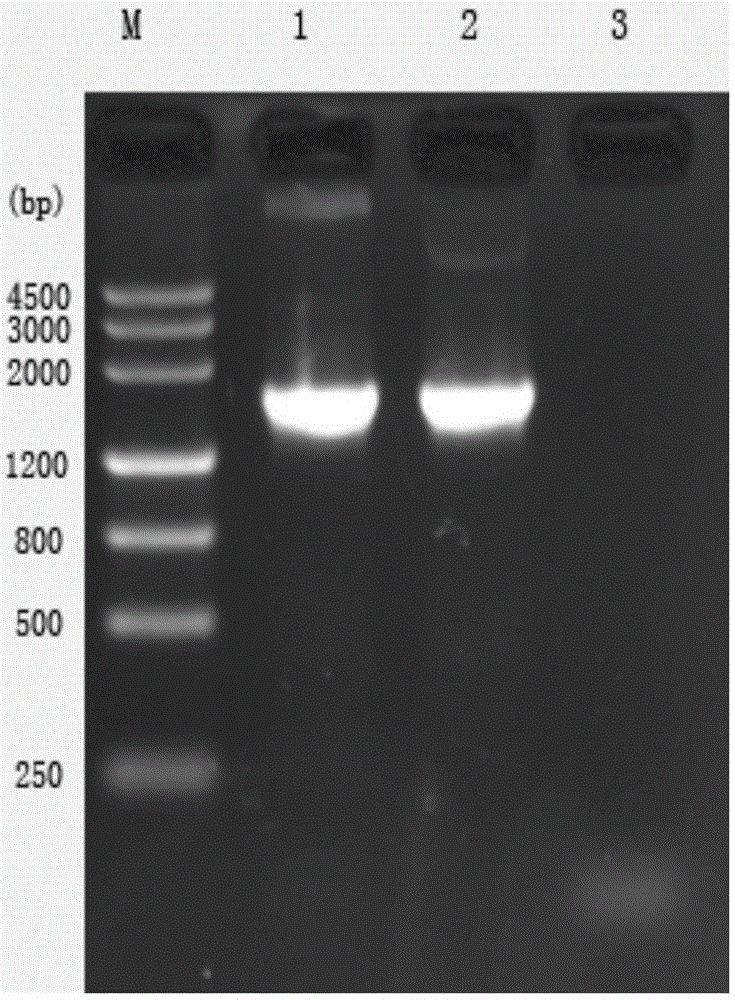
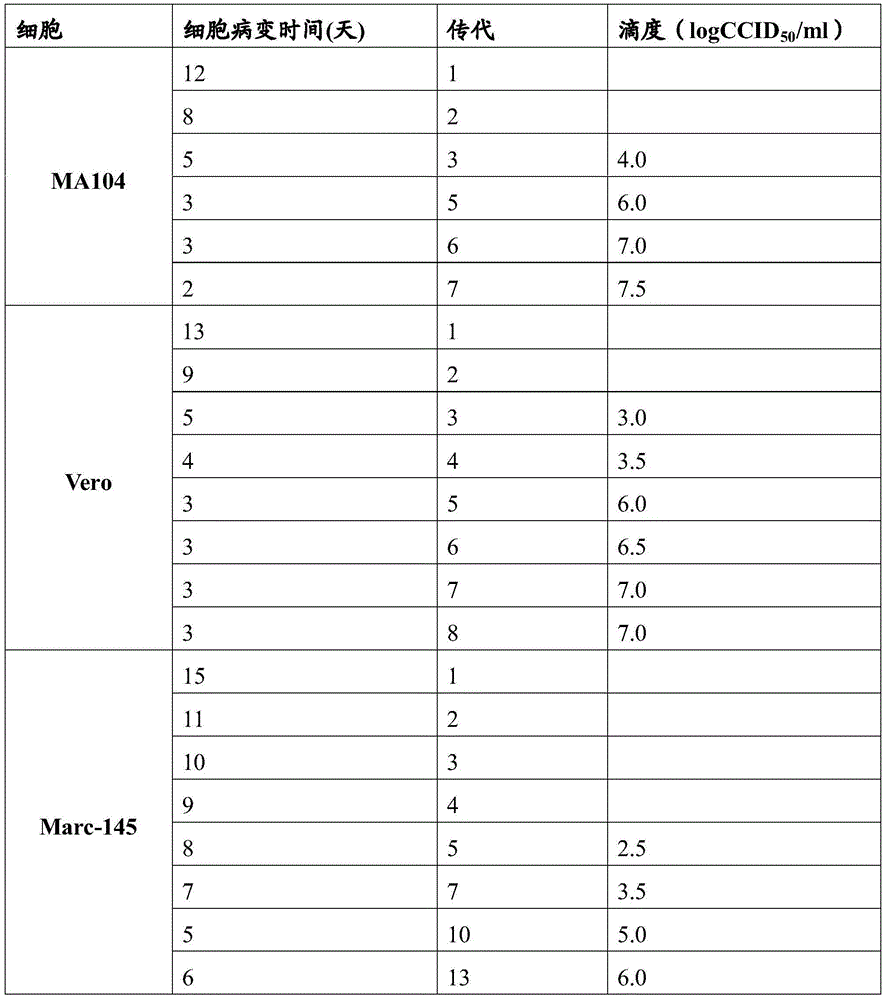
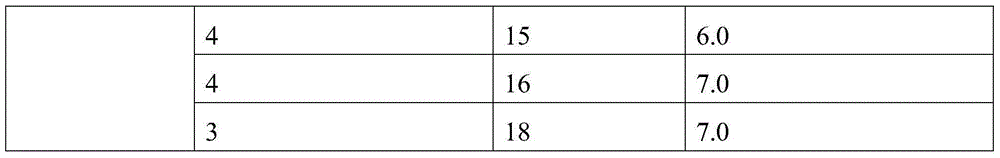
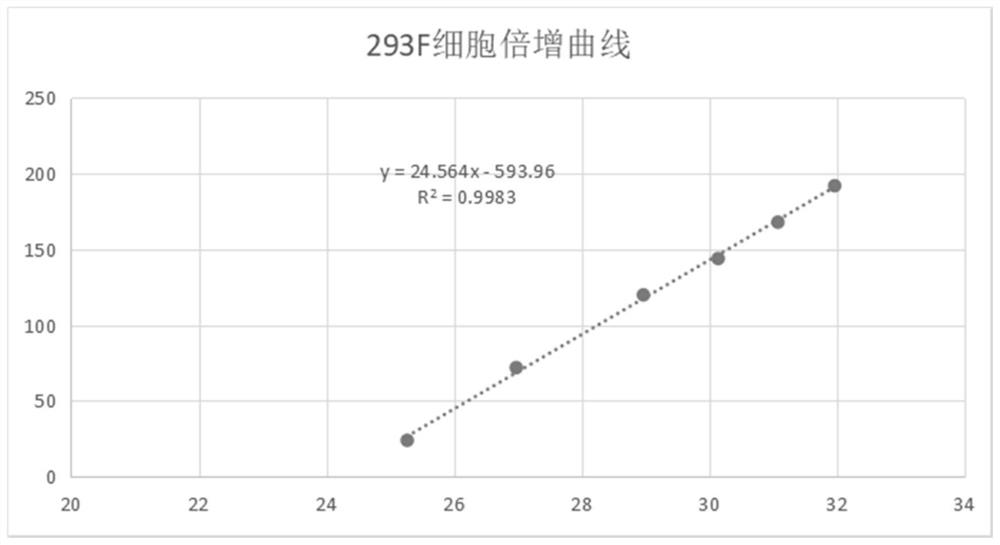
Patents
Literature
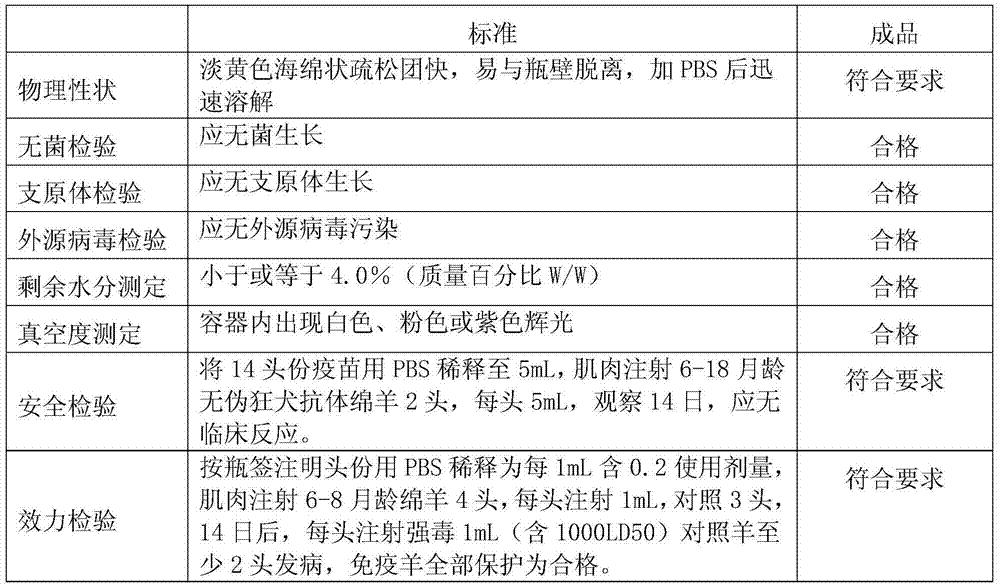
64results about How to "High viral titer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for producing virus vector
ActiveUS9102943B2High viral titerHigh transduction efficiencyAntibacterial agentsAntimycoticsHistone deacetylaseViral vector
Owner:TAKARA HOLDINGS
Method for production of porcine epidemic diarrhea virus
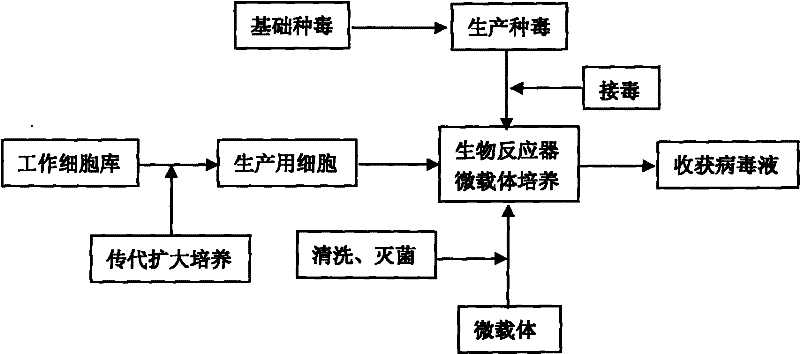
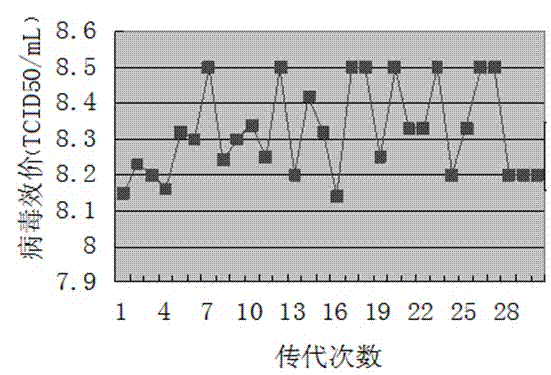
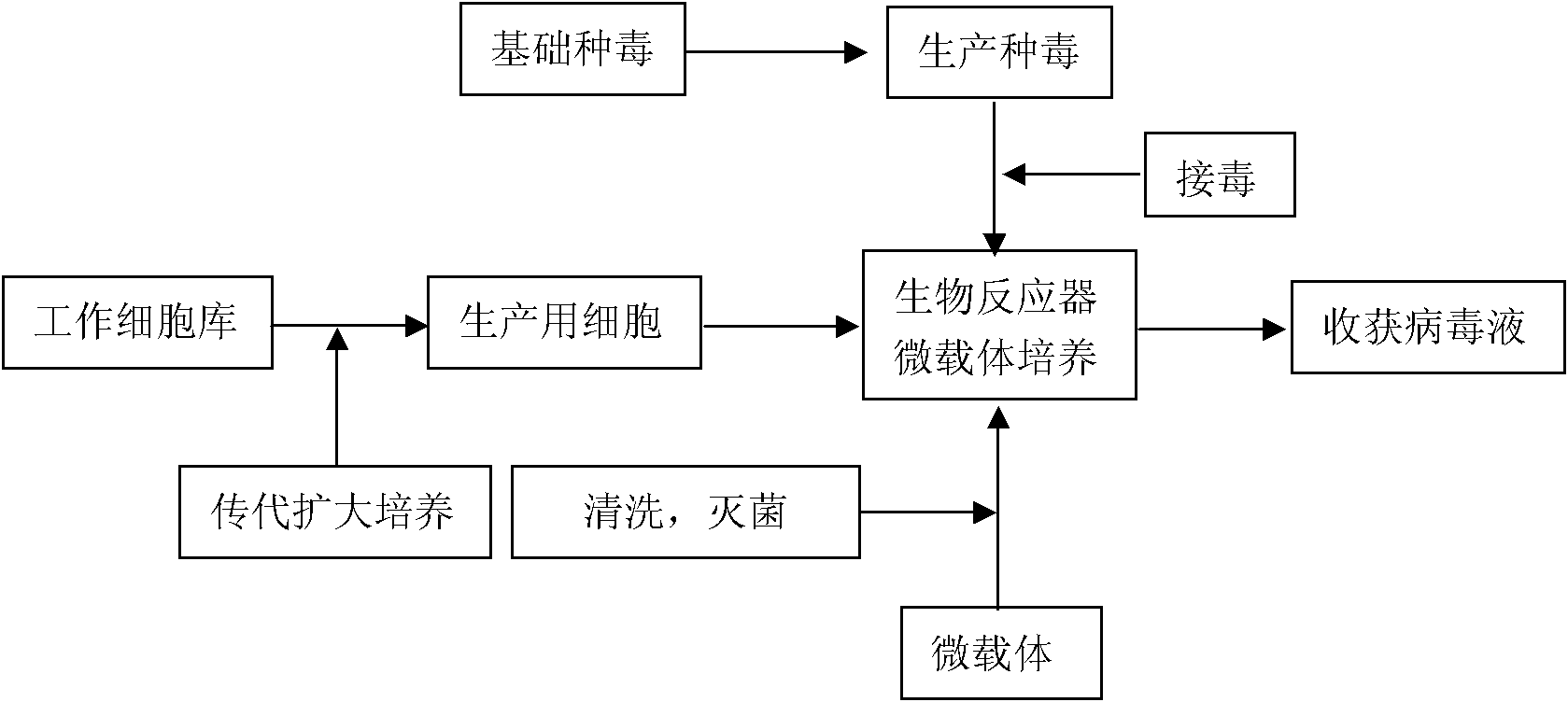
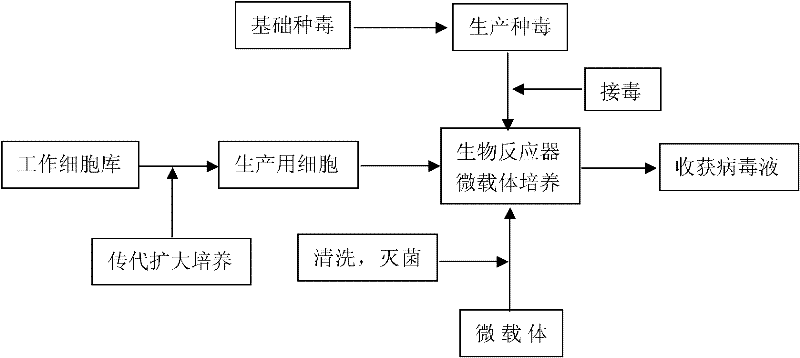
The invention discloses a technology for the production of porcine epidemic diarrhea virus by means of the microcarrier culture of VREO cells using a bioreactor, and comprises the technology for the production of different porcine epidemic diarrhea virus strains. The technology comprises the following technical steps: (1) selection of VERO cells as cell line for vaccine; (2) passage and culture of cells for vaccine; (3) propagation of seed culture of the porcine epidemic diarrhea virus; (4) microcarrier suspension culture of the VERO cells in the bioreactor; (5) propagation of porcine epidemic diarrhea virus antigen; and (6) treatment of acquired virus antigen liquid. The production method can remarkably lower production cost and enhance output-input ratio by 5 to 10 times, and has the advantages of short production period, small occupied space, great easiness for enlarging production scale rapidly, little environmental pollution, easy processing, high automation degree, a small number of staff, easy implementation of even and stable quality, obviously lowered production cost and enhanced yield and quality of vaccine.
