Patents
Literature
183 results about "Nasal Drops" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
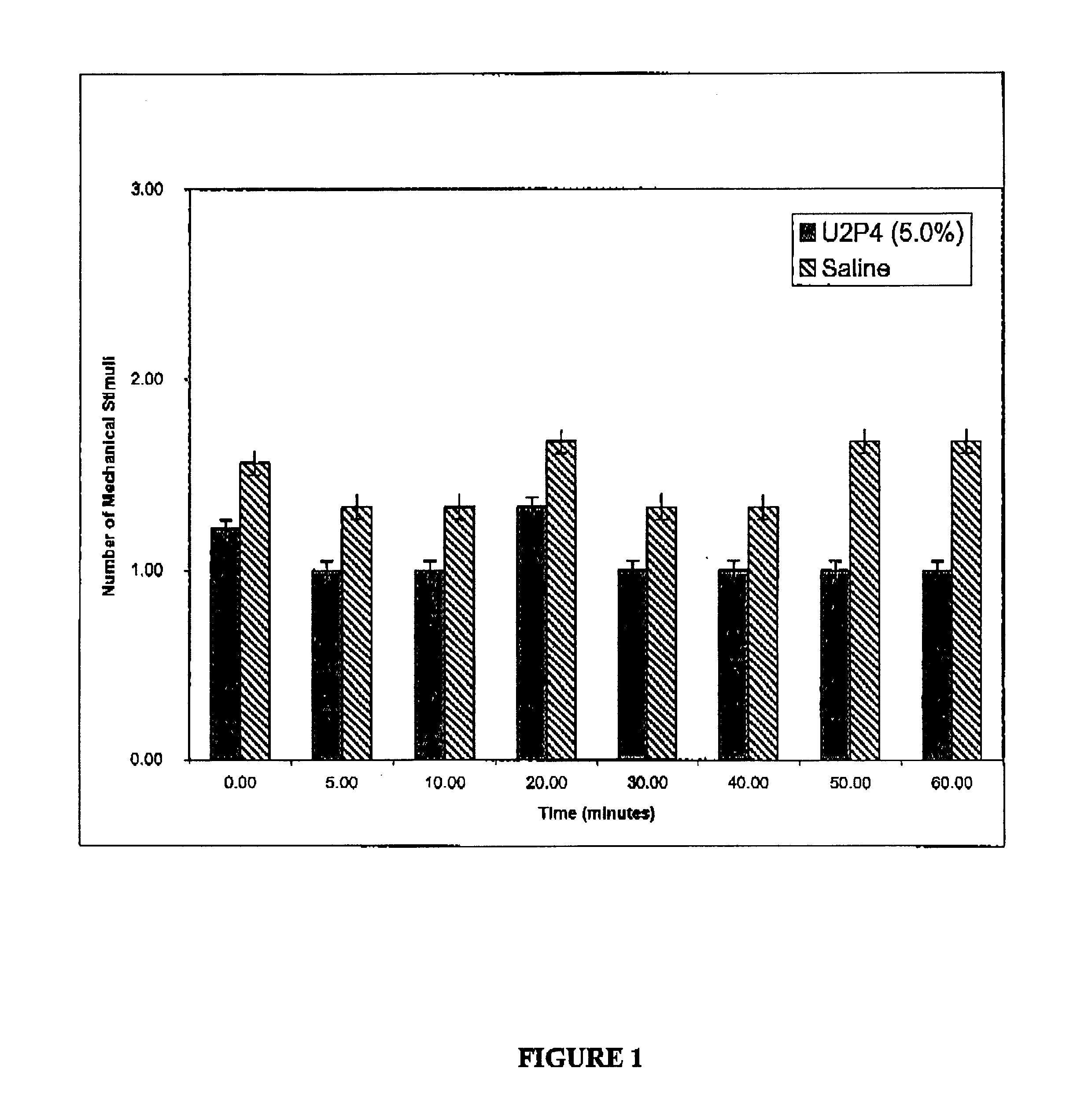
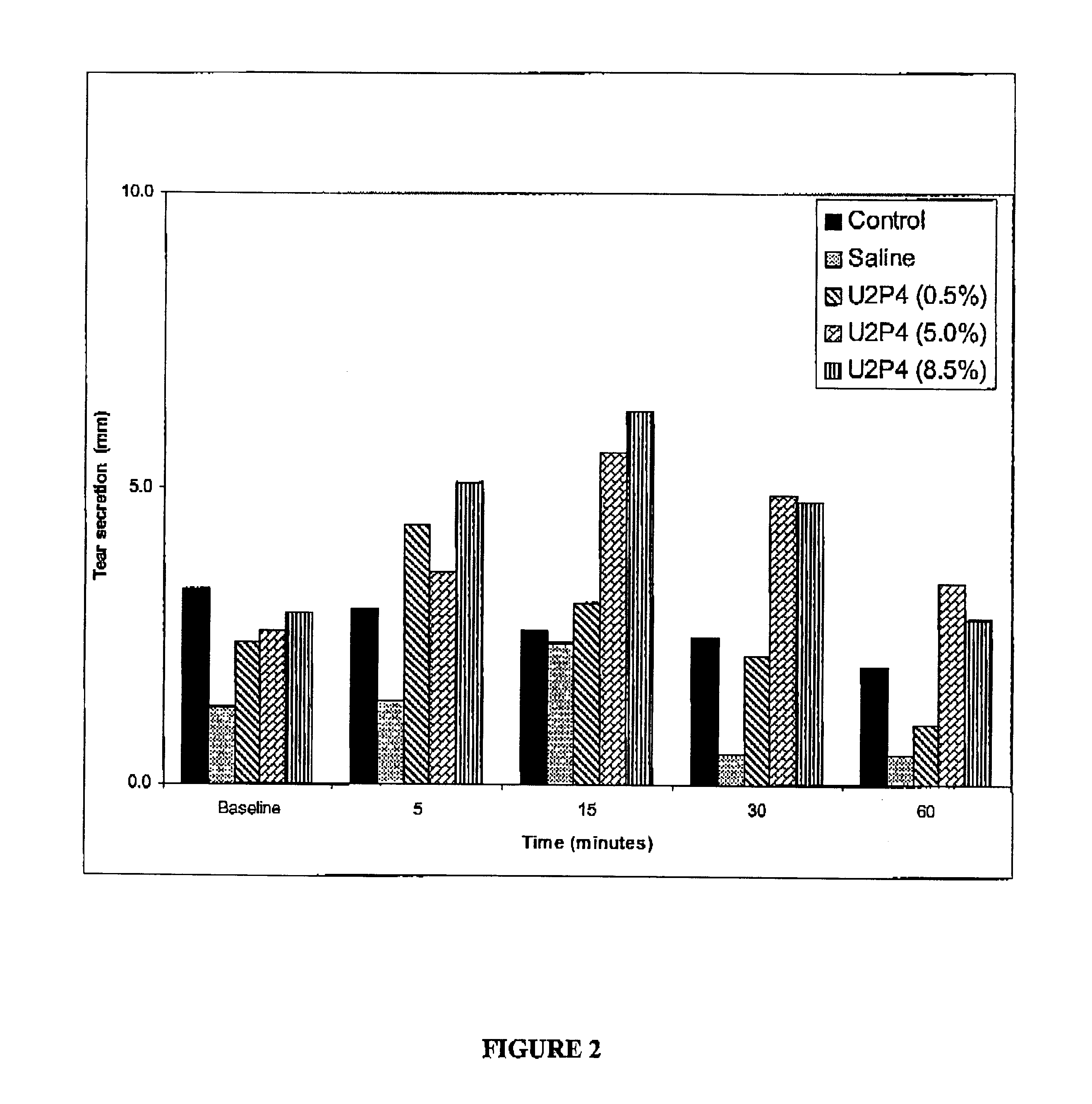
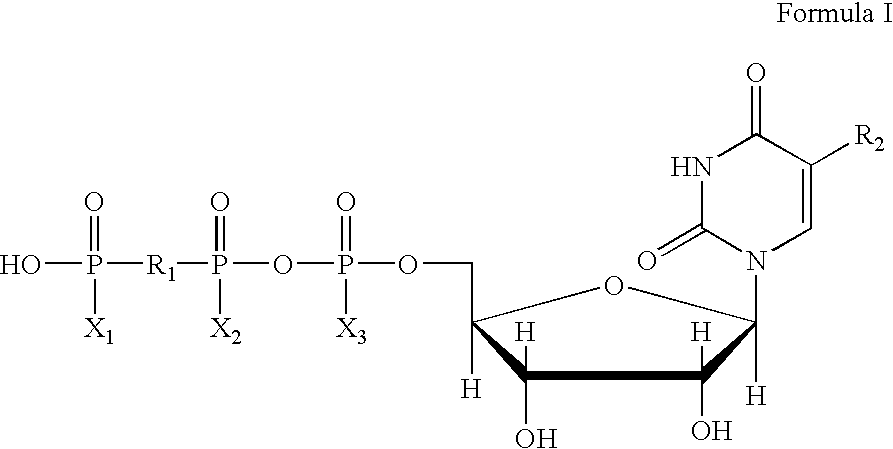
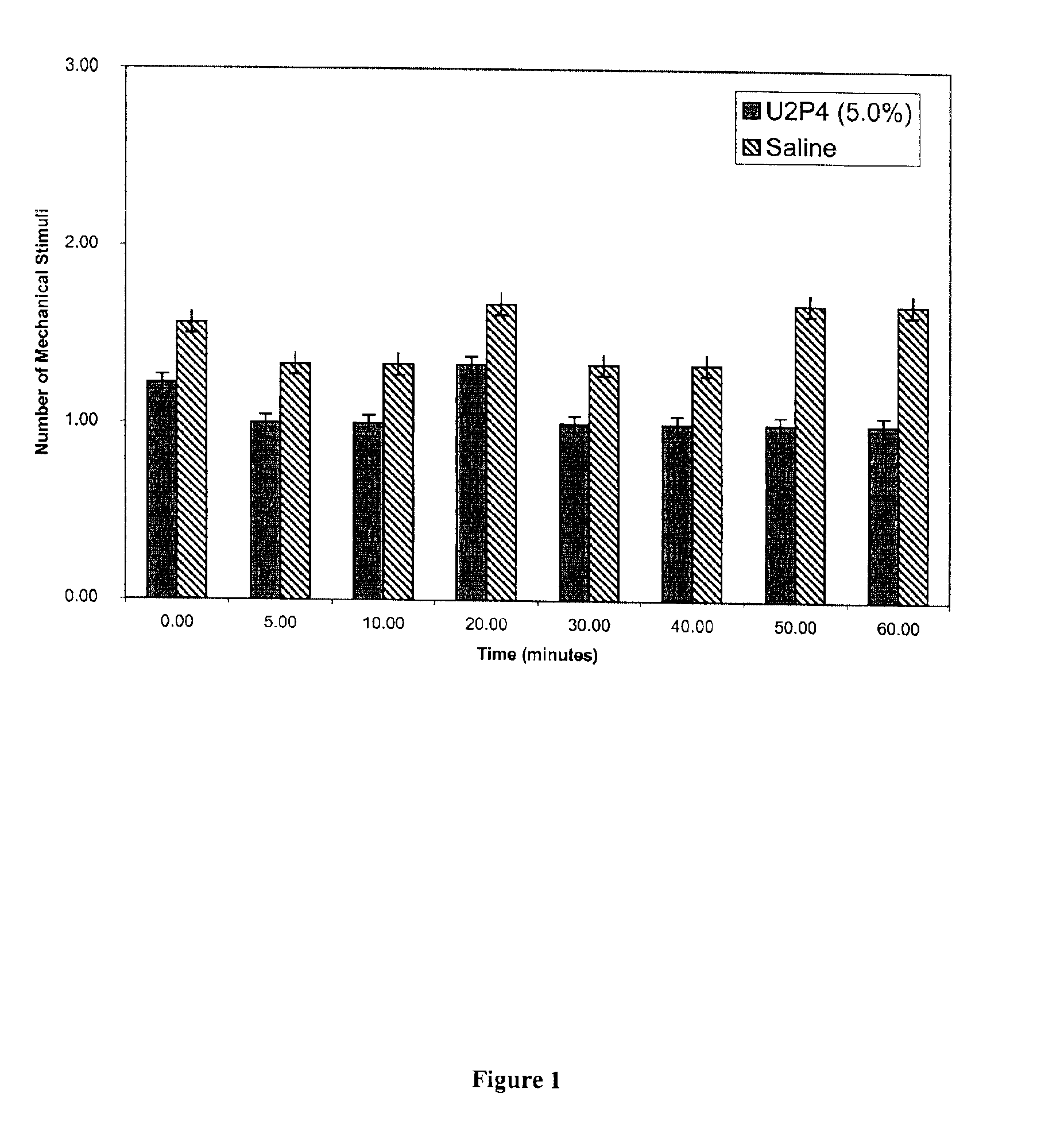
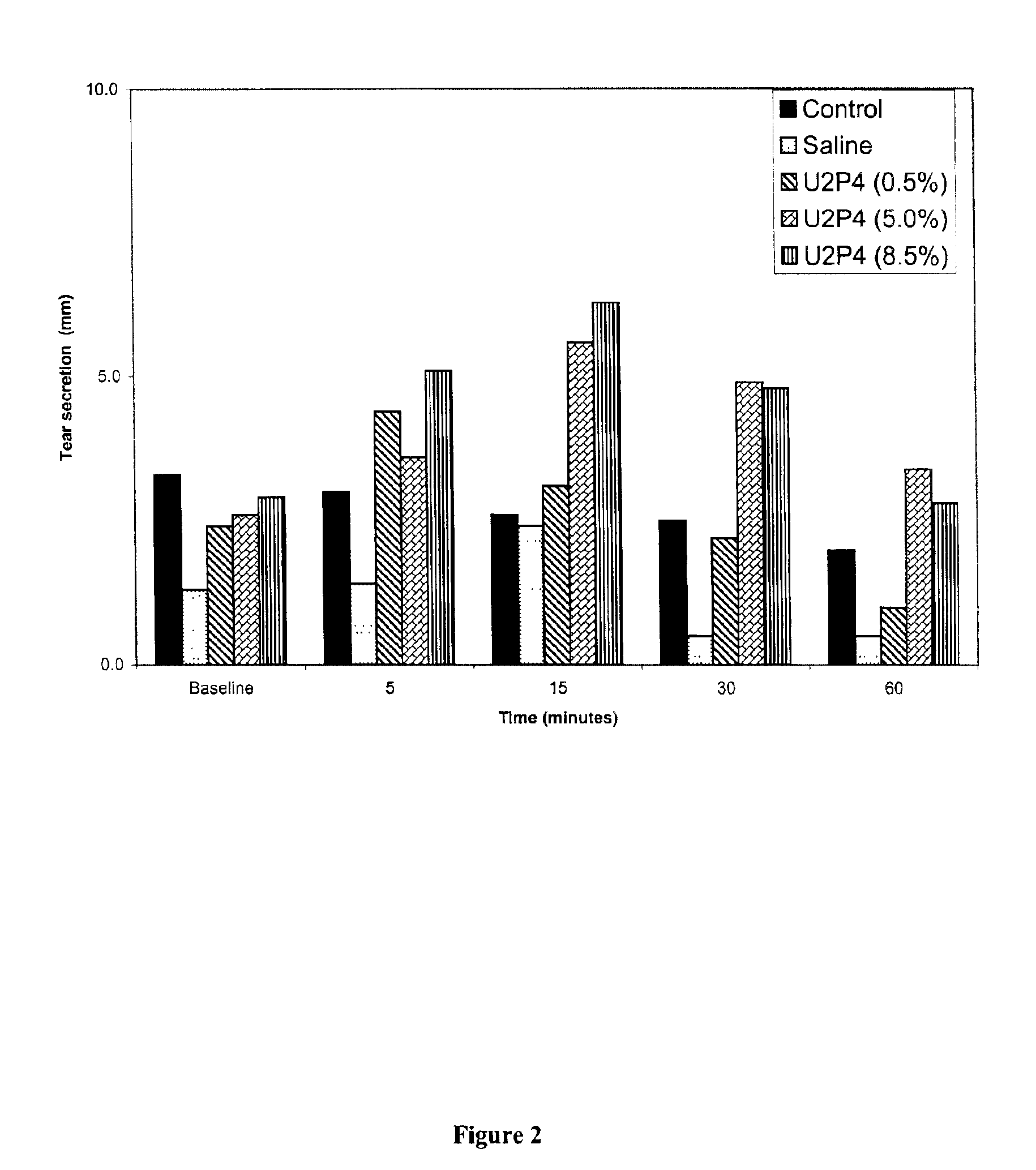
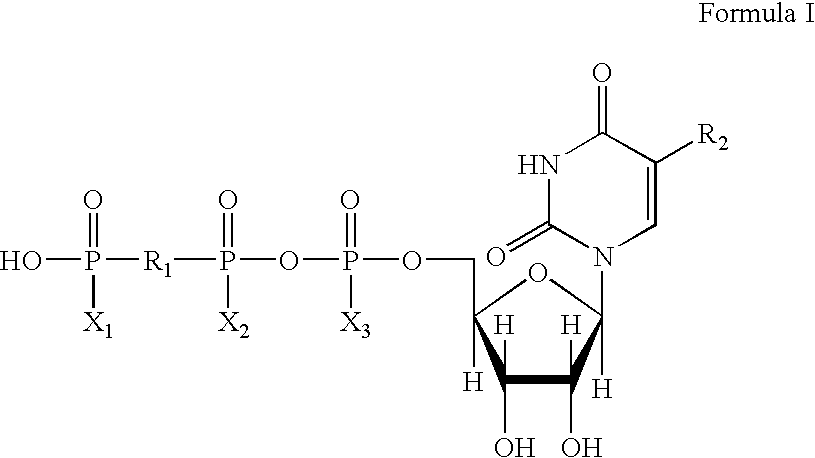
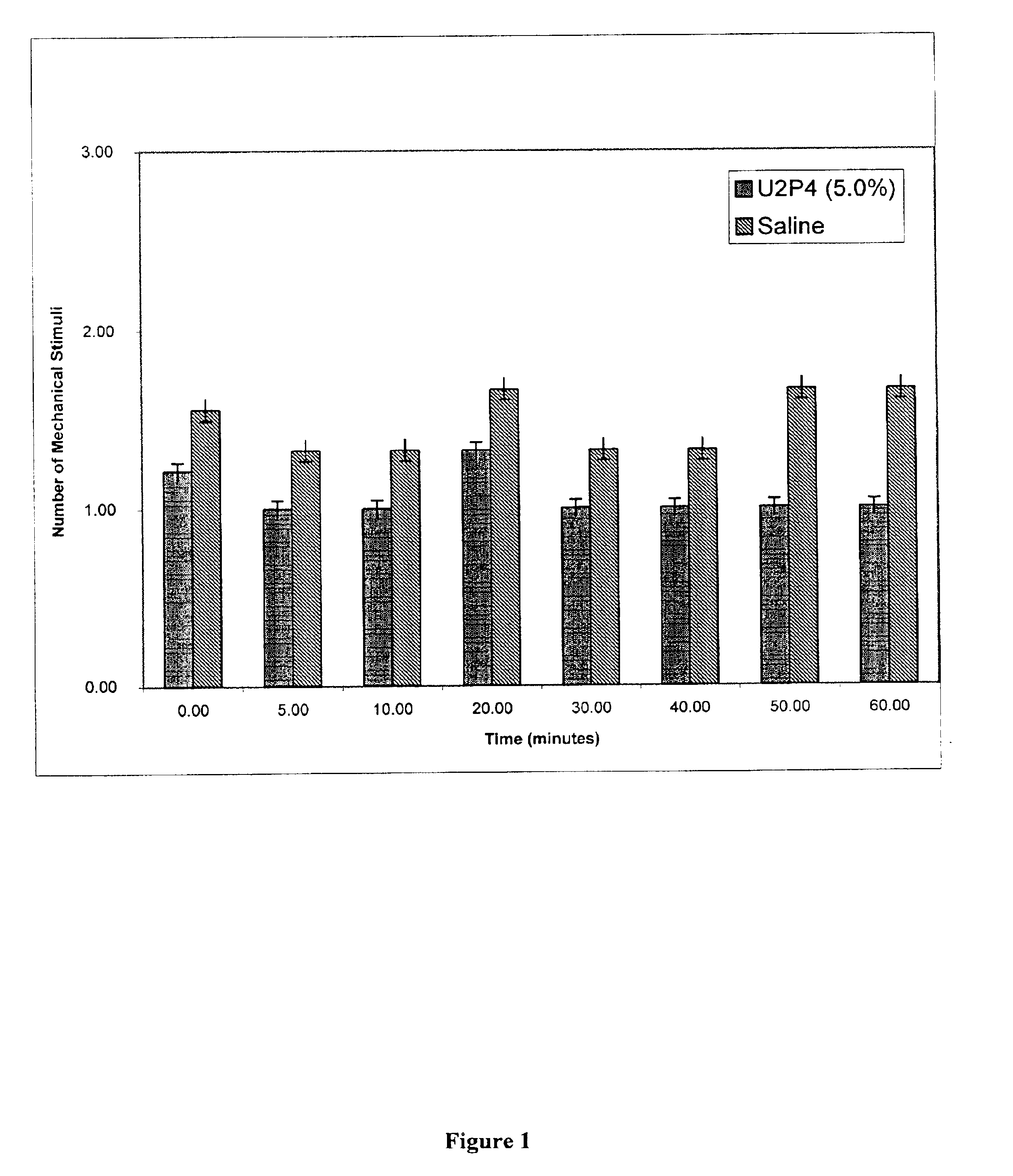
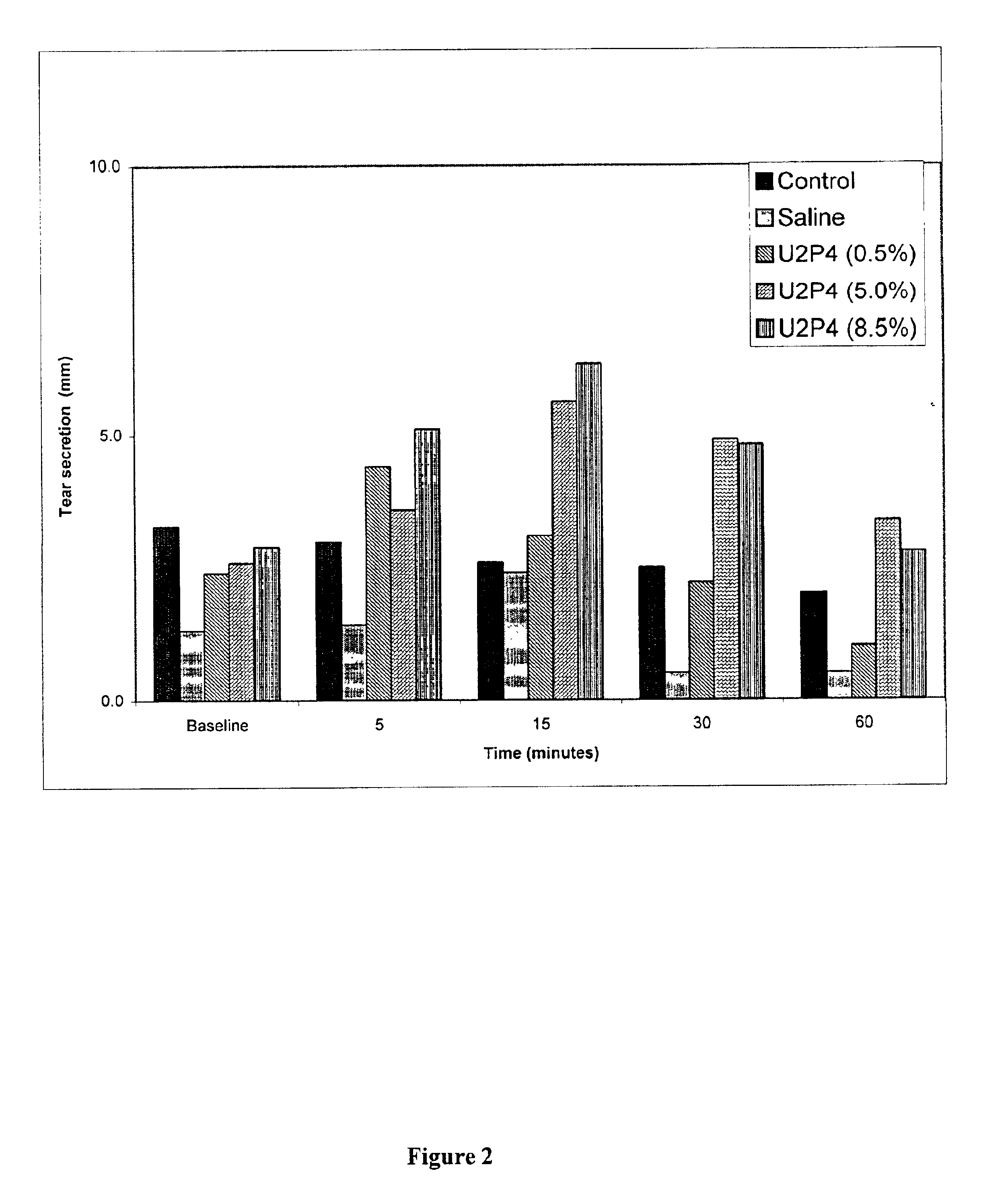
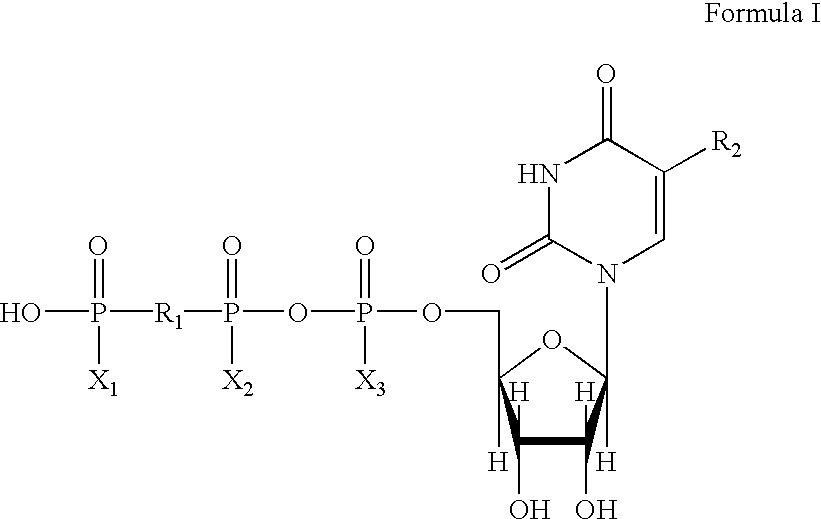
Method of treating dry eye disease with purinergic receptor agonists
A method and preparation for the stimulation of tear secretion in a subject in need of such treatment is disclosed. The method comprises administering to the ocular surfaces of the subject a purinergic receptor agonist such as uridine 5′-triphosphate [UTP], dinucleotides, cytidine 5′-triphosphate [CTP], adenosine 5′-triphosphate [ATP], or their therapeutically useful analogs and derivatives, in an amount effective to stimulate tear fluid secretion and enhance drainage of the lacrimal system. Pharmaceutical formulations and methods of making the same are also disclosed. Methods of administering the same would include: topical administration via a liquid, gel, cream, or as part of a contact lens or selective release membrane; or systemic administration via nasal drops or spray, inhalation by nebulizer or other device, oral form (liquid or pill), injectable, intra-operative instillation or suppository form.
