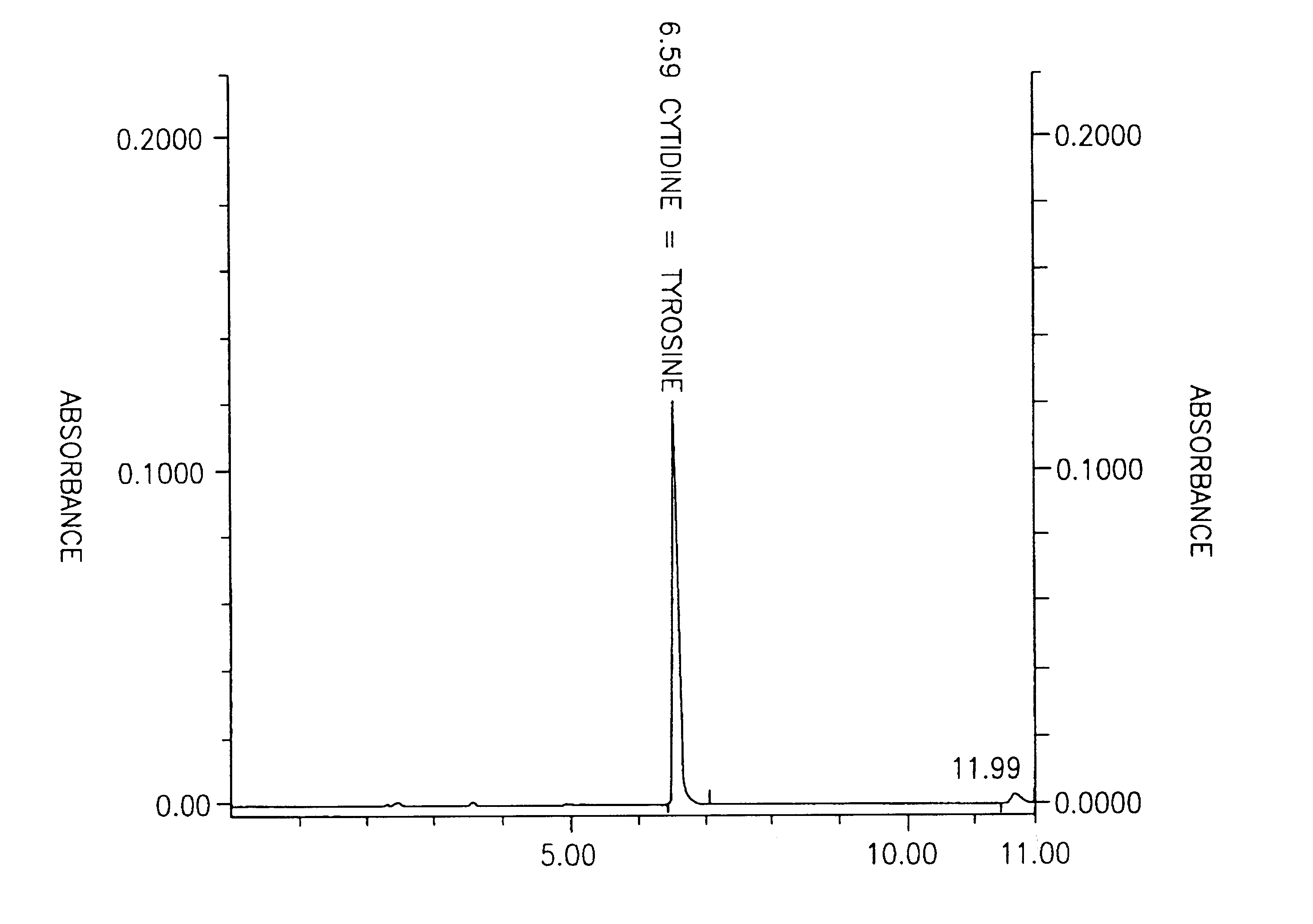
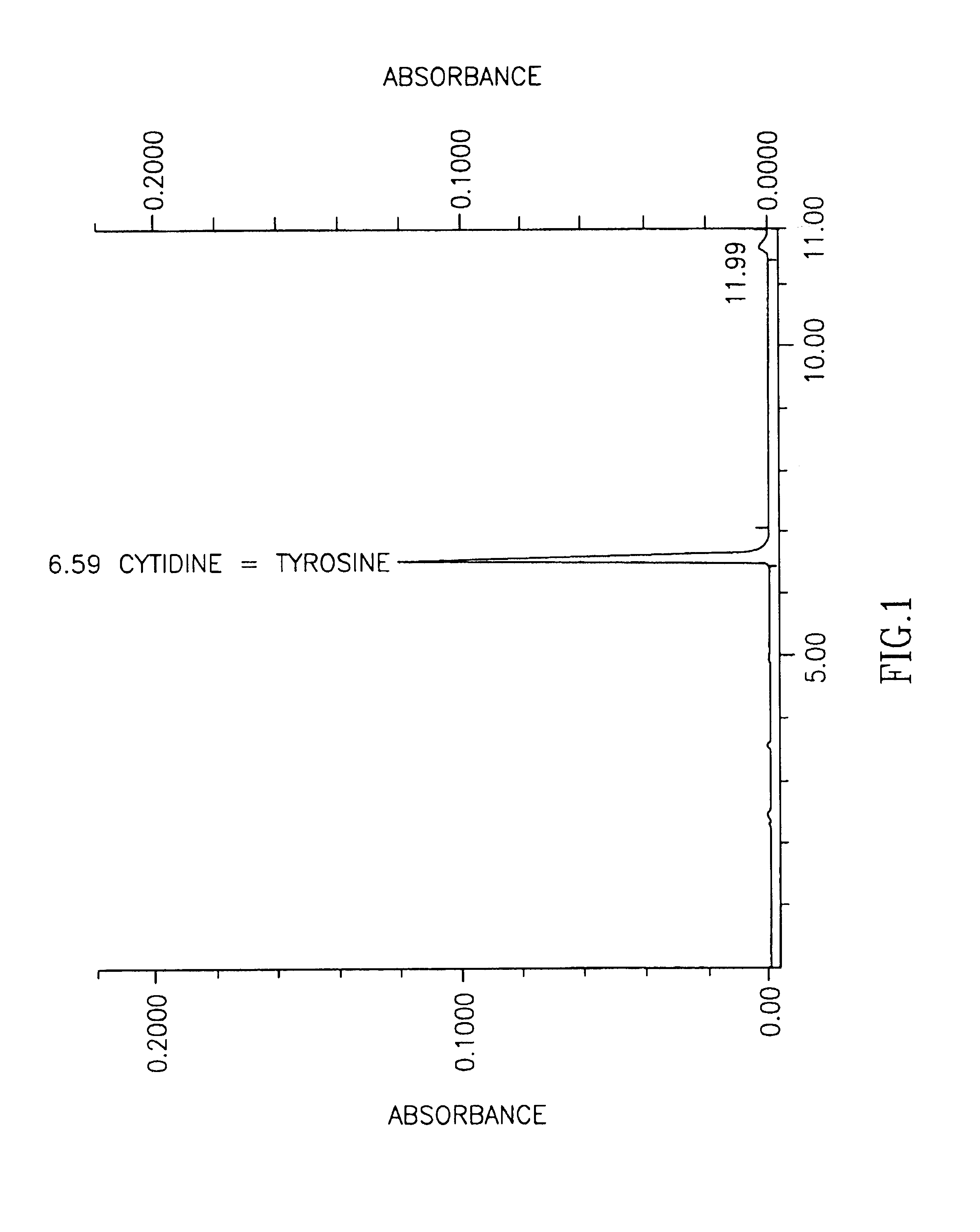
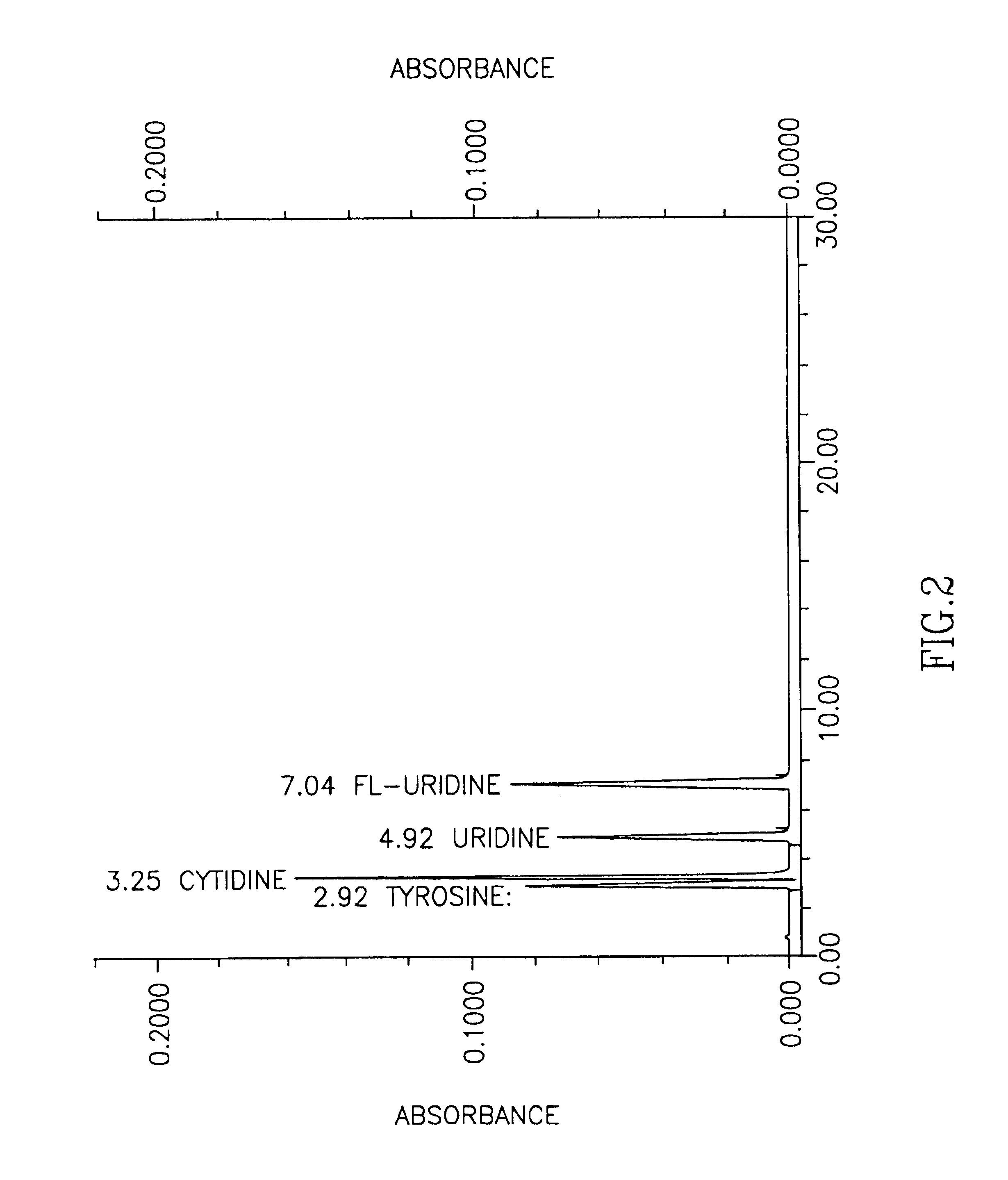
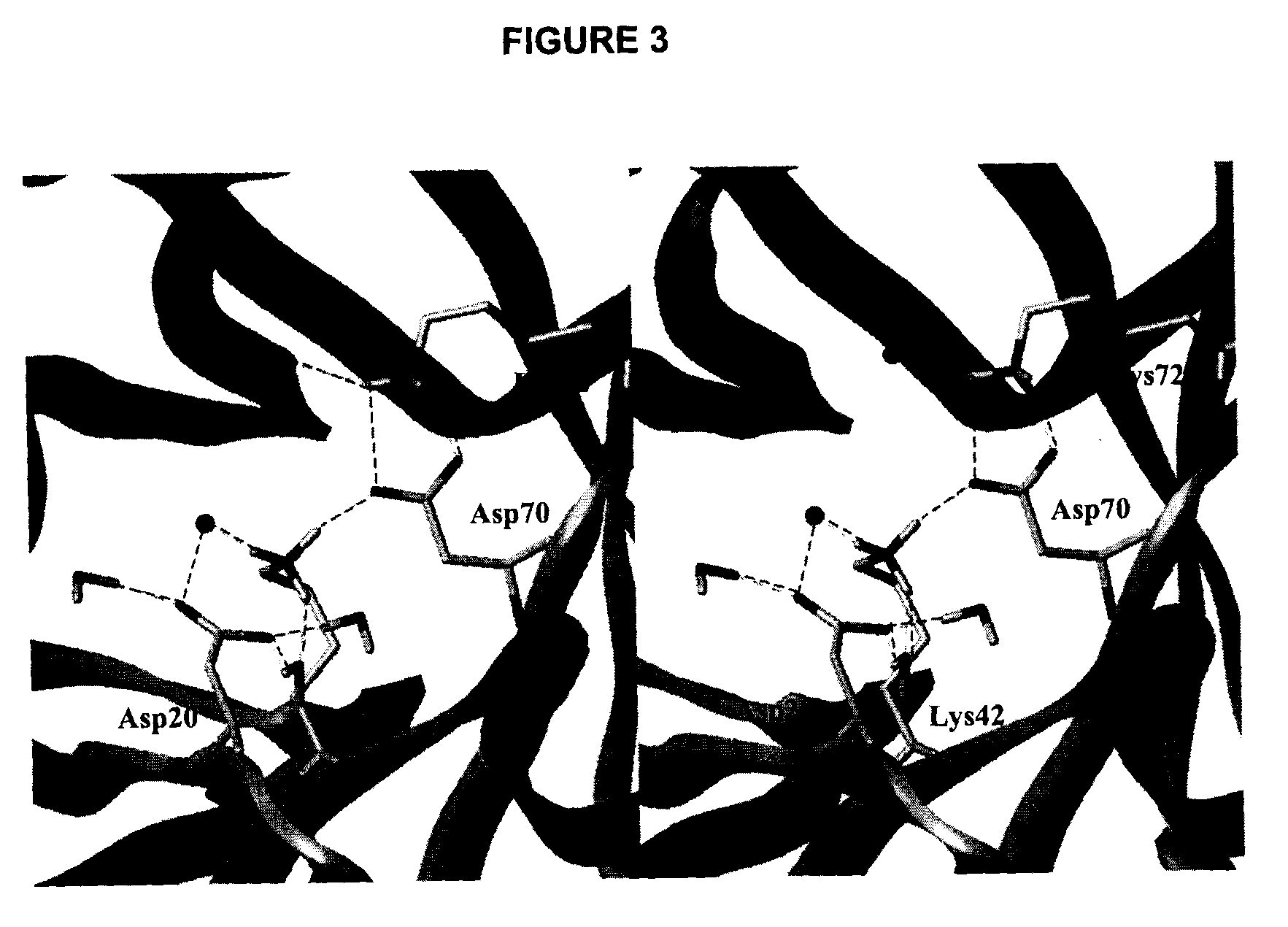
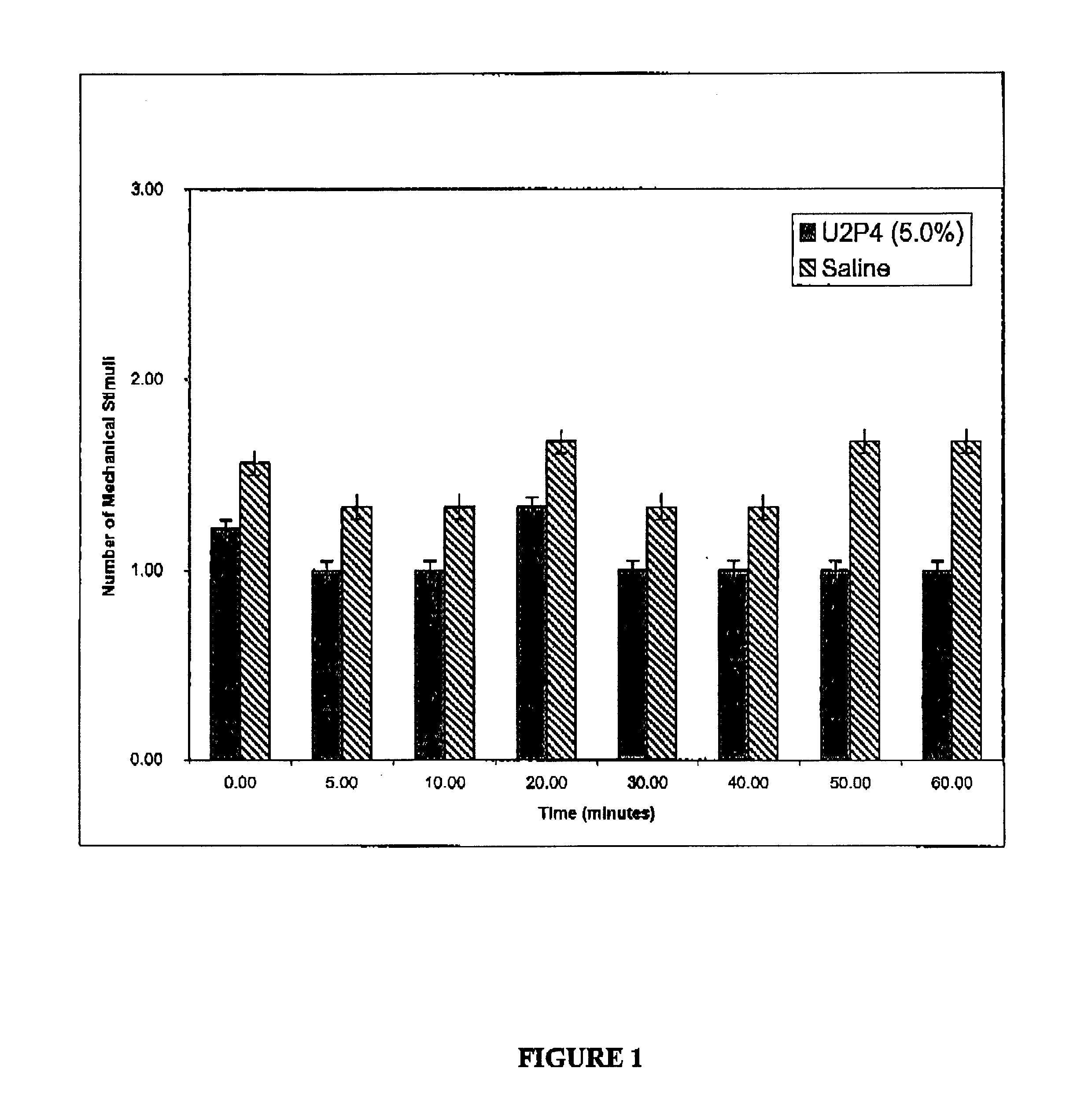
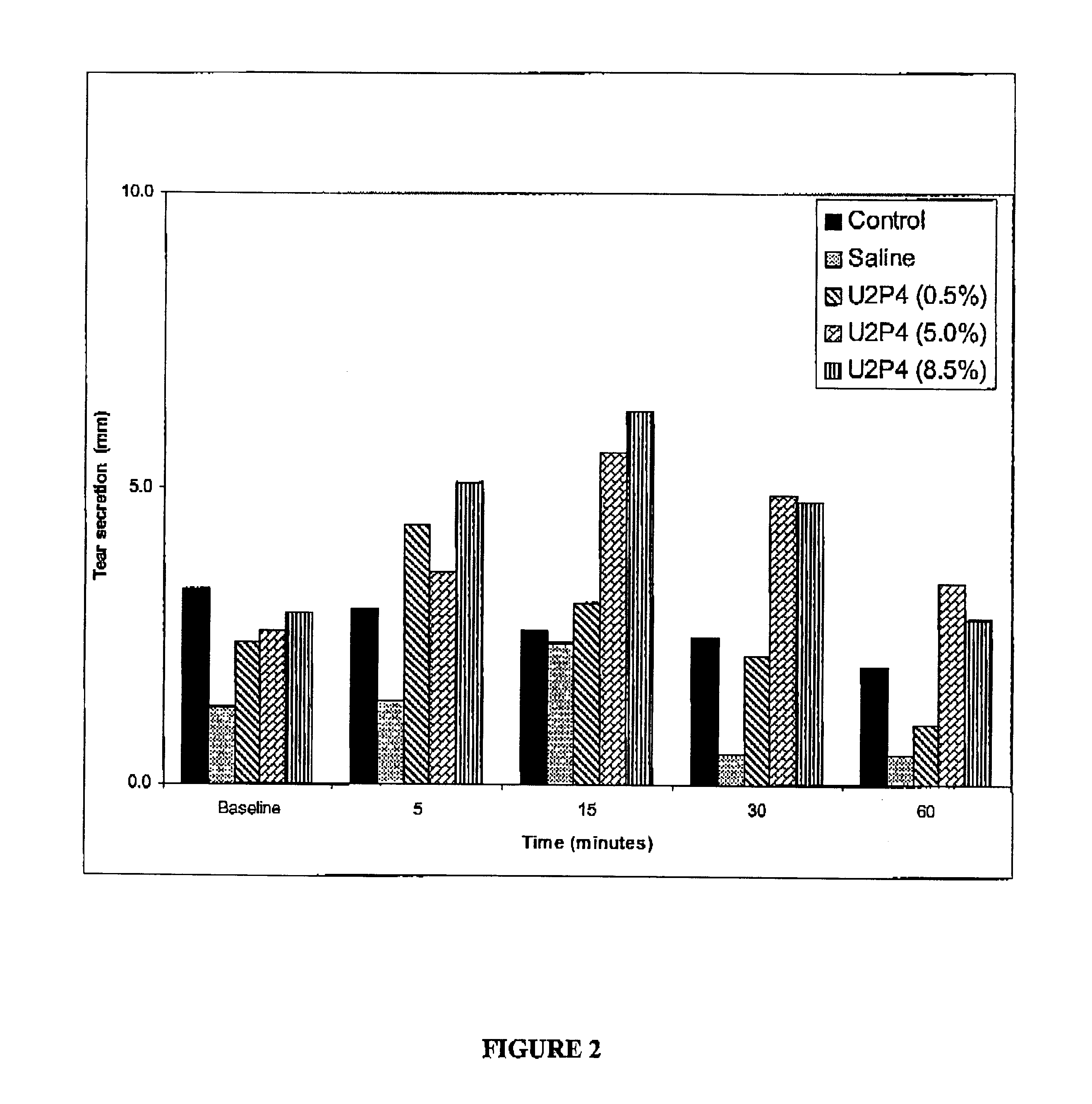
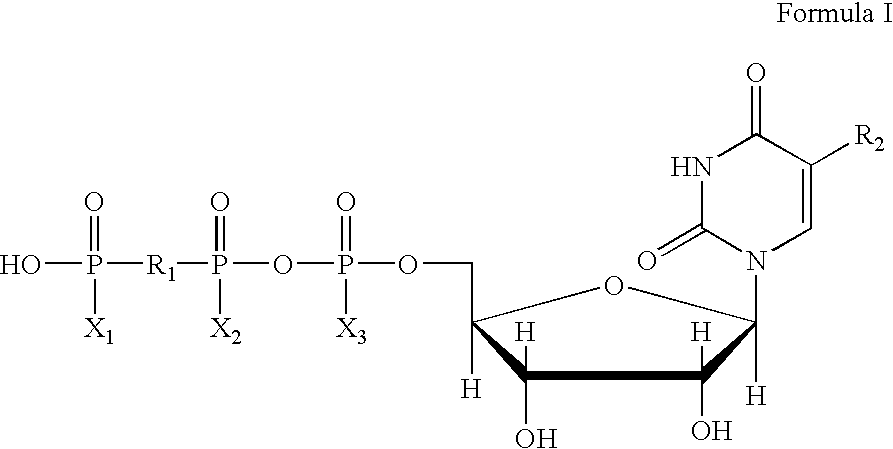
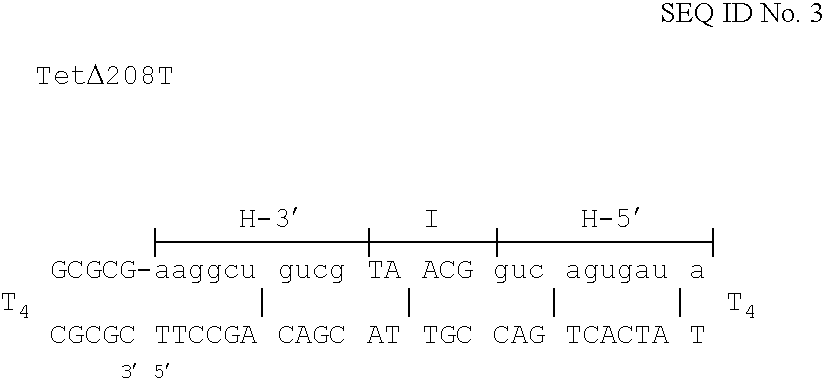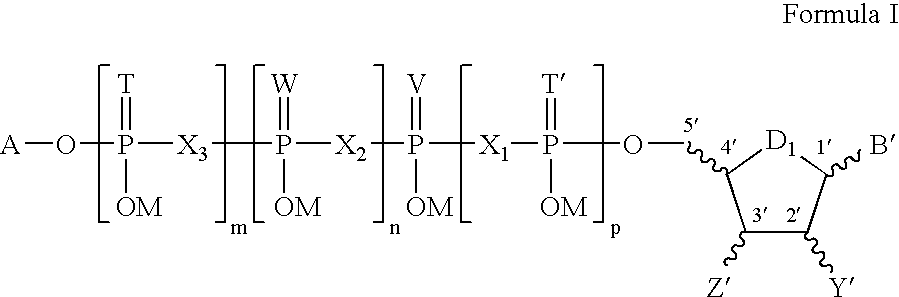Patents
Literature
191 results about "Uridine Nucleotides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
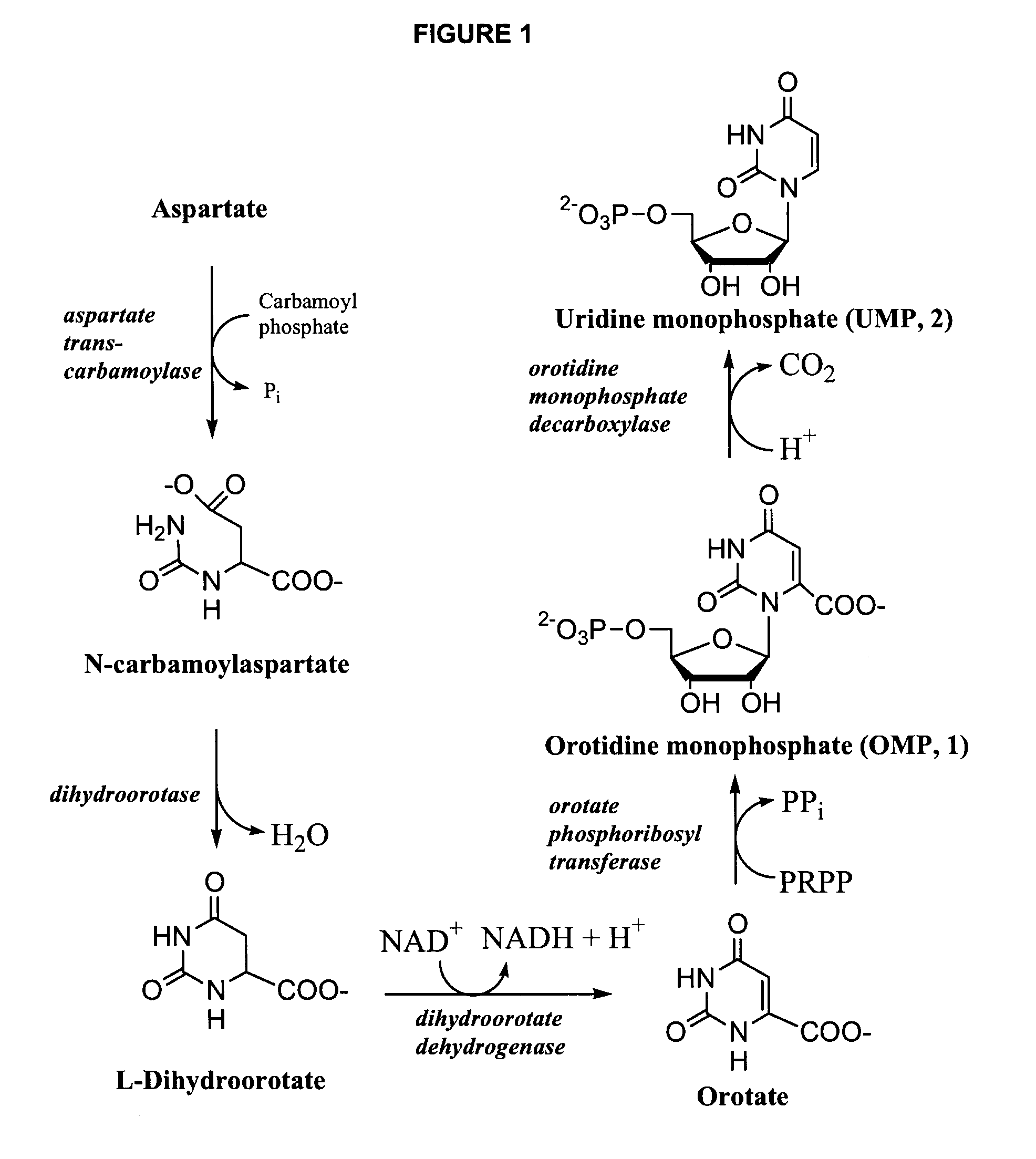
Uridine is a glycosylated pyrimidine-analog containing uracil attached to a ribose ring (or more specifically, a ribofuranose) via a β-N1-glycosidic bond. It is one of the five standard nucleosides which make up nucleic acids, the others being adenosine, thymidine, cytidine and guanosine.
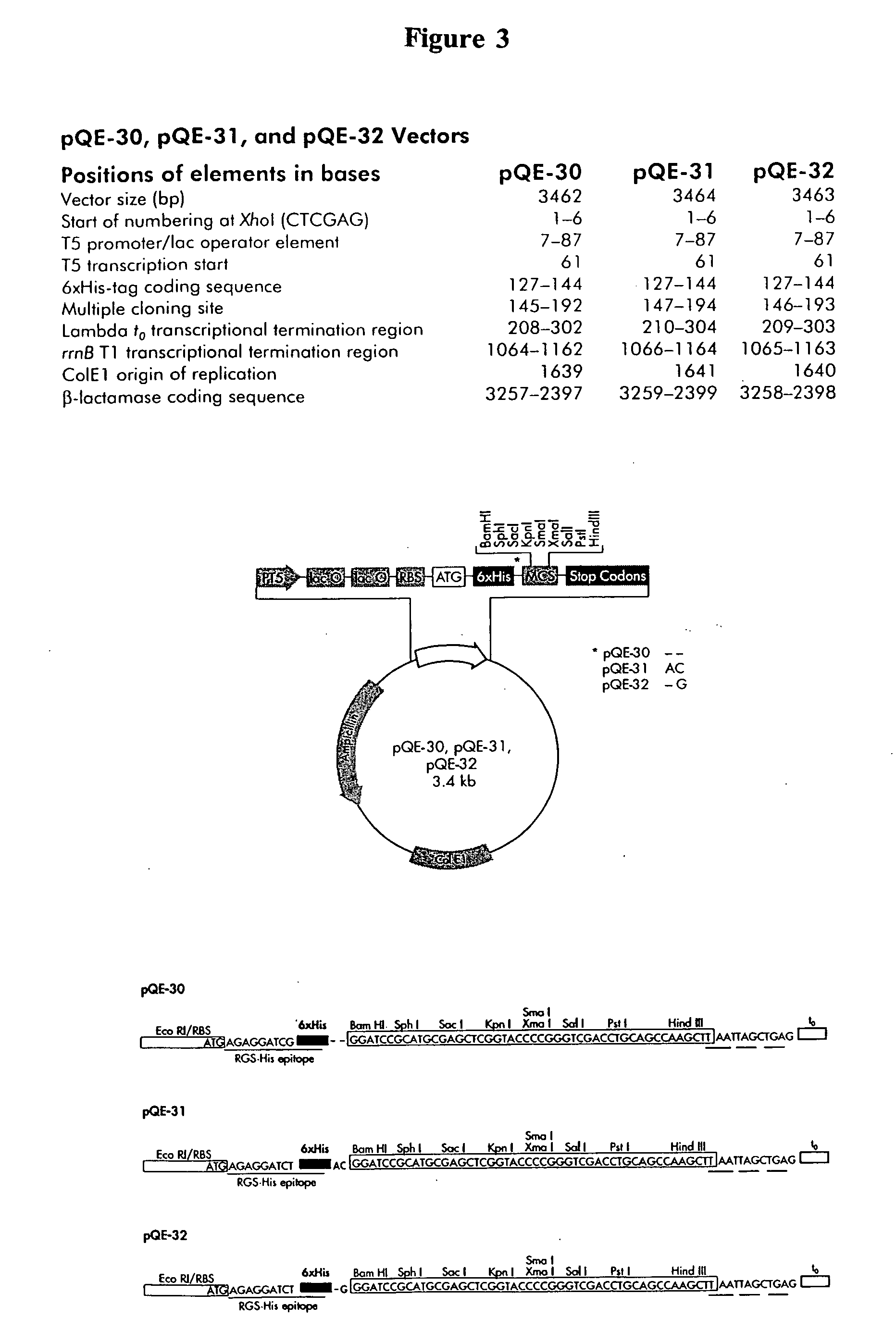
Eukaryotic use of non-chimeric mutational vectors
