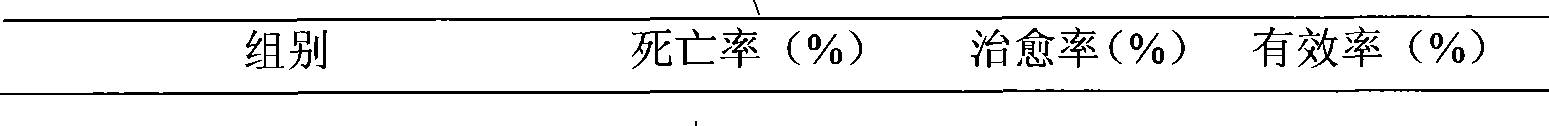Patents
Literature
43 results about "Polyinosinic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
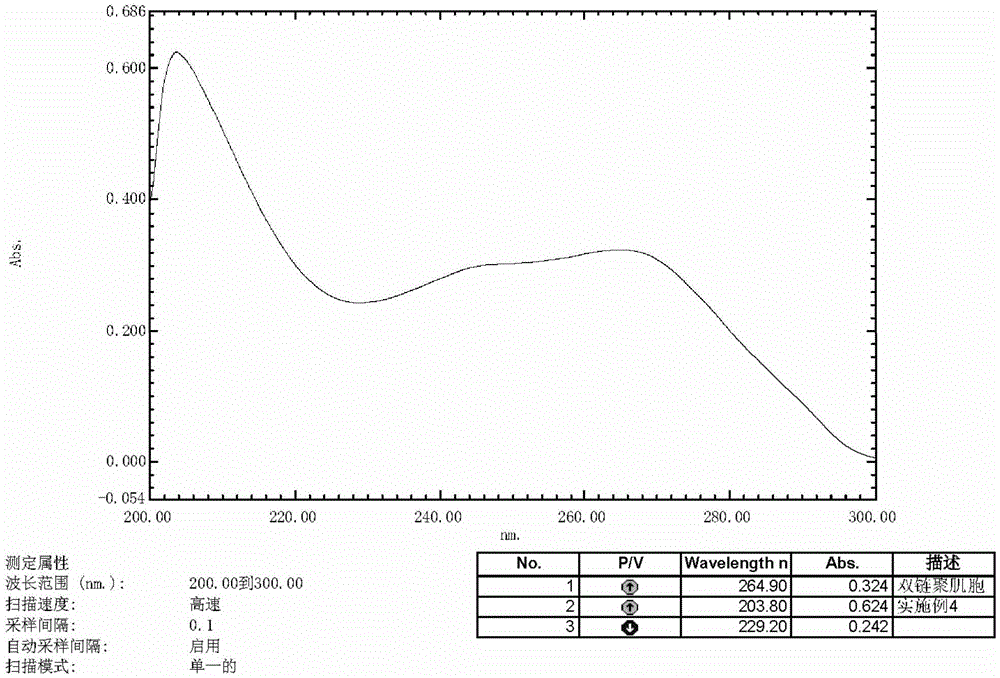
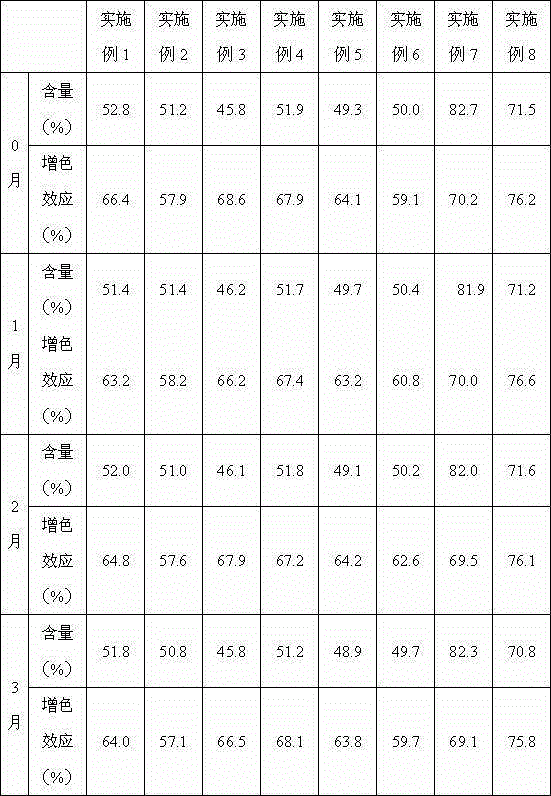
Preparation method of polyinosinic-polycytidylic acid dry powder
ActiveCN103599071AEasy to makeEasy to operatePowder deliveryCosmetic preparationsBiological bodyOrganic solvent
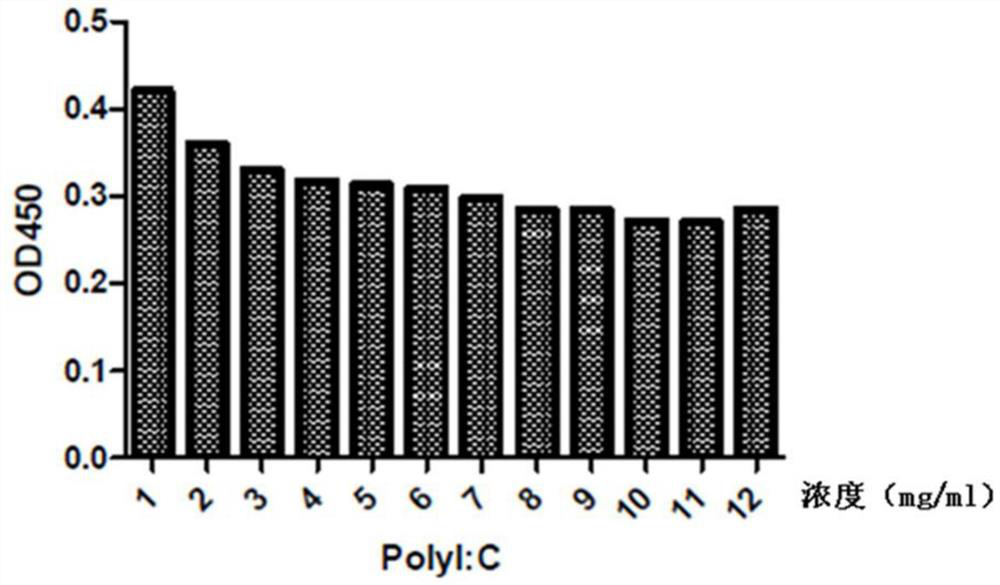
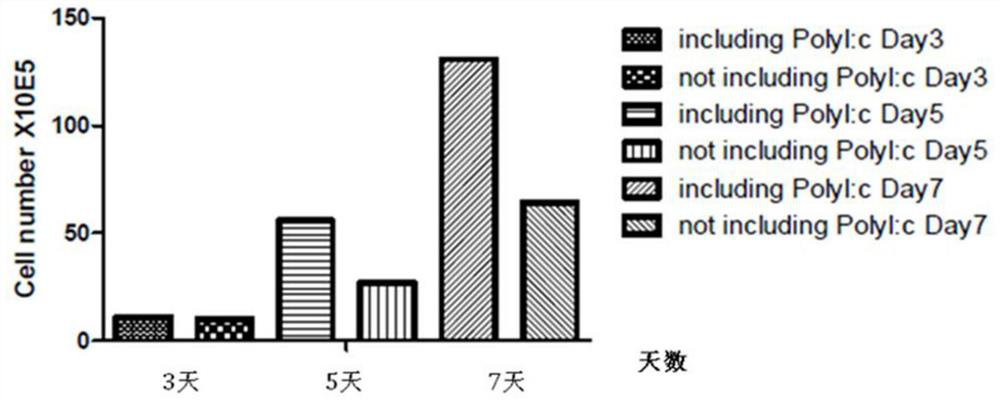
The invention provides a preparation method of polyinosinic-polycytidylic acid dry powder. The method comprises the following steps: (1) respectively dissolving polyinosinic acid and polycytidysic acid in a phosphate buffer solution, mixing the solution, adding a stabilizer, uniformly mixing the stabilizer and the solution, and performing heat preservation on the mixture at the temperature of 40-100DEG C; (2) naturally cooling the reaction liquid obtained in the step (1), mixing the reaction liquid with an organic solvent, standing for precipitating, and drying the precipitate, thereby obtaining the polyinosinic-polycytidylic acid dry powder. The preparation method has the beneficial effects that the prepared polyinosinic-polycytidylic acid dry powder has a complete double-helix structural features and physiological activity, the quality and the stability are better than those of the polyinosinic-polycytidylic acid at a solution state, a stronger enzymolysis-resisting performance can be achieved compared with the bare polyinosinic-polycytidylic acid after entering a living body, so that the curative effect can be enhanced. The polyinosinic-polycytidylic acid is purified in the process for making the polyinosinic-polycytidylic acid solution into the dry powder, a plurality of small-molecular-weight substances and impurities can be removed, the toxic and side effects can be reduced, and the quality of the polyinosinic-polycytidylic acid can be improved.
