2 type subunit vaccine for porcine circovirus as well as preparation method and application thereof
A subunit vaccine, porcine circovirus technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of unoptimized production process conditions, loss of protein immunogenicity, and difficulty in cultivation. , to achieve the effect of improving viral protein yield and quality, enhancing specific antibody response, and improving immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
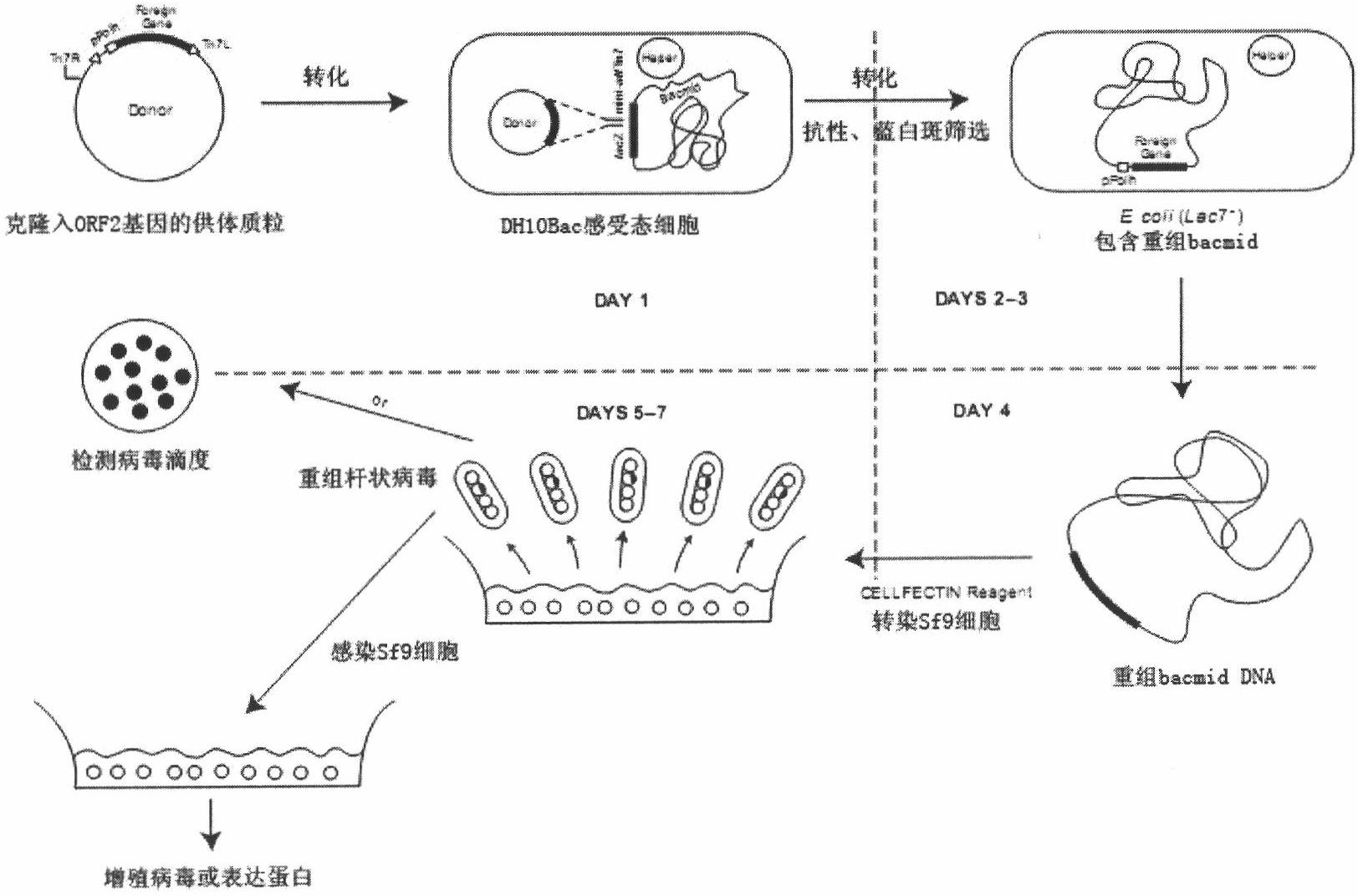
[0061] Example 1 Construction of recombinant baculovirus
[0062] Use the Bac-to-Bac system to construct recombinant baculovirus, and design the following primers according to the gene sequence published by GENBANK (NCBI accession number EU340257.1):