Owner:MERCK SHARP & DOHME CORP
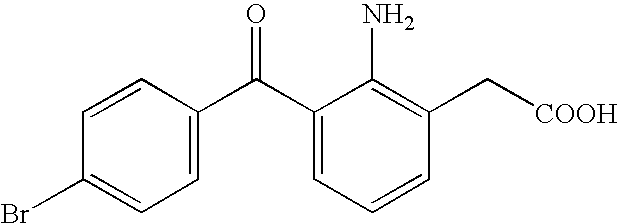
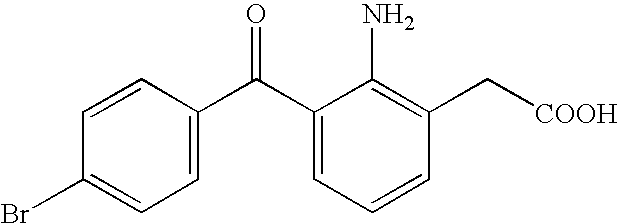
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
ActiveUS20050239895A1No irritationInhibit deteriorationBiocideOrganic active ingredientsScleritisPhenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
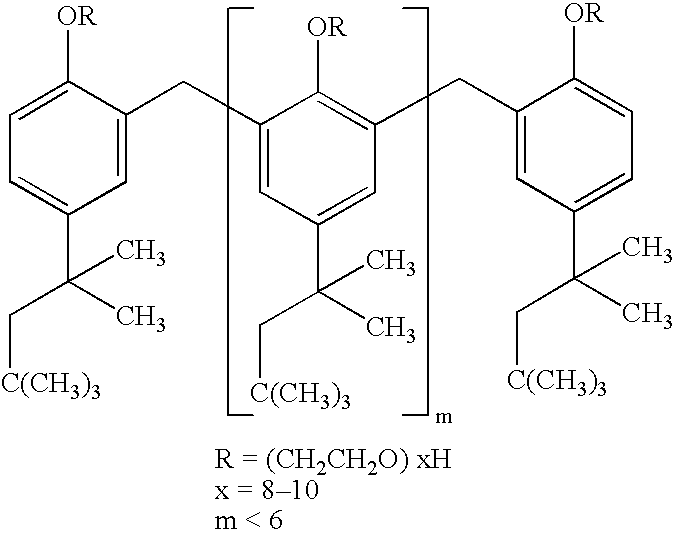
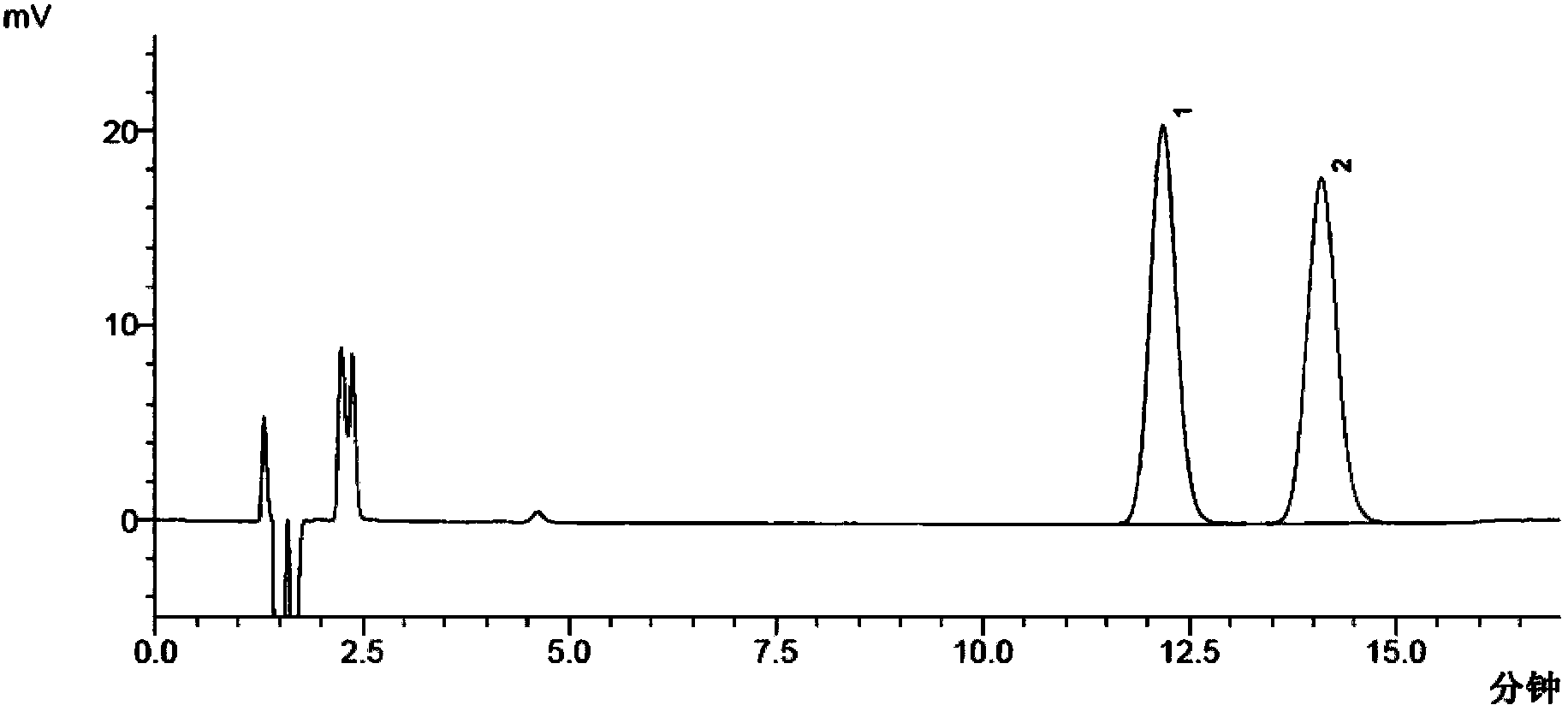
Aqueous suspension preparations with excellent redispersibility
InactiveUS6274634B1Reduce surface tensionSurface tensionBiocideSolution deliveryOral medicationLotion
The aqueous suspension can be prepared by incorporating, in an aqueous suspension of a hardly soluble drug, a water-soluble polymer within the concentration range from the concentration at which the surface tension of the aqueous suspension of the drug begins to decrease up to the concentration at which the reduction in surface tension ceases. The resulting aqueous suspension shows ready redispersibility and will not undergo aggregation of dispersed particles or caking. Because of its good redispersibility, the suspension is useful as a parenteral preparation, eye drops, nasal drops, a preparation for oral administration, a lotion or the like.
Owner:SENJU PHARMA CO LTD
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
Aqueous suspension preparations
InactiveUS20030195179A1Good redispersibilityAntibacterial agentsPowder deliverySuspended particlesWater soluble
Addition of polyvinylpyrrolidone and a water-soluble anionic macromolecular compound to an aqueous suspension of a hardly soluble drug allows to provide an aqueous suspension in which aggregation of drug particles, formation of macro crystals from suspended particles and formation of secondary particles from deposited particles are prevented, and adhesion and adsorption to containers made of plastics, e.g., polypropylene or polyethylene, are avoided. As it has a good redispersibility, the aqueous suspension is useful as eye drops, nasal drops, ear drops, injections, oral preparations, liniments and lotions.
Owner:SENJU PHARMA CO LTD
Pralmorelin-containing nasal drop preparations
InactiveUS7008927B2Promote absorptionImprove stabilitySenses disorderPeptide/protein ingredientsPralmorelinIn vivo absorption
The preparation for intranasal administration comprising D-alanyl-3-(naphthalen-2-yl)-D-alanyl-L-alanyl-L-tryptophyl-D-phenylalanyl-L-lysinamide (pralmorelin) and / or an acid addition salt thereof as an active ingredient and water permits a marked increase in the in vivo absorption of pralmorelin and hence provides adequate efficacy even if it is administered in a small dose at a time. The preparation also allows pralmorelin to be dissolved in an increased amount, so it can be formulated pharmaceutically with great ease. It also high stability over time.