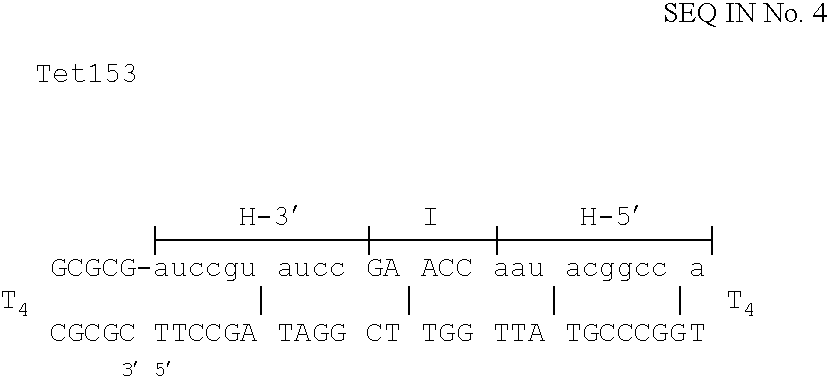
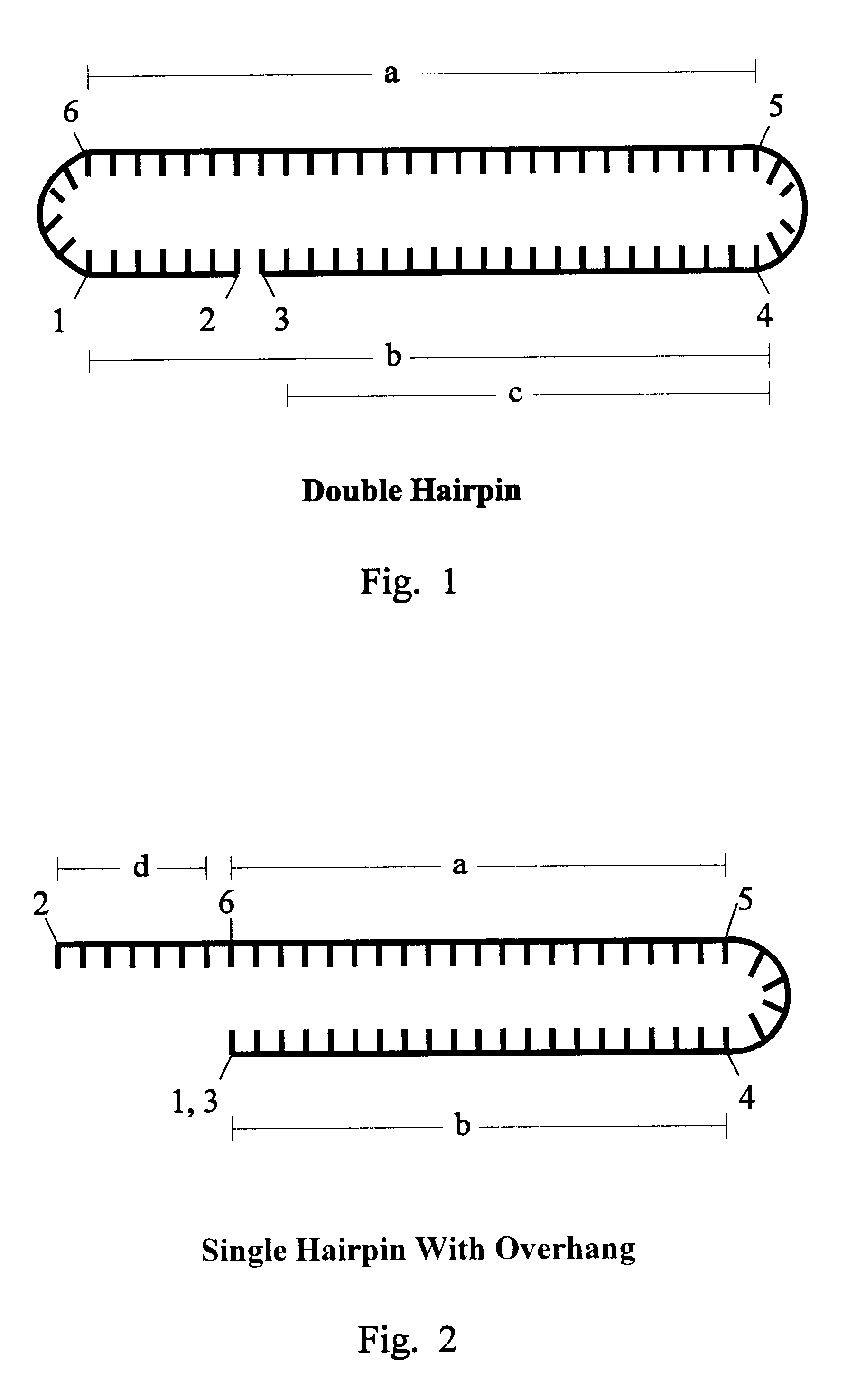
The invention is based on the reaction of Duplex Mutational Vector in a cell-free system containing a cytoplasmic cell extract and a test plasmid. The reaction specifically converts a mutant kanr gene to recover the resistant phenotype in transformed MutS, RecA deficient bacteria. Using this system a type of Duplex Mutational Vector termed a Non-Chimeric Mutational Vector, having no RNA:DNA hybrid-duplex is shown to be an effective substrate for eukaryotic enzymes. The invention concerns the use of Non-Chimeric Mutational Vectors protected from 3' exonuclease attack in eukaryotic cells. Such protection can be conferred by replacement of a tetrathymidine linker by a nuclease resistant oligonucleotide, such as tetra-2'-O-methyl-uridine, to link the two strands of the recombinagenic oligonucleobase.