[0063] P1: TCTGGATCCATGACGTATCCAAGGAGGCG
[0064] P2: GCGAAGCTTTAAGGGTTAAGTGGGGGTC
[0065] P3: TCTCTCGAGATGACGTATCCAAGGAGGCG
[0066] P4: GCGGGTACCTAAGGGTTAAGTGGGGGGTC
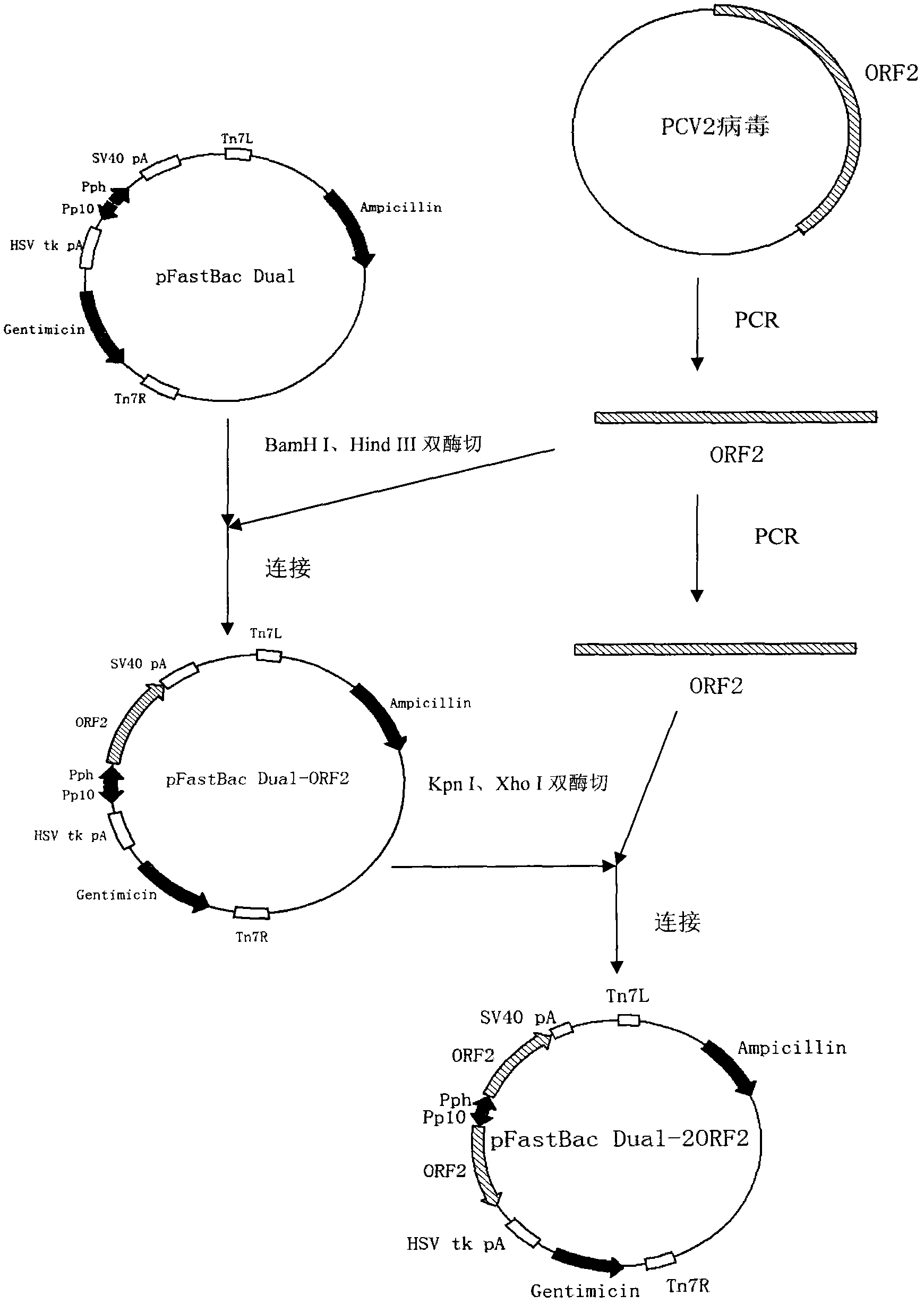
[0067] Using the PCV2b strain virus ORF2 sequence as a template (the template is synthesized according to the published PCV2b ORF2 sequence: accession number EU340257.1), P1 and P2 amplify the PCV2 ORF2 gene, and clone the gene into the pMD-19T vector to obtain The recombinant vector pMD-19T-ORF2-1 uses the PCV2b strain ORF2 sequence as a template, amplifies the PCV2 ORF2 gene at P3 and P4, and clones the gene into the pMD-19T vector to obtain the recombinant vector pMD-19T-ORF2-2 .