Owner:KAKEN PHARMA CO LTD
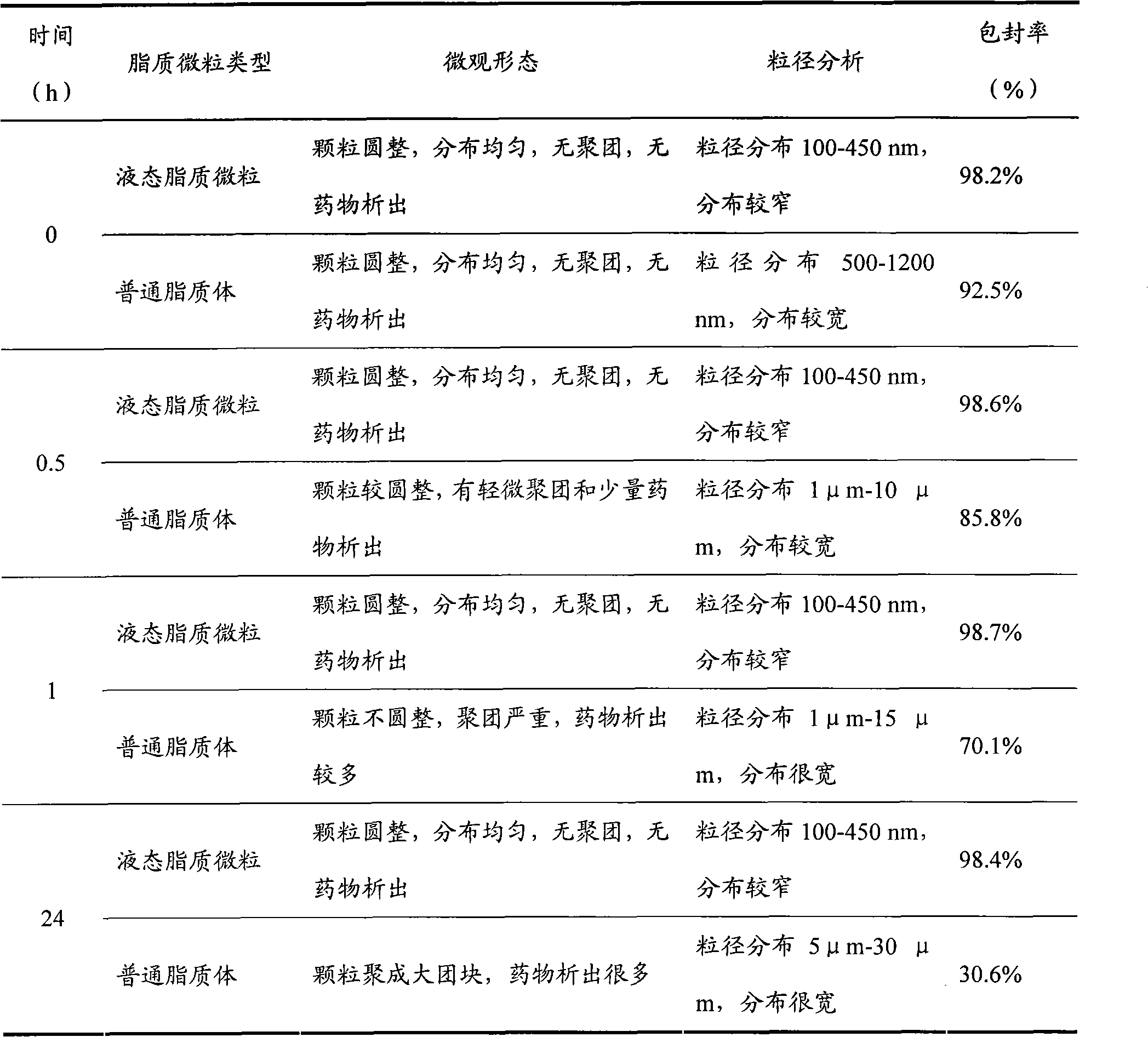
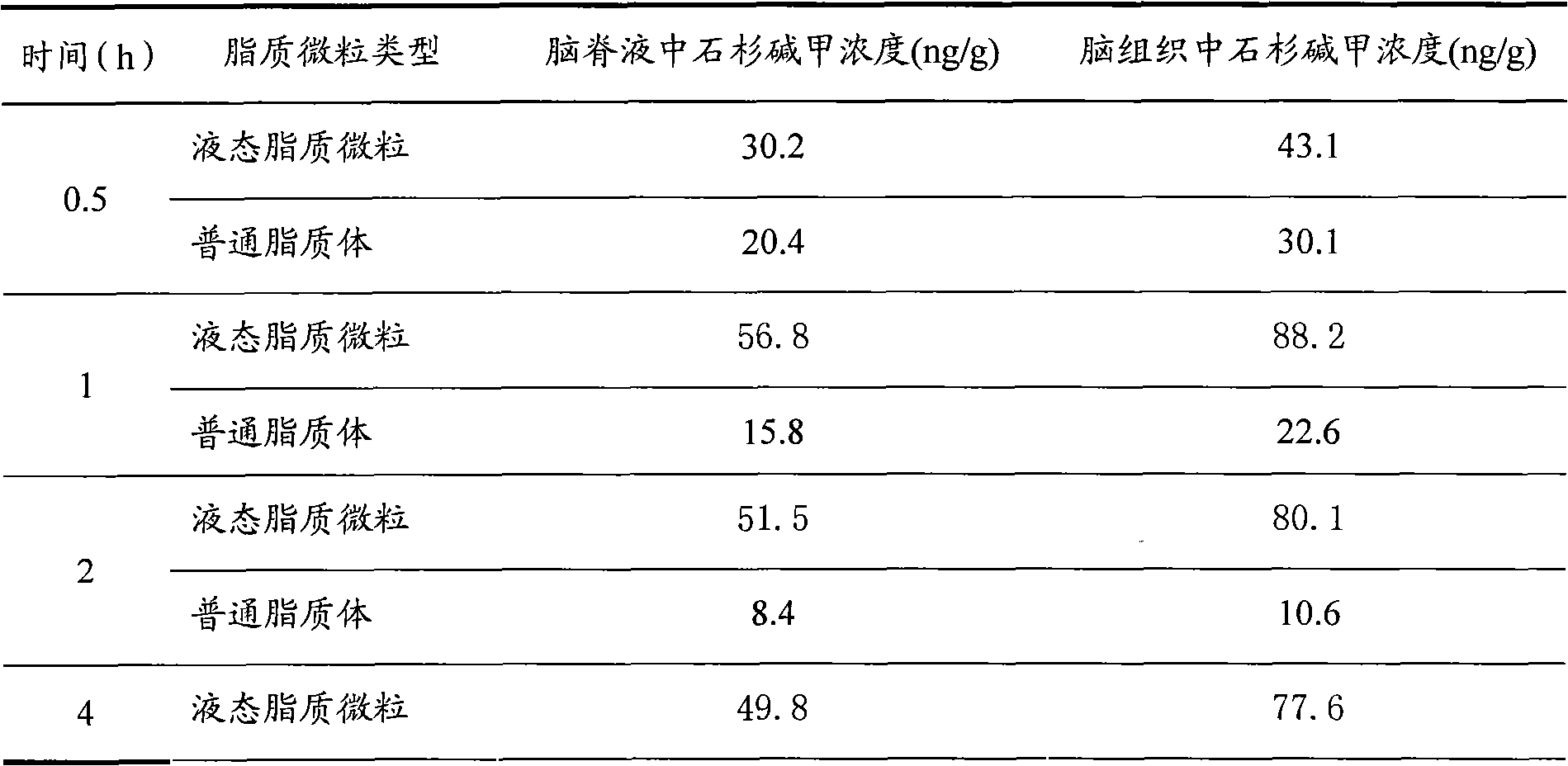
Liquid-state lipid micro-particles used for delivering cerebric medicine through olfactory pathway, preparation method thereof, and preparation thereof
InactiveCN102370623AStable structureImprove stabilityNervous disorderAerosol deliveryHydroxyethyl starchDisease
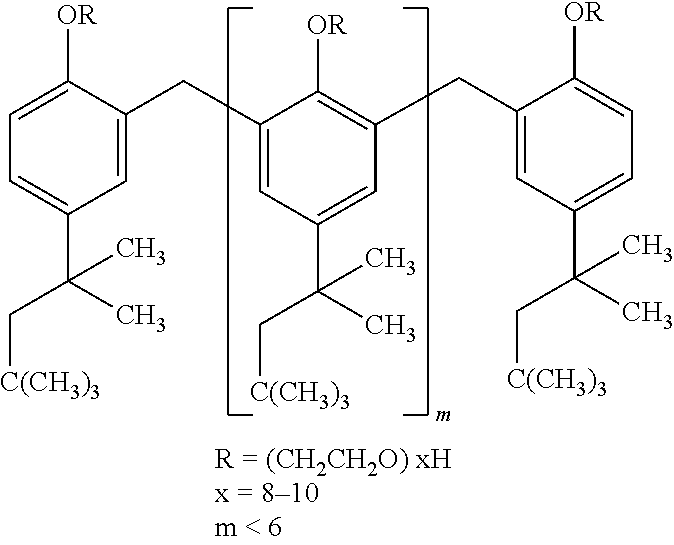
The invention relates to liquid-state lipid micro-particles used for delivering a cerebric medicine through a nose olfactory pathway, a preparation method thereof, and a preparation thereof. The liquid-state lipid micro-particles at least comprise a medicine, a lipid material, a penetrating agent, hydroxyethyl starch, propylene glycol and water. The mass ratio of the medicine to the lipid material is 1:1 to 1:10. The volume ratio of propylene glycol to water is 2:1 to 1:10. The liquid-state lipid micro-particles are advantaged in large drug load, high deformation capacity, simple preparation method, and high entrapment rate. The micro-particles are beneficial for the medicine to be delivered into the brain through the nose olfactory pathway and to provide curative effects. The range of appropriate medicines of the liquid-state lipid micro-particles is large, and the liquid-state lipid micro-particles are especially suitable for hormone, polypeptide, gene or vaccine medicines. With the liquid-state lipid micro-particles, preparations such as aerosols, nasal drops, and gels can be produced, such that requirements of cerebric disease medication can be satisfied.
Owner:ZHEJIANG HISUN PHARMA CO LTD
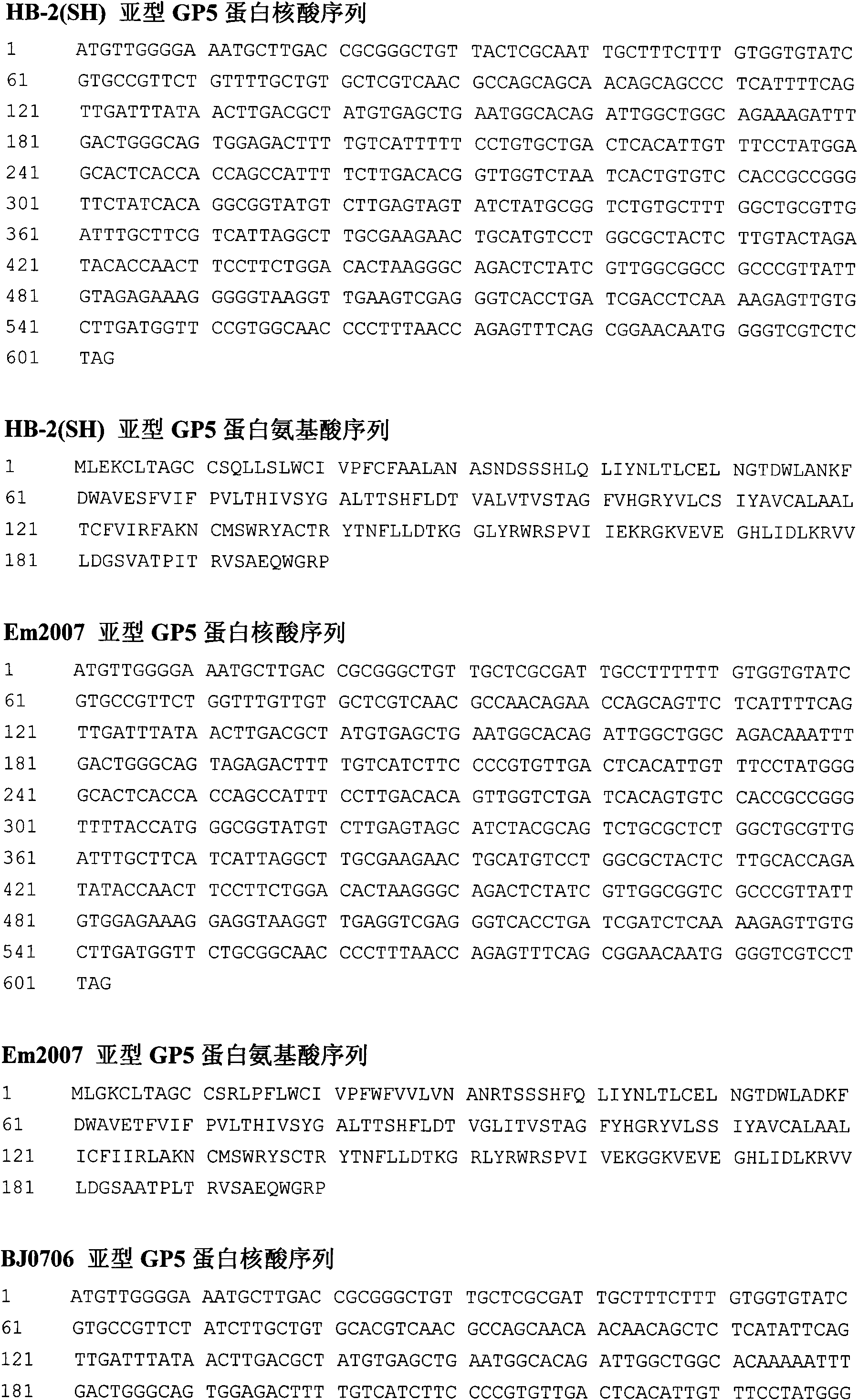
Porcine reproductive and respiratory syndrome virus-like particle vaccine and preparation method thereof
A porcine reproductive and respiratory syndrome virus-like particle vaccine and a preparation method thereof relate to the field of biological medicines and aims at disclosing the porcine reproductive and respiratory syndrome virus-like particle vaccine (PRRS VLP vaccine) and the preparation method the vaccine. The PRRS VLP vaccine contains a VLP comprising three structure proteins of porcine reproductive and respiratory viruses M, N and GP5 and can excite favorable dual cell and humoral immune response. By adding or not adding an adjuvant into the formed VLP protein antigen, the pharmacodynamic test shows the prepared injection type, nasal drop type and water agent type vaccines are immunized with different animal groups so as to safely and effectively prevent the PRRSV infection and provide ideal vaccines for safely and effectively immunizing, preventing and controlling the PRRSV infection on different groups of sows, piglets and fat pigs.
