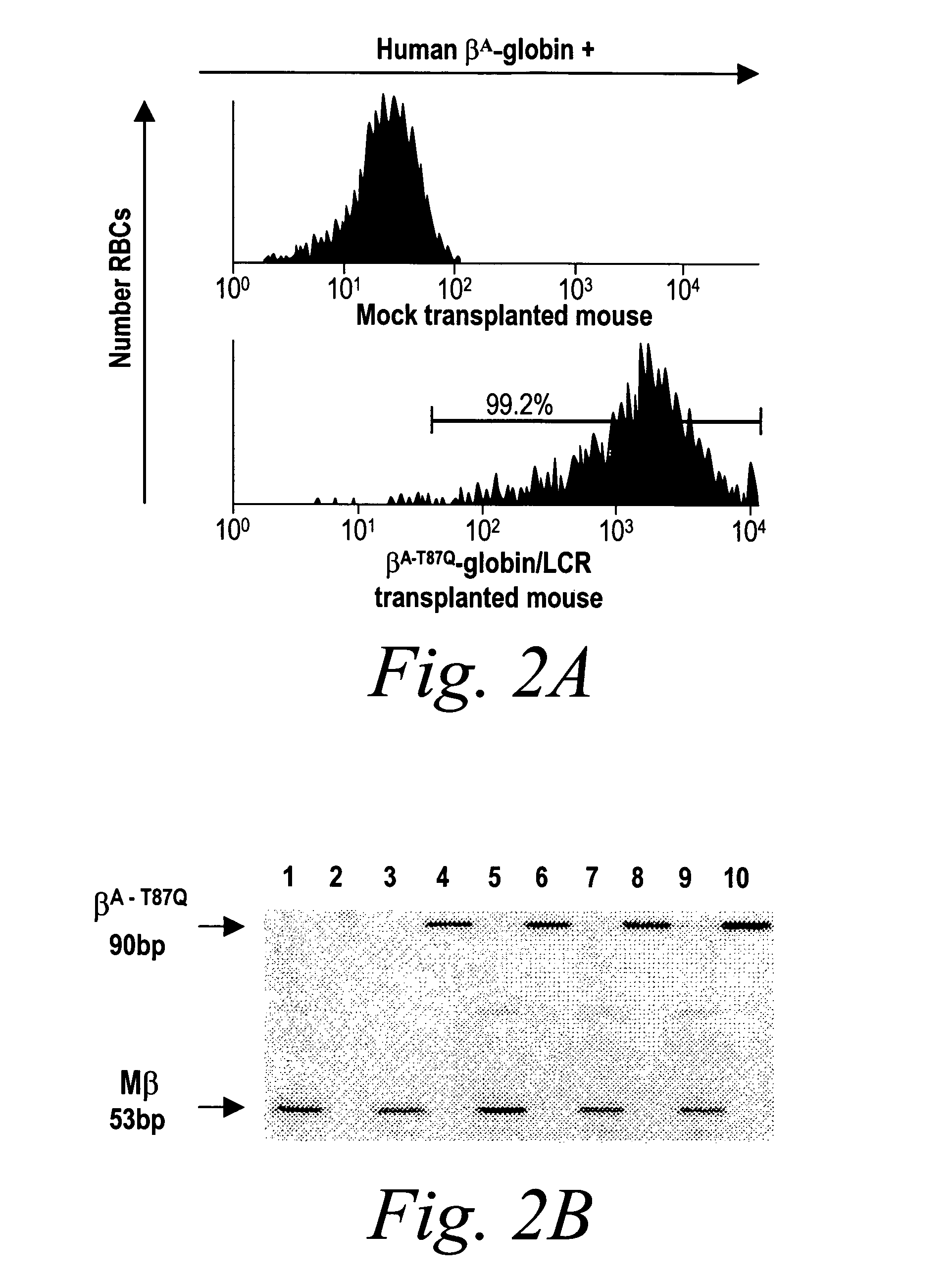
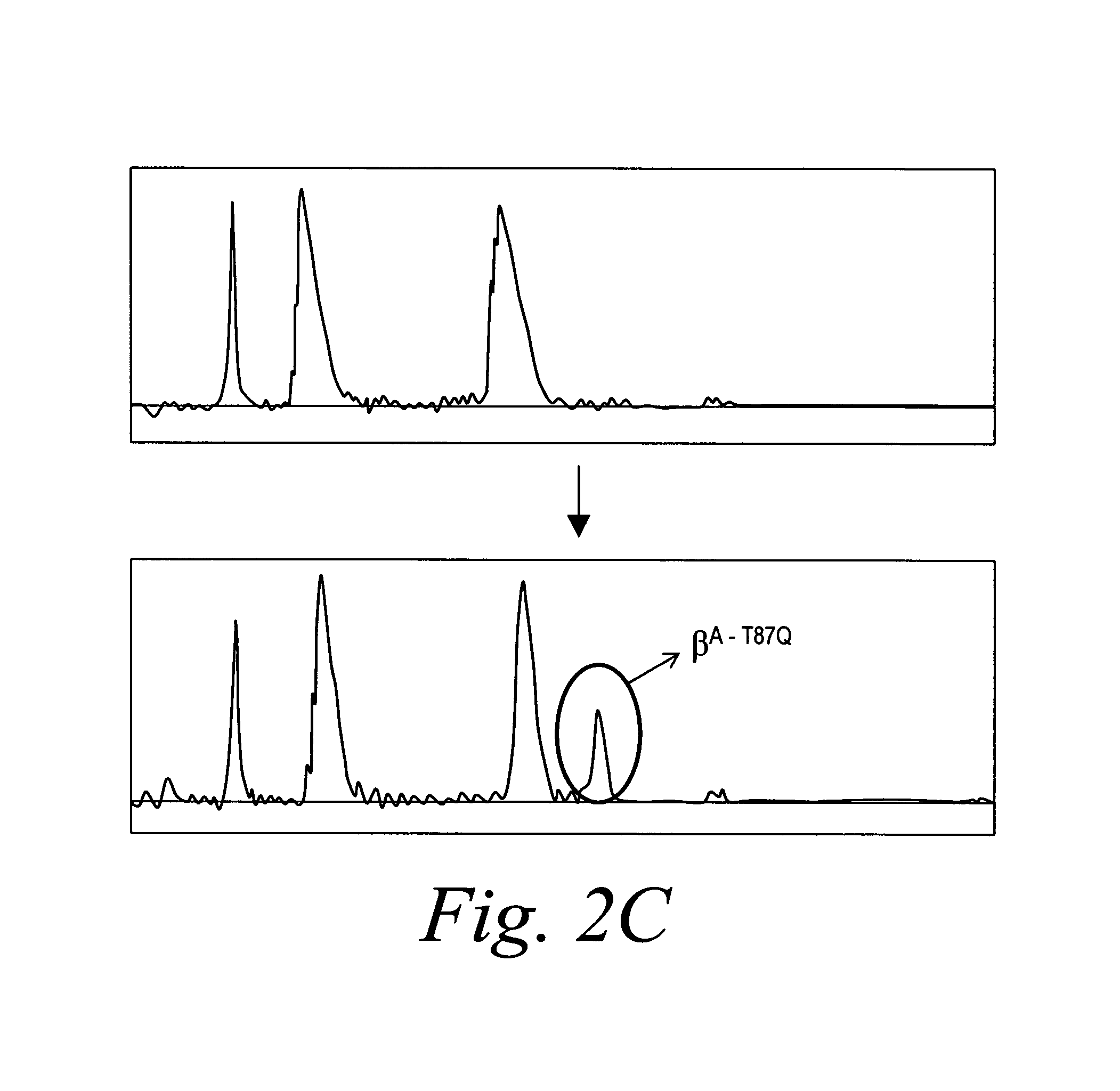
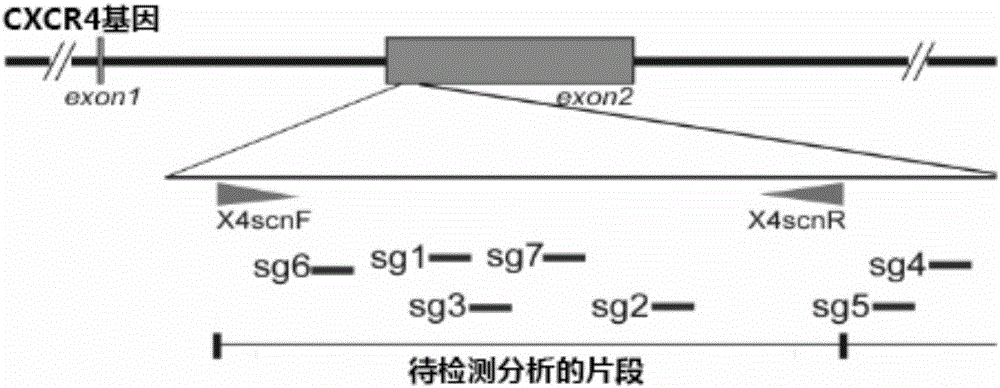
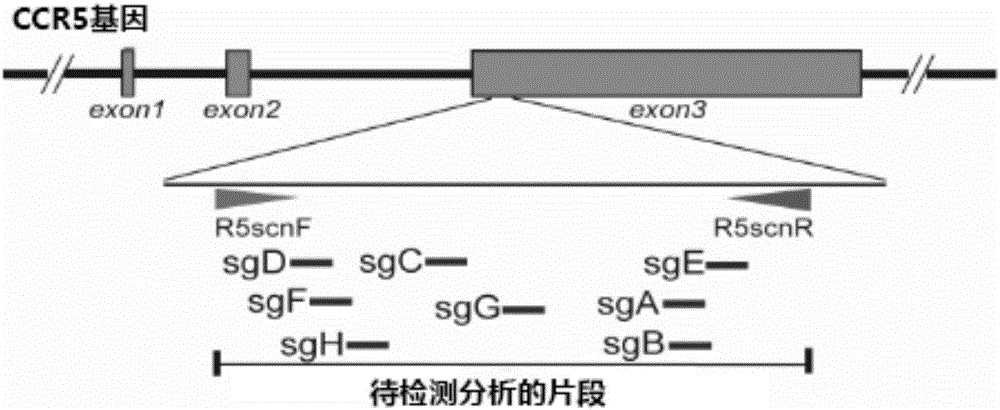
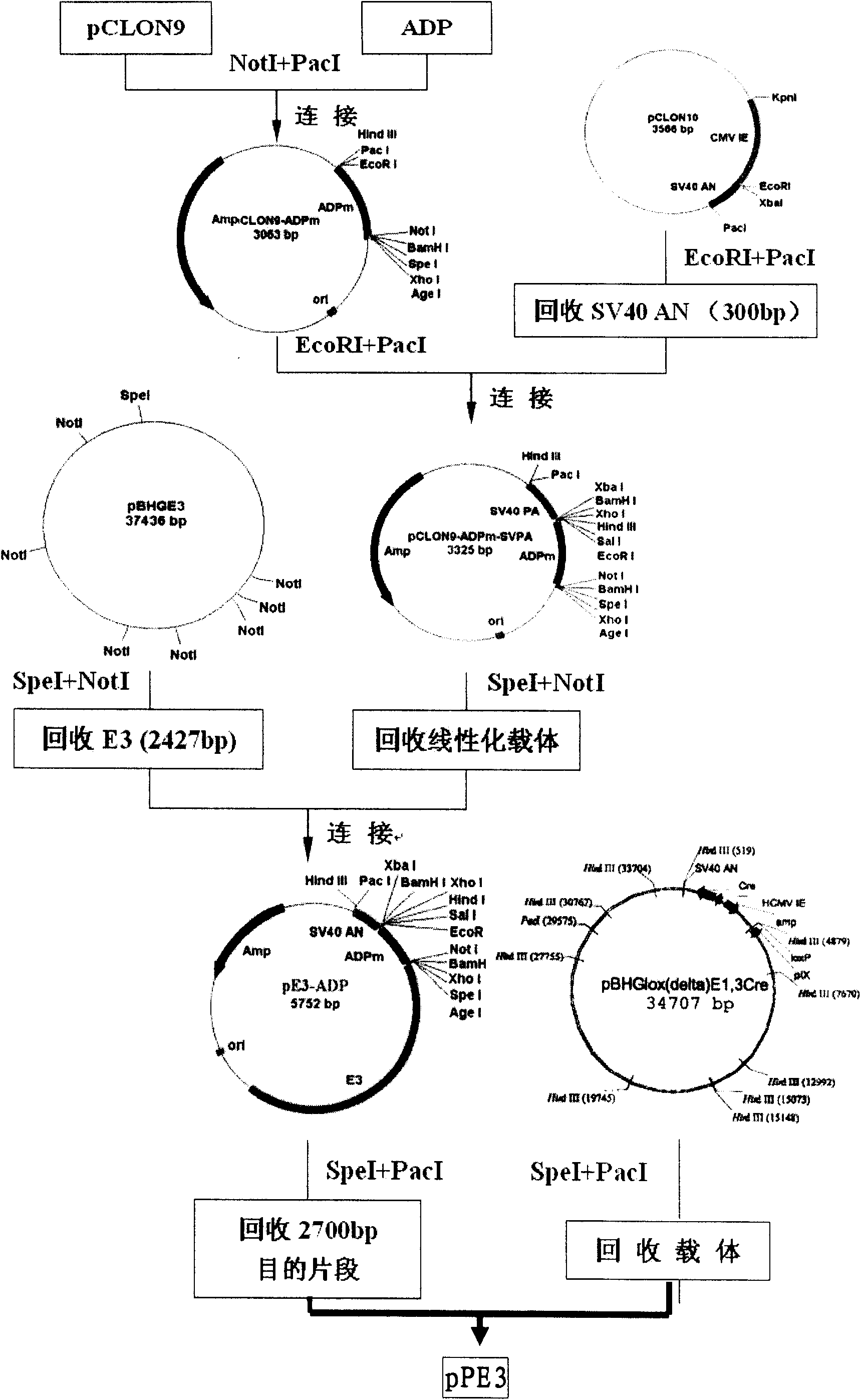
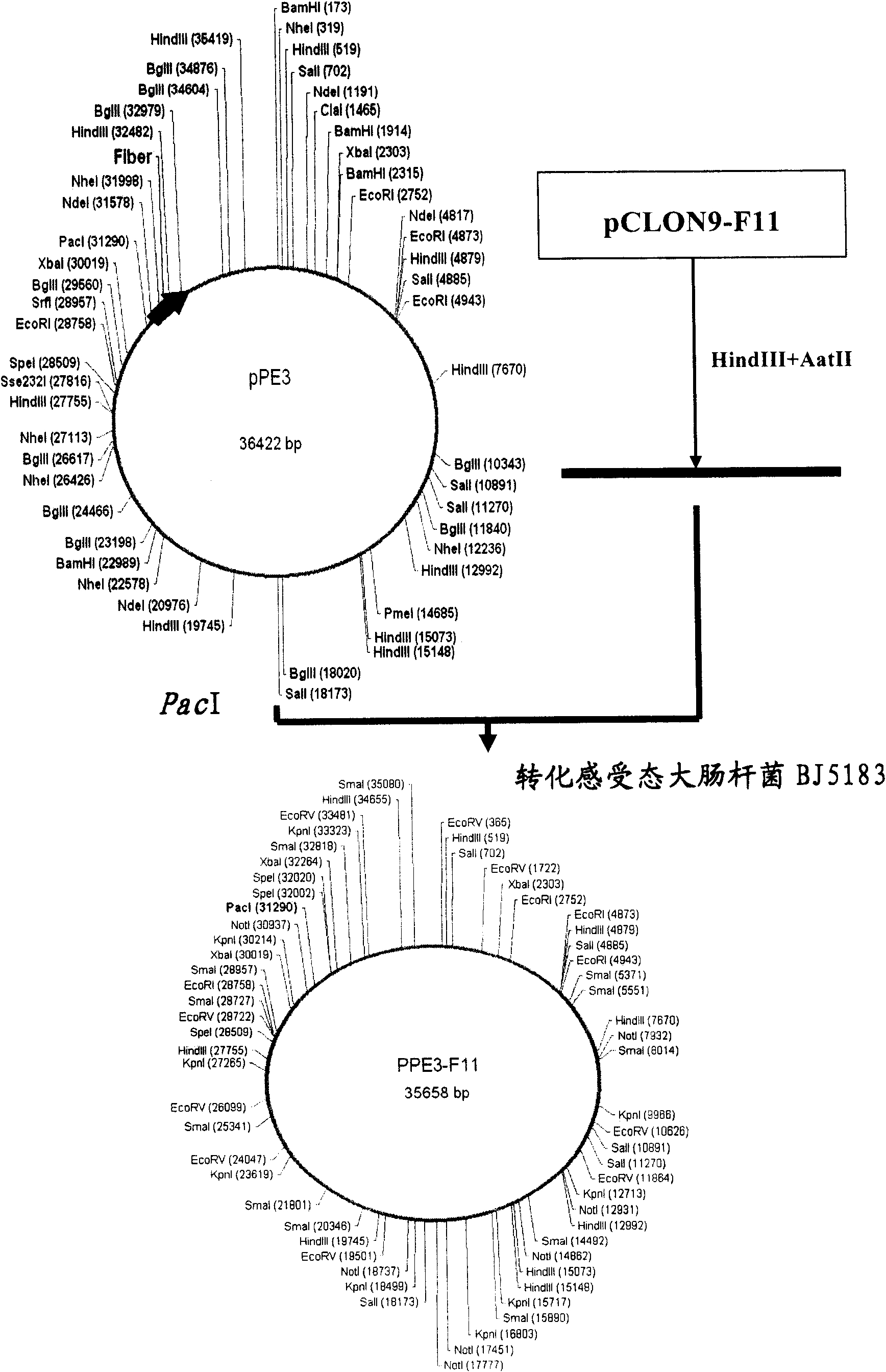
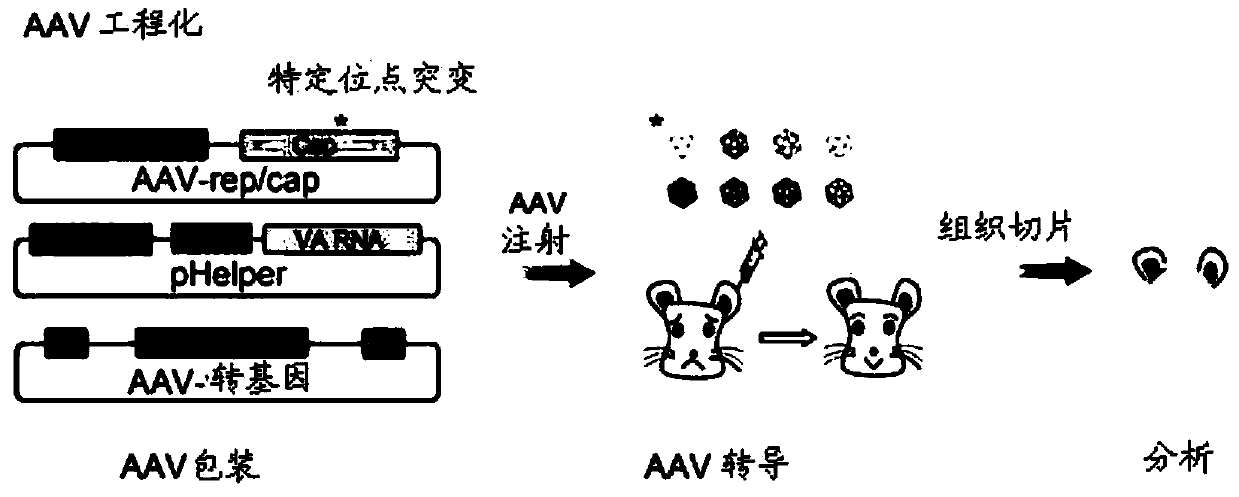
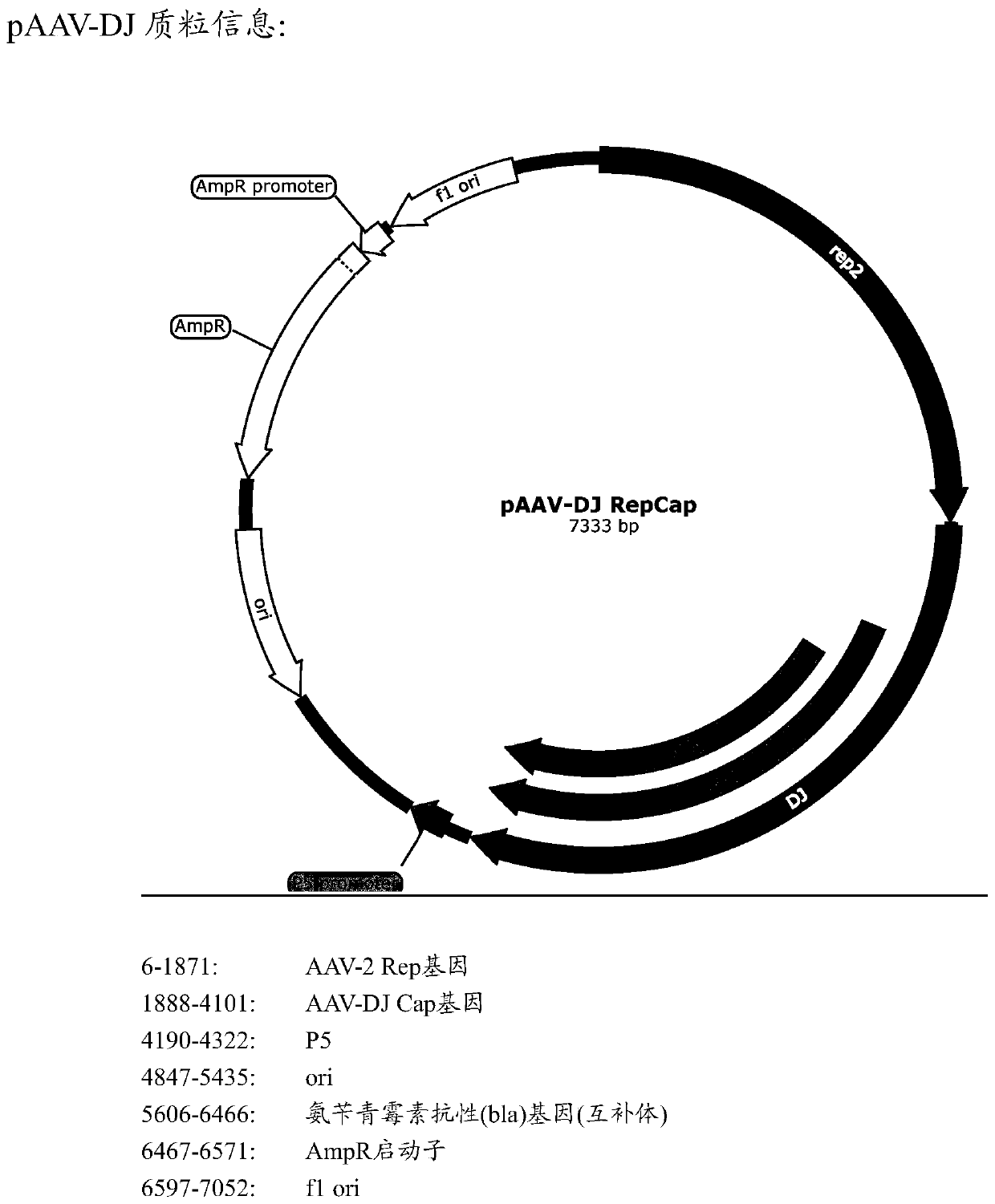
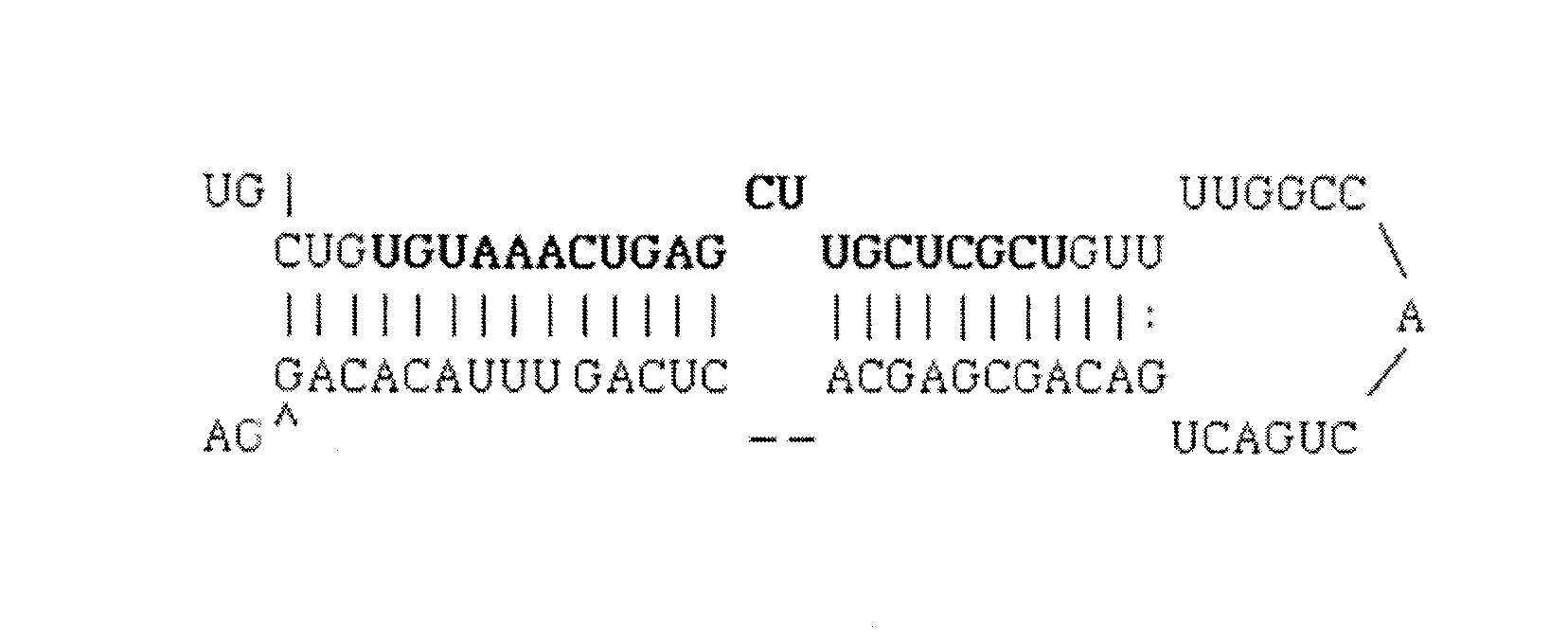Patents
Literature
158results about How to "High infection efficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