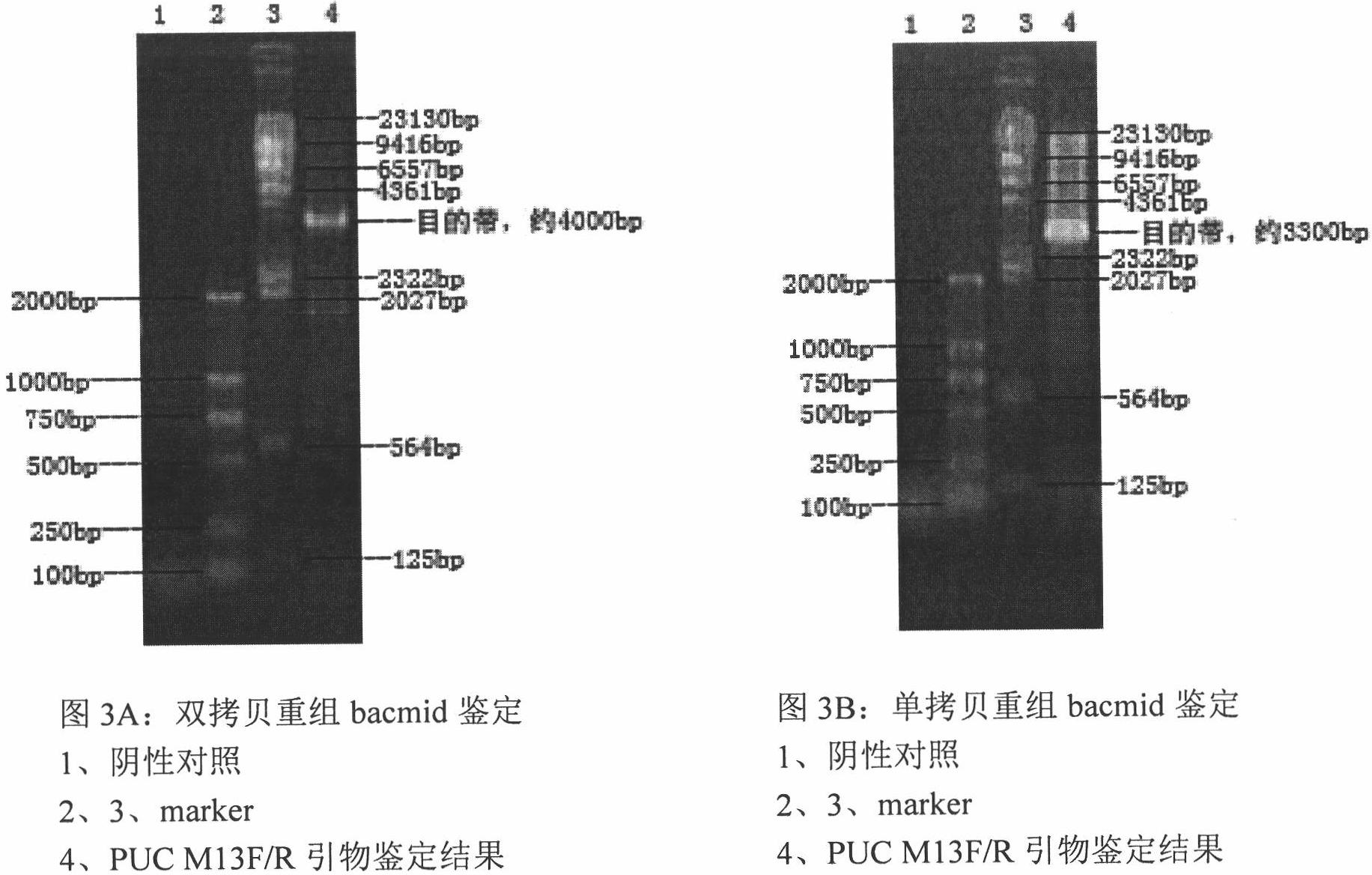
[0068] Digest pMD-19T-ORF2-1 with BamH I and Hind III, clone the ORF2 ...
Embodiment 2
[0069] Example 2 Serum-free Suspension Culture of Insect Cells in a Bioreactor and Quantification of Cap Protein Expression
[0070] Aseptically culture Sf9 insect cells in a 1000ml shake flask for 3-4 days until the concentration reaches 3-5×10 6 cells / ml, when the viability is greater than 95%, the cells were inoculated into a 5L bioreactor with an inoculation concentration of 3-8×10 5 cells / ml. When the cell concentration reaches 3-5×10 6 cells / ml, inoculate the cells into a 50L bioreactor, and wait for the cells to grow to a concentration of 3-5×10 6 cells / ml, inoculate into a 500L bioreactor until the cell concentration reaches 2-8×10 6 When cells / ml, inoculate the recombinant virus rBac-2ORF2 or rBac-ORF2, the multiplicity of infection (MOI) is 0.001-10, the reactor culture conditions are pH 6.0-6.5, temperature 25-27°C, dissolved oxygen 30-80%, stirring Speed 100-180rpm. Considering the optimal conditions for cell culture, the preferred pH is 6.2, the temperatu...
Embodiment 3
[0076] Example 3 Purification of VLP Particles and Observation with Electron Microscope
[0077] Harvest the expression cell culture, centrifuge the cell culture at 10,000 g at 4°C for 30 min, remove cell debris, take the supernatant, and centrifuge the supernatant at 31,000 rpm for 3 h (Beckman SW70 rotor), resuspend the precipitate with a small amount of PBS, and wait until the precipitate is completely dissolved , add CsCl according to 2.1g / 4.5ml solution, mix evenly, dispense into 5ml ultracentrifuge tubes, add PBS to a distance of 2-3mm from the tube mouth, after accurate balance, centrifuge at 149000g at 10°C for 24h. After centrifugation, two light yellow transparent bands can be seen. The target band (lower band) is aspirated and collected, which is the purified virus-like particles.
[0078] Under the same conditions, culture an equal amount of cell cultures expressing rBac-ORF2 and rBac-2ORF2, and treat them according to the above method, dilute the aspirated targ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com