Owner:CHONGQING UNIV
Compound and pharmaceutical composition for treating nasal oversecreation and chronic obstructive pulmonary disease
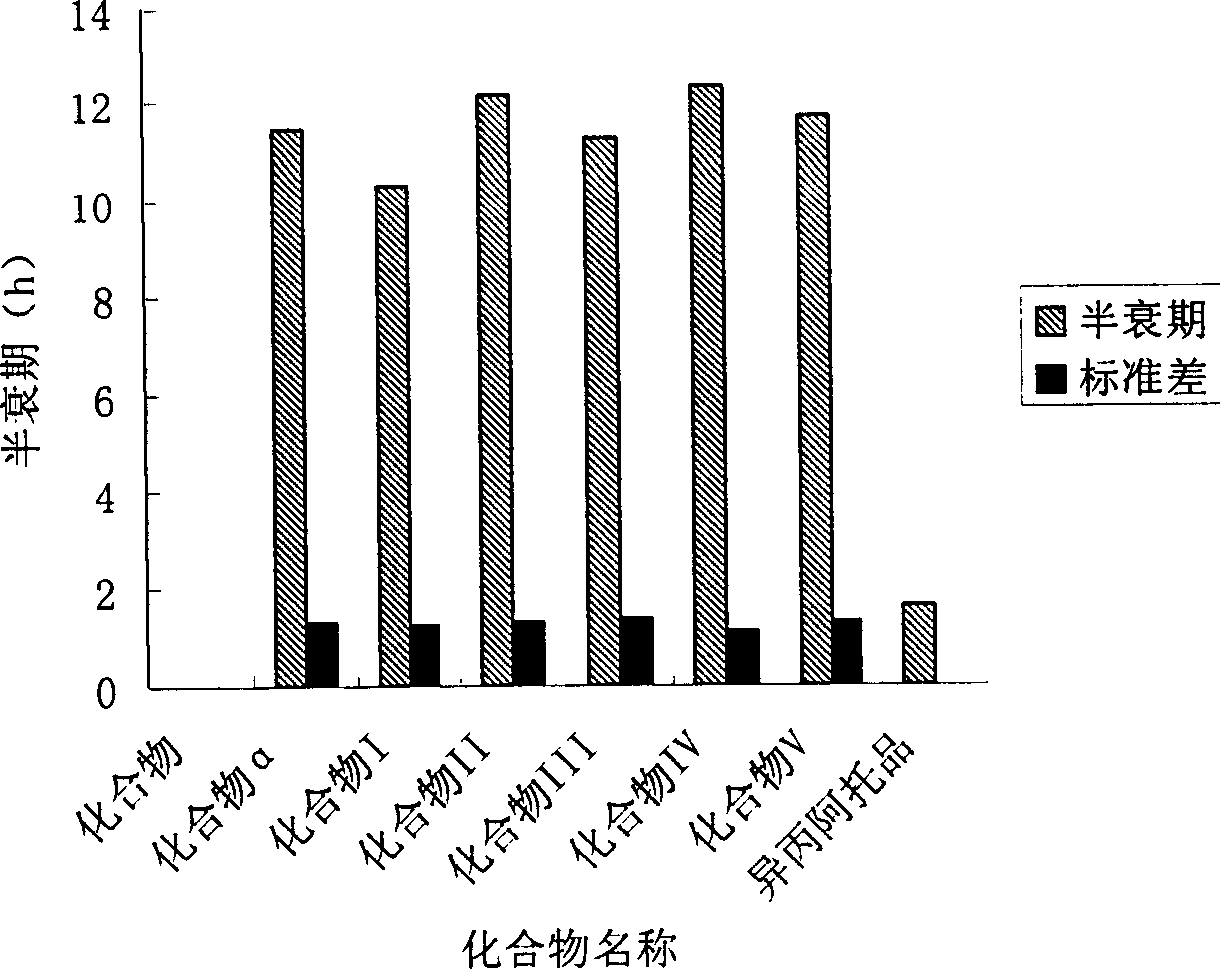
ActiveCN1769286AExtended half-lifeReduce the number of medicationsPowder deliveryOrganic active ingredientsNasal cavityObstructive Pulmonary Diseases
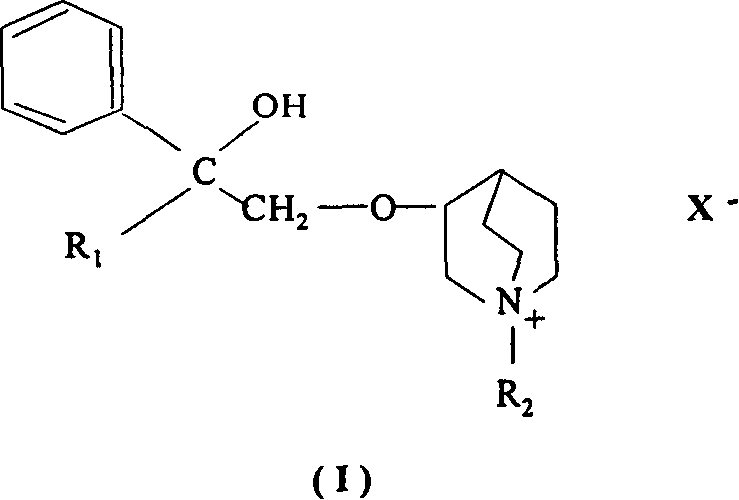
The invention discloses the drug of treating the nasal over-secretion and chronic obstructive emphysema, at the mean time, provides the drug compositions of treating the nasal over-secretion and chronic obstructive emphysema, which are aerosol, powder cloud agent, naristillae and spray. In the invention, the compositions, which are introduced quaternary ammonium group on the basis of penehyclidine hydrochloride, can't permeate membrane and enter into the circulatory system and central, so it can be used in treating the chronic obstructive emphysema. Besides, due to the low absorptivity in the mucous membrane position, the said new compositions are extended the resistance time at local, so it can be used in treating the chronic obstructive emphysema and coryza.
Owner:YINGU PHARMA
Method of treating dry eye disease with non-drying antihistamines
InactiveUS7247623B2Reducing dry eye symptomsPromote secretionBiocideSenses disorderDiseaseEpinastine Hydrochloride
A method and preparation for reducing dry eye symptoms and promoting tear secretion in a subject in need of such treatment is disclosed. The method comprises administering to the eyes of the subject a non-drying antihistamine compound, such as epinastine hydrochloride, in an amount effective to reduce dry eye symptoms and stimulate tear fluid secretion. Pharmaceutical formulations and methods of making the same are also disclosed. Methods of administering the compound include topical administration via a liquid, gel, cream, or as part of a contact lens or a continuous or selective release device; or systemic administration via nasal drops or spray, inhalation by nebulizer or other device, oral form (liquid or pill), injectable, intra-operative instillation or suppository form.
Owner:INSPIRE PHARMA
Method for separating and measuring moxifloxacin hydrochloride and enantiomer thereof
InactiveCN103543230AChiral resolution achievedAccurate detectionComponent separationControl releaseEnantiomer
The invention discloses a method for separating and measuring moxifloxacin hydrochloride and an enantiomer thereof. According to the method, high performance liquid chromatography is adopted, a reversed phase column is used, a chiral reagent serves as a mobile phase, the enantiomer in the moxifloxacin hydrochloride is positioned according to a moxifloxacin hydrochloride racemate, the enantiomer of the moxifloxacin hydrochloride existing in various dosage forms including sustained release type, controlled release type or common tablets, capsules, granules, oral liquid, injection, eye drops, nasal drops, auristilla, suppository or injection can be checked, the quality standard of the conventional moxifloxacin hydrochloride is improved, and a basis is provided for setting an enantiomer measurement standard of the moxifloxacin hydrochloride in the future.
Owner:NEW FOUNDER HLDG DEV LLC +2
A nasal drop for treating rhinitis and method for preparing same
InactiveCN1879791ASignificant effectNo irritation symptomsHydroxy compound active ingredientsMammal material medical ingredientsLicorice rootsCockleburs
Disclosed is a drop agent for treating rhinitis and its preparing process, wherein the medicament comprises cocklebur fruit 18-30g, magnolia flower 9-15g, spearmint 9-15g, dahurian angelica root 9-15g, asarum herb 2-6g, gamene 12-20g, honeysuckle flower 12-20g, wild chrysanthemum flower 12-20g, licorice root 12-20g, herba schizonepetae 12-20g, Chinese ephedra 6-12g, yellow corktree bark 9-15g, anemarrhena rhizome 9-15g, schisandra fruit 12-20g, borneo camphor 8-12g, musk 0.4-0.8g and sesame oil 500g.
Owner:SHANDONG BIGAO PHARMA
Aqueous suspension with good redispersibility
InactiveUS20010036966A1Reduce surface tensionSurface tensionBiocideSolution deliveryOral medicationWater soluble
The aqueous suspension can be prepared by incorporating, in an aqueous suspension of a hardly soluble drug, a water-soluble polymer within the concentration range from the concentration at which the surface tension of the aqueous suspension of the drug begins to decrease up to the concentration at which the reduction in surface tension ceases. The resulting aqueous suspension shows ready redispersibility and will not undergo aggregation of dispersed particles or caking. Because of its good redispersibility, the suspension is useful as a parenteral preparation, eye drops, nasal drops, a preparation for oral administration, a lotion or the like.
Owner:SENJU PHARMA CO LTD
Method of enhancing drainage of lacrimal system with purinergic receptor agonists
A method and preparation for the stimulation of tear secretion in a subject in need of such treatment is disclosed. The method comprises administering to the ocular surfaces of the subject a purinergic receptor agonist such as uridine 5′-triphosphate (UTP), dinucleotides, cytidine 5′-triphosphate (CTP), adenosine 5′-triphosphate (ATP), or their therapeutically useful analogs and derivatives, in an amount effective to stimulate tear fluid secretion and enhance drainage of the lacrimal system. Pharmaceutical formulations and methods of making the same are also disclosed. Methods of administering the same would include: topical administration via a liquid, gel, cream, or as part of a contact lens or selective release membrane; or systemic administration via nasal drops or spray, inhalation by nebulizer or other device, oral form (liquid or pill), injectable, intra-operative instillation or suppository form.
