Patents
Literature
577results about How to "Good immune protection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
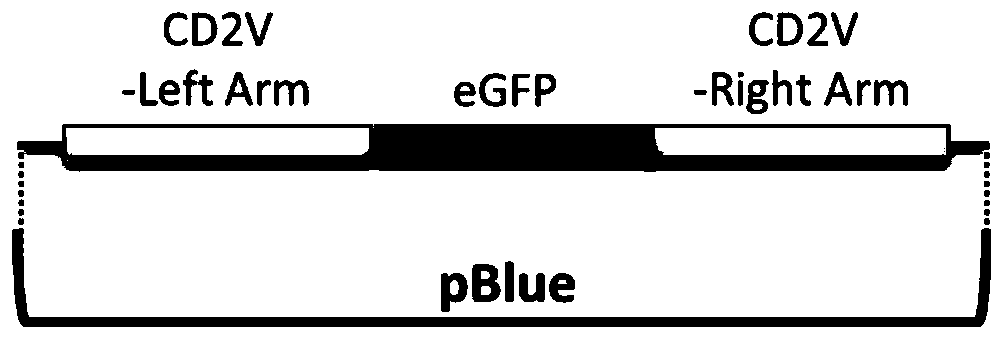
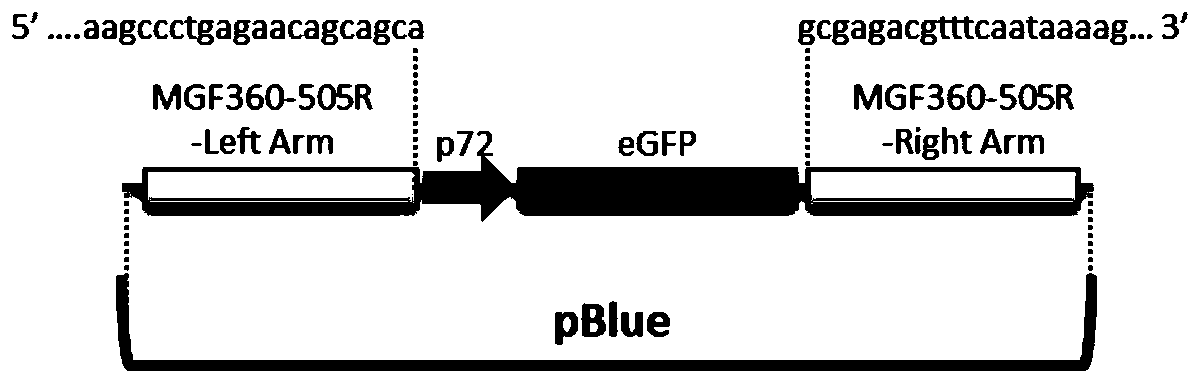
Gene deletion attenuated African swine fever virus and application thereof as vaccine
ActiveCN110093324AGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
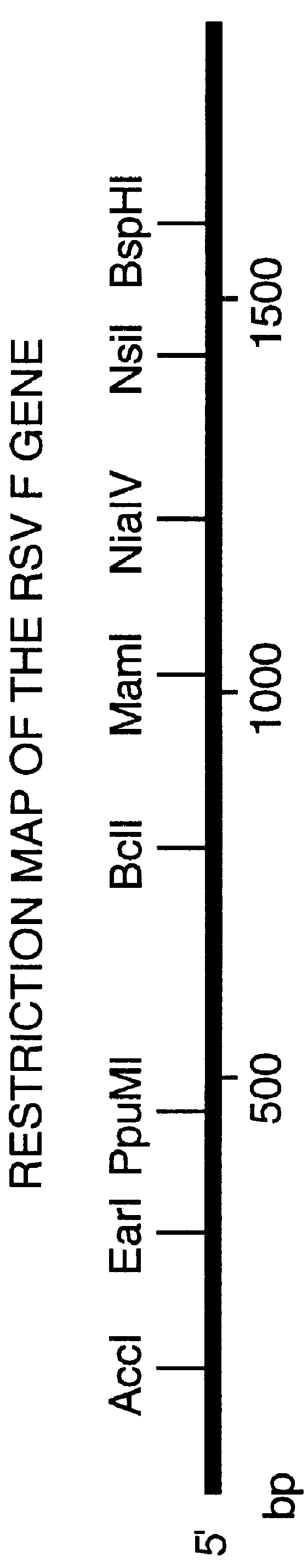
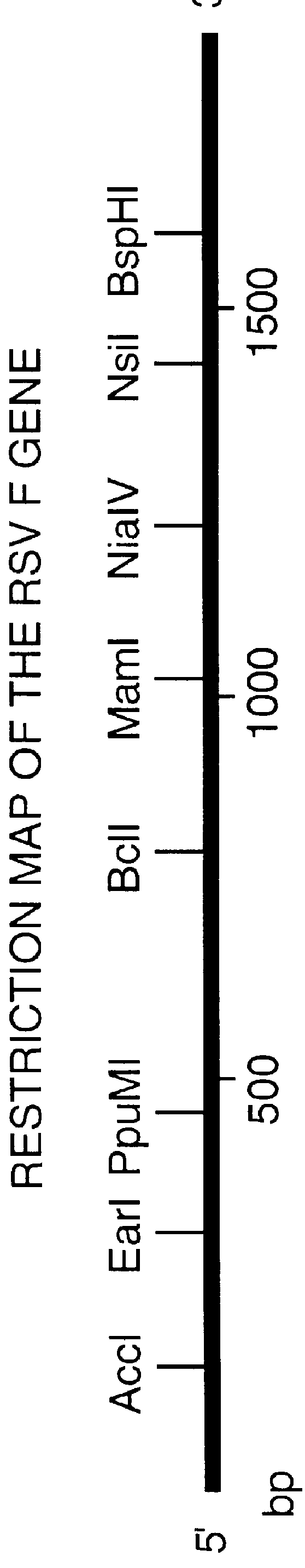
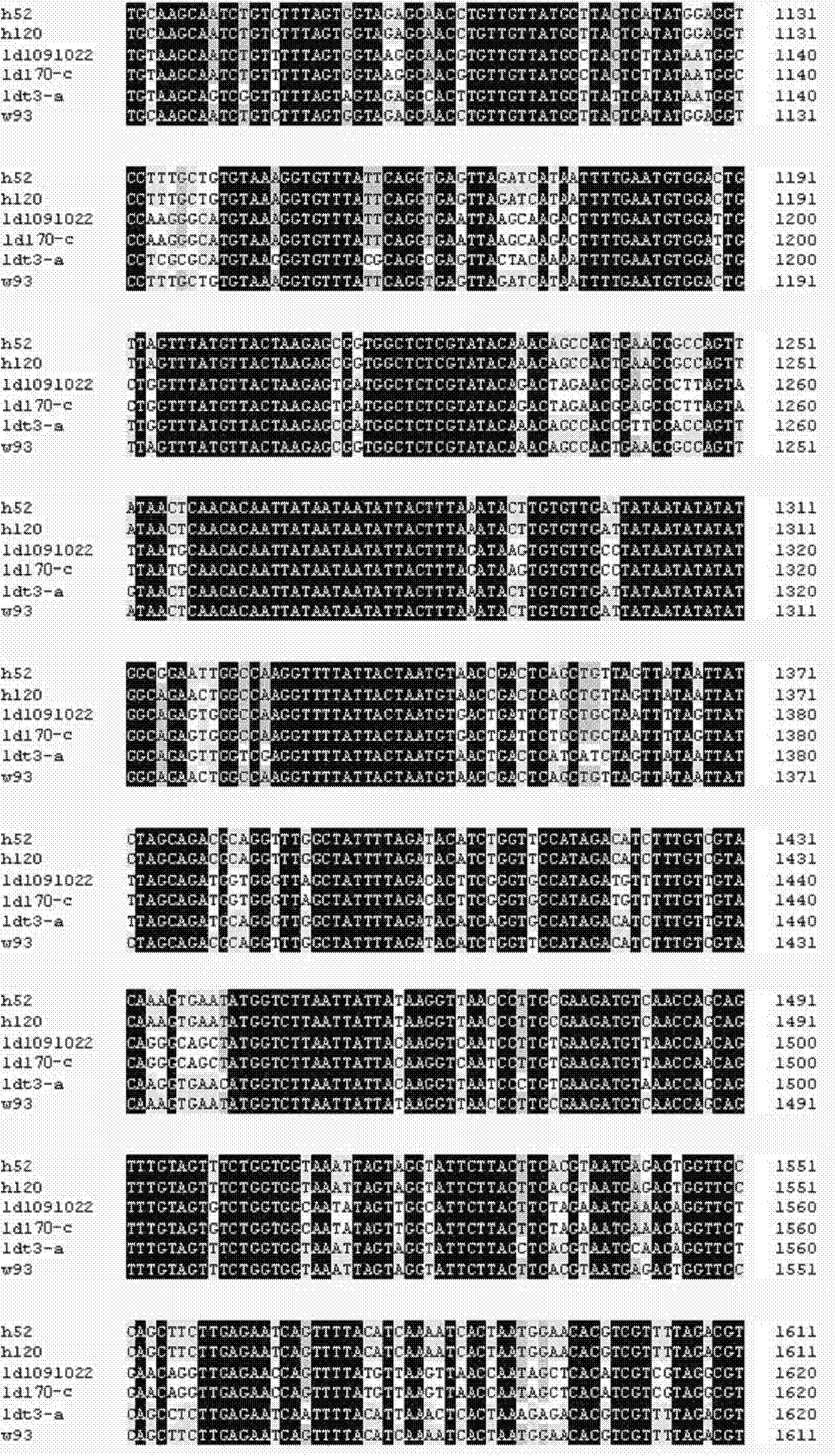
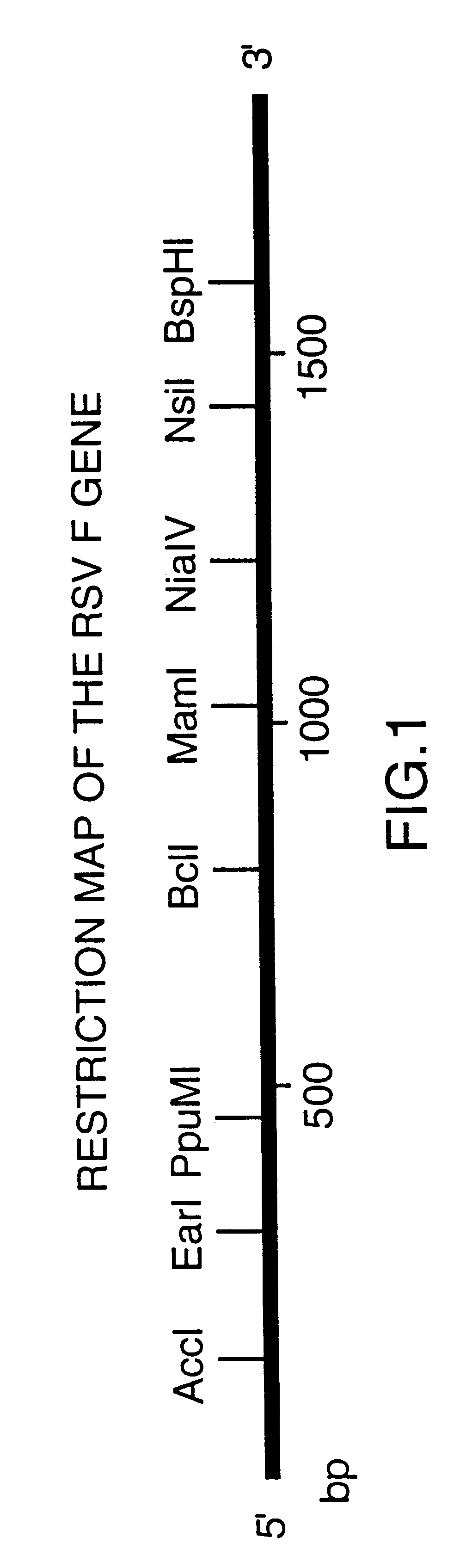
Nucleic acid respiratory syncytial virus vaccines
InactiveUS6019980AReduce backgroundGood immune protectionSsRNA viruses negative-senseGenetic material ingredientsNucleotideViral Vaccine
Vectors containing a nucleotide sequence coding for an F protein of respiratory syncytial virus (RSV) and a promoter for such sequence, preferably a cytomegalovirus promoter, are described. Such vectors also may contain a further nucleotide sequence located adjacent to the RSV F protein encoding sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such vectors may be used to immunize a host, including a human host, by administration thereto. Such vectors also may be used to produce antibodies for detection of RSV infection in a sample.
