Patents
Literature
63 results about "Adenovirus vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An adenovirus vaccine is a vaccine against Adenovirus. It was used by the United States military from 1971 to 1999, but was discontinued when the only manufacturer stopped production. This vaccine elicited immunity to serotypes 4 and 7. On March 16, 2011, the U.S. Food and Drug Administration approved an adenovirus vaccine manufactured by Teva Pharmaceuticals under contract to the U.S. Army. This vaccine is essentially the same as the one used from 1971 to 1999. On October 24, 2011, the military services began administering the new adenovirus vaccine to recruits during basic training.
Porcine reproductive and respiratory syndrome bivalence recombinant adenovirus vaccine and preparation method thereof
InactiveCN101380468AImmediately exert cellular immune functionNot pathogenicViral antigen ingredientsAntiviralsEukaryotic plasmidsAttenuated vaccine
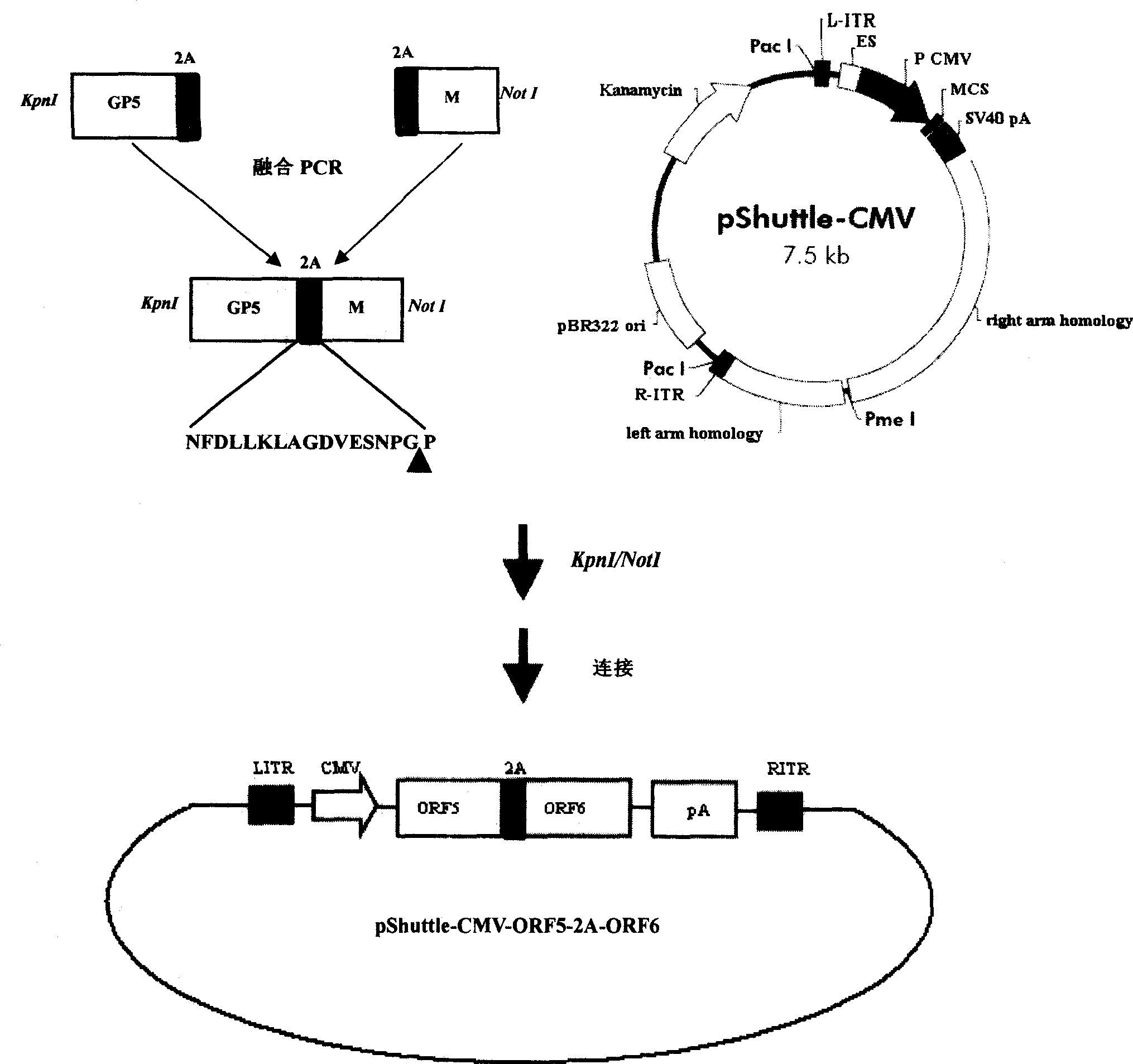
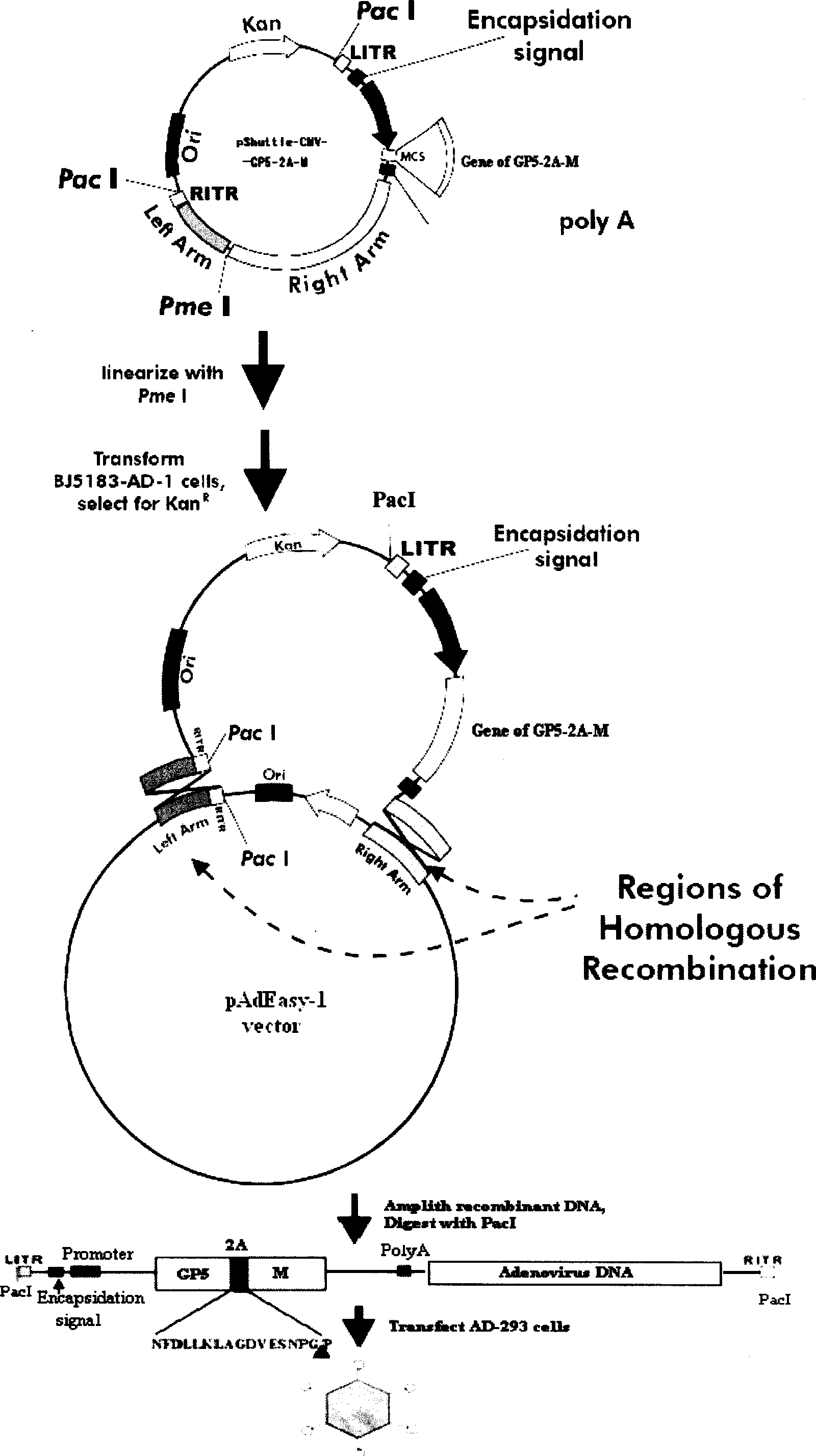
The invention discloses a porcine reproductive and respiratory syndrome divalent recombination adenovirus vaccine and the preparation method thereof. The invention belongs to the technical field of biological vaccine preparation. The vaccine can be prepared by the following steps: a GP5-2A-M fusion protein gene can be constructed by inserting an FMDV2A gene with self craking between PRRSV GP5 and M protein; homologous recombination is carried out on the GP5-2A-M fusion protein gene and adenovirus backbone plasmid pAdEasy-1; recombination adenovirus rAd-GP5-2A-M is prepared by restriction enzyme and HEK-293A cells transfection, and the divalent recombination adenovirus vaccine is prepared by the technology and the steps such as purification, amplification, and the like. After expression, the aggregate protein GP5-2A-M constructed by the invention is self cracked into GP5 and M protein, as well as exerts the viral neutralization of GP5 and the immune function of the M protein; the vaccine has stable titer with the virulent valence being 10<10.43>TCID<50> / 1.0ml, as well as has both the duplication characteristic of a routine attenuated vaccine and the safety of an inactivated vaccine; the divalent recombination adenovirus vaccine can be popularized in and applied to the control work of porcine reproductive and respiratory syndrome.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
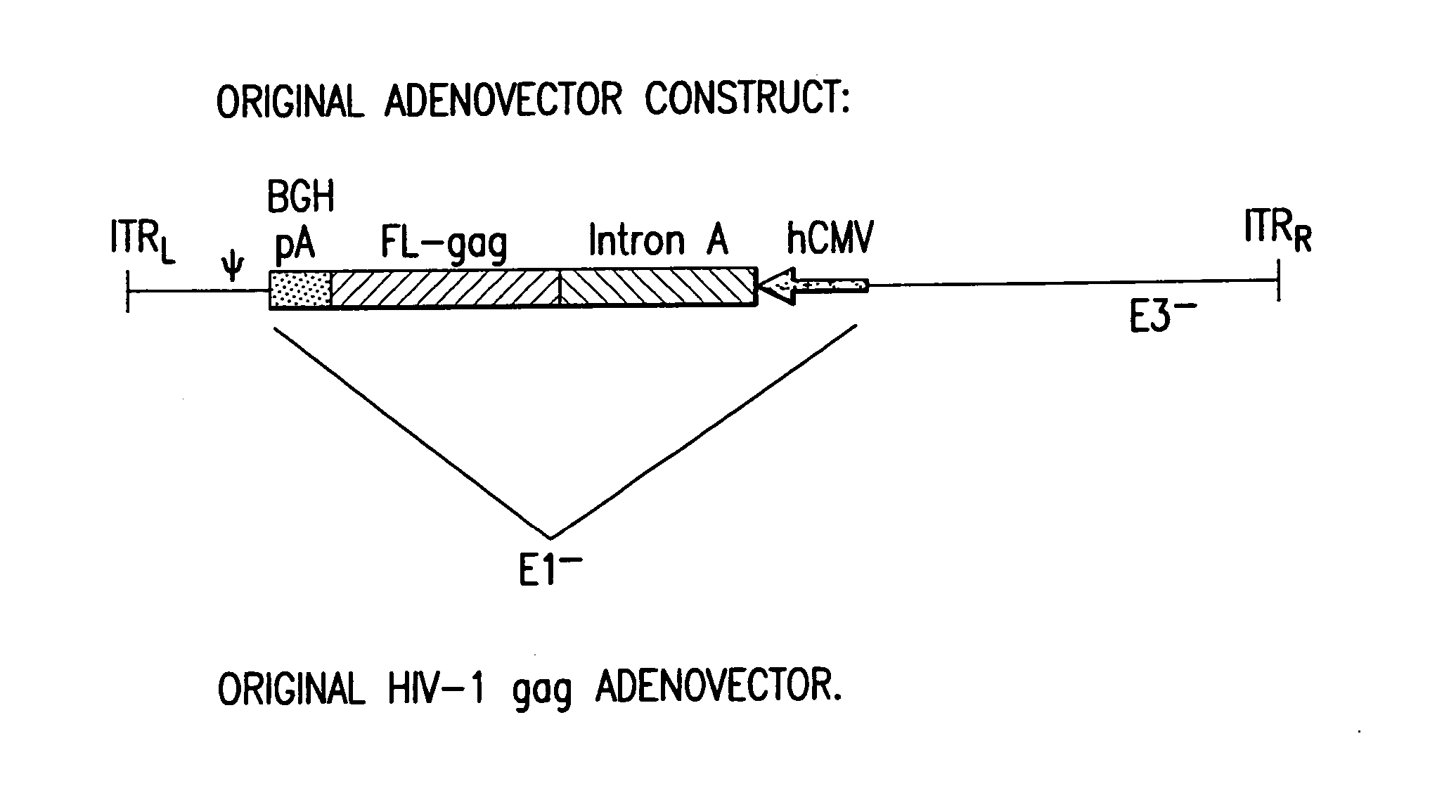
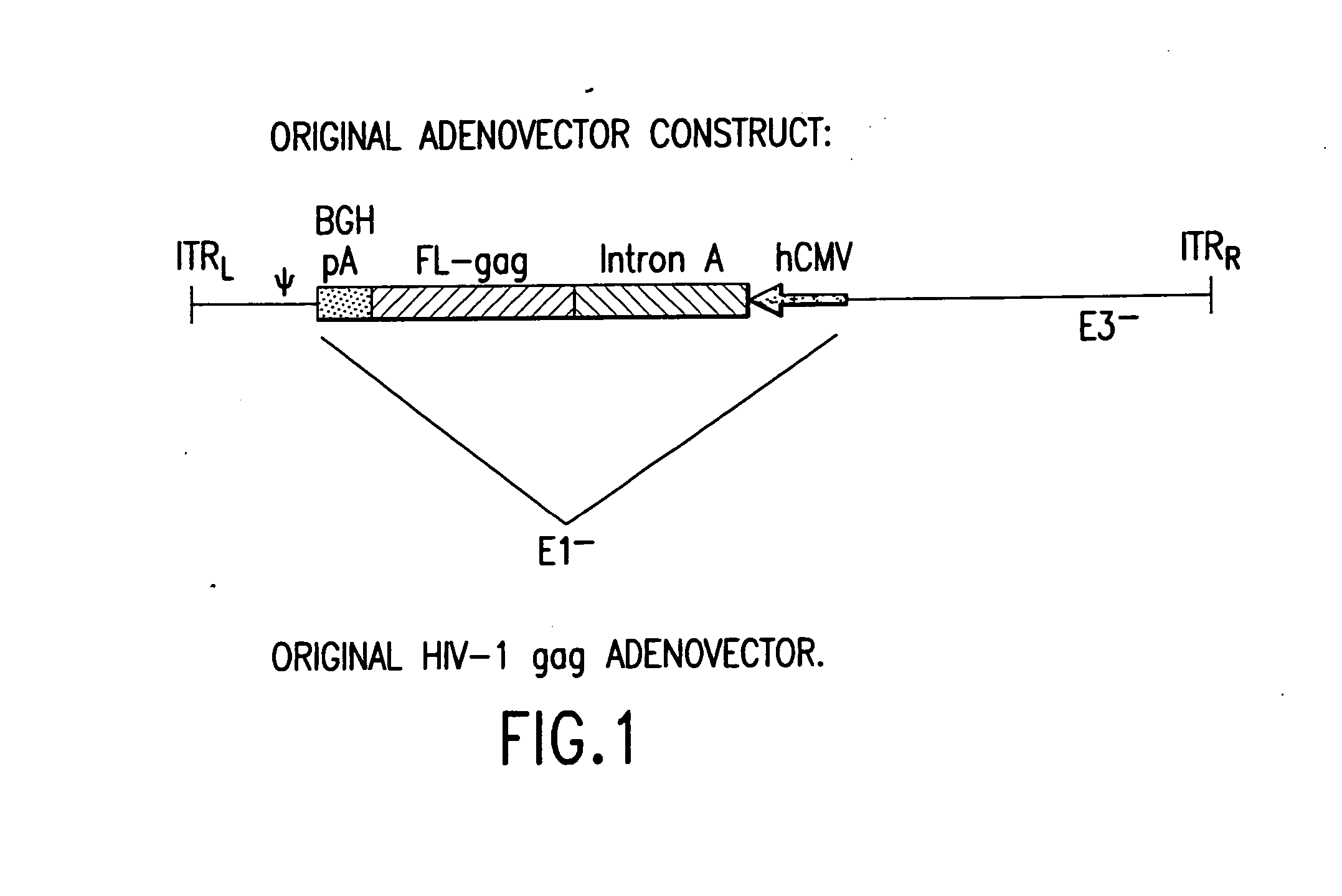
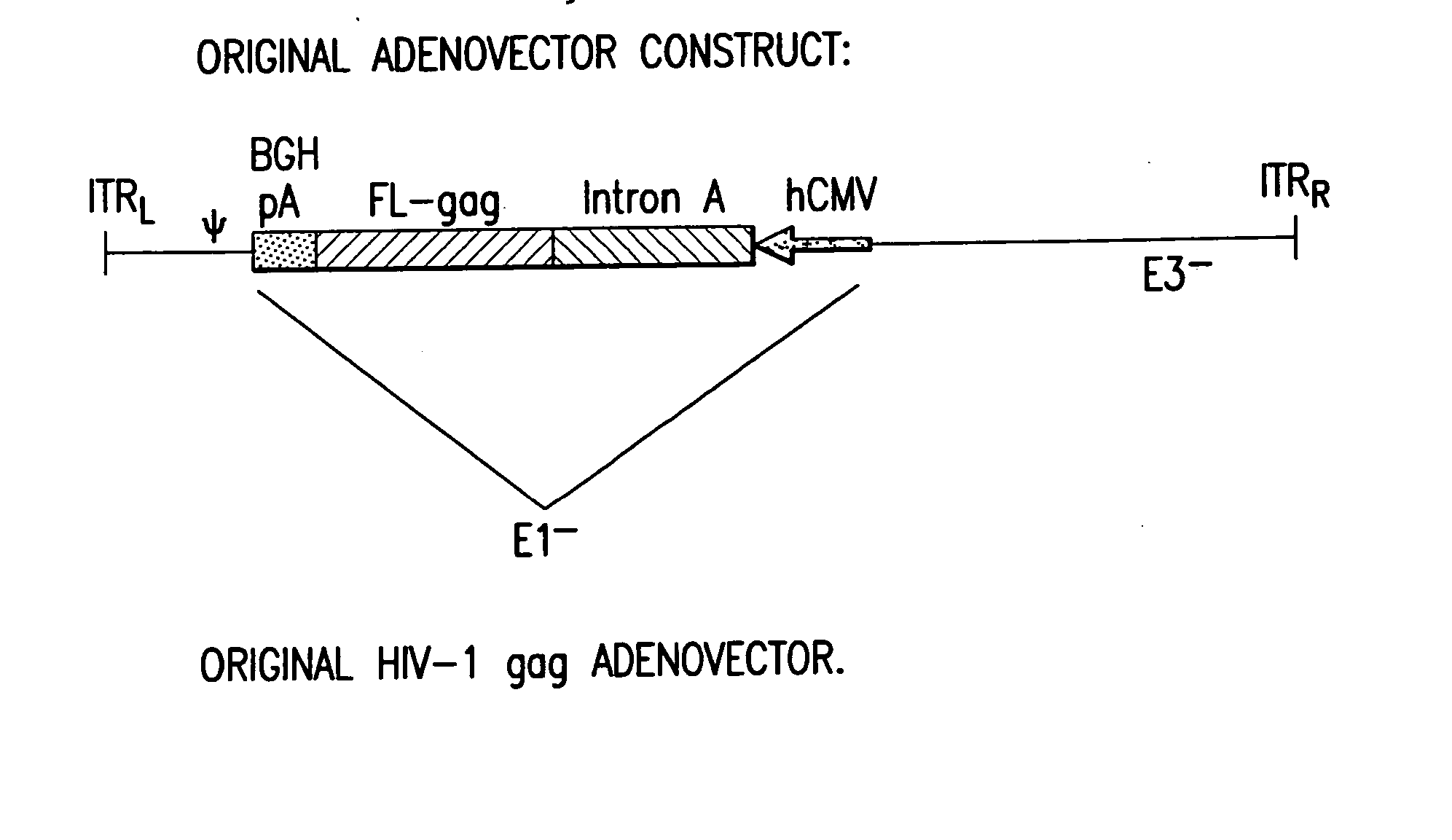
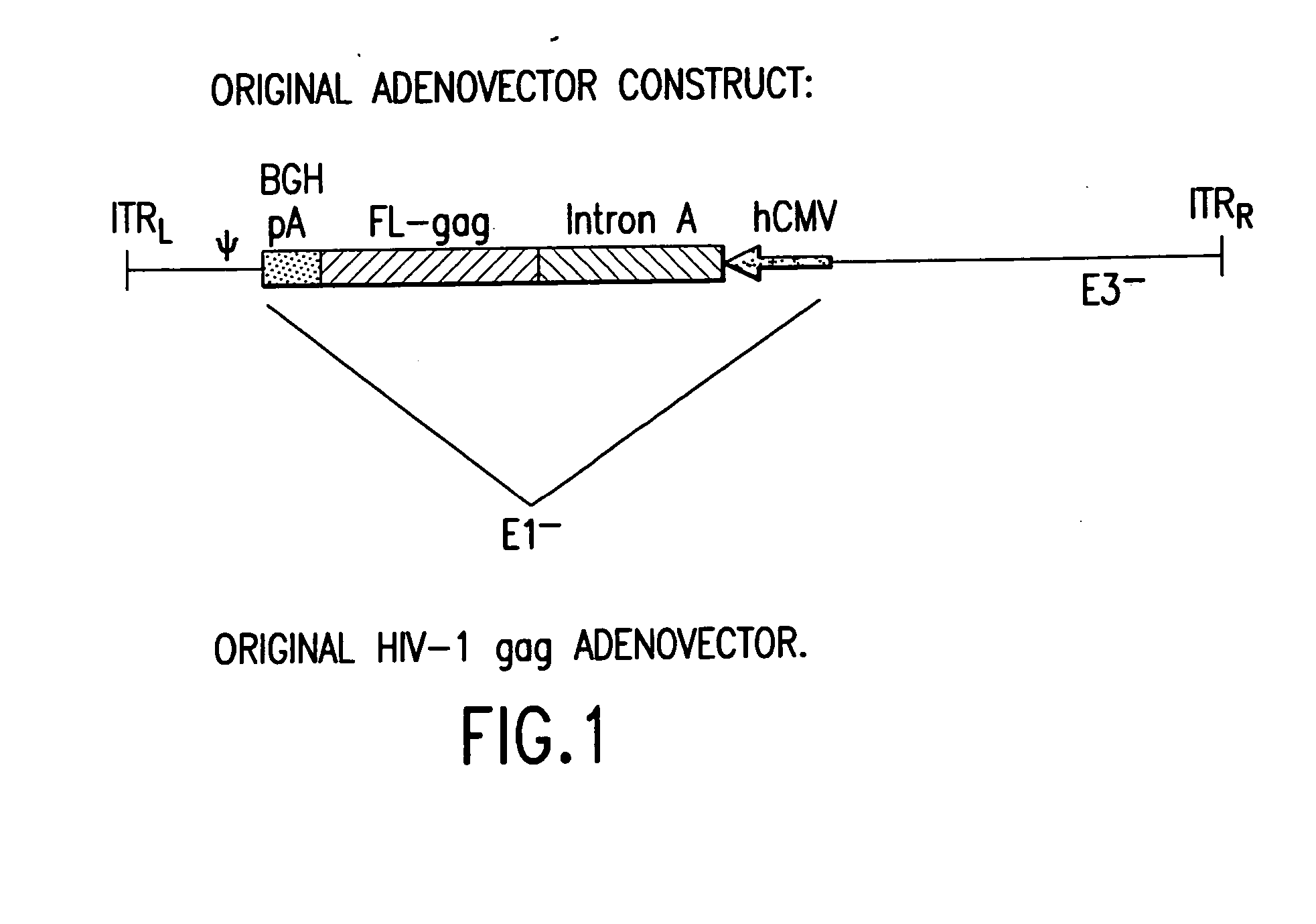
Enhanced first generation adenovirus vaccines expressing codon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070054395A1Genetically stableImprove featuresVectorsViral antigen ingredientsMammalReverse transcriptase
