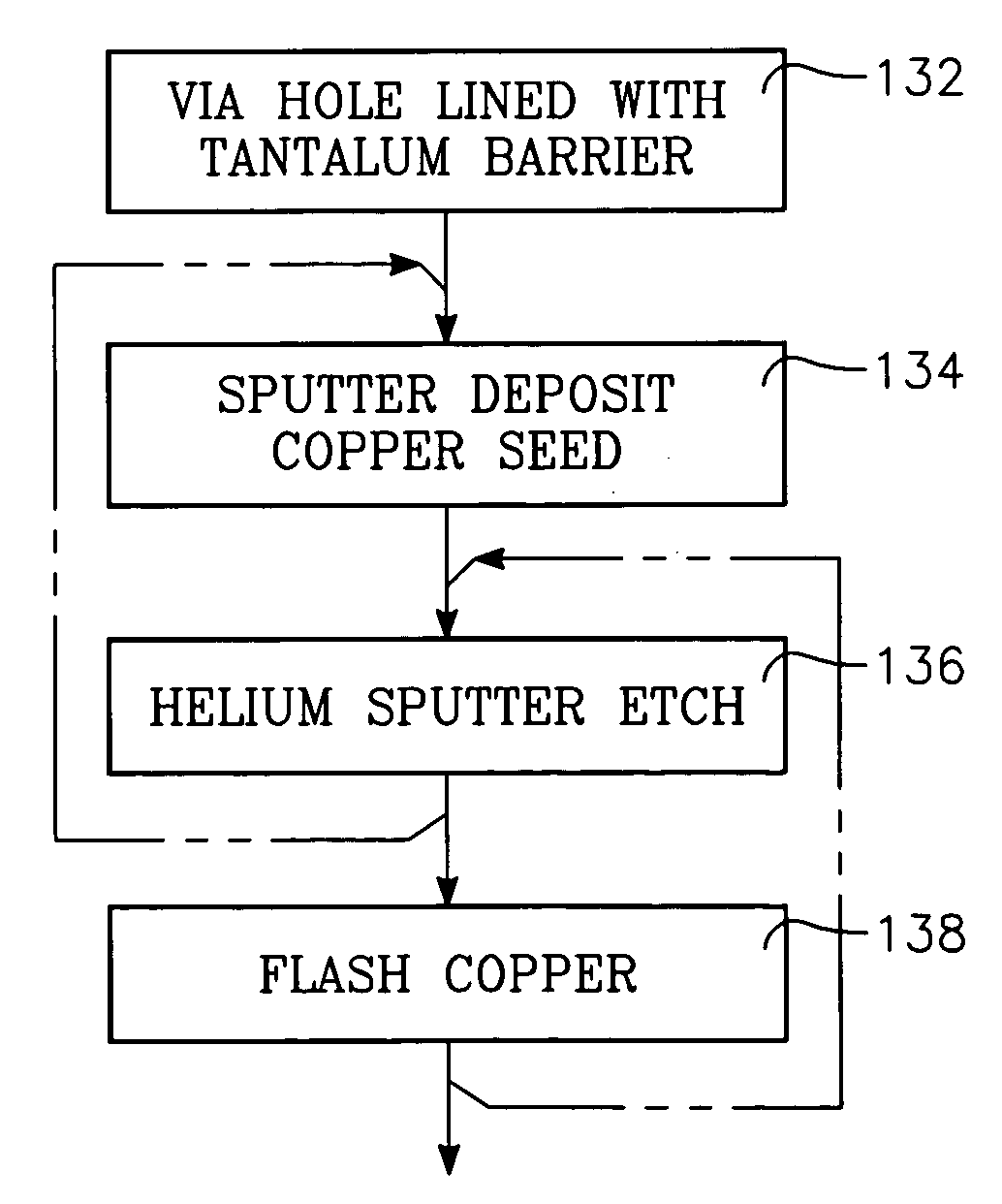
An integrated sputtering method and reactor for copper or aluminum seed layers in which a plasma sputter reactor initially deposits a thin conformal layer onto a substrate including a high-aspect ratio hole subject to the formation of overhangs. After the seed deposition, the same sputter reactor is used to sputter etch the substrate with energetic light ions, especially helium, having an energy sufficiently low that it selectively etches the metallization to the heavier underlying barrier layer, for example, copper over tantalum or aluminum over titanium. An RF inductive coil generates the plasma during the sputtering etching while the target power is turned off. A final copper flash step deposits copper over the bare barrier field region before copper is electrochemically plated to fill the hole. The invention also includes a simultaneous sputter deposition and sputter etch, and an energetic ion processing of the copper seed sidewall.
Owner:APPLIED MATERIALS INC
Hydrogen assisted hdp-cvd deposition process for aggressive gap-fill technology
InactiveUS20040146661A1Excellent gap fillingStrong Gap Filling CapabilityElectric discharge tubesVacuum evaporation coatingHigh densityHydrogen
Abstract of the Disclosure A method of depositing a silicon oxide layer over a substrate having a trench formed between adjacent raised surfaces. In one embodiment the silicon oxide layer is formed in a multistep process that includes depositing a first portion of layer over the substrate and within the trench by forming a high density plasma process that has simultaneous deposition and sputtering components from a first process gas comprising a silicon source, an oxygen source and helium and / or molecular hydrogen with high D / S ratio, for example, 10-20 and, thereafter, depositing a second portion of the silicon oxide layer over the substrate and within the trench by forming a high density plasma process that has simultaneous deposition and sputtering components from a second process gas comprising a silicon source, an oxygen source and molecular hydrogen with a lower D / S ratio of, for example, 3-10.
Owner:APPLIED MATERIALS INC
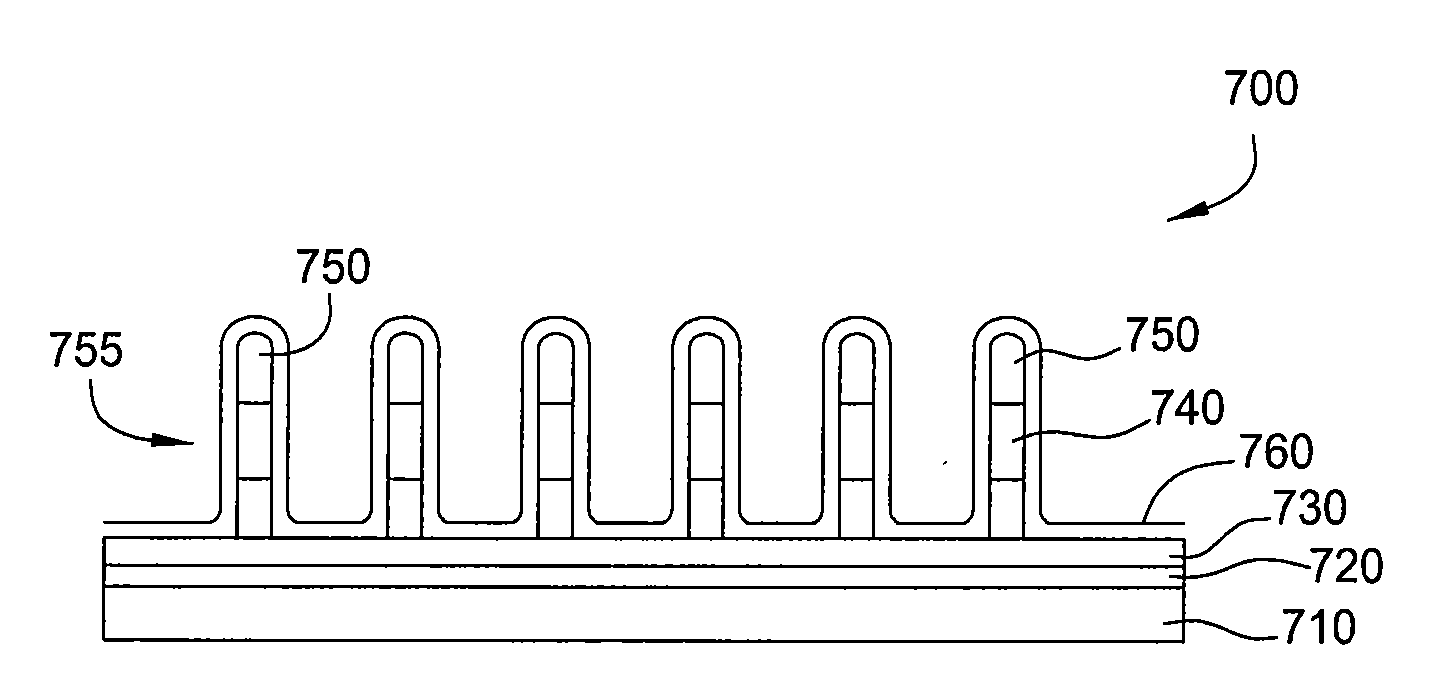
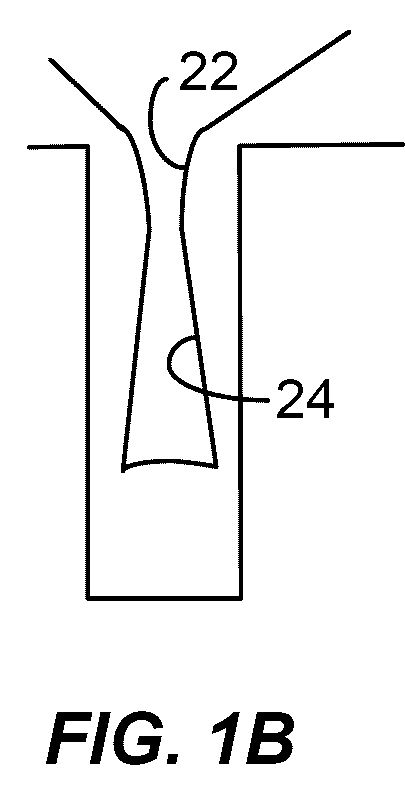
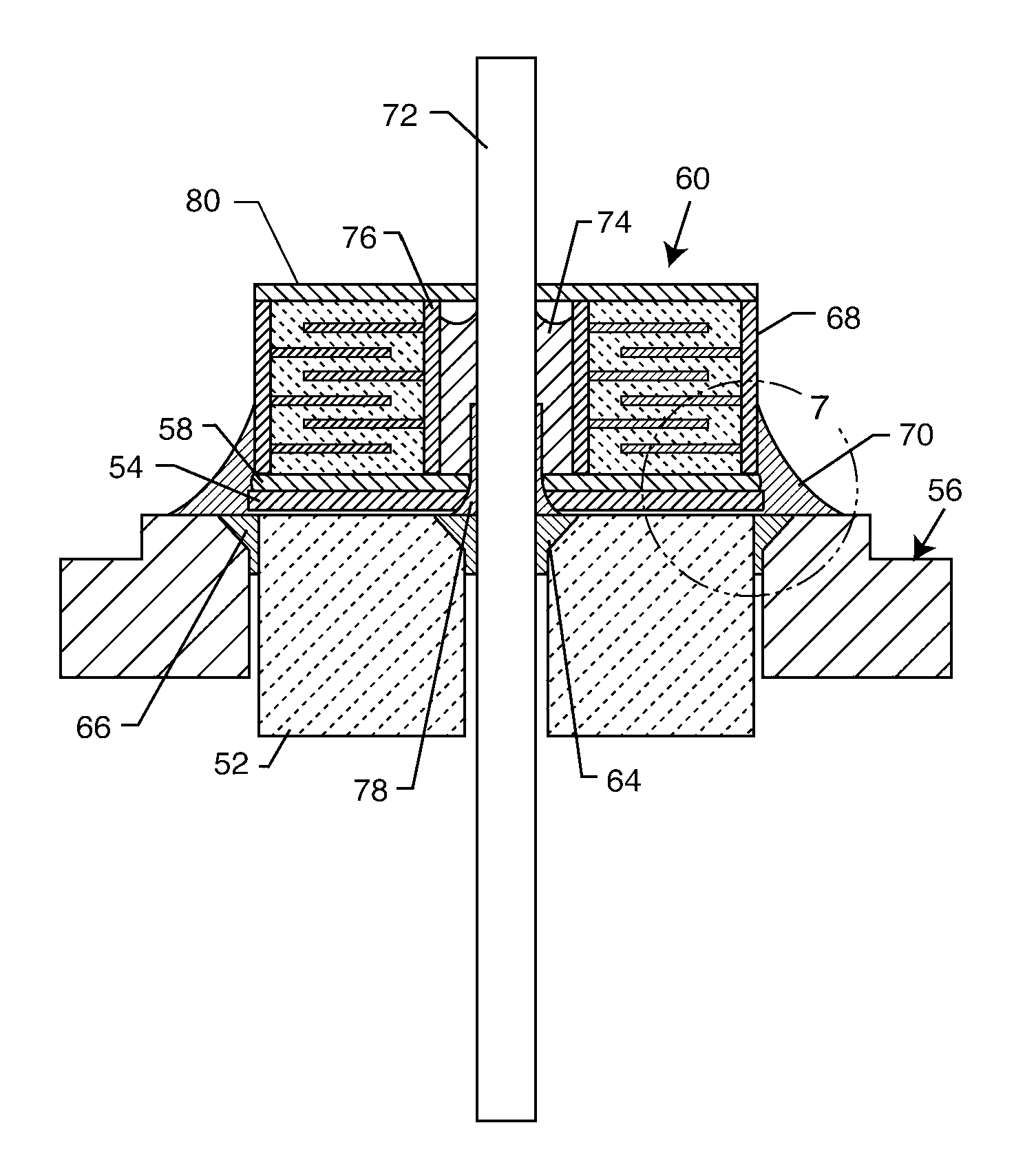
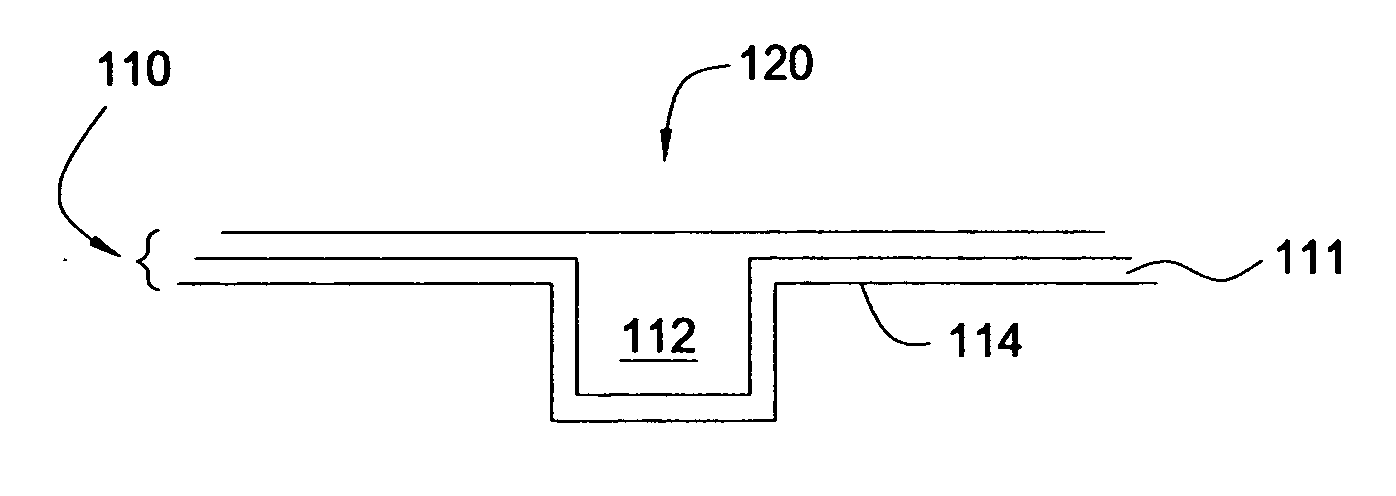
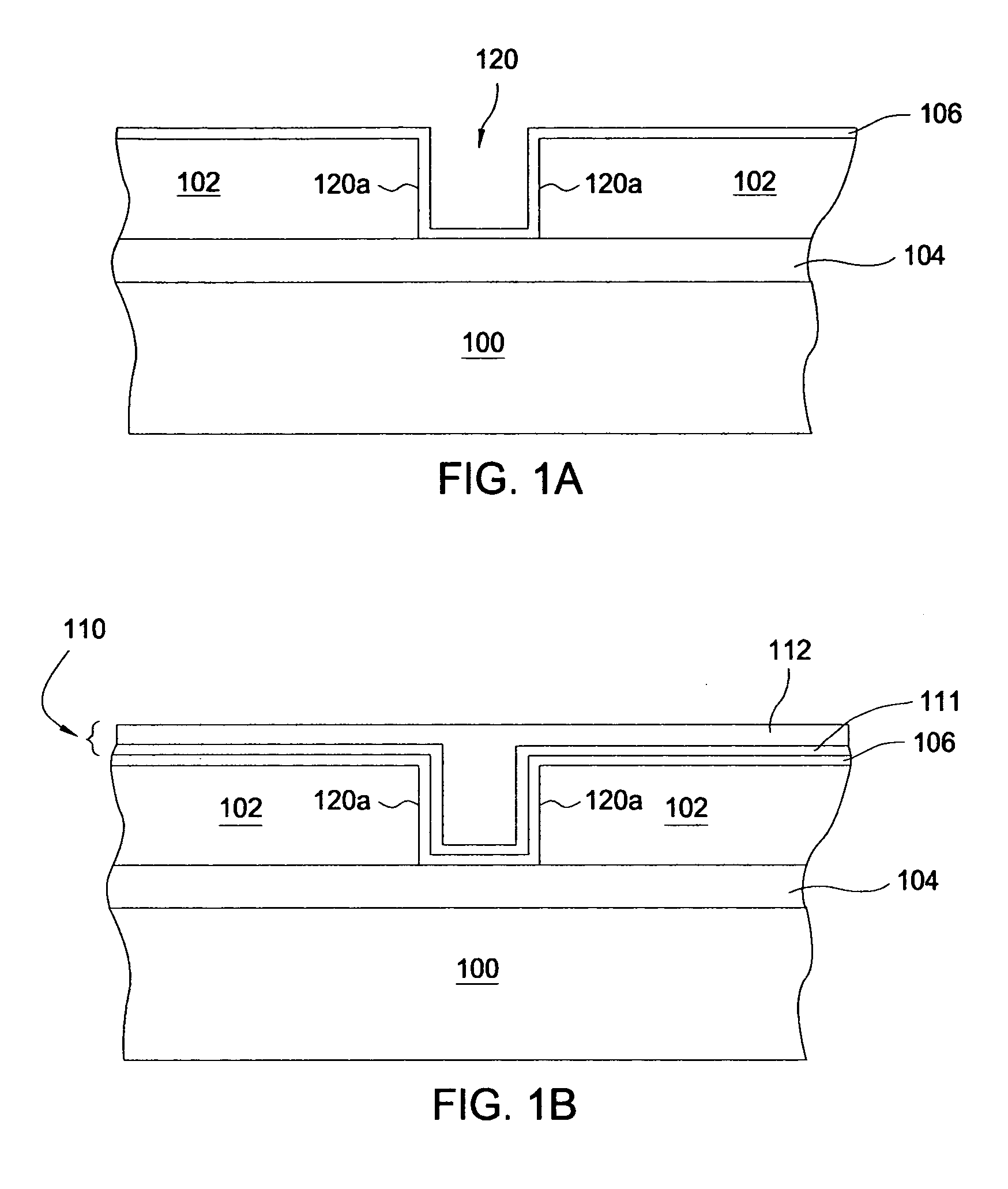
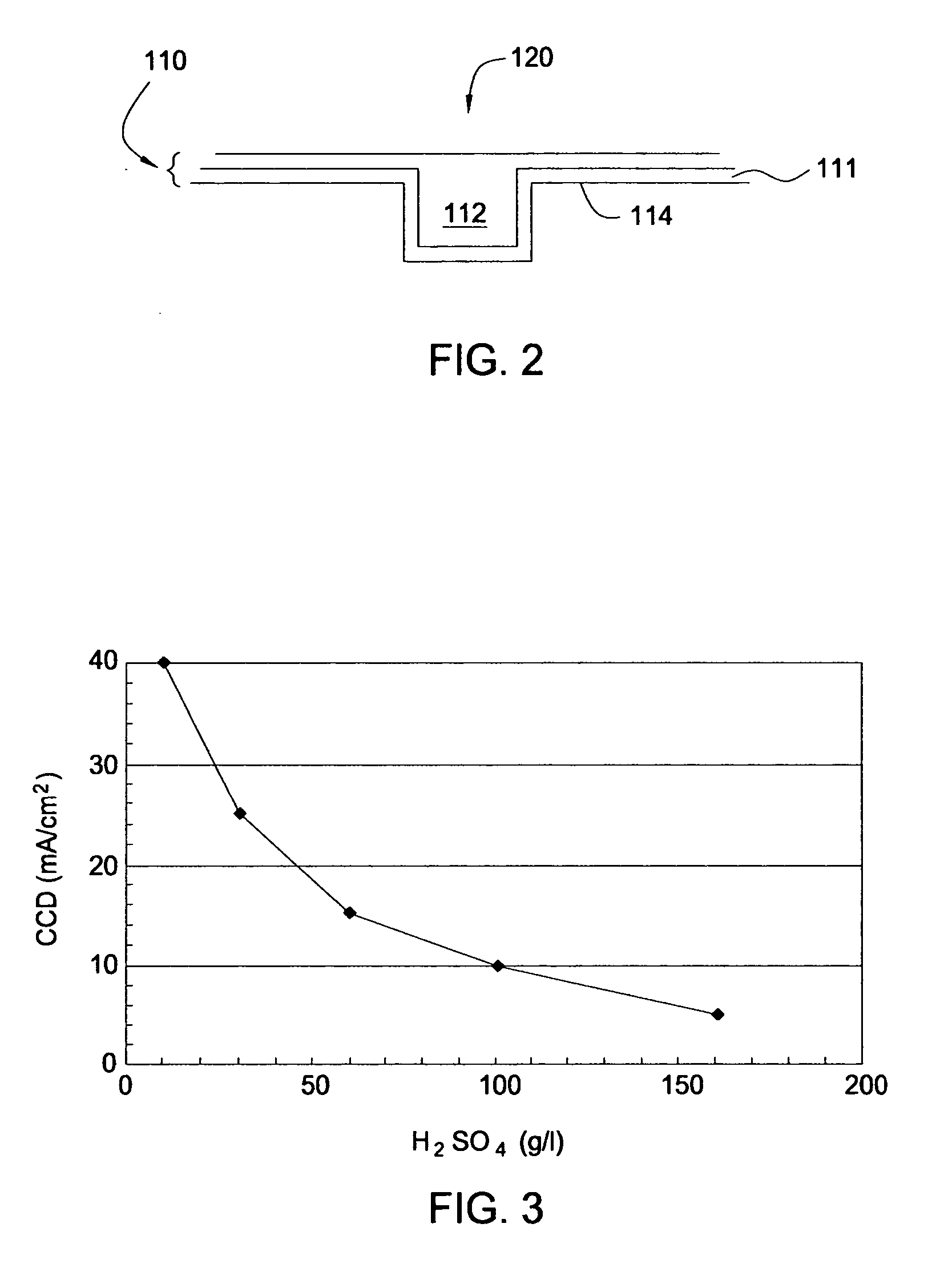
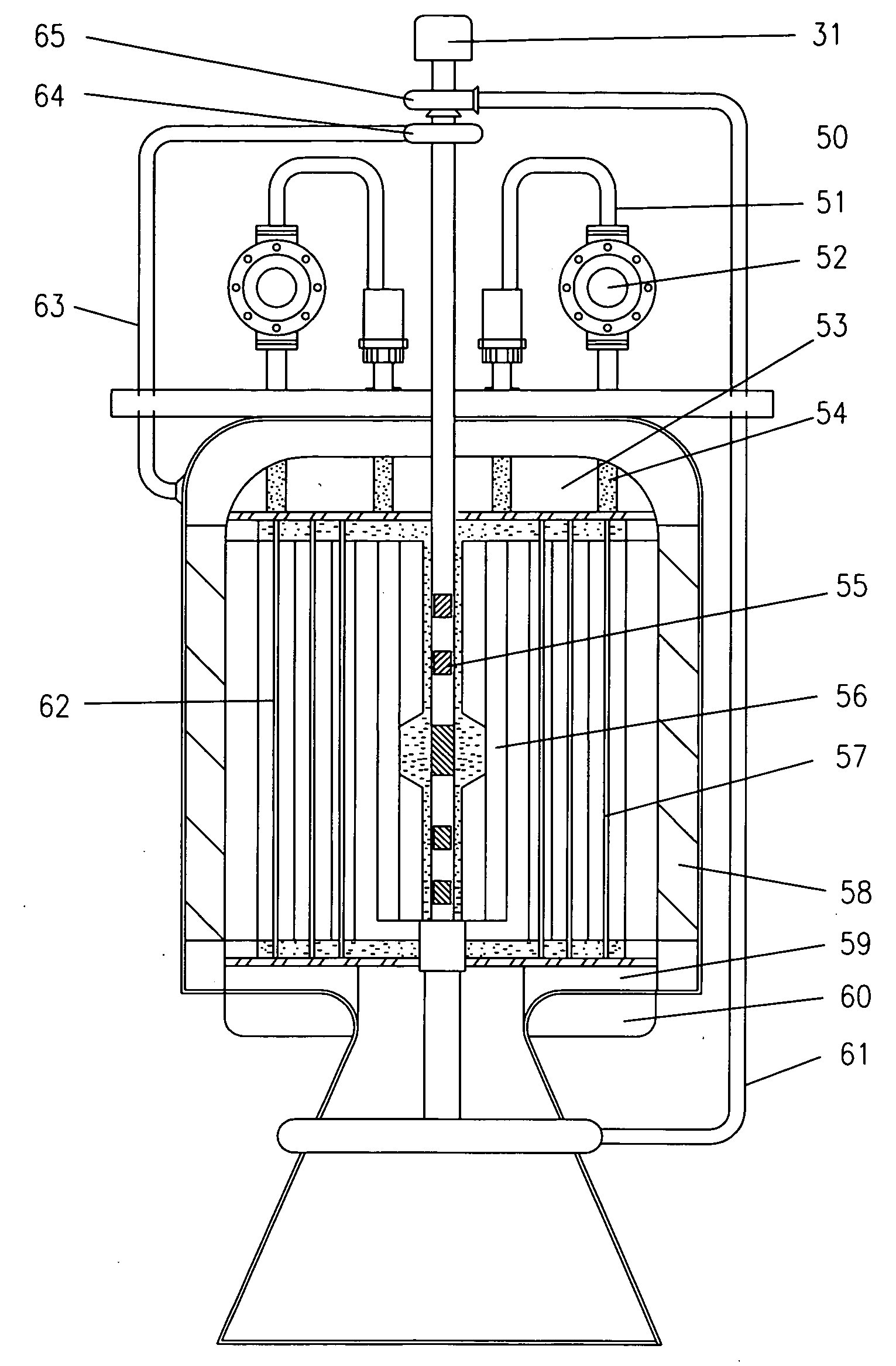
Feedthrough capacitor filter assemblies with laminar flow delaminations for helium leak detection