Owner:MERCK SHARP & DOHME CORP
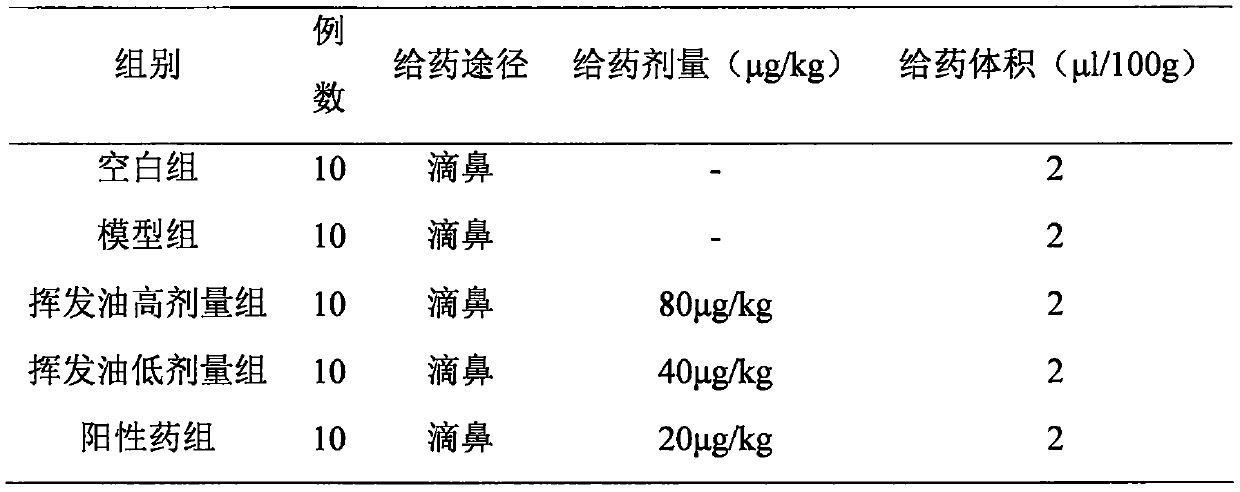
Application of maidenhair volatile oil in rhinitis resistance medicine
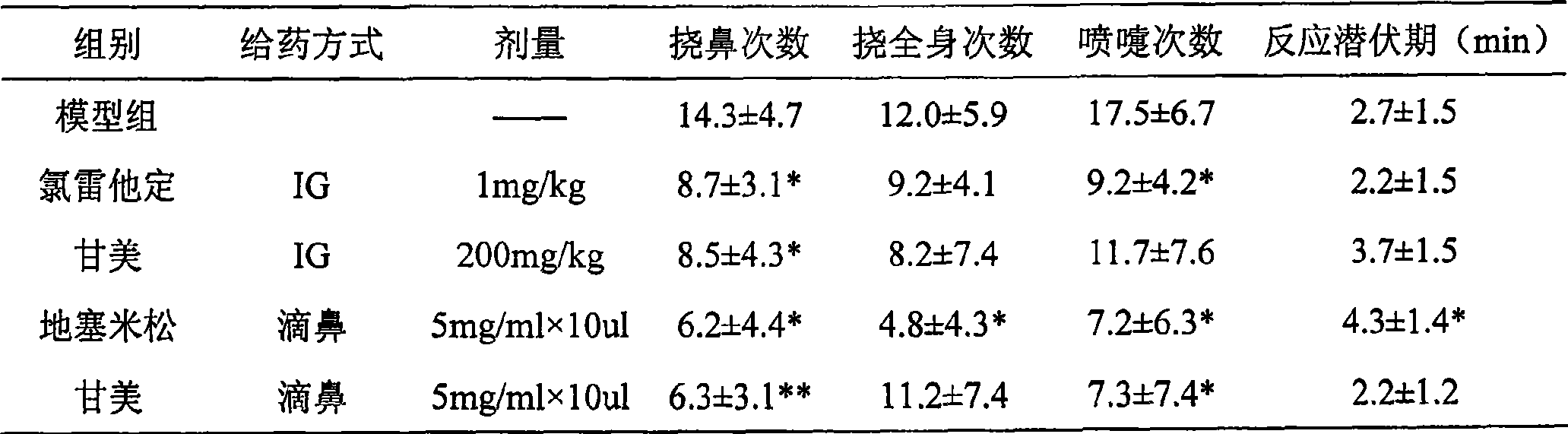
The invention relates to application of maidenhair volatile oil in a medicine for treating rhinitis. A method comprises the step of grinding a whole herb of maidenhair and then extracting the maidenhair by a steam distillation method or a carbon dioxide supercritical fluid extraction method to form the volatile oil. No toxic reagent is used by the two extraction methods, and the extracted volatile oil has the characteristics of no toxicity, no residue, simple extraction technology and high extraction efficiency. A medicinal auxiliary material can be added into the volatile oil to prepare a nasal drop or a spray as the medicine for treating the rhinitis. Pharmacological experiments show that the volatile oil has a better pharmacological action on an allergic rhinitis model of a guinea pig, can obviously alleviate symptoms of a nose of the allergic rhinitis model of the guinea pig, reduces an ethological accumulative integral, and significantly relieves edema of a nasal mucosa of the allergic rhinitis model of the guinea pig.
Owner:蔡德成 +2
Recombinant porcine alpha interferon and application thereof in preparing medicines for treating Porcine cytomegalovirus (PCMV)
InactiveCN102796758APeptide/protein ingredientsMicroorganism based processesInfected cellProtein target
The invention discloses a recombinant porcine alpha interferon, prepared by the following methods: A1, synthesizing an artificially modified porcine alpha interferon; A2, constructing a recombinant eukaryotic expression vector pGAPZ alpha-IFN alpha; and A3, highly expressing IFN alpha by an eukaryotic cell yeast expression system. The invention is characterized by taking a supernatant to purifying the porcine alpha interferon which is cultured by a lot of engineering bacteria and expressed with a chromatography column, then collecting a target protein eluate, filtering through a 0.22 mum microfiltration membrane, then determining the activity by cytopathic inhibition, and calculating the potency unit according to 50% pathology; wherein the cell used for determination is Madin-Darby bovine kidney (MDBK), and vesicular stomatitis virus (VSV) is used for attacking the virus. According to the invention, the recombinant porcine alpha interferon obtained by purification is applied in controlling PCMV, and experiments prove that the recombinant porcine alpha interferon has obvious protection effect on PCMV infected cells. The route of administration of the porcine alpha interferon is injection or mucous membrane administration, and the dosage form comprises injection or nasal drops.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Method of treating dry eye disease with purinergic receptor agonists
A method and preparation for the stimulation of tear secretion in a subject in need of such treatment is disclosed. The method comprises administering to the ocular surfaces of the subject a purinergic receptor agonist such as uridine 5′-triphosphate (UTP), dinucleotides, cytidine 5′-triphosphate (CTP), adenosine 5′-triphosphate (ATP), or their therapeutically useful analogs and derivatives, in an amount effective to stimulate tear fluid secretion and enhance drainage of the lacrimal system. Pharmaceutical formulations and methods of making the same are also disclosed. Methods of administering the same would include: topical administration via a liquid, gel, cream, or as part of a contact lens or selective release membrane; or systemic administration via nasal drops or spray, inhalation by nebulizer or other device, oral form (liquid or pill), injectable, intra-operative instillation or suppository form.
Owner:MERCK SHARP & DOHME CORP
Novel base material for pharmaceutical and/or cosmetic cream (herbal composition for itchy or infected skin)
InactiveUS20070014749A1Reduce impactPromoting faster healingBiocideCosmetic preparationsDiseaseFreeze-drying