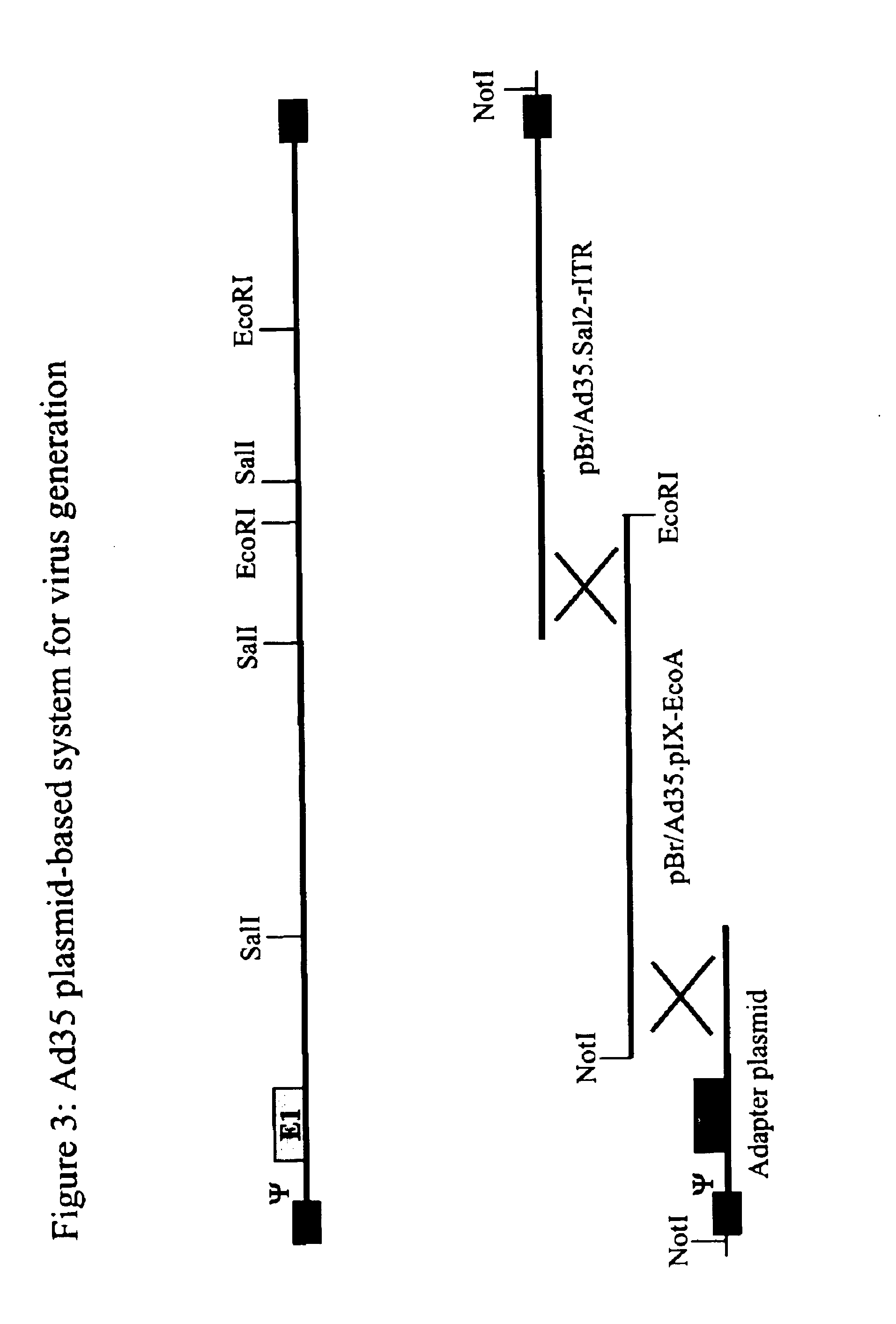
Serotype of adenovirus and uses thereof
InactiveUS6913922B1High infection efficiencyEasy to copyBiocideGenetic material ingredientsCell bindingSerotype
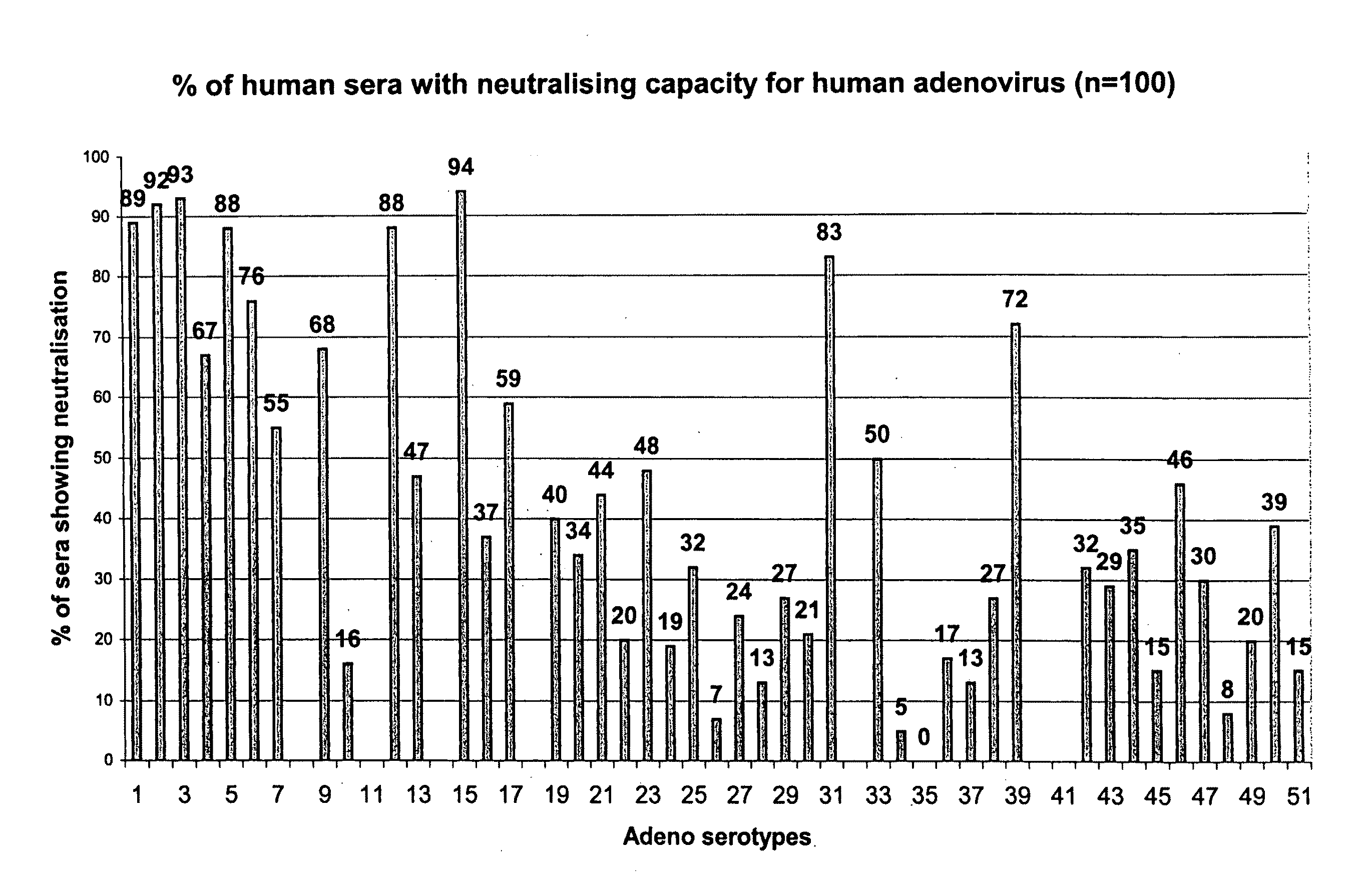
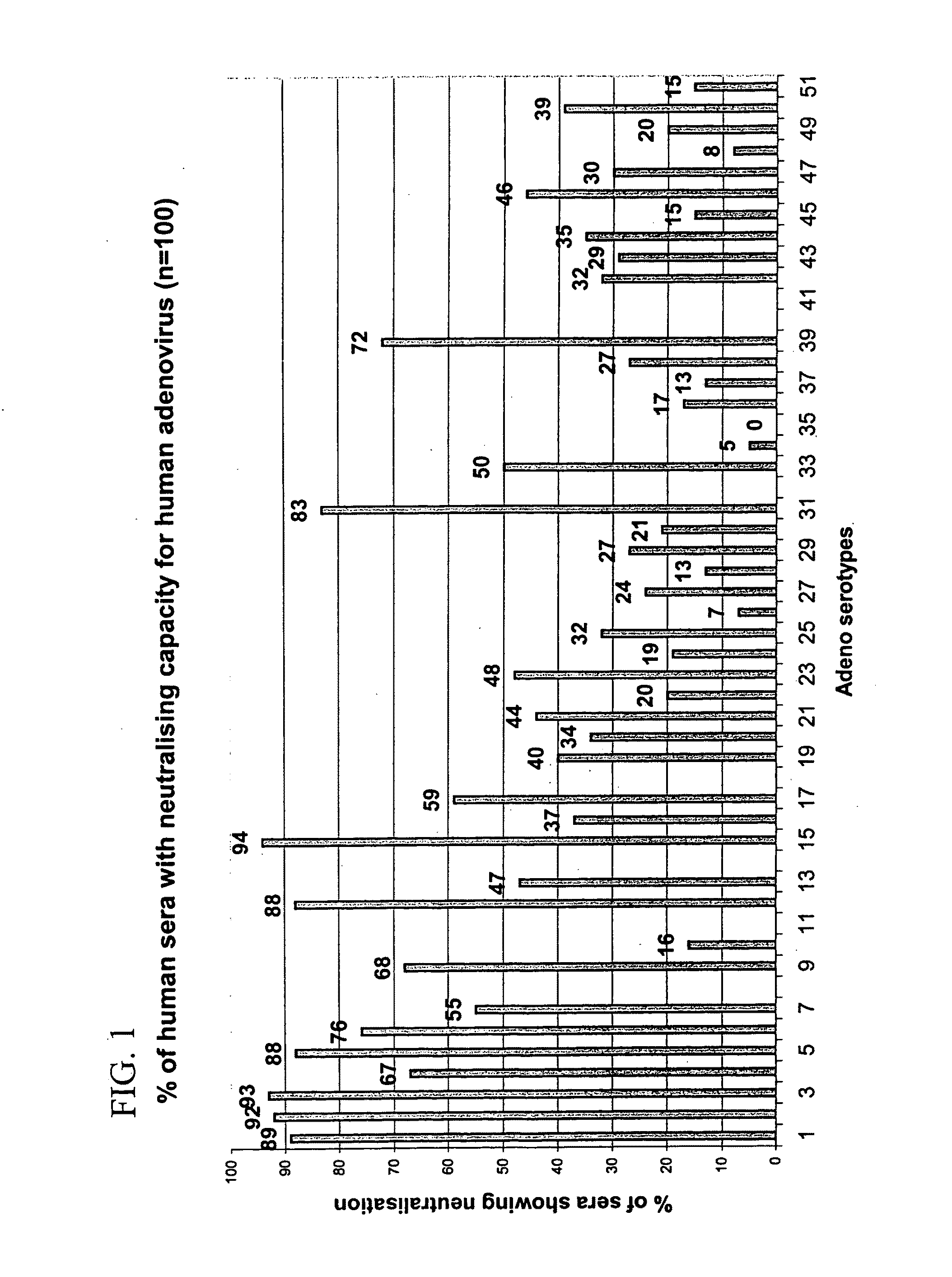
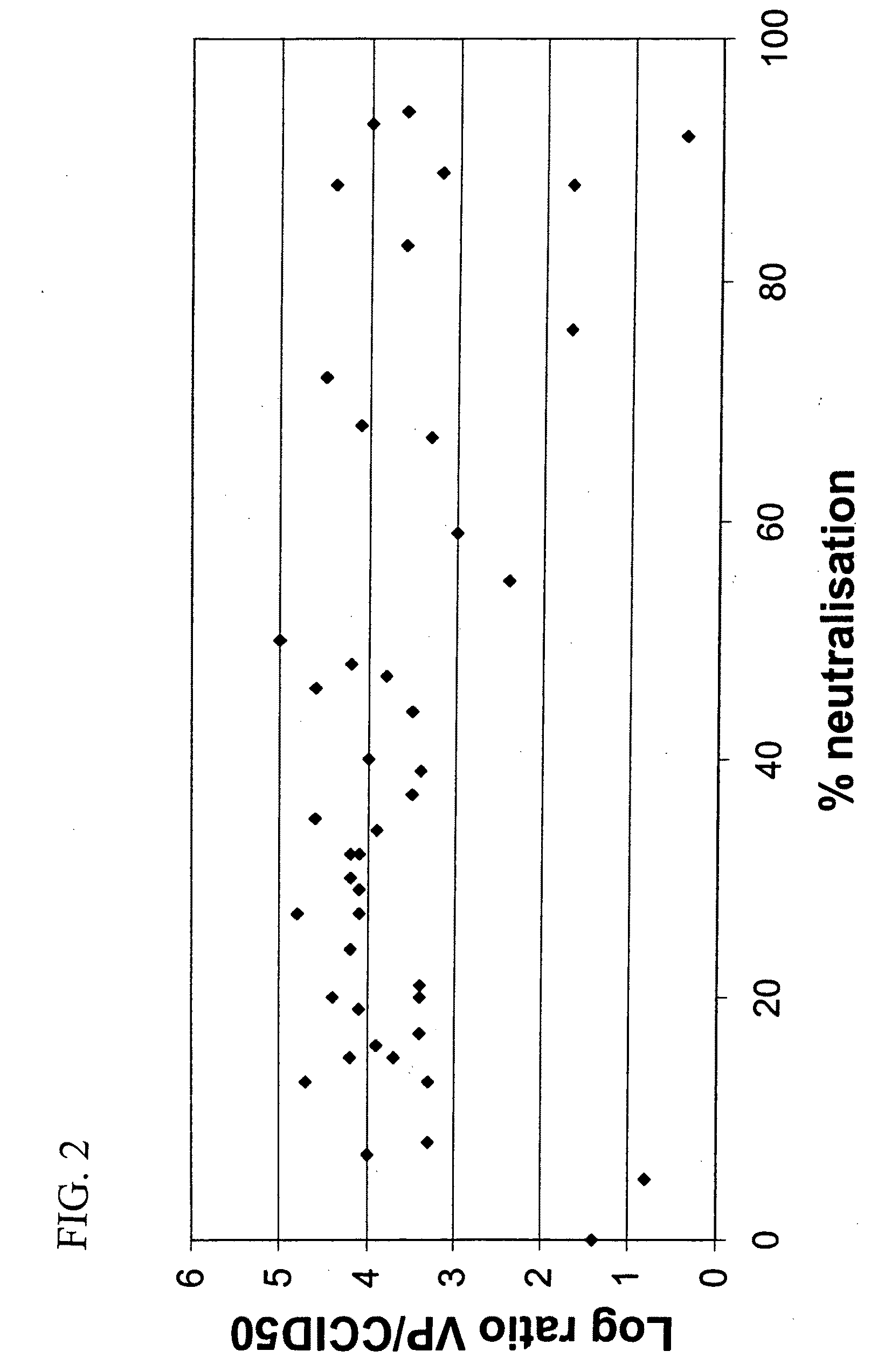
Adenovirus serotypes differ in their natural tropism. The adenovirus serotypes 2, 4, 5, and 7 all have a natural affiliation towards lung epithelia and other respiratory tissues. In contrast, serotypes 40 and 41 have a natural affiliation towards the gastrointestinal tract. The serotypes described, differ in at least capsid proteins (penton-base, hexon), proteins responsible for cell binding (fiber protein), and proteins involved in adenovirus replication. This difference in tropism and capsid protein among serotypes has led to the many research efforts aimed at redirecting the adenovirus tropism by modification of the capsid proteins.