Owner:成都史纪生物制药有限公司
Preparation method of swine pseudorabies vaccine
The invention relates to a preparation method of a swine pseudorabies vaccine, which comprises the following steps of: culturing a virus by using a swine testicle cell, and when one layer of cells grows, inoculating a swine pseudorabies virus; then adding into a cell maintenance medium, statically or rotatably culturing, when the cell suffers from more than 80 percent of pathological changes, harvesting a cell culture, repeatedly freeze-thawing to obtain a cell venom containing supernate, and mixing the cell venom qualified in toxic valence detection with formaldehyde for inactivating; and mixing with an emulsifying agent for emulsifying to obtain the swine pseudorabies vaccine. Compared with the prior art, a strain used for preparing the vaccine has the advantages of stronger toxicity and high virus valence; the swine pseudorabies vaccine has good immunogenicity and long immunization period; and the preparation method has the advantages of reasonable process and lower cost, thereby greatly lowering the cost load of the fish breeding and poultry raising industry.
Owner:SHANGHAI ELITE AGRI SCI TECH GROUP +1
Method for producing PRRS (Porcine Reproductive and Respiratory Syndrome) viruses
ActiveCN102002482AQuality improvementIncrease productionRecovery/purificationPerfusion bioreactorPerfusion Culture
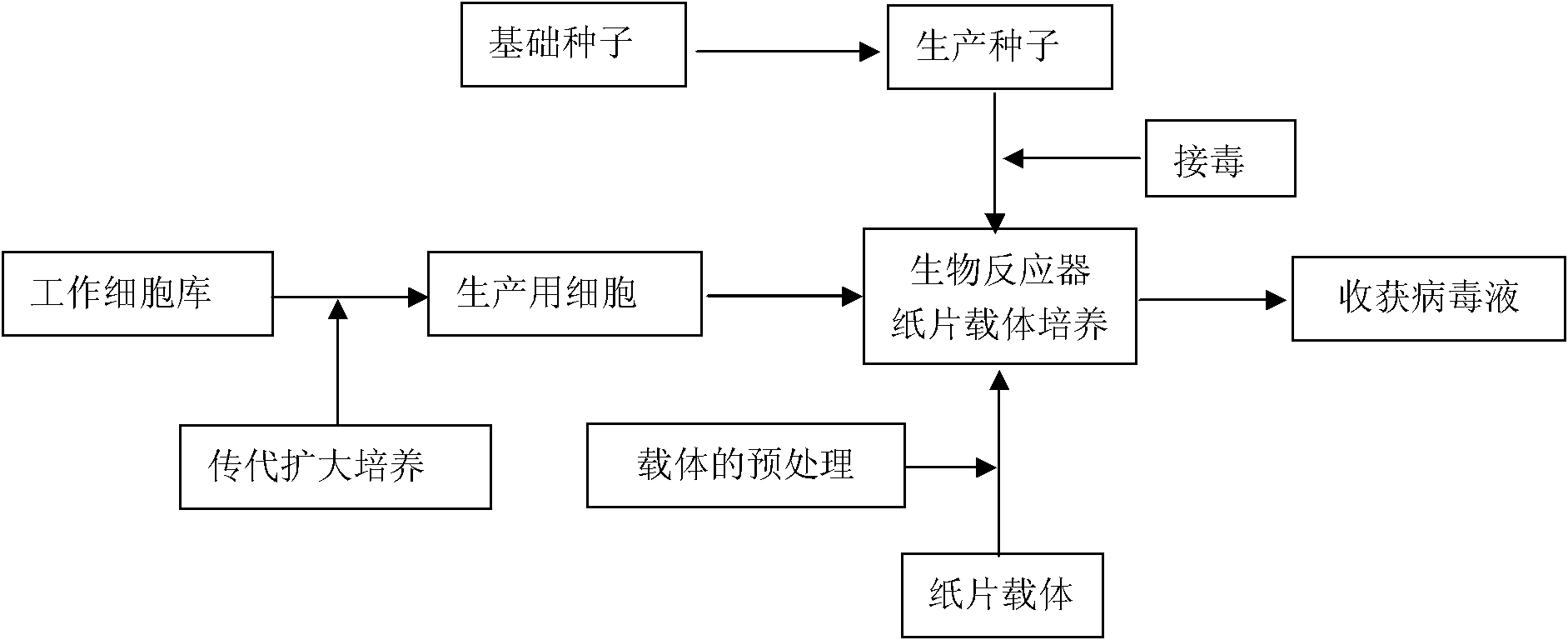
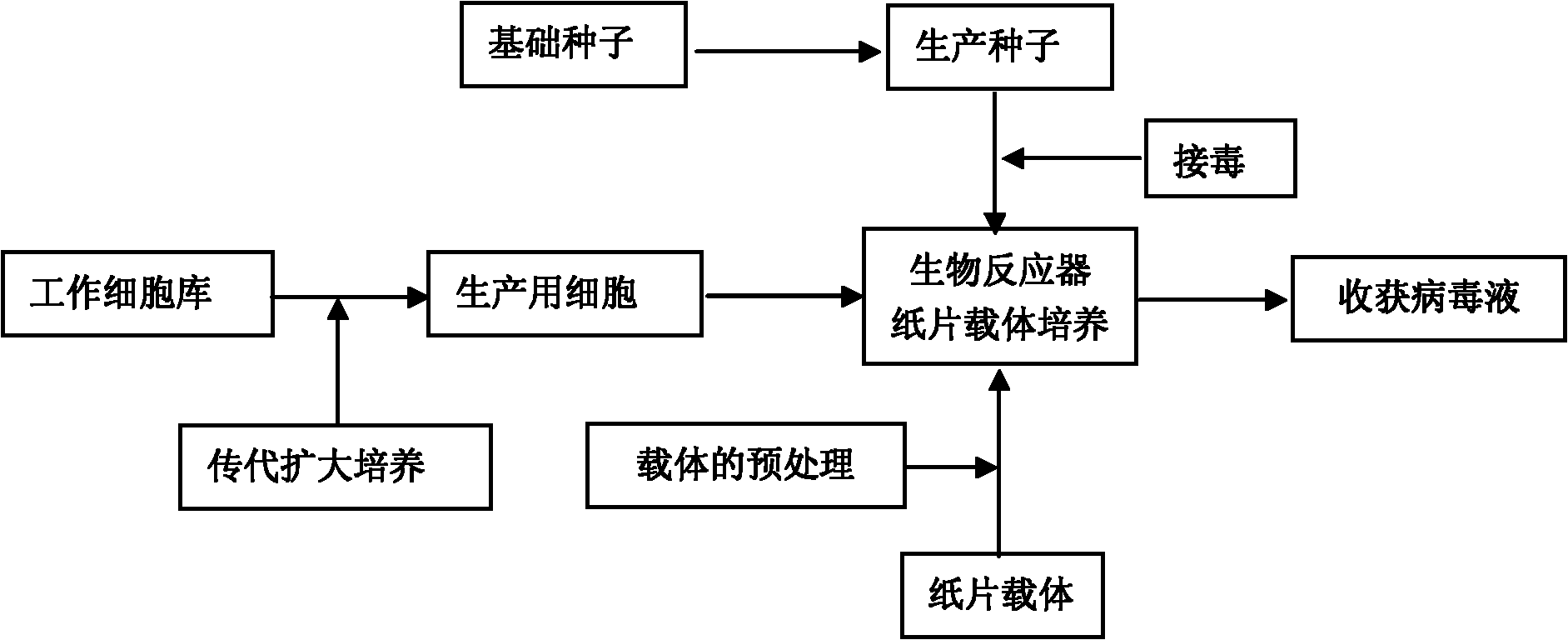
The invention discloses a method for producing PRRS (Porcine Reproductive and Respiratory Syndrome) viruses through culturing Marc-145 cells by applying a torrent-perfusion bioreactor. The method comprises the following steps of: (1) selecting Marc-145 cells as cells for culturing seedlings; (2) subculturing the Marc-145 cells; (3) reproducing virus seeds for production; (4) perfusing and culturing the Marc-145 cells on a paper carrier in the torrent-perfusion bioreactor; (5) producing the PRRS viruses in the torrent-perfusion bioreactor; and (6) treating the obtained virus antigen solution. The invention can greatly decrease the production cost, improve the input-output ratio by more than 10 times and expand the production scale easily and rapidly, has short production preparation cycle and high degree of automation, occupies less area, causes less environmental pollution which is easy to treat, consumes less labor, is easy to realize balanced and stable virus quality and can obviously improve the yield and the quality of the viruses.