Owner:CONNAUGHT LAB
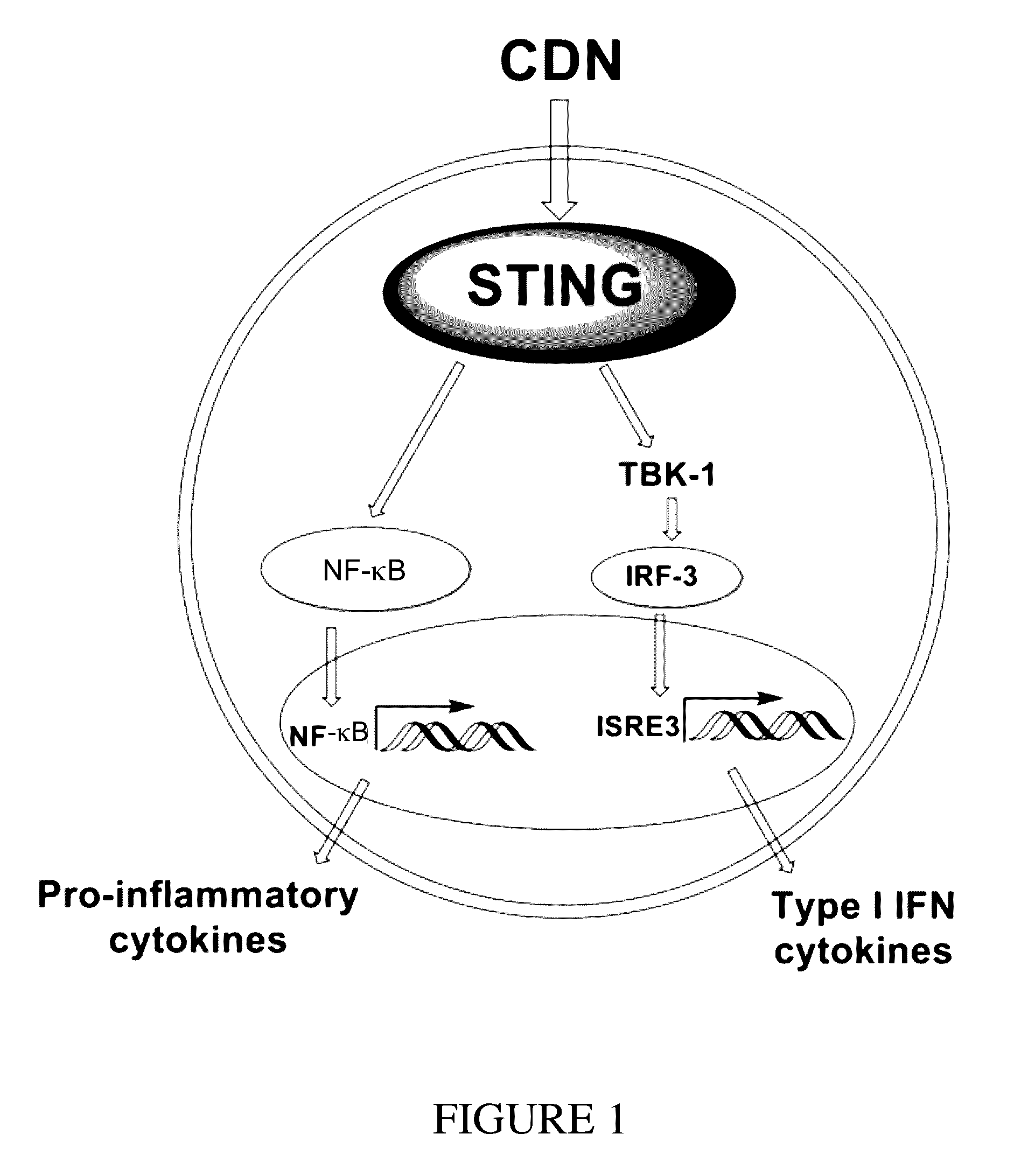
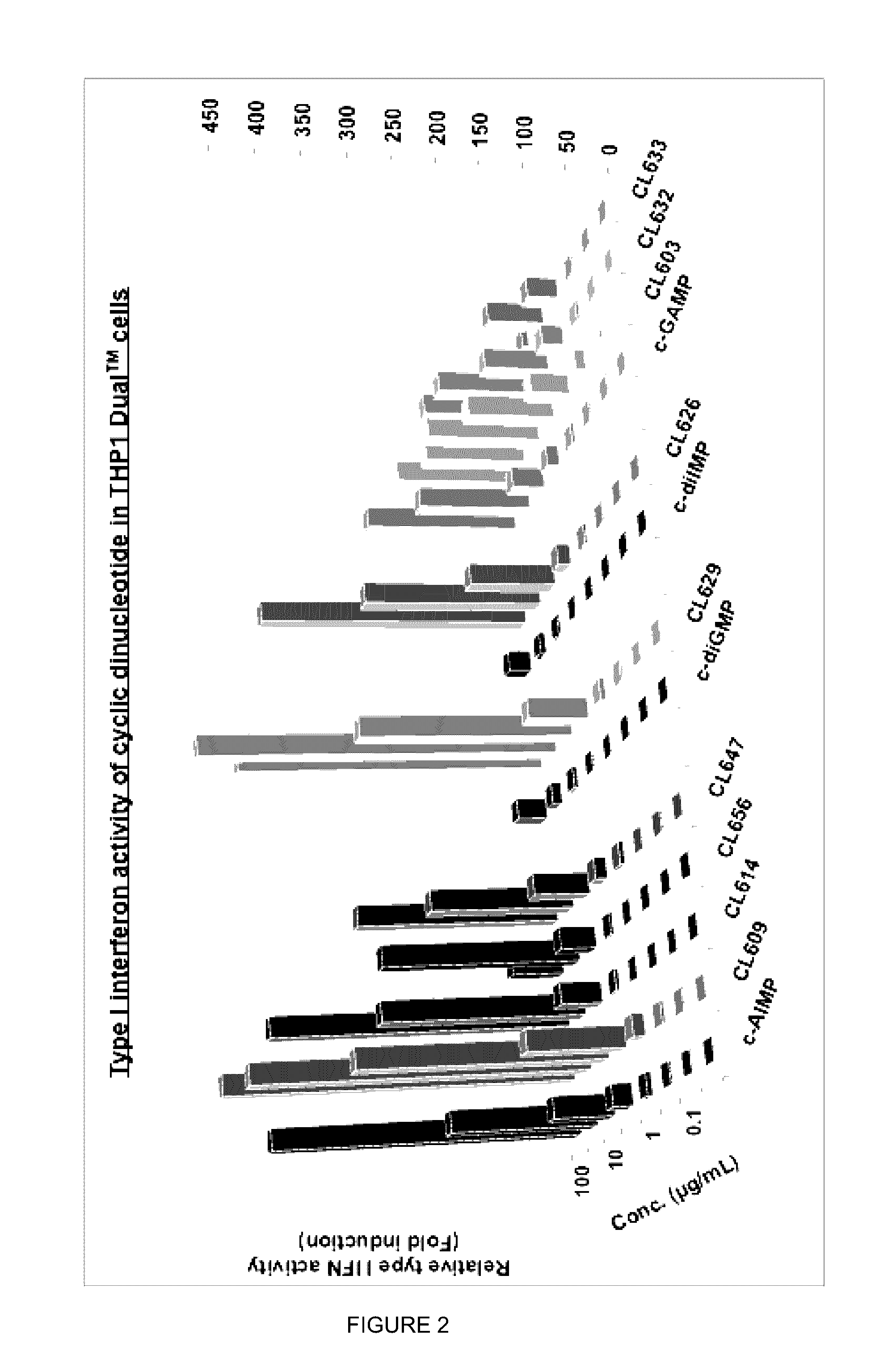
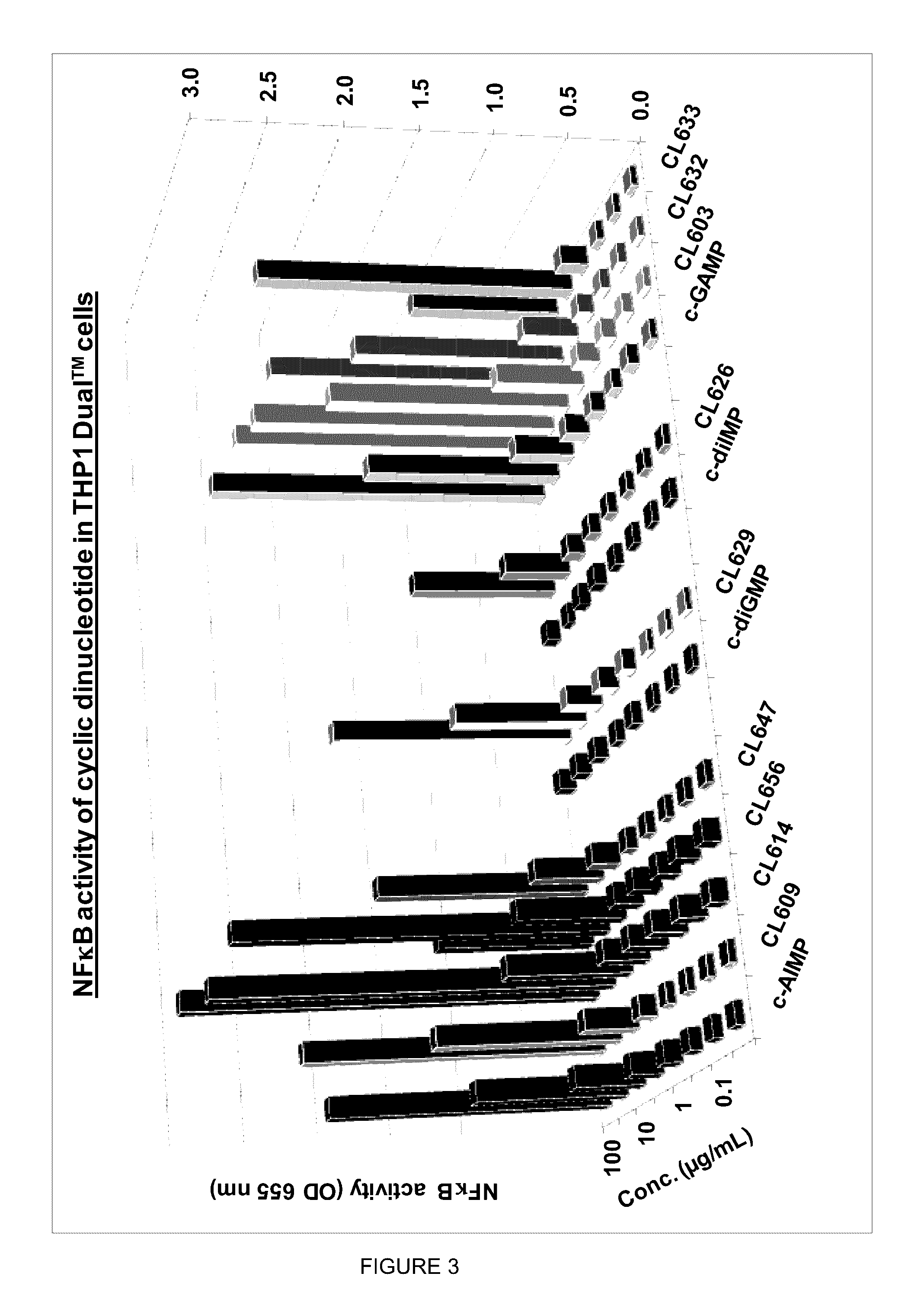
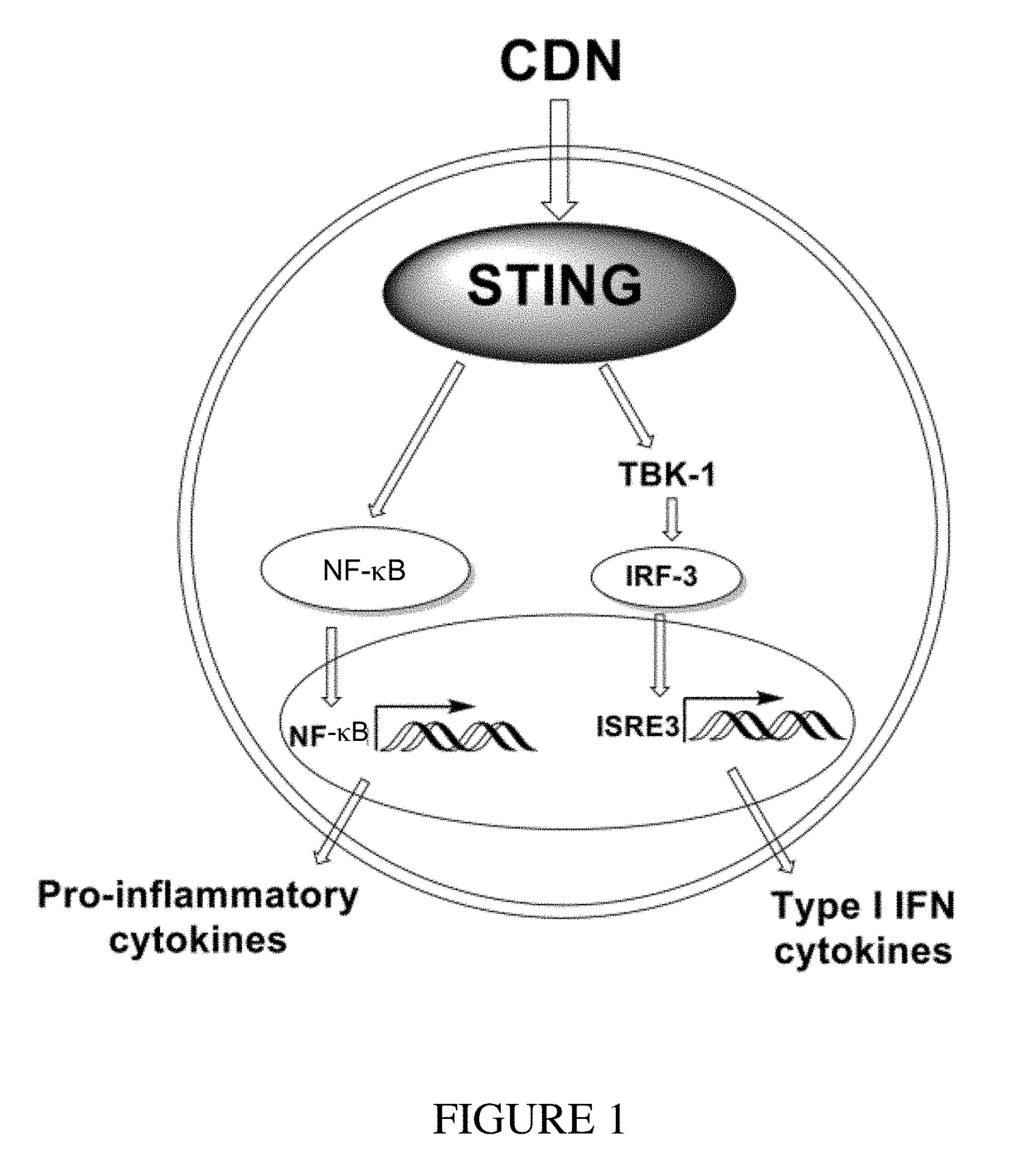
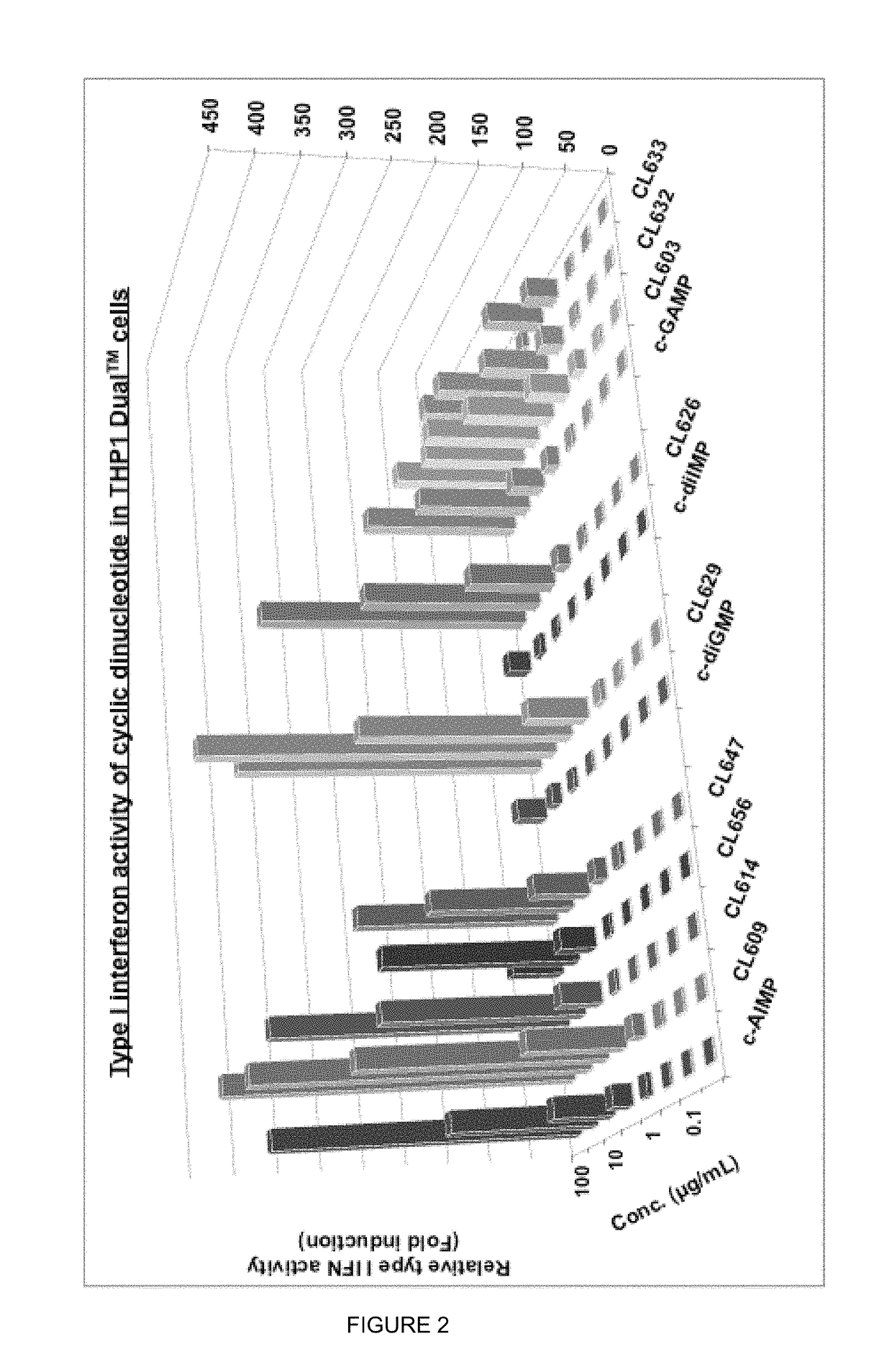
Cyclic dinucleotides for cytokine induction
ActiveUS20160362441A1Improve the immunityDelay EliminationAntibacterial agentsOrganic active ingredientsPurineIn vivo
A cyclic dinucleotide compound of Formula (I):wherein X1 is H or F; X2 is H or F; at least one among X1 and X2 is a fluorine atom; Z is OH, OR1, SH or SR1, wherein: R1 is Na or NH4, or R1 is an enzyme-labile group which provides OH or SH in vivo such as pivaloyloxymethyl; B1 and B2 are bases chosen from Adenine, Hypoxanthine or Guanine, and B1 is a different base than B2 and a pharmaceutically acceptable salt thereof. Pharmaceutical compositions including the cyclic dinucleotide, as well as their use in the treatment of a bacterial infection, a viral infection or a cancer are also described.