Owner:JANSSEN VACCINES & PREVENTION BV
Therapeutic retroviral vectors for gene therapy
InactiveUS7901671B2Maintaining therapeutic levelImprove the level ofBiocidePeptide/protein ingredientsHematopoietic cellRed blood cell
Owner:MASSACHUSETTS INST OF TECH
sgRNA (singleguide Ribonucleic Acid), lentiviral vector constructed by the same and application thereof
InactiveCN106801056AEffective knockoutExcellent anti-virus infection abilityCell receptors/surface-antigens/surface-determinantsAntiviralsTreatment fieldHIV receptor
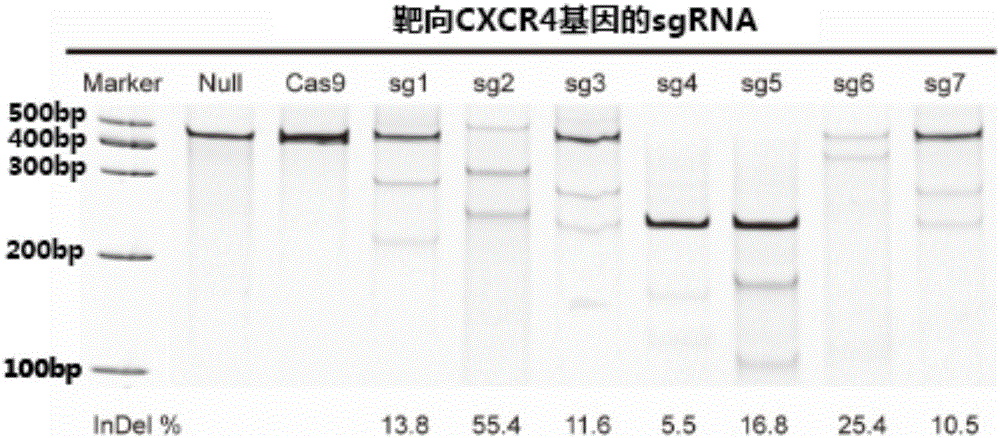
The invention relates to the field of gene therapy, in particular relates to sgRNA (singleguide Ribonucleic Acid), as well as a lentiviral vector constructed by the same and application thereof and specifically relates to sgRNA with SIVmac1A11 lentivirus as a framework to express SpCas9 protein and gene specificity. The sgRNA is applied to treating human and simian AIDS (Acquired Immune Deficiency Syndrome). A nucleotide sequence of the sgRNA is shown as SEQ ID NO.1 to 2. According to the sgRNA disclosed by the invention, a current most efficient CRISPR / Cas9 gene editing tool is utilized, a designed CXCR4 / CCR5 gene sgRNA locus has gene knockout activity superior to other loci reported by existing research, and the sgRNA is applied to gene therapy of SIV infected rhesus monkeys for the first time. Compared with ZFN and TALEN, the sgRNA has the advantages of being convenient to operate, low in cost and the like.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Serotype of adenovirus and uses thereof
InactiveUS20050232900A1Avoid and diminish immune responseImmune responseBiocideGenetic material ingredientsCell bindingSerotype
Adenovirus serotypes differ in their natural tropism. The adenovirus serotypes 2, 4, 5 and 7 all have a natural affiliation towards lung epithelia and other respiratory tissues. In contrast, serotypes 40 and 41 have a natural affiliation towards the gastrointestinal tract. The serotypes described differ in at least capsid proteins (penton-base, hexon), proteins responsible for cell binding (fiber protein), and proteins involved in adenovirus replication. This difference in tropism and capsid protein among serotypes has led to the many research efforts aimed at redirecting the adenovirus tropism by modification of the capsid proteins.
Owner:JANSSEN VACCINES & PREVENTION BV
Serotype of adenovirus and uses thereof
InactiveUS20100034774A1Immune responseLess of such a drawbackBiocideGenetic material ingredientsCell bindingSerotype
