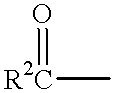Patents
Literature
65 results about "Viral growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral growth. A virus will typically spread exponentially at first if there is no immunization available. Each infected person can infect multiple new people. SARS (Severe Acute Respiratory Syndrome) and Ebola are two such viruses whose impact to affected areas can be devastating.
Method for treating enterovirus or rhinovirus infection using antisense antiviral compounds
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Picornaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Enterovirus and / or Rhinovirus infection in a mammal. The antisense antiviral compounds are substantially uncharged, morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 32 nucleotide region of the viral 5′ untranslated region identified by SEQ ID NO:7.
Owner:SAREPTA THERAPEUTICS INC
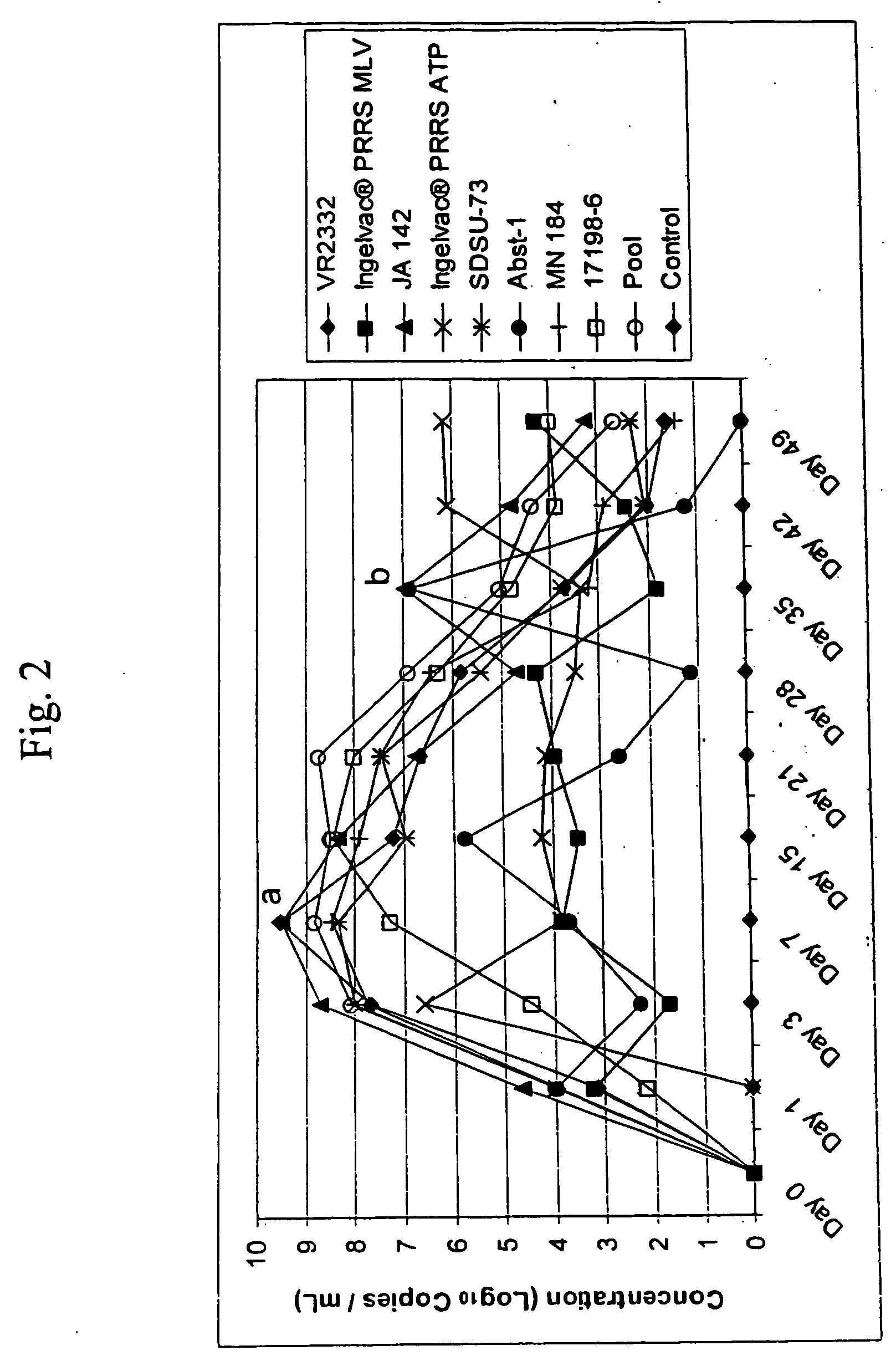
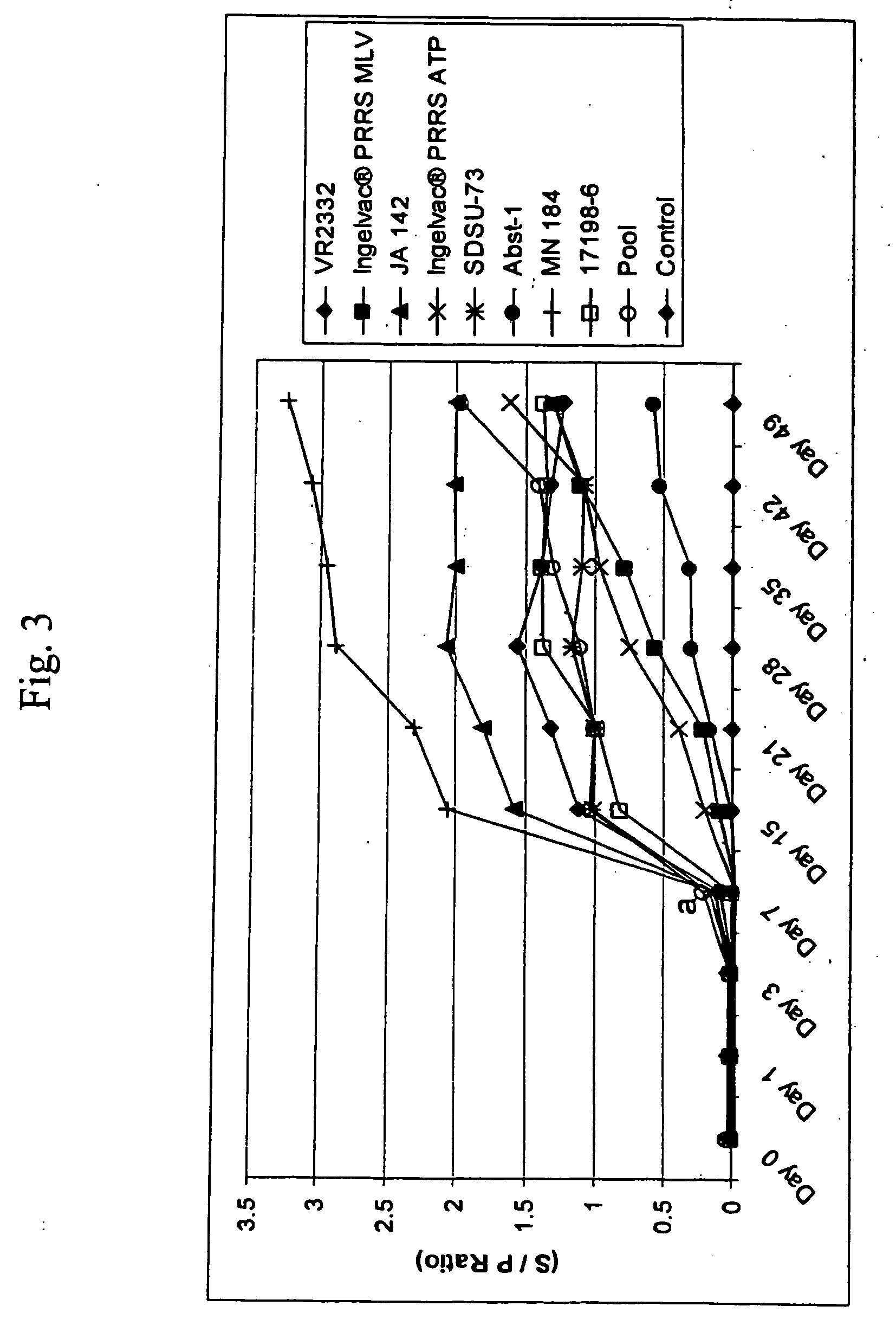
Porcine reproductive and respiratory syndrome isolates and methods of use
InactiveUS20060063151A1Improve immunityIncrease virulenceSsRNA viruses positive-senseMicrobiological testing/measurementVirulent characteristicsImmunogenicity
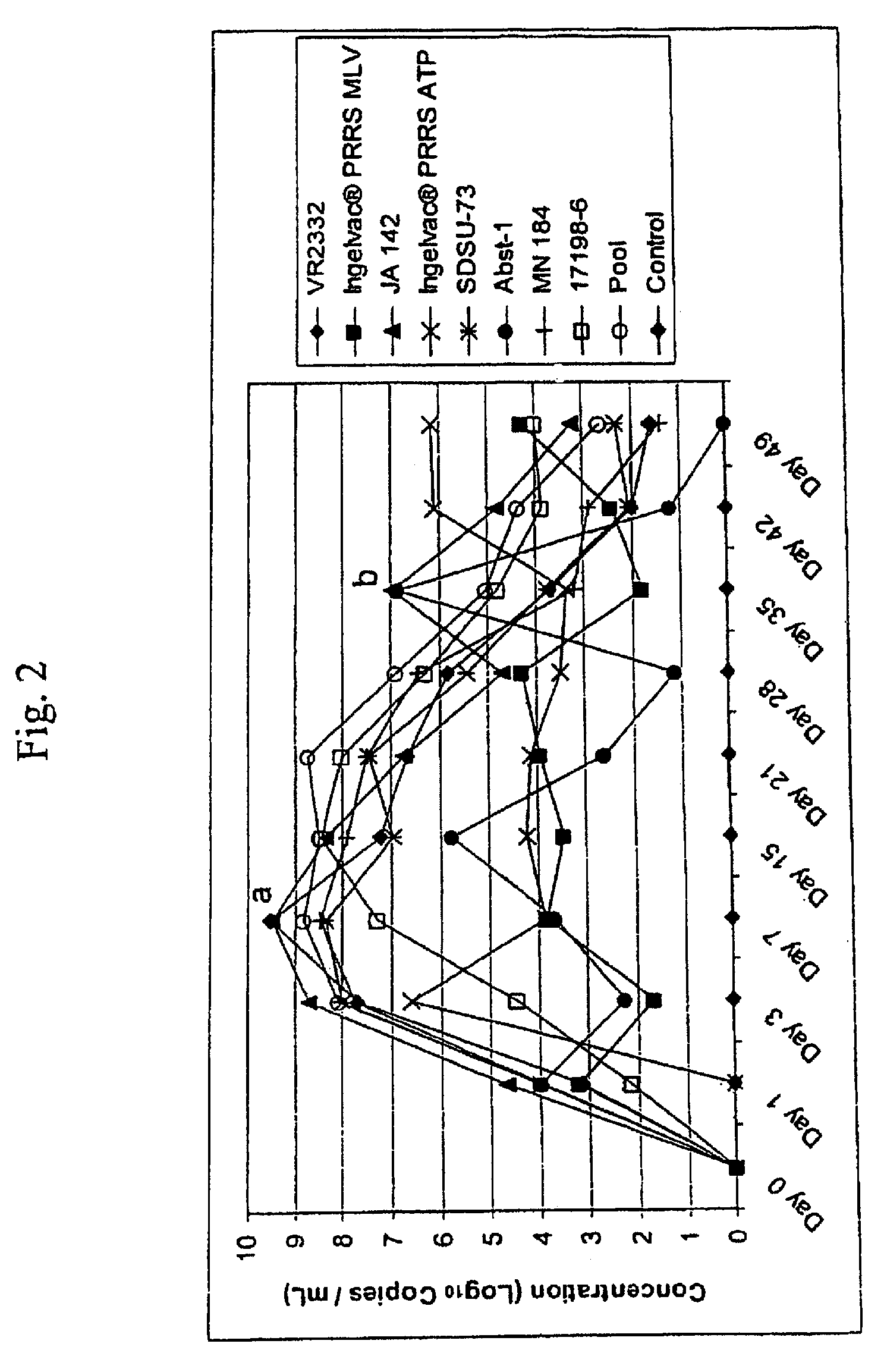
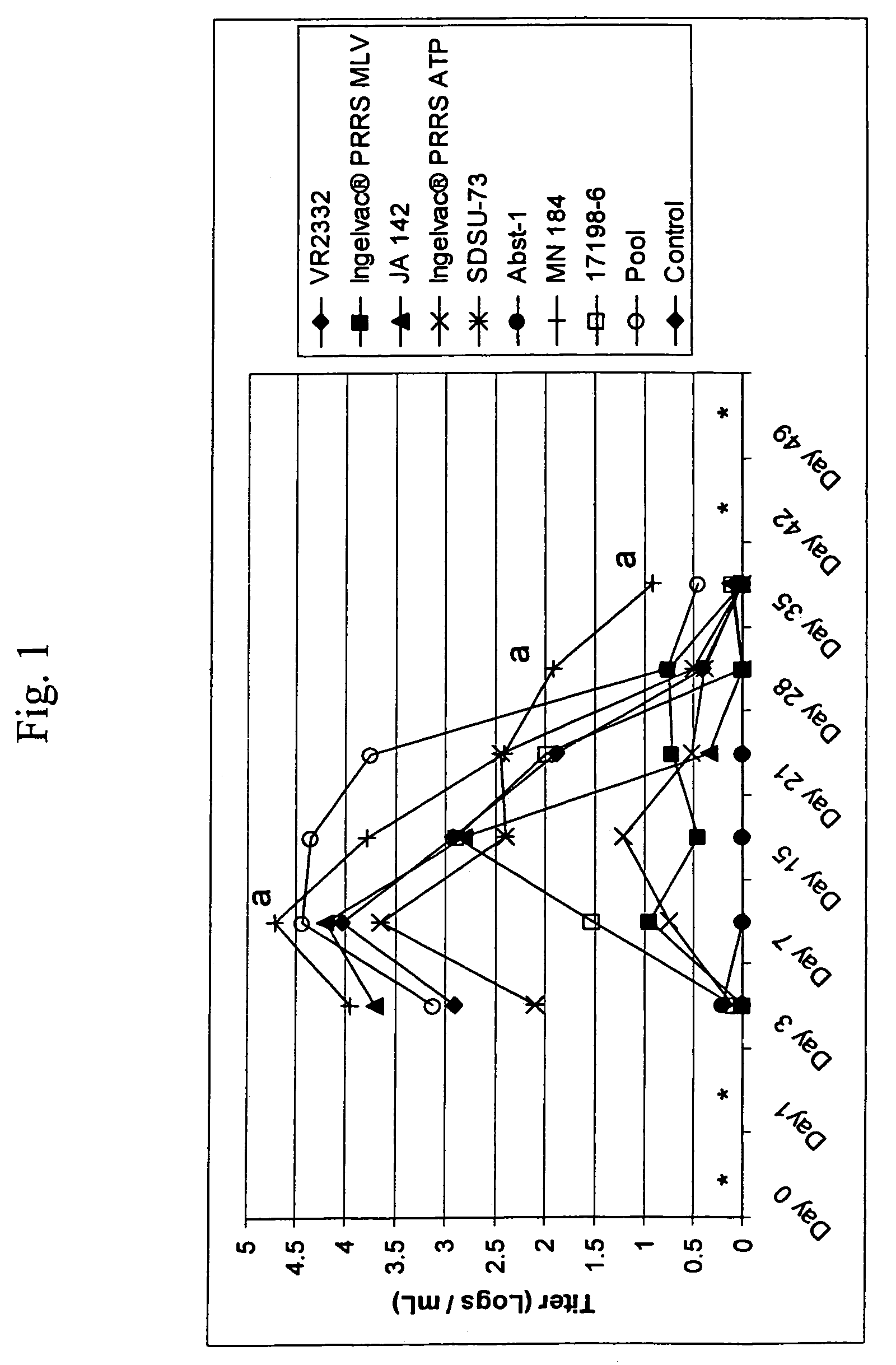
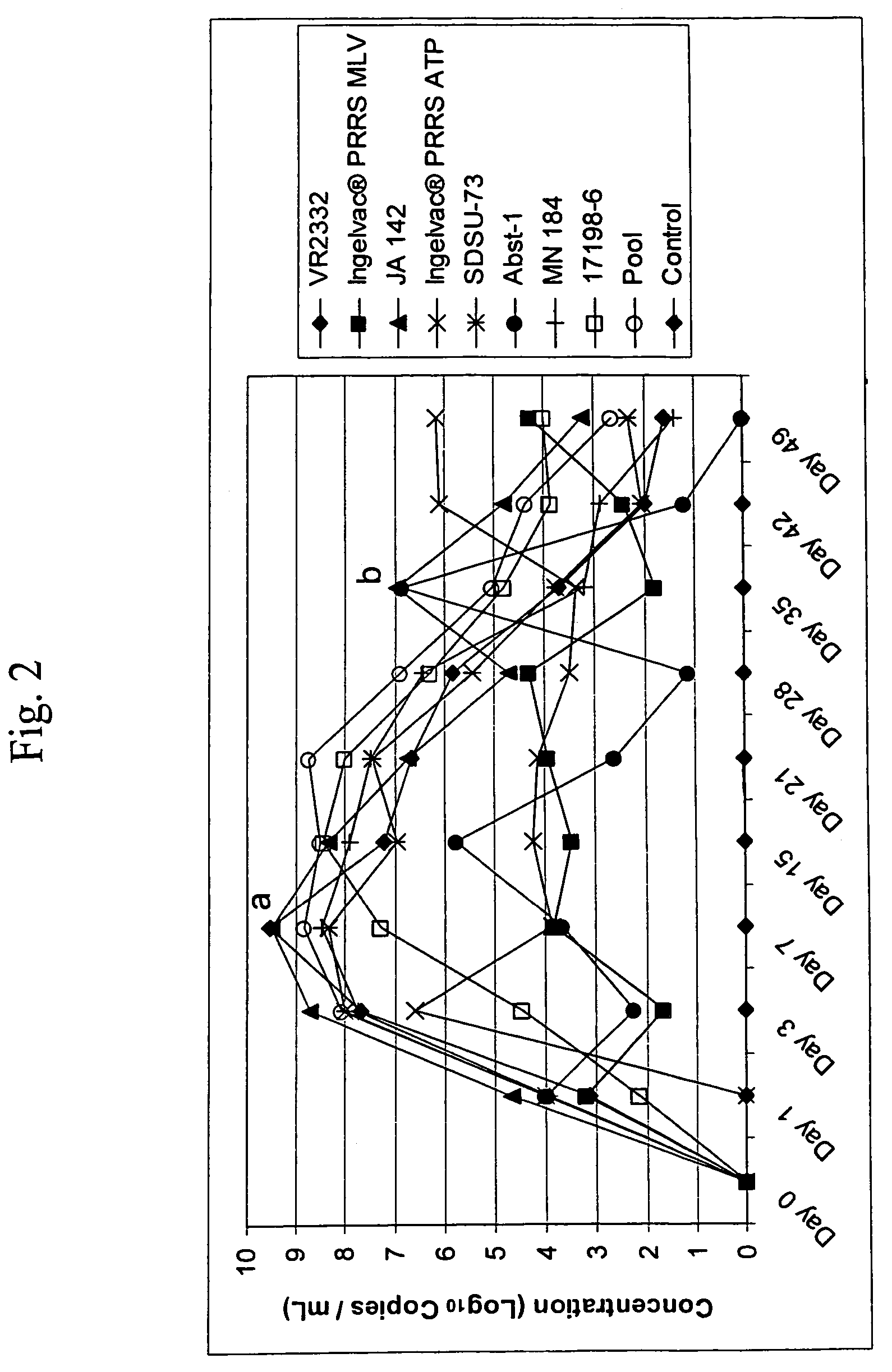
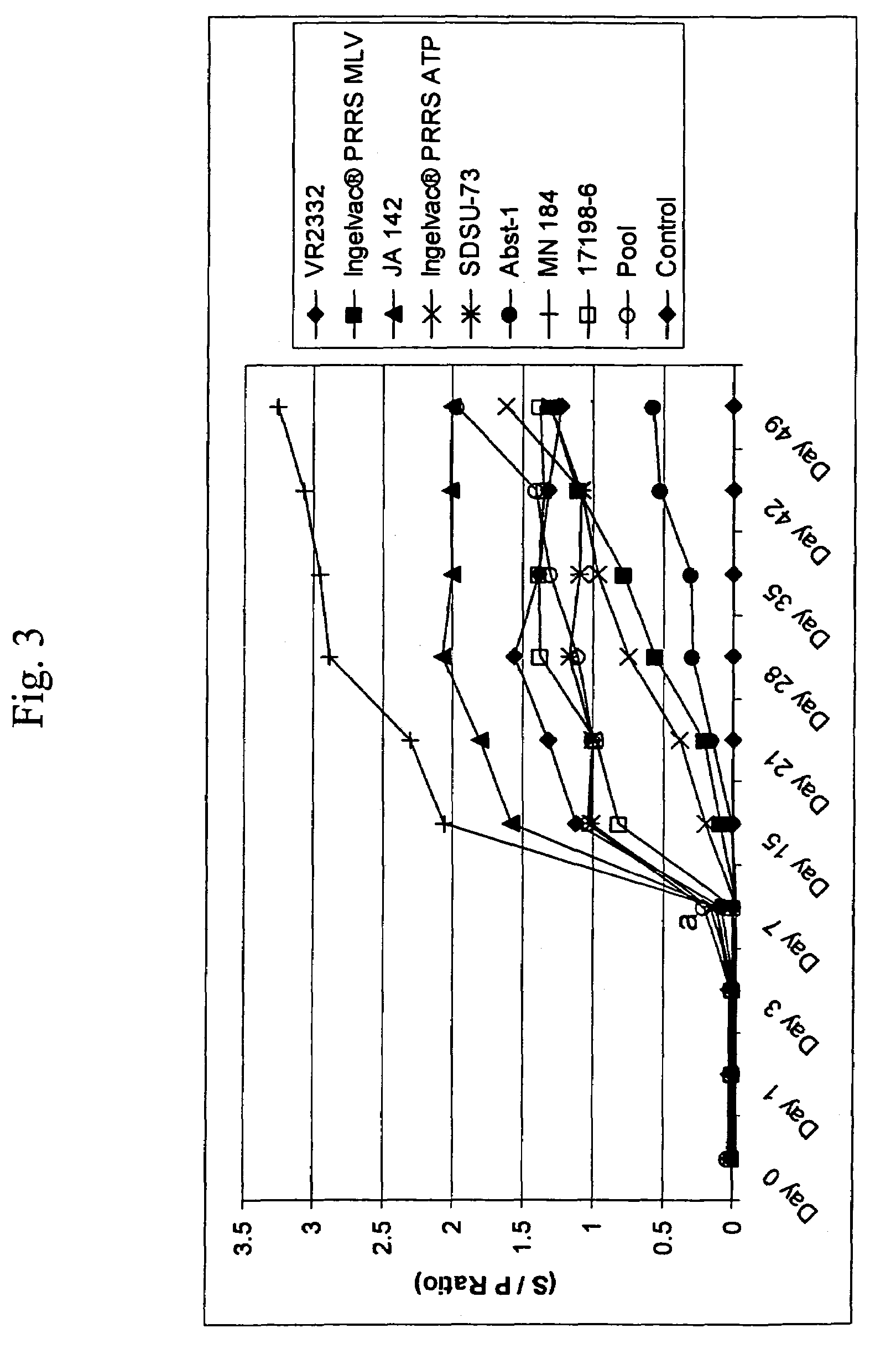
A method of predicting the virulence of a new or uncharacterized PRRS virus isolate is provided wherein the isolate is injected into swine and allowed to replicate for a period of from about 3-15 days. During this period, the rate of virus growth and / or the magnitude of viremia is determined, and this data is compared with a corresponding growth rate and / or viremia magnitude of a PRRS virus isolate of known virulence, as a measure of the virulence of the new or uncharacterized isolate. Additionally, a method of selecting an isolate for inclusion in an immunogenic composition based on the predicted virulence is also provided, together with compositions incorporating attenuated forms of viruses predicted to be virulent.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
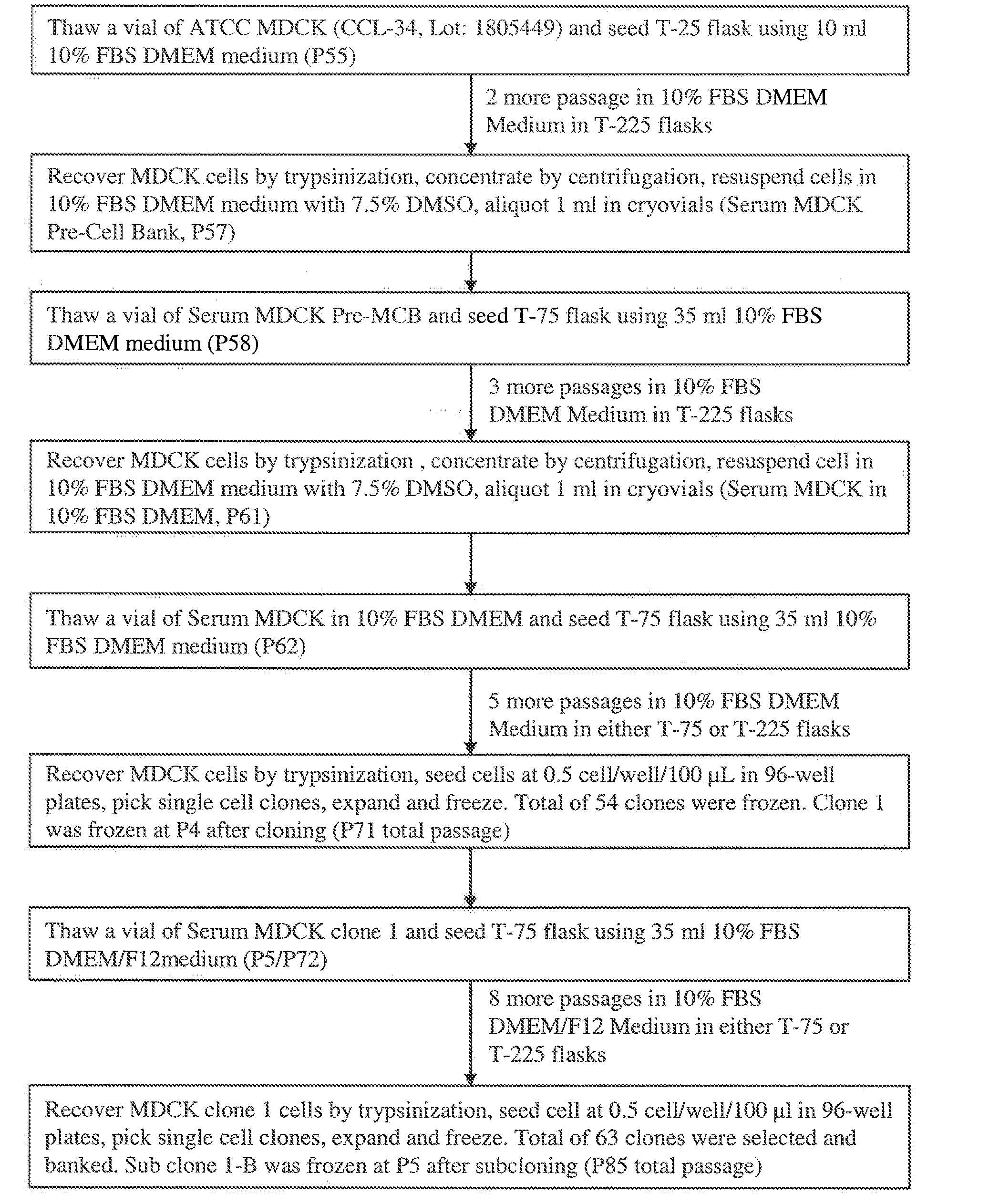
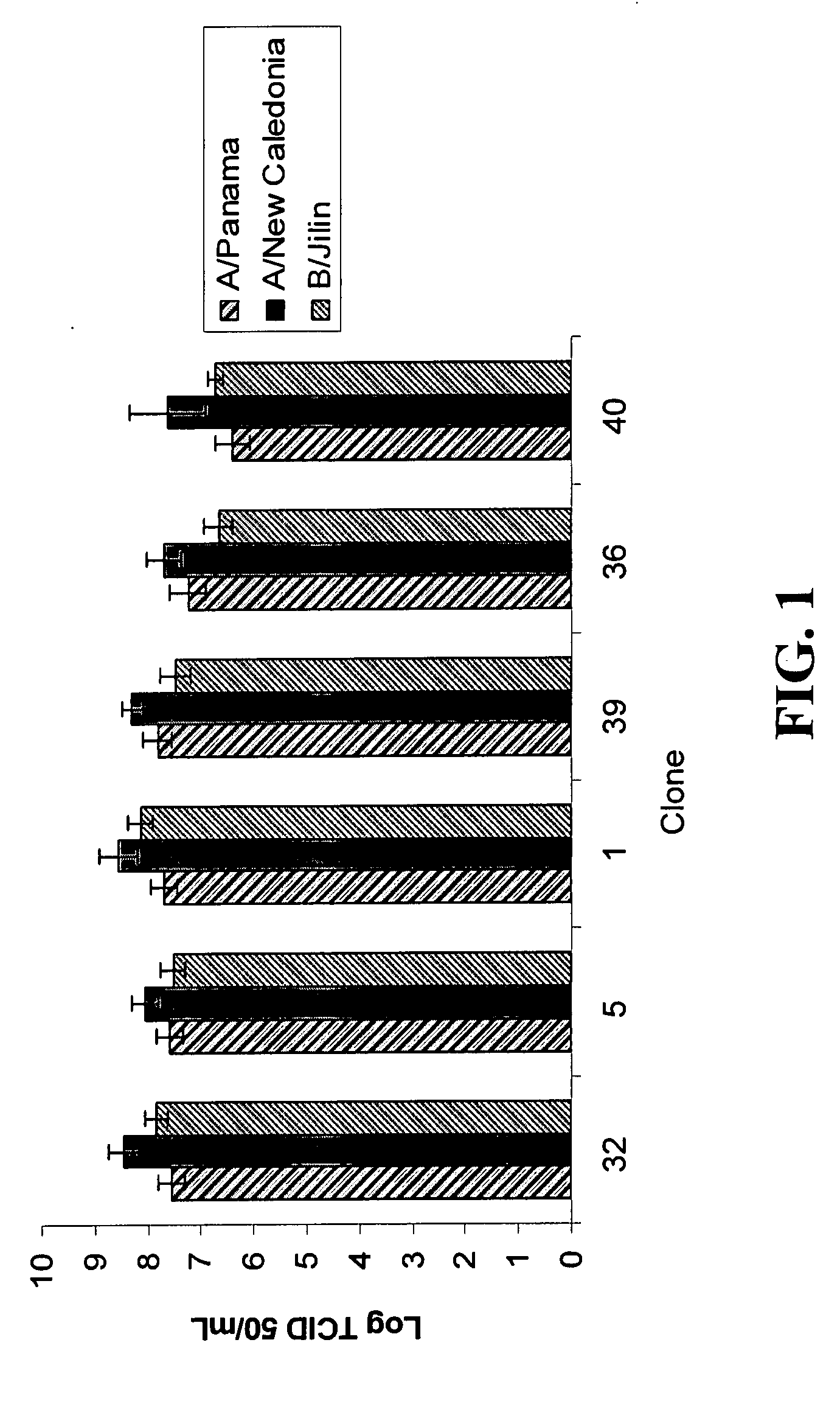
Mdck cell lines supporting viral growth to high titers and bioreactor process using the same
