Escherichia coli mutant strain with high cytidine production and method for fermentation production of cytidine
A technology of Escherichia coli and mutant strains, applied in the field of fermentation, can solve problems such as unclear amino acid positions, and achieve the effect of improving tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
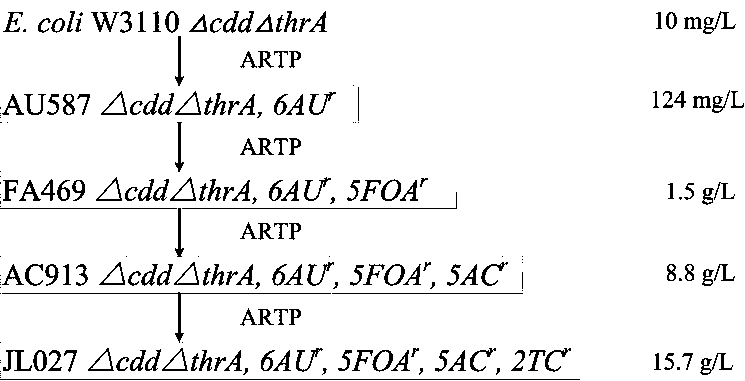
[0028] Example 1: Breeding of Escherichia coli mutant strain E.coli JL027 for cytidine production
[0029] 1. ARTP mutagenesis
[0030] The starting strain E.coli W3110ΔcddΔthrA was inoculated into a shaking tube containing LB medium, and cultured in a shaker at 37°C and 200r / min for 8h. Dilute the cultivated seed liquid with saline to OD 600 =0.8, then use a pipette to draw 10 μL of bacterial solution and apply it evenly on the circular metal sheet. Place the metal piece in the ARTP breeding machine (Beijing Siqingyuan Biotechnology Co., Ltd.), use helium as the working gas, set the gas flow rate to 10L / min, the distance between the gas injection port and the metal piece is 2mm, and the working power is 100W. The time is 30S.
[0031] 2. Initial screening of resistance plate
[0032] The treated bacterium was resuspended in 1mL of normal saline, and spread on the 5-fluoroorotic acid / 200mg / L of 6-azauracil / 150mg / L containing nucleoside structural analog 5-azacytosine / 200m...
Embodiment 2
[0040] Example 2 Fermentative production of cytidine by Escherichia coli mutant strain E.coli JL027
[0041] Use E.coli JL027 as the production strain, inoculate into a 10L fermenter containing 6L seed medium for seed culture, the inoculum size is 8%, the culture temperature is 37°C, pH 7.0, dissolved oxygen is controlled at 30%, residual sugar Control at 2%, and the culture period is 12h. After the seed culture is completed, inoculate into a 50L fermenter containing 20L fermentation medium for cytidine fermentation, the inoculum size is 9%, the culture temperature is 37°C, pH 7.0, the dissolved oxygen is controlled at 25%, and the residual sugar is controlled at 1%. , The fermentation period is 40h. After the fermentation is completed, the concentration of cytidine in the fermentation broth can reach 80g / L, and the sugar-acid conversion rate can reach 30%.
[0042] The fermenter seed medium is: glucose 30g / L, yeast powder 6g / L, citric acid 1.5g / L, peptone 2g / L, potassium di...
Embodiment 3
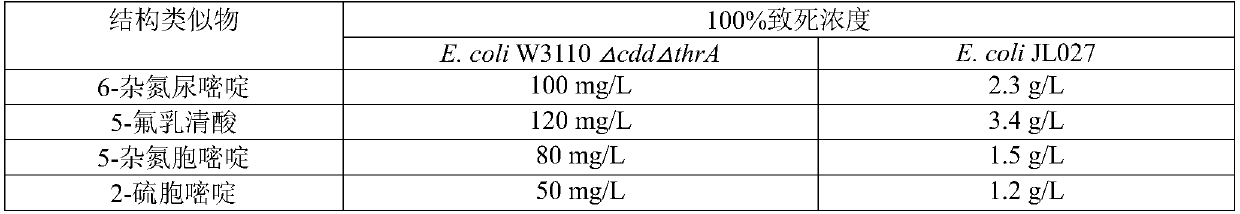
[0044] Example 3 Escherichia coli mutant strain E.coli JL027 and starting strain E.coli W3110ΔcddΔthrA tolerance test to different nucleoside structural analogues
[0045] The mutant strain E.coli JL027 and the starting strain E.coli W3110ΔcddΔthrA were inoculated in shake tubes containing LB medium, and cultured in a shaker at 37°C and 200r / min for 8h. The cultured seed solution was diluted 108 times with physiological saline, and then 10 μL of the bacterial solution was pipetted and evenly spread into LB solid medium containing different concentrations of nucleoside structural analogs. Three parallels were performed for each concentration, and the control was LB solid medium. Put all the solid medium into the incubator at 37°C for 10 h at the same time. Plate colony counting was used to calculate the lethal rate and lethal concentration, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com