Owner:成都史纪生物制药有限公司
Recombinant vaccine strain for foot-and-mouth disease type A as well as preparation method thereof and application thereof
InactiveCN102757942AIncrease production capacityHigh titerMicroorganism based processesAntiviralsAntigenDisease
The invention relates to a recombinant vaccine strain for foot-and-mouth disease type A, which is constructed by a gene recombination technology and featured with high valence, high antigen compatibility and high immune protective rate, as well as a preparation method thereof and an application thereof. An antigen nucleotide sequence of the vaccine strain is shown by SEQ ID NO. 1; a rescuing system is an efficient eukaryotic plasmid, which is manually constructed and can express an accurate foot-and-mouth disease virus genome RNA; therefore, a foot-and-mouth disease recombinant virus can be constructed and prepared; through the adoption of the plasmid, the vaccine strain with high titer and good antigen compatibility can be prepared so as to prepare inactivated vaccines; after pigs and cattle are immunized, bodies can be effectively stimulated to generate immune responses and immune protective effects on the pig and cattle bodies can be provided; an A type AISA lineage of strain is challenged with 10000 times of 50% cattle infective dose (BID50), so that the immune protective rate reaches 100% and 50% protective dose (PD50) is 10.81-13.59; and the vaccine can be used for preventing and controlling foot-and-mouth diseases type A in China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
VII type Newcastle disease virus L-gene mutation attenuated vaccine strain and preparation method thereof
ActiveCN103525777AHigh viral titerGenetically stableViral antigen ingredientsInactivation/attenuationAntigenMolecular biology
The invention relates to a VII type Newcastle disease virus L-gene mutation attenuated vaccine strain produced by using a reverse genetic manipulation technology and a preparation method of the VII type Newcastle disease virus L-gene mutation attenuated vaccine strain, and belongs to the field of biological products and biological technologies. Toxicity of the VII type Newcastle disease virus L-gene mutation attenuated vaccine strain is weakened obviously, and at the same time, the vaccine strain on a chick embryo has high titer, stable heritability and high compatibility to an antigen of a prevalent virus, is suitable for large-scale production of vaccines, and can be used for producing the vaccines. Compared with conventional vaccines (gene I and gene II types), the VII type Newcastle disease virus L-gene mutation attenuated vaccine strain has a wide application prospect in control of incidence and prevalence of the Newcastle diseases.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Seneca Valley virus recombinant plasmid and Seneca Valley virus recombinant virus, and construction methods thereof
ActiveCN109022374AHigh viral titerHigh virus proliferation capacitySsRNA viruses positive-senseVirus peptidesTiterSeneca Valley virus
The invention relates to a Seneca Valley virus recombinant plasmid and a Seneca Valley virus recombinant virus, and construction methods thereof, and belongs to the technical field of virus construction. The Seneca Valley virus recombinant virus is a Seneca Valley virus with the 177th amino acid, mutated to A from S, of a GH ring of capsid VP2. The Seneca Valley virus recombinant virus has a highvirus tilter and a high virus proliferation ability.
Owner:HUAZHONG AGRICULTURAL UNIVERSITY
SARS-CoV-2 inactivated vaccine and preparation method of vaccine
ActiveCN111569058AReduce Biosecurity RisksLow impurity contentSsRNA viruses positive-senseViral antigen ingredientsVirus inactivationTGE VACCINE
The invention relates to a SARS-CoV-2 inactivated vaccine and a preparation method of the vaccine. The preparation method comprises the following steps: step (1) recovery and large-scale culture of Vero cells; step (2) inoculation of working seeds, batch of virus seeds, virus culture and one-time harvest virus liquid, namely, the virus harvest liquid; step (3) obtaining an inactivated virus concentrated solution after a first virus inactivation, concentration and a second virus inactivation; step (4) obtaining a vaccine stock solution after purifying the inactivated virus concentrated solution; and step (5) preparation of semi-finished product and sub-package after the stock solution is verified to be qualified.
Owner:中国生物技术股份有限公司 +1
BHK-21-SC cell strain adapted to serum-free suspension culture and method for preparing vaccine antigen with cell strain
InactiveCN110093307AFast growthImprove shear resistanceSsRNA viruses negative-senseViral antigen ingredientsGolden hamsterVaccine antigen
The invention discloses a BHK-21-SC cell strain adapted to serum-free suspension culture and a method for preparing a vaccine antigen with the cell strain. The BHK-21-SC cell strain is a juvenile golden hamster kidney cell strain, and has the preservation number of CGMCC No.16817. The cell strain is cultured according to the initial cell density of 0.5-0.6*10<6> cell / ml. 72 hours later, the cellsgrow to the cell density of 8-10*10<6> cell / ml. The screened and domesticated BHK-21 cell strain adapted to serum-free full suspension culture is used for production of a high-titer Newcastle diseasevirus solution, and a domestication process for preparation of Newcastle disease virus by the BHK-21 cell strain adapted to serum-free suspension culture is established.
Owner:BEIJING VBIOSCI INC +1
Recombinant rabies virus carrying two genes G and gene VP2 and application of recombinant rabies virus
InactiveCN106244602AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsVp2 geneCanine parvovirus
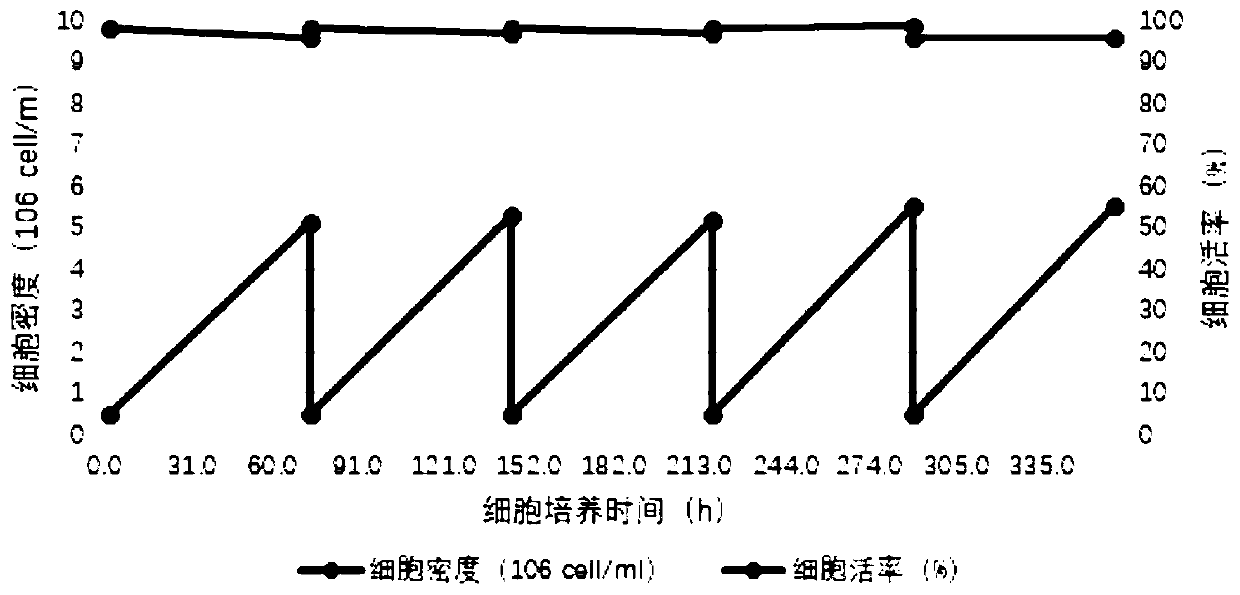
The invention discloses a recombinant rabies virus rHEP-Flury(dG-VP2) carrying two genes G and a gene VP2. According to the recombinant virus, a rabies virus HEP-Flury strain serves as a framework, the VP2 gene shown as SEQ ID NO.1 is inserted into HEP-Flury, an additional gene G of the rabies virus is inserted, and the recombinant plasmid pHEP-Flury(dG-VP2) carrying the two genes G and the gene VP2 is obtained; finally, rescuing screening is carried out, and the recombinant rabies virus rHEP-Flury(dG-VP2) is obtained. The recombinant rabies virus rHEP-Flury(dG-VP2) carries the two genes G and expresses VP2 protein, a high rabies virus resisting antibody and canine parvovirus VP2 resisting antibody level can be generated through immune induction, the cost of vaccines for dogs can be reduced, and very good application and popularization prospects are achieved.
Owner:SOUTH CHINA AGRI UNIV
Human-derived rotavirus attenuated strain with wide-spectrum immunogenicity and vaccine prepared from human-derived rotavirus attenuated strain
ActiveCN104312985AReduce pathogenicityPathogenicity NoneViral antigen ingredientsInactivation/attenuationAnimal testingImmunogenicity
The invention discloses a human-derived rotavirus attenuated strain with wide-spectrum immunogenicity and a vaccine prepared from the human-derived rotavirus attenuated strain. The human-derived rotavirus attenuated strain with wide-spectrum immunogenicity is named as HRV-LZ-2013, has a gene type of G10P8, is preserved in the China general microbiological culture collection center and has a strain preservation number of CGMCC N0. 9452. An experiment proves that the rotavirus attenuated strain keeps low pathogenicity to animals. An immunization protection experiment on animals and especially on rotavirus-caused diarrhoea model rabbit and pig proves that the attenuated live vaccine or inactivated vaccine prepared from the human-derived rotavirus attenuated strain has good immunizing protection properties. An immunizing protection test on cattle and sheep proves that the vaccine provided by the invention has a wide immunizing protection range and can be used for preventing cattle, pig, horse, sheep and dog diarrhoea caused by rotaviruses.