The present invention relates to novel MDCK cells which can be to grow viruses, e.g., influenza viruses, in cell culture to higher titer than previously possible. The MDCK cells can be adapted to serum-free culture medium. The present invention further relates to cell culture compositions comprising the MDCK cells and cultivation methods for growing the MDCK cells. The present invention further relates to methods for producing influenza viruses in cell culture using the MDCK cells of the invention.
Owner:MEDIMMUNE LLC
Cellular permissivity factor for viruses and uses thereof
ActiveUS20050271685A1High expressionMore infectionSsRNA viruses positive-senseAntiviralsViral growth
Owner:ZOETIS SERVICE LLC
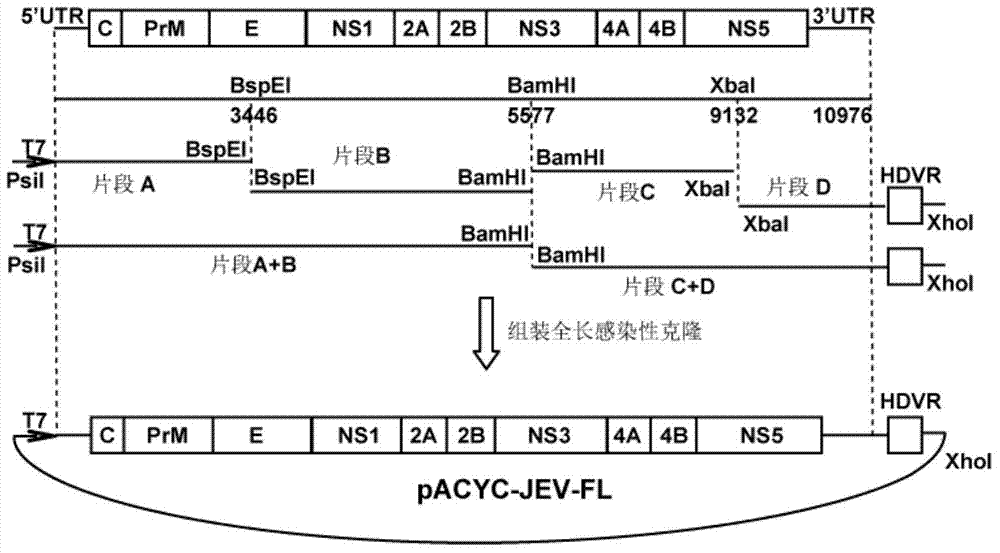
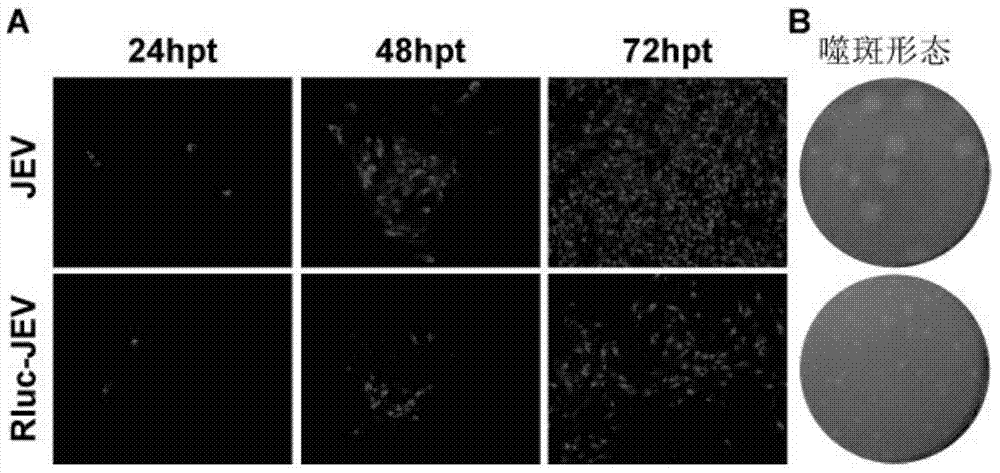
Japanese encephalitis virus (JEV) infectious clone with luciferase gene and building method and application thereof
InactiveCN103497972ASolve the phenomenon of not copyingFast growing trendMicrobiological testing/measurementFluorescence/phosphorescenceLuciferase GeneVaccine evaluation
The invention discloses a Japanese encephalitis virus (JEV) infectious clone with a luciferase gene, and a building method and application thereof. The JEV infectious clone with the luciferase gene is prepared by the following steps: (1) sectional synthesis of a JEV SA14 strain gene sequence; (2) assembling and building of the JEV infectious clone; (3) building of the JEV infectious clone with the luciferase gene. It is proved that a Rluc-JEV report virus with the same growth tendency as the JEV virus can be saved by the JEV infectious clone with the luciferase gene built by the method, and the JEV infectious clone has wide application value in the aspects of an animal model, virus replication and pathogenesis, drug screening and drug action mechanism, live animal imaging and vaccine evaluation and the like through the experiments of immunofluorescence, Rluc activity detection, virus bacteriophage plaque, drug inhibition, live animal imaging, vaccine evaluation and the like.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Sense Antiviral Compound and Method for Treating Ssrna Viral Infection
InactiveUS20080311556A1Disruption of secondary structureInhibition of replicationOrganic active ingredientsBiocideSsRNA virusesViral infection
The invention provides sense antiviral compounds and methods of their use in inhibition of growth of viruses of the Flaviviridae, Picornoviridae, Caliciviridae, Togaviridae, Coronaviridae families and hepatitis E virus in the treatment of a viral infection. The sense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of (12-40) subunits, including at least (12) subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 3′-terminal end (40) bases of the negative-sense RNA strand of the virus.
Owner:AVI BIOPHARMA