Owner:美亚药业海安有限公司
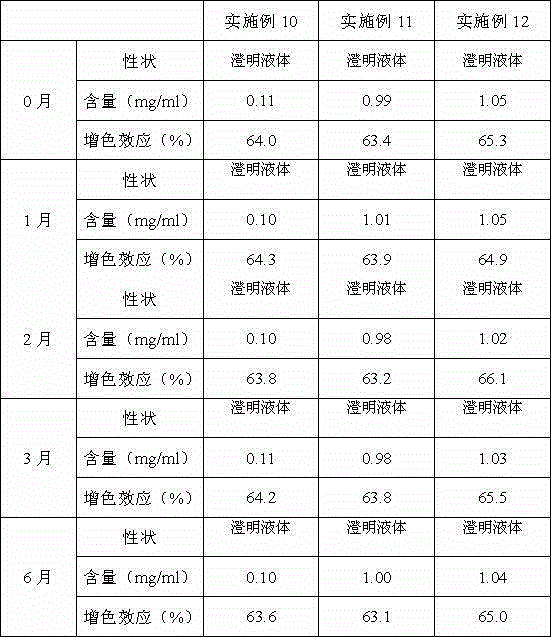
Preparation method of polyinosinic acid-polycytidylic acid lyophilized powder injection
InactiveCN102988303ASlow down the rate of oxidative degradationLong validity periodOrganic active ingredientsPowder deliverySide effectOrganic chemistry
The invention discloses a preparation method of a polyinosinic acid-polycytidylic acid lyophilized powder injection. The method comprises the preparation of a polyinosinic acid-polycytidylic acid solution and a preparation of a lyophilized powder injection thereof. According to the invention, proper amounts of polyinosinic acid and polycytidylic acid are respectively dissolved by using normal saline; the mixtures are mixed and stirred, such that the polyinosinic acid-polycytidylic acid solution is prepared; a lyophilization additive is added and well mixed; and filtering, sub-packaging, and lyophilization are carried out. The prepared injection is suitable for frost preservation under a temperature below 0 DEG C. Therefore, expiration date is postponed, oxidative degradation speed is reduced, toxic and side effect are reduced, and stability is improved.
Owner:天津泽世德生物医药有限公司
Polyinosinic acid-polycytidylic acid dominated adjuvant
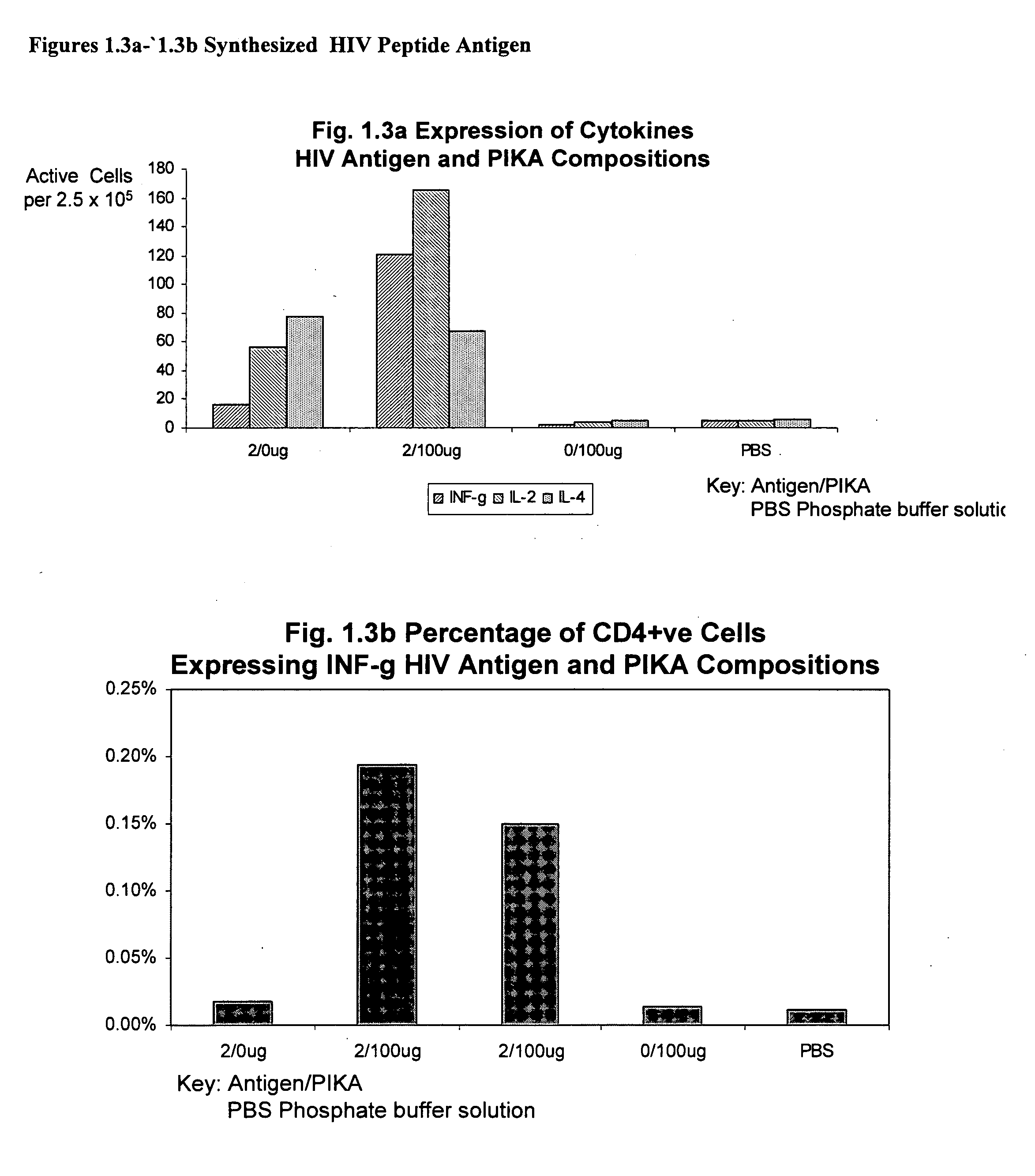
The invention provides a polyinosinic acid-polycytidylic acid dominated adjuvant. The invention provides an oligonucleotide adjuvant composition and an immunoreaction initiated application method thereof. The invention also provides an immunogen composition containing the oligonucleotide adjuvant composition and an antigen, for example, the two components are in a vaccine. The adjuvant composition disclosed by the invention has specific physical properties such as molecular weight, concentration and pH, and the properties can meet a required effective and safe adjuvant capable of inducing and strengthening immunoreaction and changing the type of immunoreaction. The invention further relates to application methods of the adjuvant compositions, and particularly relates to a method for inducing the immunoreaction for antigen compositions.
Owner:YISHENG BIOPHARMA SINGAPORE
Foot-and-mouth disease genetic engineering mixed epitope vaccine and preparation method thereof
ActiveCN103007273AGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsGenetic engineeringPolyinosinic Acids
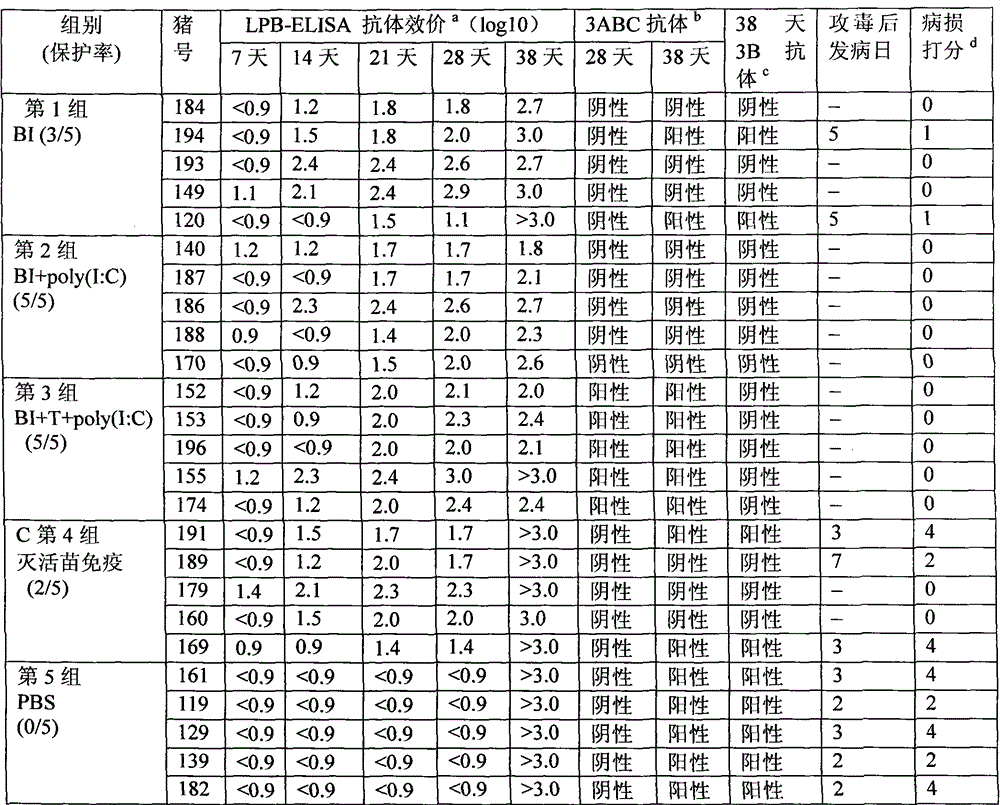
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Mucosal immunogenic substances comprising a polyinosinic acid - polycytidilic acid based adjuvant
InactiveUS20070166239A1Enhance immune responseImprove responseSsRNA viruses negative-senseOrganic active ingredientsMucosal Immune ResponsesAdjuvant
The present invention provides a polynucleotide adjuvant composition and methods of use in eliciting an immune response, in particular a mucosal immune response. The present invention also provides an immunogenic composition comprising the polynucleotide adjuvant composition together with other immunogenic compositions such as an antigen (e.g., as in a vaccine). The present invention further contemplates methods of use of such adjuvant compositions, particularly in eliciting an immune response, in particular a mucosal immune response to an antigenic compound.
Owner:YISHENG BIOPHARMA SINGAPORE
Polyinosinic dropping-pills for pet and preparation method thereof
InactiveCN101491503AEasy to useNo injectionOrganic active ingredientsGenetic material ingredientsPh bufferingMedicine
The invention relates to a polyinosinic acid drop pill for pets and a method for preparing the same. The drop pill is prepared from polyinosinic acid, polycytidylic acid, a pH buffering system, an excipient and a substrate.
Owner:TIANJIN RINGPU BIO TECH
Immunogenic substances comprising a polyinosinic acid-polycytidilic acid based adjuvant
The present invention provides a polynucleotide adjuvant composition and methods of use in eliciting an immune response. The present invention also provides an immunogenic composition comprising the polynucleotide adjuvant composition together with other immunogenic compositions such as an antigen (e.g., as in a vaccine). The present invention further contemplates methods of use of such adjuvant compositions, particularly in eliciting an immune response to an antigenic compound.