Owner:VALIGEN US +1
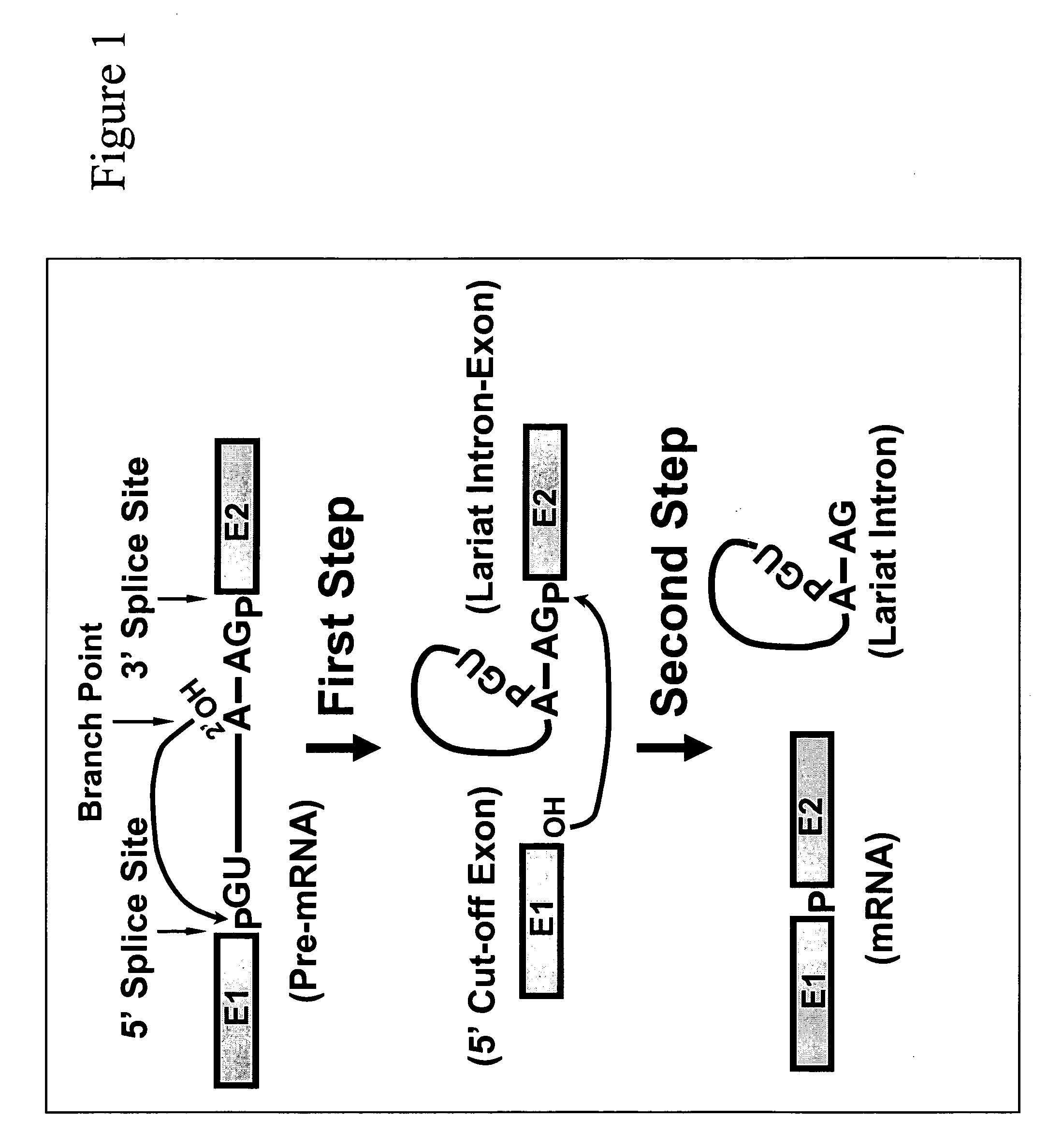
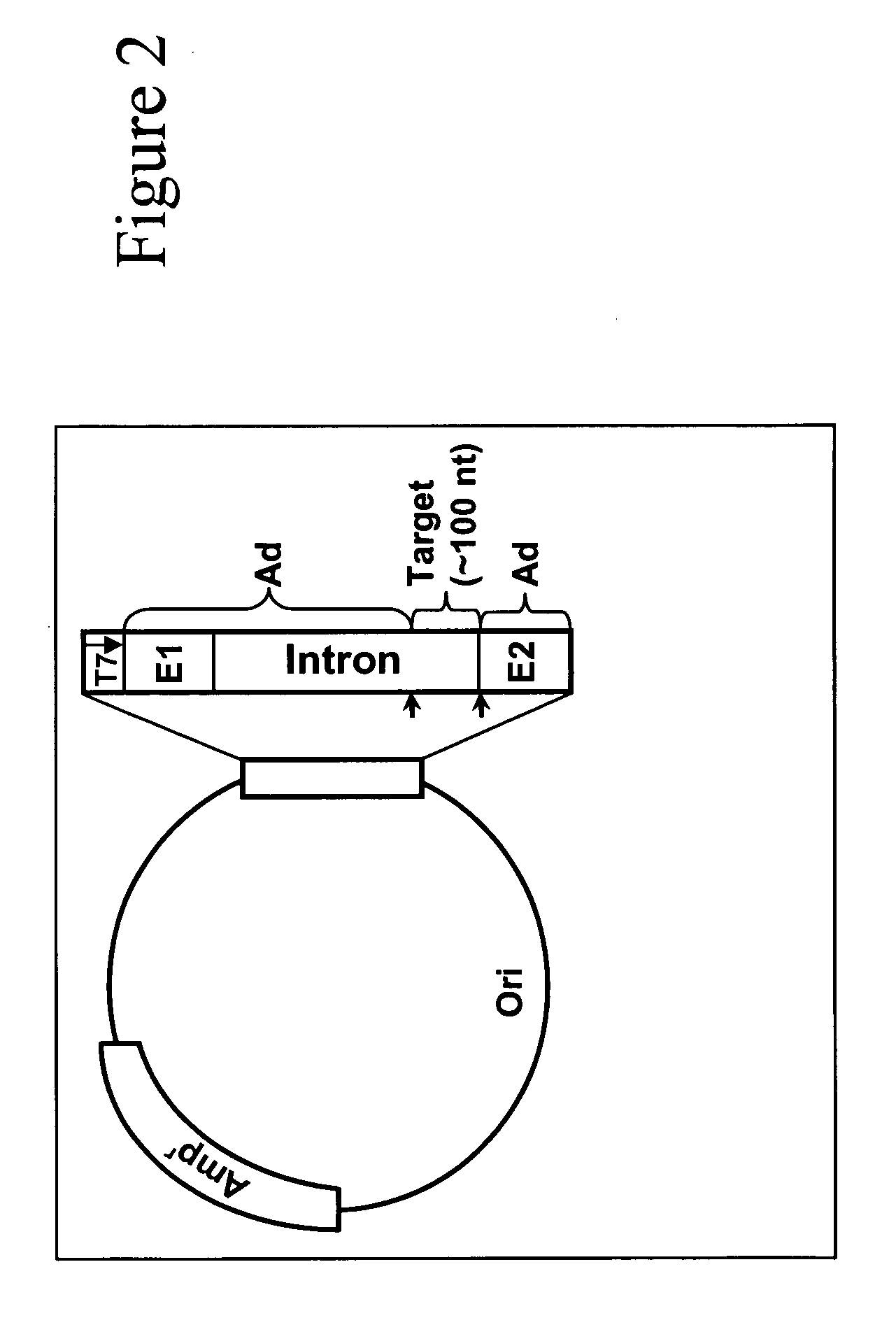
Targeted pre-mRNA/mRNA modification and gene regulation
ActiveUS20070141030A1Easy to identifyLarge productionBiocideGenetic material ingredientsAdenosinePrecursor mRNA
Methods for affecting mRNA expression or translation through the modification of pre-mRNA or mRNA transcripts are described. In one embodiment of the methods of the present invention, the branch point adenosine of a pre-mRNA transcript is 2′-O-methylated to block splicing and subsequent expression of the protein encoded by the transcript. In another embodiment, a uridine residue in a nonsense stop codon may be modified to pseudouridine, causing the translation machinery to read through the nonsense stop codon and translate a full length protein.
Owner:UNIVERSITY OF ROCHESTER
Pharmaceutical formulation comprising dinucleoside polyphosphates and salts thereof
The present invention provides a method of treating edematous retinal disorders. The method comprises administration of a pharmaceutical formulation comprising a hydrolysis-resistant P2Y receptor agonist to stimulate the removal of pathological extraneous fluid from the subretinal and retinal spaces and thereby reduce the accumulation of said fluid associated with retinal detachment and retinal edema. The P2Y receptor agonist can be administered with therapeutic and adjuvant agents commonly used to treat edematous retinal disorders. The pharmaceutical formulation useful in this invention comprises a P2Y receptor agonist with enhanced resistance to extracellular hydrolysis, such as dinucleoside polyphosphate compounds, or hydrolysis-resistant mononucleoside triphosphate salts. The present invention also provides P1-(2′-deoxycytidine 5′-)P4-(uridine 5′-)tetraphosphate, tetra-(alkali metal) salts such as tetrasodium, tetralithium, tetrapotassium, and mixed (tetra-alkali metal) salts. The present further provides a pharmaceutical formulation comprising a P1-(2′-deoxycytidine 5′-)P4-(uridine 5′-)tetraphosphate, tetra-(alkali metal) salt, in a pharmaceutically acceptable carrier.
Owner:MERCK SHARP & DOHME LLC
Methods for increasing blood cytidine and/or uridine levels and treating cytidine-dependent human diseases
InactiveUS6989376B2Enhancing uridine bioavailabilityBiocideNervous disorderMedicineUridine Nucleotides
Methods of treating certain neurological diseases using exogenous uridine or a uridine source alone as a precursor of endogenous cytidine, particularly in the human brain, are disclosed. Methods are also disclosed wherein exogenous uridine or a uridine source is combined either with drugs increasing uridine availability or with compounds that serve as a source of choline in phospholipid synthesis.
Owner:MASSACHUSETTS INST OF TECH
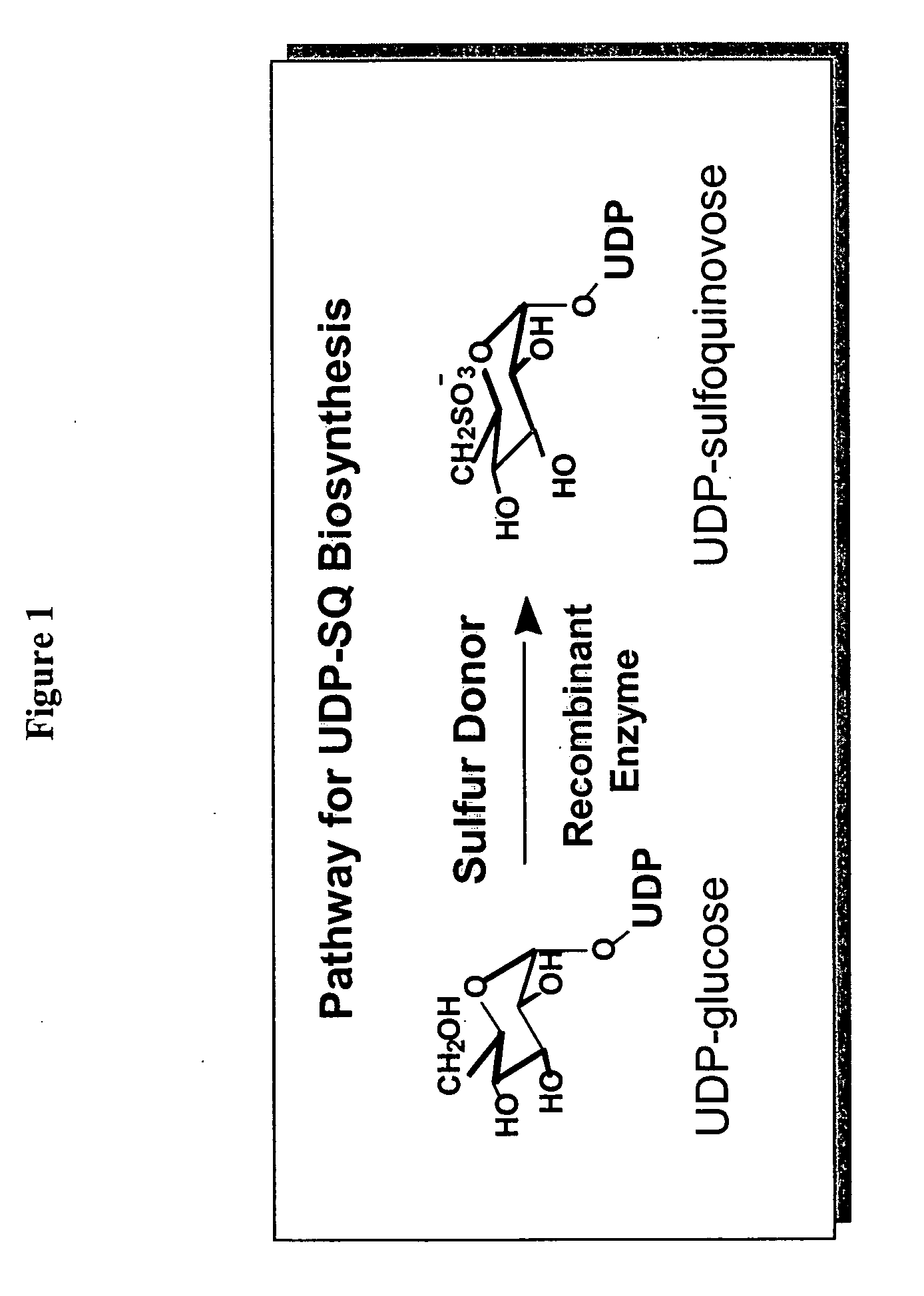
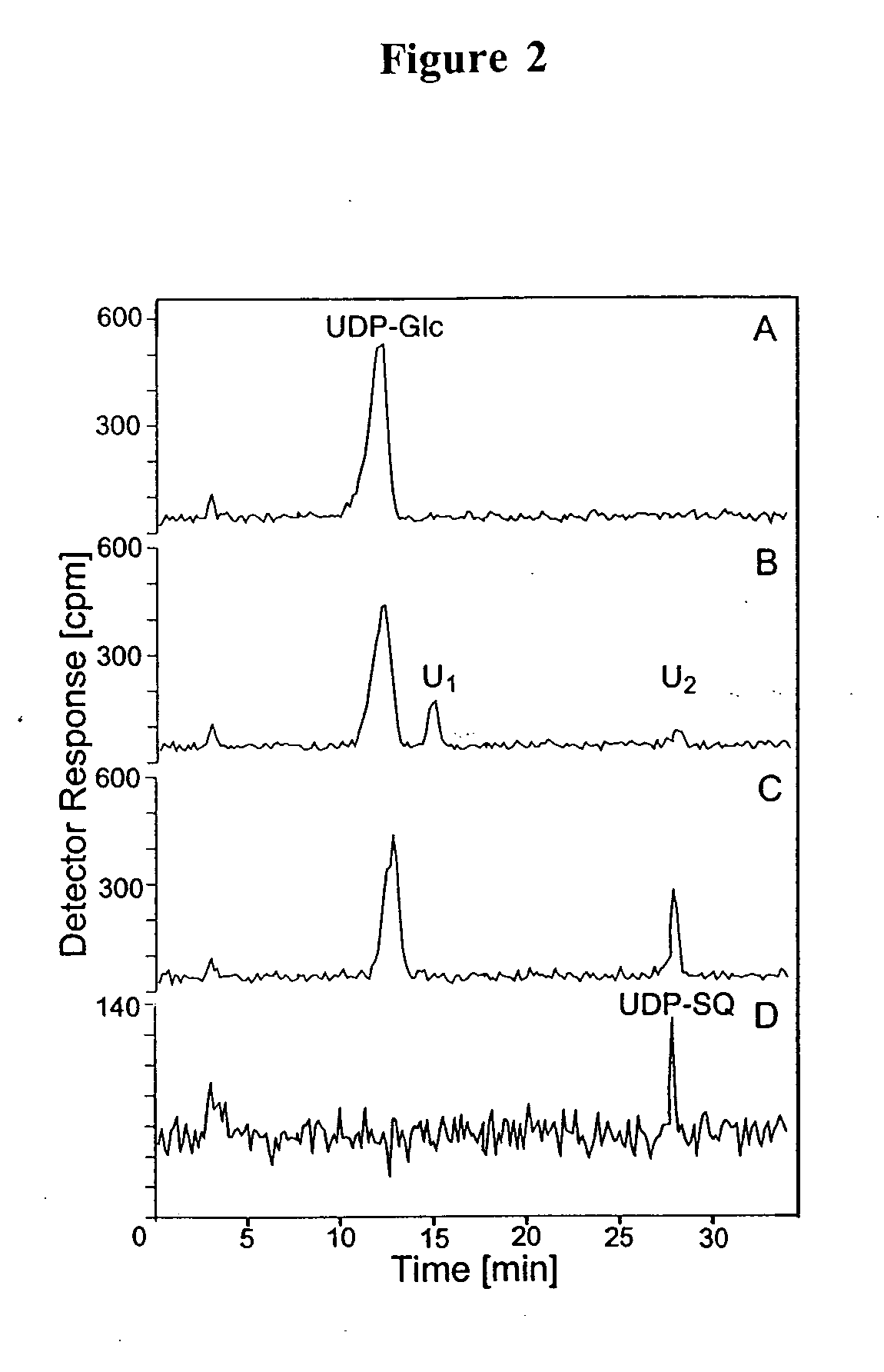
Compositions and methods for the synthesis and subsequent modification of uridine-5'-diphosphosulfoquinovose (UDP-SQ)
The present invention is directed to compositions and methods related to the synthesis and modification of uridine-5′-diphospho-sulfoquinovose (UDP-SQ). In particular, the methods of the present invention comprise the utilization of recombinant enzymes from Arabidopsis thaliana, UDP-glucose, and a sulfur donor to synthesize UDP-SQ, and the subsequent modification of UDP-SQ to form compounds including, but not limited to, 6-sulfo-α-D-quinovosyl diaclyglycerol (SQDG) and alkyl sulfoquinovoside. The compositions and methods of the invention provide a more simple, rapid means of synthesizing UDP-SQ, and the subsequent modification of UDP-SQ to compounds including, but not limited to, SQDG.