Porcine reproductive and respiratory syndrome isolates and methods of use
InactiveUS20090148474A1Improve immunityIncrease virulenceSsRNA viruses positive-senseViral antigen ingredientsVirulent characteristicsImmunogenicity
A method of predicting the virulence of a new or uncharacterized PRRS virus isolate is provided wherein the isolate is injected into swine and allowed to replicate for a period of from about 3-15 days. During this period, the rate of virus growth and / or the magnitude of viremia is determined, and this data is compared with a corresponding growth rate and / or viremia magnitude of a PRRS virus isolate of known virulence, as a measure of the virulence of the new or uncharacterized isolate. Additionally, a method of selecting an isolate for inclusion in an immunogenic composition based on the predicted virulence is also provided, together with compositions incorporating attenuated forms of viruses predicted to be virulent.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Porcine reproductive and respiratory syndrome isolates and methods of use
ActiveUS7632636B2SsRNA viruses positive-senseMicrobiological testing/measurementVirulent characteristicsVirus strain
A method of predicting the virulence of a new or uncharacterized PRRS virus strain is provided wherein the strain is injected into swine and allowed to replicate for a period of from about 3-15 days. During this period, the rate of virus growth and / or the magnitude of viremia is determined, and this data is compared with a corresponding growth rate and / or viremia magnitude of a PRRS virus strain of known virulence, as a measure of the virulence of the new or uncharacterized strain.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
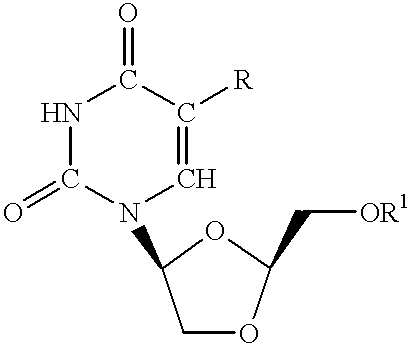
L- beta -dioxolane uridine analogs and methods for treating and preventing Epstein-Barr virus infections
InactiveUS6022876ALow toxicityMinimal toxicityBiocideGroup 5/15 element organic compoundsHigh activityUridine Nucleotides
The present invention relates to the discovery that certain beta -L-dioxolane nucleoside analogs which contain a uracil base, and preferably, a 5-halosubstituted uracil base, exhibit unexpectedly high activity against Epstein-Barr virus (EBV), Varciella-Zoster virus (VZV) and Herpes Virus 8 (HV-8). In particular, the compounds according to the present invention show potent inhibition of the replication of the virus (viral growth) in combination with very low toxicity to the host cells (i.e., animal or human tissue). Compounds are useful for treating EBV, VZV and HV-8 infections in humans.