Veterinary interferon inducer and preparation method thereof
ActiveCN104706580ALow costStrongly cationicAntibacterial agentsPharmaceutical delivery mechanismPhosphateIrritation
The invention relates to a veterinary interferon inducer. The veterinary interferon inducer is prepared from the following components: a sodium chloride-phosphate buffer solution with the pH value of 7.2, a 10-80g / L calcium chloride aqueous solution, a 0.1-5g / L chitosan hydrochloride solution, polyinosinic acid and polycytidysic acid. The invention further provides a preparation method of the veterinary interferon inducer. The veterinary interferon inducer provided by the invention is few in side reaction, low in irritation and high in safety, and the preparation method can avoid precipitates generated by calcium chloride and other components.
Owner:SOUTH CHINA AGRI UNIV +2
Compound vaccine adjuvant composition
ActiveCN107441485AEnhance cross immune protectionImprove immunitySsRNA viruses positive-senseViral antigen ingredientsMedicineCellular immunity
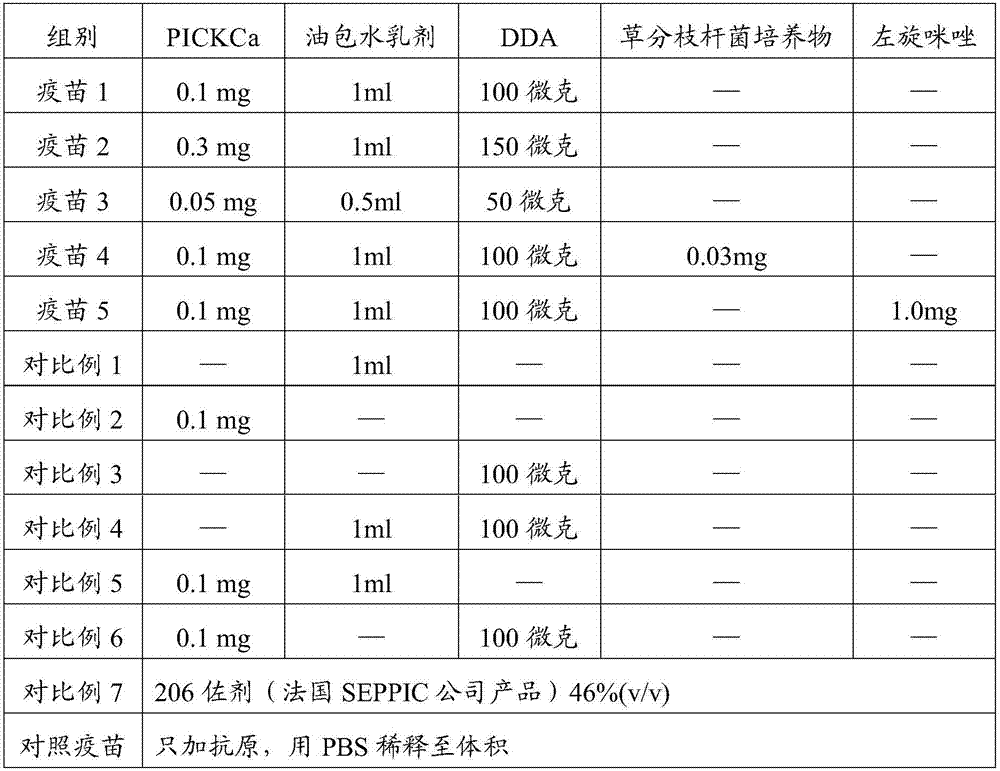
The invention relates to a compound adjuvant composition and a vaccine composition containing the adjuvant. The compound adjuvant composition consists of an oil-in-water emulsion, polyinosinic acid-polycytidysic acid or derivatives thereof and DDA. Cellular immunity of antigen can be obviously improved.
Owner:PU LIKE BIO ENG
Immunogenic Substances Comprising A Polyinosinic Acid-Polycytidilic Acid Based Adjuvant
InactiveUS20090175902A1Antibacterial agentsBacterial antigen ingredientsMucosal Immune ResponsesAdjuvant
The present invention provides a polynucleotide adjuvant (PICKCa) composition and methods of use in eliciting an immune response, in particular a mucosal immune response. The polynucleotide adjuvant comprises of a polyriboinosinic-polyribocytidylic acid (PIC), at least one antibiotic and at least one positive ion. The present invention also provides an immunogenic composition comprising the polynucleotide adjuvant composition together with other immunogenic compositions such as an antigen (e.g., as in a vaccine) selected from viral, bacterial, fungal, parasitic and / or cancer antigens. The present invention further contemplates methods of use of such adjuvant compositions, particularly in eliciting an immune response, in particular a mucosal immune response to an antigenic compound.
Owner:YISHENG BIOPHARMA SINGAPORE
Method for preparing polyinosinic preparation and application of polyinosinic preparation to tumor resistance
InactiveCN102488703APharmaceutical non-active ingredientsSynthetic polymeric active ingredientsKanamycinPhospholipid
The invention aims to provide a method for preparing a polyinosinic preparation and application of the polyinosinic preparation to tumor resistance. The invention further provides a double-stranded ribonucleic acid (RNA) medicine containing polyinosinic acid and polycytidylic acid. The polyinosinic preparation contains a carrier in which phospholipids serve as an essential component, and a complex of the polyinosinic acid, the polycytidylic acid and kanamycin.
Owner:TIANJIN JIMINGSHENG BIOLOGICAL TECH
Mucosal immunogenic substances comprising a polyinosinic acid - polycytidilic acid based adjuvant
InactiveUS20090311334A1Enhance immune responseImprove responseSsRNA viruses negative-senseOrganic active ingredientsMucosal Immune ResponsesAdjuvant
The present invention provides a polynucleotide adjuvant (PICKCa) composition and methods of use in eliciting an immune response, in particular a mucosal immune response. The polynucleotide adjuvant comprises of a polyriboinosinic-polyri-bocytidylic acid (PIC), at least one antibiotic and at least one positive ion. The present invention also provides an immunogenic composition comprising the polynucleotide adjuvant composition together with other immunogenic compositions such as an antigen (e.g., as in a vaccine). The present invention further contemplates methods of use of such adjuvant compositions, particularly in eliciting an immune response, in particular a mucosal immune response to an antigenic compound.
Owner:YISHENG BIOPHARMA SINGAPORE
Polyinosinic acid-polycytidylic acid-based adjuvant
ActiveUS7838017B2Safely and effectively administeredEasy to useSsRNA viruses negative-senseViral antigen ingredientsImmunogenicityPhysical property
The present invention provides a polynucleotide adjuvant composition and methods of use in eliciting an immune response. The present invention also provides an immunogenic composition comprising the polynucleotide adjuvant composition together with an antigen (e.g., as in a vaccine). The adjuvant compositions of the invention have particular physical properties (e.g., molecular weight, concentration, and pH) which address the need for a safe adjuvant for eliciting an enhanced immune response. The present invention further contemplates methods of use of such adjuvant compositions, particularly in eliciting an immune response to an antigenic compound.
Owner:YISHENG BIOPHARMA SINGAPORE
Subunit vaccine against Mycobacterium tuberculosis based on modification by arabinogalactan-polyinosinic acid polycytidylic acid and preparation method thereof
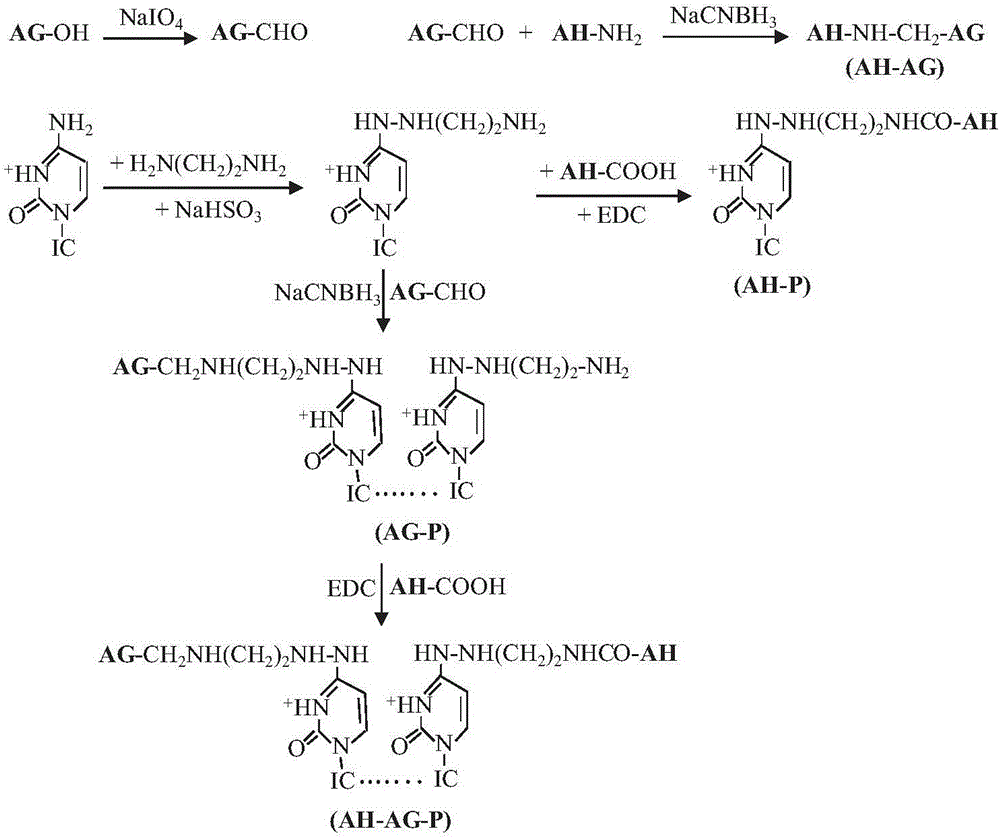
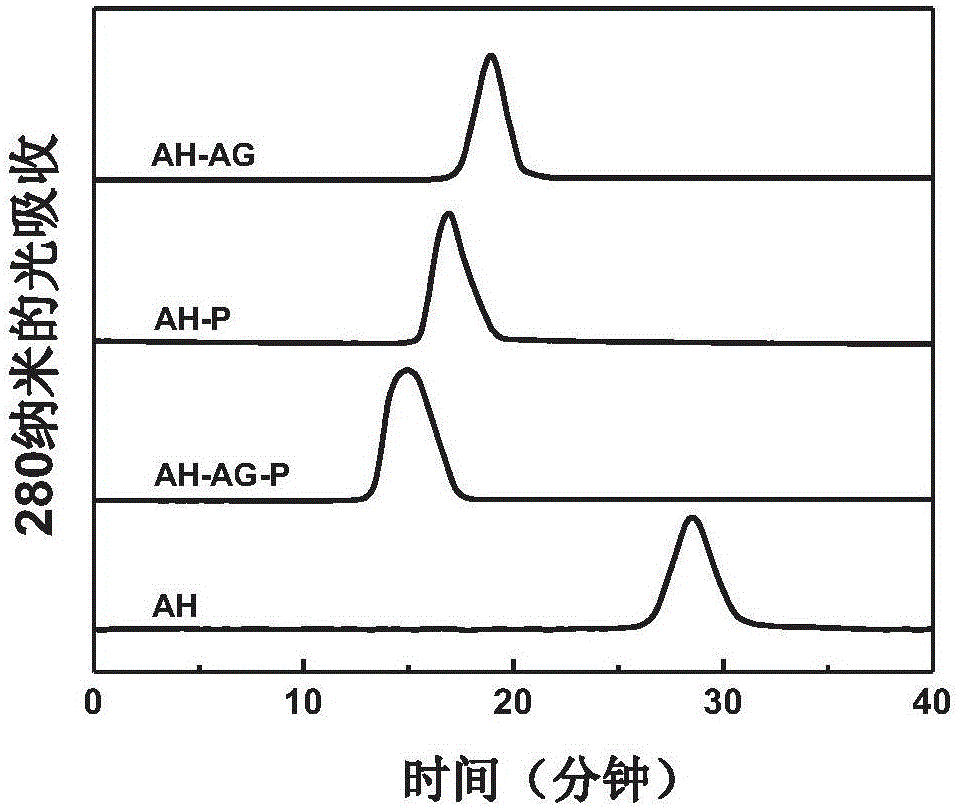
InactiveCN105903009AStrong cellular immune responseStrong humoral immune responseAntibacterial agentsBacterial antigen ingredientsEscherichia coliHalf-life
The invention provides a subunit vaccine against Mycobacterium tuberculosis based on modification by arabinogalactan-polyinosinic acid polycytidylic acid and a preparation method thereof. The preparation method comprises the following steps: subjecting arabinogalactan (AG) and polyinosinic acid polycytidylic acid (poly(I:C)) to chemical coupling so as to prepare an AG-poly(I:C) conjugate which is used as an immunoadjuvant; subjecting antigen protein Ag85B expressed in a replicative period of Mycobacterium tuberculosis and antigen protein HspX expressed in an incubation period of Mycobacterium tuberculosis to recombinant fusion expression in Escherichia coli so as to obtain Ag85B-HspX fusion protein; and modifying the Ag85B-HspX fusion protein with the AG-poly(I:C) conjugate so as to obtain the subunit vaccine against Mycobacterium tuberculosis. The vaccine has a long cycle half-life period in a body and can stimulate the body to generate high-level humoral immune response and cellullar immune response.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Pharmaceutical composition of dsRNA and astragalus polysaccharide and application thereof