A feedthrough filter capacitor assembly includes a capacitor having first and second sets of conductive electrode plates embedded within a dielectric body and mounted to the hermetic terminal of an implantable medical device. A laminar delamination gap is provided between the capacitor sealing materials and the hermetic terminal assembly to facilitate helium leak detection. At least one feedthrough terminal pin extends through the capacitor in conductive relation with the first set of electrode plates, and an outer ferrule is mounted about the capacitor in conductive relation with the second set of electrode plates. The mounting washer is spaced against the hermetic seal and is adhesively connected to the feedthrough capacitor. The mounting washer forms a laminar flow delamination through which helium molecules can flow during a helium leak detection test. Provision is made for a pre-connection to the gold braze so that the capacitor inside diameter termination is not electrically isolated from the lead wire.
Owner:WILSON GREATBATCH LTD
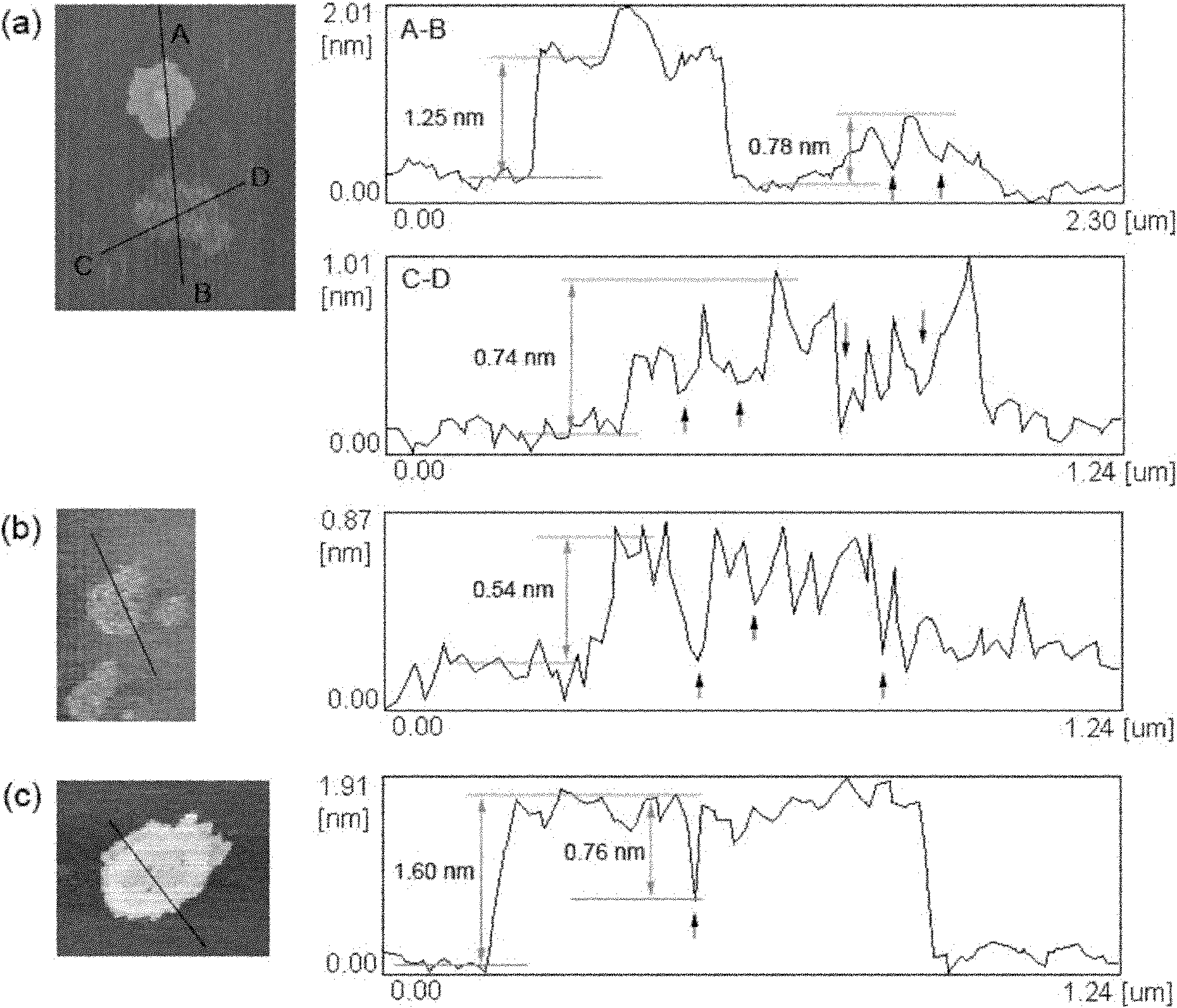
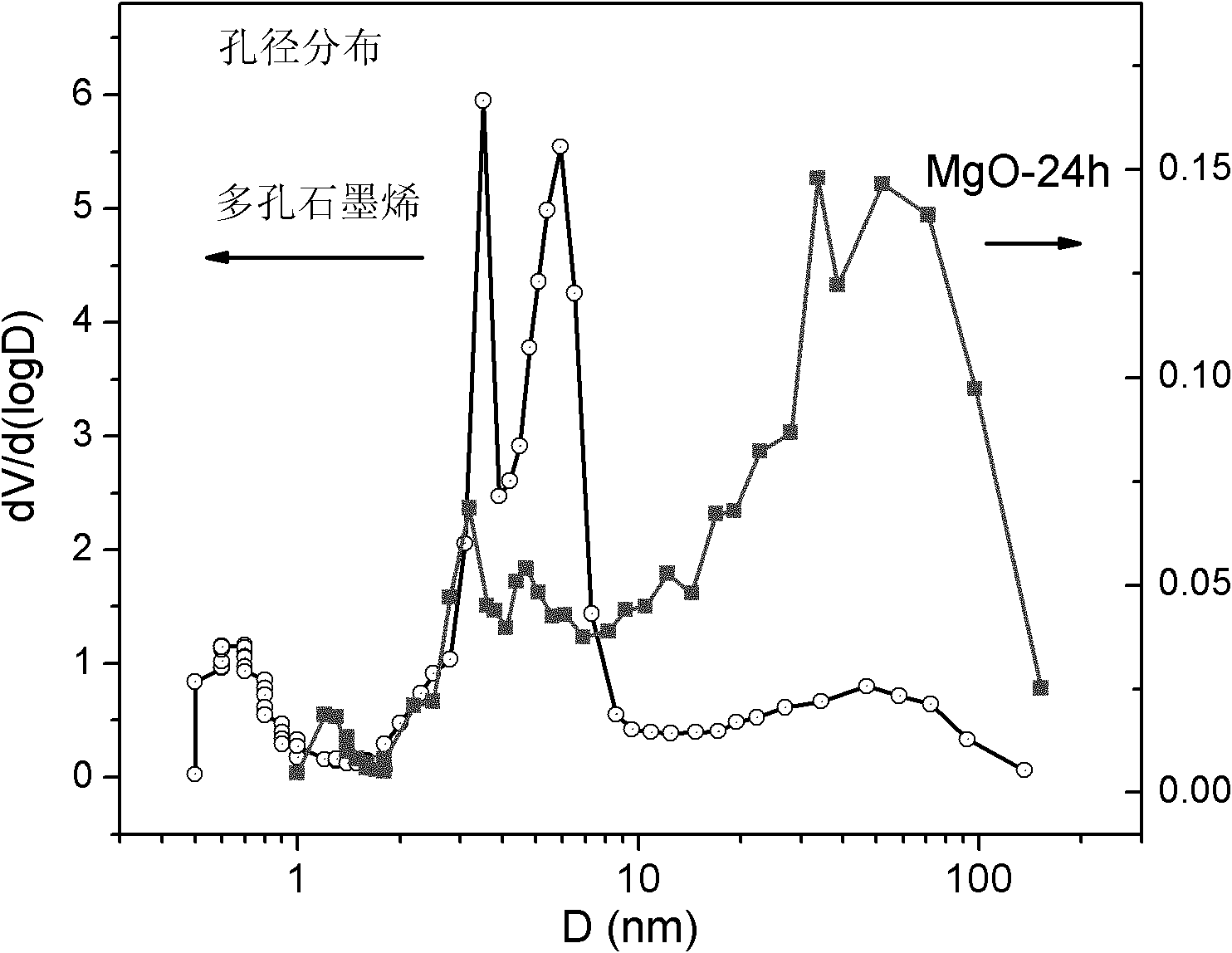
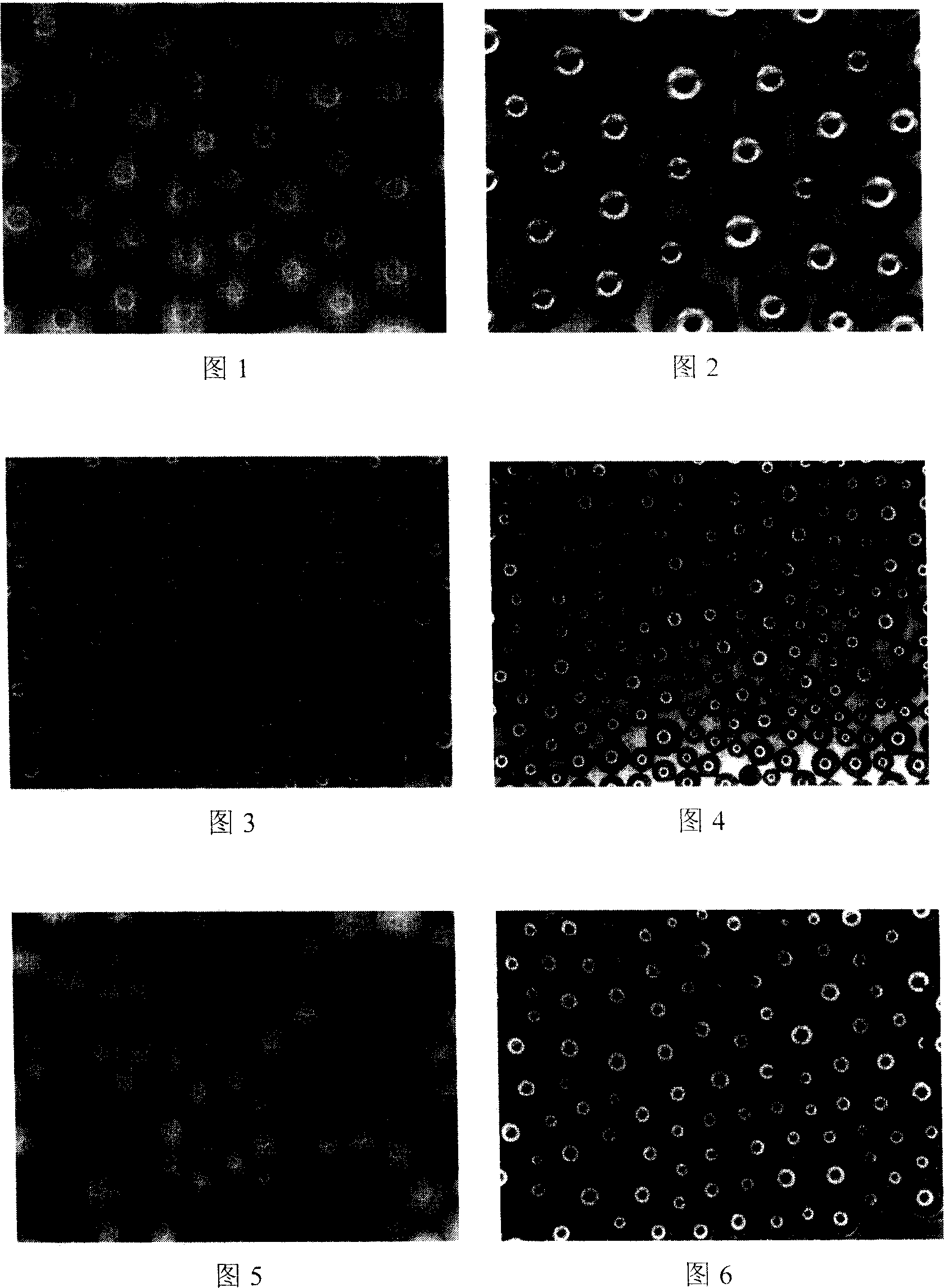
Graphene with porous structure and preparation method of graphene
The invention discloses a graphene with a porous structure and a preparation method of the graphene. The porous grapheme consists of a single-layer or multi-layer graphene structure unit, the single-layer or multi-layer grapheme structure unit has a pore-shaped structure (pore diameter is 0.1-200 nm) and a large specific surface area (300-2000 m<2> / g), thus the graphene has potential application value in the aspects of super-capacitors, conductive filling materials and the like. The preparation method of the porous grapheme is characterized in that MgO, Mg(OH)2, Al2O3, Al(OH)3, hydrotalcite compounds and / or corresponding calcined products of the substances are used as catalysts, or MgO, Mg(OH)2, Al2O3, Al(OH)3, hydrotalcite compounds and / or corresponding calcined products of the substances are used as carriers so as to further load one or more active components of Fe, Co, Ni and Mo and then the obtained substance is used as the catalyst ( the pore diameter of the catalyst is 1-200 nm, and the specific surface area is 10-300 m<2> / g); and then at the temperature of 300-1000 DEG C, the graphene is prepared by using inert gases such as nitrogen, argon, helium and the like and using a hydrocarbon chemical gas phase deposition method.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
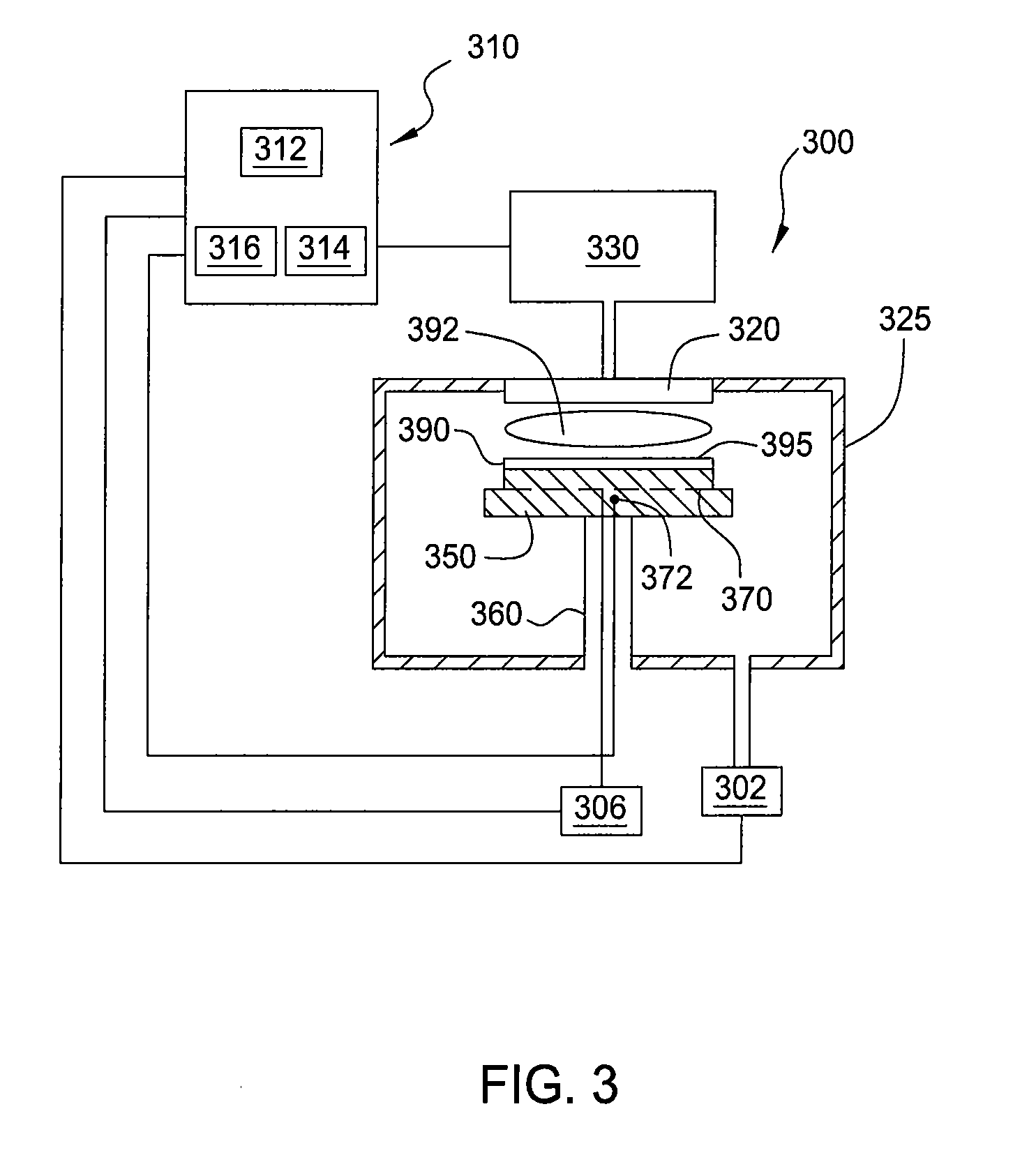
Multiple zone gas distribution apparatus for thermal control of semiconductor wafer
ActiveUS7156951B1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingControl systemProduct gas
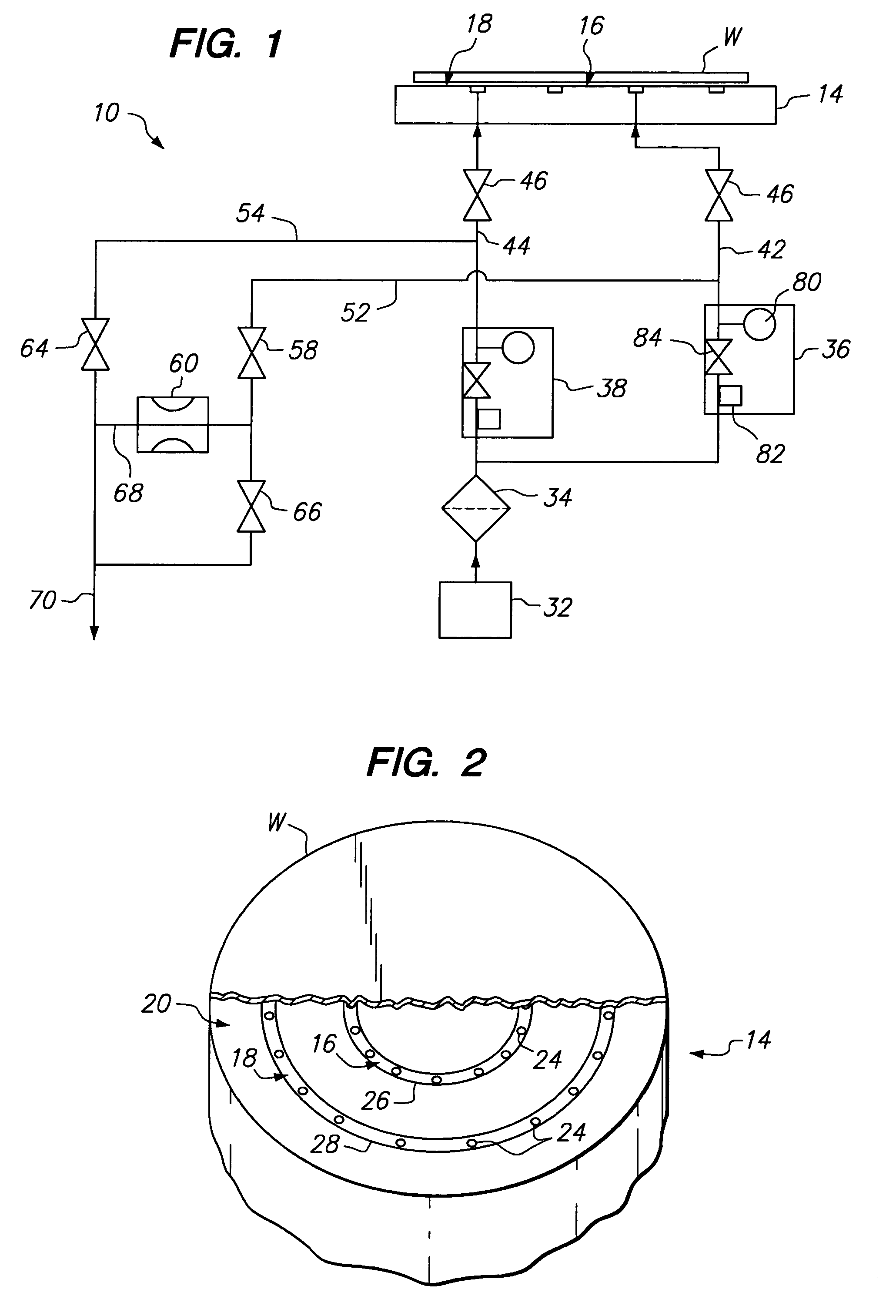
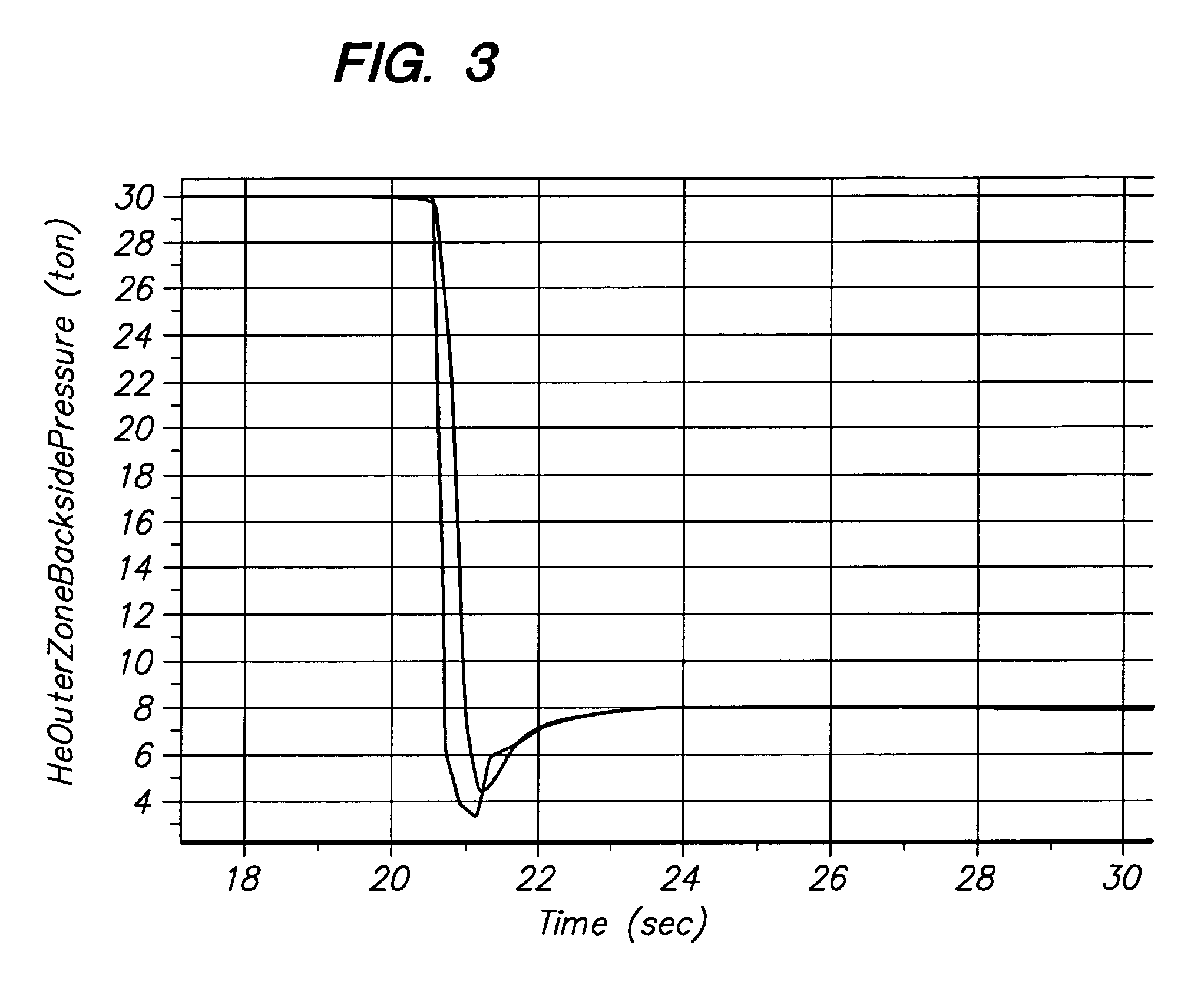
A gas distribution apparatus delivers a coolant gas, such as helium, to an upper surface of a chuck for controlling a temperature of a wafer placed on the chuck. The gas distribution apparatus allows first and second zones, such as an inner zone and an outer zone of the chuck, to be supplied with a coolant gas at different pressures for control of the temperature across the wafer. The gas distribution apparatus includes a pressure and flow control system for supplying the coolant gas at selected pressures and bleed lines which provide the dual function of allowing rapid evacuation of the inner and outer zones and preventing excess pressure from one zone from migrating to another zone.