Owner:GEORGIA UNVERSITY OF RES FOUND INC THE +1
Mdck cells lines supporting viral growth to high titers and bioreactor process using the same
The present invention relates to novel MDCK cells which can be to grow viruses, e.g., influenza viruses, in cell culture to higher titer than previously possible. The MDCK cells can be adapted to serum-free culture medium. The present invention further relates to cell culture compositions comprising the MDCK cells and cultivation methods for growing the MDCK cells. The present invention further relates to methods for producing influenza viruses in cell culture using the MDCK cells of the invention.
Owner:MEDIMMUNE LLC
Cell lines for the propagation of mutated herpes viruses
InactiveUS7262033B1Prevents repairAvoid repairsGenetic material ingredientsInactivation/attenuationNucleic acid sequencingMutation
A process for propagating a mutant herpes virus having a mutation in its endogenous HSV VP16 gene or a homologue thereof, which process comprises infecting a cell line with the mutant herpes virus and culturing the cell line, wherein the cell line comprises a nucleic acid sequence encoding a functional herpes simplex virus (HSV) VP16 polypeptide, or a homologue thereof, operably linked to a control sequence permitting expression of the polypeptide in said cell line; the nucleic acid sequence being (i) capable of complementing the endogenous gene and (ii) unable to undergo homologous recombination with the endogenous gene. In addition, the present invention provides cell lines which can be used for the growth of mutant herpes viruses which have defects in certain immediate early genes together with mutations in VP16 or homologue thereof.
Owner:BIOVEX LTD
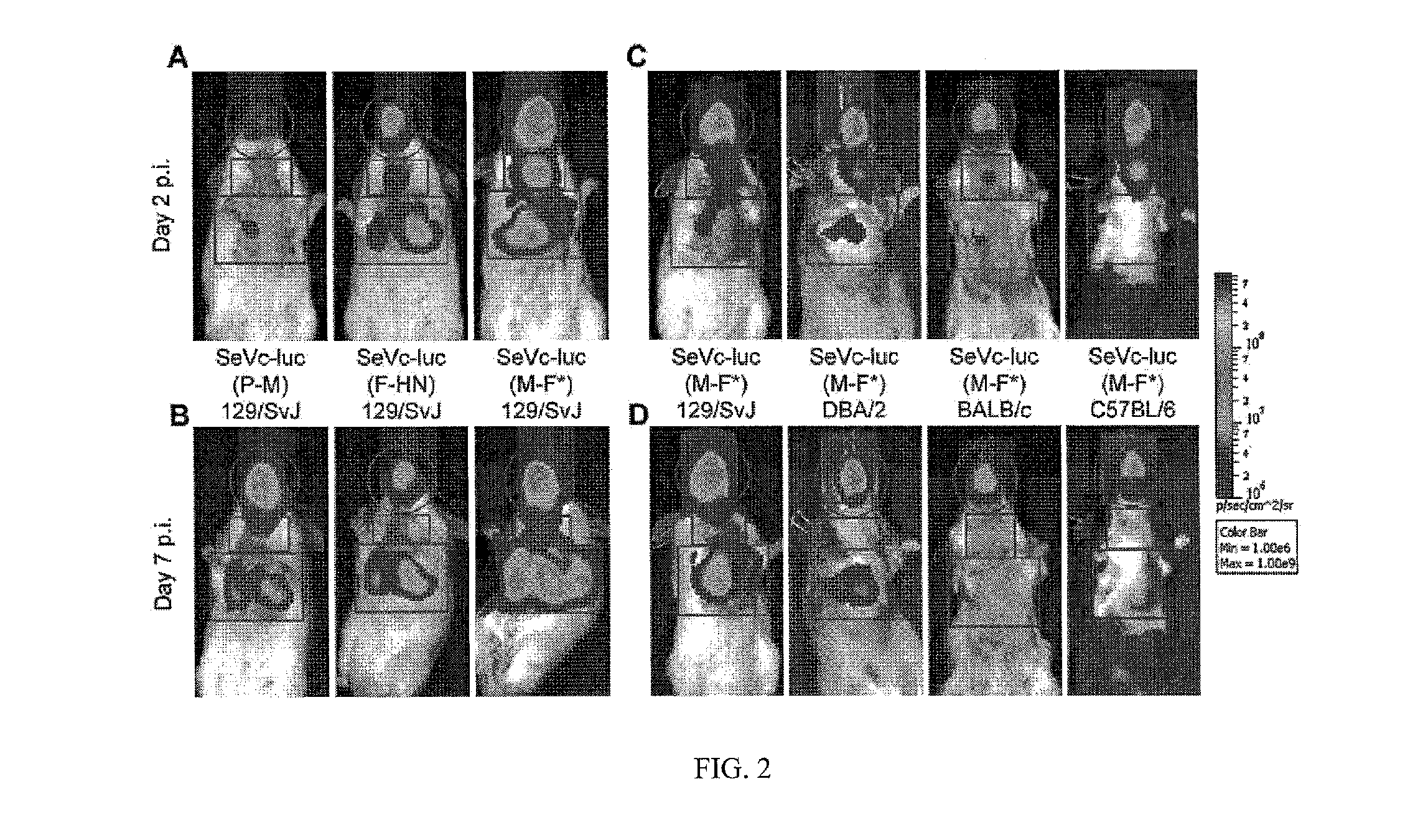
Modified sendai virus vaccine and imaging vector
ActiveUS20140186397A1Easy to trackEasy rescueSsRNA viruses negative-senseVirus peptidesViral VaccineIn vivo
The present invention relates to a Sendai virus or recombinant Sendai virus vector. In particular the present invention provides methods, vectors, formulations, compositions, and kits for a modified Enders strain Sendai viral vector. An immunogenic vector can be used in any in vitro or in vivo system. Moreover, some embodiments include vectors for imaging virus growth, location and transmission.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Goose-origin gene RIG-I (retinoic acid-inducible gene-I) with anti-Newcastle disease virus activities and application thereof
InactiveCN103173455AGrowth inhibitionCan exert anti-NDV effectFungiBacteriaEmbryoMessenger ribonucleic acid
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Non-simian cells for growth of porcine reproductive and respiratory syndrome (PRRS) virus
ActiveUS8202717B2SsRNA viruses positive-senseViral antigen ingredientsAlveolar macrophagePulmonary Macrophages
Disclosed are compositions and methods relating to growth of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) using non-simian cells. In a particular example, porcine alveolar macrophage cells are described as having a capability of supporting infectivity and reproduction by PRRSV. Cells and cell lines of the invention are disclosed in connection with applications relating to PRRS disease, including vaccine technologies.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
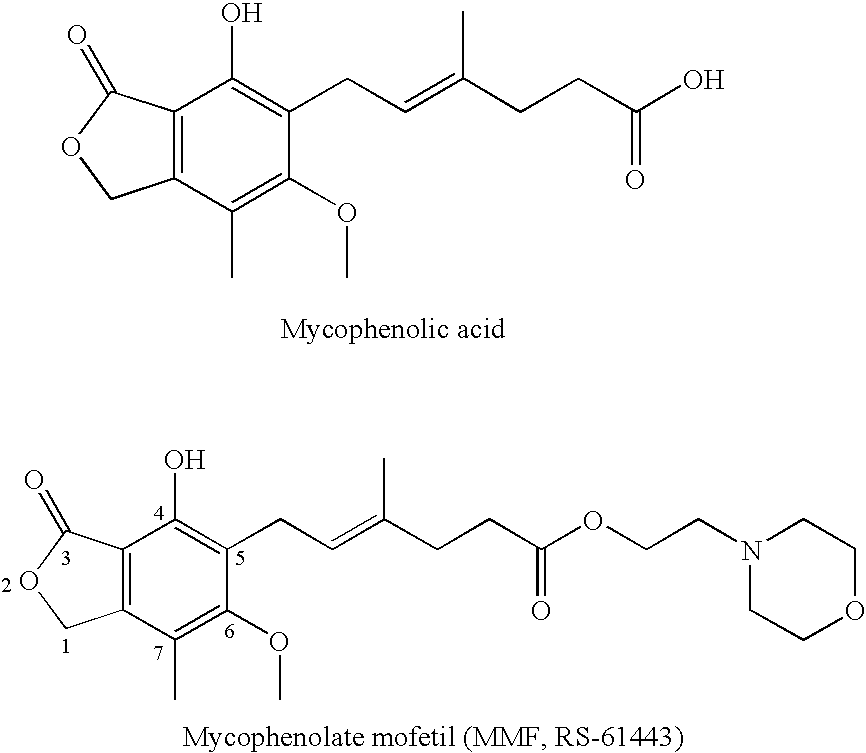
Phosphonate Derivatives of Mycophenolic Acid
Phosphorus substituted mycophenolate oxime derivatives with anti-cancer, anti-viral, anti-inflammatory anti-tissue / organ transplant rejection properties having use as therapeutics and for other industrial purposes are disclosed. The compositions inhibit tumor growth, viral growth, inflammation, and tissue / organ transplant rejection and / or are useful therapeutically for the treatment or prevention of cancer, viral infection, inflammation and tissue / organ transplant rejection, as well as in assays for the detection of cancer, viral infection, inflammation and tissue / organ transplant rejection.
Owner:GILEAD SCI INC
Chimeric strain of porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN109762792ASlow growth rateReduced viral titerViral antigen ingredientsAntiviralsDiseaseHighly pathogenic
The invention discloses a chimeric strain of a porcine reproductive and respiratory syndrome virus and application thereof. Based on a full-length infectious cDNA clone of an attenuated strain of highly pathogenic PRRSV TJM-F92 vaccine and a NADC30-like subtype strain FJ1402, a reverse genetic technology is utilized to construct the chimeric strain of the porcine reproductive and respiratory syndrome virus. It is verified by tests that the chimeric strain has a lower virus growth speed and virus titer on Marc-145 cells than the original strain TJM-F92, and the virulence is low. The chimeric strain has a good immunoprotective effect on HP-PRRSV strain BB0907 and NADC30-like strain FJ1402 and can provide effective cross-immunization protection against infections of highly pathogenic strainsand NADC30-like strains, which lays an important foundation for the development of new broad-spectrum vaccines against this disease.