Adenovirus serotypes differ in their natural tropism. The adenovirus serotypes 2, 4, 5 and 7 all have a natural affiliation towards lung epithelia and other respiratory tissues. In contrast, serotypes 40 and 41 have a natural affiliation towards the gastrointestinal tract. The serotypes described differ in at least capsid proteins (penton-base, hexon), proteins responsible for cell binding (fiber protein), and proteins involved in adenovirus replication. This difference in tropism and capsid protein among serotypes has led to the many research efforts aimed at redirecting the adenovirus tropism by modification of the capsid proteins.
Owner:JANSSEN VACCINES & PREVENTION BV
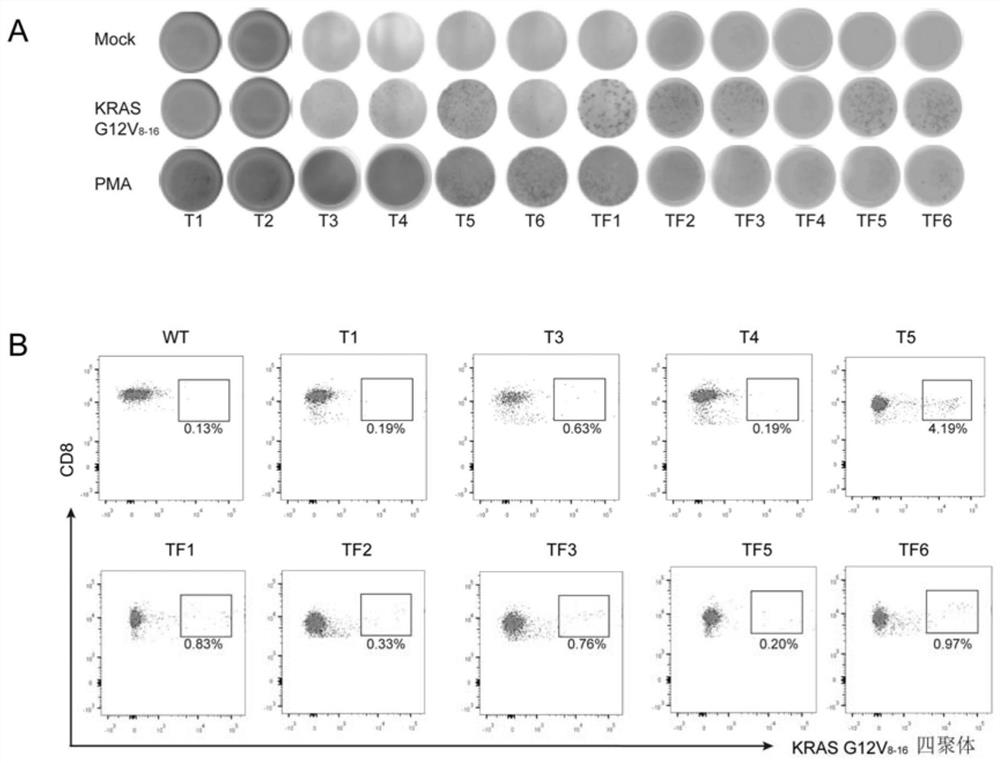
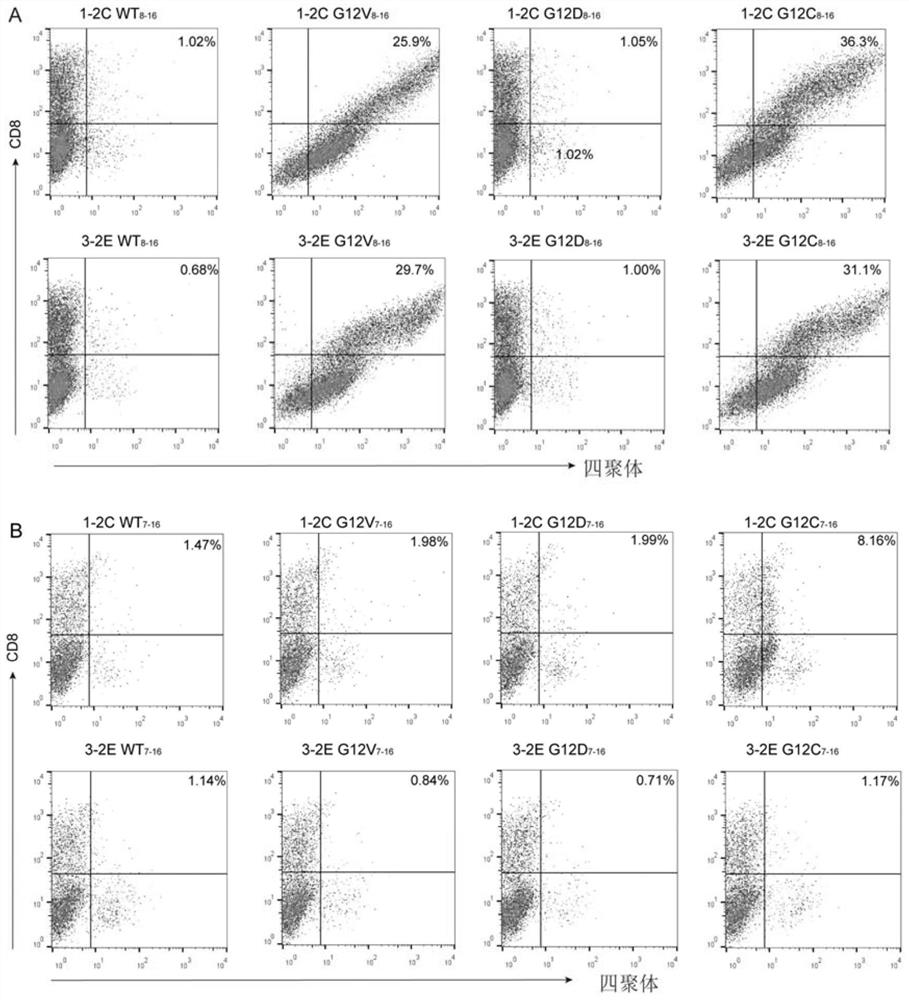
Screening and anti-tumor application of KRAS mutation specific T cell receptor
ActiveCN112300269AEffectively identify and killGrowth inhibitionNucleic acid vectorFermentationEpitopeImmune effects
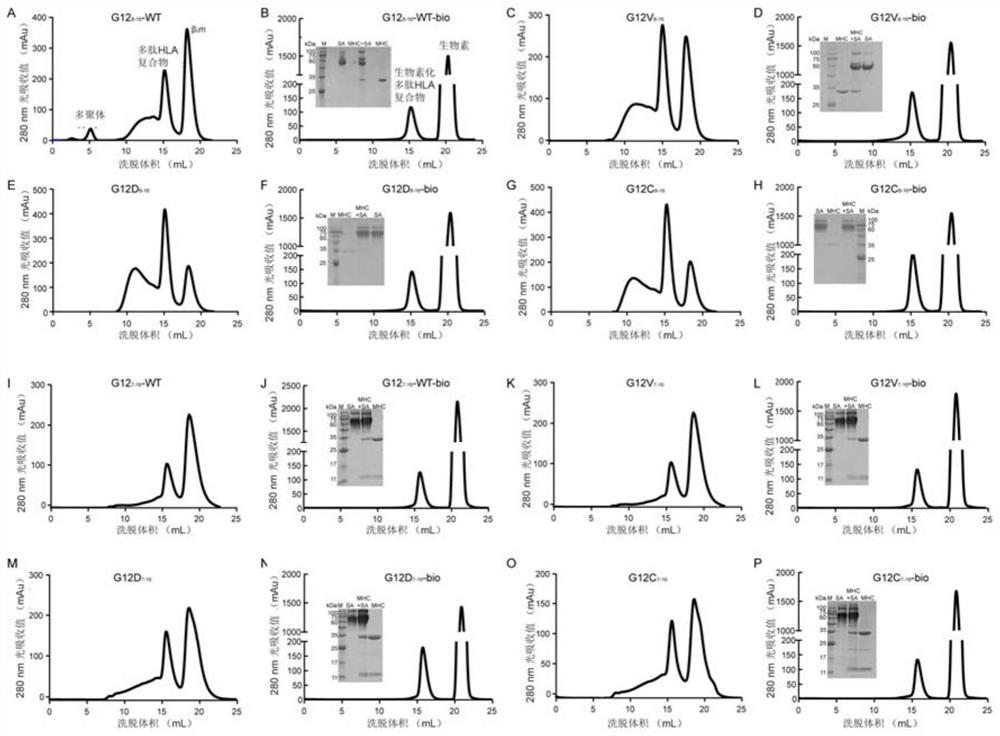
The invention provides two specific T cell receptors targeting G12V or G12C mutation epitopes of a KRAS gene and an anti-tumor application. The two T cell receptors respectively consist of an alpha peptide chain and a beta peptide chain. The invention also provides an antigen binding fragment of the T cell receptors, nucleic acid encoding the antigen binding fragment, a vector containing the nucleic acid, and a host cell containing the vector. The invention further provides a method for preparing a G12V mutation-specific T cell receptor of KRAS or an antigen-binding fragment thereof. The specific T cell receptor and the antigen binding fragment thereof can be used as immune effect activators to stimulate the immune response of the body, thereby generating the action effect of resisting tumors and other diseases.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Serotype of adenovirus and uses thereof
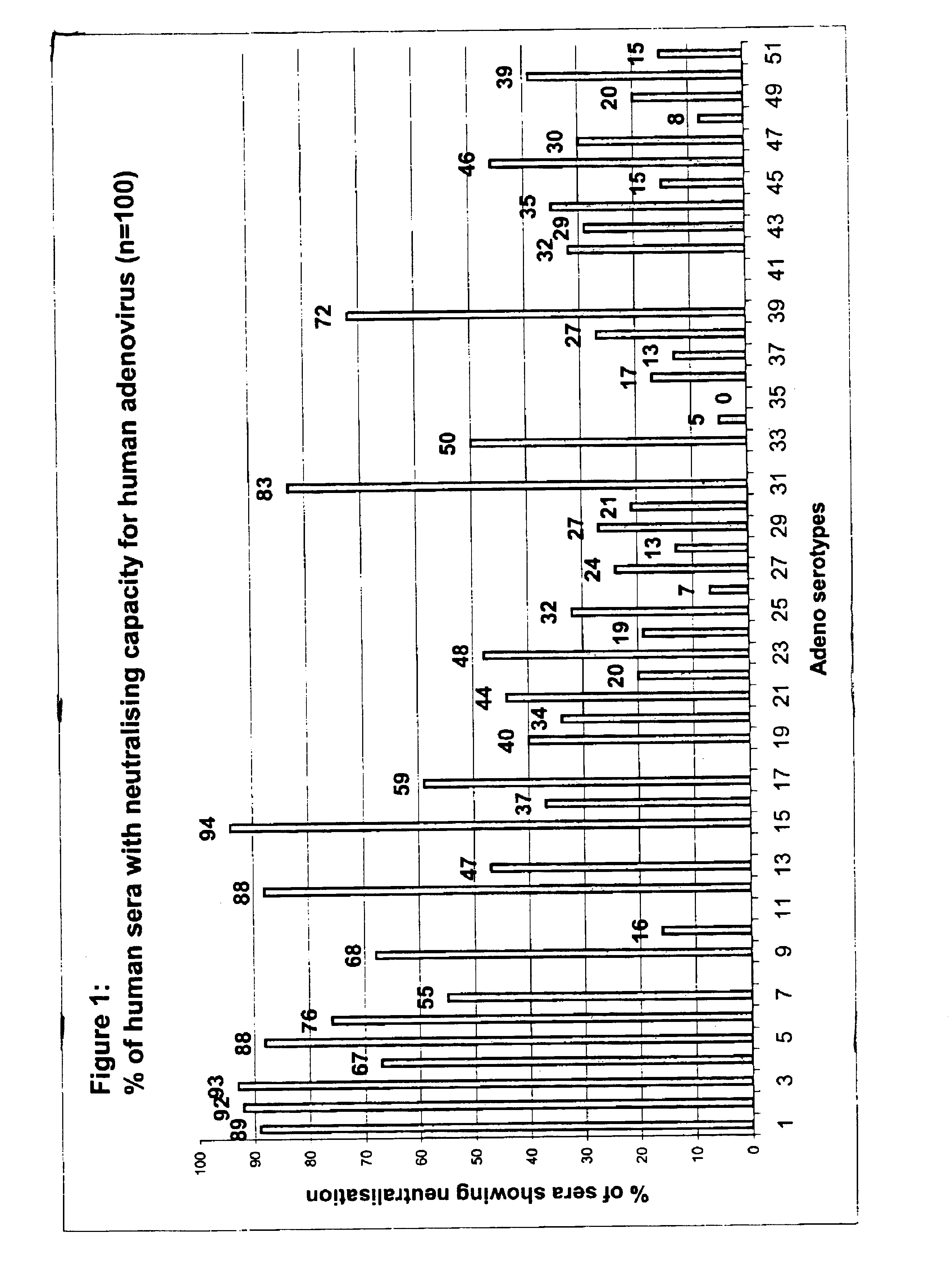
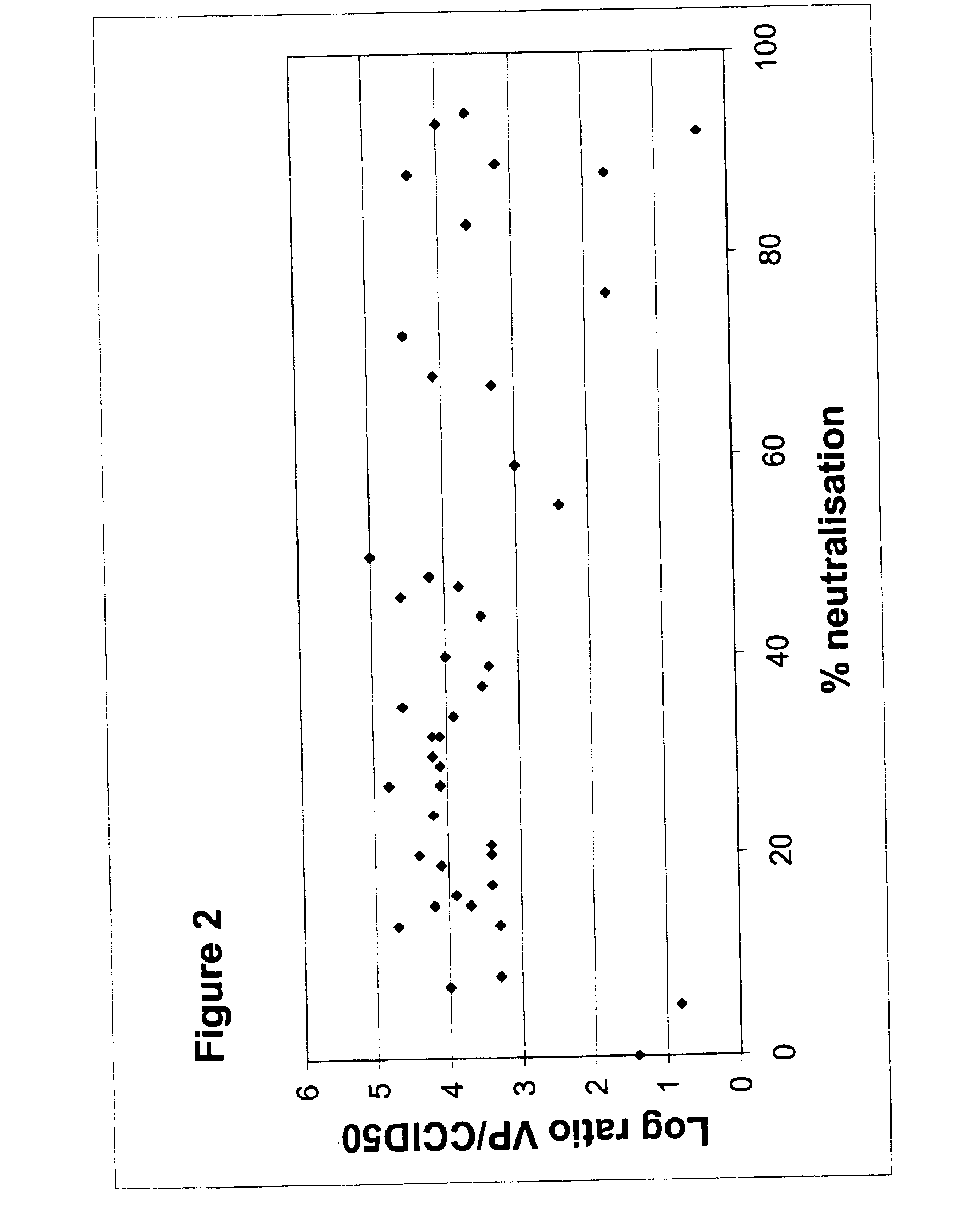
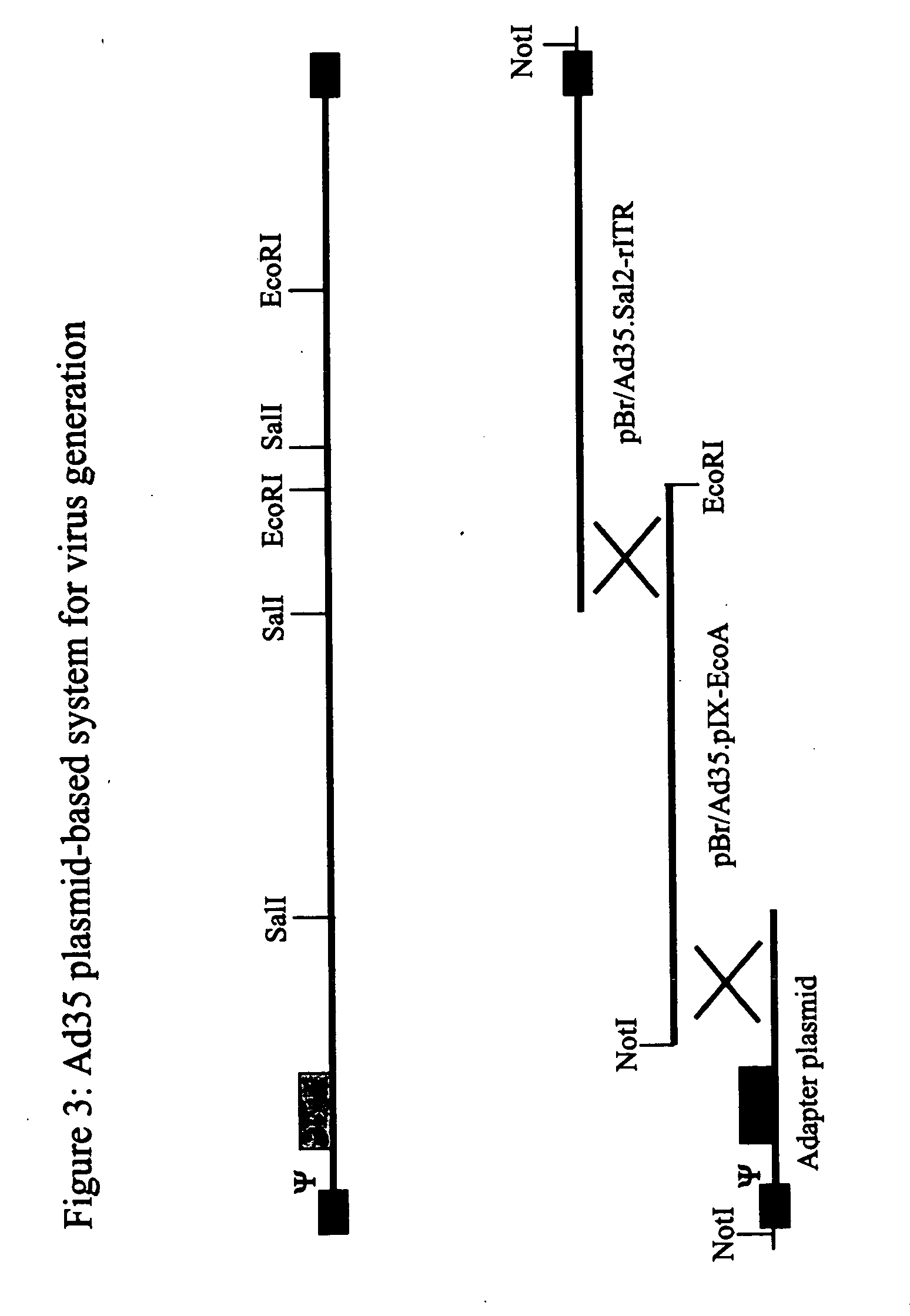
The invention provides a gene delivery vehicle and a gene of interest comprising at least one Ad35 element or a functional equivalent thereof, responsible for avoiding or diminishing neutralizing activity against adenoviral elements by the host to which the gene is to be delivered. A functional equivalent / homologue of an Ad35 (element) includes an adenovirus (element) which, like adenovirus 35, encounters pre-existing immunity in less than about 10% of the hosts to which it is administered for the first time, or which is capable in more than about 90% of the hosts to which it is administered of avoiding or diminishing the immune response.
Owner:JANSSEN VACCINES & PREVENTION BV
Therapeutic retroviral vectors for gene therapy
ActiveUS20060057725A1Maintaining therapeutic levelImprove the level ofPeptide/protein ingredientsGenetic material ingredientsHemoglobinopathyRed blood cell
Retroviral gene therapy vectors that are optimized for erythroid specific expression and treatment of hemoglobinopathic conditions are disclosed.
