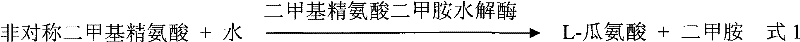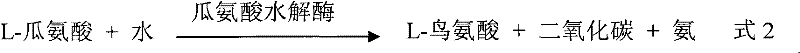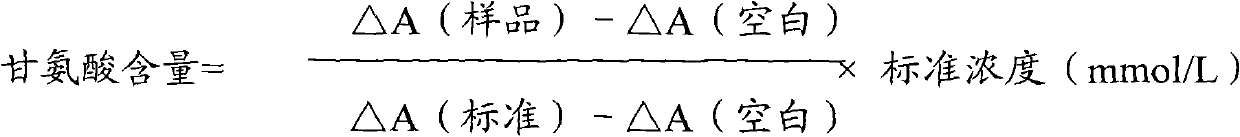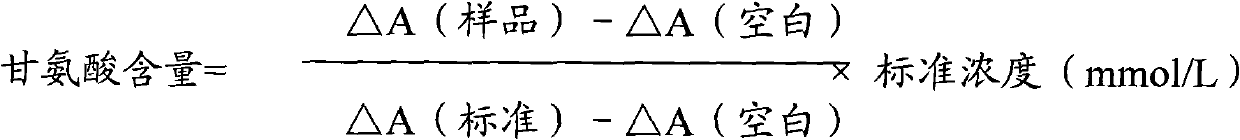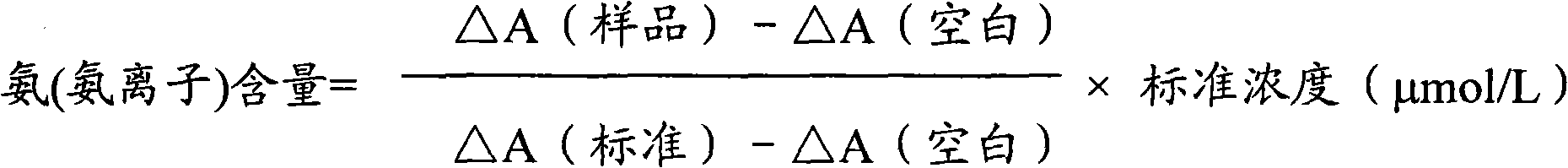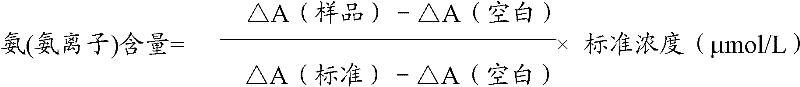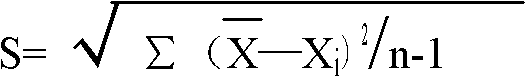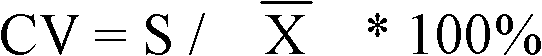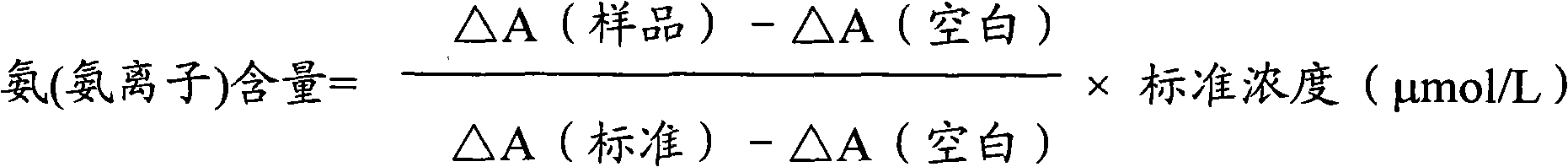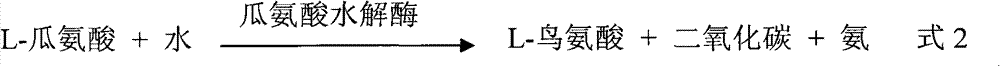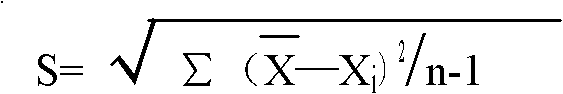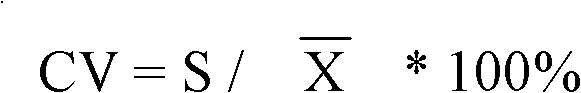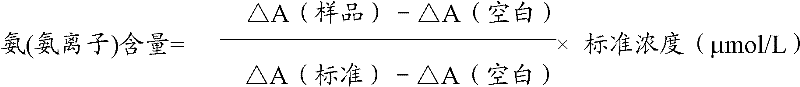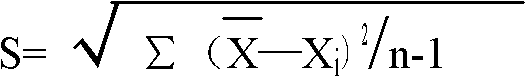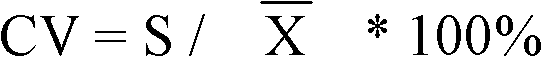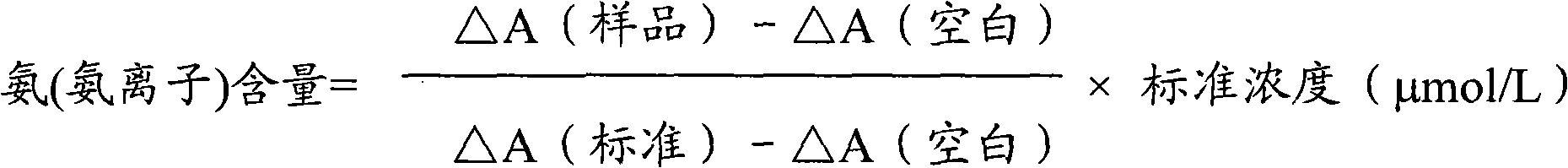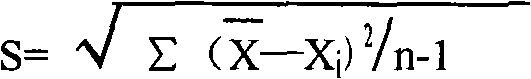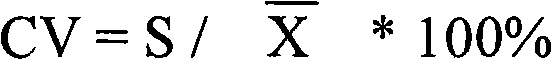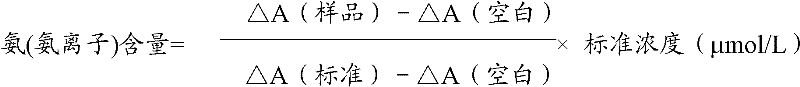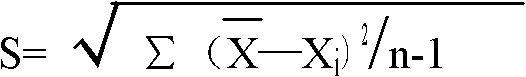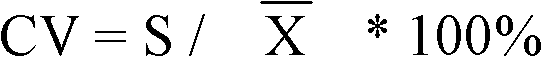Patents
Literature
53 results about "Carbamyl Phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
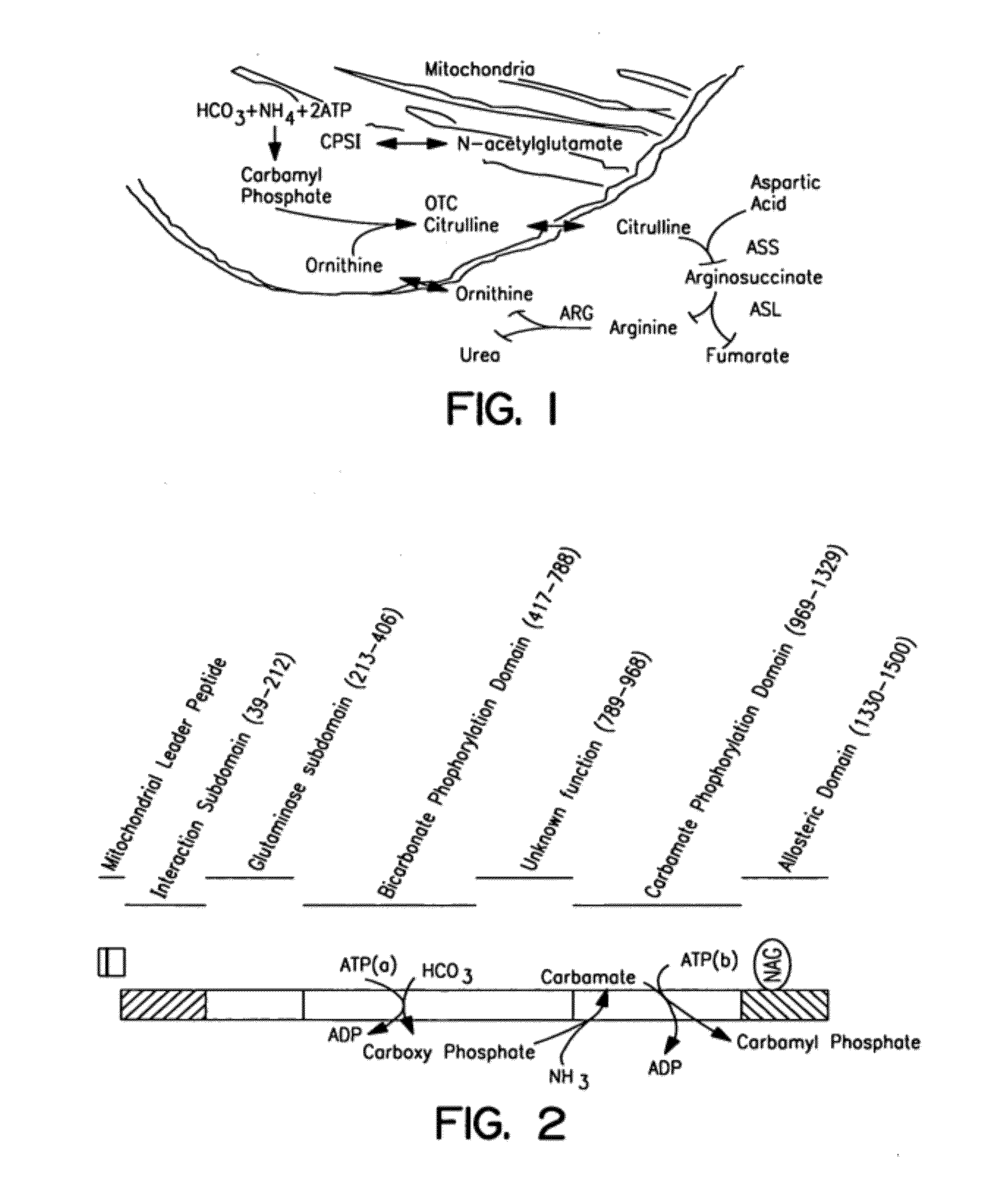
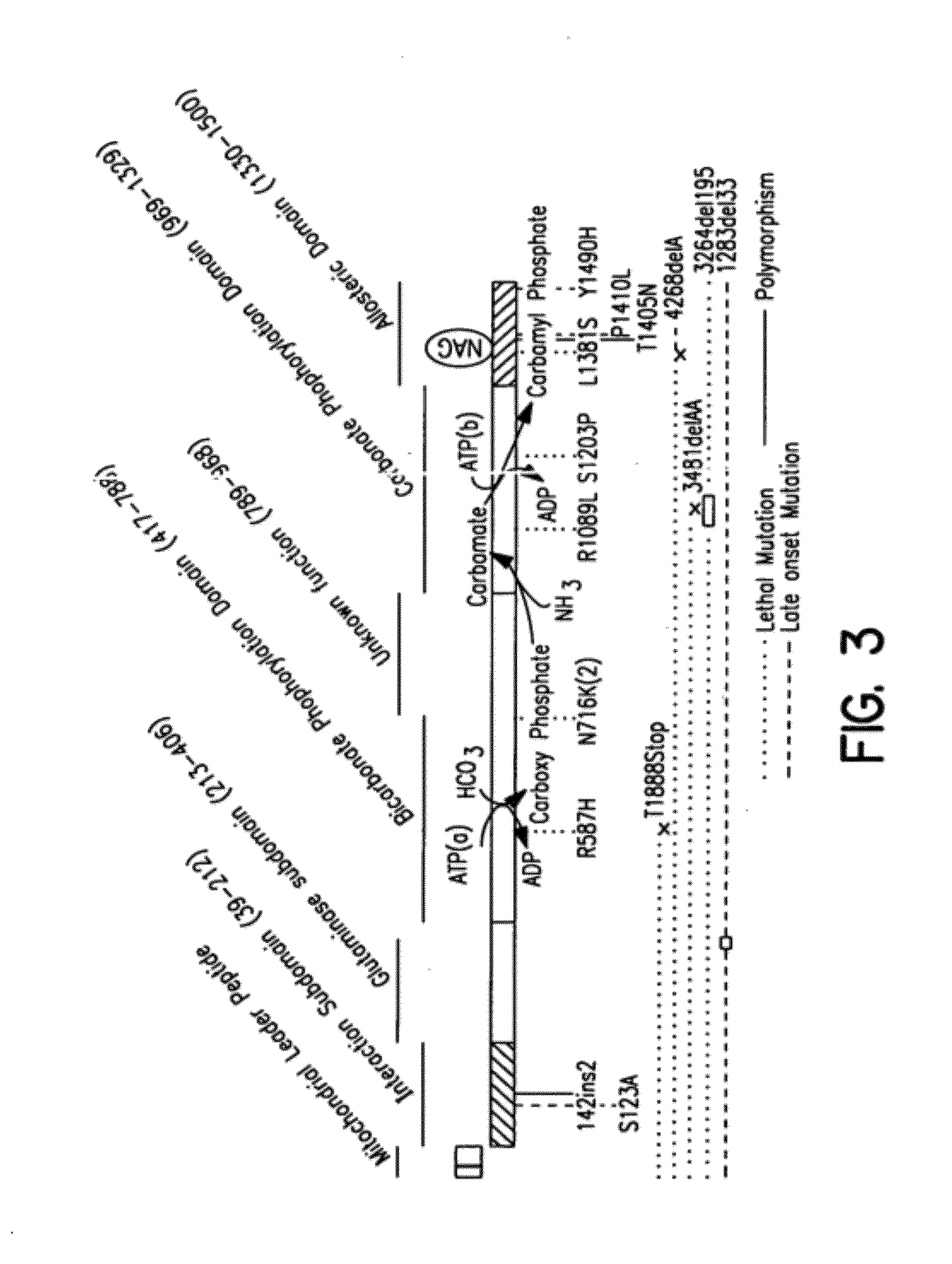
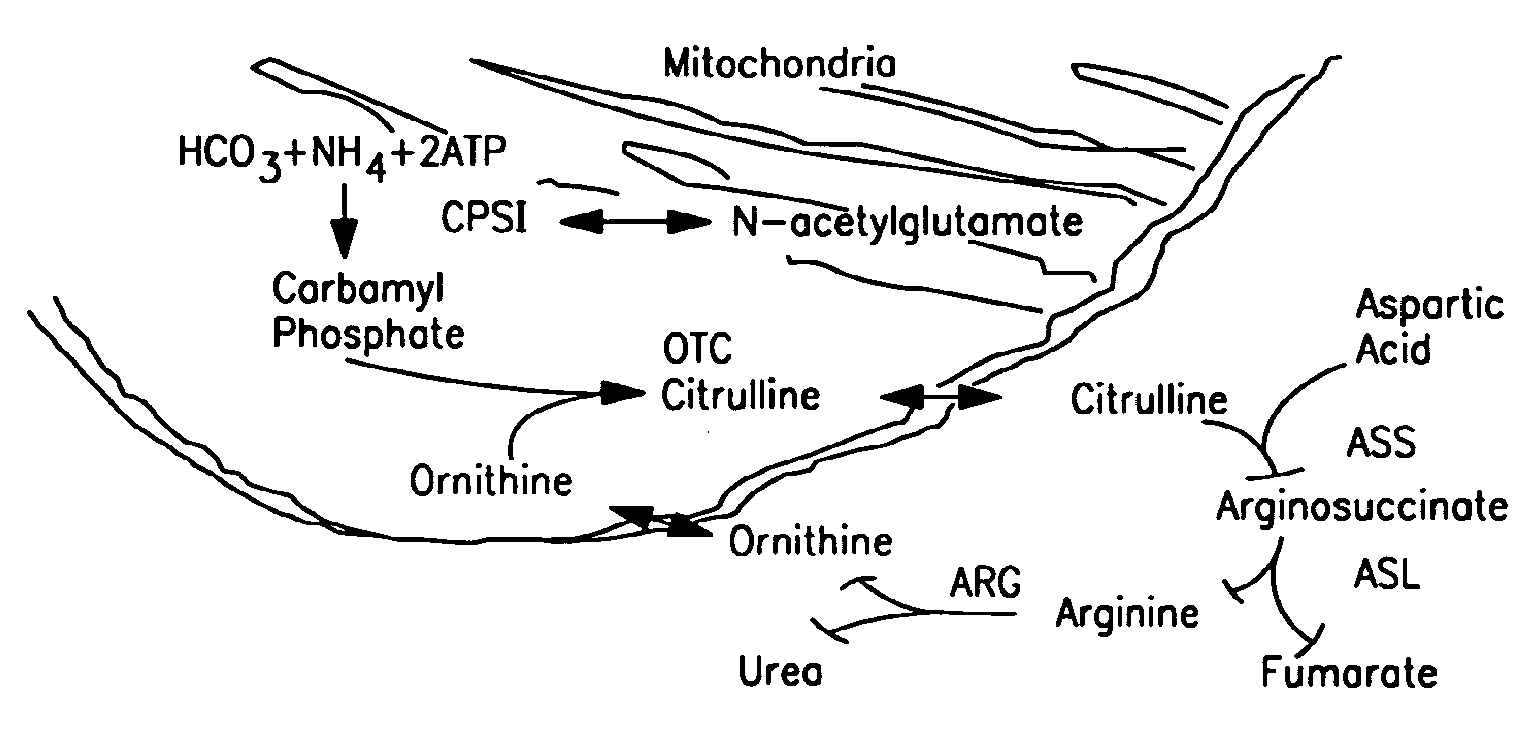
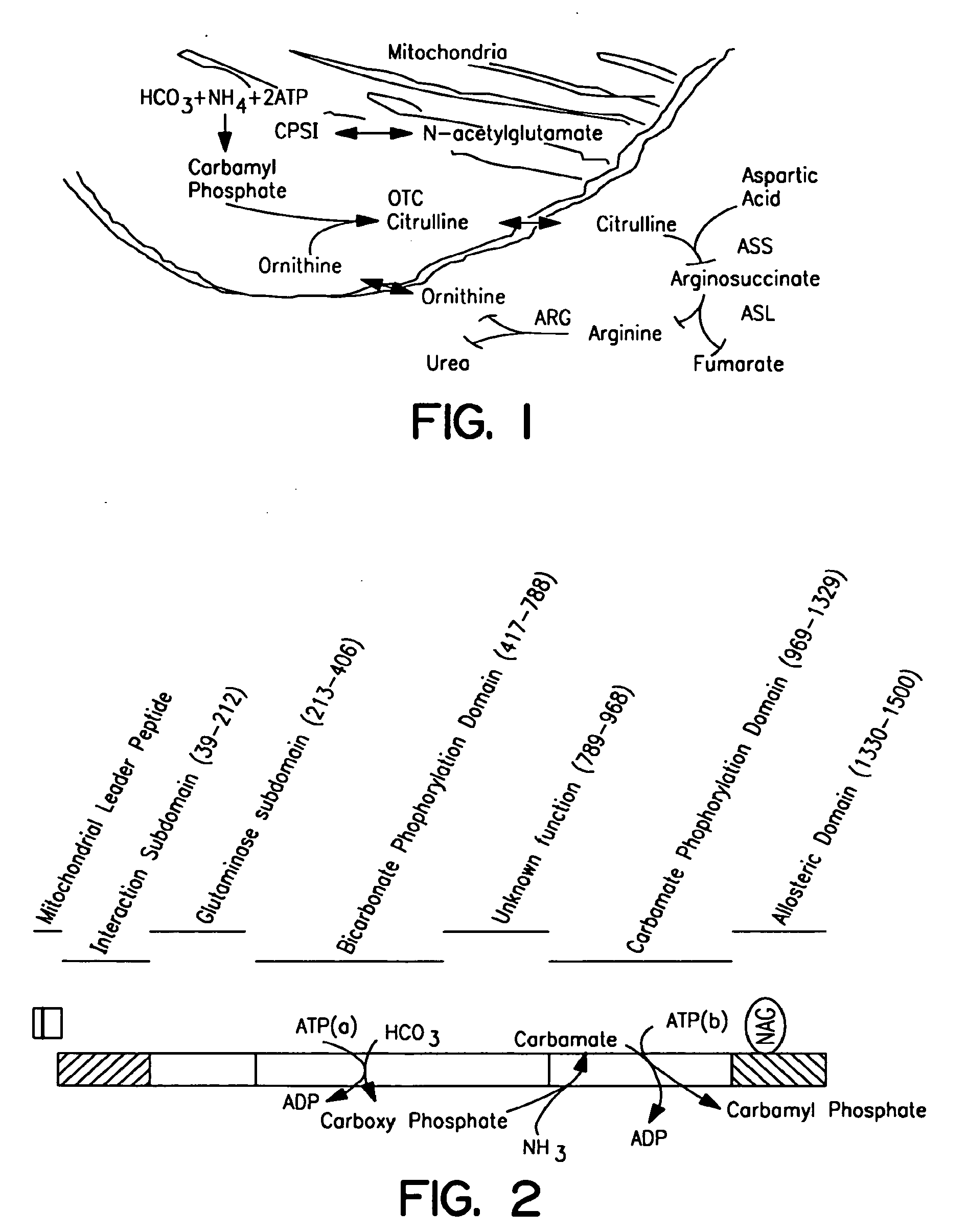
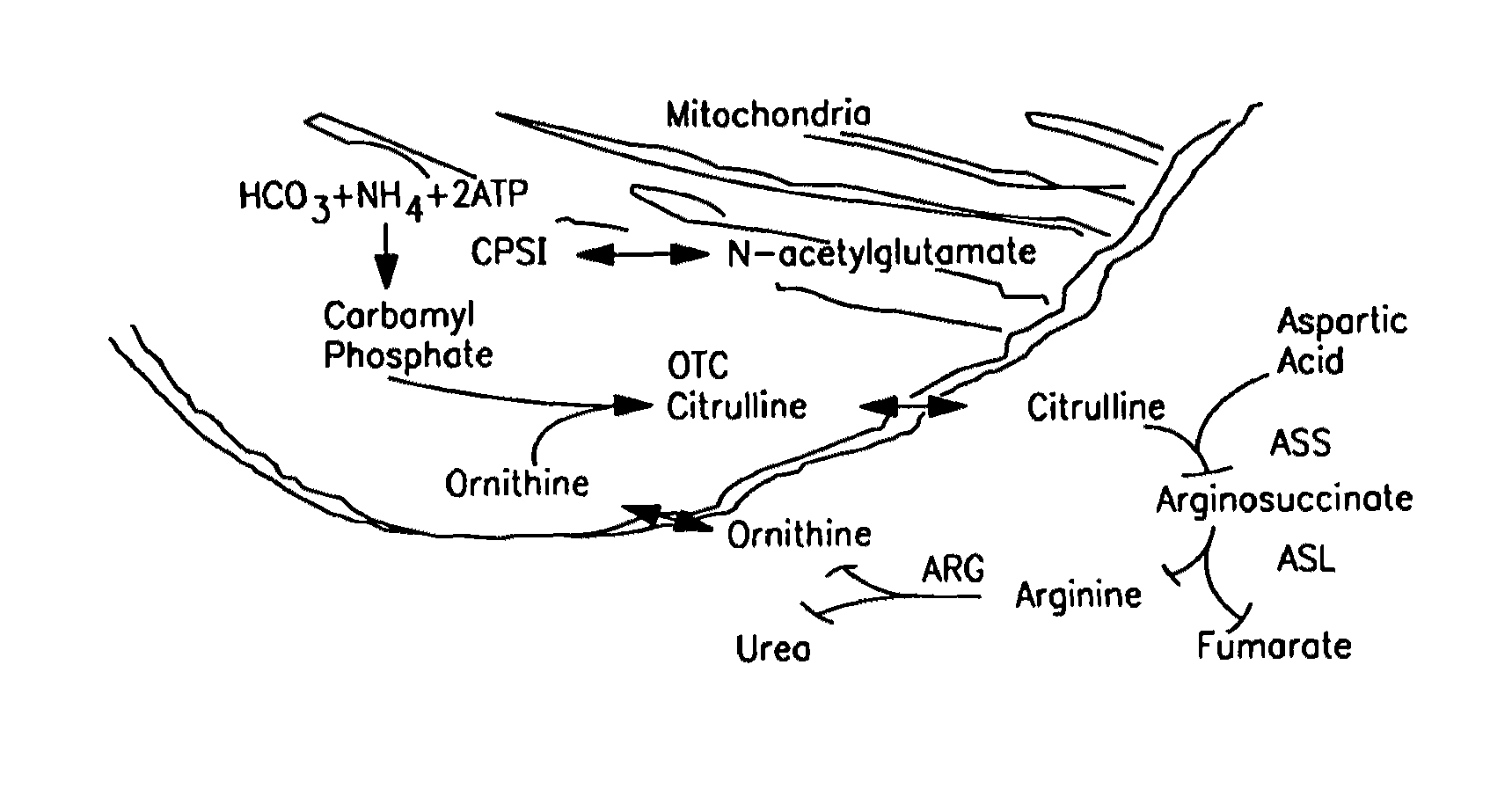
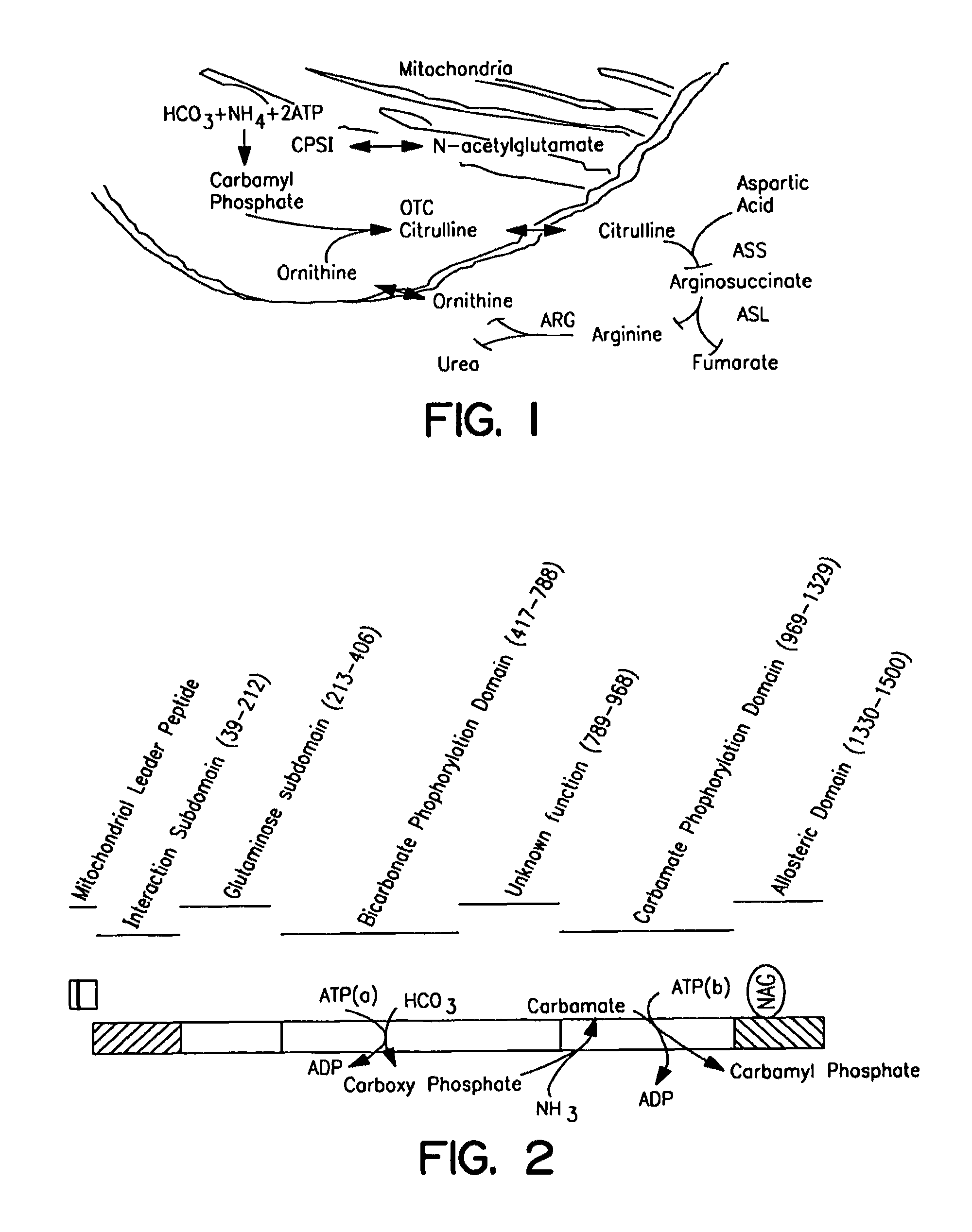
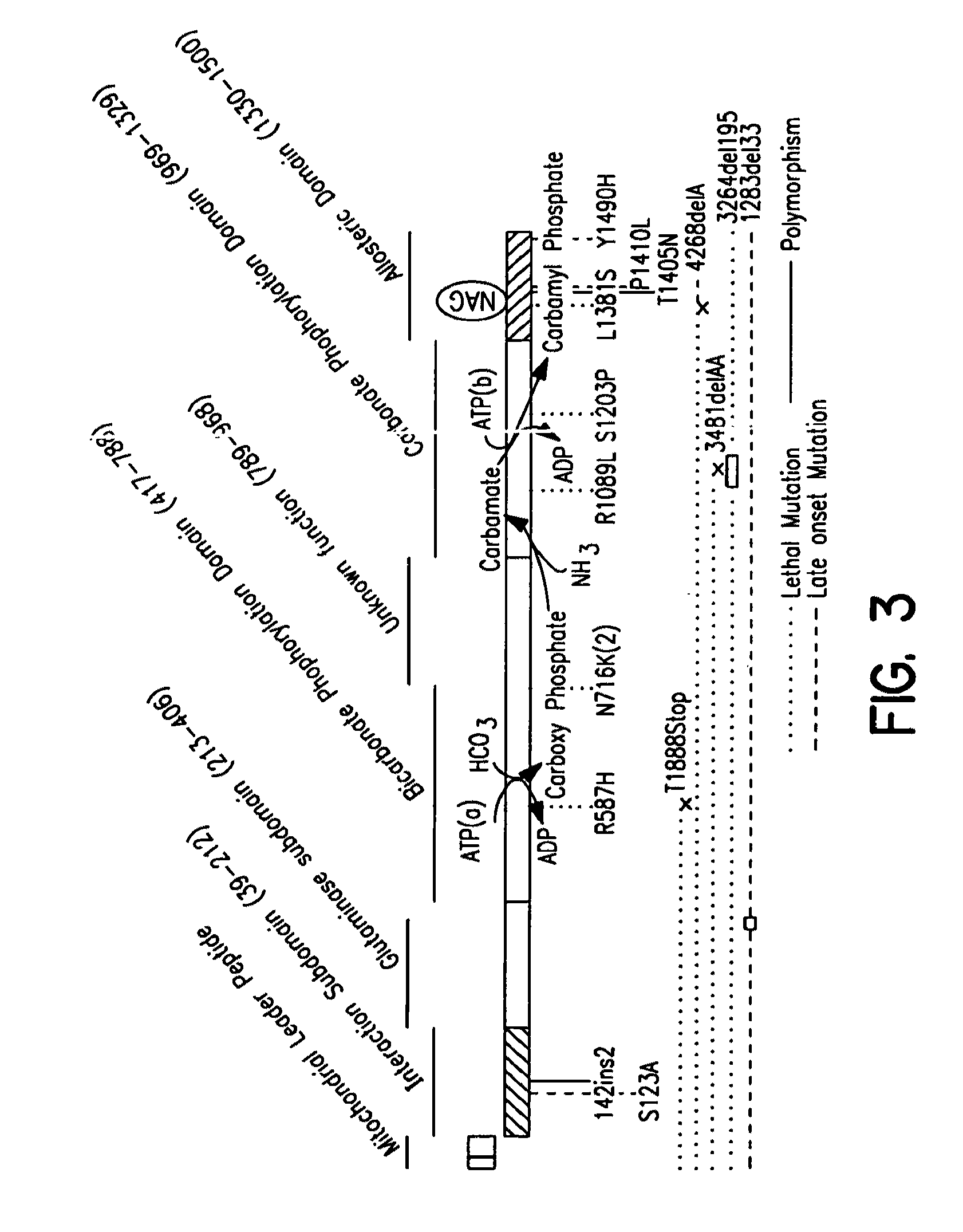
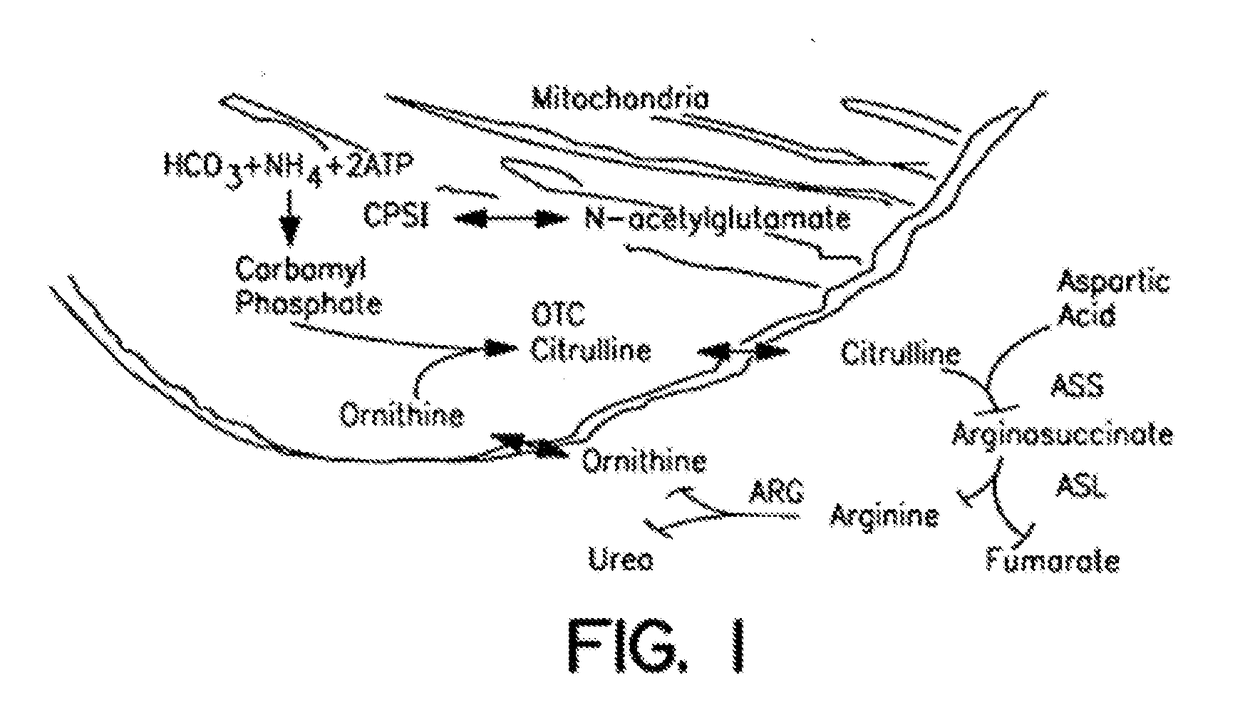
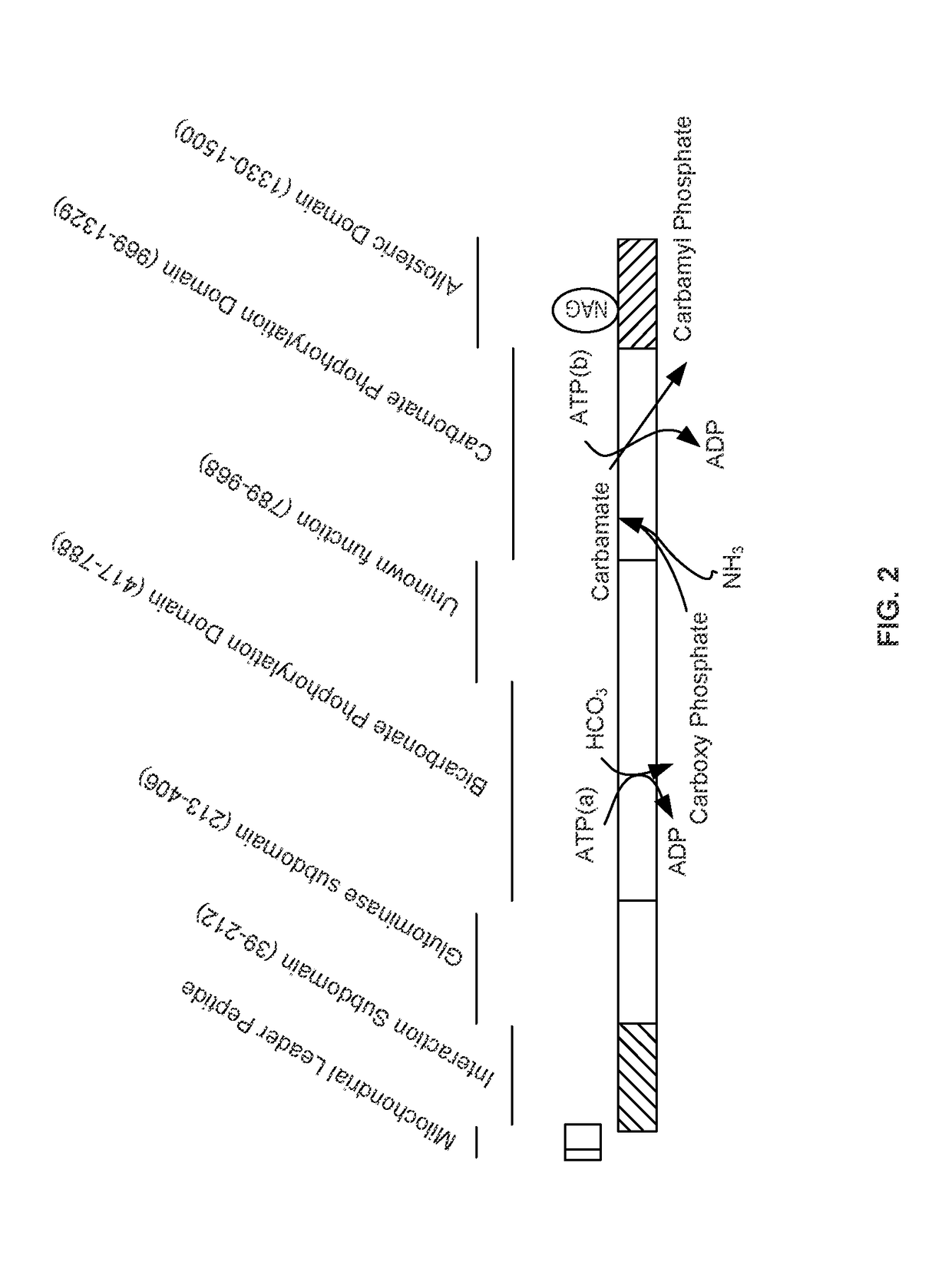
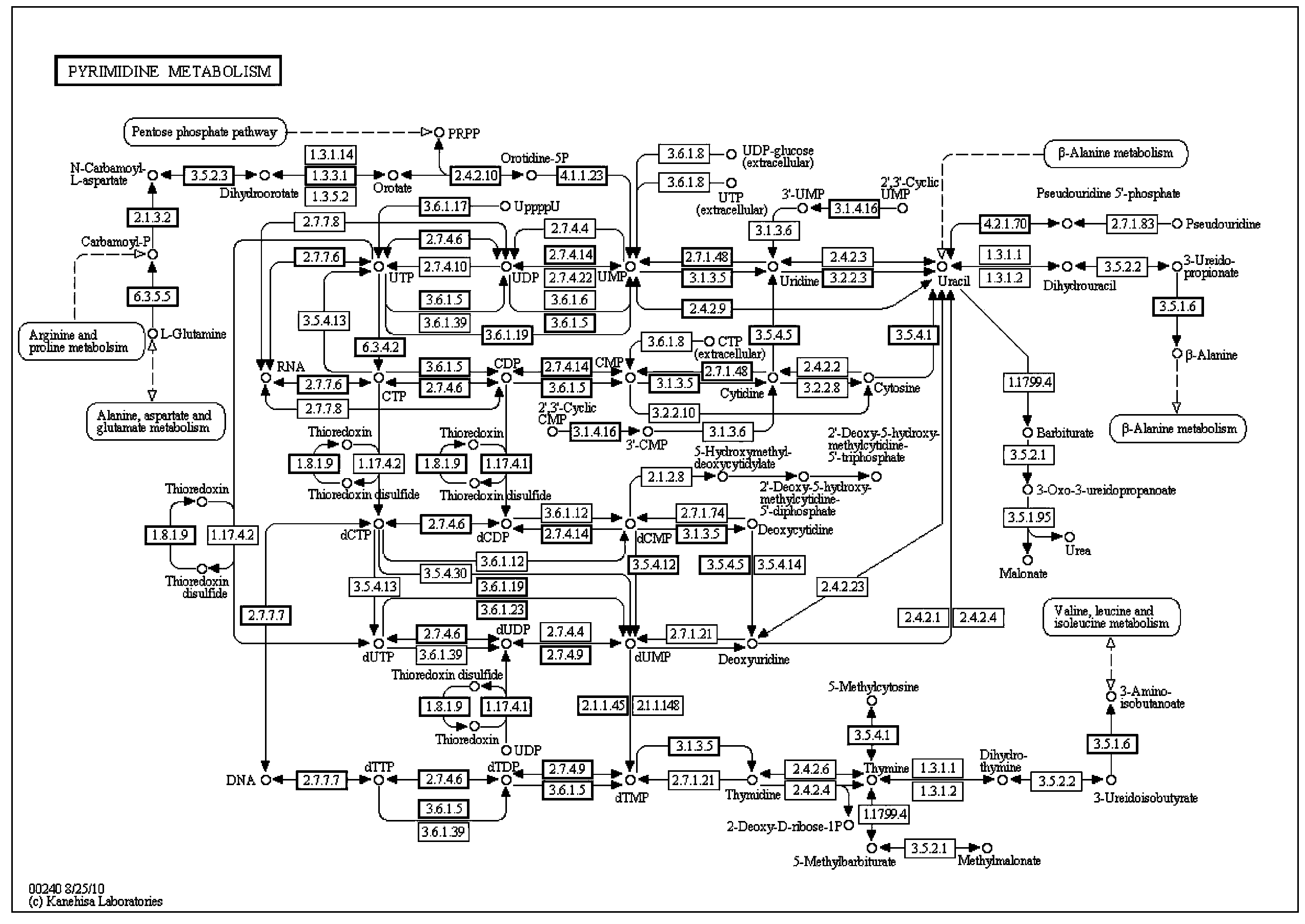