Owner:NANJING AGRICULTURAL UNIVERSITY
Micromolecule compound inhibitor for enterovirus and application of inhibitor
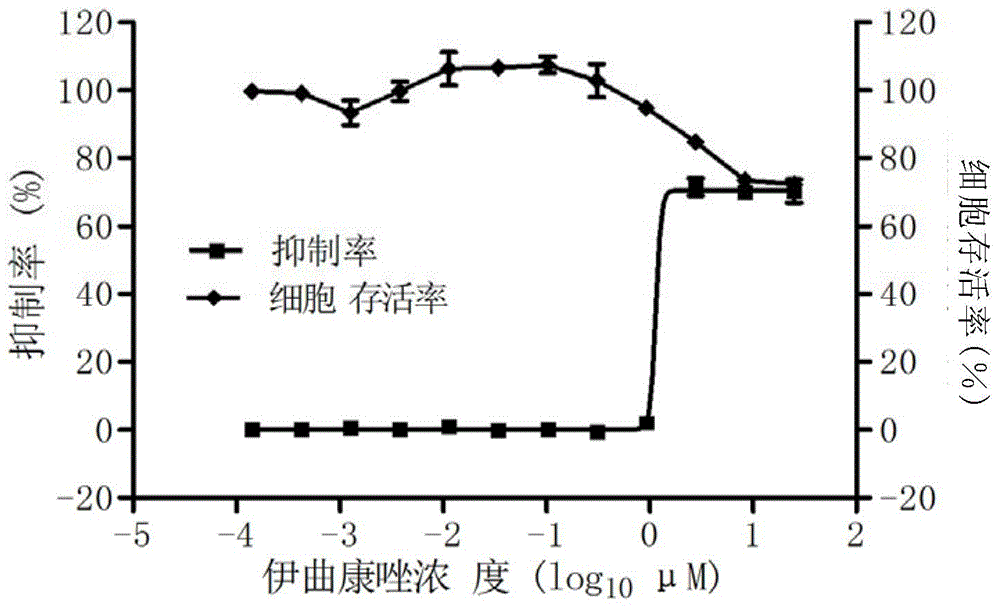
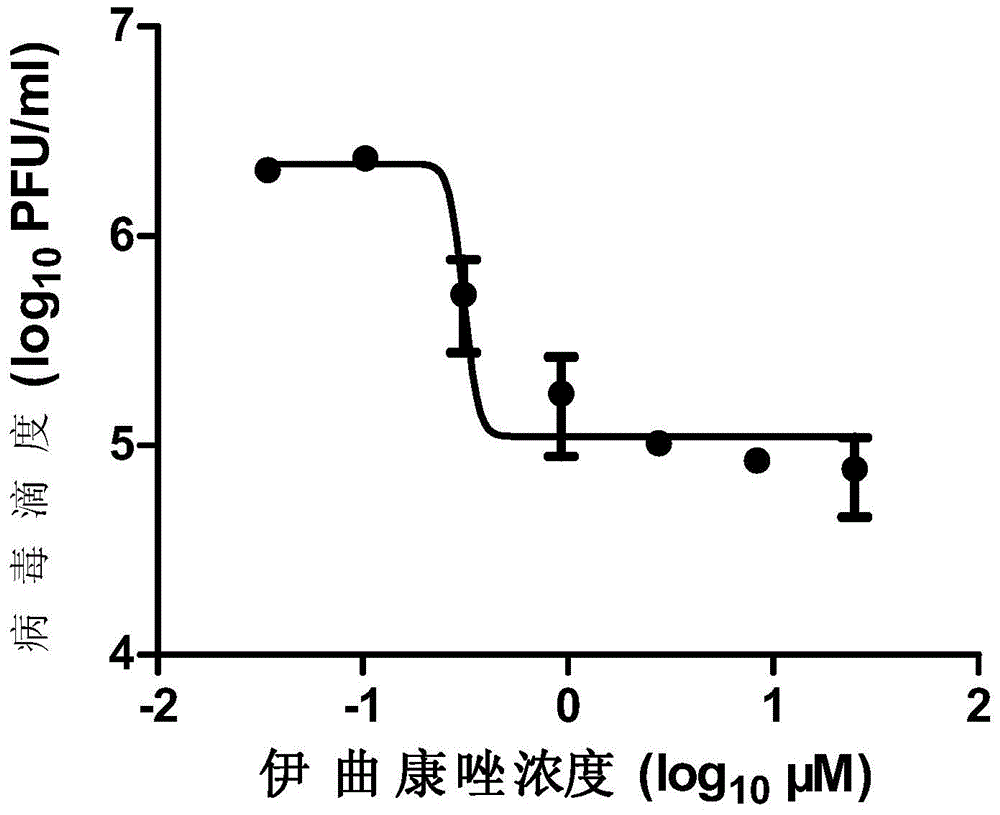
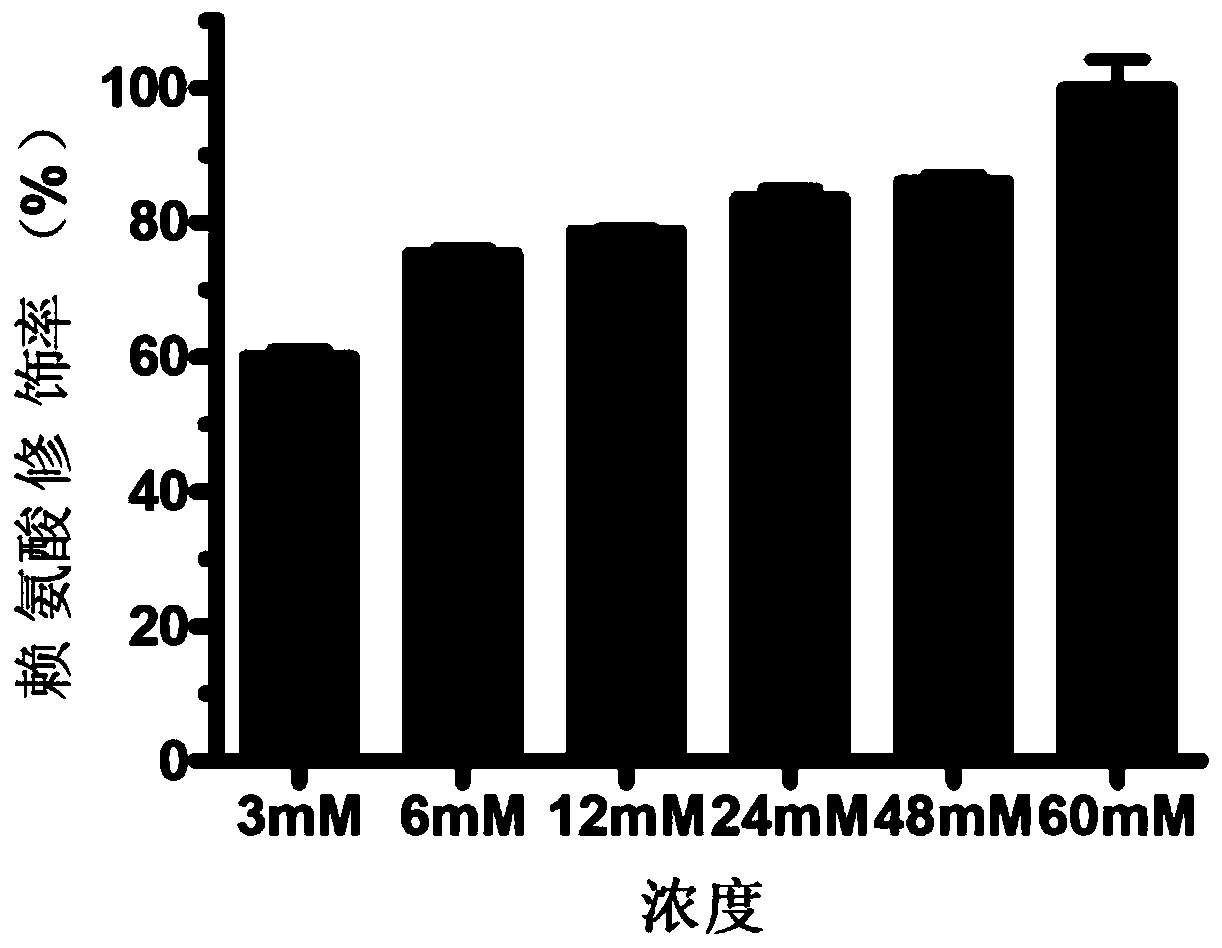
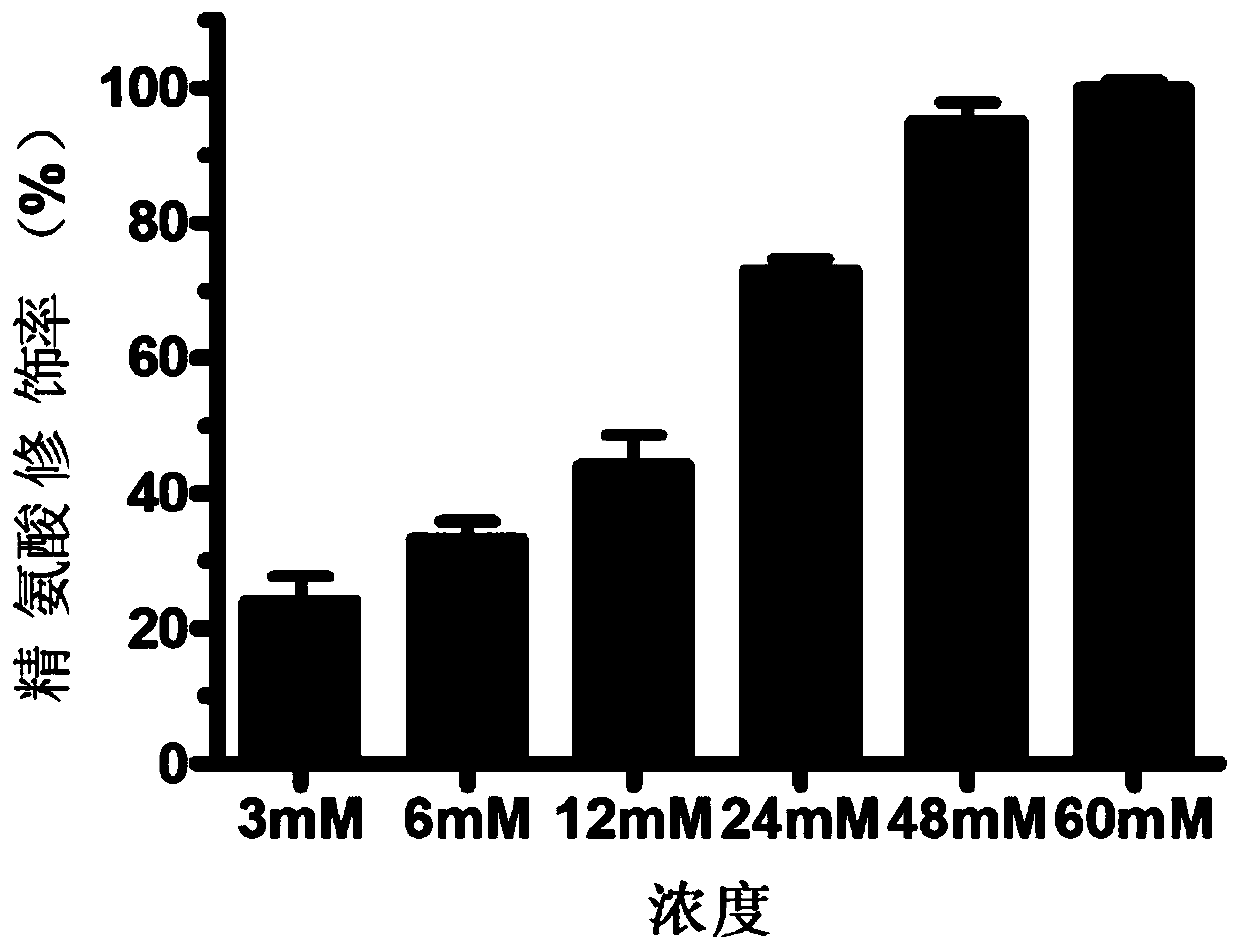
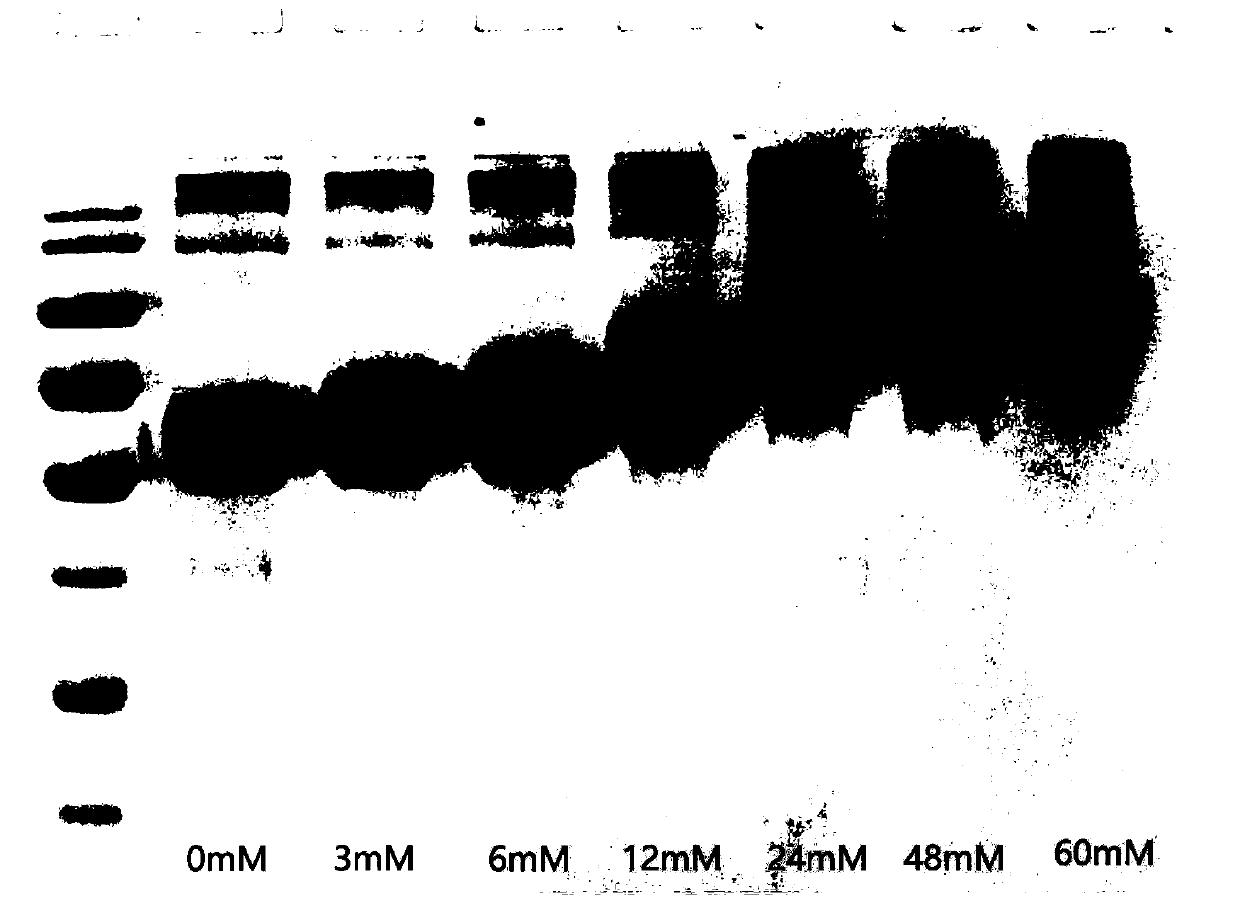
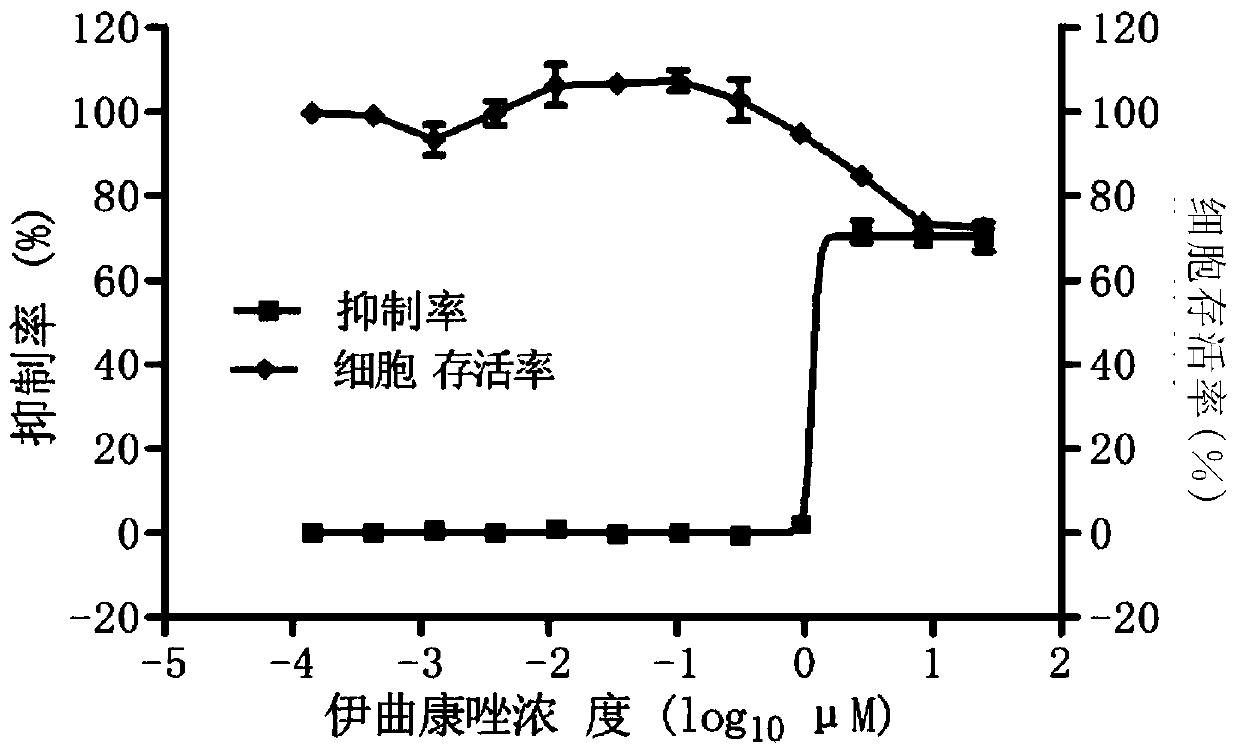
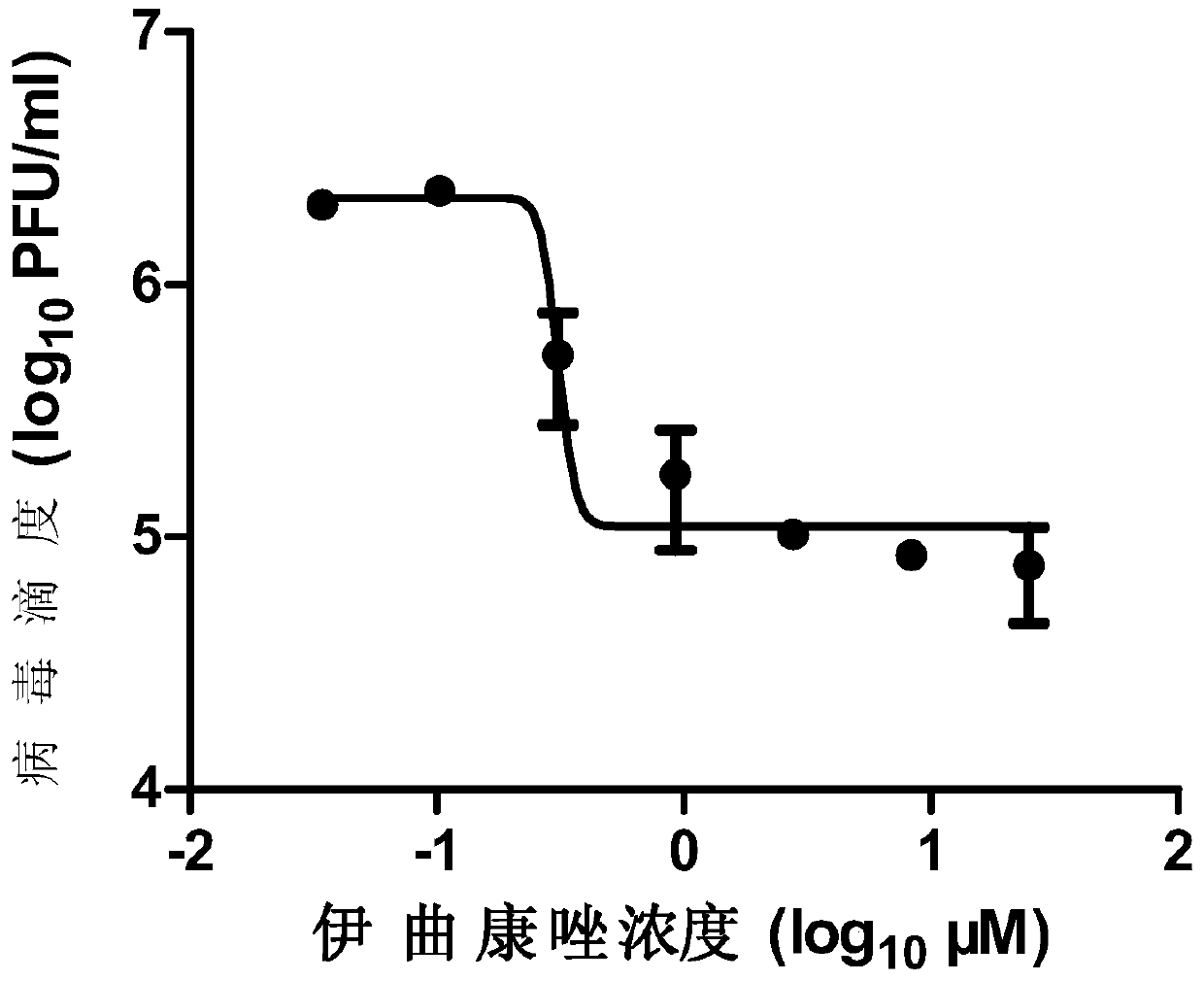
The invention provides a micromolecule compound inhibitor for enterovirus and an application of the inhibitor. Specifically, the invention provides an application of itraconazole, an itraconazole analogue, or pharmaceutically acceptable salt of itraconazole or the itraconazole analogue to preparation of a reagent, which is used for inhibiting growth or reproduction of enterovirus and / or for inhibiting synthesis of enterovirus RNA. The invention also provides an inhibitor and medicine composition containing itraconazole or the itraconazole analogue for enterovirus, and provides a method for in-vitro non-therapeutically inhibiting growth of enterovirus or killing enterovirus. Experimental results show that itraconazole and the itraconazole analogue has an excellent inhibition effect on multiple kinds of enterovirus.
Owner:中国科学院上海免疫与感染研究所
L-beta-dioxolane uridine analogs and their pharmaceutical compositions
InactiveUS6274589B1Potent inhibitionLow toxicityBiocideGroup 5/15 element organic compoundsHigh activityUridine Nucleotides
The present invention relates to the discovery that certain beta-L-dioxolane nucleoside analogs which contain a uracil base, and preferably, a 5-halosubstituted uracil base, exhibit unexpectedly high activity against Epstein-Barr virus (EBV), Varciella-Zoster virus (VZV) and Herpes Virus 8 (HV-8). In particular, the compounds according to the present invention show potent inhibition of the replication of the virus (viral growth) in combination with very low toxicity to the host cells (i.e., animal or human tissue). Compounds are useful for treating EBV, VZV and HV-8 infections in humans.
Owner:YALE UNIV +1
Compositions and methods for the production of alpha-herpesviruses
The present invention relates to virus growth media that improve the yield of alpha-herpesviruses (e.g., HSV-2) grown in cell cultures. The growth media of the invention include two additives, a disaccharide and a lipid mixture, that can be added to serum-free or serum-enriched growth media to improve the efficiency of virus production. The invention further provides methods of producing alpha-herpesviruses (e.g., HSV-2) in such growth media.
Owner:SANOFI PASTEUR BIOLOGICS CO
Compositions and Methods for the Production of Alpha-Herpesviruses
ActiveUS20110201087A1Improve yieldIncrease productionCulture processCell culture mediaCell culture mediaBlood serum