Owner:MASSACHUSETTS INST OF TECH
Triple minRNA for resisting virus infection of aids and construction method thereof
InactiveCN103184224APrevent escapeHigh infection efficiencyAntiviralsFermentationPol genesRna expression
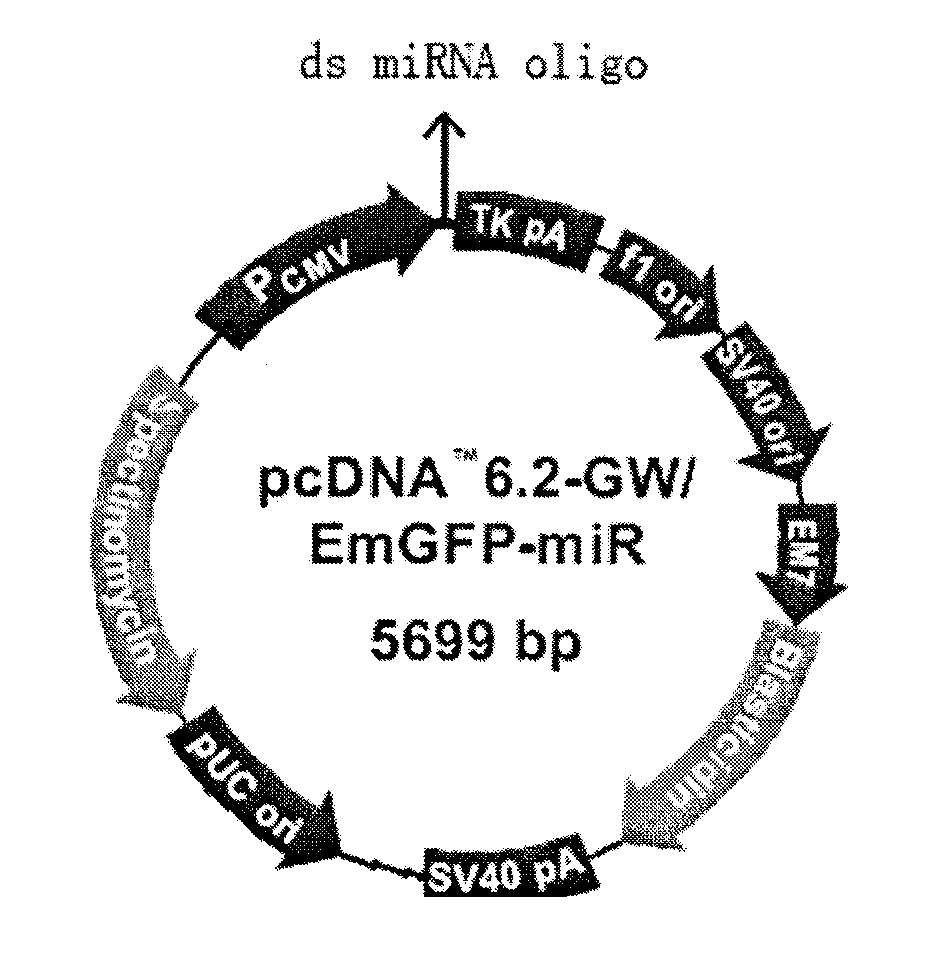
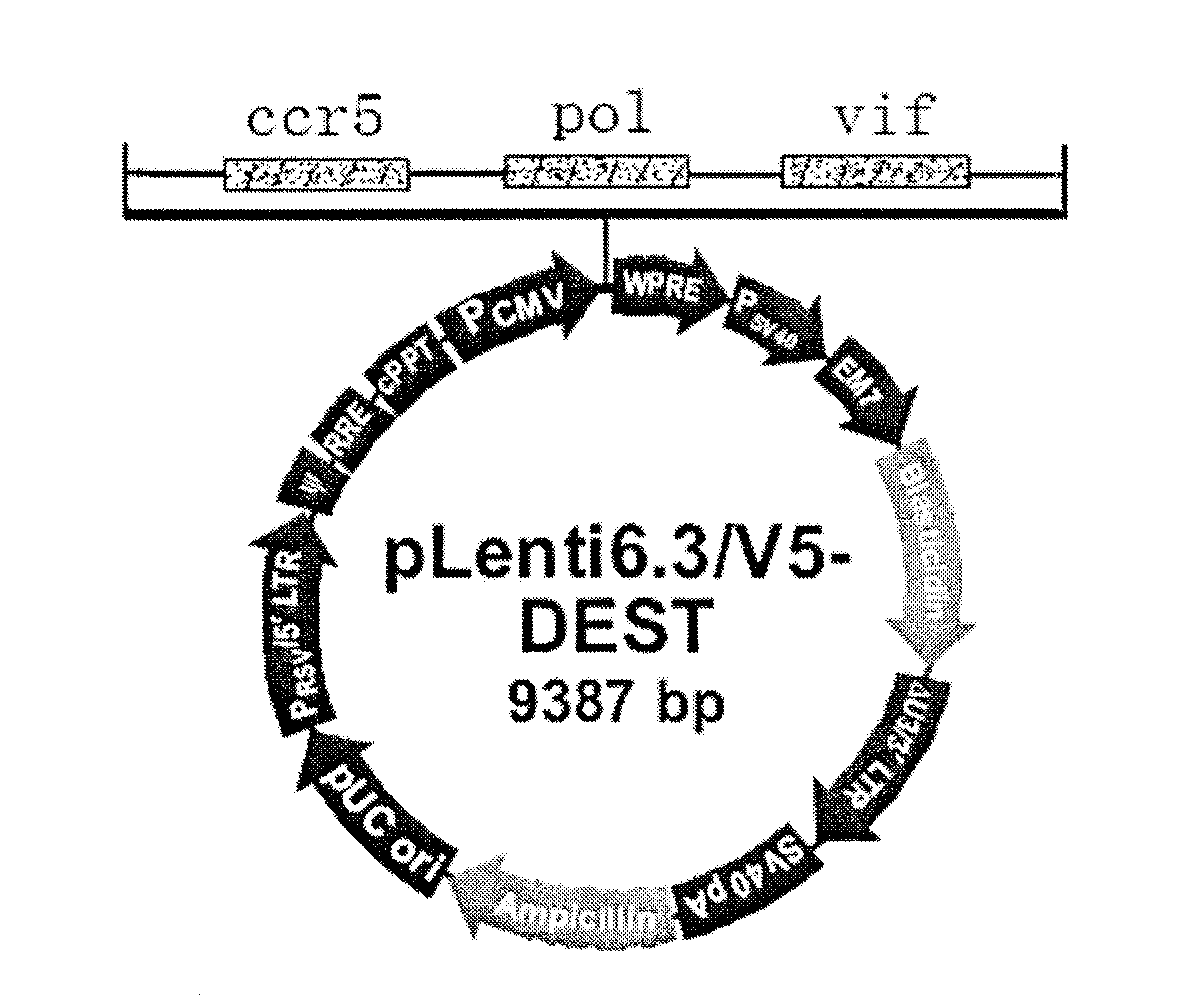
The invention discloses a triple minRNA for resisting virus infection of aids and the construction method thereof. The sequences of the triple minRNA are respectively designed according to gene sequences of ccr5, pol and vif and combined to be miRNA oligomeric and single strand DNA, then double-strand DNA is formed through annealing, then double-strand DNA is respectively inserted into the expression vector of pcDNA<TM> 6.2-GW / EmGFPmiR of miRNA to built the mi RNA expression plasmid, and the jamming effect of mi RNA expression plasmid to target gene can be detected through a Qpcr method. A miRNA string with the best gene interference effect to ccr5, vif and pol is inserted into a carrier of pcDNA 6.2 <TM>-GW / EmGFP, so as to obtain a series-wound interference vector of pcDNA6.2<TM>-GW / EmGFP-ccr5-pol-vif, and then the miR-ccr5-pol-vif in the series-wound interference vector is transferred to a lentiviral vector pLenti6.3 / V5-DEST so as to construct pLenti6.3 / V5-DEST miR-ccr5-pol-vif. The recombinant lentiviral vector can product three miRNA at the same time to cause interference effects to gene ccr5, gene pol and gene vif, so as to prevent the gene ccr5, the gene pol and the gene vif from expressing corresponding protein. Therefore, the effect of controlling Hiv infection and replication can be achieved.
Owner:HENGYANG NORMAL UNIV
Recombinant adenovirus vector for efficiently inducing pluripotent stem cell (PS cell), method for inducing PS cell by using recombinant adenovirus vector and usage of recombinant adenovirus vector
ActiveCN101792776AWide spectrum of infectionHigh infection efficiencyCosmetic preparationsToilet preparationsDiseaseSOX2
The invention relates to a recombinant adenovirus vector for efficiently inducing a pluripotent stem cell (PS cell) and method for inducing the PS cell by using the recombinant adenovirus vector. The recombinant adenovirus vector is characterized in that the fiber genes therein are B subgroup adenovirus fiber genes, and an Sox2 gene expression cassette and an Oct4 gene expression cassette are connected within the recombinant adenovirus vector in an operating way. The terminally differentiated cell or the adult stem cell of the mammal, particularly the human is infected in vitro, the Oct4 genes and the Sox2 genes are expressed in an ectopic way, and the terminally differentiated cell or the adult stem cell of the mammal, particularly the human can be induced into the PS cell efficiently and rapidly under the synergistic effect of adjusting the epigenetic-inheritance small-molecular medicament. The PS cell of the human can be used for the cell replacement therapy to treat diseases, and the PS cell of the mammal can be used for preparing the transgenic animal model and the animal disease model.
Owner:HEPATOBILIARY SURGERY HOSPITAL SECOND MILITARY MEDICAL UNIV +1
Adeno-associated virus vector and application thereof
PendingCN111349148AImprove efficiencyHigh infection efficiencySenses disorderVirus peptidesNucleic acid sequencingMutant
The present invention relates to an adeno-associated virus (AAV) capsid protein mutant, a polynucleotide containing a nucleic acid sequence encoding the AAV capsid protein mutant, a vector containingthe polynucleotide, and a host cell containing the vector. The invention also relates to an adeno-associated vector comprising the AAV capsid protein mutant, a recombinant adeno-associated virion which is constructed from the AAV vector and carries a gene expression cassette, a method for producing the adeno-associated virus vector or virion, and an application of the adeno-associated virus vectorand virion in treating diseases.
Owner:HUIGENE THERAPEUTICS CO LTD
Vaccine produced by suspended microcarrier cell culture system and method for producing vaccine
ActiveCN101869702AReduce usageReduce exposureViral antigen ingredientsMicroorganism based processesAdjuvantFreeze-drying
The invention discloses a vaccine produced by a suspended microcarrier cell culture system and a method for producing the vaccine. The method comprises the following technical steps of: (1) inoculating cells for preparing the vaccine to a culture tank which contains a culture medium and a microcarrier; (2) uniformly mixing the cells and the microcarrier to make the cells attached to the microcarrier; (3) providing sufficient nutrient and gas for the cells at an appropriate temperature to make the cells continue growing on the microcarrier; (4) preparing virus suspension from viruses for preparing the vaccine, inoculating the virus suspension to the cells and continuing culturing, and harvesting virus liquid or the cells containing the viruses and replacing culture solution at intervals of1 to 3 days; and (5) after purifying the harvested virus liquid, inactivating the virus liquid as required, adding a proper adjuvant into the inactivated virus liquid, adding a proper freeze-drying protective agent into activated virus liquid, and quantitatively packaging after fully and uniformly mixing to obtain the vaccine. The method has the advantages of simple production process and capability of obviously improving the yield and quality of the vaccine.
Owner:香港维克贸易有限公司
Recombinant adeno-associated virus as well as construction method and application thereof
InactiveCN105586320AImprove submission efficiencyEnhance antigen presentationTumor rejection antigen precursorsTumor specific antigensWilms' tumorGene
The invention relates to a recombinant adeno-associated virus. The recombinant adeno-associated virus is formed by inserting a tumor antigen gene into a shuttle expression vector pAAV-MCS. The invention also provides a construction method and an application of the recombinant adeno-associated virus. According to the invention, the recombinant adeno-associated virus carrying a tumor-associated antigen gene is utilized for infecting DC cells and expresses tumor-associated antigen protein in the DC cells, and a PD-1 gene is also combined for silencing CTL cells in the application method of the recombinant adeno-associated virus.
Owner:厚朴生物科技(苏州)有限公司
Lentivirus recombinant expression vector/recombinant lentivirus, as well as application, host cell and preparing method thereof
InactiveCN103695470AImprove transcription efficiencyHigh infection efficiencyGenetic material ingredientsSkeletal disorderEstrogen-related receptorHost gene
The invention provides a lentivirus recombinant expression vector / a recombinant lentivirus, as well as application, a host cell and a preparing method thereof. The encoding gene of an estrogen related receptor (ERR) protein can be inserted in a host gene group by the lentivirus recombinant expression vector / the recombinant lentivirus which is provided by the invention through a gene recombinant so as to continuously and stably express the ERR. The ERR can be permanently and stably expressed by the host cell which is provided by the invention, and sufficient high-quality virus liquid can be provided for animal in-vivo experiments. Besides, the lentivirus recombinant expression vector / the recombinant lentivirus which is provided by the invention has a wide host range, can infect various cells, such as nerve cells, muscle cells, liver cells, tumor cells and endothelial cells and has a very wide application range.
Owner:SHENZHEN INST OF ADVANCED TECH
Spore suspension for improving yield of white muscardine silkworms and application of spore suspension
The invention discloses spore suspension for improving the yield of white muscardine silkworms and application of the spore suspension. The spore suspension for improving the yield of the white muscardine silkworms comprises the following raw materials: muscardine spore powder with 1x106-5x106 muscardine spores per mL, 0.2 part by mass of polysorbate-80, 1-3 parts by mass of cane sugar, 0.4-0.6 part by mass of peptone, 0.05 part of auxiliary materials and the balanced sterile water. The auxiliary materials can be magnesium sulfate, chloramphenicol and streptomycin sulfate. By the spore suspension for improving the yield of the white muscardine silkworms, the concentration of the optimized muscardine suspension is increased, the stickiness and the activity of the muscardine suspension are improved, the germination rate of the muscardine suspension is increased, and the yield and the quality of the white muscardine silkworms are improved obviously.
Owner:SERICULTURE & AGRI FOOD RES INST GUANGDONG ACAD OF AGRI SCI
Efficient, rapid and stable genetic transformation method for strawberries
ActiveCN104195171AImprove transgenic efficiencyBacteria concentration decreasedGenetic engineeringFermentationFragariaTransformation efficiency
The invention discloses an efficient, rapid and stable genetic transformation method for strawberries 'beauty'. The method comprises the steps of activation of culture, preparation of plant materials, infection, co-culture, screening culture and rooting culture, solves the problems of the pollution of agrobacterium and infectious microbes in a traditional agrobacterium-mediated method, low conversion efficiency of the strawberries 'beauty' and long period of the strawberries 'beauty' from transgenosis and transgenic detection to field transplanting, shortens the period, further solves the problems of large workload and heavy work of transgenic detection, simplifies a detection method, and increases the detection speed. On the basis of the established 'beauty' strawberry leaf regeneration system, the method researches various factors for influencing agrobacterium tumefaciens transforming the 'beauty' strawberry leaves, optimizes various parameters, simplifies the operation process, and determines the best transformation conditions.