The monoanhydride of carbamic acid with PHOSPHORIC ACID. It is an important intermediate metabolite and is synthesized enzymatically by CARBAMYL-PHOSPHATE SYNTHASE (AMMONIA) and CARBAMOYL-PHOSPHATE SYNTHASE (GLUTAMINE-HYDROLYZING).
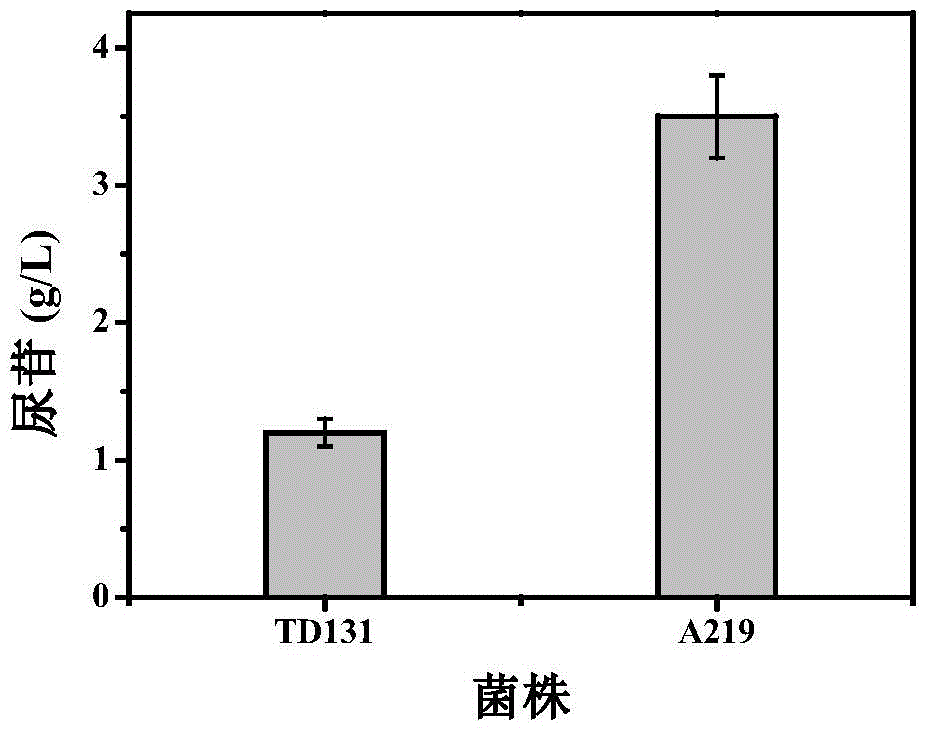
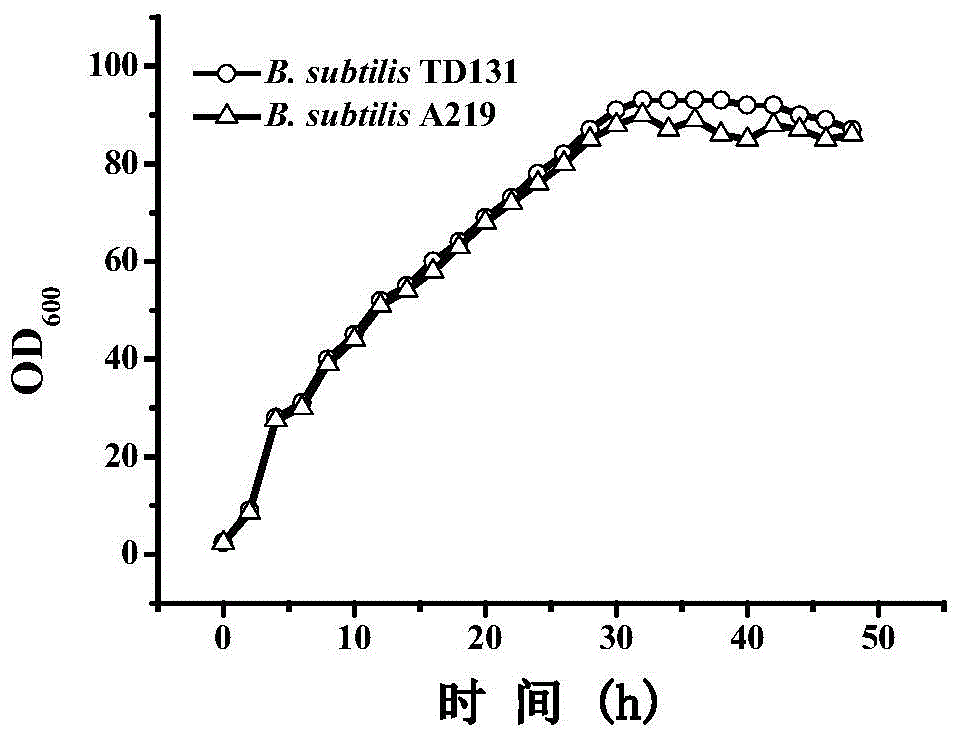
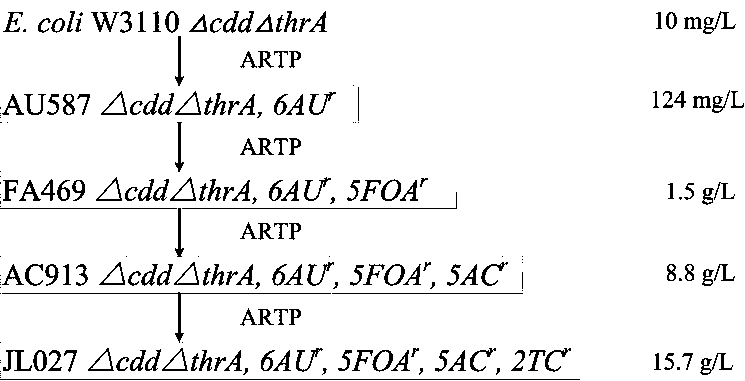
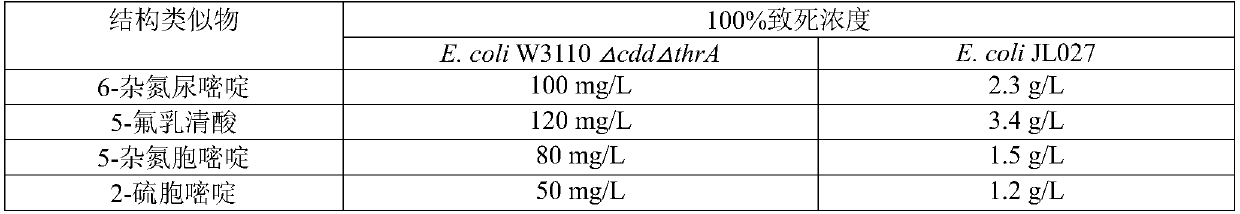
Pyrimidine nucleoside high-yielding strain and carbamyl phosphate synthetase adjusting site thereof
ActiveCN105671007AImprove toleranceBacteriaMicroorganism based processesCarbamyl PhosphateStructural analog
The invention belongs to the technical field of enzyme engineering, and concretely relates to a pyrimidine nucleoside high-yielding strain and a carbamyl phosphate synthetase adjusting site thereof. The invention provides a pyrimidine nucleoside production Bacillus subtilis mutant strain and a carbamyl phosphate synthetase encoding gene. A key regulation site related to carbamyl phosphate synthetase end product feedback inhibition is defined, and provides reference for breeding of later pyrimidine nucleoside high-yielding strains. The Bacillus subtilis mutant strain allows the output of fermentation process produced nucleoside pyrimidine uridine to reach 14-16g / L, and has substantially improved tolerance to different pyrimidine nucleoside structure analogs.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Therapeutic methods employing nitric oxide precursors
