Polyinosinic acid-polycytidylic acid dominated adjuvant
A polyribocytidylic acid and polynucleotide technology, which is used in medical preparations containing active ingredients, antibody medical ingredients, pharmaceutical formulas, etc., and can solve problems such as adverse side effects and inability to provide efficacy/safety status.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0209] Embodiment 1. Measure the molecular weight of PIKA and AV-PICKCA
[0210] This example illustrates the assay format for PIKA adjuvant and compares it with Av-PICKCa.
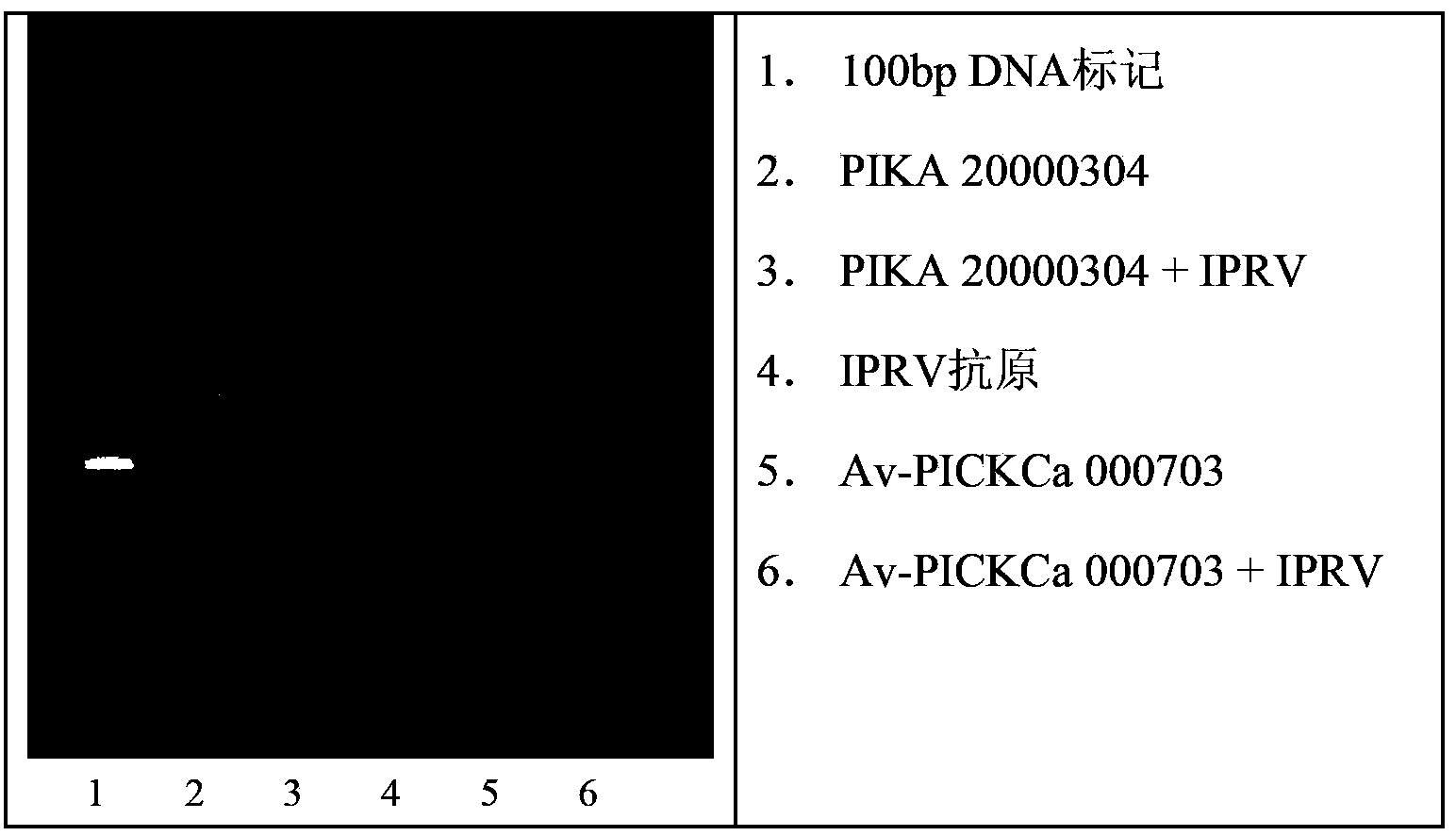
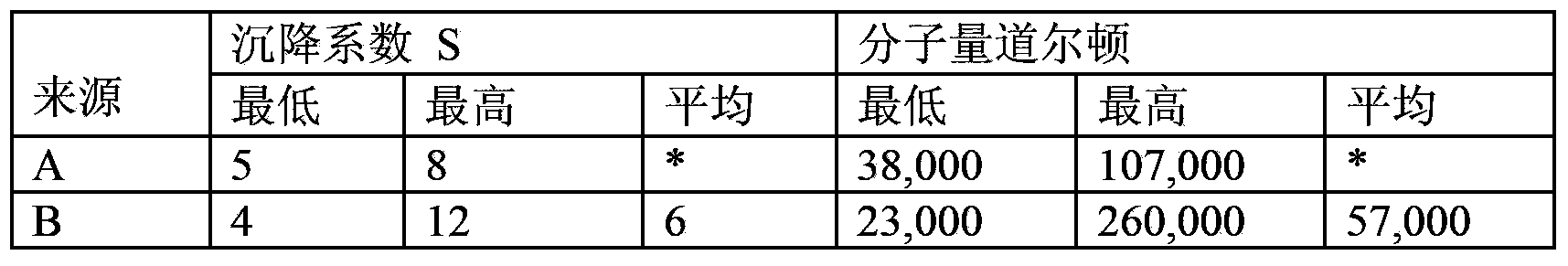
[0211] Agarose gel electrophoresis is well known to those skilled in the art, so this description only describes the features of the invention. The agarose gel used in the present invention has a concentration of 1.5% agarose. Molecular markers are 100bp DNA ladders from 100bp to 1000bp, corresponding to 6.6x10 4 up to 6.6x10 5 Dalton's molecular weight range. The concentration of the 4ul loaded sample is 1mg / ml. figure 1 Shows a representative graph of the results of an agarose gel sample obtained following the teachings of this paragraph. Five (5) different batches were tested showing a broad range of their molecular weight distributions. Their upper molecular weight limit is 2.3x10 in Av-PICKCa 5 Dalton to PIKA is 5.28x10 5 Dalton range.
Embodiment 2
[0212] Example 2. Comparison of Immunological Effectiveness of PIKA and AV-PICKCA
[0213] This example shows the difference in potency strength between Av-PICKCa with a maximum molecular weight of 230,000 Daltons and a PIKA sample with a maximum molecular weight of 528,000 Daltons.
[0214] Three batches of PIKA adjuvant with different molecular weights, and one batch with a molecular weight corresponding to the molecular weight of Av-PIKA, were combined with hamster kidney cell inactivated purified rabies antigen (HKC-IPRA). Subsequently, the resulting composition was subjected to NIH potency testing.
[0215] The NIH Efficacy Trial is a rigorous and experimentally intensive comparative study comparing a test rabies vaccine with a standard rabies vaccine. Vaccinated mice were infected with a live rabies virus strain and their survival was measured. Different dilutions of rabies vaccine were administered to different groups of mice. The potency of the experimental vaccine ...