InactiveCN101703518AImprove immunityEasy to solveOrganic active ingredientsAntiviralsBiotechnologyAstragalus polysaccharide
The invention relates to a pharmaceutical composition of dsRNA and astragalus polysaccharide and application thereof, in particular to the pharmaceutical composition applied to viral infection prevention and treatment of cultured animals. The pharmaceutical composition is formed by combining the astragalus polysaccharide and the dsRNA according to a certain proportion, wherein the astragalus polysaccharide has the content range of 20-50mg / ml; the dsRNA (polyinosinic acid) has the content range of 1-5mg / ml and the molecular weight range of 200-500bp; and the main components of the astragalus polysaccharide and the dsRNA are processed by matching liquid, filtering, filling and encapsulating as well as sterilizing according to water injection production technology, and the pharmaceutical composition can be obtained after being checked to be qualified. A certain amount of polyinosinic cytidylic acid is added into water for injection and dissolved under the condition of 20-60 DEG C; then, equimolar polycytidylic acid is added into the solution to be dissolved; heat preservation and pairing are carried out for 15-50min; and right amount of astragalus polysaccharide is added, evenly mixed and treated by filtering, filling and encapsulating as well as sterilizing. Therefore, the invention is applicable to improving the immunity of livestock, poultry and aquatic animals, and improves the prevention and treatment for virus diseases.
Owner:青岛汉河动植物药业有限公司
Method for efficiently producing DC-CIK cells through induction of polyinosinic: polycytidylic acid copolymer
PendingCN111733129AEnhance tumor killing activityEasy to killCulture processBlood/immune system cellsCancer cellSpecific immunity
The invention relates to the technical field of immunization, in particular to a polyinosinic: polycytidylic acid copolymer (Poly I: C). The polyinosinic: polycytidylic acid copolymer is a ligand of atype-3 Toll-like receptor in an animal body, can mediate a series of immune reactions of the body after TLR-3 is activated, and has a good promoting effect on specific immunity and non-specific immunity of the body. Compared with a control group, DC-CIK induced by the polyinosinic: polycytidylic acid copolymer has higher proliferation, high differentiation (especially CD3+CD4-CD8+, CTL) and hightumor killing activity. The autologous efficient DC-CIK induced by the polyinosinic: polycytidylic acid copolymer and other immune cells are applied to treatment of clinical tumor patients. The polyinosinic: polycytidylic acid copolymer aims to recover or improve the immune function of the tumor patients, and the immune system of the body is improved to kill and inhibit proliferation of tumor cells. The tumor cell load is reduced, tiny residual lesions are removed, or the proliferation mode of residual tumor / cancer cells is obviously inhibited, factors such as relapse and metastasis are eliminated, the cure possibility is increased, the survival time is prolonged, and the life quality is improved. Therefore, the purpose of treating tumors / cancers is achieved.
Owner:成都源泉生物科技有限公司 +1
Polynucleotide microcapsule for treating diseases of livestock and poultry and preparation method thereof
InactiveCN101474165AProlong the action timeNo side effectsOrganic active ingredientsSugar derivativesDiseaseFiber
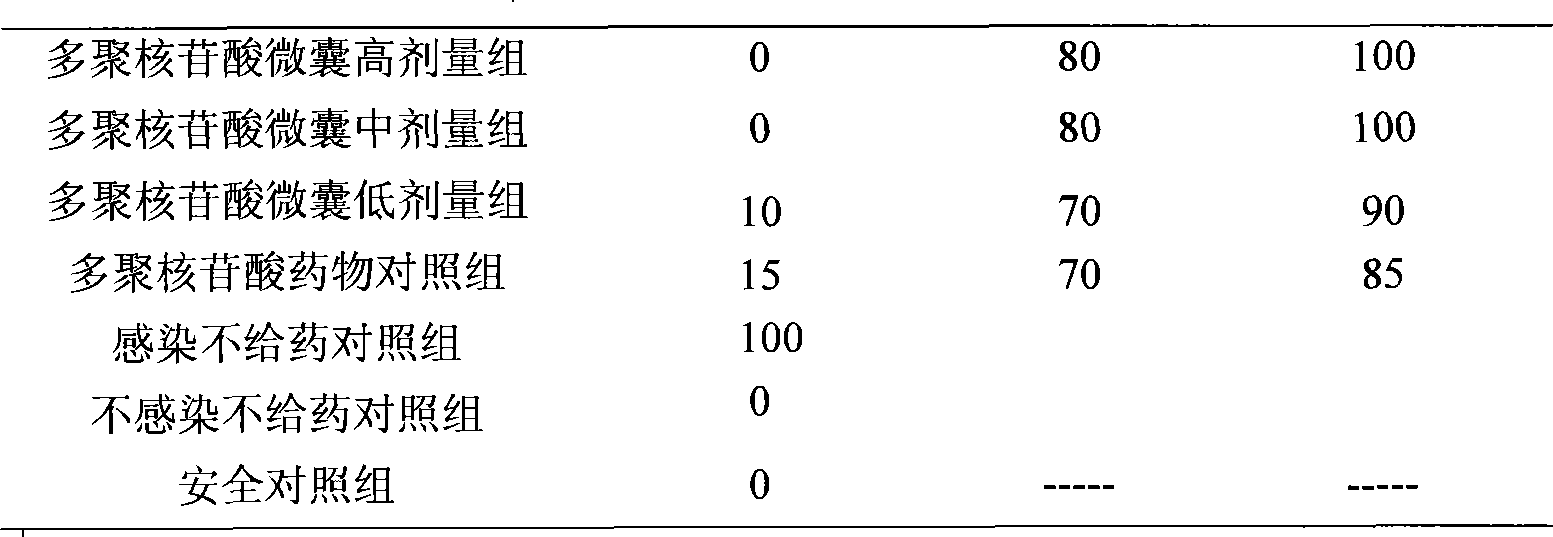
The invention relates to a polynucleotide microcapsule for treating animal disease and a preparation method thereof, belonging to the technical field of veterinary medicament preparation. Polyinosinic acid and polycytidylic acid are weighted and are added into preheated sodium chloride-phosphate buffer solution, and polynucleotide solution is prepared; the polynucleotide solution is added into ethyl cellulose to prepare soft wood and dry powder is prepared; the dry powder and magnesium stearate are added into ice-bath acetone to obtained a mixture, the mixture is stirred and slowly added into whiteruss containing 2%Span-80 surface active agent; and polynucleotide microcapsule is obtained by a method of in-liquid drying. The invention effectively prolongs the acting time of medicament within human body, decrease the applied time of the medicament and achieves the aims of long acting and sustained release. Experiments prove that the dead rate of the high dose group or the medium dose group of the polynucleotide microcapsule is obviously lower than that of an infectious control group. The cure rate is high, both are 80%, and the effective rate of the two groups are 100%. Experiments prove that the medicament of the invention is safe.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Mucosal immunity preparation capable of resisting infection and tumors
ActiveCN109125264AExtended half-lifeImprove effectivenessAntibacterial agentsPowder deliveryDiseaseHalf-life
The invention relates to the field of immunology, and particularly discloses a mucosal immunity preparation capable of resisting infection and tumors. The effective component of the preparation is a mucosal immunity substance which is formed by combining polyinosinic acid-polycytidylic acid, a non-antibiotic amino compound, metal positive ions, and PEG and / or PEI and / or PLGA and / or positive ion polymers through hydrogen bonds and other chemical bonds. The preparation is a spray or an aerosol in dosage form, but preferentially, the preparation does not contain ingredients utilized by common sprays or ingredients utilized by aerosols, and propellants. The mucosal immunity preparation can achieve the slow-release function partially or wholly, the half-life period of the mucosal immunity preparation is prolonged, the availability and effectiveness of drugs are improved, and the compliance of a patient is improved. The mucosal immunity preparation can promote mucosal immunity of the human body by means of the mucosal immunity, and then promote activation and proliferation, including whole-body nonspecific immunity, humoral immunity and cellular immunity, of all immune cells instead of only acting on the local part of a disease wound, and thus the preventing and treating effects of resisting infection and tumors are achieved.