Owner:KAYLA THERAPEUTICS
Porcine circovirus type 2 subunit vaccine and preparation method thereof
InactiveCN101884787AImprove biological activityHighly species-specificViral antigen ingredientsVirus peptidesOpen reading frameAntigenicity
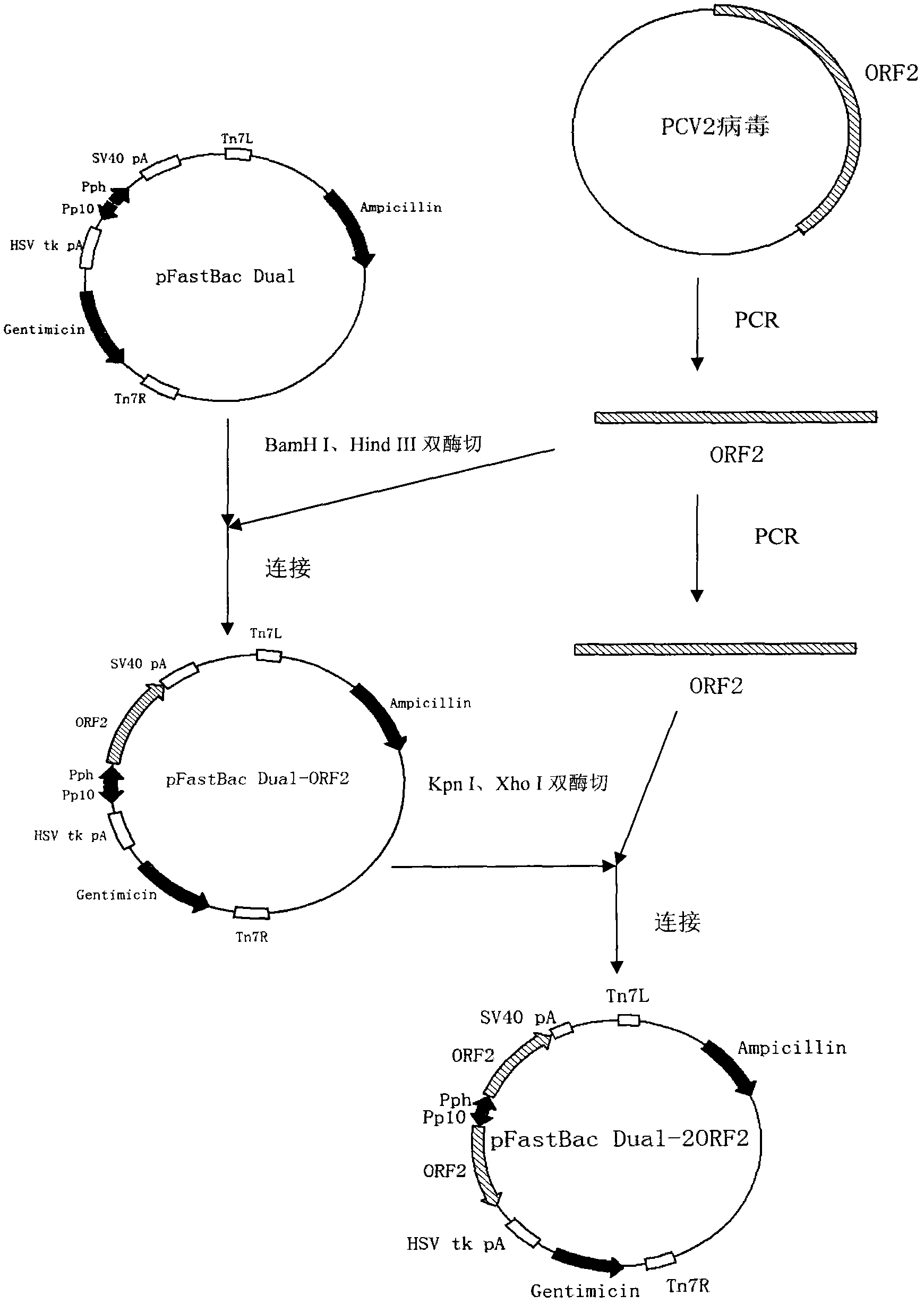
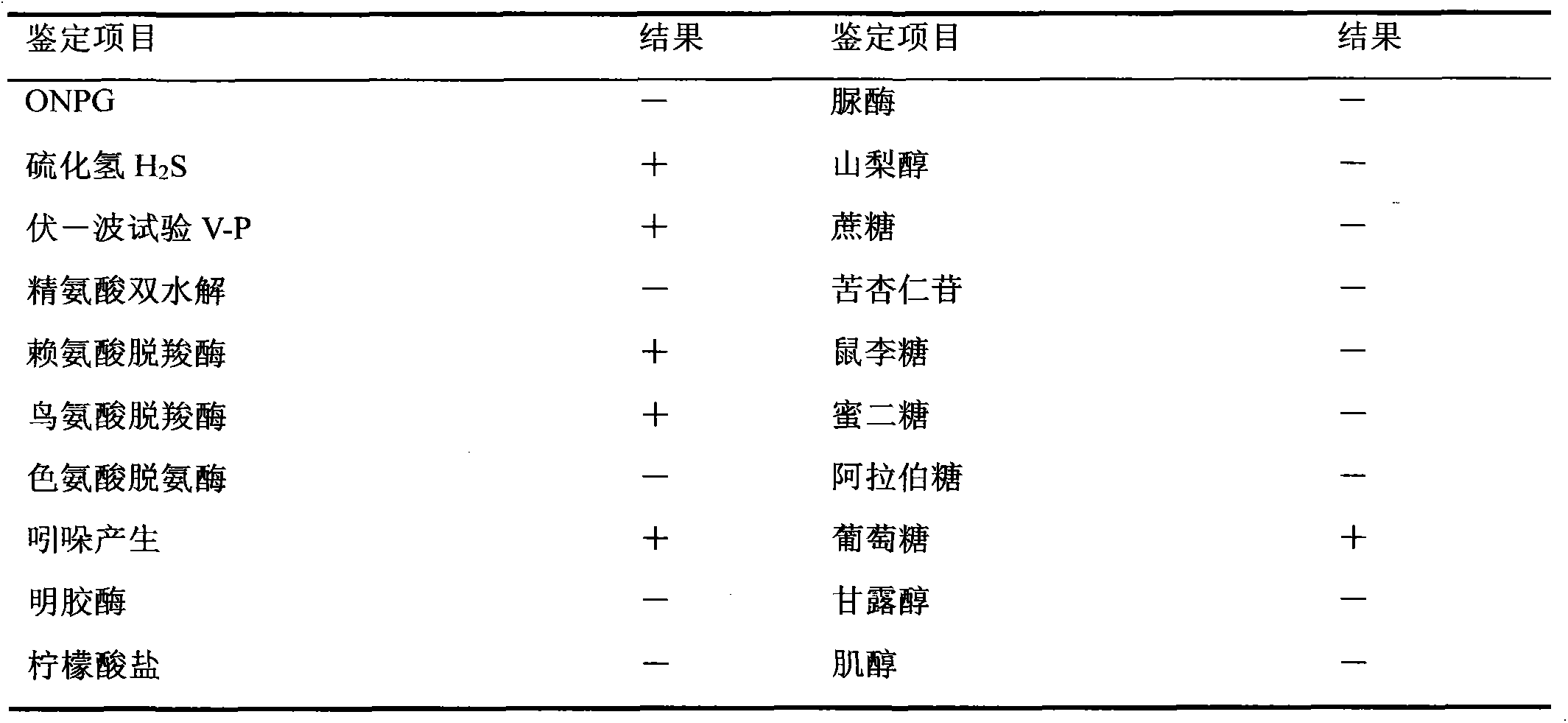
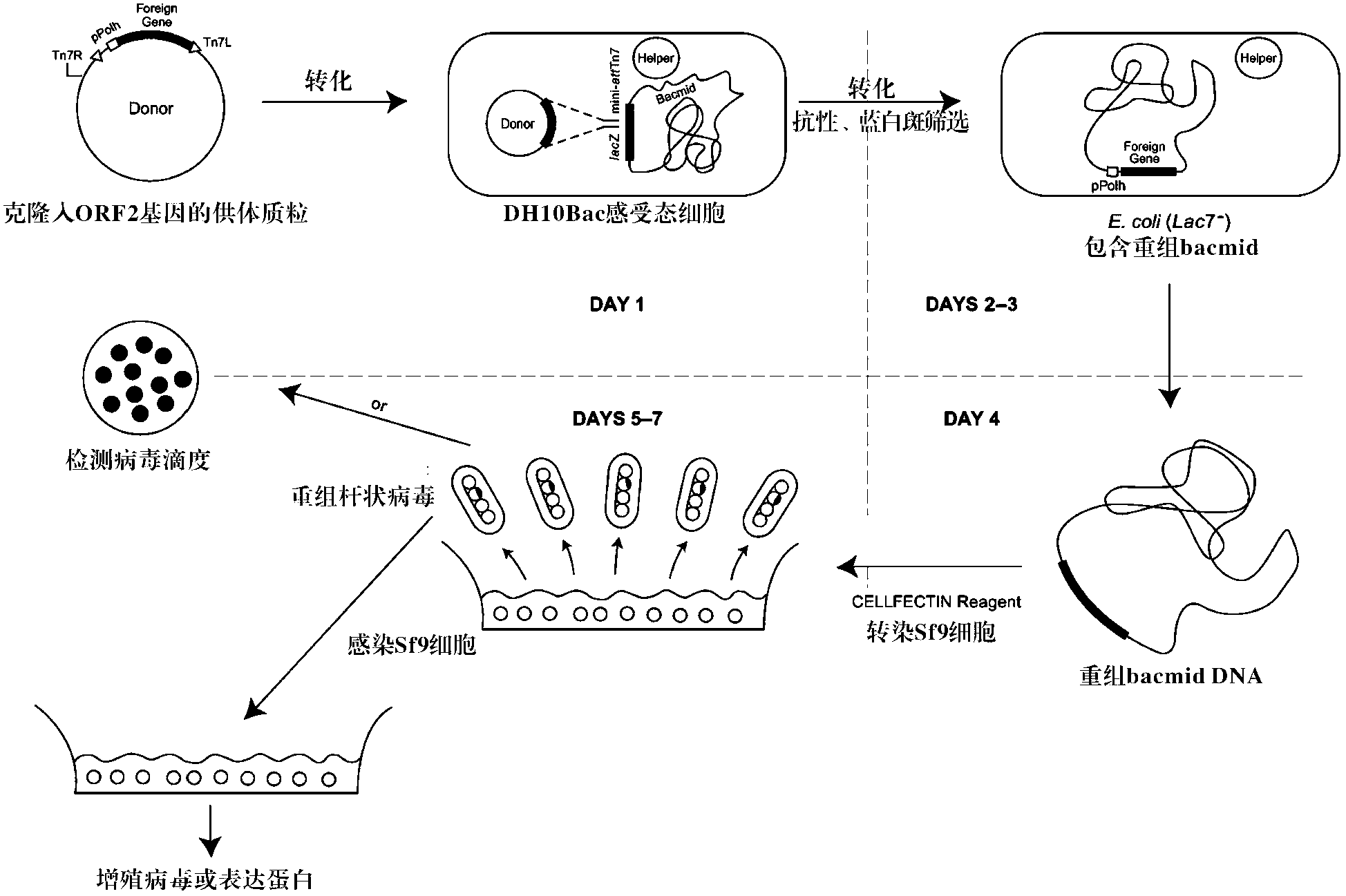
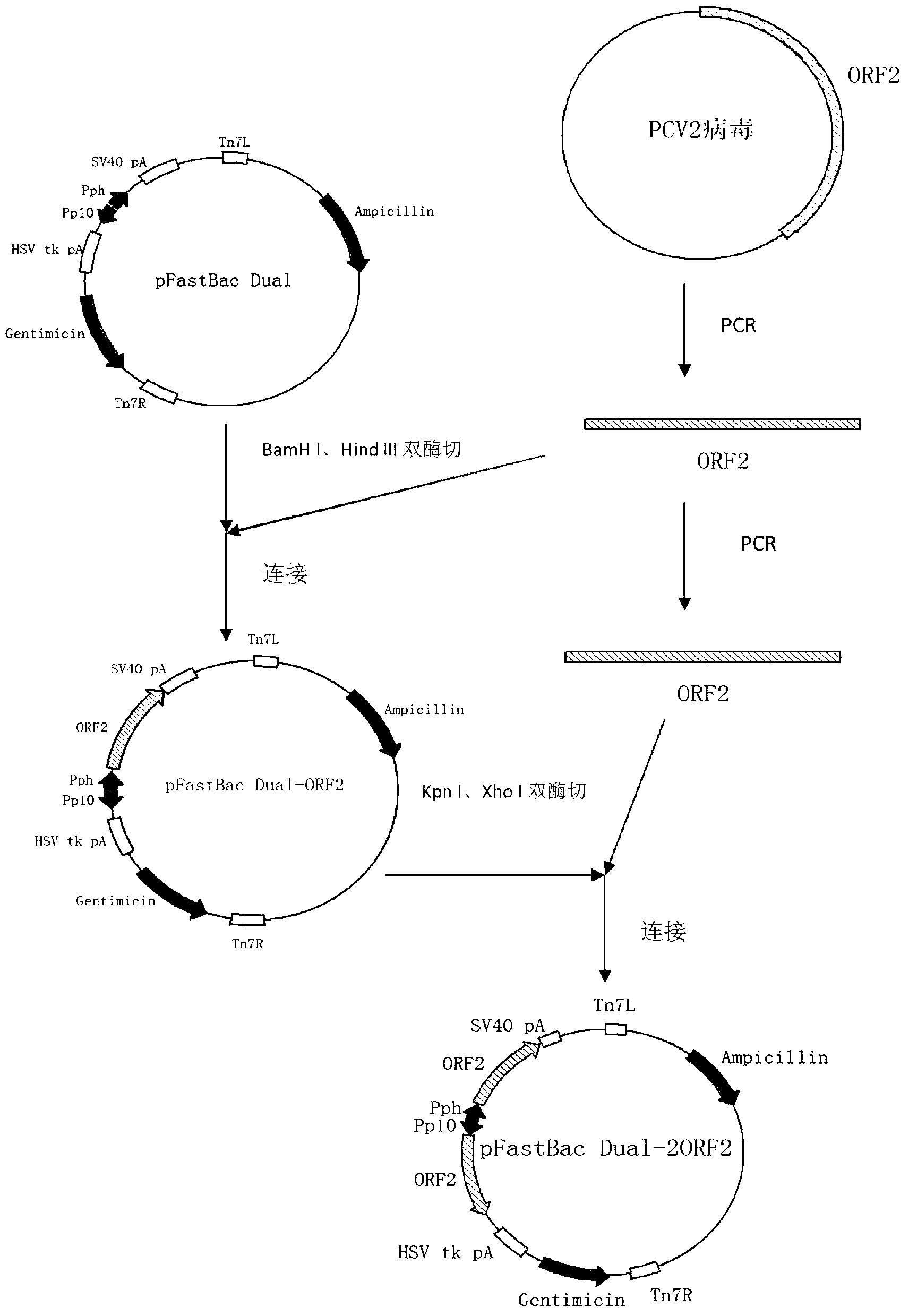
The invention mainly aims to provide a porcine circovirus type 2 (PCV2) subunit vaccine and a preparation method thereof. Particularly, a baculovirus vector expression system is utilized to express a large amount of recombinant open reading frame type 2 (ORF2) protein in insect cells, so that the subunit vaccine with good immunity effect is developed. A bac-to-bac baculovirus expression system is adopted to perform whole gene amplification on the porcine circovirus type 2 ORF2 gene, and a melittin signal peptide nucleotide sequence is introduced into a terminal 5', so that the recombinant baculovirus of an open reading frame containing the melittin signal peptide nucleotide sequence and a PVC2ORF2 gene sequence is established, wherein the infected insect cell expresses the recombinant ORF2 protein with efficient and high PCV2 antigenicity; and thus the subunit vaccine containing the PCV2 recombinant ORF2 protein is prepared. The inoculation experiments of piglets show that the subunit vaccine has a good immunity protection effect.
Owner:PU LIKE BIO ENG
Cyclic dinucleotides for cytokine induction
ActiveUS10011630B2High activityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPurineIn vivo
A cyclic dinucleotide compound of Formula (I):wherein X1 is H or F; X2 is H or F; at least one among X1 and X2 is a fluorine atom; Z is OH, OR1, SH or SR1, wherein: R1 is Na or NH4, or R1 is an enzyme-labile group which provides OH or SH in vivo such as pivaloyloxymethyl; B1 and B2 are bases chosen from Adenine, Hypoxanthine or Guanine, and B1 is a different base than B2 and a pharmaceutically acceptable salt thereof. Pharmaceutical compositions including the cyclic dinucleotide, as well as their use in the treatment of a bacterial infection, a viral infection or a cancer are also described.
Owner:KAYLA THERAPEUTICS
Nucleic acid respiratory syncytial virus vaccines
InactiveUS6083925AGood immune protectionImprove expression levelSsRNA viruses negative-senseGenetic material ingredientsHeterologousF protein
Non-replicating vectors containing a nucleotide sequence coding for an F protein of respiratory syncytial virus (RSV) and a promoter for such sequence, preferably a cytomegalovirus promoter, are described for in vivo immunization. The nucleotide sequence encloding the RSV F protein may lack a sequence encoding the homologous signal peptide but possessing a heterologous signal peptide enhancing RSV F protein expression. Such non-replicating vectors, including plasmids, also may contain a further nucleotide sequence located adjacent to the RSV F protein encoding sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such non-replicating vectors may be used to immunize a host against disease caused by infection with RSV, including a human host, by administration thereto, and may be formulated as immunogenic compositions with pharmaceutically-acceptable carriers for such purpose. Such vectors also may be used to produce antibodies for detection of RSV infection in a sample.
