Japanese encephalitis virus (JEV) infectious clone with luciferase gene and building method and application thereof
A technology of luciferase gene and infectious clone, applied in the field of preparation of JEV infectious clone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] A method for constructing a JEV virus infectious clone with a luciferase gene, the steps of which are:
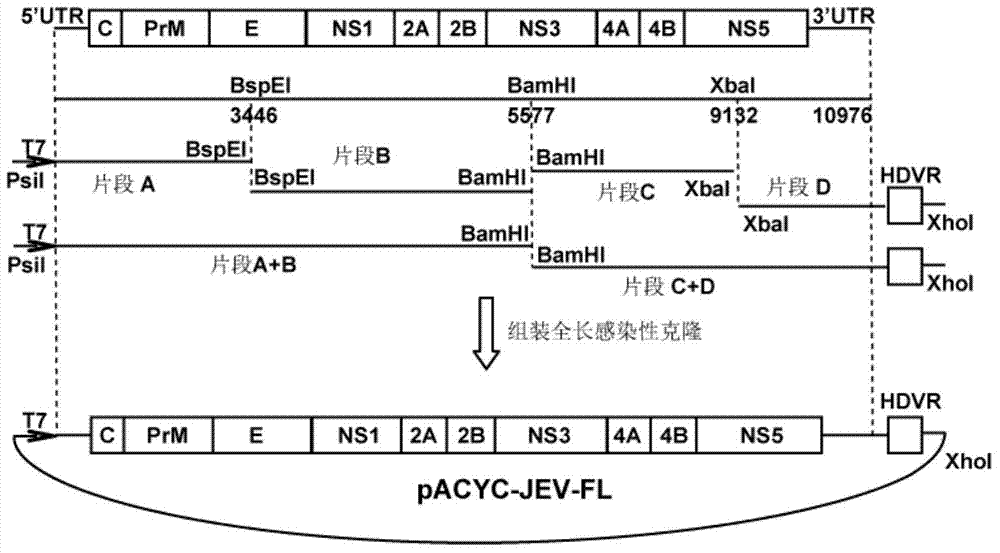
[0068] 1. Segmented synthesis of the genome sequence of JEV-SA14 virus strain:
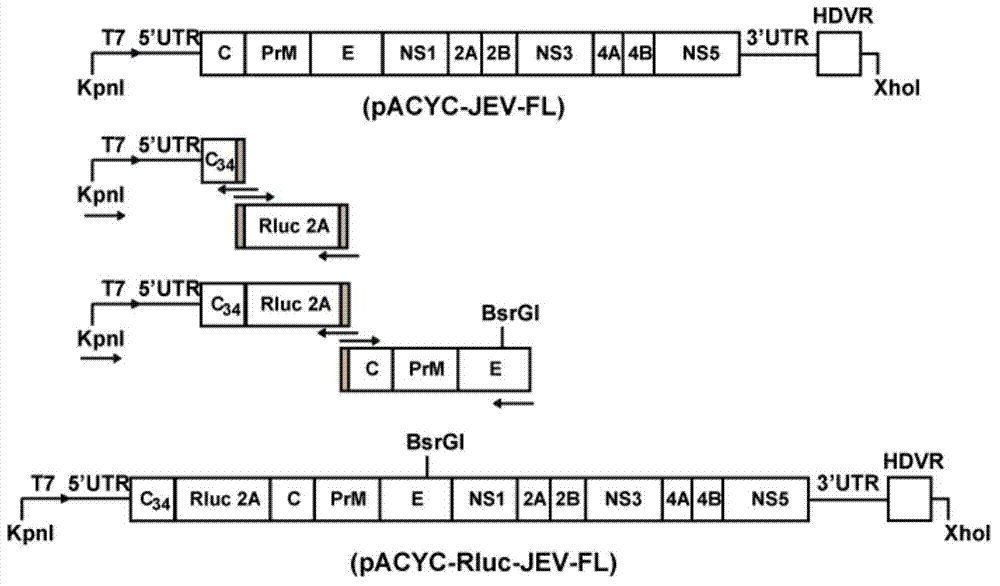
[0069] Construction of cDNA infectious clones with full-length JEV genome: select the JEV-SA14 (GenBank No.U14163.1) virus strain sequence, divide it into four fragments for gene synthesis (Golden Wisdom Biotechnology Co., Ltd.), and the four fragments are respectively Name them Fragment A, Fragment B, Fragment C, and Fragment D. Fragment A includes the sequence of "T7+genome 1-3450nt", fragment B includes the sequence of "genome 3446-5581nt", fragment C includes the sequence of "genome 5576-9136nt", and fragment D includes the sequence of "genome 9121-10976nt+HDVR" sequence. "T7" in Fragment A is a promoter, the sequence is shown in SEQ ID NO.2, and it is located before the 5'UTR of the viral genome to ensure that the constructed cDNA is transcribed in vitro to obtain genomic RNA. "H...
Embodiment 2
[0085] Infectious clones of JEV virus with luciferase gene successfully rescued the virus with the same growth tendency as wild-type virus
[0086]1. Linearization and phenol-chloroform extraction of plasmids: each take 10 μg of plasmid pACYC-JEV-FL and plasmid pACYC-Rl uc-JEV-FL, digest with XhoI (described above), digest at 37°C for two hours, and After 1% agarose gel electrophoresis to identify the complete digestion, add 100 μl of saturated phenol (purchased from Sinopharm Chemical Reagent Company) to the digested product, shake and mix, centrifuge at 17,000g for 5min, draw the upper layer into a new centrifuge tube, And add 100μl sterile water to the original centrifuge tube, shake and mix well, centrifuge at 17000g for 5min; absorb the upper layer liquid and combine with the liquid obtained in the previous step (total volume is about 200μl), add 200μl chloroform (purchased from Sinopharm Chemical Reagent Company) and mix well, 17000g Centrifuge for 5 minutes; draw the up...
Embodiment 3
[0091] A kind of application of JEV infectious clone with luciferase gene in antiviral drug screening, its steps are:
[0092] 1. Inhibitory effect of NITD008 on Rluc-JEV: Inoculate 1×10 in each well of a 12-well cell culture plate 5 BHK-21 cells at 37°C, 5% CO 2 Under culture conditions, when the confluence reached 60%, add Rluc-J EV virus solution to each well, the MOI was 0.01, 37°C, 5% CO 2 After 1 hour of adsorption in the incubator, the virus liquid in each well was discarded with a pipette tip, and NITD008 serially diluted with DMEM medium containing 2% fetal bovine serum (concentrations of 0, 0.11, 1, 3, 9 and 27μM), at 37℃, 5%CO 2 After culturing in the incubator for 48 hours, discard the supernatant in the wells, add 1ml PBS to the wells, discard the PBS in the wells, add 200μl cell lysate to treat the cells and mix the cell lysates in the wells, take 20μl in In a white 96-well plate, 50 μl of substrate was added, and the activity of Rluc was detected using a mult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com