First generation adenoviral vectors and associated recombinant adenovirus-based HIV vaccines which show enhanced stability and growth properties and greater cellular-mediated immunity are described within this specification. These adenoviral vectors are utilized to generate and produce through cell culture various adenoviral-based HIV-1 vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof. These adenovirus vaccines, when directly introduced into living vertebrate tissue, preferably a mammalian host such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV1-Gag, Pol and / or Nef protein or biologically modification thereof, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, encoding codon optimized HIV-1 Pol, derivatives of optimized HIV-1 Pol (including constructs wherein protease, reverse transcriptase, RNAse H and integrase activity of HIV-1 Pol is inactivated), HIV-1 Nef and derivatives of optimized HIV-1 Nef, including nef mutants which effect wild type characteristics of Nef, such as myristylation and down regulation of host CD4. The adenoviral vaccines of the present invention, when administered alone or in a combined modality regime, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
Preparation method of porcine epidemic diarrhea recombinant adenovirus vaccine
InactiveCN102512693AImprove abilitiesThe production is effectiveGenetic material ingredientsAntiviralsEnzyme digestionA-DNA
The invention discloses a preparation method of a porcine epidemic diarrhea recombinant adenovirus vaccine. The preparation method provided by the invention comprises the following steps of inserting a DNA sequence of a zone S1 of a porcine epidemic diarrhea virus (PEDV) into an adenovirus shuttle plasmid pShuttle-CMV to obtain pShuttle-CMV-S1, carrying out linearization of the pShuttle-CMV-S1, transforming the linear pShuttle-CMV-S1 into a BJ5183 competent cell containing pAdEasy-1, carrying out homologous recombination, carrying out enzyme digestion, carrying out AD-293 cell transfection, carrying out packaging to obtain a recombinant adenovirus rAd-S1, and carrying out purification, amplification and sub-packaging. After oral immunization, the porcine epidemic diarrhea recombinant adenovirus vaccine can induce generation of mucosal immunity thereby preventing porcine epidemic diarrhea (PED) well.
Owner:GENIFARM LAB INC
Method for establishing recombinant adenovirus vector with Africa swine fever EP153R and P54 gene coexpression and packaging adenovirus
PendingCN109735567AEnhance immune responseViral antigen ingredientsAntiviralsGene coexpressionMultiple cloning site
The invention provides a method for establishing a recombinant adenovirus vector with Africa swine fever EP153R and P54 gene coexpression and packaging adenovirus and belongs to the technical field ofgene engineering. According to the adenovirus vector of coexpression of the EP153R gene and the P54 gene, with a pShuttle-CMV eukaryotic expression vector as a basis, the CTLA4, EP153R and P54 genesare introduced at multiple cloning sites. The invention further provides recombinant adenovirus with coexpression of the EP153R and P54 genes. The established pAD-Shuttle-CMV-CTLA4-EP153R-P54 vector is used for achieving the adenovirus packaging process, the adenovirus which can directly infect animals or eukaryocyte is obtained, the aim of coexpression of the EP153R and P54 genes in eukaryocyte is achieved, and the basis is laid for research on adenovirus vaccines of coexpression of the EP153R and P54 genes.