Owner:MICHIGAN STATE UNIV
Method of treating gastrointestinal tract disease with purinergic receptor agonists
InactiveUS20020052336A1Increase secretionBiocideCarbohydrate active ingredientsDiseaseDisease irritable bowel
The invention provides a method of regulating water and mucin secretions and fluid transport in the gastrointestinal tract. The invention also provides a method for treating a gastrointestinal disease in which the mucosal barrier of the gastrointestinal system is impaired. The invention additionally provides a method for correcting disorders of fluid secretion or absorption in the gastrointestinal system. The method comprises administering to a patient a pharmaceutical composition comprising a purinergic P2Y receptor agonist, in an amount effective to regulate water and mucin secretions or to correct abnormal fluid transport in the gastrointestinal tract. The pharmaceutical composition used in this invention comprises a P2Y purinergic receptor agonist such as uridine 5'-diphosphate (UDP), uridine 5'-triphosphate (UTP), cytidine 5'-diphosphate (CDP), cytidine 5'-triphosphate (CTP), adenosine 5'-diphosphate (ADP), adenosine 5'-triphosphate (ATP), and their analogs; and dinucleotide polyphosphate compounds of general Formula (IV). Said compound is prepared in an oral form, an injectable form, or a suppository form, and administered to a patient.
Owner:INSPIRE PHARMA
Method of detecting lung disease
A method of facilitating the obtaining of a mucus sample from at least one lung of a subject comprises administering to at least one lung of the subject, in an amount effective to hydrate lung mucous secretions and / or stimulate cilia beat frequency therein, uridine 5'-triphosphate, an active analog thereof, or a pharmaceutically acceptable salt of either thereof, and, optionally, concurrently administering to said at least one lung a physiologically acceptable salt in an amount effective to hydrate lung mucus secretions therein. A sputum or mucus sample is then collected from said at least one lung, which sample can then be analyzed for lung disease. Pharmaceutical compositions useful for carrying out the method comprise UTP or a salt thereof, alone or in combination with a physiologically acceptable salt, or a pharmaceutically acceptable salt of either thereof. The composition may be a liquid / liquid suspension composition or a dry powder composition.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
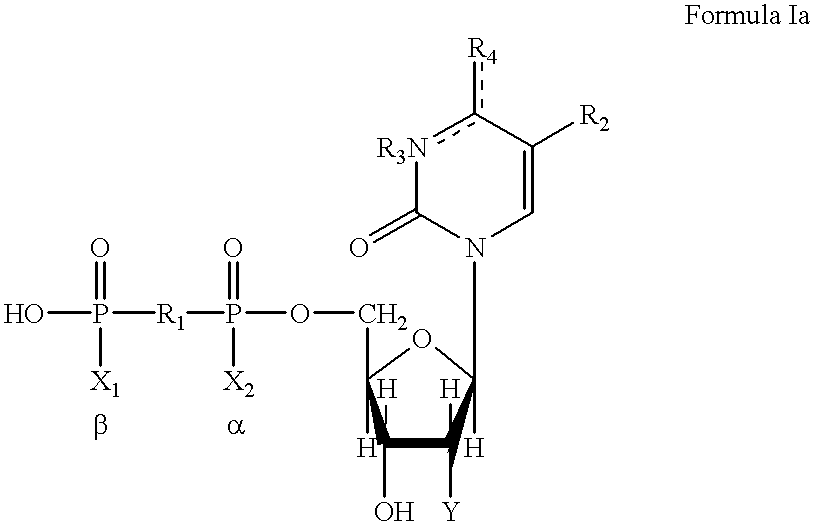
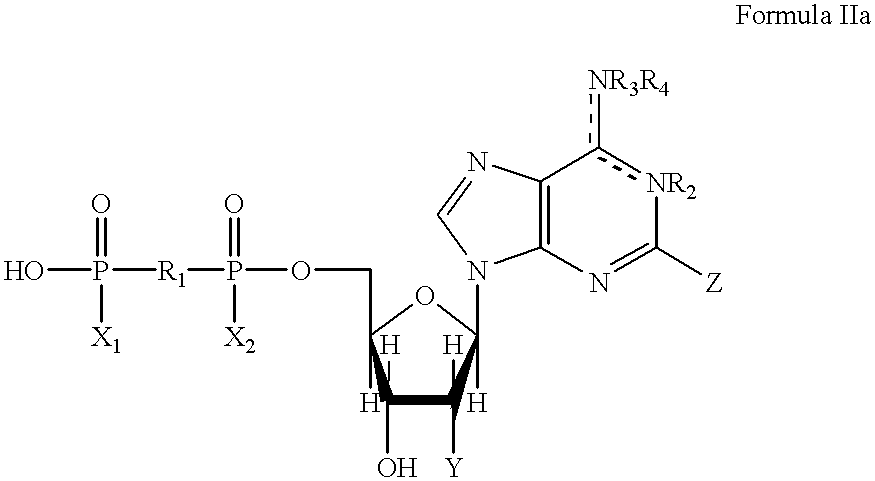
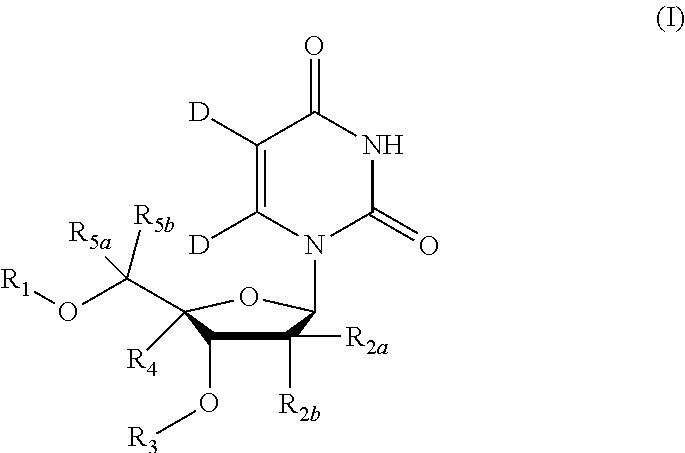
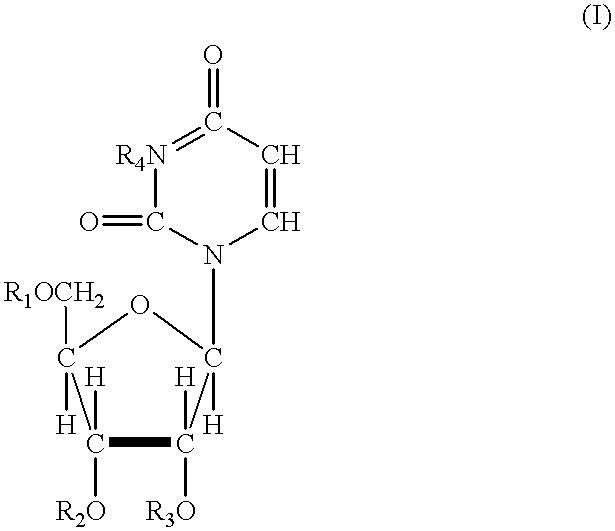
5, 6-d2 uridine nucleoside/tide derivatives
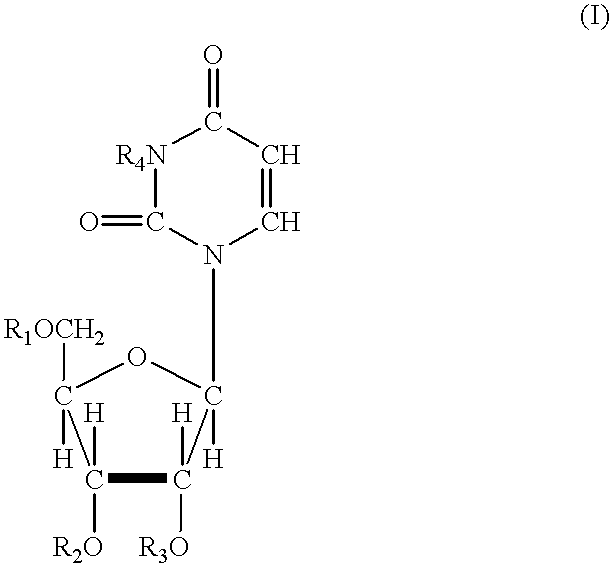
In one aspect, the invention provides compounds represented by Formula I,and pharmaceutically acceptable salts, esters, stereoisomers, tautomers, solvates, and combinations thereof, pharmaceutical compositions comprising these compounds and the use of these compounds for treating a viral infection in a subject.
Owner:ENANTA PHARM INC
Eukaryotic use of improved chimeric mutational vectors
The invention is based on the reaction of recombinagenic oligonucleotides in a cell-free system containing a cytoplasmic cell extract and a test duplex DNA on a plasmid. The reaction specifically converts a mutant kanr gene to recover the resistant phenotype in transformed MutS, RecA deficient bacteria and allows for the rapid and quantitative comparison of recombinagenic oligonucleobases. Using this system a type of Duplex Mutational Vector termed a Heteroduplex Mutational Vector, was shown to be more active in than the types of mutational vectors heretofore tested. Further improvements in activity were obtained by replacement of a tetrathymidine linker by a nuclease resistant oligonucleotide, such as tetra-2'-O-methyl-uridine, to link the two strands of the Duplex Mutational Vector and removal of the DNA-containing intervening segment. The claims concern Duplex Mutational Vectors that contain the above improvements. In an alternative embodiment the claims concern a reaction mixture containing a recombinagenic oligonucleobase, a cell-free enzyme mixture and a duplex DNA containing a target sequence. In yet an alternative embodiment, the invention concerns the use of such mixture to test improvements in recombinagenic oligonucleobases, as well as to test the effects of compounds on the activity of the cell-free enzyme mixture and also to make specific changes in the target DNA sequence.
Owner:CIBUS
Acylated uridine and cytidine and uses thereof
ActiveUS6258795B1No untoward pharmaceutical effectEfficient managementBiocideSugar derivativesDiseaseDiabetes mellitus
The invention relates to compositions comprising acyl derivatives of cytidine and uridine. The invention also relates to methods of treating hepatopathies, diabetes, heart disease, cerebrovascular disorders, Parkinson's disease, infant respiratory distress syndrome and for enhancement of phospholipid biosynthesis comprising administering the acyl derivatives of the invention to an animal.
Owner:WELLSTAT THERAPEUTICS
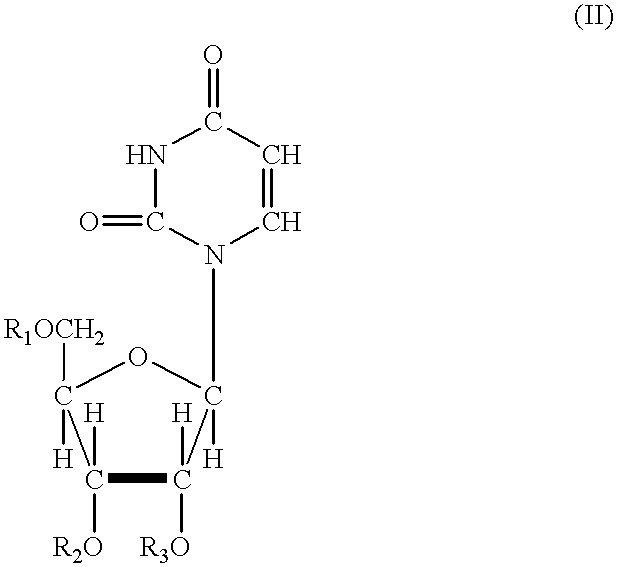
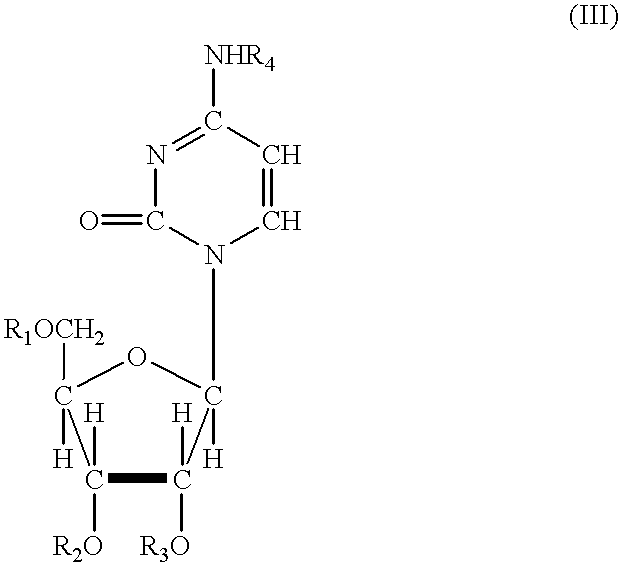
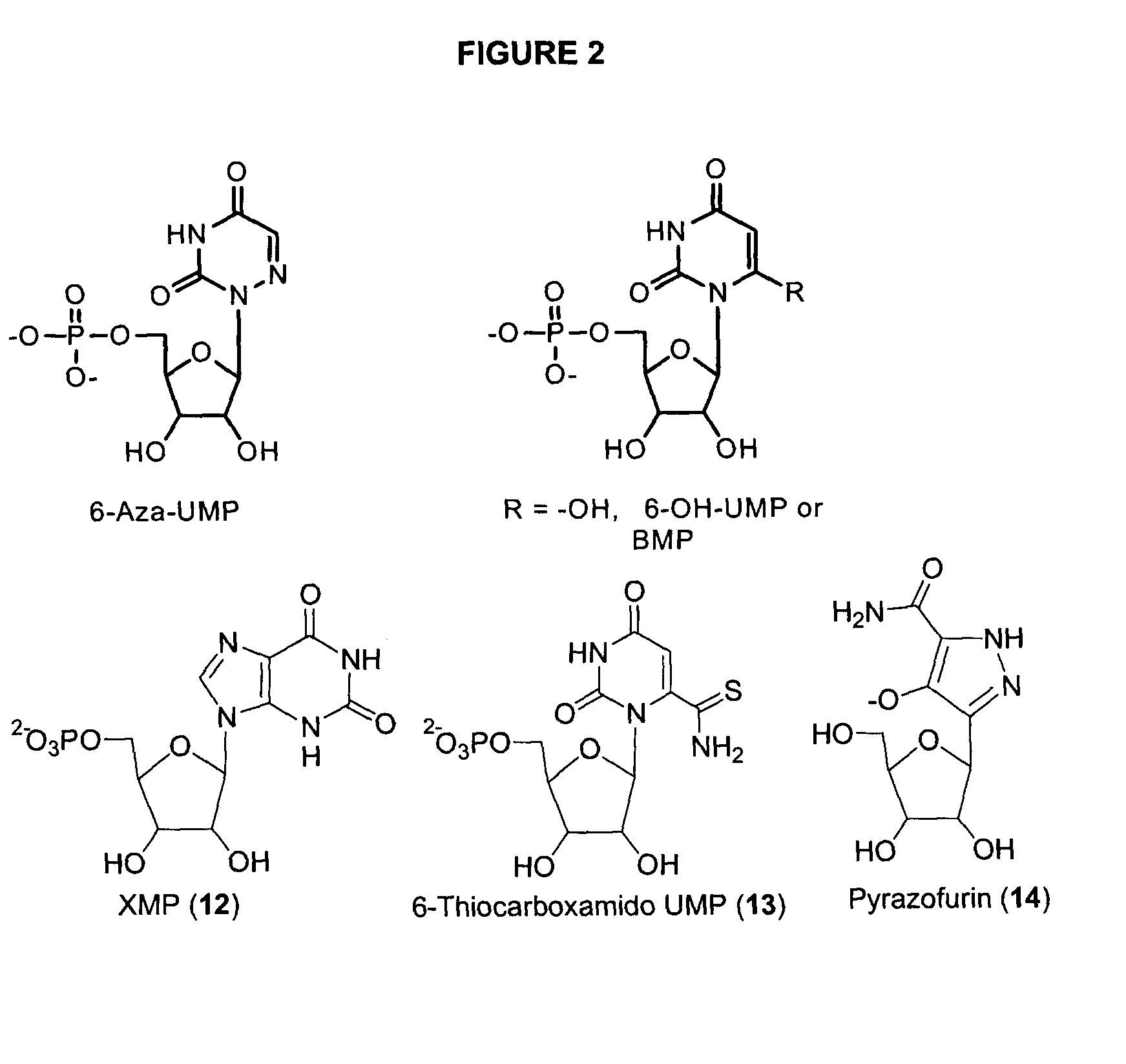
Odcase inhibitors for the treatment of malaria
The present invention includes methods of treating or preventing malaria by administering an anti-malarial effective amount of 6-substituted uridine derivatives to a subject need thereof. The invention also includes new 6-substituted uridine derivatives for use as therapeutics, in particular to treat malaria.
Owner:UNIV HEALTH NETWORK
Method of treating dry eye disease with purinergic receptor agonists
A method and preparation for the stimulation of tear secretion in a subject in need of such treatment is disclosed. The method comprises administering to the ocular surfaces of the subject a purinergic receptor agonist such as uridine 5′-triphosphate [UTP], dinucleotides, cytidine 5′-triphosphate [CTP], adenosine 5′-triphosphate [ATP], or their therapeutically useful analogs and derivatives, in an amount effective to stimulate tear fluid secretion and enhance drainage of the lacrimal system. Pharmaceutical formulations and methods of making the same are also disclosed. Methods of administering the same would include: topical administration via a liquid, gel, cream, or as part of a contact lens or selective release membrane; or systemic administration via nasal drops or spray, inhalation by nebulizer or other device, oral form (liquid or pill), injectable, intra-operative instillation or suppository form.
Owner:MERCK SHARP & DOHME CORP
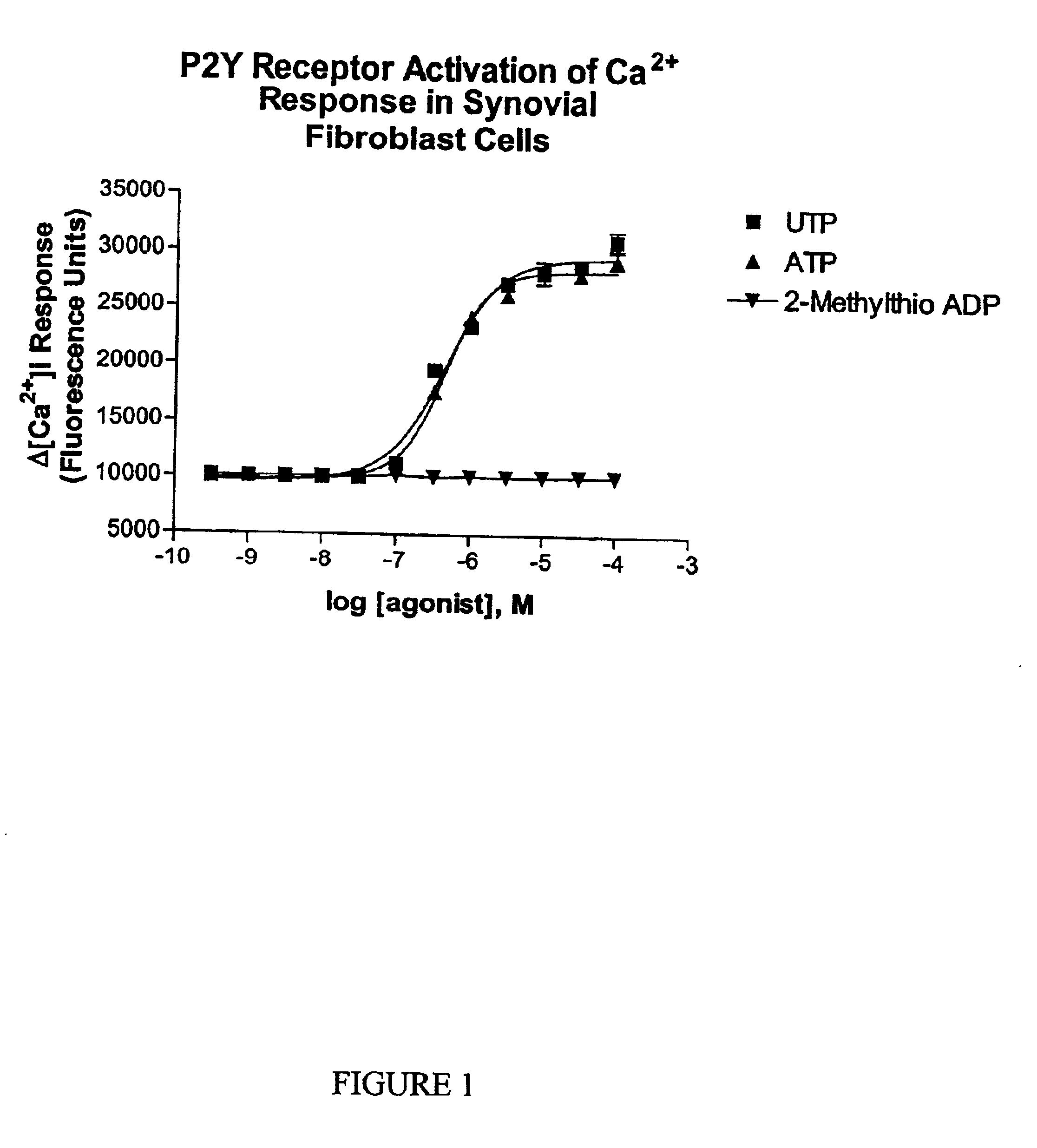
Joint lubrication with P2Y purinergic receptor agonists
InactiveUS7109181B2Enhance joint lubricationTreat osteoarthritisBiocideAerosol deliveryPhospholipidUridine Nucleotides
The present invention is directed to a method of altering the amount or composition of synovial fluids secreted from joints in a subject in need of such treatment. The method comprises administering to a subject a pharmaceutical composition comprising a P2Y purinergic receptor agonist in an amount effective to alter the amount or composition of synovial fluids. The P2Y purinergic receptor agonist is administered in an amount effective to stimulate secretion of synovial fluid, lubricin, hyaluronic acid, or surface-active phospholipids; to enhance joint lubrication; or to treat osteoarthritis. The pharmaceutical compositions useful in the present invention comprise a P2Y purinergic receptor agonist of Formula I and include, but are not limited to: uridine-, adenosine-, cytidine-5′-di- or triphosphates, dinucleoside polyphosphates, and analogs thereof. The invention is useful for treating conditions associated with reduced joint lubrication and joint stiffness, such as osteoarthritis.