Owner:JIANGSU POLYTECHNIC COLLEGE OF AGRI & FORESTRY
Recombinant gland related virus expressing human kallikrein, its preparation method and application
InactiveCN1412303AFunction increaseHigh infection efficiencyViruses/bacteriophagesAntibody medical ingredientsDiseaseHuman body
The present invention provides a recombinant glandular related virus capable of expressing human kallikrein, its preparation method and application, and medicine composition containing said recombinant glandular related virus. Said recombinant glandular related virus can drive the human kallikrein gene to make long-period expression in human body, and possesses good physiological and pathologic physiological action for effectively reducing blood pressure, improving renal function and preventing apoplexy, and can be used for clinical curing the above-mentioned diseases.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
High-efficiency genetic transformation method for soybean immaturity seed lobe regeneration system with auxiliary vacuum permeation
InactiveCN101173297AIncrease chance of intrusionHigh infection efficiencyHorticulture methodsPlant tissue cultureEmbryoPlant genetic engineering
The invention discloses an efficient genetic transformation method of vacuum permeation assistance soybean immature cotyledon regeneration system, relating to the technical field of plant genetic engineering, comprising the following steps: (a) the induction and proliferation of soybean immature cotyledon somatic embryo; (b) the vacuum permeation assistance introduction of foreign DNA; (c) the regeneration of the somatic embryo with foreign DNA into a plant. Adopting the vacuum permeation assistance agrobacterium mediating method, under a certain vacuum condition, after treat for a certain time, a plurality of micromechanical wounds are made on the soybean somatic embryo, which fully raises the intruding opportunity for agrobacterium to obviously raise the infection efficiency of agrobacterium and the conversion of the soybean compared with the common agrobacterium mediating method. Soybean immature cotyledon is used as an explant to transform genetically, which avoids the problem that chimerism easily, appears with the cotyledonary node as the explant.
Owner:JILIN ACAD OF AGRI SCI
Chimeric antigen receptor and gene and recombinant expression vector thereof, engineered CD19 targeting NKT cell and application thereof
InactiveCN105384820AHigh infection efficiencyEfficient tumoricidal activityPeptide/protein ingredientsGenetic material ingredientsAntigen receptorCD137
The invention discloses a chimeric antigen receptor and a gene and a recombinant expression vector thereof, an engineered CD19 targeted NKT cell and an application thereof. The chimeric antigen receptor is CD19ScFv-2-CD8-CD137-CD3zeta which is formed by connecting CD19ScFv-2, a hinge domain and a transmembrane domain of CD8, an intracellular signal structural domain of CD137 and an intracellular signal structural domain of CD3zeta in series. The nucleotide sequence, which encodes the gene of the CD19ScFv-2, is represented by SEQ ID NO.7. By adopting the chimeric antigen receptor CD19ScFv-2-CD8-CD137-CD3zeta modified NKT cell for treating CD19 positive acute b lymphoblastic leukemia, the cell has specific killing activity on leukemia cells.
Owner:GENERAL HOSPITAL OF PLA
Animal rabies virus and vaccine and production method thereof
ActiveCN101979515AHigh infection efficiencyHigh titerInactivation/attenuationAntiviralsFreeze thawingAdjuvant
Owner:PULIKE BIOLOGICAL ENG INC
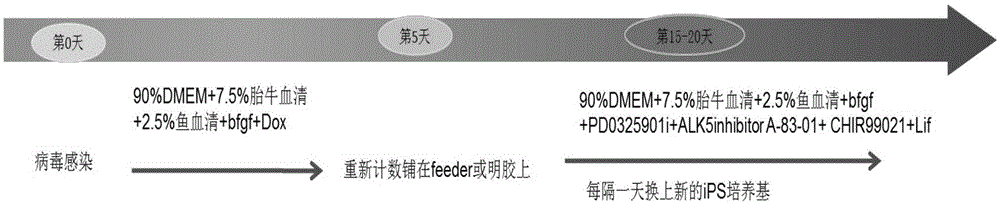
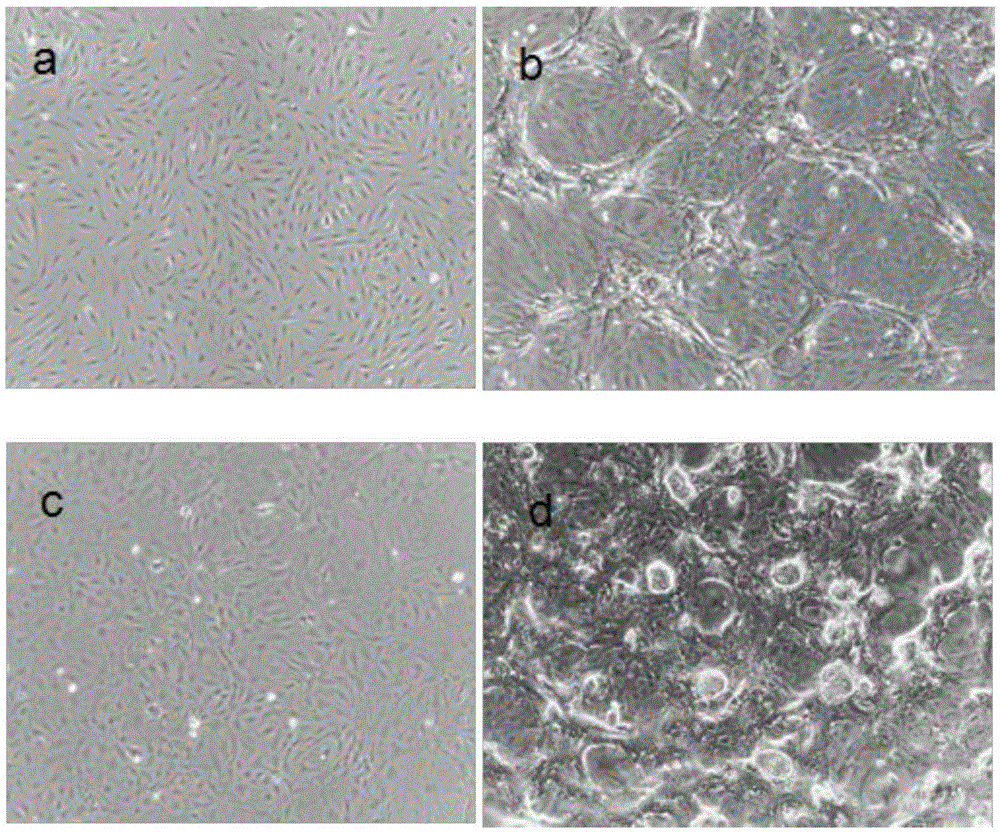
Inducing method for danio-rerio inductivity multi-potential stem cells and inductive culture medium and iPS culture medium used for the same
The invention discloses an inducing method for danio-rerio inductivity multi-potential stem cells. The induction method includes the following steps that danio-rerio tissues or danio-rerio embryos are gotten, and danio-rerio fibroblasts are obtained through in-vitro isolated culturing; the danio-rerio fibroblasts are infected through viruses, the infected danio-rerio fibroblasts are induced, and the danio-rerio inductivity multi-potential stem cells are obtained through manual selecting cloning and subculturing. Based on the same technical scheme, the invention further discloses an inductive culture medium and an iPS culture medium used for the inducing method. RT-PCR analysis indicates that iPS cells induced through the embryo fibroblasts have the potency of being differentiated into various triploblastic-source cells. By means of the method, the inductive culture medium and the iPS culture medium, the technical difficulty that the infection efficiency of slow viruses in fish cells is low is overcome, and new tool cell sources and new concepts are provided for researching a danio-rerio somatic-cell reprogramming mechanism to select cell materials.
Owner:HUNAN NORMAL UNIVERSITY
Classical swine fever virus vaccine and production method thereof
InactiveCN101926991AHigh poison priceExpand production scaleInactivation/attenuationMicroorganism based processesCulture fluidFreeze-drying
The invention discloses a method for preparing a classical swine fever (CSF) vaccine by using a cell microcarrier suspension culture system, which comprises the following steps of: (1) inoculating cells for preparing the vaccine to a carrier tank containing culture solution and a microcarrier, and uniformly mixing the cells and the microcarrier to make the cells attached to the microcarrier; (2) when the concentration after cell proliferation is 5 to 40 times of the initial inoculation concentration, inoculating CSF virus (lapinized virus) to the cells according to multiplicity of infection (M.O.I.) of the virus of 0.01-1 and reproducing the virus; and (3) mixing prepared virus liquid, adding an appropriate freeze-drying protective agent, fully and uniformly mixing, quantitatively packaging, and freeze-drying to obtain the CSF vaccine. The CSF vaccine produced by the method has the advantages of high density of cultured cells, continuous culture, high yield of the virus, high immune effect, high safety, complete immune protection on attack of violent CSF, and the like.
Owner:PU LIKE BIO ENG
Chimeric antigen receptor T cell and application thereof
PendingCN111411085ARelief of immunosuppressionImprove anti-tumor effectAntibody mimetics/scaffoldsNucleic acid vectorAntigen receptorImmune escape
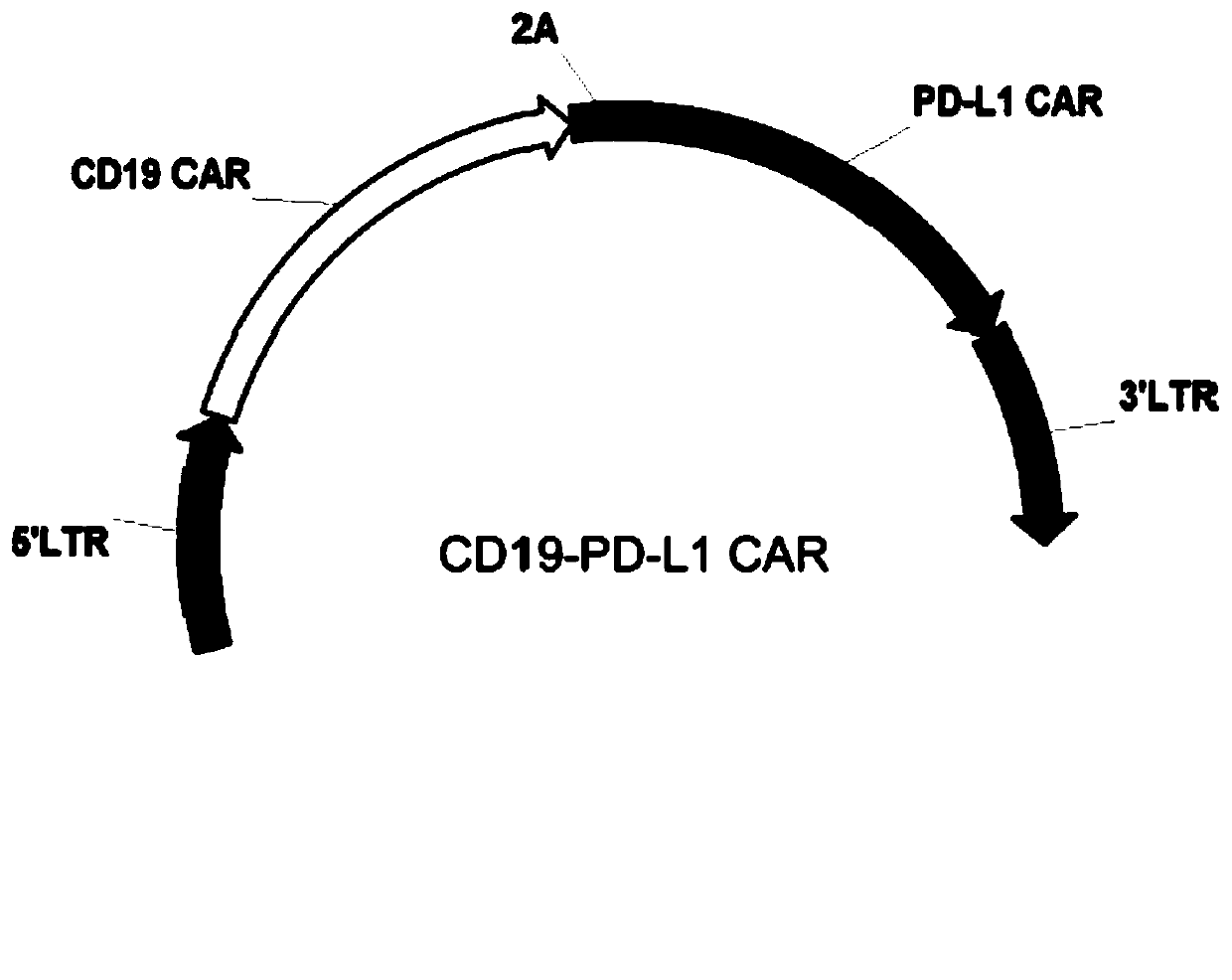
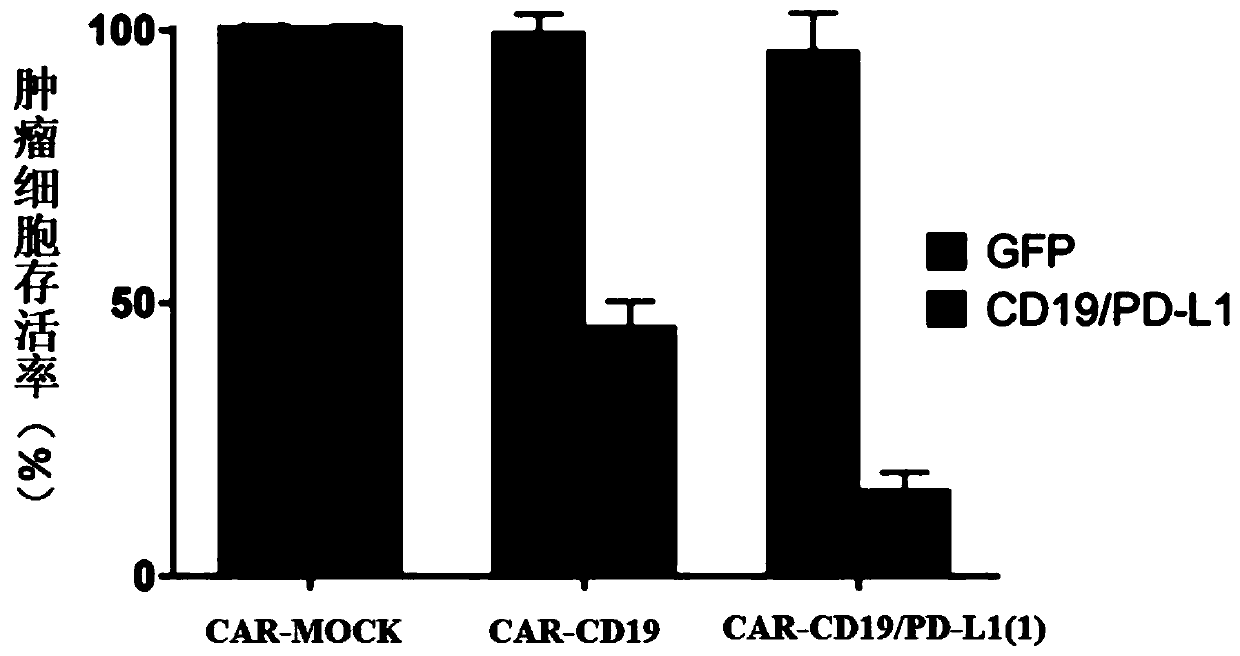
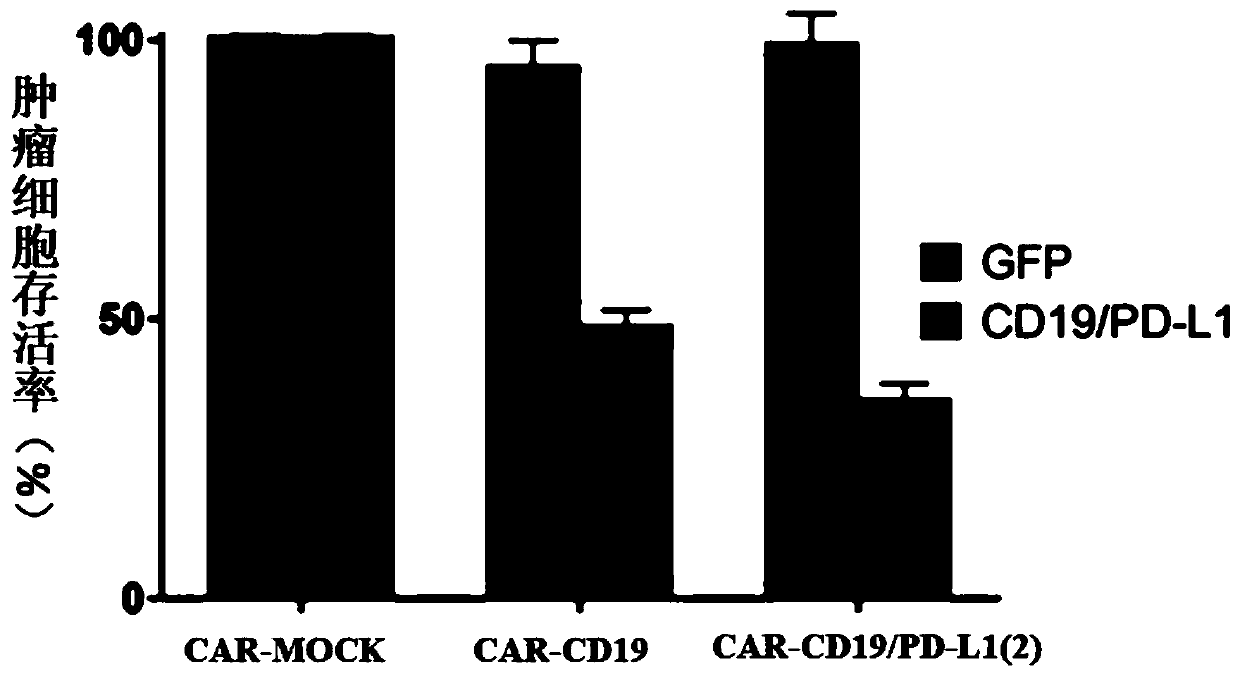
The invention provides a chimeric antigen receptor T cell and application thereof. The chimeric antigen receptor T cell expresses chimeric antigen receptors targeting CD19 and PD-L1 at the same time.The chimeric antigen receptor T cell expresses a CD19 CAR structure and a PD-L1 CAR structure at the same time, and after PD-L1 CAR is combined with PD-L1 expressed by a tumor cell, an activation signal can be transmitted intracellularly. Therefore, the chimeric antigen receptor T cell not only can recognize the tumor cells expressing CD19 in a targeted manner, but also can relieve immunosuppression of PD-1 expressed by the tumor cells on the T cells, avoid an immune escape mechanism of the tumor cells and relieve the problem of drug resistance of CAR-T treatment; and a new idea is provided for CAR-T treatment and adoptive feedback immune cell treatment.
Owner:格源致善(上海)生物科技有限公司
Oncolytic adenovirus vector for modifying and expressing two exogenous genes by fibrin, construction method and application of vector
ActiveCN102229962AIncrease the number of expressionsEnhanced inhibitory effectGenetic material ingredientsGenetic engineeringOncolytic adenovirusViral vector
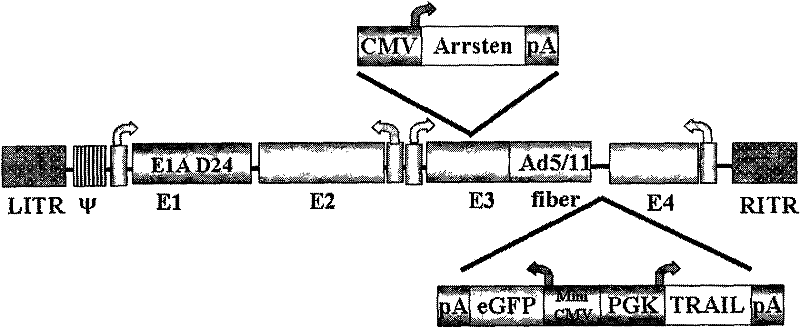
The invention provides an oncolytic adenovirus vector for modifying and expressing two exogenous genes by fibrin. The oncolytic adenovirus vector provided by the invention is characterized in that: a human adenovirus 5 type gene group has a lacking of 24bp basic group in 922bp-947bp; and an expression element for expressing a first exogenous gene is inserted into 28183bp-29906bp of the human adenovirus 5 type gene group, a fibrin chimera is inserted into 31042bp of the human adenovirus 5 type gene group and an expressing element for doubly expressing a second exogenous gene and an eGFP (Green Fluorescent Protein) is inserted into 32021bp and 32022bp of the human adenovirus 5 type gene group. A construction method of the oncolytic adenovirus vector provided by the invention comprises the following steps of: constructing a shuttle vector lacking pAd5 E1A 24bp, a pHBD24 adenovirus vector framework, a shuttle vector of a pHBDE3-first exogenous gene, a pHBDE3-first exogenous gene / SwaI condition copied adenovirus vector framework and a pshuttle Ad5-E4-fibrin chimera shuttle vector; expressing a shuttle vector / second exogenous gene-E4-fibrin embedding body sequence of the eGFP and the second exogenous gene; and preparing the oncolytic adenovirus vector for modifying and expressing two exogenous genes by fibrin. The vector provided by the invention can be used for inhibiting malignant glioma, liver cancer, stomach cancer, colon cancer, breast cancer and melanoma.
