Mucosal immunity preparation capable of resisting infection and tumors
A technology of mucosal immunity and anti-infection, applied in the field of immunology, can solve the problem of weak induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1 mucosal immune preparation and preparation method thereof
[0078] (1) Using polyinosinic acid and polycytidylic acid as raw materials, use PBS buffer to prepare polyinosinic acid solution and polycytidylic acid solution; stir at 30-60 ° C to prepare 0.5-10 mg / mL double-stranded poly Inosinic acid-polycytidylic acid solution (PIC solution);
[0079] (2) Oligochitosan solution (COS solution) with a concentration of 1.6 to 12.8 mg / mL was formulated with PBS buffer solution;
[0080] (3) Add the COS solution dropwise to the double-stranded polyinosinic acid-polycytidylic acid solution obtained in step (1), add dropwise while stirring, then add the PEI solution dropwise under stirring, and then add dropwise under stirring CaCl 2 solution to obtain a mucosal immune substance sample solution;
[0081] (4) The mucosal immune substance sample solution prepared above is sterilized and filtered and distributed into suitable spray bottles or aerosol bottles to obta...
Embodiment 2
[0082] Example 2 Mucosal immune preparation and preparation method thereof
[0083] (1) Using polyinosinic acid and polycytidylic acid as raw materials, use PBS buffer to prepare polyinosinic acid solution and polycytidylic acid solution; stir at 30-60 ° C to prepare 0.5-10 mg / mL double-stranded poly Inosinic acid-polycytidylic acid solution (PIC solution);
[0084] (2) Oligochitosan solution (COS solution) with a concentration of 1.6 to 12.8 mg / mL was formulated with PBS buffer solution;
[0085] Or use PBS buffer to prepare PEG-g-COS into a PEG-g-COS solution with a concentration of 1.6-51.2mg / mL;
[0086] (3) Add PEG-g-COS solution or chitosan oligosaccharide solution dropwise to the double-stranded polyinosinic acid-polycytidylic acid solution obtained in step (1), add dropwise while stirring, and then add dropwise under stirring CaCl 2 solution to obtain PIC-PEG-g-COS-CaCl 2 or PIC-COS-CaCl 2 Complex;
[0087] (4) Mix PIC-PEG-g-COS-CaCl on a constant temperature mag...
experiment example 1
[0089] Experimental Example 1 Nasal Drops Mucosal Immunization Preparation Alone Anti-Influenza Mice Protection Test
[0090] Influenza virus: Subtype A murine lung-adapted strain FM1, purchased from the Institute of Viral Disease Control, Chinese Academy of Preventive Medicine.
[0091] Ribavirin: positive control drug, purchased from Shenyang Yanfeng Pharmaceutical Factory.
[0092] White mice: Kunming species, 8-10g for FM1 virus subculture, 14-20g for the following formal experiments.
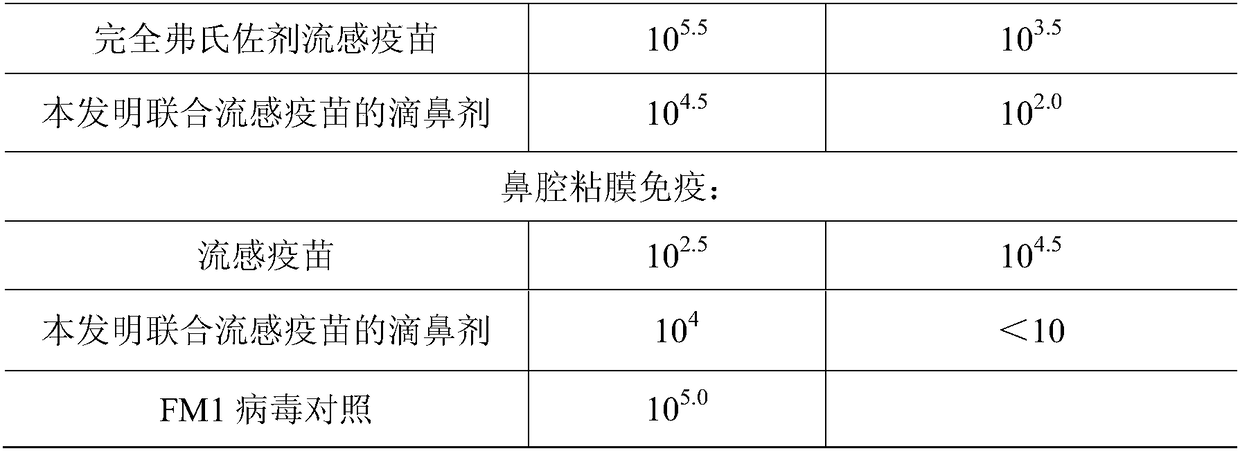
[0093] Influenza virus FM1 strain mouse lung suspension with 5LD 50 / only can cause lethal pneumonia by intranasally dripping to mice, infect earlier during the test and then administer the drug, and carry out the grouping test according to Table 1.
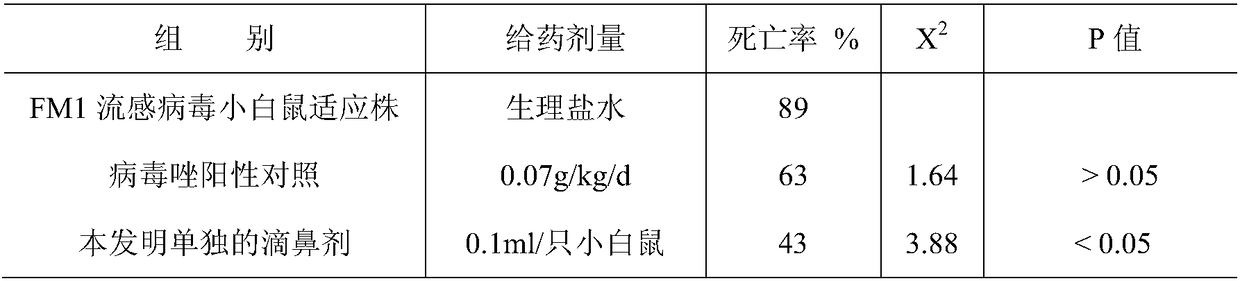
[0094] Table 1 Mucosal immune preparation of the present invention nasal drop method anti-influenza mouse protection test
[0095]
[0096] The experimental results show that in the mouse protection test, the nasal drops of the present ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com