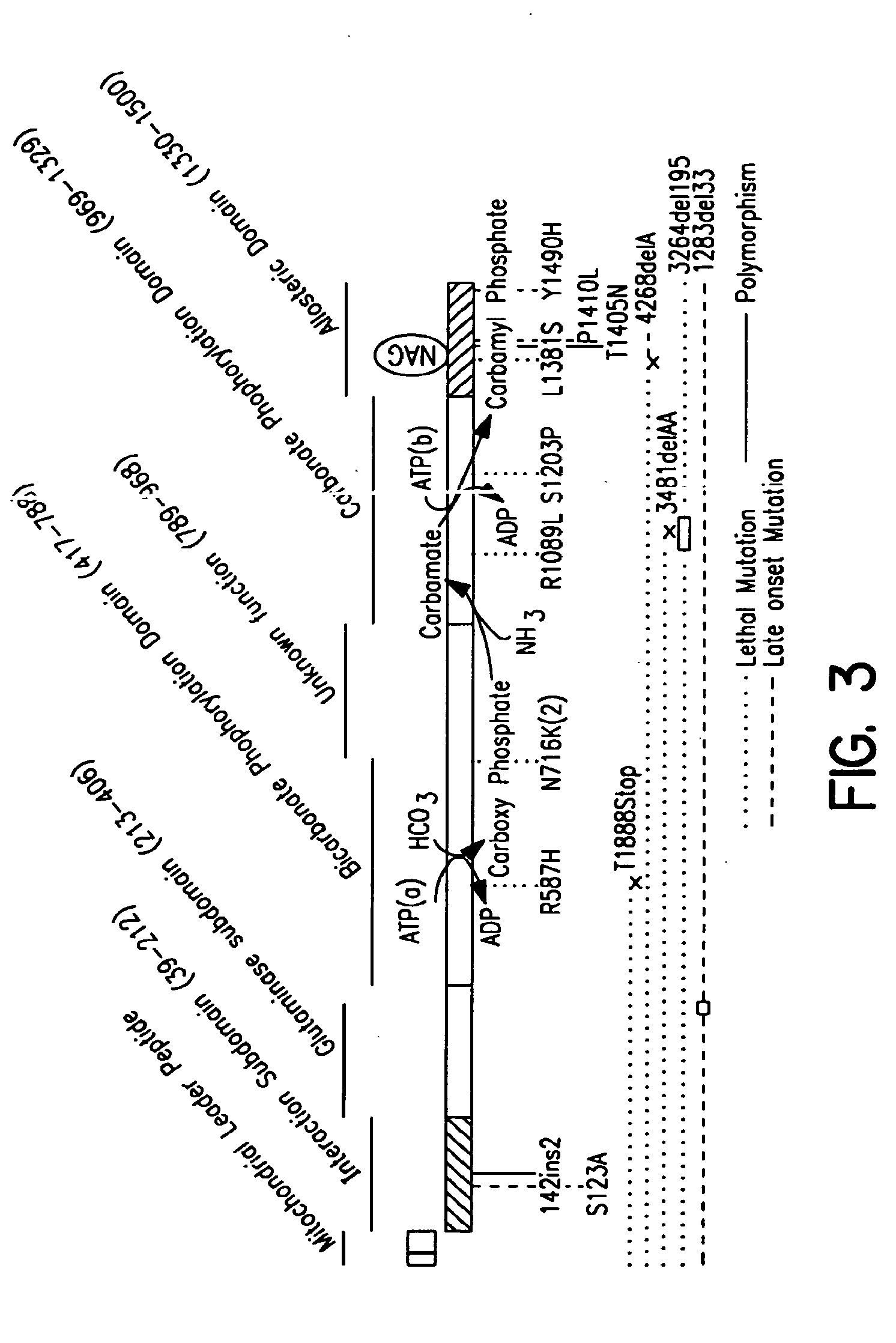
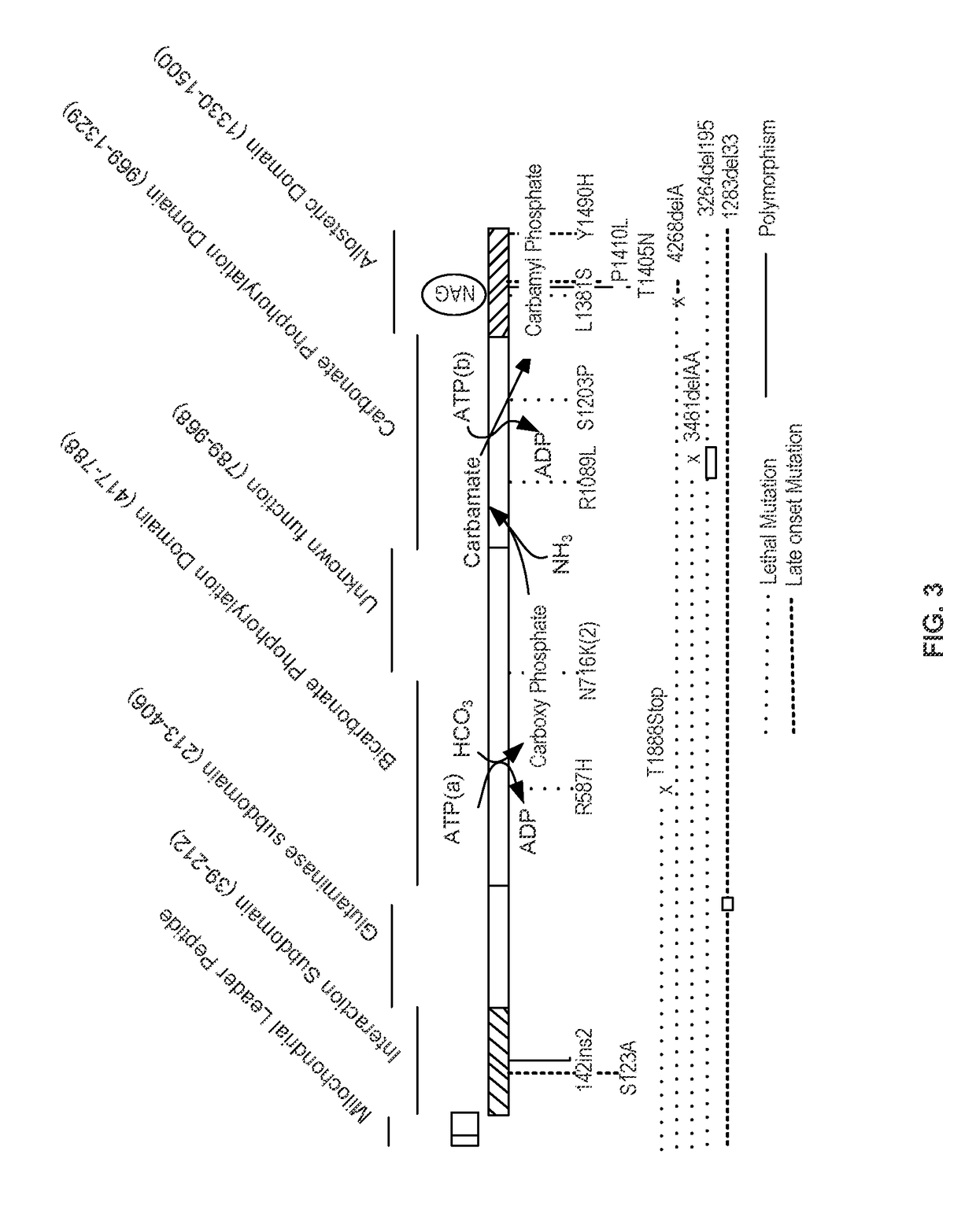
Isolated polynucleotide molecules and peptides encoded by these molecules are used in the analysis of human carbamyl phosphate synthetase I phenotypes, as well as in diagnostic and therapeutic applications, relating to a human carbamyl phosphate synthetase I polymorphism. By analyzing genomic DNA or amplified genomic DNA, or amplified cDNA derived from mRNA, it is possible to type a human carbamyl phosphate synthetase I with regard to the human carbamyl phosphate synthetase I polymorphism, for example, in the context of diagnosing and treating hepatic veno-occlusive disease (HVOD) associated with bone marrow transplants.
Owner:VANDERBILT UNIV
Genetically engineered bacterium for producing L-arginine and construction method and application thereof
ActiveCN110964683AShorten the growth cycleClear genetic backgroundBacteriaHydrolasesEscherichia coliCarbamyl Phosphate
The invention discloses a genetically engineered bacterium for producing L-arginine and a construction method and application thereof. According to the invention, a gene for encoding carbamyl phosphate synthetase and a