Owner:INSPIRE PHARMA
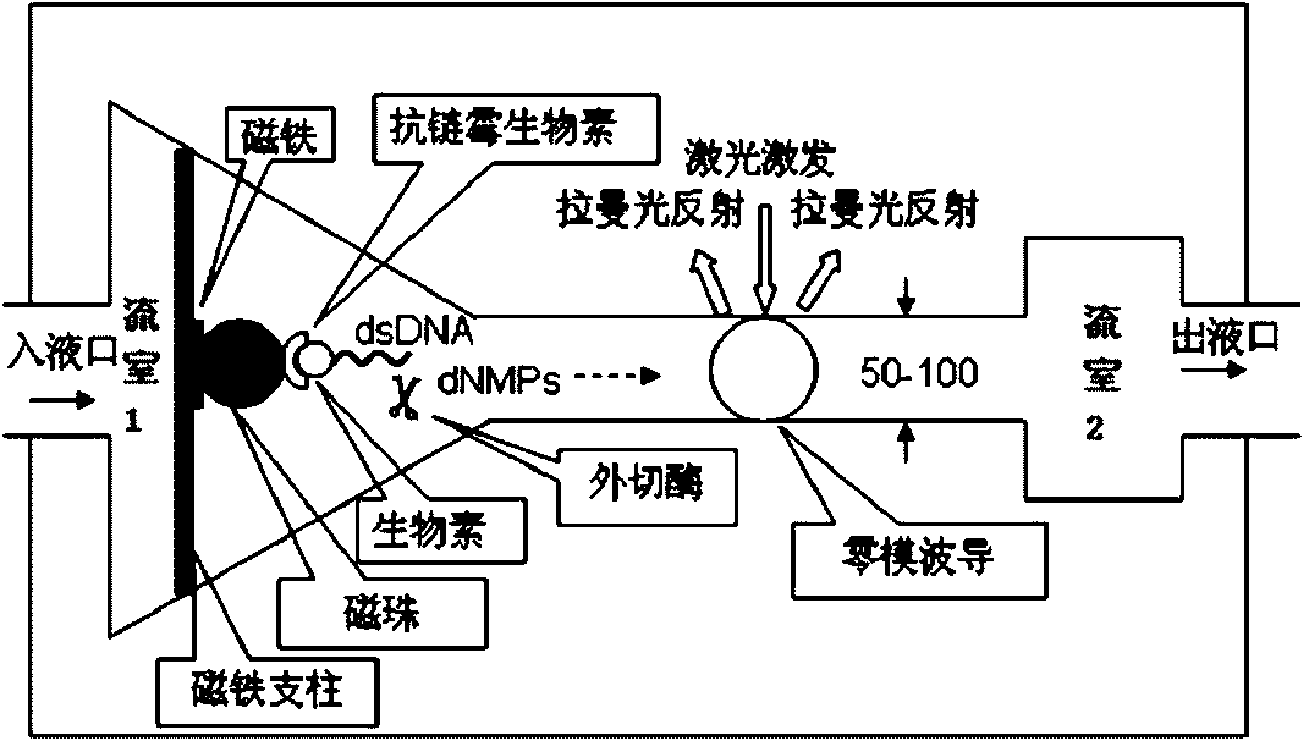
Method for testing sequence of nucleic acid single molecule
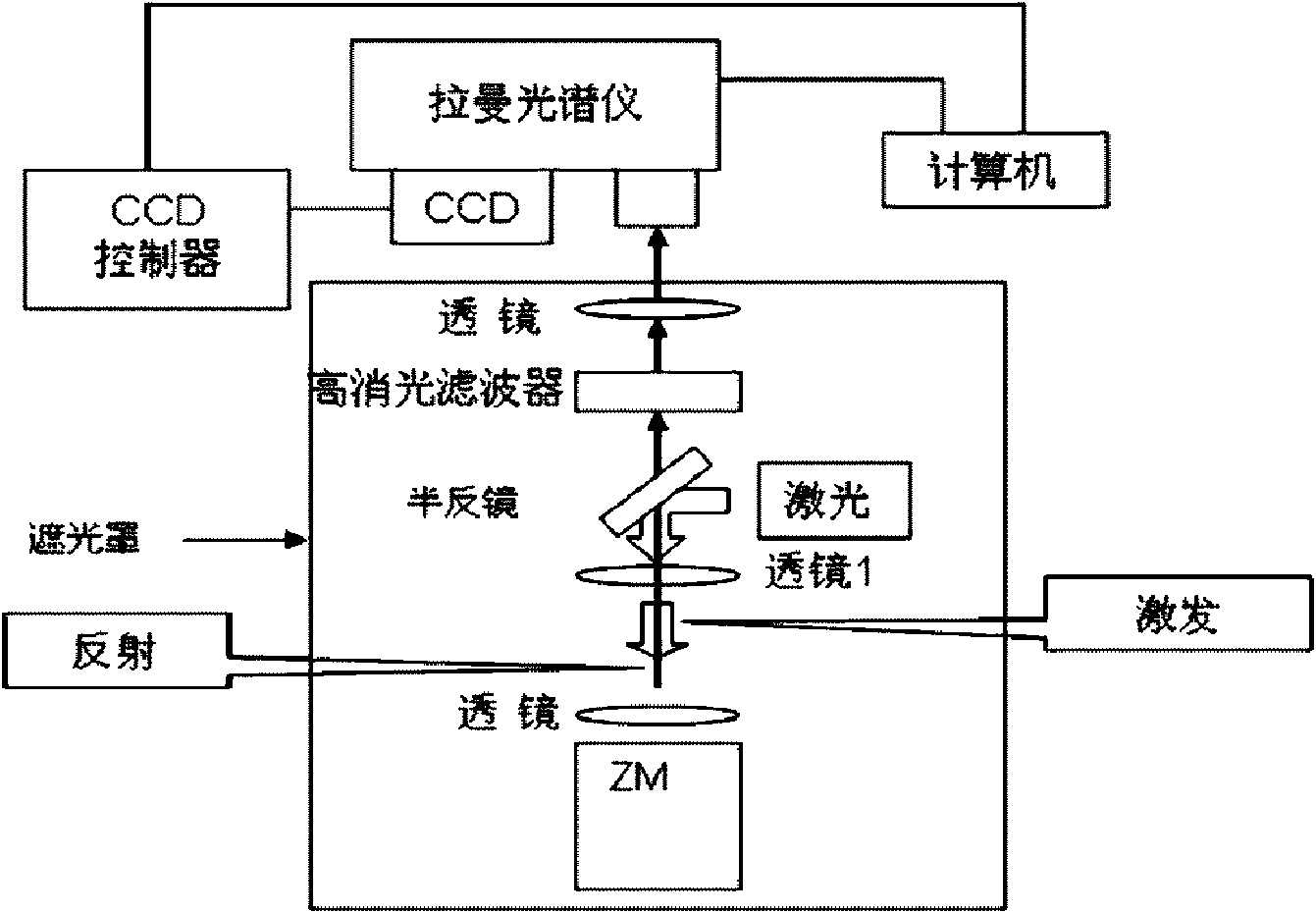
InactiveCN101654712AFast measurementHigh assay qualityMicrobiological testing/measurementRaman scatteringPhosphoric acidWaveguide
The invention relates to a method for testing a sequence of a nucleic acid single molecule in the technical field of biology, which comprises the following steps: respectively detecting Raman spectrumsignals of deoxyadenosine 5'-phosphoric acid, deoxyguanosine 5'-phosphoric acid, deoxycytidine 5'-phosphoric acid, deoxythymidine 5'-phosphoric acid, methyldeoxycytidine 5'-phosphoric acid, adenosine5'-phosphoric acid, vernine 5'-phosphoric acid, cytidine 5'-phosphoric acid and uridine 5'-phosphoric acid and establishing a standard curve; cutting a nucleic acid molecule to be detected by exonclease and detecting the Raman spectrum signal transmitted by a cut product when passing through a zero mode waveguide by the trigger of a laser; converting the Raman spectrum signal obtained in the step2 into concrete ribotide according to the standard curve obtained in the step 1 and obtaining the concrete sequence of the nucleic acid molecule to be detected by combining the cutting direction of the exonclease. The method can directly detect a natural nucleic acid sequence without a mark and has high detecting speed and detecting quality and low detecting cost.
Owner:SHANGHAI JIAO TONG UNIV
Acylated uridine and cytidine and uses thereof
InactiveUS6274563B1No untoward pharmaceutical effectEfficient managementBiocideSugar derivativesDiseaseDiabetes mellitus
The invention relates to compositions comprising acyl derivatives of cytidine and uridine. The invention also relates to methods of treating hepatopathies, diabetes, heart disease, cerebrovascular disorders, Parkinson's disease, infant respiratory distress syndrome and for enhancement of phospholipid biosynthesis comprising administering the acyl derivatives of the invention to an animal.
Owner:WELLSTAT THERAPEUTICS
Pyrimidine nucleoside high-yielding strain and carbamyl phosphate synthetase adjusting site thereof
ActiveCN105671007AImprove toleranceBacteriaMicroorganism based processesCarbamyl PhosphateStructural analog
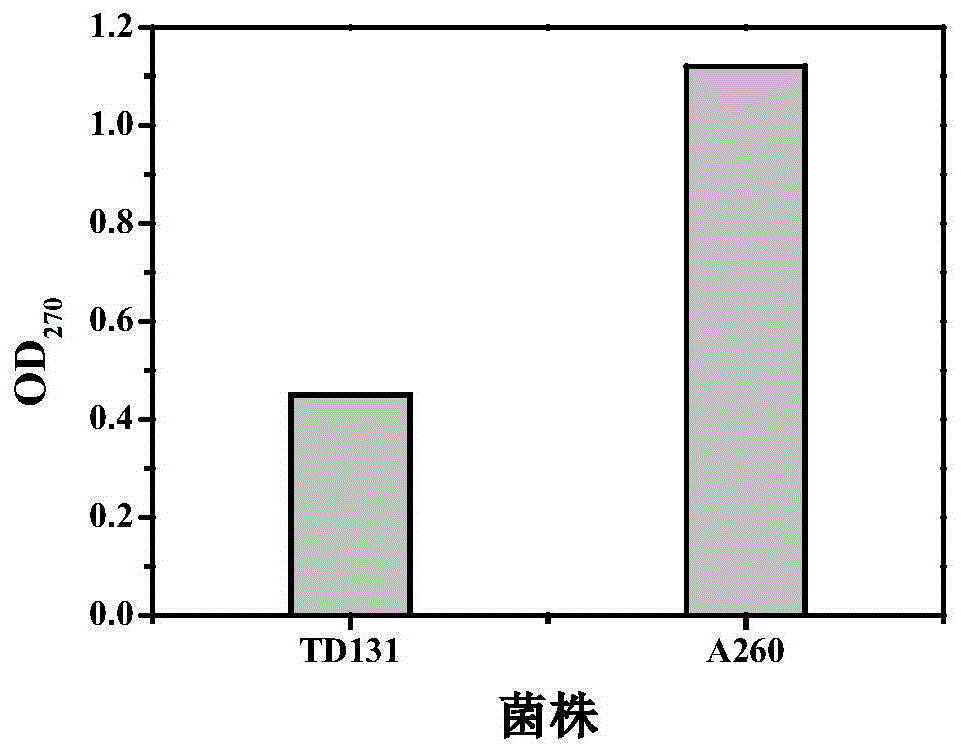
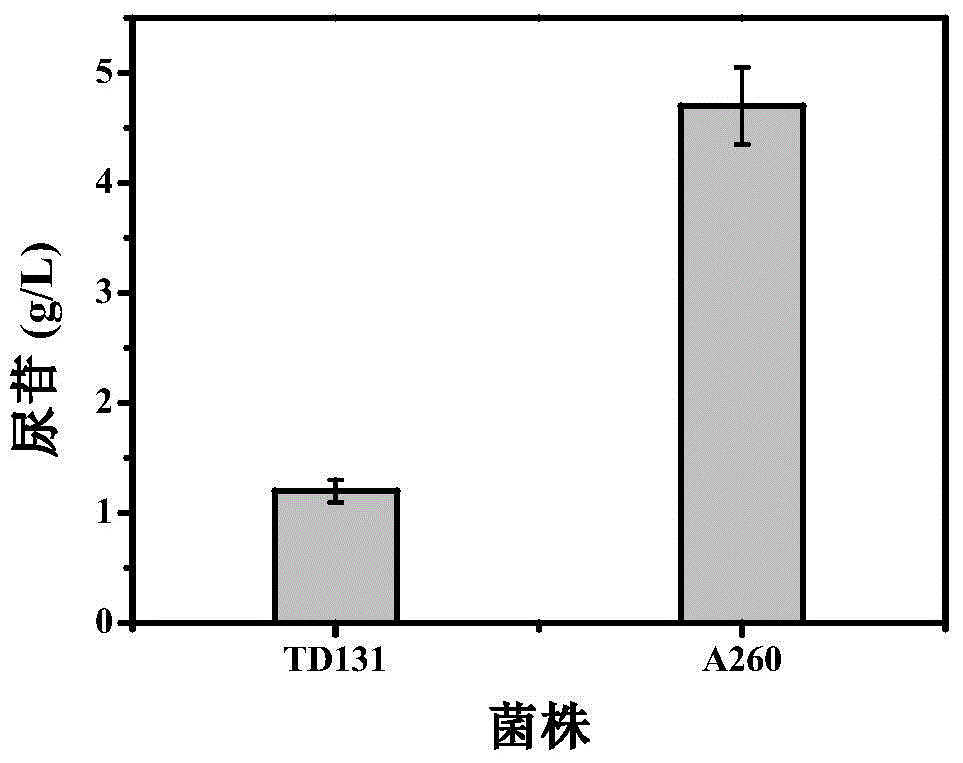
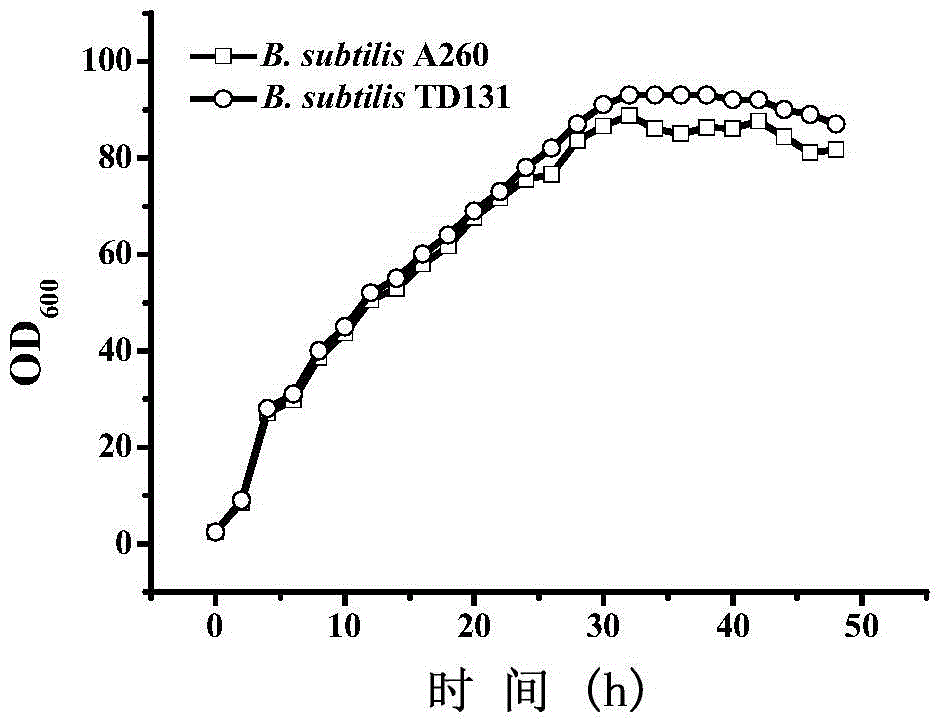
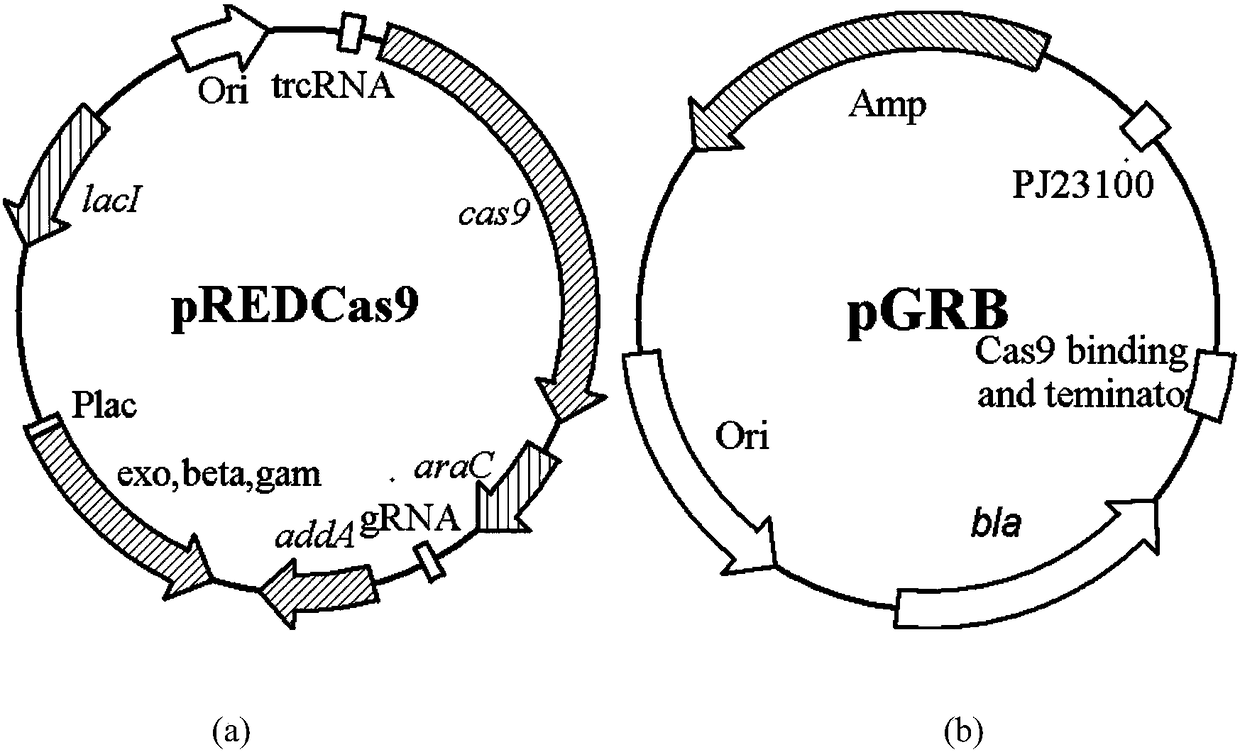
The invention belongs to the technical field of enzyme engineering, and concretely relates to a pyrimidine nucleoside high-yielding strain and a carbamyl phosphate synthetase adjusting site thereof. The invention provides a pyrimidine nucleoside production Bacillus subtilis mutant strain and a carbamyl phosphate synthetase encoding gene. A key regulation site related to carbamyl phosphate synthetase end product feedback inhibition is defined, and provides reference for breeding of later pyrimidine nucleoside high-yielding strains. The Bacillus subtilis mutant strain allows the output of fermentation process produced nucleoside pyrimidine uridine to reach 14-16g / L, and has substantially improved tolerance to different pyrimidine nucleoside structure analogs.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Genetic engineering bacterium capable of producing uridine at high yield as well as building method and application thereof
ActiveCN108130306AImprove growth traitsIncrease productivityBacteriaEnzymesEscherichia coliNucleotide
The invention provides a genetic engineering bacterium capable of producing uridine at high yield as well as a building method and application thereof. The genetic engineering bacterium is characterized in that pyrimidine nucleoside operons pyrBCAKDFE with the nucleotide sequence shown as SEQ ID NO:1 is integrated on a genome of colon bacillus; the starting is realized by a strong promoter P[trc];a uridine synthesis path is reconstituted; the self PRPP synthetase coding gene prsA on the genome is subjected to dual copying, and the starting is realized by the strong promoter P[trc]; meanwhile,the activity of udk, udp, and rihA, rihB and rihC is lacked; the thrA activity is lacked; the argF activity is lacked. The genetic engineering bacterium is applied to fermentation production of uridine; 40 to 67g / L of uridine can be produced after the fermentation is performed for 40 to 70h in a 5L fermentation tank; the maximum production intensity can reach 1.5g / (L*h); the glucoside conversionrate is 15 to 25 percent; the genetic engineering bacterium belongs to the highest level for producing the uridine by the fermentation method reported in the prior art.