Owner:YANGZHOU UNIV
Enhanced first generation adenovirus vaccines expressing condon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070077257A1Improved cellular-mediated immune responseLow transmission rateViral antigen ingredientsVirus peptidesTreatment effectNucleotide
First generation adenoviral vectors and-recombinant adenovirus-based HIV vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof are described. The adenovirus vaccines, when directly introduced into living vertebrate tissue, express the relevant proteins, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, HIV-1 Pol, HIV-1 Nef, and derivatives thereof. The adenoviral vaccines of the present invention, alone or in combination, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
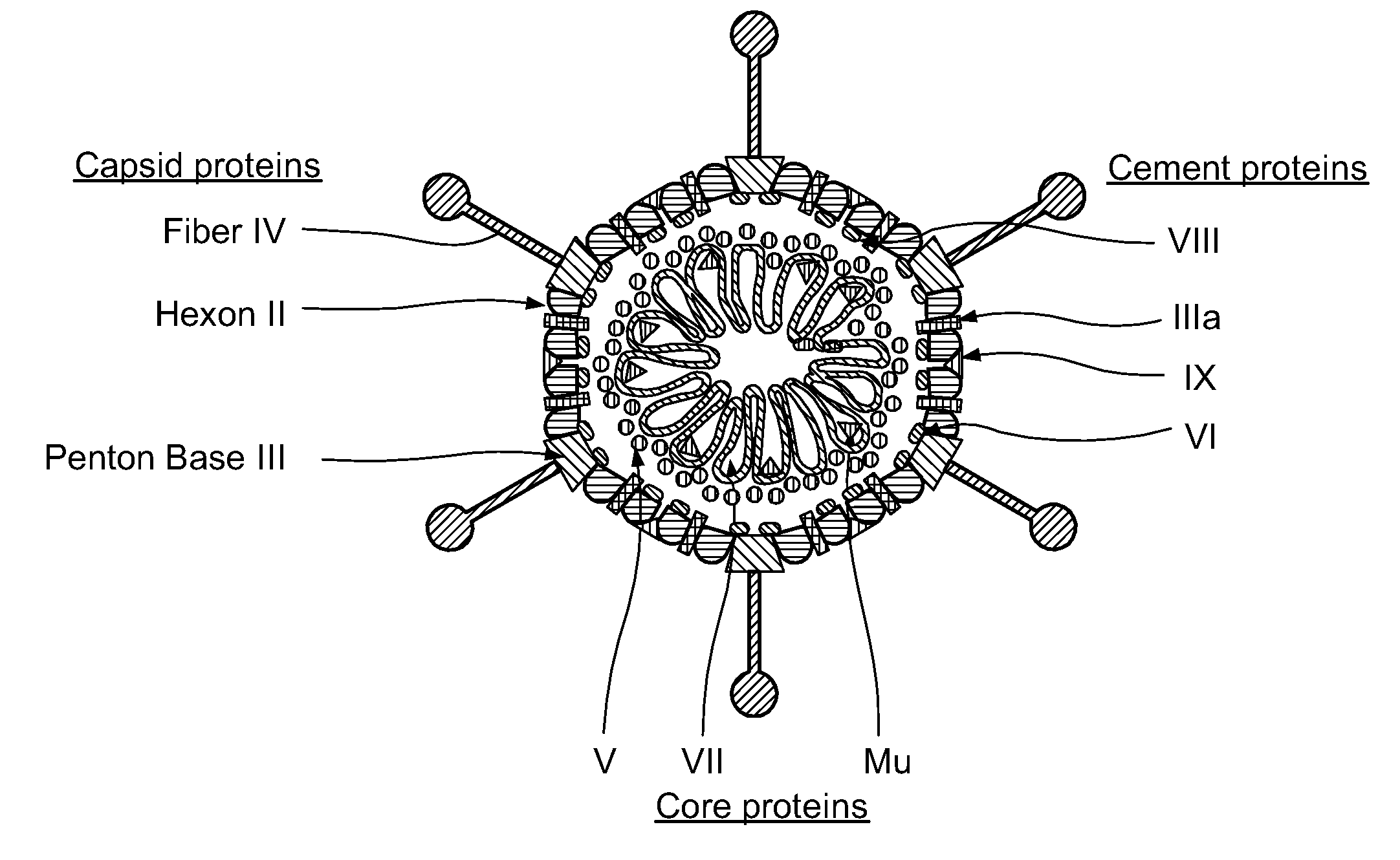
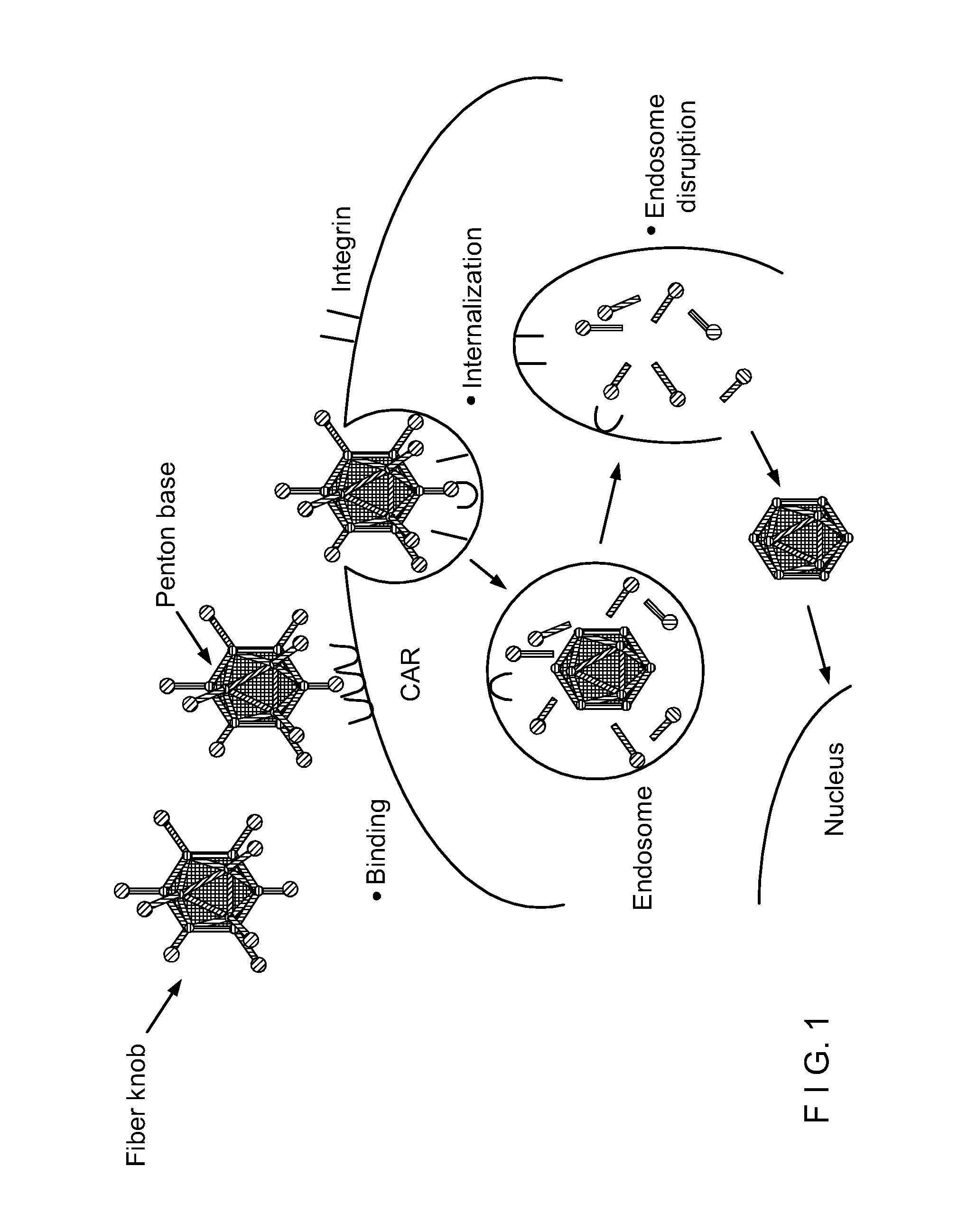
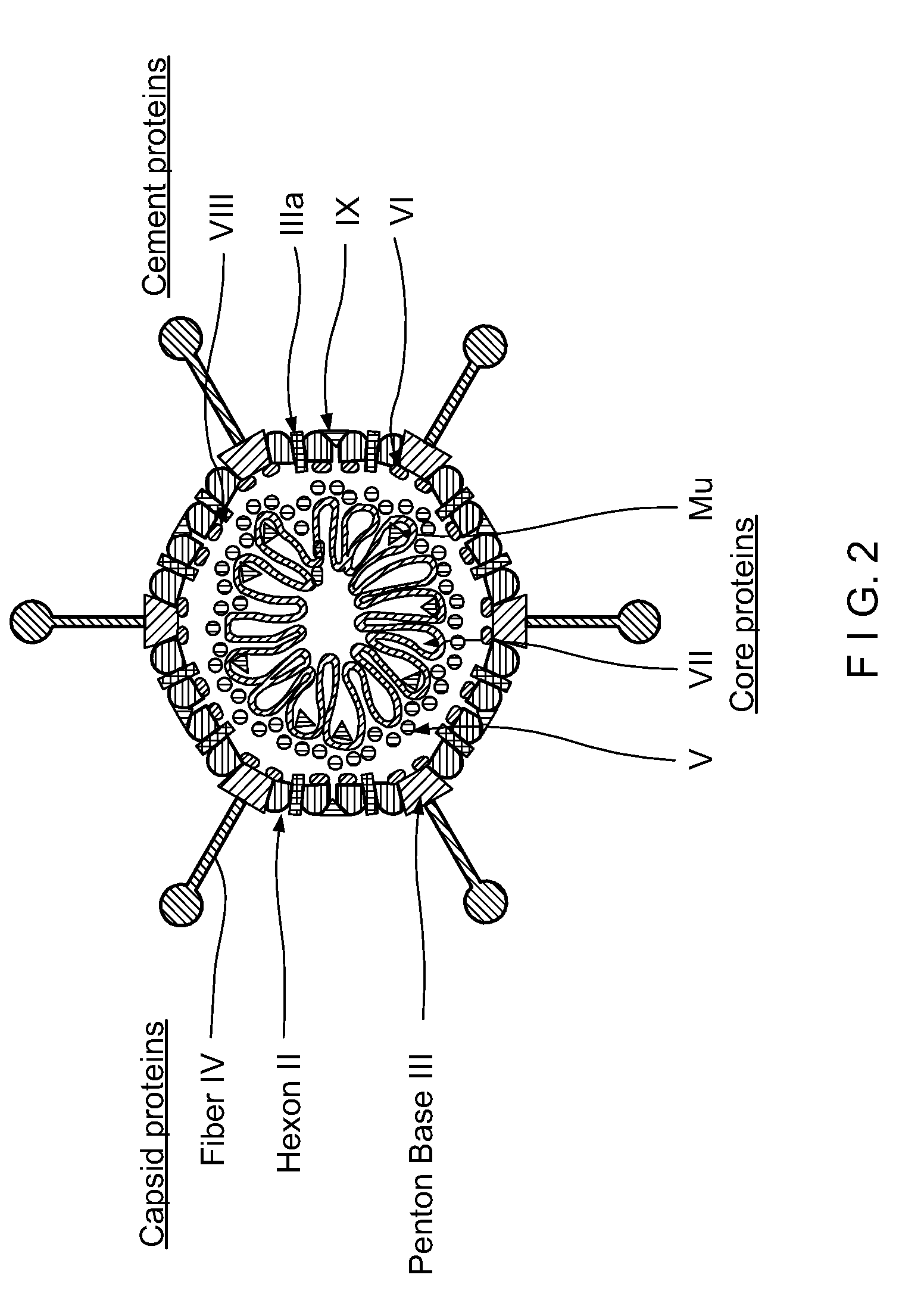
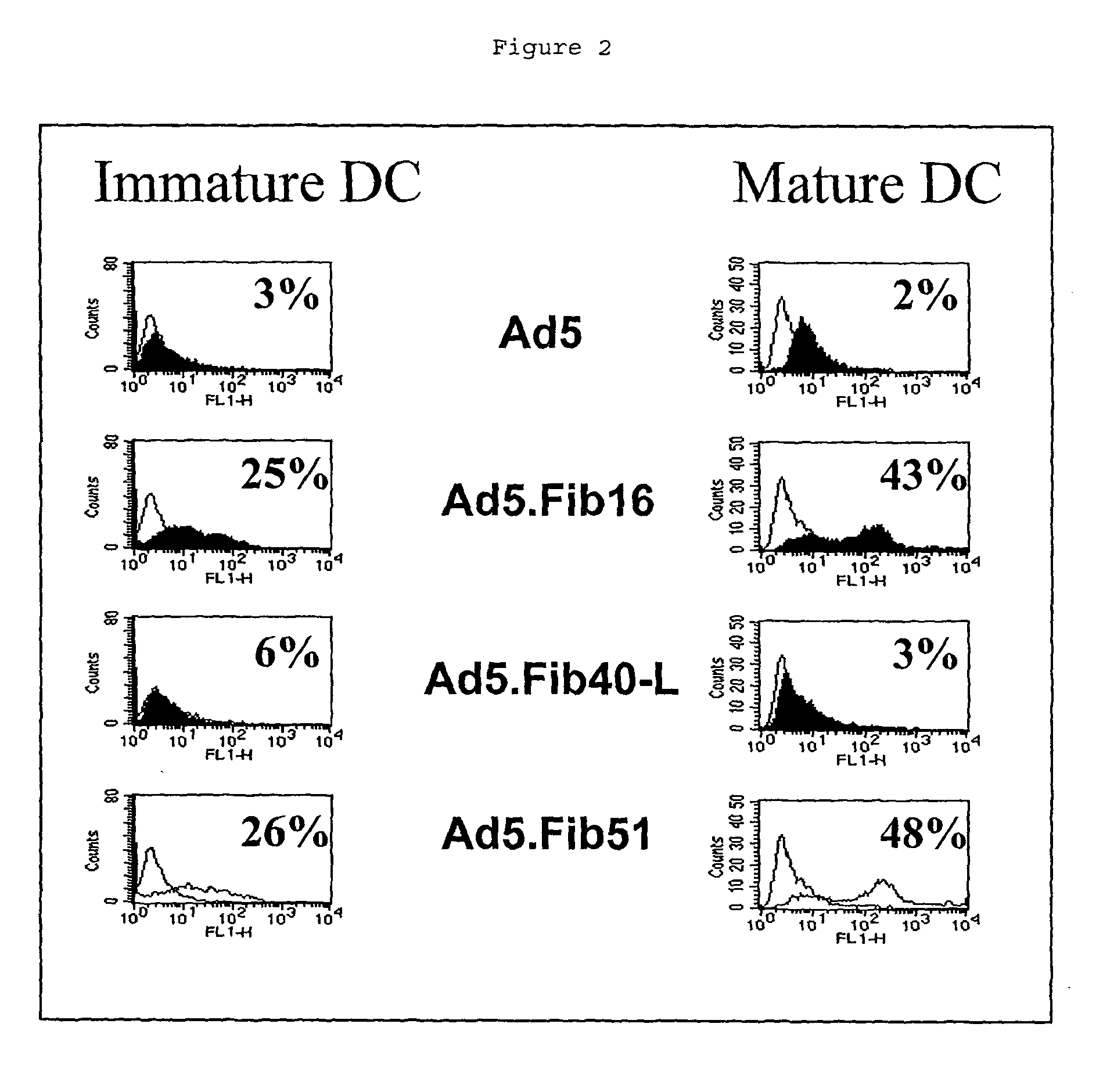
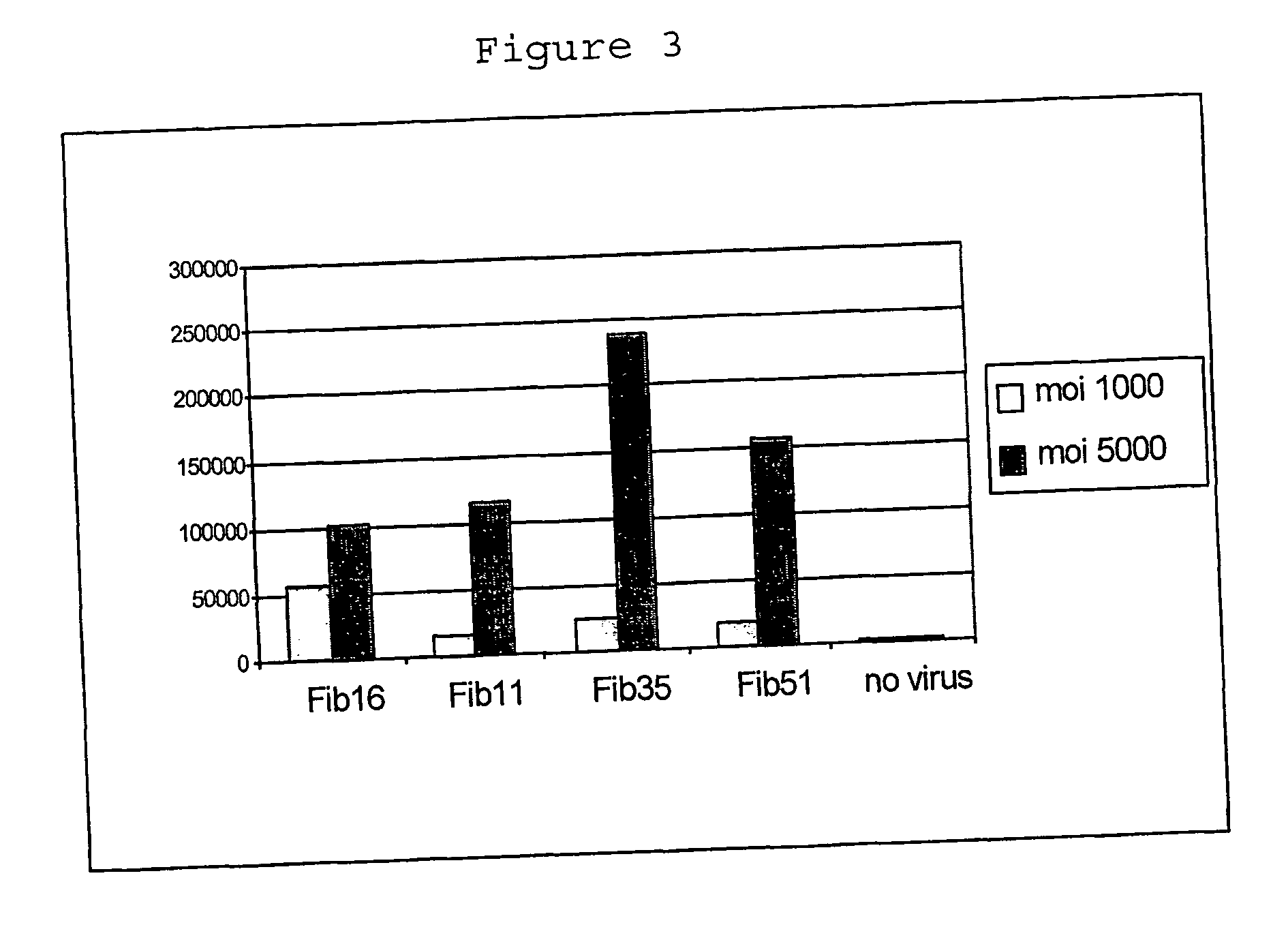
Capsid-Incorporated Antigen for Novel Adenovirus Vaccine
InactiveUS20110059135A1High affinityEnhances Ad transductionAntibody mimetics/scaffoldsAntibody medical ingredientsEpitopeAntigen delivery
This invention pertains to tropism-modified adenoviral vectors optimized for antigen delivery that induced both humoral and cellular immune responses, as well as a method of constructing and using such vectors. The vectors of the present invention may incorporate an epitope or an antigen into a capsid protein. Methods for treating of a host with an effective amount of adenovirus vector of the present invention are also provided.
Owner:KOVESDI IMRE +1
Construction of EP153R and EP402R gene co-expression recombinant adenovirus vector and adenovirus packaging method
InactiveCN109652449ADirect infectionEnhance immune responseViral antigen ingredientsVirus peptidesMultiple cloning siteCloning Site
The invention provides construction of an EP153R and EP402R gene co-expression recombinant adenovirus vector and a adenovirus packaging method, and belongs to the technical field of genetic engineering. Based on a pShuttle-CMV eukaryotic expression vector, an EP153R gene and EP402R gene overexpression adenovirus vector pAD-Shuttle-CMV-CTLA4-EP153R-EP402R is introduced with CTLA4, EP153R and EP402Rgenes at multiple cloning sites. The invention also provides recombinant adenovirus for co-expressing EP153R and EP402R genes, and the adenovirus packaging process is achieved by utilizing the constructed pAD-Shuttle-CMV-CTLA4-EP153R-EP402R vector to obtain the adenovirus capable of directly infecting animal or eukaryotic cells, thereby achieving the purpose of co-expressing EP153R and EP402R genes in eukaryotic cells, and laying a foundation for researching the adenovirus vaccine for achieving the co-expression of EP153R and EP402R genes.