The present invention relates to virus growth media that improve the yield of alpha-herpesviruses (e.g., HSV-2) grown in cell cultures. The growth media of the invention include two additives, a disaccharide and a lipid mixture, that can be added to serum-free or serum-enriched growth media to improve the efficiency of virus production. The invention further provides methods of producing alpha-herpesviruses (e.g., HSV-2) in such growth media.
Owner:SANOFI PASTEUR BIOLOGICS CO
Preparation method of recombination live vector vaccines for diseases of canid and/or feline
The invention provides a method for preparing recombination live vector vaccines for diseases of canid and / or feline based on an RNA (ribose nucleic acid) virus rescuing technique, which is characterized in that a recombination virus expression vector capable of expressing main protective antigens of diseases of the canid and / or the feline based on a reverse genetic manipulation system is established. A rabies virus is conjunctly transfected by the expression vector and assistant plasmids to copy a permissive host cell to rescue the recombination virus, so that multivalent live vector vaccines are prepared. The growth curve of the virus presents that the rescued maternal virus has no obvious difference from the growth kinetic of the recombination virus which expresses the protective antigen genes of main disease pathogens of the canid and / or the feline, and a foundation is established for successfully preparing gene recombination live vector vaccines of main diseases of the canid and / or the feline.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
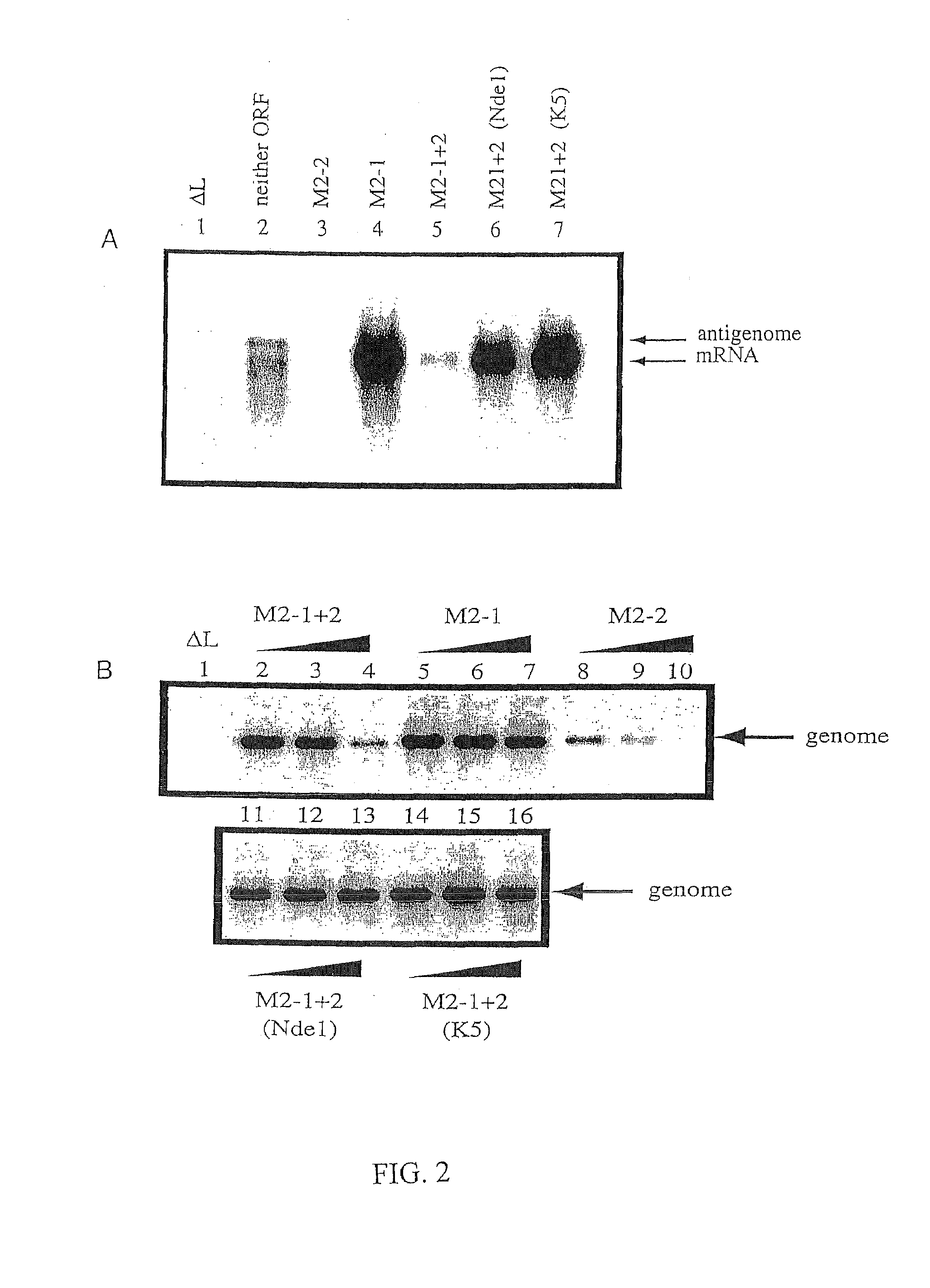
Production of attenuated respiratory syncytial virus vaccines involving modification of M2 ORF2
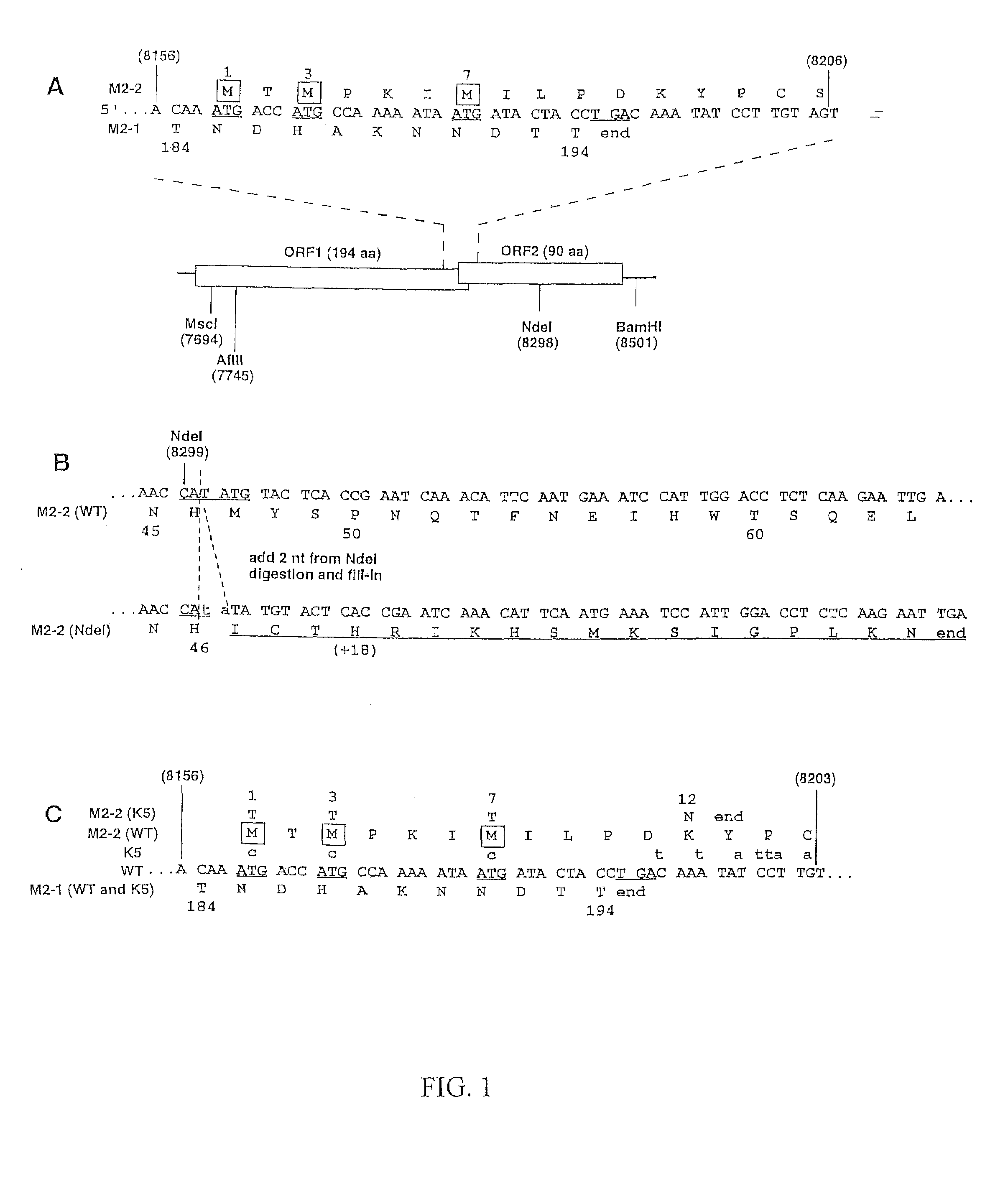
InactiveUS7485440B2Slow kineticsIncrease synthesisSsRNA viruses negative-senseSugar derivativesOpen reading frameADAMTS Proteins
Recombinant respiratory syncytial virus (RSV) are provided in which expression of the second translational open reading frame encoded by the M2 gene (M2ORF2) is reduced or ablated to yield novel RSV vaccine candidates. Expression of M2 ORF2 is reduced or ablated by modifying a recombinant RSV genome or antigenome to incorporate a frame shift mutation, or one or more stop codons in M2 ORF2. Alternatively, M2 ORF2 is deleted in whole or in part to render the M2-2 protein partially or entirely non-functional or to disrupt its expression altogether. M2 ORF2 deletion and knock out mutants possess highly desirable phenotypic characteristics for vaccine development. These changes specify one or more desired phenotypic changes in the resulting virus or subviral particle. Vaccine candidates are