Method for producing avian adenovirus inactivated vaccine through LMH clone line
An inactivated vaccine, avian adenovirus technology, applied in veterinary vaccines, biochemical equipment and methods, vaccines, etc., can solve the problems of low vaccine protection rate, low virus titer, poor cell viability, etc. High, reduce production costs, improve the effect of valence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of liposome complex:
[0037] (1) Weigh 0.2g phosphatidylcholine, 0.08g cholesterol, and 0.04g distearoylphosphatidylethanolamine, mix, dissolve in 50mL ethanol, and ultrasonic treatment for 5min;
[0038] (2) Then, the ethanol was removed by rotary evaporation under the conditions of 28℃ and 0.1MPa, and it was allowed to stand for 30min. Then, add 100mL pH 7.4 and 0.5% (m / v) catalase-containing phosphate buffer solution, and put it in a 35℃ water bath. The hydration reaction is about 1h by shaking to obtain liposome suspension;
[0039] (3) Use a syringe filter to adjust the particle size of the liposome suspension to 150 nm to obtain a liposome containing catalase inside.
Embodiment 2
[0040] Example 2 Selection of the best trypsin-EDTA concentration for digesting LMH cells and the selection of the best culture time for LMH cells
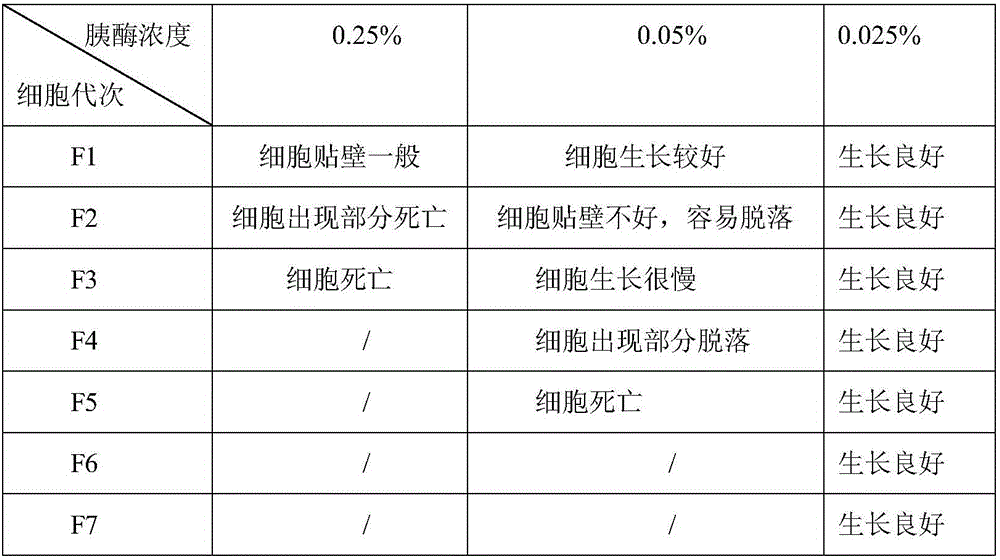
[0041] The LMH cells (ATCC LMH-CRL-2117) were digested and dispersed with trypsin-EDTA (0.02%) with mass-volume ratios of 0.25%, 0.05%, and 0.025%; added with 5-10% (v / v) newborn Bovine serum, 1.0-1.2% (v / v) bi-antibody and 0.5-2.5% (m / v) hydroxyethylpiperazine ethanesulfonic acid in DMEM culture medium at 37℃, 5% CO 2 Cultivate the cells to a monolayer in an incubator for passage.
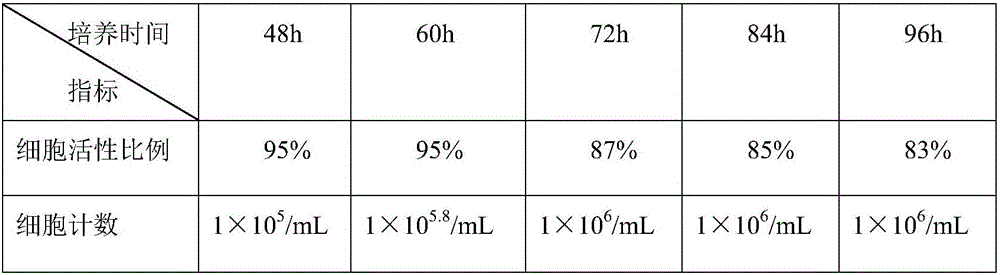
[0042] The LMH cells digested with three different concentrations of trypsin-EDTA were passaged separately, and the cell growth of different passages is shown in Table 1. Digest LMH cells with 0.025% pancreatin-EDTA (0.02%) in a mass-volume ratio at 37℃, 5% CO 2 After culturing in an incubator for different times, the cells were tested for cell viability by MTT method and counted with a red blood cell counter. The results are shown in Table 2.
[0043] Tabl...
Embodiment 3
[0049] Example 3 Screening of the optimal inoculation concentration of avian adenovirus type 4
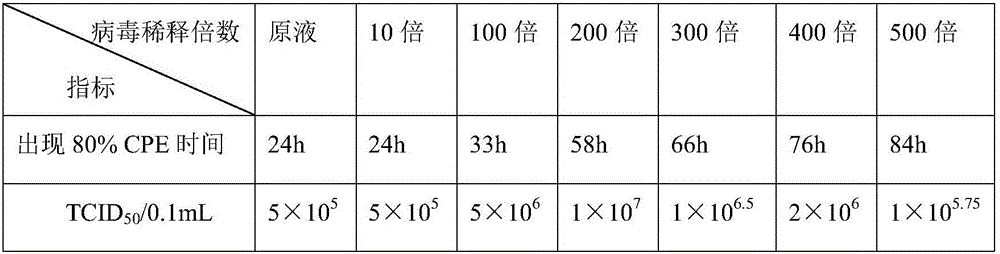
[0050] The monolayer of LMH cells were diluted at different concentrations for avian adenovirus type 4 inoculation. After inoculation to about 80% of the cells, the cell venom was harvested and freeze-thawed twice at -20°C, 5000rpm, After centrifugation at 4°C for 10 minutes, collect the supernatant, which is the virus stock, and determine its TCID 50 , And the results are shown in Table 3.
[0051] Table 3 Screening of avian adenovirus type 4 inoculation concentration
[0052]
[0053] The results show that when the virus solution with different dilution concentrations is used for inoculation, the time when the cells appear lesions are very different and the titer of the cytovenom is very different. From the above table, it can be seen that the 200-fold diluted virus was used for inoculation and cultured for 58 hours. The virus has the highest titer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com