gene for encoding L-arginine biosynthetic pathway enzyme are integrated in escherichia coli; a synthetic route of arginine in escherichia coli and metabolic flux related to argininein a whole amino acid metabolic network are analyzed and reconstructed to obtain genetically engineered bacteria which are clear in genetic background, do not carry plasmids, are not mutated and canstably and efficiently produce L-arginine; and the genetically engineered bacteria have good L-arginine production capacity.
Owner:NINGXIA EPPEN BIOTECH
Therapeutic methods employing nitric oxide precursors
Isolated polynucleotide molecules and peptides encoded by these molecules are used in the analysis of human carbamyl phosphate synthetase I phenotypes, as well as in diagnostic and therapeutic applications, relating to a human carbamyl phosphate synthetase I polymorphism. By analyzing genomic DNA or amplified genomic DNA, or amplified cDNA derived from mRNA, it is possible to type a human carbamyl phosphate synthetase I with regard to the human carbamyl phosphate synthetase I polymorphism, for example, in the context of diagnosing and treating hepatic veno-occlusive disease (HVOD) associated with bone marrow transplants.
Owner:VANDERBILT UNIV
Measuring method of asymmetric dimethylarginine concentration and measuring reagent thereof
InactiveCN102243227AHigh detection sensitivitySimple and fast operationMicrobiological testing/measurementColor/spectral properties measurementsGeneration rateOrnithine Carbamoyltransferase
The invention discloses a measuring method of asymmetric dimethylarginine concentration, comprising the steps of: subjecting a sample to be measured to a reaction in an enzymatic cycling reaction system, measuring and calculating the generation rate of ammonia from the enzymatic cycling reaction, thus obtaining the content of asymmetric dimethylarginine in the sample to be measured. The enzymatic cycling reaction system consists of dimethylarginine dimethylaminohydrolase, citrullinase, ornithine carbamoyltransferase, and carbamyl phosphate or its salt. The invention also discloses a measuring reagent corresponding to the method. In the invention, an enzymology measuring method and a measuring reagent of asymmetric dimethylarginine are established for the first time. Needing no special instrument, the method of the invention has simple operation, high sensitivity and low cost, thus being able to realize rapid and high flow sample detection in clinic. Thus, asymmetric dimethylarginine is probable to become a conventional inspection item in clinic. Therefore, the method provided in the invention is of great scientific and economic value.