Owner:YANGZHOU UNIV
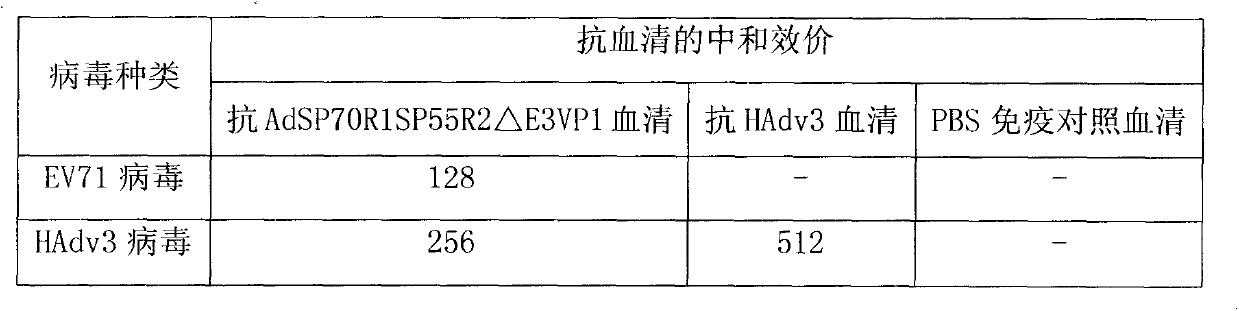
Recombinant human adenovirus 3, and preparation method and application thereof
InactiveCN103966263ARetain major antigenic activityAvoid infectionMicroorganism based processesFermentationEnterovirus 71Genome
The invention discloses a novel enterovirus 71-recombinant human adenovirus 3 vaccine candidate strain with human adenovirus 3 (HAdv3) as a carrier, and a preparation method thereof. Two EV71 neutralizing epitopes are embedded to the hexon of the human adenovirus 3, and the VP1 protein cassette of EV71 is inserted to the genome E3 region of the human adenovirus 3. The vaccine candidate strain can induce a strong anti-EV71 infection and anti-HAdv3 infection immunization reaction, and can be used for making bivalent vaccines for preventing the EV71 infection and the HAdv3 infection.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +1
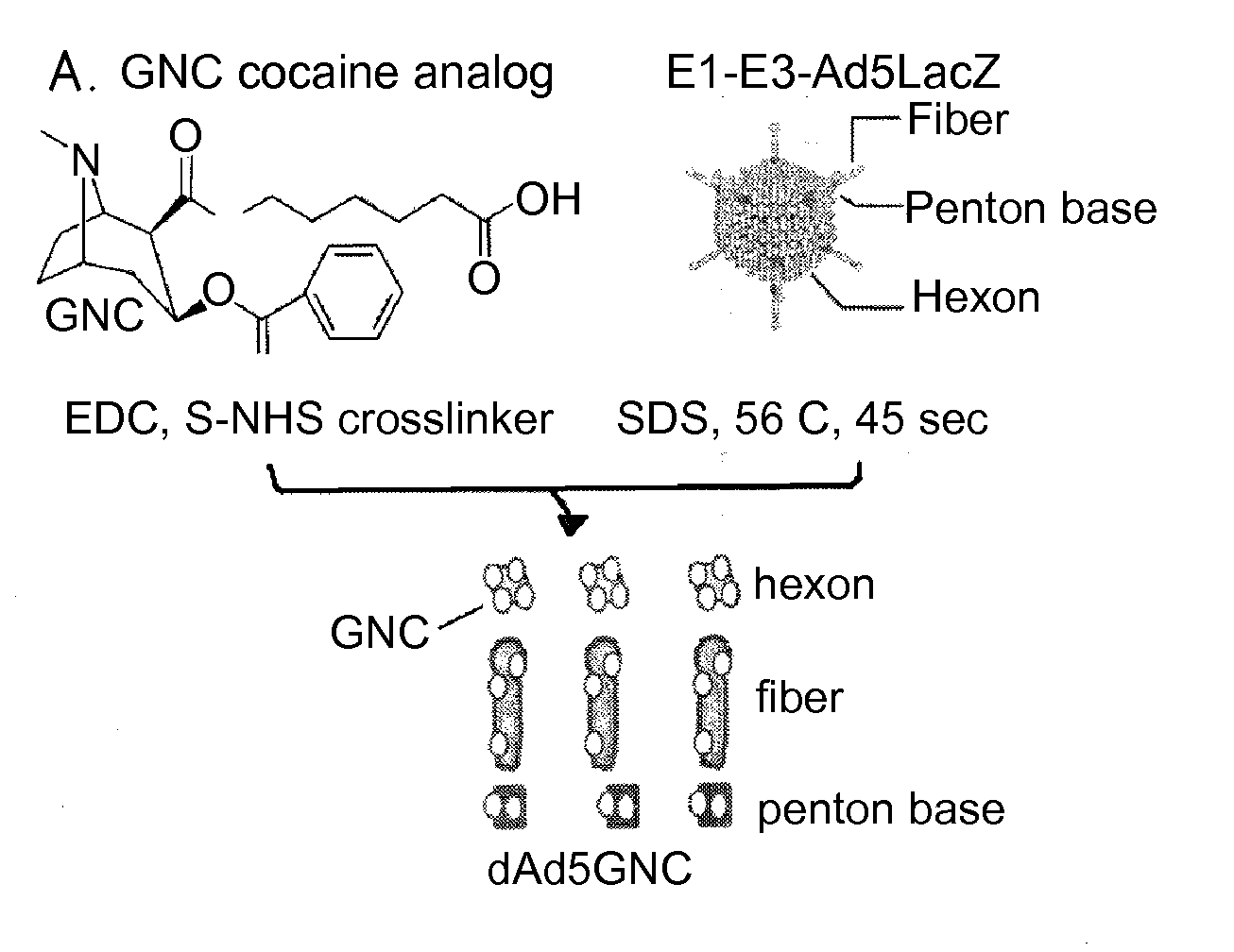
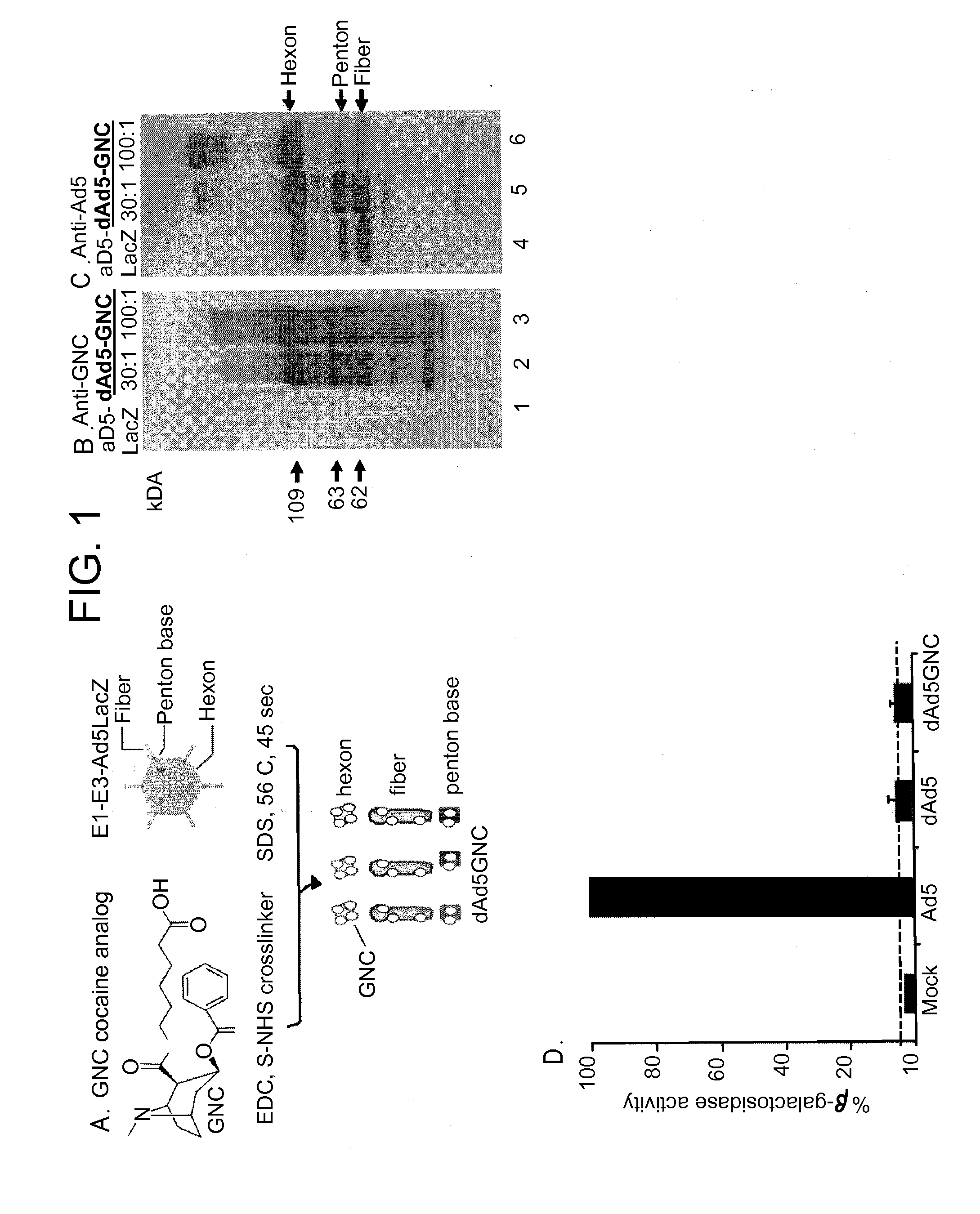
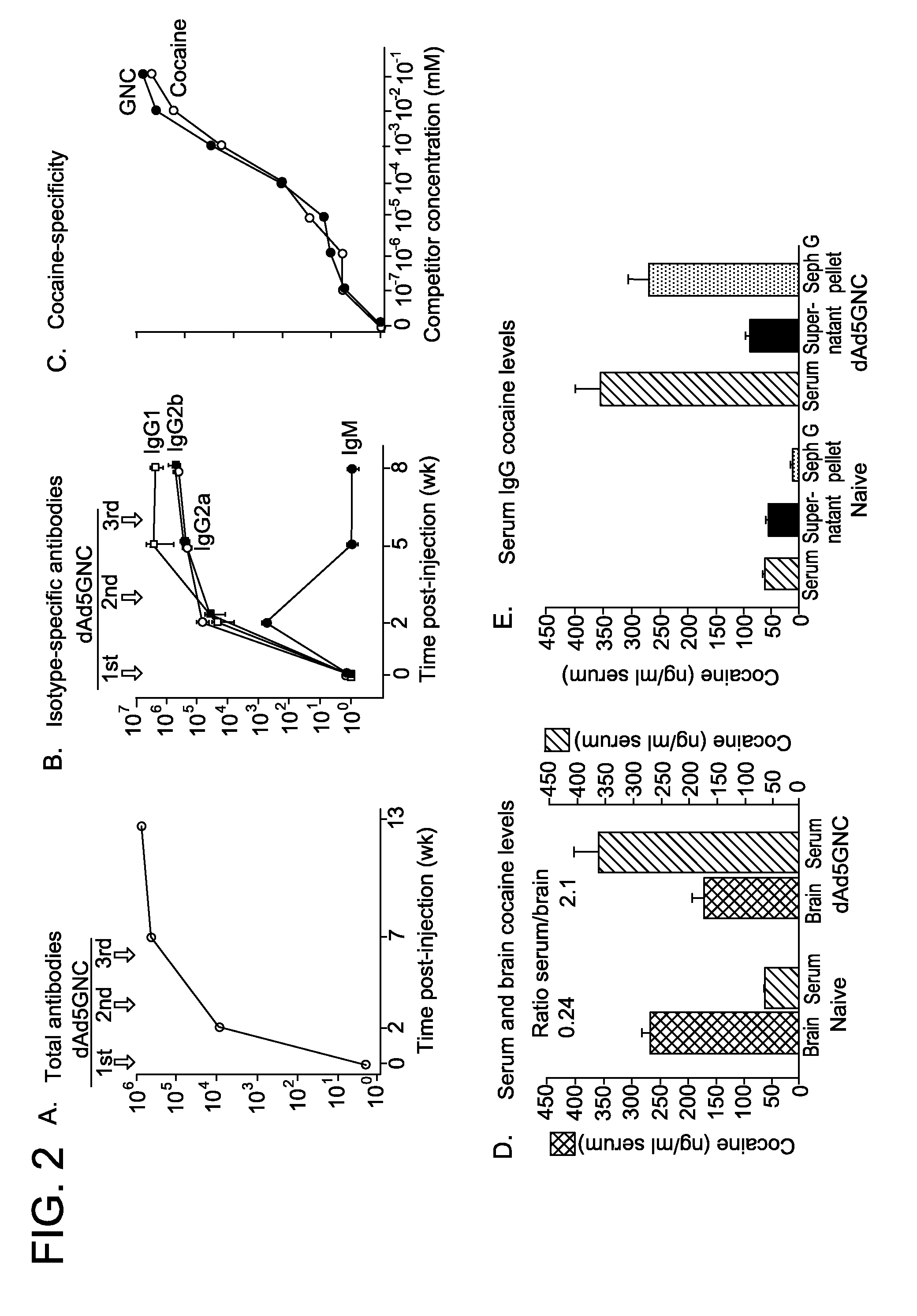
Disrupted adenovirus-based vaccine against drugs of abuse
The invention is directed to an adenovirus-antigen conjugate comprising (a) a disrupted adenovirus with a coat protein and (b) an antigen conjugated to the coat protein of the disrupted adenovirus, as well as a conjugate comprising (a) a disrupted adenovirus with a coat protein and (b) an antigen conjugated to the coat protein of the disrupted adenovirus. The invention also provides a method of inducing an immune response against an antigen in a human using the aforementioned conjugates. The invention further provides an adeno-associated viral vector comprising a nucleic acid sequence which encodes an antibody directed against cocaine.
Owner:CORNELL UNIVERSITY
Therapeutic immunization of hiv-infected individuals
InactiveUS20060216272A1Effectively maintaining low titerReceive treatment wellBiocideGenetic material ingredientsImmunodeficiency virusMedicine
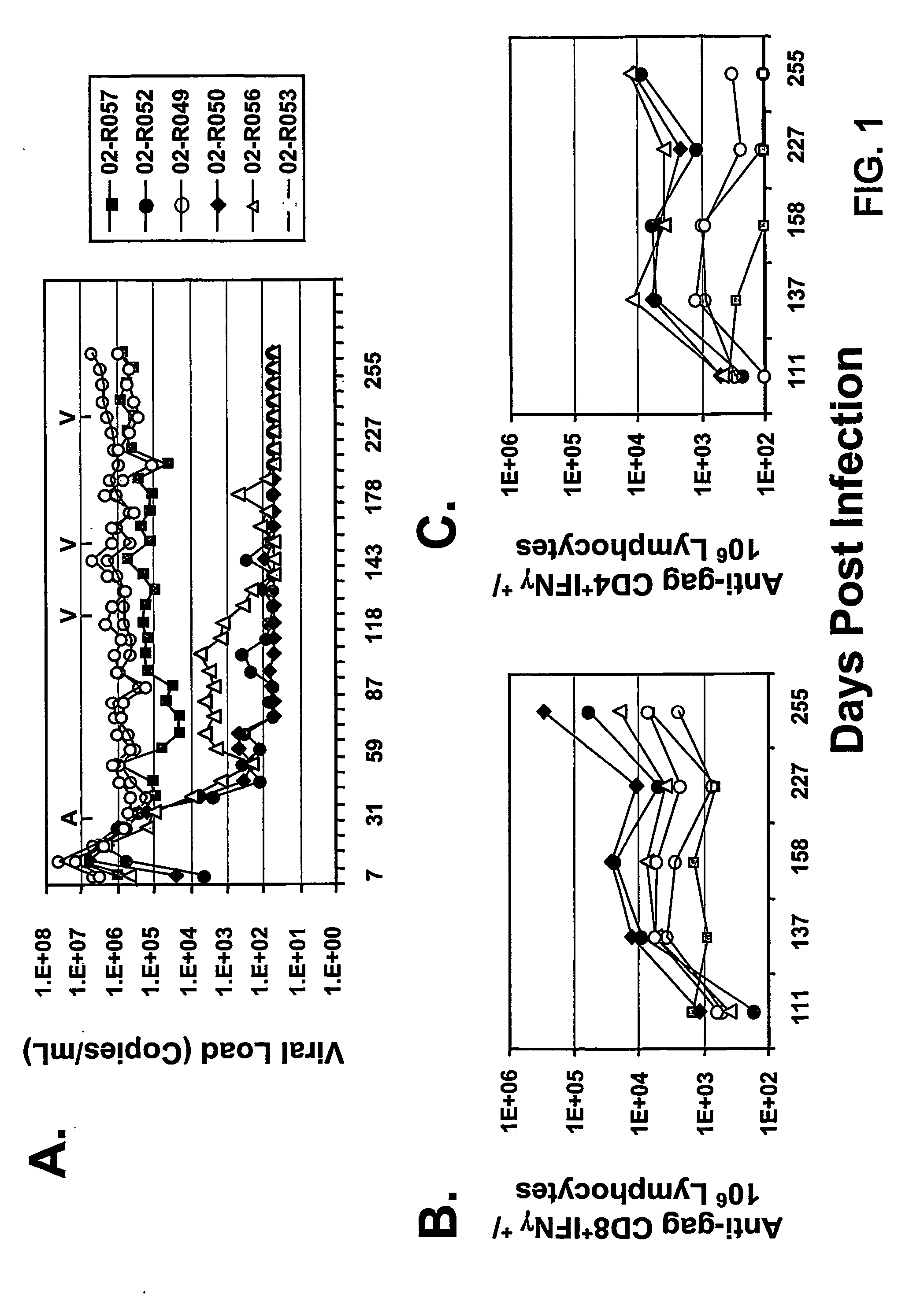
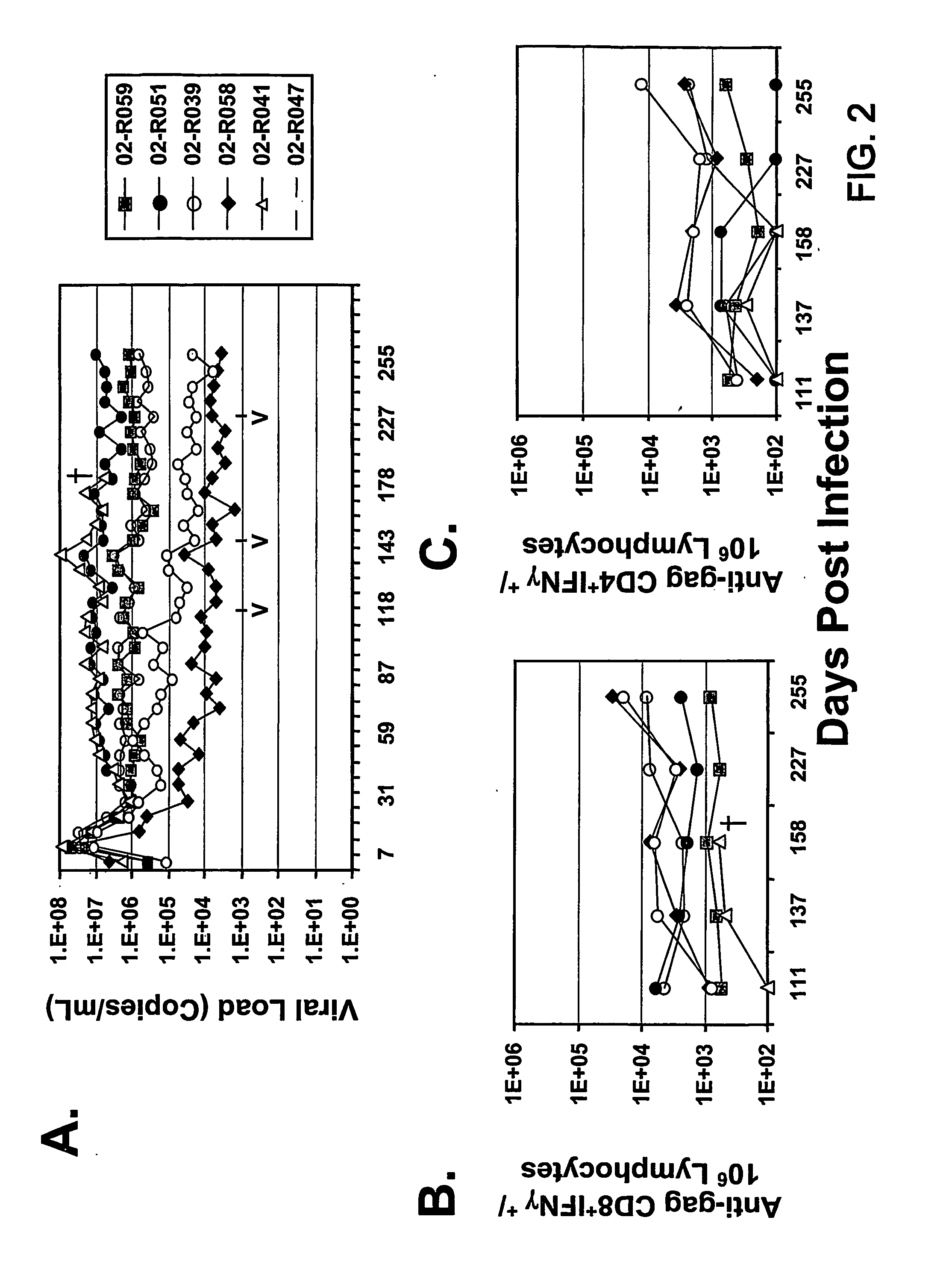
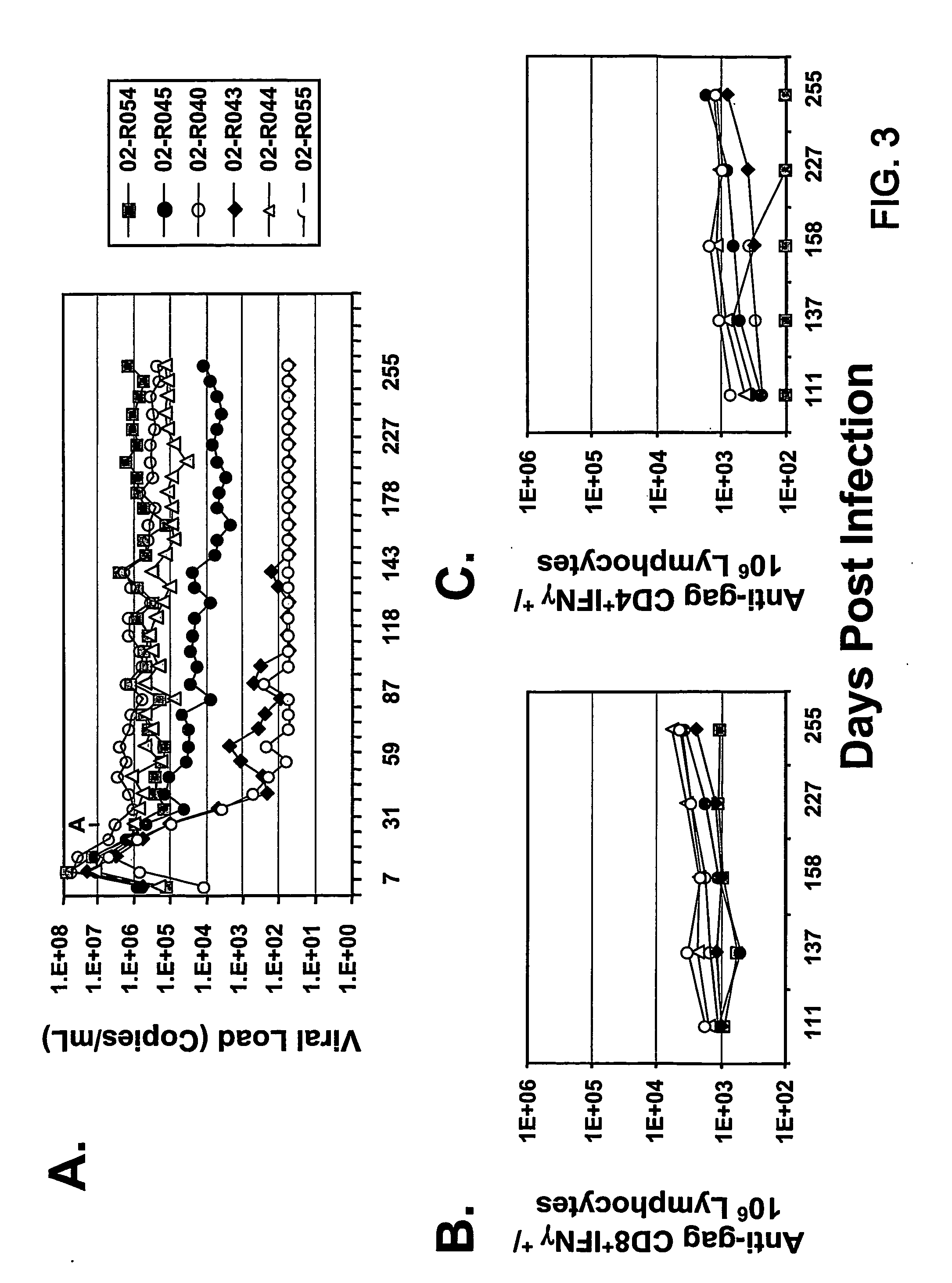
The present invention provides an improved method for eliciting a therapeutic immune response in an individual infected with human immunodeficiency virus (“HIV”). The method comprises administering an adenoviral vaccine composition expressing an HIV antigen to an individual with controlled viremia. Immunization of infected individuals in this manner elicits a cellular-mediated immune response against the virus that is significant both in the level of the response and the breadth of the response. The therapeutic immune response that ensues is capable of effectively maintaining low titers of virus and, thus, offers the prospect of reducing individual dependency on antiviral therapy.