Owner:CONNAUGHT LAB
Gene encoding hemagglutinin protein of H5 avian influenza virus and its application
ActiveCN1632124AHigh level of immune responseImproving immunogenicityViral antigen ingredientsAntibody ingredientsHemagglutininHighly pathogenic
The present invention relates to an artificially synthesized gene optiHA containing codons for chicken partial tropism. Its reading frame contains 1707 bp nucleotides and encodes a total of 568 amino acids. The gene is compatible with H5 subtype highly pathogenic avian influenza virus A / Goose / GuangDong / 1 / 96(H5N1)[GD / 1 / 96(H5N1)]hemagglutinin (HA) gene has a nucleotide homology rate of 70%, an amino acid homology rate of 100%, and encodes the H5 subtype Hemagglutinin (HA) protein of avian influenza virus GD / 1 / 96 (H5N1). The invention also relates to the application of the gene as an immunogenic gene of H5 subtype influenza DNA vaccine and other genetic engineering vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Helicobacter pylori vaccine based on urease B subunit active segment and its prepn process
InactiveCN1887349AGood immune protectionAntibacterial agentsBacterial antigen ingredientsProtective antigenAntigen
The present invention provides one kind of genetic engineering multivalent subunit vaccine for preventing and treating human helicobacter pylori infection and its preparation process. The vaccine consists of active helicobacter pylori UreB fragment UreB414 as the central antigen component, other combined protective antigens, and intramolecular or extramolecular adjuvant. Compared with univalent vaccine, the multivalent combined vaccine can stimulate the body to generate more powerful and more comprehensive helicobacter pylori resisting specific immune reaction.
Owner:ARMY MEDICAL UNIV
Porcine circovirus type 2 recombinant cap protein and subunit vaccine
ActiveCN102174086AImprove securityNot pathogenicBacteriaViral antigen ingredientsAntigenEscherichia coli
The invention belongs to the field of molecular biology, and discloses a porcine circovirus type 2 recombinant cap protein and a subunit vaccine. The porcine circovirus type 2 cap protein expressed by recombinant Escherichia coli is obtained by steps of cloning a porcine circovirus type 2 cap protein in a nuclear localization signal area of which the N terminal is cut and which is rich in arginine into a prokaryotic expression vector to obtain a recombinant expression vector, transfecting the recombinant expression vector into Escherichia coli BL21(DE3), and expressing by using the recombinant Escherichia coli BL21(DE3). Tests prove that the constructed recombinant strain expresses a foreign protein stably. When the subunit vaccine is prepared from the expressed recombinant protein, an antigen has high purity and safety, does not have pathogenicity on animals such as pigs and the like, and passes safety evaluation easily.
