Helicobacter pylori vaccine based on urease B subunit active segment and its prepn process
A technology of Helicobacter pylori and active fragments, applied in the field of genetic engineering multivalent subunit vaccines and its preparation, can solve the problems of fusion protein refolding difficulty, fusion protein expression rate low, fusion protein purification difficulty, etc., and achieve good immune protection The effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Screening of Helicobacter pylori urease B active fragments
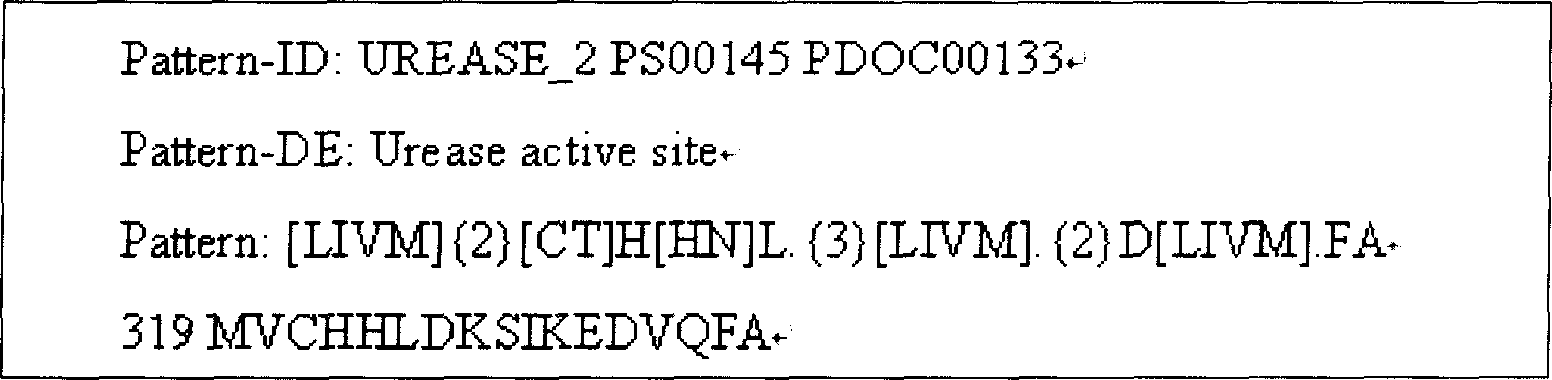
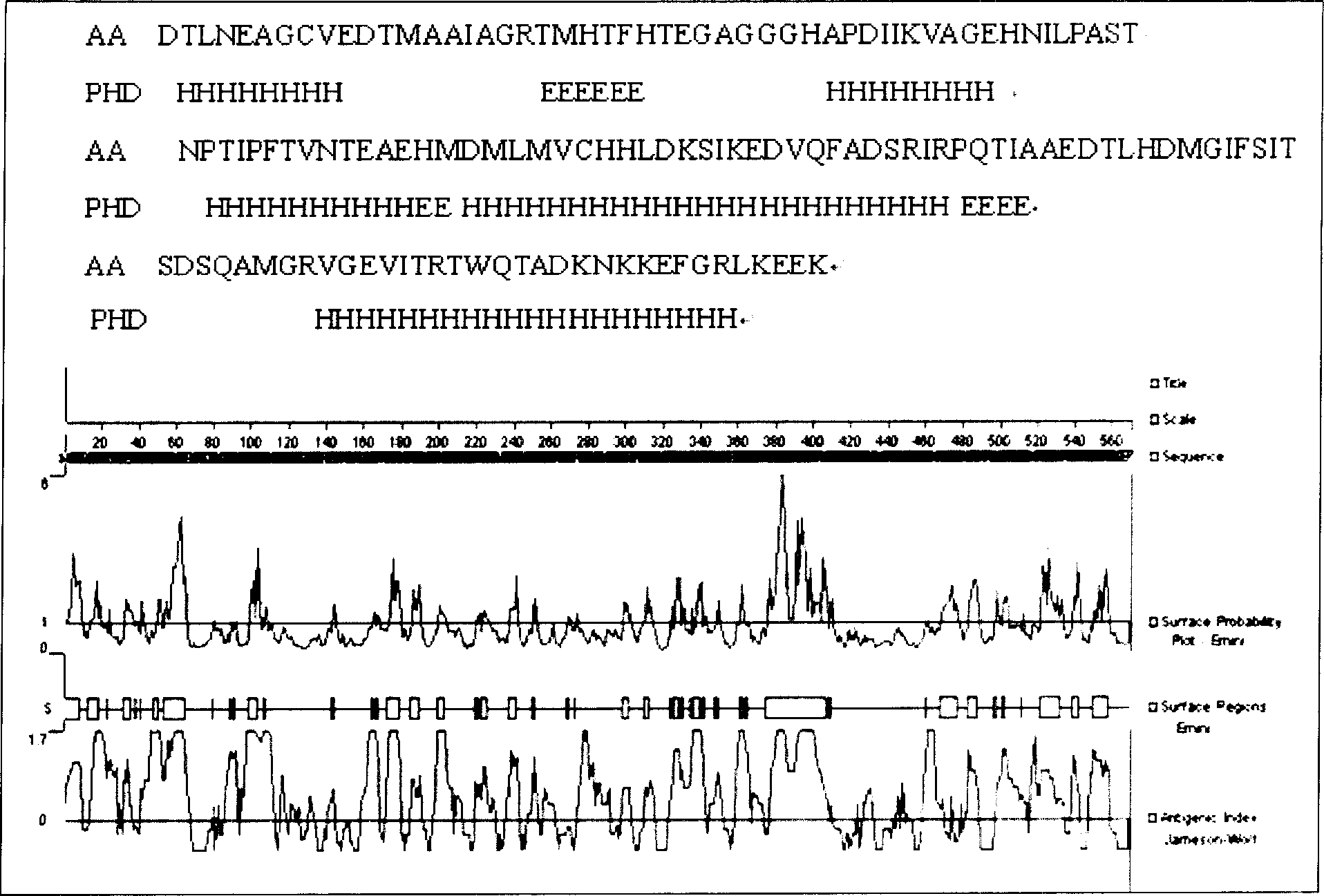
[0052] Using the http: / / cubic.bioc.columbia.edu / predictprotein protein online tool, the functional region of UreB protein and the secondary structure such as hydrophilicity, surface probability, and antigenicity of the full sequence protein were analyzed. Combined with the tertiary conformation of UreB protein X-ray crystallography, select an active fragment with a relatively stable structure and a functionally active domain, that is, the 138 amino acid sequence from the 251st amino acid to the 389th amino acid at the 5' end of the UreB protein (named UreB414) , to replace the full-length UreB protein. The nucleotide sequence of UreB414 shown in SEQ ID NO:1, and the protein structure is the amino acid sequence of UreB414 shown in SEQ ID NO:2.
Embodiment 2
[0053] Example 2 Preparation of recombinant antigenic protein, recombinant adjuvant protein and recombinant fusion protein
[0054] 1 Cloning the coding genes of HspA, HpaA, UreB414 and LTB of Helicobacter pylori according to conventional methods.