Owner:吕宏亮 +1
Virus and vaccine of porcine reproductive and respiratory syndrome and preparation method of same
ActiveCN101979514AHigh viral titerHigh poison priceViral antigen ingredientsAntiviralsFreeze-dryingCells/microL
The invention discloses a method for preparing virus of porcine reproductive and respiratory syndrome on a large scale. In the method, the virus of the porcine reproductive and respiratory syndrome is prepared in a cell microcarrier suspension culture system by a bioreactor. The method comprises the following steps of: inoculating host cells for preparing the virus to a carrier tank containing culture solution and a microcarrier, and mixing the cells and the microcarrier uniformly to ensure that the cells are attached to the microcarrier; providing sufficient nutrients and appropriate gas environment for the cells under the appropriate culture environment to ensure that the cells are grown until the cells are in an amount which are 10 to 20 times of the inoculation concentration on the microcarrier; preparing virus suspension from the virus of the porcine reproductive and respiratory syndrome by using cell maintenance culture solution to ensure that the suspension is adsorbed to the cells; culturing the virus under the appropriate culture environment; culturing continuously for 2 to 3 days to obtain virus solution; and after the virus solution passes inspection, performing freeze thawing on the virus solution twice at the temperature of -20 DEG C, and inactivating and purifying to prepare an inactivated vaccine of the porcine reproductive and respiratory syndrome or adding a freeze-drying protective agent for freeze drying to prepare a live vaccine of the porcine reproductive and respiratory syndrome. The method has large production scale, high yield of single batch and low production cost.
Owner:PU LIKE BIO ENG
Varicella attenuation live vaccine
ActiveCN101161286AImprove securityHigh viral titerAntiviralsAntibody medical ingredientsInfected cellKanamycin
The present invention relates to herpes virus family medical microorganism. The present invention is prepared by the following method: (1) serial passage MRC-5 cell; (2) inoculating to cell and continuous infecting; (3) adding culture solution; (4) washing cell surface; (5) eluting infected cell, centrifugal separating, adding vaccine fluid containing stabilizing agent to refrigerate; (6) ultrasonic crashing and centrifugal separating to acquire virus stock solution; (7) combining and diluting, and at once processing to pack and freeze-dry, preparing attenuated pox vaccine. The stabilizing agent is prepared by dissolving NaCl, KCl, KH2PO4, Na2HPO4.12H2O, saccharose, sodium glutamate, kalamycin, erythromycin and deionized water into the water with weight ratio 3:0.1:0.2:3.3:50:0.4:0.1:0.03:1000. The present invention has good advantages of safety and immunogenicity.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Infectious bursal disease virus Vero cell-adapted strain and application thereof
InactiveCN103923885AStable proliferationImprove securityViral antigen ingredientsMicroorganism based processesOil emulsionEmbryo
The invention provides an infectious bursal disease virus Vero cell-adapted strain and belongs to the field of bioengineering. The related infectious bursal disease virus Vero cell-adapted strain is named Ck / Jiangsu / NJ-23 / 2008 and has a collection No. of CGMCCNO.8852. The infectious bursal disease virus Vero cell-adapted strain which can efficiently multiply on serum-free cultured Vero cell is finally obtained through wild strain separation, chick embryo passage, Vero cell passage adaption; the infectious bursal disease virus Vero cell-adapted strain is subjected to continuous passage culture on the serum-free cultured Vero cell and TCID50 can be kept to be higher than 108.5 / mL. Virus culture solution is inactivated and prepared into oil emulsion; after the prepared oil emulsion is used to immunize chicken, detection proves that the prepared oil emulsion has good immunogenicity. The infectious bursal disease virus (IBDV) strain and the production process thereof are simple, safe and efficient and suitable for industrial culture.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Production method of porcine reproductive and respiratory syndrome virus
ActiveCN102002481AQuality improvementIncrease productionRecovery/purificationPorcine reproductive and respiratory syndrome virusVirus antigen
The invention discloses a production process of a porcine reproductive and respiratory syndrome virus (PRRSV) by using a bioreactor to culture a Marc-145 cell, which comprises the following technical steps of: (1) selecting a Marc-145 cell as a vaccine preparation cell; (2) culturing the vaccine preparation cell through passage and amplification; (3) reproducing seed virus for production; (4) culturing the microcarrier of the Marc-145 cell in the bioreactor; (5) producing an antigen of the PRRSV; and (6) treating the harvested virus antigen liquid. The invention can greatly reduce production cost and increase the input-output ratio by 5-10 times. Moreover, the invention has the advantages of short production period, small occupied land, easy and rapid expansion of production scale, less environment pollution, easy treatment, high automation degree, fewer people and easy realization of balanced and stable quality. The production cost can be obviously increased, and the yield and the quality of the vaccine can be increased.
Owner:成都史纪生物制药有限公司
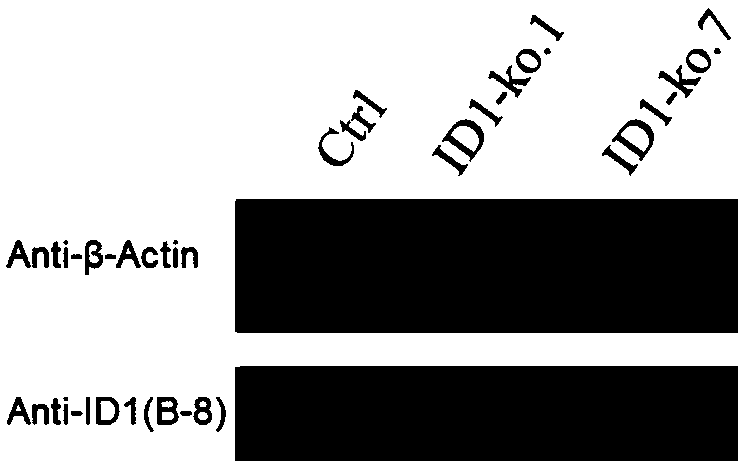
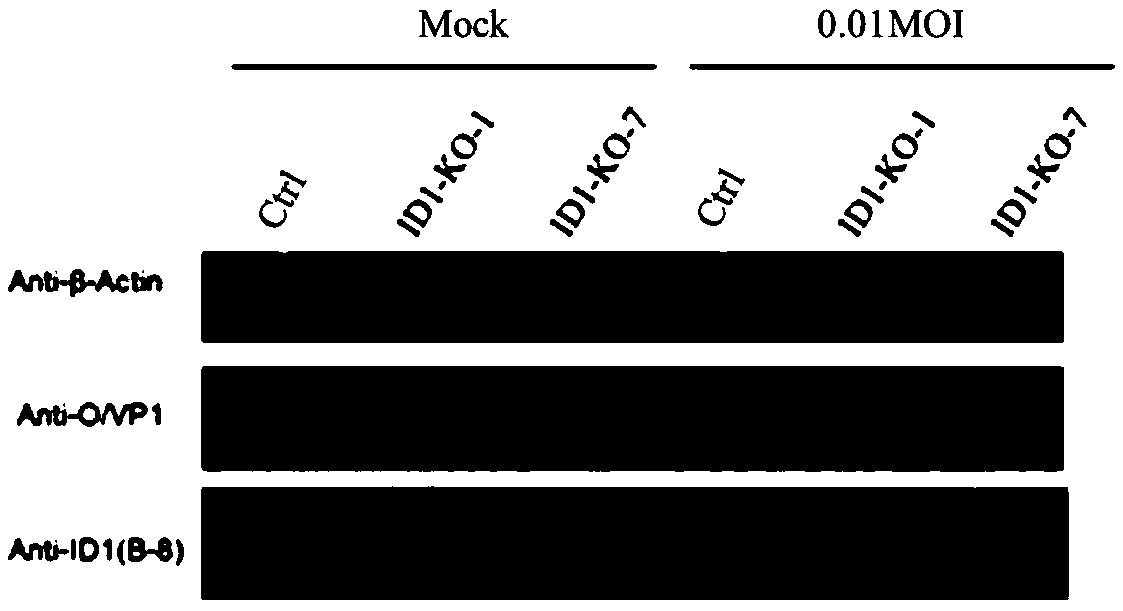
sgRNA of ID1 (Inhibitor of Differentiation 1) genes, knock-out method for ID1 genes, BHK-21 cell line and application thereof
ActiveCN108611351AHigh viral titerReduce manufacturing costSsRNA viruses positive-senseGenetically modified cellsTiterFoot-and-mouth disease virus
The invention relates to the technical field of biology and discloses sgRNA of ID1 (Inhibitor of Differentiation 1) genes, a knock-out method for ID1 genes, a BHK-21 cell line and the application thereof. Sequences of the sgRNA are shown as any pair of SEQ ID NO:1-2 or SEQ ID NO:3-4. Because the ID1 is capable of inhibiting foot and mouth disease virus multiplication, by knocking-out the ID1 genesin BHK-21 cells, the virus titer of an ID1 knock-out cell line is increased, so that the production cost of the foot and mouth disease virus is reduced.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Large-scale production method for veterinary pseudorabies attenuated live vaccines
ActiveCN104740627ASolve outputSolve efficiency problemsMicroorganism based processesAntiviralsVirology
The invention discloses a large-scale production method for veterinary pseudorabies attenuated live vaccines. The method comprises the steps of culturing pseudorabies virus attenuated strains by using a cell suspension process so that the density of BHK21-C13 cells subjected to suspension culture in a reactor is more than or equal to 2*10<6> cells per milliliter, then culturing with a virus maintenance solution containing pseudorabies suspended virus seeds, and performing clarification, seedling, packaging and freeze-drying on the harvested pseudorabies attenuated virus solution to obtain the veterinary pseudorabies attenuated live vaccines. By adopting the method, mass multiplication of pseudorabies attenuated viruses can be realized, the titer of viruses and the yield of antigen are greatly improved, process automation is realized, the quality of the vaccines is improved, the density of the BHK21-C13 cells can be improved, the optimal biochemical condition of cell growth or virus multiplication can be automatically monitored, the titer of viruses is improved, large-scale production of the process is relatively easy, and the vaccines are stable in quality and low in batch difference and have broad production and application prospect.