Owner:SHAANXI NORMAL UNIV
Capsid protein phage display particle of recombinant II porcine circovirus as well as preparation method and application thereof
InactiveCN103540605AReduce manufacturing costOvercome the deficiency of weak immunogenicityViral antigen ingredientsMicroorganism based processesNucleotideCell immune response
The invention belongs to the biological field and particularly relates to preparation and application of recombinant porcine circovirus PCV2-Cap / Phage display particle. A preparation method of the capsid protein phage display particle of recombinant II porcine circovirus specifically comprises the following four steps: (1), optimizing and coding nucleotide of the capsid protein of the recombinant II porcine circovirus; (2), constructing a recombinant plasmid; (3), constructing a recombinant phage genome; and (4), preparing the capsid protein phage display particle of recombinant II porcine circovirus. An animal experiment proves that the recombinan display plasmid has good antigenicity, can stimulate a pig body to generate good humoral immunity and cellular immunity reaction, and can prevent and control II porcine circovirus infection; time needed for copying the process is short, production cost of the recombinant display particle is low, and product is convenient to use, simple and convenient to store and high in cloning yield.
Owner:CHONGQING ACAD OF ANIMAL SCI
Dynein mosaic type recombinant human type-B adenovirus and preparation method thereof
ActiveCN107267469AHigh infection efficiencyMicroorganism based processesFermentationGolden hamsterHuman type
The invention discloses a dynein mosaic type recombinant human type-B adenovirus and a preparation method thereof. The skeleton of the dynein mosaic type recombinant human type-B adenovirus is a human type-B adenovirus genome, and a base sequence which encodes a receptor binding domain of dynein is a base sequence which encodes a corresponding domain of a human type-C adenovirus. By a molecular cloning method, Ad5-knob gene fragments are cloned and replaced to recombinant shuttle plasmids, in-vitro recombinant on the Ad5-knob gene fragments and a recombinant human type-3 adenovirus genome is realized, obtained knob gene fragments are replaced into type-5 recombinant human type-3 adenovirus genome, and therefore, dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 is obtained. The dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 can be infected with mouse primitive epithelial cells and golden hamster lung and kidney primitive cells in vitro, and the infection efficiency of the dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 is close to that of Ad5, and is much higher than that of a parent strain rAd3E, in golden hamster cells, significant copying exists, and the dynein mosaic type recombinant human type-B adenovirus can be used for small animal model research of human type-3 adenovirus vaccines and antiviral drug evaluation.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
Isolation and identification of Yunnan tomato leaf curl viral genome and agrobacterium tumefaciens-mediated infective clone construction
InactiveCN102703435AAvoid pitfalls in inoculation methodsReduce dependenceMicrobiological testing/measurementFermentationRestriction enzyme digestionGenomic DNA
The invention discloses isolation and identification of Yunnan tomato leaf curl viral genome and agrobacterium tumefaciens-mediated infective clone construction thereof. Total DNA of a genome of an infected tomato is extracted, virus total genomic DNA is cloned by using PCR (Polymerase Chain Reaction), and a total genomic sequence of a Yunnan tomato leaf curl virus is obtained through sequence measurement. 1.4 direct repeated genomes are obtained through methods of PCR and restriction enzyme digestion and are inserted into a plant expression carrier pBinPLUS with high replication capacity, a recombinant carrier is imported with agrobacterium tumefaciens strains EHA105 with strong infection capacity through an electroporation method, therefore, the agrobacterium tumefaciens-mediated infective clone has high-efficiency infection capacity to a host plant. The invention provides a matured method and system for researching interaction among the host plant, a virus infecting medium and a virus, field detection, genome structure and function research of the Yunnan tomato leaf curl virus.
Owner:ZHEJIANG UNIV
Virus and vaccine of porcine reproductive and respiratory syndrome and preparation method of same
ActiveCN101979514AHigh viral titerHigh poison priceViral antigen ingredientsAntiviralsFreeze-dryingCells/microL
The invention discloses a method for preparing virus of porcine reproductive and respiratory syndrome on a large scale. In the method, the virus of the porcine reproductive and respiratory syndrome is prepared in a cell microcarrier suspension culture system by a bioreactor. The method comprises the following steps of: inoculating host cells for preparing the virus to a carrier tank containing culture solution and a microcarrier, and mixing the cells and the microcarrier uniformly to ensure that the cells are attached to the microcarrier; providing sufficient nutrients and appropriate gas environment for the cells under the appropriate culture environment to ensure that the cells are grown until the cells are in an amount which are 10 to 20 times of the inoculation concentration on the microcarrier; preparing virus suspension from the virus of the porcine reproductive and respiratory syndrome by using cell maintenance culture solution to ensure that the suspension is adsorbed to the cells; culturing the virus under the appropriate culture environment; culturing continuously for 2 to 3 days to obtain virus solution; and after the virus solution passes inspection, performing freeze thawing on the virus solution twice at the temperature of -20 DEG C, and inactivating and purifying to prepare an inactivated vaccine of the porcine reproductive and respiratory syndrome or adding a freeze-drying protective agent for freeze drying to prepare a live vaccine of the porcine reproductive and respiratory syndrome. The method has large production scale, high yield of single batch and low production cost.
Owner:PU LIKE BIO ENG
Recombined viral vectors and uses thereof
ActiveCN101220373AHigh infection efficiencyHigh foreign gene expression levelsGenetic material ingredientsDigestive systemCell immune responseAntigen
The invention relates to a genetic engineering technology, in particular to a recombinant virus vector. The vector includes the virus vector and at least two nucleotide sequences, the nucleotide sequences respectively code the antigens of pathogenic virus, the antigen comprises a B cell epitope and a T cell epitope; the recombinant virus vector can lead immune animals to produce humoral immune and cell immune reactions, thus achieving the purpose of the prevention and treatment of diseases. The injection of the recombinant adenovirus vector of the invention has high safety at the same time.
Owner:恒瑞源正(上海)生物科技有限公司 +1
Mammalian cell non-susceptible H9N2 subtype cold-adapted avian influenza virus rescue
InactiveCN110305898AHigh infection efficiencyImprove the success rate of rescueSsRNA viruses negative-senseViral antigen ingredientsAnimals vaccinesKidney cell
The invention relates to the technical field of animal vaccines, and especially relates to a rescue method for a H9N2 subtype cold-adapted live attenuated vaccine transfected with 293T and DF-1 co-cultured cells. A H9N2 subtype cold-adapted attenuated strain TX-25-CE30 is selected to construct 8 plasmids expressing the gene, the internal gene of TX-25-CE30 is used as a skeleton, the parental strain TX strain HA and NA genes are taken as external genes, the human embryonic kidney cell 293T and chicken fibroblast cell line DF-1 co-cultured cells are transfected, the strain that has weaker ability to infect mammalian cells is rescued and named as rTXca-HA-NA, and the immune protection rate of the recombinant virus after immunization of the chicken to a homologous strain can reach 100%.
Owner:YANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com