The present invention relates to the preparation and use of compositions for the treatment of skin disorders itchy and / or infected skin such as impetigo, acne (on face, forehead scalp and on the back of the body) and fungal infection of skin and nails. Whether the infection may be acute or chronic or sub-acute or acute on chronic. As well as for the promotion of non carrier state of the human beings and animals from some pathogenic bacteria such as staphylococci. The compositions are based on extracts from the plants Cassia tora, Centratherum anthelminticum and / or Melia azadirachta. A variety of other herbal extracts may be included and the composition may take the form of a freeze dried or a spray dried powder or presence of this in a cream or ointment based on Ghee, or in a cream or ointment form developed with any other vehicle, or they may be in a powdered form without spray drying. That spray dried or freeze dried powder may be suspended in a suitable mouth wash or nasal drops. The herbs may be in a powdered form of suitable for preparing decoctions in hot water. Laboratory results are of Specimen Example 2. Specimen Example 1 I have found working on the patients clinically as broad spectrum covering pustules as well as fungal infections.
Owner:VIRAJ SHAH VARION
Drug administration preparations of ligustrazine for nasal mucosa and method for preparing the same
InactiveCN101152182AImprove distributionImprove treatment efficiencyOrganic active ingredientsAerosol deliveryNasal cavitySide effect
The invention belongs to medical technical field and discloses nasal mucosa medication agent of tetramethylpyrazine and the preparation method. The agent contains tetramethylpyrazine, pharmaceutical excipient and absorption enhancer. The weight percentage is 1:0.1 to 100:0.1 to 10. The tetramethylpyrazine includes tetramethylpyrazine prepared in various methods and salts of tetramethylpyrazine. Formula dosage of tetramethylpyrazine is put into absorption enhancer and additional pharmaceutical solutions of suitable concentration prepared in advance to produce nasal mucosa medication agent, such as nasal drops, spray, aerosol, microsphere agent, liposomes, etc. Via the special physiological structure of nasal cavity, the agent medicates via nasal mucosa and allows tetramethylpyrazine bypasses the blood-brain barrier to enhance the distribution of medicine in brain tissue. The drug can be used to treat migraine, cerebral ischemia, cerebral embolism and emergencies such as angina pectoris, coronary heart disease, myocardial infarction, etc, and the drug has the advantages of complete and rapid absorption, high treating effects, high stability, and few side effects.
Owner:SHENYANG PHARMA UNIVERSITY
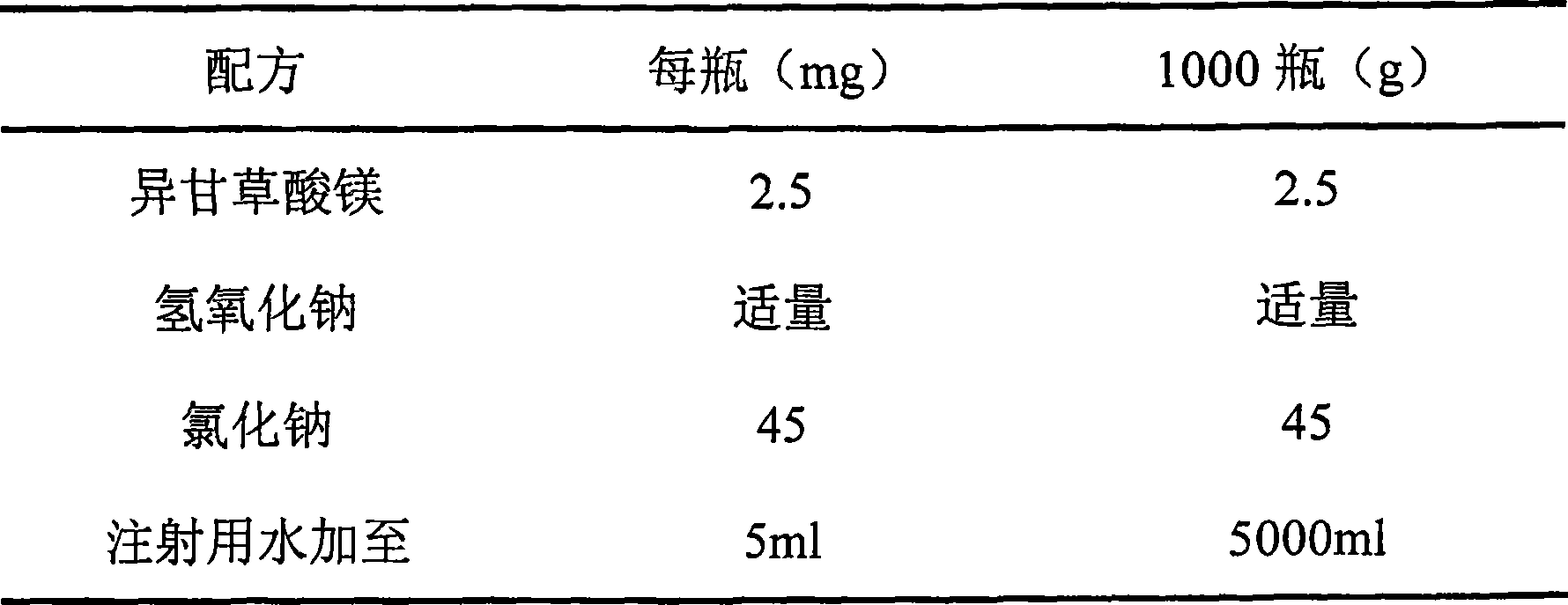
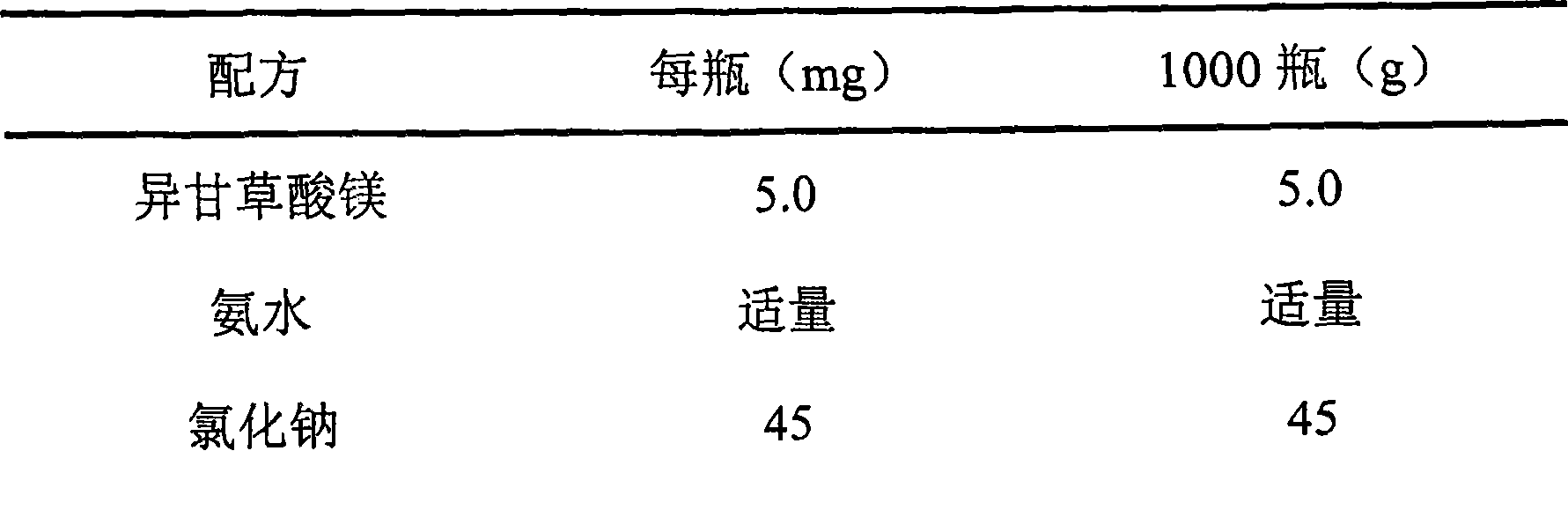
Use of iso-glycyrrhizic acid and salt thereof in treating allergic rhinitis
ActiveCN101396368ASymptoms improvedGood treatment effectOrganic active ingredientsAerosol deliveryActive componentNasal spray
The invention relates to application of iso-glycyrrhizic acid or salt in a drug for remedying allergic rhinitis and the preparation of nasal drop and nasal spray preparation with the iso-glycyrrhizic acid or salt as an active component.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
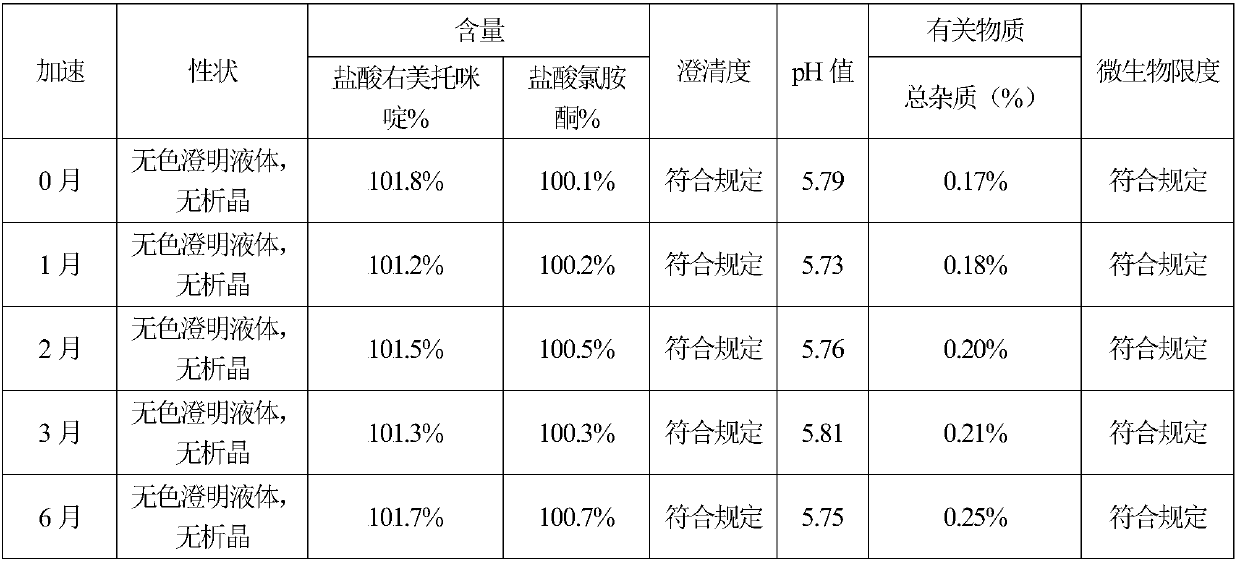
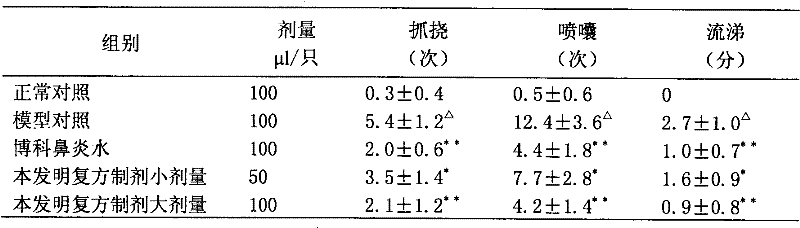
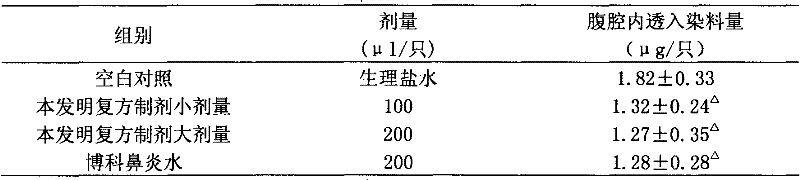
Nasal drops used for anesthesia and preparation method of nasal drops
InactiveCN107693485ANo painImprove complianceOrganic active ingredientsInorganic non-active ingredientsAntioxidantPh regulation
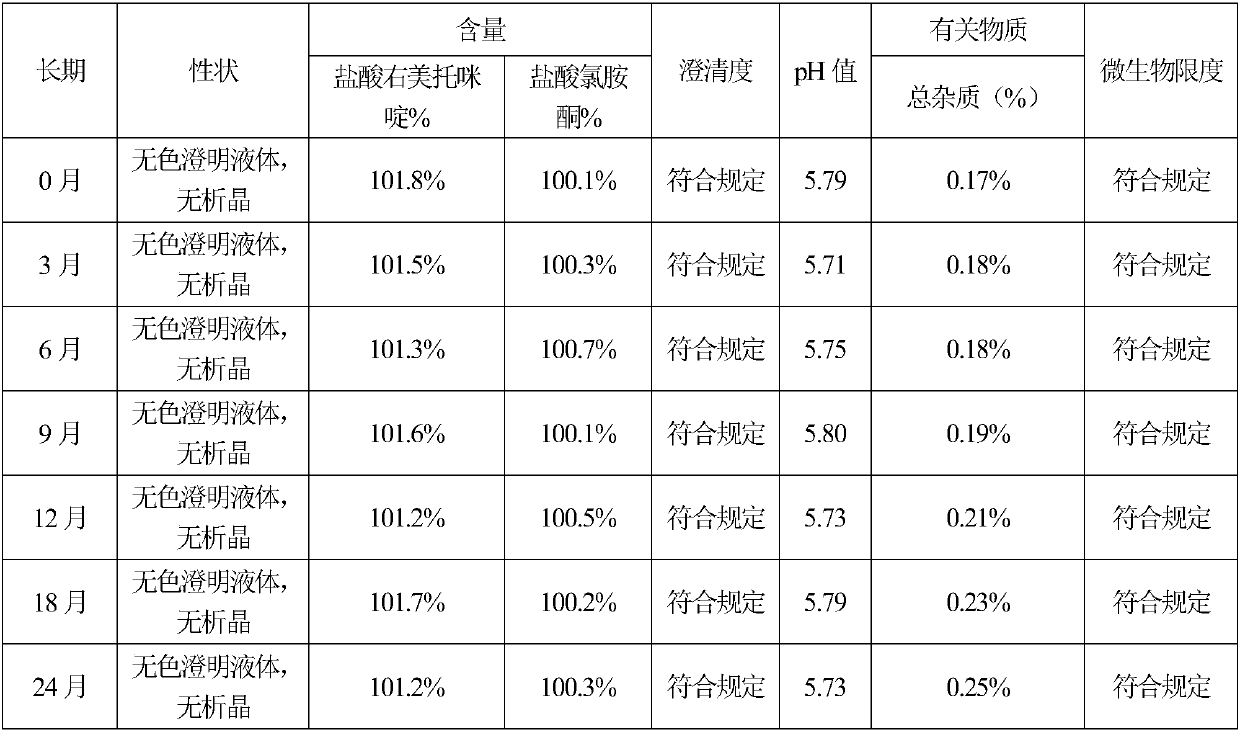
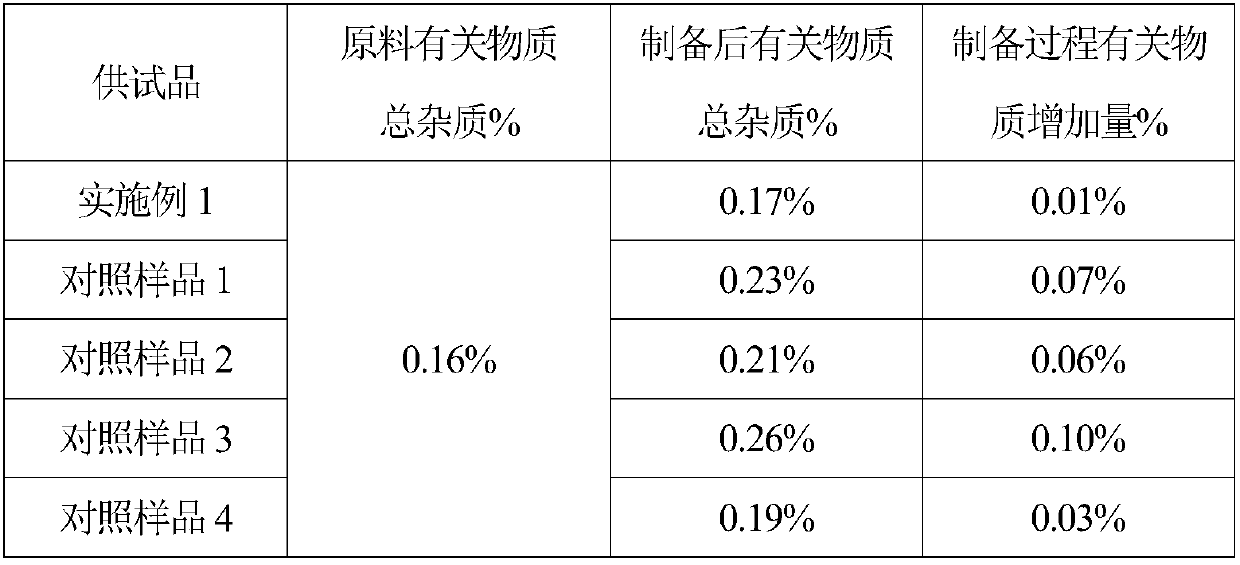
The invention provides nasal drops used for anesthesia. The nasal drops are characterized in that dexmedetomidine hydrochloride injection and ketamine hydrochloride are adopted as the raw materials, an osmotic pressure regulator, a bacteriostatic agent, an antioxidant, a cosolvent and a pH value regulator with certain amount are added, the steps including concentrated preparation, diluted preparation, filling, sterilizing and externally packaging are carried out, and thus the nasal drops are obtained. For the nasal drops used for anesthesia, a special administration channel does not need to beestablished, so that no pain exists during the medication process of a patient, the compliance is good, the stability is good during the storage process of the product, deterioration due to crystallization does not exists, within the period of validity, the pH of the solution basically has no change, the microbial limit is qualified, the validity period of the product is as long as 24 months or alonger time, the increased amount of impurities during the preparation process is small, the increased amount of impurities during the whole preparation process is only 0.01%, the preparation processis simple and feasible, and the nasal drops are worthy of market promotion.
Owner:CHONGQING YUBEIHAI TECH CO LTD
Compound nasal drop-oil, and its prepn. method
An oily nose drip is prepared from 12 Chinese-medicinal materials including xanthium fruit, asarum her, magnolia flower, astragalus root, etc through immersing in vegetable oil and decocting.