Owner:LIAONING YIKANG BIOLOGICAL CORP LTD
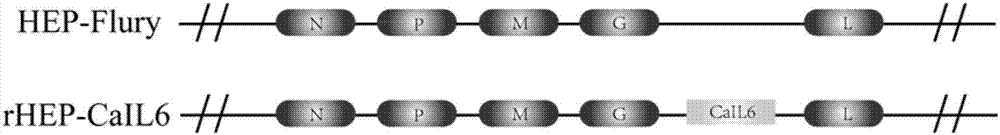
Recombinant rabies virus carrying interleukin 6 gene and application thereof
InactiveCN106967691AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsRabies virus strainEukaryotic plasmids
The invention discloses a recombinant rabies virus rHEP-CaIL6 carrying an immune enhancement factor interleukin (IL) 6 gene and application thereof. The recombinant virus takes rabies virus HEP-Flury strain as the skeleton, the IL6 gene of canine is inserted to a position between the G and L gene of HEP-Flury to obtain a recombinant plasmid pHEP-CaIL6, and finally saving screening is carried out to obtain the recombinant rabies virus strain rHEP-CaIL6. The recombinant virus carries the immune enhancement factor, can enhance the immune response and induce the production of higher rabies virus neutralizing antibody, thus better protecting the body from resisting the attack of lethal rabies virus, also can produce a neutralizing antibody with protective ability at a low dose, and lowers the cost of canine vaccines. Moreover, the IL6 gene is recombined into the rabies virus, thus achieving stable expression of IL6 protein, also avoiding the overexpression thereof, and overcoming the defect that excessive IL6 can cause pathological injury.
Owner:SOUTH CHINA AGRI UNIV
Recombinant rabies virus carrying deoptimized M gene and two G genes
ActiveCN107201371AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsRabies virus strainRecombinant virus vaccine
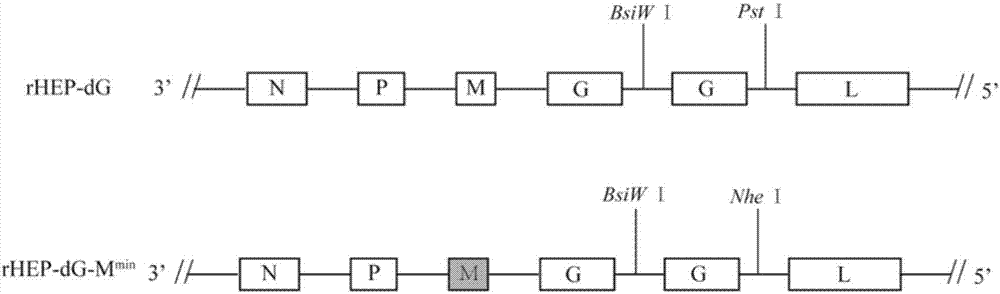
The invention discloses a recombinant rabies virus carrying a deoptimized M gene and two G genes. Firstly, a reading frame part of the M gene is deoptimized, wherein the sequence after deoptimization is as shown in SEQ ID NO.2; secondly, a rabies virus HEP-Flury strain is taken as a skeleton, an M gene in the HEP-Flury is replaced by the deoptimized M gene, and an additional rabies virus G gene is inserted to obtain recombinant rabies virus pHEP-dG-Mmin plasmid carrying the deoptimized M gene and two G genes; finally, rescuing and screening are conducted to obtain a recombinant rabies virus strain rHEP-dG-Mmin. The recombinant virus has higher virus titer, and the cost of canine vaccine can be reduced. Under the condition of multiplicity of infection, G protein with higher level can be expressed, virus replication and transcription of each structural gene are increased, and the recombinant virus has the potential of serving as a rabies vaccine candidate strain.
Owner:SOUTH CHINA AGRI UNIV
CA-193 virus strain and application thereof to preparation of inactivated vaccine
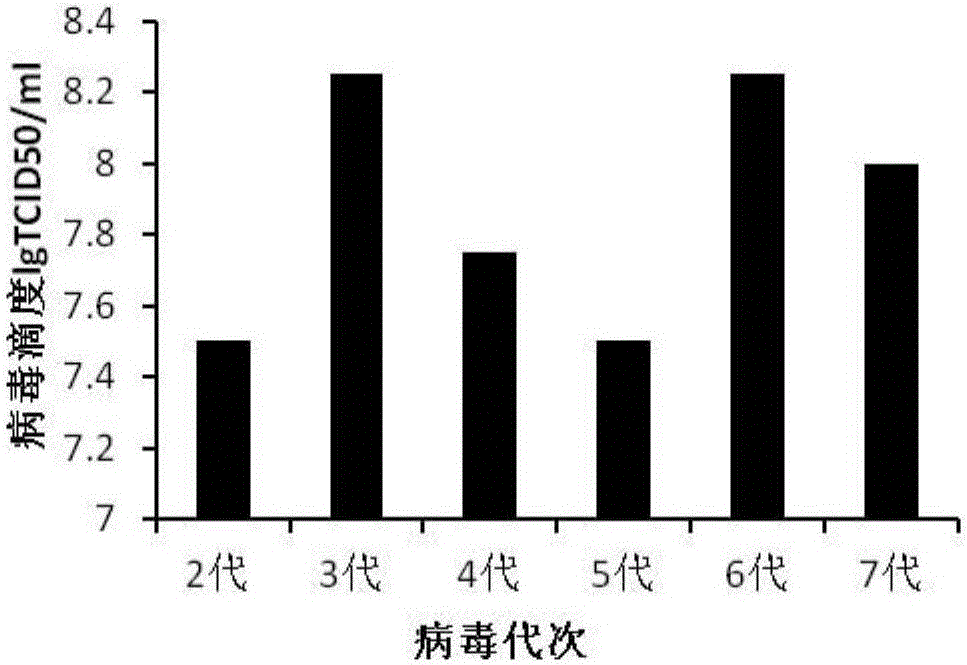
ActiveCN106367398ARich preparation methodImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsFiltrationVaccine virus
The invention discloses a CA-193 virus strain (Coxackievirus A16) and application thereof to preparation of an inactivated vaccine. For the application, MRC-5 cells are used for proliferating the CA-193 virus strain; virus proliferation liquid is subjected to centrifugation, concentration and column chromatography filtration and purification after inactivation by formaldehyde or beta-propiolactone; and a CA16 inactivated vaccine is obtained. The invention provides the vaccine virus strain capable of being used for preparing a human CA16 inactivated vaccine; the subtype of the vaccine virus strain belongs to the main epidemiological subtype B1 in Chinese Mainland; the vaccine virus strain has good growth characteristics on the MCR-5 cells; and the virus titer (7.5 to 8.251gTCID50 / ml) with high stability can be obtained. A preparation method of the vaccine is complete; the impurity content is low; the immunogenicity and the immunizing protection performance are good; meanwhile, cell culture substrates are MRC-5 cells; the safety is high; and the international and CFDA specifications and requirements on the vaccine research and development can be well met.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Preparation method for serum-free and suspension culture of HEK-293 cells and application of serum-free and suspension culture of HEK-293 cells
ActiveCN113717927AReach the standard of freezingGood dispersionCulture processMicroorganism based processesBiotechnologySerum free
The invention provides a preparation method for serum-free and suspension culture of HEK-293 cells and an application of the serum-free and suspension culture of the HEK-293 cells, and relates to the preparation method for the serum-free and suspension culture of the HEK-293 cells, a cell line obtained by the preparation method and the application of the cell line. The preparation method is few in passage times and high in domestication efficiency, and the obtained cells are high in viability, good in dispersity and stable in growth state and can meet the requirements of commercialized and large-scale production.
