Recombined viral vectors and uses thereof
A recombinant viral vector and viral vector technology, applied in the field of recombinants, can solve the problems of weak immune response, carcinogenesis of exogenous nucleic acid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, the construction of CHB4 recombinant adenovirus vaccine
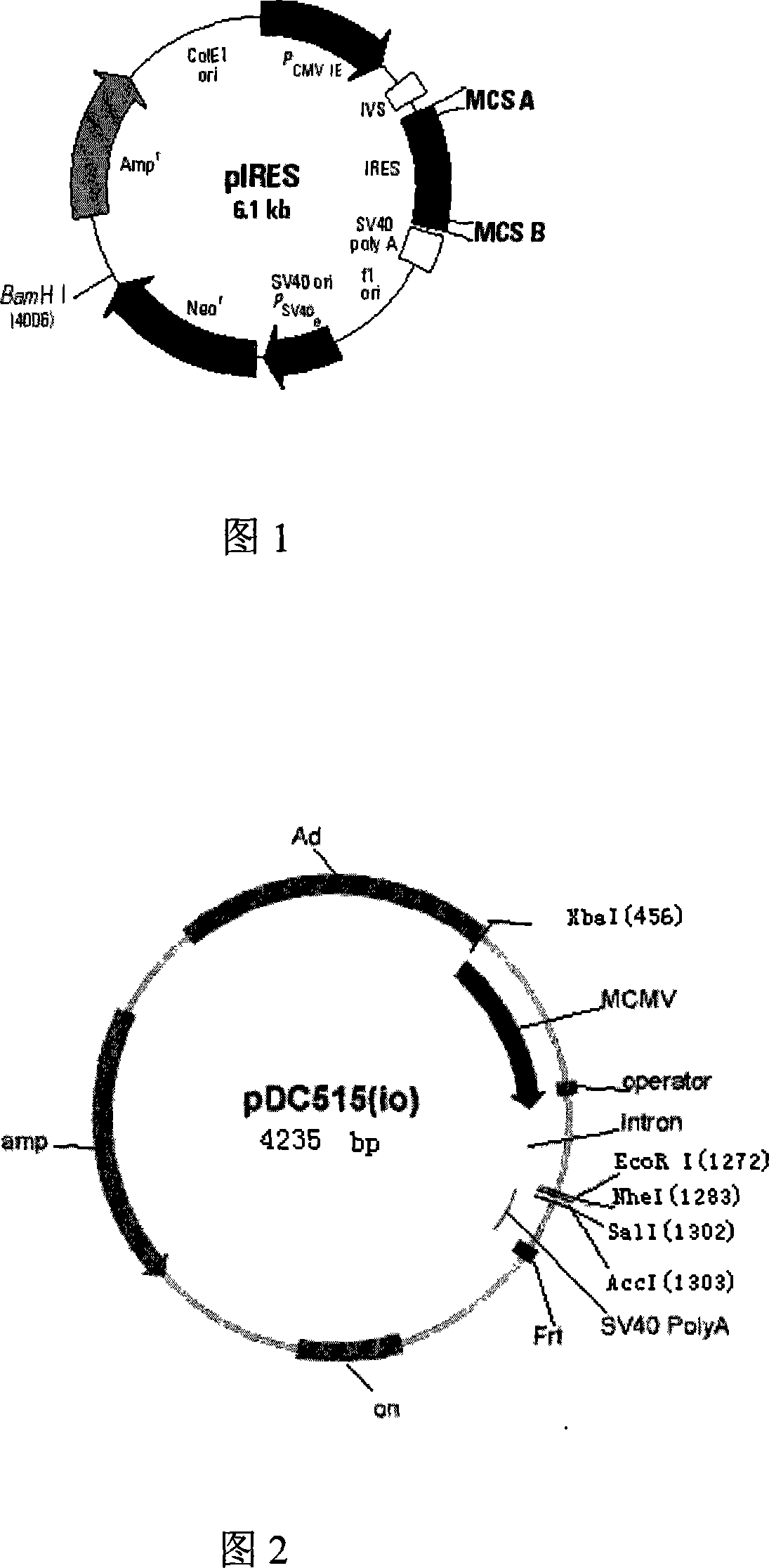
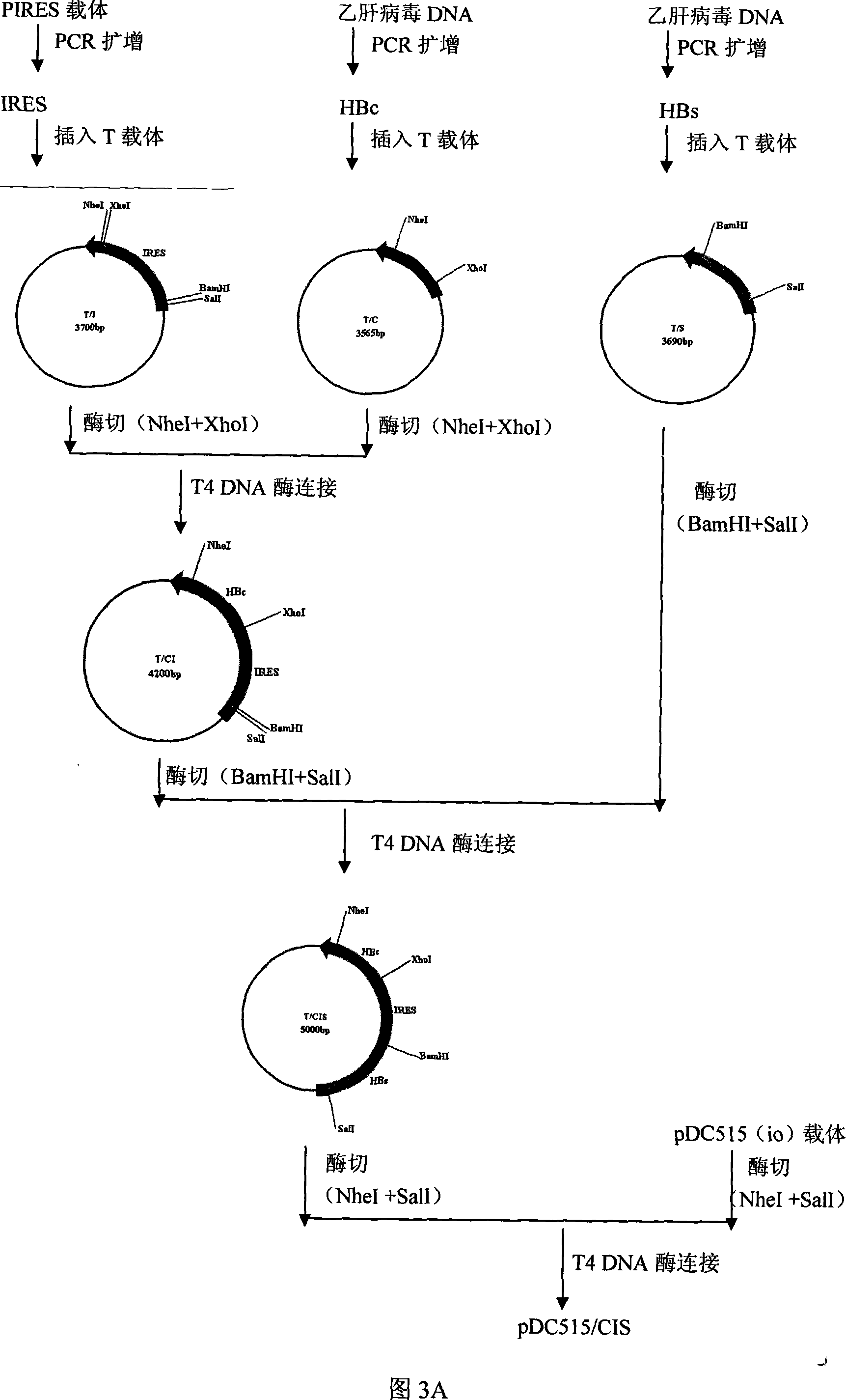
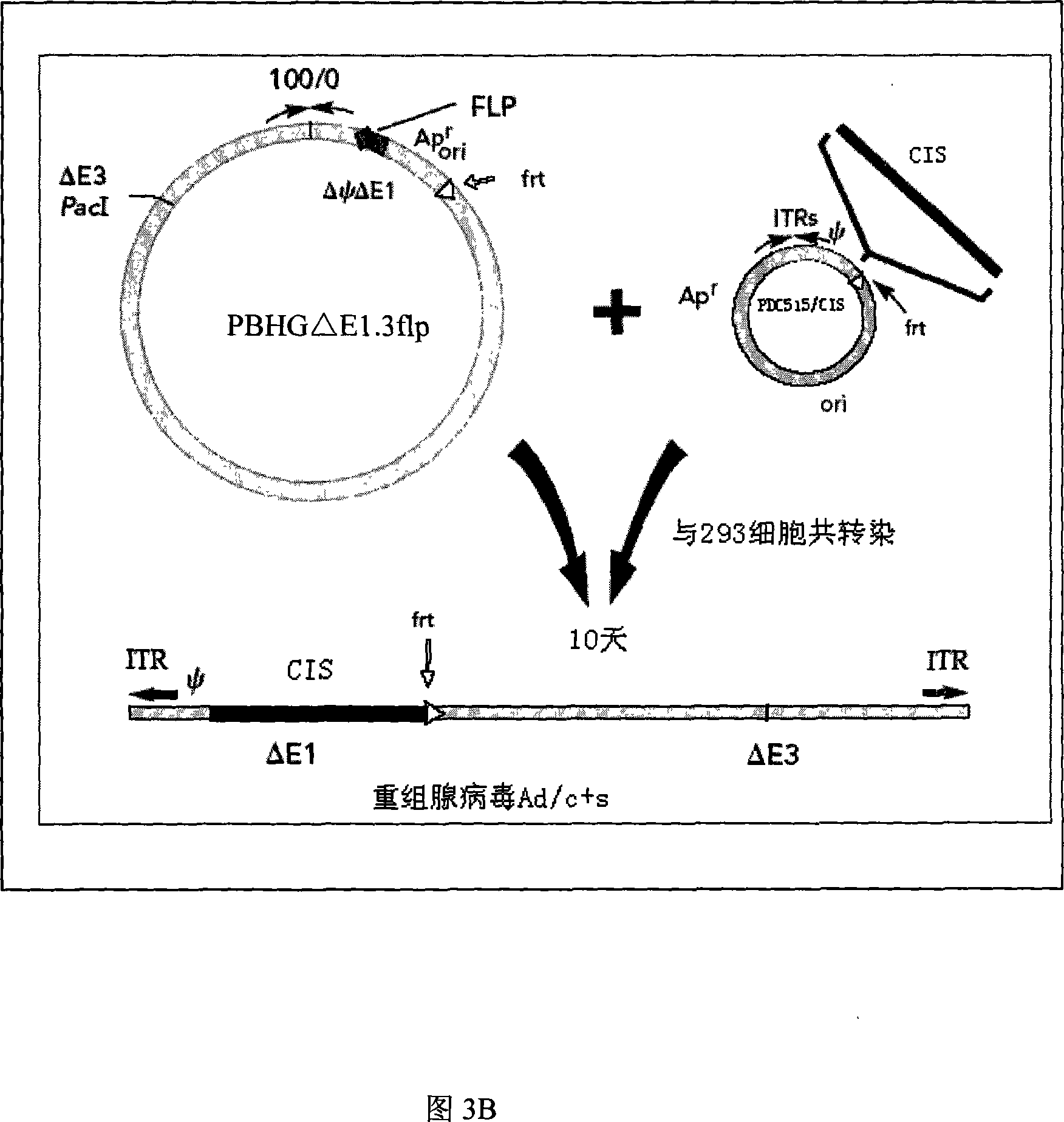
[0042] 1. Acquisition of the internal ribosome entry site (IRES, Internal Ribosome Entry Site) fragment: using the pIRES vector (purchased from BD Company, whose plasmid map is shown in Figure 1) as a template, design primers: IRES(F)5' ACG ACT CAC TAT AGG CTA 3', IRES(R) 5' TCG ACT CTA GAG GAT CC, (Synthesized by Huada Genomics Shanghai Ding'an), for PCR amplification, the amplification conditions are: the first cycle of denaturation at 94°C for 5 minutes , each subsequent cycle: denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, extension at 72°C for 1 minute, a total of 30 cycles, and extension at 72°C for 5 minutes. The IRES fragment was obtained, and its specific sequence is shown in SEQ ID NO:3. The amplified PCR fragment was inserted into a T vector (Promega Company) to obtain a T / I vector, see FIG. 3A .