Owner:NANJING AGRICULTURAL UNIVERSITY
2 type subunit vaccine for porcine circovirus as well as preparation method and application thereof
InactiveCN102517331AQuick responseHigh activityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
The invention relates to a 2 type subunit vaccine for a porcine circovirus as well as a preparation method and application thereof. A recombinant bacilliform virus contains double promoters (a polyhedrin promoter and a P10 promoter), a coding gene of a Cap protein with double copying can be expressed, and the expression efficiency of the protein is obviously enhanced; moreover, the Cap protein expressed by an inserted foreign gene does not contain an excess sequence, virus-like particles (VLPs) can be effectively formed, and the immunogenicity of an expressed protein is enhanced; furthermore, a produced antigen has high content; and according to the 2 type subunit vaccine for the porcine circovirus, which is disclosed by the invention, the productivity ratio and the quality of a viral protein of the 2 type subunit vaccine for the porcine circovirus are obviously enhanced, and a prepared vaccine composition has the advantages of stable and persistent immune effect, high safety and the like.
Owner:WUHAN CHOPPER BIOLOGY
SARS vaccine and its preparation method
InactiveCN101007168AImprove securityTo achieve the purpose of surface displayAntiviralsRespiratory disorderSurface displayCompetent cell
The invention discloses a SARS vaccine and preparing method, which comprises the following steps: 1) constructing external baculoviral surface display carrier of S protein of SARS coronary virus; 2) transmitting the carrier into susceptive cell with baculoviral genome plasmid Bacmid; obtaining recombinant baculoviral genome plasmid; 3) using the plasmid to infect insect cell; purifying the recombinant plasmid; obtaining the product.
Owner:PEKING UNIV
Foot-and-mouth disease genetic engineering mixed epitope vaccine and preparation method thereof
ActiveCN103007273AGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsGenetic engineeringPolyinosinic Acids
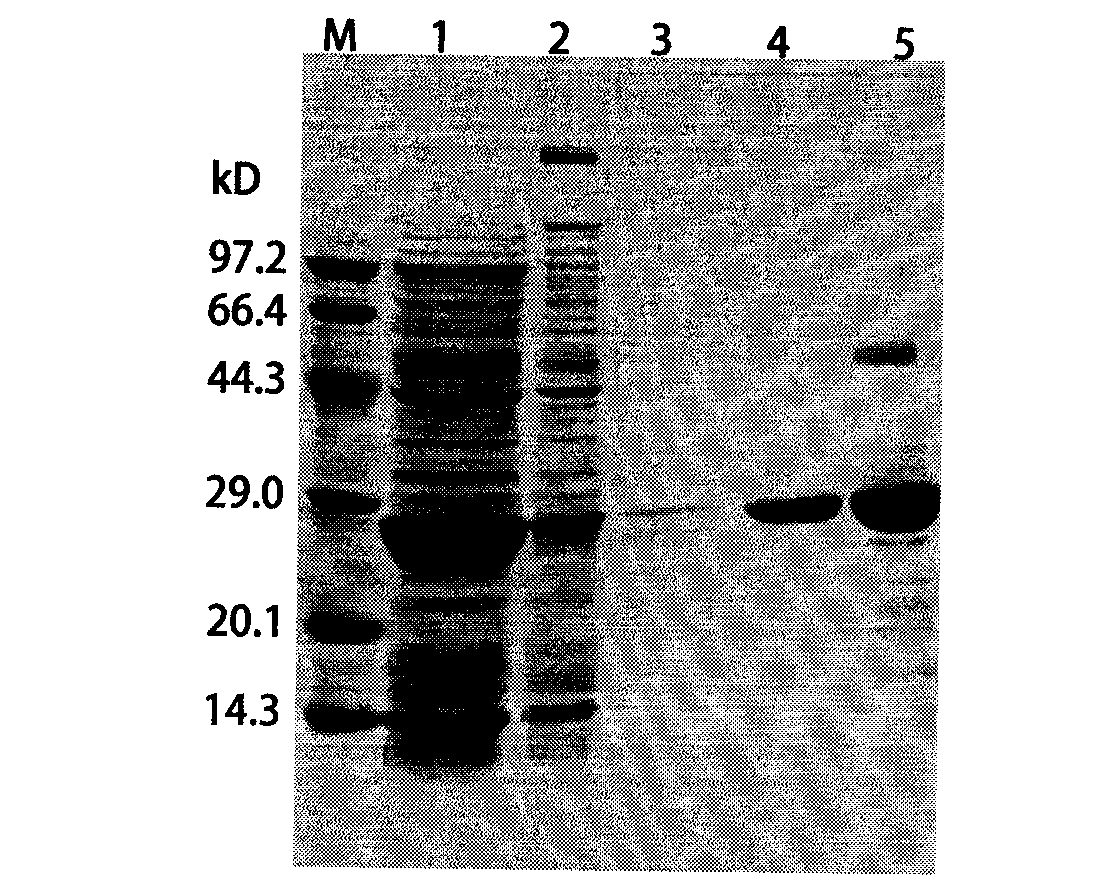
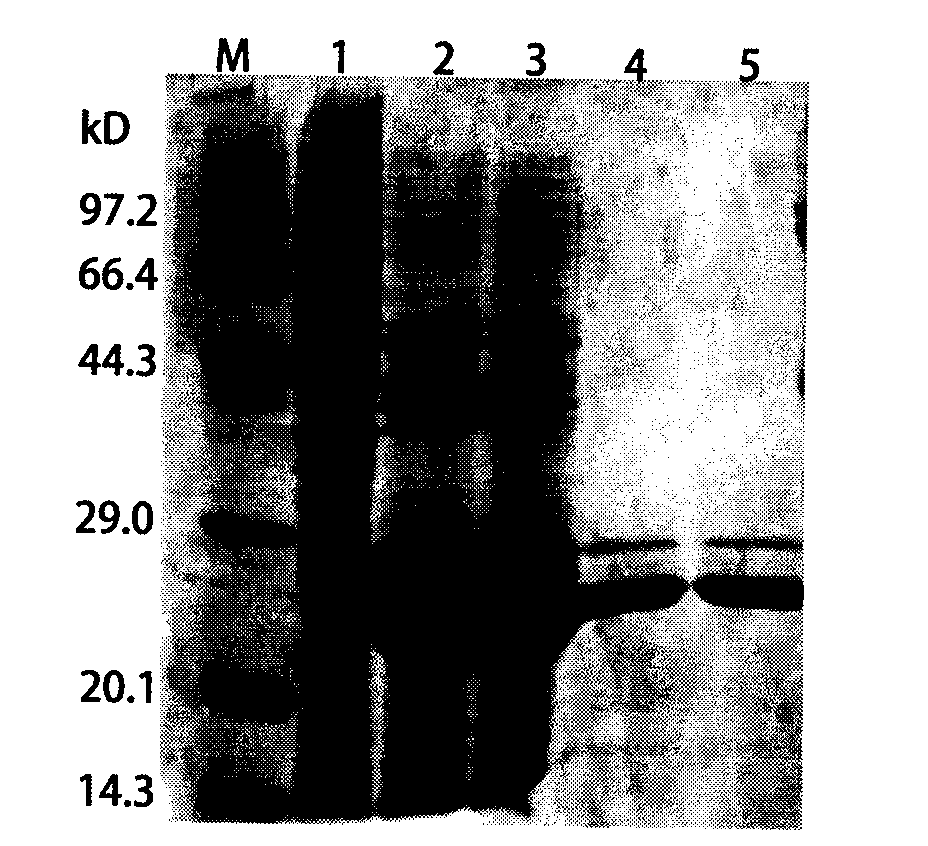
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Attenuated African swine fever virus with gene deletion and its application as a vaccine
ActiveCN110093324BGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Avian infectious bronchitis cold adaptation attenuated vaccine strain and application thereof
ActiveCN102220287AGood protective effectGood securityInactivation/attenuationMicroorganism based processesCombined VaccinesCold adaptation
The invention discloses an avian infectious bronchitis cold adaptation attenuated vaccine strain and application thereof. In the invention, an avian infectious bronchitis virus wild strong strain is subject to continuous low-temperature cold adaption passage attenuated culture so as to obtain a cold adaption weak virus vaccine strain with good immune protective efficiency, and the microbial preservation number is CGMCC No.4711. Immune efficiency evaluation experiments show that the cold adaptation attenuated vaccine strain disclosed by the invention has good immune protectiveness, the active immune protection rate is over 80%, the passive immune can get over 90% protection within 9 days, and the cold adaptation attenuated vaccine strain has good protective efficiency to avian infectious bronchitis, and can effectively protect chicken against the attack of the virulence of avian infectious bronchitis viruses. Safety experiments show that the cold adaption weak virus live vaccine disclosed by the invention is safe for chickens and layer chickens, has no side effects and has good safety. The cold adaption attenuated vaccine strain can be applied in the preparation of single vaccine or combined vaccine and the like for preventing the avian infectious bronchitis viruses.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Nucleic acid respiratory syncytial virus vaccines
InactiveUS6486135B1Good immune protectionImprove expression levelSsRNA viruses negative-senseGenetic material ingredientsHeterologousF protein
Non-replicating vectors containing a nucleotide sequence coding for an F protein of respiratory syncytial virus (RSV) and a promoter for such sequence, preferably a cytomegalovirus promoter, are described for in vivo immunization. The nucleotide sequence encloding the RSV F protein may lack a sequence encoding the homologous signal peptide but possessing a heterologous signal peptide enhancing RSV F protein expression. Such non-replicating vectors, including plasmids, also may contain a further nucleotide sequence located adjacent to the RSV F protein encoding sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such non-replicating vectors may be used to immunize a host against disease caused by infection with RSV, including a human host, by administration thereto, and may be formulated as immunogenic compositions with pharmaceutically-acceptable carriers for such purpose. Such vectors also may be used to produce antibodies for detection of RSV infection in a sample.