Owner:IMMUNE CELL BIOTECH CO LTD +2
Production method of porcine circovirus type 2
InactiveCN103550769ASolve low production efficiencyImproving vaccine quality and yieldViral antigen ingredientsInactivation/attenuationPorcine CircovirusesCulture cell
The invention discloses a production process for producing porcine circovirus type 2 by culturing cells pK-15A1# in a bioreactor. The production process comprises the technical steps of (1) selecting pK-15A1# cells for multiplying circovirus type 2; (2) utilizing the bioreactor as a cell culture tool; (3) carrying out a virus culture process of synchronous virus inoculation; (4) culturing the pK-15A1# cells on micro-carriers in the bioreactor; (5) carrying out multiplication culture on viruses of porcine circovirus type 2 in the cells on the micro-carriers in the bioreactor; and (6) harvesting virus culturing materials of porcine circovirus type 2. The production process has the advantages that the production cost can be lowered, and compared with that of a traditional spinner bottle production process, the input-output ratio of the production process is increased by 5-10 times; the production cycle is short, the occupied area is small, the production scale can be easily and rapidly expanded, the environmental pollution is slight and is easy to avoid, the automation degree is high, few workers are required, the quality is stable, the cost can be remarkably lowered, and the yield and quality of vaccines can be remarkably improved.
Owner:成都史纪生物制药有限公司
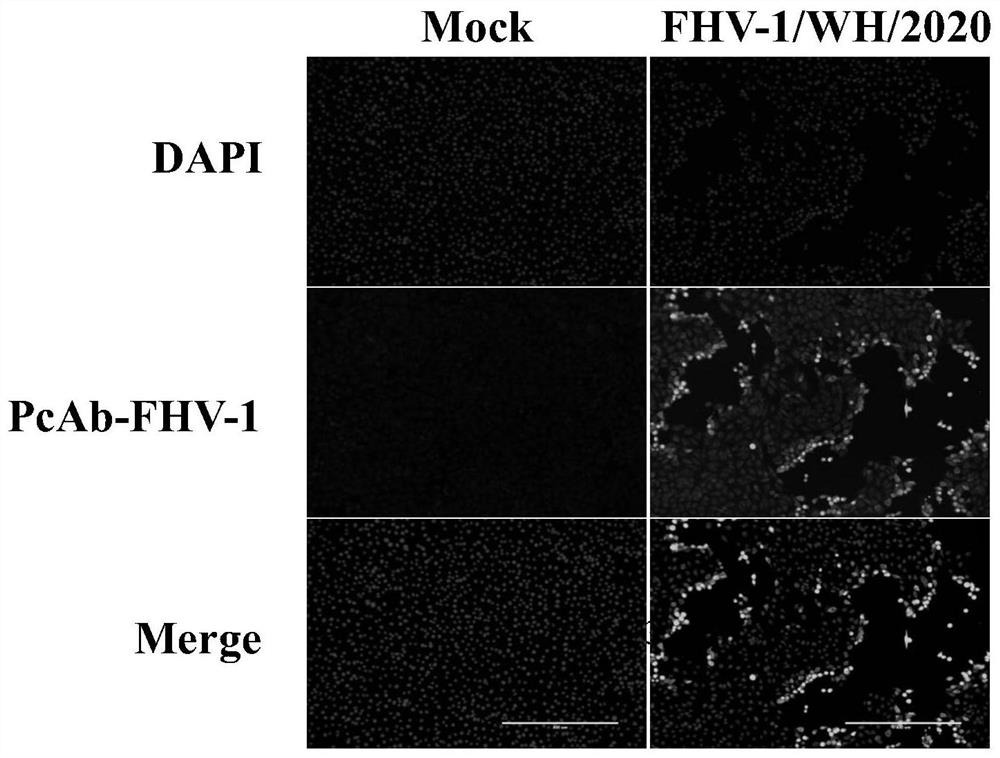
Feline herpesvirus I type virus strain and application thereof
ActiveCN113308441AAnti-epidemicHigh viral titerViral antigen ingredientsVirus peptidesTiterBiomedical engineering
The invention discloses a feline herpesvirus I type virus strain and application thereof, and belongs to the technical field of biological products. The preservation number of the virus strain is CCTCC NO: V202126, the preservation time is April 27, 2021, and the preservation address is Wuhan University, Wuhan, China. The virus strain belongs to an isolated strain in Wuhan of China, has high virus titer of 107.65 TCID50 / mL, has strong pathogenicity to domestic cats and has the basic potential of becoming a vaccine candidate strain, a vaccine prepared from the virus strain can be used for preventing and treating feline infectious rhinotracheitis diseases, an important vaccine virus source is provided for prevention and control of the feline herpesvirus I in China, and effective prevention and control of the feline herpesvirus I are achieved.
Owner:HUAZHONG AGRI UNIV
Porcine epidemic diarrhea virus as well as separation method and application thereof
InactiveCN108130316AEfficient separationHigh puritySsRNA viruses positive-senseViral antigen ingredientsImmune effectsPurification methods
The invention provides a porcine epidemic diarrhea virus as well as a separation method and application thereof, relating to the technical field of biology. According to a separation purification method of the porcine epidemic diarrhea virus, the porcine epidemic diarrhea virus is separated by virtue of polyethylene glycol 6000. The method is simple in operation and strong in practicability, the porcine epidemic diarrhea virus can be effectively separated, and the separated porcine epidemic diarrhea virus has relatively high purity and virus titer. A tissue inactivated vaccine prepared from the porcine epidemic diarrhea virus has the beneficial effects that impure proteins are few, the batch difference of the vaccines is small, the immune effect is good, and the side effect is slight.