Owner:林海祥 +3
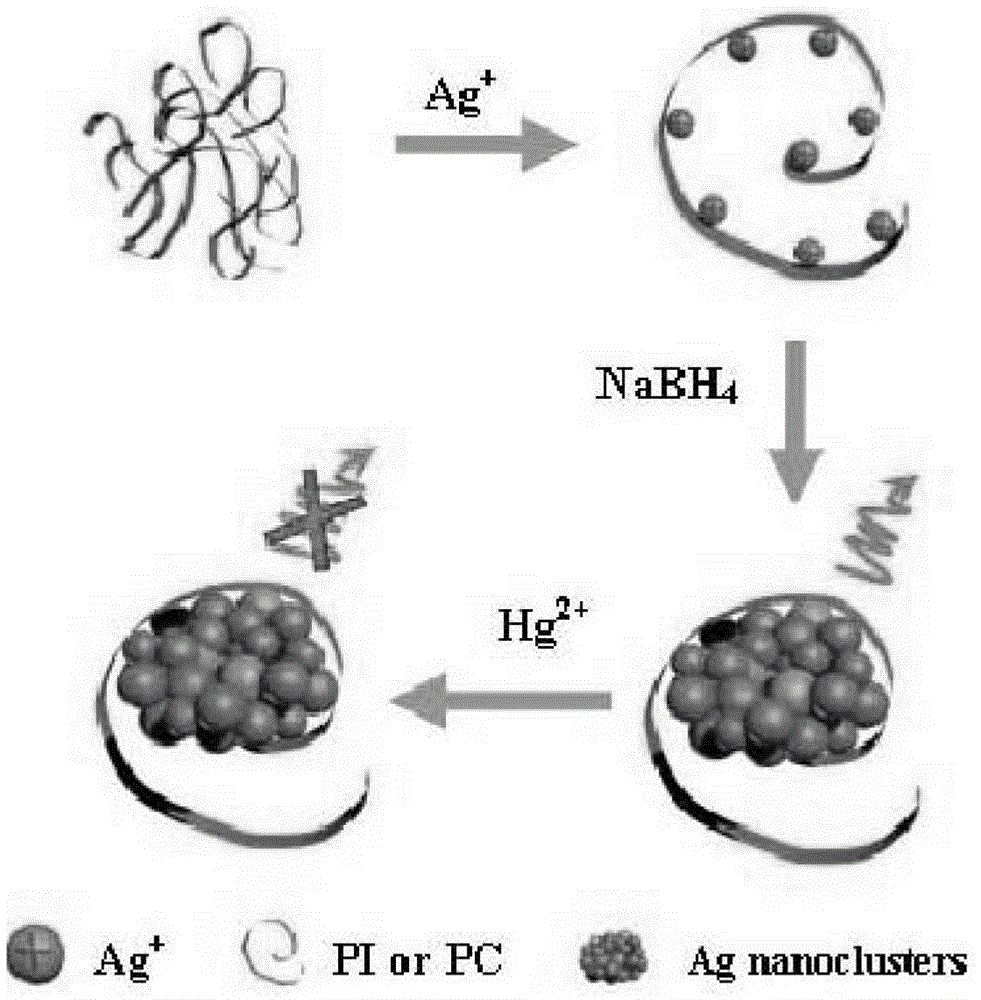
A method for synthesizing silver nanoclusters using single-stranded DNA as a template
InactiveCN103264165BEasy to synthesizeGreen synthesisFluorescence/phosphorescenceFluorescenceSpecial design
The invention provides a method for synthesizing silver nanoclusters by the aid of single-stranded DNA (deoxyribonucleic acid) used as a template, and belongs to the technical field of nano materials. By the method, problems of complicated production and excessively high cost of nano-silver for detecting mercury ions at present are solved. The method for synthesizing the silver nanoclusters by the aid of the single-stranded DNA used as the template is characterized in that the single-stranded DNA containing cytosine and guanine is used as the template and is mixed with Ag<+> solution to obtain a mixture, reducing agents are added into the mixture, and the fluorescent silver nanoclusters can be acquired after reduction reaction is performed on the mixture with the reducing agents. The method has the advantages that polyinosinic acid (PI) and polycytidylic acid (PC) which are commercially available are used as the template in the simple, low-cost, environment-friendly and reliable method for synthesizing the silver nanoclusters; fluorescence is utilized, and fluorescent silver nanocrystals are synthesized from the polyinosinic acid, the polycytidylic acid and the like without special design or preprocessing; and the novel silver nanoclusters are excellent in selectivity and sensitivity, can be applied to detecting Hg<2+>, and are feasible and have a future development prospect in the aspect of monitoring pollutants in water.
Owner:ZHEJIANG NORMAL UNIVERSITY
Riemerella anatipestifer bacterial ghost vaccine adopting chitosan oligosaccharide as adjuvant
InactiveCN104971346AImprove the level ofAvoid the stress of immunizationAntibacterial agentsBacterial antigen ingredientsPenicillinAdjuvant
The purpose of the present invention is to provide a riemerella anatipestifer bacterial ghost vaccine loaded with polyinosinic acid-polycytidylic acid having interferon inducing activity and added with chitosan oligosaccharide as an adjuvant. According to the technical scheme, a temperature control expression vector is constructed and is electrotransformed into riemerella anatipestifer protoplast, positive clones are screened and then are subjected to bacterial amplification culture at a temperature of 28 DEG C, lysis gene expression is induced at a temperature of 42 DEG C, polylysine is added to continuously act when the OD600 is no longer be reduced so as to completely lyze the live bacteria, centrifugation is performed to separate the bacteria, the dried bacteria and a 6 mg / mL Poly I:C solution according to an equal ratio so as to make the Poly I:C be embedded into the bacterial ghosts, a 2% chitosan oligosaccharide aqueous solution is added, stirring is performed to form a uniform mixture, sub-packaging is performed into penicillin bottles, and freeze-drying is performed so as to obtain the novel riemerella anatipestifer bacterial ghost vaccine. According to the present invention, the prepared riemerella anatipestifer bacterial ghost vaccine is immunized through drinking water, such that the local immunity on the respiratory tract mucous membrane and the digestive tract mucous membrane can be irritated, the humoral immunity can be stimulated, and the protection effect on the human body is not different from the injection immunity.
Owner:QINGDAO AGRI UNIV
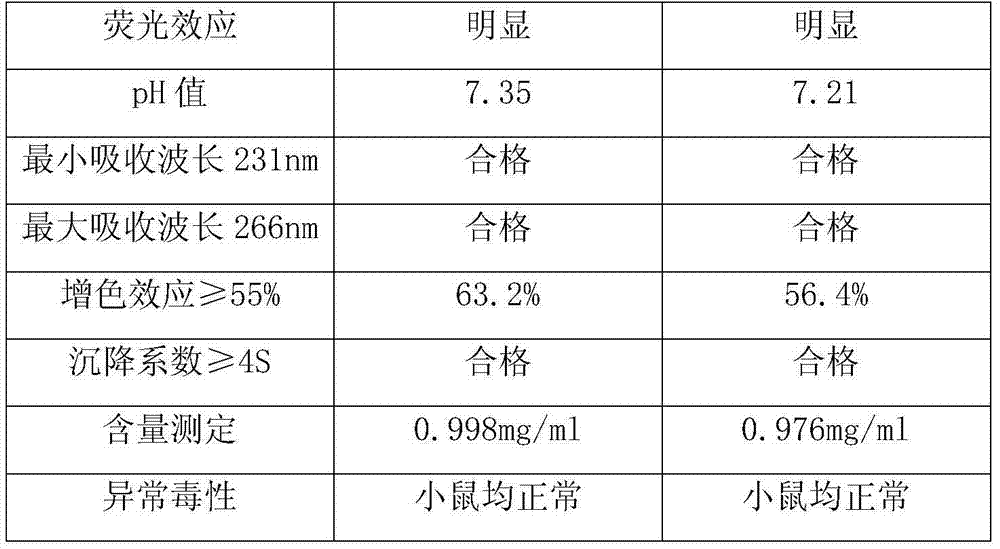
Preparation method of refined poly zic and application
InactiveCN102488704AImprove product qualityLittle side effectsSynthetic polymeric active ingredientsAntineoplastic agentsOrganic chemistryPolyinosinic Acids
Owner:TIANJIN JIMINGSHENG BIOLOGICAL TECH
Polyinosinic-polycytidylic acid injection and method for lowering toxins in polyinosinic-polycytidylic acid injection
PendingCN110433173AReduce contentControl contentOrganic active ingredientsSenses disorderPolymer solutionToxin
Owner:CHENGDU HAITONG PHARMA
Hepatitis B vaccine agonist composition and application thereof
ActiveCN105194666AImprove antigen presentation functionRaise the level of immune responseAntiviralsAntibody medical ingredientsNucleotideBiotin
The invention discloses a hepatitis B vaccine agonist composition and application thereof, and relates to a hepatitis B vaccine. The hepatitis B vaccine agonist composition comprises Lewisx-Polyacrylamide-biotin, polyinosinic acid-polycytidylic acid, and oligodeoxynucleotide, wherein the component of the Lewisx-Polyacrylamide-biotin is 30 kd polyacrylamide containing 5 mol% of biotin and 20 mol% of a carbohydrate; the sequence of the oligodeoxynucleotide is 5'-tcgacgttcgtcgttcgtcgttc-3', and the whole process is subjected to phosphorothioate modification. Through agonists of three receptors DC-SIGN, TLR3 and TLR9, high expression of corresponding acceptors are stimulated, so that the antigen presenting function of DC is improved, and Th1 immune response of a body is promoted.