generated that show a change in mRNA transcription, genomic or antigenomic RNA replication, viral growth characteristics, viral antigen expression, viral plaque size, and / or a change in cytopathogenicity. In addition, M2-2 knock out or deletion virus exhibits increased levels of synthesis of viral proteins in cell culture, providing an enriched source of viral antigen or protein for purification and use as a noninfectious subunit vaccine.
Owner:UNITED STATES OF AMERICA
Method for inhibiting coronavirus by anhydride-modified protein
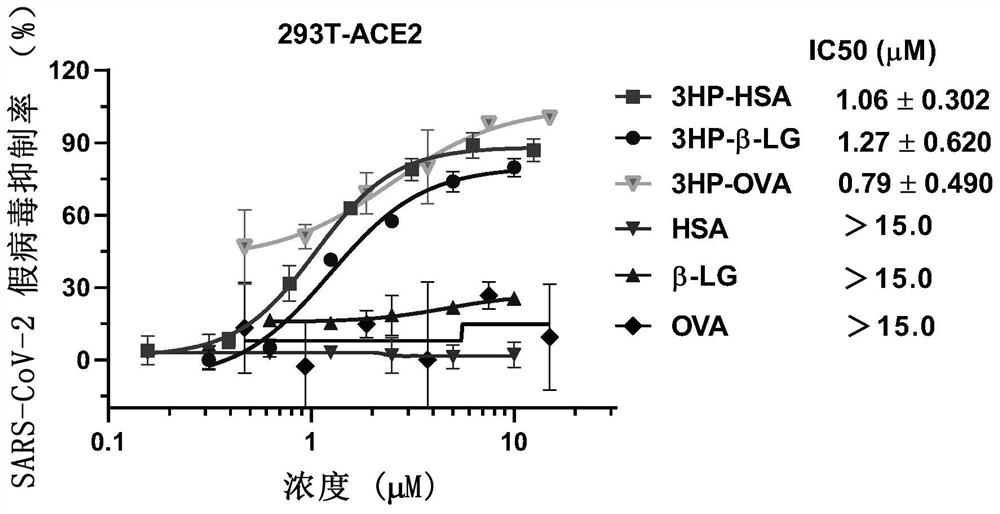
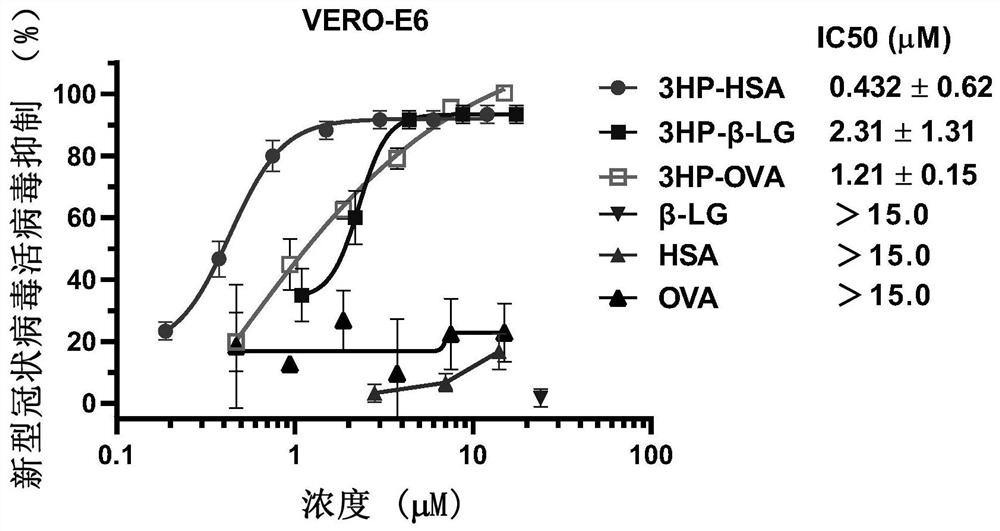
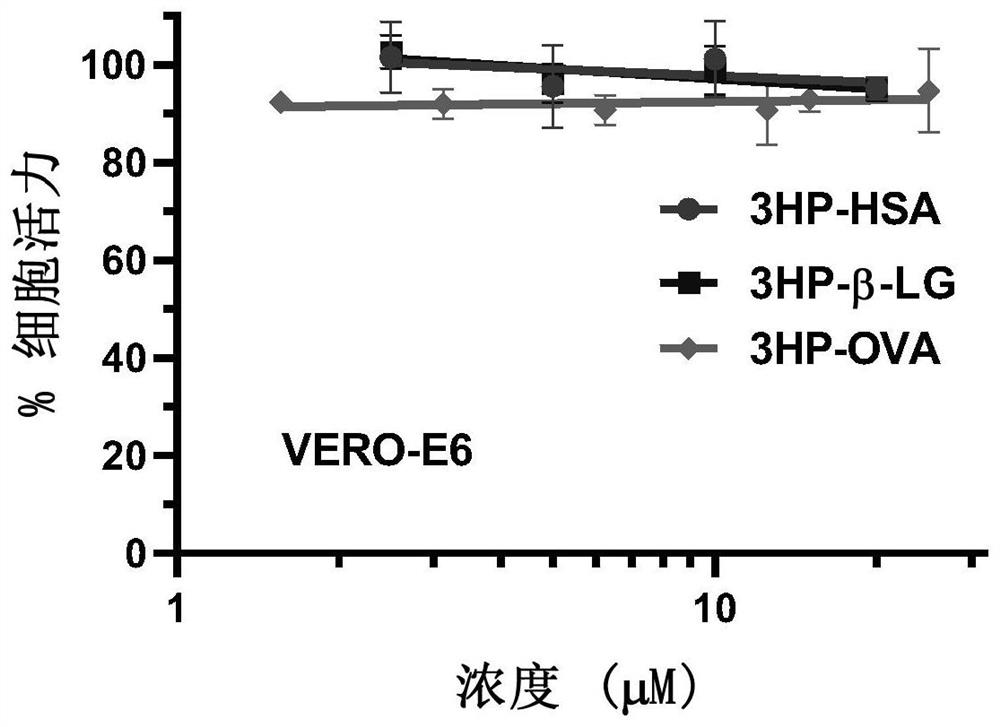
ActiveCN112316152AControlling SARS-CoV-2Activity blockingPeptide/protein ingredientsAntiviralsIn vivoProtein
The invention provides a method for inhibiting coronavirus growth, preventing, or controlling coronavirus infected cells in vitro by an anhydride-modified protein or a composition comprising an anhydride-modified protein. The invention also provides application of the anhydride-modified protein or the composition containing the anhydride-modified protein in preparation of medicines. The protein isselected from albumin, beta lactoglobulin and RNAase; and the anhydride is selected from 3-hydroxyphthalic anhydride, succinic anhydride or maleic anhydride. The anhydride-modified protein can effectively inactivate coronavirus in vivo or in vitro and is non-toxic to cells, and a method for controlling coronavirus epidemic situation is provided.
Owner:SHANXI JINBO BIO PHARMA CO LTD +3
Methods and Devices for Quantitative Viral Assays
ActiveUS20070111296A1Reflects virus replicating abilityGreat extentSsRNA viruses negative-senseSsRNA viruses positive-senseLiquid mediumCulture cell
A method for quantifying infectious particles of a virus in a sample comprises providing a layer of host cells of the virus, contacting the layer of host cells with a preparation of the sample, and culturing the cells under conditions wherein the cells are submerged in a thin layer of liquid culture medium, and wherein the virus infects host cells and releases its progeny from said infected host cells, imposing a flow of the liquid medium, wherein the spread of the viral progeny to uninfected host cells is enhanced, culturing the cells under conditions to allow further virus infection and viral gene expression, wherein infected host cells develop an observable indication of viral gene expression, and determining the number of infected host cells, whereby the number of infectious particles of the virus in the sample is quantified. The method may be used for measuring viral growth rate or for screening for antiviral compounds. Also provided are microfluidic devices suitable for the inventive method.