Embodiment 3
[0219] Interferon Production Comparison Between Example 3.PIKA and Av-PICKCA
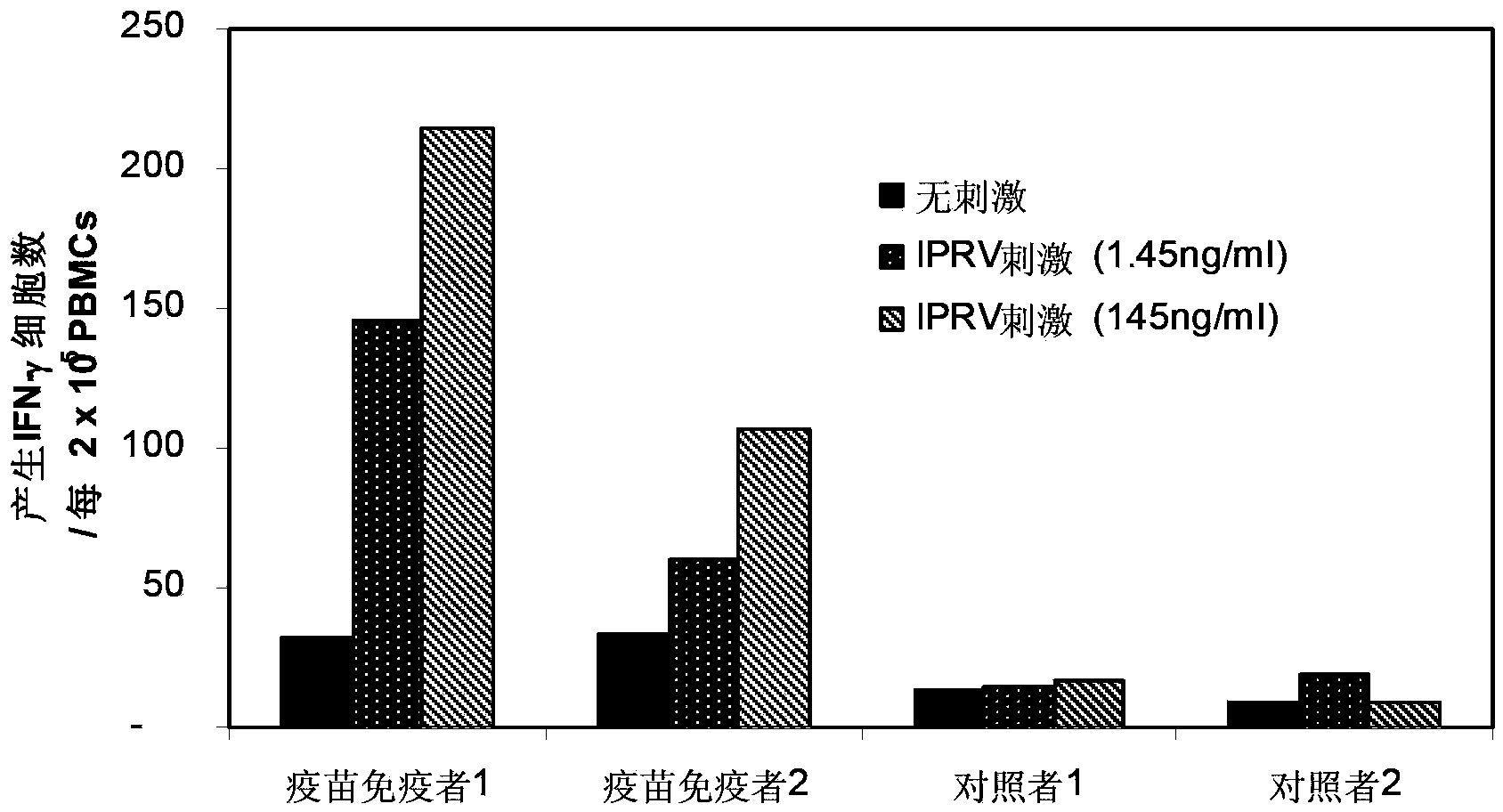
[0220] This example shows the difference in the ability to induce interferon production between Av-PICKCa samples with an upper molecular weight limit of 230,000 Daltons and PIKA samples with an upper molecular weight limit of 1,200,000 Daltons.
[0221] The second batch has an upper molecular weight limit of 1.2x10 6 Dalton and 4.6x10 5 Dalton's PIKA, and a batch with an upper molecular weight of 2.3x10 5 Dalton's Av-PICKCa for comparison.
[0222] The PIKA and Av-PICKCa compositions were combined with hamster kidney cell inactivated purified rabies antigen (HKC-IPRA). These compositions were injected subcutaneously into mice. Two hours later, the level of interferon in the serum of each mouse was measured. General procedures for measuring interferon are known to those skilled in the art. Briefly, about 30,000 L929 cells were seeded at 0.15ml / well in a 96-well culture plate. Three days later...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com