Owner:ZHEJIANG YATAI PHARMA
131I labeled anti-tumor humanized monoclonal antibody 1E2 and use thereof
InactiveCN101407545AImprove targetingGood inhibitory effectIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntigenPhage antibodies
The invention provides an anti-lung cancer human resource monoclonal antibody 1E2 marked by <131>I. The human resource 1E2 antibody, Na <131>I and chloramine T are used as raw materials, and purified 1E2 antibody marked by the <131>I can be prepared by using the chloramine T method, wherein, the human source 1E2 antibody is obtained by taking paracanser lymph nodes of a non-small cell lung cancer patient, extracting total RNA in lymph node tissues, and then a phage antibody library containing the 1E2 antibody is obtained by using the constructing technology of the phage antibody library, and the phage antibody 1E2 is adsorbed by using a carbamyl phosphate synthetase antigen, and a human source 1E2 antibody which is specific to the carbamyl phosphate synthetase is finally obtained by washing, screening and amplifying. The invention applies radiation immunity treatment technology, combines advantages of radionuclide and anti-tumor cell human source monoclonal antibody to one, improves the targeting ability and curative effect of medicines to tumor cells, reduces side effects and poor immune reactions, and is wider in application range and suitable for treatments of the non-small cell lung cancer of each stage.
Owner:CHONGQING MEDICAL UNIVERSITY
Therapeutic methods employing nitric oxide precursors
Isolated polynucleotide molecules and peptides encoded by these molecules are used in the analysis of human carbamyl phosphate synthetase I phenotypes, as well as in diagnostic and therapeutic applications, relating to a human carbamyl phosphate synthetase I polymorphism. By analyzing genomic DNA or amplified genomic DNA, or amplified cDNA derived from mRNA, it is possible to type a human carbamyl phosphate synthetase I with regard to the human carbamyl phosphate synthetase I polymorphism, for example, in the context of diagnosing and treating hepatic veno-occlusive disease (HVOD) associated with bone marrow transplants.
Owner:VANDERBILT UNIV
Pyrimidine nucleoside high-yielding strain and carbamyl phosphate synthetase adjusting site thereof
InactiveCN105671008AImprove toleranceBacteriaMicroorganism based processesCarbamyl PhosphateStructural analog
The invention belongs to the technical field of enzyme engineering, and concretely relates to a pyrimidine nucleoside high-yielding strain and a carbamyl phosphate synthetase adjusting site thereof. The invention provides a pyrimidine nucleoside production Bacillus subtilis mutant strain and a carbamyl phosphate synthetase encoding gene. A key regulation site related to carbamyl phosphate synthetase end product feedback inhibition is known, and provides reference for breeding of later pyrimidine nucleoside high-yielding strains. The Bacillus subtilis mutant strain allows the output of fermentation process produced nucleoside pyrimidine uridine to reach 12.5-14.5g / L, and has substantially improved tolerance to different pyrimidine nucleoside structure analogs.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Escherichia coli mutant strain with high cytidine production and method for fermentation production of cytidine
ActiveCN111321103AEffective deregulation of feedbackDefeedback regulationBacteriaHydrolasesEscherichia coliPhenylalanine
The present invention relates to an escherichia coli mutant strain with high cytidine production and a method for fermentation production of cytidine, and belongs to the technical field of fermentation. The escherichia coli mutant strain is obtained by mutagenic screening and has the following characteristics: 1, alanine at 182nd position of carbamyl phosphate synthetase is mutated to valine, andserine at 948th position is mutated to phenylalanine; 2, isoleucine at 86th position, glycine at 93rd position and cysteine at 109th position of aspartic acid carbamoyl transferase regulatory subunitare deleted; 3, asparagine at 72nd position of uridine kinase is mutated to alanine and aspartic acid at 159th position is mutated to asparagine; and 4, aspartic acid at 147th position of cytidine triphosphate synthase is mutated to glutamic acid, and glutamic acid at 149th position is mutated to alanine. The mutant strain is fermented in a 50-L fermentation tank, cytidine yield reaches (90 plus or minus 2) g / L, sugar-acid conversion rate reaches (30 plus or minus 1)%, and the cytidine yield and sugar-acid conversion rate are both the highest values reported.
Owner:HENAN JULONG BIOLOGICAL ENG CO LTD +1
Homocysteine diagnosing/measuring reagent (kit) and homocysteine concentration measuring method
InactiveCN101750392AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsSodium bicarbonateAdditive ingredient
The invention relates to a homocysteine diagnosing / measuring reagent (kit) using the technologies of an enzyme colorimetric method and an enzyme linkage method. At the same time, the invention also relates to a homocysteine concentration measuring method, the reagent composition and reagent ingredients, which belong to the technical field of medical detection and measurement. The reagent (kit) mainly comprises the following ingredients: a buffer solution, cozymase, sodium bicarbonate (carbon dioxide), adenyl pyrophosphate, glyceraldehyde-3-phosphate, homocysteine desulfhydrase, carbamyl phosphate synthetase, glyceraldehyde-3-phosphate dehydrogenase and stabilizing agents. The method has the following steps: mixing samples and the reagent by the certain volume percent for making the samples and the reagent take a series of enzymatic reaction, and then, placing reactants under an ultraviolet / visible light analyzer to detect the rising degree of the light absorbancy in the position with the main wave length of 340 nm, so the concentration of the homocysteine can be measured and calculated.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Genetically engineered strain for producing L-citrulline and application of genetically engineered strain
ActiveCN112280728AIncrease production intensityStable fermentationBacteriaHydrolasesEscherichia coliSuccinic acid
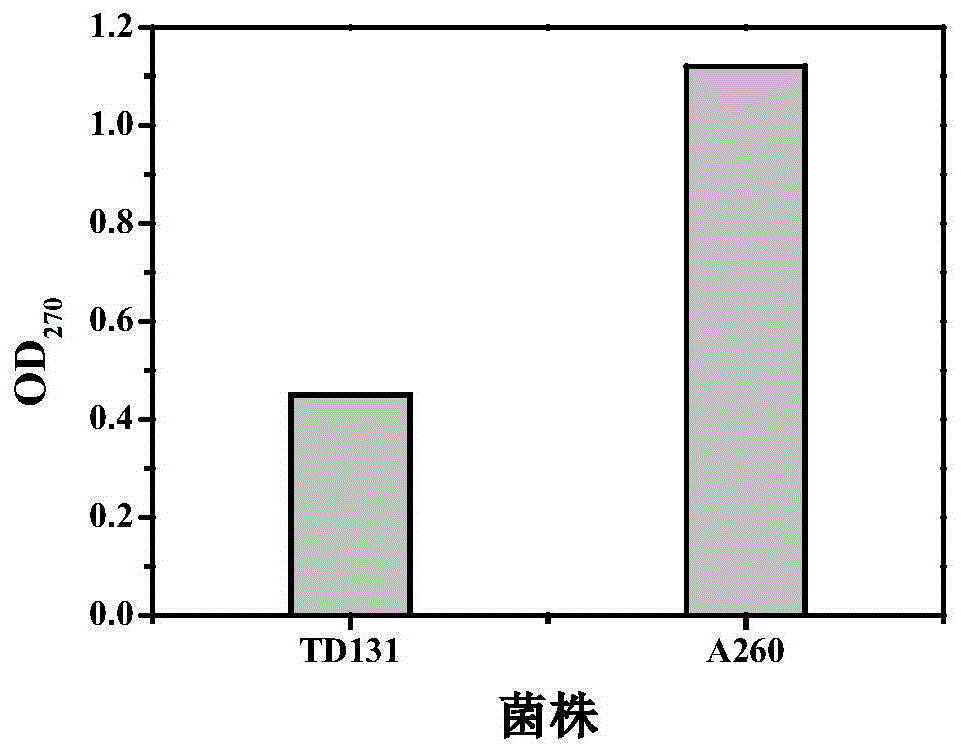
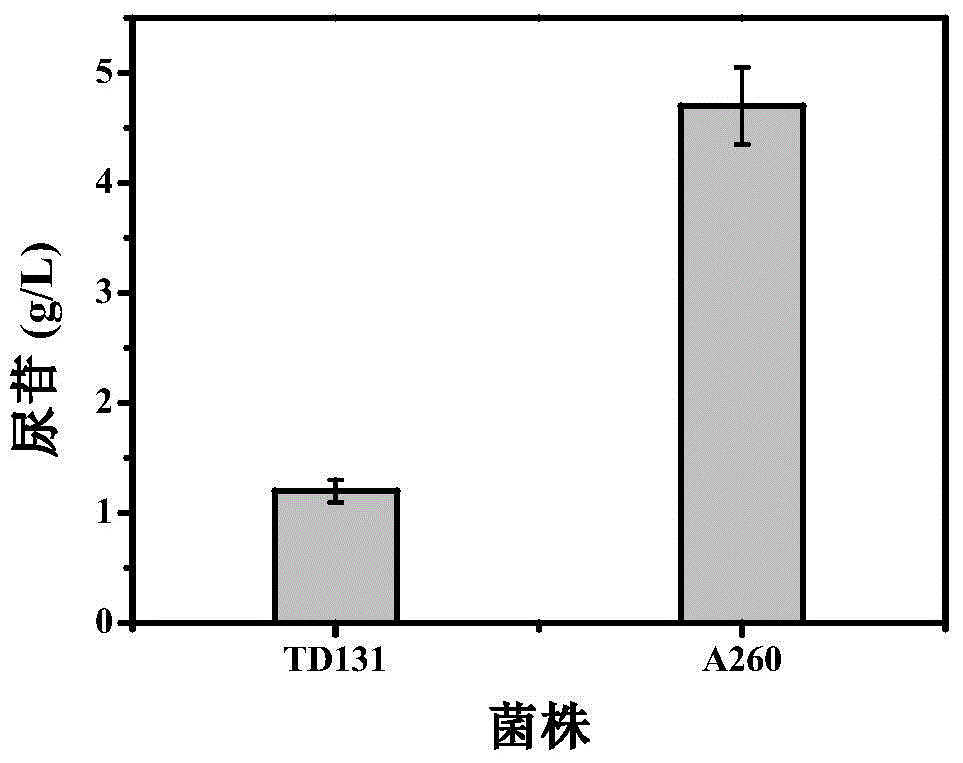
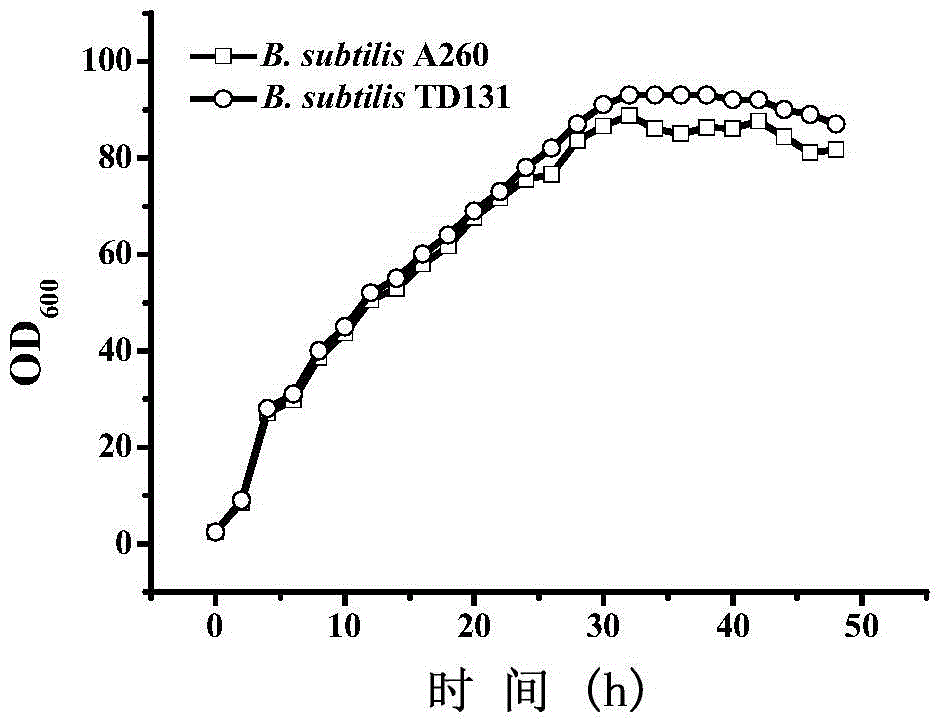