[0055] 2 Construct UreB414-LTB, HspA-UreB414, HpaA-UreB414, HspA-UreB414-LTB, HpaA-UreB414-LTB, and HspA-HpaA-UreB414 fusion genes respectively according to the following steps.
[0056] a) The fusion genes of UreB414-LTB, HspA-UreB414, HpaA-UreB414, HspA-UreB414-LTB, HpaA-UreB414-LTB, and HspA-HpaA-UreB414 were obtained by the overlap extension method, and a linker sequence was introduced between each gene.
[0057] b) PCR amplification products were separated by agarose gel electrophoresis.
[0058] c) Cloning the recovery products of the fusion genes of UreB414-LTB, HspA-UreB414, HpaA-UreB414, HspA-UreB414-LTB, HpaA-UreB414-LTB, and HspA-HpaA-UreB414 into prokaryotic cell expression vectors to obtain the above-mentioned Recomb...
Embodiment 3
[0065] Example 3 The preparation method of multivalent combination vaccine
[0066] Method 1: Physically mix recombinant UreB414 protein, at least one recombinant antigen protein (recombinant HspA, recombinant HpaA, recombinant Vac, recombinant CagA, recombinant NAP and recombinant Catalase), and recombinant adjuvant protein LTB or CTB in an appropriate proportion , to prepare the Hp multivalent combination vaccine.
[0067] Method 2: Link the nucleic acid sequence of UreB414 with the nucleic acid sequence of at least one recombinant antigen (HspA, HpaA, Vac, CagA, NAP, and Catalase) at the gene level in different combinations to construct a polynucleotide that expresses a combination of different antigens. A recombinant fusion protein, and on this basis, it is physically mixed with LTB or CTB and other adjuvant components according to an appropriate ratio to prepare genetically engineered recombinant multivalent fusion protein vaccines with different extramolecular adjuvants....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com