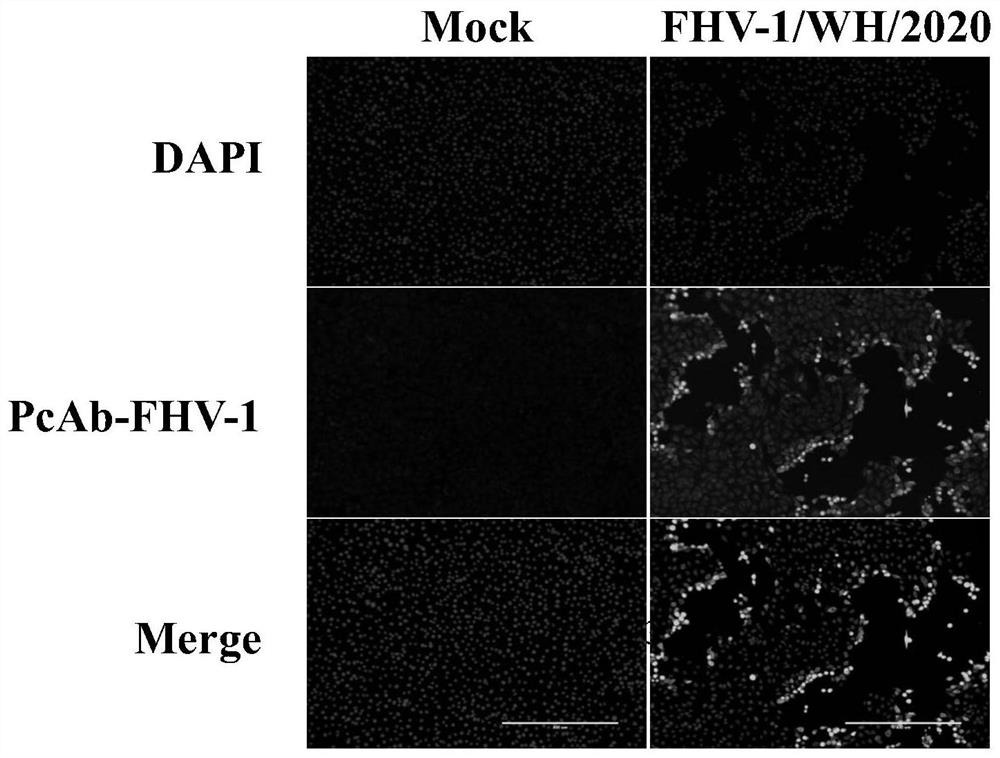
Feline herpesvirus I type virus strain and application thereof
A feline herpes virus and virus strain technology, applied in the field of biological products, can solve problems such as inability to effectively control, antibody titer not reaching the titer against virus infection, poor immune effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
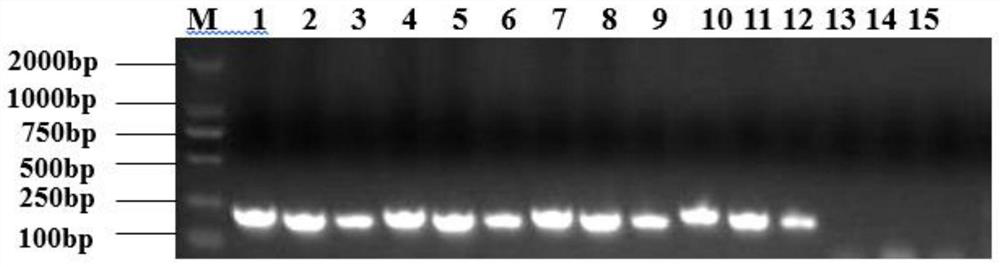
[0028] Isolation and identification of embodiment 1FHV-1 / WH / 2020 virus strain
[0029] 1. Experimental method
[0030] 1. Virus isolation
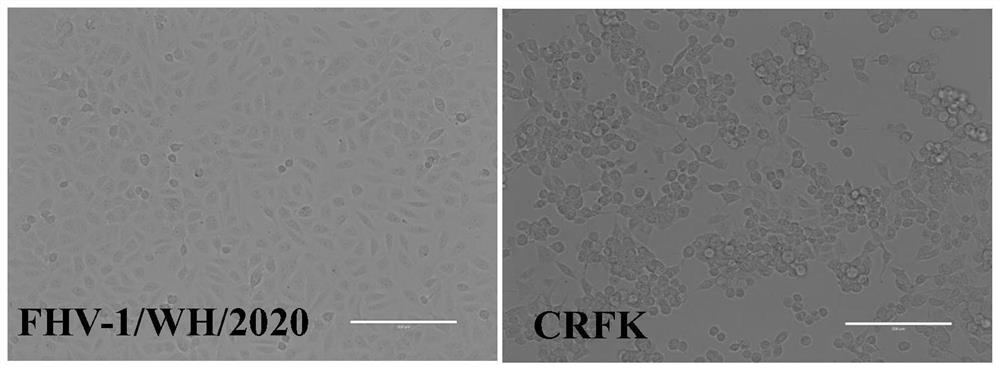
[0031] (1) The eye and nose swab samples of cats diagnosed with FHV-1 infection were collected from a pet hospital in Wuhan, China, and added with serum-free DMEM culture medium (containing 1% double antibody) to make a 1:5 suspension, centrifuged at 5000r / min for 10min, The supernatant was taken, sterilized by filtration through a 0.22 μM microporous membrane, and dispensed into sterile EP tubes as the inoculum for the isolated virus, and stored at -80°C.
[0032] (2) Cultivate FHV-1 susceptible cells CRFK to a monolayer in a 6-well culture plate, inoculate the diseased material samples treated in the previous step into CRFK according to 1 / 10 of the volume of the culture solution, and inoculate in a 37°C incubator After 1-2 hours of adsorption, the culture medium was discarded, and the CRFK cells were washed three times with PBS. Add D...
Embodiment 2
[0042] The plaque purification of embodiment 2FHV-1 / WH / 2020
[0043] 1. Experimental method