The invention provides a genetically engineered strain CIT 4 for efficiently and stably producing Lcitrulline, which takes Escherichia coli as a host, firstly, activity of arginine succinate synthaseis disabled from the host so as to prevent the Lcitrulline from being degraded into arginine succinic acid; a gene argG for encoding escherichia coli arginine succinate synthase being integrated on ahost genome, and expression regulation being carried out by a tryptophan promoter Ptrp; the host is also enabled to delete activity of the acetylornithine deacetylase; a gene argJ of corynebacterium glutamicum coded glutamate acetyltransferase is integrated on a host genome so that the metabolic flow in the process of synthesizing acetylglutamic acid from glutamic acid is enhanced; and genes PyrAAand PyrAB of two subunits of the carbamyl phosphate synthase encoded by the bacillus subtilis mutant strain A260 are also integrated on a host genome so that the feedback inhibition of arginine on the carbamyl phosphate synthase is relieved, and the supply of a precursor substance carbamyl phosphate is improved.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method and kit for measuring glycine
InactiveCN102087267AMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementGlycineCarbamyl Phosphate
The invention relates to a method for measuring glycine content by utilizing a double amplification method, an enzyme colorimetric method and an enzyme linked method technology, and composition and components of reagents. The measuring technical principle is that the measurement is finished according to a series of catalytic reactions of glycine dehydrogenase, carbamyl phosphate synthetase and glyceraldehyde-3-phosphate dehydrogenase. The invention also relates to a kit for measuring glycine. The measuring method has high sensitivity and small error; therefore, the method and the kit for measuring the glycine can be widely applied to food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
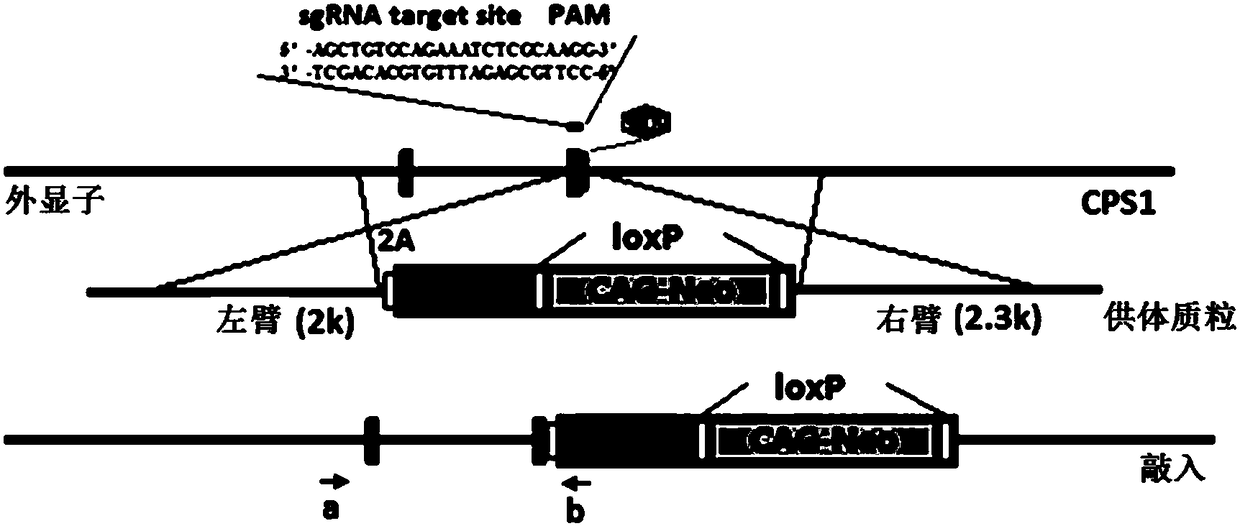
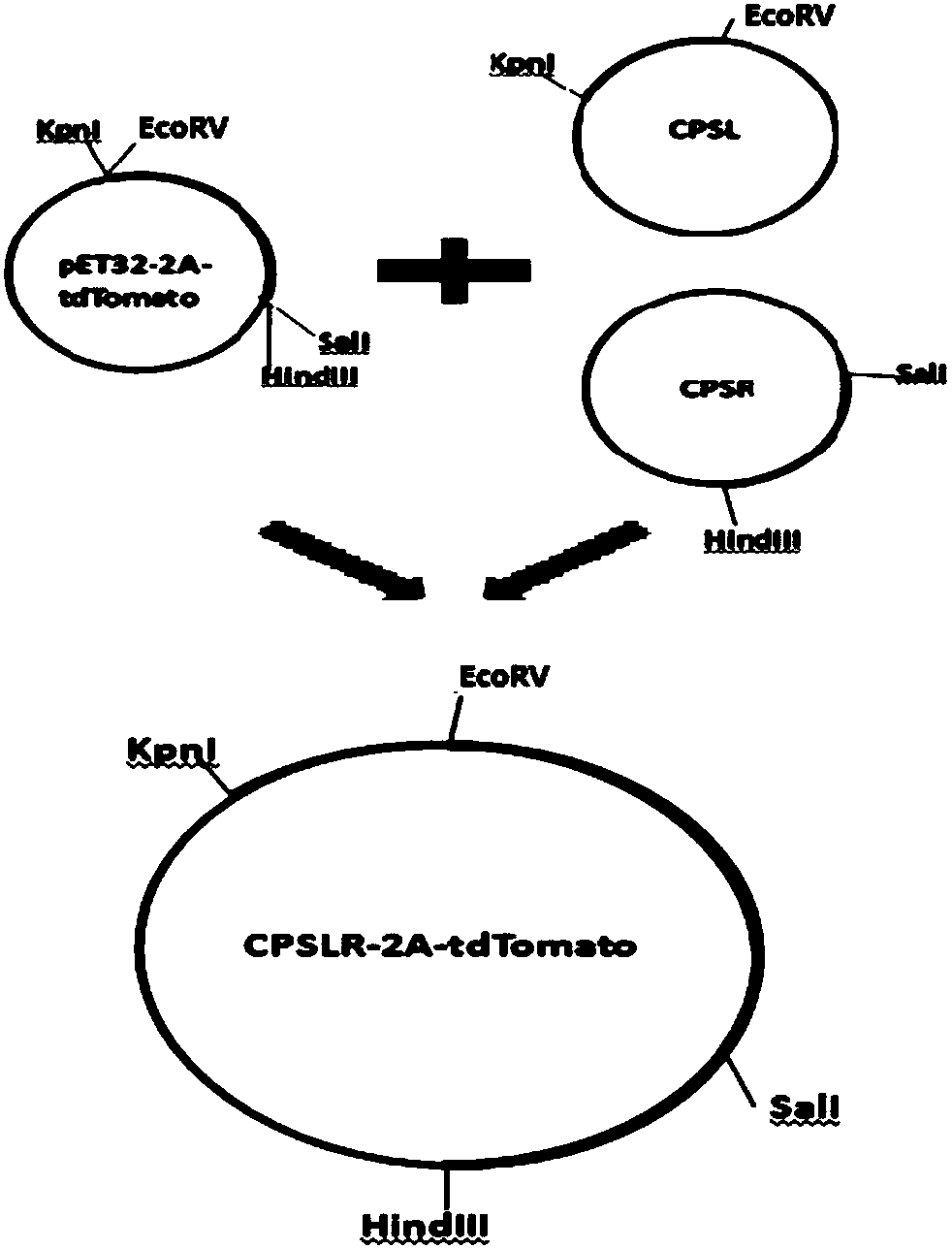
CPS1 report gene stem cell, and building method and application thereof
InactiveCN108660156AHigh ammonia metabolism capacityHigh purityMicrobiological testing/measurementVector-based foreign material introductionMetabolism studyCarbamyl Phosphate
The invention discloses a carbamyl phosphate synthetase 1 report gene stem cell (CPS1 report gene stem cell), and a building method and application thereof. Experiments prove that by using the induction scheme obtained through CPS1 report gene human embryonic stem cell (hESCs) screening, the induced differentiation to effectively enhanced hepatocyte-like cells is realized. The CPS1 report gene stem cell can be applied to the screening of induction agents in a liver direction induction differentiation system; hepatocyte-like cells obtained through induction differentiation can be applied to liver medicine metabolism study and liver medicine toxicity evaluation; and wide application prospects are realized.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Reagent (kit) for diagnosing/determining amino acid and method for determining concentration of amino acid
InactiveCN101750337AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsSodium bicarbonateEnzymatic Colorimetry
The invention relates to a reagent (kit) for diagnosing / determining amino acid by using an enzyme multiplication method, an enzyme colorimetric method and an enzyme link method, and also discloses a method for determining the concentration of the amino acid, a composition and components of the reagent, belonging to the technical field of medicine / food / environmental test determination. The reagent (kit) comprises the main components: a buffer solution, a coenzyme, sodium bicarbonate (carbon dioxide), adenosine triphosphoric acid, glyceraldehyde-3-phosphate, an amino acid dehydrogenase, a carbamyl phosphate synthetase, a glyceraldehyde-3-phosphate dehydrogenase and a stabilizing agent. A sample and the reagent are mixed according to a certain volume ratio to generate a series of enzymatic reactions, and reactants are placed under an ultraviolet / visible light analyzer for detecting the degree of absorbance rise at the dominant wavelength of 340 nm, thereby measuring and calculating the concentration of the amino acid.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Therapeutic methods employing nitric oxide precursors
Isolated polynucleotide molecules and peptides encoded by these molecules are used in the analysis of human carbamyl phosphate synthetase I phenotypes, as well as in diagnostic and therapeutic applications, relating to a human carbamyl phosphate synthetase I polymorphism. By analyzing genomic DNA or amplified genomic DNA, or amplified cDNA derived from mRNA, it is possible to type a human carbamyl phosphate synthetase I with regard to the human carbamyl phosphate synthetase I polymorphism, for example, in the context of diagnosing and treating hepatic veno-occlusive disease (HVOD) associated with bone marrow transplants.
Owner:VANDERBILT UNIV
Measurement method for ammonia (ammonia ions) and diagnosis/measurement kit for ammonia (ammonia ions)
InactiveCN102466712AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateAmmonia
The invention relates to a content measurement method for ammonia (ammonia ions), reagent composition and components. The measurement method makes use of an enzymatic colorimetric method and a couple reaction method, and has a technical principle based on a series of catalytic reactions of glutamate oxidase, carbamyl phosphate synthetase, and melate dehydrogenase. The invention also relates to a diagnosis / measurement kit for ammonia (ammonia ions). The measurement method of the invention has high sensitivity and minor errors, so that the measurement method and kit provided in the invention can be widely applied in clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Cordyceps Chinese Hirsutella carbamyl phosphate synthetase, coding gene and application thereof
ActiveCN103031281BIncrease productionEnhance expressive abilityBacteriaMicroorganism based processesNucleotideBiology
The invention relates to a carbamyl phosphate synthetase from Bailing production bacterium Cordyceps Chinese Hirsutella for synthesizing metabolic carbamyl phosphate from L-glutamic acid, a coding gene and application thereof. The amino acid sequence of the carbamyl phosphate synthetase has more than 90% of homology with sequence disclosed as SEQ ID NO.1; and the nucleotide sequence of the coding gene has more than 90% of homology with sequence disclosed as SEQ ID NO.2. The invention researches the metabolic pathway of L-glutamic acid synthesized carbamyl phosphate synthetase in details in principle. The cloned DNA (deoxyribonucleic acid) comprising the nucleotide sequence provided by the invention can be transformed into engineering bacterium by transduction, conversion and conjugal transfer. By adjusting the expression of the carbamyl phosphate biosynthesized gene, the host carbamyl phosphate is endowed with high expressivity, thereby providing an effective way for enhancing the yield of the carbamyl phosphate and having great application prospects.