Owner:MERCK & CO INC
Recombinant adenovirus and tetravalent adenovirus vaccine and preparation method thereof
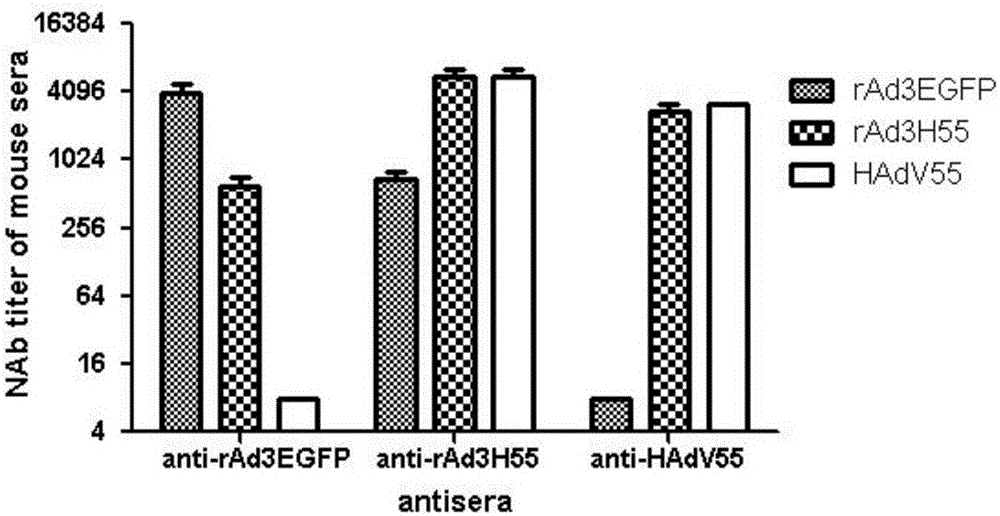
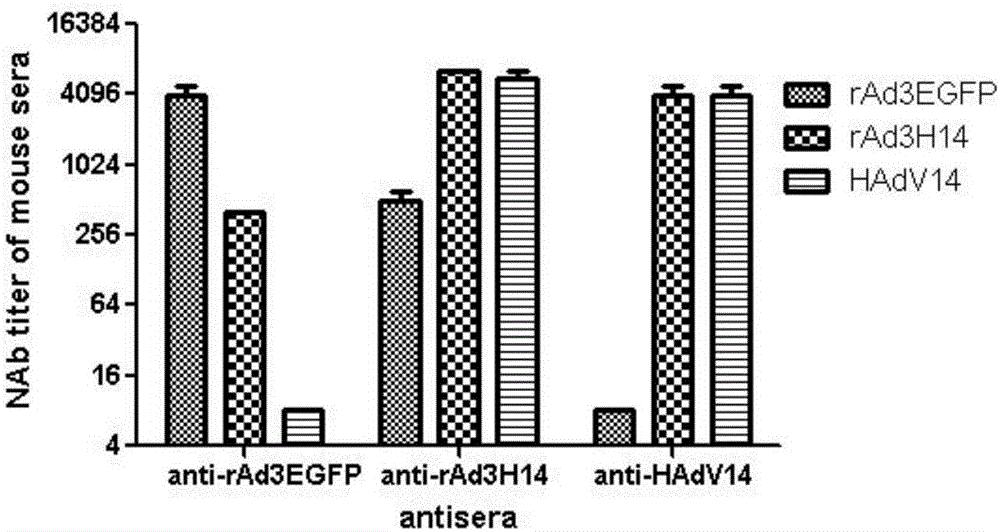
ActiveCN106318916ANo recombinationHigh neutralization potencyViral antigen ingredientsVirus peptidesHuman typeSerotype
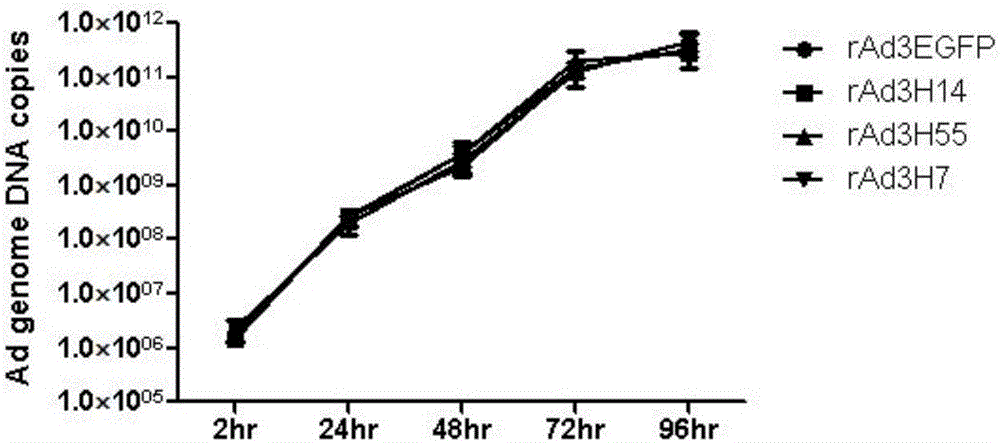
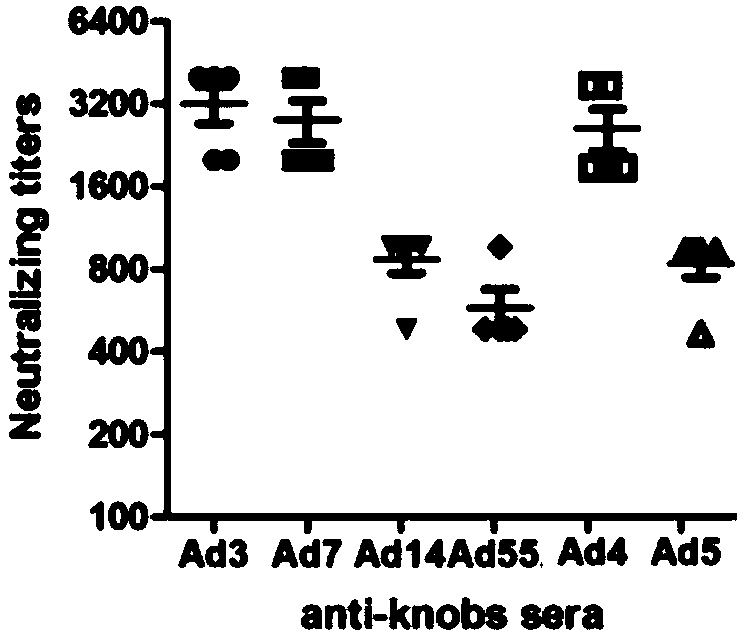
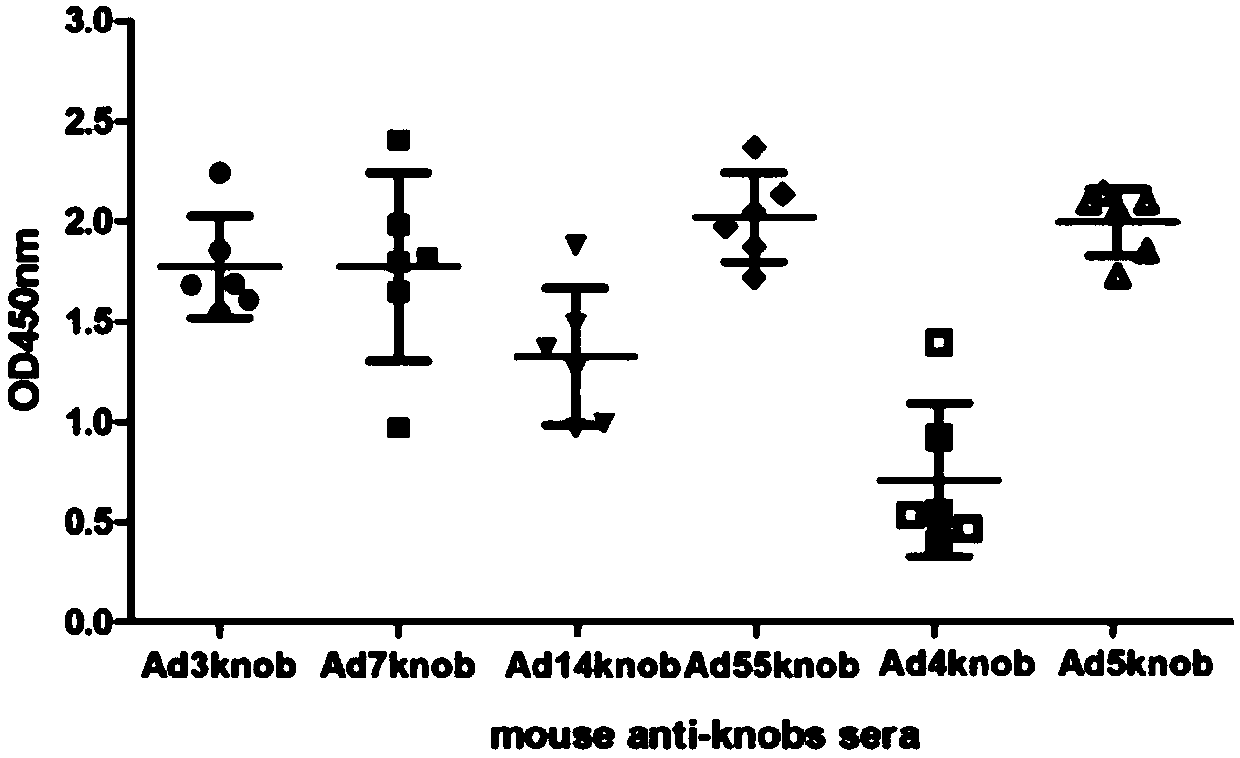
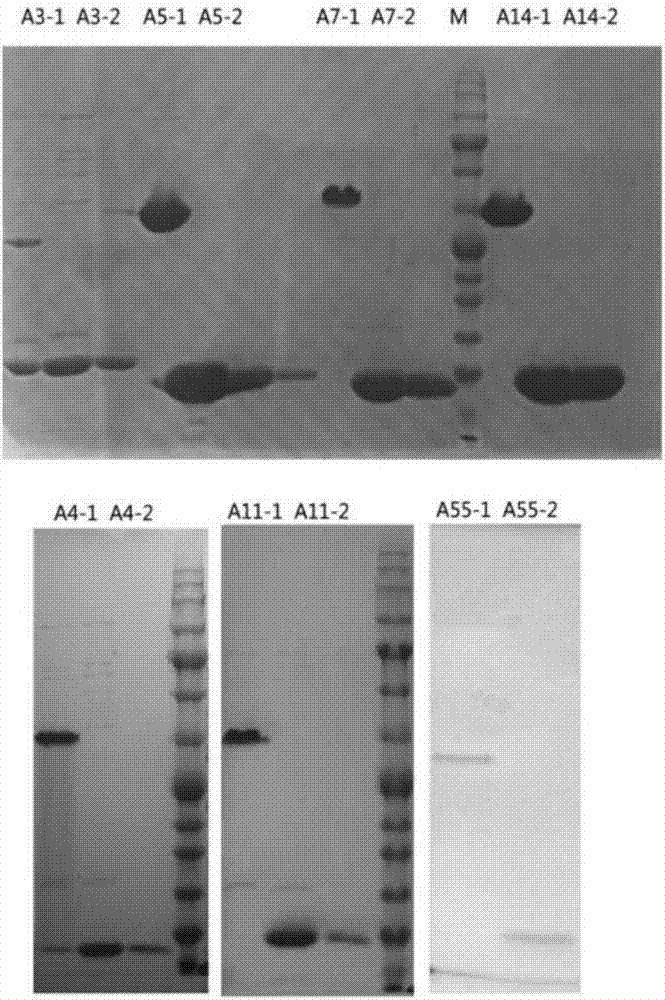
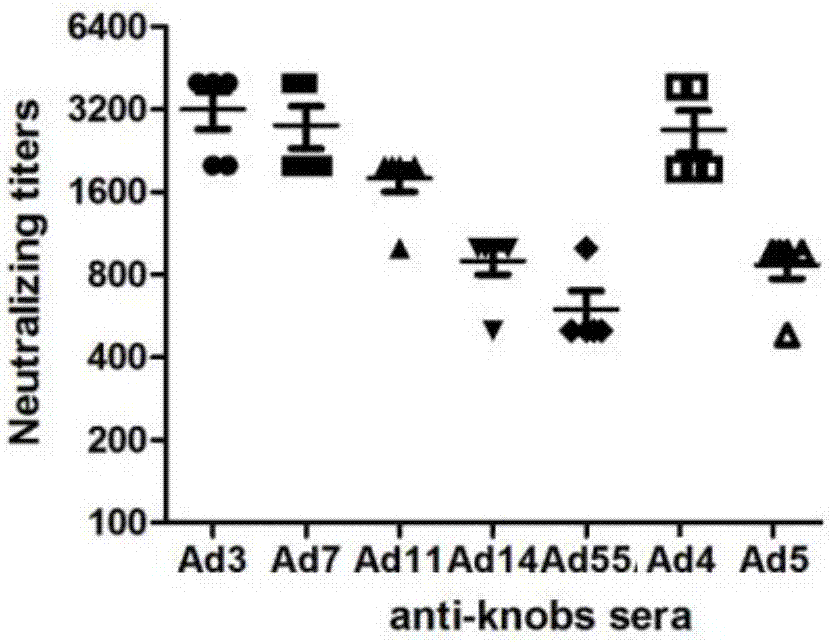
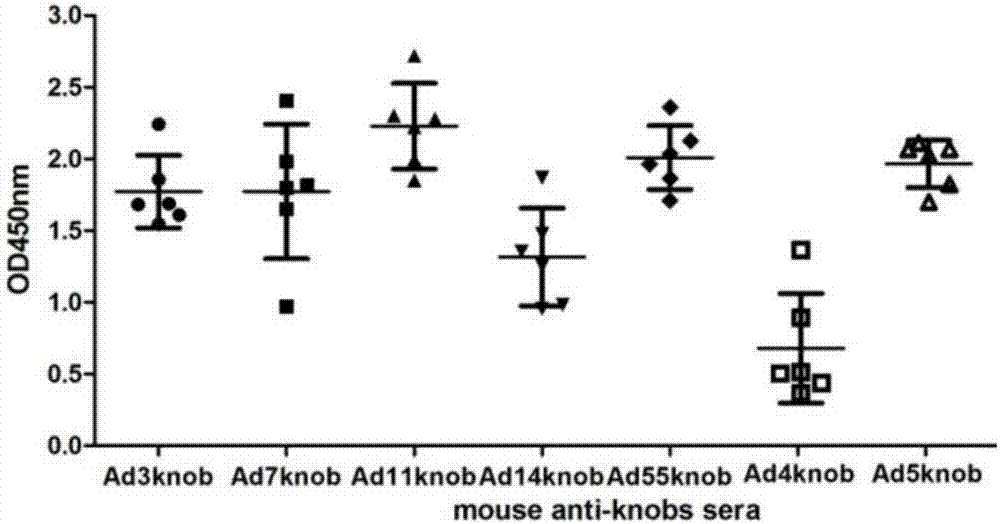
The invention discloses a recombinant adenovirus and tetravalent adenovirus vaccine and a preparation method thereof. The tetravalent recombinant adenovirus vaccine contains a recombinant type 3 adenovirus strain, a recombinant type 7 adenovirus strain, a recombinant type 14 adenovirus strain and a recombinant type 55 adenovirus strain. The preparation method disclosed by the invention comprises the following steps: preparing recombinant shuttle plasmids containing hexon gene segments, and performing in-bacteria homologous recombination with a recombinant human type 3 adenovirus strain, thereby obtaining a recombinant adenoviral genome in which the hexon gene segments are replaced by type 7, type 14 and type 55; transfecting cells, rescuing to obtain recombinant human type 3, 7, 14 and 55 recombinant adenoviruses with different main capsid protein-hexon proteins; purifying, mixing according to the same protein content, and inactivating by using beta-propiolactone, thereby obtaining the tetravalent adenovirus vaccine. The tetravalent adenovirus vaccine is capable of inducing neutralizing antibody responses to four types of serotype adenoviruses, and the neutralizing titer is 500-1000.