Owner:TIANJIN UNIV OF SCI & TECH
Memory in subjects with mini-mental state examination of 24-26
InactiveUS20100331258A1Reduce needReduce dosage of treatmentBiocideNervous disorderDocosahexaenoic acidModerate dementia
The invention thus pertains to the use of a composition comprising: (a) uridine or uridine phosphate; and (b) docosahexaenoic acid and / or eicosapentaenoic acid, for improving memory and / or the treatment or prevention of impaired memory function, in a subject with a mini-mental state examination of 24-26, wherein said composition is enterally administered to the subject. In the MMSE test, any score of 27 or higher (out of 30) is effectively normal. In the patients with dementia, 20-26 indicates mild dementia, 10-19 moderate dementia, and below 10 severe dementia. It was the present inventors' belief that within the group of 20-26, the memory impairment in the sub-group of 24-26 may even be reversible, as the pathological pathways have just started to develop. In this group of subjects the pathological pathways have just started to develop. Clinical studies show excellent results for this subgroup.
Owner:NUTRICIA
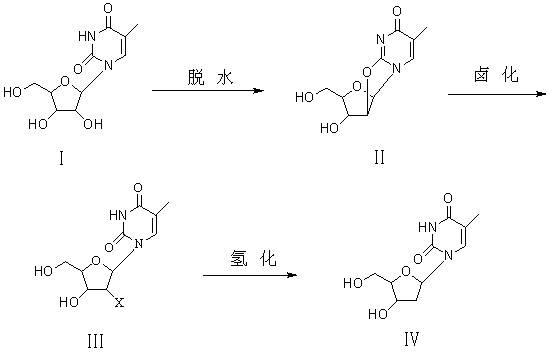
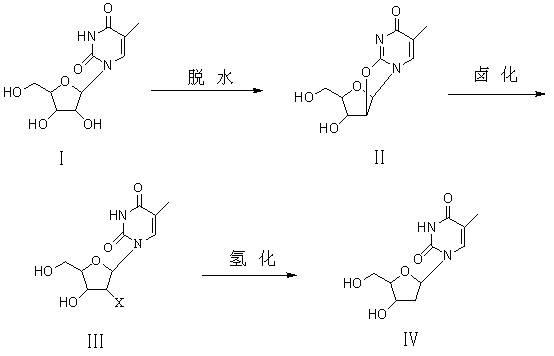
Preparation method of beta-thymidine
ActiveCN102086222ASimple processLow costSugar derivativesSugar derivatives preparationCarbonateUridine Nucleotides
The invention provides a preparation method of beta-thymidine, belonging to the technical field of fine chemical industry. The preparation method is as follows: reacting 5-methyl uridine used as a raw material with dialkyl carbonate (RO)2CO for dehydration; generating halogenation reaction with a halogenation reagent; and finally, carrying out catalytic hydrogenation by reducing metal so as to prepare the beta-thymidine. The synthesis process is simple, and the cost is low; and only twice separations and purifications are carried out, thereby greatly reducing labor intensity and avoiding the defects that propionyl bromide is used, cost is high, yield is low, and equipment corrosion is severe in the original halogenation reaction process. By using a novel halogenation reaction system, raw material cost is reduced, conversion rate is high, yield is up to above 85%, the purity of the product is high, and high-performance liquid chromatography (HPLC) is larger than 99.8%.
Owner:ZHEJIANG XIANFENG TECH
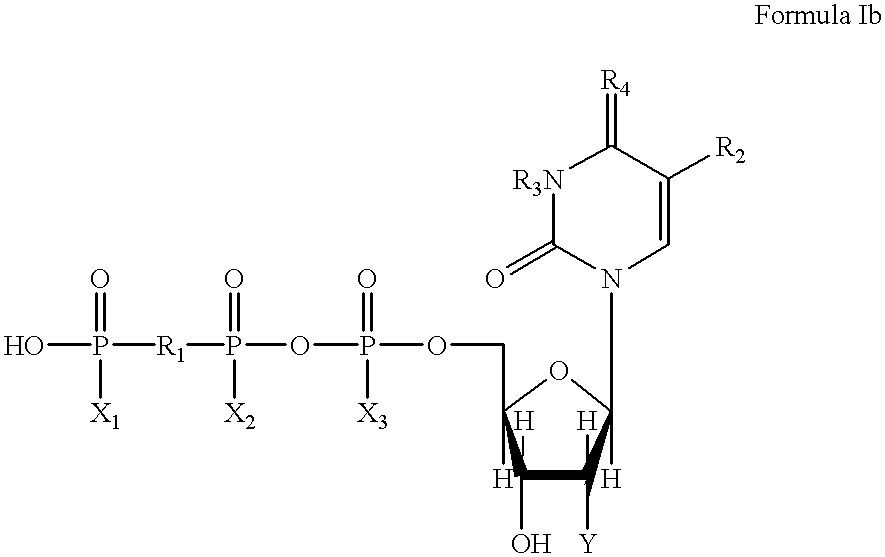
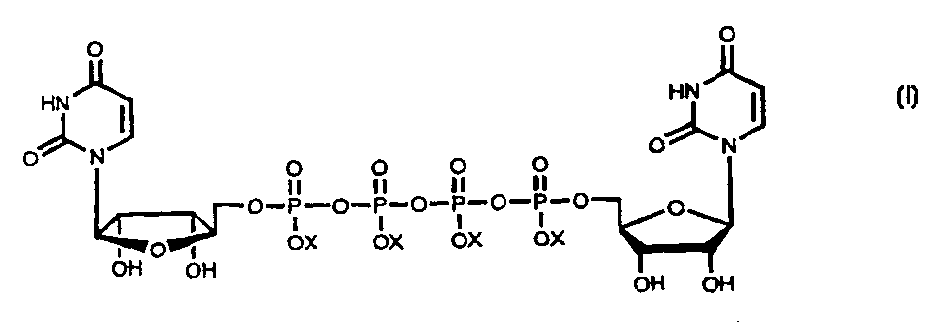
Method for large-scale production of Di (uridine 5'-tetraphosphate) and salts thereof
The present invention provides new methods for the synthesis of the therapeutic dinucleotide, P<1>,P<4>-di(uridine 5'-tetraphosphate), and demonstrates applicability to the production of large quantities. The methods of the present invention substantially reduce the time period required to synthesize diurindine tetraphosphate, preferably to three days or less. The novel tetrammonium and tetrasodium salts of P<1>,P<4>-di(uridine 5'-tetraphosphate) formula (I) prepared by these methods are stable, soluble, nontoxic, and easy to handle during manufacture. In formula I, X is Na, NH4 or H, provided that all X groups are not H.
Owner:MERCK & CO INC
Fingerprint pattern quality control method for cordyceps sinensis bacterium powder raw material in herbs medicaments for strengthening the body resistance and activating blood and dissolving stasis
ActiveCN101293002AGuarantee normal implementationHigh sensitivityFungiComponent separationHplc fingerprintRetention time
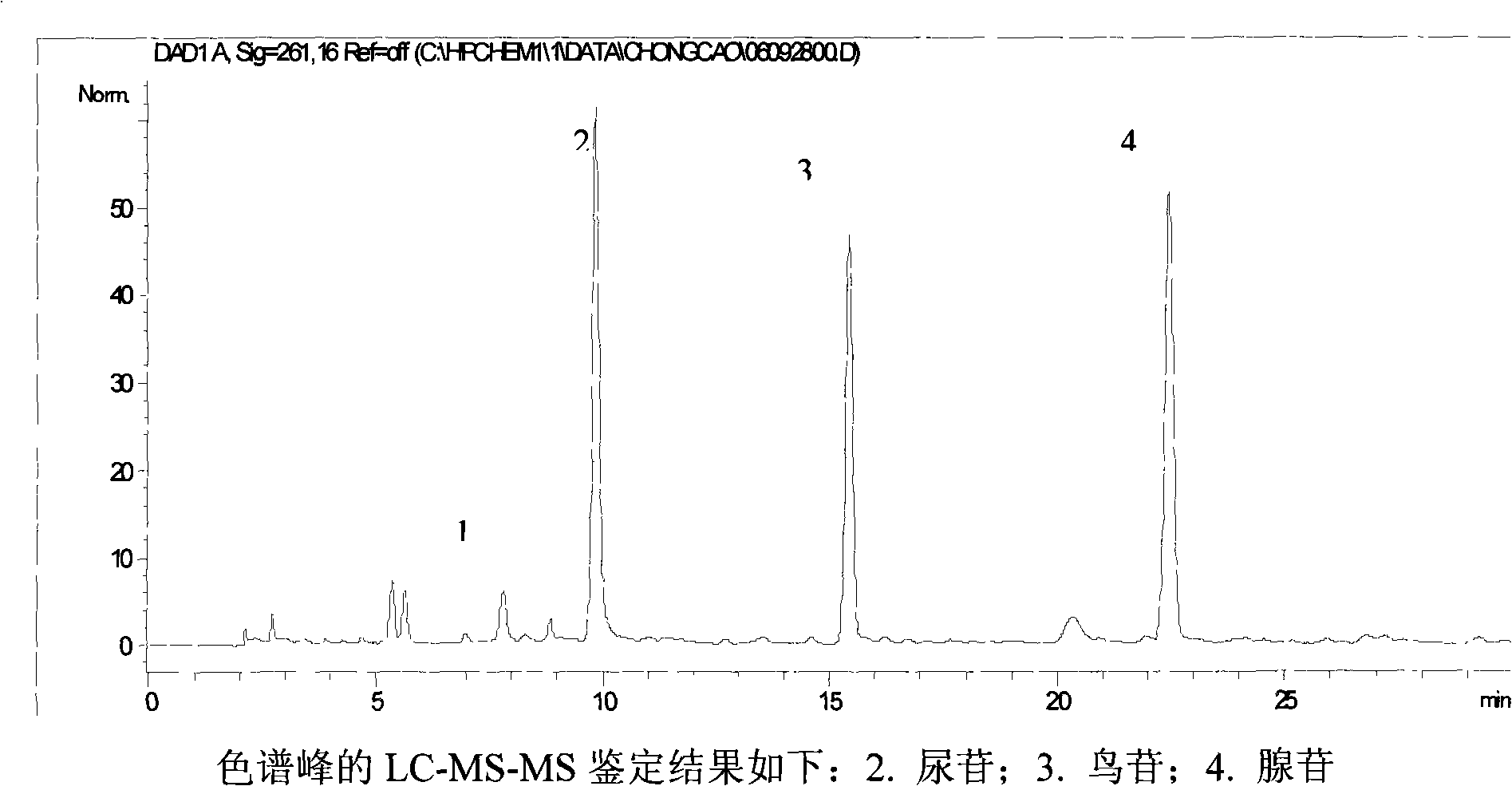
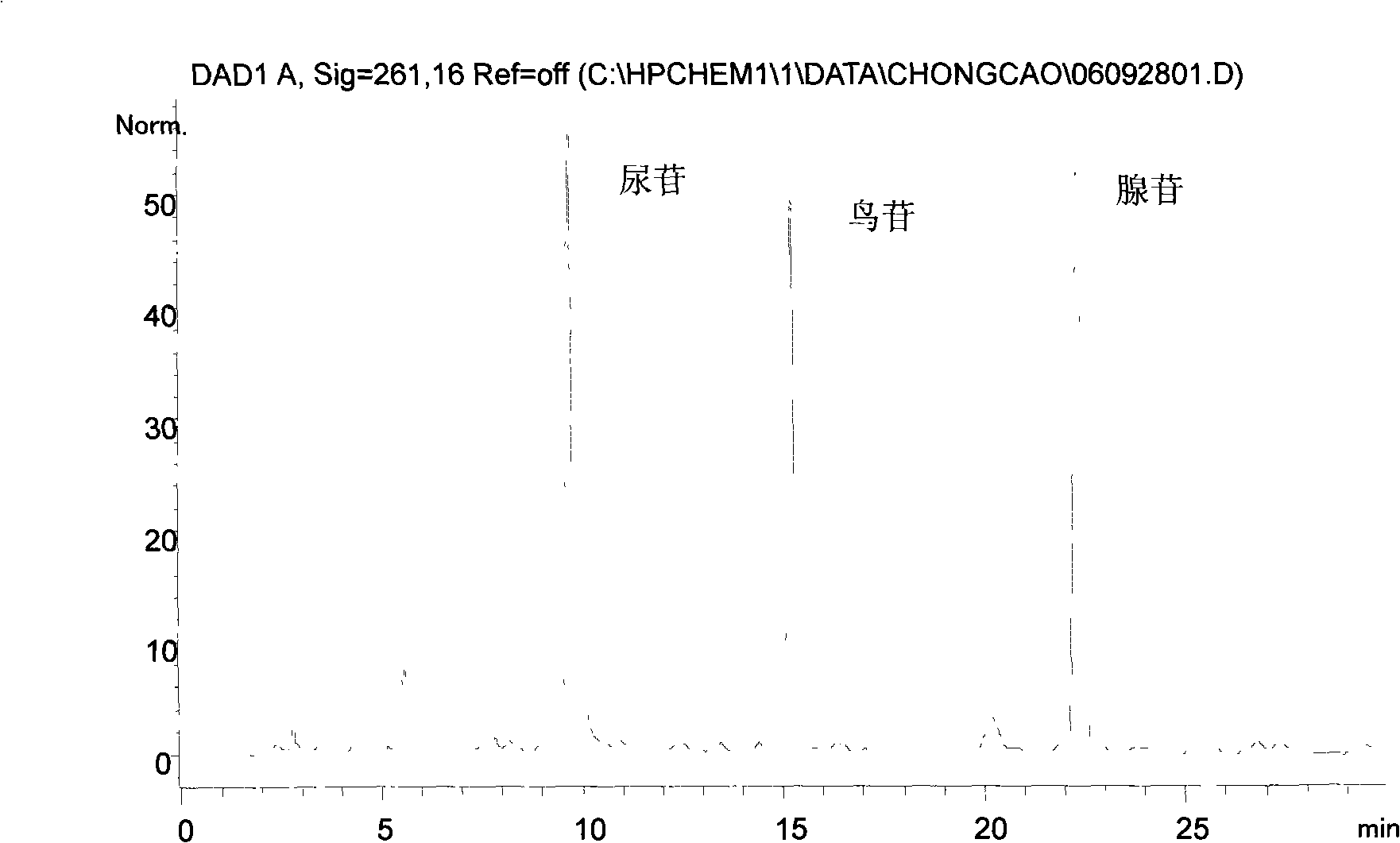
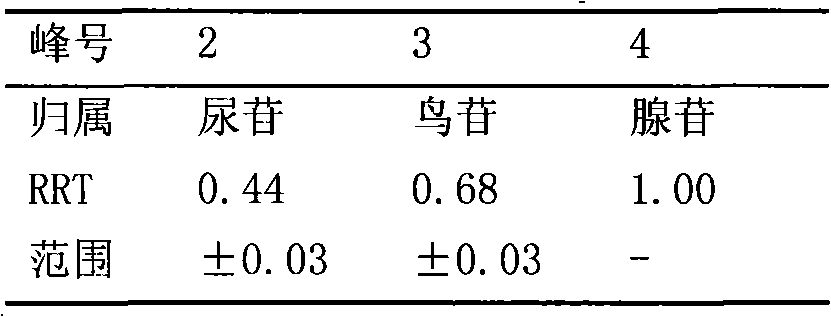
The invention relates to a control method of the fingerprint spectrum quality of cordyceps sinensis powder raw material in botanical drug for strengthening vital qi and removing blood stasis, comprising the steps that: (1) cordyceps sinensis powder is extracted: 0.100g of cordyceps sinensis powder is taken, purified water is added, the ultrasonic extraction, the filtration and the sample injection are carried out; (2) the gradient elution with mobile phase is carried out: octadecyl silane bonded silica gel is taken as a filler, water and acetonitrile are taken as mobile phase to carry out the gradient elution for 0 to 30min and 0 to 7 percent B; (3) a standard fingerprint spectrum is established: the HPLC standard fingerprint spectrum of the cordyceps sinensis powder is determined, and 3 characteristic peaks are selected; (4) the quality control of the fingerprint spectrum is carried out: the relative retention time of No.2 peak uridine, No.3 peak guanosine and No.4 peak adenosine are 0.44 plus or minus 0.03, 0.68 plus or minus 0.03 and 1.00 respectively; the HPLC fingerprint spectrum of the sample is compared with the contrast fingerprint spectrum. The similarity calculated by the 5 common peaks is not less than 0.9, (5) the preparation of the cordyceps sinensis powder raw material is carried out; the control method has good repetitivity and can fully reflect the basic characteristics of nucleoside ingredients of the cordyceps sinensis powder.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Use of UDPG pyrophosphorylase in rice
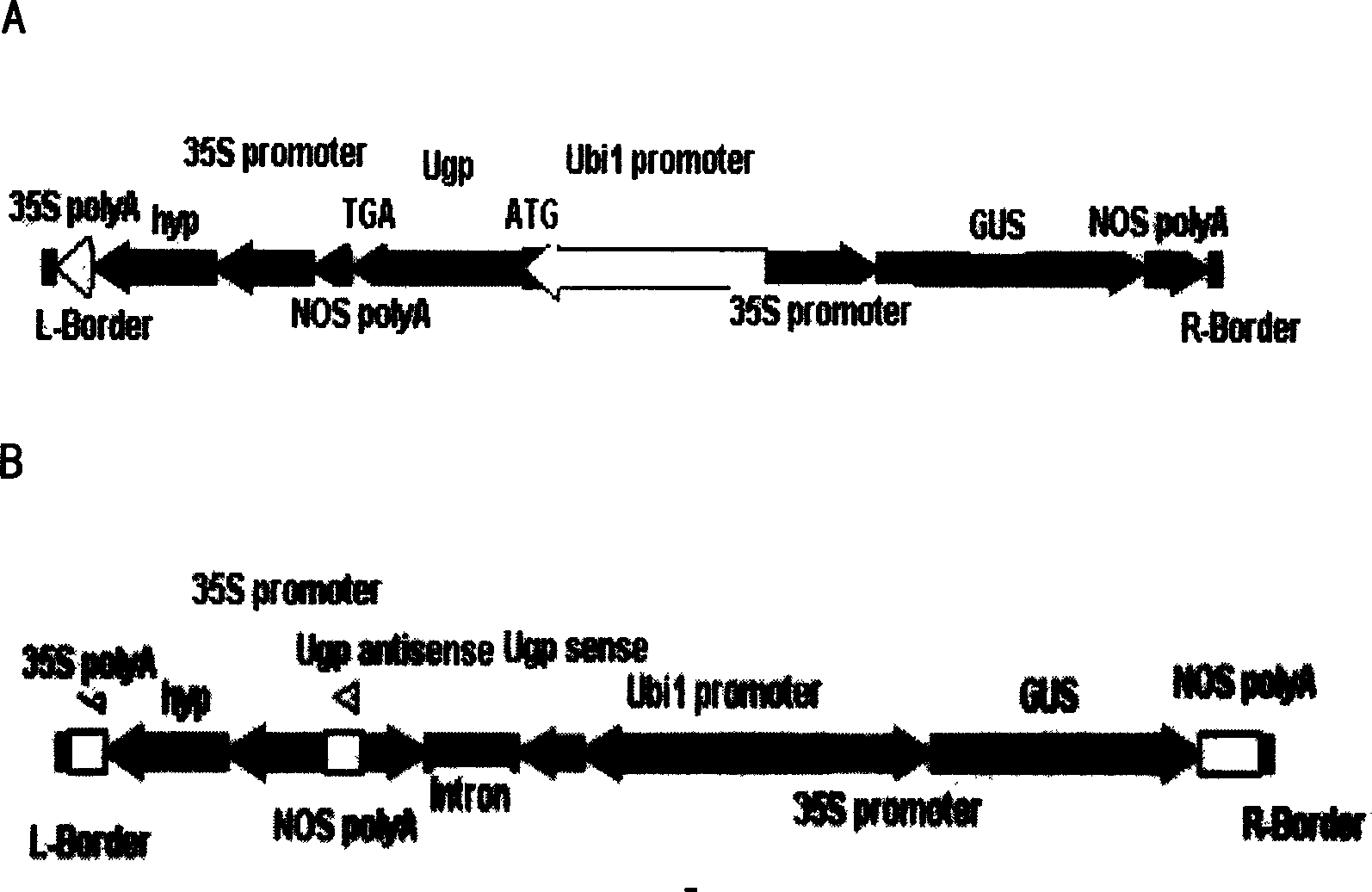
InactiveCN1614023APromote growthGrow moreFermentationVector-based foreign material introductionGenetically modified riceUridine diphosphate glucose pyrophosphorylase
The invention was involved in the application of uridine 5'diphosphate glucose pyrophosphorylase gene in the rice field. Uridine 5'diphosphate glucose pyrophosphorylase gene was connected with intensified or inhibited expression vector, which was intensified or, inhibited and transferred expression vector into rice, then, uridine 5'diphosphate glucose pyrophosphorylase gene in rice was intensified or inhibited. The rice that was intensified by uridine 5'diphosphate glucose pyrophosphorylase gene had rapid growth, long ears, many seeds and high yields per plant, while the rice that was inhibited by uridine 5'diphosphate glucose pyrophosphorylase gene showed that male sterility was barren and its pollen was with 100% sterility. It was with self-sterility while cross was normal. The invention was found to provide a new path for improving rice species applying uridine 5'diphosphate glucose pyrophosphorylase gene.