Owner:LAM RES CORP
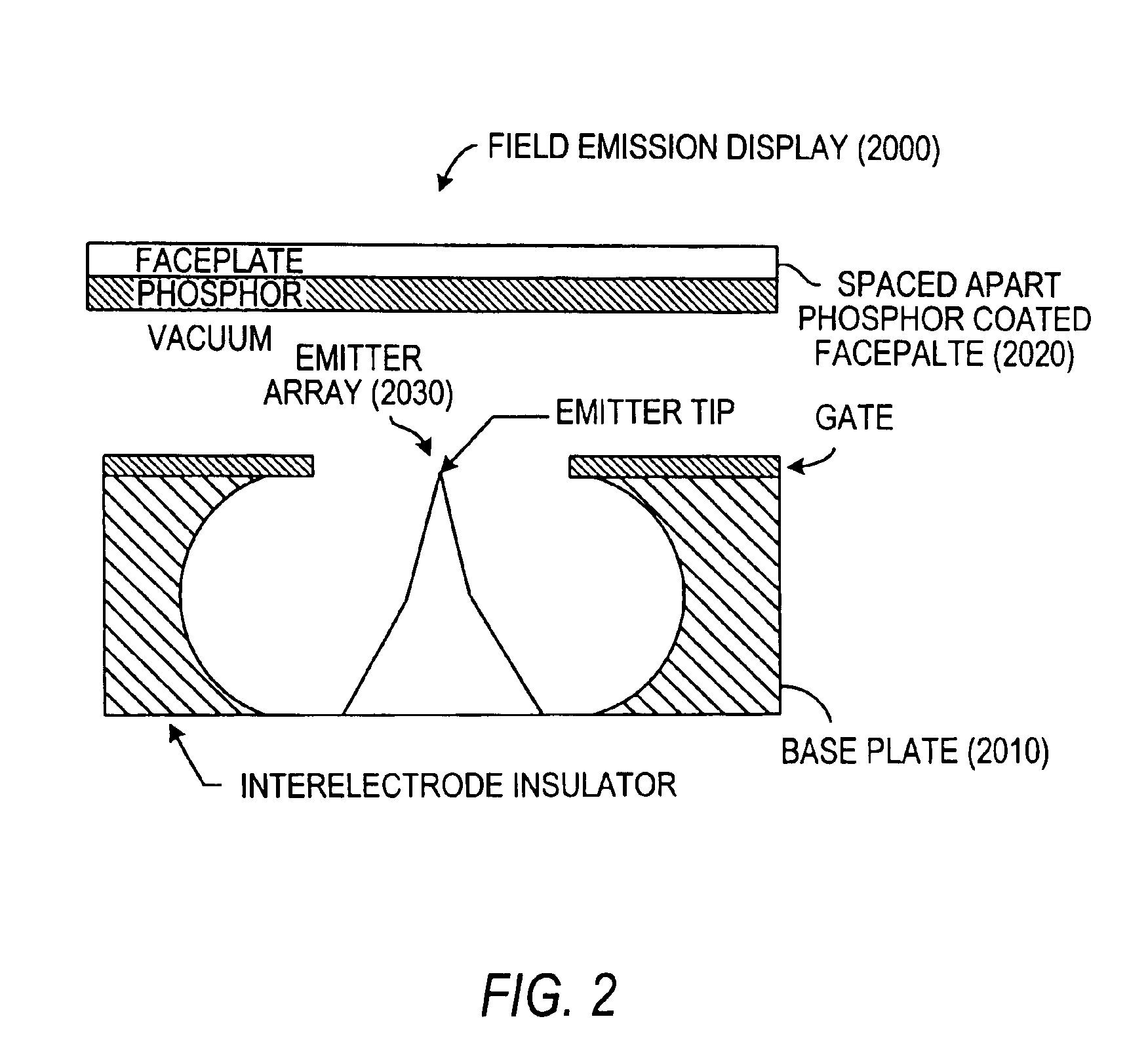
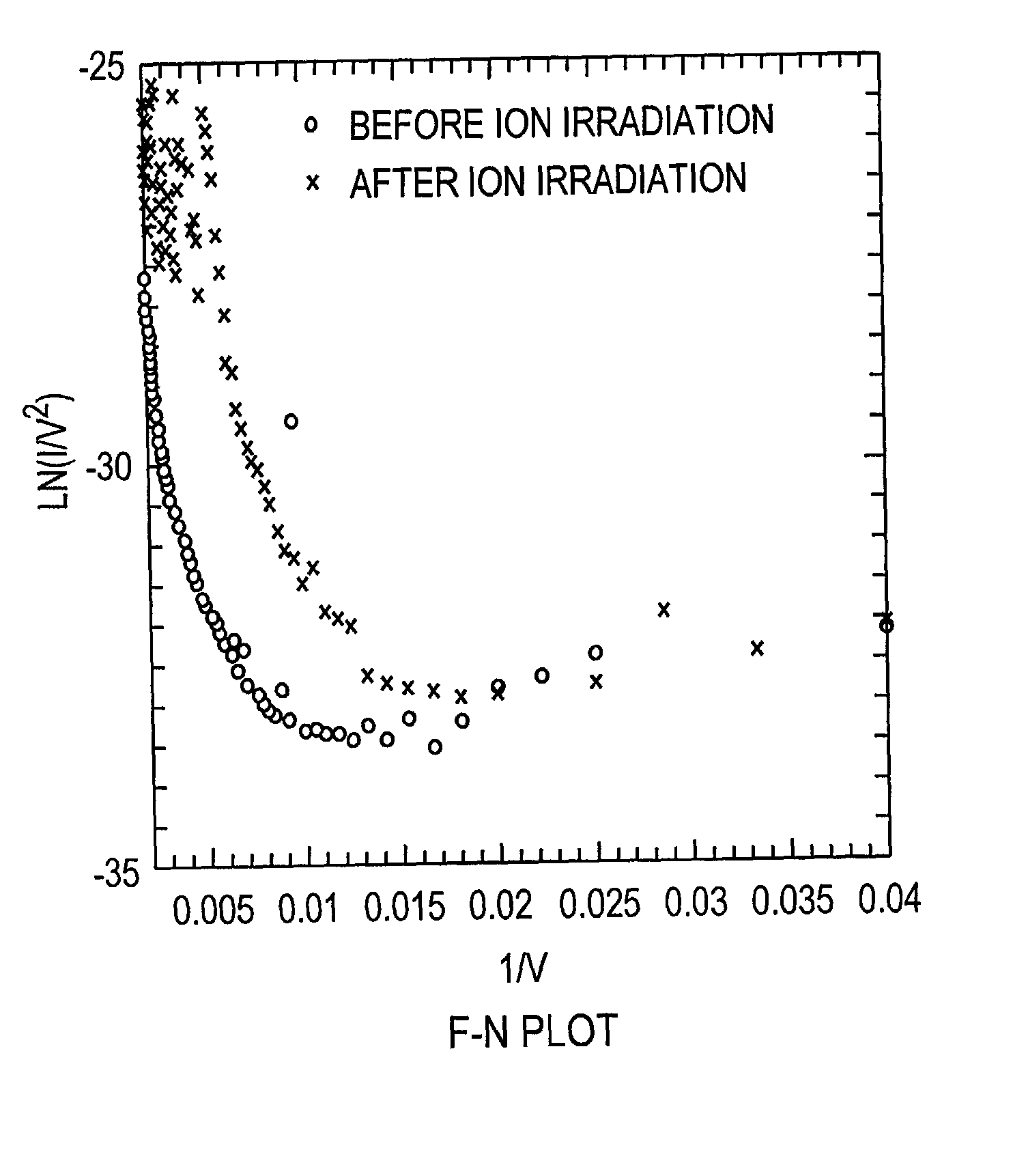
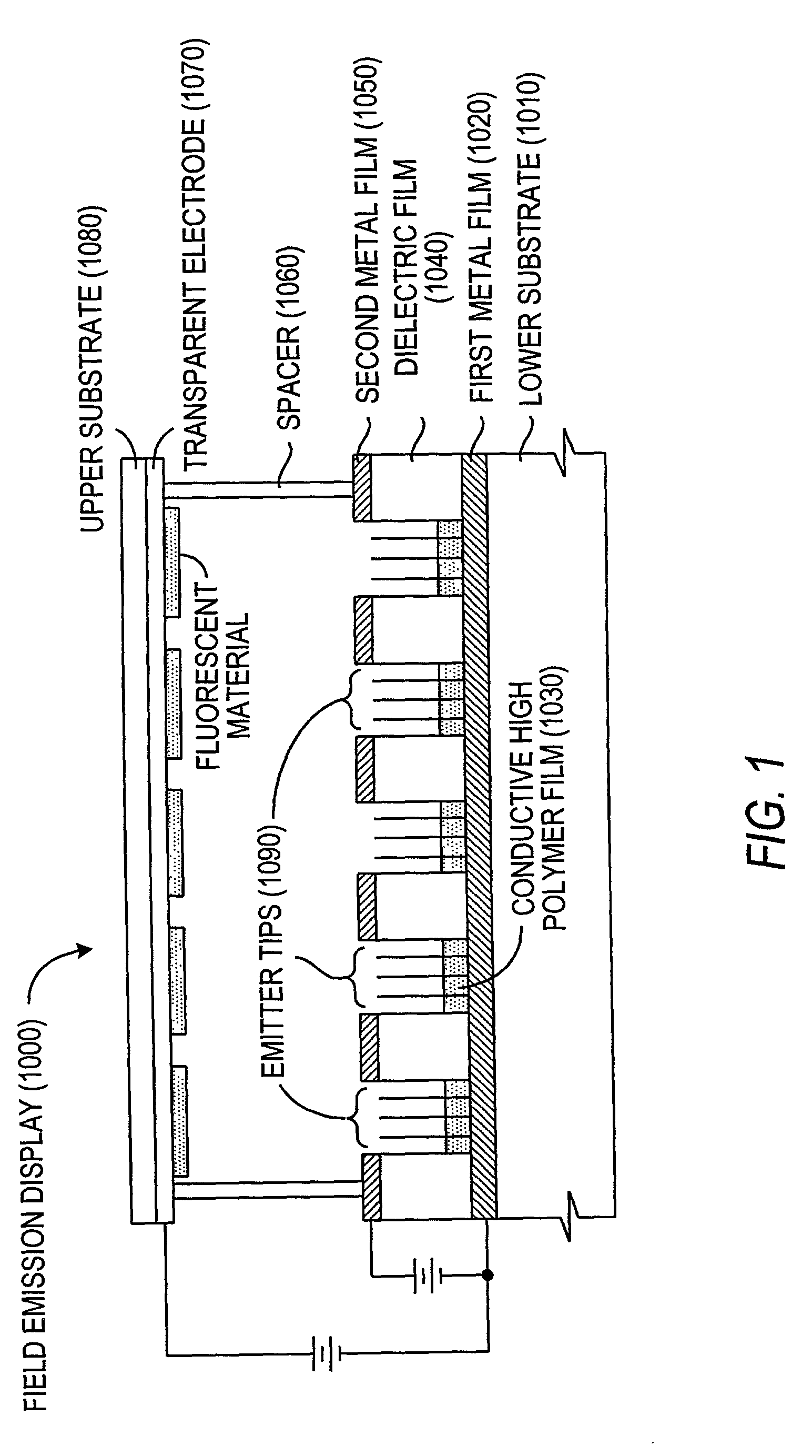
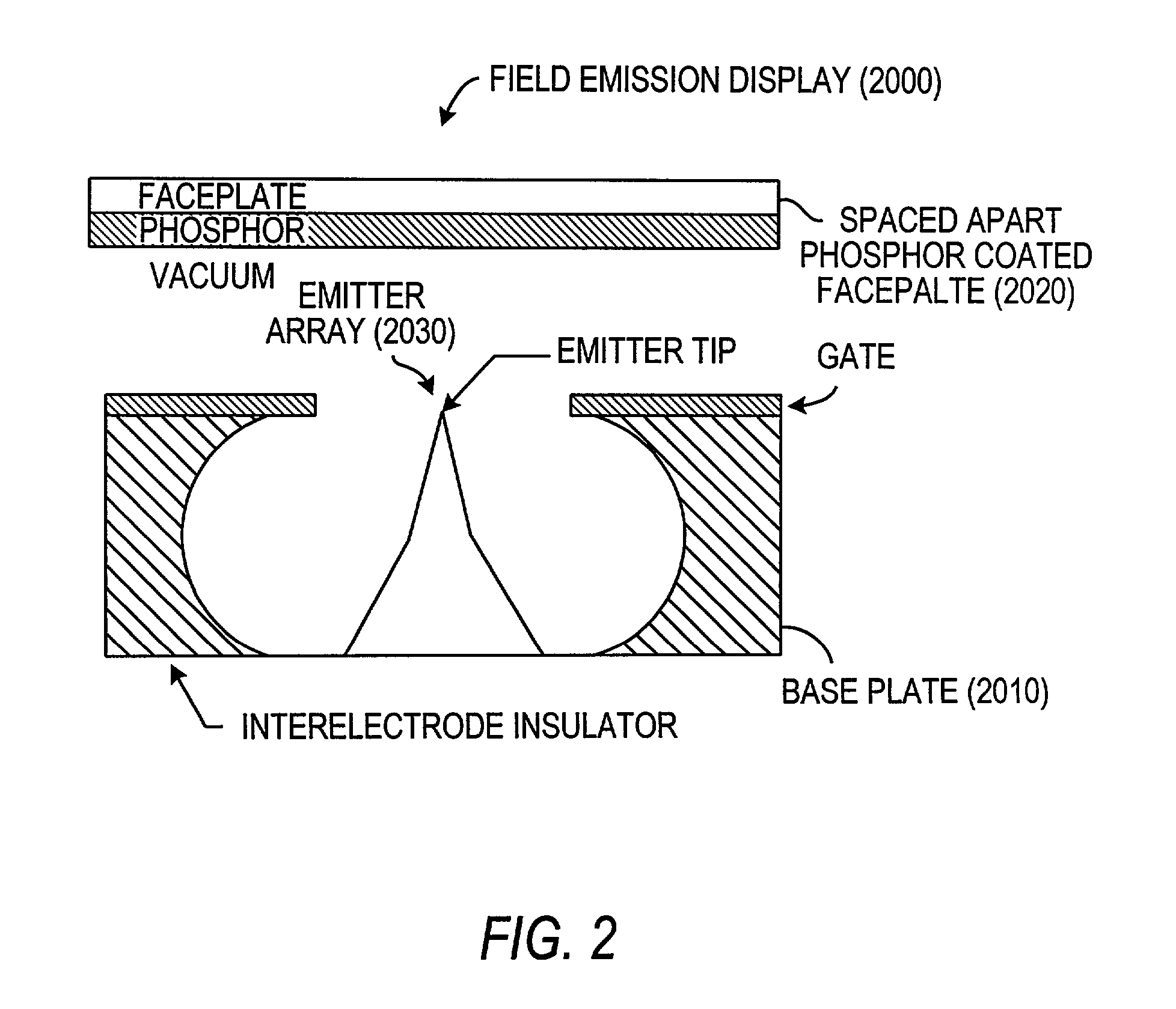
Field emission devices using ion bombarded carbon nanotubes
InactiveUS6911767B2Reduce voltageAccelerate emissionsCathode ray tubes/electron beam tubesNanoinformaticsField emission deviceOxygen
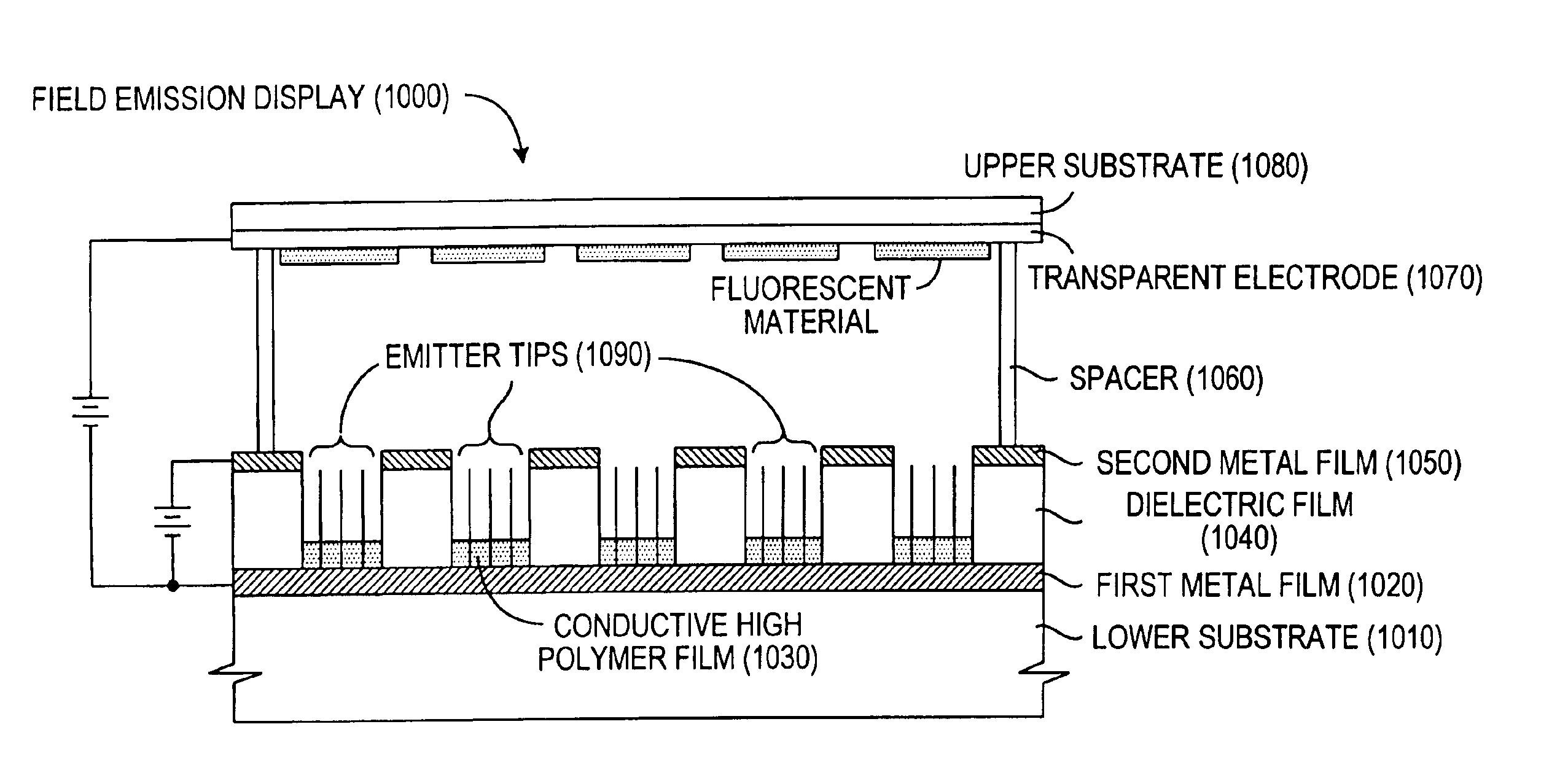
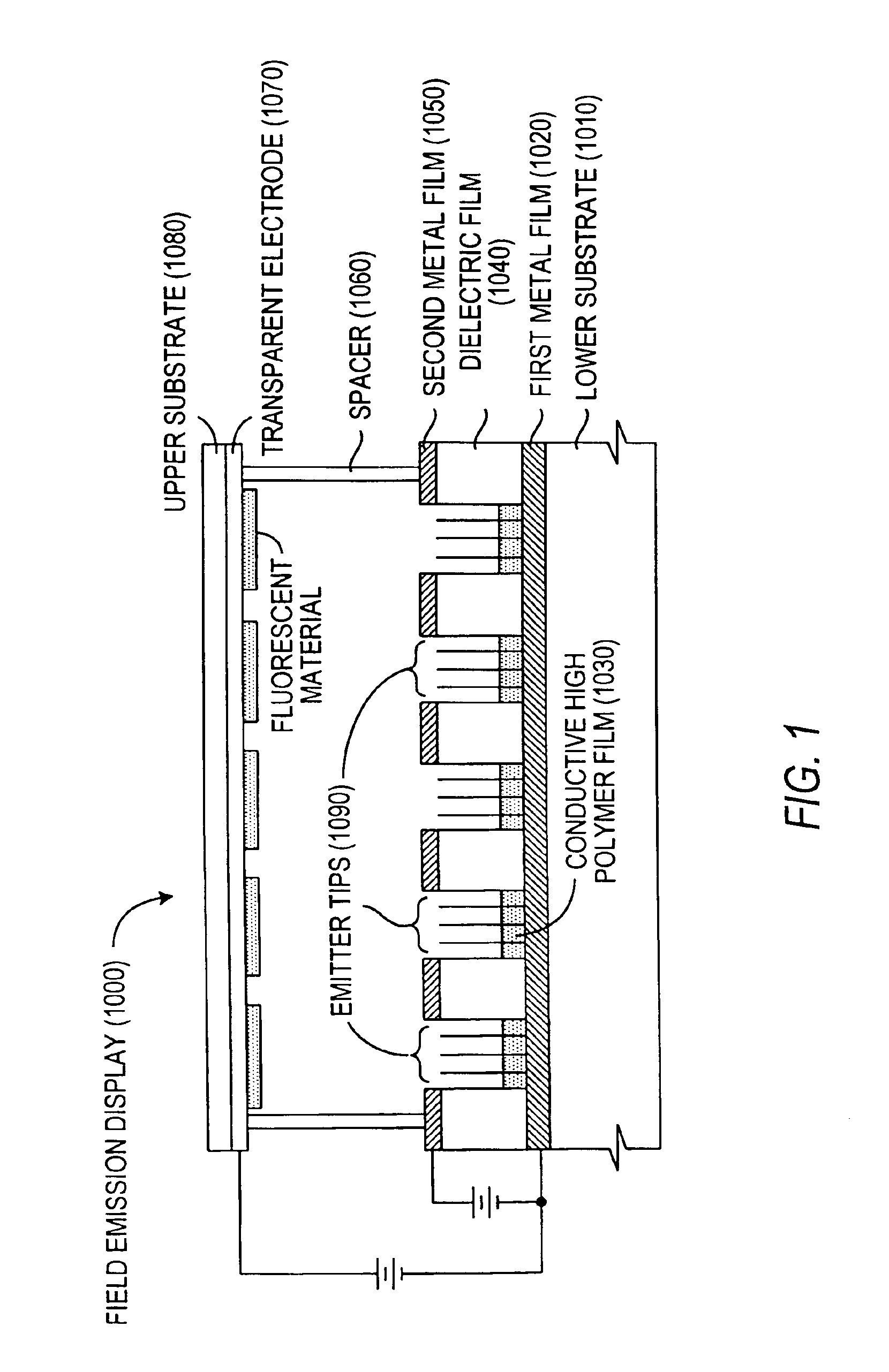
The present invention relates to a field emission device comprising an anode and a cathode, wherein said cathode includes carbon nanotubes which have been treated with an ion beam. The ion beam may be any ions, including gallium, hydrogen, helium, argon, carbon, oxygen, and xenon ions. The present invention also relates to a field emission cathode comprising carbon nanotubes, wherein the nanotubes have been treated with an ion beam. A method for treating the carbon nanotubes and for creating a field emission cathode is also disclosed. A field emission display device containing carbon nanotube which have been treated with an ion beam is further disclosed.
Owner:HYPERION CATALYSIS INT
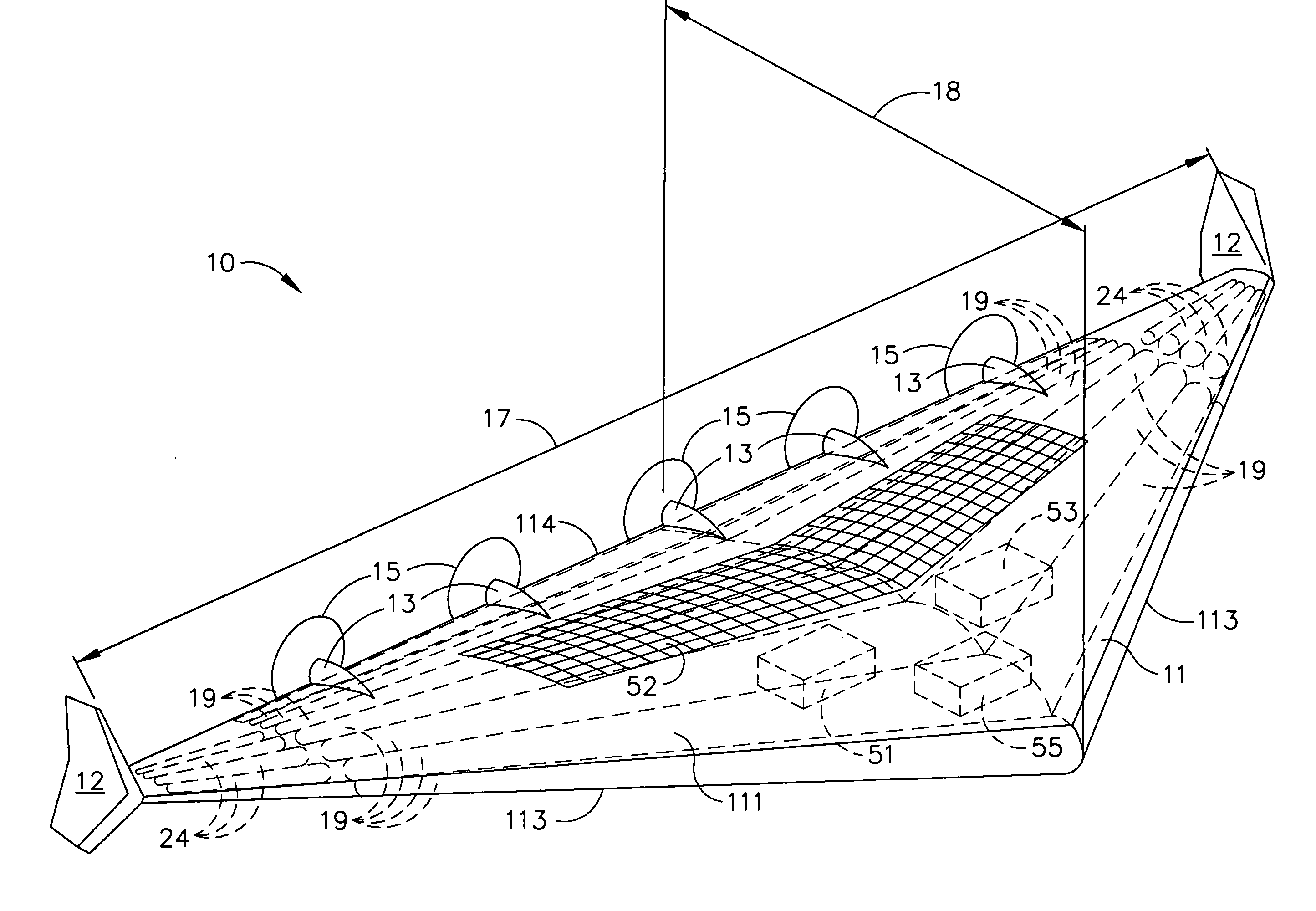
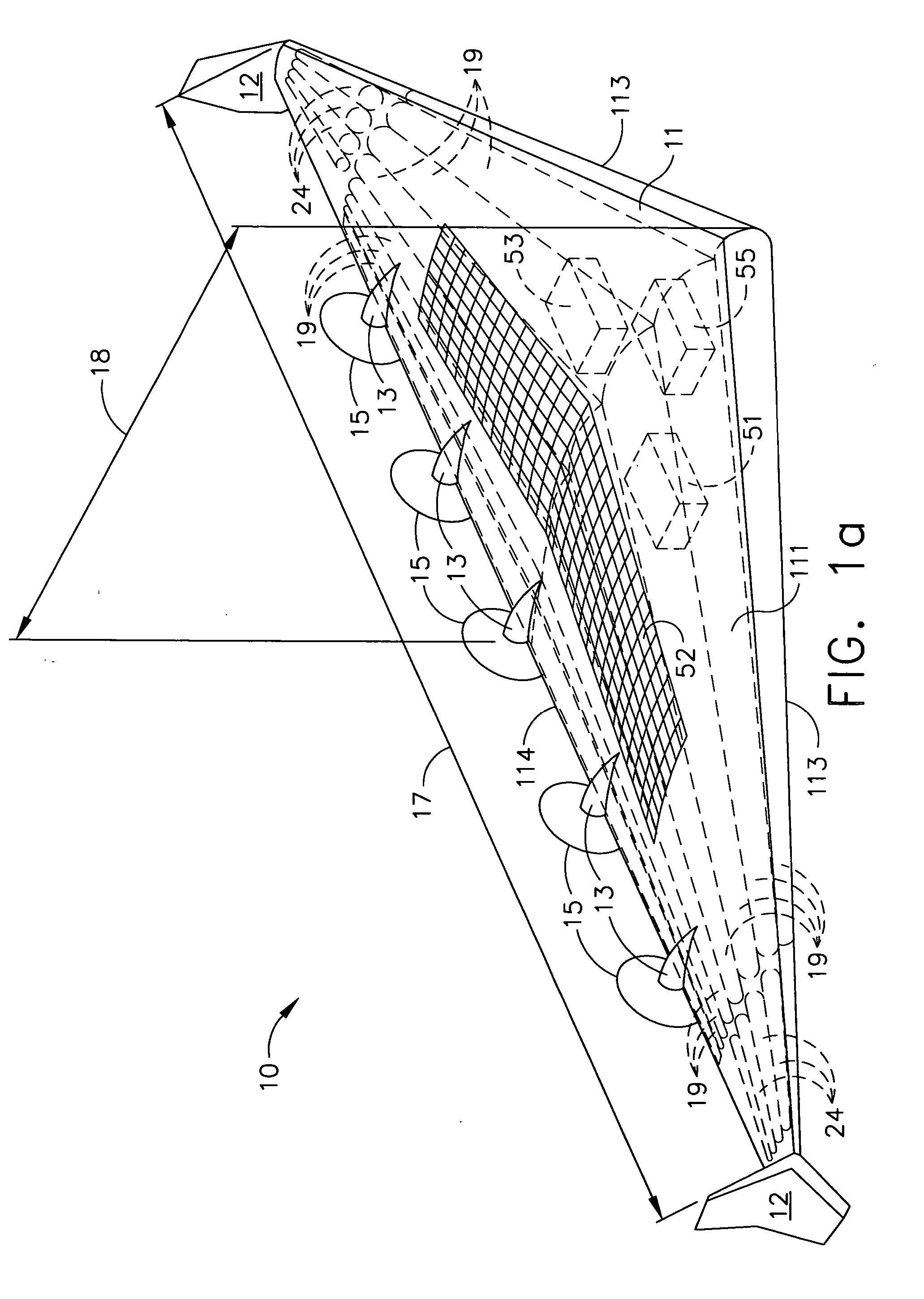
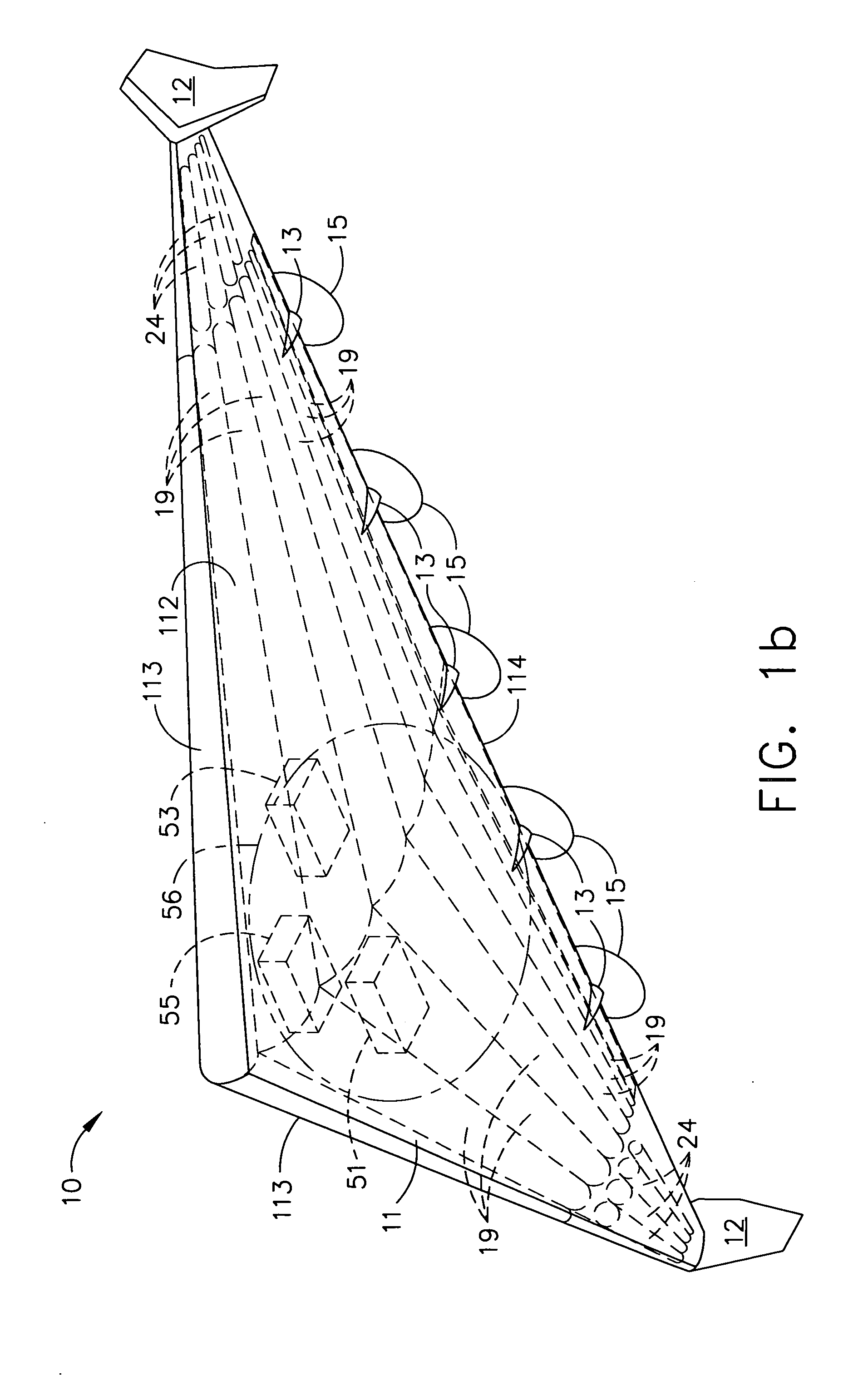
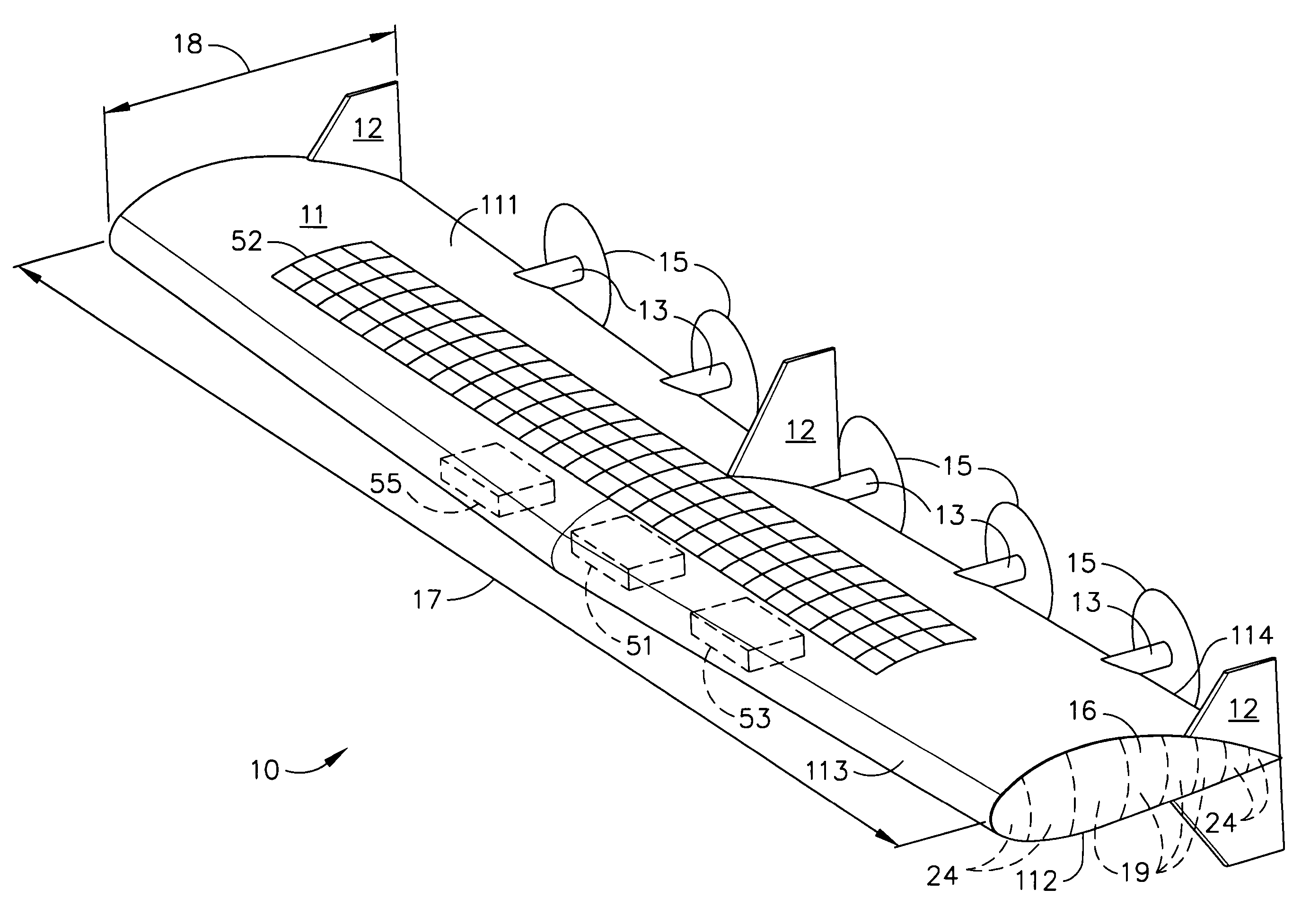
Delta-winged hybrid airship
ActiveUS20050258306A1Long flight enduranceHigh altitudeEnergy efficient board measuresAll-wing aircraftHigh energyVolumetric Mass Density
In one aspect, a hybrid airship including an outer shell, a plurality of helium filled gas envelopes, and an all-electric propulsion system may have the shape of a delta-wing. In some embodiments, the hybrid airship may be launched using buoyancy lift alone and aerodynamic lift may be provided by the all-electric propulsion system. In one aspect, a photovoltaic array and a high energy density power storage system may be combined to power the propulsion system making the propulsion system regenerative. The delta-wing shape can provide a surface area large enough to accommodate very large circular or elliptical transmission devices. By continuously recharging the power storage system, the hybrid airship in accordance with some embodiments can stay aloft at an operational altitude of at least about 85,000 ft for months or even years. The hybrid airship may function as an airborne military communications relay platform.
Owner:THE BOEING CO
Aerosol deliver apparatus IV
InactiveUS20050217667A1Avoid wastingConvenient to accommodateRespiratorsLiquid surface applicatorsVolumetric Mass DensityNon-rebreather mask