Owner:WISCONSIN ALUMNI RES FOUND
Nasal drops and preparation method thereof
InactiveCN104398579AGood curative effectGood effectHydroxy compound active ingredientsPharmaceutical delivery mechanismBacterial virusDisease
The invention relates to the technical field of rhinitis medication, which discloses nasal drops and a preparation method thereof. The preparation method comprises the following steps: 1)respectively weighing raw materials according to weight part; 2)immersing lithospermum, xanthium, dahurian angelica root and magnolia flower by sesame oil; 3)decocting; and 4)adding borneol, mint cream and liquid paraffin, continuously decocting, depositing, separating, and filtering to obtain a filtrate which is the nasal drops. The nasal drops can effectively inhibit growth of bacterial virus, promote tissue restoration of nasal cavity and increase local immunization state; the nasal drops is prepared by traditional Chinese medicinal materials, have the characteristics of no side effect, excellent curative effect, good effect and high cure rate, are suitable for various rhinopathy patients, have obvious alleviation effect for acute cold and nasal obstruction, rhinorrhea and dizziness, can shorten the course of disease, and obviously improve the life quality.
Owner:郝杰
Indoor air purifying agent containing attapulgite, and preparation method thereof
InactiveCN108421405AEasy to makeImprove the effect of adsorption and purificationGas treatmentDispersed particle separationMicroparticleSilicon dioxide
The invention provides an indoor air purifying agent containing attapulgite, and a preparation method thereof, and belongs to the technical field of air purifying agent. The indoor air purifying agentcontaining attapulgite comprises 10 to 15 parts of nanometer silica, 5 to 10 parts of nanometer titanium dioxide, 5 to 10 parts of gaseous state and liquid state sulfur dioxide, 5 to 10 parts of active carbon, 5 to 10 parts of attapulgite particle, 5 to 10 parts of a photocatalyst, 5 to 10 parts of an UV glue, 1 to 3 parts of a perfume, 1 to 3 parts of a mixed adjusting agent, and 80 to 100 partsof distilled water. According to the preparation method, attapulgite particle is added into the raw materials of the air purifying agent for the first time, so that the adsorption purifying effect ofthe air purifying agent is better, the obtained indoor air purifying agent is capable of inhibiting growth of bacteria and virus, removing peculiar smell, cleaning the air, and removing pollutants, such as particle matters, SO2, NO2, and formaldehyde in the air.
Owner:盱眙启睿矿业有限公司
Polypropylene resin for melt-blown fabric production and preparation method thereof
The invention discloses polypropylene resin for melt-blown fabric production and a preparation method thereof, and relates to the technical field of materials for melt-blown fabric production. The polypropylene resin is prepared from the following raw materials: polypropylene resin, a silver ion aqueous solution, waterborne aziridine, waterborne polyurethane, a compatilizer, a weather-proof agent and an antioxidant. The preparation method comprises the following steps: stirring and mixing raw materials in a stirrer to obtain a mixed material; putting the mixed material into a double-screw extruder for plasticizing extrusion, cooling to obtain a hardened material, granulating, sieving, cooling and drying. The melt-blown fabric has the effect of inhibiting the growth of bacteria and viruses, and solves the problem that the mask and other products made of the existing melt-blown fabric cannot be repeatedly used due to no effect of inhibiting the growth of bacteria and viruses, and therefore the resource waste is caused.
Owner:广西德福莱医疗器械有限公司
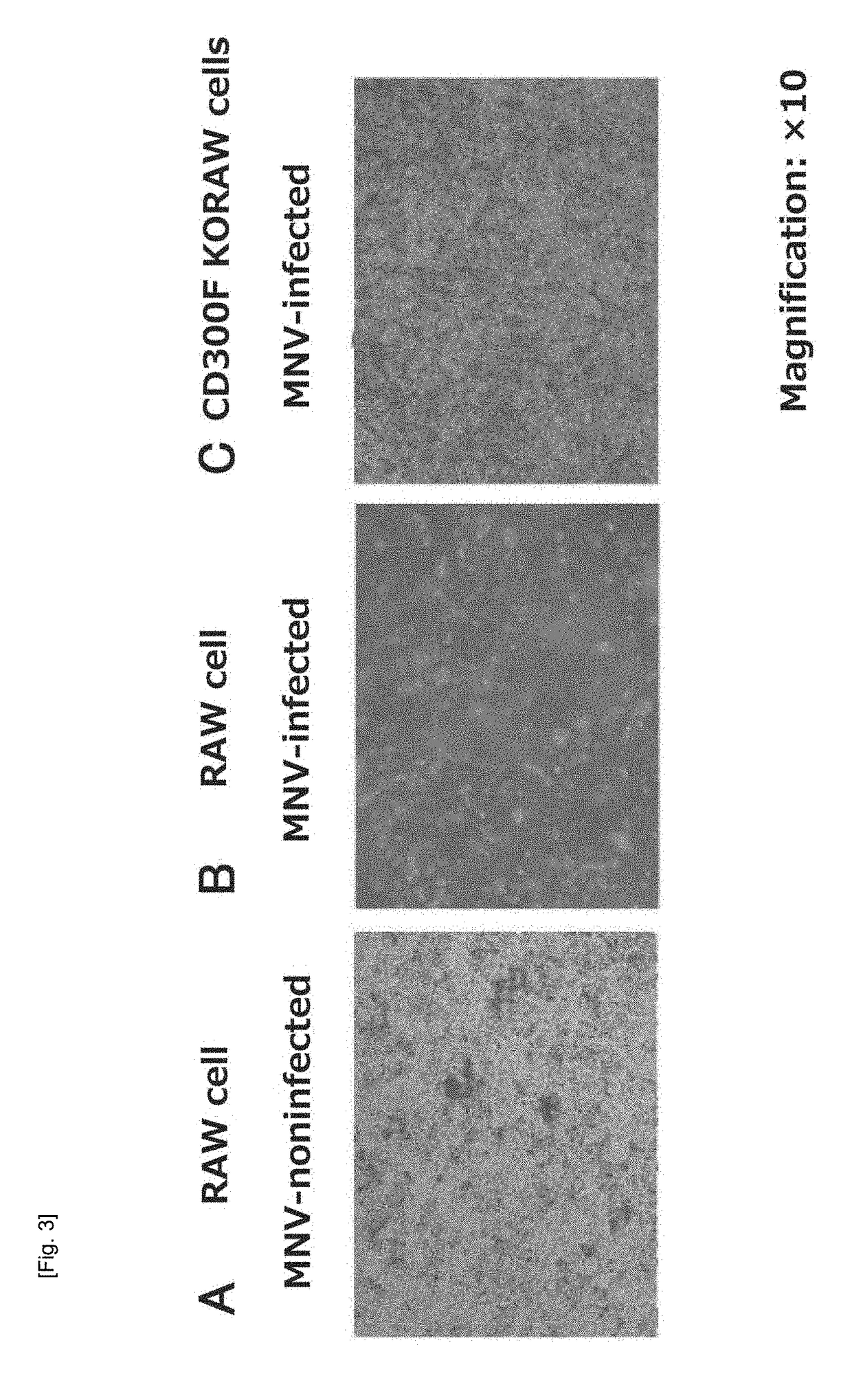
Cultured transgenic cell allowing growth of norovirus, and use thereof
ActiveUS20190040415A1Immunoglobulin superfamilyMicrobiological testing/measurementMurine norovirusMammal
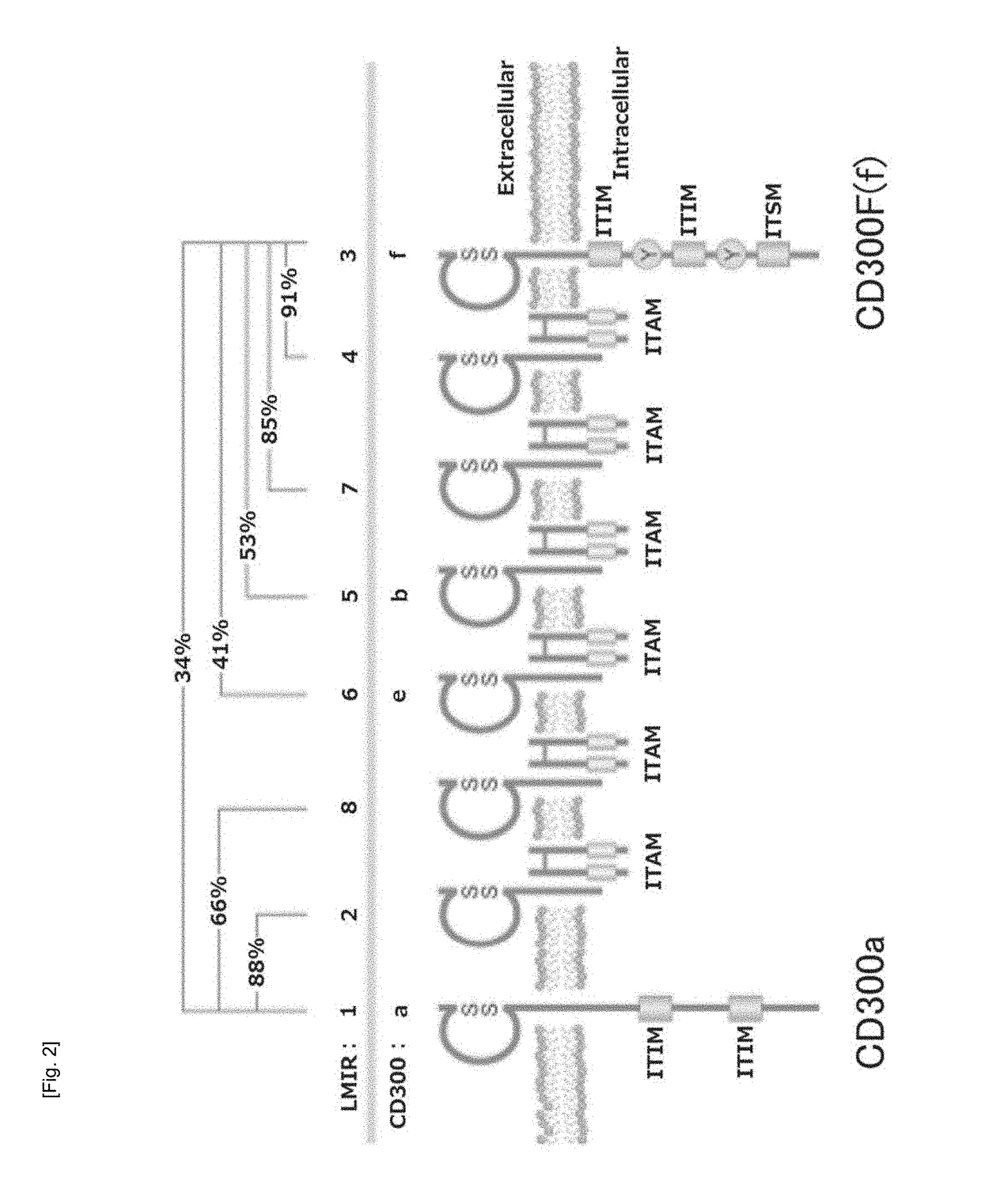
An object of the present invention is to solve problems in terms of stagnation of research on norovirus by providing a cultured transgenic cell or a transgenic animal in which murine norovirus (MNV) can be grown across the barrier of host specificity in mammalian cells, and providing a screening method that uses the cultured transgenic cell or the transgenic animal. The present inventors have found that MNV infection is determined in a cultured transgenic mammalian cell or a mammal possessing the cultured transgenic mammalian cell as its own cell, the cultured transgenic mammalian cell containing one or more species selected from the entirety or a portion of the murine CD300F gene and / or a CD300 family gene having an extracellular domain nucleotide sequence similar to that of the murine CD300F gene. The present inventors have solved the aforementioned problems by providing, for example, a norovirus-related drug screening method on the basis of this finding.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES +2
Broad-spectrum anti-enterovirus protein drug and application thereof
PendingCN111053892AHas inhibitory activityEasy to operatePeptide/protein ingredientsAntiviralsProteinEnterovirus 68
The invention relates to a broad-spectrum anti-enterovirus protein drug and application thereof, and discloses a method for inhibiting growth of enteroviruses and preventing or controlling cells infected with enteroviruses in vitro by acid anhydride modified protein or a composition containing the acid anhydride modified protein. The invention also discloses a biological agent for preventing and treating various enterovirus infections, wherein the biological agent is protein treated by acid anhydride. Specifically, the anhydridized protein is anhydridized human serum albumin and bovine beta lactoglobulin. The proteins can effectively inhibit recent massive outbreak of enterovirus 68 type, enterovirus 71 type and coxsackie virus 16 type infected cells. The inhibitor disclosed by the invention has the advantages of broad-spectrum inhibition of enterovirus invading cells, prevention of virus diffusion, stability, low cost and the like.
Owner:SHANXI JINBO BIO PHARMA CO LTD +1
A small molecular compound inhibitor of enterovirus and its application
The invention provides a micromolecule compound inhibitor for enterovirus and an application of the inhibitor. Specifically, the invention provides an application of itraconazole, an itraconazole analogue, or pharmaceutically acceptable salt of itraconazole or the itraconazole analogue to preparation of a reagent, which is used for inhibiting growth or reproduction of enterovirus and / or for inhibiting synthesis of enterovirus RNA. The invention also provides an inhibitor and medicine composition containing itraconazole or the itraconazole analogue for enterovirus, and provides a method for in-vitro non-therapeutically inhibiting growth of enterovirus or killing enterovirus. Experimental results show that itraconazole and the itraconazole analogue has an excellent inhibition effect on multiple kinds of enterovirus.
Owner:中国科学院上海免疫与感染研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com