[0043] 2. Acquisition of the nucleotide sequence HBs encoding the...
Embodiment 2
[0048] Embodiment 2, the construction of CHB5 recombinant adenovirus vector
[0049] 1. Obtaining the IRES2 fragment: artificially synthesize the IRES2 sequence, as shown in SEQ ID NO: 4, and use it as a template to design primers: IRES-MluI(F): 5'ACGCGTTATCCCTTGCGG 3'IRES-SmaI(R): 5' CCCGGGTGTGGCAGGAGT 3' (Shanghai Ding'an Synthetic Co., Ltd. of BGI) was used for PCR amplification. The amplification conditions were: denaturation at 94°C for 2 minutes in the first cycle, denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and 72°C for each cycle. Extension was performed for 40 seconds for a total of 30 cycles, followed by a 7 minute extension at 72°C. Obtain the IRES2 fragment. The amplified PCR fragment was inserted into a T vector (Promega Company) to obtain a T / IRES2 vector.
[0050] 2. Acquisition of the adenovirus shuttle vector PDC515 / CIS2: Please refer to Figure 5, digest plasmid T / IRES2 with restriction enzymes M1uI and SmaI to obtain the IRES2 fra...
Embodiment 3
[0052] Embodiment 3, the construction of HB-S'IC' recombinant adenovirus vaccine (CHB6)
[0053] 1. Obtaining the IRES fragment: Obtain the T / I vector by the same steps as in Example 1.
[0054] 2. Obtaining of HBs' fragments: extract the serum of hepatitis B patients, extract hepatitis B virus DNA from the patient's serum, and use the extracted DNA as a template to design primers: HBs-NheI (IF): 5'GCTAGCCAACCATGGAGAGCACA3' (the underline is the enhancer) , HBs-XhoI (R): 5'CTCGAGTCAAATGTATACCCA3' (Synthesized by Huada Genomics Shanghai Ding'an), PCR amplification, amplification conditions: the first cycle of denaturation at 94°C for 5 minutes, subsequent cycles: denaturation at 94°C for 30 seconds , annealing at 61°C for 30 seconds, extension at 72°C for 1 minute, a total of 30 cycles, and then extension at 72°C for 7 minutes. Obtain HBs' fragment. Insert its PCR fragment into the T vector to obtain the T / S' vector.
[0055] 3. Acquisition of HBc' fragments: Extract the ser...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com