A vaccine composition, its preparation method and application
A vaccine composition and culture technology, applied in the field of vaccine compositions, can solve the problems of lack of co-infection combined vaccine, and achieve the effects of good immune protection, good immunogenicity and resistance to infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0016] As an embodiment of the present invention, in the vaccine composition of the present invention, the antigen of the gene VII Newcastle disease virus strain or its culture is an inactivated whole virus antigen.
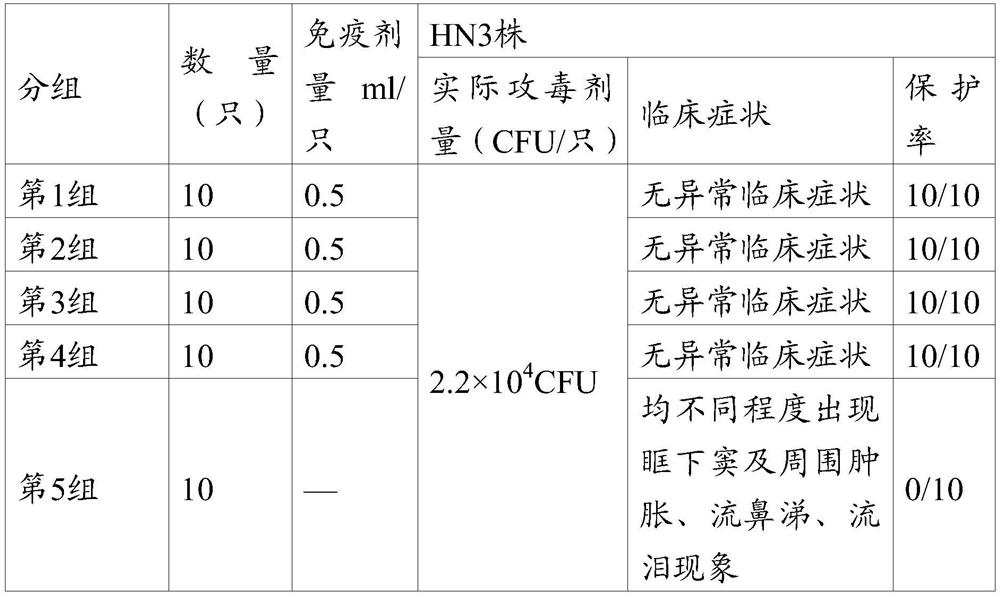
[0017] The NDV N7a strain is a gene type VII Newcastle disease virus strain, which is a Newcastle disease virus strain in which the F cleavage site 112R-R-Q-K-R-F117 of the gene type VII Newcastle disease epidemic wild strain is mutated into an attenuated cleavage site 112G-R-Q-G-R-L117.
[0018] "Vaccine composition" refers to a pharmaceutical composition containing the immunogenicity of Avibacterium paragallinarum and Newcastle disease virus type VII, which can induce, stimulate or enhance the immune response of chickens against Avibacterium paragallinarum and Newcastle disease virus . The vaccine composition can include the attenuated live vaccine, inactivated vaccine, subunit vaccine or synthetic peptide vaccine of immunizing amount of Avian bacillus paragall...
Embodiment 1
[0060] The preparation of embodiment 1 Avian bacillus paragallinarum antigen
[0061] 1. Primary seed propagation
[0062] Streak inoculated strains on chicken broth agar plate in 5%-10% CO 2 Cultivate at 37°C for 18-24 hours in the environment, select a suitable number of typical translucent dewdrop-like bacterial lawns (colonies) with strong fluorescence, inoculate the bacterial lawns into the yellow sac of chicken embryos (6-7 days old), and place them at 37 Continue to incubate at ℃, collect the yolk liquid of chicken embryos that died within 30 hours, and use them as first-class seeds after passing the pure inspection.
[0063] 2. Secondary seed propagation
[0064] Take the egg yolk fluid of infected chicken embryos, streak and inoculate the chicken broth agar plate, culture at 37°C for 18 to 24 hours in an environment containing 5% to 10% CO2, select typical colonies with strong fluorescence and inoculate them in the broth medium, place Cultivate at 37°C and 180rpm f...
Embodiment 2
[0069] The preparation of embodiment 2 chicken Newcastle disease antigen
[0070] Get chicken Newcastle disease virus N7a strain, do appropriate dilution with sterilized physiological saline (10 -4 or 10 -5 ) to inoculate 10-11-day-old susceptible chicken embryos, 0.1ml per embryo, and continue to incubate at 37°C after inoculation. Select dead and surviving chicken embryos 48 to 120 hours after inoculation, harvest the allantoic fluid, and measure the virus content, which is 10 8.0 EID 50 / 0.1ml. Add formaldehyde solution (v / v) with a final concentration of 0.1%, put it at 37°C for inactivation, stir once every 4-6 hours, inactivate for 16 hours, and set aside after complete inactivation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com