Owner:GUANGZHOU GIR MEDICINE CO LTD +1
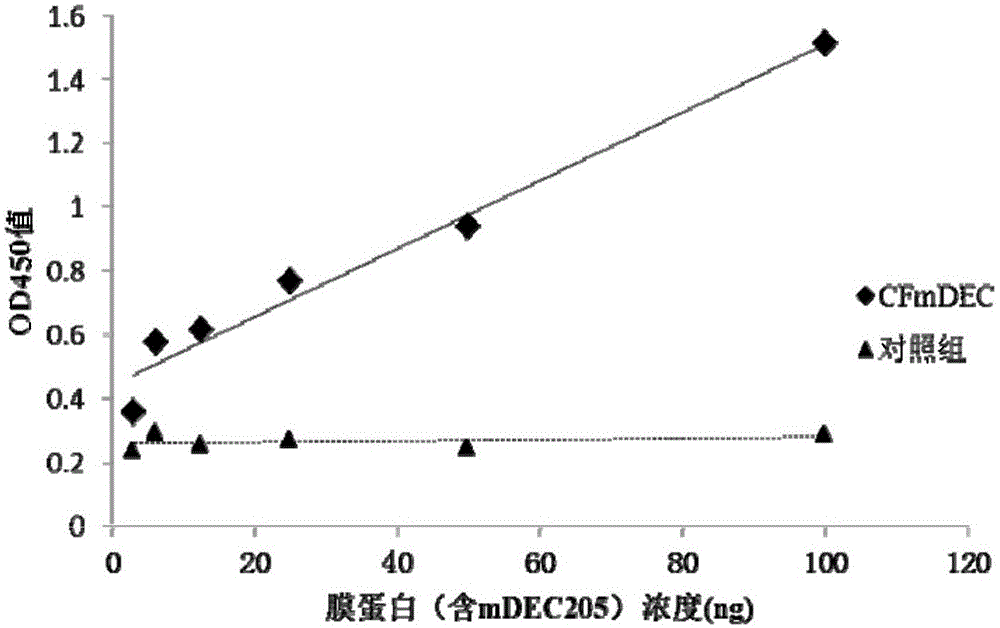
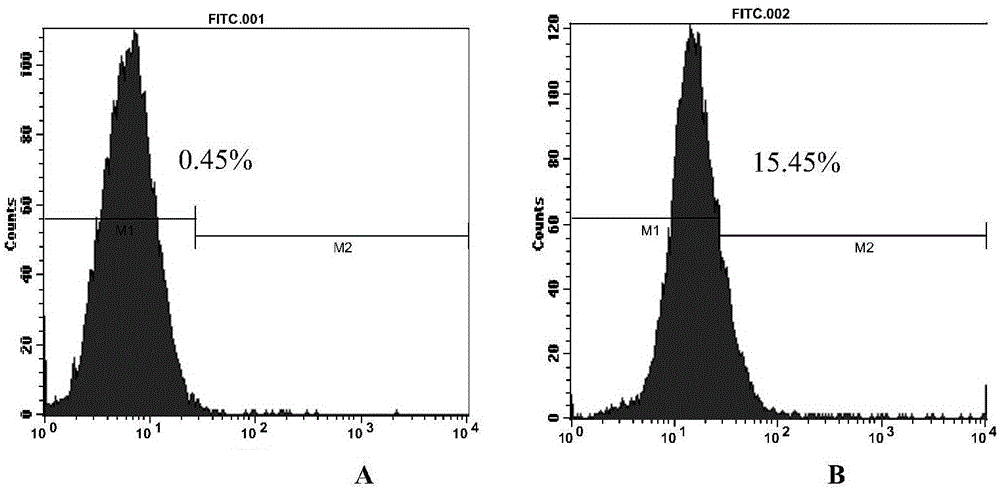
Dual-targeting fusion protein and encoding gene and application thereof
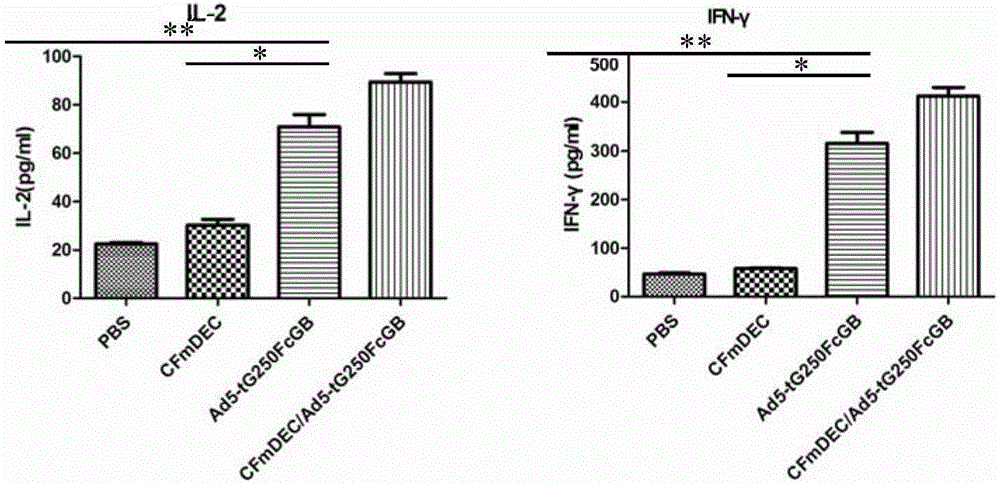
The present invention discloses dual-targeting fusion protein CFmDEC capable of mediating a type 5 adenovirus vector to be specifically bound with a surface molecule DEC205 of a dendritic cell; and by utilizing a gene fragment sig-tG250-Fc-IRES-GM-CSF-B7.1(25) of a multi-targeted anti-renal cancer compound antigen, an anti-renal cell carcinoma adenovirus vaccine-Ad5-tG250FcGB is constructed with a type 5 replication-deficient adenovirus as a vector. The dual-targeting fusion protein CFmDEC is capable of enhancing the antitumor effect of the renal cell cancer adenovirus vaccine Ad5-tG250FcGB, is effectively combined with the adenovirus, improves the DCs-targeted ability of the adenovirus vaccine, and can induce higher specific antitumor immune response in a human body and suppress the growth of renal cell cancer.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Recombinant Adenovirus Vaccines
Recombinant adenovirus vaccines comprising recombinant adenoviruses whose hexon, fiber or protein IX capsid proteins are engineered to include exogenous peptide segments, e.g. vaccines for human papillomavirus (HPV) and malaria.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Dynein mosaic type recombinant human type-B adenovirus and preparation method thereof
ActiveCN107267469AHigh infection efficiencyMicroorganism based processesFermentationGolden hamsterHuman type
The invention discloses a dynein mosaic type recombinant human type-B adenovirus and a preparation method thereof. The skeleton of the dynein mosaic type recombinant human type-B adenovirus is a human type-B adenovirus genome, and a base sequence which encodes a receptor binding domain of dynein is a base sequence which encodes a corresponding domain of a human type-C adenovirus. By a molecular cloning method, Ad5-knob gene fragments are cloned and replaced to recombinant shuttle plasmids, in-vitro recombinant on the Ad5-knob gene fragments and a recombinant human type-3 adenovirus genome is realized, obtained knob gene fragments are replaced into type-5 recombinant human type-3 adenovirus genome, and therefore, dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 is obtained. The dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 can be infected with mouse primitive epithelial cells and golden hamster lung and kidney primitive cells in vitro, and the infection efficiency of the dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 is close to that of Ad5, and is much higher than that of a parent strain rAd3E, in golden hamster cells, significant copying exists, and the dynein mosaic type recombinant human type-B adenovirus can be used for small animal model research of human type-3 adenovirus vaccines and antiviral drug evaluation.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
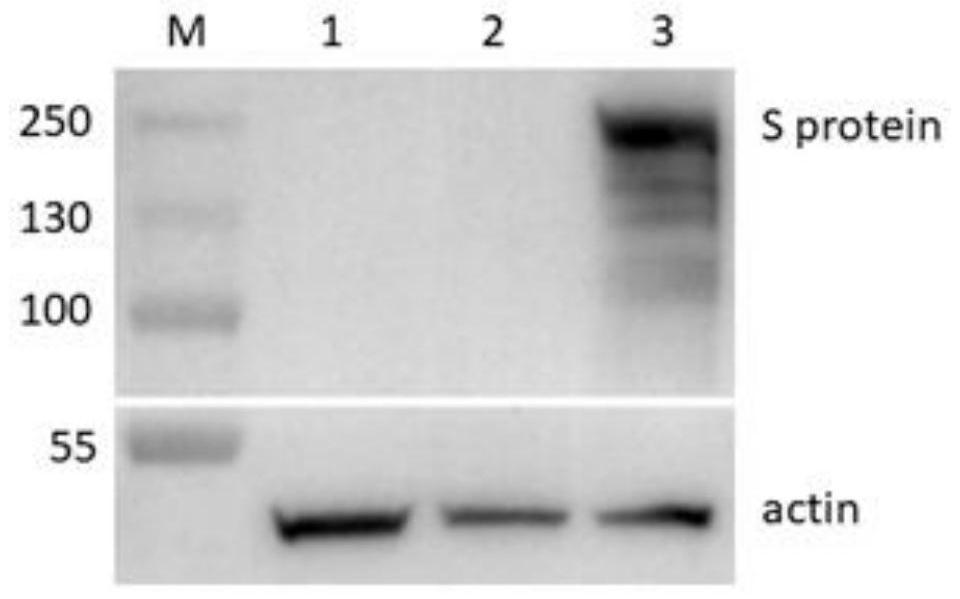
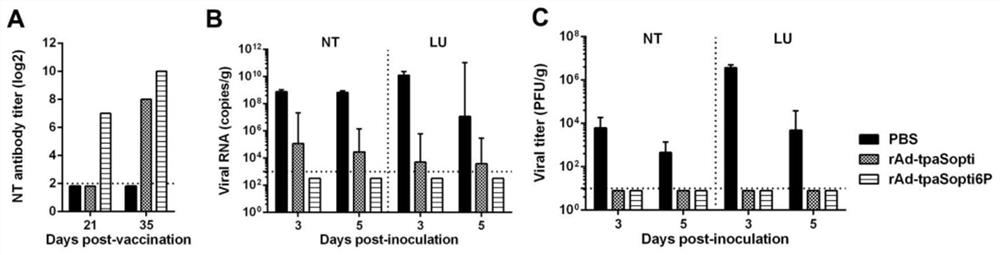
Novel coronavirus pneumonia recombinant human type 5 adenovirus vaccine
The invention provides a vaccine composition containing a modified SARS-CoV-2 spike protein. The vaccine composition is characterized in that a replication-deficient human type 5 adenovirus rAd virus strain is combined with a novel modified SARS-CoV-2 spike protein (S6P) or an immunogenic derivative thereof to construct a recombinant replication-deficient human type 5 adenovirus for preventing infection of a novel coronavirus, especially against novel coronavirus infection in mammals.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
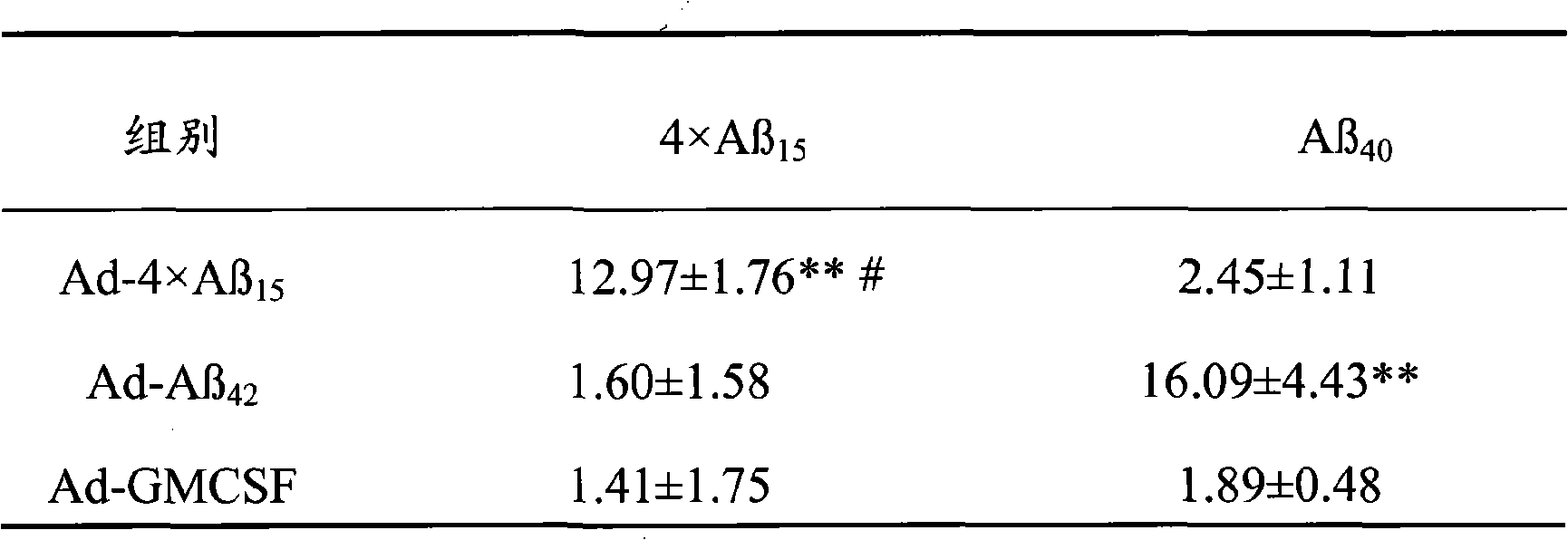
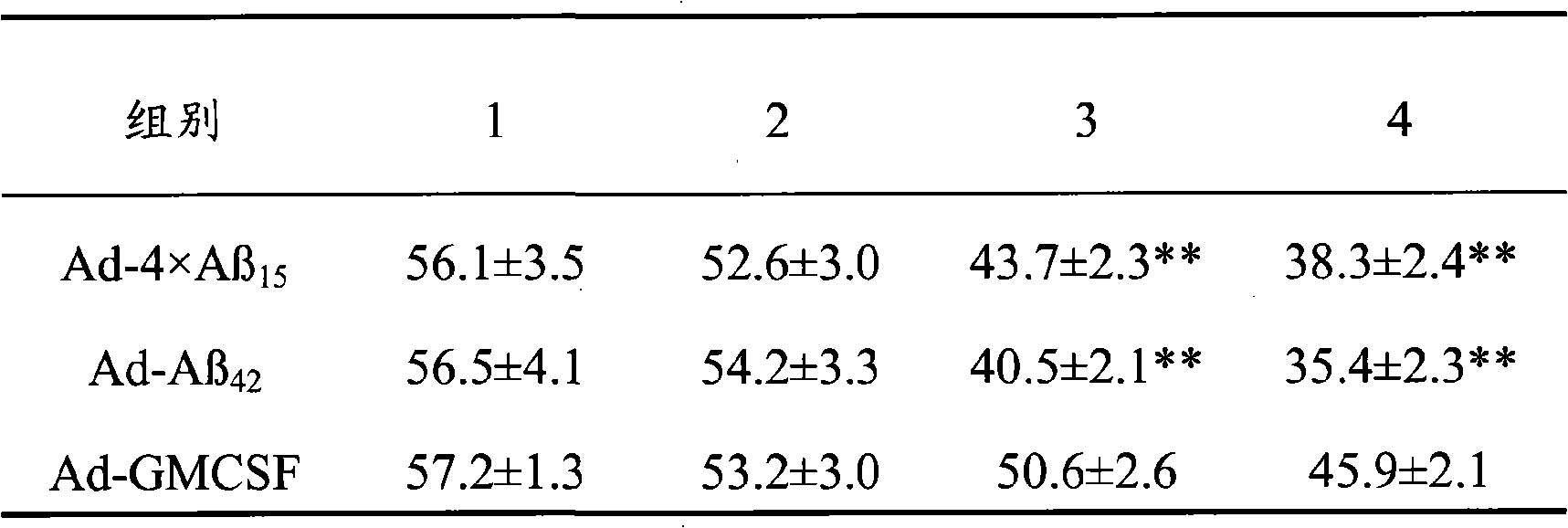
Senile dementia recombinant adenovirus gene vaccine and preparation method thereof
ActiveCN101357227AHigh molecular weightAvoid spatially rigid structuresNervous disorderPeptide/protein ingredientsChemical synthesisAmyloid
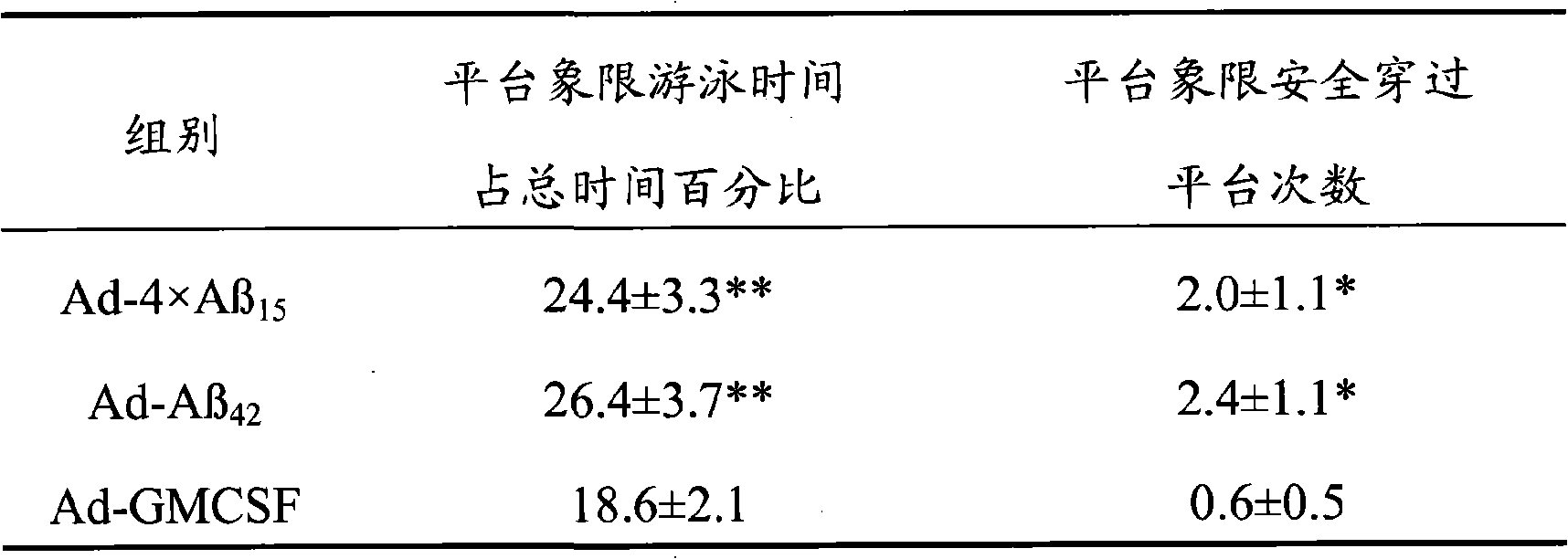
The invention discloses senile dementia Ad-GFP gene vaccine and a preparation method theerof and is Ad-GFP vaccine of flexible foldable multivalence Beta- amyloid 1-15 (ABeta1-15) of secretion co-expression and genetic adjuvant, which a flexible connecting peptide is used for connecting multivalence. The invention selects foldable multivalence ABeta1-15 as immunogen, which avoids the space rigid structure of multiple copy ABetaB cell epitopes, also ensures that ABeta15 epitopes are more easily exposed, thus enhancing immunogenicity and also increasing immunogenic molecular weight and reducing the possibility of degradation; a antigen delivery system that takes adenovirus as a carrier is selected, which avoids the defects of high challenge and high cost of chemosynthesis.
Owner:LIVZON PHARM GRP INC
Recombinant expression adenovirus cilia protein peptide, adenovirus subunit vaccine and preparation method of recombinant expression adenovirus cilia protein peptide
ActiveCN107459562AHigh expressionImproving immunogenicityViral antigen ingredientsVirus peptidesMonoclonal antibodyImmunogenicity
The invention discloses recombinant expression adenovirus cilia protein peptide, an adenovirus subunit vaccine and a preparation method of the recombinant expression adenovirus cilia protein peptide. The recombinant expression adenovirus cilia protein peptide has an amino acid sequence shown by SEQ ID No:2, and the vaccine contains the adenovirus cilia protein peptide. The adenovirus cilia protein peptide disclosed by the invention has advantages of high expression quantity, strong immunogenicity, capability of serving as a candidate of the adenovirus subunit vaccine, convenience in production and purification and low cost; by means of the adenovirus cilia protein peptide, an immune mouse can induce a high-titer neutralizing antibody; the adenovirus cilia protein peptide can induce a cross neutralizing function antibody and can serve as a candidate of a polyvalence adenovirus vaccine; when being matched with other types of knob, the adenovirus cilia protein peptide also can serve as a candidate of a polyvalence adenovirus subunit vaccine; the adenovirus cilia protein peptide can serve as immunizing antigen and detecting antigen for preparing a broad-spectrum neutralizing active monoclonal antibody or human-derived monoclonal antibody.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
HBx and human IL-12 double-gene recombinant vector and liver caner-resistant vaccine
ActiveCN102660579AGood treatment effectGenetic material ingredientsGenetic engineeringHBxLiver cancer
The invention belongs to the field of gene therapy and aims at providing a novel targeted gene vaccine for treating HBV (Hepatitis B Virus)-relevant liver cancer and application of the novel targeted gene vaccine in preparation of a targeted gene immunotherapeutic drug for the HBV-relevant liver cancer. The main active component of the liver caner-resistant vaccine disclosed by the invention is a recombinant vector of a gene coded with an HBx protein and a gene for coding a human IL-12 protein; and the recombinant vector can be used for simultaneously expressing the HBx protein and the human IL-12 protein in a eukaryotic cell. Provided by the experiment, a recombinant adenovirus vaccine disclosed by the invention can be used for selectively killing HBx-electropositive liver cancer cell and expresses a favorable liver cancer-resisting effect. The recombinant adenovirus vaccine provides a new selection for targeted gene immunization therapy of the HBV-relevant liver cancer and has a favorable application prospect.