[0044] (1) After digesting CRFK evenly, adjust the cell density to 1×10 5 Cells / mL were inoculated into a 6-well plate. When the cells grew to a single layer, the virus solution was prepared, and ten-fold serial dilutions were performed to obtain five dilutions of the virus solution. Discard the culture medium in the wells, wash the wells with PBS 3 times, inoculate 800 μL of the virus solution into each well, absorb at 37°C for 2 hours, shake the plate every 15 minutes to fully absorb the virus solution, and discard the virus solution after 2 hours , washed three times with PBS.
[0045] (2) Prepare 2% low-melting point agarose solution, melt it at 72°C, put it in a water bath at 42°C to keep it warm for use, and place 2×DMEM in a water bath at 37°C for preheating.
[0046] (3) Mix the above 2% low-melting point agarose solution with 2×DMEM maintenance solution (2% serum, 1% doubl...
Embodiment 3
[0050] The determination of embodiment 3FHV-1 / WH / 2020 growth kinetics
[0051] 1. Experimental method
[0052] (1) CRFK cells were subcultured and inoculated in 24-well plates. After the cells grew to a single layer, the cells were infected with 0.01 MOI virus, and the culture medium was replaced with 2% maintenance medium, and cultured in a 37°C incubator.
[0053] (2) Supernatant samples were collected at 12, 24, 36, 48, 60, and 72 hours after inoculation, and the virus TCID was measured with CRFK cells 50 .
[0054] (3) Cells were subcultured in a 96-well plate, and when the cell density reached about 50%, the virus was diluted 10 times with the cell maintenance solution, and 100 μL of the virus solution was added to each well, and each dilution was replicated 8 times. Put into the incubator to cultivate.
[0055] (4) Observe once every 24 hours, record the virus dilution and the number of holes where cytopathic disease occurs, stop the observation after 4-5 days, record...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com