A multipurpose aerosol medication delivery apparatus that includes a collapsible / expandable, or a fixed volume, or a combination of partially fixed volume and partially collapsible / expandable holding chamber for use with a metered dosed inhaler (MDI) and / or any standard small volume nebulizer. The holding chamber is designed to deliver-aerosol medication particles generated by an MDI; aerosol medication particles generated by a nebulizer; a single gas or a mixture of gases; a single gas or a mixture of gases that can yield a gas density that will enhance aerosol delivery of medication with both MDI and nebulizer; a single gas or a mixture of gases that will yield and deliver an oxygen concentration to a patient ranging from room air concentration to 100%. The device includes a reservoir that stores nebulized aerosol generated during exhalation to be inhaled during the next breath. The device also included a one way valve to prevent carbon dioxide generated during exhalation from rebreathing by not allowing the exhaled air from entering the holding chamber. The device includes an exit port with a second one way valve that allows the exhaled air to exit the device but closes during inhalation to prevent any entrainment of room air gas. The exit port may instead have a filter with one-way valve to trap the exhaled aerosol particles while allowing the exhaled gases to escape. The filter valve will similarly close during inhalation to prevent entrainment of room air gas. The holding chamber will allow a uniform mixture of aerosol medication and gases to flow together during inhalation to the patient via a mouthpiece or a facemask. The holding chamber is connected to a nebulizer chamber with a single or multiple connecting tubes that allow gas mixtures with varying density, viscosity, humidity and concentration of oxygen to flow into the holding chamber from the nebulizer chamber. The pattern of flow of the gas(es) does not disturb the flow of the nebulized medication from the nebulizer chamber to the holding chamber or interfere with the plume generated by an MDI. The device also serves as a facemask for delivering precise concentrations of oxygen or as a 100% non-rebreather mask. The device also serves to deliver precise concentrations of different density gases i.e. nitrogen, helium, oxygen, etc. This will allow varying fractions of inspired oxygen to deliver aerosol medication via MDI or a nebulizer. Thus, the device has the ability to deliver aerosol medication with an MDI or a nebulizer while retaining the ability to simultaneously deliver different density gas mixtures and varying fraction of inspired oxygen without interrupting one for the other.
Owner:DHUPER SUNIL +1
Delta-winged hybrid airship
ActiveUS7093789B2Long flight enduranceHigh altitudeEnergy efficient board measuresAll-wing aircraftHigh energyVolumetric Mass Density