Owner:SHANXI MEDICAL UNIV
Preparation method of poly i-c
ActiveCN110256516AEfficient removalEasy to removeSugar derivativesFermentationInosine DiphosphatePhosphoric acid
The invention belongs to a preparation method of poly i-c and belongs to the technical field of biomedicine. The preparation method includes: utilizing inosine diphosphate to prepare polyinosinic acid, and using cytidine diphosphate to prepare polycytidylic acid; utilizing the prepared polyinosinic acid to react with the polycytidylic acid to obtain a high-purity poly i-c product. Poly i-c is prepared through self-developed high-purity polyinosinic acid and polycytidylic acid, so that prepared poly i-c is high in purity, and treatment difficulty in the process of preparing is lowered.
Owner:宋龙飞
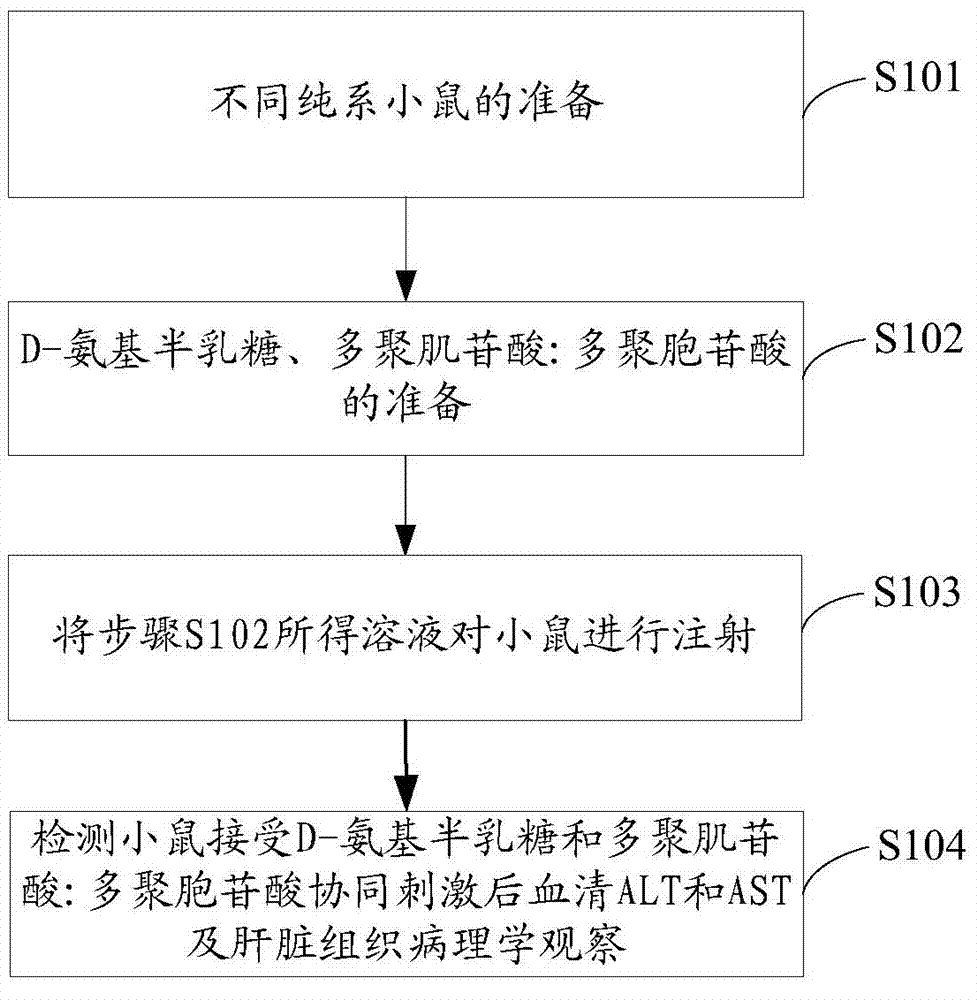
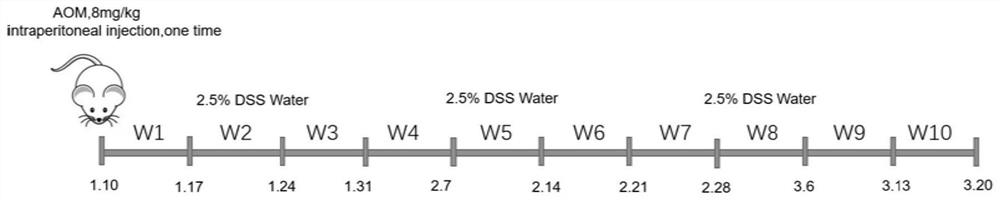
Method for establishing acute viral hepatitis animal model
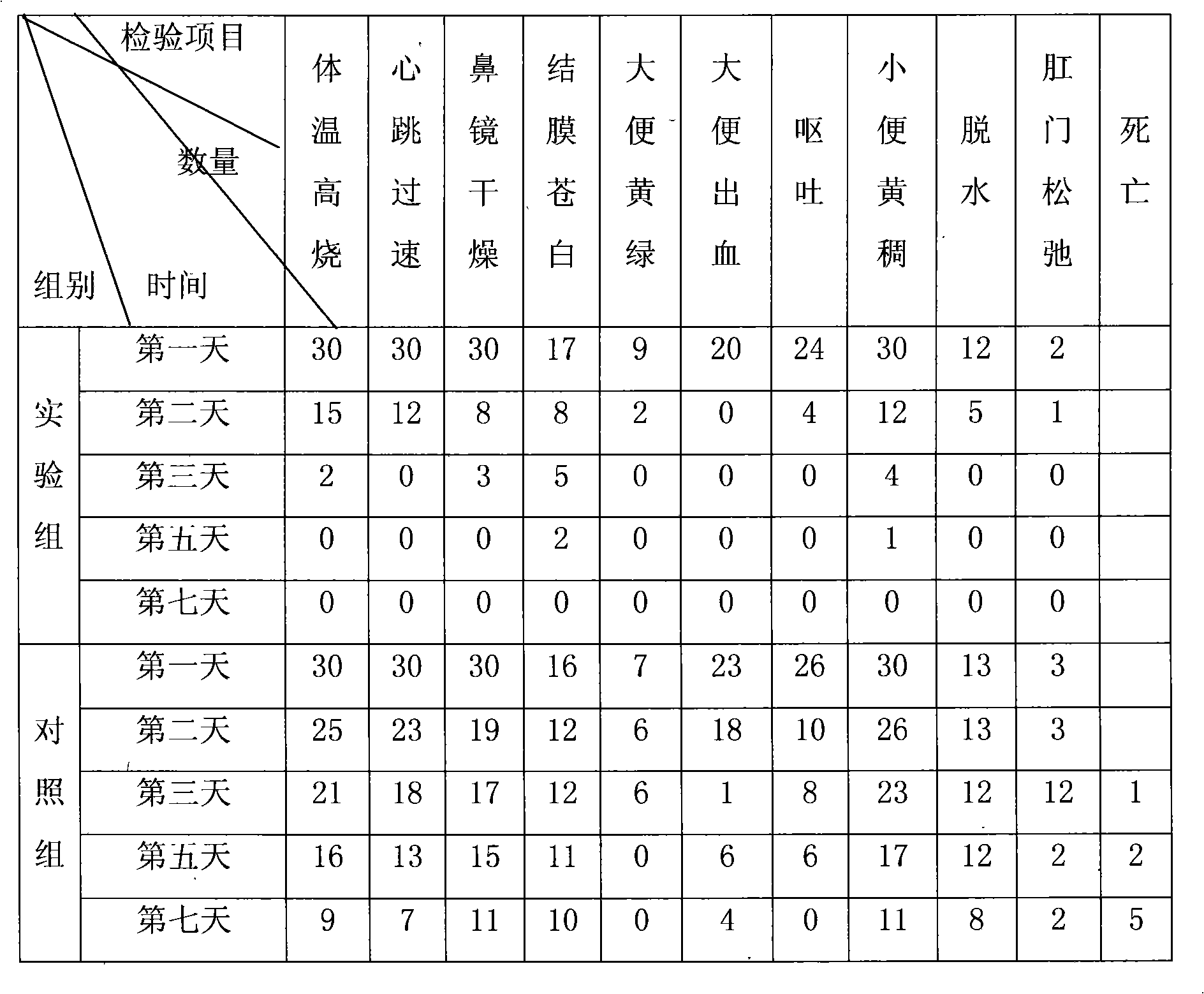
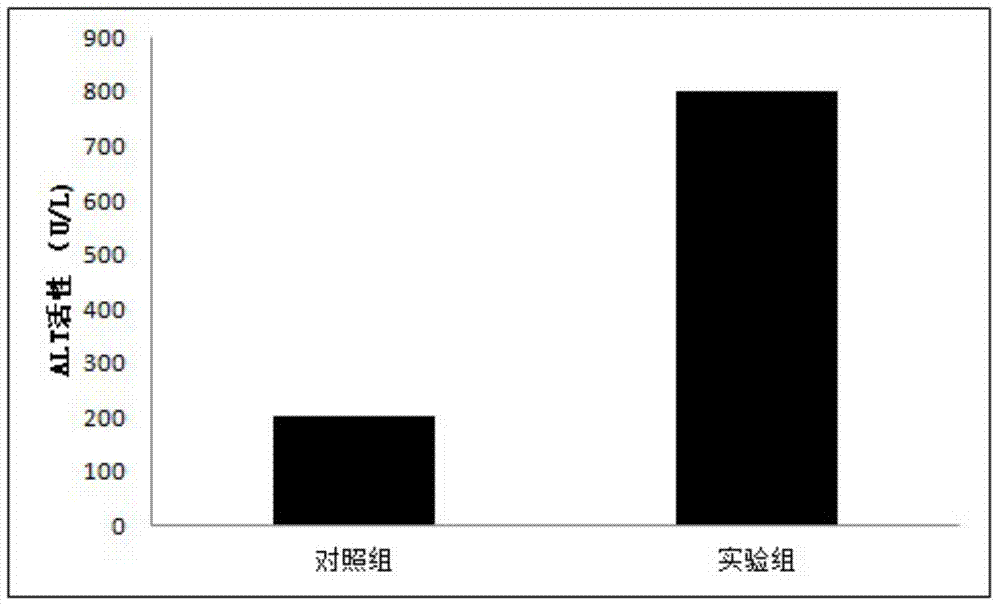
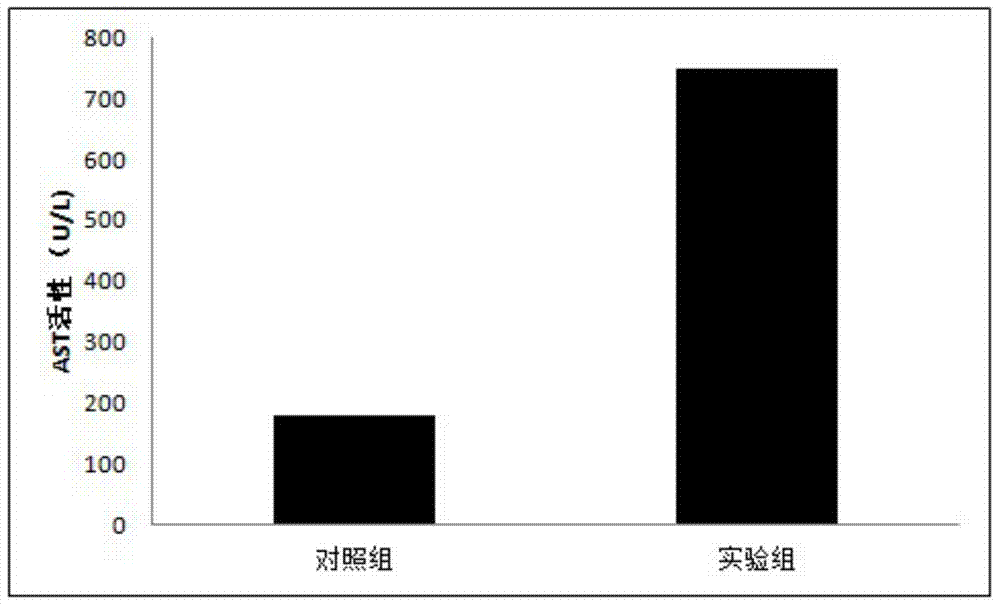
The present invention relates to the technical field of liver immunology and experimental zoology, particularly to a method for establishing an acute viral hepatitis animal model, wherein D-galactosamine and polyinosinic acid:polycytidylic acid are injected in a peritoneal injection or intravenous injection manner, the D-galactosamine and the polyinosinic acid:polycytidylic acid produce the synergy effect, the ALT activity change is detected, and the acute viral hepatitis animal model is established. With the method of the present invention, the hepatitis symptoms are rapidly induced, and the viral infection simulation effect is good.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
A kind of foot-and-mouth disease genetic engineering mixed epitope vaccine and its preparation method
ActiveCN103007273BGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsFhit geneGenetic engineering
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
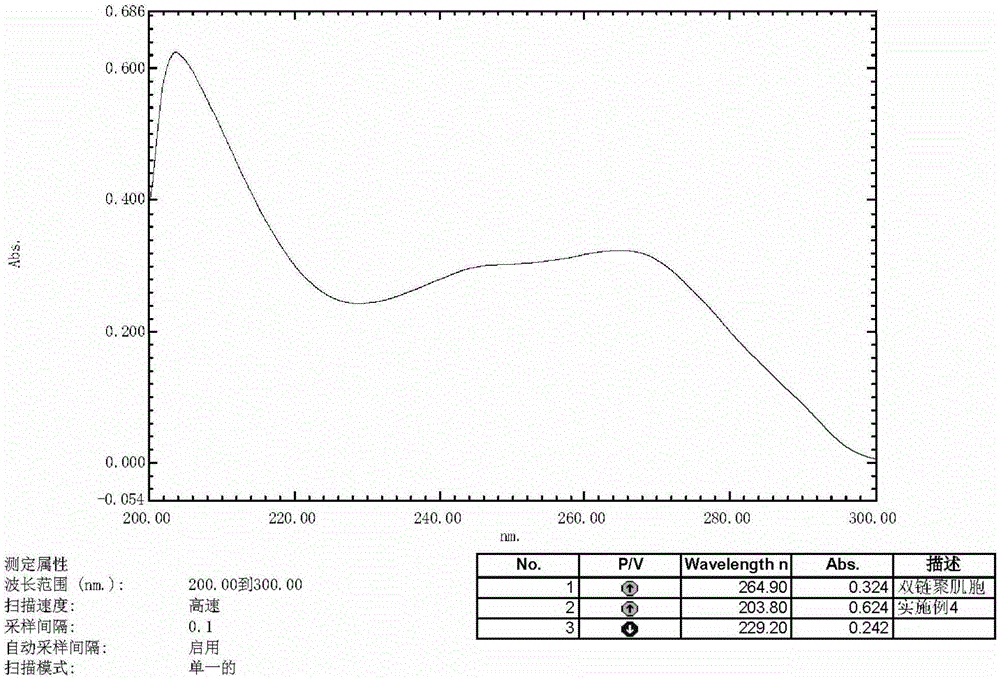
A kind of preparation method of double chain polymyocyte dry powder
ActiveCN103599071BQuality improvementImprove stabilityPowder deliveryCosmetic preparationsSide effectPhosphate