Owner:SICHUAN UNIV
Recombinant human type 3 adenovirus as well as preparation method and application thereof
InactiveCN105219740AAvoid infectionPrevention of Hand, Foot and Mouth DiseaseMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
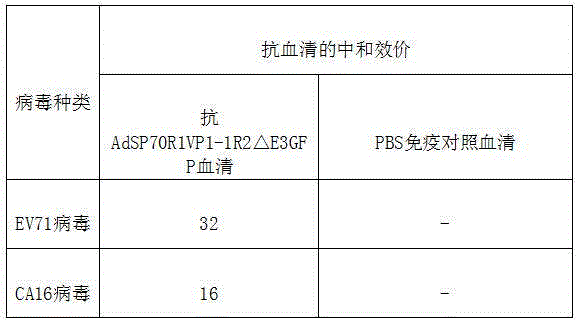
The invention belongs to the technical field of recombinant virus genetic engineering, and in particular relates to a novel human enterovirus 71 (EV71)-coxackievirus A16 (CA16)-recombinant type 3 adenovirus (Had 3) candidate vaccine strain using recombinant human type 3 adenovirus as a vector, and a preparation method of the strain; hexon of the human type 3 adenovirus is simultaneously interpolated with a neutralizing epitope SP70 of the EV71 and a neutralizing epitope VP1-1 of the CA16, wherein the amino acid sequence of the neutralizing epitope SP70 of the EV71 is shown as YPTFGEHKQEKDLEYC and the amino acid sequence of the neutralizing epitope VP1-1 of the CA16 is shown as PKPTSRDSFAWQTAT. The recombinant human type 3 adenovirus is capable of simultaneously preventing EV71 and CA16, and the adenovirus has a significant effect on preventing hand-foot-and-mouth disease.
Owner:东莞市第八人民医院
Recombinant expression adenovirus fiber peptide, adenovirus subunit vaccine and preparation method of adenovirus cilia protein peptide
ActiveCN107602672AHigh expressionImproving immunogenicityImmunoglobulins against virusesAntiviralsCross neutralizationMonoclonal antibody
The invention discloses a recombinant expression adenovirus fiber peptide, an adenovirus subunit vaccine and a preparation method of the adenovirus fiber peptide. The recombinant expression adenovirusfiber peptide has an amino acid sequence shown in SEQ ID NO:2, and the vaccine comprises the above adenovirus fiber peptide. The adenovirus fiber peptide provided by the invention has a high expression quantity, when mice are immunized, a neutralizing antibody with a high titer can be induced, immunogenicity is strong, and the adenovirus fiber peptide can be used as an adenovirus subunit vaccinecandidate, is convenient for production and purification and low in costs; the adenovirus fiber peptide can induce a cross neutralization antibody, and can be used as a multivalent adenovirus vaccinecandidate; compatability of the adenovirus fiber peptide and other type knob can also be used as a multivalent adenovirus subunit vaccine candidate; and the adenovirus fiber peptide can be used as animmune antigen and a detection antigen for preparing a broad-spectrum neutralizing activity monoclonal antibody or a humanized monoclonal antibody.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
Human papillomavirus (HPV) L1-based recombinant adenovirus for preventing and treating esophagus cancer
InactiveCN102649963AViral antigen ingredientsGenetic material ingredientsHuman papillomavirusViral Vaccine
The invention relates to human papillomavirus (HPV) L1-based recombinant adenovirus for preventing and treating esophagus cancer, which belongs to the field of biomedical technology. The invention provides an optimized genetic sequence of main capsid protein of code HPV18-type (HPV18) and replication-defective recombinant HPV18L1 adenovirus vaccine containing the genetic sequence, and provides a method for preparing the recombinant adenovirus. The recombinant HPV18L1 adenovirus and the recombinant HPV16L1 adenovirus vaccine (patent No. ZL200610056900.3) can be used for preventing and treating the esophagus cancer.
Owner:BEIJING UNIV OF TECH
Replicative recombinant human 55-type Adenovirus vector and preparation method and application thereof
ActiveCN105274142AGood effectBreakthrough of pre-existing immunityGenetic material ingredientsImmunoglobulins against virusesNeutralizing antibodyBiology
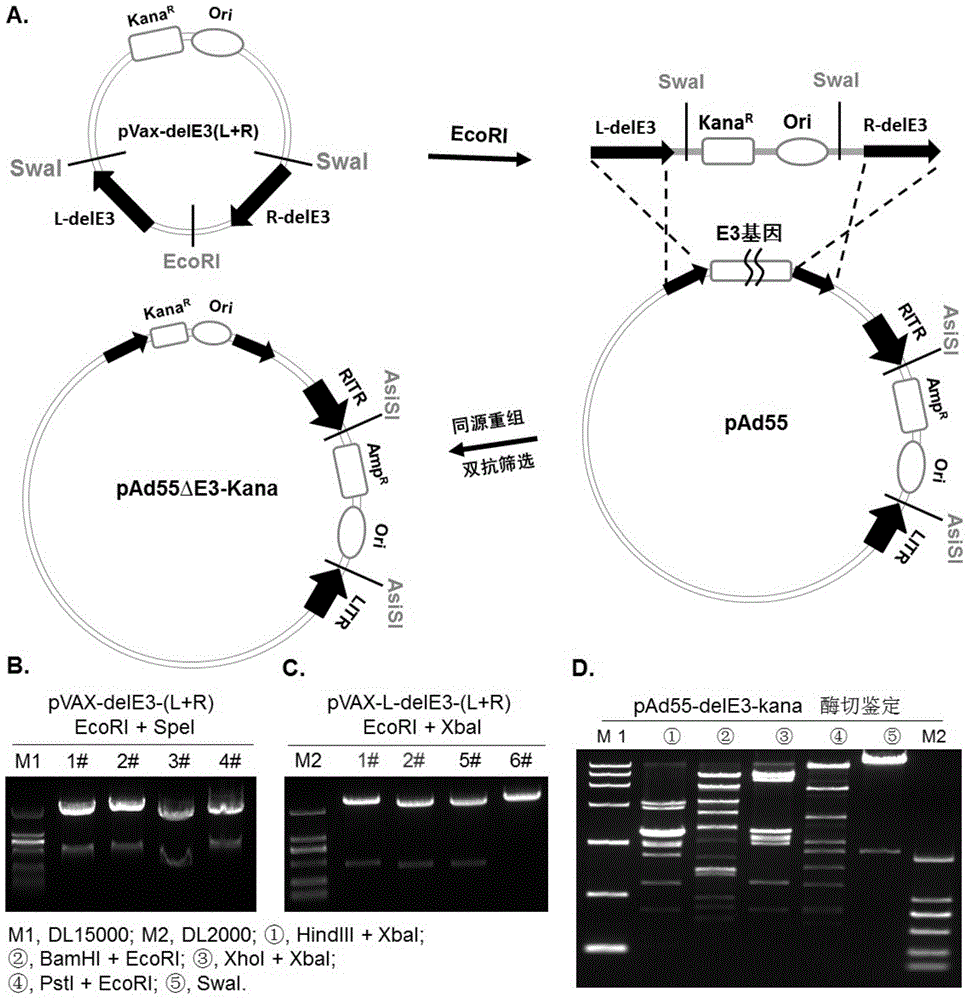
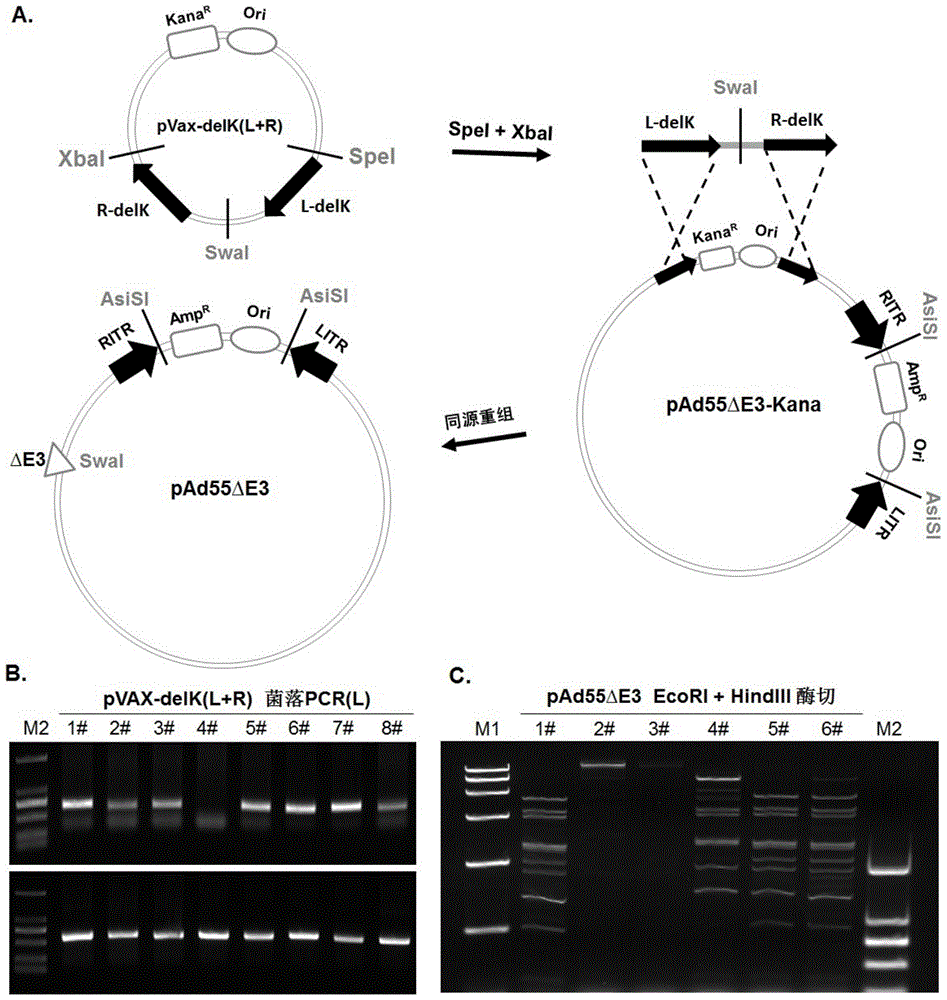
The invention discloses a replicative recombinant human 55-type Adenovirus vector and a preparation method and application thereof. A human 55-type Adenovirus genome is cyclized through homologous recombination technology, E3 genes of Ad55 are knocked out through homologous recombination and double resistance screening technology, and an exogenous gene expression cassette can be integrated in; after Ad55 vector plasmids deleted in the E3 genes are linearized, successful rescue and mass production can be realized by transfecting mammalian cells; further, a purified recombinant Ad55 vector is obtained through density gradient centrifugation, efficient expression in target cells can be realized by using the vector to carry exogenous genes, and activity of exogenous reporter genes can reflect growth characteristics of virus. The replicative recombinant vector based on human Ad55 can be potentially applied to research and development of vaccines resistant to human 55-type Adenovirus, screening of drug and neutralizing antibodies resistant to the human 55-type Adenovirus, research and development of vaccines resistant to other pathogens and development of report tracer systems for biological study.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
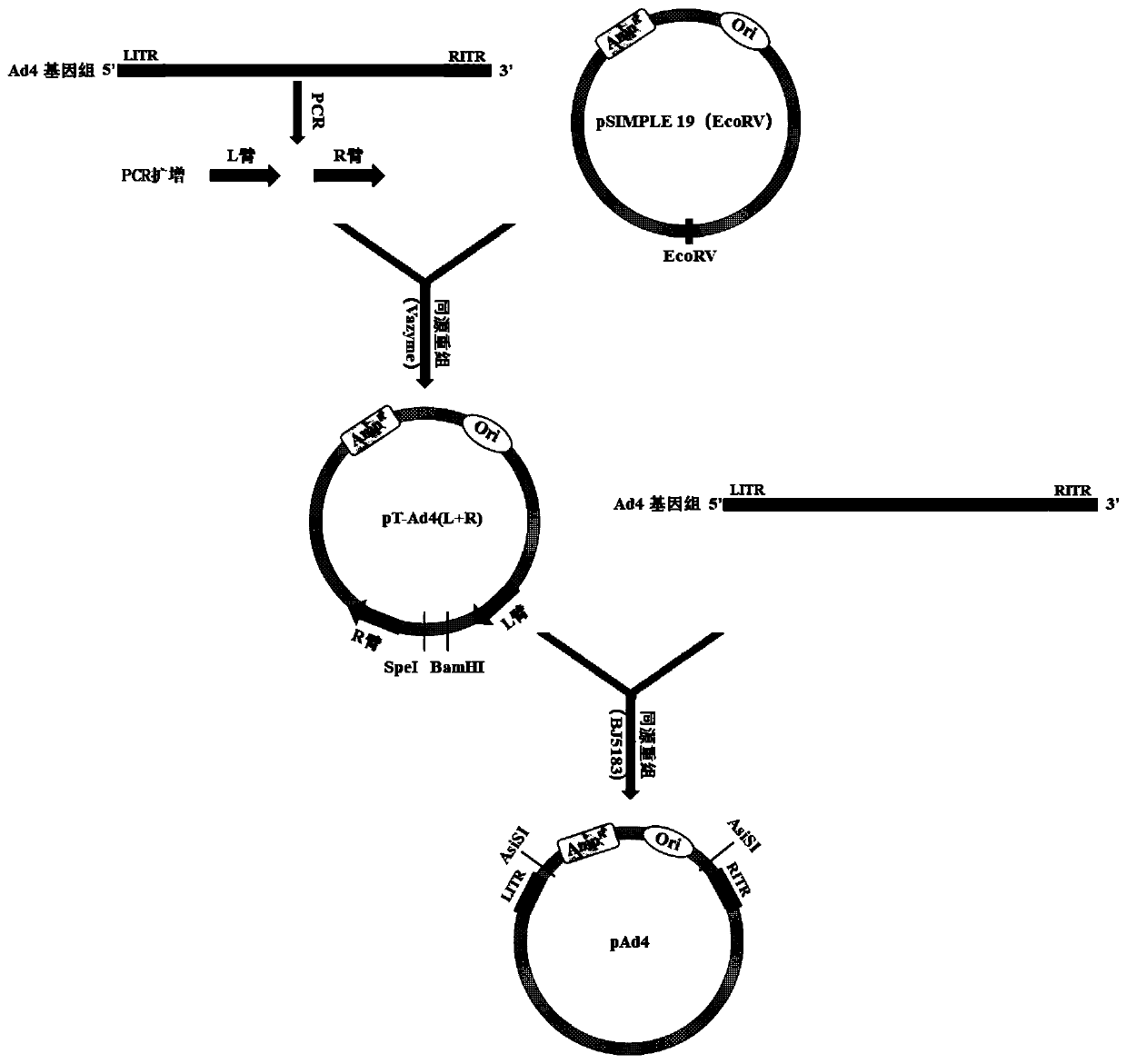
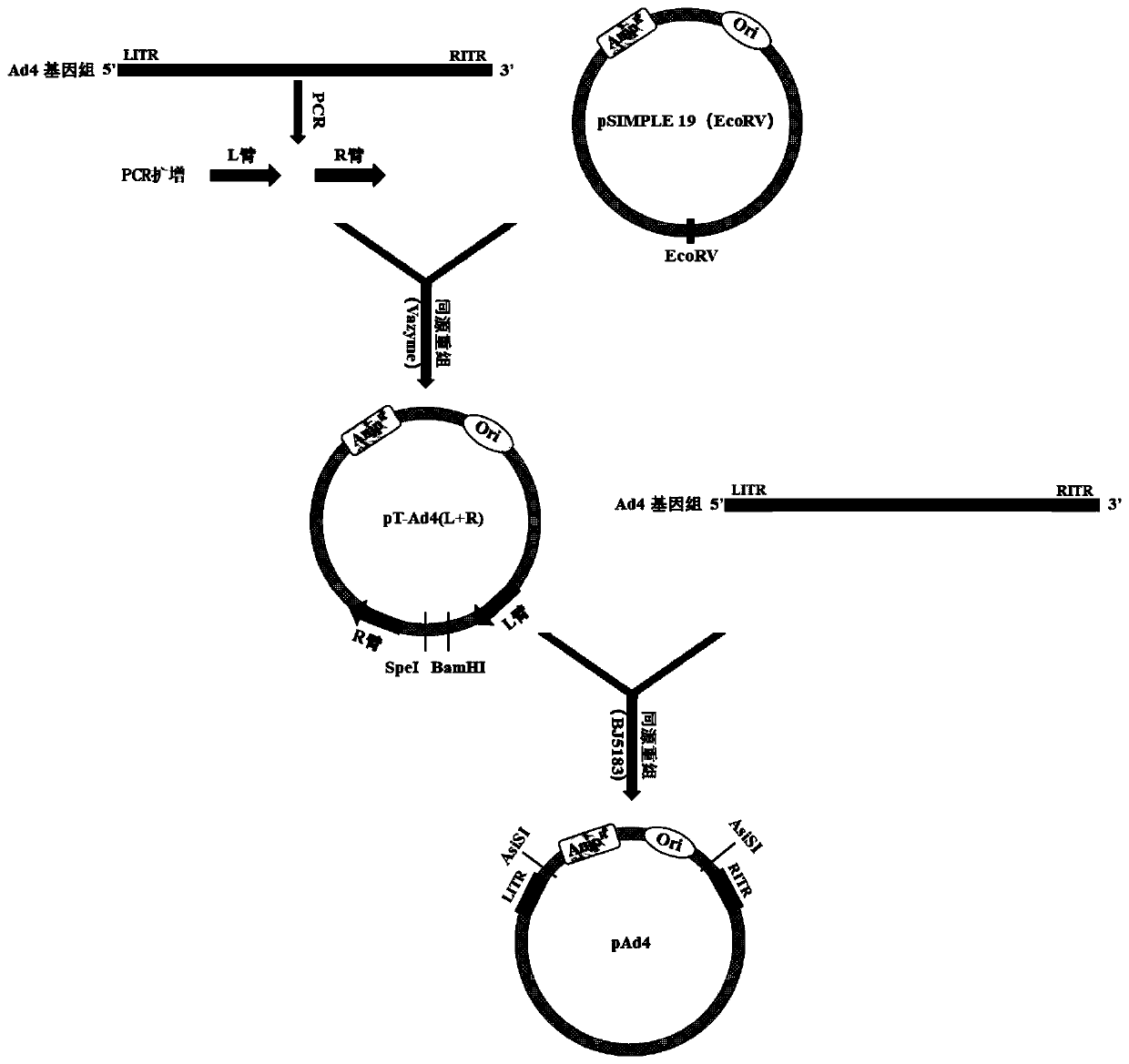
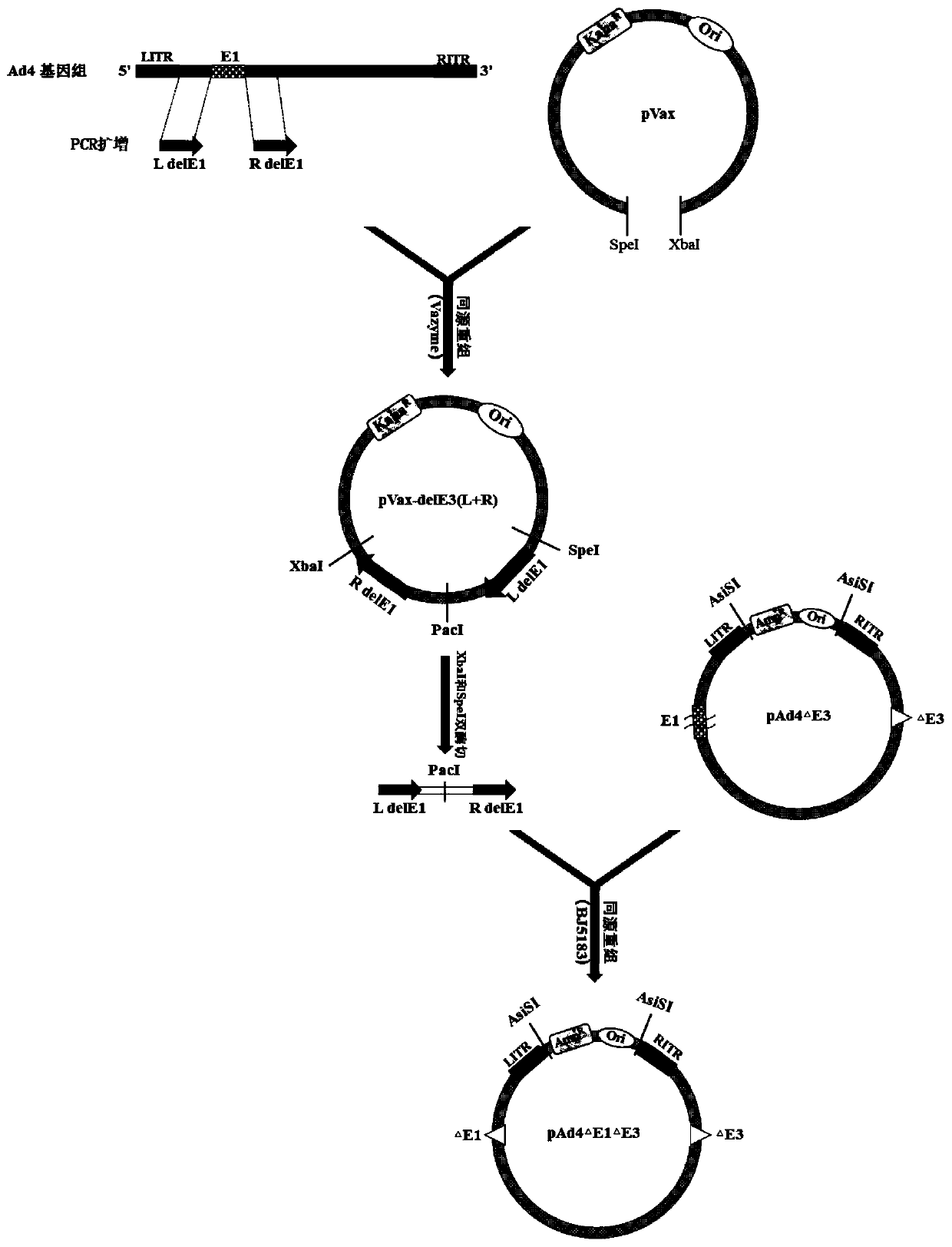
Replication-defective recombinant human-type-4 adenovirus, and preparation method and application thereof
PendingCN110551757AStrong immune responseViral antigen ingredientsImmunoglobulins against virusesOpen reading frameHuman type
The invention discloses a preparation method and application of replication-defective recombinant human-type-4 adenovirus. The replication-defective recombinant human-type-4 adenovirus is obtained bythe following method that a genome of the human-type-4 adenovirus is made to be like plasmids, genes E1 and E3 of the human-type-4 adenovirus are knocked out, and open reading frames 2, 3, 4, 5 and 6of an gene E4 of Ad4 are replaced with the corresponding open reading frames of an gene E4 of Ad5. The described replication-defective recombinant human-type-4 adenovirus vector can be potentially applied to the research and development of anti human-type-4 adenovirus vaccine, the screening of anti human type 4 adenovirus neutralizing antibody and drugs, the research and development of anti otherpathogen vaccine, report tracer system of biological research and the like.