In one aspect, a hybrid airship including an outer shell, a plurality of helium filled gas envelopes, and an all-electric propulsion system may have the shape of a delta-wing. In some embodiments, the hybrid airship may be launched using buoyancy lift alone and aerodynamic lift may be provided by the all-electric propulsion system. In one aspect, a photovoltaic array and a high energy density power storage system may be combined to power the propulsion system making the propulsion system regenerative. The delta-wing shape can provide a surface area large enough to accommodate very large circular or elliptical transmission devices. By continuously recharging the power storage system, the hybrid airship in accordance with some embodiments can stay aloft at an operational altitude of at least about 85,000 ft for months or even years. The hybrid airship may function as an airborne military communications relay platform.
Owner:THE BOEING CO
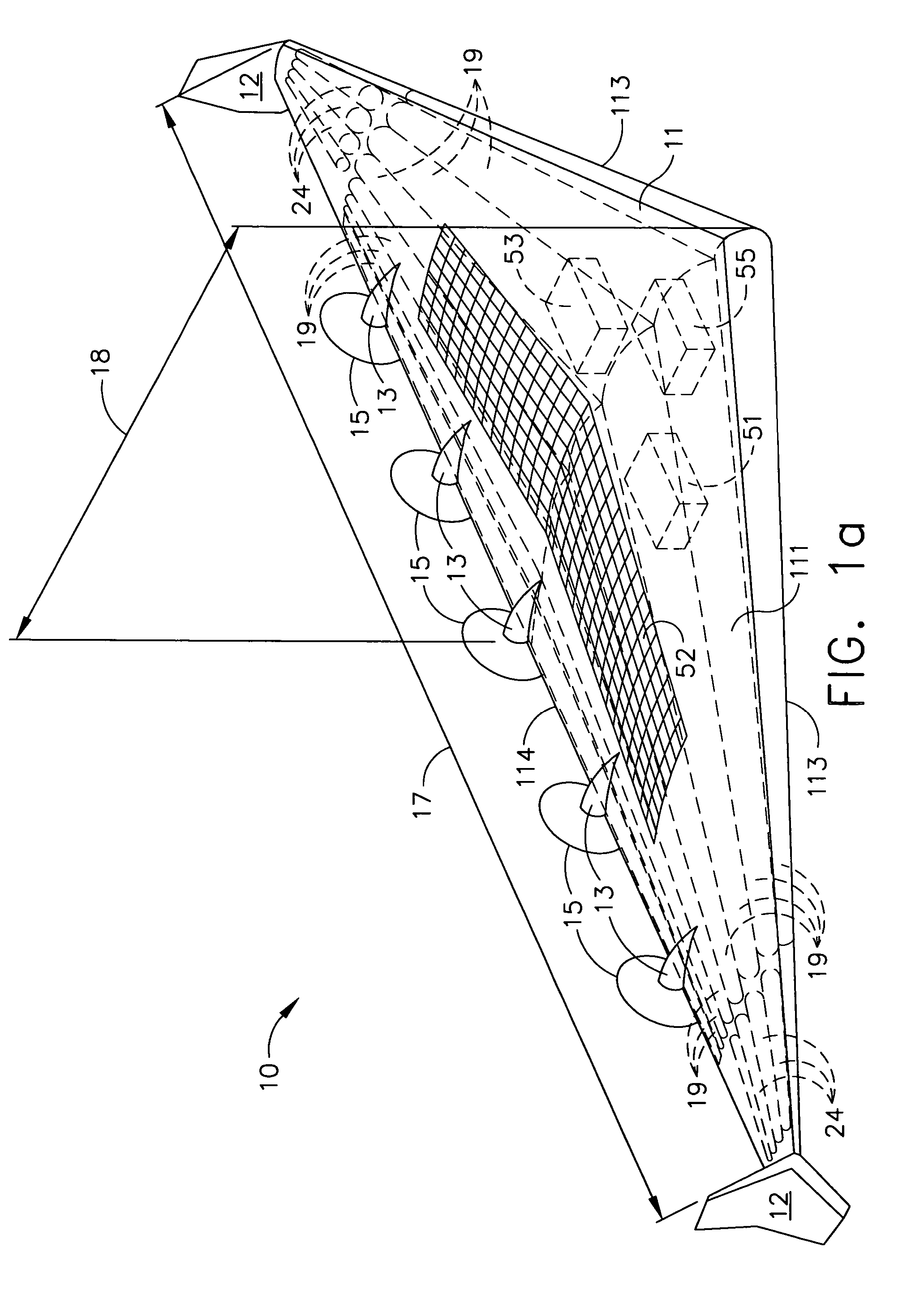
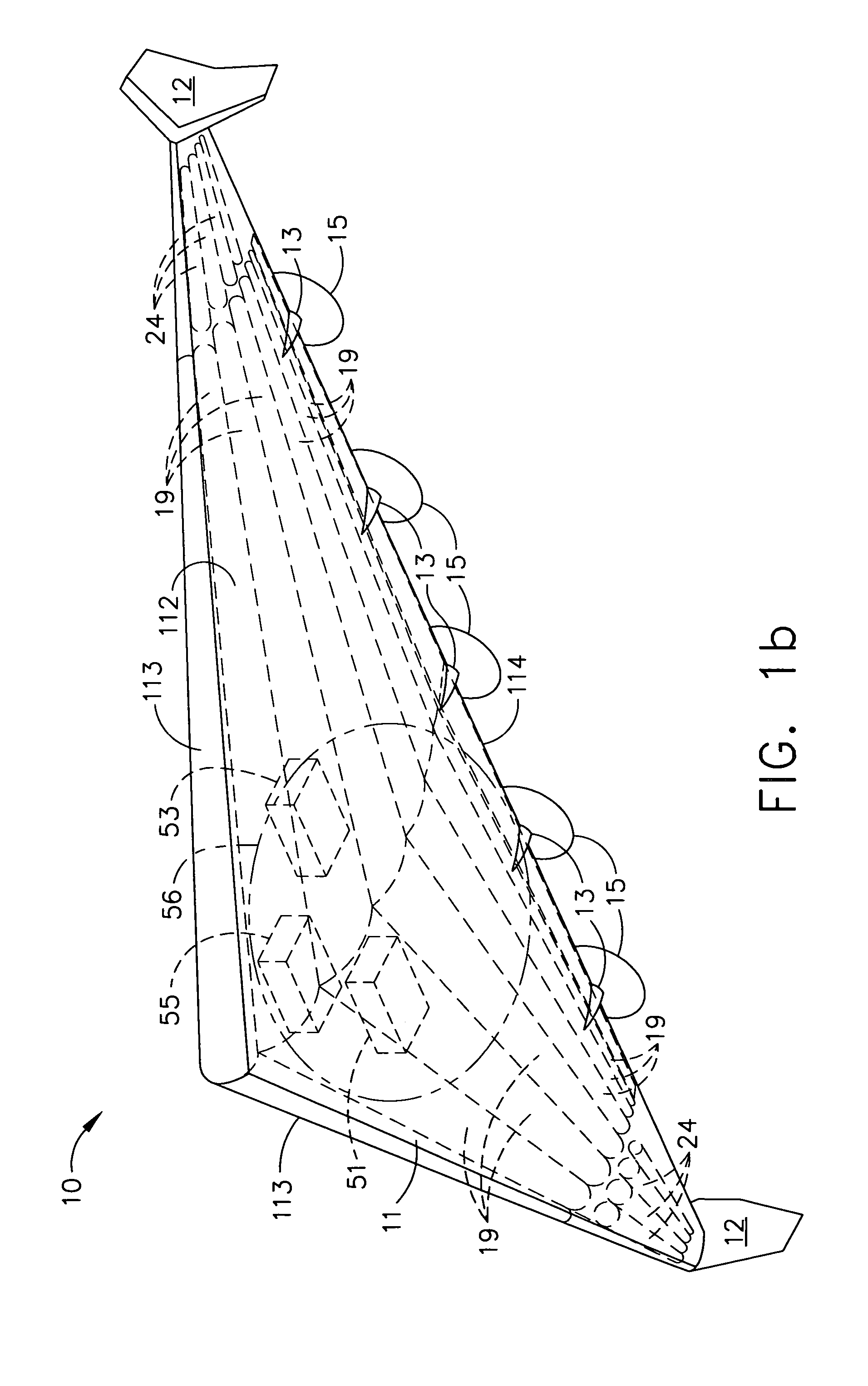
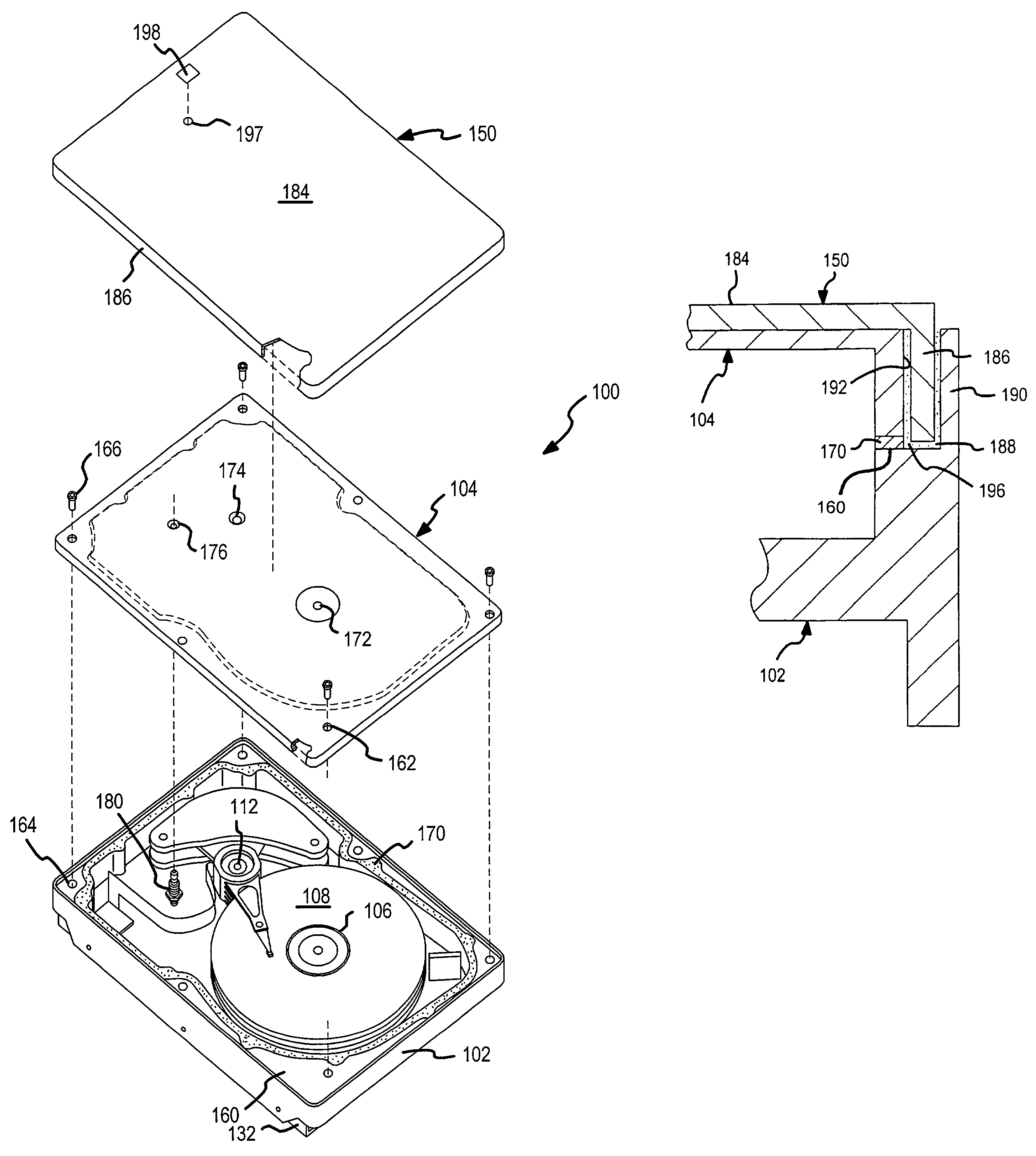
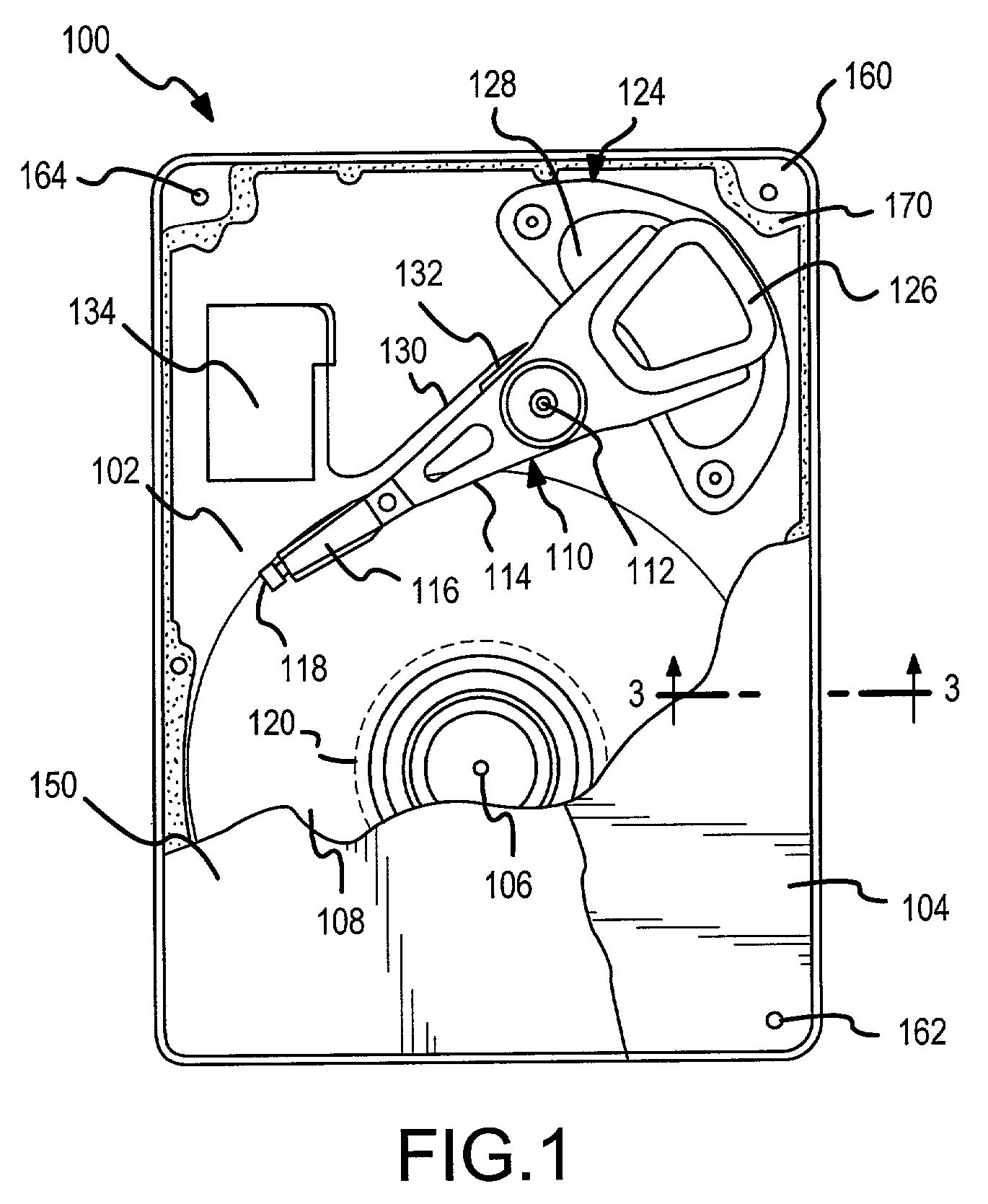
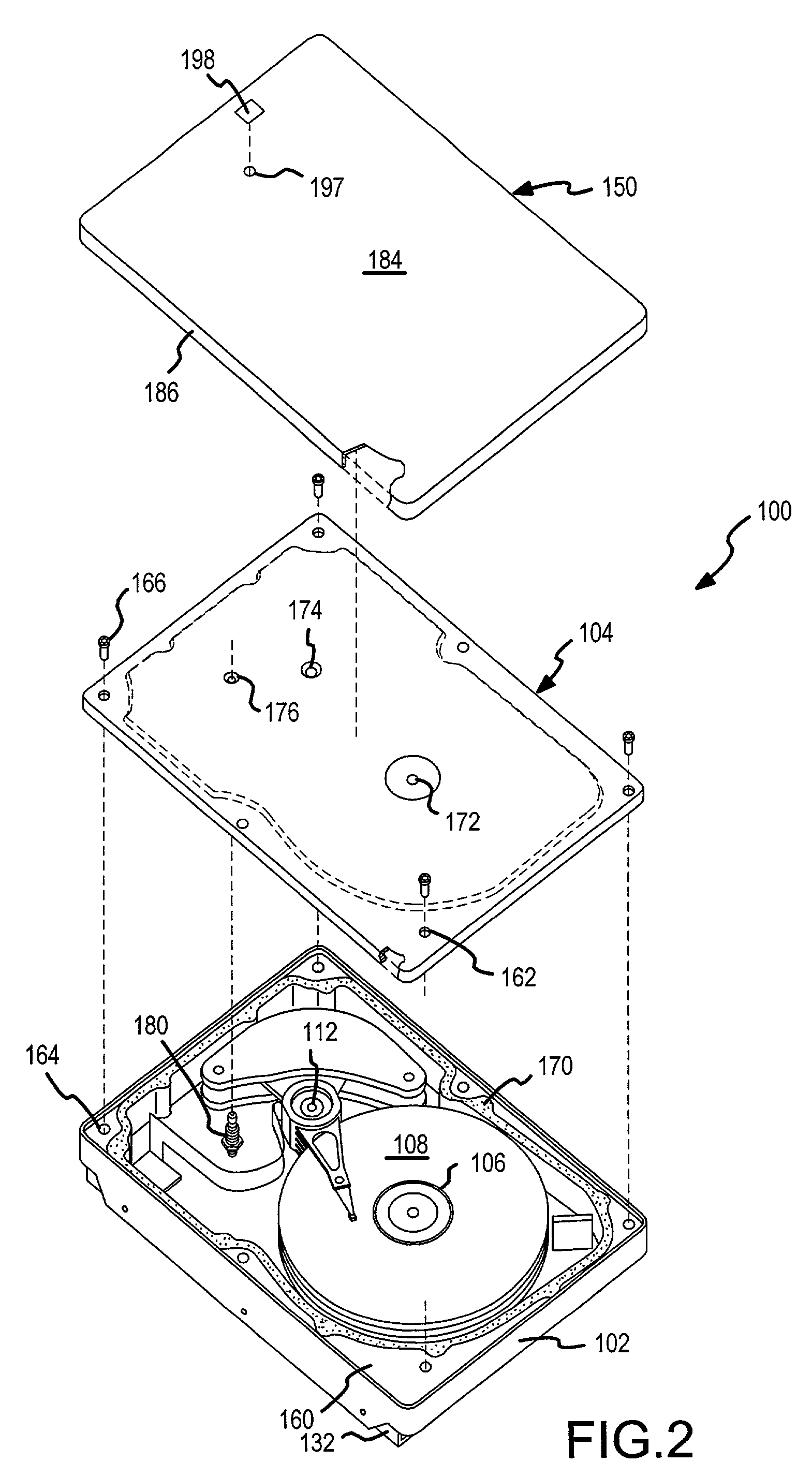
Two-stage sealing of a data storage assembly housing to retain a low density atmosphere
ActiveUS7218473B2Stable operation of driveGuaranteed uptimeElectrical transducersCarrier constructional parts dispositionHermetic sealEngineering
A disc drive includes a base plate and a structural cover removably attached to the base plate to form an internal environment within the disc drive that is filled with a low density gas such as helium. A sealing cover is permanently attached to the base plate and the structural cover to form a hermetic seal for the low density gas. A first seal secured between the base plate and the removable structural cover permits short term operation of the disc drive with minimal leakage of the low density gas so that the drive can be tested prior to permanent attachment of the sealing cover.
Owner:SEAGATE SINGAPORE INT HEADQUARTERS PTE LTD
Improved process for preparing porous microsphere active carbon
The improved process for preparing high strength porous microsphere active carbon includes the following steps: oxidizing thermosetting resin ball at 60-400 deg.c in oxygen containing atmosphere or pre-treating thermosetting resin ball at 60-300 deg.c in oxidizing acid, SO2 or SO3; carbonizing at 600-1200 deg.c in helium, nitrogen or their mixture atmosphere; and activating at 600-1200 deg.c in activating water vapor atmosphere to obtain high strength porous microsphere active carbon with grain size of 10 micron to 2 mm, BET specific surface area of 500-4000 sq m / g, and grain strength of 50-200 MPa. The process is simple, low in cost and high in yield, and the prepared microsphere active carbon has high strength and high purity and is one excellent medical active adsorption material.
Owner:EAST CHINA UNIV OF SCI & TECH
Field emission devices using ion bombarded carbon nanotubes
InactiveUS20030044519A1Easy to disassembleReduce voltageCathode ray tubes/electron beam tubesNanoinformaticsField emission deviceOxygen