Owner:刘强
Traditional Chinese medicine compound preparation for treating rhinitis and nasosinusitis and preparation process thereof
InactiveCN102641380AImprove permeabilityRespiratory disorderImmunological disordersSinusitisNasal cavity
The invention discloses a traditional Chinese medicine compound preparation for treating rhinitis and nasosinusitis and a preparation process thereof. The preparation is prepared from the following raw materials in parts by weight or in parts by volume: 100-200 parts by weight of honeysuckle, 60-120 parts by weight of cocklebur fruit, 60-120 parts by weight of radix scrophulariae, 40-80 parts by weight of liquorice and 1-2 parts by volume of dementholized peppermint oil. The raw materials are taken and conventional accessories are added to the raw materials; and then the mixture is prepared according to the conventional process into nasal delivery preparations including but not limited to nose drops, nasal spray and nasal gel. The compound traditional Chinese medicine nasal delivery preparation provided in the invention is prepared by thoroughly extracting the main active ingredients of the raw materials and has obvious curative effect for treating rhinitis (comprising acute rhinitis, chronic rhinitis and allergic rhinitis) and nasosinusitis.
Owner:谈钰元
Application of composition containing borneol and drug loaded nanoparticles in treatment of cerebral diseases
InactiveCN105879036ASpeed up entryImprove intake capacityPowder deliveryNervous disorderDiseaseTherapeutic drug action
The invention discloses an application of composition containing borneol and drug loaded nanoparticles in treatment of cerebral diseases. The weight ratio of the borneol to the drug loaded nanoparticles is (1-5):(5-100), and an oral preparation, an injection or nasal drops are prepared from the borneol and the drug loaded nanoparticles. Borneol can promote the drug loaded nanoparticles to cross the blood brain barrier to enter cells, so that the uptake function of the drug loaded nanoparticles in intracerebral cells is enhanced, and the drug treatment effect is enhanced.
Owner:CHENGDU UNIV
Pharmaceutical composition for treating nose disease and its preparation method
ActiveCN1806815AEffective treatmentHydroxy compound active ingredientsPharmaceutical delivery mechanismDiseaseClinical efficacy
The invention discloses a pharmaceutical composition for treating nasal diseases, which is prepared from the following raw material medicaments: ephedrine hydrochloride, glycoside of baikal skullcap root, lily magnolia oil, boneol and honeysuckle flower. The pharmaceutical composition can be made into any clinically acceptable preparations through conventional methods in the prior art, preferably nasal drops, aerosols and sprays.
Owner:SINOPHARM GRP DEZHONG (FOSHAN) PHARM CO LTD
Application of Platycodon grandiflorum total saponins in the treatment and prevention of mycoplasma pneumoniae infectious diseases
ActiveCN102274264AInfectious Disease Prevention and ControlAvoid Overshooting ProblemsAntibacterial agentsRespiratory disorderBALB/cTherapeutic effect
The invention relates to application of a platycodon root total saponin to medicaments for treating and preventing mycoplasma pneumoniae infectious diseases, and relates to application of platycodon root total saponin to medicaments. The platycodon root total saponin is used as the active ingredient of the medicaments for treating and preventing the mycoplasma pneumoniae infectious diseases, and is a composition of one or more of saponins of platycogenic acid, polygalic acid, platycogenic acid and platycogenic acid A lactone. The platycodon root total saponin has the effect of resisting mycoplasma pneumoniae; in-vitro experiments indicate that a minimal inhibitory concentration (MIC) value of resisting mycoplasma pneumoniae (MP) is between 16 and 64 mu g / ml, and the minimal bactericidal concentration (MBC) is between 64 to 128 mu g / ml; and in-vivo experiments indicate that after 8 mg / Kg and 16 mg / Kg of platycodon root total saponins are subjected to intragastric administration on BALB / C mice subjected to MP injection by nasal dropping for 10 days, the pathological injury of lungs of the mice can be relieved obviously, and the immunologic function of organisms is regulated, so the platycodon root total saponin has a treatment effect on the MP infection.
Owner:黑龙江省中医研究院
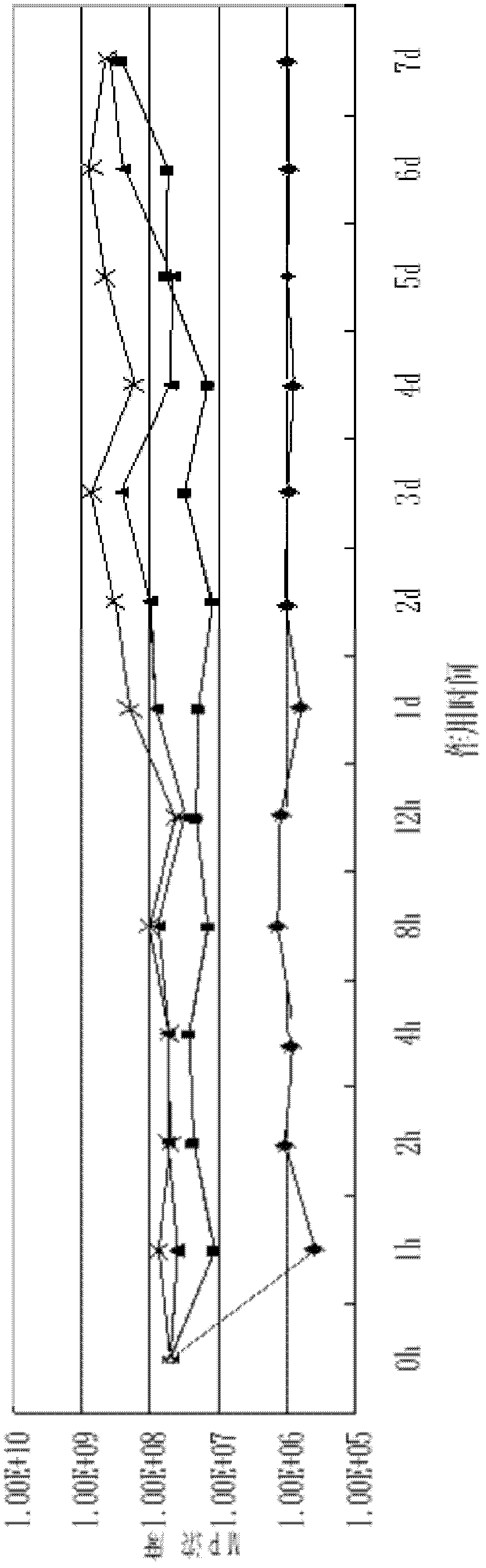
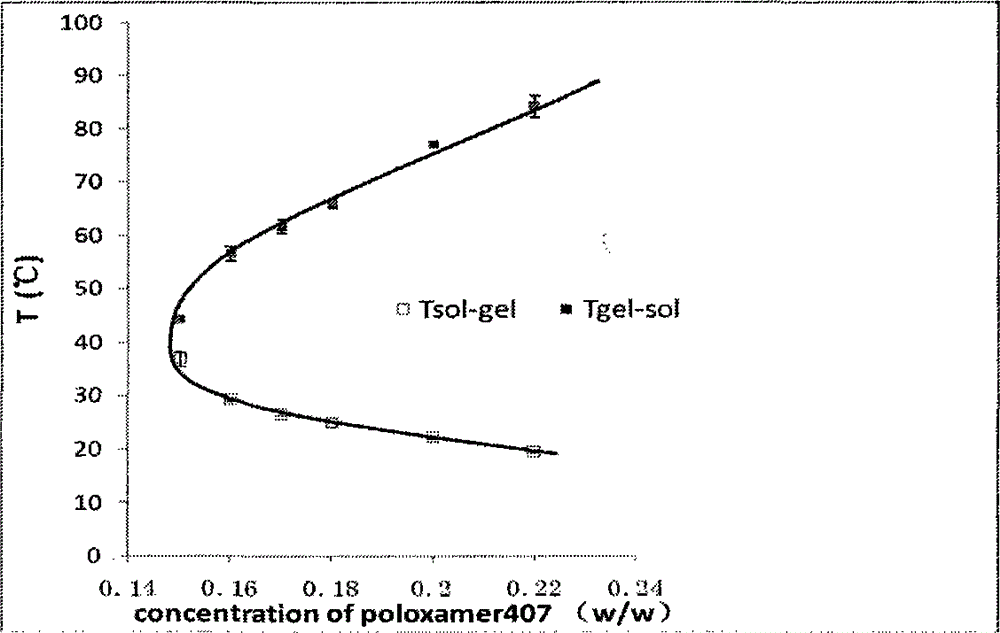
Nasal in situ gel delivery system, preparation and applications thereof
ActiveCN104997724AImprove performanceQuality is easy to controlPeptide/protein ingredientsAerosol deliveryNasal cavityGel preparation
The present invention belongs to the field of pharmaceutical formulations in medicine, and discloses a nasal in situ gel delivery system, preparation and applications thereof, wherein particularly the system has great advantages in the fields of central nervous system drugs and protein peptide drugs. According to the present invention, the process for preparing the NGF nasal in situ gel preparation is a two-bottle method, wherein one bottle is an in situ gel solution, wherein poloxamer is preferably poloxamer P407 and the content is 16-16.8%, the polyethylene glycol is preferably PEG10000 and the content is 0.2-0.6%, the polysorbate is preferably Tween80 and the content is 1-3%, the preservative is preferably benzalkonium chloride and the content is 0.001-0.002%, the other bottle is NGF lyophilized powder preparation, the NGF content is 0.05-0.1%, the albumin content is 0.1-0.2%, the mannitol content is 5%, and before the use, the gel solution is added to the NGF lyophilized powder and uniform mixing is performed so as to obtain the system. According to the present invention, the disclosed drug delivery system can be used in any nasal administration dosage forms, preferably nasal drops and sprays; and with the nasal in situ gel delivery system, the difficult problem that the protein peptide drugs are used for the central nervous system is solved, and especially the application range for the nerve growth factor is expanded.
Owner:PEKING UNIV +1
Traditional Chinese medicine composite preparation for treating acute and chronic rhinitis and preparation method thereof
InactiveCN103230513AEasy to buyLow priceMammal material medical ingredientsRespiratory disorderSalvia miltiorrhizaFritillaria thunbergii
The invention relates to a traditional Chinese medicine composite preparation for treating acute and chronic rhinitis and a preparation method thereof. The composition comprises the following raw ingredients: 10-20 parts of India madder root, 5-15 parts of salvia miltiorrhiza, 15-25 parts of Sichuan lovage rhizome, 10-20 parts of common selfheal fruit-spike, 5-15 parts of pinellia tuber, 10-20 parts of safflower, 5-15 parts of platycodon root, 10-20 parts of weeping forsythia capsule, 20-30 parts of chrysanthemum, 6-10 parts of Chinese honeylocust spine, 10-20 parts of Chinese lovage, 8-12 parts of thunberg fritillary bulb and 0.01-0.03 part of musk. The preparation method comprises the steps of mixing and then grinding the medicines, adding 75% ethanol to carry out reflux extraction for 2-3 times, merging the filtrate, concentrating the merged filtrate to thick extract, drying the thick extract to obtain powder, and adding 3 parts of camphor to prepare nose drops. The composite preparation can inhibit development of conditions of patients and relieve clinical symptoms, and has obvious treatment effects.
Owner:广州鼻祖健康管理有限公司
Butylphthalide nasal drop and preparation method thereof
ActiveCN103505414AQuick effectReduce volumeOrganic active ingredientsNervous disorderMedicineButylphthalide
The invention relates to a butylphthalide nasal drop and a preparation method thereof. The butylphthalide nasal drop prepared by adopting the method has the advantages of good stability, quick effect, small size and simple preparation process, and is convenient to use, suitable for clinical first aid and suitable for industrialized production.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Allergic Nasal Drops
ActiveCN102266522ARaw materials are easy to getLow costHydroxy compound active ingredientsPharmaceutical delivery mechanismPresent methodGram
The invention discloses anti-anaphylactic rhinitis nasal drop. The anti-allergic nasal drop is characterized by comprising the following raw materials by weight: 8 to 13 grams of small centipeda herb, 1 to 3 grams of sharp-leaf galangal fruit, 3 to 7 grams of ephedra herb, 8 to 13 grams of beautiful sweetgum fruit, 8 to 13 grams of Siberian cocklour fruit, 8 to 13 grams of honeysuckle, 8 to 13 grams of mint, 8 to 13 grams of acorus gramineus, and 0.5 to 1.5 grams of borneol, and prepared by present method. The anti-allergic nasal drop has the advantages that: the raw materials are easily obtained, the cost is low, the treatment effect is remarkable and the like.
Owner:韩必芝
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com