Owner:WUHAN UNIV
L- beta -dioxolane uridine analogs and methods for treating and preventing Epstein-Barr virus infections
InactiveUS6022876ALow toxicityMinimal toxicityBiocideGroup 5/15 element organic compoundsHigh activityUridine Nucleotides
The present invention relates to the discovery that certain beta -L-dioxolane nucleoside analogs which contain a uracil base, and preferably, a 5-halosubstituted uracil base, exhibit unexpectedly high activity against Epstein-Barr virus (EBV), Varciella-Zoster virus (VZV) and Herpes Virus 8 (HV-8). In particular, the compounds according to the present invention show potent inhibition of the replication of the virus (viral growth) in combination with very low toxicity to the host cells (i.e., animal or human tissue). Compounds are useful for treating EBV, VZV and HV-8 infections in humans.
Owner:GEORGIA UNVERSITY OF RES FOUND INC THE +1
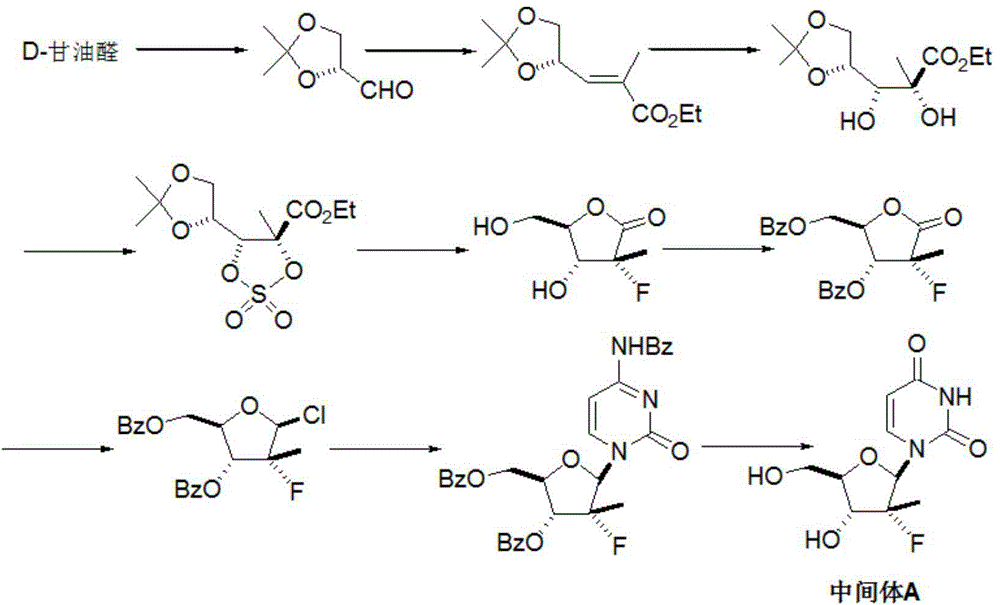
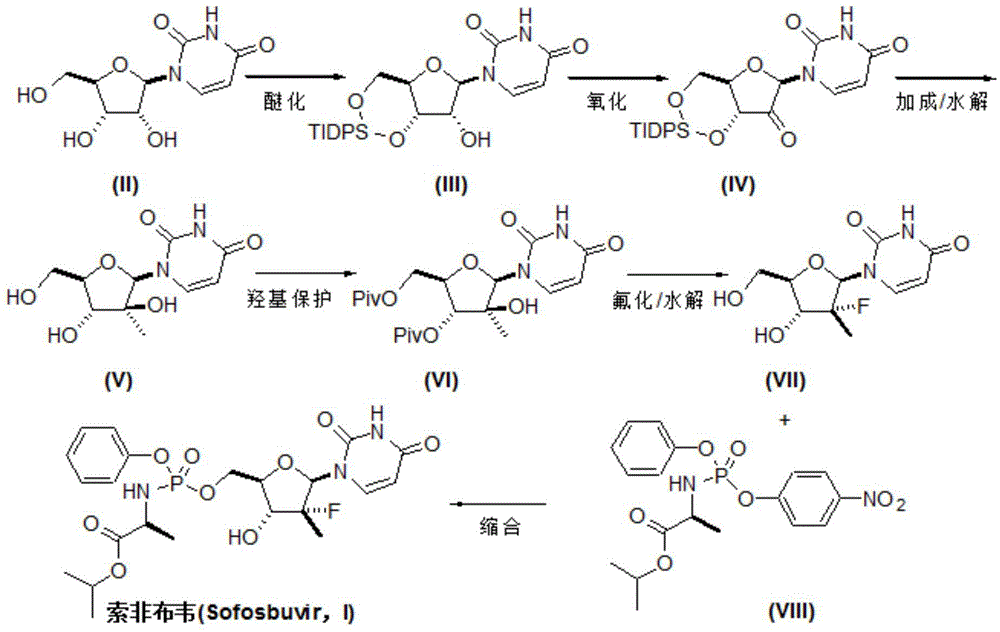
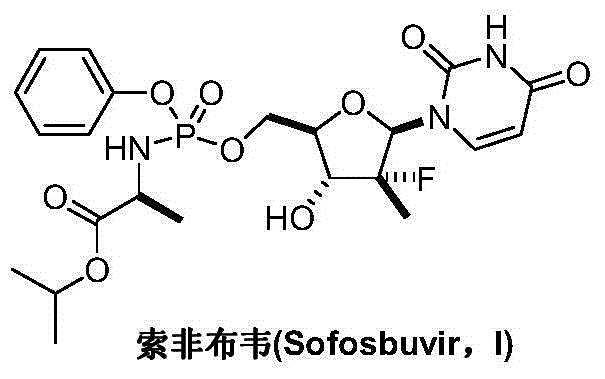
Preparation method for sofosbuvir
InactiveCN104478976AEase of industrial productionRaw materials are easy to getSugar derivativesSugar derivatives preparationUridine NucleotidesSofosbuvir
The invention discloses a preparation method for preparing sofosbuvir (Sofosbuvir,I) with uridine as a raw material and through etherification, oxidation, addition, condensation and other steps; the preparation method has the advantages of easily obtained raw materials, concise process, economy, and environmental protection, and is suitable for industrialized production.
Owner:SUZHOU MIRACPHARMA TECH
Method of preparing phosphate
The invention provides a method of preparing a phosphate. The method comprises the following step: enabling a pyrophosphate active compound expressed by formula II to react with uridine monophosphate expressed by a formula III or a salt thereof in a hydrophilic solvent under the action of a bimetallic ion composite catalyst to obtain P1,P4-bis (5'-uridine group) tetraphosphate expressed by formula I. In the formula II, X is imidazolyl, N-methyl imidazolyl, or 1, 2, 4-triazolyl; and the bimetallic ions in the bimetallic ion composite catalyst are a combination of any two of Zn2+, Mn2+, Mg2+, Fe2+, Fe3+ and Al3+. The method of preparing a phosphate employs a bimetallic catalytic system and can achieve high-efficiency and easy separation preparation of diquafosol.
Owner:CHANGCHUN PUHUA PHARMA
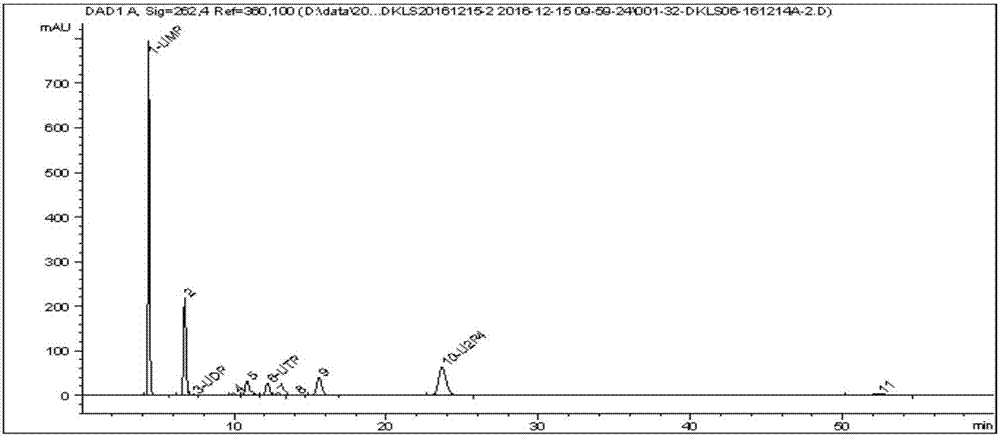
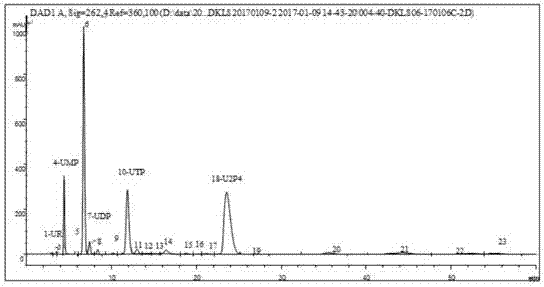
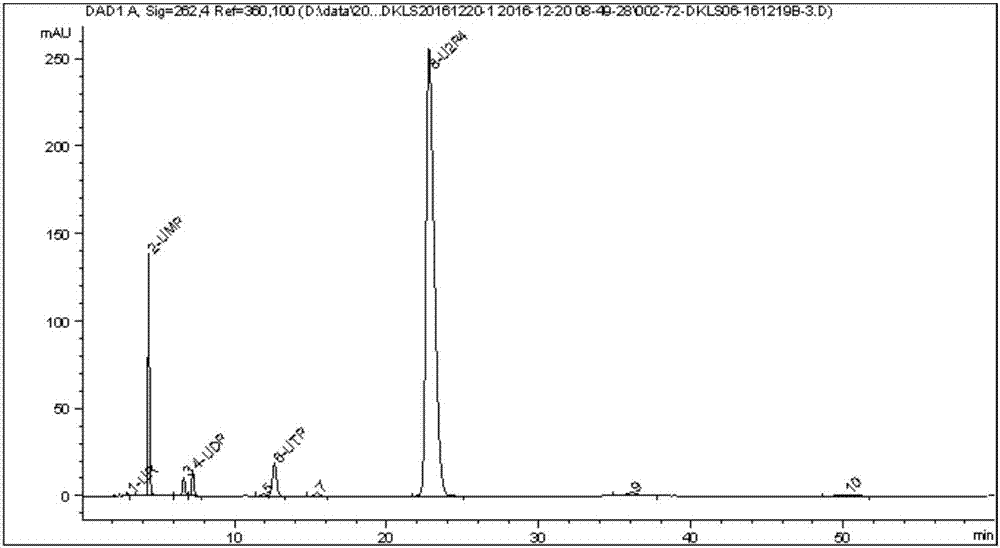
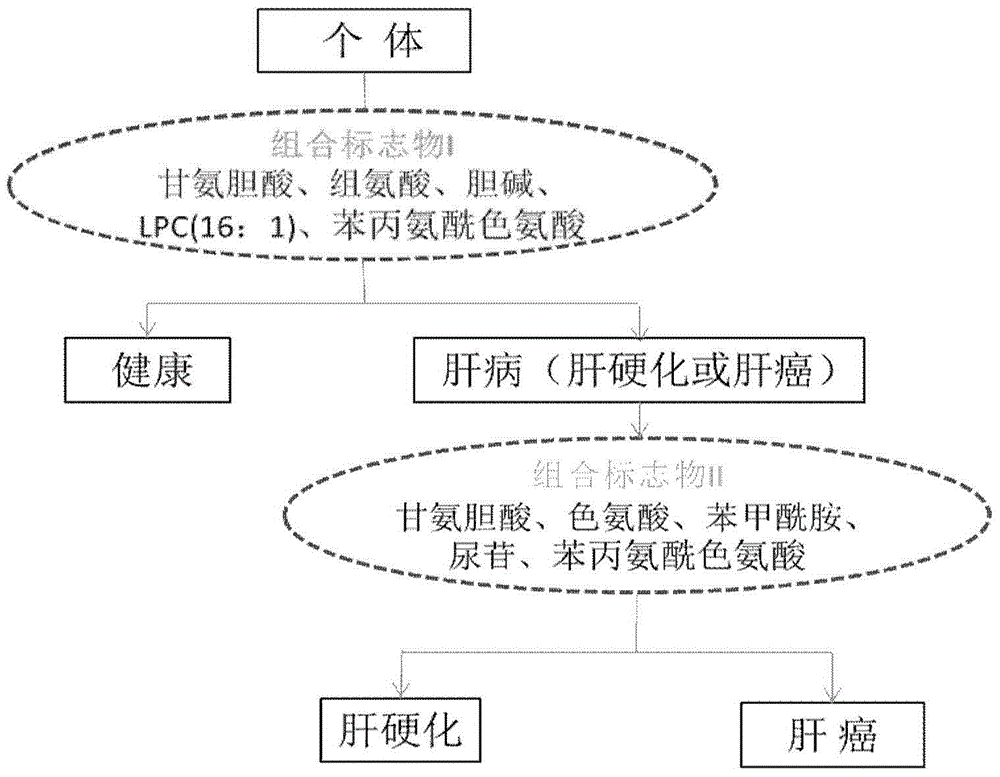
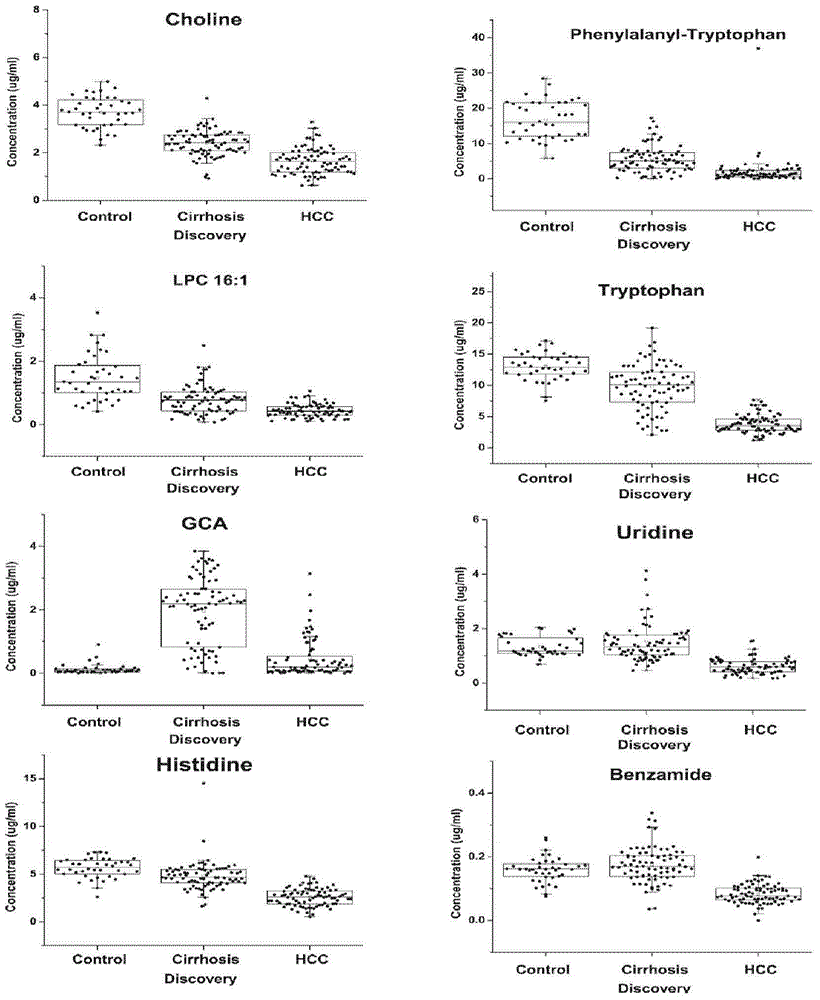
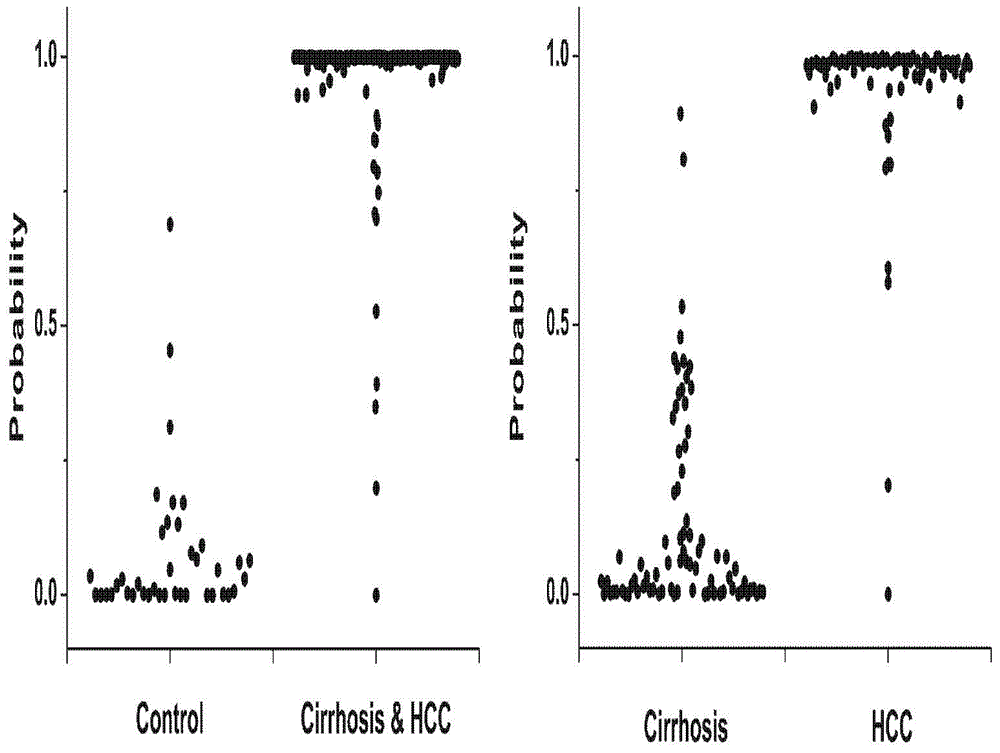
Novel serum metabolite composition and application thereof as liver cancer diagnosis marker
ActiveCN105738626AMaintain stabilityHas the value of clinical development and applicationMaterial analysisTryptophanUridine Nucleotides
The invention discloses a novel serum metabolite composition and an application thereof as a marker for preparing an early-stage liver cancer diagnosis kit. The serum metabolite composition includes: glycocholic acid, tryptophan, histidine, uridine, choline, benzamide, lysophosphatidyl choline 16:1 and phenylalanyl tryptophan. The serum metabolite composition can be used for early-stage diagnosis of liver cancer, is low in detection cost, is high in repeatability and sensitivity, and has excellent complementarity with a conventional clinical diagnosis marker serum alpha fetoprotein (AFP).