The present invention relates to a field emission device comprising an anode and a cathode, wherein said cathode includes carbon nanotubes which have been treated with an ion beam. The ion beam may be any ions, including gallium, hydrogen, helium, argon, carbon, oxygen, and xenon ions. The present invention also relates to a field emission cathode comprising carbon nanotubes, wherein the nanotubes have been treated with an ion beam. A method for treating the carbon nanotubes and for creating a field emission cathode is also disclosed. A field emission display device containing carbon nanotube which have been treated with an ion beam is further disclosed.
Owner:HYPERION CATALYSIS INT
Method for manufacturing semiconductor device
ActiveUS8193031B2Reduce the presence of impuritiesStable electrical characteristicsStatic indicating devicesSolid-state devicesNoble gasDehydrogenation
An object is to provide a semiconductor device having stable electric characteristics in which an oxide semiconductor is used. An oxide semiconductor layer is subjected to heat treatment for dehydration or dehydrogenation treatment in a nitrogen gas or an inert gas atmosphere such as a rare gas (e.g., argon or helium) or under reduced pressure and to a cooling step for treatment for supplying oxygen in an atmosphere of oxygen, an atmosphere of oxygen and nitrogen, or the air (having a dew point of preferably lower than or equal to −40° C., still preferably lower than or equal to −50° C.) atmosphere. The oxide semiconductor layer is thus highly purified, whereby an i-type oxide semiconductor layer is formed. A semiconductor device including a thin film transistor having the oxide semiconductor layer is manufactured.
Owner:SEMICON ENERGY LAB CO LTD
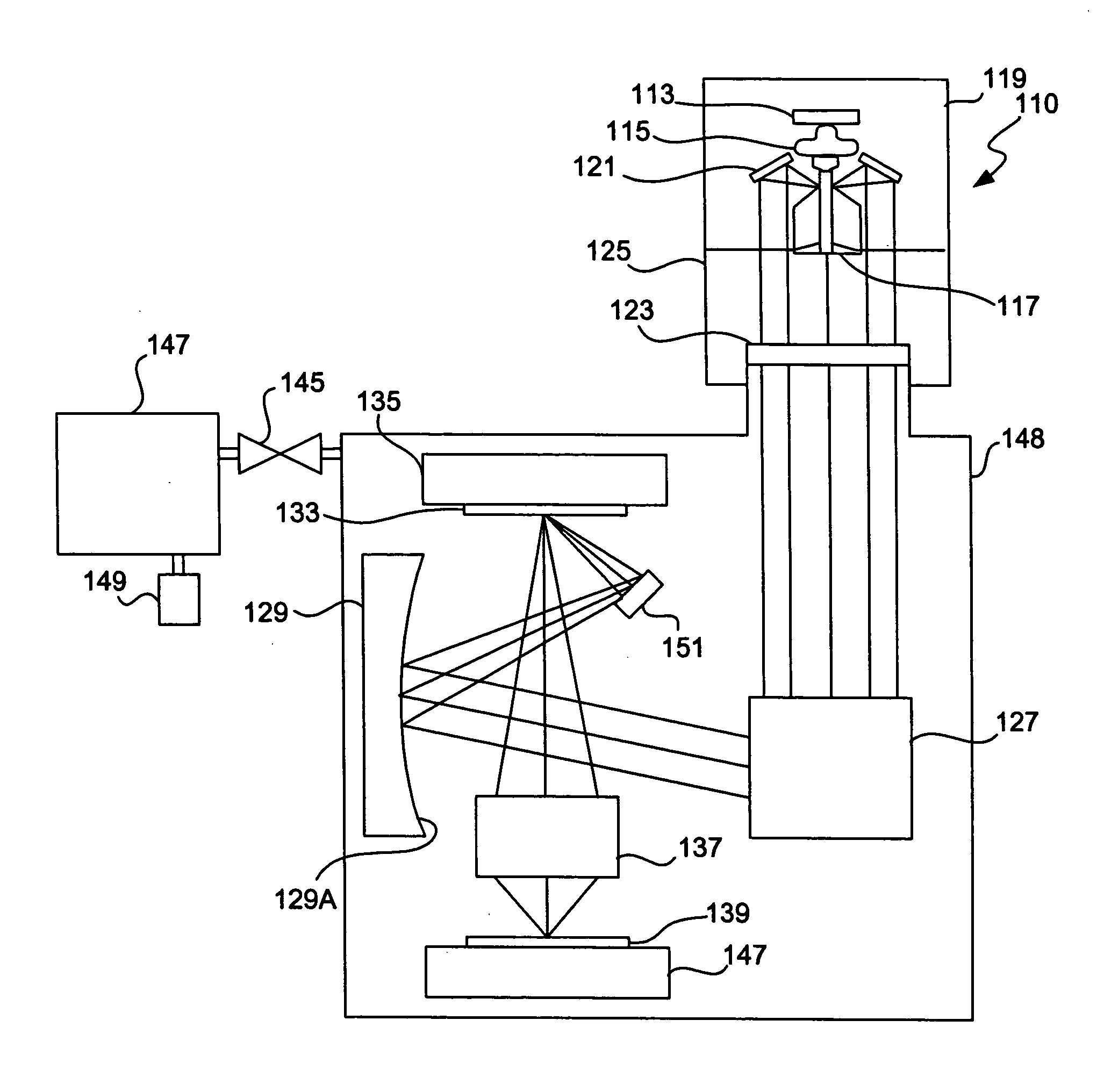
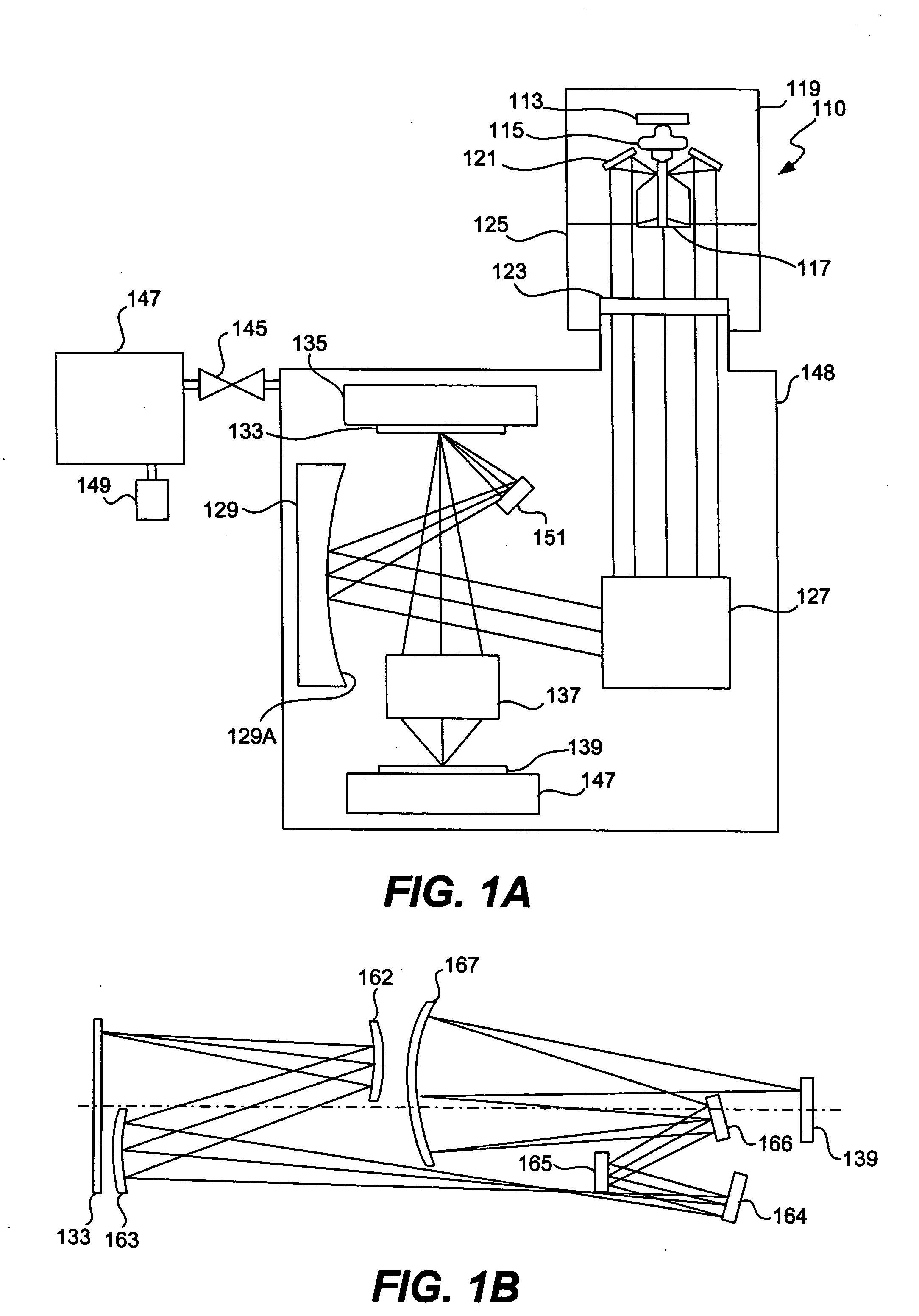
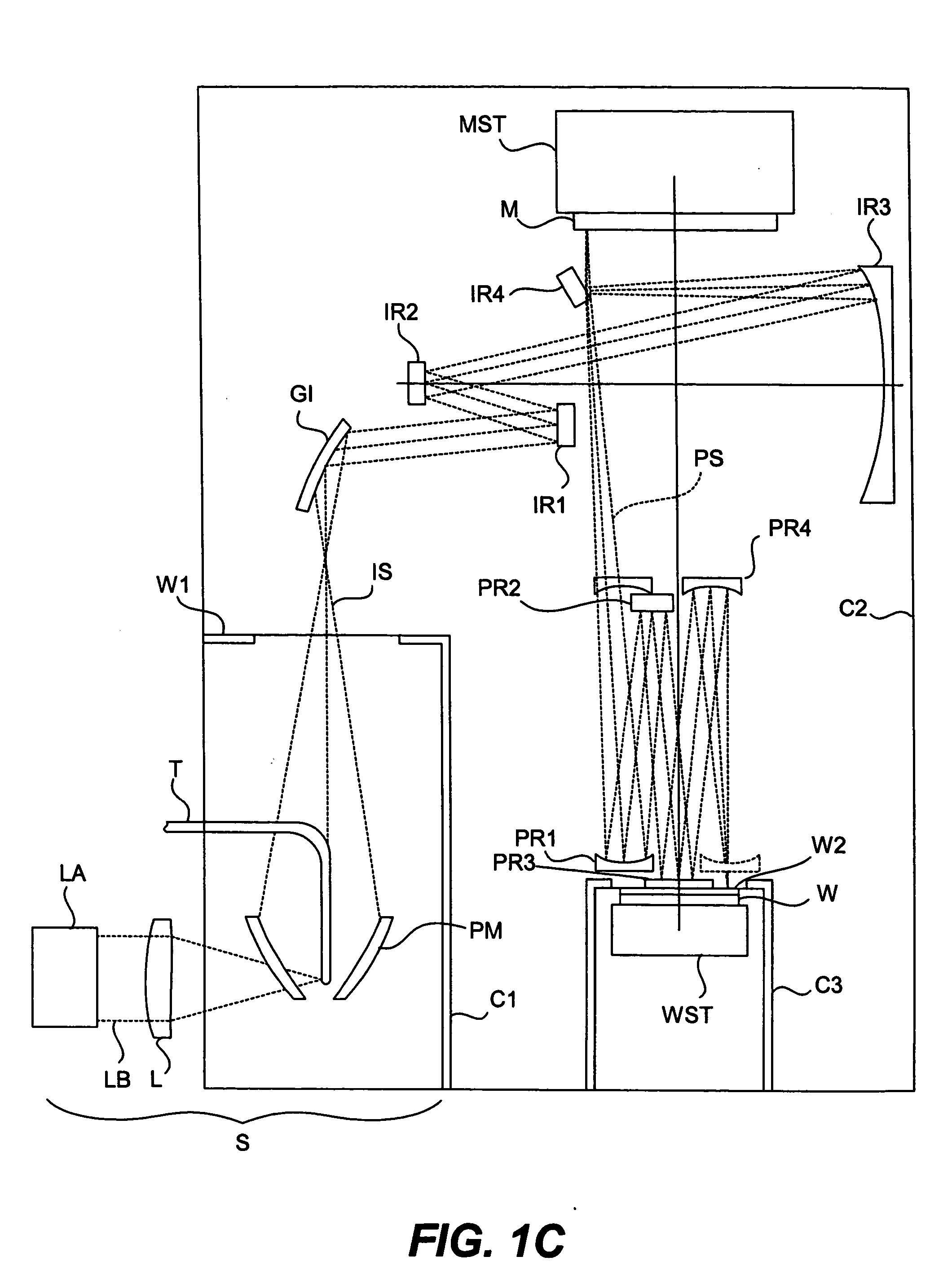
Minimizing thermal distortion effects on EUV mirror
InactiveUS20050099611A1Improve thermal conductivityImprove heat transfer performanceMirrorsSemiconductor/solid-state device manufacturingHeat conductingConductive materials
A mirror is provided with throughholes, or channels, formed through its main body and a coolant pipe of a heat-conductive material is inserted in each of the channels for passing a cooling fluid inside. The outer wall of the coolant pipe does not contact the inner wall of the channel, and there is left a gap in between. The gap contains a heat-conducting gas such as helium. The gap is of a width of less than 100 μm such that the gas has a high heat transfer coefficient even if its pressure is not too high. In some applications the gap may be filled with a heat-conductive fluid. It may be preferable, depending upon the circumstances, to form these channels proximally to the surface on which radiation is made incident. Additionally, the surface of the side of the mirror opposite the reflective side may be heated by auxiliary heat sources.