Owner:AVENTIS PASTEUR LTD
Recombinant protein coded by grass carp reovirus (GCRV) type-II S10 gene, polyclonal antibody prepared from recombinant protein and application of recombinant protein
InactiveCN103539842AImproving immunogenicityGood immune protectionViral antigen ingredientsVirus peptidesNucleotideStructural protein
The invention discloses a recombinant protein coded by a grass carp reovirus (GCRV) type-II S10 gene, a polyclonal antibody prepared from the recombinant protein and an application of the recombinant protein. The amino acid sequence of the GCRV type-II S10 gene-coded protein is shown by SEQ ID No:2, and the nucleotide sequence coding the protein is shown by SEQ ID No:1. The recombinant protein disclosed by the invention has good immunogenicity; compared with the proteins of other structures of GCRV, the specific antibody valence generated by inducing an immune animal is higher. Further tests indicate that by immunizing the grass carp with the S10 recombinant protein, the grass carp can be induced to generate high specific antibody, and certain immune protection effect can be realized against the attack of a virulent strain of GCRV. Therefore, the study and application of the GCRV type-II S10 gene-coded protein are of vitally important significance to the development of a novel vaccine for the hemorrhagic disease of grass carp and an immunological detection kit, and a new effective solution is provided for the hemorrhagic disease of grass carp.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Method for producing pseudorabies living vaccines by using subculture cell source and product thereof
ActiveCN101695573AImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesPig kidneyAntibiotic Y
The invention provides a method for producing pseudorabies living vaccines by using a subculture cell source and a pseudorabies living vaccine product thereof. The method comprises the following steps: culturing pseudorabies virus low-virulent strains by using subculture cells; harvesting the strains to obtain cell culture venom; and then adding a stabilizing agent and an antibiotic into the cellculture venom, and freezing and vacuum-drying the mixture to obtain the pseudorabies living vaccines of the subculture cell source. The subculture cells are subculture cells ST of pig testicle or subculture cells PK15 or IBRS-2 of pig kidney. The method for producing the pseudorabies living vaccines by using the subculture cell source has the advantages of simple and stable production process, easy operation, high virus content, little batch difference and controllable quality, can remarkably improve the yield and quality of the vaccines and reduce the anaphylactic reaction and the like. The pseudorabies living vaccines obtained by using the production method of the invention have good safety and high immune efficacy, and have better immune protection effect on pseudorabies virulent attack.
Owner:广东永顺生物制药股份有限公司
Application of brucellosis A19 molecular marking vaccine and immunological identification thereof
InactiveCN102772794ASolve difficult to identifyGood immune protectionAntibacterial agentsBacterial antigen ingredientsMarker vaccineVirus
The invention relates to an application of brucellosis A19 molecular marking vaccine and immunological identification of the brucellosis A19 molecular marking vaccine. The brucellosis vaccine can be applied to an enzyme-linked immunosordent assay (iELISA) method to distinguish an animal inoculated with the vaccine from a wild virus infected animal. According to the invention, a cattle is used as a test target animal antiepidemic to the brucellosis, the cattle is immune to brucellosis A19-detlaVirB12 marking vaccine so as to evaluate the immunological protection of the molecular marking vaccine. An iELISA method for distinguishing the brucellosis A19-detlaVirB12 immune animal from the wild virus infected animal is created. The brucellosis A19-detlaVirB12 marking vaccine provided by the invention not only has good immunological protection against the cattle brucellosis, and the iELISA method provided by the invention solves the problem that the immune animal is difficult to be distinguished from the clinic diseased animal, and the brucellosis A19 molecular marking vaccine has an actual application value in prevention and control, elimination and purification of the cattle brucellosis.
Owner:新疆维吾尔自治区畜牧科学院兽医研究所
Largemouth bass iridovirus disease inactivated vaccine and preparation method thereof
ActiveCN111135295AReduce manufacturing costSimple processViral antigen ingredientsAntiviralsDiseaseRanavirus
The invention belongs to the technical field of biological medicines for veterinary use and in particular relates to a largemouth bass iridovirus disease inactivated vaccine and a preparation method thereof. The vaccine comprises EPC (epithelioma papulosum cyprinid) cells and largemouth bass ranavirus LMBV. The preparation method comprises the following steps: culturing EPC cells; performing LMBVvirus amplification on the cultured EPC cells so as to obtain an LMBV virus liquid; and performing inactivation treatment on the LMBV virus liquid. The vaccine prepared by the invention is good in immune protection effect and can be applied to prophylactic immunization of largemouth bass iridovirus, and the survival rate and the breeding benefits of largemouth bass can be increased.
Owner:ZHEJIANG INST OF FRESH WATER FISHERIES
Velogenic Edwardsiella tarda vaccine strain and application thereof
ActiveCN103255089AStrong drug resistanceReduce lossesAntibacterial agentsBacterial antigen ingredientsBacteroidesProtective antigen
The invention relates to an Edwardsiella tarda strain and an application method thereof. The Edwardsiella tarda strain is separated from a turbot adult fish body and is a wild strain with strong virulence, and the preservation number of the Edwardsiella tarda strain is CGMCC No.7197. Preparation modes of an antigen of the Edwardsiella tarda strain comprise any one or more than one of an inactivated thallus, a bacteruak ghost ingredient, an attenuated strain, a protective antigen, an antigen subunit and an expression product of an antigen determinant or an antigen gene expression carrier; the produced vaccine can be a single ingredient of the antigen prepared by utilizing the Edwardsiella tarda strain and can also be a combined vaccine produced by mixing the antigen prepared by utilizing the Edwardsiella tarda strain with antigens of other bacteria, and the prepared single or combined vaccine antigen is added with an adjuvant to produce the vaccine; and an inoculation mode of the vaccine in immunization application can adopt injection immunization, wound immunization, immersion bath immunization or oral administration immunization.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Porcine circovirus type 2 subunit vaccine, and preparation method and application thereof
ActiveCN102925486AImprove expression efficiencyImproving immunogenicityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
The invention relates to a porcine circovirus type 2 subunit vaccine, and a preparation method and an application thereof. The recombinant baculovirus contains double promoters (a polyhedrin protein promoter and a P10 promoter), and can express double copies of Cap protein coding genes, such that protein expression efficiency is substantially improved. Also, Cap protein expressed by an inserted exogenous gene does not contain excess sequences, such that virus-like particles (VLPs) can be effectively formed, expressed protein immunogenicity is improved, and the content of produced antigen is high. According to the porcine circovirus type 2 subunit vaccine provided by the invention, protein yield and quality of porcine circovirus type 2 subunit vaccine are substantially improved, and prepared vaccine compositions have the advantages of stable and long-lasting immune effect, high safety, and the like.
Owner:WUHAN CHOPPER BIOLOGY
Method for producing pseudorabies attenuated vaccine by using bioreactor and pseudorabies attenuated vaccine product
ActiveCN101695572AImprove immune efficiencyIncrease growth densityMicroorganism based processesAntiviralsVaccine ProductionAntibiotic Y
The invention provides a method for producing a pseudorabies attenuated vaccine by using a bioreactor and a pseudorabies attenuated vaccine product. After being sterilized, the bioreactor and a micro carrier are inoculated with cells for producing the vaccine, and a cell growth medium is added for culture. A maintenance medium containing attenuated strains of pseudorabies viruses are inoculated into the bioreactor to continue culturing the cells. 2 to 3 days after virus inoculation, cell culture virus liquid is obtained and added with a stabilizer and antibiotics, and the cell culture virus liquid is refrigerated and dried under vacuum to obtain the pseudorabies attenuated vaccine. In the method, the cell density and virus concentration are improved greatly, the titer of the vaccine is improved, the side reactions, labor intensity and product cost are reduced, the monitoring performance of vaccine production is improved and uniform and stable product quality is guaranteed. The pseudorabies attenuated vaccine produced by the method has high safety, immune efficacy and good immune and protective effect against the attack by the virulent pseudorabies viruses.
Owner:广东永顺生物制药股份有限公司
Porcine circovirus-like particle, and vaccine and preparation method thereof
InactiveCN103436499ASafe infectionEffective infectionViral antigen ingredientsInactivation/attenuationAdjuvantStructural protein
The invention discloses a porcine circovirus-like particle, a vaccine and a preparation method thereof. The virus-like particle is composed of a main structural protein-nucleocapsid protein of 2-type porcine circovirus, can excite cell and humoral immune response, can be used as a virus-like particle vaccine (VLP vaccine) to immune different fauna and can safely and effectively prevent PCV-2 infections after being used. The VLP can be made into injections, nose drops and drinking preparations by adding adjuvants or not. An ideal vaccine is provided for security of different populations of sows, piglets, fattening pigs and for effective immune prevention and control of PCV-2 infections.
Owner:CHONGQING AULEON BIOLOGICALS
Preparation method and application of recombinant cold-adaptation attenuated influenza vaccine strain
InactiveCN105886529ARapid reorganizationControllable positioningSsRNA viruses negative-senseViral antigen ingredientsHemagglutininImmune effects
The invention discloses a preparation method and an application of a recombinant cold-adaptation attenuated influenza vaccine strain. The method comprises the following steps: importing an influenza virus hemagglutinin HA gene, an influenza virus neuraminidase NA gene as well as seven genes, namely PB2, PB1, PA, NP, M, NS1 and NS2, in an influenza virus to a host cell, and conducting cultivating, so that a recombinant virus is obtained. Tests prove that an attenuated influenza tetravalent vaccine, which is prepared by virtue of a 7+2 plasmid rescuing system, can achieve an excellent immune effect through nasal immunization; the influenza virus, which is recombined by the 7+2 plasmid rescuing system, has a property of being time-efficient; in accordance with the epidemic characteristics of influenza viruses in various years, effective targeted influenza vaccines can be prepared; and the preparation method is rapid, obvious in immunogenicity and immune protection effect, and good in safety; therefore, the problems on safety and stability of attenuated influenza vaccines which are prepared by virtue of a conventional method are solved, and a protection spectrum of the influenza vaccines is more comprehensive.
Owner:CHANGCHUN HAIJIYA BIOTECH CO LTD
Antibiotic-free piglet feed and preparation method thereof
InactiveCN106721021AKeep the original scentImprove digestibilityFood processingAnimal feeding stuffDiseaseDiarrhea
The invention discloses an antibiotic-free piglet feed and a preparation method thereof, and belongs to the fields of formula design and processing technologies of feeds. The feed is mainly prepared from, by weight, 50-60 parts of corn, 2-4 parts of steamed fish meal, 10-16 parts of soybean meal, 1-5 parts of puffed soybeans, 3-5 parts of wheat flour, 3-6 parts of whey powder, 0.5-1 part of coconut oil, 0.5-1 part of soybean oil, 0.02-0.03 parts of antibacterial peptides, 1-3 parts of probiotics, 0.012 parts of Yucca smalliana Fern. extract, 0.3-0.7 parts of stone flour, 0.3-0.5 parts of edible salt, 0.8-1.2 parts of calcium hydrogen phosphate, 0.3-0.8 parts of amino acids, 0.03-0.05 parts of compound enzyme, 0.3-0.4 parts of composite vitamins and 0.2-0.6 parts of composite trace elements. The feed meets antibiotic-free piglet daily ration nutrient standards, adopts the antibacterial peptides to substitute antibiotics, and comprehensively solves problems through adding the probiotics, the daily gain is increased by 6.17%, the average daily feed intake is increased by 5.38%, and the diarrhea rate is reduced by 8.72%. The feed makes the intestinal tracts of piglets have good health state, has high disease resistance, and lays an early foundation for early slaughtering, no medication and safe pork products.
