Genetic engineering vaccine of enterohemorrhagic escherichia coli 0157:H7 and the preparing method thereof
A genetically engineered vaccine, Escherichia coli technology, applied in antibacterial drugs, pharmaceutical formulations, medical preparations containing active ingredients, etc. Easy separation and purification, good immune protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
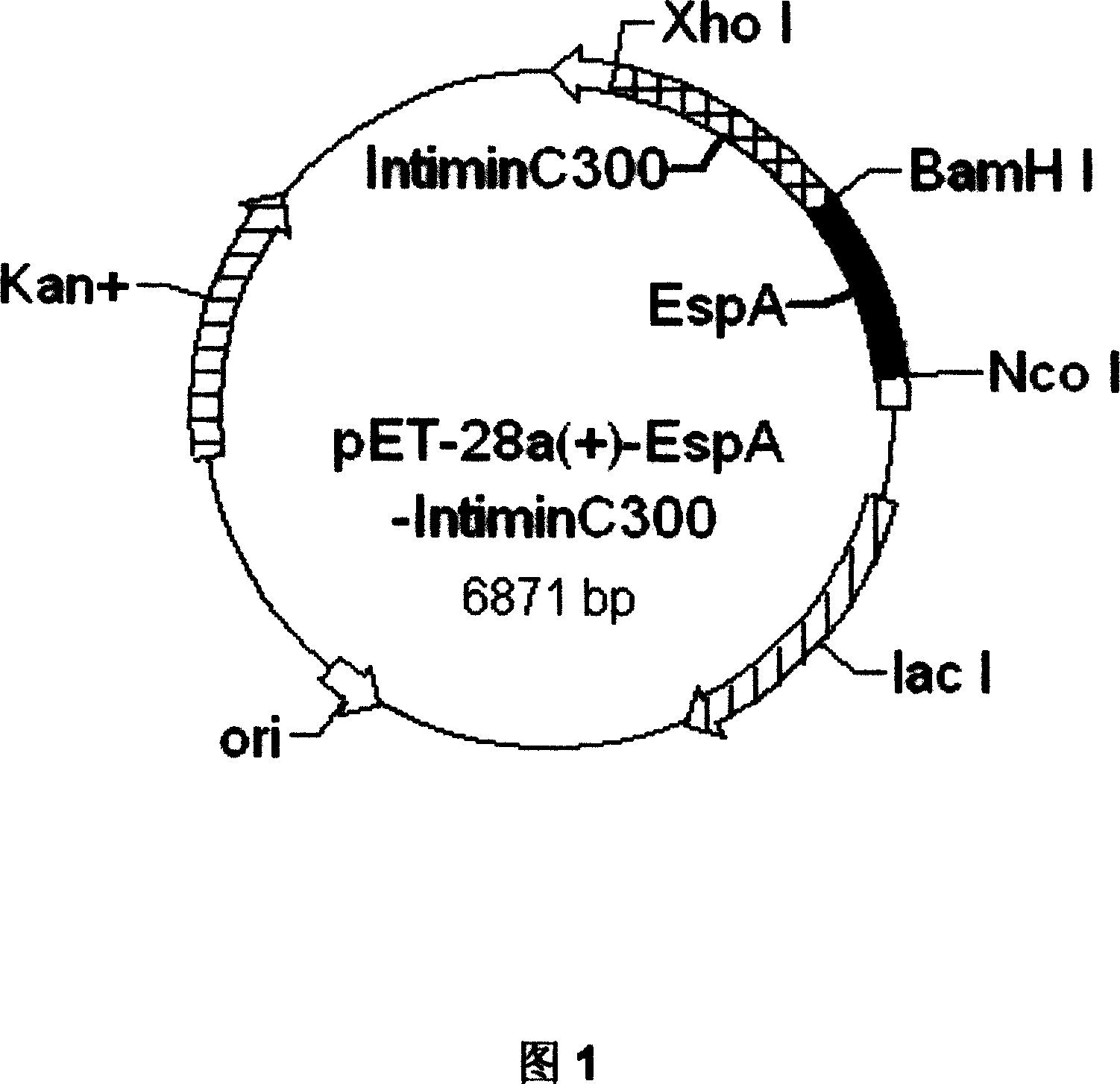
[0080] Construction of EspA-Intimin-C300 Fusion Gene of Enterohaemorrhagic Escherichia coli O157:H7
[0081] 1. Primer design and PCR amplification
[0082] 1) The primers were designed as follows: according to the genes encoding EspA and Intimin-C300 of O157:H7 (SaKai standard strain) found from GenBank, the primers were designed and analyzed using the software Primer Premier 5.0. The linker (the part in the box) sequence is designed at the 3' end of the upstream gene (EspA) and introduced into the BamHI site, and the two genes can be connected correctly with the sticky end digested with BamHI. Primers were synthesized by Shanghai Yingjun Company.
[0083] EspA:
[0084] Upstream primer P1:
[0085] 5′- CCATGG ATACATCAAATGCAAC-3′(NcoI)
[0086] Downstream primer P2:
[0087] 5′- TTACCAAGGGATATT-3'(BamHI) Intimin-C300:
[0088] Upstream primer P3:
[0089] 5′- ACTTCAGCACTTA-3' (BamHI)
[0090] Downstream primer P4:
[0091] 5′- CTCGAG TTCTACACAAACCGCATA-3' (Xho...
Embodiment 2
[0102] Construction and expression of EspA-Intimin-C300-Stx2B fusion protein of Escherichia coli O157:H7
[0103] 1. Primer design and PCR amplification
[0104] 1) The primers are designed as follows: according to the gene encoding Stx2B of O157:H7 (SaKai standard strain) found in GenBank and the fusion gene EspA-Intimin-C300 sequence constructed in Example 1, the primers are designed using the software Primer Premier5.0 and analysis. The sequence of the linker (the part in the box) was designed at the 3' end of the upstream fusion gene (EspA-Intimin-C300), and the fusion gene EspA-Intimin-C300 and the gene encoding Stx2B were connected by overlapping extension PCR method. Primers were synthesized by Shanghai Yingjun Company.
[0105] EspA-Intimin-C300:
[0106] Upstream primer P1:
[0107] 5′- CCATGG ATACATCAAATGCAAC-3′(NcoI)
[0108] Downstream primer P2:
[0109] 5′- TTCTACACAAACCG-3′
[0110] Stx2B:
[0111] Upstream primer P3:
[0112] 5′- AAGAAGATGTTTAT-3′...
Embodiment 3
[0132] Animal immunity and antibody detection
[0133] The target protein EspA-Intimin-C300-Stx2B was purified and immunized 4-5 weeks old Balb / c mice, 100ug / mouse / time, 100μL antigen was mixed with the same amount of Freund's complete adjuvant, and injected subcutaneously in the abdomen and groin of mice immunity. Once a week, blood was collected 4 days after the 3rd and 4th immunizations, and ELISA was used to detect the change of serum specific antibody titer.
[0134] Results: The antibody-positive rate of EspA-Intimin-C300-Stx2B was 90% after 3 times of immunization, and 98% after 4 times of immunization.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com