Owner:TECON BIOLOGY CO LTD
Different serotypes of vesicular stomatitis virus as expression vectors for immunization regimens
ActiveUS9248178B2Effective vaccine vectorEasily kill the cells infectedSsRNA viruses negative-senseAntibacterial agentsMammalPrime boost
Owner:UNIV OF WESTERN ONTARIO
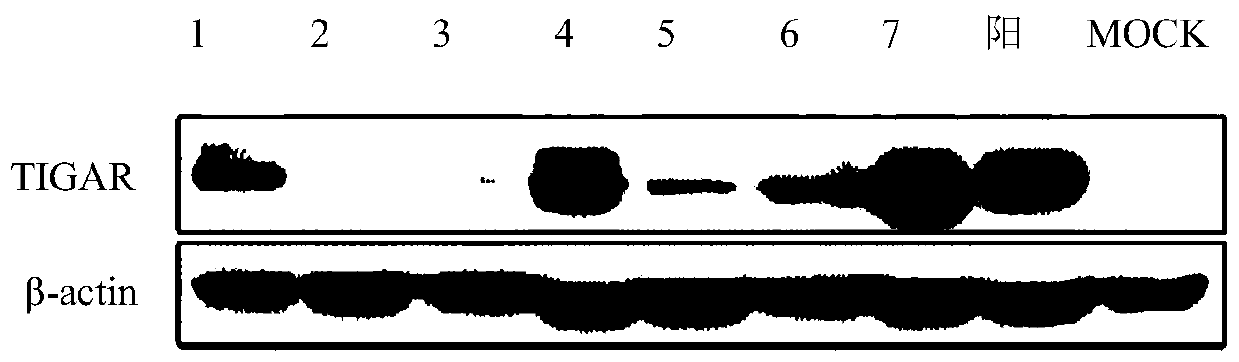
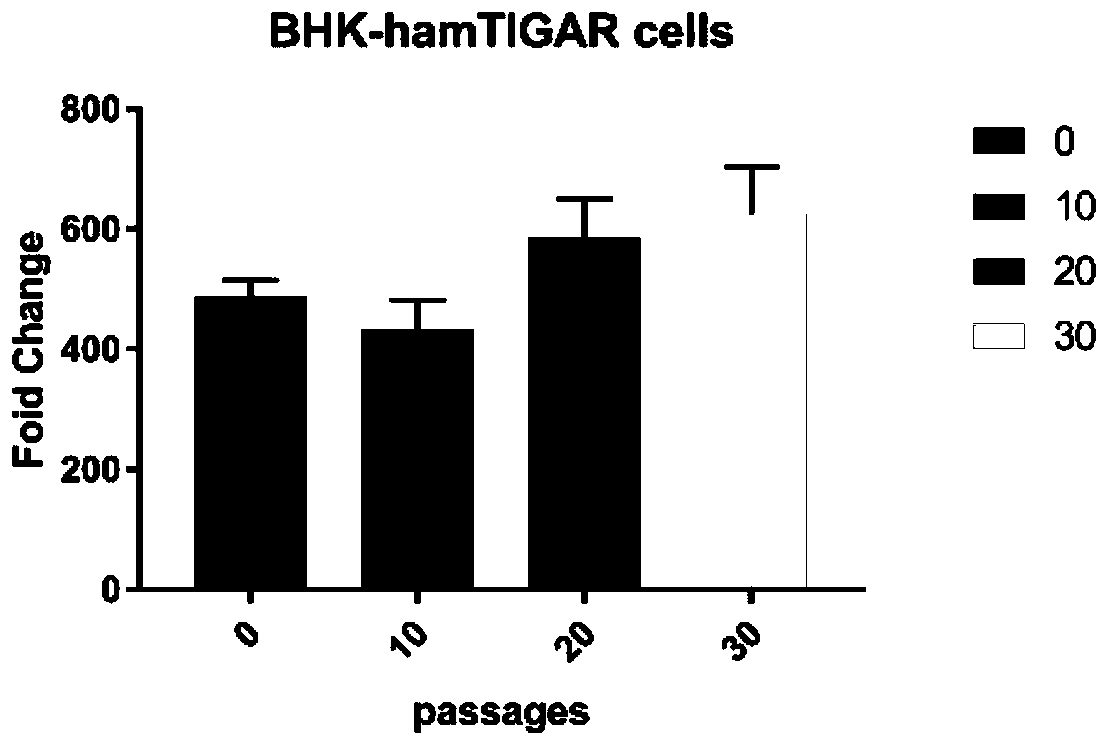
BHK cell line stably expressing hamster TIGAR gene, and construction method and application thereof
ActiveCN110093318AImprove anti-apoptotic abilityProlong survival timeSsRNA viruses negative-senseGenetically modified cellsLentivirusNewcastle disease virus NDV
The invention relates to a BHK cell line stably expressing a hamster TIGAR gene, and a construction method and an application thereof. The cell line contains the TIGAR gene and has the preservation number of CCTCC NO:C201928. The hamster TIGAR gene is amplified firstly, and a plasmid is constructed; then the constructed plasmid is transferred into 293T cells with PAX and PMD2G plasmids at the sametime to form packaged lentivirus; after the BHK cells are infected by the packaged lentivirus, drug screening is performed, and then enlarged cultivation is performed to obtain the cell line. The cell line can increase the anti-apoptotic ability of cells, prolong the survival time of the cells, increase the viral titer of Newcastle disease in the cells, and successfully construct and screen high-yield cell lines suitable for the reproduction of Newcastle disease virus.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Method for producing PRRS (Porcine Reproductive and Respiratory Syndrome) viruses
ActiveCN102002482BQuality improvementIncrease productionRecovery/purificationPerfusion CulturePerfusion bioreactor
The invention discloses a method for producing PRRS (Porcine Reproductive and Respiratory Syndrome) viruses through culturing Marc-145 cells by applying a torrent-perfusion bioreactor. The method comprises the following steps of: (1) selecting Marc-145 cells as cells for culturing seedlings; (2) subculturing the Marc-145 cells; (3) reproducing virus seeds for production; (4) perfusing and culturing the Marc-145 cells on a paper carrier in the torrent-perfusion bioreactor; (5) producing the PRRS viruses in the torrent-perfusion bioreactor; and (6) treating the obtained virus antigen solution. The invention can greatly decrease the production cost, improve the input-output ratio by more than 10 times and expand the production scale easily and rapidly, has short production preparation cycle and high degree of automation, occupies less area, causes less environmental pollution which is easy to treat, consumes less labor, is easy to realize balanced and stable virus quality and can obviously improve the yield and the quality of the viruses.
Owner:成都史纪生物制药有限公司
Production method of porcine reproductive and respiratory syndrome virus
ActiveCN102002481BQuality improvementIncrease productionRecovery/purificationPorcine reproductive and respiratory syndrome virusVirus antigen
The invention discloses a production process of a porcine reproductive and respiratory syndrome virus (PRRSV) by using a bioreactor to culture a Marc-145 cell, which comprises the following technical steps of: (1) selecting a Marc-145 cell as a vaccine preparation cell; (2) culturing the vaccine preparation cell through passage and amplification; (3) reproducing seed virus for production; (4) culturing the microcarrier of the Marc-145 cell in the bioreactor; (5) producing an antigen of the PRRSV; and (6) treating the harvested virus antigen liquid. The invention can greatly reduce production cost and increase the input-output ratio by 5-10 times. Moreover, the invention has the advantages of short production period, small occupied land, easy and rapid expansion of production scale, less environment pollution, easy treatment, high automation degree, fewer people and easy realization of balanced and stable quality. The production cost can be obviously increased, and the yield and the quality of the vaccine can be increased.
Owner:成都史纪生物制药有限公司
Cell affinity agent capable of improving duck flavivirus in-vitro infection efficiency and preparation method of cell affinity agent
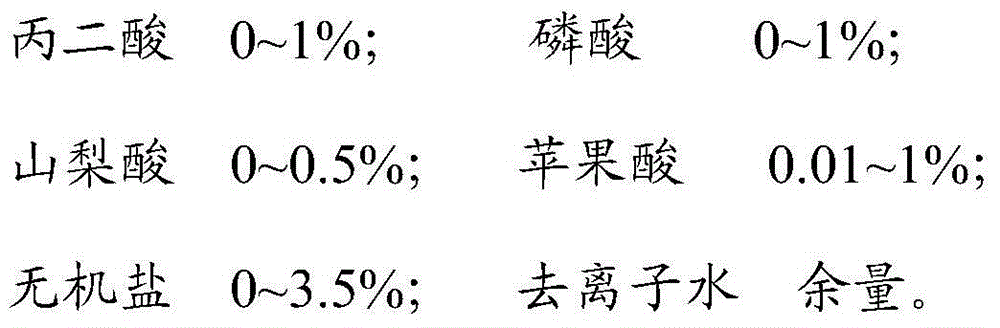
ActiveCN104611298AEasy accessImprove in vitro infection efficiencyMicroorganism based processesViruses/bacteriophagesOrganic acidInorganic salts
The invention discloses a cell affinity agent capable of improving duck flavivirus in-vitro infection efficiency and a preparation method of the cell affinity agent and belongs to the technical field of agents for microbial virus culture. The cell affinity agent is prepared from the following components in percentage by mass: 0.5-5% of organic acid, 0-1% of phosphoric acid, 0-3.5% of inorganic salt and the balance of deionized water. By the cell affinity agent, the microenvironment between the duck flavivirus and the cell can be improved, and the affinity between the duck flavivirus and the cell is increased so that the infection efficiency of the duck flavivirus to the cell is effectively increased.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST +1
Method for production of porcine epidemic diarrhea virus
ActiveCN101748102BQuality improvementIncrease productionMicroorganism based processesRecovery/purificationVirus antigenMicrocarrier
The invention discloses a technology for the production of porcine epidemic diarrhea virus by means of the microcarrier culture of VREO cells using a bioreactor, and comprises the technology for the production of different porcine epidemic diarrhea virus strains. The technology comprises the following technical steps: (1) selection of VERO cells as cell line for vaccine; (2) passage and culture of cells for vaccine; (3) propagation of seed culture of the porcine epidemic diarrhea virus; (4) microcarrier suspension culture of the VERO cells in the bioreactor; (5) propagation of porcine epidemic diarrhea virus antigen; and (6) treatment of acquired virus antigen liquid. The production method can remarkably lower production cost and enhance output-input ratio by 5 to 10 times, and has the advantages of short production period, small occupied space, great easiness for enlarging production scale rapidly, little environmental pollution, easy processing, high automation degree, a small number of staff, easy implementation of even and stable quality, obviously lowered production cost and enhanced yield and quality of vaccine.
Owner:成都史纪生物制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com