Owner:HANGZHOU HEALTH BANK MEDICAL LAB CO LTD
Method for detecting quality of traditional Chinese medicine indigowoad root granules
ActiveCN101912428AGuaranteed to be scientificGuaranteed growthComponent separationAntiviralsAdenosineElectro spray
The invention provides a method for detecting the quality of traditional Chinese medicine indigowoad root granules. The method comprises the following steps of: detecting the traditional Chinese medicine indigowoad root granules by using a soft-ionization mass spectrometry technique; performing primary evaluation on the number and strength of characteristic fingerprint peaks in an electro-spray fingerprint; measuring by high performance liquid chromatography to obtain the peak area of a chromatographic peak corresponding to each of cytidine, adenine, uridine, guanosine and adenosine serving as standard reference substances; and measuring according to a standard curve to obtain the cytidine content, adenine content, uridine content, guanosine content and adenosine content of one gram of the traditional Chinese medicine indigowoad root granules: 0.234 to 0.238 milligrams of cytidine, 0.116 to 0.118 milligrams of adenine, 0.472 to 0.474 milligrams of uridine, 0.331 to 0.334 milligrams of guanosine and 0.447 to 0.450 milligrams of adenosine. The method has the advantages of ensuring the scientificity and progressiveness of the method for detecting the quality of the traditional Chinese medicine indigowoad root granules, along with qualitative and quantitative performance.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Methods for improving frontal brain bioenergetic metabolism
InactiveUS20100041620A1Few adverse effectImprove toleranceBiocideNervous disorderMedicineFrontal lobe
The invention provides methods and compositions for augmenting bioenergetic metabolism in the frontal brain involving administration of a cytidine-containing or uridine-containing compound to a human.
Owner:THE MCLEAN HOSPITAL CORP
Method for preparing uridine diphosphate
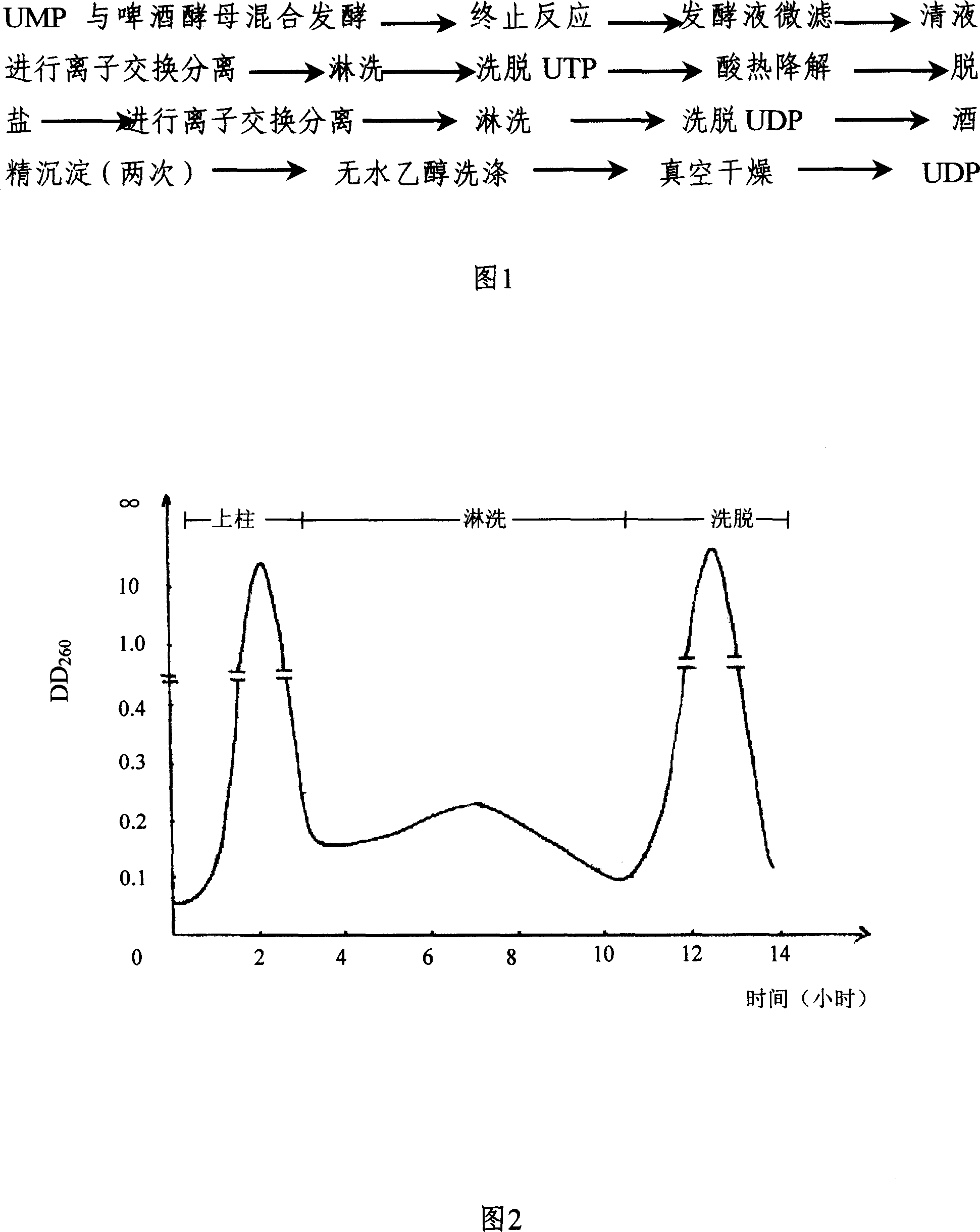
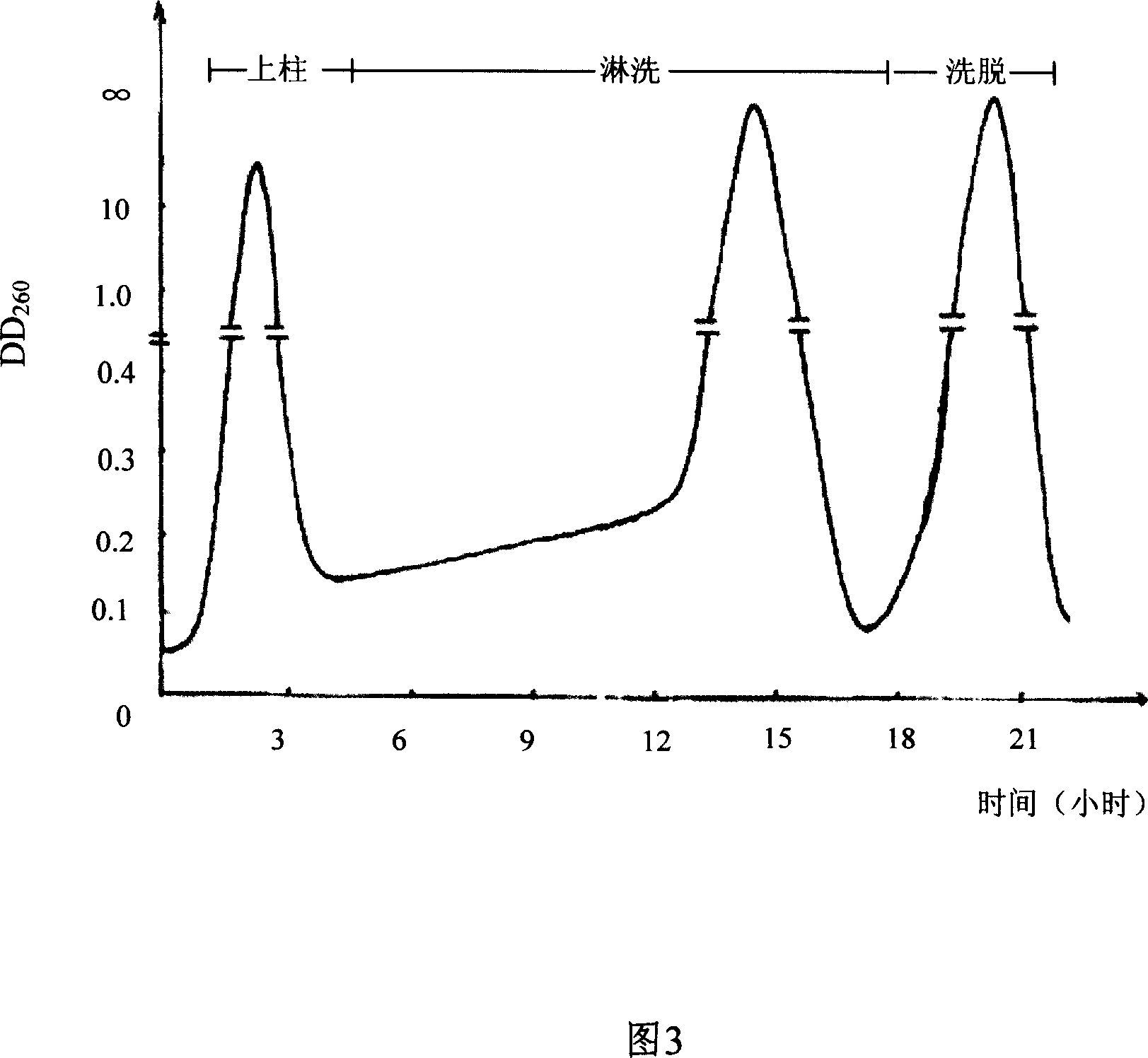
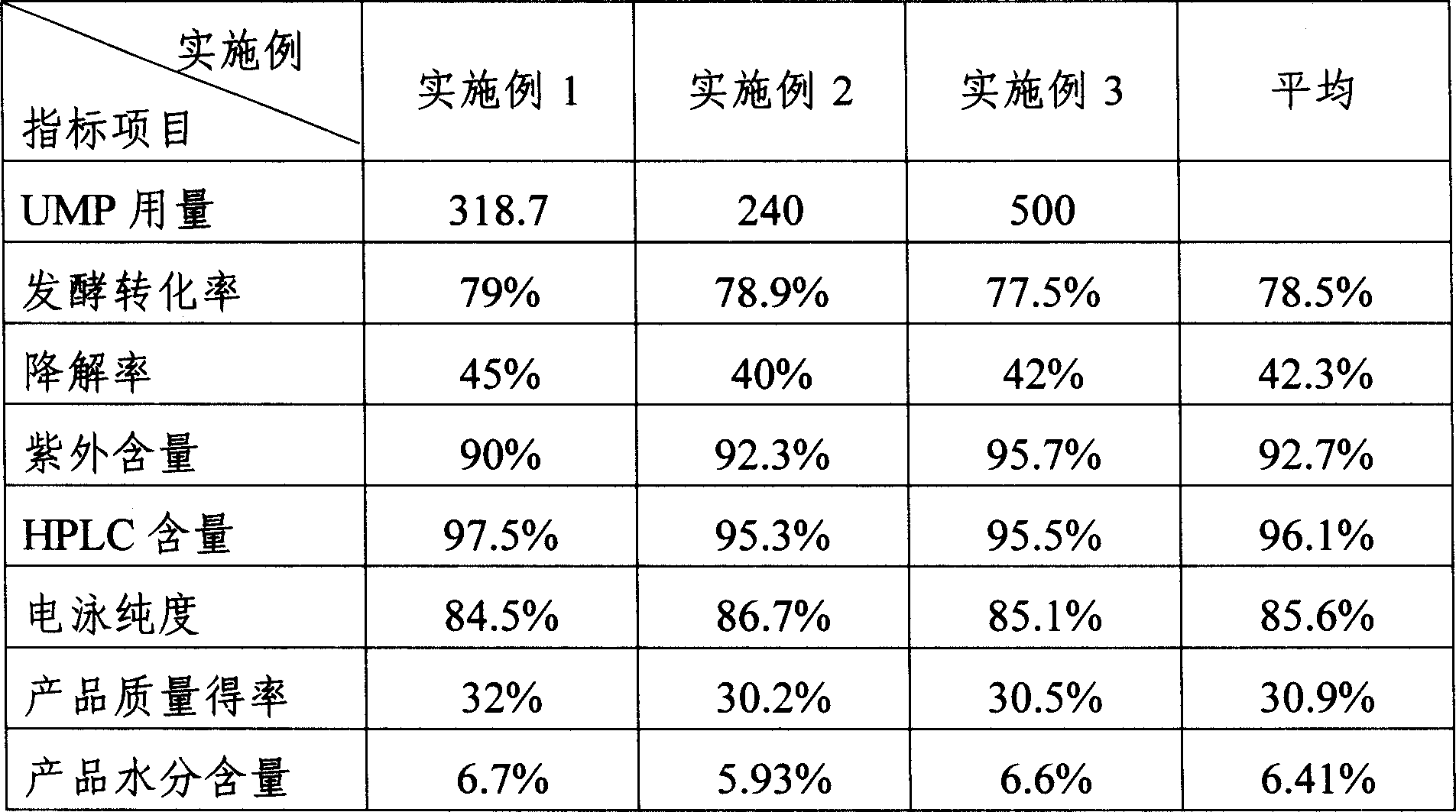
InactiveCN1962875AReduce the burden onShorten the timeMicroorganism based processesFermentationYeastPhosphoric acid
The invention discloses a preparing method of uridine diphosphite, which comprises the following steps: blending uridine monophosphate and beer yeast to ferment; terminating fermenting to obtain the ferment liquid of uridine triphosphate; predisposing ferment liquid; separating and purifying; proceeding acid heat to decompose purified uridine triphosphate; filtering; separating; refining. The invention shortens the predisposing time by two thirds and separating purifying time by one third, which makes receiving rate by over 30%.
Owner:北京燕京中科生物技术有限公司
Biocatalytic synthesis of aminodeoxy purine N9-beta-D-nucleosides containing 3-amino-3-deoxy-beta-D-ribofuranose, 3-amino-2,3-dideoxy-beta-D-ribofuranose, and 2-amino-2-deoxy-beta-D-ribofuranose as sugar moieties
Purine N9-β-D-nucleosides containing 3-amino-3-deoxy-β-D-ribofaranose, 3-amino-2,3-dideoxy-β-D-ribofuranose, and 2-amino-2-deoxy-β-D-ribofuranose as sugar moieties are synthesized by biocatalytic transglycosylation of purine bases and the respective 3′-amino-3′-deoxyuridine, 3′-amino-3′-deoxythymidine and 2′-amino-2′-deoxyuridine as donors of the carbohydrate moiety, and the cells of Escherichia coli as a biocatalyst or glutaraldehyde (GA) treated cells of Escherichia coli as a biocatalyst or a mixture of thymidine (uridine) phosphorylase and purine nucleoside phosphorylase.
Owner:METKINEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com