The invention provides a preparation method of polyinosinic-polycytidylic acid dry powder. The method comprises the following steps: (1) respectively dissolving polyinosinic acid and polycytidysic acid in a phosphate buffer solution, mixing the solution, adding a stabilizer, uniformly mixing the stabilizer and the solution, and performing heat preservation on the mixture at the temperature of 40-100DEG C; (2) naturally cooling the reaction liquid obtained in the step (1), mixing the reaction liquid with an organic solvent, standing for precipitating, and drying the precipitate, thereby obtaining the polyinosinic-polycytidylic acid dry powder. The preparation method has the beneficial effects that the prepared polyinosinic-polycytidylic acid dry powder has a complete double-helix structural features and physiological activity, the quality and the stability are better than those of the polyinosinic-polycytidylic acid at a solution state, a stronger enzymolysis-resisting performance can be achieved compared with the bare polyinosinic-polycytidylic acid after entering a living body, so that the curative effect can be enhanced. The polyinosinic-polycytidylic acid is purified in the process for making the polyinosinic-polycytidylic acid solution into the dry powder, a plurality of small-molecular-weight substances and impurities can be removed, the toxic and side effects can be reduced, and the quality of the polyinosinic-polycytidylic acid can be improved.
Owner:美亚药业海安有限公司
Application of poly inosinic acid cytidine monophosphate combined with anti-CD47 antibody in tumor treatment
InactiveCN111840541AImprove the activation effectImprove response rateAntibody ingredientsAntineoplastic agentsAntiendomysial antibodiesTumor therapy
The invention discloses an application of polyinosinic acid combined with an anti-CD47 antibody in tumor treatment, which can promote the innate immune function of macrophages so as to improve the activation performance of the anti-CD47 antibody, promote the macrophages to kill tumor cells and improve the response rate of tumor patients to the anti-CD47 antibody. Meanwhile, when the polyinosinic acid is combined with the anti-CD47 antibody to treat tumors, adverse reactions such as anemia and the like caused in the treatment process can be remarkably reduced, and the life quality of a patientand the compliance in the treatment process are improved; more importantly, solid tumors including colon cancer can be treated by combining the Poly I: C immunologic adjuvant with the anti-CD47 antibody for immunotherapy, so that the restriction that the anti-CD47 antibody is difficult to realize an effective treatment effect on the solid tumors in the prior art is broken through; a new research direction is provided for immunotherapy and colon cancer treatment in the prior art, and development of the field of tumor treatment is promoted.