Owner:长春禾丰饲料有限责任公司
Adjuvant for improving immunization effect of Edwardsiella vaccine and use method of adjuvant
ActiveCN102988981AGood immune protectionSafe to useImmunological disordersAntibody medical ingredientsProtective antigenAdjuvant
The invention relates to an adjuvant for improving the immunization effect of Edwardsiella vaccine and a use method of the adjuvant. The adjuvant is characterized by being extracted from raw materials including grain, yeast and a part of fungi or algae. The effective component of the adjuvant is beta-1,3-glucan or the natural, artificially modified or synthetic product of the beta-1,3-glucan. The adjuvant can be used for increasing the specific immune protection ratio of any one or more inculcated Edwardsiella vaccine antigens including the inactivated bacteria, the bacteria disintegration component, the less-virulent strain, the attenuated strain, the protective antigen, the antigen subunit, the antigenic determinant clusters or the expression product of the antigen cell expression vector of the Edwardsiella, can be used together or not together with the vaccine antigen and can be prepared into a single preparation which is used together with the vaccine antigen.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Mutant strain of Brucella bacterin with weak poison, constructing method, and application
InactiveCN101092605AGood immune protectionNo increase in virulenceAntibacterial agentsBacterial antigen ingredientsAntigenMutant strain
This invention discloses attenuated Brucella mutant, its construction method and its application. The attenuated Brucella mutant is constructed by deleting bp26, wboA, omp31, and P39 or pgm gene from attenuated Brucella. It is proven by experiments that attenuated Brucella mutant has good immune protection effects and the same virulence as attenuated Brucella. Because certain antigen is delected in the attenuated Brucella mutant, vaccine immunity and natural infection can be differentiated according to the immunobiology of the deleted gene. The attenuated Brucella mutant can be used to manufacture preventative Brucella vaccine and marked attenuated vaccine, and prevent epidemic diseases caused by Brucella.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Vaccine composition for resisting swine fever virus and porcine circovirus 2 infection, and preparation and application thereof
ActiveCN103623403AGood immune protectionSimple production processViral antigen ingredientsAntiviralsTotivirusVaccine Production
The invention relates to a bivalent vaccine composition for resisting swine fever virus and porcine circovirus 2 infection, and preparation and application thereof. According to the method, a porcine circovirus 2 subunit antigen and a swine fever virus antigen are mixed and lyophilized to prepare the bivalent vaccine composition for resisting swine fever virus and porcine circovirus 2 infection; or a porcine circovirus 2 totivirus antigen inactivated vaccine is used as a diluent to dilute a swine fever virus vaccine, so as to prepare a bivalent vaccine composition kit for resisting swine fever virus and porcine circovirus 2 infection. The bivalent vaccine composition does not produce immunity interference; after immunization, the swine fever virus antigen speeds up in immunoreaction; the protective antibodies provided by the vaccine composition reach an immunization protective dose one week earlier than a single vaccine antibody; and the antibodies produced by the porcine circovirus vaccine in the bivalent vaccine have similar effects as that produced by single vaccine immune. The method has the advantages of simple vaccine production process and high production efficiency. The animal experiments prove that the effect of the vaccine composition is better that that of single vaccine immune, therefore the invention has great application value.
Owner:PU LIKE BIO ENG
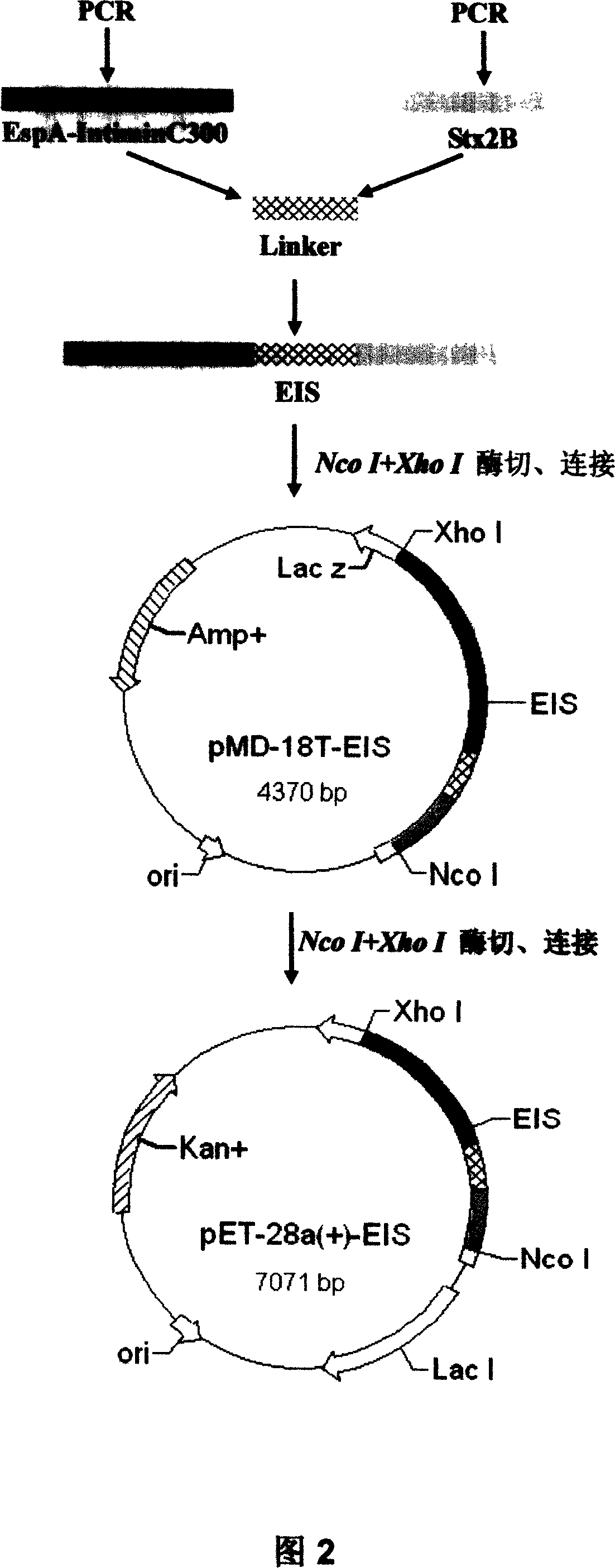
Genetic engineering vaccine of enterohemorrhagic escherichia coli 0157:H7 and the preparing method thereof
InactiveCN101062410AGood immune protectionEasy to separate and purifyAntibacterial agentsAntibody medical ingredientsHigh densityProtein molecules
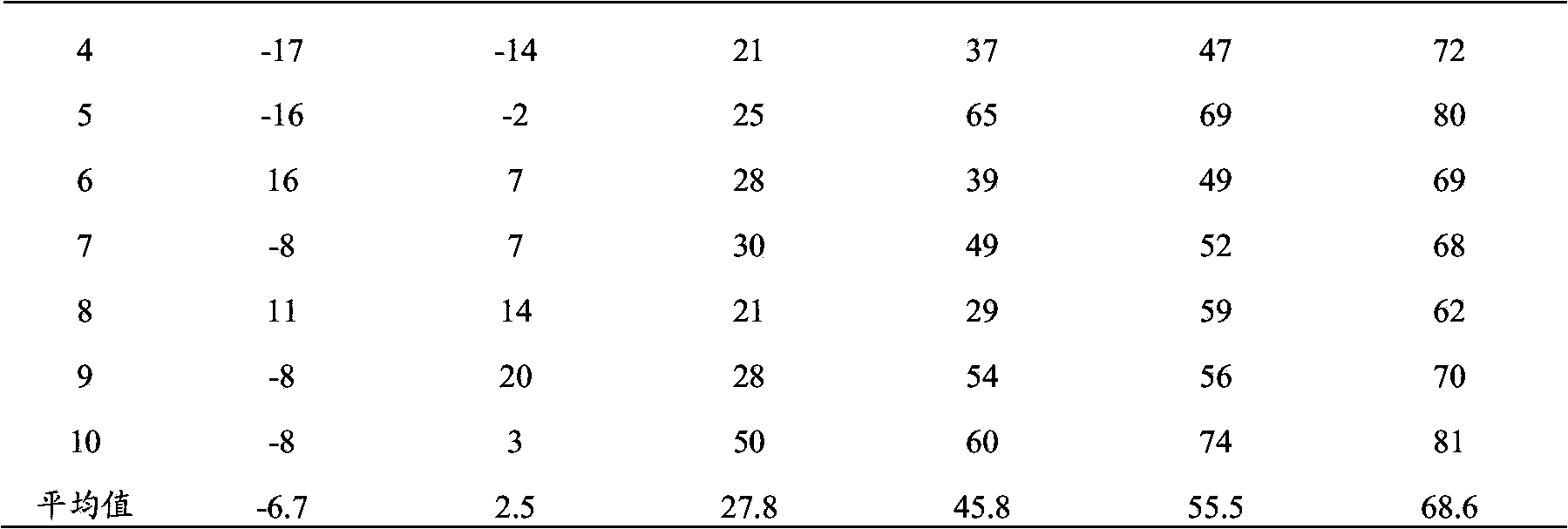
The invention discloses a poly valency fuse type enterorrhagia property bacillus coli O157:H7 gene engineering vaccine, which comprises the following steps: adopting enterorrhagia property bacillus coli O157:H7 Vi antigen Shiga's toxin II; combining subunit, compact sticking element and III type secretory protein A; constructing fuse engineering bacteria through gene retooling method; proceeding high density ferment; proceeding a series of purity course; getting fuse protein molecule vaccine with high purity. This invention possesses simple craft, good repeatability and higher protein purity.
Owner:ARMY MEDICAL UNIV
Pig viral infectious disease gene recombined live vaccine using canine II type adenovirus as carrier and preparation process thereof
InactiveCN1827172AGood genetic stabilityEasy to storePowder deliveryGenetic material ingredientsAntigenVp4 gene
This invention supplies a series of production techniques of gene recombination live vaccine of swine virus contagion with canine ó� adenovirus as carrier and finished goods. The viral live vectors vaccine takes swine important virus zymad protective antigens gene as object gene, which are chosen from HCV-E1, E2 gene, FMDV-VP1íóVP2íóVP3íóVP4 gene, TGEV-SíóNíóM gene, PEDV-SíóNíóM geneú¼SIV-HAíóNA geneú¼RV-GíóN gene, etc. The produced vaccines contain recombined swine influenza virus HA gene adenovirus carrier live vaccine, swine plague virus E2 gene adenovirus carrier live vaccine and recombination swine AsiaI foot-and-mouth disease virus VPI gene adenovirus carrier live vaccine. Recombination virus has good inheritance stability, and vaccine immunization can induct pig develop differential antiviral neutralization antibody. It has good immune protection effect and has no toxic side effect. The goods are facilitating for preserve and transportú”it has long storage life and simple technics, and it fits for commercial manufacture.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com