Owner:NIKON CORP
High-aspect ratio hybrid airship
InactiveUS7137592B2Long flight enduranceHigh operating requirementsAircraft stabilisationEnergy efficient board measuresHigh energyVolumetric Mass Density
Owner:THE BOEING CO
Hermetically sealed disk drive with fill port valve
A novel disk drive includes an enclosure and a disk rotatably mounted to and within the enclosure. The disk drive enclosure has a fill port opening with an internally threaded section and an internal annular seating surface. A fill port valve is disposed within the fill port opening. The fill port valve includes an externally threaded section that is engaged with the internally threaded section of the fill port opening, an external annular seating surface in contact with and forming a continuous annular seal with the internal annular seating surface of the fill port opening, and a gas flow channel spanning the externally threaded section of the fill port valve. The disk drive may be helium-filled, and may also include a metal foil seal that is externally adhered to the disk drive enclosure and covers the fill port opening and the fill port valve.
Owner:WESTERN DIGITAL TECH INC
Method for producing a nanostructured funcitonal coating and a coating that can be produced according to said method
InactiveUS20050011748A1Increase ion densityHigh materialPigmenting treatmentVacuum evaporation coatingOxygenNanostructure
A method for producing a nanostructured, in particular a ceramic-like functional coating on a substrate is described. To that end, using at least one plasma source, a pulsed plasma is produced with which a matrix phase and at least one nano-scale interstitial phase embedded in it are deposited on the substrate via a material input. Preferably a plurality of pulsed plasma sources that are time-correlated or synchronized with each other are used. Also proposed is a nanostructured functional coating, in particular one producible by this method, which is free of chlorine and / or sulfur, and which contains at least one metal and / or at least one element selected from the group oxygen, hydrogen, nitrogen, carbon, helium, argon or neon.
Owner:ROBERT BOSCH GMBH +1
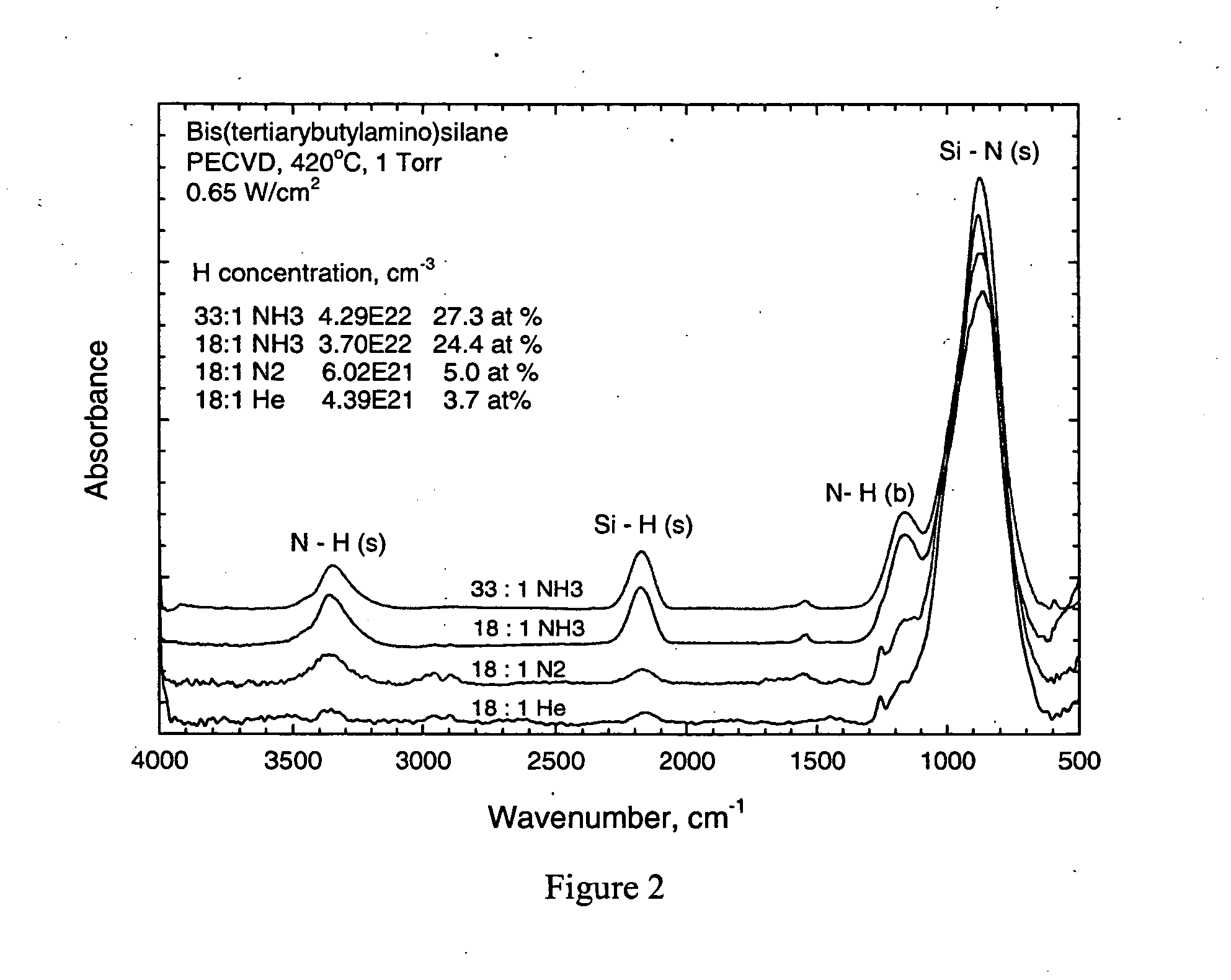
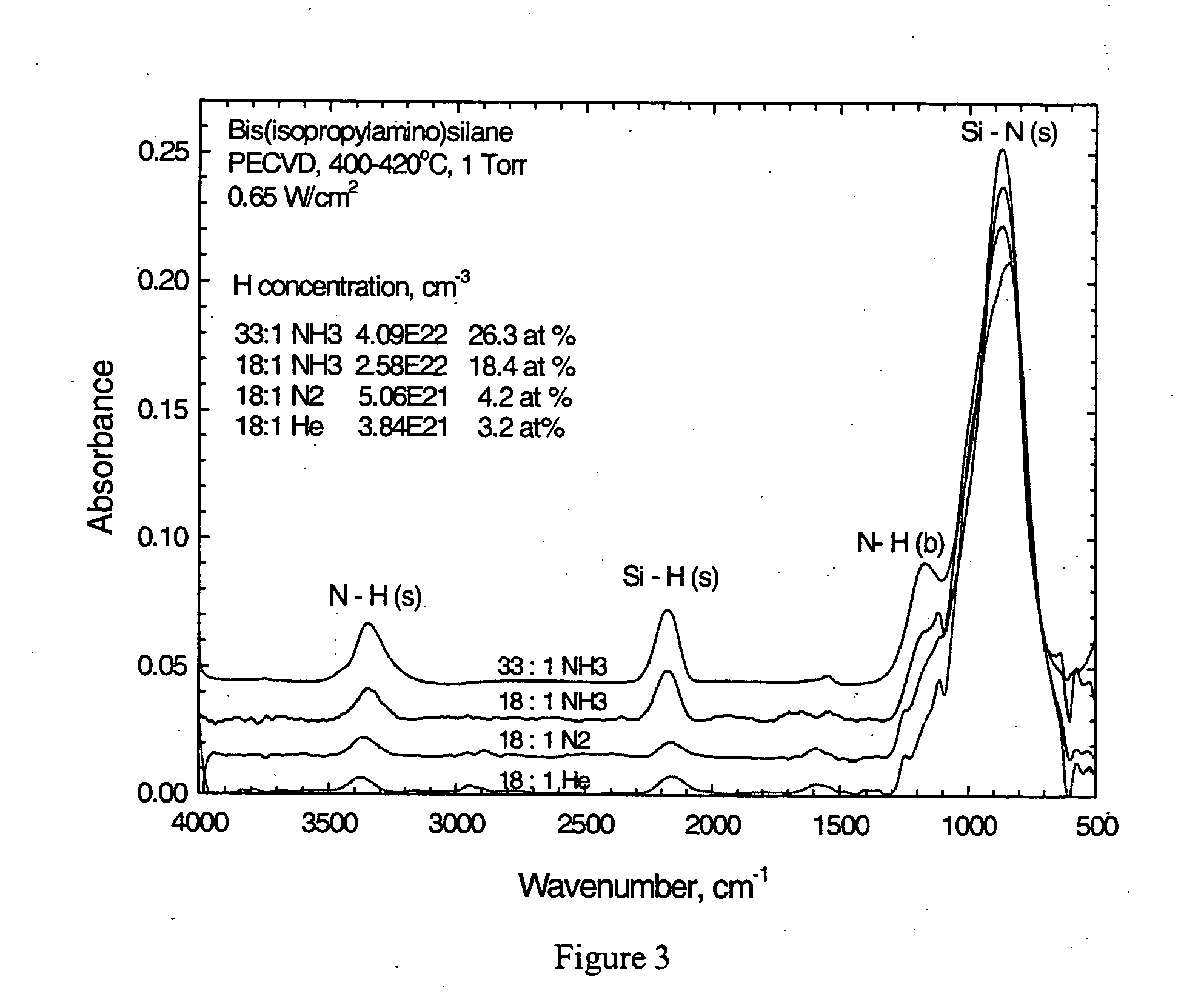
Silicon nitride from aminosilane using PECVD
InactiveUS20060045986A1Enhanced vapor depositionHigh densitySemiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseSilanes
A process for the plasma enhanced chemical vapor deposition of silicon nitride films from nitrogen, argon, xenon, helium or ammonia and an aminosilane, preferably of the formula: (t-C4H9NH)2SiH2 that provides improved properties, particularly etch resistance and low hydrogen concentrations as well as stress control, of the resulting film for use in the semiconductor industry.
Owner:VERSUM MATERIALS US LLC
Inflatable wing flight vehicle
InactiveUS20090108135A1Improved lift characteristicThin atmosphereCosmonautic vehiclesCosmonautic partsNatural satelliteLow speed
The invention is an aircraft having an inflatable wing connected to a base unit, with the inflatable wing inflated with a lifting gas such as helium. The inflatable wing has a series of cell structures, and may be configured with ballonets to selectively introduce and expel outside air within the inflatable wing to vary the buoyancy and / or airfoil properties of the inflatable wing. The aircraft is particularly useful at low speeds and in thin atmospheres (such as at high Earth altitudes and on Mars), and can be used for interplanetary missions to explore planetary bodies, such as moons and planets, having atmospheres.