Owner:GUANGZHOU N BIOMED LTD
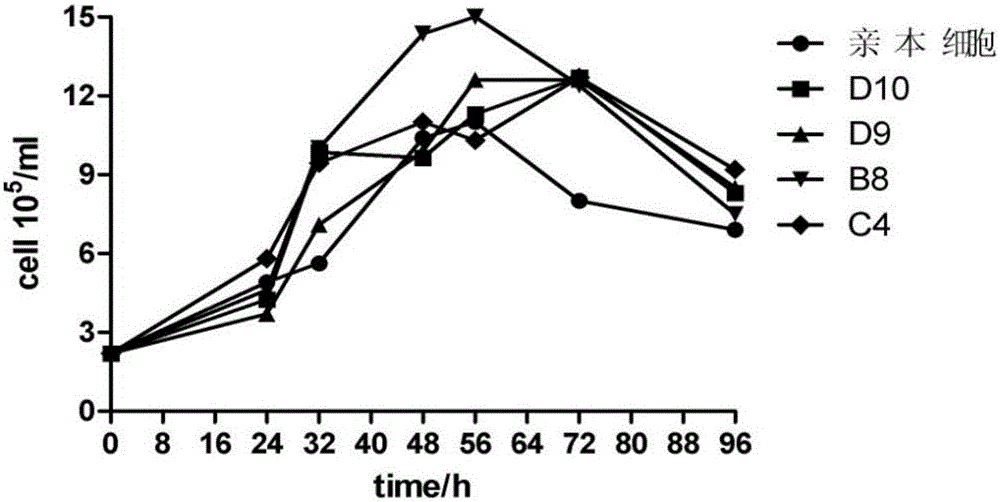
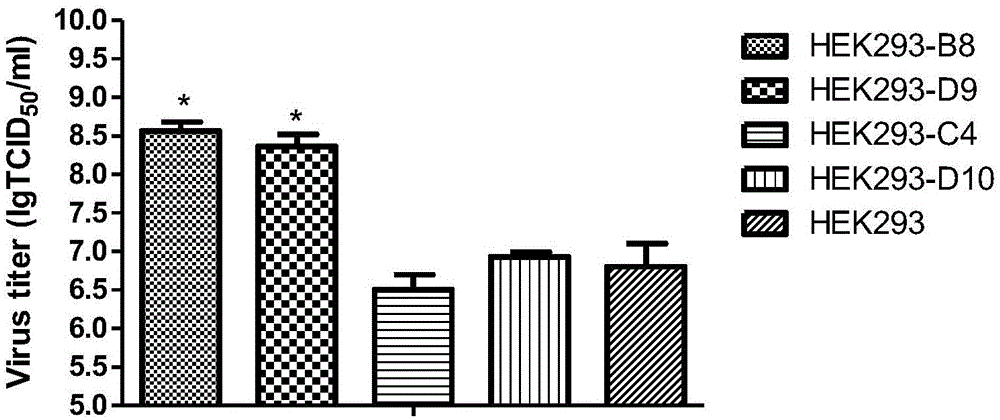
High-adaptability HEK293 clonal cell strains for recombinant adenovirus vaccine strains and application of high-adaptability HEK293clonal cell strains
ActiveCN105154390AEfficient ProliferationHigh titerMicroorganism based processesViruses/bacteriophagesMicroorganismMicrobiology
The invention discloses high-adaptability HEK293 clonal cell strains for recombinant adenovirus vaccine strains and application of the high-adaptability HEK293 clonal cell strains, which belongs to the field of HEK293 clonal cell strains. The high-adaptability HEK293 clonal cell strains and the application thereof are characterized in that a limiting dilution method is adopted for cloning HEK293 single cells; and two monoclonal cell strains with excellent performance are finally screened by subcloning the cloned HEK293 single cells, with microbial preservation numbers of CGMCC (China General Microbiological Culture Collection Center) No. 11096 and CGMCC No. 11097. Compared with a female cell, the two HEK293 monoclonal cell strains provided by the invention have the advantages that the growth speed is remarkably improved, recombinant adenovirus rAdV-SFV-E2 strains can be better replicated, the replication level of viruses is obviously improved, and the better stability is obtained; the two HEK293 monoclonal cell strains are used for replicating the recombinant adenovirus rAdV-SFV-E2 strains, so that virus bulk with higher titer can be obtained, and the virus titer is significantly improved.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Tumor-treatment adenovirus vaccine taking hTERT (human telomerase reverse transcriptase) as target point
InactiveCN103952443AGenetic material ingredientsViruses/bacteriophagesShuttle vectorReverse transcriptase
The invention discloses a tumor-treatment adenovirus vaccine taking hTERT (human telomerase reverse transcriptase) as a target point. Firstly, a hTERT composite gene eTERTFcGB is inserted to the downstream of a CMV (cytomegalovirus) promoter in a pShuttle-CMV shuttle vector to obtain a recombinant shuttle vector with the hTERT composite gene eTERTFcGB; then, the recombined shuttle vector and a virus framework vector AdEasy-1 / F11p co-transform bacteria to obtain a recombinant adenovirus vector. The recombinant adenovirus vector can inhibit growth of transplantation tumors, and can be applied to immunopotentiation of malignant tumor biological immune therapy.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Recombinant expression type adenovirus dynein peptide, adenovirus subunit vaccine and preparation method thereof
ActiveCN107365365AHigh expressionImproving immunogenicityViral antigen ingredientsVirus peptidesCross neutralizationMonoclonal antibody
The invention discloses recombinant expression type adenovirus dynein peptide, an adenovirus subunit vaccine and a preparation method thereof. The recombinant expression type adenovirus dynein peptide has an amino acid sequence shown as SEQ ID No: 2; the vaccine contains the adenovirus dynein peptide. The adenovirus dynein peptide, disclosed by the invention, has a high expression quantity, can be used for inducing a high-titer neutralizing antibody when being used for immunizing a mouse and has strong immunogenicity; the adenovirus dynein peptide can be used as a candidate of the adenovirus subunit vaccine, is convenient to produce and purity and is low in cost; the adenovirus dynein peptide can be used for inducing a cross neutralization effect antibody and can be used as a candidate of a multivalent adenovirus vaccine; the adenovirus dynein peptide can be matched with other types of knob to be used as a candidate of a multivalent adenovirus subunit vaccine; the adenovirus dynein peptide can also be used as an immune antigen and a detection antigen which are used for preparing broad-spectrum neutralization activity monoclonal antibody or a human-derived monoclonal antibody.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
Adenovirus vector recombinant new coronavirus B.1.617.2 variant vaccine and application thereof
ActiveCN114106116AImproving immunogenicityStrong and broadly neutralizing antibody responseSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
The invention discloses a novel crown variant vaccine taking human type 5 replication-deficient adenovirus as a vector. The vaccine takes E1 and E3 combined deletion replication-deficient human type 5 adenovirus as a carrier, and a novel coronavirus B.1.617.2 variant spike protein mutant coding gene (Ad5-nCoV-B.1.617.2) which is subjected to optimization design is integrated in a genome of the E1 and E3 combined deletion replication-deficient human type 5 adenovirus vaccine. The vaccine can effectively express protective antigen protein in host cells. The specific antibody reaction aiming at the B.1.617.2 variant can be excited by using the vaccine for single immunization. When the novel coronavirus vaccine is combined with a 2019 wild type novel coronavirus vaccine for use, a strong and broad-spectrum new coronavirus variant neutralizing antibody reaction can be stimulated after vaccine booster immunization, and the novel coronavirus vaccine has a remarkable application advantage, particularly has an outstanding advantage in heterotype booster immunization and coping with a new coronavirus B.1.617.2 variant epidemic situation, can be used as a vaccine candidate strain, and can be used for preparing a new coronavirus vaccine for preventing and treating the new coronavirus B.1.617.2 variant epidemic situation. The method is used for coping with continuously-spreading new crown variant epidemic situations.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Gene delivery vectors provided with a tissue tropism for dendritic cells
InactiveUS20040033605A1Safe deliveryImprove efficiencyVectorsGenetic material ingredientsGene deliveryDendritic cell
Adenoviral vectors can be used in vaccines to cause antigen-presenting cells to display desired antigens. Disclosed is a vector and associated means and methods which transduce antigen-presenting cells better than currently available vectors, enabling the vector to be delivered in lower doses, and thus improving the efficiency of adenoviral vaccines technology.
Owner:HAVENGA MENZO +2
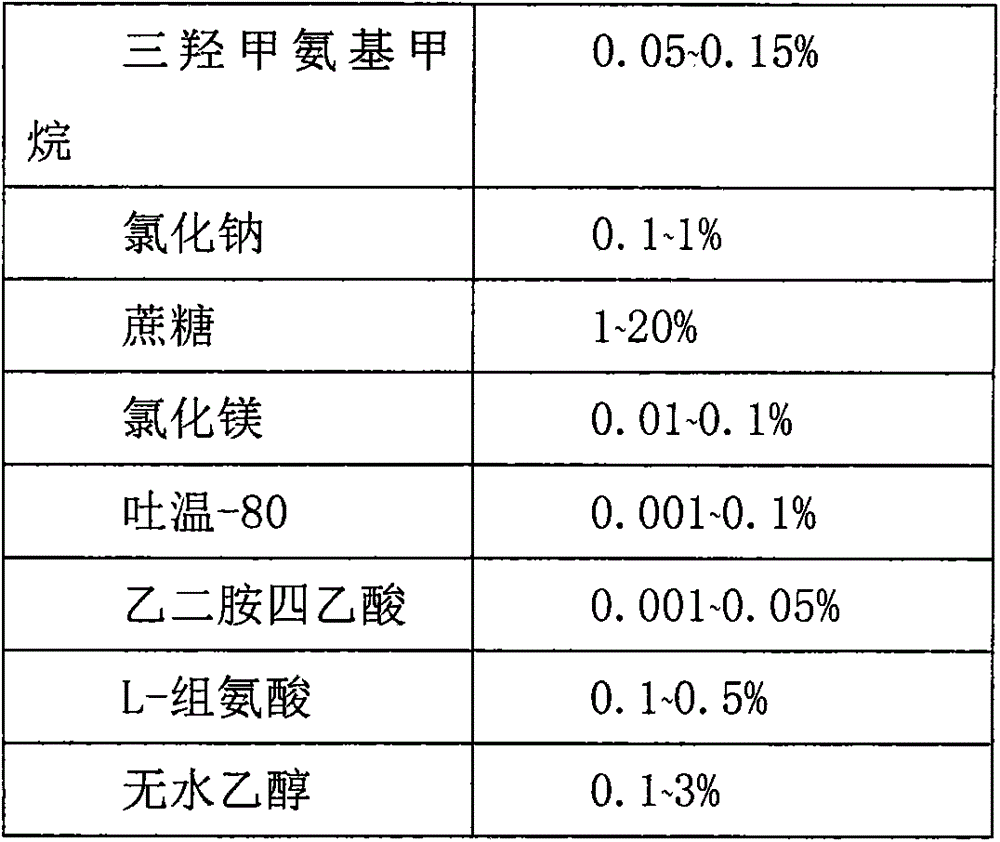
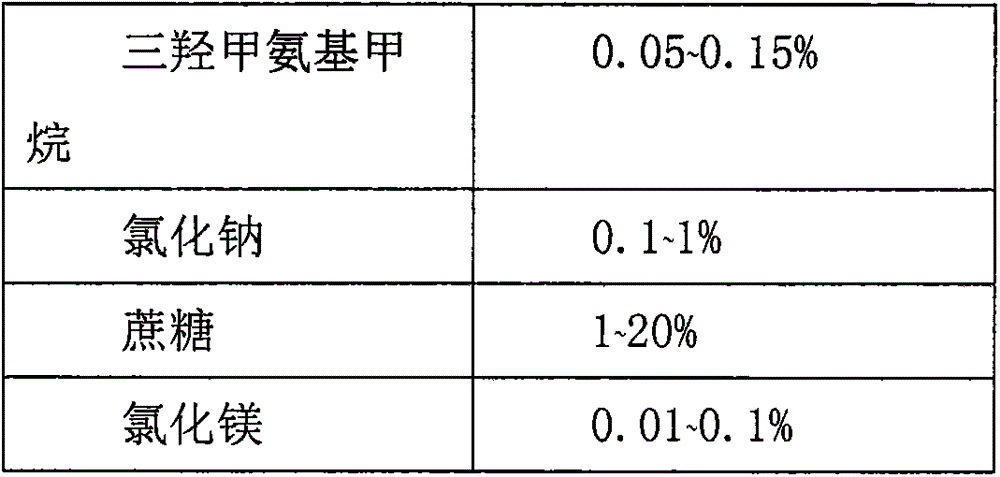
Recombinant adenovirus vaccine preparation and preparation method thereof
InactiveCN105267960AKeep aliveImprove stabilityGenetic material ingredientsPharmaceutical non-active ingredientsPenicillinFiltration
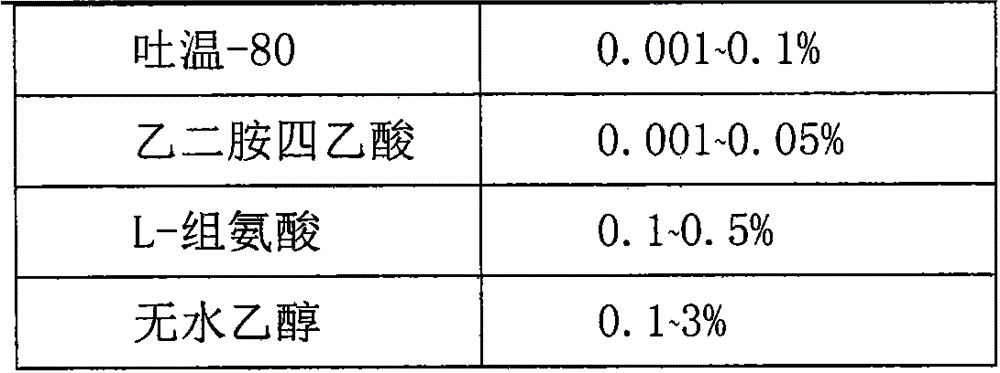
The invention relates to a preparation method of a recombinant adenovirus vaccine preparation in the field of biotechnology. The invention aims to provide a recombinant adenovirus vaccine preparation. According to the technical scheme, the recombinant adenovirus vaccine preparation contains a recombinant adenovirus stock solution and a preparation prescription. The method for producing the above recombinant adenovirus vaccine preparation comprises the following steps: (1) preparing a buffer solution according to the preparation prescription, finally adding the virus stock solution, uniformly mixing, carrying out aseptic filtration, and subpackaging in penicillin bottles; filling nitrogen and tamponing and capping; and (2) carrying out light inspection on the subpackaged recombinant adenovirus vaccine preparation, and preserving the recombinant adenovirus vaccine preparation at minus 20 DEG C. The recombinant adenovirus vaccine preparation can be preserved for at least 24 months.
Owner:亚宝药业太原制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com