Owner:ZHEJIANG UNIV OF TECH +1
Ammonia (ammonia ion) content determination method and ammonia (ammonia ion) diagnosis/determination kit
InactiveCN102565378AMicrobiological testing/measurementColor/spectral properties measurementsAssayCarbamyl Phosphate
The invention relates to an ammonia (ammonia ion) content determination method, and composition and ingredients of reagents adopted by the ammonia (ammonia ion) content determination method. The ammonia (ammonia ion) content determination method adopts an enzymatic colorimetry and an enzyme-linked immunosorbent assay technology. A technical principle of the ammonia (ammonia ion) content determination method comprises that determination is realized by a series of catalytic reactions of ammonia kinase, carbamyl phosphate synthetase and hydrogen cyanide synthetase. The invention also relates to an ammonia (ammonia ion) diagnosis / determination kit. The ammonia (ammonia ion) content determination method provided by the invention has high sensitivity and a small error. The ammonia (ammonia ion) content determination method and the ammonia (ammonia ion) diagnosis / determination kit can be widely utilized for clinical medicine and food examination.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Ammonia (ammonia ion) determination method and ammonia (ammonia ion) diagnosis/determination reagent kit
InactiveCN102539677AMicrobiological testing/measurementColor/spectral properties measurementsFood inspectionAmmonia kinase
The invention relates to a method for determination of ammonia (ammonia ion) content by using an enzymatic colorimetric method and an enzyme couple reaction technique and composition and components of a reagent. A determination technology principle of the method lies in that the determination is completed according to series catalytic reactions of ammonia kinase, carbamyl phosphate synthetase and malonic acid-semialdehyde dehydrogenase. The invention further relates to an ammonia (an ammonia ion) diagnosis / determination reagent kit. The determination method is high in sensitivity and small in error. Therefore, the determination method and the reagent kit can be widely applied to clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Reagent (kit) for diagnosing/determining amino acid and method for determining concentration of amino acid
InactiveCN101762491AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsSodium bicarbonatePhosphoric acid
The invention relates to a reagent (kit) for diagnosing / determining amino acid by utilizing the techniques of an enzyme colorimetric method and an enzyme coupling method, and also relates to a method for determining the concentration of amino acid and a composition and components of the reagent, belonging to the technical field of detection and determination of medicine / foodstuff / environment. The reagent (kit) mainly comprises the following components: a buffer solution, coenzyme, sodium hydrogen carbonate (carbon dioxide), adenosine triphosphoric acid, glyceraldehyde-3-phosphoric acid, amino acid oxidase, carbamyl phosphate synthetase, glyceraldehyde-3-phosphoric acid dehydrogenase and a stabilizer. A sample and the reagent are mixed according to a certain volume ratio to generate a series of enzymic reactions, then a reactant is placed under an ultraviolet / visible light analyzer, and the rising degree of absorbance in the position of 340 nm of main wave length is detected so that the concentration of the amino acid is measured and calculated.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination method of ammonia (ammonia ions) and diagnosis/determination kit for ammonia (ammonia ions)
InactiveCN102565332AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateEnzymatic Colorimetry
The invention relates to a determination method of ammonia (ammonia ions) content by means of technologies of enzymatic colorimetry and enzyme-linked assay, and composition and components of a reagent. The technical principle of determination is based on a series of catalytic reactions of ammonia kinase, carbamyl phosphate synthetase, and malic dehydrogenase. The invention further relates to a diagnosis / determination kit for ammonia (ammonia ions). The determination method provided by the invention has high sensitivity and small error. Therefore, the determination method and the kit provided by the invention can be widely applied to clinical medical / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Assay method of ammonia (ammonia ion) and ammonia (ammonia ion) diagnosis/assay kit
InactiveCN102466716AMicrobiological testing/measurementColor/spectral properties measurementsBenzoic acidAssay
The invention relates to an assay method for assaying ammonia (ammonia ion) content with an enzymatic colorimetry and an enzyme-linked immunosorbent assay technology, and component and composition of a reagent, the technology principle of the assay is completed according to the series of catalytic reactions of glutamate oxidase, carbamyl phosphate synthetase, chlorobenzoic acid-1 and 2-dioxygenase, and the invention also relates to an ammonia (ammonia ion) diagnosis / assay kit. The assay method disclosed by the invention is high in sensitivity, and is small in error, so the assay method and the kit, disclosed by the invention, can be widely applied to clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Measuring method of asymmetric dimethylarginine concentration and measuring reagent thereof
InactiveCN102243227BSimple and fast operationHigh sensitivityMicrobiological testing/measurementGeneration rateOrnithine Carbamoyltransferase
The invention discloses a measuring method of asymmetric dimethylarginine concentration, comprising the steps of: subjecting a sample to be measured to a reaction in an enzymatic cycling reaction system, measuring and calculating the generation rate of ammonia from the enzymatic cycling reaction, thus obtaining the content of asymmetric dimethylarginine in the sample to be measured. The enzymatic cycling reaction system consists of dimethylarginine dimethylaminohydrolase, citrullinase, ornithine carbamoyltransferase, and carbamyl phosphate or its salt. The invention also discloses a measuring reagent corresponding to the method. In the invention, an enzymology measuring method and a measuring reagent of asymmetric dimethylarginine are established for the first time. Needing no special instrument, the method of the invention has simple operation, high sensitivity and low cost, thus being able to realize rapid and high flow sample detection in clinic. Thus, asymmetric dimethylarginine is probable to become a conventional inspection item in clinic. Therefore, the method provided in the invention is of great scientific and economic value.
Owner:ZHEJIANG YATAI PHARMA
Ammonia (ammonia ion) determination method and ammonia (ammonia ion) diagnosis/determination kit
InactiveCN102565353AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateEnzymatic Colorimetry
The invention relates to a method for determining the content of ammonia (ammonia ion) by using technologies of enzymatic colorimetry and enzyme-linked immunosorbant assay, and compositions of a reagent. A technical principle for determination is that the determination is finished according to a series of catalytic reactions of ammonia kinase, carbamyl phosphate synthetase and fatty acid synthase. The invention also relates to an ammonia (ammonia ion) diagnosis / determination kit. The determination method provided by the invention is high in sensitivity and small in error, so that the determination method and the kit can be widely applied to clinical medical / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
A high-yielding pyrimidine nucleoside strain and its carbamoyl phosphate synthase regulatory site
InactiveCN105671008BImprove toleranceBacteriaMicroorganism based processesCarbamoyl phosphate synthesisRegulatory site
The invention belongs to the technical field of enzyme engineering, and concretely relates to a pyrimidine nucleoside high-yielding strain and a carbamyl phosphate synthetase adjusting site thereof. The invention provides a pyrimidine nucleoside production Bacillus subtilis mutant strain and a carbamyl phosphate synthetase encoding gene. A key regulation site related to carbamyl phosphate synthetase end product feedback inhibition is known, and provides reference for breeding of later pyrimidine nucleoside high-yielding strains. The Bacillus subtilis mutant strain allows the output of fermentation process produced nucleoside pyrimidine uridine to reach 12.5-14.5g / L, and has substantially improved tolerance to different pyrimidine nucleoside structure analogs.
Owner:TIANJIN UNIV OF SCI & TECH
Ammonia (ammonia ion) determination method and ammonia (ammonia ion) diagnosis/determination reagent kit
InactiveCN102539664AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateEnzymatic Colorimetry
The invention relates to a method for determination of ammonia (ammonia ion) content by using an enzymatic colorimetric method and an enzyme couple reaction technique and composition and components of a reagent. A determination technology principle of the method is completed according to series catalytic reactions of ammonia kinase, carbamyl phosphate synthetase and formate dehydrogenase. The invention further relates to an ammonia (ammonia ion) diagnosis / determination reagent kit. The determination method is high in sensitivity and small in error. Therefore, the determination method and the reagent kit can be widely applied to clinical medical science / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
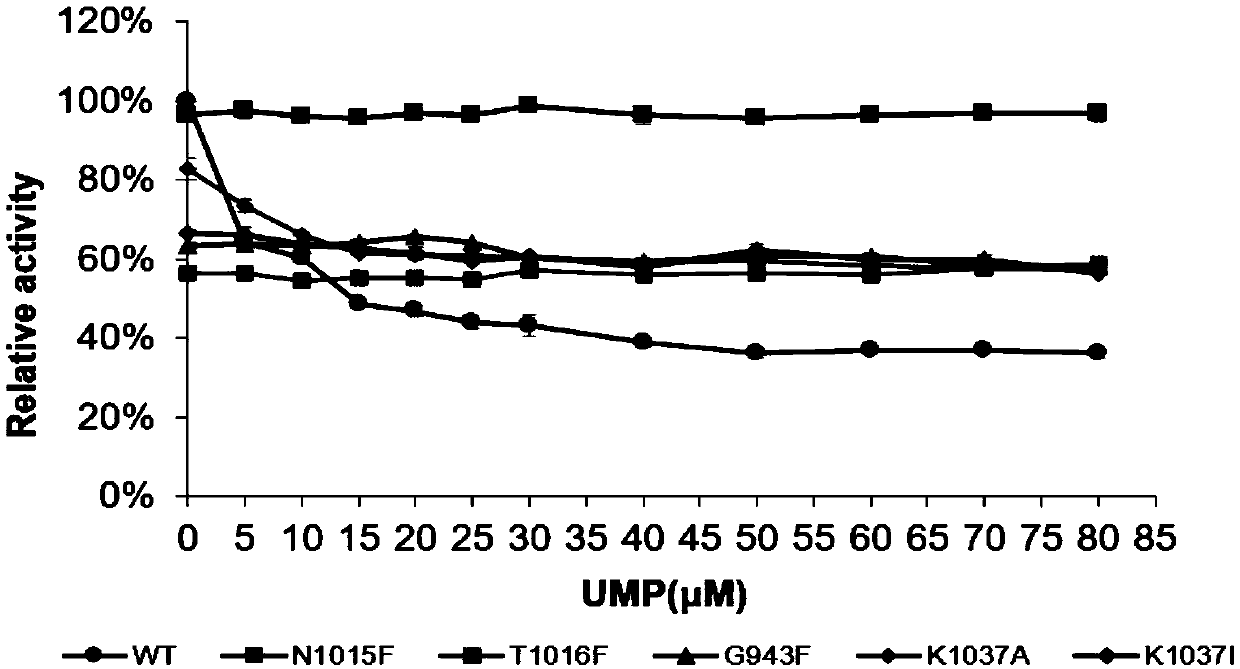
Carbamyl phosphate synthetase mutant with effect of uridylic acid feedback inhibition resistance and application thereof
ActiveCN110343672AInhibitory activityStrong inhibitory activityBacteriaMicroorganism based processesAlanineCarbamyl Phosphate
The invention provides a carbamyl phosphate synthetase mutant with an effect of uridylic acid feedback inhibition resistance and application thereof. The mutant is a mutant K1006A obtained by mutatingthe 1006 lysine into alanine or a mutant N1015F obtained by mutating the 1015 asparagine into phenylalanine, based on the carbamyl phosphate synthetase having an amino acid sequence as shown in the SEQ ID NO.1. Compared with a wild type Ec-CarAB, the mutant enzyme N1015F and 1006A have the advantages of respectively showing relief of feedback inhibition of the inhibitor UMP and improvement of catalytic activity. The initial enzyme activity of the N1015F is not variable in comparison with that of WT, and the activity of the N1015F is invariant along with increase of the concentration of the inhibitor UMP. The initial enzyme activity of the K1006A is increased by 12 percent than that of the wild type enzyme, the enzyme activity of the K1006A is gradually reduced along with increase of the concentration of the UMP, the K1006A is saturated under 20mM UMP concentration, and the relative enzyme activity of the K1006A still can be kept at 65 percent or more.
Owner:EAST CHINA UNIV OF SCI & TECH
Determination method of ammonia (ammonia ion) and ammonia (ammonia ion) diagnosis/determination kit
InactiveCN102539723AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateAcyl group
The invention relates to an ammonia (ammonia ion) content determination method by an enzymic colorimetric method and enzyme-linked technology, a reagent composition and components; the technical principle of the determination is based on a series of catalytic reactions of ammonia kinases, carbamyl phosphate synthetases, acyl-Co A synthetases; the invention also relates to an ammonia (ammonia ion) diagnosis / determination kit. The determination method of the invention has high sensitivity and small errors, and thus the determination method and the kit of the invention are widely applicable to clinical medicine / food examination.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination method of ammonia (ammonia ion) and ammonia (ammonia ion) diagnosis / determination kit
InactiveCN102466717AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateAmmonia
The invention relates to a determination method of ammonia (ammonia ion) content utilizing enzymatic colorimetric method and couple reaction, and reagent composition and components. Technical principles of the determination method are in the light of a series of catalytic reactions of glutamate oxidase, carbamyl phosphate synthetase and malonate-semialdehyde dehydrogenase. The invention also relates to an ammonia (ammonia ion) diagnosis / determination kit. The determination method of the present invention has high sensitivity and little error, so the determination method and the kit of the invention can be widely applied to clinic medicine / food examination.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Detection method for ammonia (ammonia ions), and ammonia (ammonia ion) diagnosis/detection kit
InactiveCN102539718AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateCoupling reaction
The invention relates to a detection method for ammonia (ammonia ion) content by using an enzymic colorimetric method and an enzyme couple reaction technology, a composition of regents for detecting the ammonia (ammonia ions), and components of the regents for detecting the ammonia (ammonia ions). The technical principle for detecting the ammonia (ammonia ions) is that: the detection of the ammonia (ammonia ion) content is completed according to a series of catalytic reactions of ammonia kinase, carbamyl phosphate synthetase and 6-methylsalicylic acid synthase. The invention further relates to an ammonia (ammonia ion) diagnosis / detection kit. The detection method of the present invention has high sensitivity and small error, such that the detection method and the kit of the present invention can be widely applicable for the clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com