Owner:SHAW DONALD ORVAL
System for determing the integrity of a package or packaging material based on its transmission of a test gas
InactiveUS6892567B1Detection of fluid at leakage pointPermeability/surface area analysisWater vaporTransmittance
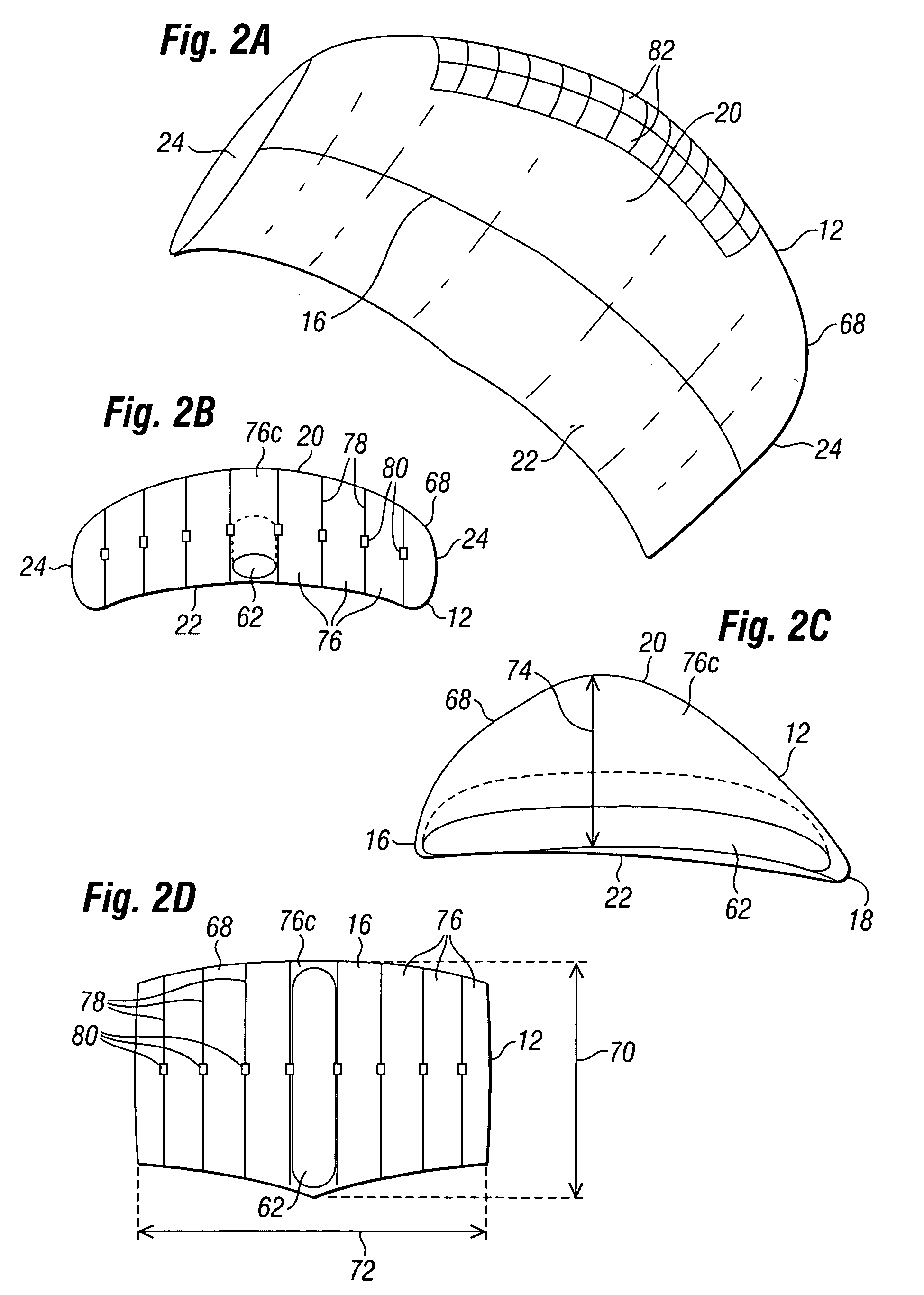
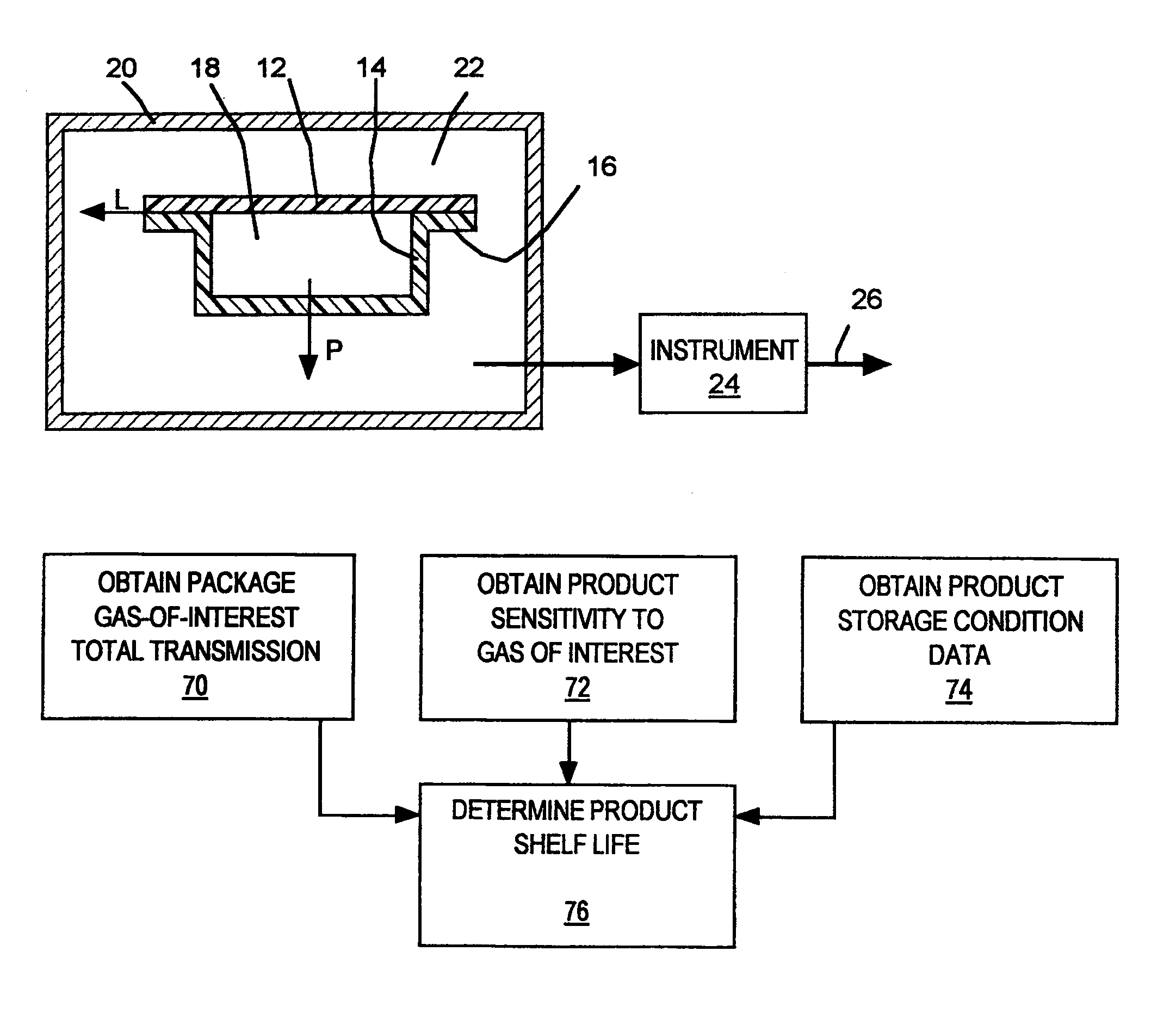
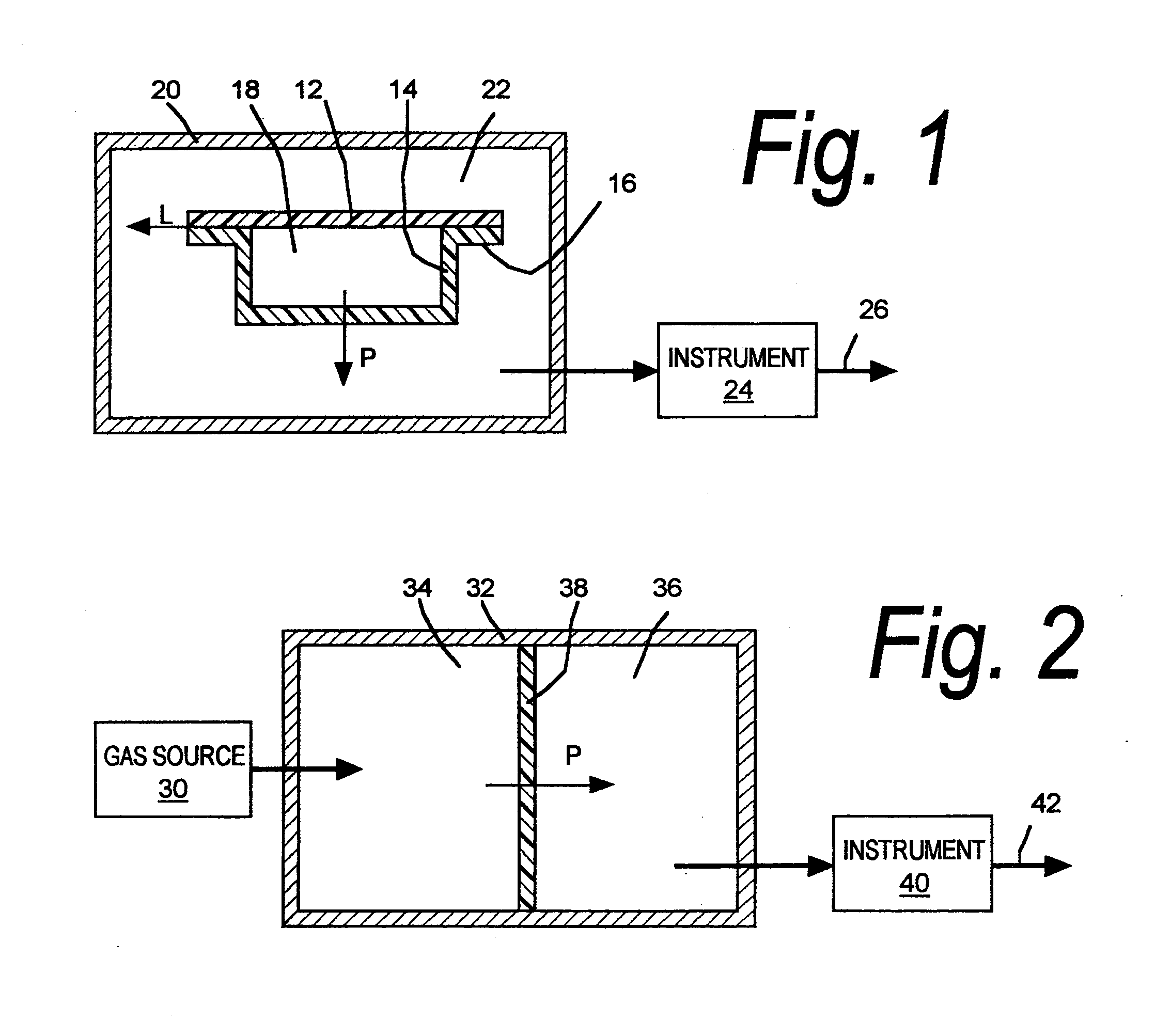
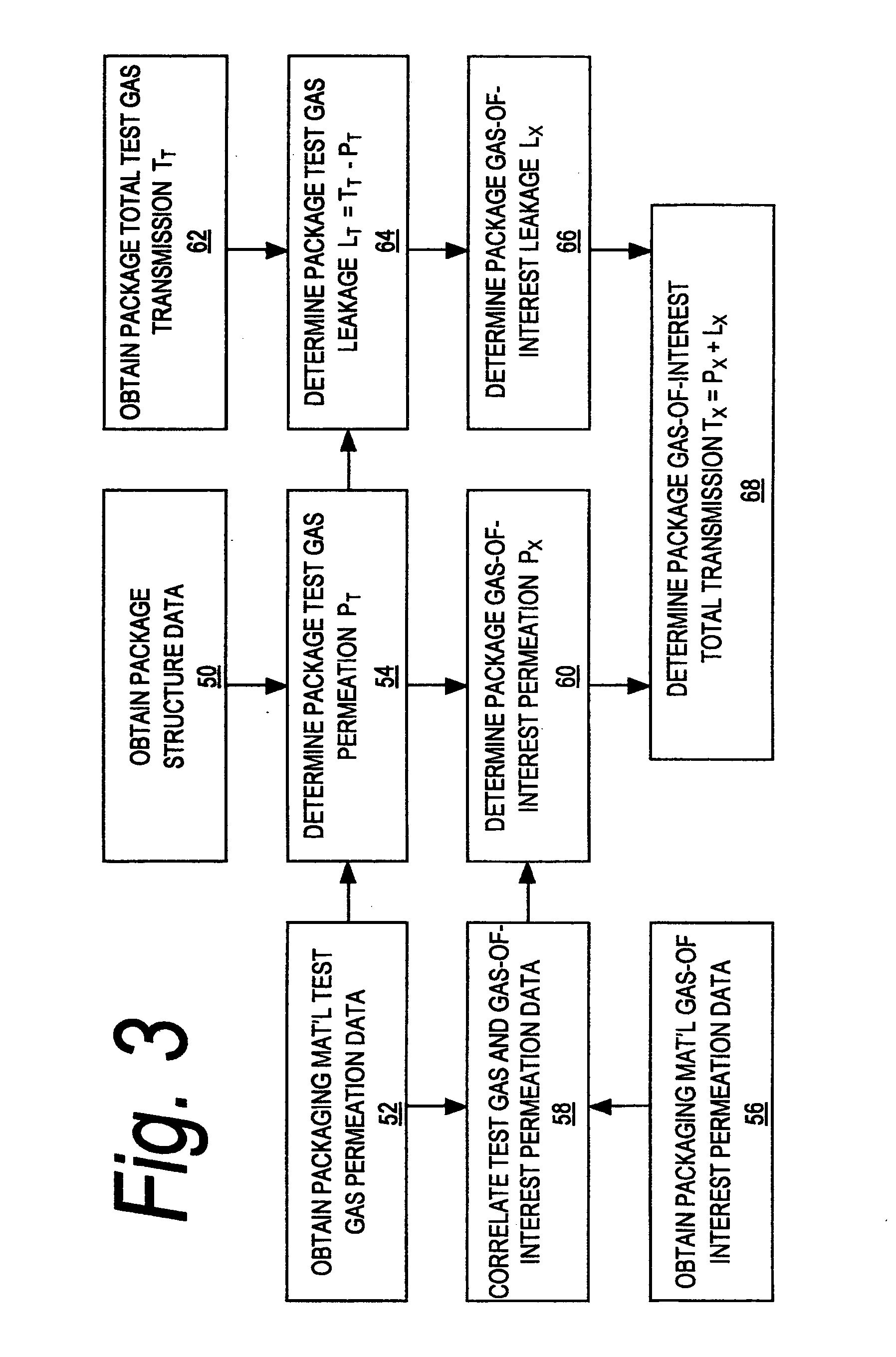
A sealed package's transmission of oxygen, water vapor, carbon dioxide, or other gas or vapor that is of interest because of its potential adverse effects on the package contents is determined indirectly, based upon the package's transmission of a different gas selected as a test gas. Helium is preferred as a test gas. The package's total transmission of the test gas is separated into its components of leakage through the package seals and permeation through the packaging material itself. The package's leakage of the gas of interest is determined based on its leakage of the test gas, in accordance with the molecular weights of the gases. The package's permeation of the gas of interest is determined based on its permeation of the test gas, in accordance with data correlating the permeation of the gas of interest and permeation of the test gas for the materials from which the package is made, and with package structure data relating to the size, shape, and disposition of the materials from which the package is made. The package's total transmission of the gas of interest is determined by adding its leakage and permeation components so determined. Such data may be used with other data to determine a packaged product's shelf life or its sensitivity to a gas of interest.
Owner:MORROW DARRELL R
Method of direct plating of copper on a ruthenium alloy
InactiveUS20060283716A1Semiconductor/solid-state device detailsSolid-state devicesAlloyDirect plating
A method is disclosed for depositing a copper seed layer onto a substrate surface, generally onto a barrier layer that is an alloy of a group VIII metal and a refractory metal. In one aspect, the alloy consists of at least 50% ruthenium and the balance a copper diffusion barrier material. A copper layer is electroplated on the alloy directly. In one aspect, the surface of the barrier layer is conditioned prior to plating to improve adhesion and reduce the critical current density for plating on the barrier layer. The conditioning may include cathodic pre-treatment or a plasma pre-treatment in a hydrogen or hydrogen / helium mixture. In one aspect, the substrate surface is immersed in an acidic plating bath and a nucleation waveform is applied to form a seed layer. In another aspect, the substrate is immersed in a neutral or alkaline copper solution that includes complexed copper ions.
Owner:APPLIED MATERIALS INC
High flux sub-critical reactor for nuclear waste transmulation
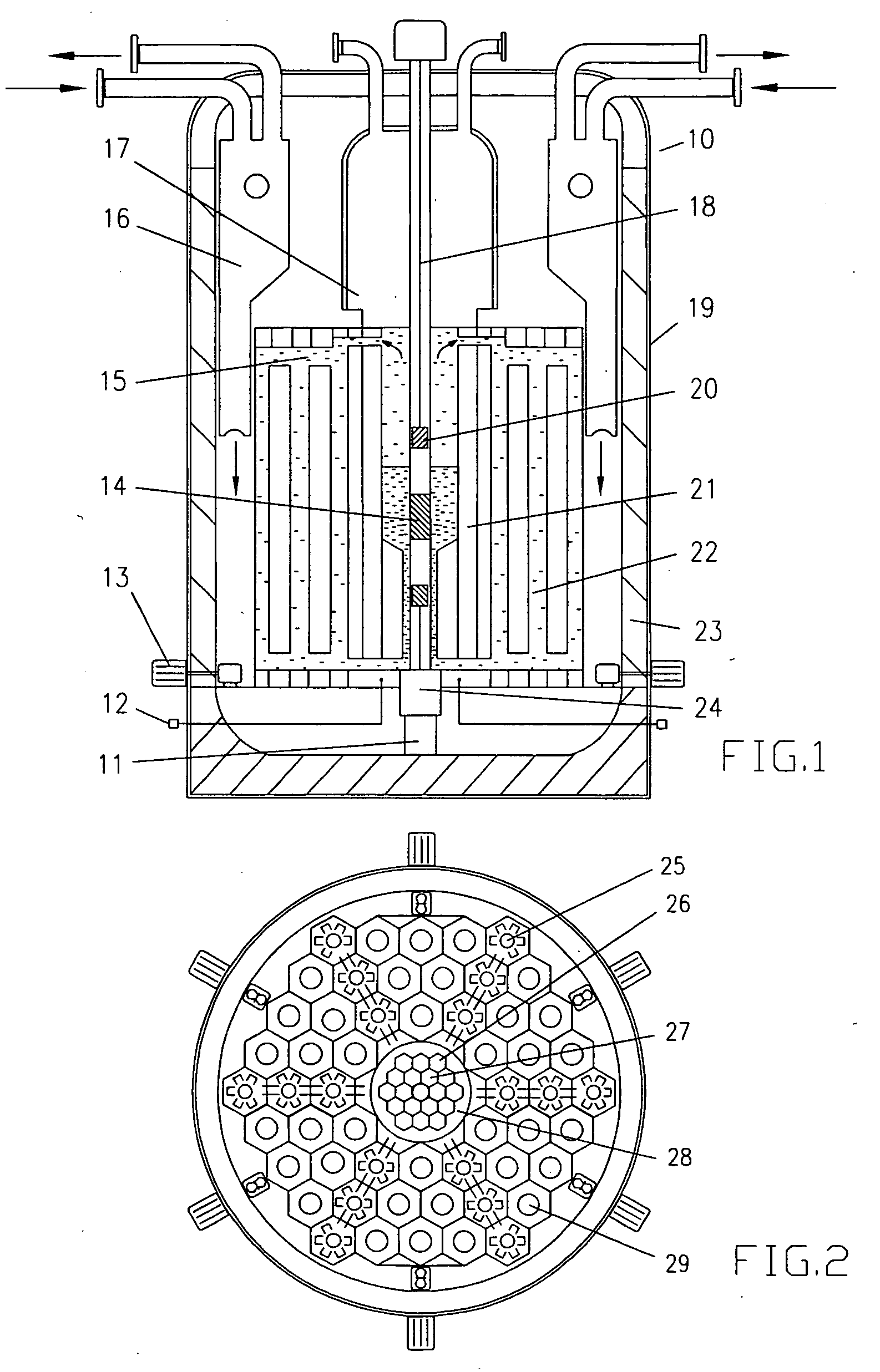
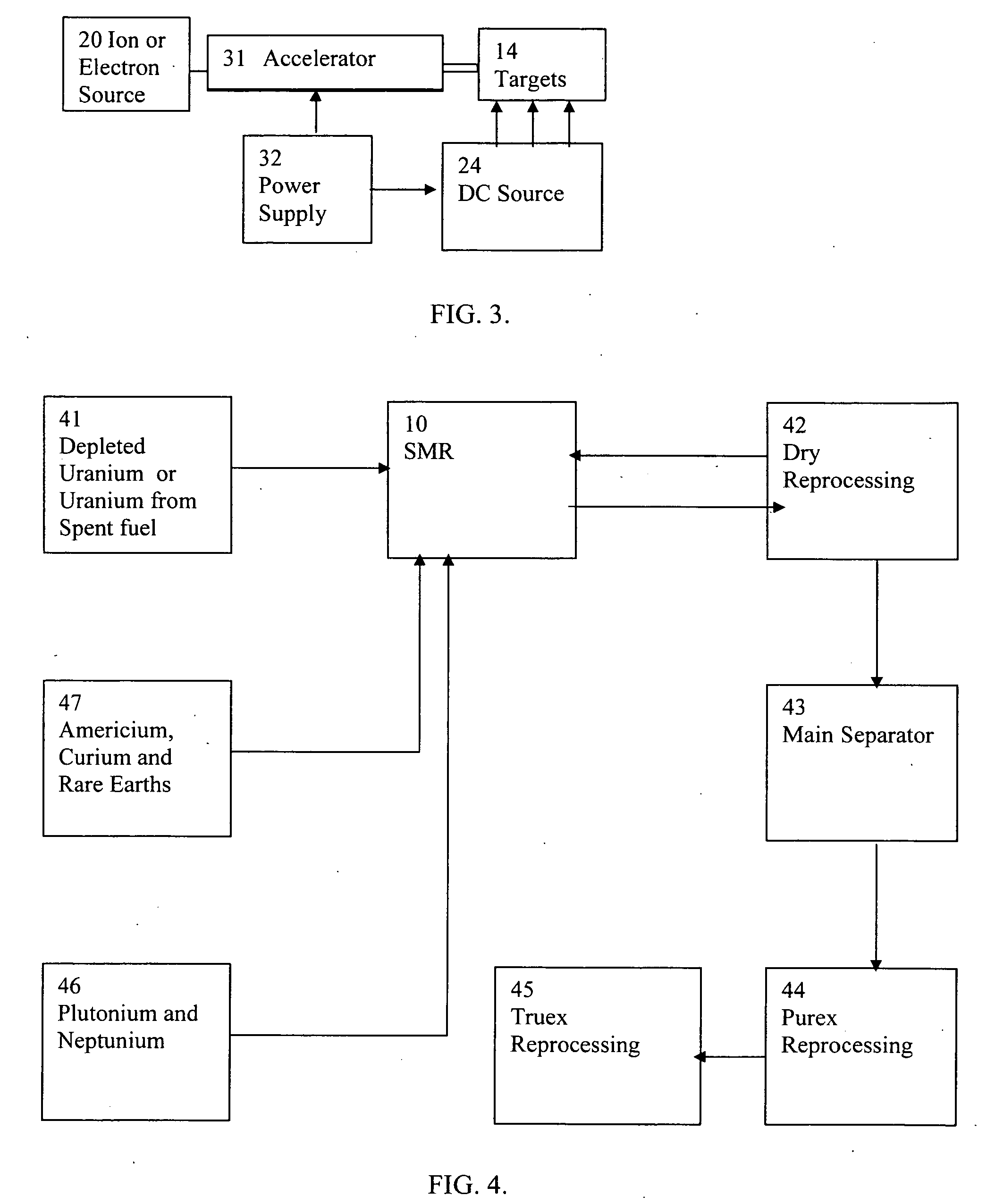
InactiveUS20080232533A1Improve distributionImprove economyConversion outside reactor/acceleratorsNuclear energy generationHigh fluxPu element
A process to safely convert about 95% of the nuclear waste into a usable fuel source is disclosed. The process, involving a sub-critical power reactor and a proliferation-resistant fuel cycle, consumes depleted uranium or thorium fuel with fissionable fuel, including reactor or weapons-grade plutonium. The reactor is comprised of coaxial neutron and energy-amplifying regions separated by moderating and thermal neutron absorbing layers. Control of the water or gas-cooled reactor is provided by plutonium-helium loops with a variable volume flow rate and an external source of neutrons that quickly reacts to any fluctuations of the reactor parameters. A second embodiment of the invention is a compact sub-critical propulsion reactor utilizing fission electric cell and thermo-acoustic technology for electrical power generation.
Owner:BLANOVSKY ANATOLY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com