Owner:SUN YAT SEN UNIV
EV71 vaccine containing adjuvant combination
InactiveCN108853488AEfficient activationReduce morbiditySsRNA viruses positive-senseViral antigen ingredientsAdjuvantVirus-like particle
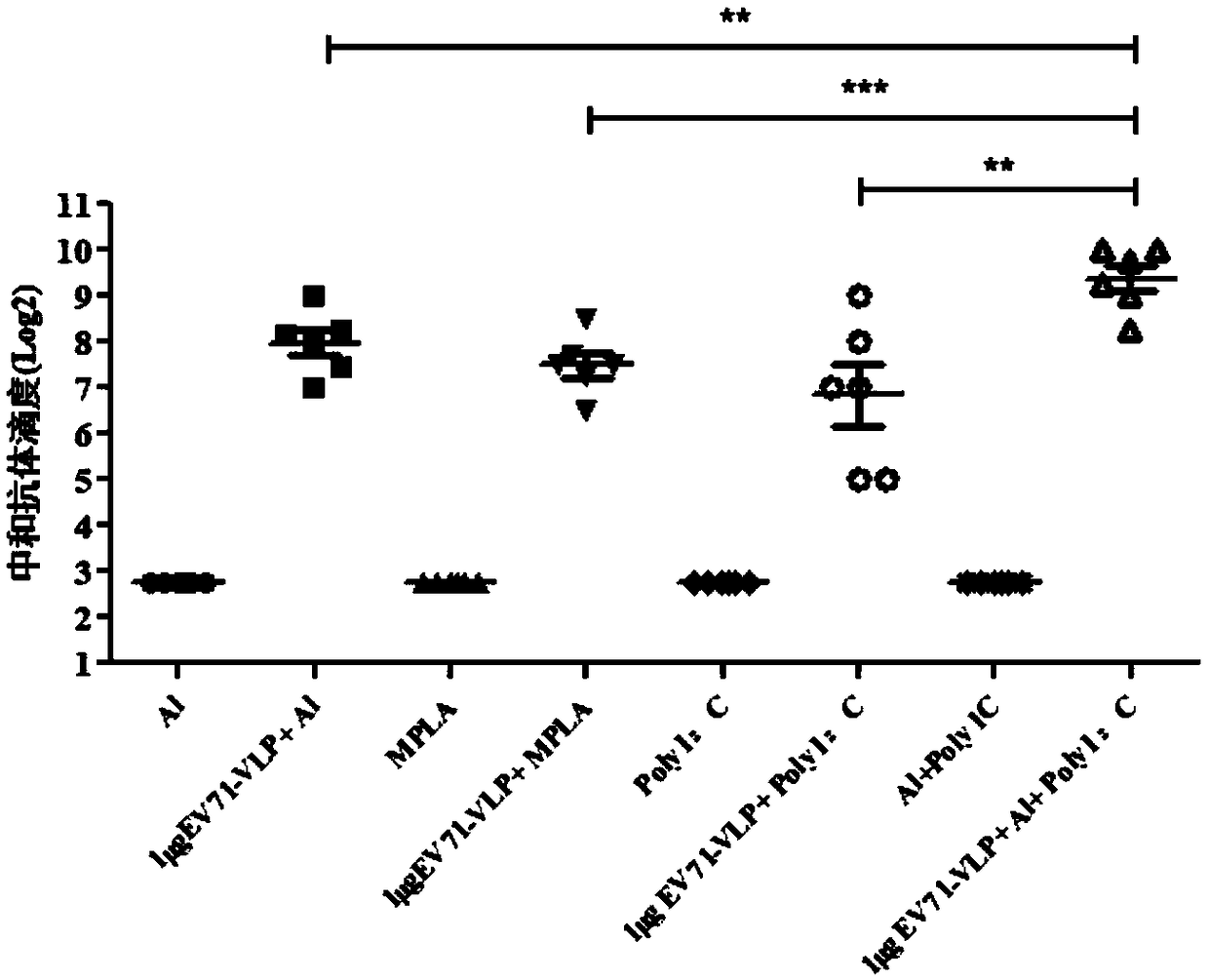
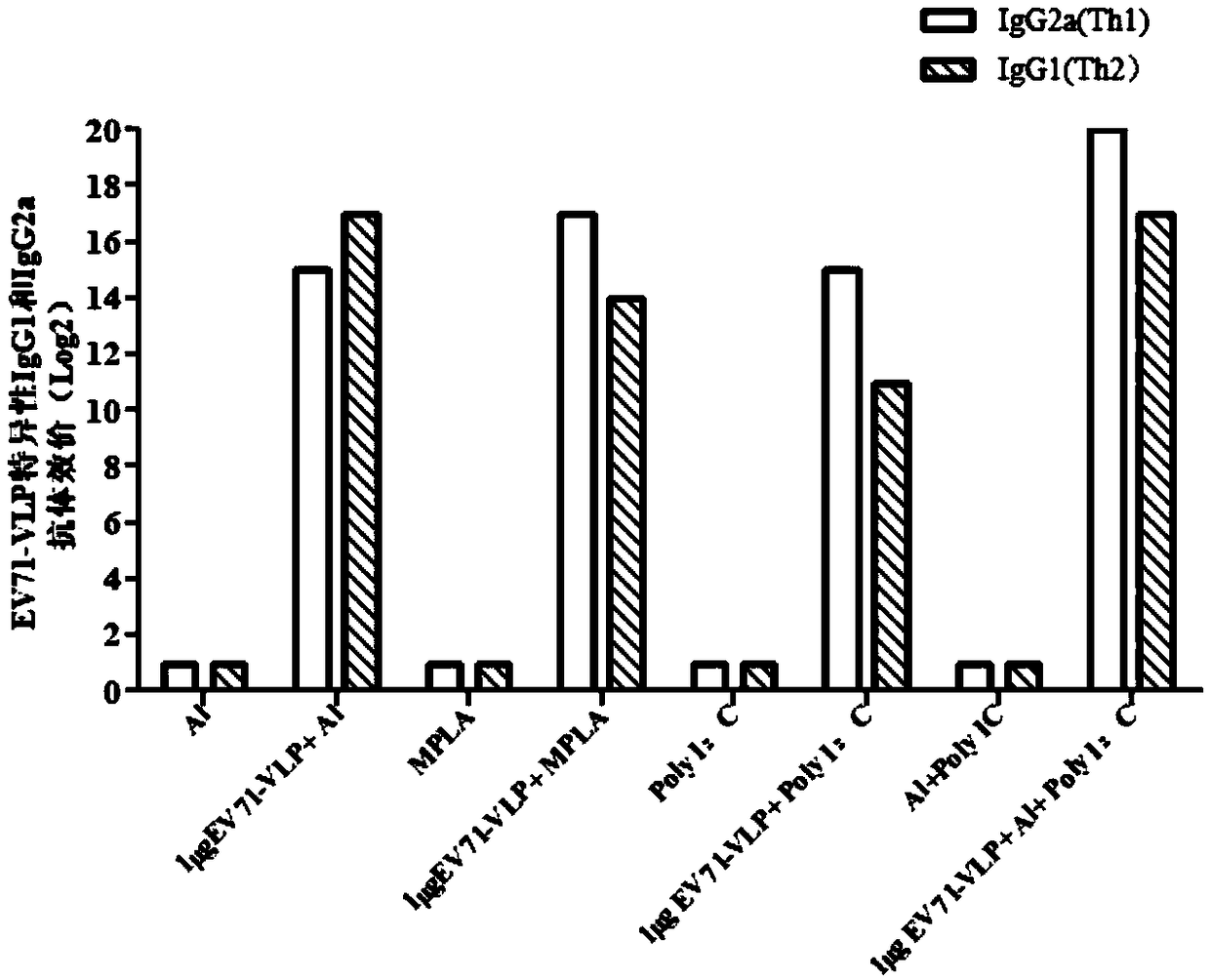
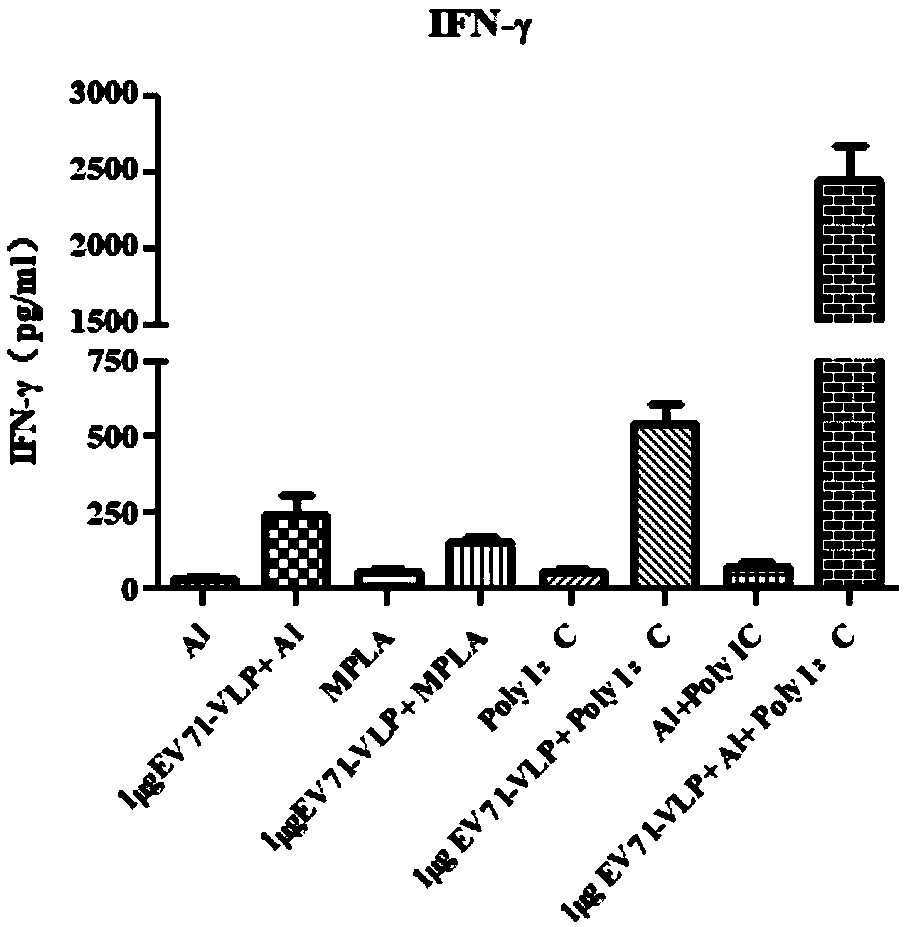
The invention discloses an EV71 vaccine containing an adjuvant combination. The EV71 vaccine is prepared from the following main components: EV71 virus-like particles (EV71-VLPs), an aluminum adjuvantand polyinosinic acid cytidylic acid (poly I: C). The immunogenicity of the EV71 protein vaccine provided by the invention is superior to that of monophosphate lipids A (MPLA), Al or a poly I: C adjuvant vaccine which is singly used currently; the EV71 vaccine not only can well induce humoral immune response with antigen specificity, but also can induce high-level cellular immune response; the vaccine can effectively prevent related diseases caused by EV71 virus infection.
Owner:SOUTH CHINA UNITED VACCINE INST
A kind of compound vaccine adjuvant composition
ActiveCN107441485BEnhance cross immune protectionImprove immunitySsRNA viruses positive-senseViral antigen ingredientsTGE VACCINEMedicinal chemistry
Owner:PU LIKE BIO ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com